Note: Descriptions are shown in the official language in which they were submitted.
CA 02496467 2005-02-21
WO 2004/018359 PCT/EP2003/008333
1
Stabilized, acdueous silicon dioxide dispersion
The invention relates to an aqueous dispersion containing
silicon dioxide, which is stable in the acid pH range, its
production and use. The invention further relates to a
powder that can be used to produce the dispersion.
Silicon dioxide dispersions are generally not stable in the
acid pH range. A possibility for stabilizing such
dispersions is offered for example by the addition of
aluminium compounds.
WO 00/20221 discloses an aqueous silicon dioxide solution
that is stable in the acid range. It is produced by
bringing silicon dioxide particles into contact with
aluminium compounds in an aqueous medium. The quantity of
aluminium compound required to produce the dispersion
claimed in the '221 application can be tracked by
increasing the zeta potential and is achieved at the point
where the rise in the zeta potential curve moves towards
zero or a plateau is reached. The '221 application also
claims dispersions in which the zeta potential achieves
only 50~ of the maximum achievable value. In every case,
the zeta potential of the claimed dispersions has strongly
positive values of up to 30 mV. This means that the
originally negatively-charged silicon dioxide particles
have been completely cationized by the addition of the
aluminium compound. Although the claimed dispersion has
good stability, it is no longer a silicon dioxide
dispersion as the surface is covered with positively-
charged aluminium species. This is a disadvantage in
applications in which the dispersion is brought into '
contact with anionic substances or dispersions. This can
lead, for example, to unwanted flocculation or
sedimentation.
US 2,892,797 on the other hand, discloses an aqueous
silicon dioxide dispersion, which is stabilized by
CA 02496467 2005-02-21
WO 2004/018359 PCT/EP2003/008333
2
treatment with an alkali metalate. Sodium aluminate is
preferred in particular. Stabilization takes place through
the anion, for example [A1(OH)4]-. The dispersions are
normally stable in a pH range of 5 to 9. The zeta potential
of the powder thus treated is negative. The subsequent
removal of the ration, by ion exchange processes for
example, can be a disadvantage of this process. For special
applications, such as for example chemical-mechanical
polishing, alkali rations are generally undesirable. A
further disadvantage is the low stability in more acid
media below pH 5.
The object of the invention is to provide a silicon dioxide
dispersion that is stable in the acid range, without
changing the properties of the silicon dioxide powder by
reversing the charge on the particle surface.
The invention provides an aqueous dispersion containing
silicon dioxide powder with a silicon dioxide content of 10
to 60 wt.~, wherein
- the dispersion is stable in a pH range of 2 to 6,
- the dispersion additionally contains at least one
compound, which is at least partially soluble in
aqueous solution in the pH range 2 to 6 in the form
of polyvalent rations, the rations being stable in
a silicate-like environment as an anionic component
of the particle surface of the silicon dioxide
powder,
- the quantity of ration-providing compound in
relation to the surface of the silicon dioxide is
0.001 to 0.1 mg ration-providing compound/m2 silicon
dioxide surface, the ration-providing compound
being calculated as oxide and
the zeta potential of the dispersion has values of
less than or equal to zero.
CA 02496467 2005-02-21
WO 2004/018359 PCT/EP2003/008333
3
The zeta potential is the outwardly-active potential of the
particles and represents a measure of the electrostatic
interaction between individual particles. It plays a part
in the stabilization of suspensions and in particular of
dispersions containing dispersed, ultra-fine particles. The
zeta potential can be determined, for example, by measuring
the colloidal vibration current (CVI) of the dispersion or
determining its electrophoretic mobility.
Cation-providing compounds according to the invention are
understood always to be those that are at least partially
soluble in aqueous solution in the pH range 2 to 6 in the
form of polyvalent rations, these rations being stable in a
silicate-like environment as an anionic centre. These are
compounds having Ca, Sr, Ba, Be, Mg, Zn, Mn, Ni, Co, Sn,
Pb, Fe, Cr, A1, Sc, Ce, Ti and Zr as ration.
A silicate-like environment is understood to mean that the
rations of the above-mentioned metals are present in the
form of metal-oxygen bonds with the silicon atoms of the
silicon dioxide surface. They can also replace silicon
atoms in the silicon dioxide structure.
An anionic component is understood to be a component, which
does not change the negative charge of the surface of a
silicon dioxide powder, measured as zeta potential, or
which shifts it towards more negative values.
Stable is understood to mean that the particles of the
silicon dioxide powder do not agglomerate further in the
dispersion and the viscosity of the dispersion does not
change or changes only slightly (increase in viscosity of
less than 10~) within a period of at least one week.
Preferred ration-providing compounds are amphoteric
compounds with Be, Zn, Al, Pb, Fe or Ti as ration and
mixtures of these compounds.
CA 02496467 2005-02-21
WO 2004/018359 PCT/EP2003/008333
4
Amphoteric compounds are those that, at a given pH, act as
a base in relation to a stronger acid and as an acid in
relation to a stronger base.
Cation-providing compounds preferred in particular are
aluminium compounds, such as for example aluminium
chloride, aluminium hydroxychlorides of the general formula
A1(OH)XC1 in which x=2-8, aluminium chlorate, aluminium
sulfate, aluminium nitrate, aluminium hydroxynitrates of
the general formula Al(OH)XN03 in which x=2-8, aluminium
acetate, alums such as aluminium potassium sulfate or
aluminium ammonium sulfate, aluminium formiate, aluminium
lactate, aluminium oxide, aluminium hydroxide acetate,
aluminium isopropylate, aluminium hydroxide, aluminium
silicates and mixtures thereof. Aluminium silicate can, for
example, be Sipernat 820 A from Degussa AG, which is a
fine-particle aluminium silicate containing ca 9.5 wt.~
aluminium as A1203 and ca 8 wt.~ sodium as Na20, or a sodium
aluminium silicate in the form of a zeolite A.
There is no restriction on the type of silicion dioxide
powder in the dispersion according to the invention. Thus
silicon dioxide powders produced by sol-gel processes,
precipitation processes or pyrogenic processes can be used.
It can be a metal oxide powder completely or partially
encased in silicon dioxide, provided that its zeta
potential is equal to or less than zero in the pH range
2 to 6.
Pyrogenically-produced silicion dioxide powder is
preferred.
Pyrogenically according to the invention is understood to
mean the formation of silicon dioxide by flame hydrolysis
of a compound or compounds containing silicon in the gas
phase in a flame produced by the reaction of a combustion
gas and an oxygen-containing gas, preferably air. Suitable
silicon-containing compounds are for example silicon
tetrachloride, methyltrichlorosilane, ethyltrichlorosilane,
CA 02496467 2005-02-21
WO 2004/018359 PCT/EP2003/008333
propyltrichlorosilane, dimethyldichlorosilane,
alkoxysilanes and mixtures thereof. Silicon tetrachloride
is preferred in particular. Suitable combustion gases are
hydrogen, methane, ethane, propane, hydrogen being
5 preferred in particular. During flame hydrolysis, highly-
disperse, non-porous primary particles are formed first,
which grow together as the reaction progresses to form
aggregates, which can further combine to form agglomerates.
The surface of the pyrogenically-produced silicon dioxide
particles has silanol groups(Si-OH) and siloxane groups
(Si-O-Si).
Pyrogenically-produced silicon dioxide powders also include
doped silicon dioxide powders and pyrogenically-produced
silicon-metal mixed oxide powders, provided that their zeta
potential is less than or equal to zero in the pH range
2 to 6.
The production of doped powders is disclosed for example in
DE-A-196 50 500. Typical doping components are for example
aluminium, potassium, sodium or lithium. The content of the
doping component is generally no greater than 1 wt.~.
Pyrogenically-produced mixed oxide powders are understood
to mean those in which both precursors of the mixed oxide
are hydrolyzed together in the flame. Typical mixed oxide
powders are silicon-aluminium mixed oxides or silicon-
titanium mixed oxides.
According to a particular embodiment, the ratio of the
cation-providing compound to the surface of the silicon
dioxide is preferably 0.0025 to 0.04, and in particular
0.005 to 0.02 mg cation-providing compound/mz silicon
dioxide surface.
The silicon dioxide surface corresponds to the specific
surface area of the silicon dioxide powder determined
according to DIN 66131. The BET specific surface area can
CA 02496467 2005-02-21
WO 2004/018359 PCT/EP2003/008333
6
be 5 to 600 m2/g, the range 30 to 400 m2/g being preferred
and the range 50 to 300 m2/g being preferred in particular.
The pH value of the dispersion according to the invention
is 2 to 6. It is preferably 3 to 5.5. In particular, at a
BET specific surface area of the silicon dioxide powder of
up to 50 m2/g it can be 3 to 4, at a BET specific surface
area of 50 to 100 mz/g it can be 3.5 to 4.5, at a BET
specific surface area of 100 to 200 m2/g 4 to 5 and at a
BET specific surface area of more than 200 m2/g 4.5 to 5.5.
The pH value can, if necessary, be set using acids or
bases. Preferred acids are hydrochloric acid, sulfuric
acid, nitric acid or carboxylic acids, such as for example
acetic acid, oxalic acid or citric acid. Preferred bases
are alkalihydroxides, such as KOH or NaOH, ammonia,
ammonium salts or amines. If necessary, buffer systems can
be formed by adding salts.
According to a particular embodiment, at a shear energy of
1.28 s-1, the viscosity of the dispersion according to the
invention can be at least 10~ lower than the viscosity of a
dispersion of the same composition, which does not,
however, contain a cation-providing compound. The viscosity
is preferably 25~, in particular 50~, lower than that of a
dispersion of the same composition, which does not,
however, contain a cation-providing compound.
According to a particular embodiment, the number of
agglomerates over 1 ~m in size in the dispersion according
to the invention can be at least 50~ lower than the number
in a dispersion of the same composition, which does not,
however, contain a cation-providing compound. The number of
agglomerates over 1 ~m in size is preferably 75~, in
particular 90~, lower than in a dispersion of the same
composition, which does not, however, contain a cation-
providing compound.
CA 02496467 2005-02-21
WO 2004/018359 PCT/EP2003/008333
7
The dispersion according to the invention can further
contain preservatives. Suitable preservatives are, for
example, benzylalcohol mono(poly)hemiformal,
tetramethylolacetylened'iurea, formamide monomethylol,
trimethylolurea, N-hydroxymethylformamide, 2-bromo-2-
nitropropane-1,3-diol, 1,6-dihydroxy-2,5-dioxahexane,
chloromethylisothiazolinone, orthophenylphenol,
chloroacetamide, sodium benzoate, octylisothiazolone,
propiconazol, iodopropinyl butylcarbamate, methoxycarbonyl
aminobenzimidazole, 1,3,5-triazine derivatives,
methylisothiazolinone, benzoisothiazolinone and mixtures of
these.
The invention further provides a process for the production
of the dispersion according to the invention, wherein the
silicon dioxide powder and at least one cation-providing
compound in a quantity of 0.001 to 0.1 mg cation-providing
compound/mz silicon dioxide surface are brought into
contact whilst moving in an aqueous solution.
Bringing into contact whilst moving is understood to mean,
for example, stirring or dispersing. Dissolvers, toothed
gear disks, rotor-stator machines, ball mills or
mechanically agitated ball mills, for example, are suitable
for dispersal. Higher energy inputs are possible with a
planetary kneader/mixer. However the effectiveness of this
system depends on the mixture processed having a
sufficiently high viscosity to incorporate the high shear
energies required to disperse the particles. High-pressure
homogenizers can be used to obtain aqueous dispersions with
aggregate sizes in the dispersion of less than 200 nm.
With these devices at least two pre-dispersed suspension
streams under high pressure are released through a nozzle.
The~two dispersion jets collide with each other exactly and
the particles mill themselves. In another embodiment the
pre-dispersion is also placed under high pressure, but the
collision of the particles takes place against armoured
CA 02496467 2005-02-21
WO 2004/018359 PCT/EP2003/008333
8
areas of wall. The operation can be repeated as often as
desired to obtain smaller particles.
The process for the production of the dispersion according
to the invention can be carried out in such a way that the
cation-providing compound, in solid form or as an aqueous
solution, is added to an aqueous dispersion of silicon
dioxide.
It can also be carried out in such a way that the silicon
dioxide powder is added to an aqueous solution of the
cation-providing compound at once or in portions.
Furthermore, it is possible to add the silicon dioxide
powder and the cation-providing compound to the liquid
dispersion phase at the same time, in portions or
continuously.
In this case, "at the same time" is understood to mean that
the silicon dioxide powder and the cation-providing
compound can be pre-mixed in the form of a physical or
chemical mixture.
The invention further provides a powder of this type,
containing at least one cation-providing compound and
silicon dioxide powder, the content of cation-providing
compound, calculated as oxide, being 0.001 to 0.1 mg
cation-providing compound/m2 silicon dioxide surface.
Included in this are typical impurities of the starting
materials and impurities introduced during production. The
content of impurities is less than 1 wt.~, and normally
less than 0.1 wt.~.
The cation-providing compound is preferably an aluminium
compound and the silicon dioxide a pyrogenically-produced
silicon dioxide powder.
The powder according to the invention can be incorporated
rapidly into aqueous media.
CA 02496467 2005-02-21
WO 2004/018359 PCT/EP2003/008333
9
In the simplest case, it can be produced by physical mixing
of silicon dioxide powder and at least one cation-providing
compound. Here it is useful to use individual packages
completely. Consequently it is not necessary to have
present a homogeneous distribution of silicon dioxide and
cation-providing compound.
The powder according to the invention can further be
obtained also by spraying onto the silicon dioxide powder
at least one compound that is soluble in the pH range of <6
or that provides cations by chemical reaction in the pH
range <6. The solution of the cation-providing compound can
be sprayed on in heated mixers and dryers with spray
devices, either continuously or in batches. Suitable
devices are, for example: plough mixers, disk- or fluidized
bed dryers.
The solution of the cation-providing compound can be
sprayed on with an ultrasound nozzle or atomized. The mixer
can optionally also be heated.
Furthermore, the powder according to the invention can be
obtained by separating a cation-providing compound, for
example aluminium chloride, from vapour in a fluidized bed
or mixer.
The invention further provides the use of the dispersion
according to the invention for chemical-mechanical
polishing of metal surfaces, in particular polishing of
copper surfaces, for the production of ink-jet papers, for
gel batteries, for clarifying/fining wine and fruit juices,
for water-based dispersion paints to improve the suspension
behaviour of pigments and fillers and to increase scratch-
resistance, to improve the stability and "-blackness" of
carbon black dispersions for ink-jet inks, to stabilize
emulsions and dispersions in the field of biocides, as a
reinforcing agent for natural latex and synthetic latexes,
to produce latex/rubber articles such as gloves, condoms,
infant soothers or foamed rubber, in the sol-gel field, to
CA 02496467 2005-02-21
WO 2004/018359 PCT/EP2003/008333
remove surface stickiness (anti-blocking), to achieve an
anti-slip effect in paper and cardboard, to improve slip
resistance, to produce optical fibres, to produce quartz
glass.
5 It is,surprising that a silicon dioxide dispersion brought
into contact with a cation-providing compound has good
stability in the acid range and at the same time the
surface of the silicon dioxide particle retains, or even
strengthens, its negative surface charge.
10 The mechanism of this stabilization has not yet been
explained. However, it must differ from that disclosed in
WO 00/20221. Here, the charge of silicon dioxide particles
is completely reversed by positively-charged aluminium
species, giving the particles a positively-charged shell.
The mechanism must also differ from that disclosed in
US 2,892,797. Here, a negatively-charged metalate ion is
incorporated into the surface of a silicon dioxide
particle. Although the particles thus changed, like the
particles in the dispersion according to the invention,
have a negative surface charge, they have little stability
in the acid pH range.
CA 02496467 2005-02-21
WO 2004/018359 PCT/EP2003/008333
11
Examples
Analysis methods
The zeta potential is determined with a DT-1200 type device
from Dispersion Technology Inc., by the CVI method.
The viscosity of the dispersions was determined with a
Physica MCR 300 rotation rheometer and CC 27 measuring
beaker, measurement taking place at shearing rates of 0.01
to 500 s-1 and 23°C. The viscosity is given at a shearing
rate of 1.28 s-1. This shearing rate lies in a range in
which structurally viscous effects have a clear impact.
The particle/agglomerate sizes were determined with Horiba
LB 500 and LA 300 devices, or a Malvern Zetasizer 3000 Hsa.
Dispersal
The dispersion devices used were, for example, a Dispermat
AE-3M type dissolver from VMA-GETZMANN with a dissolver
disk diameter of 80 mm or an Ultra-Turrax T 50 type
rotor/stator dispersing unit from IKA-WERKE with S50N -
G45G dispersing tools. When using rotor-stator devices, the
charge container is cooled to room temperature.
Dispersal can also be carried out using a high-energy mill.
For charges containing 50 kg silicon dioxide powder each, a
portion of the DI water is placed into a 60 1 special steel
charge container. The corresponding quantity of Aerosil
powder is sucked in using an Ystrahl Conti-TDS 3 dispersion
and suction mixer and roughly pre-dispersed. During powder
intake, a pH value of 3.5 +- 0.3 is maintained by adding
sodium hydroxide solution and aluminium chloride solution.
After powder intake, dispersion is completed with the Conti
TDS 3 (stator slit width of 4 mm) with a closed suction
nozzle at maximum speed. Before rotor/stator dispersal the
pH of the dispersion was set at 3.5 by adding more sodium
hydroxide solution and this remained the same after
CA 02496467 2005-02-21
WO 2004/018359 PCT/EP2003/008333
12
dispersing for 15 minutes. By adding the remaining quantity
of water, an Si02 concentration of 20 wt.~ was set. This
pre-dispersion is milled in an HJP-25050 Ultimaizer System
high-energy mill from Sugino Machine Ltd., at a pressure of
250 Mpa and a diamond nozzle diameter of 0.3 mm and two
passes through the mill.
Chemicals
The aerosil types 50,90,200 and 300 from Degussa AG were
used as the silicon dioxide powder. A1C13 in the form of
the hexahydrate was used as the water-soluble aluminium
compound. A 1 wt.~ solution, in relation to A1203, was used
to simplify dosing and homogenization. A 1 N NaOH solution
or a 1 N HCL solution was used to correct the pH.
To allow comparisons between the viscosity of the
dispersions, a uniform pH value of 3.5 is optionally set by
adding a further 1 N NaOH.
Dispersioas
The various charge sizes and the properties of the
dispersions obtained are given in Tables 1 and 2.
Example 1a (Refereace example)
100 g Aerosil 50 (BET specific surface area ca 50 m2/g)
were incorporated in portions into 385 g DI water using a
dissolver at a setting of ca 1800 rpm. This produced a pH
value of 3.5. The remaining 15 g DI water were then added
to achieve a 20 percent dispersion and this was then
dispersed for 15 minutes at 2000 rpm and 15 minutes using
an Ultra Turrax at ca 5000 rpm.
CA 02496467 2005-02-21
WO 2004/018359 PCT/EP2003/008333
13
Examples lb-g
100 g Aerosil 50 were incorporated in portions into 385 g
DI water and 1.25 g of a 1 wt.~ aqueous aluminium chloride
solution (in relation to aluminium oxide), using a
dissolver at a setting of ca 1800 rpm. This produced a pH
value of 3.4, which was set at pH 3.5 by adding 0.7 g 1N
NaOH. The remaining 13.1 g water were then added to achieve
a 20 wt.~ dispersion and this was then dispersed for 15
minutes at 2000 rpm and 15 minutes with an Ultra Turrax at
ca 5000 rpm.
Examples lc-g were carried out in the same way as 1b.
Example 2a (Refereace example)
100 g Aerosil 90 were incorporated in portions into 370 g
Dl.water using a dissolver at a setting of ca 1800 rpm and
were then dispersed for 15 minutes at 2000 rpm. The pH
value was then set at 3.5 using 1N HC1 and the dispersion
was dispersed for 1f minutes with an Ultra Turrax at ca
5000 rpm. The remaining water was then added to achieve a
20 wt.~ dispersion and the pH value was re-set to 3.5.
Example 2b
100 g Aerosil 90 were incorporated alternately, in
portions, into 370 g DI water using a dissolver at a
setting of ca 1800 rpm and then dispersed at a setting of
ca 2000 rpm. 2.50 g of a 1 wt.~ solution (in relation to
aluminium oxide) of aluminium chloride were then added
whilst dispersing with an Ultra Turrax at ca 5000 rpm and
this was dispersed for 15 minutes. 26.3 g DI water and
1.24 g 1N NaOH were then added to obtain a 20 wt.~
dispersion with a pH value of 3.5.
CA 02496467 2005-02-21
WO 2004/018359 PCT/EP2003/008333
14
Example 3a (Reference exaaSple)
250 g DI water and 20 g of a 1 wt.~ aqueous aluminium
chloride solution (in relation to aluminium oxide) were
provided. Aerosil 90 was added in portions using a
dissolver. The pH value was maintained at 3.5 during this
process. After adding ca 40 g Aerosil 90 powder, the
dispersion thickened very strongly making further additions
impossible.
Examples 3b,3c
100 g Aerosil 90 were incorporated into 250 g DI water
using a dissolver and 10 g of a 1 wt.~ aqueous aluminium
chloride solution (in relation to aluminium oxide) and 1N
NaOH were added alternately in portions so that the pH
value was 3.3 to 4.2. A further 100 g Aerosil 90 and a
further 10 g of a 1 wt.~ aluminium chloride solution (in
relation to aluminium oxide) and sufficient 1N NaOH were
then added alternately in portions using an Ultra-Turrax at
5000 rpm to produce a pH value of 3.5 at the end of
addition.
In example 3c a pH value of 4.0 was set.
Example 4 (Reference example)
50 g Aerosil 90 were incorporated in portions into 350 g DI
water using a dissolver at a setting of ca 1800 rpm and
were then dispersed for 15 minutes at 2000 rpm. 100 g of a
1 wt.~ solution (in relation to aluminium oxide) of
aluminium chloride were then added whilst dispersing in an
Ultra Turrax at ca 5000 rpm and dispersed for 15 minutes
and the pH value of 2 was increased to a pH of 3.5 using
30~ sodium hydroxide solution.
Examples 5
Example 5a was carried out in the same way as 2a. Examples
5b-d were carried out in the same way as 2b. For examples
CA 02496467 2005-02-21
WO 2004/018359 PCT/EP2003/008333
5e-g, ca 100 ml of the dispersion of example 5d was brought
up to the pH value given in Table 2 with 30~ sodium
hydroxide solution added drop-wise, homogenized for ca 5
minutes with a magnetic stirrer and the zeta potential of
5 each was measured.
Examples 6
Example 6a was carried out in the same way as 2a. Examples
6b-d were carried out in the same way as 2b.
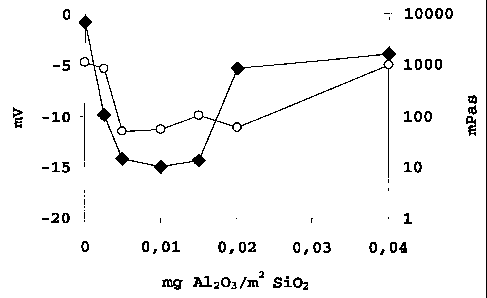
10 Fig. 1 shows the zeta potential in mV (~) and the viscosity
in mPas (o) of examples 1a-g as a function of mg A1203/m2
SiOz-surface .
CA 02496467 2005-02-21
WO 2004/018359 PCT/EP2003/008333
16
Example 7 - powder production
500g silicon dioxide powder (Aerosil 200, Degussa) were
added to a 20 1 Lodige mixer. 20g of a 5 wt.~ (in relation
to A1203) aluminium chloride solution with a spray output
of ca 100 ml/h were applied at a speed of 250 rpm within
10-15 min. The powder has 0.01mg A1203/m2 silicon dioxide
surface, a BET specific surface area of 202 m2/g and a
tamped density of ca 60 g/1. The water content is ca 4~ and
can, if desired, be reduced by heating the wiper or by
subsequent drying in a drying cabinet, revolving tube or
fluidized bed. An aqueous dispersion (20 wt.-~) has a pH
value of 2.6.
CA 02496467 2005-02-21
WO 2004/018359 PCT/EP2003/008333
17
Table 1: Aqueous Aerosil dispersions~l~
Example BET SiOa A1a03~a~ DI-Ha0 Total 1N NaOH
provided DI_Ha0
[m2/gl [mgl [gl [gl [g)
la 50 0 375 400 -
1b 50 12.5 375 398.1 0.7
1c 50 25 375 395.8 1.7
ld 50 50 375 393.1 1.9
1e 50 75 375 389.1 2.0
if 50 100 375 387.9 2.1
1g 50 200 375 376.8 2.5
2a 90 0 370 399 . 8 0 . 2 ~3~
2b 90 25 370 396.3 1.2
2c 90 100 370 385.0 5.0
2d 90 200 370 370.9 9.1
3a 90 200 250 - -
3b 90 200 250 268.8 11.2
3c 90 200 250 268.3 11.7
4 90 1000 350 - 7 4~
5a 200 0 360 399.9 0.1~3~
5b 200 25 360 396.0 1.5
5c 200 100 360 382.9 7.1
5d 200 200 360 367.2 12.8
5e 200 200 360 367.2 12.5 5'
5f 200 200 360 367.2 12.5 5
5g 200 200 360 367.2 12.5.5'
6a 300 0 330 399 . 8 0.2 ~3~
6b 300 200 330 368.6 11.4
6c 300 300 330 352.0 18.0
6d 300 400 330 331.2 28.8
1) in all trials 100 g Si02 each, except 3: 200 g and 4: 50 g; Ex. 1:
Aerosil 50, Ex. 2,3,4: Aerosil 90, Ex. 5: Aerosil 200, Ex.6:
Aerosil 300, all Degussa AG;
2 ) A1z03 used as A1C13;
3) 1N HC1 instead of 1N NaOH;
4) 30$ sodium hydroxide solution;
5) additionally a few drops of 30~ sodium hydroxide solution to
achieve the corresponding pH value in Table 2.
CA 02496467 2005-02-21
WO 2004/018359 PCT/EP2003/008333
18
Table 2: Analytical data of the dispersioas~l~
Example SiOa A1a03/SiOa pH of Zeta- Visco-
Content Dispersion Potential sity~'~
[wt.~] [mglm ] [mV] [mPas]
1a 20 0 3.6 -0.8 1142
1b 20 0.0025 3.5 -9.8 846
1c 20 0.0050 3.4 -14.2 50
1d 20 0.0100 3.3 -15.0 55
1e 20 0.0150 3.4 -14.4 104
1f 20 0.0200 3.0 -5.3 60
1g 20 0.0400 3.1 -3.9 970
2a 20 0 3.3 -0.5 246
2b 20 0.0028 3.2 -10.0 95
2c 20 0.0111 3.1 -9.9 40
2d 20 0.0222 3.0 -6.5 82
3a 0.0111 - - -
3b 40 0.0111 3.2 -11.4 205
3c 40 0.0111 4.0 -13.3 n.d.
4 10 0.222 3.3 + 25 n.d.
5a 20 0 3.5 3 -0.4 3
5b 20 0.0013 3.4 -4.1 1080
5c 20 0.0050 3.0 -6.2 672
5d 20 0.0100 3.1 -6.3 864
5e 20 . 0.0100 3.4 -7.75 n.d:
5f 20 0.0100 5.0 -9.5 n.d.
5g 20 0.0100 6.0 -11.3 n.d.
6a . 20 0 - - (5)
6b 20 0.0067 3.3 -6.6 1520
6c 20 0.0100 3.3 -6.7 537
6d 20 0.0133 3.4 -5.2 1160
1) measured after one week;
2) shearing rate of 1.28 s-1;
3) no 40 percent dispersion could be produced;
4) n.d. = not determined;
5) dispersion gelled