Note: Descriptions are shown in the official language in which they were submitted.
CA 02497064 2006-11-16
PROCESS AND APPARATUS FOR CONVERTING SPENT POTLINERS INTO A
GLASS FRIT, AND RESULTING PRODUCTS
FIELD OF THE INVENTION
The present invention relates generally to processing spent potliners from
aluminium manufacturing or other hazardous materials. In particular, the
invention
relates to a pyrometallurgical process and apparatus for converting potliners
into a
glass frit, and to products obtained by the process.
BACKGROUND OF THE INVENTION
In the process of aluminum production, alumina is dissolved in cryolite in
electrolytic cells, or pots, which are steel shells protected by refractory
material and
lined with carbon. A number of pots, usually more than 100, are arranged in
series to
form a potline. The pots contain a molten electrolyte consisting primarily of
cryolite
(Na3AIF6) and operate at approximately 930 to 1000 C. Other materials are
added to
the electrolyte to improve the efficiency of the operation or to reduce power
consumption, such as alumina, aluminium fluoride, sodium fluoride, soda ash,
calcium
fluoride, lithium carbonate and magnesium oxide.
The hearth or lining of the cell is composed of carbon, which is backed by
insulation and contained within a steel container called a potshell. The
carbon portion
of the lining serves as the cathode and contains the molten electrolyte. The
carbon
lining is composed of prefabricated carbon blocks joined together by a carbon
paste,
which is hydraulically rammed in the seams between the carbon blocks. The
sidewalls
of the lining are typically formed with carbon paste, but may contain
prefabricated
carbon blocks.
Over the life of the cathode and its cell lining, the carbon and insulating
materials become impregnated with fluoride-containing salts. Failure can occur
by
cracking or excessive heaving of the lining. When these failures occur, the
cell is taken
off-line and the cathode lining material is removed from the potshell by
mechanized
digging equipment. This spent cathodic material is referred to as spent
potliner (SPL).
In addition to containing fluoride salts, as mentioned above, SPL contains
cyanides that are formed by the ingress of air through openings in the
potshell and
subsequent reaction of nitrogen with the carbon lining.
- 1 -
CA 02497064 2006-11-16
Spent potliner was listed by the Environmental Protection Agency (EPA) on
Sep. 13, 1988 (53 Fedl. Reg. 35412) as a hazardous waste (K088) under 40
C.F.R.,
Part 261, Subpart D because it may contain significant amounts of iron cyanide
complexes and free cyanide. Thus there is a need in the aluminium industry for
an
economical process for detoxifying spent potliner such that the treated
residue is not a
hazardous waste. This is important because of the need for alternatives to
land
disposal of hazardous waste, established as national policy in the RCRA
Hazardous
and Solid Waste Ameridments (HSWA) of 1984, and the anticipated lack of
hazardous
waste treatment capacity.
The composition of SPL is highly variable. One range of analyses is given in
Table I. A process for the treatment of SPL should be versatile enough to
treat SPL
generated while using different cell designs, electrolyte compositions, and
insulation
packages, and any residues generated should meet anticipated EPA-defined
limits for
all constituents of concern (e.g., cyanide, fluoride, organics and metals).
The
components of SPL of greatest concern environmentally are cyanide and soluble
fluoride salts. Of environmental and safety concern is the presence of water
reactive
materials (carbides) created during the pot life and resulting in explosive
gases
(hydrogen, methane and ammonia) upon exposure to water or even moisture of the
air.
Table 1. Chemical Composition of Spent Potliners
C Al Ca Mg Na Si F Fe CN S
Weight 10- 5- 1- 0.2- 7- 1- 10- 0.3- 0.2- 0.01-
% 50 22 8 1 20 20 19 5 0.5 0.3
The aluminum industry has long recognized the environmental liability of SPL
and has pursued many options for treatment and/or disposal. These options
include
landfilling, recycling as a feedstock in other industries, such as the steel,
cement,
aluminum, or mineral wool industries, fluidized bed combustion, cryolite
recovery,
pyrohydrolysis, pyrosulfolysis, and others. Landfilling is an option that is
presently
available but will becorne increasingly expensive, and eventually may be
prohibited,
since hazardous waste landfills are required.
Certain existing treatment processes are described below, and are grouped into
two categories based on the amount of carbon combusted in the described
process.
-2-
CA 02497064 2006-11-16
The first three processes operate under conditions in which little, if any,
carbon is
combusted. In these three processes, the carbon remains in the final product.
U.S. Patent No. 5,164,174 (Reynolds Metals Company) describes a method for
detoxifying SPL by thermal treatment in a counter-current rotary kiln.
Limestone and
metal siiicates are added to destroy cyanides by oxidation, and convert the
soluble
fluoride salts to relatively insoluble calcium fluoride and fluoride-bearing
minerals. Air
emissions contain very little fluorine and cyanide because of the low kiln
operating
temperature (6000 to 870 C). Ash and particulate matter is removed and
recycled or
land-filled. The discharge material is air cooled and contains the carbon.
However,
because of the relatively low processing temperatures, the conversion of NaF
to CaF2
is inefficient.
U.S. Patent No. 4,497,464 (Ogden Environmental Services) describes a
process for the treatment of ground SPL to reduce cyanide content to
environmentally
non-hazardous levels. Ground SPL is roasted in a stream of air or nitrogen at
a
temperature between about 260 and 760 C (500 and 1400 F). Cyanide levels are
reduced without combustion of a major portion of carbonaceous material,
resulting in
an end product rich in carbon and fluorine. Because no calcium oxide source is
added
the following chemical reactions do not occur:
CaCO3 -> CaO + CO2;
2NaF + CaO -> CaF2 + Na20; and
2AIF3 + 3CaO -> 3CaF2 + A1203.
Therefore, the fluoride salts are not converted to a stable, calcium fluoride.
The
Ogden discharge is essentially a landfill material.
U.S. Patent No. 4,993,323 (Tabery et al.) describes a method for thermal
destruction of SPL by Fluidized Bed Combustion (FBC) in a mixture of lignite
and
limestone. Fluoride salts are converted to calcium fluoride. Significant
carbon would
remain in the final product.
The following processes for processing SPL result in the combustion of large
amounts of carbon.
U.S. Patent No. 4,763,585 (Ogden Environmental Services) describes a
process for the combustion of ground SPL using about 1 to about 20 weight
percent of
a powdered inert anti-agglomeration additive. The SPL is burned at between
1400 F to
-3-
CA 02497064 2006-11-16
about 22000 F(760 to 1204 C). The contaminated components are bound or
encapsulated into a solid glassy slag, described as a glassy sodium metal (Al)
silicate
matrix encapsulating fluorine residues. Silica is added to the feed.
Conditions are
adjusted to produce hy'drogen fluoride, which is scrubbed with water to
produce a
hydrogen fluoride solution. The high combustion temperatures cause much of the
carbon to be combusted. Since no calcium oxide source is added, the following
chemical reactions do inot occur:
CaCO3 -> CaO + C02;
2NaF + Ca0 -> CaF2 + Na20; and
2AIF3 + 3CaO -> 3CaF2 + AI203.
In fact, aluminium fluoride and hydrogen fluoride are produced. Also, this
process is not be able to convert substantially all of the fluorides salts to
calcium
fluoride. In fact, much sodium fluoride would be evaporated, and fluorine
merely
encapsulated as a calcium salt.
U.S. Patent No. 5,711,018 (Alcoa) describes an industrial waste management
facility (IWMF) for disposing of SPL using a co-current gas-fired rotary kiln.
The IWMF
recovers and recycles fluorides from discharge gases to the molten bath and
collects
and withdraws glass frit residue. The operating temperature is between 1150 C
and
1250 C, and cannot exceed 1250 C to avoid generating fluoride gas. A solid
oxygenating and gasifying agent (e.g. limestone) is added to form CO2 which
reacts
with C to form CO. The CO is burned above the molten bath. Siliceous material
is
added to the bath. Flucirides are fixed in the frit. Carbon is burnt, as it is
not desired in
the frit because of its deleterious effect on porosity and leachability.
Silicon oxide is
added to help convert the fluorides to a stable form. Due to poor kinetics,
conversion of
the fluorides salts to calcium fluoride is poor.
U.S. Patent No. 5,286,274 (Elkem Technology) describes a method for
treatment of SPL for use as a filler, or as a raw material. Crushed SPL is
supplied
optionally with an SiO2 source, to a closed electrothermic smelting furnace.
The SPL is
melted at a temperature between 1300 C and 1750 C. An oxidation agent is
supplied
to the furnace to oxidize carbon and other oxidizable components, such as
metals,
carbides and nitrides. Further, a source of calcium oxide is supplied to the
smelting
furnace in an amount necessary to react with all fluoride present to form CaF2
and to
-4-
CA 02497064 2006-11-16
form a calcium aluminate or calcium aluminate silicate slag containing CaF2.
The slag
is liquid at the bath temperature in the furnace, and it, and optionally a
metal phase,
are tapped from the furnace and cooled to blocks or granules. Cyanide is
combusted,
and the conversion of the fluoride salts to calcium fluoride is poor.
U.S. Patent No. 5,245,115 (Aluminium Pechiney) describes a thermal shock
treatment of ground SF'L mixed with a mineral additive (selected from the
group
consisting of anhydrous CaSO4, CaO and mixtures thereof). The mineral additive
combines, with or without melting or fusion, with fluorine-containing
compounds to form
CaF2, binary, ternary or quaternary compounds of NaF, CaF2, Si02, A1203,
CaSO4, Na2
SO4, of the nephelite, hauynite or similar type. The conversion of the
fluorides salts to
calcium fluoride is poor.
U.S. Patent No. 4,113,832 (Kaiser Aluminum & Chemical Corporation)
describes a treatment process for waste materials, such as SPL, channel and
trench
cleanings, floor sweepings and spent alumina from offgas purifying dry
scrubbers. The
waste material is pyrohydrolyzed at elevated temperature. Fluorides, such as
NaF and
HF can be recovered from the offgas generated by pyrohydrolysis, while alumina
and
Na20, or if desired, sodium aluminate, can be reclaimed from the solid residue
of
pyrohydrolysis. Na+ and fluorine are volatized in this process.
U.S. Patent No. 4,735,784 (Morrison-Knudsen Company, Inc.) describes a
process for treating solid substantially non-volatile waste contaminated with
a heat
sensitive contaminant. At least a portion of the contaminating components
(i.e. fluorine)
are either decomposed or evolve from the melt as a gas. The slag is cooled and
any
remaining contaminating compounds are bound or encapsulated into a solid
glassy
slag. The residue from the slagging reaction is a glassy solid sodium metal
silicate
matrix. Substantially all of the fluorine is volatized and captured as HF.
U.S. Patent No. 6,074,623 (Vick et al.) describes a process of converting SPL
by gasification technology to produce an inert vitreous frit and useful gases
including
hydrogen fluoride, hydrogen and carbon monoxide. Substantially all of the
fluorine is
volatized and captured.
The first processes, described in U.S. Patents Nos. 5,164,174 (Reynolds);
4,497,464 (Ogden) and 4,993,323 (No Assignee), relate to processes in which
little, if
any, carbon is combusted. In all instances, the carbon remains in the final
product.
Therefore, the described processes fail to provide a process where the carbon
is
-5-
CA 02497064 2006-11-16
removed. Likewise, these processes fail to provide a product from which carbon
has
been removed.
The next processes, described in U.S. Patents Nos. 4,763,585 (Ogden);
5,711,018 (Alcoa); 5,286,274 (Elkem) and 5,245,115 (Aluminum Pechiney); and
4,113,832 (Kaiser), relate to processes in which a large portion of the carbon
is
combusted and an attempt is made to capture fluorine in the final product.
These
processes do not perrriit recycling of carbon and have poor conversion of the
fluoride
salts to calcium fluoride.
The final three processes, described in U.S. Patents Nos. 4,113,832 (Kaiser);
4,735,784 (Morrison-Knudson) and 6,074,623 (No Assignee), describe processes
in
which a large amount of fluorine is volatized and a large amount of carbon is
combusted. These patents fail to provide processes in which fluorine is
efficiently
captured in the frit and carbon is separated by physical means.
It is, therefore, desirable to provide a process for converting SPL into a
glass frit
which obviates or mitigates at least one of the disadvantages of the prior
art.
SUMMARY OF THE INVENTION
It is an object of the present invention to obviate or mitigate at least one
disadvantage of previous processes, apparatuses and products.
In a first aspect, the present invention provides a process comprising the
steps
of: combining and heating spent potliner, a calcium oxide source and a silica
source to
destroy cyanides, convert fluoride salts to calcium fluoride and form a
generally
homogeneous amorphous liquid material; physically separating carbon from the
material; and vitrifying the material.
In one embodiment, about 9 to about 19% of a total feed is aluminium. In one
embodiment, the heatirig is effected in counter-current fashion. In one
embodiment, the
process further comprises, after the combining and heating step, a glass
finishing step
in which the material is held at a temperature sufficient to enhance matrix
formation
and to facilitate separation of carbon from the material. In one embodiment,
the spent
potliner is crushed to less than 6mm. In one embodiment, vitrifying the
material is
effected by quenching. In one embodiment, a sufficient amount of calcium oxide
is
used to result in a complete conversion of: CaCO3 and NaF to CaF2, Na20 and
C02,
according to: CaCO3 + 2 NaF 4 CaF2 + Na20 + CO2. In one embodiment, an amount
-6-
CA 02497064 2006-11-16
of calcium oxide is added to achieve a CaO to vitrified material ratio of
between about
0.04 and 0.10. In one embodiment, an amount of silica is added to achieve a
Na20/SiO2 ratio in the vitrified material of between about 0.21 and about
0.36. In one
embodiment, the material is heated to between about 800 and about 1200 C. In
one
embodiment, the heating is at a temperature which retains fluorine and carbon
in the
material. In one embodiment, glass is added to a molten portion of the
material to
increase production and to improve cementitious properties of the vitrified
material. In
one embodiment, the rnaterial, after vitrification, is ground to between about
4000 and
8000 m3/kg.
In further aspect, the present invention provides an apparatus comprising: (a)
a
first vessel for heating a mixture of spent potliner, silica source and a
calcium oxide
source to destroy cyanides, convert fluoride salts to calcium fluoride and
form a
homogeneous liquid miaterial, (b) a second vessel for physically separating
carbon
from the material; and (c) a third vessel for vitrifying the material. In one
embodiment,
the first vessel is a rotary kiln for heating in a counter-current fashion. In
one
embodiment, the second vessel is a glass finishing furnace for holding the
material at a
temperature sufficient 'to enhance matrix formation and to facilitate
separation of
carbon from the material. In one embodiment, the third vessel is a quench
basin.
In a further aspect, the present invention provides a vitrified material made
by
the above-described process.
In a further aspect, the present invention provides a generally amorphous
solid
matrix of calcium and sodium fluoro-aluminosilicate with a carbon content of
less than
1 weight percent and a calcium fluoride content of between about 5 and about
35
weight percent having cementitious properties when finely ground that is
produced
from spent potliners. In further embodiments, the solid is at least about 95%,
at least
about 99% amorphous, or at least about 99.8% amorphous. In one embodiment, the
solid is in granular forrn. In one embodiment, the solid has a Blaine value of
from about
2000 to 8000cm2/g. In one embodiment, the solid has the following composition
by
weight: CaF2 of about 5-35%, Si02 of about 30-60%, Na20 of about 5-25%, AI203
of
about 10-25% and less than about 1% carbon. In one embodiment, the solid has
the
following composition by weight: CaF2 of about 10-25%, Si02 of about 35-45%,
Na20
of about 10-15%, AI203 of about 18-23% and less than about 1% carbon.
-7-
CA 02497064 2006-11-16
In a further aspect, the present invention provides a use of the generally
amorphous solid described above together with an alkaline activator as a
binder for the
manufacture of concrete.
In a further aspect, the present invention provides a composition comprising
Portland cement, the generally amorphous solid described above, silica fume,
and
slag. In one embodiment, the composition comprises about 30-40% Portland
cement,
about 5-35% of the generally amorphous solid, about 2-8% silica fume, and
about 20-
30% slag.
In a further aspect, the present invention provides a use of between about 20
to
30% by weight of the generally amorphous solid described above as a Portland
cement
substitute.
In a further aspect, the present invention provides a use of more than about
90% of the generally amorphous solid described above in cement.
In a further aspect, the present invention provides a use of the generally
amorphous solid described above as a hydraulic binder.
Other aspects and features of the present invention will become apparent to
those ordinarily skilled in the art upon review of the following description
of specific
embodiments of the invention in conjunction with the accompanying figures.
BRIEF DESCRIPTION OF THE DRAWINGS
Embodiments of the present invention will now be described, by way of
example only, with reference to the attached Figures, wherein:
Fig. 1 is a schematic of an apparatus according to an embodiment of the
present invention;
Fig. 2 is a bloc diagram of a process according to an embodiment of the
present invention;
Fig. 3 is a flow chart illustrating a process according to an embodiment
of the present invention;
Fig. 4 is a schematic of a gas circuit of a process according to an
embodiment of the present invention;
Fig. 5 is a schematic of a solid circuit of a process according to an
embodinient of the present invention;
-8-
CA 02497064 2006-11-16
Fig. 6 is a schematic of a water circuit of a process according to an
embodiment of the present invention;
Fig. 7 is a diffractogram of a product according to an embodiment of the
present invention.
Fig. 8 is a graph showing chloride ion-permeability of blended
concretes, some of which contain a product according to an
embodiment of the present invention; and
Fig. 9 is a graph showing the expansion obtained on concretes, some of
which contain a product according to an embodiment of the present
invention.
DETAILED DESCRIPTION
Generally, the present invention provides a pyrometallurgical process and
apparatus for convertirig spent potliners (SPL) from aluminium manufacturing,
or other
hazardous materials, irito commercially saleable amorphous calcium and sodium
fluoroaluminosilicate glass frit (an example of which currently carries the
trade mark
CAISiFrit ) and a high carbon material (an example of which currently carries
the trade
mark CAISiCoke ). Spent potliners, a calcium oxide source, and a silicate
source are
fed into a non-burner end of a counter-current rotary kiln. The material is
heated to
destroy cyanides, convert fluoride salts to calcium fluoride and form a
homogeneous
liquid material. Carbon is then physically separated from the material in a
fixed-bed
furnace in series with the rotary kiln and the material is then vitrified.
Both the rotary
kiln and the fixed-bed furnace are linked to a flue gas treatment system.
The term "spent potliners" is known in the art. The word "spent" is not
intended
to limit the expression "spent potliners" to potliners which are completely
spent as
manufacturers may determine when a potliner should be replaced according to
different criteria. Potliners are replaced well before their useful life ends
in certain
instances, according to plant operations. Other hazardous materials,
especially those
containing aluminium, calcium, or silicon can be treated according to the
present
process, such as catalysts from petroleum refineries, polycyclic aromatic
hydrocarbons, a contarninated refractories, water purifying muds, and carbon
anodes
from aluminium smelteirs.
-9-
CA 02497064 2006-11-16
i
The apparatus according to the present invention will now be described with
reference to Figure 1. SPL, energy and additives are fed into a long body
rotary kiln
(101) at a non-burner end thereof (i.e. at the right hand side in Figure 1).
The material
passes through the kiln to a glass finishing furnace (103) and to a quench
basin (105).
Gas and water treatment systems are integrated with the apparatus as detailed
below.
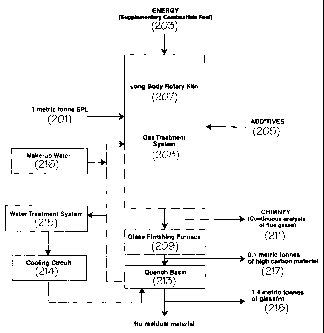
Referring to Figure 2 for a general description of the process, SPL (201),
energy (203), and additives (205) are added to the long body rotary kiln (207)
where
heating occurs. Gases are treated by a gas treatment system (208) and are
partially re-
circulated into the kiln (207) and treated gases are discharged through a
chimney
(211). At the exit of the kiln, the material falls into glass finishing
furnace (209) where
the high carbon material, (217) is separated and the remaining material passes
to a
quench basin (213) where it is vitrified into a glass frit (218). A portion of
the water from
the quench basin (213) is fed to the gas treatment system (208) and the
balance is fed
to the cooling circuit (214) and water treatment system (215), where it is
cooled and
returned to the quench basin (213). Since the process consumes water, make-up
water
(216) is added.
A process flow chart is provided in Figure 3. The feed comprising SPL and
additives is combined and heated (301) to form a liquid material. Carbon is
physically
separated from the material (303). The material is vitrified to produce a
glass frit (304).
Between steps (301) and (303) the material may be held at a high temperature
to
enhance matrix formation (302). The process is described in greater detail
below.
A) Crushing and preparation of the mixture (i) The SPL is crushed to a
dimension of, on average, 6 mm and continuously sampled during stockpiling. In
an
integrated system, sampling takes place during silo filling so that a computer
has the
information at all times of which material corresponds to which analysis. As
an
example, eight silos can simultaneously be used to correctly homogenize the
SPL
destined to feed the furnace. Stockpiling inside specially a designed silo
comprising air
intake and filtering ventilation is preferred over large reclaiming stockpiles
due to the
reactivity of the material with moisture in the air. (ii) The crushed SPL is
quarantined
awaiting analysis. (iii) After receipt of the chemical composition data of the
SPL,
additives and reagents are added during its feeding accordingly to the
analysis. The
quantities of additives and reagents are determined by a software program to
provide a
consistent feedstock. Alumina bearing material such as recycled material is
added to
-10-
CA 02497064 2006-11-16
the SPL to present a total Al content of about 9 to 19% of the total feed,
preferably
13.5%. Alternatively, SPL is crushed after been mixed to form the recipe.
Enough
calcium (such as CaCO3, limestone, calcium oxide (or quicklime) hydrated lime,
possibly dolomite, and calcium containing waste) is added to complete the
following
simplified reaction:
CaCO3 + 2 NaF --) CaF2 + Na20 + COZ
Additional calcium is added over the above stochiometric reaction amount for a
ratio CaO/glass frit of between about 0.02 to 0.12, preferably about 0.07.
Sufficient
silica is added according to the sodium of the SPL to offer a final Na20/SiO2
ratio of
about 0.16 to 0.36, preferably about of 0.26 in the glass frit.
B) The furnace is fed. The furnace is preferably a long body rotary furnace
(LBRF) or kiln. The material progresses from upstream to downstream passing
through
different thermal and physical phases. The feedstock is fed to the furnace by
rate
controlled conveyors and/or a chute.
C) Operation of the long body rotating furnace (LBRF). (i) The LBRF has a
diameter of 3 m and a!length of 40 m. It should be understood that while
specific
dimensions or shapes may be described or shown throughout this description,
this is
intended to be illustrati've and not limiting. Infrared temperature detectors
are disposed
along its entire length to measure the exterior temperature of the furnace
and,
consequently, the temperature variations in the furnace. Once control is
established it
may be sufficient to monitor the cold end temperature of the process gases and
the
temperature of the exiting material. (ii) The material is progressively
heated, starting
from ambient, as it moves within the furnace. The heat source is downstream,
making
the process counter-current. Figure 1 shows the various phases through which
the
material passes. Zone 1 (1001) is the cold zone where material enters the
kiln. Zone 2
(1002) is where the material is heated to between 800 and 1200 C and chemical
reactions occur. In Zone 3 (1003), solid material slowly melts while
continuing to
undergo chemical reactions. In Zone 4 (1004), material is liquid and final
chemical
liaison are taking place. Zone 5 (1005) represents the secondary glass
finishing
furnace where separation of carbon takes place. The secondary glass finishing
furnace
should afford effectively separate carbon from the matrix. Zone 6 (1006)
represents the
quench basin. (iii) Increasing temperature favours the breaking of certain
existing
-11-
CA 02497064 2006-11-16
chemical bonds, and the creation of the bonds which define new compositions.
This
causes the destruction of cyanides by oxidation, and the capture of fluorides
which
pass from a sodium bond to a calcium bond. The primary reactions are as
follows:
2CN + 202 -> 2CO2 + N2
CaCO3 -> CaO + COZ
2NaF + CaO -> CaF2 + Na20
2AIF3 + 3CaO -> 3CaF2 + A1203
(iv) The material, which at the entrance of the LBRF was composed
heterogeneously
of solid materials, gradually becomes a homogeneous liquid composition. In
Zone 4
(1004), the matrix is formed and the following two reactions occur:
CaF2 + Si02 + Na20 + A1203 4 vitrified matrix
C + 03 4 CO2
D) Vitrification of siliceous matrix. (i) At the exit of the LBRF, the
material, now
in an amorphous state, falls into a secondary non-rotating furnace, or a glass
finishing
furnace (GFF), to prolorig the time of retention at high temperature, thereby
ensuring
the complete formation of a complex matrix of fluroaluminosilicate of sodium
and
calcium. The GFF also permits the gravitational separation of carbon, and. by
raking it
or blowing it out, the production of a matrix with very little carbon. The
carbon floating
on the matrix is recovered instead of being burnt. Analysis of this high
carbon material
gives a carbon content as high as most metallurgical cokes found on the
market.
Chemically, it consists of a pyrometallurgical coke containing greater than 80
percent
carbon. In the less than 20 percent of ashes, 2 to 4 percent is calcium
fluoride, a level
which presents a marked advantage with respect to the fluidization and the
lowering of
eutectic points of clinkers and of metallurgical slags. The recovery of
residual carbon in
this process increases the global co-efficient of recycling of the spent
potliners and
reduces as much the greenhouse gas effect by tonne of spent potliners used.
(iii) The
glass liquid thus extracted is quickly immersed in water to rapidly reduce the
temperature and to conserve its amorphous character, one of its essential
qualities. (iv)
The resulting glass frit, is dried and ground. The glass frit is preferably
ground to a
Blaine value of from about 2000 to 8000cmZ/g and more preferably about 4000 to
6000
cmZ/g. The glass frit in rriatrix form is composed of (CaF2 5-35% or
preferably 10-25%),
Si02 (30-60% or preferably 35-45%), Na2O (5-25 or preferably 10-15%) and A1203
(10-
-12-
CA 02497064 2006-11-16
25%). Smaller amounts of CaO (3-12%) and Fe203 (1-3%) are also present. Less
than
1% carbon is present. 'The ratio of glass frit to SPL is about 1.4 or 1.55.
E) Treatment of gases. Figure 4 illustrates the gas circuit. (i) In order to
capture
all the gases generated during the production of the glass frit the LBRF (403)
and the
GFF (401) are connected by a mobile head of high temperature type. The LBRF
burner
is shown as (402) while the burner of the GFF is shown as (412). This assures
a
sealed system, which, under negative pressure, directs all the gases of
combustion
and gases of production (decomposition of carbonates and ferrocyanides, etc.)
to an
after-burner and purging system (404), before their emission to the chimney
(410). (ii)
A first purging takes place in the relaxation chamber (403a) of the furnace,
through the
vertical exit of gases to a high temperature cyclone (not shown). This avoids
massive
entrainment of dust downstream. The cyclone is not necessary. Once separated
from
the flux gas, the dust is re-fed to the LBRF via a solid feed circuit
(discussed below with
respect to Figure 5). (iii) The temperature in the after-burner system is
raised to about
775-950 C, preferably 875 C to combust CO, and eliminate hazardous air
pollutants
such as polycyclic aromatic hydrocarbons. (iv) The gases are then cooled to a
temperature compatible with the filter bags (407). This also neutralizes acid
gases to
be further polished in a dry reactor (406). (v) The dry reactor (406)
eliminates, by
injection of calcium hydroxide, HF and other acids, which may be found in the
gas
stemming from the after-burner (411). If the detection system signals the
presence of
fluorine gases to the chimney, the dry reactor (406) automatically increases
the
injection of calcium hydroxide in order to assure the quality of the emissions
into the
atmosphere. (vi) The filter bags (407) capture the solid dust coming from the
dry
reactor (406). The presence of an adequate cake on the filtrating sleeves
helps to
assure that the acidic molecules are absorbed by the Ca(OH)2 and, therefore,
plays a
role similar of that of the dry reactor (406). A further burner (412) is
present for the GFF
(401). Combustion of natural gas and/or other combustibles occurs at (413).
Continuous natural gas measurement occurs at (414). Other combustibles, liquid
or
solid, can be used with the kiln burner. Natural gas is preferred at the after-
burner to
ensure complete burning of all elements which is the purpose of the after-
burner. A
post combustion chamber (405) is also provided.
F) Verification of atmospheric emissions. Figure 4 illustrates the gas
circuit. (i)
After filtering, the gases are directed to the chimney (410). Continuous
measurement of
-13-
CA 02497064 2006-11-16
the atmospheric emissions: HF, CO, C02, 02, SO2, NOx and opacity, are
performed in
the analysis and sampling atmospheric station (409).
G) Treatment of dust and returns. Figure 5 illustrates the solid circuit. (i)
All dust
coming from the gas treatment is returned into the solid feed and re-injected
into the
furnace. Feed debris, quench-water treatment mud and the like such as spent
kiln
refractories are also recycled back into the process. The SPL comes from SPL
container (501) via a hopper (502) and conveyor (503) although conveyors are
not
necessary. Alternatively and more preferred is to crush the SPL and homogenize
it into
the bank of silos. Additive silos (504) hold the additives. Recipe hopper
(505) and
furnace hopper (506) are used for feeding. Feed debris (507), post combustion
and
conditioning recycles (508), and recycles from filter bags (509) are re-fed
into the
furnace hopper (506). Also shown are the LBRF (510), LBRF burner (511), GFF
(512),
high carbon material container (513), glass frit quench basin (514), automatic
glass frit
sampler (515), and the glass frit (516).
H) Water treatment. Figure 6 illustrates the water circuit. (i) Lime is added
to the
treatment water to prevent its acidification. The water is then cooled before
being
recycled back to the quench basin. (ii) A portion of the water feeds the
conditioning
tower of the after-burner. Water cooling is done through evaporation. Overall,
the
process consumes water and, therefore, there is no waste water expelled.
Referring to
Figure 6, glass frit (609) is shown coming from quench basin (601). Water from
quench
basin (601) flows through holding basin (603) and pump basin (604) and
therefore a
portion is added to the conditioning and post-combustion tower (605) and the
balance
is fed to cold water treatment basin (608) from which water evaporates (606).
Additional water (607) is added to the water basin (608). Waste water is
collected in
catch basin (602) and using collection basin pump is pumped to holding basin
(603).
Water basin pump (611) is used to pump water to the quench basin (601).
Temperature profile in the kiln
The material temperature at the feed end is approximately ambient and at the
exit end is 950-1250 C and preferably 1050-1125 C. Using the state of the
material
and the liquidus material temperature, the temperature at ten to twelve meters
from the
burner end of the furnace has been calculated to be 860 C. At one meter from
the
burner, the temperature has been measured to be 1125-1150 C. This temperature
can
-14-
CA 02497064 2006-11-16
be controlled by increasing or decreasing the amount of fuel used by the front
burner.
Additional correction involving the varying of the flame type from axial to
radial is done
after observing the location and state of the annulus at approximately ten to
twelve
meters from the burner end.
The gas temperature at the burner end of the kiln (exit of the kiln) is 1450 C
and the gas temperatur-e and flow rate at the feed end of the kiln is
measured. In
relation to the feed rate, these measures indicate whether the profile is
being raised or
cooled. This is confirmed by the shell temperatures being measured
continuously at
some 20 sections, which trends will indicate in which direction the process is
proceeding. These shell readings are affected by the external temperature,
wind, rain
etc. and therefore are useful in a relative way to indicate a trend not
absolute values.
The shell temperature r-eadings are also affected by the thickness and state
of the
refractory lining augmented by the thickness of present accretions. As
discussed
above, measurement along the shell can help but it not necessary.
If the profile is not controlled, the accretions inside the kiln will get
rapidly to a
point where you cannol: feed more material. To remove these accretions, the
operation
is stopped to enter the kiln to physically break away the accretion with jack-
hammers.
Radial COZ blasting, axial gun shooting and superheating have proved
themselves
quite ineffective ways to remove/control the accretions.
Kinetics
Counter current kiln favors preheating of feed material and cooling of gas.
The
temperature inside the kiln is controlled by adjusting the ventilator (408)
(see in Figure
4) situated before the escape chimney and the energy input by the primary
burner.
The use of a LBRF (long body rotary kiln) in a counter-current set-up permits
operators to properly, dynamically and relatively slowly raise the temperature
of the
material as it moves down the kiln towards the hot zone. The rotation of the
kiln
ensures an intimate contact between the different materials. The rotation also
promotes rubbing of the different compositions one against the other in an
eroding
action. The necessary reactions can then take place in a catalytic and
successive
manner.
The use of a LBRF in a counter-current set-up ensures that most of the
involuntarily release of volatized NaF and produced Na20 and HF will condense
inside
-15-
CA 02497064 2006-11-16
the LBRF with its cool gas exit and be brought back into the process stream
due to the
rubbing action of the feed material onto the walls.
Thermodynamic simulations have shown the necessity to minimize the amount
of H20 vapor inside the kiln to prevent the reaction
2 NaF + H20 4 Na20 + 2 HF
The simulations also showed the necessity to finalize the process at less than
1200 C to reduce the vapor pressure of NaF and therefore the creation of HF
due to
the water vapor created by the combustion of natural gas.
CH4 + 02 4 C02 + 2H20
The process of the present invention employs these thermodynamic features.
The process of the present invention thus differs from the others which heat
rapidly the
SPL and provoke the creation of HF.
An exemplary time for the material to pass through the LBRF is 1.5 to 2.5
hours.
Auxiliary Feed Material
In one embodinient, an auxiliary feed material is used to increase the
production of the kiln and to improve cement properties. The counter-current
LBRF has
its cold zone at the feed section (upper section). Material is added in the
hot zone right
into the molten basin iri the lower section of the kiln. This material is
injected into the
kiln at 60 feet/sec to reach 4 to 6 meters into the kiln. The added material
then has time
to melt and partially or totally mix with the glass matrix. Up to about 7 tons
per hour is
expected to be able to be added in this manner therefore greatly increasing
the kiln
output.
The injection into the hot zone of the kiln is also done because the material
being injected is broken glass (cullet) which would negatively affect the
melting (and
chemical reactions) of the recipe if it was fed into the kiln in the cold
upper zone. Cullet
is presently preferred but others materials could be used.
These additions modify the structural liaison between the different components
of the glass matrix which in turn affect the reactivity of the amorphous
produced glass
and thus, its cementitious properties. Lowering the ratios of Ca and Al/glass
frit
reduces its susceptibility to sulfate and carbonate attacks rendering the
concretes
-16-
CA 02497064 2006-11-16
made out of the glass frit more durable. These additions result in a product
offering
some of the properties actually brought to concretes by silica fumes.
The glass frit can make concretes at high temperatures without reactions
creating deleterious ettringite. High temperature reduces strengthening time
required
by concretes therefore increasing the turn-over capability of pre-fab plants.
An activator
is used to turn the glass frit into a high performance binder for the
manufacture of
resistant and durable cements (an example of which currently carries the trade
mark
CAISiBinderHP ).
Mass Balance
A study of the mass balance of this process during the recycling of 1000
metric
tonnes of spent potliners revealed the following: a fluorine mass balance of
99.26%
(the loss of fluorine by atmospheric emission represents only 0.003% of the
total mass
of fluorine fed in the forrn of potliner, a COz mass balance of 99.02% (or
100% taking
into account the admissible tolerance of the various instruments of
measurement), a
solid mass balance of 99.8% (or 100% taking into account the admissible
tolerance of
the various instruments of measurement). The fluorine mass balance confirms
that
fluorine is not lost somewhere in the process. The quantity of carbon feed as
potliner
was measured as 324.99 4 2.03 metric tonnes and the carbon found in the glass
frit
and high carbon material respectively was 126.30 { 0.51 and 102.12 +8.17
metric
tonnes indicating that the quantity of carbon consumed during the process in
the kiln
and found as carbon dioxide gas in the chimney is 96.57 8.43 metric tonnes.
Only
0.84 tonnes of C02 are produced per tonne of potliner which favourably
compares to
1.19 tonnes in another known process.
The Glass Frit
The glass frit is an amorphous siliceous material forming a calcium and sodium
fluoroaluminosilicate matrix. This homogenous solid substance possesses a high
reactivity potential and shows superior cementitious properties when finely
ground. The
glass frit once quenche<i is finely ground into a powder, of, for instance 410
mZ/kg.
Figure 7 is an X-ray diffraction analysis of the glass frit shows that the
amorphous
character of character of the glass frit. Although a 95% amorphous character
is
desired, at least 98%, is preferred, and at least 99% more preferred. Table 2
shows a
-17-
CA 02497064 2006-11-16
chemical composition of the glass frit using one specific measurement. The
values
shown under the "normal" heading are the limits over which the material is
declared a
"Hazardous Material". TCLP is the standard practice defining the method to
analyse
lixiviation using an acidic medium.
Table 2. Chemical Composition of a Glass Frit
Parameter Normal Result
Total Analysis
Al, % 12.0
Ca, % 12.1
Mg, % 0.5
Si, % 15.4
Fe, % 1.6
K,% 1.55
F total, % 6.2
Na, % 10.2
Lixiviation TCLP
As < 5 mg/I < 0.01
Ba < 100 mg/I 0.38
B < 500 mg/I 0.09
Cd < 0.5 mg/I 0.34
Cr < 5.0 mg/I 0.02
Hg < 0.1 mg/I < 0.0004
Pb < 5.0 mg/I 3.3
Se < 1.0 mg/I < 0.01
U < 2.0 mg/I < 0.005
Nitrates+Nitrites < 1000 mg/I < 0.02
Fluorides < 150 mg/I 11.9
Nitrites < 100 mg/I < 0.02
-18-
CA 02497064 2006-11-16
The glass frit is useful as a cementitious addition and hydraulic binder. When
used as a cement enhancer, it has been demonstrated by a report prepared by
the
University of Sherbrooke that the glass frit improves the flowability of the
blended
concretes. This rheology allows for a reduction of the water to binder (W/B)
ratio.
Besides reducing the chemical admixture dosage requirements (especially in
water
reducer and superplaticizers), this improved rheology permits a reduction of
the binder
content without sacrificing either the workability or the physical properties
of the
concretes. The glass frit is a chloride-ion permeability reducer that enhances
the
durability of concrete. Figure 8 shows the results of chlorine ion
permeability tests
carried out after 91 days of curing in 100% RH and at a temperature of 23t2 C,
water
to binder ratio (W/B) of 0.35, according to ASTM C1202 standard. The cements
of
Figure 8 are defined as follows: Control is 100% Portland cement. Binary is
75%
Portland cement and 25% blast furnace slag. Binary CF is 75% Portland cement
and
25% glass frit. Ternary is 75% Portland cement, 20% of the glass frit, and 5%
silica
fume. Quat/FA is 50% Portland cement, 25% of the glass frit, 5% silica fume,
and 20%
flyash. Quat/slag is 401)/0 Portland cement, 25% of the glass frit, 5% silica
fume, and
30% slag.
Figure 8 shows that the permeability of concrete to chloride-ion decreases by
more than 50% when using 25% of the glass frit as a Portland cement
substitute. 20%-
30% is presently preferred. Figure 8 shows that using the glass frit is a
quaternary
mixture resulted in a permeability reduction to 1/15'h the level of a control
concrete
using a cement made of 100% Portland Type 10. Concrete's resistance to
chlorine-ion
attack is one of the most important factors to consider when determining its
durability.
This is precisely where the glass frit excels as an additive. Due to its
discontinuous
pore network, it ensures better resistance to salt, acid and water attack.
Figure 9 shows the expansion obtained on concrete prisms of 75 x 75 x 300
mm cured for longer than two years in 100% RH and at a temperature of 38 C,
according to CSA A23.2-14A standard. Replacing 25% of the cement with the
glass frit
reduced expansion. Following standardized curing for 853 days, the expansion
noted
in concrete using 100% Portland cement was 0.23%; while it was 0.13% with
cement
containing 25% of the glass frit. To evaluate a material's effectiveness in
controlling
alkali-aggregate reaction, article 6 of CSA standard A23.2-28A stipulates a
limit to the
expansion of concrete cDntaining additives to 0.04% after two years of
-19-
CA 02497064 2006-11-16
curing. The analysis of the graph allows us to conclude that although the
glass frit did
not meet the standard, its expansion is still far less than that caused by
commercial
Portland Type 10 cement. However, even though the glass frit has a significant
alkali
content, when used in concentrations above 90%, it meets CAS norm A23, 2-28A
article 6.
The glass frit being more than 99.8% amorphous, boasts strong binding
potential as a cement. When ground to 4000 Blaine and when combined with an
alkaline activator at hydration, it becomes an effective hydraulic binder
enabling its use
in RPC-type concretes. As shown by American laboratory F.L. Smidth,
pulverization of
the glass frit to 4000 Blaine required less energy than that of clinker or
slag.
Resistance and other mechanical characteristics of concrete are improved
when the glass frit is used. All tests performed to date by the University of
Sherbrooke
and St. Lawrence Cement corroborate this. More specifically, compressive,
tensile and
flexile strengths of blended concrete are improved, as is durability. By
substituting, as
the principle durability agent, 20% the glass frit to the Portland cement used
in the
preparation of general use concretes, not only are the tensile, flexile and
compressive
strengths increased their permeability is also greatly reduced. Their
workability is
improved while smaller quantities of rheology enhancing agents are needed.
The paleness of finely ground Glass frit is another quality. This is
especially true
when the aesthetics of a finished product is perceived as a component of its
quality
and durability. The glass frit also shows potential as an element in the
formulation of
reactive powder concrete.
The glass frit allows for the reduction of greenhouse gas emissions (GHG).
When used as a substitute of clinker in the manufacture of cement, it
generates a
credit equivalent to 450 kgs/mt of replaced Portland. This GHG credit reaches
650
kgs/mt of substituted Portland when cullet is added to the silica matrix. Thus
is a more
environmentally friendly and valuable material within a sustainable
development
framework. On an environmental and sustainable development level, the addition
of
The glass frit to cement is particularly beneficial. It eliminates an
environmental risk for
the aluminium smelters by transforming a hazardous waste into a commercially
viable
product. There will no longer be a need to landfill spent pot-liners, thus the
major
environmental benefit of this process. There are major socio-ecological
benefits in the
-20-
CA 02497064 2006-11-16
reclamation of residual hazardous materials into commercial products by the
use of a
clean, residue-less recycling process.
The high carbon materual
Another product of the glass frit process is a high carbon material or coke
(an
example of which currently carries the trade mark CAISiCoke . The high carbon
material has a carbon content as high as most pyrometallurgical cokes found on
the
market. Minor metal oxides contained give the material additional
metallurgical
properties sought by cement and metallurgical industries. Primary users of the
high
carbon material may be cement and steel makers, who require coke to supply
energy,
or as a means of reducing oxides and recarbonising steel.
-21 -
CA 02497064 2007-02-05
Table 3 shows the chemical composition of the high carbon material.
Table 3. Chemical Composition of the High Carbon Material
Parameter Units Results
Carbon % 80.1
Sulphur % < 0.1
Ca total % 1.5
Cd total % < 0.01
P total mg/kg 290
CN total mg/kg 1.3
Fluorine total % 2.9
Pb total % 0.01
Fe total % 0.4
Mg total % 0.05
Na total % 3.4
Sitotal % 1.5
Altotal % 3.3
LIXIVIATION
TCLP
As mg/I < 0.1
B mg/I 1.2
Ba mg/I < 0.01
Cd mg/I 0.01
Cr mg/I 0.04
Hg mg/I < 0.001
F mg/I 74.6
Pb mg/I < 0.02
Se mg/I < 0.1
The above-described embodiments of the present invention are intended to be
examples only. Alterations, modifications and variations may be effected to
the
particular embodiments by those of skill in the art without departing from the
scope of
the invention, which is defined solely by the claims appended hereto.
-22-