Note: Descriptions are shown in the official language in which they were submitted.
CA 02499058 2005-03-15
WO 2004/025265 PCT/US2003/029102
TEST STRIP AND METHOD FOR DETERMINING LDL
CHOLESTEROL CONCENTRATION FROM WHOLE BLOOD
REFERENCE TO RELATED APPLICATION
The present application claims priority to co-pending provisional application
Serial No. 60/411,209, bearing the same title, which was filed on September
16,
2002. The disclosure of this application is incorporated herein by reference.
FIELD OF THE INVENTION
The present invention relates generally to testing of body fluids for
concentration of analytes and more particularly to test strips for determining
concentration of analytes in whole blood.
BACKGROUND
The level of cholesterol in blood is a significant indicator of risk of
coronary
heart disease. "Total cholesterol" includes low density lipoproteins (LDL),
very low
density lipoproteins (VLDL) and high density lipoproteins (HDL). It is well-
established from epidemiological and clinical studies that there is a positive
correlation between levels of LDL cholesterol ("bad" cholesterol) and coronary
heart disease and a negative correlation between levels of HDL cholesterol
("good"
cholesterol) and coronary heart disease. Standing alone, the level of total
cholesterol in blood, which is a measure of the sum total of HDL, LDL, VLDL
and
chylomicrons, is not generally regarded as an adequate indicator of the risk
of
coronary heart disease because the overall level of total cholesterol does not
reveal
the relative proportions of HDL, LDL and VLDL. To better assess the risk of
heart
disease, it is desirable to determine the amount of LDL cholesterol in
addition to
total cholesterol.
The most common approach to determining LDL cholesterol in the clinical
laboratory is the Friedewald calculation, which estimates LDL cholesterol from
measurements of total cholesterol, HDL cholesterol and triglycerides. Although
convenient, the Friedewald calculation suffers from several well-established
drawbacks. Nauck et al., Methods for Measurement of LDL-Cholesterol: A
Critical
Assessment of Direct Measurement by Homogeneous Assays versus Calculation,
Clin. Chem. 4~:2 (2002) (citation omitted). For example, since the Friedewald
1718-0004
CA 02499058 2005-03-15
WO 2004/025265 PCT/US2003/029102
calculation involves measurements other than LDL cholesterol, it is subject to
potential compounded inaccuracies from the determinations of the other lipids
in the
equation. Further, its usefulness is limited when assaying blood samples with
triglycerides levels above 400 mg/dl.
Ultracentrifugation is a known technique to separate LDL cholesterol, but it
is tedious, time consuming, and the highly labile lipoproteins can be
substantially
altered by the high salt concentrations and centrifugal forces. "Furthermore,
a
plethora of different types of equipment and tubes are used, making conditions
difficult to reproduce from one laboratory to another and consistent
separations
highly dependent on the skills and care of the technician." Id. at 238.
Other techniques for measuring LDL cholesterol include electrophoresis,
which requires a fresh agarose gel specimen, is only semi-automatic, and
depends at
least in part on the technique of the technician performing the test. Other so-
called
homogeneous methods have recently become available.
One homogeneous method for determining LDL is disclosed in U.S. Patent
No. 5,888,827 (I~ayahara, Sugiuchi, et al.; assigned to Kyowa Medex Co.,
Japan).
The '827 patent discloses a two-stage liquid phase reaction to quantify LDL
concentration in a fluid sample. In the first step, the sample containing LDL
cholesterol is placed in a first reagent which includes trirnethyl j3-
cyclodextrin as a
sugar compound, polyoxyethylene monolaurate as a protein solubilizing agent,
EMSE (N-ethyl-N-(3-methylphenyl)-N'-succinylethylenediamene) and Tris buffer.
The sample is then heated to 37 °C, and after 5 minutes the absorbance
is read. A
second reagent including cholesterol esterase, cholesterol oxidase,
peroxidase, 4-
aminoantipyrine and Tris buffer is then added and after another 5 minutes the
absorbance is again measured at the same wavelength. LDL cholesterol is then
calculated by separately subjecting a standard solution of cholesterol to the
same
procedure and comparing the respective absorbance values. This method suffers
from the drawback of requiring conducting the reaction at a temperature of 37
°C.
Further, this method requires individual reagents to be added in two distinct
steps at
two different times
Another two-stage homogeneous assay is disclosed in U.S. Patent No.
6,194,164 (Matsui et al.; assigned to Denke Seiken, Ltd. Japan). In the first
stage,
HDL, VLDL and chylomicrons in the test sample are "erased," and in the second
step, the cholesterol remaining in the test sample viz., LDL) is quantified.
In the
1718-0004
CA 02499058 2005-03-15
WO 2004/025265 PCT/US2003/029102
3
first step, cholesterol esterase and cholesterol oxidase act on the test
sample in the
presence of a surfactant which acts on lipoproteins other than LDL ("non-
LDLs").
The hydrogen peroxide thereby generated is decomposed to water and oxygen by
catalase. Alternatively, a phenol-based or an aniline-based hydrogen donor
compound is reacted with the hydrogen peroxide to convert it to a colorless
quinone.
Preferred surfactants which act on the non-LDLs include polyoxyethylene lauryl
ether, polyoxyethylene cetyl ether, polyoxyethylene oleyl ether,
polyoxyethylene
higher alcohol ether, polyoxyethylene octyl phenyl ether, polyoxyethylene
nonylphenyl ether, and the like. In the second reaction disclosed in the ' 164
patent,
cholesterol remaining in the test sample, which should theoretically contain
only
LDL, is quantified. The second step may be carried out by adding a surfactant
which acts on at least LDL and quantifying the hydrogen peroxide by the action
of
the cholesterol esterase and the cholesterol oxidase added in the first step.
Like the '827 patent, one disadvantage of the method taught by the '164
patent is that it requires conducting the reaction at a temperature of 37
°C, and it has
been found that the test is not accurate at lower temperatures. Another
disadvantage
of the '164 patent similar to the '827 patent is that the '164 patent requires
individual reagents to be added in two distinct steps at two different times.
A more
general disadvantage of these liquid phase LDL tests is that they are not
easily
adaptable to point of care ("POC"), much less over the counter ("OTC")
applications.
What is needed, then, is a convenient, easy to use, diagnostic test for
determining LDL cholesterol which overcomes the drawbacks noted above.
1718-0004
CA 02499058 2005-03-15
WO 2004/025265 PCT/US2003/029102
4
SUM1V1ARY OF THE INVENTION
The present invention provides a dry phase test strip and method for
determining the concentration of LDL in whole blood or plasma. The test strip
directly measures concentration of total cholesterol and directly measures
concentration of non-LDLs, the difference therebetween being equal to the
concentration of LDL cholesterol. Dry phase test strips of the present
invention
function at room temperature and all test results are produced from pseudo
endpoint
reflectance measurements, such that the test method does not require timing.
In one form thereof, the present invention provides a method of determining
concentration of non-LDL cholesterol in a whole blood sample using a dry phase
test strip. The whole blood sample is contacted with a blood separation layer
of the
test strip and the blood cells are separated from the sample, thereby
producing
plasma. The plasma so produced is then contacted with a test layer and the non-
LDL fraction reacts substantially faster than the LDL fraction to produce
color
substantially in proportion to the concentration of LDL cholesterol in the
sample.
The color produced is measured and corresponds to the concentration of non-LDL
cholesterol. The test layer employs a surfactant which acts on lipoproteins
other
than LDL, "non-LDLs," such that the non-LDLs react to produce color and the
result is read photometrically before any LDLs have substantially reacted. In
this
manner, the reflectance or colorimetric response is a function of non-LDL
concentration, substantially unaffected by LDL concentration.
By reading the concentration of non-LDLs and also reading concentration of
total cholesterol, the concentration of LDL cholesterol can be easily
calculated as the
difference therebetween.
In another form thereof, the present invention provides a test strip for
determining the concentration of LDL cholesterol in a sample of whole blood or
plasma. The test strip includes a test strip matrix having at least two
stacks. A first
one of the stacks has reagents incorporated therein to produce a colorimetric
response in proportion to the amount of total cholesterol in the sample. A
second
one of the stacks has reagents incorporated therein to produce a color
response in
proportion to the amount of non-LDL cholesterol in the sample. The value of
non-
LDL cholesterol obtained from the second stack can be subtracted from the
value of
total cholesterol obtained from the first stack to yield the value of LDL
cholesterol
in the sample.
1718-0004
CA 02499058 2005-03-15
WO 2004/025265 PCT/US2003/029102
One advantage of the present invention is that it provides a dry phase test
strip that produces reliable measurements of LDL cholesterol without relying
on the
Friedewald equation. As noted above, the Friedewald equation has serious
drawbacks.
Another advantage of the present invention is that the inventive test strips
can be used over a range of room temperatures, quite unlike the known
homogeneous liquid LDL assays, which require heating to 37°C. This
temperature
independence of the present invention is a significant advantage because test
strips
required to be heated to a specified temperature would be largely unmarketable
in
the over the counter ("OTC") and point of care ("POC") markets and, of course,
inconvenient. The temperature independence of the present invention was a
surprising result, in that the prior art liquid LDL assays and the testing of
the same
strongly suggests that temperature must be tightly controlled to produce
reliable and
accurate results in the liquid phase.
Yet another advantage of the present invention is that, even though a two
stage reaction occurs in the non-LDL stack or panel of the strip, the first
stage being
production of color in proportion to non-LDLs and the second being the
production
of color in proportion to LDL cholesterol, the reactions need not be timed.
Instead,
a "pseudo endpoint" is produced at the completion of the first stage,
whereupon
color production in proportion to non-LDL cholesterol concentration is read by
an
optoelectronic instrument. Not having to time the reaction is a significant
advantage
that allows the test strips to have much wider applicability, ease of use, and
reliability than would otherwise be possible. The time independence of the
present
invention was quite surprising, in view of the prior art liquid LDL assays all
teaching timed reactions.
Still another advantage of the present invention is that the non-LDL test is
completed and the result read in less than 11/2 minutes. Thus, with test
strips in
accordance with the present invention that read multiple analytes, e.g_, HDL,
total
cholesterol and non-LDL, the three results are all obtained at about the same
time.
Waiting for the non-LDL result is unnecessary. The present single measurement
approach is in contrast to the prior art LDL assays noted above that teach two-
stage
reactions and a result measured after both stages.
Still another advantage of the present invention is that it provides a dry
phase
"lipid panel" which measures total cholesterol, HDL cholesterol and LDL
1718-0004
CA 02499058 2005-03-15
WO 2004/025265 PCT/US2003/029102
cholesterol results without reliance upon the Friedewald equation, and without
the
need to measure triglycerides, which was hitherto not possible in a dry phase
test
strip.
To summarize, the present invention provides a dry-phase test strip for
determining LDL concentration in whole blood or plasma that is inarguably
quicker,
more convenient, and is essentially time and temperature independent. This
breakthrough technology makes possible for the first time the potential for
point-of
care and patient self testing of LDL cholesterol without relying upon the
Friedewald
equation.
1718-0004
CA 02499058 2005-03-15
WO 2004/025265 PCT/US2003/029102
BRIEF DESCRIPTION OF DRAWINGS
The above-mentioned and other advantages of the present invention, and the
manner of obtaining them, will become more apparent and the invention itself
will
be better understood by reference to the following description of the
embodiments of
the invention taken in conjunction with the accompanying drawings, wherein:
Fig. 1 is a perspective view looking down on an assembled and closed test
strip in accordance with the present invention;
Fig. 2 is an exploded perspective view of a test strip holder in accordance
with the present invention, the view being taken from the bottom of the test
strip
holder;
Fig. 3 is perspective view of a test strip holder in accordance with the
present
invention, the test strip holder having its top and bottom portions unfolded
and the
underside thereof being shown;
Fig. 4 is an exploded perspective view of a test strip holder in accordance
with the present invention illustrating the layers and stacks of the test
matrix and
their relationship with the top and bottom portions of the test strip holder;
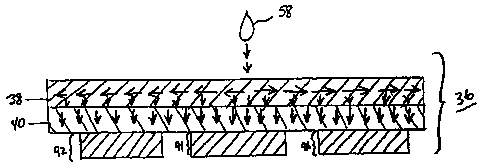
Fig. 5 is a side sectional view of an exemplary test matrix in accordance with
one embodiment of the present invention;
Fig. 6 is a graph illustrating color production versus time for the two stage
reaction that occurs in the non-LDL stack of panel of test strips in
accordance with
the present invention;
Fig. 7 is a perspective view illustrating the vertical flow scheme utilized by
the stacks and blood separation layer of the present invention;
Figs. 8, 9 and 10 are cross-sectional views of the layers of test strips used
in
certain of the examples given hereinbelow; and
Figs. 11 and 12 are cross-sectional views of the layers of test strips in
accordance with alternate embodiments of the present invention.
Corresponding reference characters indicate corresponding parts throughout
the several views.
1718-0004
CA 02499058 2005-03-15
WO 2004/025265 PCT/US2003/029102
,.,.. " . ..... .,.., ,.~, ..n" , ....... ... ....... ..... ...,...
DETAILED DESCRIPTION
The embodiments of the present invention described below are not intended
to be exhaustive or to limit the invention to the precise forms disclosed in
the
following detailed description. Rather, the embodiments are chosen and
described
so that others skilled in the art may appreciate and understand the principles
and
practices of the present invention.
Definitions
"HDL" refers to high density lipoprotein.
"LDL" refers to low density lipoprotein.
"VLDL" refers to very low density lipoprotein.
"NonHDL" refers to LDL, VLDL and chylomicrons, i.e., lipoproteins other
than HDL that will react with a conventional cholesterol reaction membrane.
"Non-LDL" refers to HDL, VLDL and chylomicrons, i.e., lipoproteins other
than LDL that will react with a conventional cholesterol reaction membrane.
"Plasma" refers to the non-cellular portion of blood from which cellular
components such as red blood cells are excluded.
"Serum" technically differs from plasma, in that it does not include
fibrinogen. However, for purposes of this application "serum" and "plasma" are
sometimes used interchangeably.
"Room Temperature" means from about 17°C to about 30°C.
Test Device
Referring now to Fig. 1, test strip 20 includes test strip holder 22 which is
preferably formed by injection molding. Test strip holder 22 includes handle
24 and
top portion 26 (Figs. 2 and 3) which is preferably hingedly attached by hinge
portion
28 to bottom portion 30, shown exploded away in Fig. 2. With reference to Fig.
3,
top portion 26 is foldable about hinge portion 28 over bottom portion 30 as
shown.
Top portion 26 includes an opening 32 while bottom portion 30 includes three
spaced openings 34. Opening 32, while shown as round, can be formed as an
elongated oval shape to facilitate disbursement of blood. When top portion 26
is
folded over bottom portion 30, opening 32 is aligned centrally over openings
34. In
its folded position, opening 32 in holder 22 defines an area for depositing a
body
fluid sample while openings 34 define areas in which optoelectronic
measurements
1718-0004
CA 02499058 2005-03-15
WO 2004/025265 PCT/US2003/029102
of chemistry test reactions are conducted. Optionally, openings 34 can be
configured with transparent windows, although such is not necessary.
The particular test strip described herein is suitable for use with a modified
optoelectronic instruments sold under the trademarks BioScanner and Cardio
Chek,
available from Polymer Technology Systems, Inc., Indianapolis, IN.
Referring now to Fig. 4, top and bottom portions 26 and 30 of strip holder 22
sandwich a test matrix 36 therebetween. Test matrix 36 is made up of a top
disbursement layer 38, a blood separation layer 40, stacks 42, and adhesive
layer 44
having openings 46 that align with openings 34 and the bottoms of respective
stacks
42 when the layers are assembled. Stacks 42 are further made up of one or more
vertically aligned layers, the function and specifics of which are described
in further
detail hereinbelow. The second layer of the "stacks" 42 in Fig. 4 is shown in
phantom to indicate that this second layer is not used in all embodiments
disclosed
in this application. When assembled and closed, the layers of stacks 42 and
layers
38, 40 and 44 are all pressed together. Opening 32 exposes a part of
disbursement
layer 38 and openings 34 and 46 expose the bottom surface of the bottom layers
of
stacks 42.
It has been found that only a minimally compressive force provided by strip
holder 22 is necessary to sandwich the layers of test matrix 36. To this end,
portions
26 and 30 have complementary I-shaped indentations or recesses 48 (Figs. 2 and
3)
in which the corresponding I-shaped matrix 36 is received. Recesses 48 allow
portions 26 and 30 to be snapped together in a snap-tight engagement as shown
in
Fig. 1 while still exerting a minimally compressive force on matrix 36. As
shown in
Figs. 2 and 3, top portion 26 includes oval shaped receptacles 50 that receive
complementary shaped bosses 52 disposed on portion 30. Receptacles 50 include
pegs 54 that fit via friction fit into mating cylindrical openings 56 formed
in bosses
52. Stacks 42 all include the same number of layers or at least have about the
same
thickness, such that the bottom surfaces of stacks 42 are substantially
coplanar. This
coplanar structure helps maintain the proper compressive pressure on matrix 36
by
holder 22.
It should be understood that at the time of this writing, it is believed that
a
minimally compressive force exerted upon matrix 36 is preferable. However, the
amount of pressure with which matrix 36 is to be pressed together is a design
variable that can be adjusted by (1) adjusting the depth of recesses 48; (2)
adjusting
1718-0004
CA 02499058 2005-03-15
WO 2004/025265 PCT/US2003/029102
the engagement between receptacles 50 and bosses 52; or (3) adjusting the
height of
pegs 54 and/or the depth of cylindrical openings 56.
Referring to Fig. 5, the individual layers and the diagnostic chemistries of
matrix 36 can be appreciated. The top layer of matrix 36 is a disbursement or
spreader layer capable of rapidly spreading the blood sample 58 rapidly
through
layer 38 as shown by the reference arrows. It has been found that layers used
as
conjugate pads in pregnancy test kits perform quite well as layer 38. Layer 38
is an
open cell layer capable of rapidly and effectively spreading the fluid sample.
One
suitable material for layer 38 is available under the name "Accuflow Plus-P"
from
Schleicher & Schuell, Inc. Another suitable material for layer 38 is available
under
the name "Accuwik" from Pall Biochemicals. Both of these layers are made of
polyester and provide excellent movement of blood sample 58 through layer 38.
As will become clearer with reference to the discussion below, substantial
lateral movement occurs only in disbursement layer 38 of matrix 36. As shown
by
the reference arrows in Fig. 5, however, the delivery of blood from layer 38
to layer
40 occurs substantially vertically, or normal to the plane of layer 40. In the
remaining layers, the net direction of fluid flow is believed to be
substantially
vertical, or normal to the plane of the layers. For example, with reference to
Fig. 7,
fluid sample drop 60 is deposited onto layer 62 (which could be blood
separation
layer 40 or one of the layers from one of stacks 42). Layer 62 defines a plane
64
that is substantially parallel therewith. Transfer of fluid through layer 62
is normal
or perpendicular to plane 64, or in the direction of vector V, shown at
reference
numeral 66. Thus, there is no substantial migration of fluid from one side of
layer
62 to the other. Fluid flow is through layer 62, not across it.
Returning now to Fig. 5, layer 40 is a blood separation layer that is adjacent
to and in fluid communication with layer 38. Blood separation layer 40
separates
blood cells from plasma and passes the plasma therethrough, retaining the
blood
cells. Blood separation layer 40 is generally a glass fiber membrane. A
suitable
commercial membrane for layer 40 is Ahlstrom Grade 144, thickness 0.378mm,
available from Ahlstrom Filtration, Inc., Mt. Holly Springs, PA. Other glass
fiber
matrices could be substituted as demonstrated in the examples that follow
hereinbelow. More specifics regarding the blood separation membrane are given
in
co-pending U.S. provisional patent applications serial nos. 60/344,300 and
1718-0004
CA 02499058 2005-03-15
WO 2004/025265 PCT/US2003/029102
11
60/342,790, commonly owned by the assignee of the present invention, the
disclosures of which are hereby incorporated by reference herein in their
entirety.
Total Cholesterol Measurement Stack
With further reference to Fig. 5, the stack 92 is formed of a single layer and
is spaced from stack 94 and is adjacent to and in fluid communication with
layer 40.
Stack 92 takes plasma from layer 40 and produces a color response proportional
to
the concentration of total cholesterol in sample 58. In the embodiment
depicted in
Fig. 5, stack 96 is also a "total cholesterol" stack; identical to stack 92.
The
preparation of reagents for the total cholesterol stacks (also called
"panels") is set
forth in detail in the examples hereinbelow.
Non-LDL Stack
Still referring to Fig. 5, middle stack or layer or panel 94 forms a color
response that is proportional to the amount of non-LDL cholesterol in sample
58, at
room temperature and without requiring the reaction to be timed, as will be
explained below. Layer 94 is loaded with reagents such that non-LDL
cholesterol
produces a color response much faster than does LDL cholesterol. The
preparation
of reagents for the non-LDL stack or panels is set forth in detail in the
examples
hereinbelow.
The non-LDL cholesterol layer 94 differs from the cholesterol layer
primarily in that layer 94 includes a surfactant which acts on non-LDLs, i.e.,
lipoproteins other than LDL. A commercially available and suitable surfactant
is
sold under the trade name Emulgen B66. Generally, however, it is believed that
many other polyalkylene oxide derivatives having HLB values of between 13 and
1 S
would work suitably as the surfactant. Examples include condensation products
with higher alcohols, condensation products with higher fatty acids,
condensation
products with higher fatty acid amides, condensation products with higher
alkylamines, condensation products with higher alkylmercaptane and
condensation
products with alkyl phenols.
Test Methodology and Theory
By taking the total cholesterol concentration derived from one or the average
of
stacks 92 and 96 and subtracting therefrom the amount of non-LDL cholesterol
measured in stack 94, the amount of LDL cholesterol can be obtained.
1718-0004
CA 02499058 2005-03-15
WO 2004/025265 PCT/US2003/029102
l~
More particularly, the reaction that produces color from non-LDL cholesterol
is significantly faster than the reaction that produces color from LDL
cholesterol,
particularly at the lower room temperatures used by the present invention.
Surprisingly, it has been found that an optoelectric instrument which uses a
pseudo
end-point algorithm, as disclosed in U.S. patent no. 5,597,532 effectively
detects
such endpoint after the non-LDLs in the plasma provided to layer 94 have
reacted,
but before the LDL cholesterol has significantly contributed to color
production.
That is, an end-point can be detected while the reaction producing color from
LDL
cholesterol is ongoing. The "pseudo end-point" is reached when there is only a
small change in color density per time interval, e.g_, five (5) seconds. For
example,
the algorithm can be programmed to reach a pseudo endpoint when change in
reflectance drops to less than 1% over 5 seconds. This pseudo endpoint
chemistry
allows measuring the reflectance and thus non-LDL concentration without timing
the reaction, which is a significant advantage.
With reference to Fig. 6. At time t°, layer 94 becomes wetted with
plasma
and the non-LDLs in the sample begin to produce color quickly as shown by the
curve in Fig. 6. At time tl, (pseudo endpoint shown on the curve), the nonLDLs
have substantially completely reacted to form color, but the LDL cholesterols
have
not. Nonetheless, the reaction of the LDLs after tl is much slower.
Consequently,
the algorithm detects an endpoint at the time the slope flattens as shown at
tl.
The difference in reaction rates of non-LDLs versus LDLs produces a
"pseudo endpoint" sufficient to form a cut-off point for the algorithm, which
is a
significant and surprising advantage. It is significant in that there is no
need to
establish a predetermined time which corresponds to the completion of non-LDL
color production. It is surprising because the liquid phase tests, from which
the dry
phase tests were adapted, required strict control of the time at which the non-
LDL
measurement was taken, which is consistent with the homogeneous prior art
assays
described above that require the first phase to be timed.
Without wishing to be tied to any specific theory, it has been found that the
pseudo endpoint is enhanced by conducting the test at lower temperatures,
viz.,
room temperature, in stark contrast to the prior art teachings of heating the
liquid
reagents to 37°C. Lower temperatures are believed to inhibit the slow
phase (LDL
color production) sufficiently such that the slope of the LDL production curve
shown in Fig. 6 is sufficiently flat. Yet, at the same time, the first phase
of the
1718-0004
CA 02499058 2005-03-15
WO 2004/025265 PCT/US2003/029102
13
reaction, in which the non-LDLs are expended to produce color, is nonetheless
sufficiently fast and ends sufficiently abruptly such that the pseudo endpoint
shown
in Fig. 6 is always detected at room temperature.
On the other hand, if the test strip in accordance with the present invention
were used at elevated temperatures, e.~., 37°C, as taught by prior art
liquid phase
tests, the second stage LDL reaction would take place more quickly, as
reaction
kinetics are typically enhanced by higher temperatures. A faster second phase
is
desirable in the liquid phase tests discussed above because it shortens the
total test
time, which even at 37°C can be quite long. However, with the present
invention, at
elevated temperatures, it has been found that the pseudo endpoint is not as
prominent and can therefore be missed by the algorithm. See dashed line
corresponding to elevated temperatures in Fig. 6. Thus, the elevated
temperatures
taught by prior art liquid phase LDL assays teach away from the present
invention.
Indeed, testing of commercially available prior art homogeneous LDL
measurement
devices has demonstrated that their accuracy is significantly reduced if the
test is
conducted at temperatures even a few degrees lower than 37°C. Further,
the precise
times required to perform the tests in the liquid phase also teach away from
the
invention, whose end-point for non-LDL production can be determined by an
endpoint algorithm, without having to time the reaction In view of the
unacceptable
results produced by the homogeneous LDL tests at temperatures lower than
37°C, it
was quite surprising that the inventive dry phase test strip can be used at
room
temperature and over a range of temperatures.
Quite unlike the prior art liquid phase tests, which teach two separate
measurements at two subsequent times, the present inventive dry phase test
strips
never measure LDL concentration directly. Thus, the length of time required to
complete the second phase of the reaction, in which LDLs react to produce
color, is
not important, whether it be 2 minutes or 20 minutes. Further advantageously,
since
this novel test strip does not require the LDL concentration to be directly
measured,
only a single step of the two phase reaction occurring in test layer 94 is
measured,
thereby completely eliminating one of the two sequential measurements taught
and
indeed required by certain of the prior art homogeneous assays discussed
above.
Another important discovery is that the selectivity of layer 94 for non-LDLs
is dependent upon pH of the solution which impregnates layer 94 to a greater
extent
than in the liquid solutions used in homogeneous liquid phase tests. As
detailed in
1718-0004
CA 02499058 2005-03-15
WO 2004/025265 PCT/US2003/029102
m
the examples hereinbelow, pH of the impregnating solution of layer 94 should
be pH
7. Selectivity for non-LDLs decreases as the pH becomes lower than 7.
Examples
Specific examples embodying the technology described above are set forth
below.
Example 1
Example 1 illustrates the adaptation of the test from the liquid phase and the
reliance on pH.
Spectrophotometric assay of LDL Cholesterol: pH 5 vs. pH 7
Formulations:
Reagent 1 a 100 mM Citric Acid, pH S
0.5% Triton X-100
0.56 mM MAOS
Reagent lb 100 mM Citric Acid, pH 5
0.5% Emulgen B66*
0.56 mM MAOS
Reagent 1 c 100 mM MOPS Buffer, pH 7.0
0.5% Emulgen B66*
0.56 mM MAOS
Reagent 2 100 mM Citric Acid, pH 5
1.5 kU/mL Cholesterol Oxidase
4 kU/mL Peroxidase
4.8 kU/mL Cholesterol Esterase
4 mM 4-Amino antipyrine
*Emulgen B66 is a nonionic surfactant available from I~ao Corporation (1-3,
Bunk 2-Chome; Sumida-Ku; Tokyo 131-8501, Japan). Emulgen B66 has previously
been shown to have a selective action for the reactivity of Non-LDL
Cholesterol
(cited in US; US 6,194,164). Triton X100 is used as a nonselective surfactant
to
give a Total Cholesterol Reaction.
In the assay, 8 ~.L EDTA plasma were added to 750 ~,L of Reagent 1 and
incubated
for 5 minutes at 37°C. The reaction was initiated with 250 p.L Reagent
2 and
scanned on a Bio-Spec1601 spectrophotometer (Shimadzu) for 200 seconds at 630
nm. A 200 mg/dL calibrator was used with Reagent la (pH 5.0/Triton X100) and
Reagent 2 to obtain a factor for the calculation of concentration
171-0004
CA 02499058 2005-03-15
WO 2004/025265 PCT/US2003/029102
m
Total cholesterol was measured with Reagent la (pH 5/Triton X100) and non-LDL
cholesterol was measured separately using either Reagent lb (pH 5/Emulgen B66)
or lc (pH 7.0/Emulgen B66). Measurements were made at various pre-selected
times after initiating the reaction with Reagent 2. The optimum Reaction time
was
determined to be 75 seconds. LDL Cholesterol was calculated as the difference
between Total and Emulgen B66 reactive Cholesterol.
Reference LDL Cholesterol was measured using a commercially available Kit: LDL
Direct Liquid Select Cholesterol Reagent. This kit was run according to the
manufacturer's directions using a Cobas Mira clinical analyzer. This reaction
is
performed in two steps . In the first step sample is mixed with a reagent that
solubilizes only non-LDL Cholesterol. During this step, non-LDL is removed
with a
reaction that does not produce color. Then a second reagent is added to
produce
color with the remaining LDL Cholesterol.
nH 5 Data with 75 Second Measnremc:nt TntPrval
Sample Reference LDL Measured LDL
1 75 _
58
2 _ . 70
127
3 128 85
4 101 68
5 182 111
nH 7 T)ata with 75 .C'Prnnrl MPaa»ramant TntPrcral
Sample Reference LDL Measured LDL
1 75 86
2 127 138
3 128 117
4 101 98
5 182 180
These results show that pH is critical to achieving selectivity for LDL
Cholesterol.
Emulgen B66 gives selectivity for non-LDL at pH 7 but does not give
selectivity at
pH 5. Agreement between Reference and Measured LDL decreased if other
reaction time intervals were used. It was found that at 75 seconds,
substantially all
of the non-LDL Cholesterol had reacted but little or no LDL Cholesterol had
reacted. At longer measurement intervals, the measured values of LDL
Cholesterol
decreased due to the slow but significant reaction of LDL Cholesterol in the
1718-0004
CA 02499058 2005-03-15
WO 2004/025265 PCT/US2003/029102
16
presence of Emulgen B66. Thus, in the spectrophotometric assay, control of
reaction time is critical to the measurement of LDL Cholesterol
Example 2
S
Spectrophotometric Assay of Elevated LDL Samples in Liquid
We extended the observations in Example 1 to additional samples with
elevated LDL Cholesterol. Total Cholesterol was run as above with Reagent 1 a
(pH
5/Triton X100 ) and Reagent 2. Non-LDL was measured with Reagent lc (pH
7/TritonX100) and Reagent 2. Readings were taken 75 seconds after initiating
the
reactions with Reagent 2.
Sample Reference LDL Measured LDL
1 117 111
2 174 187
3 162 ~ 161
4 182 168
Excellent agreement was obtained between the Measured and Reference Total
Cholesterol.
Example 3
LDL Assay with Test Strips: Single Strips for Total and Non-LDL Cholesterol
Formulations for impregnation of reaction membranes were made according
to the Tables below. A Foundation Solution containing a portion of the
ingredients
was made and used in both the Total and Non-LDL formulations.
Test strips were assembled using test strip holders as described in co-pending
provisional patent application serial no. 60/342,790. The layers in the strip
holders
from top to bottom are shown in Figs. 8 and 9 and are spreading mesh 200
(Tetko);
blood separation layer 202; Cytosep 1660 (untreated blank) 204 and Biodyne A
membrane impregnated with the either Total Cholesterol (206) or non-LDL
Cholesterol formulation (208).
Strips were assembled and tested using whole blood (EDTA anticoagulated)
with a Bioscanner 2000 reader, available from Polymer Technology Systems, Inc.
Indianapolis, Indiana. Cholesterol concentrations were calculated using a
curve set
prepared using total cholesterol strips. This curve was applied to readings
from both
1718-0004
CA 02499058 2005-03-15
WO 2004/025265 PCT/US2003/029102
1/
total 206 and non-LDL 208 layers. Photometric readings were made at ten second
intervals over the course of the reaction.
Total cholesterol measurements were made in duplicate and averaged. Non-
LDL Cholesterol measurements were performed with ten replicates and averaged.
LDL Cholesterol was calculated as the difference between the Total and the Non-
LDL Cholesterol.
Total Cholesterol Formulation
Deionized Water 200 g
Triton X-100 0.771
g
Cholesterol Foundation** 532 g
BSA 13.88
g
10% Gantrez AN-139 (w/v) 95.61
g
CHAPS (3-{(3-Cholamidopropyl)dimethylammonio}-1-propane-sulfonate)19.82
g
Sucrose 37.01
g
pH 4.9-5.1
Potassium Ferrocynanide 0.116
g
TOOS 0.37
g
MAOS 4.63
g
Cholesterol Oxidase 148 KU
Peroxidase 462.6
KU
Cholesterol Esterase 92.5
KU
4-Amino antipyrine 4.163
g
Final pH 5.3-5.5
Q.S. to 1000mL with D.I. Water
**Cholesterol Foundation:
D.I. Water 800 g
Sodium Citrate, dehydrate 30 g
PVP K-30 60 g
Benzoic Acid 2 .g
BSA 4 g
EDTA, disodium, dehydrate 1.47 g
pH 5.4-5.6
Q.S. to 1000mL with D.I. Water
Catalase 0.05 KU
1718-0004
CA 02499058 2005-03-15
WO 2004/025265 PCT/US2003/029102
1 tS
Non LDL Cholesterol Formulation:
Lholesterol Foundation** 0.532 g/mL
Emulgen B66 g ~
BSA 1.388
Gantrez AN-139 (w/v) 0.956
Sucrose 3.7
MOPS 25 mM
pH 7.4-7.6
Potassium Ferrocyanide 0.275 mM
MAOS 0.00463 g/mL
Cholesterol Oxidase 0.074 KU/mL
Peroxidase 0.2313 U/mL
Cholesterol Esterase ~ 0.24 KU/mL
4-Amino antipyrine 0.00416 g/mL
Final pH 7.4-7.6
Assay of LDL Cholesterol with Separate Total and Non-LDL Cholesterol Test
, ~t,.;"~
Sample ~ Reference LDL ~ Measured LDL
1 124 115
2 94 102
3 107 115
4 83 109
127 114
6 89 87
7 140 130
8 78 83
111 95
10 112 92
11 49 72
12 91 8g
13 130 142
14 136 113
15 154 127
16 102 g7
17 188 172
From the above date, it can be appreciated that there was excellent agreement
between the Reference and Measured LDL Cholesterol values. To determine the
best time for endpoint of the first stage reaction, LDL cholesterol levels
were taken
at several different times over the course of the reaction. Remarkably, it was
determined that the best results were consistently obtained when both total
and non-
LDL Cholesterol reactions were allowed to reach a pseudo endpoint as
determined
by the Bioscanner software and as explained above. As noted above, this is
very
i7r~-ooo4
CA 02499058 2005-03-15
WO 2004/025265 PCT/US2003/029102
19
surprising since the data with the spectrophotometric assay required strict
control of
read time and temperature.
Example 4
LDL Assay with Test Strips: Two Hole Test Strips for Total and Non-LDL
Cholesterol
We used the same formulation for non-LDL Cholesterol as in Example 3. A
modified Total Cholesterol Formulation was used:
Cholesterol Foundation** 0.532 g/mL
CHAPS 1.982
Emulgen B66 8
BSA 1.388
Gantrez AN-139 (w/v) 0.956
Sucrose 3,7 %
MOPS 25 ~
pH 7.4-7.6
Potassium Ferrocynanide 0.275 mM
MAOS 0.00463 g/mL
Cholesterol Oxidase 0.074 kU/mL
Peroxidase 0.2313 kU/mL
Cholesterol Esterase 0.24 kU/rnL
4-Amino anti-pyrine 0.00416 g/mL
Final nH 7.4-7.6
Strip holders with two reaction zones were used to assemble test strips with
both Total and Non-LDL Cholesterol reaction pads. A cross section of the
layers is
shown in Fig. 10. The layers from top to bottom were a spreading layer 300
consisting of Accuflow PS (Schleicher~zSchuell), blood separation layer 302
and
reaction membranes 304 and 306 composed of Biodyne A impregnated with either
Total Cholesterol Reagent (304) or Non-LDL Cholesterol Reagent (306). The
spreading layer 300 spread the sample evenly and delivered it vertically to
the blood
separation layer 302, which in turn retained red blood cells before delivering
plasma
to reaction layers 304 and 306.
Whole blood samples (EDTA anticoagulated) were tested using the
Bioscanner 2000. Separate standard curves were set for total and non-LDL
cholesterol. Reference non-LDL cholesterol was determined by measuring both
total and LDL cholesterol with automated methods and then subtracting the two
1718-0004
CA 02499058 2005-03-15
WO 2004/025265 PCT/US2003/029102
~U
measured values. LDL cholesterol was calculated with the test strips by
subtracting
the measured non-LDL from the measured total cholesterol.
Assay of LDL Cholesterol with Two Hole Total and Non-LDL Cholesterol Test
Strips
Sample Reference LDL Measured LDL
1 118 123
2 118 118
3 117 117
4 156 145
156 160
As shown above, there was excellent agreement between the LDL
cholesterol values obtained with the two hole test strips and the Reference
LDL
values. This approach greatly simplifies the assay and improves precision
relative to
the separate test strips.
Example 5
LDL Assay with Test Strips: Three Hole Test Strips for Total and Non-LDL
Cholesterol
Strip holders with three reaction zones were used to assemble test strips with
both total and non-LDL Cholesterol reaction pads or stacks as shown in Fig. S.
The
layers from top to bottom were a spreading layer 38 made from Accuflow PS
(Schleicher & Schuell), blood separation layer 40 (as described above with
reference
to Fig. 5) and reaction membranes 92, 94 and 96 made from Biodyne A
impregnated
with either Total Cholesterol Reagent (92 and 96) or Non-LDL Cholesterol
Reagent
(94). The spreading and blood separation layers 38 and 40 covered all three
stacks
92, 94 and 96. Layer 38 spreads the sample evenly and delivered it vertically
downward to the blood separation layer 40, which separated red blood cells.
The
functions of layers 38 and 40 are described in more detail in co-pending
application
serial no. 60/344,300, incorporated herein by reference.
Whole blood samples (EDTA anticoagulated) were tested using the
Cardiochek PA. Separate standard curves were set for total and non-LDL
Cholesterol. Reference non-LDL Cholesterol was determined by measuring both
total and LDL Cholesterol with automated methods and then subtracting the two
measured values. LDL Cholesterol was calculated with the test strips by
subtracting
1718-0004
CA 02499058 2005-03-15
WO 2004/025265 PCT/US2003/029102
21
the measured Non-LDL from the measured Total Cholesterol. Non-LDL
cholesterol reaction membrane 94 was positioned as the center panel or stack
and the
two outer panels 92 and 96 were impregnated with the total cholesterol
solution.
Results were computed with by averaging the two total cholesterol obtained
from
photometrically reading the color production from layers 92 and 96 or by using
a
single total cholesterol value from layer 92 or 96. In either case, only a
single non-
LDL value was obtained from the color production in layer 94.
Assay of LDL Cholesterol with Three Hole Total and Non-LDL Cholesterol Test
~tY7YlC
Sample Reference LDL Measured LDL (Duplicate
TC)
1 118 126
2 118 112
3 117 116
4 156 146
5 156 163
Sample Reference LDL Measured LDL one
TC
1 118 123
2 118 117
3 117 115
. 4 156 144
5 156 165
As shown above, there was excellent agreement between the LDL
cholesterol values obtained with the three hole test strips and the reference
LDL
values. This format holds great promise for improved lipid panel results.
Substitution of one of the Total Cholesterol Reaction stacks with an HDL
cholesterol stack would provide estimates of total, LDL and HDL Cholesterol in
a
single test. An HDL stack suitable for use with the present invention is
taught and
disclosed in co-pending and commonly owned provisional application serial no.
60/344,300.
Alternate Embodiments
Matrix 36' shown in Fig. 11 includes three stacks that are spaced apart and
are adjacent to and in fluid communication with disbursement layer 38. Each
stack
has its own blood separation layer 440 as its top layer. The difference
between
matrix 36' and matrix 36 (Fig. 5) is that matrix 36' has separate blood
separation
1718-0004
CA 02499058 2005-03-15
WO 2004/025265 PCT/US2003/029102
22
layers 440 for each stack. Otherwise, matrix 36' is the same as matrix 36
described
with reference to Fig. 5. The embodiment shown in Fig. 11 is advantageous in
that
layers 440 collectively consume less blood than all of layer 40 (Fig. 5),
which may
help reduce the quantity of blood required to complete the test. Experimental
data
show that either the separate blood separation layers as shown in Fig. 11 or a
single
blood cell separation layer as shown in Fig. 5 produce accurate results.
Fig. 12 illustrates a panel including 4 stacks. One embodiment for this panel
could include a total cholesterol stack, an HDL stack, a non-LDL stack and a
triglycerides stack. The top layer is a spreader layer 536, as described above
with
reference to Fig. S, except slightly longer so as to accommodate four stack.
The first
(total cholesterol), third (non-LDL) and fourth (triglycerides) stacks include
a spacer
layer 502, as taught in co-pending provisional application serial no.
60/344,300.
The spacer layers can be formed from CytoSep 1660 and serve to keep the stacks
even, since layer 512 is needed in the HDL stack to precipitate and separate
non-
HDLs, as also taught in co-pending application serial number 60/344,300. The
HDL
and triglycerides stacks are fully disclosed and taught in co-pending
application
serial number 60/344,300, as is the total cholesterol stack. The non-LDL stack
is
only disclosed in this application.
Advantageously, the four-stack system just described can incorporate an
error checking system by incorporating the Friedewald equation described
above.
For example, an opto-electronic instrument such as Cardio-Chek, available from
Polymer Technology Systems, Inc., can be programmed to calculate the values of
total cholesterol, LDL, HDL and triglycerides in a sample being tested by
strip 536.
Then, using the Friedewald equation, the instrument can calculate the value of
LDL
and compare it to the measured value, the measured value being the measure
total
cholesterol value less the measured non-LDL. If the two values differ by a
predeternuned percentage, an error signal can be produced. For example, the
display on the instrument can instruct the user to repeat the test.
Other calculations are also possible. For example, the value of VLDL
cholesterol plus chylomicrons can be determined by taking the measured non-LDL
value and subtracting therefrom the measured value of HDL.
As noted in co-pending provisional application 60/344,300, additional stacks
for ketones, creatinine and other analytes could also be added to the test
strip.
1718-0004
CA 02499058 2005-03-15
WO 2004/025265 PCT/US2003/029102
23
While a preferred embodiment incorporating the principles of the present
invention has been disclosed hereinabove, the present invention is not limited
to the
disclosed embodiments. Instead, this application is intended to cover any
variations,
uses, or adaptations of the invention using its general principles. Further,
this
application is intended to cover such departures from the present disclosure
as come
within known or customary practice in the art to which this invention pertains
and
which fall within the limits of the appended claims.
1718-0004