Note: Descriptions are shown in the official language in which they were submitted.
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
COMPOSITIONS AND METHODS OF THERAPY FOR CANCERS
CHARACTERIZED BY EXPRESSION OF THE TUMOR-ASSOCIATED
ANTIGEN MN/CA IX
FIELD OF THE INVENTION
The present invention is directed to compositions and methods of therapy for
the treatment of cell proliferative disorders, more particularly antibodies
and small
molecules that target MN/CA IX enzymatic activity on neoplastic cells that
express
this protein and methods of their use in treatment of cancers characterized by
expression of this tumor-associated antigen.
BACKGROUND OF THE INVENTION
Carbonic anhydrases (CAs) catalyze the interconversion of carbon dioxide and
bicarbonate. These enzymes play a key role in physiological,processes
involving
water and electrolyte balance, COa and HC03- transport, and pH regulation. The
novel membrane-associated CA isoenzyme CA IX was initially described as the
tumor-associated membrane antigen designated MN. Recognition of MN as being a
putative cell-adhesion molecule that has an extracellular domain with the
essential
structural features and activity of CAs (Pastorek et al. (1994) Oncogene
9:2877-2888;
Opavsky et al. (1996) Gezzonzics 33:480-487; Zavada et al. (1997) Izztl. J.
Oncol.
10:857-863) resulted in the classification of MN as the ninth member of the
carbonic
anhydrase family, renamed CA IX. Since then, a kidney-cancer-associated
antigen
designated 6250 has been cloned and identified as a transmembrane protein
identical
to the tumor-associated antigen MNICA IX (Grabmaier et al. (2000) Izat. J.
Cazzce~
85:865-870).
Though originally detected in the human cervical carcinoma cell line HeLa
and in a number of human carcinomas, CA IX (MN/G250) is also present in normal
gastric, intestinal, and biliary mucosa (Pastorekova et al. (1997)
Gastz°oerzte>"ology
112:398-408), more notably in rapidly proliferating normal cells in the small
intestine
(Saarnio et al. (1998) J. Histoclzezzz. C,ytoclzezn. 46:497-504). The presence
of CA IX
protein is almost 100% associated with cervical carcinomas (Liao et al. (1994)
Am J.
-1-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
Pathol. 145:598-609), esophagus carcinomas (Turner et al. (1997) Hum. Patlzol.
28:740-744), and with renal clear cell carcinomas (Liao et al. (1997) Cancer
Res.
57:2827-2831) in human patients. It has also been detected in a high
percentage of
colorectal (Saarnio et al. (1998) Am. J. Pathol. 153:279-285) and lung
(Vermylen et
al. (1999) Eu.~. Respir. J. 14:806-811) carcinomas of human patients.
Expression of CA IX in vitro is upregulated by cell density in HeLa cells and
correlates with tumorigenicity in HeLa cell/fibroblast cell hybrids (Zavada et
al.
(1993) Int. J. Cancer 54:268-274). Further, its expression in NIH3T3 cells
promotes
cell proliferation (Pastorek et al. (1994) Ohcogehe 9:2877-2888). Conditions
of
hypoxia induce expression of CA IX in tumors and cultured tumor cells,
indicating
that this protein may be a biomarker for hypoxia in some tumors (Ivanov et al.
(2001)
Am. J. Pathol. 158(3):905-919; Beasley et al. (2001) Cancer Res. 61(13):5262-
5267).
This correlation between CA IX expression, hypoxia, and extracellular
acidification
have led to the suggestion that expression of CA IX may help to maintain the
extracellular acidic pH in tumors, thereby providing a conducive environment
for
tumor growth and proliferation as well as enhancing tumor resistance to
radiotherapy
and chemotherapy (Ivanov et al. (2001) Am. J. Pathol. 158(3):905-919; Beasley
et al.
(2001) Cahcef°Res. 61(13):5262-5267).
Given its key role in tumor proliferation and progression, compositions and
methods for inhibiting the activity of CA IX are needed to provide effective
treatment
for cancers that are characterized by expression of this protein.
BRIEF SUMMARY OF THE INVENTION
Compositions and methods useful in inhibiting proliferation of CA IX+
preneoplastic or neoplastic cells in a mammal are provided. The compositions
are
antagonist anti-CA IX antibodies and other inhibitory agents that target
carbonic
anhydrase activity of CA IX on these cells. The antagonist anti-CA IX
antibodies or
antigen-binding fragments thereof specifically react with an inhibitory
epitope of CA
IX or biologically active variant thereof such that an antibody-antigen
complex is
formed, whereby the formation of this complex results in inhibition of
carbonic
anhydrase activity of CA IX or biologically active variant thereof. This
activity is
essential for transformation and proliferation of preneoplastic and neoplastic
cells of
cancers and other proliferative disorders characterized by expression of this
tumor-
-2-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
associated antigen. In one embodiment, the antagonist anti-CA IX antibodies
are
monoclonal antibodies. Suitable monoclonal antibodies are those having human
constant regions, those also having wholly or partially humanized framework
regions,
and those that are fully human antibodies, or antigen-binding fragments
thereof that
specifically bind to the inhibitory epitope of interest on CA IX or
biologically active
variant thereof such that the formation of the antibody-antigen complex
results in
inhibition of carbonic anhydrase activity of the CA IX protein of interest,
for
example, human CA IX. Other small molecule agents that inhibit carbonic
anhydrase
activity of CA IX or biologically active variant thereof and screening assays
for
identifying such agents are also provided. The antagonist anti-CA IX
antibodies,
antigen-binding fragments thereof, and other CA IX inhibitory agents
identified
herein are useful in the treatment of cancers characterized by the expression
of the CA
IX tumor-associated antigen.
Methods for treating a cancer that is characterized by expression of carbonic
anhydrase IX (CA IX) in a mammal are also encompassed. The method comprises
administering a therapeutically effective amount of an agent that inhibits
carbonic
anhydrase activity of CA IX in a mammal in need thereof. Agents of the
invention
include antagonist anti-CA IX antibodies, and antigen-binding fragments of
said
antagonist anti-CA IX antibody, peptides, peptoids, and small organic
molecules. The
anti-CA IX antibodies or antigen binding fragments thereof specifically react
with an
inhibitory epitope of CA IX to form an antibody-antigen complex, whereby the
formation of said complex results in inhibition of carbonic anhydrase activity
of said
CA IX. The agents of the invention find use in inhibiting proliferation of
neoplastic
cells that are characterized by expression of CA IX protein. The agents are
administered in a therapeutically effective dose to inhibit carbonic anhydrase
activity
of CA IX.
The invention further comprises methods for screening and identifying agents
that inlubit carbonic anhydrase activity of a CA IX polypeptide having a
functional
carbonic anhydrase domain. The agents are identified by combining an agent to
be
tested with a cell expressing a CA IX polypeptide under conditions suitable
for
detecting carbonic anhydrase activity and assessing the ability of an agent to
inhibit
carbonic anhydrase activity. Inhibition of carbonic anhydrase activity by the
agent
indicates that the agent is an inhibitor. Agents may also be identified by
combining
-3-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
an agent to be tested with a composition comprising an isolated or
recombinantly
produced CA IX polypeptide under conditions suitable for detecting carbonic
anhydrase activity and assessing the ability of the agent to inhibit carbonic
anhydrase
activity. These agents may be used in compositions or formulated into
pharmaceutical compositions for use in the methods of the invention.
BRIEF DESCRIPTION OF THE DRAWINGS
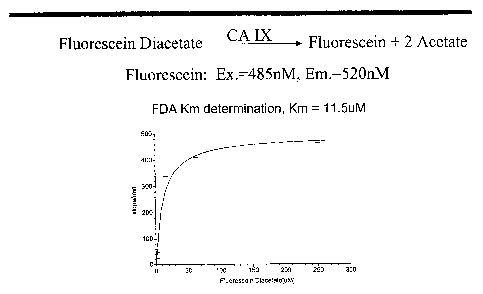
Figure 1 shows a fluorescein diacetate assay used to detect carbonic anhydrase
activity of CA IX in a cell-free high-throughput screening assay.
DETAILED DESCRIPTION OF THE INVENTION
The present invention is directed to compositions and methods for treating a
mammal having a cancer characterized by expression of carbonic anhydrase IX
(also
known as MN and 6250; referred to herein as CA IX). Compositions include anti-
CA IX antibodies and antigen-binding fragments thereof that specifically react
with
an epitope of CA IX such that an antibody-antigen complex is formed, whereby
the
formation of this complex results in inhibition of carbonic anhydrase activity
of CA
IX. Such epitopes are referred to herein as "inhibitory epitopes." Inhibitory
epitopes
can comprise contiguous amino acid residues (i.e., residues within the epitope
are
arranged sequentially one after another in a linear fashion), discontinuous
amino acid
residues (referred to herein as "discontinuous epitopes; residues within these
epitopes
are not arranged sequentially), or both contiguous and discontinuous amino
acid
residues of the CA IX polypeptide or variant thereof, and the residues making
up the
epitope can reside within the CA IX carbonic anhydrase domain and/or outside
the
CA IX carbonic anhydrase domain, so long as the formation of the antibody-
antigen
complex with the anti-CA IX antibody or antigen-binding fragment thereof
results in
reduction or inhibition of the carbonic anhydrase activity of CA IX. In some
embodiments, the inhibitory epitopes are epitopes comprising contiguous amino
acid
residues of the carbonic anhydrase domain of CA IX, epitopes comprising
discontinuous amino acid residues of the carbonic anhydrase domain of CA IX,
and
epitopes comprising both contiguous and discontinuous residues of the carbonic
anhydrase domain. Binding of an anti-CA IX antibody or antigen-binding
fragment
thereof to inhibitory epitopes of CA IX as described herein may prevent CA IX
from
-4-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
assuming an appropriate structural form or conformation necessary for
mediating
carbonic anhydrase activity, may physically inhibit CA IX from complexing with
inorganic co-factors necessary for carbonic anhydrase activity, or may
physically
inhibit CA IX from complexing with the appropriate enzymatic substrate, though
other mechanisms of inhibition are also encompassed. For purposes of the
present
invention, the anti-CA IX antibodies of the invention are hereinafter referred
to as
"antagonist anti-CA IX antibodies." These antagonist anti-CA IX antibodies and
other
inhibitory agents as noted elsewhere herein are useful in treating a subject
having a
cancer that is characterized by expression of the CA IX tumor-associated
antigen.
CA IX, also known as the MN and 6250 tumor-associated antigens, is an N-
glycosylated transmembrane protein encoded by the MN gene. The coding sequence
for human CA IX (see SEQ m NO:1) and the translated amino acid sequence for
this
protein (SEQ m N0:2) are known in the art. See, for example, U.S. Patent Nos.
6,204,370 and 5,989,838, herein incorporated by reference. Human CA IX
comprises
a signal peptide, a proteoglycan-like domain, a carbonic anhydrase domain, a
transmembrane domain, and an intracellular (also referred to as
intracytoplasmic)
domain at the C-terminus. With the exception of the starting position of the
signal
peptide (i.e., residue 1), the residue positions denoting the location of
these domains
within the CA IX protein vary within the reported literature, generally by ~1-
3
residues at either end of any given domain. For purposes of the present
invention, this
variation is accounted for with the use of the term "about." In this manner,
the term
"about" with respect to the residue denoting the beginning or end of each of
these
domains encompasses 1-3 residues on either side of the designated position.
Thus, for
example, where a domain is designated a location of about residues 38-134 of
the
human CA IX amino acid sequence, it is intended that the domain encompasses a
starting location at residue 381-3 residues and encompasses an ending location
at
residue 1341-3 residues. In this example, the starting location would
encompass
residue 35, 36, 37, 38, 39, 40, or 41 of the human sequence, and the ending
location
would encompass residue 131, 132, 133, 134, 135, 136, or 137 of the human
sequence. For purposes of the present invention, the residue locations of the
various
domains of the human CA IX protein are defined in accordance with this
foregoing
description.
-5-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
The human CA IX protein consists of a signal peptide (corresponding to
residue 1 to about residue 37 of SEQ m N0:2), a proteoglycan-like domain
(corresponding to about residues 38-134 of SEQ m N0:2), a carbonic anhydrase
domain (corresponding to about residues 135-414 of SEQ m N0:2), a
transmembrane
domain (corresponding to about residues 415-433 of SEQ m N0:2), and an
intracellular C-terminus (corresponding to about residues 434-459 of SEQ m
N0:2).
The functional region of the proteoglycan-like domain resides at about
residues 53-
111, while the functional region of the carbonic anhydrase domain resides at
about
residues 141-389. Three zinc-liganded histidine residues obligatory for
carbonic
anhydrase activity are located within the carbonic aWydrase (CA) domain (at
residues
226, 228, and 251 of SEQ m N0:2). Antigenic region analysis of the full-length
human CA LX protein predicts an antigenic region within the carbonic anhydrase
domain, with the region of antigenicity residing at about residues 229-256 of
SEQ m
N0:2.
The CA IX tumor-associated antigen was initially identified as being
expressed in some human tumor cell lines i~ vitf~o, for example, by T24
(bladder
carcinoma), HeLa (cervical carcinoma), SIB-Mel 1477 (melanoma), and T47D
(mammary carcinoma). CA IX is also produced by cells of some human cancers ih
vivo, for example, by cells of ovarian and endometrial carcinomas, uterine
cervical
carcinomas, renal cell carcinomas (RCC), colorectal cancer (CRC), and lung
cancer,
as well as cells of some benign neoplasias such as marmnary papillomas. CA IX
is
not found in non-tumorigenic hybrid cells, and is generally not found in the
cells of
normal tissues. An exception resides in its expression within normal gastric,
intestinal, and biliary mucosa. The CA IX gene is strongly correlated with
tumorigenesis and may be a causative agent.
"Tumor", as used herein, refers to all neoplastic cell growth and
proliferation,
whether malignant or benign, and all pre-cancerous and cancerous cells and
tissues.
The terms "cancer" and "cancerous" refer to or describe the physiological
condition in mammals that is typically characterized by unregulated cell
growth,
including tumors and neoplastic growth (i.e., neoplasms). Examples of cancer
include
but are not limited to, carcinoma, lymphoma, blastoma, sarcoma, and leukemia.
More
particular examples of such cancers include breast cancer, prostate cancer,
colon
cancer, squamous cell cancer, small-cell lung cancer, non-small cell lung
cancer,
-6-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
gastrointestinal cancer, pancreatic cancer, glioblastoma, cervical cancer,
ovarian
cancer, liver cancer, bladder cancer, hepatoma, colorectalcancer, endometrial
carcinoma, salivary gland carcinoma, kidney cancer, liver cancer, vulval
cancer,
thyroid cancer, hepatic carcinoma, and various types of head and neck cancer.
"Treatment" is an intervention performed with the intention of preventing the
development or altering the pathology of a disorder. Accordingly, "treatment"
refers
to both therapeutic treatment and prophylactic or preventative measures. Those
in
need of treatment include those already with the disorder as well as those in
which the
disorder is to be prevented. In tumor (e.g., cancer) treatment, a therapeutic
agent may
directly decrease the pathology of tumor cells, or render the tumor cells more
susceptible to treatment by other therapeutic agents, e.g., radiation and/or
chemotherapy. The "pathology" of cancer includes all phenomena that compromise
the well being of the patient. This includes, without limitation, abnormal or
uncontrollable cell growth, metastasis, interference with the normal
functioning of
neighboring cells, release of cytokines or other secretory products at
abnormal levels,
suppression or aggravation of inflammatory or immunological response, etc.
The CA IX protein and gene are described in e.g., U.S. Patent Nos. 6,204,370
and 5,989,838. Although evidence to date pointed to CA IX as having a role in
transforming cells, it has been unknown prior to the present invention which
functional domain of this protein is responsible for its role in cell
transformation and
tumorigenesis. The present invention is based upon the discovery that this
role
resides within the carbonic anhydrase domain. Thus, as shown in the examples
disclosed herein, cells expressing a functional carbonic anhydrase domain of
the CA
IX protein develop a transformed phenotype. By "functional" carbonic anhydrase
domain is intended that the domain is expressed and has fractional carbonic
anhydrase activity. Development of a transformed phenotype is exemplified by
one
or more activities selected from the group consisting of promotion of cell
proliferation, faster doubling times, enhanced DNA synthesis, induced spindle-
shaped
morphology, increased refractility, decreased adherence, lost capacity for
growth
arrest, chaotic growth pattern with higher saturation densities, decreased
growth factor
dependence, growth in soft agar using the soft agar assay described herein (a
measure
of anchorage independence), increased expression of CA IX as cell density
increases,
and ability to grow in nude mice (i.e., tumorigenicity).
_7_
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
Compositions and methods of the invention are directed to the inhibition of
carbonic anhydrase activity of CA IX protein or biologically active variant
thereof on
preneoplastic or neoplastic cells as described herein below. When administered
to a
subject in need of treatment, compositions of the invention effectively reduce
or
inhibit carbonic anhydrase activity of CA IX or biologically active variant
thereof by
5%, 10%, 15%, 20%, 25%, 30%, 35%, preferably 40%, 45%, 50%, 55%, 60%, more
preferably 70%, 75%, 80%, 85%, and most preferably 90%, 95%, 99%, or 100%.
Methods for measuring the ability of a candidate antibody or candidate test
agent to
inhibit carbonic anhydrase activity of a CA IX protein are known in the art.
See, for
example, the assays described herein below, though any assay available in the
art for
measuring COZ release or conversion in the presence of the candidate
inhibitory agent
can be used to detect the ability of the candidate agent to inhibit CA IX
carbonic
anhydrase activity. Reduction or inhibition of this activity on preneoplastic
cells
prevents their phenotypic transformation into tumorogenic cells. Reduction or
inhibition of this activity on neoplastic cells can inhibit their continued
proliferation
or result in overall reduction in tumor size and tumor burden.
Thus, compositions of the invention can be used to inhibit carbonic anhydrase
activity of CA IX at the cell surface of preneoplastic or neoplastic cells
expressing
this cell-surface antigen, thereby limiting carbonic anhydrase activity in the
extracellular environment. Where the composition is an antagonist antibody of
the
invention, such inhibition occurs by the formation of an antibody-antigen
complex as
described elsewhere herein. Depending upon the location of the epitope within
the
CA IX protein, formation of the antibody-antigen complex between the epitope
and
an antagonist anti-CA IX antibody of the invention can result in a complex
that
resides extracellularly. Alternatively, formation of the antibody-antigen
complex can
result in internalization of the CA IX cell-surface protein, thereby
sequestering the
CA IX molecule inside the cell. Where internalization occurs, the antagonist
anti-CA
IX antibody can also be used as a delivery mechanism to deliver antibody-
conjugated
therapeutic moieties to the inside of CA IX-bearing tumor cells. Suitable
therapeutic
moieties that can be conjugated to the antagonist anti-CA IX antibodies of the
invention for subsequent delivery to the inside of CA IX-bearing tumor cells
are
described herein below and include, but are not limited to, cytotoxins,
chemotherapeutics, radio-metal ions, and the like.
_g_
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
Any agent that inhibits carbonic anhydrase activity of CA IX or biologically
active variant thereof on CA IX+ preneoplastic or neoplastic cells can be used
in the
methods of the present invention to prevent phenotypic transformation into
tumorogenic cells and/or to inhibit proliferation of these cells. These agents
find use
in the treatment of subjects having a cancer characterized by expression of
this tumor-
associated antigen. Suitable inhibitory agents can be identified using a
variety of
screening assays, including these disclosed herein. In one embodiment,
suitable
agents are identified by monitoring their ability to inhibit cells expressing
the CA IX
molecule of interest from displaying the transformed phenotype using the soft
agar
assay described herein below. Suitable inhibitory agents include the
antagonist anti-
CA IX antibodies disclosed herein, and peptides, peptoids, and small organic
molecules that target activity of the carbonic anhydrase (CA) domain of CA IX.
Antagonist Anti-CA IX Antibodies
Suitable anti-CA IX antibodies of the invention are referred to herein as
"antagonist" anti-CA IX antibodies in view of their ability to inhibit
carbonic
anhydrase activity of a CA IX polypeptide as noted above. In this manner, the
antagonist anti-CA IX antibodies of the invention and antigen-binding
fragments
thereof are specifically reactive with an inhibitory epitope of CA IX such
that an
antibody-antigen complex is formed, whereby the formation of this complex
results in
inhibition of carbonic anhydrase activity of CA IX. As noted above, inhibitory
epitopes can comprise contiguous amino acid residues, discontinuous amino acid
residues, or both contiguous and discontinuous amino acid residues of the CA
IX
polypeptide or variant thereof, and the residues making up the epitope can
reside
within the CA IX carbonic anhydrase domain and/or outside the CA IX carbonic
anhydrase domain, so long as the formation of the antibody-antigen complex
with the
anti-CA IX antibody or antigen-binding fragment thereof results in reduction
or
inhibition of the carbonic anhydrase activity of CA IX. In some embodiments,
the
inhibitory epitopes are epitopes comprising contiguous amino acid residues of
the
carbonic anhydrase domain of CA IX, epitopes comprising discontinuous amino
acid
residues of the carbonic anhydrase domain of CA IX, and epitopes comprising
both
contiguous and discontinuous residues of the carbonic anhydrase domain.
-9-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
By "specifically reactive" or "specifically reacts" is intended that the
antibody
that recognizes a particular region of the CA IX protein that comprises the
inhibitory
epitope of interest forms a specific antibody-antigen complex with that
portion of the
CA IX protein corresponding to the inhibitory epitope of interest when the
antibody
comes into contact with that protein, either in an ih vitro or ifz vivo
setting. Methods
for detecting the formation of such antibody-antigen complexes are well known
in the
art. For example, candidate antagonist anti-CA IX antibodies that have been
prepared
against a CA IX protein or biologically active portion thereof that comprises
the CA
domain of the CA IX protein of interest can be tested for formation of an
antibody-
antigen complex using a number of well-defined diagnostic assays, such as
conventional immunoassay formats to detect and/or quantitate antigen-specific
antibodies. Such assays include, for example, enzyme immunoassays, e.g.,
enzyme-
linked immunosorbant assays (ELISA), cell-based assays, flow cytometry,
radioimmunoassays, and immunohistochemical staining. Numerous competitive and
non-competitive protein binding assays are known in the art and many are
commercially available. A representative assay to detect anti-CA IX antibodies
specific to the inhibitory epitopes identified herein, for example inhibitory
epitopes
comprising residues within the carbonic anhydrase domain, is a competition
assay in
which labeled CA IX polypeptide comprising residues within the CA domain is
precipitated by candidate antibodies in a sample, for example, in combination
with
monoclonal antibodies raised against one or more epitopes comprising residues
within
this domain. Anti-CA IX antibodies that specifically react with an epitope of
interest,
i.e., an inhibitory epitope of CA IX, can be identified by screening a series
of
antibodies prepared against a CA IX protein or fragment of the protein
comprising the
particular epitope of the CA IX protein of interest. For example, for human CA
IX,
epitopes of interest include inhibitory epitopes comprising contiguous and/or
discontinuous amino acid residues of human CA IX of SEQ ID N0:2. Such
inhibitory epitopes of human CA IX include, for example, those inhibitory
epitopes
comprising contiguous and/or discontinuous amino acid residues residing within
the
CA domain corresponding to about residues 135-414 of SEQ ID NO:2.
Alternatively,
competition assays with previously identified suitable antagonist anti-CA IX
antibodies could be used to select monoclonal antibodies comparable to the
previously identified antibodies.
-10-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
Antibodies employed in such immunoassays may be labeled or unlabeled.
Unlabeled antibodies may be employed in agglutination; labeled antibodies may
be
employed in a wide variety of assays, employing a wide variety of labels.
Detection
of the formation of an antibody-antigen complex between an anti-CA IX antibody
and
an epitope of interest can be facilitated by attaching a detectable substance
to the
antibody. Suitable detection means include the use of labels such as
radionuclides,
enzymes, coenzymes, fluorescers, chemiluminescers, chromogens, enzyme
substrates
or co-factors, enzyme inhibitors, prosthetic group complexes, free radicals,
particles,
dyes, and the like. Examples of suitable enzymes include horseradish
peroxidase,
alkaline phosphatase, (3-galactosidase, or acetylcholinesterase; examples of
suitable
prosthetic group complexes include streptavidin/biotin and avidin/biotin;
examples of
suitable fluorescent materials include umbelliferone, fluorescein, fluorescein
isothiocyanate, rhodamine, dichlorotriazinylamine fluorescein, dansyl chloride
or
phycoerythrin; an example of a luminescent material is luminol; examples of
bioluminescent materials include luciferase, luciferin, and aequorin; and
examples of
suitable radioactive material include lash 1311, 3sS, or 3H. Such labeled
reagents may
be used in a variety of well-known assays, such as radioimmunoassays, enzyme
immunoassays, e.g., ELISA, fluorescent immunoassays, and the like. See for
example, U.S. Patent Nos. 3,766,162; 3,791,932; 3,817,837; and 4,233,402.
Where the CA IX protein is human CA IX, the antagonist anti-CA IX
antibodies of the invention are specifically reactive with an inhibitory
epitope
comprising contiguous and/or discontinuous residues of the human CA IX
protein,
and can comprise residues within the carbonic anhydrase (CA) domain of human
CA
IX (i.e., about residues 135-414 of SEQ ID N0:2), residues outside the CA
domain,
or residues residing inside and outside the CA domain of human CA IX. Such
inlubitory epitopes can correspond to a length of at least 5 residues, at
least 8 residues,
at least 10, 15, 20, 25, 30, or at least 35 residues of the human CA IX
protein.
In some embodiments, the antagonist anti-CA IX antibodies are specifically
reactive with an inhibitory epitope comprising contiguous and/or discontinuous
residues of the carbonic anhydrase (CA) domain of human CA IX, which is
located at
about residues 135-414 of SEQ ID N0:2 and encompasses the functional region of
this domain, i.e., about residues 141-389 of SEQ ID N0:2. In one embodiment,
the
antagonist anti-CA IX antibodies are specifically reactive with an inhibitory
epitope
-11-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
corresponding to at least 5 residues, at least 8 residues, at least 10, 15,
20, 25, 30, or at
least 35 residues of the CA domain of human CA IX protein, i.e., about
residues 135-
414 of SEQ ID N0:2. In these embodiments, the residues making up the
inhibitory
epitope can all be contiguous, can all be discontinuous, or can be a mixture
of
contiguous and discontinuous residues. Examples of such inhibitory epitopes
are
those inhibitory epitopes corresponding to a group of contiguous and/or
discontinuous
amino acid residues selected from residues 200 to 300, residues 210 to 260,
residues
220 to 255, and residues 229 to 256 of SEQ ID N0:2. In one embodiment, the
inhibitory epitope has a length of about 5, 8, 10, 15, 20, 25, 30, or 35
residues, and
comprises at least one of the three essential zinc-liganded histidine residues
of the CA
domain, i.e., at least one of residues 226, 228, and 251 of SEQ ID N0:2. In
other
embodiments, the inhibitory epitope has a length of about 5, 8, 10, 15, 20,
25, 30, or
35 residues, and comprises at least one of residues 229 to 256 of SEQ ID NO:2.
In
such embodiments, the inhibitory epitope can comprise, for example, 1, 3, 5,
8, 10,
12, 15, 18, 20, 22, 25, or even 28 of the residues within residues 229 to 256
of SEQ
ID N0:2. Where the inhibitory epitope comprises more than one of, but less
than 28
of, residues 229 to 256 of SEQ ID N0:2, these residues can all be contiguous,
can all
be discontinuous, or can be a mixture of contiguous and discontinuous
residues.
Where the CA IX protein is a biologically active variant of human CA IX as
defined herein below, the antagonist anti-CA IX antibodies of the invention
are
specifically reactive with an inhibitory epitope comprising contiguous and/or
discontinuous residues of the CA IX polypeptide variant. Such an inhibitory
epitope
can comprise residues within the functional CA domain of the CA IX polypeptide
variant (i.e., the functional CA domain that is homologous to the functional
domain of
human CA IX residing at about residues 135-414 of SEQ ID N0:2 when the amino
acid sequence for the CA domain of the CA IX polypeptide variant is optimally
aligned against the amino acid sequence for the CA domain of human CA IX using
sequence alignment methods noted herein below), residues outside the
functional CA
domain, or residues residing inside and outside the functional CA domain of
the CA
IX polypeptide variant. Such inhibitory epitopes can correspond to a length of
at least
5 residues, at least 8 residues, at least 10, 15, 20, 25, 30, or at least 35
residues of the
CA IX polypeptide variant.
-12-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
In some embodiments, the antagonist anti-CA IX antibodies of the invention
are specifically reactive with an inhibitory epitope of the CA IX polypeptide
variant
that comprises contiguous and/or discontinuous residues of the functional
carbonic
anhydrase (CA) domain that is homologous to the functional carbonic CA domain
in
human CA IX (i.e., about residues 135-414 of SEQ m N0:2) when the amino acid
sequence for the CA domain of the CA IX polypeptide variant is optimally
aligned
against the amino acid sequence for the CA domain of human CA IX using
sequence
alignment methods noted herein below. As with human CA IX, the antagonist anti-
CA IX antibodies are specifically reactive with an inhibitory epitope
corresponding to
at least 5 residues, at least 8 residues, at least 10, 15, 20, 25, 30, or at
least 35 residues
of the functional CA domain of the CA IX polypeptide variant. In these
embodiments, the residues making up the inlubitory epitope can all be
contiguous, can
all be discontinuous, or can be a mixture of contiguous and discontinuous
residues.
Examples of such inlubitory epitopes are those inhibitory epitopes
corresponding to a
group of contiguous and/or discontinuous amino acid residues selected from the
region of the functional CA domain that is homologous to residues 200 to 300,
residues 210 to 260, residues 220 to 255, and residues 229 to 256 of SEQ m
N0:2
when 'the amino acid sequence for the CA domain of the CA IX polypeptide
variant is
optimally aligned against the amino acid sequence for the CA domain of human
CA
IX using sequence alignment methods noted herein below. In one embodiment, the
inhibitory epitope of the CA IX polypeptide variant has a length of about 5,
8, 10, 15,
20, 25, 30, or 35 residues, and comprises at least one of the three essential
zinc-
liganded histidine residues of the functional CA domain, i.e., at least one of
the
residues homologous to residues 226, 228, and 251 of SEQ m N0:2 when the amino
acid sequence for the functional CA domain of the CA IX polypeptide variant is
optimally aligned against the amino acid sequence for the CA domain of human
CA
IX using sequence aligmnent methods noted herein below. In other embodiments,
the
inhibitory epitope of the CA IX polypeptide variant has a length of about 5,
8, 10, 15,
20, 25, 30, or 35 residues, and comprises at least one of the residues of the
region of
the functional CA domain that is homologous to residues 229 to 256 of SEQ m
N0:2
when the amino acid sequence for the functional CA domain of the CA IX
polypeptide variant is optimally aligned against the amino acid sequence for
the CA
domain of human CA IX using sequence alignment methods noted herein below. In
-13-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
such embodiments, the inhibitory epitope can comprise, for example, 1, 3, 5,
8, 10,
12, 15, 18, 20, 22, 25, or even 28 of the residues within the region of the
functional
CA domain of the CA IX polypeptide variant that is homologous to residues 229
to
256 of SEQ ID N0:2. Where the inhibitory epitope of the CA IX polypeptide
variant
comprises more than one of, but less than 28 of, the residues in the region of
the
functional CA domain that is homologous to residues 229 to 256 of SEQ ID NO:2,
these residues can all be contiguous, can all be discontinuous, or can be a
mixture of
contiguous and discontinuous residues.
Any antagonist anti-CA IX antibody or antigen-binding fragment thereof
having the binding characteristics and specificity noted herein is suitable
for use in the
methods of the present invention. Thus, suitable antagoiust anti-CA IX
antibodies are
specifically reactive with an epitope of CA IX such that an antibody-antigen
complex
is formed, whereby formation of the complex results in inhibition of CA IX
carbonic
anhydrase activity by 5%, 10%, 15%, 20%, 25%, 30%, 35%, preferably 40%, 45%,
50%, 55%, 60%, more preferably 70%, 75%, 80%, 85%, and most preferably 90%,
95%, 99%, or 100%. Antagonist anti-CA IX antibodies that are specifically
reactive
with an inhibitory epitope of interest of human CA IX protein or variant
thereof can
be prepared against human CA IX protein or the variant CA IX polypeptide, for
example, antigenic peptides comprising all or a portion of the carbonic
anhydrase
domain of a CA IX protein of interest, and identified using immunoassays for
detecting antibody-antigen complexes and epitope mapping tecluuques l~nown in
the
art. Such immunoassays and epitope mapping techniques include those described
in
U.S. Patent Nos. 4,708,871 and 5,635,182, herein incorporated by reference in
their
entirety, and noted elsewhere herein.
As used herein, "antagonist anti-CA IX antibody" thus encompasses any
antibody that specifically recognizes and binds to an epitope of the CA IX
tumor-
associated antigen whereby formation of the antibody-antigen complex results
in
inhibition of carbonic anhydrase activity of CA IX. As noted above, epitopes
that are
recognized by the antagonist anti-CA IX antibodies of the invention are
referred to
herein as "inhibitory epitopes," as formation of an antibody-antigen complex
between
the antagonist anti-CA IX antibody and the inhibitory epitope results in
inhibition of
carbonic anhydrase activity of CA IX. The antagonist anti-CA IX antibodies of
the
invention include polyclonal antibodies, monoclonal antibodies, single-chain
-14-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
antibodies, and fragments thereof such as Fab, F(ab')2, F,,, and other
fragments that
retain the antigen binding function of the parent antagonist anti-CA IX
antibody, i.e.,
binding to an inhibitory epitope of CA IX, whereby carbonic anhydrase activity
of
this protein is inhibited. Polyclonal sera may be prepared by conventional
methods.
In general, a solution containing the CA IX antigen is first used to immunize
a
suitable animal, preferably a mouse, rat, rabbit, or goat. Rabbits or goats
are preferred
for the preparation of polyclonal sera due to the volume of serum obtainable,
and the
availability of labeled anti-rabbit and anti-goat antibodies. Polyclonal sera
can be
prepared in a transgenic animal, preferably a mouse bearing human
immunoglobulin
loci. In one embodiment, S~ cells or Tn5 cells expressing CA IX are used as
the
immunogen. Immunization can also be performed by mixing or emulsifying the
antigen-containing solution, preferably in an adjuvant such as Freund's
complete
adjuvant, and injecting the mixture or emulsion parenterally (generally
subcutaneously, intraperitoneally, or intramuscularly). A dose of about 10-
200, up to
about 500 ~g/injection is typically sufficient. Immunization is generally
boosted 2-6
weeks later with one or more injections of the protein, preferably emulsified
with
Freund's incomplete adjuvant. One may alternatively generate antibodies by i~
vitro
immunization using methods known in the art, which for the purposes of this
invention is considered equivalent to ifz vivo immunization. Polyclonal
antisera are
obtained by bleeding the immunized animal into a glass or plastic container,
incubating the blood at 25°C for one hour, followed by incubating at
4°C for 2-18
hours. The serum is recovered by centrifugation (e.g., 1,000 x g for 10
minutes).
About 20-50 ml per bleed may be obtained from rabbits.
Preferably the antibody is monoclonal in nature. By "monoclonal antibody" is
intended an antibody obtained from a population of substantially homogeneous
antibodies, i.e., the individual antibodies comprising the population are
identical
except for possible naturally occurring mutations or modifications that may be
present
in minor amounts. Monoclonal antibodies are lughly specific, being directed
against a
single antigenic site, i.e., for purposes of the present invention, an epitope
of CA TX
described herein that when bound to an antagonist antibody of the invention
forms an
antibody-antigen complex, whereby carbonic anhydrase activity of CA IX is
inhibited. Furthermore, in contrast to conventional (polyclonal) antibody
preparations
that typically include different antibodies directed against different
determinants
-15-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
(epitopes), each monoclonal antibody is directed against a single determinant
on the
antigen. The modifier "monoclonal" indicates the character of the antibody as
being
obtained from a substantially homogeneous population of antibodies, and is not
to be
construed as requiring production of the antibody by any particular method.
For
example, the monoclonal antibodies to be used in accordance with the present
invention may be made by the hybridoma method first described by I~ohler et
al.
(1975) Nature 256:495, or may be made by recombinant DNA methods (see, e.g.,
U.S. Patent No. 4,816,567). The "monoclonal antibodies" may also be isolated
from
yeast display (Hudson and Souriau (2001) Expert. Opih. Biol. They. 1(5):845-
855),
ribosomal display (Hudson and Souriau (2001) Expert. Opih. Biol. Ther.
1(5):845-
855), or phage antibody libraries using the techniques described in, for
example,
Clackson et al. (1991) Nature 352:624-628; Marks et al. (1991) J. Mol. Biol.
222:581-
597, and U.S. Patent No. 5,514,548; herein incorporated by reference.
Monoclonal antibodies can be prepared using the method of I~ohler et al.
(1975) Nature 256:495-496, or a modification thereof. Typically, a mouse is
immunized with a solution containing an antigen. linmunization can be
performed by
mixing or emulsifying the antigen-containing solution, preferably in an
adjuvant such
as Freund's complete adjuvant or Freund's incomplete adjuvant, and injecting
the
mixture or emulsion parenterally. Any method of immunization known in the art
may
be used to obtain the monoclonal antibodies of the invention. After
immunization of
the animal, the spleen (and/or optionally, several large lymph nodes) is
removed and
dissociated into single cells. The spleen cells may be screened by applying a
cell
suspension to a plate or well coated with the antigen of interest. The B cells
expressing membrane bound immunoglobulin specific for the antigen bind to the
plate
and are not rinsed away. Resulting B cells, or all dissociated spleen cells,
are then
induced to fuse with myeloma cells to form hybridomas, and are cultured in a
selective medium. The resulting cells are plated by limiting dilution and are
assayed
for the production of antibodies that specifically bind the antigen of
interest (and that
do not bind to unrelated antigens). The selected monoclonal antibody (mAb)-
secreting hybridomas are then cultured either in vitro (e.g., in tissue
culture bottles or
hollow fiber reactors), or ih vivo (as ascites in mice).
As an alternative to the use of hybridomas, antibody can be produced in a cell
line such as a CHO cell line or myeloma cell line, as disclosed in U.S. Patent
Nos.
-16-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
5,545,403; 5,545,405; 5,998,144; and 5,807,715; incorporated herein by
reference.
Examples of myeloma cell lines include, but are not limited to, Sp2 and NSO
cell
lines, and cell lines derived therefrom. Briefly the cell line is transfected
with vectors
capable of expressing a light chain and a heavy chain, respectively. By
transfecting
the separate vectors encoding the two proteins, chimeric antibodies can be
produced.
Another advantage is the correct glycosylation of the antibody. Alternatively,
the
antibody can be produced by transfecting the cell line with a bicistronic
single
plasmid encoding the light and heavy chains.
Additionally, the term "anti-CA IX antibody" as used herein encompasses
chimeric anti-CA IX antibodies. By "chimeric" antibodies is intended
antibodies that
are most preferably derived using recombinant deoxyribonucleic acid techniques
and
which comprise both human (including immunologically "related" species, e.g.,
chimpanzee) and non-human components. Thus, the constant region of the
clumeric
antibody is most preferably substantially identical to the constant region of
a natural
human antibody; the variable region or portion thereof of the chimeric
antibody is
most preferably derived from a non-human source and has the desired antigenic
specificity to the region of the CA IX protein of interest, for example, human
CA IX
or biologically active variant thereof, that comprises an inhibitory epitope
described
therein. The non-human source can be any vertebrate source that can be used to
generate antibodies to a human CA IX or material comprising a human CA IX, or
more particularly an antigenic peptide of human CA IX comprising an inhibitory
epitope described herein, for example, an inhibitory epitope residing within
the CA
domain. Such non-human sources include, but are not limited to, rodents (e.g.,
rabbit,
rat, mouse, etc.; see, for example, U.S. Patent No. 4,816,567, herein
incorporated by
reference) and non-human primates (e.g., Old World Monkey, Ape, etc.; see, for
example, U.S. Patent Nos. 5,750,105 and 5,756,096; herein incorporated by
reference). As used herein, the phrase "immunologically active" when used in
reference to chimeric anti-CA IX antibodies means a chimeric antibody that
binds
human CA IX, more specifically binds to an inhibitory epitope described
herein, for
example, an inhibitory epitope comprising contiguous and/or discontinuous
residues
residing within the CA domain of human CA IX or biologically active variant
thereof.
Humanized anti-CA IX antibodies are also encompassed by the term anti-CA
IX antibody as used herein. By "humanized" is intended forms of anti-CA IX
-17-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
antibodies that contain minimal sequence derived from non-human immunoglobulin
sequences. For the most part, humanized antibodies are human immunoglobulins
(recipient antibody) in which residues from a hypervariable region (also known
as
complementarity determining region or CDR) of the recipient are replaced by
residues
from a hypervariable region of a non-human species (donor antibody) such as
mouse,
rat, rabbit, or nonhuman primate having the desired specificity, affinity, and
capacity.
Humanization can be essentially performed following the method of Winter and
co-
workers (Jones et al. (1986) Nature 321:522-525; Riechmann et al. (1988)
Nature
332:323-327; Verhoeyen et al. (1988) Science 239:1534-1536), by substituting
rodent
or mutant rodent CDRs or CDR sequences for the corresponding sequences of a
human antibody. See also U.S. Patent Nos. 5,225,539; 5,585,089; 5,693,761;
5,693,762; 5,859,205; herein incorporated by reference. In some instances,
residues
within the framework regions of one or more variable regions of the human
immunoglobulin are replaced by corresponding non-human residues (see, for
example, U.S. Patent Nos. 5,585,089; 5,693,761; 5,693,762; and 6,180,370).
Furthermore, humanized antibodies may comprise residues that are not found in
the
recipient antibody or in the donor antibody. These modifications are made to
further
refine antibody performance (e.g., to obtain desired affinity). In general,
the
humanized antibody will comprise substantially all of at least one, and
typically two,
variable domains, in which all or substantially all of the hypervariable
regions
correspond to those of a non-human immunoglobulin and all or substantially all
of the
framework regions are those of a human immunoglobulin sequence. The humanized
antibody optionally also will comprise at least a portion of an immunoglobulin
constant region (Fc), typically that of a human immunoglobulin. For further
details
see Jones et al. (1986) Nature 331:522-525; Riechmann et al. (1988) Nature
332:323-
329; and Presta (1992) Cu~~. Op. St~uct. Biol. 2:593-596; herein incorporated
by
reference. Accordingly, such "humanized" antibodies may include antibodies
wherein substantially less than an intact human variable domain has been
substituted
by the corresponding sequence from a non-human species. In practice, humanized
antibodies are typically human antibodies in which some CDR residues and
possibly
some framework residues are substituted by residues from analogous sites in
rodent
antibodies. See, for example, U.S. Patent Nos. 5,225,539; 5,585,089;
5,693,761;
5,693,762; 5,859,205. See also U.S. Patent No. 6,180,370, and International
-18-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
Publication No. WO 01/27160, where humanized antibodies and techniques for
producing humanized antibodies having improved affinity for a predetermined
antigen are disclosed.
Also encompassed by the term anti-CA IX antibodies are xenogeneic or
modified anti-CA IX antibodies produced in a non-human mammalian host, more
particularly a transgenic mouse, characterized by inactivated endogenous
immunoglobulin (Ig) loci. In such transgenic animals, competent endogenous
genes
for the expression of light and heavy subunits of host immunoglobulins are
rendered
non-functional and substituted with the analogous human immunoglobulin loci.
These transgenic animals produce human antibodies in the substantial absence
of light
or heavy host immunoglobulin subunits. See, for example, U.S. Patent Nos.
5,877,397 and 5,939,598, herein incorporated by reference.
Fragments of the antagonist anti-CA IX antibodies are suitable for use in the
methods of the invention so long as they retain the desired affinity and
activity of the
full-length antibody. Thus, a fragment of an anti-CA IX antibody will (1)
retain the
ability to bind to an inhibitory epitope of CA IX as defined elsewhere herein,
for
example, an inhibitory epitope comprising contiguous and/or discontinuous
residues
of the CA domain of the CA IX protein of interest, for example, human CA IX or
biologically active variant thereof; and (2) exhibit antagonist activity when
bound to
such an inhibitory epitope of CA IX, thereby inhibiting carbonic anhydrase
activity of
the CA IX protein. Such fragments are referred to herein as "antigen-binding"
fragments.
Suitable antigen-binding fragments of an antibody comprise a portion of a
full-length antibody, generally the antigen-binding or variable region
thereof.
Examples of antibody fragments include, but are not limited to, Fab, F(ab~2,
and Fv
fragments and single-chain antibody molecules. By "single-chain Fv" or "sFv"
antibody fragments is intended fragments comprising the VH and VL domains of
an
antibody, wherein these domains are present in a single polypeptide chain.
See, for
example, U.S. Patent Nos. 4,946,778; 5,260,203; 5,455,030; 5,856,456; herein
incorporated by reference. Generally, the Fv polypeptide further comprises a
polypeptide linker between the VH and VL domains that enables the sFv to form
the
desired structure for antigen binding. For a review of sFv, see Pluckthun
(1994) in
-19-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
The PhaYmacology of Monoclonal Antibodies, Vol. 113, ed. Rosenburg and Moore
(Springer-Verlag, New York), pp. 269-315.
Antibodies or antibody fragments can be isolated from antibody yeast
libraries, ribosomal expression libraries, or phage libraries generated using
the
techniques described in, for example, McCafferty et al. (1990) Nature 348:552-
554
(1990) and U.S. Patent No. 5,514,548. Clackson et al. (1991) Nature 352:624-
628
and Marks et al. (1991) J. Mol. Biol. 222:581-597 describe the isolation of
marine and
human antibodies, respectively, using phage libraries. Subsequent publications
describe the production of high affinity (nM range) human antibodies by chain
shuffling (Marks et al. (1992) BiolTechsZOlogy 10:779-783), as well
as~combinatorial
infection and in vivo recombination as a strategy for constructing very large
phage
libraries (Waterhouse et al. (1993) Nucleic. Acids Res. 21:2265-2266). Thus,
these
techniques are viable alternatives to traditional monoclonal antibody
hybridoma
techniques for isolation of monoclonal antibodies.
Various techniques have been developed for the production of antibody
fragments. Traditionally, these fragments were derived via proteolytic
digestion of
intact antibodies (see, e.g., Morimoto et al. (1992) .Iouf°raal
ofBioehemical and
Biophysical Methods 24:107-117 (1992) and Brennan et al. (1985) Science
229:81).
However, these fragments can now be produced directly by recombinant host
cells.
For example, the antibody fragments can be isolated from the antibody yeast,
ribosomal, or phage libraries discussed above. Alternatively, Fab'-SH
fragments can
be directly recovered from E. eoli and chemically coupled to form F(ab')Z
fragments
(Carter et al. (1992) BiolTechnology 10:163-167). According to another
approach,
F(ab')2 fragments can be isolated directly from recombinant host cell culture.
Other
tech~uques for the production of antibody fragments will be apparent to the
skilled
practitioner.
The invention also encompasses de-immunized antagonist anti-CA IX
antibodies, which can be produced as described in, for example, International
Publication Nos. WO 98/52976 and WO 0034317; herein incorporated by reference.
In this manner, residues within the antagonist anti-CA IX antibodies of the
invention
are modified so as to render the antibodies non- or less immunogenic to humans
while
retaining their antagonist activity toward carbonic anhydrase activity of CA
IX,
wherein such activity is measured by assays noted elsewhere herein. Also
included
-20-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
within the scope of the invention are fusion proteins comprising an antagonist
anti-CA
IX antibody of the invention, or a fragment thereof, which fusion proteins can
be
synthesized or expressed from corresponding polynucleotide vectors, as is
known in
the art. Such fusion proteins are described with reference to conjugation of
antibodies
as noted below.
Antagonist anti-CA IX antibodies identified as having the binding
characteristics and specificity described herein for use in the methods of the
present
invention can have sequence variations produced using methods described in,
for
example, Patent Publication Nos. EP 0 983 303 Al, WO 00/34317, and WO
98/52976, incorporated herein by reference. For example, it has been shown
that
sequences within the CDR can cause an antibody to bind to MHC Class II and
trigger
an unwanted helper T cell response. A conservative substitution can allow the
antibody to retain binding activity yet lose its ability to trigger an
unwanted T cell
response. Any such conservative or non-conservative substitutions can be made
using
art-recognized methods, such as those noted elsewhere herein, and the
resulting
antibodies will fall within the scope of the invention. The variant antibodies
can be
routinely tested for antagonist activity, affinity, and specificity using
methods
described herein.
The antibodies of this invention can also have ADCC and CDC activity in
addition to neutralizing activity. Antibodies that have ADCC activity interact
with
"human effector cells" such as leukocytes that express one or more FcRs and
perform
effector functions. Preferably, the cells express at least FcyRIII and carry
out
antigen-dependent cell-mediated cyotoxicity (ADCC) effector function. Examples
of
human leukocytes that mediate ADCC include peripheral blood mononuclear cells
(PBMC), natural killer (NK) cells, monocytes, cytotoxic T cells, and
neutrophils; with
PBMCs and NK cells being preferred. Antibodies that have ADCC activity are
typically of the IgGl or IgG3 isotype. Note that in addition to isolating IgG1
and
IgG3 antibodies, such ADCC-mediating antibodies can be made by engineering a
variable region from a non-ADCC antibody or variable region fragment onto an
IgGl
or IgG3 isotype constant region.
The terms "Fc receptor" or "FcR" are used to describe a receptor that binds to
the Fc region of an antibody. The preferred FcR is a native sequence human
FcR.
Moreover, a preferred FcR is one that binds an IgG antibody (a gamma receptor)
and
-21-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
includes receptors of the FcyRI, FcyRII, and FcyRIII subclasses, including
allelic
variants and alternatively spliced forms of these receptors. FcyRII receptors
include
FcyRIIA (an "activating receptor") and FcyRIIB (an "inhibiting receptor"),
which
have similar amino acid sequences that differ primarily in the cytoplasmic
domains
thereof. Activating receptor FcyRIIA contains an immunoreceptor tyrosine-based
activation motif (ITAM) in its cytoplasmic domain. Inhibiting receptor FcyRIIB
contains an immunoreceptor tyrosine-based inhibition motif (ITIM) in its
cytoplasmic
domain (see Daeron (1997) Anzzu. Rev. Izzzznuzzol. 15:203-234). FcRs are
reviewed in
Ravetch and I~inet (1991) Anzzu. Rev. Immuzzol 9:457-92; Capel et al. (1994)
Immu>zozzZethods 4:25-34; and de Haas et al. (1995) J. Lab. Clin. Meel.
126:330-341.
Other FcRs, including those to be identified in the future, are encompassed by
the
term "FcR" herein. The term also includes the neonatal receptor, FcRn, which
is
responsible for the transfer of maternal IgGs to the fetus (Guyer et al.
(1976) J.
Iznznuhol. 117:587; and Kim et al. (1994) J. Immu>zol. 24:249).
"Complement dependent cytotoxicity" or "CDC" refers to the ability of a
molecule to lyse a target in the presence of complement. The complement
activation
pathway is initiated by the binding of the first component of the complement
system
(Clq) to a molecule (e.g., an antibody) complexed with a cognate antigen. To
assess
complement activation, a CDC assay, e.g., as described in Gazzano-Santoro et
al.
(1996) J. Immuhol. Methods 202:163, may be performed.
Any of the previously described antagonist anti-CA IX antibodies or antibody
fragments thereof may be conjugated prior to use in the methods of the present
invention. Methods for producing conjugated antibodies are known in the art.
Thus,
the antagonist anti-CA IX antibody may be labeled using "indirect labeling" or
an
"indirect labeling approach. By "indirect labeling" or "indirect labeling
approach" is
intended that a chelating agent is covalently attached to an antibody and at
least one
radionuclide is inserted into the chelating agent. See, for example, the
chelating
agents and radionuclides described in Srivagtava and Mease (1991) Nucl. Med.
Bio.
18:589-603, herein incorporated by reference. Alternatively, the antagonist
anti-CA
IX antibody may be labeled using "direct labeling" or a "direct labeling
approach",
where a radionuclide is covalently attached directly to an antibody (typically
via an
amino acid residue). Preferred radionuclides are provided in Srivagtava and
Mease
(1991) supz"a. The indirect labeling approach is particularly preferred. See
also, for
_22_
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
example, International Publication Nos. WO 00/52031 and WO 00/52473, where a
linker is used to attach a radioactive label to antibodies; and the labeled
forms of anti-
CA IX antibodies described in U.S. Patent No. 6,015,542; herein incorporated
by
reference.
Further, an antagonist anti-CA IX antibody (or antigen-binding fragment
thereof) may be conjugated to a therapeutic moiety such as a cytotoxin, a
therapeutic
agent, or a radioactive metal ion. A cytotoxin or cytotoxic agent includes any
agent
that is detrimental to cells. Examples include taxol, cytochalasin B,
gramicidin D,
ethidium bromide, emetine, mitomycin, etoposide, tenoposide, vincristine,
vinblastine, colchicin, doxorubicin, daunorubicin, dihydroxy anthracin dione,
mitoxantrone, mithramycin, actinomycin D, 1-dehydrotestosterone,
glucocorticoids,
procaine, tetracaine, lidocaine, propranolol, and puromycin and analogs or
homologs
thereof. Therapeutic agents include, but are not limited to, antimetabolites
(e.g.,
methotrexate, 6-mercaptopurine, 6-thioguanine, cytarabine, 5-fluorouracil
decarbazine), alkylating agents (e.g., mechlorethamine, thioepa chlorambucil,
melphalan, carmustine (BSNU) and lomustine (CCNL~, cyclothosphamide, busulfan,
dibromomannitol, streptozotocin, mitomycin C, and cis-dichlorodiamine platinum
(II)
(DDP) cisplatin), anthracyclines (e.g., daunorubicin (formerly daunomycin) and
doxorubicin), antibiotics (e.g., dactinomycin (formerly actinomycin),
bleomycin,
mithramycin, and anthramycin (AMC)), and anti-mitotic agents (e.g.,
vincristine and
vinblastine). The conjugates of the invention can be used for modifying a
given
biological response; the drug moiety is not to be construed as limited to
classical
chemical therapeutic agents. For example, the drug moiety may be a protein or
polypeptide possessing a desired biological activity. Such proteins may
include, for
example, a toxin such as abrin, ricin A, pseudomonas exotoxin, or diphtheria
toxin; a
protein such as tumor necrosis factor, interferon-alpha, interferon-beta,
nerve growth
factor, platelet derived growth factor, tissue plasminogen activator; or,
biological
response modifiers such as, for example, lymphokines, interleukin-1 ("IL-1"),
interleukin-2 ("IL-2"), interleukin-6 ("IL-6), granulocyte macrophase colony
stimulating factor ("GM-CSF"), granulocyte colony stimulating factor ("G-
CSF"), or
other growth factors.
Techniques for conjugating such therapeutic moiety to antibodies are well
known. See, for example, Arnon et al. (1985) "Monoclonal Antibodies for
-23-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
Immunotargeting of Drugs in Cancer Therapy," in Monoclonal Antibodies and
Cancer Therapy, ed. Reisfeld et al. (Alan R. Liss, Inc.), pp. 243-256;
Hellstrom et al.
eds. (1987) "Antibodies for Drug Delivery," in Controlled Drug Delivefy, ed.
Robinson et al. (2d ed; Marcel Dekker, Inc.), pp. 623-653; Thorpe (1985)
"Antibody
Carriers of Cytotoxic Agents in Cancer Therapy: A Review," in Monoclonal
Antibodies '84: Biological and Clinical Applications, ed. Pinchera et al.
(Editrice
I~urtis, Milano, Italy, 1985), pp. 475-506; "Analysis, Results, and Future
Prospective
of the Therapeutic Use of Radiolabeled Antibody in Cancer Therapy," in
Monoclonal
Antibodies for Cancer Detection and Therapy, ed. Baldwin et al. (Academic
Press,
New York, 1985), pp. 303-316; and Thorpe et al. (1982) Immunol. Rev. 62:119-
158.
Alternatively, an antibody can be conjugated to a second antibody to form an
antibody heteroconjugate as described by Segal in U.S. Patent No. 4,676,980.
In
addition, linkers may be used between the labels and the antibodies of the
invention
(see U.S. Patent No. 4,831,175). Antibodies or, antigen-binding fragments
thereof
may be directly labeled with radioactive iodine, indium, yttrium, or other
radioactive
particle known in the art (U.S. Patent No. 5,595,721). Treatment may consist
of a
combination of treatment with conjugated and nonconjugated antibodies
administered
simultaneously or subsequently (WO 00/52031 and WO 00/52473).
The anti-CA IX antibodies of the present invention can bind to amino-acid-
residues-specific-epitopes, carbohydrate-specific epitopes, or epitopes formed
by both
amino acid residues and carbohydrate portions of the molecule, as expressed by
the
target-bearing tumor cells. If the anti-CA IX antibody carries a cytotoxic or
cytostatic
agent, it might be preferable that the antibody binds to an epitope that
internalizes the
antibody-target receptor complex. If the anti-CA IX antibody is to work
through
ADCC and CDC, then it is preferable that the anti-CA IX antibody remains on
the
surface of the target tumor cell until the antibody Fc region binds to
effector cells.
Methods for determining whether an antibody bound to a cognate cell surface
antigen
remains on a cell surface or is internalized are well known in the art.
Other Inhibitory Agents
Having identified the importance of the functional carbonic anhydrase domain
of CA IX in its role in tumorigenesis, any agent that specifically inhibits
carbonic
anhydrase activity of CA IX on CA IX+ tumorigenic cells can be utilized in the
-24-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
methods of the present invention. Candidate agents that inhibit carbonic
anhydrase
activity of a CA IX protein of interest may be derived from almost any source
of
chemical libraries, naturally occurring compounds, or mixtures of compounds.
Exemplary sources of candidate inhibitors, synthesis of libraries of peptides,
peptoids,
and small organic molecules are described below. Any agent that is an
inhibitor or
antagonist of carbonic anhydrase activity of CA IX, for example, human CA IX
or
biologically active variant thereof, can be used in the treatment methods of
the present
invention. The inhibitor of carbonic anhydrase activity can be a peptide
antagonist, a
peptoid antagonist, or a small organic molecule antagonist. It is expected
that some
inhibitors will act at one or more of the three zinc-binding histidine
residues within
the carbonic anhydrase domain of the CA IX polypeptide (for example, at
residues
226, 22~, and 251 of human CA IX shown in SEQ ID N0:2), which are obligatory
for
the catalytic activity of the CA domain (Sly and Hu (1995) A~cfau. Rev.
Bioclaem.
64:375-401). However, the use and appropriateness of such inhibitors of CA IX
carbonic anhydrase activity for the purposes of the invention are not limited
to any
theories of mechanism of action of the inhibitor. It is sufficient for
purposes of the
invention that an inhibitor inhibit the carbonic anhydrase activity of CA IX,
for
example, human CA IX or biologically active variant thereof as defined herein
below.
Analogs of peptides as used herein include peptides having one or more
peptide mimics, for example peptoids that possess protein-like activity.
Included
within the definition are, for example, peptides containing one or more
analogs of an
amino acid (including, for example, unnatural amino acids), peptides with
substituted
linkages, as well as other modifications known in the art, both naturally
occurnng and
not naturally occurring.
The term "small molecule" includes any chemical or other moiety that can act
to affect biological processes. Small molecules can include any number of
therapeutic
agents presently known and used, or can be small molecules synthesized in a
library
of such molecules for the purpose of screening for function. Small molecules
are
distinguished from polymers and macromolecules by size and lack of
polymerization.
Small molecules can include peptides, peptoids, and small organic molecules.
The candidate inhibitors of CA IX carbonic anhydrase activity and libraries of
candidate inhibitors for screening by the assays disclosed herein can be
derived from
any of the various possible sources of candidate inhibitors, such as for
example,
-25-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
libraries of peptides, peptoids, and small molecules. The inhibitor could be a
polypeptide presented by phage display. In general an inhibitor of CA IX
carbonic
anhydrase activity can be any molecule that may be capable of inhibiting CA IX
carbonic anhydrase activity, or carbonic anhydrase activity of a variant CA IX
polypeptide. Some libraries for screening can be subdivided into library pools
for
assaying inhibition of CA IX carbonic anhydrase activity by the assays
disclosed
herein. Some of each pool is assayed and some is saved for reassay, or to
further
subdivide into subpools, should a positive be identified. Generation of some
of the
possible libraries suitable for assay by the methods of the invention is
described
herein below.
Libraries that are peptide and peptoid inhibitors of CA IX carbonic anhydrase
activity are made as follows. A "library" of peptides may be synthesized and
used
following the methods disclosed in U.S. Patent No. 5,010,175, (the '175
patent) and
in International Publication No. WO 91/17823. In the method of the '175
patent, a
suitable peptide synthesis support, for example, a resin, is coupled to a
mixture of
appropriately protected, activated amino acids. The method described in WO
91/17823 is similar but simplifies the process of determining which peptides
are
responsible for any observed alteration of gene expression in a responsive
cell. The
methods described in WO 91/17823 and U.S. Patent No. 5,194,392 enable the
preparation of such pools and subpools by automated techniques in parallel,
such that
all synthesis and resynthesis may be perfornzed in a matter of days.
Further alternative agents include peptide analogs and derivatives that can
act
as inhibitors of gene expression, or as ligands or antagonists. Some general
means
contemplated for the production of peptides, analogs or derivatives are
outlined in
Weinstein, ed. (1983) Che~r2ist~y and Biochenaist~y ofAmino Acids, Peptides,
and
P~oteihs- A Survey of Receht Developments (Marcell Dekker, Inc., New Yorlc),
herein
incorporated by reference. Moreover, substitution of D-amino acids for the
normal L-
stereoisomers can be carried out to increase the half lives of the molecules.
Peptoids, polymers comprised of monomer units of at least some N-substituted
moieties, can act as small molecule inhibitors herein and can be synthesized
as
described in International Publication No. WO 91/19735. Presently preferred
amino
acid substitutes are N-alkylated derivatives of glycine, which are easily
synthesized
and incorporated into polypeptide chains. However, any monomer units that
allow for
-26-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
the sequence specific synthesis of pools of diverse molecules are appropriate
for use
in producing peptoid molecules. The benefits of these molecules for the
purpose of
the invention are that they occupy different conformational space than a
peptide and
are more resistant to the action of proteases because their amide linkages are
N-
substituted.
Peptoids are easily synthesized by standard chemical methods. The preferred
method of synthesis is the "submonomer" technique described by Zuckermann et
al
(1992) J. Am. Chem. Soc. 114:10646-7. Synthesis by solid phase techniques of
heterocyclic organic compounds in which N-substituted glycine monomer units
form
a backbone is described in copending application entitled "Synthesis of N-
Substituted
Oligomers" filed on June 7, 1995 and is herein incorporated by reference in
full.
Combinatorial libraries of mixtures of such heterocyclic organic compounds can
then
be assayed for the ability to inhibit enzyme activity, specifically carbonic
anhydrase
activity of CA IX or biologically active variant thereof.
Synthesis by solid phase of other heterocyclic organic compounds in
combinatorial libraries is also described in copending application U.S. Serial
No.
08/485,006 entitled "Combinatorial Libraries of Substrate-Bound Cyclic Organic
Compounds" filed on June 7, 1995, herein incorporated by reference in its
entirety.
Highly substituted cyclic structures can be synthesized on a solid support by
combining the submonomer method with powerful solution phase chemistry. Cyclic
compounds containing one, two, three or more fused rings are formed by the
submonomer method by first synthesizing a linear backbone followed by
subsequent
intramolecular or intermolecular cyclization as described in the same
application.
As with the antagonist anti-CA IX antibodies of the invention, the other CA
IX inhibitory agents of the invention interact with CA I~ or biologically
active
variant thereof resulting in inhibition of CA IX carbonic anhydrase activity
by 5%,
10%, 15%, 20%, 25%, 30%, 35%, preferably 40%, 45%, 50%, 55%, 60%, more
preferably 70%, 75%, 80%, 85%, and most preferably 90%, 95%, 99%, or 100%.
Assays for detecting inhibition of carbonic anhydrase activity are known in
the art,
including those assays disclosed elsewhere herein.
_27_
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
Screening Assays for Detection of Other Inhibitory~ents and Antibodies Capable
of
Inhibiting CA IX Carbonic Anhvdrase Activity
The present invention thus provides methods for identifying other agents that
can serve as therapeutic agents for the treatment of cancers that are
characterized by
expression of the CA IX protein. The methods comprise screening assays whereby
the ability of a test agent to inhibit carbonic anhydrase activity of the CA
IX protein
of interest is assessed. Where a candidate test agent is identified as having
the ability
to inhibit carbonic anhydrase activity in an in vitro assay, the agent can be
fuxther
tested for its ability to inhibit phenotype transformation of cell lines
expressing the
CA IX protein of interest using cell-based assays known in the art, including
the soft
agar assay described herein in the Experimental section below. Inhibitory
agents
identified using ifa vitro assays can also be further tested using in vivo
assays.
Thus, the invention provides a method (also referred to herein as a "screening
assay") for identifying inhibitors, i.e., candidate or test compounds or
agents (e.g.,
peptides, peptidomimetics, small organic molecules, or other drugs; and
suitable
inhibitory antibodies) that bind to the CA IX protein of interest for example,
human
CA IX or biologically active variant thereof, and which have an inhibitory
effect on
carbonic anhydrase activity of this protein.
The test compounds of the present invention can be obtained using any of the
numerous approaches in combinatorial library methods known in the art,
including
biological libraries, spatially addressable parallel solid phase or solution
phase
libraries, synthetic library methods requiring deconvolution, the "one-bead
one-
compound" library method, and synthetic library methods using affinity
chromatography selection. The biological library approach is limited to
peptide
libraries, while the other four approaches are applicable to peptide,
nonpeptide
oligomer, or small molecule libraries of compounds (Lam (1997) Anticanee~ Drug
Des. 12:145).
Examples of methods for the synthesis of molecular libraries can be found in
the art, for example in DeWitt et al. (1993) P~oc. Natl. Acad. Sci. USA
90:6909; Erb
et al. (1994) Ps°oc. Natl. Acad. Sci. USA 91:11422; Zuckermann et al.
(1994). J. Med.
Clzem. 37:2678; Cho et al. (1993) Sciehce 261:1303; Carrell et al. (1994)
Ahgew.
Clzem. Iht. Ed. Engl. 33:2059; Carell et al. (1994) Ahgew. Chem. Int. Ed.
Ehgl.
33:2061; and Gallop et al. (1994) J. Med. Chem. 37:1233.
_28_
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
Libraries of compounds may be presented in solution (e.g., Houghten (1992)
BiolTechhiques 13:412-421), or on beads (Lam (1991) Nature 354:82-84), chips
(Fodor (1993) Natuf°e 364:555-556), ribosbmes (Hudson and Souriau
(2001) Expert.
Opih. Biol. Ther. 1(5):845-855), bacteria (U.S. Patent No. 5,223,409), spores
(IJ.S.
Patent Nos. 5,571,698; 5,403,484; and 5,223,409), plasmids (Cull et al. (1992)
P~oc.
Natl. Acad. Sci. USA 89:1865-1869), phage (Scott and Smith (1990) Sciehce
249:386-
390; Devlin (1990) Sciehce 249:404-406; Cwirla et al. (1990) Proc. Natl. Acad.
Sci.
USA 87:6378-6382; and Felici (1991) J. Mol. Biol. 222:301-310), and yeast
(Hudson
and Souriau (2001) Expert. Opin. Biol. They. 1(5):845-855).
Determining the ability of the test compound to bind to the CA IX protein can
be accomplished, for example, by coupling the test compound with a
radioisotope or
enzymatic label such that binding of the test compound to the CA IX protein,
more
particularly to the carbonic anhydrase (CA) domain of this protein, can be
determined
by detecting the labeled compound in a complex. For example, test compounds
can
be labeled with lash 3sS~ i4C, or 3H, either directly or indirectly, and the
radioisotope
detected by direct counting of radioemmission or by scintillation counting.
Alternatively, test compounds can be enzymatically labeled with, for example,
horseradish peroxidase, alkaline phosphatase, or luciferase, and the enzymatic
label
detected by determination of conversion of an appropriate substrate to
product.
In another embodiment, an assay of the present invention is a cell-free assay
comprising contacting a CA IX protein, or biologically active portion thereof
comprising the CA domain, with a test compound and determining the ability of
the
test compound to bind to the CA IX protein or biologically active portion
thereof,
more particularly to the CA domain. Binding of the test compound to the CA IX
protein can be determined either directly or indirectly as described above. In
a
preferred embodiment, the assay includes contacting the CA IX protein or
biologically active portion thereof comprising the CA domain with a known
compound that binds CA IX protein to form an assay mixture, contacting the
assay
mixture with a test compound, and determining the ability of the test compound
to
preferentially bind to CA IX protein or biologically active portion thereof as
compared to the known compound.
In the above-mentioned assays, it may be desirable to immobilize a CA IX
protein to facilitate separation of complexed from uncomplexed forms of the
protein,
-29-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
as well as to accommodate automation of the assay. In one embodiment, a fusion
protein can be provided that adds a domain that allows the CA IX protein to be
bound
to a matrix. For example, glutathione-S-transferase/CA IX fusion proteins can
be
adsorbed onto glutathione sepharose beads (Sigma Chemical, St. Louis, MO) or
glutathione-derivatized microtitre plates, which are then combined with the
test
compound or the test compound and the nonadsorbed CA IX protein, and the
mixture
incubated under conditions conducive to complex formation (e.g., at
physiological
conditions for salt and pH). Following incubation, the beads or microtitre
plate wells
are washed to remove any unbound components and complex formation is measured
either directly or indirectly, for example, as described above. Alternatively,
the
complexes can be dissociated from the matrix, and the level of CA 1X binding
or
activity determined using standard techniques.
In yet another embodiment, an assay is a cell-free assay comprising contacting
CA IX protein or biologically active portion thereof comprising the CA domain
with a
test compound and determining the ability of the test compound to inhibit the
carbonic anhydrase activity of the CA IX protein or biologically active
variant
thereof. Determining the ability of the test compound to inhibit the carbonic
anhydrase activity of a CA IX protein or biologically active portion thereof
can be
accomplished, for example, by determining the ability of the CA IX protein or
portion
thereof to bind to a CA IX substrate molecule and inhibit enzymatic activity,
as
described, for example, in the Experimental section below.
In the above-mentioned assays, it may be desirable to immobilize a CA IX
protein to facilitate separation of complexed from uncomplexed forms of the
protein,
as well as to accommodate automation of the assay. In one embodiment, a fusion
protein can be provided that adds a domain that allows the CA IX protein to be
bound
to a matrix. For example, glutathione-S-transferase/CA IX fusion proteins can
be
adsorbed onto glutathione sephaxose beads (Sigma Chemical, St. Louis, MO) or
glutathione-derivatized microtitre plates, which are then combined with the
test
compound or the test compound and the nonadsorbed CA IX protein, and the
mixture
incubated under conditions conducive to complex formation (e.g., at
physiological
conditions for salt and pH). Following incubation, the beads or microtitre
plate wells
are washed to remove any unbound components and complex formation is measured
either directly or indirectly, for example, as described above. Alternatively,
the
-30-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
complexes can be dissociated from the matrix, and the level of CA IX binding
or
activity determined using standard techniques.
Other techniques for immobilizing proteins on matrices can also be used in the
screening assays of the invention. For example, CA IX protein can be
immobilized
utilizing conjugation of biotin and streptavidin. Biotinylated CA IX molecules
can be
prepared from biotin-NHS (N-hydroxy-succinimide) using techniques well known
in
the art (e.g., biotinylation kit, Pierce Chemicals, Rockford, IL), and
immobilized in
the wells of streptavidin-coated 96-well plates (Pierce Chemicals).
Alternatively,
antibodies reactive with a CA IX protein but which do not interfere with the
catalytic
action of the CA IX protein on its substrate molecule can be derivatized to
the wells
of the plate, and unbound CA IX protein trapped in the wells by antibody
conjugation.
Methods for detecting such complexes, in addition to those described above for
the
GST-immobilized complexes, include immunodetection of complexes using
antibodies reactive with the CA IX protein, as well as enzyme-linked assays
that rely
on detecting an enzymatic activity associated with the CA IX protein or
biologically
active portion thereof.
In another embodiment, inhibitors of CA IX carbonic anhydrase activity are
identified in a method in which a cell is contacted with a candidate compound
and the
carbonic anhydrase activity of CA IX protein in the cell is determined
relative to
carbonic anhydrase activity of CA IX protein in a cell in the absence of the
candidate
compound. When carbonic anhydrase activity is less (statistically
significantly less) in
the presence of the candidate compound than in its absence, the candidate
compound
is identified as an inhibitor of CA IX protein carbonic anhydrase activity.
The level
of CA IX protein carbonic anhydrase activity in the cells can be determined by
methods described elsewhere herein for detecting such enzymatic activity. The
cells
expressing CA IX protein can be derived from a CA IX+ cancer cell line that is
positive for expression of this tumor-associated antigen. Alternatively, any
suitable
host cell can be utilized for recombinant expression of the particular CA IX
protein of
interest, for example, human CA IX of SEQ ID N0:2 (encoded by SEQ ID NO:1), or
biologically active variant thereof as noted elsewhere herein. The CA IX
protein of
interest can alternatively be expressed as part of a fusion protein as noted
herein
below. Methods for recombinant expression of CA IX protein or biologically
active
variants thereof are known in the art. See, for example, the host cell
expression
-31-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
systems disclosed in U.S Patent No. 6,051,226, herein incorporated by
reference in its
entirety. Such cell-based assays provide a method for assaying an antibody for
the
ability to inhibit carbonic anhydrase activity of a CA IX protein of interest.
In this
manner, a candidate antibody of interest that is to be tested for its
inhibitory activity
can be combined with a cell expressing CA IX protein or biologically active
variant
thereof under conditions suitable for detecting carbonic anhydrase activity,
and then
the level of the carbonic anhydrase activity of CA IX protein in the cell is
determined
relative to carbonic anhydrase activity of CA IX protein in a cell in the
absence of the
candidate antibody. Inhibition of carbonic anhydrase activity by the antibody
would
be indicative that the antibody is an inhibitor of CA IX carbonic anhydrase
activity.
Such candidate antibodies that can be tested using this method include
existing
antibodies as well as candidate antibodies yet to be identified. By "existing"
antibodies is intended those antibodies that have been previously identified,
including,
but not limited to, those antibodies already known in the art. The cell-based
screening
methods of the present invention provide a means for assaying such antibodies
for
their ability to inhibit CA IX carbonic anhydrase activity.
In yet another aspect of the invention, the CA IX proteins can be used as
"bait
proteins" in a two-hybrid assay or three-hybrid assay (see, e.g., U.S. Patent
No.
5,283,317; Zervos et al. (1993) Cell 72:223-232; Madura et al. (1993) J. Biol.
Chem.
268:12046-12054; Bartel et al. (1993) BiolTechhiques 14:920-924; Iwabuchi et
al.
(1993) On.cogefZe 8:1693-1696; and PCT Publication No. WO 94/10300), to
identify
other proteins, which bind to or interact with CA IX protein ("CA IX-binding
proteins" or "CA IX-by"). Then one can test for inhibition of CA IX carbonic
anhydrase activity.
Thus, the invention includes generating cRNA and cDNA libraries for
screening for inhibition of carbonic anhydrase activity of CA IX, can require
expression of recombinant CA IX polypeptides, which can alternatively be
expressed
as a fusion protein as noted elsewhere herein, and can also involve
transforming a cell
with the gene for a CA IX polypeptide, or fusion protein comprising a CA IX
polypeptide, for expression in a screening assay. However, it is not necessary
to
overexpress CA IX in all the cell-based assays, as CA IX is endogenously
expressed
in cancer cell lines (i.e., CA IX+ neoplastic cells) derived from cancers that
are
characterized by expression of CA IX. Exemplary systems for generating
-32-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
polypeptides or libraries useful for the screening methods of the invention
would
include, for example, any standard or useful mammalian, bacterial, yeast, or
insect
expression system, many of which are described in U.S Patent No. 6,204,370
with
regard to production of recombinant CA IX polypeptides. Thus any CA IX
polypeptide or peptide useful in the invention can be made by these or other
standard
methods.
Other items not specifically exemplified, such as plasmids, can be constructed
and purified using standard recombinant DNA techniques described in, for
example,
Sambrook et al. (1989), Molecular Cloning, A Laboratory Manual (2d ed., Cold
Spring Harbor Laboratory Press, Plainview, NY), and Ausubel et al. (1994)
Current
Protocols in Molecular Biology (Greene Publishing Associates and John Wiley &
Sons, New York, NY) under the current regulations described in United States
Department of Health and Human Services (HHS), National Institute of Health
(NLH)
Guidelines for Recombinant DNA Research. These references include procedures
for
the following standard methods: cloning procedures with plasmids,
transformation of
host cells, cell culture, plasmid DNA purification, phenol extraction of DNA,
ethanol
precipitation of DNA, agarose gel electrophoresis, purification of DNA
fragments
from agarose gels, and restriction endonuclease and other DNA-modifying enzyme
reactions.
Methods of Treatment
The antagonist anti-CA IX antibodies, antigen-binding fragments thereof, and
other agents that inhibit carbonic anhydrase activity of CA IX or biologically
active
variant thereof are collectively referred to herein as CA IX inhibitory
agents. These
CA IX inhibitory agents are useful in the treatment of cancers that are
characterized
by expression of the CA IX protein. Such cancers include, but are not limited
to,
carcinomas, such as mammary, bladder, ovarian, uterine, cervical, endometrial,
squamous cell and adenosquamous carcinomas; and head and neck cancers;
mesodermal tumors, such as neuroblastomas and retinoblastomas; sarcomas, such
as
osteosarcomas and Ewing's sarcoma; and melanomas. Of particular interest are
head
and neck cancers, gynecologic cancers including ovarian, cervical, vaginal,
endometrial and vulval cancers as well as gynecologic precancerous conditions,
such
as metaplastic cervical tissues and condylomas; gastrointestinal cancer, such
as,
-33-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
stomach, colon and esophageal cancers; urinary tract cancer, such as, bladder
and
kidney cancers; skin cancer; liver cancer; prostate cancer; lung cancer; and
breast
cancer.
Thus, the invention provides methods for treating a subject having a cancer
that is characterized by expression of the tumor-associated antigen CA IX. By
"subject" is intended mammals, e.g., humans, dogs, cattle, horses, and the
like.
Preferably the subject undergoing treatment with the methods of the invention
is
human.
Subj ects having a cancer characterized by expression of the CA IX protein can
be determined by standard assays known in the art. The presence of CA IX
antigen
can be detected and/or quantitated using a number of well-defined diagnostic
assays.
Those in the art can adapt any of the conventional immunoassay formats, for
example
radioimmunoassays, immunohistochemical staining, immunoelectron and scanning
microscopy using immunogold, among other techniques, to detect and/or
quantitate
MN antigen. Many other formats for detection of CA IX antigen are available.
Those
can be Western blots, ELISAs (enzyme-linked immunosorbent assays), RIAs
(radioimmunoassay), competitive EIA or dual antibody sandwich assays, among
other
assays all commonly used in the diagnostic industry. W such immunoassays, the
interpretation of the results is based on the assumption that the antibody or
antibody
combination will not cross-react with other proteins and protein fragments
present in
the sample that is unrelated to CA IX. Exemplary immunoassays that are
suitable for
detecting a serum antigen include those described in U.S. Patent Nos.
3,791,932;
3,817,837; 3,839,153; 3,850,752; 3,850,578; 3,853,987; 3,867,517; 3,879,262;
3,901,654; 3,935,074; 3,984,533; 3,996,345; 4,034,074; and 4,098,876; herein
incorporated by reference.
Exemplary monoclonal antibodies that can be used for detection of CA IX
expression in a sample obtained from a subject that is suspected of having
existing
neoplastic (tumor) cells or newly formed neoplastic cells that express this
tumor-
associated antigen are the monoclonal antibodies designated M75 and 6250. A
complete discussion of the M75 antibody is disclosed in U.S. Patent No.
6,051,226,
herein incorporated by reference in its entirety. A hybridoma that produces
this
representative CA IX-specific antibody was deposited at the American Type
Culture
Collection [ATCC; 10801 University Blvd., Manassas, Virginia 20110-2209
(LTSA)]
-34-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
under ATCC Number HB 11128. The 6250 antibody is well known to those of skill
in the art. See, for example, Oosterwijk et al. (1996) Pnoc. Natl. Acad. Sci.
USA
37:461; Uemura et al. (1994) Int. J. Cancer 56:609-614; Oosterwijk et al.
(1995)
Semin. Oncol. 22:34-41; herein incorporated by reference in their entirety.
These
MN/CA IX-specific monoclonal antibodies can be used to identify the CA IX
protein
and can be used to readily identify CA IX antigen in Western blots, in
radioimmunoassays, and immunohistochemically, for example, in tissue samples
that
are fresh, frozen, or formalin-, alcohol-, acetone- or otherwised fixed and/or
paraffin-
embedded and deparaffinized. Such samples include tissue specimens, body
fluids,
tissue extracts, and cell extracts obtained from the subj ect who is a
candidate for
treatment with the methods of the invention. Preferred tissue specimens to
assay by
immunohistochemical staining include cell smears, histological sections from
biopsied tissues or organs, and imprint preparations among other tissue
samples.
Biopsied tissue samples can be, for example, those samples removed by
aspiration,
bite, brush, cone, chorionic villus, endoscopic, excisional, incisional,
needle,
percutaneous punch, and surface biopsies, among other biopsy techniques.
The treatment methods of the invention comprise administering to a subject in
need thereof a therapeutically effective dose or amount of an antagonist anti-
CA IX
antibody, antigen-binding fragment thereof, or other agent that inhibits
carbonic
anhydrase activity of CA IX or biologically active variant thereof. When
administered in accordance with the methods of the present invention, these CA
IX
inhibitory agents exhibit anti-tumor activity. "By "anti-tumor activity" is
intended
that the individual undergoing therapy with the CA IX inhibitory agents of the
invention will exhibit a favorable response with respect to the neoplastic or
preneoplastic condition for which the individual is being treated. Examples of
favorable responses include, but are not limited to a reduction in the rate of
cell
proliferation, and hence a decline in growth rate of an existing tumor or in a
tumor
that arises during therapy, and/or destruction of existing neoplastic (tumor)
cells or
newly formed neoplastic cells, and hence a decrease in the overall size of a
tumor
during therapy. Thus, these agents that target CA IX carbonic anhydrase
activity can
be used to promote a positive therapeutic response with respect to a cancer
that is
characterized by expression of this protein. By "positive therapeutic
response" is
-35-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
intended an improvement in the disease in association with the anti-tumor
activity of
these agents, and/or an improvement in the symptoms associated with the
disease.
Factors influencing the respective amount of a particular agent to be
administered to a subject to achieve inhibition of carbonic anhydrase activity
of CA
IX, thereby inhibiting proliferation of neoplastic or phenotype transformation
of
preneoplastic cells expressing this antigen (i.e., CA IX+ neoplastic or
preneoplastic
cells) include, but are not limited to, the particular cancer or proliferative
disorder or
disorder undergoing therapy, the severity of the disease, the history of the
disease, and
the age, sex, height, weight, health, and physical condition of the individual
undergoing therapy. The amount of CA IX inhibitory agent that will constitute
an
inhibitory amount will also vary depending on such parameters as the
particular
inhibitory agent and its potency, the half life of the inhibitory agent in the
body, the
rate of progression of the cancer or proliferative disorder being treated, the
responsiveness of the condition to the dose of treatment or pattern of
administration,
the formulation, the attending physician's assessment of the medical
situation, and
other considerations such as prior administration of other therapeutics, or co-
administration of any therapeutic that will have an effect on the inhibitory
activity of
the inhibitory agent or that will have an effect on carbonic anhydrase
activity of CA
IX.
In all cases, routine experimentation in clinical trials can determine
specific
ranges for optimal therapeutic effect, for each therapeutic agent and each
administrative protocol, and administration to specific subjects can also be
adjusted to
within effective and safe ranges depending on the subject's condition and
responsiveness to initial administrations.
In some embodiments of the invention, the method comprises administration
of multiple doses of antagonist anti-CA IX antibody or antigen-binding
fragment
thereof, or multiple doses of a small molecule inhibitory agent (e.g.,
peptide, peptoid,
or other small molecule). The method may comprise administration of 1, 2, 3,
4, 5, 6,
7, 8, 9, 10, 15, 20, 25, 30, 35, 40, or more therapeutically effective doses
of a
pharmaceutical composition comprising an antagonist anti-CA IX antibody or
antigen-binding fragment thereof or a pharmaceutical composition comprising a
small
molecule inhibitory agent identified herein. The frequency and duration of
administration of multiple doses of the particular pharmaceutical composition
-36-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
comprising an inhibitor of CA IX carbonic anhydrase activity can be readily
determined by one of skill in the art. Moreover, treatment of a subject with a
therapeutically effective amount of such an inhibitory agent can include a
single
treatment or, preferably, can include a series of treatments.
It will also be appreciated that the effective dosage of antibody or antigen-
binding fragment thereof or other CA IX inhibitory agent used for treatment
may
increase or decrease over the course of a particular treatment. Changes in
dosage may
result and become apparent from the results of diagnostic assays.
"Co-administration" as used herein means administration of an antagonist
anti-CA IX antibody, an antigen-binding fragment thereof, or other agent that
inhibits
carbonic anhydrase activity of CA IX or biologically active variant thereof
according
to the treatment methods of the invention in combination with a second
therapeutic
agent. The second therapeutic agent can be any therapeutic agent useful for
treatment
of the subject's condition. Suitable second therapeutic agents include, but
are not
limited to, methotrexate, tamoxifen, nelandron, nilutamide, adriamycin, SFU,
interferons, chemokines such as secondary lymphoid-tissue chemokine (CCL21),
interferons, and cytokines, including interleukin-2 (IL-2), IL-12, IL-13, and
IL-15,
and granulocyte-macrophage colony stimulating factor (GM-CSF), and the like.
The
CA IX inhibitory agents can also be administered in combination with radiation
therapy.
For example, inhibition of neoplastic cell proliferation and/or tumor growth
using a tumor-associated antigen for the CA IX+ cancer undergoing treatment,
or
using a vaccine comprising such a tumor-associated antigen, as a second
therapeutic
agent used in conjunction with a therapeutic agent of the invention that
inhibits
carbonic anhydrase activity of CA IX or variant thereof is contemplated.
Additionally, for example, a first therapeutic agent can be a small molecule
inhibitor
of CA IX carbonic anhydrase activity, and a second therapeutic agent can be an
antisense or ribozyme molecule against CA IX that, when administered in a
viral or
nonviral vector, will facilitate a transcriptional inhibition of CA IX that
will
complement the inhibitory activity of the small molecule. Peptides that are
useful for
generating a CA IX+ tumor-specific cytotoxic T-lymphocyte (CTL) response, and
vaccines comprising these peptides, are known in the art. See, for example,
International Publication Nos. WO 01/98363 and WO 01/60317, herein
incorporated
-37-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
by reference. Also see LT.S. Patent No. 6,204,370, where antisense sequences
specific
for CA IX are disclosed. Co-administration may be simultaneous, for example,
by
administering a mixture of the therapeutic agents, or may be accomplished by
administration of the agents separately, such as within a short time period.
Co-
administration also includes successive administration of an inhibitor of CA
IX
carbonic anhydrase activity and one or more of another therapeutic agent. The
second
therapeutic agent or agents may be administered before or after the inhibitor
of CA IX
carbonic anhydrase activity. The second therapeutic agent may also be an
inhibitor of
CA IX carbonic anhydrase activity, which has particular advantages when
administered with the first inhibitor. Dosage treatment may be a single dose
schedule
or a multiple dose schedule.
Therapeutically effective doses of agents that inhibit carbonic anhydrase
activity of CA IX on neoplastic or preneoplastic cells expressing this protein
can be
administered using any medically acceptable mode of administration that
results in an
effective amount of the inhibitory agent being presented to the cells that are
expressing this protein without incurring unacceptable adverse side effects.
Examples
of routes of administration include parenteral, e.g., intravenous, infusion,
intradermal,
subcutaneous, intramuscular, oral (e.g., inhalation), transdermal (topical),
transmucosal, and rectal administration. In one embodiment, therapeutically
effective doses of these therapeutic agents are injected directly into the
tumor
(intratumoral) or into a peritumor site. By "peritumor site" is meant a site
less than
about 15 cm from an outer edge of the tumor. Admiiustration may be to one or
more
sites. Thus, the therapeutically effective doses of these agents can be
administered at
multiple sites within a tumor and/or surrounding a tumor.
The antagonist anti-CA IX antibodies and other CA IX inhibitory agents of the
invention can be incorporated into an appropriate pharmaceutical composition
that
includes a pharmaceutically acceptable carrier for the agent. The
pharmaceutical
carrier for the agents may be the same or different for each agent. Suitable
carriers
may be large, slowly metabolized macromolecules such as proteins,
polysaccharides,
polylactic acids, polyglycolic acids, polymeric amino acids, amino acid
copolymers,
and inactive viruses in particles. Such carriers are well known to those of
ordinary
skill in the art. Pharmaceutically acceptable salts can be used therein, for
example,
mineral acid salts such as hydrochlorides, hydrobromides, phosphates,
sulfates, and
-3 8-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
the like; and the salts of organic acids such as acetates, propionates,
malonates,
benzoates, and the like. A thorough discussion of pharmaceutically acceptable
excipients is available in Remington's Pharmaceutical Sciences (Mack
Publishing
Co., New Jersey, 1991). Pharmaceutically acceptable Garners in therapeutic
compositions may contain liquids such as water, saline, glycerol, and ethanol.
Additionally, auxiliary substances, such as wetting or emulsifying agents, pH
buffering substances, and the like, may be present in such vehicles.
Typically, the
therapeutic compositions are prepaxed as injectables, either as liquid
solutions or
suspensions; solid forms suitable for solution in, or suspension in, liquid
vehicles
prior to injection may also be prepared. Liposomes are included within the
definition
of a pharmaceutically acceptable Garner. Liposomes are described in U.S.
Patent Nos.
5,422,120 and 4,762,915, International Publication Nos. WO 95/13796, WO
94/23697, and WO 91/144445, and EP 524,968, and in Starner (1975) Biochemistry
(W. H. Freeman, San Francisco, California), pages 236-240; Szoka et al. (1980)
Biochina. Biophys. Acta. 600:1-18; Bayer et al. (1979) Biochim Biophys Acta.
550:46-
473; Rivnay et al. (1987) Methods Enzymol. 149:119-123; Wang and Huang (1987)
P~oc. Natl. Acad. Sei. ZISA 84:7851-7855; and Plant et al. (1989) Anal
Bioclaem.
176:420-426.
The pharmaceutically acceptable carrier or diluent may be combined with
other agents to provide a composition either as a liquid solution, or as a
solid form
(e.g., lyophilized), which can be resuspended in a solution prior to
administration. As
previously noted, the composition can be administered by parenteral or
nonparenteral
routes.
The CA IX inhibitory agents of the present invention can be prepared and/or
identified using the naturally occurring, full-length CA IX protein of
interest, for
example, human CA IX (SEQ ID N0:2, encoded by SEQ ID NO:l). Alternatively,
these inhibitory agents can be prepared andlor identified using a biologically
active
variant of the CA IX protein of interest, for example, a biologically active
variant of
human CA IX. For purposes of the present invention, a biologically active
variant of
a naturally occurring CA IX protein is referred to as a "CA IX polypeptide
variant," a
"polypeptide variant of the CA IX protein," or a "CA IX protein variant." CA
IX
polypeptide variants refer to polypeptides or proteins derived from the native
or
naturally occurring CA IX protein, for example, human CA IX protein of SEQ ID
-39-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
N0:2. CA IX polypeptide variants can be naturally occurring or can be
generated by
deletion or addition of one or more amino acids to the N-terminal and/or C-
terminal
end of the native protein; deletion or addition of one or more amino acids at
one or
more sites in the native protein; or substitution of one or more amino acids
at one or
more sites in the native protein. Such variants include CA IX fragments
comprising a
functional carbonic anhydrase domain, allelic variants, muteins, homologous
orthologues, analogues and fusions of native CA IX polypeptide sequences. By
"analogues" is intended analogues of either a native CA IX protein or a
fragment of a
native CA IX protein that comprise a native CA IX sequence and structure
having one
or more amino acid substitutions, insertions, or deletions. Peptides having
one or
more peptoids (peptide mimics) are also encompassed by the term analogues (WO
91104252).
Of particular interest are polypeptide variants of human CA IX of SEQ ID
N0:2, which may be naturally occurring (e.g., allelic variants that occur at
the CA IX
locus) or recombinantly produced (for example, muteins). Polypeptide variants
of
human CA IX also encompass homologues or orthologues of this native protein.
Any CA IX protein or CA IX polypeptide variant that is biologically active as
noted below can be used to prepare and/or identify the inhibitory agents of
the present
invention. For purposes of the present invention, the term "CA IX polypeptide"
encompasses the native or naturally occurring CA IX protein, for example,
human CA
IX, and biologically active variants of the native or naturally occurnng CA IX
protein,
including CA IX fragments as noted below, where the biologically active
variants
have the functional and structural characteristics defined herein below.
Fragments or so-called "truncated forms" of a native or naturally occurring
CA IX protein or of a CA IX polypeptide variant can be used to prepare and/or
identify the inhibitory agents of the present invention as long as the
fragments have a
functional carbonic anhydrase domain as noted below. Functional fragments or
truncated forms of a CA IX protein, or of a CA IX polypeptide variant, are
referred to
as "CA IX fragments," or "CA IX polypeptide fragments." These fragments or
truncated forms of a CA IX protein or of a CA IX polypeptide variant are
generated
by removing amino acid residues from the full-length CA IX amino acid sequence
(i.e., the sequence for the native CA IX protein or the sequence for the CA IX
polypeptide variant), for example, using recombinant DNA techniques well known
in
-40-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
the art and described elsewhere herein, and include N-terminal and C-terminal
deletions of the full-length CA IX sequence. Because CA IX fragments are
directly
or indirectly derived from a native or naturally occurring CA IX protein, they
represent one type of CA IX polypeptide variant as noted herein above.
For purposes of screening candidate inhibitory agents, including candidate
antagonist anti-CA IX antibodies, for their ability to inhibit CA IX carbonic
anhydrase activity, the full-length CA IX polypeptide variants, and fragments
or
truncated forms of a full-length CA IX protein or of a full-length CA IX
polypeptide
variant, should retain a functional carbonic anhydrase domain that provides
for a
carbonic anhydrase activity that is at least 20%, 25%, 30%, 35%, 40%, 45%,
preferably at least 50%, 55%, 60%, 65%, 70%, 75%, more preferably at least 80%
of
the carbonic anhydrase activity of the naturally occurring CA IX protein, for
example,
human CA IX shown in SEQ m N0:2. Fragments of a CA IX protein or fragments
of a CA IX polypeptide variant suitable for preparing the anti-CA IX
antibodies of the
invention will comprise at least one inhibitory epitope that can be recognized
by an
antagonist anti-CA IX antibody, and thus form an antibody-antigen complex in
the
presence of this antibody whereby carbonic anhydrase activity of the CA IX
polypeptide fragment is inhibited. Suitable epitopes to be included in a CA IX
polypeptide fragment include those inhibitory epitopes comprising contiguous
and/or
discontinuous residues of the CA IX polypeptide fragment, for example,
inhibitory
epitopes comprising contiguous and/or discontinuous residues within the
carbonic
anhydrase domain. The CA IX inhibitory agents of the invention identified
using the
methods of the invention are suitable for treating a subject having a cancer
characterized by expression of CA IX, or expression of a naturally occurring
allelic
variant of CA IX.
Native or naturally occurring CA IX proteins and CA IX polypeptide variants,
including CA IX fragments (derived from the native or naturally occurnng CA IX
protein or derived from a full-length CA IX polypeptide variant) that are
useful in the
methods of the invention may be modified further so long as they retain CA IX
carbonic anhydrase activity as noted below. Further modifications include, but
are
not limited to, phosphorylation, substitution of non-natural amino acid
analogues, and
the like. For the purposes of the present invention, CA IX polypeptide
variants have
an amino acid sequence that shares at least 70%, generally at least 75%, 80%,
85%,
-41-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
preferably about 90% to 95% or more, and more preferably about 98% or more
sequence identity to the native or naturally occurnng CA IX protein, where
sequence
identity is determined as noted herein below. Thus, for example, where the CA
IX
polypeptide variant is a variant of human CA IX, the CA IX polypeptide variant
has
an amino acid sequence that shares at least 70%, generally at least 75%, 80%,
85%,
preferably about 90% to 95% or more, and more preferably about 98% or more
sequence identity to human CA IX of SEQ ID N0:2. Furthermore, suitable CA IX
polypeptide variants for purposes of the present invention will have a
functional
carbonic anhydrase domain that shares at least 70%, generally at least 75%,
80%,
85%, preferably about 90% to 95% or more, and more preferably about 98% or
more
sequence identity to the carbonic anhydrase domain of human CA IX, i.e.,
residues
135-414 of SEQ ID N0:2. A variant of the CA IX protein useful in preparing the
compositions of the invention may differ from the native or naturally
occurring CA
IX protein, for example human CA IX of SEQ ID NO:2, by as few as 1-15, as few
as
1-10, such as 6-10, as few as 5, as few as 4, 3, 2, or even 1 amino acid
residue, so long
as the functional carbonic anhydrase domain of the variant retains at least
70%
sequence identity with residues 135-414 of SEQ ~ NO:2. As previously noted,
the
CA IX polypeptide variant will comprise a functional carbonic anhydrase domain
that
provides for a carbonic anhydrase activity that is at least 20%, 25%, 30%,
35%, 40%,
45%, preferably at least 50%, 55%, 60%, 65%, 70%, 75%, more preferably at
least
80% of the carbonic anhydrase activity of the native CA IX protein.
Methods for calculating sequence identity and similarity are known in the art.
See, for example, Conaputer Analysis of Sequence Data, Past 1, ed. Griffin and
Griffin
(Humana Press, New Jersey, 1994), von Heinj a (1987) Sequence Analysis in
Molecular
Biology (Academic Press, New York); and Gribskov and Devereux, eds. (1991)
Sequence Analysis Prinae~ (M Stockton Press, New York). In general, to
determine the
percent identity of two amino acid sequences, the sequences are aligned for
optimal
comparison purposes. The percent identity between the two sequences is a
function
of the number of identical positions shared by the sequences (i.e., percent
identity =
number of identical positions/total number of positions (e.g., overlapping
positions) x
100). For example, by a CA 1X variant polypeptide having an amino acid
sequence at
least 95% "identical" to a reference CA IX amino acid sequence is intended
that the
amino acid sequence of the CA IX variant polypeptide is identical to the
reference CA
-42-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
IX polypeptide sequence except that the polypeptide sequence of the CA IX
variant
can include up to five amino acid alterations per each 100 amino acids of the
reference CA IX amino acid sequence. These alterations of the CA IX reference
sequence may occur at the amino or carboxy terminal positions of the reference
CA
IX polypeptide sequence or anywhere between those terminal positions,
interspersed
either individually among residues in the reference CA IX polypeptide sequence
or in
one or more contiguous groups within the reference CA IX sequence.
The determination of percent identity between two sequences can be
accomplished using a mathematical algorithm. For the purposes of the present
invention, percent sequence identity is determined using the Smith-Waterman
homology search algorithm using an affine gap search with a gap open penalty
of 12
and a gap extension penalty of 2, BLOSUM matrix of 62. The Smith-Waterman
homology search algorithm is taught in Smith and Waterman (1981) Adv. Appl.
Math.
2:482-489.
The CA IX polypeptide variants useful in preparing the compositions of the
invention may be obtained by amino acid substitutions, deletions, truncations,
and
insertions. Preferred CA IX polypeptides variants have one or more
conservative
amino acid substitutions of the polypeptide of SEQ ID N0:2. For example,
conservative amino acid substitutions may be made at one or more amino acid
residues. Preferably, substitutions are made at nonessential amino acid
residues, and
preferably involve 1-15 residues, 1-10 residues, including 1, 2, 3, 4, 5, 6,
7, 8, 9, or 10
residue substitutions.
A "nonessential" amino acid residue is a residue that can be altered from the
wild-type sequence of a CA IX protein (e.g., the sequence of SEQ ID N0:2)
without
altering one of the biological activities, whereas an "essential" amino acid
residue is
required for a given biological activity.
A "conservative amino acid substitution" is one in which the amino acid
residue is replaced with an amino acid residue having a similar side chain.
Families
of amino acid residues having similar side chains have been defined in the
art. These
families include amino acids with basic side chains (e.g., lysine, arginine,
histidine),
acidic side chains (e.g., aspartic acid, glutamic acid), uncharged polar side
chains
(e.g., glycine, asparagine, glutamine, serine, threonine, tyrosine, cysteine),
nonpolar
side chains (e.g., alanine, valine, leucine, isoleucine, proline,
phenylalanine,
-43-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
methionine, tryptophan), beta-branched side chains (e.g., threonine, valine,
isoleucine) and aromatic side chains (e.g., tyrosine, phenylalanine,
tryptophan,
histidine). See, for example, Bowie et al. (1990) Science 247:1306, herein
incorporated by reference. Preferably, such substitutions would not be made
for
conserved cysteine residues, such as the amino terminal contiguous cysteine
residues.
With respect to optimal alignment of two amino acid sequences, the
contiguous segment of the variant amino acid sequence may have additional
amino
acid residues or deleted amino acid residues with respect to the reference
amino acid
sequence. The contiguous segment used for comparison to the reference amino
acid
sequence will include at least 20 contiguous amino acid residues, and may be
30, 40,
S0, or more amino acid residues. Corrections for sequence identity associated
with
conservative residue substitutions or gaps can be made (see Smith-Waterman
homology search algorithm, taught in Smith and Waterman (1981) Adv. Appl.
Matla.
2:482-489).
The CA IX polypeptide variants useful in preparing and/or screening for the
compositions of the invention may be isolated as naturally occurnng variants,
isolated
after mutagenesis or recombinant manipulation, or be synthetically produced.
Naturally occurnng allelic variants can be identified with the use of well-
known
molecular biology techniques, such as, for example, with polyrnerase chain
reaction
(PCR) and hybridization techniques. Methods for such manipulations are
generally
known in the art. In addition, variants of the CA IX proteins can be prepared
by
mutagenesis or recombinant manipulations. Methods for mutagenesis and
nucleotide
sequence alterations are well known in the art. See, for example, I~unkel
(1985) Proc.
Natl. Acad. Sci. USA 82:488-492; I~unkel et al. (1987) Methods Enzynaol.
154:367-
382; U.S. Patent No. 4,873,192; Walker and Gaastra, eds. (1983) Techniques in
Molecular Biology (MacMillan Publishing Company, New York), and the references
cited therein. Obviously, the mutations that will be made in the DNA encoding
the
variant must not place the sequence out of reading frame and preferably will
not
create complementary regions that could produce secondary mRNA structure. See,
EP Patent Application Publication No. 75,444. Guidance as to appropriate amino
acid
substitutions that do not affect biological activity of the protein of
interest may be
found in the model of Dayhoff et al. (1978) Atlas of Protein Sequence and
Structure
(Natl. Biomed. Res. Found., Washington, D.C.), herein incorporated by
reference.
-44-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
Thus, the CA IX polypeptides useful in preparing the antagonist anti-CA IX
antibodies and identifying other inhibitory agents encompass both naturally
occurring
proteins as well as variations and modified forms thereof. Such variants will
continue
to possess the desired CA IX activity, i.e., carbonic anhydrase activity, so
that
specificity of these inhibitory agents can be tested against the variant
polypeptide.
For the purposes of the present invention, a "CA IX polypeptide variant,"
including
CA IX polypeptide fragments, will exhibit at least 20%, 25%, 30%, 35%, 40%,
45%,
preferably at least 50% of the CA IX carbonic anhydrase activity of the native
CA IX
polypeptide. Thus, for example, a variant of the CA IX polypeptide of SEQ ~
N0:2
will exhibit at least 20%, 25%, 30%, 35%, 40%, 45%, preferably at least 50% of
the
carbonic anhydrase activity of the corresponding native CA IX polypeptide of
SEQ
ID N0:2. More typically, variants exhibit at least 60% of the native CA IX
carbonic
anhydrase activity; even more typically, variants exhibit at least 80% of the
native CA
IX carbonic anhydrase activity.
The deletions, insertions, and substitutions of the CA IX polypeptide
sequences useful in preparing the CA IX inhibitory compositions disclosed
herein are
not expected to produce radical changes in the characteristics of the
particular CA IX
protein. However, when it is difficult to predict the exact effect of the
substitution,
deletion, or insertion in advance of doing so, one skilled in the art will
appreciate that
the effect will be evaluated by routine screening assays. That is, the
carbonic
anhydrase activity can be evaluated by standard assays known to those skilled
in the
art. See, for example, the assays disclosed in the Experimental section below.
Specific amino acids witlun the CA domain that are involved in carbonic
anhydrase activity of the CA IX polypeptide that are essential for carbonic
anhydrase
activity of CA IX can be identified by methods known in the art. Such methods
include alanine-scanning mutagenesis, molecular evolution (Crameri et al.
(1996)
Nat. BioteclZhol. 14(3):315-319; Crameri et al. (1998) Natu~~e 15:288-291;
Patten et
al. (1997) Cu~~. Opih. Biotechhol. 8:724-733; Stemmer (1994) P~oc. Natl. Acad.
Sci.
USA 91:10747-51; Stemmer (1994) Nature 370:389-391), or site-directed
mutagenesis. See, Cunningham et al. (1989) Science 244:1081. Resulting mutants
can be tested for biological activity. Sites critical for activity can be
determined by
structural analysis such as crystallization, photoaffinity labeling, or
nuclear magnetic
resonance. See, deVos et al. (1992) Science 255:306 and Smith et al. (1992) J.
Mol.
-45-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
Biol. 224:899. For example, the three histidine residues residing at positions
226,
228, and 251 of SEQ ID N0:2 are known to be critical for this enzymatic
activity in
human CA IX.
The following examples are offered by way of illustration and not by way of
limitation.
EXPERIMENTAL
Example 1: Glu-tagged CA IX Purification Procedure
The glu-tagged CA domain portion of the human CA IX protein used in the
Examples below was expressed in a soluble form in Baculovirus-infected Tn5
cells.
Media containing the secreted, glu-tagged CA IX protein (CA IX-glu) was
harvested
and stored at -80°C. Baculovi~us media containing CA IX-glu was thawed
(at room
temperature) and made 1 mM PMSF. It was concentrated 6 to 7 fold on ice, on a
10
kD Minisette (Filtron) membrane.
- An Anti Glu-tag Affi-Gel 10 (Biorad) affinity chromatography column was
prepared in advance according to the manufacturer's instructions, using
Protein G
purified monoclonal antibodies. At 4°C, this column was equilibrated in
the
following equilibration buffer: PBS / 10% Glycerol / 1% Octyl Glucoside. The
concentrated media was filtered on a 0.22 ~,M CN membrane (Nalgene). Complete,
EDTA-free Protease Inhibitor Cocktail (Roche Diagnostics #1873580)
supplemented
with 5 ~.g/ml of Leupeptin, and 10 ~.g/ml of E-64, was added. This material
was
loaded on the Anti Glu-tag Affi-Gel 10 column.
This column was washed with 10 CV of the equilibration buffer. It was
subsequently eluted with 10 CV of the following elution buffer: 0.1 mg/ml
EYMPTD
peptide, custom manufactured by ResGen (#K0121 008) in PBS / 10% Glycerol / 1
Octyl Glucoside. The eluate was dialyzed vs PBS / 10% Glycerol / 1% Octyl
Glucoside on a YM-10 Millipore membrane to eliminate the elution peptide and
to
concentrate the protein. Finally, the concentrated eluate was sterile filtered
on a 0.22
~,M Millipore (Durapore) membrane.
-46-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
Example 2: Glu-tagged, Full-Length CA IX Purification Procedure
The full-length, glu-tagged human CA IX protein used in Examples below was
expressed internally in Baculovirus-infected Tn5 cells. Cells were harvested
and
stored at -80°C. Cells were thawed (at room temperature) and diluted
1/3 with the
following lysis buffer: PBS / 10% Glycerol / 0.5% Triton-X, pH 7.4, with
Complete,
EDTA-free Protease Inhibitor Cocktail (Roche Diagnostics #1873580)
supplemented
with 5 ~,g/ml of Leupeptin, and 10 ~,g/ml of E-64.
Cells were then lysed in a glass homogenizer on ice. Lysate was centrifuged at
15,OOOxg for 1 hour at 4°C. The centrifuged supernatant of the lysate
was processed
further as follows. The lysate, diluted 1/3 with the lysis buffer, was applied
on a p-
Aminomethyl-benzenesulfonamide Agarose gel matrix (Sigma# A-0796) equilibrated
in the above lysis buffer at 4°C. (Lysis buffer is equilibration buffer
#1.) The column
was washed with 10 CV of equilibration buffer #1 and, subsequently eluted with
10
CV of the following elution buffer #1: PBS / 10% Glycerol / 0.1 mM
Acetazolamide
(Sigma A-9842). After concentration on a YM-30 Millipore membrane, the eluate
was diafiltered into the following equilibration buffer #2: PBS / 10% Glycerol
/ 1%
Octyl Glucoside, pH 7.4.
An Anti Glu-tag Affi-Gel 10 (Biorad) affinity chromatography column was
prepared in advance according to the manufacturer's instructions, using
Protein G
purified monoclonal antibodies. At 4°C, this column was equilibrated in
equilibration
buffer #2. The diafiltered eluate was loaded on the Anti Glu-tag Affi-Gel 10
column.
It was washed with 10 CV of equlibration buffer #2, and then eluted with 10 CV
of
the following elution buffer #2: 1 mg/ml EYMPTD peptide, custom manufactured
by
ResGen (#K0121 008) in PBS / 10% Glycerol / 1% Octyl Glucoside. The eluate was
dialyzed vs PBS / 10% Glycerol / 1% Octyl Glucoside on a YM-30 Millipore
membrane to eliminate the elution peptide and to concentrate the protein.
Finally, the
concentrated eluate was sterile filtered on a 0.22 ~.M Millipore (Durapore)
membrane.
-47-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
Example 3: Low-throughput Assay for Carbonic Anhydrase Activity of CA IX
The following low-throughput assay can used to screen candidate test agents
for their ability to inhibit carbolic anhydrase activity of CA IX. This assay
can be
conducted using the full-length CA IX protein, or a partial-length CA IX
polypeptide
that includes a functional carbonic anhydrase domain. The CA IX protein can be
glu-
tagged as well.
Prepare 4X buffer and filter:
100 mM HEPES, pH 7.5
400 mM phenol red
400 mM NaS04
Bubble C02 into H20 (C02-H20).
In a 96-well UV transparent plate:
aliquot 50 p,l 4X buffer/well;
add 25 ~,1/well CA standard (bovine carbonic anhydrase [Sigma]) or CA IX
diluted
in PBS or glu-tagged CA IX diluted in PBS;
add 25 ~,1/well test sample diluted in media or media alone;
include wells with 50 ~14X buffer + 25 ~1 PBS + 25 ~,1 media as background.
Start reaction by adding 100 ~,1/well COz-HZO.
Read at 562 nm in kinetic mode for 15.0 min with mixing.
CA Assay (mini)
Prepare phenol buffer:
40 mM HEPES, pH 8.3 at 4°C
400 mM phenol red (Tissue Culture Grade)
-48-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
Reformulate mAbs in 40 mM HEPES final (need 25 ~.1 sample/well).
Bubble C02 into HZO on ice for a minimum of 30 min ("CO2-Ha0").
Resuspend Mafenide to 50 mg/ml in 40 mM HEPES = 203 mM (must be freshly
prepared).
Dilute samples with 40 mM HEPES:
As a negative control, make one of the samples the monoclonal antibody M75
(should not inhibit)
As a positive control, make one of the samples 200 ~,M Mafenide (50 ~,M
final)
(should inhibit)
Dilute CA-glu to g0 nM in phenol buffer (20 nM final).
Add equal volume of diluted CA-glu to samples (1:2 dilution).
Degas on ice for 20 min.
Incubate 96-well UV Star plate on ice.
Set-up reader for 560 nm for 5 minute kinetic read with 3 second shaking in
between.
Aliquot 50 ,ul of sample into wells on ice.
Pour C02-H20 into reservoir.
Click "Read" on plate reader controls and begin counting.
Before plate reader door closes, add COZ-H20 (50 ~,l/well).
Read plate.
-49-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
Assay Details:
Can test up to 12 wells per run
Need to include "without CA-glu" and "with CA-glu" controls with every run
Each sample should be tested in triplicate or quadruplet
CA Assay (macrolmidi)
Prepare buffer:
40 mM HEPES, pH 8.3 at 4°C
Reformulate mAbs in 40 mM HEPES buffer.
Dilute CA-glu to 80 nM in HEPES buffer (20 nM final).
Resuspend Mafenide to 50 mg/ml in 40 mM HEPES = 203 mM (must be freshly
prepared).
Dilute samples with 40 mM HEPES:
Include as a sample the monoclonal antibody M75 (should not inhibit)
Include as a sample 200 ~,M Mafenide (50 ~,M final) (should inhibit)
Include a sample with no Ab or Mafenide (HEPES only) to determine
spontaneous rate of acidification
Mix samples and CA-glu 1:1 on ice.
Include a control set with no CA-glu.
Degas on ice for 20 min.
Bubble COZ into H20 on ice for a minimum of 30 min on ice.
Add equal volume C02 saturated water to CA-glu/mAb samples on ice.
-50-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
Start monitoring pH immediately. Monitor pH every 10 sec., and chart pH vs.
time.
Expected results:
The control set with no enzyme should not show a change in pH over the time
of the assay.
Isotype control sets and the M75 set should show no change in acidification
kinetics compared to the spontaneous acidification sample (water + enzyme
only; no Abs or inhibitors).
The Mafenide sample should inhibit (slow down) the rate of acidification.
Example 4: High-throughput Assay for Carbonic Anhydrase Activity of CA I~
The following high-throughput assay can be used to screen candidate test
agents for their ability to inhibit carbonic anhydrase activity of CA IX. The
glu-
tagged CA IX (CA IX-glu) is purified as noted in the examples above. As with
the
low-throughput assay, the full-length CA IX protein or a partial-length CA IX
polypeptide that includes a functional carbonic anhydrase call be used. The
substrate
used in the reaction is fluorescein diacetate (FDA), catalog # F-103
(available from
Molecular Probes). The reference standard is Acetazolamide, catalog #A-9842
(available from Sigma Chemicals). The assay buffer is 50 mM MES, pH=6.5, 0.05%
Tween-20, O.lmM EDTA.
Protocol
The following assay is carried out in a 384-well black polypropylene plate.
1) 1.25 pl of test sample in 100% Dimethyl sulfoxide is added to each well.
2) Dilute CA IX-glu enzyme stock to 83.3 nM in assay buffer. Add 30 p,l of
dilute enzyme stock to each well. Mix. For background wells, add 30 ~1 of
assay
buffer - no enzyme.
3) Dilute FDA substrate stock to 62.5 p,M in assay buffer. Add 20 ~,1 of
dilute
substrate stock to each well. Mix.
4) Incubate 2 hr at room temperature.
-51-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
5) Read fluorescence at Excitation=485nm, Emission=520nm.
Example 5: Soft Agar Assay for Detection of Inhibition of Carbonic Anhydrase
Activity
The following protocol can be used to test the ability of a candidate
inhibitory
agent for its ability to inhibit carbonic anhydrase activity of CA IX.
1) Coat each well of a non-tissue culture 96-well plate with 1.2% poly(HEMA).
When the coating is dry, sterilize the plate.
2) Seed cells in a 96-well plate at a density of 350 to 500 cells in 100 ~1
per well.
If desired, prior to plating, the cells can be pre-incubated with an antibody
to be tested
for inhibitory activity.
3) Add 300 ~,1 of melted 4% noble agar (60°C) to 840 ~1 of media
(37°C) and
mix.
4) Dispense 50 ~.1 of mixture to each well, doing one column of wells at a
time,
and mix to create a homogeneous suspension of cells in agar.
5) Allow agar to solidify for 10 to 15 minutes at room temperature.
6) Overlay agar with 100 ~,l of media per well and incubate plate at
37°C for 7 to
14 days (depending on how fast the cells grow). If desired, chemical compounds
or
antibodies to be tested for inhibitory activity can be included in the media.
7) After 7 to 14 days, add 20 ~,1 of Alamar Blue to each well and gently shake
the
plate at room temperature for 10 to 15 minutes.
8) Return the plate to the incubator and monitor every hour early in the
incubation period.
9) Alamar Blue is metabolized by the cells and reduced over time.
10) Reduced Alamar dye is read at 530 nm Excitation, 590 nm Emission and the
fluorescence units are used as, an indicator of cell growth.
-52-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
Example 6: Carbonic Anhydrase Activity Is Required for the Ability of CA IX to
Contribute Toward the Transformed Phenotype
The first obj ective of this study was to determine if expression of the
proteoglycan-like (PG) domain of human CA IX (residues 38-134 of SEQ m N0:2)
alone or the carbonic anhydrase (CA) domain of human CA IX (residues 135-414)
alone is sufficient to confer a transformed phenotype to NIH-3T3 cells. If the
CA
domain alone was identified as being sufficient, the second objective was to
determine if enzymatic activity is required to confer a transformed phenotype
to NIH-
3T3 cells.
cDNA expression constructs encoding full-length human CA IX (SEQ ID
N0:2; encoded by SEQ ID NO:1), the PG domain of human CA IX (residues 38-134
of SEQ ID N0:2), the CA domain of human CA IX (residues 135-414 of SEQ ID
NO:2), or the CA domain of CA IX bearing mutations abrogating carbonic
anhydrase
enzymatic activity were made and transfected into NIH-3T3 cells. The complete
constructs were as follows:
Full-length CA IX construct (CA-full). This construct encoded the full-length
human CA IX. The coding and translated polypeptide sequences are shown in SEQ
ID NOS:1 and 2, respectively.
Proteoglycan-like (PG) domain construct (CA-PG). This construct encoded
the signal peptide (residues 1-37 of SEQ ID N0:2), the PG domain (residues 38-
131
of SEQ ID N0:2, which includes most of the PG domain of SEQ ID N0:2 and
encompasses the entire functional region of the PG domain, i.e.; residues 53-
111 of
SEQ ID N0:2), a tri-peptide serine linker (included for technical reasons),
the
transmembrane domain (residues 415-433 of SEQ ID N0:2), and the intracellular
or
intracytoplasmic domain (residues 434-459 of SEQ ID N0:2). The coding and
translated polypeptide sequences are shown in SEQ ID NOS:3 and 4,
respectively.
Carbonic anhydrase (CA) domain construct (CA-CAH). This construct
encoded the signal peptide (residues 1-37 of SEQ ID N0:2), the CA domain
(residues
135-414 of SEQ ID N0:2), the transmembrane domain (residues 415-433 of SEQ ID
N0:2), and the intracellular or intracytoplasmic domain (residues 434-459 of
SEQ ID
N0:2). The coding and polypeptide sequences are shown in SEQ ID NOS:S and 6,
respectively.
-53-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
Glu-tagged CA domain construct (CA-CAHgIu). This construct encoded the
signal peptide (residues 1-37 of SEQ ID N0:2), a short series of residues from
the
non-functional region of the PG domain (residues 38-45 of SEQ ID N0:2), a
single
alanine linker (included for technical reasons), residue 47 of SEQ ID N0:2
(from the
non-functional region of the PG domain), the glu tag (EYMPME), a short series
of
residues from the non-functional region of the PG domain (residues 123-134 of
SEQ
ID N0:2), the CA domain (residues 135-414 of SEQ ID N0:2), the transmembrane
domain (residues 415-433 of SEQ ID N0:2), and the intracellular or
intracytoplasmic
domain (residues 434-459 of SEQ ID NO:2). The coding and polypeptide sequences
are shown in SEQ m NOS:7 and 8, respectively.
Glu-tagged mutant CA domain construct (CA-CAHglumut). This construct
encoded the same amino acid sequence as the glu-tagged CA domain construct
with
the exception of having the three lustidine residues obligatory for carbonic
anhydrase
activity (i.e., residues 226, 228, and 251 of SEQ ID N0:2) mutated to three
glutamine
residues. The coding and polypeptide sequences are shown in SEQ 117 NOS:9 and
10,
respectively.
These transfected cells, an untransfected negative control cell line, and an
src-
transfected positive control cell line were then tested for their ability to
grow in soft
agar using the soft agar assay described in Example 5. Results are shown in
Table 1
below.
The results confirm that full-length CA IX confers upon NIH-3T3 cells the
ability to grow in soft agar (transformed phenotype). These results further
demonstrate that the carbonic anhydrase domain of CA IX alone is sufficient to
confer
this transformed phenotype (the ability to grow in soft agar), while the
proteoglycan-
like (PG) domain alone is not sufficient. Furthermore, mutants of the CA
domain
engineered to lack carbonic anhydrase enzymatic activity do not confer a
transformed
phenotype onto transfected NIH-3T3 cells, suggesting that in addition to
expression of
the CA domain, carbonic anhydrase activity is also necessary for conferring
the
transformed phenotype.
These results indicate that targeting carbonic anhydrase activity of CA IX is
likely to be beneficial therapeutically. Thus, protein-based therapeutics such
as
antibodies which inhibit CA IX carbonic anhydrase activity, or small molecule
based
-54-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
therapeutics that inhibit CAIX carbonic anhydrase activity, may be effective
therapeutic agents for cancers expressing CA IX.
-55-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
56
~°
0
0
A
b
V~ ~ ~ r '~'r ~ ~' 'd'~ r
r O ~ rO r 'Od'm N
r
N r ~r N N N M
~ M MN
InN O f~I~ tn d' a0O)M
N o~07tn N r oo d:
M O NO ~ O ~ VM'~ 00 ~ ~ ~ N O ch 00 1~
OD N d' I~ N O c0 a0 O O O a0 OD
W
V Or M _N N M M M CM O
ct
O
U O In N LnIn O 07 N (p(A
0 ~ O N NN ~ M N ~ m M M I~O In
y. N h CM N O l()OJ ~ ~ ~ ~ h ~ GO OO M
., O
M
O O M M~ r M d'N n O O .
0 0 0
o ~ d0' dd
'7 ~ O O '
N
COa0 (ONe0 c0 O Inh O
d'N triM(()~ O O 00cY ~ r O M O t~ d; Od:CO
O~0 ~ 007COO ~ O O a~ON O O)d'OD N d' OO d'
S=I ~ M Mr r M 'd'N N ~ V ~ ~ O 'Od'
N M O>a0h N ~ N
N W O N
M M t~h(O M CO M N d:
N N
, (V r ~O N C()N ~ r O) O r (n r O OI~h
~ C~O~ ~ N M Ire.N (~ ~ M h N CO O07CV
N ~ COf0 N M r ~ ~ ~ M O COO
N m ~ N N o0N
t()(b d'lf>00 In M 00O
O m O 0O O ~ O 'O ~ N O N (A In In O07(A
0C 'd' I~C O O
0~ ~
M a0
c _ pip ~ 0~7M d0' N N
O
~ ~ N M 'd'
tt)CO GOOh Q (O M O O
In '
n
N CO CVOIn fn lij InCOd
C'~~ 07COM M ~ N COf~ ~. Cp r <AO d' t~ Nt~
N
'b D
N 0 M N ~ ~ N O0
a c N
o O d' O0
d'
V tnN r WfJ In CO tn(OM ~
M N ~ Nr M r O f7N
O N r tn
M M d:f~~ M r O)~tO
00 r r h NM 00
Ind' N hCA tn O t~h I~ 07 t~h LO Cn I~ CO
N O NM N M i~N W ' O due' M ~~ N
r O) t a N o
r O p
~
~
r N ~l7 tnOOd' N h COO O
r In O)COIO r lO (ACOCO 0 M O I Nr
. ( ChC l O
d ~ ~~ M M O O ) O0
M
c O ( d' O 'd'O O O
O N M tntf~
V N
'
O
r O O)Ln~Y r h t~l1)r
O tnM M Or tn f~ fvfst(7
O d' (nMtn O CV C~~ h O ~ ~ O m N~ M
NN M cM0_ dM' N C09N N O M 'd'
'~i'
i
N N M(O r M CO'd:h r dp ~ ~ r r ~ rr O
O ~ ~ LOd' O 'd' ~ O)h O M O r r
M O tn d M In
~ M ue~ M ' N
O
'3 MM d N d N N M
' '
vi
tpfp M ~ ' tn M ~ L tn M ~ ~ fn M7
M M M M
~ I I U~
U= D Q = US D Q ? = UZ ~ Q = UZ DQ
Z ~ U= Z ~ U = Z ~ = Z ~U
UU U U U U U
U
U
U
U
<IMG>
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
Example 7: ELISA Test to Detect Antibodies Against the CA Domain of CA IX
The antigen used in the ELISA test was a glu-tagged recombinant protein
representing the CA domain portion of human CA IX ("CA-glu" protein), isolated
as
described in Example 1. The CA-glu protein was suspended in phosphate-buffered
saline (PBS) at a concentration of 1 ~,g/ml, and 100 pl/well of this solution
was
dispensed into 96-well, flat-bottomed plates (Immulon 4 HBX). After incubation
of
the plates overnight at 4°C, the protein solution was removed and the
wells were
washed with PBST (PBS + 0.05% Tween-20). The wells received 200 wl/well of
Blocking Buffer (3% Carnation non-fat dry milk in PBS + 0.01% Tween-20), and
the
plates were incubated for 1- 2 hours at room temperature. The wells were again
washed with PBST, and each well then received 100 ~1 of a solution containing
an
antibody or hybridoma supernatant to be tested. In the case of the hybridoma
supernatants, the solutions consisted of 5 - 50 pl of supernatant brought up
to a final
volume of 100 ~,1 with Blocking Buffer. After incubation of the plates for 1
hour at
room temperature, the wells were washed with PBST. Each well received 100 ~,1
of a
secondary antibody solution (horse radish peroxidase-conjugated rabbit anti-
mouse
IgG (gamma); Zymed #61-6020; diluted 1:4000 in Blocking Buffer), and the
plates
we reincubated for 0.5 -1 hour at room temperature. After a final wash of the
wells
with PBST, 100 ~,l/well of ODP peroxidase substrate (Sigma #P-9187; prepared
according to the manufacturer's instructions) was added, and the plates were
incubated at room temperature for 5 - 30 min to allow color development. The
peroxidase reaction was stopped by the addition of 50 ~,1/well of 4 M H2SO4,
and the
reaction in each well was quantified as the optical density at 490 nm minus
the optical
density at 540 nm.
Example 8: Fluorescence-Activated Cell Sorting (FACS) Assay to Detect
Antibody Reactivity Against Cell-Surface CA IX
To determine whether 'anti-CA-glu antibodies could bind the CA domain when
the domain was present as part of the native molecule on the surface of cells,
fluorescence-activated cell sorting was performed. The cell type utilized in
the assay
was the HT29 cell line (ATCC #HTB-38), derived from a human colorectal
-58-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
adenocarcinoma. For the assay, the cells were detached from tissue culture
plates by
treatment for approximately 30 minutes with 3 mM EDTA. The cells were
collected
by centrifugation and then resuspended in cold incubation buffer (PBS
containing 1%
bovine serum albumin). Antibodies or hybridoma supernatants to be tested were
mixed with 2 - 5x105 cells in cold incubation buffer in a final volume of 100
~1. As a
negative control, cells were mixed with 10 ~g/ml of nonspecific mouse IgG2b;
for a
positive control, the cells were mixed with 10 ~g/ml of the anti-CA IX
monoclonal
antibody M75 (Pastorekova et al. (1992) hirology 187:620-626). After
incubation for
30 min at 4°C, the cells were collected by centrifugation, washed three
times with
cold incubation buffer, and mixed with a detecting antibody (goat anti-mouse
IgG
antibody conjugated with phycoerythrin; CALTAG M30004-4; 0.2 dug in 100 p,l
cold
incubation buffer per cell sample). After incubation of the cells with the
secondary
antibody for 30 min at 4°C, the cells were again collected by
centrifugation and
washed three times with cold incubation buffer. Each cell sample was
resuspended in
300 - 500 p,l of incubation buffer containing 0.5 ~1/sample of propidium
iodide
solution (Roche #1 348 639). The samples were then analyzed utilizing a
FACScaliber FAGS machine (Becton Dickinson).
Example 9: Generation of Hybridomas
Female BALB/c mice were first immunzed with cells expressing full-length
recombinant human CA IX. To produce the cells for the immunization, Tn5 insect
cells were infected with a recombinant baculovirus encoding the human CA IX
protein. Two days after the infection, the cells were collected by
centrifugation and
resuspended in PBS to a final concentration of 5x107 cells/ml. The cell
suspension
was emulsified with an equal volume of Freund's Incomplete Adjuvant (Sigma, F-
5506), and 0.2 ml of the emulsion was injected intraperitoneally into each
mouse.
The mice were then boosted every 2 - 3 weeks with an intraperitoneal injection
of 30
~.g/mouse of recombinant CA-glu protein. For the first boost, the CA-glu
protein was
emulsified with an equal volume of Freund's Complete Adjuvant (Sigma, F-5881)
prior to injection. In all subsequent intraperitoneal boosts, the protein was
emulsified
with Freund's Incomplete Adjuvant prior to injection. Eight days following
each
boost, blood was collected from each animal, serum was generated from the
blood
-59-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
samples, and the sera were tested for the level of antibodies recognizing the
CA-glu
protein (titer) using the ELISA described in Example 7.
Mice with the highest serum titers against CA-glu after the third boost were
given a final boost of 10 ~,g of the CA-glu protein. For this boost, the
protein was
injected intravenously into the tail vein as a suspension in PBS, without any
added
adjuvant. Three days later, the mice were sacrificed and the spleens removed.
Splenocytes were isolated and fused with Sp210 myeloma cells, and the fusion
mixes
were plated in 96-well plates under conditions selective for growth of
hybridomas.
Conditioned media from the wells were screened for the presence of anti-CA IX
antibodies using the ELISA described in Example 7. Control wells in the ELISA
received either nonspecific mouse IgG2b rather than hybridoma supernatant
(negative
control for assay background), or samples of hybridoma supernatants previously
shown to contain anti-CA-glu antibodies (positive controls). Selected
supernatants
were also assayed utilizing the FAGS protocol described in Example 8.
Example 10: Antigenic Region Analysis of Human CA IX
The full-length human CA IX protein and the carbonic anhydrase domain (i.e.,
residues 135-414 of SEQ ID N0:2) were subjected to antigenic region analysis
using
the Bioannotator program of Vector NTI (see, for example, Vector TI Advance
(VNTI Suite 8.0) available from InforMax). The antigencity plots for both
sequences
predicted an antigenic region within the carbonic anhydrase domain, where the
28-
residue region of antigenicity corresponded to residues 229 to 256 of SEQ ID
N0:2
(data not shown).
All publications and patent applications mentioned in the specification are
indicative of the level of those skilled in the art to which this invention
pertains. All
publications and patent applications are herein incorporated by reference to
the same
extent as if each individual publication or patent application was
specifically and
individually indicated to be incorporated by reference.
Although the foregoing invention has been described in some detail by way of
illustration and example for purposes of clarity of understanding, it will be
obvious
that certain changes and modifications may be practiced within the scope of
this
invention.
-60-
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
SEQUENCE LISTING
<110> Worig,
Justin
Winter, Jill
Lalehzadeh,
Guita
Warne, Robert
<120> Compositions
and Methods
of Therapy
for
Cancers Characterized by theTumor-Associated
Expression
of
Antigen MN/CA
IX
<130> PP19155.003
<150> 60/405,577
<151> 2002-08-23
<160> 10
<170> FastSEQ
for Windows
Version 4.0
<210> 1
<211> 1380
<212> DNA
<213> Homo
Sapiens
<220>
<221> CDS
<222> (1)...(1380)
<400> 1
atg get ccc cccagcccctggctccctctgttgatcccggcc 48
ctg tgc
Met Ala Pro ProSerProTrpLeuProLeuLeuIleProAla
Leu Cys
1 5 10 15
cct get cca actgtgcaactgctgctgtcactgctgettctg 96
ggc ctc
Pro Ala Pro ThrValGlnLeuLeuLeuSerLeuLeuLeuLeu
Gly Leu
20 25 30
atg cct gtc cagaggttgccccggatgcaggaggattccccc 144
cat ccc
Met Pro Val GlnArgLeuProArgMetGlnGluAspSerPro
His Pro
35 40 45
ttg gga gga tctggggaagatgacccactgggcgaggaggat 192
ggc tct
Leu Gly Gly SerGlyGluAspAspProLeuG1yGluGluAsp
Gly Ser
50 55 60
ctg ccc agt gattcacccagagaggaggatccacccggagag 240
gaa gag
Leu Pro Ser AspSerProArgGluGluAspProProGlyGlu
Glu Glu
65 70 75 80
gag gat cta gaggaggatctacctggagaggaggatetacct 288
cct gga
Glu Asp Leu GluGluAspLeuProGlyGluGluAspLeuPro
Pro Gly
85 90 95
gaa gtt aag tcagaagaagagggctccctgaagttagaggat 336
cct aaa
Glu Val Lys SerGluGluGluGlySerLeuLysLeuGluAsp
Pro Lys
100 105 110
cta cct act getcctggagatcctcaagaaccccagaataat 384
gtt gag
Leu Pro Thr AlaProGlyAspProGlnGluProGlnAsnAsn
Val Glu
115 120 125
gcc cac agg gaaggggatgaccagagtcattggcgctatgga 432
gac aaa
Ala His Arg GluGlyAspAspGlnSerHisTrpArgTyrGly
Asp Lys
130 135 140
1
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
ggc gac ccg ccc'tgg ccc cgg gtg tcc cca gcc tgc gcg ggc cgc ttc 480
Gly Asp Pro Pro Trp Pro Arg Val Ser Pro Ala Cys Ala Gly Arg Phe
145 150 155 160
cag tcc ccg gtg gat atc cgc ccc cag ctc gcc gec ttc tgc ccg gcc 528
Gln Ser Pro Val Asp Ile Arg Pro Gln Leu Ala Ala Phe Cys Pro Ala
165 170 175
ctg cgc ccc ctg gaa ctc ctg ggc ttc cag ctc ccg ccg ctc cca gaa 576
Leu Arg Pro Leu Glu Leu Leu Gly Phe Gln Leu Pro Pro Leu Pro Glu
180 185 190
ctg cgc ctg cgc aac aat ggc cac agt gtg caa ctg acc ctg cct cct 624
Leu Arg Leu Arg Asn Asn Gly His Ser Val Gln Leu Thr Leu Pro Pro
195 200 205
ggg eta gag atg get ctg ggt ccc ggg cgg gag tac egg get etg cag 672
Gly Leu Glu Met Ala Leu Gly Pro Gly Arg Glu Tyr Arg Ala Leu Gln
2l0 215 220
ctg cat ctg cac tgg ggg get gca ggt cgt ecg ggc teg gag cac act 720
Leu His Leu His Trp Gly Ala Ala Gly Arg Pro Gly Ser Glu His Thr
225 230 235 240
gtg gaa ggc cac cgt ttc cct gcc gag atc cac gtg gtt cac ctc agc 768
Val Glu Gly His Arg Phe Pro Ala Glu Ile His Val Val His Leu Ser
245 250 255
acc gcc ttt gcc aga gtt gac gag gcc ttg ggg cgc ccg gga ggc ctg 8i6
Thr Ala Phe Ala Arg Val Asp Glu Ala Leu Gly Arg Pro Gly Gly Leu
260 265 270
gcc gtg ttg gcc gcc ttt ctg gag gag ggc ccg gaa gaa aac agt gcc 864
Ala Val Leu Ala Ala Phe Leu Glu Glu Gly Pro Glu Glu Asn Ser Ala
275 280 285
tat gag cag ttg Ctg tCt cge ttg gaa gaa ate get gag gaa ggc tca 912
Tyr Glu Gln Leu Leu Ser Arg Leu Glu Glu Ile Ala Glu Glu Gly Ser
290 295 300
gag act cag gtc cca gga ctg gac ata tct gca ctc ctg ccc tct gac 960
Glu Thr Gln Val Pro Gly Leu Asp Ile Ser Ala Leu Leu Pro Ser Asp
305 310 315 320
ttc agc cgc tac ttc caa tat gag ggg tct ctg act aca ccg ccc tgt 1008
Phe Ser Arg Tyr Phe Gln Tyr Glu Gly 5er Leu Thr Thr Pro Pro Cys
325 330 335
gcc cag ggt gtc atc tgg act gtg ttt aac cag aca gtg atg ctg agt 1056
Ala Gln Gly Val Ile Trp Thr Val Phe Asn Gln Thr Va1 Met Leu Ser
340 345 350
get aag cag ete cac acc ctc tct gac acc etg tgg gga cct ggt gac 1104
Ala Lys Gln Leu His Thr Leu Ser Asp Thr Leu Trp Gly Pro Gly Asp
355 360 365
tct cgg cta cag ctg aac ttc cga gcg acg cag cct ttg aat ggg cga 1152
Ser Arg Leu Gln Leu Asn Phe Arg Ala Thr Gln Pro Leu Asn Gly Arg
370 375 380
gtg att gag gce tec tte cct get gga gtg gac age agt cct cgg get 1200
Val Ile Glu Ala Ser Phe Pro Ala Gly Val Asp Ser Ser Pro Arg Ala
385 390 395 400
2
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
getgagccagtccagctgaattcctgcctggetgetggtgacatccta 1248
AlaGluProValGlnLeuAsnSerCysLeuAlaAlaGlyAspIleLeu
405 p 410 415
gccctggtttttggcctcctttttgetgtcaccagcgtcgcgttcctt 1296
AlaLeuValPheGlyLeuLeuPheAlaValThrSerValAlaPheLeu
420 425 430
gtgcagatgagaaggcagcacagaaggggaaccaaagggggtgtgagc 1344
ValGlnMetArgArgGlnHisArgArgGlyThrLysGlyGlyValSer
435 440 445
taccgcccagcagaggtagccgagactggagcctag 1380
TyrArgProAlaGluValAlaGluThrGlyAla
450 455
<210> 2
<2ll> 459
<212> PRT
<213> Homo Sapiens
<400> 2
Met Ala Pro Leu Cys Pro Ser Pro Trp Leu Pro Leu Leu Ile Pro Ala
1 5 10 15
Pro Ala Pro Gly Leu Thr Val Gln Leu Leu Leu Ser Leu Leu Leu Leu
20 25 30
Met Pro Val His Pro Gln Arg Leu Pro Arg Met Gln Glu Asp Ser Pro
35 40 45
Leu Gly Gly Gly Ser Ser Gly Glu Asp Asp Pro Leu Gly Glu Glu Asp
50 55 60
Leu Pro Ser Glu Glu Asp Ser Pro Arg Glu Glu Asp Pro Pro Gly Glu
65 70 75 80
Glu Asp Leu Pro Gly Glu Glu Asp Leu Pro Gly Glu Glu Asp Leu Pro
85 90 95
Glu Val Lys Pro Lys Ser Glu Glu Glu Gly Ser Leu Lys Leu Glu Asp
100 105 110
Leu Pro Thr Val Glu Ala Pro Gly Asp Pro Gln Glu Pro Gln Asn Asn
115 120 125
Ala His Arg Asp Lys Glu Gly Asp Asp Gln Ser His Trp Arg Tyr Gly
130 135 140
Gly Asp Pro Pro Trp Pro Arg Val Ser Pro Ala Cys Ala Gly Arg Phe
145 150 155 160
Gln Ser Pro Val Asp Ile Arg Pro Gln Leu Ala Ala Phe Cys Pro Ala
165 170 175
Leu Arg Pro Leu Glu Leu Leu Gly Phe Gln Leu Pro Pro Leu Pro Glu
180 185 190
Leu Arg Leu Arg Asn Asn Gly His Ser Val G1n Leu Thr Leu Pro Pro
195 200 205
Gly Leu Glu Met Ala Leu Gly Pro Gly Arg Glu Tyr Arg Ala Leu Gln
210 215 220
Leu His Leu His Trp Gly Ala Ala Gly Arg Pro Gly Ser Glu His Thr
225 230 235 240
Val Glu Gly His Arg Phe Pro Ala Glu Ile His Val Val His Leu Ser
245 250 255
Thr Ala Phe Ala Arg Val Asp Glu Ala Leu Gly Arg Pro Gly Gly Leu
260 265 27p
Ala Val Leu Ala Ala Phe Leu Glu Glu Gly Pro Glu Glu Asn Ser Ala
275 280 285
Tyr Glu Gln Leu Leu Ser Arg Leu Glu Glu Ile Ala Glu Glu Gly Ser
290 295 300
Glu Thr Gln Val Pro Gly Leu Asp Ile Ser Ala Leu Leu Pro Ser Asp
305 310 315 320
Phe Ser Arg Tyr Phe Gln Tyr Glu Gly Ser Leu Thr Thr Pro Pro Cys
325 330 335
Ala Gln Gly Val Ile Trp Thr Val Phe Asn Gln Thr Val Met Leu Ser
3
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
340 345 350
Ala Lys Gln Leu His Thr Leu Ser Asp Thr Leu Trp Gly Pro Gly Asp
355 360 365
Ser Arg Leu Gln Leu Asn Phe Arg Ala Thr Gln Pro Leu Asn Gly Arg
370 375 380
Val Ile Glu Ala Ser Phe Pro Ala Gly Val Asp Ser Ser Pro Arg Ala
385 390 395 400
Ala Glu Pro Val Gln Leu Asn Ser Cys Leu Ala Ala Gly Asp Ile Leu
405 410 415
Ala Leu Val Phe Gly Leu Leu Phe Ala Val Thr Ser Val Ala Phe Leu
420 425 430
Val Gln Met Arg Arg Gln His Arg Arg Gly Thr Lys Gly Gly Val Ser
435 440 445
Tyr Arg Pro Ala Glu Val Ala Glu Thr Gly Ala
450 455
<210> 3
<211> 540
<212> DNA
<213> Artificial Sequence
<220>
<223> Coding sequence for human CA IX proteoglycan
domain construct
<221> CDS
<222> (1)...(540)
<400> 3
atg get ccc ctg tgc ccc agc ccc tgg ctc cct ctg ttg atc'ccg gcc 48
Met Ala Pro Leu Cys Pro Ser Pro Trp Leu Pro Leu Leu Ile Pro Ala
1 5 10 l5
ctt get cca ggc ctc act gtg caa ctg ctg ctg tca ctg ctg ctt ctg 96
Leu Ala Pro Gly Leu Thr Val Gln Leu Leu Leu Ser Leu Leu Leu Leu
20 25 30
atg cct gtc cat ccc cag agg ttg ccc cgg atg cag gag gat tcc ccc 144
Met Pro Val His Pro Gln Arg Leu Pro Arg Met Gln Glu Asp Ser Pro
35 40 45
ttg gga gga ggc tct tct ggg gaa gat gac cca ctg ggc gag gag gat 192
Leu Gly Gly Gly Ser Ser Gly Glu Asp Asp Pro Leu Gly Glu Glu Asp
50 55 60
ctg ccc agt gaa gag gat tca ccc aga gag gag gat cca ccc gga gag 240
Leu Pro Ser Glu Glu Asp Ser Pro Arg Glu Glu Asp Pro Pro Gly Glu
65 70 75 80
gag gat cta cct gga gag gag gat cta cct gga gag gag gat cta cct 288
Glu Asp Leu Pro Gly Glu Glu Asp Leu Pro Gly Glu Glu Asp Leu Pro
85 90 95
gaa gtt aag cet aaa tca gaa gaa gag ggc tcc ctg aag tta gag gat 336
Glu Val Lys Pro Lys Ser Glu Glu Glu Gly 5er Leu Lys Leu Glu Asp
100 105 110
cta cct act gtt gag get cct gga gat cct caa gaa ccc cag aat aat 384
Leu Pro Thr Val Glu Ala Pro Gly Asp Pro Gln Glu Pro Gln Asn Asn
115 120 125
gcc cat agg agc tcg agc atc cta gcc ctg gtt ttt ggc ctc ctt ttt 432
4
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
Ala His Arg Ser Ser Ser Ile Leu Ala Leu Val Phe Gly Leu Leu Phe
130 135 140
get gtc acc agc gtc gcg ttc ctt gtg cag atg aga agg cag cac aga 480
Ala Val Thr Ser Val Ala Phe Leu Val Gln Met Arg Arg Gln His Arg
145 150 155 160
agg gga acc aaa ggg ggt gtg agc tac cgc cca gca gag gta gcc gag 528
Arg Gly Thr Lys Gly Gly Val Ser Tyr Arg Pro Ala Glu Val Ala Glu
165 170 l75
act gga gcc tag 540
Thr Gly Ala
<210> 4
<211> 179
<212> PRT
<213> Artifical Sequence
<220>
<223> Polypeptide encoded by coding sequence for human
CA IX proteoglycan domain construct
<400> 4
Met Ala Pro Leu Cys Pro Ser Pro Trp Leu Pro Leu Leu Ile Pro Ala
1 5 10 15
Leu Ala Pro Gly Leu Thr Val Gln Leu Leu Leu Ser Leu Leu Leu Leu
20 25 30
Met Pro Val His Pro Gln Arg Leu Pro Arg Met Gln Glu Asp Ser Pro
35 40 45
Leu Gly Gly Gly Ser Ser Gly Glu Asp Asp Pro Leu Gly Glu Glu Asp
50 55 60
Leu Pro Ser Glu Glu Asp Ser Pro Arg Glu Glu Asp Pro Pro Gly Glu
65 70 75 80
Glu Asp Leu Pro Gly Glu Glu Asp Leu Pro Gly Glu Glu Asp Leu Pro
85 90 95
Glu Val Lys Pro Lys Ser Glu Glu Glu Gly Ser Leu Lys Leu Glu Asp
100 105 l10
Leu Pro Thr Val Glu Ala Pro Gly Asp Pro Gln Glu Pro Gln Asn Asn
ll5 120 125
Ala His Arg Ser Ser Ser Ile Leu Ala Leu Val Phe Gly Leu Leu Phe
130 135 140
Ala Val Thr Ser Val Ala Phe Leu Val Gln Met Arg Arg Gln His Arg
145 150 155 160
Arg Gly Thr Lys Gly Gly Val Ser Tyr Arg Pro Ala Glu Val Ala Glu
165 170 175
Thr Gly Ala
<210> 5
<211> 1089
<212> DNA
<213> Artificial Sequence
<220>
<223> Coding sequence for human CA IX carbonic anhydrase
domain construct
<221> CDS
<222> (1)...(1089)
<400> 5
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
atg get ccc ctg tgc ccc agc ccc tgg ctc cct ctg ttg atc ccg gco 48
Met Ala Pro Leu Cys Pro Ser Pro Trp Leu Pro Leu Leu Ile Pro Ala
1 5 10 15
cct get cca ggc ctc act gtg caa ctg ctg ctg tca ctg ctg ctt ctg 96
Pro Ala Pro Gly Leu Thr Val Gln Leu Leu Leu Ser Leu Leu Leu Leu
20 25 30
atg cct gtc cat coc ggg gat gac cag agt cat tgg cgc tat gga ggc 144
Met Pro Val His Pro Gly Asp Asp Gln Ser His Trp Arg Tyr Gly Gly
35 40 45
gacccgccctggccccgggtgtccccagcctgcgcgggccgcttccag 192
AspProProTrpProArgValSerProAlaCysAlaGlyArgPheGln
50 55 60
tccccggtggatatccgcccccagctcgccgccttctgcccggccctg 240
SerProValAspIleArgProGlnLeuAlaAlaPheCysProAlaLeu
65 70 75 80
cgccccctggaactcctgggcttccagCtCCCgccgctcccagaactg 288
ArgProLeuGluLeuLeuGlyPheGlnLeuProProLeuProGluLeu
85 90 95
cgcctgcgcaacaatggccacagtgtgcaactgaccctgcctcctggg 336
ArgLeuArgAsnAsnGlyHisSerValGlnLeuThrLeuProProGly
100 105 110
ctagagatggetctgggtcccgggcgggagtaccgggetctgcagctg 384
LeuGluMetAlaLeuGlyProGlyArgGluTyrArgAlaLeuG1nLeu
115 120 125
catctgcactggggggetgcaggtcgtccgggctcggagcacactgtg 432
HisLeuHisTrpGlyAlaAlaGlyArgProGlySerGluHisThrVal
130 135 140
gaaggccaccgtttccctgccgagatccacgtggttcacctcagcacc 480
GluGlyHisArgPheProAlaGluIleHisValVa1HisLeuSerThr
145 150 155 160
gcctttgccagagttgacgaggccttggggcgccegggaggcctggcc 528
AlaPheAlaArgValAspGluAlaLeuGlyArgProG1yGlyLeuAla
165 170 175
gtgttggccgcctttctggaggagggcccggaagaaaacagtgcctat 576
ValLeuAlaAlaPheLeuGluGluGlyProGluGluAsnSerAlaTyr
180 l85 190
gagcagttgctgtctcgcttggaagaaatcgetgaggaaggctcagag 624
GluGlnLeuLeuSerArgLeuGluGluIleAlaGluGluGlySerGlu
195 200 205
actcaggtcccaggactggacatatctgcactcctgccctctgacttc 672
ThrGlnValProGlyLeuAspIleSerAlaLeuLeuProSerAspPhe
210 215 220
agccgctacttccaatatgaggggtctctgactacaccgccctgtgcc 720
SerArgTyrPheGlnTyrGluGlySerLeuThrThrProProCysAla
225 230 235 240
cagggtgtcatctggactgtgtttaaccagacagtgatgctgagtget 768
GlnGlyValIleTrpThrValPheAsnGlnThrValMetLeuSerAla
245 250 255
6
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
aag cag ctc cac acc ctc tct gac acc ctg tgg gga cct ggt gac tct 816
Lys Gln Leu His Thr Leu Ser Asp Thr Leu Trp Gly Pro Gly Asp Ser
260 265 270
cggctacagctgaacttccgagcgacgcagcctttgaatgggcgagtg 864
ArgLeuGlnLeuAsnPheArgAlaThrGlnProLeuAsnGlyArgVal
275 280 285
attgaggcctccttccctgetggagtggacagcagtcctcgggetget 912
IleGluAlaSerPheProAlaGlyValAspSerSerProArgAlaAla
290 295 300
gagccagtccagctgaattcctgcctggetgetggtgacatcctagcc 960
GluProValGlnLeuAsnSerCysLeuAlaAlaGlyAspIleLeuAla
305 310 315 320
ctggtttttggcctcetttttgetgtcaccagcgtcgcgttccttgtg 1008
LeuValPheGlyLeuLeuPheAlaValThrSerValAlaPheLeuVal
325 330 335
cagatgagaaggcagcacagaaggggaaccaaagggggtgtgagctac 1056
GlnMetArgArgGlnHisArgArgGlyThrLysGlyGlyValSerTyr
340 , 345 350
cgcccagcagaggtagccgagactggagcctag 1089
ArgProAlaGluValAlaGluThrGlyAla
355 360
<210> 6
<211> 362
<212> PRT
<213> Artificial Sequence
<220>
<223> Polypeptide encoded by coding sequence for human
CA IX carbonic anhydrase domain construct
<400> 6
Met Ala Pro Leu Cys Pro Ser Pro Trp Leu Pro Leu Leu Ile Pro Ala
1 5 10 15
Pro Ala Pro Gly Leu Thr Val Gln Leu Leu Leu Ser Leu Leu Leu Leu
20 25 30
Met Pro Val His Pro Gly Asp Asp Gln Ser His Trp Arg Tyr Gly Gly
35 40 45
Asp Pro Pro Trp Pro Arg Val Ser Pro Ala Cys Ala Gly Arg Phe Gln
50 55 60
Ser Pro Val Asp I1e Arg Pro Gln Leu Ala Ala Phe Cys Pro Ala Leu
65 70 75 80
Arg Pro Leu Glu Leu Leu Gly Phe Gln Leu Pro Pro Leu Pro Glu Leu
85 90 95
Arg Leu Arg Asn Asn Gly His Ser Val Gln Leu Thr Leu Pro Pro Gly
100 105 110
Leu Glu Met Ala Leu Gly Pro Gly Arg Glu Tyr Arg Ala Leu Gln Leu
115 120 125
His Leu His Trp Gly Ala Ala Gly Arg Pro Gly Ser Glu His Thr Val
130 135 140
Glu Gly His Arg Phe Pro Ala Glu Ile His Val Val His Leu Ser Thr
145 150 155 160
Ala Phe Ala Arg Val Asp Glu Ala Leu Gly Arg Pro Gly Gly Leu Ala
165 170 175
Val Leu Ala Ala Phe Leu Glu Glu Gly Pro Glu Glu Asn Ser Ala Tyr
180 185 190
Glu Gln Leu Leu Ser Arg Leu Glu Glu Ile Ala Glu Glu Gly Ser Glu
195 200 205
7
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
Thr GlnValProGlyLeuAspIleSerAlaLeuLeuProSerAspPhe
210 215 220
Ser ArgTyrPheGlnTyrGluGlySerLeuThrThrProProCysAla
225 230 235 240
Gln GlyValIleTrpThrValPheAsnGlnThrValMetLeuSerAla
245 250 255
Lys GlnLeuHisThrLeuSerAspThrLeuTrpGlyProGlyAspSer
260 265 270
Arg LeuGlnLeuAsnPheArgAlaThrGlnProLeuAsnGlyArgVal
275 280 285
Ile GluAlaSerPheProAlaGlyValAspSerSerProArgAlaAla
290 295 300
Glu ProValGlnLeuAsnSerCysLeuAlaAlaGlyAspIleLeuAla
305 310 315 320
Leu ValPheGlyLeuLeuPheA1aValThrSerValAlaPheLeuVal
325 330 335
Gln MetArgArgGlnHisArgArgGlyThrLysGlyGlyValSerTyr
340 345 350
Arg ProAlaGluValAlaGluThrGlyAla
355 360
<210>
7
<211>
978
<212>
DNA
<213> Sequence
Artificial
<220>
<223>
Coding
sequence
for
glu-tagged
human
CA
IX
carbonic construct
anhydrase
domain
<221>
CDS
<222> (978)
(1)...
<400>
7
atg getcecctgtgceccagcccetggeteectetgttgatcecggcc48
Met AlaProLeuCysProSerProTrpLeuProLeuLeuIleProAla
1 5 10 15
cet getccaggcetcactgtgcaaetgctgctgtcaetgetgcttctg96
Pro AlaProGlyLeuThrValGlnLeuLeuLeuSerLeuLeuLeuLeu
20 25 30
atg cctgtccatccccagaggttgccccggatgcaggaggetagcgaa144
Met ProValHisProGlnArgLeuProArgMetGlnGluAlaSerGlu
35 40 45
tac atgccaatggaacaagaaccccagaataatgcccacagggacaaa192
Tyr MetProMetGluGlnGluProGlnAsnAsnAlaHisArgAspLys
50 55 60
gaa ggg gat gac cag agt cat tgg cgc tat gga ggc gac ccg ccc tgg 240
Glu Gly Asp Asp Gln Ser His Trp Arg Tyr Gly Gly Asp Pro Pro Trp
65 70 75 80
ccc cgg gtg tcc cca gcc tgc gcg ggc cgc ttc cag tcc ccg gtg gat 288
Pro Arg Val Ser Pro Ala Cys Ala Gly Arg Phe Gln Ser Pro Val Asp
85 90 95
atc cgc ccc cag ctc gcc gcc ttc tgc ccg gcc ctg cgc ccc ctg gaa 336
Ile Arg Pro Gln Leu Ala Ala Phe Cys Pro Ala Leu Arg Pro Leu Glu
100 105 110
ctc ctg ggc ttc cag ctc ccg ccg ctc cca gaa ctg cgc ctg cgc aac 384
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
Leu LeuGlyPheGlnLeuProProLeuProGluLeuArgLeuArgAsn
115 120 125
aat ggccacagtgtgcaactgaccctgcctcctgggctagagatgget432
Asn GlyHisSerValGlnLeuThrLeuProProGlyLeuGluMetAla
l30 135 140
ctg ggtcccgggcgggagtaccgggetctgcagctgcatctgcactgg480
Leu GlyProGlyArgGluTyrArgAlaLeuGlnLeuHisLeuHisTrp
145 150 155 160
ggg getgcaggtcgtccgggctcggagcacactgtggaaggccaccgt528
Gly AlaAlaGlyArgProGlySerGluHisThrValGluGlyHisArg
165 170 175
ttc cctgccgagatccacgtggttcacctcagcaccgcctttgccaga576
Phe ProAlaGluIleHisValValHisLeuSerThrAlaPheAlaArg
180 185 190
gtt gacgaggccttggggcgcecgggaggcctggccgtgttggccgcc624
Val AspGluAlaLeuGlyArgProGlyGlyLeuAlaValLeuAlaAla
195 200 205
ttt ctggaggagggcccggaagaaaacagtgcctatgagctccacacc672
Phe LeuGluGluGlyProGluGluAsnSerAlaTyrGluLeuHisThr
210 215 220
ctc tctgacaccctgtggggacctggtgactctcggctacagctgaac720
Leu SerAspThrLeuTrpGlyProGlyAspSerArgLeuGlnLeuAsn
225 230 235 240
ttc cgagcgacgcagcctttgaatgggcgagtgattgaggcctccttc768
Phe ArgAlaThrGlnProLeuAsnGlyArgValIleGluAlaSerPhe
245 250 255
cct getggagtggacagcagtcctcgggetgetgagccagtccagctg816
Pro AlaGlyValAspSerSerProArgAlaAlaGluProValGlnLeu
260 265 270
aat tcctgcctggetgetggtgacatcctagccctggtttttggcctc864
Asn SerCysLeuAlaA1aGlyAspIleLeuAlaLeuValPheGlyLeu
275 280 285
ctt tttgetgtcaccagcgtcgcgttccttgtgcagatgagaaggcag912
Leu PheAlaValThrSerValAlaPheLeuValGlnMetArgArgGln
290 295 300
cac aga agg gga acc aaa ggg ggt gtg agc tac cgc cca gca gag gta 960
His Arg Arg Gly Thr Lys Gly Gly Val Ser Tyr Arg Pro Ala Glu Val
305 310 315 320
gcc gag act gga gcc tag 978
Ala Glu Thr Gly Ala
325
<210> 8
<211> 325
<2l2> PRT
<213> Artificial Sequence
<220>
<223> Polypeptide encoded by coding sequence for
glu-tagged human CA IX carbonic anhydrase domain
construct
9
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
<400> 8
Met Ala Pro Leu Cys Pro Ser Pro Trp Leu Pro Leu Leu Ile Pro Ala
1 5 10 15
Pro Ala Pro Gly Leu Thr Val Gln Leu Leu Leu Ser Leu Leu Leu Leu
20 25 30
Met Pro Val His Pro G1n Arg Leu Pro Arg Met Gln Glu Ala Ser Glu
35 40 45
Tyr Met Pro Met Glu Gln Glu Pro Gln Asn Asn Ala His Arg Asp Lys
50 55 60
Glu Gly Asp Asp Gln Ser His Trp Arg Tyr Gly Gly Asp Pro Pro Trp
65 70 75 80
Pro Arg Val Ser Pro Ala Cys Ala Gly Arg Phe Gln Ser Pro Val Asp
85 90 95
Ile Arg Pro Gln Leu Ala Ala Phe Cys Pro Ala Leu Arg Pro Leu Glu
100 105 110
Leu Leu Gly Phe Gln Leu Pro Pro Leu Pro Glu Leu Arg Leu Arg Asn
115 120 l25
Asn Gly His Ser Val Gln Leu Thr Leu Pro Pro Gly Leu Glu Met Ala
130 135 140
Leu Gly Pro Gly Arg Glu Tyr Arg Ala Leu Gln Leu His Leu His Trp
145 150 155 160
Gly Ala Ala Gly Arg Pro Gly Ser Glu His Thr Val Glu Gly His Arg
165 170 175
Phe Pro Ala Glu Ile His Val Val His Leu Ser Thr Ala Phe Ala Arg
180 185 190
Val Asp Glu Ala Leu Gly Arg Pro Gly Gly Leu Ala Val Leu Ala Ala
195 200 205
Phe Leu Glu Glu Gly Pro Glu Glu Asn Ser Ala Tyr Glu Leu His Thr
210 215 220
Leu Ser Asp Thr Leu Trp Gly Pro Gly Asp Ser Arg Leu Gln Leu Asn
225 230 235 240
Phe Arg Ala Thr Gln Pro Leu Asn Gly Arg Val Ile Glu Ala Ser Phe
245 250 255
Pro Ala Gly Val Asp Ser Ser Pro Arg Ala Ala Glu Pro Val Gln Leu
260 265 270
Asn Ser Cys Leu Ala Ala Gly Asp Ile Leu Ala Leu Val Phe Gly Leu
275 280 285
Leu Phe Ala Val Thr Ser Val Ala Phe Leu Val Gln Met Arg Arg Gln
290 295 300
His Arg Arg Gly Thr Lys Gly Gly Val Ser Tyr Arg Pro Ala Glu Val
305 310 315 320
Ala Glu Thr Gly Ala
325
<210> 9
<211> 978
<212> DNA
<213> Artificial Sequence
<220>
<223> Coding sequence for glu-tagged mutant human CA IX
carbonic anhydrase domain construct
<221> CDS
<222> (1)...(978)
<400> 9
atg get ccc ctg tgc ccc agc ccc tgg ctc cct ctg ttg atc ccg gcc 48
Met Ala Pro Leu Cys Pro Ser Pro Trp Leu Pro Leu Leu Ile Pro Ala
1 5 10 15
cct get cca ggc ctc act gtg caa ctg ctg ctg tca ctg ctg ctt ctg 96
Pro Ala Pro Gly Leu Thr Val Gln Leu Leu Leu Ser Leu Leu Leu Leu
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
20 25 30
atgcctgtccatccccagaggttgccccggatgcaggaggetagcgaa 144
MetProValHisProGlnArgLeuProArgMetGlnGluAlaSerGlu
35 40 45
tacatgccaatggaacaagaaccccagaataatgcccacagggacaaa 192
TyrMetProMetGluGlnGluProGlnAsnAsnAlaHisArgAspLys
50 55 60
gaaggggatgaccagagtcattggcgctatggaggcgacccgccctgg 240
GluGlyAspAspGlnSerHisTrpArgTyrGlyGlyAspProProTrp
65 70 75 80
ccccgggtgtccccagcctgcgcgggccgcttccagtccccggtggat 288
ProArgValSerProAlaCysAlaGlyArgPheGlnSerProValAsp
85 90 95
atccgcccccagctcgccgccttctgcccggccctgcgccccctggaa 336
IleArgProGlnLeuAlaAlaPheCysProAlaLeuArgProLeuGlu
100 l05 110
ctcctgggcttccagctcccgccgctcccagaactgcgcctgcgcaac 384
LeuLeuGlyPheGlnLeuProProLeuProGluLeuArgLeuArgAsn
115 120 125
aatggccacagtgtgcaactgaccctgcctcctgggctagagatgget 432
AsnGlyHisSerValGlnLeuThrLeuProProGlyLeuGluMetAla
l30 135 140
ctgggtcecgggcgggagtaccgggetctgcagctgcaactgcagtgg 480
LeuGlyProGlyArgGluTyrArgAlaLeuGlnLeuGlnLeuGlnTrp
145 150 155 160
ggggetgcaggtcgtccgggctcggagcacactgtggaaggccaccgt 528
GlyAlaAlaGlyArgProGlySerGluHisThrValGluGlyHisArg
165 170 175
ttccctgccgagatccaagtggttcacctcagcaccgcctttgccaga 576
PheProAlaGluIleGlnValValHisLeuSerThrAlaPheAlaArg
l80 185 190
gttgacgaggccttggggcgcccgggaggcctggccgtgttggccgcc 624
ValAspGluAlaLeuGlyArgProGlyGlyLeuAlaValLeuAlaAla
195 200 205
tttctggaggagggcccggaagaaaacagtgcctatgagctccacacc 672
PheLeuGluGluGlyProGluGluAsnSerAlaTyrGluLeuHisThr
210 215 220
ctctctgacaccctgtggggacctggtgactctcggctacagctgaac 720
LeuSerAspThrLeuTrpGlyProGlyAspSerArgLeuGlnLeuAsn
225 230 235 240
ttccgagcgacgcagcctttgaatgggcgagtgattgaggcctccttc 768
PheArgAlaThrGlnProLeuAsnGlyArgValIleGluAla5erPhe
245 250 255
cctgetggagtggacagcagtcctcgggetgetgagccagtccagctg 816
ProAlaGlyValAspSerSerProArgAlaAlaGluProValGlnLeu
260 265 270
aattcctgcctggetgetggtgacatcctagccctggtttttggcctc 864
AsnSerCysLeuAlaAlaGlyAspIleLeuAlaLeuValPheGlyLeu
275 280 285
11
CA 02496572 2005-02-23
WO 2004/017923 PCT/US2003/026612
ctt ttt get gtc acc agc gtc gcg ttc ctt gtg cag atg aga agg cag 912
Leu Phe Ala Val Thr Ser Val Ala Phe Leu Val Gln Met Arg Arg Gln
290 295 300
cac aga agg gga acc aaa ggg ggt gtg agc tac cgc cca gca gag gta 960
His Arg Arg Gly Thr Lys Gly Gly Val Ser Tyr Arg Pro Ala Glu Val
305 310 315 320
gcc gag act gga gcc tag 978
Ala Glu Thr Gly Ala
325
<210> 10
<211> 325
<212> PRT
<213> Artificial Sequence
<220>
<223> Polypeptide encoded by coding sequence for
glu-tagged mutant human CA IX carbonic anhydrase
domain construct
<400> 10
Met Ala Pro Leu Cys Pro Ser Pro Trp Leu Pro Leu Leu Ile Pro Ala
1 5 10 15
Pro Ala Pro Gly Leu Thr Val Gln Leu Leu Leu Ser Leu Leu Leu Leu
20 25 30
Met Pro Val His Pro Gln Arg Leu Pro Arg Met Gln Glu Ala Ser Glu
35 40 45
Tyr Met Pro Met Glu Gln Glu Pro Gln Asn Asn Ala His Arg Asp Lys
50 55 60
Glu Gly Asp Asp Gln Ser His Trp Arg Tyr Gly Gly Asp Pro Pro Trp
65 70 75 80
Pro Arg Val Ser Pro Ala Cys Ala G1y Arg Phe Gln Ser Pro Val Asp
85 90 95
Ile Arg Pro Gln Leu Ala Ala Phe Cys Pro Ala Leu Arg Pro Leu Glu
100 105 110
Leu Leu Gly Phe Gln Leu Pro Pro Leu Pro Glu Leu Arg Leu Arg Asn
115 l20 l25
Asn Gly His Ser Val Gln Leu Thr Leu Pro Pro Gly Leu Glu Met Ala
130 135 140
Leu Gly Pro Gly Arg Glu Tyr Arg Ala Leu Gln Leu Gln Leu Gln Trp
145 150 155 160
Gly Ala Ala Gly Arg Pro Gly Ser Glu His Thr Val Glu Gly His Arg
165 170 175
Phe Pro Ala Glu Ile Gln Val Val His Leu Ser Thr Ala Phe Ala Arg
180 185 190
Val Asp Glu Ala Leu Gly Arg Pro Gly Gly Leu Ala Val Leu Ala Ala
195 200 205
Phe Leu Glu Glu Gly Pro Glu Glu Asn Ser Ala Tyr Glu Leu His Thr
210 215 220
Leu Ser Asp Thr Leu Trp Gly Pro Gly Asp Ser Arg Leu Gln Leu Asn
225 230 235 240
Phe Arg Ala Thr Gln Pro Leu Asn Gly Arg Val Ile Glu Ala Ser Phe
245 250 255
Pro Ala Gly Val Asp Ser Ser Pro Arg Ala Ala Glu Pro Val Gln Leu
260 265 270
Asn Ser Cys Leu Ala Ala Gly Asp Ile Leu Ala Leu Val Phe Gly Leu
275 280 285
Leu Phe Ala Val Thr Ser Val Ala Phe Leu Val Gln Met Arg Arg Gln
290 295 300
His Arg Arg Gly Thr Lys Gly Gly Val Ser Tyr Arg Pro Ala Glu Val
305 310 315 320
Ala Glu Thr Gly Ala
325
12