Note: Descriptions are shown in the official language in which they were submitted.
CA 02496286 2005-02-07
30877-7
-I-
POLYTHIOPHENE COMPOSITIONS FOR IMPROVING ORGANIC LIGHT-
S EMITTING DIODES
FIELD OF THE INVENTION
The invention relates to compositions / formulations comprising polythiophenes
and
further polymers, their use and electroluminescent arrangements comprising
hole-
injecting layers comprising these formulations.
BACKGROUND OF ~ INVENTION
An electroluminescent arrangement (EL arrangement) is characterized in that
when an
electrical voltage is applied, with flow of current, it emits light. Such
arrangements have
been laiown for a long time under the name "light-emitting diodes" (LEDs). The
emission of light arises by positive charges ("holes") and negative charges
("electrons")
recombining with emission of light.
The LEDs customary in the art are all predominantly made of inorganic
semiconductor
materials. However, EL arrangements in which the essential constituents are
organic
materials have been known for some years.
These organic EL arrangements as a rule comprise one or more layers of organic
charge
transportation compounds.
The main layer build-up of an EL arrangement is e.g. as follows:
1. Carrier, substrate
2. Base electrode
3. Hole-injecting layer
4. Hole-transporting
layer
5. Emitter layer
6. Electron-transporting
layer
7. Electron-injecting
layer
8. Top electrode
CA 02496286 2005-02-07
30877-7
-2-
9. Contacts
10. Casing, encapsulation
This build-up represents the most detailed case and can be simplified by
omitting
individual layers, so that one layer takes over several tasks. In the simplest
case an EL
arrangement comprises two electrodes, between which is an organic layer which
fulfils all
functions - including that of emission of light.
However, it has been found in practice that electron- and/or hole-injecting
layers are
particularly advantageous in electroluminescent constructions in order to
increase the
luminous density.
EP-A-686 662 discloses specific mixtures of conductive organic polymeric
conductors,
such as poly(3,4-ethylenedioxythiophene), and, for example, polyhydroxy
compounds or
lactams as electrodes in electroluminescence displays. However, it has been
found in
practice that these electrodes have an inadequate conductivity, especially for
large-area
displays. On the other hand, the conductivity is sufficient for small displays
(luminous
area < 1 cm2).
DE-A-196 27 071 discloses the use of polymeric organic conductors, e.g.
poly(3,4-
ethylenedioxythiophene), as hole-injecting layers. By this means the luminous
intensity
of the electroluminescent displays can be increased significantly compared
with
constructions without the use of polymeric organic intermediate layers. By
reducing the
particle size of the poly(3,4-alkylenedioxythiophene) dispersions, the
conductivity can be
adjusted in a controlled manner. It is thus possible to prevent electrical
crosstalk of
adjacent address lines, especially in passive matrix displays (EP-A-1227 529).
However, the life of these displays is still not sufficient for many practical
uses.
There therefore continued to be a need for the production of EL arrangements
which
have, in addition to a high luminous intensity (luminous strength), a longer
life than
known EL arrangements.
ShfMMARY OF THE INVENTION
The present invention provides suitable formulations for the production of
such EL
arrangements. Further, the present invention produces improved EL arrangements
from these materials.
CA 02496286 2005-02-07
STA 246-US
-3-
It has been found, surprisingly, that hitherto unknown formulations comprising
optionally
substituted polythiophenes or optionally substituted polyanilines or
polypyrroles and
further polymers are outstandingly suitable for the production of hole-
injecting layers for
EL arrangements, and the EL arrangements obtained have significantly longer
lives than
known EL arrangements.
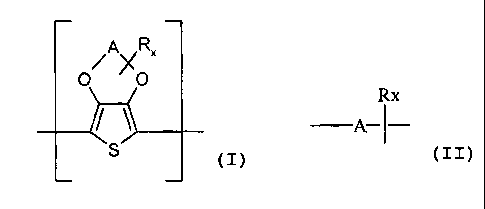
The present invention therefore provides compositions / formulations
comprising
A) at least one polythiophene containing recurring units of the general
formula (n
(I)
wherein
A represents an optionally substituted C,-CS-alkylene radical, preferably an
optionally substituted ethylene or propylene radical, particularly
preferably a 1,2-ethanediyl radical,
R represents a linear or branched C~-C~$-alkyl radical, preferably a linear or
branched C,-C~4-alkyl radical, particularly preferably a methyl or ethyl
radical, a CS-C,2-cycloalkyl radical, a C6-C14-aryl radical, a C~-C,g-aralkyl
radical, a C~-Ca-hydroxyalkyl radical or a hydroxyl radical,
x represents an integer from 0 to 8, preferably 0, 1 or 2, particularly
preferably 0 or 1 and
in the case where several radicals R are bonded to A, these can be
identical or different,
B) at least one polymer containing S03 M+ or COO-M+ groups, wherein M+
represents H+, Li+, Na+ K+, Rb+, Cs+ or NH4+, preferably H+, Na+ or K+, and
CA 02496286 2005-02-07
30877-7
-4-
C) at least one partly fluorinated or perfluorinated polymer containing S03'M+
or
COO'M~ groups, wherein 1VI~ represents H+, Li'", Na+ K+, Rb+, Cs+ or NH4+,
preferably H+, Na+ or K+.
The general formula (>] is to be understood as meaning that the substituent R
can be
S bonded to the alkylene radical A x times.
Polymers (B) and (C) are different from each other and are each different than
polythiophene (A).
Unless otherwise indicated, all numbers or expressions, such as those
expressing
quantities of ingredients, process conditions, etc., used in the specification
and claims are
understood as modified in all instances by the term "about."
DETAILED DESCRIPTION OF THE INVENTION
Formulation within the meaning of the invention may be any mixture of
components A),
B) and C) as solids, in solution or in dispersion.
Instead of Polythiophene A) any other known conducting polymer A) can be used
in the
mixture, in particular, optionally substituted polyaniline or polypyrrole.
These different conducting polymers A) can be used alone or in any mixture.
Here and below the term substituted means if not otherwise indicated a
substitution with
chemical group selected from the group consisting of
alkyl, in particular C1-C20-alkyl, cycloalkyl, in particular C3-C20-
cycloalkyl, aryl, in
particular C6-C14-aryl, halogen, in particular Cl, Br, I, ether, thioether,
disulfide,
sulfoxide, sulfone, amino, aldehyde, keto, carboxylic acid ester, cyano,
alkylsilane and
alkoxysilane groups as well as carboxylamide groups.
In preferred embodiments of the formulation according to the invention, at
least one
polythiophene
A) containing recurring units of the general formula (>) is one containing
recurring
units of the general formula (Ia)
CA 02496286 2005-02-07
STA 246-US
-5-
(Ia)
wherein
R and x have the abovementioned meaning.
In very particularly preferred formulations according to the above
description, x
represents 0 or 1. In the case where x is 1, R particularly preferably
represents methyl or
hydroxymethyl.
In further preferred embodiments of the formulation according to the
invention, at least
one polythiophene containing recurring units of the general formula (I) is one
containing
recurring units of the general formula (Iaa)
(Iaa).
In the context of the invention, the prefix poly- is to be understood as
meaning that more
than one identical or different recurring unit is contained in the polymer or
polythiophene.
The polythiophenes contain a total of n recurring units of the general formula
()7, wherein
n can be an integer from 2 to 2,000, preferably 2 to * 100. The recurring
units of the
1 S general formula (I) can in each case be identical or different within a
polythiophene.
Polythiophenes containing in each case identical recurring units of the
general formula ())
are preferred.
CA 02496286 2005-02-07
STA 246-US
-6-
In the context of the invention, recurring units are units of the general
formulae (I), (Ia) or
(Iaa), summarized as recurnng units of the general formula (I) in the
following, regardless
of whether they are contained once or several times in the polythiophene. That
is to say,
units of the general formula (I) are also to be understood as recurring units
if they are
$ contained in the polythiophene only once.
Formulations according to the invention can also be those which comprise in
the mixture,
in addition to at least one of the polythiophenes A) described above
containing recurring
units of the general formula (I), further conductive polymers A), such as, for
example,
polyanilines or polypyrroles.
The polythiophenes A) preferably in each case carry H on the end groups.
The polythiophenes A) contain a total of n recurring units of the general
formula (I),
wherein n preferably is an integer from 2 to 1,000, preferably 3 to 100,
particularly
preferably 4 to 15.
In the context of the invention, C1-CS-alkylene radicals A are particularly
methylene,
ethylene, n-propylene, n-butylene or n-pentylene. In particular, C,-C~8-alkyl
represents
linear or branched C~-C,$-alkyl radicals, such as, for example, methyl, ethyl,
n- or iso-
propyl, n-, iso-, sec- or tent-butyl, n-pentyl, 1-methylbutyl, 2-methylbutyl,
3-methylbutyl,
1-ethylpropyl, l,l-dimethylpropyl, 1,2-dimethylpropyl, 2,2-dimethylpropyl, n-
hexyl, n-
heptyl, n-octyl, 2-ethylhexyl, n-nonyl, n-decyl, n-undecyl, n-dodecyl, n-
tridecyl, n-
tetradecyl, n-hexadecyl or n-octadecyl, CS-C,2-cycloalkyl represents CS-C~2-
cycloalkyl
radicals, such as, for example, cyclopentyl, cyclohexyl, cycloheptyl,
cyclooctyl,
cyclononyl or cyclodecyl, CS-C,4-aryl represents CS-C,4-aryl radicals, such
as, for
example, phenyl or naphthyl, and C,-C,8-aralkyl represents C,-Cl$-aralkyl
radicals, such
as, for example, benzyl, o-, m- or p-tolyl, 2,3-, 2,4-, 2,5-, 2,6-, 3,4- or
3,5-xylyl or
mesityl. The above list serves to explain the invention by way of example and
is not to be
regarded as conclusive.
The preparation of the polythiophenes A) described above containing recurnng
units of
the general formula (I) is described in principle in EP-A 440 957.
The polymerization of the corresponding monomeric starting compounds is
carried out
with suitable oxidizing agents in suitable solvents. Examples of suitable
oxidizing agents
CA 02496286 2005-02-07
STA 246-US
are iron(IIl) salts, in particular FeCl3 and iron(III) salts of aromatic and
aliphatic sulfonic
acids, HZO2, KZCr20~, KZS20g, Na25208, KMn04, alkali metal perborates and
alkali metal
or ammonium persulfates or mixtures of these oxidizing agents. Further
suitable
oxidizing agents are described, for example, in Handbook of Conducting
Polymers (ed.
Skotheim, T.A.), Marcel Dekker: New York, 1986, vol. 1, 46-57. Particularly
preferred
oxidizing agents are FeCl3, NaZSZ08 and KzS20g or mixtures thereof. The
polymerization is preferably corned out at a reaction temperature of -20 to
100 °C.
Reaction temperatures of 20 to 100 °C are particularly preferred. If
appropriate, the
reaction solution is then treated with at least one ion exchanger.
Suitable solvents for the above mentioned reaction are e.g. polar solvents,
such as, for
example, water, alcohols, such as methanol, ethanol, 2-propanol, n-propanol, n-
butanol,
diacetone alcohol, ethylene glycol, glycerol or mixtures of these. Aliphatic
ketones, such
as acetone and methyl ethyl ketone, aliphatic nitriles, such as acetonitrile,
aliphatic and
cyclic amides, such as N,N-dimethylacetamide, N,N-dimethylformamide (DMF) and
1-
methyl-2-pyrrolidone (NMP), ethers, such as tetrahydrofuran (THF), and
sulfoxides, such
as dimethylsulfoxide (DMSO), or mixtures of these with one another or with the
abovementioned solvents are also suitable.
The corresponding monomeric compounds for the preparation of polythiophenes A)
containing recurnng units of the general formula (I) are lrnown. Their
preparation is
possible, for example, by reaction of the alkali metal salts of 3,4-
dihydroxythiophene-2,5-
dicarboxylic acid esters with the corresponding alkylene dihalides and
subsequent
decarboxylation of the free 3,4-(alkylenedioxy)thiophene-2,5-dicarboxylic
acids (see e.g.
Tetrahedron 1967, 23, 2437 - 2441 and J. Am. Chem. Soc. 1945, 67, 2217 -
2218).
The resulting polythiophenes are very readily soluble or dispersible in the
polar solvents
or solvent mixtures.
The formulations according to the invention comprise, in addition to at least
one partly
fluorinated or perfluorinated polymer C), at least one further polymer C)
containing S03
M+ or COO-M+ groups. Polymers B) containing S031VI+ or COO'M+ groups which are
suitable are preferably those which contain no completely conjugated main
chain, also
abbreviated to non-conjugated in the following. Examples which may be
mentioned of
suitable polymers B) containing S03 M+ or COO-M+ groups are polymeric
carboxylic
acids, such as polyacrylic acids, polymethacrylic acid or polymaleic acids, or
polymeric
CA 02496286 2005-02-07
STA 246-US
_g_
sulfonic acids, such as polystyrenesulfonic acids and polyvinylsulfonic acids.
Copolymers of vinylcarboxylic and vinylsulfonic acids with other polymerizable
monomers, such as acrylic acid esters and styrene, are furthermore also
possible.
Polystyrenesulfonic acid, poly-(styrenesulfonic acid-co-malefic acid) or poly-
(vinylsulfonic acid) are particularly suitable. Very particularly suitable
formulations are
characterized in that they comprise polystyrenesulfonic acid (PSS) as at least
one polymer
B) containing S03 M+ or COO-M+ groups.
These polymers B) are preferably soluble or dispersible in polar solvents,
such as water,
alcohols, such as methanol, ethanol, 2-propanol, n-propanol, n-butanol,
diacetone alcohol,
ethylene glycol and glycerol, aliphatic ketones, such as acetone and methyl
ethyl ketone,
aliphatic nitrites, such as acetonitrile, aliphatic and cyclic amides, such as
N,N-
dimethylacetamide, N,N-dimethylformamide (DMF) and I-methyl-2-pyrrolidone
(NMP),
ethers, such as tetrahydrofuran (THF), and sulfoxides, such as
dimethylsulfoxide
(DMSO), or mixtures containing these, preferably in water, alcohols, such as
methanol,
ethanol, 2-propanol and n-butanol, or mixtures of these.
Particularly suitable formulations according to the above description are
characterized in
that they comprise as at least one partly fluorinated or perfluorinated
polymer C)
containing S03 M+ or COO-M+ groups, for example, those containing recurring
units of
the formulae (II-a) and (II-b)
F F
i I
O-Rf C-C-S03H
F F F F F
---
F F F ~F
(II-a) (II-b)
wherein Rf represents a radical having at least one, preferably 1 to 30
recurring units) of
the formula (II-c)
F CF3
C-O--~- (Il-c)
F F
CA 02496286 2005-02-07
30877-7
-9-
Such perfluorinated polymers C) are, for example, the polymers which are
commercially
obtainable under the trade-mark Nafionm (copolymer of tetrafluoroethylene and
of the
trifluorovinylether of poly{hexafluoro propylene oxide~nono(tetrafluoro vinyl
sulfonic
acid)ethers)or in dissolved form under the trade-mark Liquion~.
S In particularly preferred embodiments, the new formulation according to the
invention
comprises Nafion°° as at least one polymer C) containing S03'M'
or C001VI+ grpups.
Formulations which comprise polystyrenesulfonic acid (PSS) as the polymer B)
containing S03M+ or C0011~f'~ groups and Nafion~ as the partly fluorinated or
perfluorinated polymer C) containing S03 M+ or C001VI~ groups are particularly
preferred.
The molecular weight of the poly-acids is preferably 1,000 to 2,000,000,
particularly
preferably 2,000 to 500,000. The poly-acids or their alkali metal salts are
commercially
obtainable, e.g. polystyrenesulfonic acids and polyacrylic acids, or can be
prepared by
known processes (see e.g. Houben Weyl, Methoden der organischen Chemie, vol. E
20
Makromolekulare Stoffe, part 2, (1987), p. 1141 et seq.).
Very particularly preferred are formulations, in which the weight ratio of
polythio-
phene(s) A) to polymers) B) containing S03'M~ or COO'M+ groups is from 1 to 2
( 1:2)
to 1 to 25 (1:25), preferably 1 to 2 (1:2) to 1 to 10 (1:10).
Furthermore very particularly preferred are formulations, in which the weight
ratio of
polythiophene(s) A) to partly fluorinated or perfluorinated polymers) C)
containing S03
M+ or COO'M+ groups is from 1 to 1 (1:1) to 1 to 15 (1:15), preferably 1 to 2
(1:2) to 1 to
10 (1:10).
All desired combinations of the two weight ratios described above for
polythiophene(s)
A) to polymers) B) containing S03 M+ or COO~M+ groups and polythiophene(s) A)
to
partly fluorinated or perfluorinated polymers) C) containing S03 M~ or COO-M+
groups
can be realized in the preferred formulations and are regarded as disclosed
herewith.
The new formulations can furthermore additionally comprise at least one polar
diluent D)
(polar solvent). In the context of the invention, polar diluents D) (polar
solvents) are to
be understood as meaning diluents having a solubility parameter 8 of 16 MPa'~
and
above, preferably 19 MPa"~ and above. Solubility parameters are as a rule
measured at
CA 02496286 2005-02-07
STA 246-US
- 10-
the standard temperature (20 °C). For measurement and calculation of
solubility
parameters, see J. Brandrup et al., Polymer Handbook, 4th ed., 1999, VI1/675 -
VI1/688.
Solubility parameters are given in tabular form e.g. in J. Brandrup et al.,
Polymer
Handbook, 4th ed., 1999, VII/688 - VII/697. Preferred polar diluents are
water, alcohols,
such as methanol, ethanol, 2-propanol, n-propanol, n-butanol, diacetone
alcohol, ethylene
glycol and glycerol, aliphatic ketones, such as acetone and methyl ethyl
ketone, aliphatic
nitrites, such as acetonitrile, aliphatic and cyclic amides, such as N,N-
dimethylacetamide,
N,N-dimethylformamide (DMF) and 1-methyl-2-pyrrolidone (NMP), ethers, such as
tetrahydrofuran (THF), and sulfoxides, such as dimethylsulfoxide (DMSO), or
mixtures
containing these. Particularly preferred polar diluents D) are water, alcohols
or mixtures
containing these, and water, methanol, ethanol, n-propanol, 2-propanol or n-
butanol or
mixtures containing these are very particularly preferred. In preferred
embodiments, the
new formulations comprise mixtures of water and at least one alcohol as the
polar diluent
D).
Such new preferred formulations comprising at least one polar diluent D)
preferably
comprise 99.99 to 80 wt.%, particularly preferably 99.8 to 95 wt.% of polar
diluent(s) D)
and have a solids content of 0.01 to 20 wt.%, particularly preferably 0.2 to 5
wt.%, i.e.
comprise in total 0.01 to 20 wt.%, particularly preferably 0.2 to 5 wt% of
polythiophene(s) A), polymers B) and C) containing S03-M+ or COO-M+ groups and
optionally further components, such as e.g. binders, crosslinking agents
and/or
surfactants, in dissolved and/or dispersed form.
The viscosity at 20°C of the new preferred formulations comprising at
least one polar
diluent D) is between the viscosity of the diluent and 200 mPas, preferably <
100 mPas.
To establish the desired solids content and the required viscosity, the
desired amount of
diluent can be removed from the formulations by distillation, preferably in
vacuo, or by
other processes, e.g. ultrafiltration.
Organic, polymeric binders and/or organic, low molecular weight crosslinking
agents or
surfactants can moreover be added to the formulations according to the
invention.
Corresponding binders are described e.g. in EP-A-564 911. Examples which may
be
mentioned here are polyvinylcarbazole as binder, silanes, such as Silquest~
A187 (OSi
specialities) as crosslinking agent, or surfactants, such as the
fluorosurfactant FT 248
(Bayer AG).
CA 02496286 2005-02-07
STA 246-US
-11-
'The formulations can preferably comprise only small amounts of ionic
impurities in the
limits such as are described in EP-A-991 303. The formulations preferably
comprise less
than 1,000 ppm of ionic impurities.
The formulations according to the invention can be prepared in a simple
manner. For
example, it is possible to mix an already finished mixture comprising at least
one polymer
B) containing S03 M~ or COO-M+ groups and at least one polythiophene A) with
at least
one partly fluorinated or perfluorinated polymer C) containing S03 M+ or
C001vI~
groups and optionally to add at least one diluent to this mixture, preferably
to completely
or partly dissolve or disperse this mixture in at least one diluent. It is
also possible to add
to an already finished mixture comprising a polymer B) containing S03 M+ or
COO~M+
groups and at least one polythiophene A) at least one diluent D) beforehand,
preferably to
completely or partly dissolve or disperse this finished mixture in at least
one diluent D),
to dissolve or disperse at least one partly fluorinated or perfluorinated
polymer C)
containing S03'M~ or COO-M+ groups in a diluent D) and then to mix the
solutions)
and/or dispersion(s). If appropriate, all or some of the diluent or diluent
mixture D) can
be removed again from this mixture, e.g, by distillation or other processes.
Surprisingly, the formulations according to the invention are outstandingly
suitable for
the production of hole-injecting or hole-transporting layexs in EL
arrangements, organic
solar cells, organic laser diodes, organic thin film transistors or organic
field effect
transistors, for the production of electrodes or electrically conductive
coatings.
The present invention therefore also provides the use of the formulations
according to the
invention for the production of hole-injecting layers in EL arrangements, for
the
production of electrodes or electrically conductive coatings.
These EL-Arrangements can be used as displays, e.g. in flat screens in lap-
tops, pagers,
mobile phones, navigation systems, (car-)radios, (car)-controlpanels, or as
planar beamer,
e.g. in lamps, background lightings of LCD-displays or signboards.
EL arrangements having a hole-injecting layer comprising a formulation
according to the
invention are distinguished in particular by a high luminous intensity
(luminous strength)
and a significantly longer life than lrnown EL arrangements.
CA 02496286 2005-02-07
STA 246-US
-12-
The present invention therefore also provides EL arrangements, in particular
light
emitting diodes comprising a hole-injecting layer comprising a formulation
according to
the invention. These are preferably those EL arrangements comprising at least
two
electrodes, of which optionally at least one is applied to an optionally
transparent
substrate, at least one emitter layer between the two electrodes and at least
one hole-
injecting layer between one of the two electrodes and the emitter layer,
characterized in
that the hole-injecting layer comprises a formulation according to the
invention.
In the production of many EL arrangements of large area, e.g.
electroluminescent display
elements of large area, it is advantageous if at least one of the current-
carrying electrodes
is made of a transparent and conductive material. Examples of suitable such
transparent
and conductive electrode materials are
a) metal oxides, e.g. indium tin oxide (ITO), tin oxide (NESA), doped tin
oxide,
doped zinc oxide etc.,
b) semi-transparent metal films, e.g. Au, Pt, Ag, Cu etc.,
c) semi-transparent conductive polymers, e.g. polythiophenes, polyanilines,
polypyrroles etc.
An electrode which is not made of one of the abovementioned transparent and
conductive
materials is preferably a metal electrode, in particular a metal cathode.
Suitable materials for metal cathodes are customary for electrooptical
constructions and
are known to the expert. Possible metal cathodes are, preferably, those of
metals of low
work of emission, such as Mg, Ca or Ba, or metal salts, such as LiF.
Suitable optionally transparent substrates are, for example, glass, extra-thin
glass (flexible
glass) or plastics, preferably films of plastic.
Particularly suitable plastics for the substrate are: polycarbonates,
polyesters, such as e.g.
PET and PEN (polyethylene terephthalate or polyethylene-naphthalene
dicarboxylate),
copolycarbonates, polyacrylate, polysulfone, polyether sulfone (PES),
polyimide,
polyethylene, polypropylene or cyclic polyolefins or cyclic olefin copolymers
(COC),
hydrogenated styrene polymers or hydrogenated styrene copolymers.
CA 02496286 2005-02-07
STA 246-US
-13-
Suitable polymer substrates can be, for example, films, such as polyester
films, PES films
from Sumitomo or polycarbonate films from Bayer AG (Mala~ofol~.
An adhesion promoter layer can be located between the substrate and the
electrode.
Suitable adhesion promoters are, for example, silanes. Epoxysilanes, such as,
for
example, 3-glycidoxypropyltrimethoxysilane (Silquest~ A187, OSi specialities)
are
preferred. Other adhesion promoters with hydrophilic surface properties can
also be used.
Thus e.g. a thin layer of PEDT:PSS is described as a suitable adhesion
promoter for
PEDT (Hohnholz et al., Chem. Common. 2001, 2444-2445).
The emitter layer of the EL arrangement according to the invention comprises
at least one
emitter material. Suitable emitter materials are those which are customary for
electrooptical constructions and known to the expert. Preferred possible
emitter materials
are conjugated polymers, such as polyphenylene-vinylene and/or polyfluorenes,
such as
the polyparaphenylene-vinylene derivatives and polyf<uorene derivatives
described, for
example, in WO-A 90/13148, or emitters from the class of low molecular weight
emitters,
also called "small molecules" in technical circles, such as aluminium
complexes, e.g.
tris(8-hydroxyquinolinato)aluminium (Alq3), fluorescent dyestuffs, e.g.
quinacridones, or
phosphorescent emitters, e.g. Ir(ppy)3. Emitter materials are described e.g.
in DE-A 196
27 071.
In addition to the abovementioned layers, further functional layers can be
contained in
such an electroluminescent layer build-up (EL arrangement), such as e.g.
further charge-
injecting, e.g, electron-injecting, charge-transporting or charge-blocking
intermediate
layers. Such layer constructions are known to the expert and are described,
for example,
in J.R. Sheats et al., Science 273, (1996), 884. One layer can also take over
several tasks.
For example, the abovementioned emitter materials can be employed in
combination with
a hole-transporting intermediate layer between the hole-injecting and emitter
layer (cf.
e.g. US 4,539,507 and US 5,150,006).
The production in principle of such EL arrangements is known to the expert.
For
example, they can be produced by applying an electrode to a substrate from
solution or
dispersion or by vapour deposition. For example, metal oxide or semi-
transparent metal
film electrodes are preferably applied to the substrate by vapour deposition,
while semi-
transparent, conductive polymer electrodes are preferably applied from
solution or
dispersion. If appropriate, an adhesion promoter can be applied - by vapour
deposition or
CA 02496286 2005-02-07
STA 246-US
- 14-
from solution or dispersion - before application of the electrode material to
the substrate.
Some such substrates coated with electrode material are also already
commercially
obtainable (e.g. K glass, ITO-coated glass substrates). The hole-injecting
layer can then
be applied to the electrode, which in the case of the EL arrangements
according to the
S invention with a hole-injecting layer comprising a formulation according to
the invention
advantageously takes place from solution or dispersion. The further layers are
then
applied to the hole-injecting layer in the sequence given in the introduction -
taking into
account that individual layers can be omitted - from solution or dispersion or
by vapour
deposition, depending on the material employed. The layer arrangement is
contacted and
encapsulated.
The production of the hole-injecting layer comprising a formulation according
to the
invention is carned out by known technologies. For this, a formulation
according to the
invention - optionally in a solvent - is applied as a film to an electrode,
preferably the
base electrode. Suitable solvents are the abovementioned polar diluents D),
preferably
water, alcohols or mixtures of these. Suitable alcohols are e.g. methanol,
ethanol, n-
propanol, 2-propanol and n-butanol.
The use of these solvents has the advantage that further layers can be applied
from
organic solvents, such as aromatic or aliphatic hydrocarbon mixtures, without
the hole-
injecting layer being attacked.
The formulation according to the invention - optionally in a solvent - can be
distributed
uniformly on the electrode, for example, by techniques such as spin-coating,
casting,
knife-coating, printing, curtain casting etc. The layers can then be dried at
room
temperature or temperatures up to 300 °C, preferably 100 to 200
°C.
The formulation according to the invention - optionally in a solvent - can
moreover
preferably be applied in structured form by printing techniques such as ink
jet. This
technique is known to the expert and, with the use of water-soluble and
dispersed
polythiophenes, such as 3,4-polyethylenedioxythiophene:polystyrenesulfonic
acid
(PEDT:PSS), is described e.g. in Science, vol. 279, 1135, 1998 and DE-A 198 41
804.
The formulations according to the invention - if appropriate in a solvent -
are preferably
filtered through a filter before the application.
CA 02496286 2005-02-07
STA 246-US
-15-
Formulations which can be filtered for cleaning purposes particularly easily
are obtained
for example if, in a solvent D) based on one part by weight of
polythiophene(s) A)
containing recurring units of the general formula (I), preferably 1 to 30
parts by weight,
particularly preferably 2 to 25 parts by weight of the polymers) B) containing
S03-M+ or
COO~1VI+ groups are used.
1'he thickness of the hole-injecting layer is, for example, 3 to 500 nm,
preferably 10 to
200 nm.
The influence of a hole-injecting layer comprising a formulation according to
the
invention on the properties of the EL arrangement can be tested in a specific
build-up of
such an EL arrangement according to the invention. For this, the hole-
injecting layer is
applied by means of a spin coater to an TTO substrate which has been cleaned
by wet
chemistry. The layer is then dried at 100-200 °C for 5 min. The layer
thickness is 20-300
nm, depending on the spinning speed. A 1 wt.% strength solution of a
polyfluorene-
based emitter material (Green 1300 LUMATIONTr'' from Dow Chemical Company) in
xylene is spun on as the emitter layer. The thiclrness of the emitter layer is
typically 60-
120 nm. Finally, a Ba layer 5 nm thick and on this an Ag layer 200 nm thick
are vapour-
deposited as the cathode. By contacting of the indium tin oxide (ITO) anode
and the
metal cathode, current/voltage/luminous density characteristic lines are
plotted by means
of a characteristic line recorder and a calibrated photodiode and the lives
are recorded.
For this, the arrangement is charged with a constant electric current or an
alternating
current and the voltage and the luminous density are monitored as a function
of time.
1'he organic light-emitting diodes according to the invention are
distinguished by a long
life, high luminous intensity, low use voltages and a high rectification
ratio. In contrast to
known light-emitting diodes with hole-injecting layers produced from a
poly(3,4-
ethylenedioxythiophene):polystyrenesulfonic acid (PEDT:PSS) dispersion
(Baytron P,
H.C. Starck GmbH), it has been found, surprisingly, that the lives of organic
light-
emitting diodes according to the invention with a hole-injecting layer
comprising a
formulation according to the invention are significantly longer.
CA 02496286 2005-02-07
STA 246-US
EXAMPLES
Example 1
-16-
Preparation of a formulation from poly(3,4-ethylenedioxythiophene)Ipoly-
styrenesulfonic acid and a perfluorinated polymer.
40 g of a 1.32 % strength poly(3,4-ethylenedioxythiophene)/polystyrenesulfonic
acid
solution (H.C. Starck GmbH, Baytron~ P, trial product TP AI 4083, weight ratio
of
PEDT/PSS is 1:6) are mixed with 9.96 g of a 5.30 wt.% strength solution of
Nafion~ in a
mixture of lower aliphatic alcohols and water (Nafion~ perfluorinated ion-
exchange resin,
5 wt.% solution in lower aliphatic alcoholslH20, CAS no. 66796-30-3, Aldrich
order no.
27,470-4, verified solids content 5.30 wt.%). The weight ratio of
PEDTlPSS/Nafion~ is
1:6:7.
Example 2
The formulation according to the invention from example 1 is used to build up
an organic
light-emitting diode (OLED). The procedure for production of the OLED is as
follows:
1. Preparation of the ITO-coated substrate
ITO-coated glass (Merck Balzers AG, FL, part no. 253 674 XO) is cut into
pieces 50 mm
x 50 mm in size (substrates). The ITO layer is structured with the
conventional
photoresist technique and subsequent etching away in FeCl3 solution. The ITO
strips
isolated have a width of 2.0 mm. The substrates are then cleaned in 3 %
strength aqueous
Mucasol solution in an ultrasonic bath for 15 min. Thereafter, the substrates
are rinsed
with distilled water and spun dry in a centrifuge. This rinsing and drying
operation is
repeated 10 times. Directly before the coating, the ITO-coated sides are
cleaned for 10
min in a UV/ozone reactor (PR-100, WP Inc., Cambridge, GB).
2. Application of the hole-injectine laver
About 10 ml of the formulation according to the invention from example 1 are
filtered
(Millipore HV, 0.45 pm). 'The cleaned TTO-coated substrate is placed on a
lacquer spin-
coater and the filtered solution is distributed over the TTO-coated side of
the substrate.
The supernatant solution is then spun off by rotating the plate at 800 rpm
over a period of
CA 02496286 2005-02-07
STA 246-US
-17-
30 s with the lid closed. Thereafter, the substrate coated in this way is
dried for 5 min at
200 °C on a hot-plate. The layer thickness is 85 nm (Tencor, Alphastep
500).
3. Application of the emitter layer
ml of a 1 wt.% strength xylene solution of the emitter Green 1300 LLTMATION~
5 (Dow Chemical Company) are filtered (Millipore HV, 0.45 Nxn) and distributed
over the
dried hole-injecting layer. This and all the further process steps are carried
out in pure NZ
atmosphere (Inert Gas Glovebox System, M. Braun, Garching). The hole injection
layer
is after-dried beforehand in the glove box for a further 5 min at 200
°C. The supernatant
solution of the emitter is then spun off by rotating the plate at 400 rpm for
30 s with the
lid closed. Thereafter, the substrate coated in this way is dried for 15 min
at 130 °C on a
hot-plate. The total layer thickness is 185 nm.
4. Application of the metal cathode
A metal electrode is vapour-deposited an to the emitter layer. The substrate
is placed
with the emitter layer downwards on a strip mask with strips 2.0 mm wide,
which is
orientated perpendicular to the ITO strips. A Ba layer 5 nm thick and then an
Ag layer
200 nm thick are vapour-deposited in succession from two vapour deposition
boats under
a pressure of p = 10-3 Pa. The vapour deposition rates are 10 .$/s for Ba and
20 A/s for
Ag. The active luminous area at the crossing point of the two electrodes is 4
mm2.
5. Encapsulation of the OLEDs
The readily oxidizable cathodes are protected from corrosion by encapsulation.
For this,
the polymeric layers are removed manually at the edge of the substrate using a
scalpel
and a metal cap (35 mm x 35 mm x 2 mm) is glued on with an epoxy adhesive (UHU
Plus, UHU, D) as protection. A moisture absorber (GDO/CA118x10x0.4, SAES
Letters
S.p.A., Italy) is additionally placed in the metal cap.
6. Characterization of the OLED
The two electrodes of the organic LED are connected (contacted) to a voltage
source via
electrical leads. The positive pole is connected to the ITO electrode and the
negative pole
is connected to the metal electrode. The dependence of the OLED current and
the
electroluminescence intensity (detection is with a photodiode (EG&G C30809E))
on the
CA 02496286 2005-02-07
STA 246-US
-18-
voltage is recorded. The lives are then determined by allowing a constant
current of I =
0.32 mA (8 mA/crn2) to flow through the arrangement and monitoring the voltage
and
light intensity as a function of time.
Comparison example 2.1
Production of an OLED with poly(3,4-
ethylenedioxythiophene)/polystyrenesulfonic acid
as the hole-injecting layer:
The procedure is as in example 2, with the following deviation in process step
2.:
2. Application of the hole injection layer
About 10 ml of a 1.3 % strength poly(3,4-
ethylenedioxythiophene)/polystyrenesulfonic
acid solution (H.C. Starck GmbH, Baytron~ P, TP AI 4083) are filtered
(Millipore HV,
0.45 prn). The ITO-coated substrate is then placed on a lacquer spin-coater
and the
filtered solution is distributed over the ITO-coated side of the substrate.
The supernatant
solution is then spun off by rotating the plate at 600 rpm over a period of 30
s with the lid
closed. Thereafter, the substrate coated in this way is dried for 5 min at 200
°C on a hot-
plate. The layer thickness is 85 nm.
The metal cathodes were applied in accordance with process step 4 together
with the layer
construction from example 2 in order to ensure comparability.
Results of the measurements of the lives of the arrangements from example 2
and
comparison examples 2.1 at a constant current (I = 8 mA/cm2).
t=0 t=260
h
U/[V) L/[rel. U/[V) L/[rel.
unit) unit]
OLED from example 3.66 6.81 3.88 6.61
2
OLED from comparison3.71 4.66 4.13 2.59
example 2.1
The EL arrangement according to the invention with the hole-injecting layer
comprising
the formulation according to the invention (example 1 ) is more efficient and
has a
significantly longer life compared with the EL arrangement which is built up
with a hole-
injecting layer of a known material (PEDT:PSS from comparison example 2.1).
After a
CA 02496286 2005-02-07
STA 246-US
-19-
long-term test of 260 h, not only the decrease in the electroluminescence
intensity but
also the increase in voltage is lower.
Example 3.1:
Preparation of a formulation from poly(3,4-ethylenedioxythiophene)/poly-
S styrenesulfonic acid and a perfluorinated polymer.
15 g of a desalinated 1.36 % strength
polyethylenedioxythiophene/polystyrenesulfonic
acid solution (H.C. Starck GmbH, Baytron~ P, TP AI 4083 desalinated) are mixed
with
4.09 g Nafiori solution (Liquion~ 1000, 5 wt.% strength solution in 2-
propanollHzO,
1000 eq., Ion Power Inc., US). The weight ratio of PEDT/PSS to Nafion~
corresponds to
1:1.
Example 3.2:
Preparation of a formulation from poly(3,4-ethylenedioxythiophene)/poly-
styrenesulfonic acid and a perfluorinated polymer.
12 g of a desalinated 1.36 % strength
polyethylenedioxythiophene/polystyrenesulfonic
acid solution (H.C. Starck GmbH, Baytron~ P, TP AI 4083 desalinated) are mixed
with
3.42 g Nafion~ solution (Liquiom 1100, 5 wt.% strength solution in 2-
propanollH20,
1100 eq., Ion Power Inc., US). The weight ratio of PEDTlPSS to Nafion~
corresponds to
1:1.
Example 4.1:
The formulation according to the invention from example 3.1 is used to build
up an
organic light-emitting diode (OLED). The procedure for the production of the
OLED is
as in example 2, with the following deviation in process step 2.:
2. Application of the hole injection layer
About 10 ml of the formulation according to the invention from example 3.1 are
filtered
(Millipore HV, 0.45 N.m). The cleaned ITO-coated substrate is placed on a
lacquer spin-
coater and the filtered solution is distributed over the ITO-coated side of
the substrate.
The supernatant solution is then spun off by rotating the plate at 800 rpm
over a period of
CA 02496286 2005-02-07
STA 246-US
-20-
30 s with the lid closed. Thereafter, the substrate coated in this way is
dried for 5 min at
200 °C on a hot-plate. The layer thickness is 85 nm (Tencor, Alphastep
500).
Examgle 4.2:
The formulation according to the invention from example 3.2 is used to build
up an
organic light-emitting diode (OLED). The procedure for the production of the
OLED is
as in example 2, with the following deviation in process step 2.:
2. Application ofthe hole injection la'rer
About 10 ml of the formulation according to the invention from example 3.2 are
filtered
(Millipore HV, 0.45 pm). The cleaned ITO-coated substrate is placed on a
lacquer spin-
coater and the filtered solution is distributed over the ITO-coated side of
the substrate.
The supernatant solution is then spun off by rotating the plate at 800 rpm
over a period of
30 s with the lid closed. Thereafter, the substrate coated in this way is
dried for 5 min at
200 °C on a hot-plate. The layer thickness is 85 nm (Tencor, Alphastep
500).
Comparison example 4.3:
Production of an OLED with poly(3,4-
ethylenedioxythiophene)/polystyrenesulfonic acid
as the hole-injecting layer:
The procedure is as in example 2, with the following deviation in process step
2.:
2. Application of the hole injection layer
About 10 ml of a desalinated 1.36 % strength poly(3,4-
ethylenedioxythiophene)/poly-
styrenesulfonic acid solution (H.C. Starck GmbH, Baytron~ P TP AI 4083) are
filtered
(Millipore HV, 0.45 Eun). The ITO-coated substrate is then placed on a lacquer
spin-
coater and the filtered solution is distributed over the ITO-coated side of
the substrate.
The supernatant solution is then spun off by rotating the plate at 600 rpm
over a period of
s with the lid closed. Thereafter, the substrate coated in this way is dried
for 5 min at
25 200 °C on a hot-plate. The layer thickness is 85 nm.
The metal cathodes were applied in accordance with process step 4 together
with the layer
constructions from examples 4.1 and 4.2 in order to ensure comparability.
CA 02496286 2005-02-07
STA 246-US
-21 -
Results of the measurements of the lives of the arrangements from examples
4.1, 4.2 and
comparison example 4.3 at a constant current (I = 24 mA/cmz).
t=0 t= 100h
U![V] L/[rel. U/[V] L![rel.
unit] unit]
OLED from example 4.19 7.58 4.40 6.95
4.1
OLED from example 4.30 7.67 4.51 7.02
4.2
OLED from comparison4.02 6.29 4.43 4.75
example 4.3
The EL arrangements according to the invention with the hole-injecting layer
comprising
the formulations according to the invention (examples 4.1 and 4.2) are more
efficient and
have significantly longer lives compared with the EL arrangement which is
built up with
a hole-injecting layer of a known material (PEDT:PSS from comparison example
4.3).
After a long-term test of 100 h at a high device current, not only the
decrease in the
electroluminescence intensity but also the increase in voltage is lower in the
EL
arrangements according to the invention.
Although the invention has been described in detail in the foregoing for the
purpose of
illustration, it is to be understood that such detail is solely for that
purpose and that variations .
can be made therein by those skilled in the art without departing from the
spirit and scope of
the invention except as it may be limited by the claims.