Note: Descriptions are shown in the official language in which they were submitted.
CA 02495097 2005-02-09
WO 2004/016302 PCT/US2003/024510
SAFETY SYRINGE SEALING SYSTEM AND METHOD OF USE
[0001] BACKGROUND OF INVENTION
The Needlestick Prevention Act was enacted by the United States Congress and
went
into effect on November 6, 2000 with a requirement of compliance by April 18,
2001. The
U.S. Congress directed the Occupational Safety and Health Administration,
hereinafter
"OSHA", to administer the Act. In accordance therewith, OSHA then promulgated
revised
regulations relating to the control of bloodborne pathogens (Bloodborne
Pathogens Standard,
29 C.F.R. Section 1910.1030). This revised Bloodborne Pathogens Standard
applies to all
occupational exposure to blood or other potentially infectious material. The
Needlestick
Prevention Act is an attempt to prevent the 600,000 to 800,000 inadvertent
exposures to
bloodborne pathogens that occur each year. Needlesticks occur at a rate of 300
inadvertent
exposures per 100 healthcare treatment beds each year. The Act requires
healthcare facilities
under the jurisdiction of OSHA to use safer medical devices, such as sharps
with engineered
sharps injury protection and needleless systems. This Act and other OSHA
directives require
an annual review of a facility's exposure control plan and the use of safer
medical devices to
help reduce needlesticks and other sharps injuries. The Needlestick Safety and
Prevention
Act, as well as other state rules and regulations, have encouraged the
development of "safety
syringes". The purpose of the safety syringe is to keep the tip of the needle
or fluids in the
syringe from inadvertently contacting anyone before and after a medication has
been
administered to a patient.
0002] There are a number of safety syringe designs such as those that have
retractable or
sheathed needles. One particular type of safety syringe includes an
extendable, sliding sheath
design where the sheath moves from a retracted position to an extended
position that
surrounds the needle after it has been used. Examples include the Monoject~
safety syringe,
manufactured by Kendall-LTP, also know as The Ludlow Company LP, a business of
Tyco
CA 02495097 2005-02-09
WO 2004/016302 PCT/US2003/024510
International Ltd., having a place of business at Two Ludlow Park Drive,
Chicopee,
Massachusetts 01022, "The Safety-LokTM" from Becton-Dickinson and Company,
having a
place of business at 1 Becton Drive, Franklin Lakes, New Jersey 07417, the
Gettig Guard~
syringe from Gettig Technologies, Inc., having a place of business at 1
Streamside Place,
Spring Mills, Pennsylvania 16875 and the Univec Sliding Sheath~ syringe from
Univec Inc.,
having a place of business at 22 Dubon Court, Farmingdale, New York, among
others.
0003] Safety syringes with an extendable sheath design are also disclosed in:
U.S. Patent
No. 4,994,045, issued to Ranford on February 19, 1991, which is incorporated
herein by
reference; U.S. Patent No. 4,998,924, issued to Ranford on March 12, 1991,
which is
incorporated herein by reference; U.S. Patent No. 4,743,233, issued to
Schneider on May 10,
1988, which is incorporated herein by reference; U.S. Patent No. 5,403,287,
issued to Talonn
et al. on April 4, 1995, which is incorporated herein by reference; U.S.
Patent No. 5,163,916,
issued to Sunderland on November 17, 1992, which is incorporated herein by
reference; U.S.
Design Patent No. 313,470, issued to Talonn et al. on January 1, 1991, which
is incorporated
herein by reference; and U.S. Design Patent No. 344,355, issued to Talonn et
al. on February
15, 1994, which is incorporated herein by reference.
0004] A radiopharmacy typically dispenses a liquid radiopharmaceutical in a
conventional
syringe, which is then placed in a pharmaceutical pig for transport to a
medical facility. The
pharmaceutical pig reduces unwanted exposure from the radioactive material and
protects the
conventional syringe from damage during transport. After delivery, the
pharmaceutical pig is
opened, the conventional syringe is removed and the liquid
radiopharnlaceutical is
administered to a patient. The used syringe is put back in the pharmaceutical
pig and
returned to the radiopharmacy for disposal. Residual blood and liquid
radiopharmaceutical
can leak from the conventional needle to contaminate the inside of the
pharmaceutical pig
and create an increased exposure risk. Moreover, before and during the
securing of this
2
CA 02495097 2005-02-09
WO 2004/016302 PCT/US2003/024510
contaminated conventional needle within the pharmaceutical pig, accidental
exposure to
bloodborne pathogens can occur. In fact, the "danger zone" is specifically
defined as the
time period from the point of patient injection to the point of disposing of
the syringe. Fifty
percent (50%) of all inadvertent exposures to bloodborne pathogens occur after
the patient is
injected and twenty percent (20%) occur when disposing of the syringe into a
sharps
container.
0005] Some radiopharmacies are independently owned and others are owned and
operated
in nationwide networks by Cardinal Health, Inc., having a place of business at
7000 Cardinal
Place, Dublin, Ohio 430017 and Mallinckrodt Inc., a business of Tyco
International, Ltd.,
having a place of business at 15 Hampshire Street, Mansfield, Massachusetts
02048.
Pharmaceutical pigs currently used with syringes are elongate devices sized to
enclose a
single conventional syringe that holds a dose for a single patient.
Conventional
pharmaceutical pigs are available from Biodex Medical Systems, Inc., having a
place of
business at 20 Ramsay Road, Shirley, New York 11967-0702 and are available
from Cardinal
Healthcare Ltd.
0006] SUMMARY OF INVENTION
In one aspect of this present invention, a safety syringe sealing system is
disclosed.
The system includes a combination safety syringe and plug. The safety syringe
includes a
barrel, a needle, a plunger and a tubular needle sheath. The needle sheath is
movable
between a retracted position where the needle is exposed, to an extended
position where the
needle is protected and the needle sheath defines an open end. In the
preferred embodiment,
the plug engages the outer circumference of the open end of the tubular needle
sheath. In an
alternative embodiment, the plug engages the inner circumference of the open
end of the
tubular needle sheath.
3
CA 02495097 2005-02-09
WO 2004/016302 PCT/US2003/024510
0007] In another aspect of this present invention, a safety syringe sealing
system is
disclosed. The system includes a combination pharmaceutical pig, safety
syringe and plug.
The safety syringe includes a barrel, a needle, a plunger and a needle sheath.
The needle
sheath is movable between a retracted position where the needle is exposed, to
an extended
position where the needle is protected and the needle sheath defines an open
end. The plug is
engageable with the open end of the needle sheath when the needle sheath is in
the extended
position. As mentioned above, the plug may engage the outer circumference or
the inner
circumference as well as the open end of the tubular needle sheath. The
pharmaceutical pig
includes a base and cap with the base and cap defining a hollow center section
that is capable
of receiving the safety syringe.
0008] In yet another aspect of this present invention, a method for sealing a
safety syringe is
disclosed. The method includes sliding a needle sheath longitudinally from a
retracted
position where a needle is exposed to an extended position where the needle is
protected.
The needle sheath defining an open end and engaging a plug with the open end
of the needle
sheath when the needle sheath is in the extended position. In the preferred
method, the plug
engages the outer circumference of the open end of the tubular needle sheath.
In an
alternative method, the plug engages the inner circumference of the open end
of the tubular
needle sheath.
0009] In still another aspect of this present invention, a method for plugging
a safety syringe
is disclosed. The method includes sliding a tubulax needle sheath
longitudinally from a
retracted position where a needle is exposed to an extended position where the
needle is
protected. The tubular needle sheath defining an open end, locking the tubular
needle sheath
into the extended position, and engaging a plug, having a cylindrical portion
with at least one
flange extending radially therefrom with the open end of the needle sheath in
the extended
4
CA 02495097 2005-02-09
WO 2004/016302 PCT/US2003/024510
position. As mentioned above, the plug may engage the outer circumference or
the inner
circumference of the needle sheath.
0010] In another aspect of this present invention, a method for sealing a
safety syringe is
disclosed. This method includes inserting a plug in a hollow center section of
a base of a
pharmaceutical pig having a cap, sliding a needle sheath longitudinally from a
retracted
position to an extended position where the needle is protected with the needle
sheath defining
an open end, inserting the safety syringe with the needle sheath into the
hollow center section
of the base of the pharmaceutical pig, and engaging the plug with the open end
of the needle
sheath in the extended position.
0011] In yet another aspect of this present invention, a method for sealing a
safety syringe is
disclosed. The method includes inserting a plug in a hollow center section of
a base of a
pharniaceutical pig having a cap, sliding a needle sheath longitudinally from
a retracted
position to an extended position where the needle is protected with the needle
sheath defining
an open end, engaging the plug with the open end of the needle sheath in the
extended
position, and inserting the safety syringe with the needle sheath into the
hollow center section
of the base of the pharmaceutical pig. Again, the plug may engage the outer
circumference of
the needle sheath or it may engage the inner circumference of the needle
sheath.
0012] These are merely some of the innumerable illustrative aspects of this
present
invention and should not be deemed an all-inclusive listing. These and other
aspects will
become apparent to those skilled in the art in light of the following
disclosure and
accompanying drawings.
0013] BRIEF DESCRIPTION OF DRAWINGS
For a better understanding of the present invention, reference may be made to
the
accompanying drawings in which:
CA 02495097 2005-02-09
(0014]
WO 2004/016302 PCT/US2003/024510
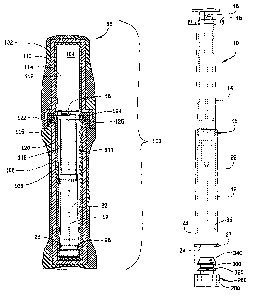
FIG. 1 is a sectional view of a medium size safety syringe, a barrel, a
needle, a
plunger and a needle sheath, wherein the needle sheath is movable between a
retracted
position where the needle is exposed to an extended position where the needle
is protected
and the needle sheath defining an open end when the extendable sheath is fully
extended. In
FIG. 1, the needle sheath is in the retracted position.
[0015] FIG. 2 is a sectional view of the safety syringe with the needle sheath
in the fully
extended position. The needle sheath and the plug are not engaged. The safety
syringe in
FIG. 2 is an example of a medium size safety syringe.
[0016] FIG. 3 is a sectional view of the safety syringe with the needle sheath
in the fully
extended position. The needle sheath and the plug are engaged. The safety
syringe in FIG. 3
is an example of a medium size safety syringe.
[0017] FIG. 4 is an enlarged side elevational view of the preferred embodiment
of the plug of
FIGS. 2 and 3.
[0018] FIG. 5 is an enlarged top view of the preferred embodiment of the plug
of FIG. 4.
[0019] FIG. 6 is an enlarged bottom view of the preferred embodiment of the
plug of FIG. 4.
[0020] FIG. 7 is an enlarged sectional view of the preferred embodiment of the
plug along
the line 7-7 of FIG. 5.
[0021] FIG. 8 is a sectional view of the safety syringe with the needle sheath
in the fully
extended position. A side elevational view of the preferred embodiment of the
plug is shown.
The needle sheath and the plug are not engaged. The safety syringe in FIG. 8
is an example of
a small size syringe.
[0022] FIG. 9 is a sectional view of the safety syringe with the needle sheath
in the fully
extended position. The needle sheath and the plug are engaged. The safety
syringe in FIG. 9
is an example of a small size syringe.
6
CA 02495097 2005-02-09
WO 2004/016302 PCT/US2003/024510
[0023] FIG. 10 is a sectional view of the safety syringe with the needle
sheath in the fully
extended position. The needle sheath and the plug are not engaged. The safety
syringe in
FIG. 10 is an example of a large size syringe.
[0024] FIG. 11 is a sectional view of the safety syringe with the needle
sheath in the fully
extended position. The needle sheath and the plug are engaged. The safety
syringe in FIG.
11 is an example of a large size syringe.
[0025] FIG. 12 is a sectional view of an assembled pharmaceutical pig, with
the safety
syringe and plug as shown in FIG. 11 positioned inside the pharmaceutical pig.
[0026] FIG. 13 is a sectional view of the safety syringe with the needle
sheath in the fully
extended position. The needle sheath and an alternative embodiment of the plug
are not
engaged.
[0027] FIG. 14 is a sectional view of a safety syringe with the needle sheath
in the fully
extended position. The needle sheath and an alternative embodiment of the plug
are engaged.
[0028] FIG. 15 is a sectional view of an assembled pharmaceutical pig, with
the safety
syringe and plug as shown in FIG. 14 positioned inside the pharmaceutical pig.
[0029] DETAILED DESCRIPTION
In the following detailed description, numerous specific details are set forth
in order
to provide a thorough understanding of the invention. However, it will be
understood by
those skilled in the art that the present invention may be practiced without
these specific
details. For example, the invention is not limited in scope to a particular
brand of safety
syringe, plug or pharmaceutical pig depicted in the Drawings.
0030] Safety syringes are produced in a wide variety of different sizes
including, but not
limited to: 1 cubic centimeter (0.06 cubic inches); 3 cubic centimeters (0.18
cubic inches); S
cubic centimeters (0.31 cubic inches); 6 cubic centimeters (0.37 cubic
inches); 10 cubic
7
CA 02495097 2005-02-09
WO 2004/016302 PCT/US2003/024510
centimeters (0.61 cubic inches); and 12 cubic centimeters (0.73 cubic inches).
An
illustrative, but nonlimiting, example of a safety syringe is the Monoject~
safety syringe,
manufactured Kendall-LTP, also know as The Ludlow Company LP, a business of
Tyco
International Ltd., having a place of business at Two Ludlow Park Drive,
Chicopee,
Massachusetts 01022. However, other types of safety syringes may be utilized
with the
present invention provided they have a needle sheath with an open end when the
needle
sheath is in the extended position. These safety syringes include, but are not
limited to "The
Safety-LokTM" from Becton-Dickinson and Company, the Gettig Guard~ syringe
from
Gettig Technologies, Inc. and the Univec Sliding Sheath~ syringe from Univec
Inc.
0031] To illustrate this invention, a Monoject~ safety syringe having a 3
cubic centimeters
(0.18 cubic inches) capacity was selected from the wide field of suitable
safety syringes and
has been generally identified by the numeral 10 as shown in Figs. 1-3. The
safety syringe 10
may sometimes hereinafter be simply referred to as the medium size safety
syringe. A
Monoject~ safety syringe having a 1 cubic centimeter (0.06 cubic inches) has
also been
selected to illustrate that the invention can be used with safety syringes of
different sizes.
The 1 cubic centimeter (0.06 cubic inches) capacity safety syringe has been
generally
identified by the numeral 9 as shown in Figs. 4 and 5. The safety syringe 9
may sometimes
hereinafter be simply referred to as the small safety syringe. To further
illustrate that this
invention can be used with a wide variety of different safety syringes, a 6
cubic centimeters
(0.37 cubic inches) Monoject~ safety syringe has also been selected and is
generally
identified by the numeral 11. The 6 cubic centimeters (0.37 cubic inches)
safety syringe 11
may sometimes hereinafter be simply referred to as the large safety syringe,
which is best
seen in Figs. 10 and 11. Because the small safety syringe 9, the medium safety
syringe 10
and the large safety syringe 11 have many similar components, similar numerals
will be used
to identify these common components throughout the drawings.
8
CA 02495097 2005-02-09
WO 2004/016302 PCT/US2003/024510
0032] As shown in FIG. 1, the medium safety syringe 10 includes a needle 12,
shown in
phantom, a barrel 14, which is also shown in phantom, a plunger 16 and finger
grips 18,
which are also known as wings. The finger grips 18 may be hexagonal, circular
or polygonal
and may fully or partially surround the barrel 14. There is a removable needle
cover 20 that
surrounds and protects the needle 12. There is an extendable, tubular sheath
22 that is in the
retracted position and surrounds the barrel 14. The extendable, tubular sheath
22 includes an
open end 24 having an outer circumference 23.
0033] FIG. 2 is a sectional view of the medium safety syringe 10 with the
extendable,
tubular sheath 22 in the fully extended position. A side elevational view of
the preferred
embodiment of the plug 26 is shown. The extendable, tubular sheath 22 and the
plug 26 are
not engaged. Prior to administration of a radiopharmaceutical to a patient,
the medium safety
syringe 10 is placed in a radiation/injection shield (not shown) and the
needle cover 20, as
shown in FIG. 1, is then removed. After administration of the
radiopharmaceutical to the
patient, the extendable, tubular sheath 22 is moved from the fully retracted
position of FIG. 1
to the fully extended position of FIG. 2, wherein the needle 12 is fully
surrounded by the
extendable, tubular sheath 22. The extendable, tubular sheath 22 is then
locked and
prevented from returning to the retracted position with a locking mechanism,
which is
generally indicated by numeral 15, by rotating the extendable, tubular sheath
22 about the
barrel 14 of the medium safety syringe 10. A locking mechanism 15 is described
in
previously referenced U.S. Patent No. 5,403,287, issued to Talonn et al. on
April 5, 1995,
which is again incorporated herein by reference.
0034] FIG. 3 is a sectional view of the medium safety syringe 10 with the
extendable,
tubular sheath 22 in the fully extended position. The extendable, tubular
sheath 22 and the
plug 26 are engaged. The outer circumference 23 of the extendable, tubular
sheath 22 has
engaged the plug 26.
CA 02495097 2005-02-09
WO 2004/016302 PCT/US2003/024510
0035] The preferred embodiment of a plug 26 will be described with reference
to FIGS. 4
and 7. This plug 26 can be of a wide variety of shapes and sizes, which
operate to close the
open end 24 of the extendable, tubular sheath 22. The preferred, but
nonlimiting,
embodiment of the plug 26 includes an end portion 28, a first cylindrical
portion 32, a second
cylindrical portion 33 and a third cylindrical portion 34. Preferably, but not
necessarily, the
first cylindrical portion 32 has the smallest diameter and the greatest
height. The first
cylindrical portion 32 can be utilized with a small safety syringe 9 having a
relatively small
volume, e.g., 1 cubic centimeter (0.06 cubic inches). Moreover, preferably but
not
necessarily, the second cylindrical portion 33 has a medium diameter and a
medium height.
The second cylindrical portion 33 can be utilized with a medium safety syringe
10 having a
medium volume, e.g., 3 cubic centimeters (0.18 cubic inches). Finally,
preferably but not
necessarily, the third cylindrical portion 34 has the largest diameter and the
lowest relative
height. The third cylindrical portion 34 can be utilized with a large safety
syringe 11 having
a relatively laxge volume, e.g., 6 cubic centimeters (0.37 cubic inches). More
than three sizes
of safety syringes and associated portions may be used in a single plug 26 and
there is no
necessity that the height of each cylindrical portion 32, 34 and 36 vary in
relation to other
cylindrical portions in any direction positive or negative.
0036] Referring now to FIGS. 4, 6 and 7, a base 28 for the plug 26 is shown.
Preferably, but
not necessarily, a biohazard symbol 37 is formed in the base 28, as shown in
FIG. 6. The
first cylindrical portion 32 defines a first u-shaped receptacle 38, which has
a first inner
circumferential wall 40, as shown in FIG. 7, that is sized to engage the outer
circumference
23 of a small safety syringe 9, as better seen in FIGS. 8 and 9. The second
cylindrical portion
33 forms a second u-shaped receptacle 42 which has a second inner
circumferential wall 44,
as shown in FIG. 7, that is sized to engage the outer circumference 23 of a
medium safety
syringe 10, as better seen in FIGS. 2 and 3. The third cylindrical portion 34
forms a third u-
CA 02495097 2005-02-09
WO 2004/016302 PCT/US2003/024510
shaped receptacle 46, which has a third inner circumferential wall 48, as
shown in FIG. 7,
that is sized to engage the outer circumference 23 of a large safety syringe
11, as better seen
in Figs. 10 and 11. Those skilled in the art will recognize that the size and
shape of the
cylindrical portions 32, 33 and 34 can be arranged to accommodate a wide
variety of safety
syringes having different sizes and the terms small, medium and large are used
merely to
illustrate that syringes of different sizes can be sealed with a single plug
26.
0037] The first cylindrical portion 32 defines a first circular lip 50 which
is sized to receive
the extendable, tubular sheath 22 of a small safety syringe 9, as better seen
in FIG. 9. The
second cylindrical portion 33 defines a second circular lip 52 which is sized
to receive the
extendable, tubular sheath 22 of the medium safety syringe 10, as better seen
in FIG. 3. The
third cylindrical portion 34 defines a third circular lip 54 which is sized to
receive the
extendable, tubular sheath 22 of a large safety syringe 1 l, as better seen in
FIG. 11. Those
skilled in the art will recognize that the respective diameters of the
circular lips 50, 52 and 54
can be arranged to accommodate a wide variety of different size syringes and
the terms small,
medium and large are used merely to illustrate that syringes of various shapes
and sizes can
be used with a single plug.
0038] The first inner circumferential wall 40 of the first u-shaped receptacle
38 of the first
cylindrical portion 32 is relatively smooth to engage the smooth outer
circumference 23 of
the needle sheath of the small safety syringe 9.
0039] The second inner circumferential wall 44 of the second u-shaped
receptacle 42 of the
second cylindrical portion 33 includes a first sealing shoulder 56 and a first
recess 58. The
first recess 58 is sized to receive and lock a circumferential shoulder 27, as
shown in FIG. 1,
extending from the extendable, tubular sheath 22 of the medium safety syringe
10 in the plug
26. The first sealing shoulder 56 is sized to engage and seal against the
outer circumference
23 of the extendable, tubular sheath 22. The purpose of the first sealing
shoulder 56 and the
11
CA 02495097 2005-02-09
WO 2004/016302 PCT/US2003/024510
first recess 58 is to lock the plug 26 and the medium safety syringe 10
together to prevent
leakage.
0040] Likewise, the third inner circumferential wall 48 of the third u-shaped
receptacle 46 of
the third cylindrical portion 34 includes a second sealing shoulder 60 and a
second recess 62.
The second recess 62 is sized to receive and lock the circumferential shoulder
27, as shown in
FIG. 1, extending from the extendable, tubular sheath 22 of the large safety
syringe 11. The
second sealing shoulder 60 is sized to engage and seal against the outer
circumference 23 of
the extendable, tubular sheath 22. Again, the purpose of the sealing shoulder
60 and the
recess 62 is to lock the plug 26 and the large safety syringe 11 together to
prevent leakage.
0041] FIG. 8 is a sectional view of a small safety syringe 9 with the
extendable, tubular
sheath 22 in the fully extended position. The extendable, tubular sheath 22
and the plug 26
are not engaged in this drawing. The outer circumference 23 of the extendable,
tubular sheath
22 of this small safety syringe 9 does not have a shoulder 27 like the medium
safety syringe
and the large safety syringe 11. This illustrates that safety syringes of
different
configurations are suitable for use with this present invention.
0042] FIG. 9 is a sectional view of the small safety syringe 9 with the
extendable, tubular
sheath 22 in the fully extended position. The extendable, tubulax sheath 22
and the plug 26
are now fully engaged.
0043] FIG. 10 is a sectional view of a large safety syringe 11 with the
extendable, tubular
sheath 22 in the fully extended position. The extendable, tubular sheath 22
and the plug 26
are not engaged in this drawing.
0044] FIG. 11 is a sectional view of the large safety syringe 11 with the
extendable, tubular
sheath 22 in the fully extended position. The extendable, tubular sheath 22
and the plug 26
are now fully engaged.
12
CA 02495097 2005-02-09
WO 2004/016302 PCT/US2003/024510
0045] FIG. 12 is a sectional view of an assembled pharmaceutical pig 36, with
the large
safety syringe 11 and plug 26, as shown in FIG. 11, positioned inside the
pharmaceutical pig
36. The pharmaceutical pig 36 includes a cap 102, having a first hollow center
section 104,
and a base 106 having a second hollow center section 108. The cap 102 and the
base 106
have a twist closure, not shown. The first hollow center section 104 and the
second hollow
center section 108 define a hollow center section 111 for the pharmaceutical
pig 36. The
needle 12, the extendable, tubular sheath 22 and the plug 26 are positioned in
the second
hollow center section 108 of the base 106. At least a portion of the plunger
16 is positioned
in the first hollow center section 104 of the cap 102.
0046] The cap 102 has an outer shell 110, an inner shell 112 and an
intermediate radiation
shield 114. The base 106 has an outer shell 116, an inner shell 118 and an
intermediate
radiation shield 120. Preferably the outer and inner shells, 110 and 112,
respectively, are
formed from plastic and the intermediate radiation shield 114 is formed from
lead or
tungsten. A seal 122 is positioned about the neck 124 of the base 106. The
seal 122 is
positioned in a channel 126 of the base 106 to provide a fluid tight seal
between the cap 102
and the base 106.
0047] FIG. 13 is a sectional view of the medium safety syringe 10 with the
extendable,
tubular sheath 22 in the fully extended position. The extendable, tubular
sheath 22 and an
alternative embodiment of the plug 260 are not engaged. The plug 260 of the
alternative
embodiment includes an end cap 280, a cylindrical portion 300, a first flange
320 and a
second flange 340. The safety syringe shown in this drawing is the same as the
safety syringe
shown in FIG. 1; therefore, the numerals used to describe the safety syringe
in FIG. 1 are the
same as those used to describe the safety syringe in this drawing. The primary
difference
between the plug 26 of the preferred embodiment (FIGS. 4-7) and the plug 260
of this
alternative embodiment is the manner in which the plugs 26, 260 engage the
extendable,
13
CA 02495097 2005-02-09
WO 2004/016302 PCT/US2003/024510
tubular sheath 22. As previously described, the plug 26 of the preferred
embodiment engages
the outside circumference 23 of the extendable, tubular sheath 22. The plug
260 of the
alternative embodiment engages the inside circumference 35 of the extendable,
tubular sheath
22 as will be better seen in FIG. 14.
0048] FIG. 14 is a sectional view of the medium safety syringe 10 of FIG. 13
with the
extendable, tubular sheath 22 in the fully extended position. The extendable,
tubular sheath
22 and the plug 260 are engaged. The inside circumference 35 of the open end
24 of the
extendable, tubular sheath 22 engages a portion of the plug 260. The outside
circumference
321 of the first flange 320 and the outside circumference 341 of the second
flange 340 engage
the inside circumference 35 of the open end 24 of the extendable, tubular
sheath 22. The end
cap 280 is mounted flush against the open end 24 to provide a liquid-tight
seal. The end cap
280 preferably has a substantially similar diameter as the extendable, tubular
sheath 22. The
combination of the medium safety syringe 10 and the plug 26 is generally
indicated by
numeral 600. This will result in the medium safety syringe 10 being sealed to
prevent
leakage of liquid radiopharmaceuticals andlor bloodborne pathogens and reduce
exposure
occurring after administration of the radiopharmaceutical to the patient.
Although not
necessary, the needle 12 is preferably removable from the medium safety
syringe 10.
0049] Refernng now to FIG. 15, which illustrates a sectional view of an
assembled
pharmaceutical pig, which is generally indicated by numeral 39. The medium
safety syringe
and the plug 260 of FIG. 14 are positioned inside the pharmaceutical pig 39.
The
combination of the pharmaceutical pig 39, the medium safety syringe 10 and the
plug 26 is
generally indicated by numeral 200.
0050] The pharmaceutical pig 39 can hold safety syringes of different volumes
of fluid, e.g.,
1 cubic centimeter (0.06 cubic inches), 3 cubic centimeters (0.18 cubic
inches), 5 cubic
centimeters (0.31 cubic inches), 6 cubic centimeters (0.37 cubic inches), 10
cubic centimeters
14
CA 02495097 2005-02-09
WO 2004/016302 PCT/US2003/024510
(0.61 cubic inches) and 12 cubic centimeters (0.73 cubic inches). Therefore,
the
pharmaceutical pig 39 can be built to accommodate the various sizes and
volumes of safety
syringes, or in the alternative, pharmaceutical pigs 39 of differing sizes can
be selected to
accommodate the various sizes and volumes of safety syringes. For example, one
size of
pharmaceutical pig 39 could accommodate the medium safety syringes 10 of 6
cubic
centimeters (0.37 cubic inches) of volume and another size of pharmaceutical
pig, not shown,
could accommodate the 10 cubic centimeters (0.61 cubic inches) and 12 cubic
centimeters
(0.73 cubic inches) safety syringes.
0051] After administration of the radiopharmaceutical to the patient, the
plunger 16 may be
fully depressed. The extendable, tubular sheath 22 is then moved to the fully
extended
position to surround the needle 12. The extendable, tubular sheath 22 is then
locked and
prevented from returning to the retracted position with the locking mechanism
15 by rotating
the extendable, tubular sheath 22 about the barrel 14 of the medium safety
syringe 10. The
plug 260 is then engaged with the inner circumference 35 of the open end 24 of
the
extendable, tubular sheath 22. The preferred, but nonlimiting, embodiment of
the plug 260
includes the cylindrical portion 300 having at least one flange extending
therefrom, e.g., the
first flange 320 and the second flange 340. The illustrative first flange 320
and illustrative
second flange 340 preferably, but not necessarily, abut the inside
circumference of the open
end 24 of the extendable, tubular sheath 22. The end cap 280 preferably has a
larger diameter
than the cylindrical portion 300 and is mounted flush against the open end 24
of the
extendable, tubular sheath 22 to provide sealing and then is placed within the
pharmaceutical
pig 39, as shown in FIG. 15.
0052] The pharmaceutical pig 39 includes a cap 420, having a first hollow
center section
380, and a base 400, having a second hollow center section 410. The first
hollow center
section 380 and the second hollow center section 410 define a hollow center
section 450 for
CA 02495097 2005-02-09
WO 2004/016302 PCT/US2003/024510
the pharmaceutical pig 39. The needle 12, extendable, tubular sheath 22 and
plug 260 are
positioned in the second hollow center section 410 of the base 400 of the
pharmaceutical pig
39. At least a portion of the plunger 16 is positioned in the first hollow
center section 380 of
the cap 420. The cap 420 threadably engages the base 400 through a series of
threaded
grooves generally indicated by numeral 440 to secure the cap 420 to the top of
the base 400.
There is an o-ring 460 that is under compression that provides a fluid-tight
seal between the
cap 420 and the base 400. The base 400 includes a radiation shield 480 and the
cap includes
a radiation shield 490. Preferably, the pharmaceutical pig 39 is a container
that has been
approved by the United States Department of Transportation.
0053] Method of Use for the Combination of Safety Syringe and Plug 26
Although the combination safety syringe 9, 10, 11 and plug 26, as shown in
FIGS. 2-3
and 8-11 indicated by numeral 60 can be utilized in any application of
medicine, it is
especially applicable to nuclear medicine. For example, with nuclear medicine,
a
prescription is provided to a radiopharmacy with the pharmacist filing the
safety syringe 9, 10
or 11 with the liquid radiopharmaceutical in accordance with the prescription.
The filled
safety syringe 9, 10 or 11 is assayed by measuring the activity of the liquid
radiopharmaceutical in the safety syringe 9, 10 or 11 with a dose calibrator
to verify
compliance with the prescription. The liquid radiopharmaceutical is then
administered to the
patient by snuggly fitting the safety syringe 9, 10 or 11 into a
radiation/injection shield (not
shown). This process includes removing the removable needle cover 20, as shown
in FIG. 1
and inserting the needle 12 into a needle port or the patient and depressing
the plunger 16.
The needle 12 can also be removed from the safety syringe 9, 10 or 11 and the
safety syringe
9, 10 or 11 can be connected to a needleless injection port. The plunger 16
does not
16
CA 02495097 2005-02-09
WO 2004/016302 PCT/US2003/024510
necessarily need to be depressed completely to the forger grips 18 although
the full dose is
often given to the patient and the grips 18 are fully depressed.
0054] After administration of the radiopharmaceutical, as shown in Figs. 2, 8
and 10, the
extendable, tubular sheath 22 is then moved to the fully extended position to
surround the
needle 12 and is then locked and prevented from returning to the retracted
position with the
locking mechanism 15 by rotating the extendable, tubular sheath 22 about the
barrel 14 of the
safety syringe 9, 10, 11.
0055] The plug 26 is then aligned with the open end 24 of the extendable,
tubular sheath 22.
As shown in FIG. 8, the open end 24 of the extendable, tubular sheath 22 of
the small safety
syringe 9 is positioned over the plug 26 so that the first cylindrical portion
32 can receive and
seal with the outer circumference 23 of the extendable, tubular sheath 22. As
shown in Figs. 2
and 3, the medium safety syringe 10 is also positioned over the plug 26 so the
second
cylindrical portion 33 can receive and seal with the outer circumference 23 of
the extendable,
tubular sheath 22. As shown in Figs. 10 and 11, the large safety syringe 11 is
likewise
positioned over the plug 26 so the third cylindrical portion 34 can receive
and seal with the
outer circumference 23 of the extendable, tubular sheath 22. Regardless of the
size of the
safety syringe 9, 10, 11, the plug 26 completely seals the needle 12 in a
leakproof relationship
to prevent any liquid radiopharmaceutical and/or bloodborne pathogen from
leaking out of
the safety syringe 9, 10, 11.
0056] This is believed to provide compliance with the revised Bloodborne
Pathogens
Standard (29 C.F.R. Sectional 1910.1030) promulgated by the Occupational
Safety and
Health Administration by fully meeting their definition of a "safe needle" by
providing a
complete and total barrier from the needle 12 that remains safe after
disposal. However the
safety syringe 9, 10, 11 utilized must be one that: provides a barrier from
the needle 12;
allows the hands of the healthcare provider to remain behind the needle 12;
has the
17
CA 02495097 2005-02-09
WO 2004/016302 PCT/US2003/024510
extendable, tubular sheath 22 function as part of the safety syringe 9, 10, 11
and not as an
accessory; and is simple to operate.
0057] Furthermore, it is also believed that the combination safety syringe 9,
10, 11 and plug
26 as indicated by numeral 60 also meets the criteria for a "safety syringe"
as defined by the
Council on Radionuclides and Radiopharmaceuticals by providing a safety
syringe 9, 10, 11
that does not leak at any time as long as the selected safety syringe 9, 10,
11: fits snuggly
within a radiation/injection shield (not shown); can be operated without
displacing the
radiation/injection shield; has a removable needle 12; and the safety syringe
9, 10, 11 can fit
within a shipping container approved by the United States Department of
Transportation.
0058] Moreover, the combination safety syringe 9, 10, 11 and plug 26 as
indicated by
numeral 60 is believed to comply with the revised Bloodborne Pathogens
Standard (29 C.F.R.
Sectional 1910.1030(d)(2)) promulgated by the Occupational Safety and Health
Administration by fully meeting their definition of a "sharps container" by
providing a
container that is: puncture resistant; capable of labeling or color-coding;
leakproof on the
sides and bottom; and does not require a healthcare provider to reach by hand
into the
container where the sharp has been placed.
0059] Method of Use for the Combination of Pharmaceutical Pig 36, Safety
Syringe and Plug
26
Although the combination of pharmaceutical pig 36, safety syringe 9, 10 and 11
and
plug 26, as shown in FIG. 12 and indicated by numeral 100, can be utilized in
any application
of medicine, it is especially applicable to nuclear medicine. For example,
with nuclear
medicine, a prescription is provided to a radiopharmacy with the pharmacist
filling the safety
syringe 9, 10, 11 with the liquid radiopharmaceutical in accordance with the
prescription.
The filled safety syringe 9, 10, 11 is assayed by measuring the activity of
the liquid
18
CA 02495097 2005-02-09
WO 2004/016302 PCT/US2003/024510
radiopharmaceutical in the safety syringe 9, 10, 11 with a dose calibrator to
verify
compliance with the prescription. The filled safety syringe 9, 10, 11 is then
placed in the
base 106 of the pharmaceutical pig 36. The cap 102 is then threaded onto the
base 106
utilizing the threaded grooves, not shown.
0060] The pharmaceutical pig 36 is then closed and wipe tested for unwanted
nuclear
activity. If the pharmaceutical pig 36 passes the wipe test, it is placed in a
delivery container
(not shown). A delivery container typically has an interior padding of rubber
foam. A
plurality of receptacles are formed in the rubber foam and each is shaped to
receive a
pharmaceutical pig 36. Several pharmaceutical pigs 36 may be placed in a
single delivery
container. Before leaving the radiopharmacy, the delivery container and the
pharmaceutical
pigs 36 are wipe tested and surveyed. If the delivery container passes, a
United States
Department of Transportation "DOT" label is affixed to the outside of the
delivery container.
The DOT label contains the radioactivity symbol and the word "Radioactive".
The delivery
container is delivered to a medical treatment facility.
0061] Upon receipt of a delivery container by the medical treatment facility,
the
pharmaceutical pig 36 is then opened and typically, the filled safety syringe
9, 10, 11 is
placed in an injection sheath (not shown). The liquid radiopharmaceutical is
then
administered to the patient. This process includes removing the removable
needle cover 20,
as shown in FIG. 1, and depressing the plunger 16. The plunger 16 does not
necessarily need
to be depressed completely to the finger grips 18, but is often fully
depressed to administer a
full dose.
0062] As shown in Figs. 2, 8 and 10 the extendable, tubular sheath 22 is then
moved to the
fully extended position to surround the needle 12 then locked and prevented
from returning to
the retracted position with the locking mechanism 15 by rotating the
extendable, tubular
sheath 22 about the barrel 14 of the safety syringe 9, 10, 11.
19
CA 02495097 2005-02-09
WO 2004/016302 PCT/US2003/024510
0063] A first method is to align the plug 26 with the open end 24 of the
extendable, tubular
sheath 22. As shown in FIG. 8, the open end 24 of the extendable, tubular
sheath 22 of the
small safety syringe 9 is positioned over the plug 26 so that the first
cylindrical portion 32
can receive and seal with the outer circumference 23 of the extendable,
tubular sheath 22. As
shown in FIGS. 2 and 3, the medium safety syringe 10 is also positioned over
the plug 26 so
the second cylindrical portion 33 can receive and seal with the outer
circumference 23 of the
extendable, tubular sheath 22. As shown in FIGS. 10 and 11, the large safety
syringe 11 is
likewise positioned over the plug 26 so the third cylindrical portion 34 can
receive and seal
with the outer circumference 23 of the extendable, tubular sheath 22.
Preferably, this is done
in a manner where the health care provider can keep his or her hands behind
the extendable,
tubular sheath 22 and not in front of the needle 12. This completely seals the
needle 12 in a
fluid-tight relationship to prevent any liquid radiopharmaceutical and/or
bloodborne pathogen
' from leaking out of the safety syringe 9, 10, 11.
0064] A second and preferred method is to insert the plug 26 into the base 106
of the
pharmaceutical pig 36. The end cap 28 will then rest against the inner shell
118 of the base 40
with the first, second and third cylindrical portions 32, 33 and 34,
respectively, pointing
upward toward the cap 102 of the pharmaceutical pig 36. The used safety
syringe 10 is then
inserted into the base 106 the pharmaceutical pig 36 so that the outer
circumference 23 of the
extendable, tubular sheath 22 engages the plug 26. The plug 26 is then
positioned flush
against the open end 24 of the extendable, tubular sheath 22. This completely
seals the
needle 12 in a fluid-tight relationship to prevent any liquid
radiopharmaceuticals and/or
bloodborne pathogens from leaking out of the safety syringe 9, 10, 11 as well
as completely
eliminating any opportunity for this used safety syringe 9, 10, 11 to
inadvertently stick
another patient. This second method is preferred since the healthcare
professional does not
have to get anywhere near the tip of the needle 12 for the safety syringe 9,
10, 11.
CA 02495097 2005-02-09
WO 2004/016302 PCT/US2003/024510
0065] After all the pharmaceutical pigs 36 have been opened and the liquid
radiopharmaceuticals have been administered, the delivery case with the
pharmaceutical pigs
36 and used safety syringes 9, 10, 11 are then returned to the radiopharmacy.
The used safety
syringes 9, 10, 11 are removed from the pharmaceutical pig 36 and placed in a
disposal bin.
The pharmaceutical pig 36 is then ready to be reused.
0066] This is believed to provide full compliance with the revised Bloodborne
Pathogens
Standard (29 C.F.R. Sectional 1910.1030) promulgated by the Occupational
Safety and
Health Administration by fully meeting their definition of a "safe needle" by
providing a
complete and total barrier from the needle 12 that remains safe after disposal
by sealing the
safety syringe 9, 10, 11 with both the plug 26 and the pharmaceutical pig 36.
However, the
safety syringe 9, 10, 11 selected must be one that: provides a barner from the
needle 12;
allows the hands of the healthcare provider to remain behind the needle 12;
has the
extendable, tubular sheath 22 that functions as part of the safety syringe 9,
10, 11 and not as
an accessory; and is simple to operate.
0067] Furthermore, it is believed that the combination safety syringe 9, 10, 1
l, plug 26 and
pharmaceutical pig 36 as indicated by numeral 100 also meets the criteria for
a safety syringe
as defined by the Council on Radionuclides and Radiopharmaceuticals by
providing a safety
syringe 9, 10, 11 that does not leak at any time as long as the selected
safety syringe 9, 10,
11: fits snuggly within a radiation/injection shield (not shown); can be
operated without
displacing the radiation/injection shield; has a removable needle 12; and can
fit within the
pharmaceutical pig 36, where the pharmaceutical pig 36 has been approved by
the U.S.
Department of Transportation.
0068] Moreover, the combination safety syringe, plug 26 and pharmaceutical pig
36 as
indicated by numeral 100 is believed to comply with the revised Bloodborne
Pathogens
Standard (29 C.F.R. Sectional 1910.1030(d)(2)) promulgated by the Occupational
Safety and
21
CA 02495097 2005-02-09
WO 2004/016302 PCT/US2003/024510
Health Administration by fully meeting their definition of a "sharps
container" by providing a
container that is: puncture resistant; capable of being labeled or color-
coded; leakproof on the
sides and bottom; and does not require a healthcare provider to reach by hand
into the
container where the sharp has been placed.
0069] A preferred embodiment of a plug is generally indicated by numeral 26 in
FIGS. 2-3,
8-11. This plug 26 can be of a wide variety of shapes and sizes, which operate
to close the
open end 24 of the extendable, tubular sheath 22.
0070] Method of Use for the Combination of Safety Syringe and Plug 260
The combination safety syringe 9, 10, 11 and plug 260 as shown in FIGS. 13 and
14
and as indicated by numeral 600 can be utilized in any application of
medicine. However,
this combination is especially applicable to nuclear medicine. For example,
with nuclear
medicine, a prescription is provided to a radiopharmacy with the pharmacist
filing the safety
syringe 9, 10, 11 with the liquid radiopharmaceutical in accordance with the
prescription. As
previously discussed, the size of the safety syringe 9, 10, 11 is typically
dependent on the
prescription. The filled safety syringe 9, 10, 11 is assayed by measuring the
activity of the
liquid radiopharmaceutical in the safety syringe 9, 10, 11 with a dose
calibrator to verify
compliance with the prescription. The safety syringe 9, 10, 11 is then put
into a
radiation/injection shield (not shown). The liquid radiopharmaceutical is then
administered
to the patient by depressing the plunger 16. The plunger 16 does not
necessarily need to be
depressed completely to the finger grips 18 but is often fully depressed to
administer a full
dose.
0071] The extendable, tubular sheath 22 is then moved to the fully extended
position to
surround the needle 12 and is then locked and prevented from returning to the
retracted
position with the locking mechanism 15 by rotating the extendable, tubular
sheath 22 about
the barrel 14 of the safety syringe 9, 10, 11.
22
CA 02495097 2005-02-09
WO 2004/016302 PCT/US2003/024510
0072] The plug 260 is then aligned with the open end 24 of the extendable,
tubular sheath 22
as shown in FIG. 13. The open end 24 of the extendable, tubular sheath 22 is
positioned over
the cylindrical portion 300 of the plug 260 so that the first flange 320 and
second flange 340
create a seal against the inside circumference 35 of the extendable, tubular
sheath 22. The
end cap 280 is then positioned flush against the open end 24 of the
extendable, tubular sheath
22. As required by regulation, this is done in a manner where the health care
provider can
keep his or her hands behind the extendable, tubular sheath 22 and not in
front of the needle
12. This completely seals the needle 12 in a leakproof relationship to prevent
any liquid
radiopharmaceutical and/or bloodborne pathogen from leaking out of the safety
syringe 9, 10,
11.
0073] This is believed to provide compliance with the revised Bloodborne
Pathogens
Standard (29 C.F.R. Sectional 1910.1030) promulgated by the Occupational
Safety and
Health Administration by fully meeting their definition of a "safe needle" by
providing a
complete and total barrier from the needle 12 that remains safe after
disposal. However the
safety syringe 9, 10, 11 utilized must be one that: provides a barrier from
the needle 12;
allows the hands of the healthcare provider to remain behind the needle 12;
has the
extendable, tubular sheath 22 function as part of the safety syringe 9, 10, 11
and not as an
accessory; and is simple to operate.
0074] Furthermore, it is also believed that the combination safety syringe 9,
10, 11 and plug
260 as indicated by numeral 600 also meets the criteria for a "safety syringe"
as defined by
the Council on Radionuclides and Radiopharmaceuticals by providing a safety
syringe 9, 10,
11 that does not leak at any time as long as the selected safety syringe: fits
snuggly within a
radiation/injection shield (not shown); can be operated without displacing the
radiation/injection shield; has a removable needle 12; and the safety syringe
9, 10, 11 can fit
within a shipping container approved by the United States Department of
Transportation.
23
CA 02495097 2005-02-09
WO 2004/016302 PCT/US2003/024510
0075] Moreover, the combination safety syringe 9, 10, 11 and plug 260 as
indicated by
numeral 600 is believed to comply with the revised Bloodborne Pathogens
Standard (29
C.F.R. Section 1910.1030 (d)(2)) promulgated by the Occupational Safety and
Health
Administration by fully meeting their definition of a "sharps container" by
providing a
container that is: puncture resistant; capable of labeling or color-coding;
leakproof on the
sides and bottom; and does not require a healthcare provider to reach by hand
into the
container where the sharp has been placed.
0076] Method of Use for the Combination of Pharmaceutical Pig 39, Safety
Syringe and Plug
260
Although the combination of pharmaceutical pig 39, safety syringe 9, 10, 11
and plug
260, as shown in FIG. 15 and indicated by numeral 200, can be utilized in any
application of
medicine, it is especially applicable to. nuclear medicine. For example, with
nuclear
medicine, a prescription is provided to a radiopharmacy with the pharmacist
filling the safety
syringe with the liquid radiopharmaceutical in accordance with the
prescription. Depending
on the prescription, the pharmacist may select a medium safety syringe 10, a
small safety
syringe 9 or a large safety syringe 11. For purposes of this example, the
pharmacist has
selected a medium size syringe 10. The filled safety syringe is assayed by
measuring the
activity of the liquid radiopharmaceutical in the safety syringe with a dose
calibrator to verify
compliance with the prescription. The filled medium safety syringe 10 is then
placed in the
base 400 of the pharmaceutical pig 39. The cap 420 is then threaded onto the
base 400
utilizing the threaded grooves 440 with the filled safety syringe 10 secured
within the second
hollow center section 410 of the base 400. The pharmaceutical pig 39 is then
closed and
wipe tested for unwanted nuclear activity. If the pharmaceutical pig 39 passes
the wipe test,
it is placed in a delivery container (not shown). A delivery container
typically has an interior
24
CA 02495097 2005-02-09
WO 2004/016302 PCT/US2003/024510
padding of rubber foam. A plurality of receptacles are formed in the rubber
foam and each is
shaped to receive a pharmaceutical pig 39. Several pharmaceutical pigs 39 may
be placed in a
single delivery container. Before leaving the radiopharmacy, the delivery
container and the
pharmaceutical pigs 39 are wipe tested and surveyed. If the delivery container
passes, a
United States Department of Transportation "DOT" label is affixed to the
outside of the
delivery container. The DOT label contains the radioactivity symbol and the
word
"Radioactive". The delivery container is delivered to a medical treatment
facility.
[0077] Upon receipt of a delivery container by the medical treatment facility,
the
pharmaceutical pig 39 is then opened and typically, the filled safety syringe
10 is placed in an
injection sheath (not shown). The liquid radiopharmaceutical is then
administered by
depressing the plunger 16. The plunger 16 does not necessarily need to be
depressed
completely to the forger grips 18 but is often fully depressed to administer a
full dose of the
radiopharmaceutical.
[0078] As shown in FIG. 13, the extendable, tubular sheath 22 is then moved to
the fully
extended position to surround the needle 12 then locked and prevented from
returning to the
retracted position with the locking mechanism 15 by rotating the extendable,
tubular sheath
22 about the barrel 14 of the safety syringe 10.
[0079] A first method is to align the plug 260 with the open end 24 of the
extendable, tubular
sheath 22. As shown in FIG. 14, the cylindrical portion 300 of the plug 260 is
then engaged
with the open end 24 of the extendable, tubular sheath 22 with the first
flange 320 and second
flange 340 creating a seal against the inside circumference 35 of the
extendable, tubular
sheath 22. The end cap 280 is then positioned flush against the open end 24 of
the
extendable, tubular sheath 22. Preferably, this is done in a manner where the
health care
provider can keep his or her hands behind the extendable, tubular sheath 22
and not in front
of the needle 12. This completely seals the needle 12 in a fluid-tight
relationship to prevent
CA 02495097 2005-02-09
WO 2004/016302 PCT/US2003/024510
any liquid radiopharmaceutical and/or bloodborne pathogen from leaking out of
the safety
syringe 10.
[0080] A second and preferred method is to insert the plug 260 into the base
400 of the
pharmaceutical pig 39. The end cap 280 will then rest against the radiation
shield 480 of the
base 400 with the cylindrical portion 300 pointing upward toward the cap 420
of the
pharmaceutical pig 39. The used safety syringe 10 is then inserted into the
base 400 of the
pharmaceutical pig 39 so that the open end 24 of the extendable, tubular
sheath 22 encloses
the cylindrical portion 300 of the plug 260 so that the first flange 320 and
second flange 340
that extend outwardly from the cylindrical portion 300 abut and form a seal
against the inside
circumference 35 of the extendable, tubular sheath 22 through the open end 24.
The end cap
280 is then positioned flush against the open end 24 of the extendable,
tubular sheath 22.
This completely seals the needle 12 in a fluid-tight relationship to prevent
any liquid
radiopharmaceuticals andlor bloodborne pathogens from leaking out of the
safety syringe 10
as well as completely eliminating any opportunity for this used safety syringe
10 to
inadvertently stick another patient. This second method is preferred since the
healthcare
professional does not have to get anywhere near the tip of the needle 12 for
the safety syringe
10.
[0081] After all the pharmaceutical pigs 39 have been opened and the liquid
radiopharmaceuticals have been administered, the delivery case with the
pharmaceutical pigs
39 and used safety syringes 10 are then returned to the radiopharmacy. The
used safety
syringes 10 are removed from the pharmaceutical pigs 39 and placed in a
disposal bin. The
pharmaceutical pig 39 is then ready to be reused.
[0082] This is believed to provide full compliance with the revised Bloodborne
Pathogens
Standard (29 C.F.R. Sectional 1910.1030) promulgated by the Occupational
Safety and
Health Administration by fully meeting their definition of a "safe needle" by
providing a
26
CA 02495097 2005-02-09
WO 2004/016302 PCT/US2003/024510
complete and total barrier from the needle 12 that remains safe after disposal
by sealing the
safety syringe 10 with both the plug 260 and the pharmaceutical pig 39.
However, the safety
syringe 10 selected must be one that: provides a barner from the needle 12;
allows the hands
of the healthcare provider to remain behind the needle 12; has the extendable,
tubular sheath
22 that functions as part of the safety syringe 10 and not as an accessory;
and is simple to
operate.
[0083] Furthermore, it is believed that the combination safety syringe 10,
plug 260 and
pharmaceutical pig 39 as indicated by numeral 200 also meets the criteria for
a safety syringe
as defined by the Council on Radionuclides and Radiopharmaceuticals by
providing a
safety syringe 10 that does not leak at any time as long as the selected
safety syringe 10: fits
snuggly within a radiation/injection shield (not shown); can be operated
without displacing
the radiation/injection shield; has a removable needle 12; and can fit within
the
pharmaceutical pig 39, where the pharmaceutical pig 39 has been approved by
the U.S.
Department of Transportation.
[0084] Moreover, the combination safety syringe 10, plug 260 and
pharmaceutical pig 39 as
indicated by numeral 200 is believed to comply with the revised Bloodborne
Pathogens
Standard (29 C.F.R. Sectional 1910.1030(d)(2)) promulgated by the Occupational
Safety and
Health Administration by fully meeting their definition of a "sharps
container" by providing a
container that is: puncture resistant; capable of being labeled or color-
coded; leakproof on the
sides and bottom; and does not require a healthcare provider to reach by hand
into the
container where the sharp has been placed.
[0085] Although a preferred embodiment of the safety syringe sealing system
and method of
use has been illustrated in the accompanying Drawings and described in the
foregoing
Detailed Description, it is understood that the invention is not limited to
the embodiment
disclosed, but is capable of numerous rearrangements, modifications and
substitutions
27
CA 02495097 2005-02-09
WO 2004/016302 PCT/US2003/024510
without departing from the spirit for the invention as set forth and defined
by the following
claims.
28