Note: Descriptions are shown in the official language in which they were submitted.
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
HAND-HELD INVENTORY TRACHING AND
AUTOMATED ORDER TRANSMISSION SYSTEM
Field of the Invention
The present invention relates to inventory tracking systems and methods for
products and particularly, to a method and system for simplifying the tracking
and
control of optical lens products, particularly, revenue and diagnostic contact
lenses,
maintained in inventory at an eye care professional's office, and for
automating the lens
product ordering process for eye care professionals and their customers.
Discussion of the Prior Art
.The advent of new manufacturing technologies has allowed new contact lens
designs and materials to be offered by manufacturers. For example,
manufacturers can
now introduce contact lenses which designs provide correction for myopia,
hyperopia,
astigmatism and presbyopia as well as vision enhancement features such UV
protection, tinted lenses and others. This proliferation of designs and
features translate
to an exponential growth on stock keeping units ("SKU's"). For example, for
spherical
lenses (correction for myopia) a single design parameter is specified. For
bifocal
contact lenses (presbyopia) two design parameters are specified. Each one of
these
parameters can vary according to the user. Thus, a spherical patient could
have a -2.5
diopter correction while another one might need a -9 diopter correction. The
incremental diopters could be in half diopters or quarter diopter. If there
are 9 diopters
offered in quarter increments then there are 36 SKU's to keep in inventory.
With the
advent of astigmatic correction (tonic lenses) there are 3 design parameters
specified.
These are a spherical power correction, a cylindrical power correction, and an
axis
correction. The combinations of spherical powers, cylindrical and axis degrees
for the
tonic lens exponentially multiplies the SKU's in the thousands of numbers.
Once tinted
lenses are added to the SKU's the reader can appreciate the proliferation of
SKU and
the associated logistics to keep inventory. Presently, many thousands of lens
products
are available and are now being introduced by ECP's to their customers for
diagnostic
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
purposes (hereinafter "diagnostic" lens products) and prescriptive purposes
(hereinafter
"revenue" lens products).
For example, ECPs are expected to fit their patients with diagnostic lenses to
assure a highly successful fitting rate. These diagnostic lenses are stored in
the ECP's
office or wherever the ECP's lens product inventory is located. Typically, the
diagnostic lens products are either in a single package or in the form of a
package strip
comprising three or more primary packages connected by a lidstock. Other
diagnostic
lenses are stored in their revenue package but dispensed as diagnostic.
Revenue lenses products, on the other hand, are filled by prescription only.
Typically, revenue lens products are ordered by phone by the office of an
optometrist
or other licensed ECP who performs eye exams and patient fittings. Most ECPs
are
expected to initiate orders for the specific product SKUs whenever the patient
needs
them. Some offices may choose to keep a small inventory, stock inventory, for
high
demand SKUs and replenish them periodically by placing a stock order.
The current practice is to offer only a portion of the total number of SKU's
for
diagnostic purposes. Whether diagnostic or revenue the lenses are typically
packaged
in primary packages, blisters, which in turn are packaged in multiples in a
secondary
package, paperboard cartons. Each carton might include multiples of 6, 12, 30
or 90
lenses, for example, individually packaged in their primary packages. Whether
diagnostic or revenue the primary packages are labeled with their printed lens
parameters, product name, expiration date, and lot number as well as the lens
brand.
This printed information can be found on a per blister basis or across
predetermined
number of blisters. Similarly, the secondary package includes the above
information as
well as FDA regulated content statements, international symbols for recycling
and UV
protection, as well as the product code (UPC-A type) and the lot number.
Currently,
only the secondary package contains barcodes for the product code and the lot
number.
Some primary packages might contain a barcode but it is not common practice
across
all products. The lens parameters might encompass any combination of
spherical,
cylindrical and add power as well as the axis according to the prescribed
product..
Currently, the ECP office maintains an inventory of revenue lens product for
those SKUs used by ECP's customers and periodically will receive and maintain
in
2
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
W ventory an amount of diagnostic lenses. However, there is currently no
systematic or
efficient way to control the amount of lenses maintained in inventory, much
less track
the usage of inventoried revenue or diagnostic lenses. For instance, the task
of
inventory management is typically performed by the ECP or a contact lens
technician,
S who, on an ad-hoc basis, assesses the inventory levels across products,
brands and
companies. Inventory level requirements on a new product are determined with
the help
of a sales representative at the time of the first order, but ongoing levels
are determined
based on the contact lens technician's or ECP staffer's judgment, i.e., ECP
staff may
haphazardly perform a manual count of the packages and/or blister packs for
specific
SKUs maintained in inventory for data processing and internal tracking
purposes. An
inventory log, however, is not routinely kept. The tracking and maintenance of
revenue
and diagnostic lens product inventories and any associated data is a difficult
task,
especially given the proliferation of new lens SKUs. Vistakon, a division of
Johnson &
Johnson, Inc., and current assignee of the present invention, offers a
customer service
program where the conflicting goals of minimal inventory and constant
availability of
product to fulfill patient orders are balanced with the aid of Doctor
Controlled Patient
Delivery ("DCPD") if the ECP office happens to run out of stock. It is
estimated that
the technician spends a minimum of four to six hours a week on the tasks of
inventory
management and order placement.
With specific regard to order placement, the re-ordering of revenue lenses
from
the manufacturer by the ECP for a specific customer, for example, requires a
verbal
description of the lens product to be ordered or manual order entry of the
patient
information including the lens product order information. This process is
tedious and
subject to human error. With the proliferation of SKU's the order process is
lengthened
to specify more parameters. This information has to be manually entered for
transmission to the order entry system of the manufacturer with increased
likelihood of
error as the number of parameters per SKU increase. Further, since the
diagnostic
lenses are provided to the ECP free of charge to fit new patients, their
quantity are
limited by the accumulated revenue lenses ordered by the ECP account.
Currently, there is a need for a uniform system that enables manufacturers to
efficiently track contact lens products disseminated to the ECPs and, enable
ECPs to
simplify their inventory control procedures for the myriad of diagnostic and
revenue
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
lenses, thus, facilitating the determination of lens availability for
dispensation to
patients. Furthermore, there is a need for a uniform system that enables ECPs
to
efficiently order lens products and reduce the occurrence of errors that would
be
prevalent by manual order entry processes.
It would thus be highly desirable to provide an efficient inventory tracking
system of revenue and diagnostic lens products for ECPs.
It would be highly desirable to provide an inventory tracking system that
enables manufacturers to track contact lens products disseminated to the ECPs
and,
enable ECPs to maintain an inventory and simplify their inventory control
procedures
for the myriad of diagnostic and revenue generating lens products available
for
dispensation to patients.
It would additionally be highly desirable to provide an efficient lens product
order entry system that simplifies and streamlines the customer service order
entry
process by automatically generating orders for diagnostic lenses and provide a
user
option for receiving a low inventory warning on revenue lenses or an automatic
order
generation based on a predetermined patient usage function.
It would additionally be desirable to provide a system an efficient lens
product
order entry system that simplifies and streamlines the customer service order
entry
process and enables the generation of lens product orders so that inventory
levels of
lens products may be automatically maintained and optimized.
It would be further highly desirable to provide ECPs with an intelligent
portable
or handheld PDA device capable of scanning unique bar-code identifiers
provided on
lens packages for use in the above desired inventory tracking system and lens
product
order entry system.
It would moreover be highly desirable to provide the above desired inventory
tracking system and automated lens product order entry system for ECPs that
utilizes
an intelligent portable or handheld bar-code scanning system in conjunction
with a
modem transmission device that interfaces with existing telephonic and
networked
communication infrastructures.
4
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
SUMMARY OF THE INVENTION
Accordingly, it is an object of the present invention to provide a dynamic and
automated inventory tracking system of revenue and diagnostic contact lenses
that is
designed to streamline the inventory tracking process.
It is another object of the present invention to provide an efficient lens
product
order entry system that simplifies and streamlines the customer service order
entry
process by automatically generating orders for revenue and diagnostic lenses
so that
inventory levels of lens products maintained by the local ECP may be
automatically
maintained and optimized.
It is a further object of the present invention to provide a system and method
for dynamic inventory tracking of revenue product inventory levels including a
customer service feature that automatically warns ECPs of low inventory levels
and
expediting initiation of a product re-order process by offering the user a
feature to
cancel or approve an order based on low inventory levels.
It is yet another object of the present invention to provide a system and
method
for dynamic inventory tracking of diagnostic product inventory which provides
a
customer service feature to automatically replenish/ship new diagnostic
product to local
ECP premises and effectively control the product diversion to unauthorized
usage.
It is yet another object of the present invention to provide a system and
method
for dynamic inventory tracking of diagnostic product inventory which provides
a
customer service feature to automatically replenish/ship new diagnostic
products to
local ECP premises and effectively control the product diversion to
unauthorized usage.
It is yet a further object of the present invention to provide a web-enabled
dynamic and automated inventory tracking system of revenue and diagnostic
contact
lenses that is designed to streamline the inventory tracking and inventory
product
ordering process.
It is still another object of the present invention to provide an efficient
lens
product order entry system that simplifies and streamlines the customer
service order
entry process by providing users with the option to receive either o~ 1) a
warning when
revenue lens products in inventory are below a pre-defined threshold, and/or
2) an auto
replenishment of a patient order based on lens usage, and/or 3) a pre-
formatted order
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
for low inventory SKU's ready for customer approval or cancellation so that
inventory
levels of lens products maintained by the ECP may be automatically maintained
and
optimized.
It is yet still another object of the present invention to provide an
inventory
tracking system that enables efficiency and improvement for tracking and
documentation of product information (after manufacturing) through its
distribution
and shipping logistics cycle, e.g., from the point of manufacturing lens
transfer to the
ECP or dispensing revenue and diagnostic lenses.
It is yet still a further object of the present invention to provide an
automated
order transmission system and methodology that is capable of assisting the
customer in
placing refill orders for lens products and to provide an automatic reordering
system to
replenish diagnostic products and provide a user feature to receive either of:
1) a low
inventory warning of revenue lens products, and/or 2) an auto replenishment of
a
patient order based on lens usage, and/or 3) an already set up order of low
inventory
SKU's ready for customer approval or cancellation by the ECP.
Thus, according to at least one embodiment of the invention, there is provided
a dynamic inventory tracking and management system comprising: a server device
for
enabling access to a database for storing lens product information, the lens
product
information including inventory data comprising types (diagnostic or revenue)
and
amounts of lens products maintained in inventory at various locations
responsible for
dispensing lenses to customers, the manufactured lens products comprising
packages
having readable indicia for directly indicating the lens product information
or providing
reference to associated lens product information included in the database;
a device provided at each location for scanning indications provided on
packages of lens products dispensed to customers at the respective location
and
obtaining inventory tracking information including a lens product type
identifier and a
quantity dispensed for each lens product type associated with a location;
a mechanism for enabling direct communication of scanned inventory tracking
information obtained at each location to the database server over a
communication
network; and,
a mechanism for tracking each inventory maintained at the various locations
based on the scanned inventory tracking information, said tracking mechanism
6
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
updating quantities of lens products dispensed at each location for each lens
product
type with quantities maintained in said inventory levels for each location,
and reporting
updated inventory lens product inventory level information for use at each
location.
Further implemented is a mechanism for authenticating the location prior to
S receiving directly communicated inventory tracking information from the
device at
each location. Additionally, a mechanism is implemented for confirming
inventory
received or shipped at the various locations based on the scanned inventory
tracking
information, the tracking mechanism updating quantities of lens products
received at or
shipped from each location for each lens product type with quantities
maintained in
said inventory levels for each location, and reporting updated inventory lens
product
inventory level information for use at each location.
Preferably, the contact lens products include revenue and diagnostic lenses
provided in packages of the following types: a primary package, a blister
pack, vial, a
secondary package, carton, tray, or plastic bag. The readable indicia provided
on the
package may comprise a unique barcode identifier indicating and/or
electronically
referencing in the database the lens product, its lens parameters, package
quantity, lot
number and SKU and product code. Preferably, the device provided at each
location for
scanning indications provided on packages of revenue and diagnostic lens
products
dispensed in the system is accomplished by means of a hand-held PC or PDA
device
equipped with scanner. Besides obtaining inventory tracking information
including a
lens product type and a quantity dispensed for each lens product type, the PDA
device
is enabled to track information including a reason for dispensing the product.
Advantageously, the automated order transmission system reduces the
customer's ECP office labor and provides better order accuracy than the
current process
of verbally describing the lens product SKU's and its parameters to the
manufacturer's
customer service representatives. Furthermore, the automated system and method
for
dynamic inventory tracking of inventory optimizes human labor in counting and
tracking product, reconciling inventory physical inventory with logical
inventory,
streamlines documentation product information to a one time 'point of entry',
and
minimizes human error during electronic documentation of inventory, lens
information
and FDA regulated information.
7
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
BRIEF DESCRIPTION OF THE DRAWINGS
Details of the invention disclosed herein shall be described below, with the
aid
of the figures listed below, in which:
Figure 1 is an example system architecture underlying the dynamic inventory
and tracking and management system of the invention;
Figure 2 is a more detailed view of the order entry processing component
including the re-ordering process steps in response to selection of a "Place
New Order"
button depicted in Figures 5(b) and 5(c) according to the invention;
Figures 3(a)-3(d) depict the process steps for inventory tracking and
management according to the principles of the invention including the process
flow for
an ECP Office dispensing lenses and, the system algorithms for inventory
update,
inventory management, processing of ECP's ordering preferences, and processing
of
orders and order fulfillment;
Figure 4 depicts the initial system setup and product storage occurring after
manufacturing of the lens product;
Figure 5(a) is an exemplary diagram depicting the GUI interface provided by
the PDA equipped with bar code scanner enabling an ECP personnel to select the
reason for dispensing lenses; diagnostic or revenue lenses;
Figure 5(b) is an exemplary diagram depicting the GUI interface provided by
the PDA equipped with bar code scanner for displaying tracked inventory levels
for
product types and enabling ECP personnel to review low inventory warning
levels;
8
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
Figure 5(c) is an exemplary diagram depicting the GIJI interface provided by
the PDA equipped with bar code scanner for displaying tracked inventory levels
for
product types and enabling ECP personnel to initiate re-orders or place new
orders;
Figure 6 is a detailed block diagram depicting an example process 160 for
inventory tracking and automated order entry according to the principles of
the
invention after the user selects the "Place New Order" button depicted in
Figures 5(b)
and S(c) according to the invention;
Figures 7(a) through 7(d) depict the various message formats with Figures 7(a)
and 7(b) depicting example inventory order entry records for respective stock
order
entry transaction (Figure 7(a)) and DCPD order entry transaction (Figure
7(b)),
respectively, Figure 7(c) depicting an example inventory usage reporting
transaction,
and Figure 7(d) depicting an example format for an invoice summary request
message;
Figure 8 is a block diagram depicting the dynamic inventory tracking process
300 according to the invention for orders that are placed automatically by the
system
and to display warnings for the user on low inventory as indicated in Figures
3(c) and
3 (d); and,
Figure 9 is a block diagram depicting one embodiment of the dynamic inventory
tracking process.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The present invention is a dynamic inventory tracking system and method that
enables ECP's to manage their diagnostic and revenue lens product inventories.
The
system is coupled with an electronic order entry system enabling ECPs to
efficiently
order lens products for their customers and to order lenses for inventory
maintenance
purposes so that a proper level of diagnostic and revenue lenses in the ECP's
inventory
may be maintained. This system provides an automated order generation feature
that is
enabled to automatically generate replenishment orders and automatically
initiate the
9
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
processing and fulfillment of generated diagnostic and revenue (when
preferred) orders
at the lens product manufacturer site, e.g., the assignee Vistakon, a division
of Johnson
& Johnson, Inc. To accomplish this, in the preferred embodiment, the inventory
management system implements a hand-held computer or Personal Digital
Assistance
(PDA) equipped with barcode scanning capability that may read and store the
unique
product identifying information, e.g., UPC, SKU, lot numbers, etc., provided
as a bar
code on the lens package label. Advantageously, the information gathered by
the PDA
is efficiently transmitted over existing telephonic communication
infrastructures, e.g.,
wired telephony networks, directly to the lens product manufacturers order
entry
system where they may be expediently processed. According to the invention,
the
dynamic inventory tracking and management system implements the PDA equipped
with a barcode scanner to dynamically track lens product usage, i.e.,
digitally establish
every time a lens product is dispensed for: 1) diagnostic or 2) revenue
reasons.
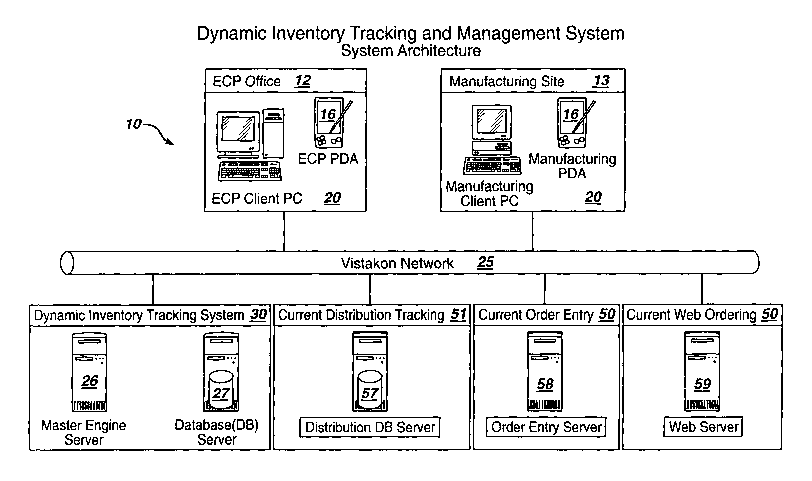
Figure 1 is an example system architecture~underlying the dynamic inventory
and tracking and management system of the invention. As shown in Figure 1, the
Inventory Tracking and Management System 10 of the invention comprises three
components: 1) a lens tracking infrastructure 20 located at the local ECP
office 12
which includes implementation of a computer or Personal Digital Assistance
(PDA)
equipped with a hand-held barcode scanner that may read and store information
associated with unique bar-codes provided on the primary or secondary lens
package
labels, for instance, for the purposes of dynamic inventory tracking and order
entry; 2)
a lens dynamic tracking and inventory management system 30 including at least
a
master engine server 26 and database server 27 implementing software for
enabling
logical inventory management and lens re-ordering functions as will be
described in
greater detail herein; and, 3) an enterprise customer service order entry and
web server
infrastructure 50 including one or more enterprise servers which may be
existing
enterprise order entry servers 58 and web-order servers 59 which receive,
maintain and
process all the information for re-ordering lens products out of a
Distribution system 51
such as implemented by the assignee, Vistakon, which currently maintains a
product
Distribution tracking infrastructure including a distribution database server
element 57.
As shown in Figure 1, the lens tracking infrastructure 20 includes a component
located
at the product manufacturing site 13 which likes implements a computer or
Personal
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
Digital Assistance (PDA) equipped with a hand-held barcode scanner that may
read and
store information associated with unique bar-codes provided on the primary or
secondary lens package labels, for instance, for the purposes of dynamic
inventory
tracking and order entry. According to the invention the existing web
ordering, order
entry 50 and distribution tracking infrastructures systems include a
communications
infrastructure represented by a network element 25, for example, an intranet
or Internet,
enabling communication of order-entry and account information among the
various
enterprise servers. As shown, the components of the dynamic inventory tracking
system 30 in addition to the lens tracking infrastructure 20 located at the
local ECP
office 12 and the manufacturing site 13 are likewise interfaced for
communication
across network element 25.
More particularly, as shown in Figure 1, to enable lens tracking functionality
according to the invention, the lens tracking infrastructure 20 at each ECP
office 12 and
manufacturing site 13 preferably implements a hand-held PC or Personal Digital
Assistant device (PDA) equipped with a bar code scanner device 16 such as may
be
implemented in conjunction with a personal computer (PC) 14. Preferably, the
PDA
equipped with a barcode scanner device 16 includes an operating system, touch
screen
interface or GUI display, and, may be equipped with custom software to
facilitate data
entry, storage and communication to and from the Dynamic Inventory Tracking
and
Management System 30. For example, a hand-held scanner such as a Denso BHT-600
series or, like equivalent such as the SPT 1500 model manufactured by Symbol
Technologies, Inc. may be provided with TCP/IP and FTP stacks firmware
including
software and entire scanner application for guaranteeing FTP functionality for
communicating with manufacturer/enterprise routers. It should be understood
that,
according to conventional techniques, the hand-held bar-code scanner 16 is
enabled to
communicate with server devices in the Dynamic Inventory Tracking and
Management
System 30 in either of two ways: 1) in real time through wireless connection
to an
access point within the local vicinity where the access point comprises a
server (not
shown) which is hard-wired to a communications network 25 such as the
Internet, for
example, via a 10/100 Base-T Ethernet (in the case that a LAN connection is
available)
or, an analog phone line (via a Public Switched Telephone Network); and, 2)
via a
batch-mode cradle connection to a modem (not shown) for interfacing with the
11
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
communication network 25 such as a wired telephony system for example, via a
RJ-11
standard telephone line connection. The communication between the hand-held
units 16
and the Dynamic Inventory Tracking and Management System infrastructure
servers
26, 27 may be modem direct dial or though a communication network 25
application,
wireline or wireless data transfer.
Figure 2 illustrates an underlying architecture of the enterprise Order Entry
processing and inventory tracking infrastructure 50 according to the
invention. As
shown in Figure 2, the enterprise Order Entry and inventory tracking
infrastructure 50
includes a first File Transfer Protocol (FTP) server device 56 for initially
receiving lens
product order entries communicated from ECP clients 12 via the PDA equipped
with a
barcode scanner device through the Dynamic Inventory Tracking and Management
System 30, and, in turn, formats the order entry requests for receipt by and
transfers the
requests to the enterprise Order Entry server device 58, such as an IBM
AS/400.
Preferably, secure communications to a proxy and order entry servers are
enabled via a
secure communications protocol. The secure servers 56, 58 incorporate defined
business rules and procedures into a customized Internet accessible database
system
and are designed with a set of dedicated computers which provide firewall,
application
serving, and database management functions. It should be understood that the
client
portion of the system (PDA, desktop PC or laptop) may equally communicate to
the
Dynamic Inventory Tracking and Management System server via Hypertext
Transmission Protocol (HTTP) and Open Secure Socket Layer (OpenSSL) on either
the
Worldwide Web or TCP/IP.
As further shown in Figure 2, central to the enterprise Order Entry and order
processing infrastructure 50 is a business document processing and electronic
data
interchange ("EDI") processing facilitator 35. Preferably, the EDI processing
facilitator includes an EXTOL Integrator device 35 that provides data
exchanges and
transaction at greater speeds and high volumes, and implements software
enabling
communication, translation and document management facilities to/from EDI
driven
platforms, e.g., the IBM AS/400. The Integrator device 35 provides tremendous
flexibility in organizing and retrieving data and integrates seamlessly with
any EDI user
application software, regardless of platform, and may take data from any
system, in any
standard, and process it automatically in real time. The Integrator EXTOL
integrator
12
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
particularly interfaces with an enterprise order processing facility 45
including
server/database systems 47 and 49 for holding orders, generating orders and
invoices,
and providing EDI order processing. As will be explained in greater detail,
the order
entry system further interfaces with the Dynamic Inventory Tracking and
Management
System 30 including server/database systems 27 for updating and tracking
inventory
usage for the particular ECP and manufacturer, and maintaining a master
inventory for
the ECP office.
Figures 3(a) through 3(d) are conceptual block diagrams depicting the process
for inventory tracking and management according to the principles of the
invention.
Figure 3(a) particularly depicts the Process Flow 100 for an ECP, and the
system
algorithms for inventory update, inventory management, processing of ECP's
ordering
preferences, processing of order and order fulfillment. As shown in Figure
3(a), a first
step 101 depicts an ECP representative scanning each product received from the
manufacturing distribution center, e.g., in fulfillment of an order for stock
lens
products, and, at step 102, storing the products in inventory maintained by
the local
office. Particularly, as shown in Figure 3(a), at step lOla, personnel at the
ECP office
utilizes the PDA equipped with the bar code scanner 16 to scan the product
into the
Dynamic Inventory Tracking and Management System 30 to update the logical
inventory for that ECP. In response to the scanning, at step 105, an algorithm
115 is
implemented in the Dynamic Inventory Tracking and Management System 30 servers
26, 27 for updating and tracking the logical inventory for that ECP.
Particularly, the
algorithm 115 as shown in Figure 3(a) is invoked to increment the existing
amount of
product for a particular SKU associated with the inventory of the local ECP
with the
quantity of product scanned by the ECP.
Subsequently, as shown at step 103 in Figure 3(a), the ECP office 12 will
dispense product in any one of three ways: 1) for diagnostic purposes, e.g.
for fitting a
particular patient, as indicated at step 104a; and 2) for revenue purposes,
i.e., for filling
a patients prescription for a particular SKU (lens product) as indicated at
step 104b. In
each of the three ECP product dispensation scenarios, the ECP office utilizes
the PDA
equipped with the bar code scanner 16 to scan the product package to be
dispensed so
that the product information may be used by Dynamic Inventory Tracking and
Management System 30 to update the logical inventory for that ECP. Figure 5(a)
°13
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
particularly exemplifies the use of the PDA 16 equipped with bar code scanner
providing a display 150 including a lens dispensing option 152 selectable by
the ECP
personnel that enables the ECP to select the reason for dispensing lenses,
i.e., for
diagnostic or revenue purposes. For purposes of explanation, the diagnostic
lenses are
to be dispensed as shown by the highlighted radio button 154. Refernng back to
Figure
3(a), in response to the scanning for diagnostic lenses dispensed, at step
104a, an
algorithm 116 is implemented in the Dynamic Inventory Tracking and Management
System 30 servers 26, 27 for updating and tracking the logical inventory of
diagnostic
lens products for that ECP. Particularly, the algorithm 116 as shown in Figure
3(a) is
invoked to decrement the existing amount of diagnostic lens product for a
particular
SKU maintained in the inventory of the local ECP with the quantity of product
dispensed as scanned by the ECP. Likewise, in response to the scanning for
revenue
lenses dispensed, at step 104b, an algorithm 117 is implemented in the Dynamic
Inventory Tracking and Management System 30 servers 26, 27 for updating and
tracking the logical inventory of revenue lens products for that ECP.
Particularly, the
algorithm 117 as shown in Figure 3(a) is invoked to decrement the existing
amount of
revenue lens product for a particular SKU maintained in the inventory of the
local ECP
with the quantity of product dispensed as scanned by the ECP.
Preferably, as will be described in greater detail, from the standpoint of
inventory tracking as enabled by the EDI inventory management and order entry
functionality, a "flat file" format has been defined comprising a multiple of
non-
delimited segments or record formats including, but not limited to: a record
indicating a
transaction start, a header record, one or more inventory management detail
records,
and a transaction end record. Thus, with more particularity as illustrated in
Figure 7(c)
there is depicted an example EDI formatted inventory usage transaction
reporting
record 275 illustrating the minimum data supplied to the Dynamic Inventory
Tracking
and Management System server 26 for inventory management/update purposes
including: an (EDI) Interchange Start segment 277 including an (EDI)
Interchange Start
segment identifier @VKS, for example; an EDI Message ID ("VKN852"), a Data
Origin which includes the sender ID or the unique id of the PDA, an optional
message
sequence number (a sequentially assigned Control number) and a transaction
date
representing the date of the inventory control transmission, e.g., in D
14
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
format; an Inventory Management Header segment 279, including text 'HDR'
indicating
the start of a header record, a transaction type, e.g., a 'V' for indicating a
Vendor
Inventory, an optional PO number, a Ship-to account number and location
indicating
the account number and location associated with the inventory management
information being submitted; and, one or more Inventory Management Detail
records
280, each of which includes data indicating: a'PRD' field indicating the start
of a detail
line item, an optional Trading Partner Number, a Transaction Date, the
manufactured
lens UPC, a Transaction Quantity, an account location from where the
Transfer/Sold/Received From/To, a unit of measure, an activity code, and a
unique
manufactured lens identification code, e.g., a product identifier code 281.
The
inventory management transaction end record or Interchange End segment 285
includes
an @VKE wrapper indication indicating the interchange end and the total number
of
detailed records provided.
Figure 3(b) depicts the management and lens product re-order functionality
provided by the Dynamic Inventory Tracking and Management System 30 of the
invention. Particularly, after updating the inventory levels in the Dynamic
Inventory
Tracking and Management System 30 servers 26, 27 for any of the respective
types of
lenses dispensed at the local ECP office 12 in accordance with Figure 3(a), a
step 106 is
further implemented for managing and optimizing the physical versus logical
inventory
maintained for the respective lens type. For instance, after algorithm 115 is
invoked for
updating stock lens products received, a further algorithm 125 is invoked for
determining if the number of lenses in inventory decremented by the amount of
lenses
dispensed is lens than a threshold stock inventory level limit. If the amount
of lenses
dispensed is lower than a threshold stock inventory level, then a responsive
action is
taken at step 107. For the case of stock lenses, a new order for stock lenses
may be
generated as indicated at step 135. Likewise, after the algorithm 116 is
invoked for
updating the amount of diagnostic lens products in inventory after
dispensation of
diagnostic lenses, a further algorithm 126 is invoked for determining if the
number of
diagnostic lenses in inventory decremented by the amount of diagnostic lenses
dispensed is lens than a threshold diagnostic lens inventory level limit. If
the amount of
lenses dispensed is lower than a threshold diagnostic lens inventory level,
then a
responsive action is taken at step 107, in this case, an automatic generation
of an order
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
for diagnostic lenses as indicated at step 136. Furthermore, as shown in
Figure 3(b),
after the algorithm 117 is invoked for updating the amount of revenue lens
products in
inventory after dispensation of revenue lenses, a further algorithm 127 is
invoked for
determining if the number of revenue lenses in inventory decremented by the
amount of
revenue lenses dispensed is lens than a threshold revenue lens inventory
level. If the
amount of lenses dispensed is lower than a threshold revenue lens inventory
level, then
a responsive action is taken at step 107, in this case, action taken in
accordance with
preferences previously indicated by the ECP office as indicated at step 137 as
will
explained in further detail with respect to Figure 3(c).
Figure 3(c) indicates in further detail how the orders are processed in
response
to actions 135-137 taken in response to management and optimization of
inventories
via the Dynamic Inventory Tracking and Management System 30. For example, in
response to generating a new order for stock lenses as indicated at step 135,
the ECP
PDA device 16 will receive a confirmation from the Dynamic Inventory Tracking
and
Management System 30 that the order for stock lenses has been placed as
indicated at
step 145. Likewise, in response to the automatic generation of a new order for
diagnostic lenses as indicated at step 136, the ECP PDA device will receive a
confirmation from the Dynamic Inventory Tracking and Management System 30 that
the order for diagnostic lenses has been placed as indicated at step 146. As
previously
mentioned, the action taken by the Dynamic Inventory Tracking and Management
System 30 with respect to new revenue lenses is dictated preferences
previously
communicated by the ECP. As shown in Figure 3(c), these preferences include
actions
indicated at step 108 including: an option 137a for the Dynamic Inventory
Tracking
and Management System 30 to generate a Low Inventory Warning message to be
communicated to the ECP PDA that the inventory for revenue lenses is below the
threshold limit; an option 137b for the Dynamic Inventory Tracking and
Management
System 30 to generate a Low Inventory Warning message to be communicated to
the
ECP PDA that the inventory for revenue lenses is below the threshold limit
and, further
to automatically initiate the setting up of an order for the ECP to either
approve,
disapprove or specify new particulars such as quantity ordered; or, an option
137c for
the Dynamic Inventory Tracking and Management System 30 to generate a Low
Inventory Warning message to be communicated to the ECP PDA that the inventory
for
16
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
revenue lenses is below the threshold limit and, further to automatically
generate a
revenue lens re-order to be fulfilled by manufacturing and distribution
without further
ECP involvement. In response to action taken by the Dynamic Inventory Tracking
and
Management System 30 in accordance with the options 137a-137c, the ECP will
respectively 1) receive a low inventory warning only for display in the ECP's
PDA
device as indicated at step 147a; 2) receive a low inventory warning for
display in the
ECP's PDA device and be prompted to review an order setup for either approval
or
cancellation as indicated at step 147b; or, 3) receive a low inventory warning
for
display in the ECP's PDA device and additionally, receive a confirmation that
the order
for new revenue lenses has been placed as indicated at step 147c..
Figure 3(d) depicts the order fulfillment processes and functionality provided
by
the Dynamic Inventory Tracking and Management System 30 of the invention. As
shown in Figure 3(d), step 109 depicts the order process functionality
performed by the
master engine server 26 of the Dynamic Inventory Tracking and Management
System
1 S 30. Thus, for orders placed for stock SKUs, diagnostic SKUs, and those
revenue lens
re-orders generated automatically in accordance with ECP preference at step
147c, the
master engine server 26 initiates order fulfillment particularly by bundling
all records
electronically (received by ECPs over the network) according to the ECPs and
sends
them to the web ordering server 59 where the fulfillment order process step
110
commences. To fulfill the lens orders for these types of lenses, the bundled
lens orders
lenses are received by web ordering server 59 and transmitted to the order
entry server
58 which currently receives orders for lenses received via the telephone, for
example.
All of these lens orders are bundled according to the ordering ECP, and a
"pick" ticket
is generated which encompasses all of the orders. The pick ticket is processed
by a
distribution server 57, which identifies the type of products and locates in
the inventory
maintained at the distribution center 60 how the order is to be processed at
the actual
inventory racks. For instance, the pick ticket may identify certain lens
orders, e.g., a
lens of power -9.0 diopters with a gray tint, as being special orders that
have to be
manually retrieved, for instance, while other more common prescriptive lenses,
e.g., a
lens of power -2.5 may be retrieved automatically for placement in a bin prior
for ease
of distribution. These lenses are finally gathered for the particular order
and shipped as
indicated at step 112 where they are received by the ECP representative back
at step
17
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
101 (See Figure 3(a)) and scanned in the system to increment the inventory
level
maintained for that ECP office. Referring back to Figure 3(c), in accordance
with ECP
preferences at step 147a and 147b, the master engine server 26 will invoke a
process for
generating the "Low Inventory" warning message for receipt by the PDA 16
located at
S the ECP office.
Figure 4 depicts a process 200 for setting up the system and storing the lens
products after manufacturing in the distribution center 60. As shown in Figure
4, a first
step 201 involves actually inputting the initial order information into the
Inventory
Tracking system including the lens type and parameters, quantities, etc. These
products
may be manufactured in anticipation of receipt of orders generated for any of
the
purposes described herein. After the information is entered into the system,
the
products are manufactured, as indicated at step 202. Then, the lenses are
packaged at
step 203 with the barcode 64 or other identifying indicia placed on the
package as
indicated at step 204. Then, at step 205, prior to placement in the inventory
maintained
at the distribution center 60, the PDA equipped with the bar code scanner is
used to
update the data associated with that product in the inventory tracking system
30 servers.
Finally, at step 206, the package containing new manufactured lenses is
physically
transferred to the inventory racks located at the distribution center 60.
Other functionality provided by the Dynamic Inventory Tracking and
Management System 30 includes the automatic processing of invoice summary
requests
from ECPs. The dynamic inventory tracking and order entry system of the
invention is
provided with functionality for enabling an ECP to request an invoice summary
using
the hand held PDA. Particularly, in the manner described herein, the PDA
operator
maybe instructed to enter the date from which the invoice will start. In
response, the
system will build a file in a pre-specified EDI file format referred to as
"VKNISR".
Utilizing a default of the current date, the operator may quickly request a
confirmation
of the most recently sent orders of the day. Confirmation will start from the
current
date and will continue until one of the following is reached: a default of
current date
(current days transmissions) or an operator entered date (e.g., maximum of 30
days).
Figure 7(d) depicts an example format for an invoice summary request message
290 generated by the PDA. Generally, the exemplary invoice summary request
message format includes: an (EDI) Interchange Start segment 292 which includes
the
18
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
Interchange start identifier @VKS; an EDI Message ID ("VK1VISR"), a Data
Origin
which includes the sender ID or the unique id of the PDA, an optional message
sequence number (or sequentially assigned Control number ) and, a transaction
date
representing the date of the inventory control transmission, e.g., in YYYYMMDD
format; an Invoice summary request Header segment 294, including text
indicating the
start of a header record, a transaction type, e.g., a 'F' for indicating a
facsimile invoice
request, an optional PO number, a Ship-to account number and location
indicating the
account number and location associated with the invoice being requested, and
the text
"PDA" indicating the requesting party; and, one or more Fax Request Detail
records
295, each of which includes data indicating: a FAX field indicating the start
of a detail
line item, a "From Date" indicating the From date of the request and the "To
Date"
indicating the To date of the request, the area code of the ECP location and
the ECP fax
number to whom the request is being sent. The invoice summary request
transaction
end record or Interchange End segment 298 includes @VKE wrapper indication
1 S indicating the interchange end and the total number of detailed records
provided.
The process 160 for automated order entry according to the principles of the
invention, is now described in further detail with respect to Figure 6. Figure
6 depicts
example menu choices presented to the user via the ECP's Personal Digital
Assistant
(PDA) 16 screen display. These menu choices include: an option 162 for reading
and
entering order data for storage therein; an option 164 for transmitting stored
order
and/or inventory management data to the manufacturer order entry system; an
option
166 for requesting facsimile confirmation of orders received; utilities
options for setting
up a user 167, or a system setup option 168; and, an LD. information option
169. With
respect to the option 162 for reading and entering order data, Figure 6
generally depicts
a first step 170 of instructing the ECP or operator to scan a bar code of the
particular
lens product to be ordered. It should be understood that users of the PDA
device may
include: internal Eye Care Professionals (ECP's) or their agents performing
optical
duties within the ECP's premises; external ECP's requiring revenue product or
diagnostic product. Generally, when ordering revenue lenses according to the
invention, the ECP or office staff preferably scans UPC barcodes on packages
or
cartons, or scans them from a product barcode book. It is understood that lens
products
are arrayed in different quantities when packaged, and their packaging
characteristics
19
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
vary. Preferably, the system is enabled to track products packaged in cases,
secondary
packages ('cartons' or 'packs'), primary packages (single 'blisters',
'vials'), or plastic
bags, e.g., when provided for diagnostic purposes. Additionally, bar codes may
be of
the one dimensional and/or two-dimensional types. Briefly, all lens order and
dispensing data entered during the course of a day is stored in the PDA and,
on a
periodic basis, e.g., daily or upon request by user, all of the order data is
communicated
to the manufacturer/enterprise Dynamic Inventory Tracking and Management
System
infrastructure 30 (Figure 1). For the case of diagnostic lenses to be ordered
for specific
customers, a peelable label may be provided on a primary package with a bar
code
representing the SKU. The ECP may peel and place the label on a patient form
after
fitting and patient forms may be gathered and each of the bar-codes labels may
be
scanned. . As in the case of revenue lenses, all diagnostic lens order and
dispensing data
entered during the course of a day is stored in the PDA and, on a periodic
basis, e.g.,
daily or upon request by the user, all of the order data is communicated to
the
manufacturer/enterprise order entry infrastructure for fulfillment. It should
be further
understood that besides utilizing the PDA equipped with a bar code scanner as
the
vehicle to generate lens product "stock" orders for inventory level
maintenance, the
PDA equipped with a bar code scanner may be used to generate lens product re-
orders
for specific customers, i.e., so-called doctor controlled patient delivery or
"DCPD"
orders. For instance, there may be a bar-code identifier associated with a
specific
patient or customer, and this bar code may be scanned during the order
generation
process for initiating orders for customers to be directly communicated from
the
Dynamic Inventory Tracking and Management System 30 to the order entry
infrastructure 50. As a result of this order processing, lens products
supplies may
replenished for specific customers or patients.
Figure S(c) particularly exemplifies the use of the PDA 16 equipped with bar
code scanner providing a display 450 including functionality for placing
product orders
including: an option 453 for specifying the type of lens product to be
ordered, and an
option 456 for specifying a specific SKU of a lens product to be ordered.
After
entering this information, the user may select the place order option 458 or,
edit an
existing order stored in the PDA device as indicated as option 460. Further,
the user
may select an option 465 to view a summary of those inventory levels having an
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
amount below a warning threshold as determined by the Dynamic Inventory
Tracking
and Management System 30. Once an inventory level is determined to be low
according to the low inventory count sheet, the user may select option 462 for
initiating
a re-order for those lenses.
Referring back to Figure 6, at step 172, a determination is made as to whether
the scanned bar code is for diagnostic or revenue via a customer selectable
screen 150
as depicted in Figure 5(a). Further the user is allowed to enter the quantity
if a one
dimensional barcode, if the barcode is two dimensional it is assumed that the
quantity is
included in the scanned data. Further, step 172 includes the step of
validating the
scanned (if two dimensional) code against a Diagnostic Product Table (not
shown)
which includes data such as Sub-Pack ID, Sub-Pack UOM , Sub-Pack Description
(e.g.,
00033P TA 8.7 0.00 -0.75 +70 D) and will initiate display of an error message
if not
found. Otherwise, the scanned code may be further validated against a Revenue
Product Table (not shown) which includes data such as the Product SKU, Product
Code UOM and a Product Code Description (e.g., 73390550790 12P TA 8.7 0.00 -
0.75 +10 ). Preferably, an error message is displayed if not found. Next, at
step 176, a
determination is made as to whether the scanned bar code is UPC-A.
Particularly, to
identify a valid manufacturer, a table of manufacturer ID's is checked. If UPC-
A is that
of an invalid manufacturer, then the PDA will display "Invalid Manufacturer",
for
example, otherwise, the PDA equipped with a barcode scanner will verify that
the
UPC-A is that of a valid manufacturer. As a result of validating the scanned
code and
verifying the manufacturer, a data file is built in a format detailing the
specifics of the
order and as indicated at step 180 will prompt the user to enter the quantity
of revenue
lenses desired to be ordered. It is understood that the operator has the
ability to change
the quantity at any time before submitting the order. The above scanned bar
codes,
description, and associated quantities ordered, will be displayed in a
scrolling list
format located on the screen (not shown) that instructs the operator to scan
the bar
code. It should be understood that the operator will additionally have the
ability to
delete entire records from the list as well as the ability to change
quantities on order for
the UPC-A scanned bar codes.
The option available to the ECP for sending the order and inventory tracking
data utilizing the hand held PDA as indicated as option 164 in Figure 6 is now
21
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
described. Preferably, the PDA device first instructs the operator to place
the PDA
device 16 on the base unit and press enter to start transmission. This will
initiate the
following sequence of events: Initially, the PDA will automatically dial the
location of
the lens manufacturer as indicated at step 174, and transmit a verification of
information including the account number and password, for verification by the
manufacturer. If the account information is valid, then the manufacturer will
send an
"OK to transmit" the information. Otherwise, the manufacturer will transmit
that the
account was invalid. It is understood that the PDA may display a message for
the user
indicating an "Invalid account information". If the PDA receives a message
from the
manufacturer that it is OK to transmit the information to the manufacturer,
i.e., there
was a successful connection, then the PDA will transmit all data located in
the scanned
barcode list. In one embodiment, the data is transferred by FTP from the
Dynamic
Inventory Tracking and Management System 30 to the FTP server 56 (Figure 2).
If the
transmission is successful, then manufacturer will transmit a "Successful
Transmission
Confirmation Number: xxxxxx" message with xxxxxx being a transmission
confirmation number. That is, as each transmitted file includes a unique name
and
unique message sequence number, this file will be used as the confirmation
number
displayed to the user. After PDA display of the Successful Transmission
message, a
transmission confirmation number and an instruction for the operator to press
"enter" to
return to the main menu. The transmission number will, at that point, be
deleted from
the PDA. It should be understood that possible errors that may be encountered
during
the transmission include: a failure to authenticate to the dial-up router/PPP
in which
case a "Failed to connect to Comm. server" user message will be displayed;
and, a
Failure to authenticate to the FTP server in which case a "Failed to connect
to the FTP
server" user message will be displayed. If connected successfully, the PDA
FTPs the
data to the FTP servers) with detail data for each type of file format
consolidated with
the appropriate Start, Header, and End segments as will be described in
greater detail
herein. It should be understood that if the FTP fails a "Data did not transfer
successfully" user message will be displayed. Preferably, a sequence number is
generated for each segment that is sent. Also, as will be explained in greater
detail,
each document (e.g., all the segments) are "packaged" at transmission time
with @VKS
and @VKE wrappers. In the event of a failed transmission, the "package"
generated is
22
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
kept as a unique "package" with it's own sequence number and subsequent scans
should
not be appended to the "package" that failed. The next time a transmission is
attempted
a new "package" should be generated. Both "packages" may be sent together. The
scrolling list of scanned bar codes will be deleted from the list after a
successful
transmission. The invoice summary request dates should also be cleared. The
packaged file will also be removed. In one embodiment, the PDA equipped with a
bar
code scanner will store information associated with each package scan whether
it be for
inventory tracking or ordering purposes and accumulate and delimit all of the
information for later transmission as a single file to the order
entry/inventory tracking
infrastructure. Preferably, the transmission is performed periodically, e.g.,
several
times per day as specified by the ECP office or, at the end of a business day
or upon
user request.
Returning to Figure 6, the option 166 available to the ECP for requesting
confirmation of orders by fax utilizing the hand held PDA is now described
with
respect to step 186. In this step, the PDA device instructs the operator to
enter the date
from which the confirmation will start. Preferably, a default of the current
date will be
utilized so that the operator may quickly request a confirmation of the most
recently
sent orders of the day. Typically, the confirmation will commence from the
current
date and continues until one of the following is reached: a default of current
date
(current days transmissions) or, an operator entered date (maximum of 30 days,
for
example). In response, the PDA device will display a message requiring the
user to
place the hand held PDA in its cradle and press "Enter" to request a faxed
confirmation.
In response to user entry of the request, the following sequence will occur
for
transmission: The PDA will automatically dial the location of the lens
manufacturer,
and transmit a verification of information including the account number and
password,
for verification by the manufacturer. If the account information is valid,
then the
manufacturer will send an "OK to transmit" the information. Otherwise, the
manufacturer will transmit that the account was invalid. It is understood that
the PDA
may display a message for the user indicating an "Invalid account
information". If the
PDA receives a message from the manufacturer that it is OK to transmit the
information to the manufacturer, i.e., there was a successful connection, then
the PDA
will transmit the request for a fax confirmation for the entered dates. If the
23
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
transmission is successful, then the manufacturer/enterprise will transmit a
transmission
confirmation number and display a "Successful Transmission" message including
the
transmission confirmation number, and instruct the operator to press "Enter"
to return
to the main menu. At that point, the transmission number will be deleted from
the
PDA.
The inventory tracking and order entry system of the invention is provided
with
the ability to automatically upgrade the operating system and application
software.
Particularly, the PDA device is enabled to check for the existence of new
application or
operating system software. These files reside on a remote location in the
enterprise
network and if present, will be downloaded accordingly. Thus, returning to
Figure 6,
the option 168 available to the ECP for enabling system set-up utilizing the
PDA
equipped with a barcode scanner is now described with respect to step 187. In
step
187, the menu selection enables the user to view the current version of
software loaded
on the PDA and also enable the operator to upgrade to the latest software
version. The
following sequence will occur if the operator request upgrade: Initially, the
PDA
instructs the operator to place the PDA in a cradle and press "enter" to begin
upgrade.
The PDA will automatically dial the location of the lens manufacturer, and
transmit a
verification of information including the account number and password, for
verification
by the manufacturer. If the account information is valid, then the
manufacturer will
send an "OK to transmit" the information. Otherwise, the manufacturer will
transmit
that the account was invalid. It is understood that the PDA may display a
message for
the user indicating an "Invalid account information". If the PDA receives a
message
from the manufacturer that it is OK to transmit the information to the
manufacturer, i.e.,
there was a successful connection, then the PDA will transmit the request for
a software
version upgrade. In response, the manufacturer/enterprise will transmit the
latest
software version. After the PDA receives the upgrade, it will indicate to the
manufacturer/enterprise that the upgrade was successful. If the transmission
of the
upgrade is not successful, the PDA will be required to revert back to its
previous
version of software and display to the operator that the upgrade was
unsuccessful. In a
preferred embodiment, the manufacturer includes the ability to automatically
upgrade
the software during the electronic ordering process.
24
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
Returning to Figure 6, the option 169 available to the ECP for enabling system
entry of LD. information utilizing the PDA equipped with a barcode scanner is
now
described with respect to step 189. In step 189, a menu item is provided which
requires
the user to enter a password or provide a PDA key sequence to begin entry of
ECP
identification information for enabling PDA access to the manufacturer order
entry
system. It is understood that information located in this area may be changed
from a
remote location via modem, e.g., for purposes of changing an ECP's account
number
during order transmission. At step 190, the identification information to be
entered
includes: an ID number or account number unique to the manufacturer; a
password
enabling access to the AS/400; and, a phone number for dialing the
manufacturer.
According to the invention, from the standpoint of automated order entry
functionality, a "flat file" format may be defined for electronic stock and
doctor
controlled patient delivery ("DCPD") orders which comprises a multiple of non-
delimited segments or record formats including, but not limited to: a record
indicating a
transaction start; a header record; an optional order comment ("COM") record;
a patient
information
("PAT") segment which is optional for stock orders but required for DCPD
orders only;
an optional address information segment ("ADD") including city state and zip
code
information which is optional for stock orders but required for DCPD orders
only; one
or more order entry Detail line item segments ("DET"); and, a transaction end
record.
It should be understood that there may only be one patient per DCPD
order.Figures 7(a)
and 7(b) depict example inventory order entry records 250, 260 each
respectively
illustrating the minimum data supplied for a respective stock order entry
transaction
250 (Figure 7(a)) and DCPD order entry transaction 260 (Figure 7(b))
including:
respective (EDI) Interchange Start segment 252, 262 which includes the EDI
Interchange start identifier @VKS, for example, EDI Message ID ("VKN850"), a
Data
Origin which includes the sender ID (e.g., "PDA) or other unique id of the bar
code
PDA, an optional message sequence number (a sequentially assigned Control
number
254, 264) and, a transaction date representing the date of the inventory
control
transmission, e.g., in YYYYMMDD format; respective Order Header segment 255,
265
including the text 'HDR' indicating the start of a header record, a
transaction type, e.g.,
an 'S' for indicating a stock replenishment order as shown in segment 255
(Figure
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
7(a)), or, a D for indicating a DCPD order type as shown in segment 265
(Figure 7(b)),
an optional purchase order number ("PO"), a Ship-to account number and
location
indicating the account number and location associated with the order
information being
submitted, and a "PDA/FAX" field indicating that the order was placed by a
"PDA"
either with a fax order confirmation request (as shown in Figure 7(a)) or
without a fax
order confirmation request (as shown in Figure 7(b)), and, optional fields
specifying
information including credit card number, diagnostic lens account number,
diagnostic
lens account location, a requested ship method and a requested future ship
date; and,
respective one or more Inventory Management Detail record segments 256, 266,
each
of which includes data indicating: a PRD field indicating the start of a
detail line item,
an optional Trading Partner Number, a Transaction Date, the manufactured lens
UPC or
unique manufactured lens identification code (product identifier code 257,
267), a
Transaction Quantity, an account location from where the
Transfer/Sold/Received
From/To, a unit of measure, and an activity code.
As shown in Figure 7(b), the DCPD order (Figure 7(b)) transaction further
includes a patient segment 268 including a "PAT" field indicating the start of
a patient
record, an optional field indicating the number of wear days, e.g., 1 -7 days
(not
shown), an optional Postcard message id including a code "A" for initiating
generation
of a message to contact the ECP office for re-ordering, or "B" for initiating
generation
of a message to call the office to set up an appointment; an optional Packing
slip
message id including codes for initiating generation of one or more messages
including
a code "A" for generating a message for announcing the importance of
contacting the
office at least one week before these lenses run out so that user wear will
not be
interrupted, a code "B" for generating a message indicating that the ordered
lenses
should be worn only in accordance with the wear regimen prescribed for that
user, but
not to exceed one day, a code "C" for generating a message indicating that
compliance
with the prescribed wear regimen is essential to maintaining proper eye health
for the
user, a code "D" for generating a message indicating that the lenses are for
daily wear
single use only and are to be never re-used, a code "E" for generating a
message serving
as a reminder that the user is wearing 1-day lenses and that these lenses are
intended to
be disposed of after no more than one day's usage, and a code "F" for
generating an
advertisement message indicating the health benefits of 1-day lenses; a
Patient id , e.g.,
26
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
if the patient is a current patient or otherwise a new Vistakon patient ID or -
1 if new
patient and, the Patient's last name, Patient first name, and a Patient middle
initial.
As further shown in Figure 7(b), the DCPD order (Figure 7(b)) transaction
further includes an address segment 268 including an "ADD" field indicating
the start
of a patient address record and one or more fields for the address
information, and, an
city/zip code/state segment 269 including the "CSZ" field indicating the start
of the
city/zip code/state record and including one or more fields for indicating the
City, State,
5 digit zip code and optional 4 digit zip code extension, an area code and 7
digit phone
number, and optional 5 digit phone number extension.
The example order entry records 250, 260 each illustrating the minimum data
supplied for a respective stock order entry transaction (Figure 7(a)) and DCPD
order
(Figure 7(b)) each further includes a detail line item segment comprising: a
DET field
indicating the start of detail line item; a PO line number field for setting
forth a
sequential line item number; a quantity, i.e., an order quantity; a Unit of
measure; a
product identifier code; a product usage including R (right eye) or L (left
eye) lens
product required for DCPD order; and an optional order detail line. Each of
the
respective stock order entry transaction (Figure 7(a)) and DCPD order (Figure
7(b))
transactions further include a respective stock 259 or DCPD order 270
transaction end
record (Interchange End segment) each of which includes the EDI Interchange
end
identifier @VKE and the total number of detailed records (DET) provided.
Figure 8 is a general block diagram depicting an example methodology 300 for
providing dynamic inventory management and tracking of revenue and/or
diagnostic
lenses in the system 10 of the invention. With regard to inventory usage and
inventory
update, as shown with respect to Figure 2, and in detail in the block diagram
of Figure
8, a first step 302 involves receiving the data transmitted over the
communication
network from the PDA device 16. As mentioned, the data sent from the PDA may
comprise either inventory usage data, or alternately, order request data.
Particularly,
data is sent from PDA via FTP from the Dynamic Inventory Tracking and
Management
System 10 to the first FTP server 56 (e.g., a Sun 2000) as shown in Figure 2.
The FTP
Sun 2000 server in response, executes a script that will bundle the PDA data
along with
any orders from lens products obtained via the Internet or via phone into a
pre-specified
file format, which file is sent via FTP to the AS/400 system 58 as shown in
Figure 2. A
27
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
program is executed that processes the EDF013 using the Extol EDI translator
35
(Figure 2). In response to importing the file EDF013 into the Extol translator
as
indicated at step 304 in Figure 8, the data input is placed in an unwrapped
connection
and the data is translated by message class. That is, as shown in Figure 8 at
step 306,
data provided in the VKN850 pre-specified message format as described herein
with
respect to Figure 7(a), is translated, i.e., mapped into EDI order detail
formats referred
to as EDF001, EDF002 file formats (Figure 2). Likewise, using the Extol
translator,
data included in the VKN852 message format described herein with respect to
Figure
7(c), is mapped into inventory update file formats referred to as VMF001 470,
as will
be explained with respect to one embodiment of the dynamic inventory tracking
process described with respect to Figure 9, and data included in the VI~NISR
message
format described herein is mapped into Invoice request file format referred to
as
VMF004 (as shown in Figure 2). Refernng back to Figure 8, the next step 308 is
to
verify that the fields in the translated EDFOOlA, EDF002A, VMF001, VMF004
formats are populated with valid data, i.e., EDF001, EDF002 includes accurate
order
header fields, VMF001 includes accurate inventory usage fields, and/or VMF004
includes accurate Invoice request fields. For purposes of discussion, assuming
inventory update data is received, the next step is to update the inventory
associated
with the ECP with usage values. That is, as indicated at step 309, a program
is called to
read usage data reported by the PDA and provided in the input VMF001 inventory
update file and, at step 310, to update an inventory master file VMF002 which
tracks
the individual ECP account's inventory count values. The algorithms invoked
are
described herein with respect to Figure 3(b) at steps 115-118. To map into the
VMF002 master inventory file, the VMF001 includes a 4-byte sub-pack identifier
which is used to chain to a product usage database GBF 472 (shown in Figure 9)
to
obtain the UPC. Using the account number, location, UPC, and unit of measure
as a
key, the data may be chained to the VMF002 master inventory file. Initially,
all of the
individual ECP user inventory counts maintained in the inventory master file
VMF002
476 (as shown in Figure 9) for tracking start with an amount of lenses "On-
hand"
inventory level = XX lenses; a "Usage" amount = YY; and "Re-order point"
threshold
(e.g., low limit threshold = ZZ lenses for each respective lens product
(diagnostic or
revenue). As information is received from the ECP PDAs, these accounts are
updated
28
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
with the lens product usage reported from the file VMF001. Particularly,
accounts with
activity from VMF001 will have on-hand decremented by the amount reported used
from VMF001 while a "Used" quantity will be incremented by the amount reported
used. For instance, the usage quantity provided in the VMF001 file is
subtracted from
S the on-hand quantity in databaseVMF002 476. If on-hand < 0 then on-hand
value is set
to 0. Once verification is completed and no errors are reported, the process
continues
to call a program VMR002 which evaluates inventory and either initiates the
generation
of warnings or orders for accounts with on-hand inventory levels less than or
equal to
the re-order point, i.e., XX<=ZZ, according to ECP preferences, or
automatically
generates re-orders, e.g., for diagnostic lenses. That is, as shown in Figure
9, the
program VMR002 478 is invoked to analyze VMF002 and tracked inventory levels
for
each ECP. As a result of this process, the automatic order generating process
is
performed, which as shown in Figure 2 invokes steps of: generating one or more
files
EDFOOIA which is the order header holding file for PDA ordering for each ECP
and
verifying that it has been written correctly only for those ECP accounts with
on-hand
inventory level less than or equal to the re-order point threshold (i.e., XX
<= ZZ);
generating a file EDF002A which is the order detail holding file for PDA
ordering and
verifying that the EDF002A detail has been written correctly for only accounts
with on-
hand inventory level <= re-order point threshold.
More particularly, refernng to Figure 9, a program VMR002 478 reads the
VMF002 until end of file (EOF) and determines if the on-hand inventory level
<= re-
order point threshold as shown in Figure 8 at step 312. If this criterion is
met, then the
process chains to the EDFOOlA file (Figure 2) with the account number and
location. If
the EDFOOIA file is not found, then the EDFOOIA file header file is created to
include
the account number, location, EDI sequence number ( = 0), the Order type (_
'S'), the
Order origin (_ 'P') and who placed the order (e.g., _ 'PDA/FAX') . Once the
EDFOOIA fields are written, fields for EDF002A are written to include the ECP
Account number, location, EDI sequence # = 0, UPC, Unit of measure, and order
quantity (e.g., = minimum quantity). Finally, as a further result of this
processing, the
following inventory tracking values are further updated in the master
inventory file
VMF002 as described herein with respect to Figures 3(b) steps 125-127 for the
respective ECP accounts. It should be understood that, as part of this VMR002
process
29
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
478, if the on-hand inventory level is not less than the re-order point
threshold, the on-
hand inventory level for the ECP may be compared against a second "Warning"
threshold "WW" which will indicate to the ECP that certain inventory levels
are low
and that the customer or ECP may want to initiate a re-order. That is, as
indicated at
step 314, Figure 8, the program VMR002 478 may additionally read the VMF002
and
determine if the on-hand inventory level <= warning threshold level (i.e., XX
<= WW)
and if so, initiate a warning message to the ECP user as indicated at step
320, Figure 8.
Otherwise the process continues to step 302 to wait for the next order.
Figure 5(b) is an example depiction of a PDA screen display 400 for a local
ECP illustrating a snapshot of that ECP's on-hand inventory levels for
revenue, and
diagnostic lenses. As depicted, upon selection of inventory tracking function
implementing software provided for ECP use in the PDA 16, there is displayed
menu
options including: a menu option 403 for reviewing inventory by product
family; an
option 406 for reviewing inventory by SKU number; an option 408 for initiating
placement of new orders; an option 410 for reviewing inventory by product
type; and
an option 412 for reviewing the overall inventory. Preferably, when viewing a
snap-
shot of the overall inventory as depicted in Figure 5(b), for each type of
lens there is
provided bar chart markers 415 indicating the physical quantities on hand;
markers 420
indicating quantities dispensed; and, markers 425 indicating logical
quantities desired
to meet expected demand or usage. As shown in Figure 5(b), there is shown an
indication 430 that an order has been placed for diagnostic lenses. Further,
as a result
of warning threshold comparisons, a low inventory warning indication 435 is
provided
with markers 440 indicating lenses and possible amounts to be re-ordered for
the
example inventoried lens products shown in the display.
In a further embodiment, triggers for automatically ordering lens products for
individual customers may be implemented. For instance, after providing a
supply of
lenses for particular customers, e.g. a 90 day supply, the number of days may
be
tracked by the ECP and/or order entry system such that an order for a new
supply may
be automatically generated for that particular patient. Thus, rather than the
ECP having
to enter the order, a trigger may be built into the ECP PDA software which
tracks a
number of days elapsed after dispensing a particular supply for a customer.
After a pre-
set time has elapsed, then, the ECP may be automatically notified that a
patient's
CA 02495106 2005-02-09
WO 2004/017156 PCT/US2002/026002
supply may have depleted to the extent that a new lens order should be
generated or,
alternately, an order may be automatically generated and fulfilled in the
manner
described herein so that a particular patient may automatically receive a new
lens
shipment.
Referring back to Figures S(b) and 8, as a further part of the dynamic
inventory
tracking process is the step 318 of automatically generating orders from order
holding
file to EDI order interface files either as a result of a determination of low
inventory
levels at step 312, or as a result of initially receiving an order request as
indicated by
broken line 319 in Figure 8. Thus, in the next step 318, a process VMR003 (as
shown
in Figure 2) is invoked to create the orders from EDFOOIA and EDF002A.
Preferably,
at a specified time, e.g., daily, the program VMR003 executes to generate
orders for the
day's activity. Particularly, VMR003 reads the EDFOOlA file until the EOF is
encountered and chain to the EDF001 file with account number, location and EDI
sequence number. If not found, then the fields from the order header created
EDFOOlA
are moved to EDF001 and are written to EDF001. Then, the program sets and
reads
EDF002A on account number, location and EDI sequence number until not found.
If
found, then the fields from EDF002A are moved to EDF002 and the EDF002 file is
written. This is a loop process, and continues until EOF is encountered in the
EDFOOlA. Upon successful program completion, EDFOOlA and EDF002A are
cleared. As further shown in Figure 2, at steps 49, a program VMR004 is
executed to
generate an invoice summary request utilizing the VMF004 EDI file format which
request is generated and placed on a fax queue. This request is a confirmation
to the
ECP to inform the ECP of the quantity of product ordered and the delivery
date.
While the invention has been described in connection with a preferred
embodiment, it is not intended to limit the scope of the invention to the
particular form
set forth, but on the contrary, it is intended to cover such alternatives,
modifications,
and equivalents as may be included within the spirit and scope of the
invention as
defined by the appended claims.
31