Note: Descriptions are shown in the official language in which they were submitted.
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
METHODS FOR MODIFYING ELECTRICAL PROPERTIES OF
PAPERMAKING COMPOSITIONS USING CARBON DIOXIDE
BACKGROUND
This invention is directed to papermaking processes and systems.
More particularly, this invention is directed to adjustment of electrical
properties of papermaking compositions.
Paper is made by mixing a number of colloidal, polymeric, and solution
components and then allowing the colloidal suspension to flow through a
narrow slit onto wire gauze. The paper pulp is a pseudoplastic material with a
well-defined yield value. The magnitude of the yield stress and the way in
which the viscosity changes with shear rate are important in producing a
smooth outflow of the pulp and an appropriate thickness on the moving wire
gauze. Those flow characteristics should be monitored and adjusted if
necessary.
The colloid science covers a wide range of seemingly very different
systems. Many natural and man-made products and processes can be
characterized as being colloidal systems. For example, commercial products
such as shaving cream and paints, foods and beverages such as mayonnaise
and beer, and natural systems such as agriculture soils and biological cells
are all colloidal systems.
Colloids in simple terms are an intimate mixture of two substances.
The dispersed or colloidal phase in a finely divided state is uniformly
distributed through the second substance called the dispersion or dispersing
medium. The dispersed phase can be a gas, liquid or solid. The size of
colloidal substance present in dispersing medium can vary in size
approximately between 10 to 10,000 angstroms (1 to 1000 nanometers)(The
American Heritage Dictionary, fourth edition, Houghton Miflin Company,
p.365, 2000). The distribution of electric charge and electrostatic potential
in
the immediate neighborhood of the surface of a colloidal particle is
important.
The reason for this is that many transport properties, such as electrical
CONFIRMATION COPY
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
2
conductivity, diffusion coefficient and the flow of many systems are
determined by charge distribution.
As indicated above, a papermaking composition (or paper furnish) is
generally made up of materials (fiber, filler, etc.) and a bulk phase,
normally
water, containing dissolved and colloidally dispersed materials (salts,
polymers, dispersants, etc.). Although the overall, or average charge of the
total furnish (particulate and water phases) must be neutral (principle of
electro-neutrality). However, individual components can be positive
(cationic),
negative (anionic), or neutral. Morerover, each particle will have a specific
average charge, derived from many individual cationic and anionic sites, and
the water phase will have an "average" charge from dissolved and colloidal
matter.
The surface chemical properties of the fibers and fines depend on
chemical composition of the surface of the fiber or fine. For example, pulp
fibers resulting from mechanical and/or chemical pulping processes, when
dispersed in water, acquire a certain charge. There are several ionizable
groups that are present in wood pulp, such as hemicellulose and lignin
carboxyl groups, lignin phenolic OH groups, sugar alcohol groups, hemiacetal
groups, and lignosulphonate groups.
Fiber and fines can also acquire charge, depending upon type and
concentration of dissolved substances in the water. For example, dissolved
salts tend to have an ion-exchange behavior and resulting charge on pulp
fibers can either be negative (or) positive (or) neutral. The strength of
attraction (ion adsorption) by the carboxyl groups is a function of ion
valency
and species. The strength of attraction of wood fibers for various ions occurs
in the following order: Na+<K+<Ag+<Ca2+=Mg2+=Ba2+<AI3+ (William E.Scott,
Wet End Chemistry, TAPPI, Ed.1996, page 16.).
Additives ae equally important with respect to the above issues. Many
of the additives listed in Table I have a surface charge. The type and
intensity of charge vary based on the additive used. These chemical include
retention aids, flocculants, drainage aids, resins, dispersants, chelants,
scale
inhibitors, corrosion inhibitors, slimicides, and the like.
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
3
Table 1: Wet End Chemical Additives
= Internal sizes = Cationic flocculants
= External sizes = Alum (papermakers alum), and
= Rosins(colophony), alum substitutes such as
typically fatty organic polyaluminimum chloride,
acids, such as abietic polyaluminium hydroxychloride,
acid and polyaluminium silicate
= Rosin soaps (for sulfate
example sodium = Dyes
abietate) = Acid dyes, typically used with a
= Starch sizes dye fixing agent
= Cereal starch (corn, = Basic dyes
wheat) = Direct dyes
= Tuber starch(for = Pigment dispersions
example potato, = Liquid sulfur dyes
tapioca) = Optical brightening agents
= Unmodified starches (OBA)
= Modified starches = Diaminostilbene disulfonic acid
= Oxidized starches derivatives
= Starch (cationic/anionic) = OBA quenchers
= Amphoteric starches = Quaternary polyamides
= Starch esters = Retention aids, drainage aids
= Hydrophobic starches = Single polymer systems
= Acid modifided starches = Polyacrylamides
= Hydrolyzed starches = Polydiallyldimethylammonium
= Alklaine (neutral) sizes chloride
= Alkyl ketene dimmer = Polyethyleneimine
(AKD) = Acrylic acid/acrylamide
= Alykenyl succinic polymers
anydrided (ASA) = Dual polymer systems
= Neutral rosin sizes
= Wax(either paraffin or
microcrystalline)
= Fluorochemicals
= Dry strength resins
(such as styrene-
acrylate copolymers,
styrene-maleic anydride
copolymers,
polyacrylamides,
polyurethane, and
ol in I alcohols
The type of water used, and variations in process conditions employed,
can also influence the amount and quantity of ions present. The current
industrial trend is to minimize the use of fresh water during papermaking and
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
4
recycle more and more of the process water. Recycling the process water
increases ions built up in the system. The dissolved charges in water are
mainly due to the presence of various soluble salts present in their ionic
form,
such as sodium, calcium, chloride and sulfates.
A common method of evaluating surface charge is by determining the
zeta potential (rather tham measuring the actual surface charge). Zeta
potential is explained as the charge potential at the interface plane between
the Stern Layer and Gouy-Chapman region of an electrical double layer. The
strength of these potentials and the distance involved determine the
resistance of hydrophobic suspensions to coagulate or flocculate (William
E.Scott, Wet End Chemistry, TAPPI, Ed.1992, page 3-4). Zeta potential is
frequently used by papermakers as an indication of the state of electrokinetic
charge in the system.
The use and measurement of zeta potential offers several benefits to a
papermaker. It can provide adsorbing capacity of pulp fibers to a given
additive. It can also help to choose the type of additive required to achieve
a
charge balance. Moreover, it can be used to predict upsets by flagging
deviations from a set point.
Some representative disclosures of zeta measurement and its
advantages to papermakers include: WO 99/54741 Al (Goss et al.), EP 0 079
726 Al (Evans et al.), WO 98/12551 (Tijero Miguel), and U.S. 4,535,285
(Evans et al.), "Wet-End Chemistry of Retention, Drainage, and Formation
Aids", Pulp and Paper Manufacture, Vol. 6: Stock Preparation (Hagemeyer, R.
W., Manson, D. W., and Kocurek, M. J., ed.), Unbehend, J. E., Chap. 7: 112-
157 (1992), "Use of Potentiometric Titration and Polyelectrolyte Titration to
Measure the Surface Charge of Cellulose Fiber", Gill, R. I. S., Fundamentals
Pmkg. (Baker & Punton, ed.) Trans. 9th Fundamental Res. Symp.
(Cambridge), Vol. 1: 437-452 (Sept. 1989), "Adsorption of Ions at the
Cellulose/Aqueous Electrolyte Interface", Harrington, T. M.; Midmore, B. R,
JCS Faraday I 80, no. 6: 1525-1566 (June 1984), "SURFACE PHENOMENA",
Clark, J. d'A, Pulp Technol. & Trmt. for Paper (Miller Freeman Publns.), Chap.
4: 87-105 (1978), "ADSORPTION AND FLOCCULATION MECHANISMS IN
PAPER STOCK SYSTEMS", Britt, K. W.; Dillon, A. G.; Evans, L. A., TAPPI
Papermakers Conf. (Chicago) Paper IIA-3: 39-42 (April 18-20, 1977), and
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
ZETA-POTENTIAL MEASUREMENTS IN PAPER MANUFACTURE",
Lindstrom, T.; Soremark, C., Papier 29, no. 12: 519-525 (Dec. 1975).
The zeta potential values measured during papermaking are system
dependent and change due to process variations and upsets. Considerable
5 deviations in zeta potential from a system's optimum will affect the
production
and quality of cellulose products. Generally speaking, many have proposed
that a zeta close to zero or slightly negative is desirable. However, a
targeted
zeta potential value for a specific paper machine is a function of several
factors, such furnish type, production rates, product grades, the ambient
conditions, the particular operator on duty, the particular starting
materials,
and additives.
One way of avoiding or rectifying zeta deviations or flagged upsets is
by adjusting the papermaking process by introducing additives to various
portions/stages thereof. However, introduction of additives has significant
drawbacks. '
First, introducing additives to the process presents unknown chemical
interactions with the papermaking composition. Unforeseen chemical
reactions may result in reaction products whose effect upon the process is
undesirable. Without more knowledge of these chemical reactions, it is
difficult to adjust the process conditions to rectify the undesirable effect.
Secondly, introducing additives to the process over time creates a
buildup of the additives and of the known reaction products of the additives
and components of the papermaking composition. Once an upper limit of
concentration(s) for any or more of these is reached, the process must be
shut down. In that case, the operator may be forced to discard pulp or treat
it
so that it may be recycled. The operator may also have to drain the process
of the aqueous components of the papermaking compositions, and replenish
them with fresh water and additives. Most importantly, production is
significantly decreased.
Thirdly, introducing additives to the process also complicates the
physical interactions of fibers, colloidal species and dissolved species
within
the papermaking composition. For example, if colloids having a significant
surface charge are not suitably neutralized, they may agglomerate with
oppositely charged species, thereby resulting in flocculation at an
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
6
inappropriate time during the process. Conversely, agglomeration and
flocculation may not occur at the appropriate time, or at all, if the colloids
do
not have a sufficient charge, i.e., they remain suspended in the aqueous
phase.
Fourthly, some additives may undesirably react with various
mechanical parts in the process. Corrosion of these parts over time may lead
to mechanical breakdowns. As a result, the process must be shut down and
the part at issue repaired or replaced. This is often very costly.
Despite the above drawbacks, many have proposed addition of cationic
or anionic chemical additives. Several have proposed various strategies for
this type of modification.
U.S. 6,072,309 (Watson et al.) suggests the use of electrolytes such as
cations (including dissolved aluminum and iron cations) in order to adjust the
zeta potential.
U.S. 5,365,775 (Penniman) discloses adjustment of the zeta potential
via addition to the papemaking process of an appropriate polymer.
The abstract from "INTERFACIAL PROPERTIES OF
POLYELECTROLYTE-CELLULOSE SYSTEMS; ELECTROKINETIC
PROPERTIES OF CELLULOSE FIBERS WITH ADSORBED MONOLAYERS
OF CATIONIC POLYELECTROLYTE", Onabe, F., J. Appl. Polymer Sci. 23,
no. 10: 2909-2922 (May 15, 1979) discloses zeta-potential measurements on
acetate-grade dissolving pulp fibers with and without irreversibly adsorbed
monolayers of cationic polyelectrolyte, viz., poly(dimethyl diallyl ammonium
chloride). As the amount of adsorbed polymers increased, the negative zeta-
potential of the fibers decreased until the polarity of the zeta-potential was
reversed to the positive side. A marked change in the value of zeta-potential
was not observed when the formation of the saturated monolayer was
completed. The abstract suggests that the charge of the cellulose fibers can
be controlled until formation of a saturated monolayer of cationic
polyelectrolytes if the number of adsorbed segments per unit area of fiber
surface at saturated monolayer formation is greater than the number of
carboxyl groups per unit area of fiber surface.
The abstract for "COMPARATIVE EVALUATION OF
ELECTROKINETIC BEHAVIOR OF POLYELECTROLYTE-CELLULOSE
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
7
SYSTEMS", Onabe, F., J. Soc. Fiber Sci. Technol. Japan (Sen-i Gakkaishi)
34, no. 11: T494-504 (Nov. 1978) discloses studies conducted to elucidate the
mechanism of electrostatic charge control in pulp fibers by cationic wet-end
additives and the function of counterions in controlling the surface electric
charge. In systems with irreversibly adsorbed polymer layers, the negative
zeta-potential of fibers with monolayers reversed polarity to a positive
value,
whereas the zeta-potential for multilayers remained negative with increased
salt concentrations. Among systems containing counterions of various
valencies, the polarity of both positively and negatively charged fibers
reversed upon increase of salt concentration. Of the two systems simulating
paper-machine wet-end operation, negatively charged fibers remained
negative with increased alum additions, but reverted to a positive charge upon
increased dosage of the polyelectrolyte. Electric double-layer models are
proposed to account for the electrokinetic behavior of the systems. The
significance of specific adsorption of poiyvalent counterions for effective
charge control on the fibers is demonstrated.
The abstract for "DRAINAGE AND RETENTION MECHANISMS OF
PAPERMAKING SYSTEMS TREATED WITH CATIONIC POLYMERS",
Moore, E. E., Tappi 58, no. 1: 99-101 (Jan., 1975) discloses that optimum
drainage or retention of a papermaking system in which a drainage and
retention aid is used does not necessarily correlate with the point of charge
neutralization of the substrate surface. In a bleached pulp suspension
containing alum, drainage or retention can increase greatly with increasing
amounts of cationic polyacrylamide, even though the fiber surface has been
charge reversed. The lack of correlation of these props. with zero zeta-
potential shows that mechanisms other than charge neutralization may
predominate.
The abstract for "IMPORTANCE OF ELECTROKINETIC PROPERTIES
OF WOOD FIBER FOR PAPERMAKING", Lindstrom, T.; Soremark, C.;
Heinegard, C.; Martin-Lof, S., Conference: TAPPI Papermakers Conf.
(Boston), TAPPI Papermakers Conf. (Boston): 77-84 (June 3-6, 1974)
discloses varing of the zeta potential and thus the tendency for flocculation
by
adding cationic polyacrylamides (PAA) to dispersions of cellulosic matl.
(microcryst. cellulose sol). Optimum flocculation occurred at a zeta potential
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
8
of ca. zero. Mill trials to determine a correlation between zeta potential and
single pass retention on the wire showed increased retention as the zeta
potential was lowered.
The abstract for "RETENTION AND RETENTION AIDS", Ninck Blok, C.
J. J.; Klein, B. de, Papierwereld 22, no. 3: 69-81 (March, 1967) discloses a
clear relation of cationic retention aids adsorption to exposed fiber surface.
Zeta-potential measurements of pulp fibers as a function of adsorbed amount
of cationic retention aids show a change from negative to positive charge
values. It suggests that increased retention is probably due to changes in
zeta-potential.
The abstract for "Online Cationic-Demand Measurement for Wet-End
Papermaking", Veal, C., 1997 Engineering & Papermakers: Forming Bonds
for Better Papermaking Conference, (TAPPI Press): 287-296 (October 6,
1997; TAPPI Press) discloses optimized control of cationic materials
enhances strength properties and improves runnability, drainage, and
formation through measurement of colloidal and dissolved charge demand to
determine or detect changes in furnish charge characteristics before the stock
reaches the paper machine.
The abstract from "Starches for Surface Sizing and Wet-End Addition",
Brouwer, P. H., Wochenbl. Papierfabr. 124, no. 1: 19-23 (January 15, 1996)
discloses that paper-machine wet-end operation gives the best results when
electric charges at both the fiber surface (zeta potential) and in the aqueous
phase (soluble charge) are near zero, and suggests that suitable cationic
additives (such as polyacrylamide) be used.
Still others have proposed addition of other additives.
The abstract from "Interactions Between Cationic Starches and
Papermaking Fibers; Effect of Starch Characteristics on Fiber Surface Charge
and Starch Retention", Gupta, B. Scott, W., 1995 Papermakers Conference:
Proceedings (TAPPI): 85-96 (April 26, 1995; TAPPI Press) discloses that, in
terms of time-dependent behavior, starch DS and dosage level were the most
significant factors affecting surface charge, and suggests that, when
selecting
a starch for a particular application, starch-retention measurements should be
carried out and that starch DS and dosage levels should be the variables
manipulated.
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
9
The abstract for "INFLUENCE OF ALUM AND pH ON THE ZETA
POTENTIAL OF FIBERS AND ADDITIVES", McKenzie, A. W.; Balodis, V.;
Milgrom, A., Appita 23, no. 1: 40-4 (July, 1969) discloses that the negative
charge normally found on fibers, on starch, and on titanium dioxide could be
reversed in the presence of the Al sulfate. In most cases, the reversal of
charge resulted from the adsorption of colloidal alumina on the surface of the
fiber or the additives.
Outside of the above area of electrical properties, some have proposed
adding carbon dioxide (C02) to papermaking processes for a variety of
reasons.
WO 99/24661 Al discloses improvement of drainage of a pulp
suspension by treating it with carbon dioxide just before a dewatering device.
US 2002/0092636 Al and US 6,599,390 B2 disclose addition of carbon
dioxide in several reactors containing pulps including calcium hydroxide or
calcium oxide in order to precipitate different forms of calcium carbonate.
US 2002/0148581 Al discloses regulation of broke pH with addition of
carbon dioxide.
US 2002/0162638 Al discloses precipitation of additives in pulp
suspensions with carbon dioxide having lowered purity.
US 2002/0134519 Al discloses eliminating detrimental substances by
forming metal hydroxides through pH control with carbon dioxide.
US 6,251,356 B1 discloses precipitation of calcium carbonate from a
pressurized reactor containing calcium hydroxide or calcium oxide.
US 6,436,232 B1 and US 6,537,425 B2 disclose addition of carbon
dioxide to pulps containing calcium hydroxide in order to precipitate calcium
carbonate.
Despite these disclosures, none have recognized interaction between
carbon dioxide and electrical properties of the papermaking composition, such
as zeta potential, conductivity and electrical charge demand. None of them
have disclosed addition of carbon dioxide to papermaking compositions based
upon measurement of electrical properties of a papermaking composition,
such as zeta potential, conductivity and electrical charge demand. None have
appreciated the advantages of adding carbon dioxide upon the electrical
properties of papermaking compositions.
CA 02495006 2007-12-28
Thus, those skilled in the art will appreciate that there is a need for
more suitable additives for papermaking systems in order to adjust electrical
properties of papermaking compositions such as zeta potential, conductivity,
electrical charge demand, and streaming potential. They will also appreciate
5 that there is a need for an additive that will not tend to build up over
time such
that the papermaking process must be shut down undesirably frequently.
They will further appreciate that there is a need for an additive that will
not
adversely affect the mechanical parts of a papermaking machine. They wili
still further appreciate that there is a need for an additive that will
improve
10 properties of pulp fiber slurries, diluted pulp fiber slurries, broke,
whitewater,
paper webs and paper sheets when added to papermaking processes.
SUMMARY OF THE INVENTION
It is anaspectof the invention to provide improved methods of adjusting
electrical properties of papermaking compositions, such as zeta potential,
electrical charge demand and conductivity. It is anotheraspectto provide
improved methods of adjusting electrical properties of papermaking
compositions that employ a more suitable additive that will not tend to build
up
over time such that the papermaking process must be shut down undesirably
frequently. It is yet anotheraspectof the invention to provide improved
methods of adjusting electrical proper4es of papermaking compositions that
employ an additive that will not adversely affect the mechanical parts of a
papermaking machine. It is a furtheraspectto provide improved methods of
adjusting electrical properties of papermaking compositions that employ an
additave that will improve properties of pulp fiber sfunies, diluted pulp
fiber
slurries, broke, whitewater, paper webs and paper sheets when added to
papermaking processes.
i,n order to meet these needs and others, a method for adjusting
electrical properties of papermaking composi4ons is provided that includes
the following steps. At least one papermaking composition is provided that
includes a colloid phase, an aqueous phase, and optionally pulp fibers. Each
of the colloid phase, aqueous phase, and optional pulp fibers of one of the at
least one papermaking composition has an electrical property and an
associated value based upon the electrical property. Carbon dioxide is
CA 02495006 2007-12-28
11
introduced into at least one of the at least one papermaking composition in an
amount such that the associated electrical property value is substantially
adjusted.
Also, a method for reducing an amount of chemical additives
introduced to a papermaking composition is provided that includes the
following steps. At least one papermaking composition is provided that
includes a colloid phase, an aqueous phase, and optionally pulp fibers. Each
of the colloid phase, aqueous phase, and optional pulp fibers of one of the at
least one papermaking composition has an electrical property and an
associated value based upon the electrical property. An amount of chemical
additives is introduced into at least one of the at least one papermaking
composition. An amount of amount of carbon dioxide is introduced into the at
least one of the at least one papermaking composition into which the chemical
additives are introduced while at the same time reducing the amount of the
chemical additives. The amount of carbon dioxide is such that the associated
electrical property value is substantially adjusted.
CA 02495006 2007-12-28
lla
In accordance with an aspect of the present invention, there is provided a
method for modifying electrical properties of papermaking compositions, said
method comprising the steps of: providing at. least one papermaking
composition
comprising pulp fibers, a colloid phase and an aqueous phase, wherein each of
the fibers, the colloid phase, and the aqueous phase of one of the at least
one
papermaking composition has an electrical property and an associated value
based upon the electrical property; introducing carbon dioxide into at least
one of
the at least one papermaking composition in an amount such that the associated
electrical property value is adjusted by at least 1%; selecting a
predetermined
value or predetermined range of values based upon the electrical property;
measuring the electrical property of at least one of the fibers, the colloid
phase,
and the aqueous phase of at least one of the at least one papermaking
composition thereby obtaining a measured value; comparing the measured value
to the predetermined value or range of values; selecting the amount of the
introduced carbon dioxide based upon said comparing step, wherein the adjusted
value is closer to the predetermined value or range of values than the
measured
value.
In accordance with an aspect of the present invention, there is provided a
method of the present invention, wherein the at least one papermaking
composition further comprises solid calcium carbonateat least a portion of the
solid calcium carbonate is dissolved upon said step of introducing carbon
dioxide.
In accordance with an aspect of the present invention, there is provided a
method of the present invention, further comprising the steps of: selecting
first,
second, and third papermaking compositions as the at least one papermaking
composition, wherein the first papermaking composition is a pulp slurry, the
second papermaking composition is broke, the third papermaking composition is
whitewater which does not include a substantial amount of pulp fibers, and
CA 02495006 2007-12-28
11b
allowing the pulp fibers of the first papermaking composition to be dewatered
on
a papermaking wire downstream of the vessel, wherein the second and third
papermaking compositions are produced at the papermaking wire.
In accordance with an aspect of the present invention, there is provided a
method of the present invention, further comprising the steps of: selecting
the
first papermaking composition as the at least one papermaking composition into
which carbon dioxide is introduced.
In accordance with an aspect of the present invention, there is provided a
method of the present invention, further comprising the steps of: selecting
the
second papermaking composition as the at least one papermaking composition
into which carbon dioxide is introduced.
In accordance with an aspect of the present invention, there is provided a
method of the present invention, further comprising the steps of: selecting
the
third papermaking composition as the at least one papermaking composition into
which carbon dioxide is introduced.
In accordance with an aspect of the present invention, there is provided a
method of the present invention, further comprising: diluting the first
papermaking
composition thereby resulting in a fourth papermaking composition; selecting
the
fourth papermaking composition as the at least one papermaking composition
into which carbon dioxide is introduced.
In accordance with an aspect of the present invention, there is provided a
method of the present invention, wherein the associated electrical property
value
is based upon zeta potential.
CA 02495006 2007-12-28
Ilc
In accordance with an aspect of the present invention, there is provided a
method of the present invention, wherein the associated electrical property
value
is based upon conductivity.
In accordance with an aspect of the present invention, there is provided a
method of the present invention, wherein the associated electrical property
value
is based upon electrical charge demand.
In accordance with an aspect of the present invention, there is provided a
method of the present invention, wherein the associated zeta potential value
of
the colloid phase of at least one of the first, second, and third papermaking
compositions is negative and adjustment thereof renders it less negative.
In accordance with an aspect of the present invention, there is provided a
method of the present invention, wherein the associated zeta potential value
of at
least one of the colloid phase and the pulp fibers of at least one of the
first,
second, and third papermaking compositions is positive and adjustment thereof
renders it less positive.
In accordance with an aspect of the present invention, there is provided a
method of the present invention, wherein the associated conductivity value of
at
least one of the colloid phase and the pulp fibers of at least one of the
first,
second, and third papermaking compositions is increased by the adjustment.
In accordance with an aspect of the present invention, there is provided a
method of the present invention, wherein the associated electrical charge
demand value of at least one of the colloid phase and the pulp fibers of at
least
one of the first, second, and third papermaking compositions is decreased by
the
adjustment.
CA 02495006 2007-12-28
lid
In accordance with an aspect of the present invention, there is provided a
method of the present invention, wherein the at least one papermaking
composition into which carbon dioxide is introduced includes pulp fibers
present
at a consistency of at least 3%.
In accordance with an aspect of the present invention, there is provided a
method of present invention, wherein the at least one papermaking composition
into which carbon dioxide is introduced includes pulp fibers present at a
consistency of at least 3%.
In accordance with an aspect of the present invention, there is provided a
method of the present invention, wherein the at least one papermaking
composition into which carbon dioxide is introduced includes pulp fibers
present
at a consistency of at least 3%.
In accordance with an aspect of the present invention, there is provided a
method of the present invention, wherein the at least one papermaking
composition into which carbon dioxide is introduced includes pulp fibers
present
at a consistency of at least 3%.
In accordance with an aspect of the present invention, there is provided a
method of the present invention, further comprising the step of: controlling
the
amount of carbon dioxide introduced with a regulating device, the regulating
device perForming said comparing step.
In accordance with an aspect of the present invention, there is provided a
method of the present invention, wherein the regulating device includes a
programmable logic controller.
CA 02495006 2007-12-28
lle
In accordance with an aspect of the present invention, there is provided a
method of the present invention, further comprising the step of: diluting the
first
papermaking composition thereby resulting in a fourth papermaking composition;
providing a pulp chest for providing a supply of the first papermaking
composition; providing a headbox which receives the fourth papermaking
composition and distributes the pulp fibers therein across an upper surface of
the
paperwire, the headbox being downstream of the pulp chest; and selecting a
point whereat the carbon dioxide is introduced, the selected point being at or
downstream of the pulp chest and upstream of the headbox but not adjacent to
the headbox.
In accordance with an aspect of the present invention, there is provided a
method of the present invention, further comprising the steps of: selecting
zeta
potential as the electrical property; selecting the first papermaking
composition
as the at least one papermaking composition into which carbon dioxide is
introduced; and selecting a consistency of fibers for the first papermaking
composition of at least 3.
In accordance with an aspect of the present invention, there is provided a
method of the present invention, further comprising the steps of: diluting the
first
papermaking composition thereby resulting in the fourth papermaking
composition; selecting zeta potential as the electrical property; and
selecting the
fourth papermaking composition as the at least one papermaking composition
into which carbon dioxide is introduced.
In accordance with an aspect of the present invention, there is provided a
method of the present invention, further comprising the steps of: selecting
zeta
potential as the electrical property; and selecting the second papermaking
composition as the at least one papermaking composition into which carbon
dioxide is introduced.
CA 02495006 2007-12-28
llf
In accordance with an aspect of the present invention, there is provided a
method of present invention, further comprising the steps of: selecting zeta
potential as the electrical property; and selecting the third papermaking
composition as the at least one papermaking composition into which carbon
dioxide is introduced.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a schematic of a system suitable for performing the inventive
20 method.
FIG. 2 is a graph showing the effect upon zeta potential by CO2 and
HZSO4 for various pH ranges.
FIG. 3 is a graph showing the effect upon zeta potential of various
concentrations of various salts.
25 FIG. 4 is a graph showing the effect upon zeta by CO2 for various salt
additions.
FIG. 5 is a graph showing the effect upon zeta by the addition of CO2
and calcium carbonate.
FIG. 6 is a graph comparing the effect upon zeta by GCC and PCC at
30 various flow rates of CO2.
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
12
FIG. 7 is a graph showing the effect upon zeta by various calcium salts
in the presence of CO2.
FIG. 8 is a graph showing the effect upon zeta by a repulped
composition not containing calcium carbonate.
DETAILED DESCRIPTION OF THE INVENTION
We have surprisingly discovered that introduction of carbon dioxide into
papermaking compositions may be used to modify various electrical
properties of components in the composition. Adjustment of these electrical
properties yield many benefits for papermaking processes and systems,
paper webs, and sheet paper produced by them.
An important benefit of this invention is that it minimizes the use of
additional chemicals such as starch, polymer, etc. that are necessary to
modify the zeta potential. It also helps in minimizing additional chemical
buildup in the system. For example, if introduced in such a manner as to
minimize variations in the electrokinetic properties of pulp slurries and/or
furnishes, the addition of CO2would be beneficial. It is a well established
fact
that the electrokinetic properties of a furnish can have a significant impact
on
retention, drainage (during web formation), and paper properties. Variations
in parameters such as retention and drainage can have an immediate effect
on the tension control of the machine. This would affect dimensional stability
and can lead to non-uniform web properties and possibly web breaks (i.e.,
down time).
In the inventive method, carbon dioxide is introduced into at least one
papermaking composition, wherein each of the papermaking composition(s)
includes a colloid phase, an aqueous phase and optionally fibers. At least
one of the a colloid phase, aqueous phase and optional fibers of one of the
papermaking composition(s) has an electrical property and an associated
electrical property value based upon the electrical property. The carbon
dioxide is then introduced in an amount such that the measured electrical
property value is substantially adjusted.
The phrase, "substantially adjusted", means that the electrical property
value is adjusted at least about one percent for a an aqueous slurry of
bleached pulp fibers or two percent for an aqueous slurry of bleached pulp
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
13
fibers blended with components found in white water. It is also within the
scope of the invention for the property value to be adjusted more than
"substantially", such as an adjustment greater than about five percent.
Preferably, practice of the invention involves up to four papermaking
compositions. The first papermaking composition includes a slurry of pulp
fibers, a colloid phase and an aqueous phase. The second and third
papermaking compositions are broke and whitewater, respectively. The
fourth (optional) papermaking composition is a diluted version of the first
papermaking composition. Preferably, the first papermaking composition is
diluted to provide the fourth papermaking composition.
Any one of the papermaking compositions may be the one whose
component's electrical property is measured, and which also receives the
introduced carbon dioxide. Alternatively, the papermaking composition
(whose component's electrical property is measured) is different from the
papermaking composition that receives the carbon dioxide. Alternatively, the
carbon dioxide is introduced into at least two papermaking compositions, one
of which may or may not be the one whose component's electrical property is
measured. Preferably, the second papermaking composition is the one that
receives the carbon dioxide. Preferably, the second papermaking
composition is the one in which its component(s) electrical properties are
measured.
The electrical property includes, without limitation, zeta potential,
conductivity, electrical charge demand, streaming potential, and the like.
Preferably, the electrical property is selected from the group comprising zeta
potential, conductivity, electrical charge demand, streaming potential, and
combinations of two or three thereof. More preferably, the electrical property
is zeta potential or electrical charge demand. Most preferably, it is zeta
potential.
The electrical property and adjustments thereof may be measured by a
measuring device that reports a value based upon the electrical property.
Carbon dioxide may be introduced into any papermaking composition,
including but not limited to: a slurry of bleached pulp fibers (whether
diluted or
not); a slurry of bleached pulp fibers (whether diluted or not) combined with
whitewater; a slurry of bleached pulp fibers (whether diluted or not) combined
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
14
with broke; a slurry of bleached pulp fibers (whether diluted or not) combined
with whitewater and broke; broke; and whitewater. Also, the measuring
device may be in-line or off-line.
Since each of the components of each of the papermaking
composition(s) has an electrical property, each of these components has a
value based upon the electrical property. The phrase, "based upon", includes
without limitation, values directly reported by a measuring device (analog
values) and values mathematically derived from the analog values. In other
words, the value is an expression of the quality of the electronic property.
For
example, the electrical property of zeta potential has a value expressed in
units of mV, while the electrical property of electrical charge demand has a
value that is often expressed in terms of mL of cationic or anionic titrant.
As
another example, conductivity typically has a value expressed in units of
milliSiemens (mS), microSiemens (pS), millimhos or microhmos. As a further
example, streaming potential typically has a value expressed in units of mA or
streaming potential units (SPUs).
Each electrical property for each component of each composition is not
necessarily the same. Rather, the phrase, "wherein each of a colloid phase,
aqueous phase, and optional pulp fibers, of each of the at least one
papermaking composition has a corresponding electrical property value based
upon the electrical property" is considered to be quite inclusive of a
plurality of
combinations/permutations. It means that for each papermaking composition,
each one of the components (suspended solids, aqueous phase, and pulp
fibers (if included)) has a value for an electrical property associated with
that
component. It does not require that a same electrical property apply to each
of the components of the papermaking composition at issue. For example,
the electrical property for the pulp fibers could be zeta potential, while the
electrical property of the aqueous phase could be electrical charge demand.
As another example, the electrical property for the pulp fibers and that for
the
aqueous phase could also be the same. It also means that different
papermaking compositions (if more than one is included) need not have the
same electrical property for corresponding components. For example, in a
first papermaking composition, the electrical property of the aqueous phase
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
could be conductivity, while the electrical property of the aqueous phase in a
second papermaking composition could be electrical charge demand.
Pulp included in the invention is lignocellulosic raw material that has
undergone a pulping process. Preferably, it is bleached. Fibers are long,
5 cylindrical lignocellulosic cells, including fiber tracheids with bordered
pits and
libriform fibers with simple pits. Fibers have a length that may be
differentiated from fines. Those skilled in the art will appreciate that fines
include very short fibers, fiber fragments, ray cells or debris from
mechanical
treatment that will pass through a standard mesh screen, such as 200 mesh.
10 Types of papermaking composition contemplated by the invention
include, without limitation: a slurry of bleached pulp fibers; a slurry of
bleached
pulp fibers combined with whitewater; a slurry of bleached pulp fibers
combined with broke; a slurry of bleached pulp fibers combined with
whitewater and broke; broke; and whitewater. The slurry of bleached pulp
15 fibers, whether or not combined with whitewater and/or broke may also be
one that is diluted. Dilution may occur at any one or more of a pulp chest, a
blending chest, a machine chest, a wire pit, a refiner (such as a deaerator, a
screener and/or a cleaner), a headbox, and points therebetween. While
dilution can also occur in the short circuit of a papermaking process, it may
also occur during stock preparation.
Each of the above types of papermaking compositions includes pulp
fibers, a colloid phase and an aqueous phase, except for the whitewater
which comprises a colloid phase and an aqueous phase.
Colloids are an intimate mixture of a solid in an aqueous phase. The
colloid phase is uniformly distributed in an aqueous phase in a finely divided
state. The aqueous phase is' sometimes called the dispersion or dispersing
medium. The size of the substances in the colloid phase can vary in size
between 10 to 10,000 angstroms or larger. The colloid phase includes,
without limitation, solid inorganic compounds, solid calcium carbonate
associated with surfactants and/or crystalline modifiers, solid organic
compounds, such as polymers, liquid organic compounds insoluble with
water, fiber fines, other fines, filler particles, and sizing particles.
Crystalline
modifiers include materials which act as "seeds" around which dissolved
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
16
calcium carbonate precipitates during the process in which the solid calcium
carbonate is produced.
The aqueous phase of the papermaking composition includes various
species dissolved in water, such as cations, anions, and non-charged
species. A typical cation includes Ca++. A typical anion includes HC03 and
C032 .
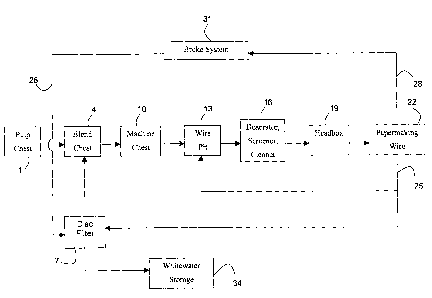
As best illustrated in FIG. 1, a typical short circuit of a papermaking
process includes the following components. Pulp from a pulp chest 1 is
provided to a blend chest 4. It should be noted that the pulp is not in dried
form, but rather exists in a slurry of pulp fibers, a colloid phase and an
aqueous phase. Thus, it is included within the meaning of "papermaking
composition". Also, while only one pulp chest is depicted, use of more than
one type of pulp or more than one pulp chest is included in the invention.
Other pulp fibers, another colloid phase containing fines, as well as an
aqueous phase from disc filter 7 are also provided to blend chest 4. The
various pulps, colloid phases and aqueous phases are blended to result in a
fiber consistency slightly lower than that of the pulp slurry in the pulp
chest.
The resultant diluted slurry is then provided to the machine chest 10, where
it
is further diluted and provided to wire pit 13 where it is even further
diluted.
This more diluted slurry is then provided to the refiner 16 where it is
deaerated, screened, and/or cleaned. From there, the refined slurry is
provided to headbox 19, where it is further diluted.
At headbox 19, the flow of diluted, refined slurry is horizontally
distributed such that when it reaches the papermaking wire 22, the flow of
diluted, refined slurry covers the entire upper surface of papermaking wire
22.
At papermaking wire 22, the diluted, refined slurry is dewatered to provide a
wet web of paper for further processing.
Much of the aqueous phase and at least some of the colloid phase is
not retained by the papermaking wire 22, but instead is collected from a lower
surface of papermaking wire 22 as whitewater 25. Whitewater 25 is recycled
back to the wire pit 13 and disc filter 7. At least some of the aqueous phase
and colloid phase from the whitewater 25 exits disc filter 7 to whitewater
storage 34, where it is used in various portions of a papermaking facility,
including pulp stock preparation. At least some of the aqueous phase and
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
17
colloid phase from the white water exits disc filter 7 to be blended with pulp
at
blend chest 4. Whitewater 25 includes a colloid phase (including fines) and
an aqueous phase.
Portions of the wet, web of paper, or a dried web of paper that are
found unsuitable are combined in mill water and/or whitewater to provide
broke 28. The broke 28 is collected at broke system 31 where it is further
refined and then provided to disc filter 7 and to blend chest 4. At least a
portion of the broke exits disc filter 7 to be blended with pulp at blend
chest 4.
Broke 28 includes pulp fibers, a colloid phase and an aqueous phase.
Those skilled in the art will recognize the method of the invention may
be performed in many other papermaking systems in which adjustment of the
electrical properties of papermaking compositions would be beneficial.
If desired, the electrical property may be measured by a suitable
measuring device. The measuring device may be off-line, such as in a
laboratory, or on-line. If an on-line measuring device is used, it may be
placed at any point in the process and system described above. Similarly, if
an off-line device is used, samples may be taken from any papermaking
composition from any point in the process and system described above. For
example, an electrical property of the pulp fibers of the broke may be
measured by placing an on-line measuring device anywhere broke is found,
or by taking a sample of the broke at any point.
There are several types of devices suitable for measuring zeta
potential. Many of these devices use any one of electrophoresis, streaming
current, streaming potential, and electro osmosis. Zeta potential
measurement devices based upon the streaming potential principle, which
include laboratory and industrial online ones, operate in the following
manner.
During the measurement, liquid is forced through a plug formed from pulp
fibers, fines and other furnish components using a pressure gradient. The
streaming potential is measured across the plug established by the flowing
liquid using electrodes placed on either side of the plug. The zeta potential
is
calculated using the following formula:
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
18
(71 * IS*x)/(Eo*6*AP)
where:
~ = Zeta Potential
IS = Streaming potential (potential between two electrodes)
x= Conductivity of the liquid
71 = Viscosity of the flowing solution
so = Electric field constant
s= Dielectric constant of the liquid
AP = Liquid pressure drop across the pad
There are several suitable devices available for measuring electrical
charge demand. As one skilled in the art will understand, electrical charge
demand is the amount of electrically charged titrant that is needed to titrate
a
sample to a zero potential. The electrical charge demand may measure any
one or more of the charged properties of polymers, colloids, and fine
particles
in a sample, as well as dissolved anions or cations.
One suitable device for measuring electrical charge demand is the
Particle Charge Detector PCD-03. It should be noted that while this device
and measurement method refer to "particle" charge, the device and method
actually measure the charge demand of the sample, in many instances, that
of dissolved ionic species. While the PCD may be used for all types of
papermaking compositions, it is often used for measurement of samples in
which the pulp fibers have been filtered out, such as pulp slurry filtrates,
broke
filtrates, and whitewater.
Measurements made with the PCD 03 are based on the following
principle. The central element is a plastic measuring cell with a fitted
displacement piston. If an aqueous sample is filled into the measuring cell,
molecules will adsorb at the plastic surface of the piston and on the cell
wall
under the action of Van der Wall forces. The counter-ions remain
comparatively free. A defined narrow gap is provided between cell wall and
piston. Driven by a motor, the piston oscillates in the measuring cell and
creates an intensive liquid flow that entrains the free counter-ions, thus
separating them from the adsorbed sample material. At the built-in
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
19
electrodes, the counter-ions induce a current which is rectified and amplified
electronically. The streaming current is shown on the display with the
appropriate sign.
For quantitative charge measurements of the sample, a Polyelectrolyte
titration has to be conducted which uses the streaming current to identify the
point of zero charge (0 mV). Available titrators include the Mutek Titrator
PCD-02 Version 1.
\With use of a titrator, an oppositely charged polyelectrolyte of known
charge density is added to the sample as a titrant. The titrant charges
neutralize existing charges of the sample. Titration is discontinued as soon
as
the point of zero charge (0 mV) is reached. Titrant consumption in mL is the
actual measured value which forms the basis for further calculations. For
anionic samples the titrant used is such as polydimethyl diallyl ammonium
chloride (Poly-Dadmac) 0.001 N .
The specific charge quantity q [eq/g] is calculated according to the
formula:
q=(V*c)/wt
where:
V = consumed titrant volume (L)
c = titrant concentration [eq/L]
wt = weight of the sample [g]
If several identical samples are to be compared, the charge quantity q
does not have to be calculated provided the samples are titrated under
identical conditions, i.e., at the same sample weight and titrant
concentrations.
In this case, the measured volume of consumed titrant in mL may be directly
used and the values obtained are directly comparable. In this context, the
terms anionic and cationic demand of a sample are in common use.
Whichever type of measuring device is selected, it may be used to
monitor the value of the electrical property in order to maintain or improve
quality production with minimal raw materials costs. However, even if the
electrical properties are carefully monitored, these measurements are less
useful if there are unsuitable methods for adjusting the values based upon the
electrical properties. In order to solve this problem, we have surprisingly
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
found that carbon dioxide may be introduced into any of the papermaking
compositions in order to adjust the electrical property value at hand. It may
be advantageously used to adjust a value that is undesirable for some reason
towards a value that is more acceptable. It can also be used to adjust an
5 electrical property value to a predetermined value or range of values, such
as
for example, a value or values that have been identified as optimal by skilled
artisans or via models.
When gaseous carbon dioxide (CO2 (g)) is introduced to an aqueous
system, such as a papermaking composition, a portion of the CO2 (g) will be
10 solubilized into free CO2 (aq), as shown in the following reaction:
CO2(9as) t-* (.02(aq)
When CO2 dissolves in water, it hydrates to yield carbonic acid (H2C03). It
should be noted that this reaction is slow (Ionic Equilibrium- Solubility and
pH
Calculations " by J. N. Butler, John Wiley & Sons, INC., 1998, chapter 10, p.
15 365). H2CO3 can dissociate into H+ and HC03 ions, as shown in the following
reaction:
H2CO3 +-* H+ + HC03
Generation of these ions is important in adjusting electrical properties of
the
pulp fibers, pulp fines, and colloids.
20 The carbon dioxide may be introduced by any method suitable for
introducing gases into papermaking compositions, including without limitation,
by pressurization or sparging.
As an example of practice of the invention, a positive zeta potential
may be made less positive. Without being bound by any particular theory, we
believe that dissolved HCO3 ions produced by hydration of carbon dioxide in
water and their subsequent disassociation thereof become attracted to
positively charged pulp fibers and/or colloids, thus lowering the positive
zeta.
Theoretically, this may continue until a zero zeta potential is reached.
Introduction of carbon dioxide is advantageous in light of prior attempts to
solve the zeta potential control problem, because it lessens the need to add
chemical additives designed to adjust the zeta potential. If carbon dioxide is
not introduced and the additive need is not decreased, these additives will
often build up in a papermaking process with the disadvantages described
above.
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
21
As another example, a negative zeta potential may be made less
negative. Often, those skilled in the art will observe that a zeta potential
at
some point in the papermaking process is unacceptably low. This is often
considered a deviation, upset or cause for attention. In that case, carbon
dioxide may be used to efficiently and effectively raise such overly negative
zeta potentials.
Additionally, with control over the amount of carbon dioxide introduced,
one skilled in the art may adjust the zeta potential towards a desired zeta
potential range or even a discrete zeta potential. Surprisingly, we have found
that, for a given pH change, the zeta potential may be adjusted by a greater
amount through carbon dioxide introduction, than by conventional additives.
In light of this disclosed invention, those skilled in the art will appreciate
and understand how to control and/or adjust a zeta potential in a portion or
portions of a papermaking process by using knowledge developed while
running papermaking processes. They will similarly be able to diagnose a
zeta potential deviation or system upset.
Practice of the invention is equally applicable with respect to an
electrical charge demand. If it is unacceptably high, introduction of carbon
dioxide into the papermaking composition unexpectedly decreases the overall
demand by a surprising amount.
Similarly, the invention may be practiced with respect to conductivity.
Surprisingly, introduction of carbon dioxide into the papermaking composition
increases it by an unexpected amount.
One skilled in the art will also understand that the streaming potential
can similarly be adjusted or controlled.
These adjustments may be achieved in an even more surprising
manner when calcium salts are present, especially calcium carbonate. The
results obtained when calcium carbonate is present do not significantly
change if the form of the calcium carbonate is different, such as precipitated
calcium carbonate (PCC) vs. ground calcium carbonate (GCC).
Furthermore, practice of this invention has also achieved startling
adjustments to pulp slurries when carbon dioxide is introduced to calcium
carbonate slurries before the calcium carbonate slurries are combined with
the pulp slurries. When this is performed, the resulting zeta potential
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
22
adjustment is much more desirable in comparison to when the calcium
carbonate is introduced without carbon dioxide.
EXAMPLES
Sample Preparation
In a first set of experiments, two different pulp slurries were used and
identified as slurry Type 1 and slurry Type 2.
Slurry Type 1: The chemically pulped and bleached hardwood (HW)
and softwood (SW) pulps used to produce this slurry were obtained from
Econotech Service, Derwent, B.C., Canada. Pulp species used included
northern hardwood, namely Aspen, and northern softwoods. The obtained
pulp sheets were refined using a Valley beater based on TAPPI test method
no (T 200 sp-96). The hardwood and softwood were refined to a freeness of
450 and 430 Canadian Standard Freeness (CSF), respectively.
The 0.5% consistency (Cy) pulp slurry Type 1, was prepared using in a
proportion of 60% HW and 40% SW. The pulp slurry was prepared using
deionized water. The mixer used to prepare the slurry was the "Square D"
mixer from IEC Controls. The resulting mixed pulp slurry was stored at 3 C.
Samples of this slurry were equilibrated to room temperature (20 2 C) before
proceeding with experimentation.
The initial properties of the Type 1 slurry are shown in Table 2A.
Table 2A: Slurry Compositions
Pulp Slurry Type 1 Type 2
Consistency 0.5 % 4.0 %
HW content 60 % 69 %
SW content 40 % 31 %
Recycled 0% 100%
Ash Content na 16.5% (525 C)-, 9.8% (900 C)*
Determined by Econotech Services.
Slurry Type 2 was generated by repulping virgin standard copy paper.
One package of 500 sheets of Office Max Premium Quality Copy Paper was
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
23
repulped in a Lamort Pulper de Laboratoire. The specifications of the copy
paper were:
~ 3-Hole Punch
~ 8.5X11 Letter size white
~ 20# Basis Weight
~ 84 Brightness
~ Acid free.
Slurry Type 2 was prepared by introducing 1,503 g of the copy paper,
and a total of 12.0 liters of hot tap water to the Lamort repulper. During the
repulping process, two mixing speed settings were used: (1) high (total mixing
time: 2 min) and (2) low (total mixing time: 8 min). The mixing speed
sequences were varied during the repulping process. What does this mean?
The repulped slurry was diluted with deionized water to produce the slurry
Type 2 having a consistency of 4.0 %.
The initial properties of the Type 2 slurry are shown in Table 2B.
Table 2B: Measured Slurry Properties
Pulp Slurry Type 1 Type 2
pH 5.35 8.90
Conductivity 0.0121 mS 0.089 mS
Zeta Potential -127.3 mV -45.3 mV
Inlet Potential 8.44 mV 3.04 mV
Pressure 0.201 bar 0.219 bar
* Initial pulp slurry properties indicated in this table (average values)
correspond to pulp slurry properties measured at different times (i.e., not
successive measurement of the same pulp slurry). When deionized water is
used to prepare the slurries, there is almost no conductivity. Therefore, this
results in very negative zeta potentials.
In the second set of experiments, two different solutions were used.
The first solution consisted of a wet end filtrate stream (filtered through
200
mesh). The second solution consisted of a 5X dilution of mill white water
(deionized water was used for dilution). Both types of solutions/filtrates
were
supplied by Abitibi-Consolidated in Beaupre, Quebec. The undiluted white
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
24
water had an extremely high conductivity and anionic charge associated with
it. Because a relatively lower conductivity and anionic charge are more
appropriately measured by the associated measuring devices the white water
was diluted 5x. Consult Table 2C for solution properties.
-
Table 2C: Mill Filtrate and Diluted White Water Properties
Mill Filtrate Diluted White Water
Properties Properties
Temperature 21.5 C * 22.6 C *
pH 7.92 8.18
Conductivity 5420 S/cm 1391 S/cm
TDS 4500 ppm (442) 962.4 ppm (442)
PCD (10.0 mL 11.976 mL Poly-Dadmac 8.594 mL Poly-Dadmac
sample) [diluted 5x] (0.001 N) (0.001 N)
* Temperature of sample during analysis.
Testing Conditions: Repeatability and Reproducibility
The zeta potential measuring device used for the testing was a "Mutek-
model no. SZP 06" meter, available from BTG Industries, Norcross, GA. In an
effort to evaluate the repeatability of the Mutek device (SZP-06), five
measurements of the same sample (500.0g) were taken. For this
repeatability test, Type 1 slurry was pH adjusted to 10.65 using NaOH (1.019
N concentration) supplied by Aldrich. The results are shown in Table 3.
Table 3: Repeatability of the Mutek SZP-06
Reading Zeta Potential Conductivity Pressure Inlet Potential
(mV) (mS) (Bar) (mV)
1 -102.5 0.151 0.195 5.79
2 -101.9 0.150 0.195 5.76
3 -102.3 0.149 0.195 5.79
-102.4 0.148 0.197 5.86
5 -100.4 0.147 0.196 5.72
avg. -101.9 0.149 0.196 5.78
std. dev. 0.87 0.002 0.001 0.051
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
In an effort to evaluate the reproducibility of the Mutek device, different
samples (5) of the same slurry preparation (slurry Type 1) were measured
5 using the Mutek device. For this particular sample, CaCO3 (Precipitated
Calcium Carbonate - PCC) was added to the pulp slurry. The slurry was
mixed in the IEC mixer at 900 rpm for a total time of 90 minutes. 15% of PCC
was added to the pulp slurry based on the initial oven dry weight of the
fiber.
10 Table 4A: Reproducibility of the Mutek SZP-06 With Slurry I
Reading Zeta Potential Conductivity Pressure Inlet Potential
(mV) (mS) (Bar) (mV)
1 -44.0 0.101 0.209 2.79
15 2 -43.6 0.102 0.207 2.73
3 -43.2 0.102 0.207 2.71
-44.2 0.101 0.206 2.76
5 -42.9 0.101 0.213 2.77
avg. -43.6 0.101 0.208 2.75
20 std. dev. 0.54 0.0005 0.003 0.032
In a further effort to evaluate the reproducibility of the Mutek device,
five portions of a pulp diluted in mill white water were measured. The pulp
25 was an 80/20 mixture of the chemically pulped and bleached HW and SW
pulps described above. The result pulp slurry was mixed in the IEC mixer at
900 rpm for a total time of 10 minutes.
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
26
Table 4B: Reproducibility of the Mutek SZP-06 With Pulp Diluted in White
Water
Beaker Zeta Conductivity Pressure Streaming Mean mV Mean
Potential Signal Pressure
mV Variation Variation
1 -29.4 4.49 203 -0.361 0.004 0.966
2 -32.5 4.44 204 -0.405 0.004 0.931
3 -33.7 4.44 201 -0.415 0.005 1
4 -36.1 4.29 202 -0.459 0.006 0.724
-32.1 4.23 203 -0.415 0.003 1.172
6 -32.5 4.31 204 -0.416 0.003 0.897
Avg -32.717 4.37 202.83 -0.41 0.004 0.948
Standard Dev 2.1858 0.104 1.17 0.03 0.001 0.146
5 Effect of pH Variations of the Pulp SlurrY
Two types of experiments were performed to investigate the effect of
pH variations on the zeta potential of the pulp slurry. For both types of
experiments, slurry Type 1 was pH adjusted to 10.20 using 1.019 N sodium
hydroxide (NaOH).
First, incremental additions of 0.1 N sulfuric acid (H2SO4) (from Aldrich)
were added to 500g of pulp slurry (slurry Type 1). After each incremental acid
addition, the pulp slurry was mixed at 700 rpm for 2 minutes using a Caframo
mixer (Model RZR-2000). Once the sample was well mixed, the pH was
measured, and the Mutek device was used to determine the zeta potential,
conductivity, inlet potential, and pressure.
Secondly, gaseous carbon dioxide (CO2) (from Air Liquide) was used to
vary the pH of the slurry. The carbon dioxide flow rate was regulated using a
mass flow controller (model MKS type 246B from MKS Instruments) and
supplied to the solution by using a'/4 inch stainless steel "dip" tube. The
pulp
was mixed using a laboratory mixer (Model RZR-2000) at 200 rpm for varying
amounts of time and CO2 flow rates (see Table 5 for CO2 flow rates and
sparging time).
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
27
Table 5: Effect of CO2 Addition on the Zeta Potential
C02 ime COZ Zeta
Sample (variable Flow Rate) (cumulative) pH Potential
(mL/min) (min) (mL) (mV)
1 na na na 10.20 -113.0
2 50 2 100 6.53 -111.4
3 50 3 250 6.08 -107.7
100 3.25 565 5.76 -106.8
350 5 2315 5.50 -106.2
6 1000 6.5 8815 5.10 -103.5
7 1400 .5 15115 .70 -96.7
8 2000 1.5 24115 1.68 -92.3
9 2000 1.5 33115 .65 -91.0
From the results in FIG. 2, it is seen that when H2SO4 was used to
acidify the slurry, a sudden modification in the zeta potential occurred at a
5 pulp slurry pH of approximately 5Ø However, previous acidification (from
pH
10.20 to pH -5.0) had an insignificant effect on the zeta potential.
Adding C02 to pulp slurry also modified the zeta potential. However, in
these experiments, it was only possible to decrease the pH from 10.20 to pH
4.65, because carbonic acid is a weak acid. CO2 addition after a pH of 4.65
did not decrease the pH, and no increase in the zeta potential was observed.
Surprisingly, the results, as best shown in FIG. 2, show that in the pH
range of 10.20 to 4.65, CO2 was more effective than H2SO4, with respect to
modifying the zeta potential of the pulp slurry. In the pH range of interest
to a
papermaker (4 to 8), the zeta potential modification was greater for a unit
change in pH when C02 was used vs. use of H2 SO4. Also, for a same zeta
potential obtained by H2SO4 in comparison to C02, a much greater drop in pH
was required by the adjustment with H2SO4. This is important because pH
changes affect many other conditions in the wet end, or short circuit, of the
papermaking process. Thus, it is evident that practice of the invention
produces results that would be greatly unexpected in comparison to those
obtained by conventional methods of addition H2S04.
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
28
To examine the effect of initial pH of the pulp slurry, an experiment
similar to the two above was performed using non-pH adjusted slurry Type I.
Incremental modifications to pH were done using CO2. The experimental
conditions used for the experiments were identical to the conditions used for
pH adjusted slurry as described earlier. Comparative results are shown in
Table 6. It should be noted that results from Table 5 are included again, in
Table 6, to show the difference in initial pH adjusting.
As the data show in Table 6, the advantageous effect of carbon dioxide
addition upon zeta potential does not depend upon an intial pH or upon pH
ranges.
Table 6: Effect of Initial pH when Supplying CO2 to Pulp Slurries
Sample CO2 (mL) pH eta CO2 (mL) pH Zeta
Potential Potential
(mV) (mV)
1 0 5.04 -129.0 0 10.20 -113.0
2 200 .65 -121.0 100 6.53 -111.4
3 400 .60 -125.5 250 6.08 -107.7
600 .53 -125.3 565 5.76 -106.8
5 1200 .41 -122.3 2315 5.50 -106.2
6 2400 .27 -122.1 8815 5.10 -103.5
7 800 .19 -116.0 15115 .70 -96.7
8 ------ ------ ------ 24115 .68 -92.3
9 ------ ------ ------ 33115 .65 -91.0
Effect of Salt Addition to the Slurry
An experiment was also performed to investigate the effect of salt
addition upon the zeta potential. Salt solutions of potassium chloride (KCI),
sodium chloride (NaCI), calcium chloride (CaC12), and aluminum chloride
(AICI3) were added to the pulp slurry Type I. To prepare the KCI, NaCL and
CaCI2 solutions, reagent grade chemicals supplied by Fisher Scientific were
dissolved in deionized water. The AICI3 solution was supplied by LabChem.
The Al concentration of the AICI3 solution was determined by Graphite
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
29
Furnace Atomic Absorption Spectrometer (GFAA), model SIMAA 6000 from
Perkin Elmer. The concentrations of the prepared solutions are shown in
Table 7.
Table 7: Concentration of Prepared Salt Solutions
Concentration of
Compound
Salt Solution
KCI 0.5 mol/L
NaCI 0.5 moI/L
CaCI2 0.5 mol/L
AICI3 13500 ppm (as Al)
The prepared solutions were added to 500.Og samples of pulp slurry
(Type 1), and mixed at 700 rpm for 5 min., using a Caframo mixer (Model
RZR-2000). After mixing, the Mutek device used to determine the zeta
potential. The results are graphically displayed in FIG. 3.
As best shown in FIG. 3, the zeta potentials of the pulp slurries vary
depending on the type of salt used, or more specifically the valency of the
corresponding cation. These results are in accordance with similar types of
experiments performed by others (A.M Scallan and J.Grignon, Svensk
Papperstidning nr2, 1979, page 40). Some have been proposed that the
cations are attracted to the negatively-charged outer surfaces of the fibers
in
suspension and, depending upon their charge and hydrated diameters, either
contract or expand the thickness of the double layer (Cohen, W.E., Farrant, G.
and Watson, A.J.: Proc. Aust. Pulp Paper Ind. Tech. Assoc.3 (1949) 72.
An experiment was also performed to investigate the effect of CO2 and
salt addition (of NaCI and CaC12) upon the zeta potential. In these
experiments, 8.8 mL of the 0.5mol/L NaCI and CaCI2 solutions were added to
the Type I slurry (corresponding to 0.0044 mol of NaCI and CaCI2). The
combination was then mixed using the Caframo mixer at 700 rpm for 5 min.
Carbon dioxide gas was introduced into the pulp slurry containing salt using a
1/4 inch stainless steel "dip" tube. The flow rate of CO2 was maintained at
500 mL/min. The slurry was mixed at 200 rpm while the CO2 was added. The
results are shown in FIG. 4. It should also be noted that in Figure 4, the
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
experiment coded as control corresponds to a pulp slurry to which no salt was
added.
The results show that the zeta potential may be adjusted by addition of
carbon dioxide whether or not salts are present.
5
Effect of Calcium Carbonate Addition
These experiments were performed using slurry Type 1. The initial pH
of the pulp slurry was adjusted to 10.65 using 1.019 N NaOH. The slurry was
pH adjusted to minimize CaCO3 dissociation in the slurry. The CaCO3 was
10 added to 500.0 g. of pulp slurry (at 0.5% consistency). The slurry was then
mixed at 700 rpm for 5 min using a Caframo mixer. Subsequently,
measurements were performed using the Mutek SZP-06 meter; also, pH was
measured. The GCC and PCC amount was added based on the oven dry
weight of pulp. The results are shown in Table 8. Ground calcium carbonate
15 (GCC) was obtained from OMYA (Omyafil), and precipitated calcium
carbonate (PCC) was obtained from Specialty Minerals Inc (Albacar HO # (A-
8-124-32)).
Table 8: Effect of Calcium Carbonate the Zeta Potential
Inlet
Ash, Zeta Conductivity Pressure
Type o0 pH mV (mS) (Bar) Potential
(mV)
Initial 0 10.65 -101.9 0.149 0.196 5.78
5 10.29 -88.7 0.154 0.200 5.11
10 10.21 -96.2 0.151 0.201 5.61
GCC
15 10.03 -100.6 0.112 0.207 6.17
30 9.88 -102.4 0.132 0.207 6.20
5 9.90 -98.2 0.113 0.207 6.06
PCC 10 np np np np np
15 10.06 -108.5 0.114 0.203 6.59
30 9.90 -111.7 0.114 0.196 6.55
20 np: not performed
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
31
As seen above, the addition of PCC or GCC initially tends to increase
the zeta potential, then decrease it. Similarly, addition of PCC or GCC
initially
tends to increase the conductivity, then decrease it. As such, addition of PCC
or GCC to a papermaking process may introduced an undesirable amount of
uncertainty in the zeta potential or conductivity.
Effect of Calcium Carbonate and Carbon Dioxide
These experiments were performed using slurry Type 1. Two different
types of calcium carbonate (CaCO3) were used for experiments to determine
the effect on zeta potential. Ground calcium carbonate (GCC) was obtained
from OMYA (Omyafil), and precipitated calcium carbonate (PCC) was
obtained from Specialty Minerals Inc (Albacar HO # (A-8-124-32)).
The CaCO3 was added to 500.0 g. of pulp slurry (at 0.5% consistency),
and the slurry was mixed at 700 rpm for 5 min using a Caframo mixer. The
calcium carbonate (GCC) 15% on pulp was based on the initial oven dry
weight of the pulp and the entire amount of calcium carbonate was added
prior to CO2 addition. Carbon dioxide gas was introduced at a flow rate of 500
mL/min. using a 1/4 inch stainless steel "dip" tube. During CO2 addition to
the
slurry, the sample was mixed at 200 rpm using a Caframo mixer. Subsequent
measurements were performed using the Mutek SZP-06 meter. Also, the pH
was measured. The results are shown in Figure 5.
In order to compare the type and source of calcium carbonate upon
CO2 addition, a comparative experiment was performed using PCC. The
carbon dioxide addition flow rate was fixed at 500 mL/min., and the initial
concentration of PCC was fixed at 15% on the oven dry weight of pulp. The
comparative results between GCC and PCC are presented in Figure 6.
To investigate the effect of introducing COZ into slurries containing
different PCC and GCC levels, the previously discussed samples (see Table
8) were utilized for experimentation. During the experiments, CO2 was added
at two different levels: 200 mL and 2400 mL. For the experiments in which
200 mL of CO2 was introduced to the slurry samples, the flow rate was 250
mL/min.; whereas for the experiments in which 2400 mL of CO2 were
introduced to the slurry samples, a flow rate of 500 mL/min was used. To mix
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
32
the slurries a Caframo mixer was used. As previously mentioned the mixing
speed during CaCO3 addition was 700 rpm for 5 min.. During CO2 addition
the mixing speed was fixed at 200 rpm. Results for these experiments are
indicated in Tables 9 and 10.
As the data show, not only have we found that carbon dioxide
introduction may be used to advantageously and surprisingly adjust the zeta
potential of a slurry, we have further found that when the slurry contains
solid
calcium carbonate, the results are even more unexpected. In this instance, it
is increased. Moreover, despite the lowering effect upon the zeta potential by
the addition of solid calcium carbonate, carbon dioxide reverses that lowering
effect and then some. Also, we have found that the effect of carbon dioxide
upon zeta potential in the presence of solid calcium carbonate does not
depend upon the form of the solid calcium carbonate, such as PCC vs. GCC.
Table 9: Effect of CO2 Addition in the Presence of PCC
Inlet
Zeta Cond. Pressure
Samples PH Potential
(mV) (mS) (Bar)
(mV)
Pulp at 0.5 % Cy 10.65 101.9 0.149 0.196 5.78
dd CaCO3- PCC (5% ash) 9.90 -98.2 0.113 0.207 6.06
200 mL C02 7.74 -55.1 0.139 0.207 3.34
2400 mL C02 6.16 -20.3 0.345 0.206 1.05
dd CaCO3- PCC (15%
ash) 10.06 -108.5 0.114 0.203 6.59
200 mL CO2 7.88 -54.7 0.146 0.205 3.26
2400 mL C02 6.62 -13.0 0.599 0.199 0.54
dd CaCO3- PCC (30%
ash) 9.90 -111.7 0.114 0.196 6.55
200 mL C02 8.00 -40.4 0.156 0.199 2.32
2400 mL CO2 6.53 -10.1 0.635 0.203 0.42
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
33
Table 10: Effect of CO2 Addition in the Presence of GCC
Inlet
Zeta Cond. Pressure
Samples PH Potential
(mV) (mS) (Bar)
(mV)
Pulp at 0.5 % Cy 10.65 101.9 0.149 0.196 5.78
dd CaCO3- GCC (5%
10.29-88.7 0.154 0.200 5.11
ash)
200 mL CO2 8.16 -73.0 0.110 0.198 .34
2400 mL C02 6.16 -24.6 0.328 0.204 1.26
dd CaCO3- GCC (10%
10.21-96.5 0.151 0.201 5.61
ash)
200 mL C02 8.60 -77.1 0.113 0.201 .64
2400 mL C02 6.45 -22.6 0.411 0.204 1.09
dd CaCO3- GCC (15% -
Na 100.6 0.112 0.207 6.20
ash)
200 mL CO2 8.02 -56.0 0.131 0.206 3.40
2400 mL C02 6.32 -18.6 0.488 0.204 0.87
dd CaCO3- GCC (30% -
9.88 102.4 0.132 0.207 6.20
ash)
200 mL CO2 8.32 -66.8 0.135 0.205 .02
2400 mL C02 na -20.7 0.558 0.207 0.92
We also investigated the effect of "reduced" CO2 addition to the slurry.
The following experiments were performed using slurry Type 1. A fixed
dosage of PCC at 15% on oven dry weight of pulp was added to the slurry.
The PCC and slurry (1 0,000g) were mixed at 900 rpm for 90 minutes using
the IEC mixer. On the slurry was prepared, CO2 was added to 500 g.
samples of the slurry/PCC mixture. The CO2 flow rate was fixed at 50 mL/min
(through ~/ inch "dip" tube) and mixing during CO2 addition was performed at
200 rpm using a Caframo mixer.
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
34
Table 11: Zeta Potential Variations in the Presence of Calcium Carbonate
Zeta Inlet Residual Ca++
Time Potential Conductivity Pressure Potential Concentration
(sec) (mV) (mS) (bar) (mV) (ppm)
0 -43.6 0.101 0.208 2.75 ---
-36.3 0.121 0.199 2.15 15
-35.1 0.132 0.198 2.05 18
-34.3 0.145 0.206 1.94 20
-33.7 0.148 0.202 1.98 22
-33.2 0.154 0.202 1.94 24
-31.7 0.163 0.202 1.84 26
90 -28.5 0.186 0.202 1.62 28
120 -25.9 0.211 0.202 1.44 31
Residual Ca2+ concentration was measured using an calcium ion
5 selective electrode (ISE) (#24502-08) distributed by Cole-Parmer
Instruments;
and the IONS 5 meter from Oakton. It should be noted that the samples were
filtered using 0.45 micron filters (from Pall Gelman Laboratory) before using
the calcium ISE. Surprisingly, the results show that when the volume of CO2
increased (as indicated by time), the zeta potential and conductivity also
10 increased. Also, the residual Ca2++ concentration increased.
Effect of Various Calcium Salts in the Presence of CO2~
In Figure 7, all previously discussed experimental data, in which calcium
15 containing salts were used, are plotted. In addition, the results of an
experiment in which calcium acetate (0.5 mol/L solution) was added to the
slurry, is also plotted. It should be noted that the one variable that was
fixed
in these experiments was the amount (concentration) of calcium added to the
500 g sample of slurry Type 1. In all the experiments show in Figure 7, the
20 amount of calcium added to a 500 g slurry sample was 0.0044 mole. As in
the previous experiments, the flow rate of CO2 was fixed at 500 mL/min. After
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
calcium acetate addition, the mixture was mixed for 5 min. at 700 rpm using a
Caframo mixer. During CO2 addition, the mixture was mixed at 200 rpm.
As shown in FIG. 7, addition of carbon dioxide unexpectedly
adjusted/increased the zeta potential when calcium salts were present. Even
5 more unexpected is the significant increase in zeta potential when solid
calcium carbonate (PCC or GCC) is present.
Effect of CO2 Addition on Recycled Furnishes:
To investigate the effect of adding CO2 to repulped slurries, slurry Type
10 2 was used. It is important to note that CaCO3 was not added to these
samples. In these experiments, CO2 was added to the slurry by using a 1/4
inch stainless steel "dip" tube. The CO2 flow rate was 750 mL/min. The first
observation that can be made, is that the zeta potential of the system is
relatively low compared to slurry Type 1 (-127.3. mV avg. vs. -45.3 mV avg).
15 This is understandable, since the repulped slurry contains a considerable
quantity of ash (i.e., CaCO3 filler). Moreover, tap water (hardness) was used
for the repulping process (i.e., to generate the 10% Cy slurry). The data are
shown in FIG. 8.
Surprisingly, addition of carbon dioxide not only adjusts/increases zeta
20 potential in slurries made from pulp, pulp containing calcium salts, pulp
containing calcium carbonate, but also does so for recycled furnishes, such as
broke.
C02 Addition to CaCO3 Prior to Mixing with the Pulp Slurries:
25 We also tested the effect of addition of carbon dioxide to calcium
carbonate slurries prior to introduction of the calcium carbonate slurry to a
pulp slurry.
First a 60/40 HW/SW blend was prepared (see Table 2B for
Properties). Next, a 10% CaCO3 (PCC) slurry was prepared (using PCC in
30 deionized water) and divided into five 200 mL samples. Next, a constant CO2
flow rate of 500 mL/min was added to each of the 200 mL PCC slurry
samples. During the CO2 addition the PCC slurry was mixed at 400 rpm
(using the Caframo mixer model RZR-2000). The carbon dioxide flow rate
was regulated using a mass flow controller (model MKS type 246B from MKS
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
36
Instruments) and was supplied to the solution by using a'/ inch stainless
steel "dip" tube. For four of the samples, the volume of carbon dioxide added
was investigated. The fifth sample was used as a control and did not receive
any carbon dioxide. The CO2 volumes were 500 mL CO2 , 2500 mL C02,
7500 mL CO2 and, 14000 mL CO2.
2.5 mL of the PCC/CO2 slurries were then added to four 500 g samples
of the pulp slurry at 0.5% Cy. After the PCC addition, the resultant slurry
was
mixed at 700 rpm for ten minutes (using the Caframo mixer). Next, the Mutek
SZP device was used to analyze the samples (pulp slurries). The pH and
temperature were also measured. The results are presented in Table 12.
Unexpectedly, the data show that the zeta potential may be increased
and the conductivity decreased from an initial pulp slurry when carbon dioxide
is first added to a calcium carbonate slurry that is later added to the pulp
slurry. Indeed, the invention is not limited to addition of carbon dioxide to
pulp
or pulp fines -containing compositions. Rather addition of carbon dioxide
may be performed upon calcium carbonate slurries which are later introduced
to the pulp or pulp fines -containing compositions with adjustments of their
electrical properties.
Table 12: CO2 Addition to CaCO3 Prior to Mixing with the Pulp Slurries
CO2 Zeta
Sample Temperature pH Conductivity
volume Potential
# (mL) ( C) (mV) (mS)
1 0 22.5 9.20 -106.0 0.0456
2 500 22.5 9.19 -103.1 0.0469
3 2500 22.5 8.85 -96.7 0.0546
4 7500 22.5 8.95 -95.9 0.0559
5 14000 22.5 8.91 -90.5 0.0627
Effect of CO2 on the PCD of a Diluted White Water Solution
To investigate the effect of adding CO2 to diluted white water (see
Table 2C for diluted white water properties), experiments were conducted in a
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
37
glass vessel reactor in which a hollow shaft mixer (i.e., hollow shaft and
hollowed Rushton turbine for gas recirculation) was used. The reactor, which
can be sealed, has an exact volume of 2,620 mL and is manufactured by
Verre- Labo Mula (France). CO2 is added through 1/4 tube immersed in the
solution (or slurry) to which a sparger has been fixed.
For each experiment, 1,000 g of the diluted (5X) white water was
introduced into the reactor. It should be noted that for these experiments,
CaCO3 was not added to the diluted white water sample. The reactor was
sealed, and then the contents were mixed at 1500 rpm. Once the reactor
contents had been mixed for 5 minutes, the CO2 was introduced to the reactor
and the contents mixed for 15 minutes at 1500 rpm. Three different CO2
dosages were investigated during this brief study. Results are shown in Table
13.
As shown in Table 13, the data surprisingly show that CO2 addition can
effectively lower the electrical charge demand even on white water.
Table 13: Effect of CO2 on the PCD and Conductivity of a Diluted White
Water Solution
CO2 Dosage (g)
Diluted White 0.1612 g 0.3240 g 1.6223 g
Water
Properties
Temperature22.6 C 22.5 C 21.8 C 21.9 C
pH 8.18 6.63 6.38 5.76
Conductivity 1391 S/cm 1374 S/cm 1364 S/cm 1372 S/cm
TDS 962.4 ppm 1005 ppm 999 ppm 1005 ppm
PCD (10.0 8.594 mL Poly- 7.578 mL Poly- 7.213 mL 6.988 mL Poly-
mL sample) Dadmac (0.001 Dadmac (0.001 Poly-Dadmac Dadmac (0.001
N) N) (0.001 N) N)
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
38
Effect of CO2 on the PCD of a Diluted White Water Solution Containing
Caco3
To investigate the effect of adding CO2 to diluted white water (see
Table 2C for diluted 1/4 tube immersed in the solution (or slurry) to which a
sparger has been fixed.
For each experiment, 990 g of the diluted (5X) white water and 10.0 g
of PCC (Albacar HO, from Specialty Minerals Inc.) were introduced into the
reactor. The reactor was sealed, and then the contents were mixed at 1500
rpm. Once the reactor contents had been mixed for 5 minutes, the CO2 was
introduced to the reactor, and the reactor contents were mixed for 15 minutes.
Three different white water properties) to which PCC has been added (prior to
carbon dioxide addition), experiments were conducted in a glass vessel
reactor in which a hollow shaft mixer (i.e., hollow shaft and hollowed Rushton
turbine for gas recirculation) was used. The reactor, which can be sealed,
has an exact volume of 2620 mL and is manufactured by Verre- Labo Mula
(France). CO2 is added through dosages were investigated during this brief
study. The results are shown in Table 14.
As shown in Table 14, the data surprisingly show that addition of CO2
to CaCO3-spiked white water will significantly raise the conductivity and
lower
the PCD. In comparison to non-CaCO3 spiked white water, addition of CO2
will lower the PCD by a much greater amount.
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
39
Table 14: Effect of CO2 on the PCD, Conductivity of diluted white water
"spiked" with CaCO3 (PCC).
C02 Dosage (g)
Diluted White 0.1621 g 0.3188 g 1.6197 g
Water
Properties (with
CaCO3)
Temperature20.8 C * 22.3 C 22.6 C 22.5 C
PH 8.77 7.57 7.28 6.70
Conductivity 1356 S/cm 1561 S/cm 1708 S/cm 2106 S/cm
TDS 994.9 ppm 1157 ppm 1264 ppm 1598 ppm
PCD (10.0 6.791 mL Poly- 5.104 mL Poly- 4.874 mL 3.060 mL Poly-
mL sample) Dadmac (0.001 Dadmac (0.001 Poly-Dadmac Dadmac (0.001
N) N) (0.001 N) N)
Effect of CO2 Comparison with HqS04:
These experiments were performed using the hollow shaft reactor
(previously described). In these experiments, CO2 was added to 1000 g of
the diluted white water and mixed for 10 minutes at 1500 rpm (using the
hollow shaft configuration). The pH, temperature, conductivity, TDS, and
PCD were recorded. Afterwards, 10.0 g. of the white water was removed
from the reactor and 10.0 g of PCC were added. The pH adjusted white
water/PCC mixture was mixed for 10 minutes and then the sample was
analyzed for pH, temperature, conductivity, TDS, and PCD.
this brief study are presented. In the table, it is shown that 0.573 g of
4.0 Normal H2SO4 were needed to get a pH of 6.39. The exact same
experiment was performed, with the exception that the CO2 was replaced by
H2SO4. In other words, the acid was used to achieve same target pH as that
obtained after CO2 addition (i.e., pH = 6.39). In Table 17, the results from
The results show that when using CO2 compared to H2SO4 to reach
same target pH level, the PCD of the sample was slightly lower when the acid
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
was used. After PCC addition, the sample in which CO2 was used for initial
pH adjustment had a much lower PCD then the acid pH adjusted sample.
The sulfuric acid used for these experiments was provided by Fisher
Scientific certified ACS at a concentration of 4 N.
5 As the data show in Table 15, addition of C02 will increase the
conductivity much more so than H2SO4 for additions reaching a same pH.
Also, addition of CO2 will increase the conductivity much more so than H2SO4
for additions reaching a same pH. Also, addition of CO2 will decrease the
electrical charge demand much more so than H2SO4 for additions reaching a
10 same pH.
Table 15: Effect of pH change agent.
Temp ( C) Cond ( S/cm) TDS(ppm) pH PCD (mL)
Baseline 22.6 1391 962.4 8.188.594
CO2 (0.3228 g C02) 20.8 1388 1020 6.39 7.848
PCC (10.0 g PCC added) 20.9 1503 1113 7.97 5.007
H2SO4 (0.573 g 4N) 21.5 1504 1114 6.39 7.491
PCC (10.0 g PCC added) 21.8 1541 1142 8.676.097
Effect of C02 on the Zeta Potential and PCD of "Dirty" Pulp Slurries
15 This experiment was performed to determine to what extent introducing
C02 to a CaCO3 containing so-called "dirty" pulp slurry would modify both the
Zeta potential and the PCD. The pulps used to prepare both slurries was
chemically pulped and bleached hardwood (HW) and softwood (SW) obtained
from an unidentifiable source located in British Columbia, and prepared by
20 Econotech Service, Derwent, B.C., Canada. Pulp species used were northern
hardwood, namely Aspen, and northern softwoods. The purchased market
pulp sheets were refined using a Valley beater based on TAPPI test method
no. T 200 sp-96. The hardwood and softwood were refined to a freeness of
461 and 451 Canadian Standard Freeness (CSF), respectively.
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
41
The pulp slurry consistency used in this experiments was 2.5%
consistency (Cy). The pulp slurry was prepared using a proportion of 80%
HW and 20% SW. The pulp slurry was prepared using a 10X dilution of the
previously mentioned white water (from mill situated in Beaupre, Quebec,
Canada [Abitibi-Consolidated]). It should be also noted that the mixer used
to prepare the slurry was the "Square D" mixer from IEC Controls.
First, the white water was diluted by ten times, i.e., 10X dilution. Next,
a pulp slurry was prepared at 2.5% Cy with a 80/20 HW/SW blend with the
dilute white water. 1300 g of the pulp slurry were added to the reactor and
mixed at 1500 rpm for 30 minutes. Baseline measurements were then taken.
13.93 g of PCC was then added and the combination mixed for 15 minutes.
The zeta potential, pH, temperature, conductivity, TDS, and PCD were
measured and recorded. Also 25 mL of sample (filtered through 200 mesh)
was taken to perform the PCD test. A CO2 dosage equivalent to 10 kg/ton
fiber were then added and mixed for 15 minutes. The zeta potential, the pH,
temperature, conductivity, TDS, and PCD were then measured recorded.
This experiment was conducted in a glass vessel reactor in which a
hollow shaft mixer (i.e., hollow shaft and hollowed Rushton turbine for gas
recirculation was used). The reactor has an exact volume of 2,620 mL and is
manufactured by Verre- Labo Mula (France). For this experiment, the reactor
was sealed during CO2 adelivery and subsequent mixing. As the data in
Table 16 show, addition of CO2 to slurries approximating those found in
papermaking processes will lower the electrical charge demand and increase
the zeta potential.
CA 02495006 2005-02-07
WO 2004/029359 PCT/IB2003/003997
42
Table 16: Effect of Carbon Dioxide upon the Zeta Potential, Conductivity, and
Electrical Charge Demand for "Dirty" Pulp Samples
Temperature Conductivity TDS Zeta PCD
( C) ( S/cm) (ppm) pH PmV ntial (mL)
~/tap water 23.4 878.4 629.2 8.20 na 2.835
10%ww
Baseline 22.3 824.7 590 7.12 -26 2 2 824
80/20 pulp slur
PCC 22.3 861.2 617.5 8.50 1.791
30% 13.93 -32.3
C02 22.4 1065.0 772.4 7.46 20.7 1.033
(10 kg/ton)
As shown above, the data unexpectedly show that slurries containing
pulp, calcium carbonate and white water had their electrical properties of
conductivity, zeta potential and electrical charge demand significantly
adjusted. In particular, the electrical charge demand and zeta potential were
lowered, while the conductivity was increased in a surprising manner.
As seen in the foregoing examples, introduction of carbon dioxide into
papermaking compositions surprisingly adjusts electrical properties of the
constituent components, and thus those of papermaking compositions. This
results in many benefits to papermakers. First, addition of carbon dioxide
will
not tend to build up over time such that the papermaking process will need to
be shut down for an undesirable amount of time. Second, addition of carbon
dioxide lessens, or maybe even eliminates, the need for costly additives
whose chemical reactivity is not known to a desirable degree of certainty.
Third, addition of carbon dioxide may be performed at many different points in
the papermaking process, such as in stock preparation, points in the short
circuit, and in calcium carbonate slurries before introduction of them into
pulp
slurries.
Those skilled in the art will understand that the scope of the invention is
not limited to the specific embodiments or examples above.