Note: Descriptions are shown in the official language in which they were submitted.
CA 02499516 2005-03-07
IMPROVED CARD-TYPE POINT-OF-CARE DIAGNOSTIC DEVICE
FIELD OF THE INVENTION
The present invention relates to the field of diagnostics.
BACKGROUND OF THE INVENTION
In the field of point-of-care drugs-of-abuse diagnostics, two technologies are
widely
utilized.
The first technology is exemplified by the panel sold by Biosite Inc. under
the trade-
mark TRIAGE. This panel includes a covered receptacle which holds chemicals.
The
panel also includes a membrane. In use, the cover is removed, a measured
amount of
urine is transferred by pipette into the receptacle and a period of time is
allowed to
elapse, to permit the urine to be conditioned by the chemicals. After the
allotted time
has elapsed, a measured amount of urine is transferred by pipette from the
receptacle
to the membrane, and a further period of time is allowed to elapse. Several
drops of a
wash buffer are added to the membrane and then a period of time is allowed to
elapse.
The presence of drugs of abuse is indicated by the appearance/disappearance of
markings on the membrane. The results are stable for only a relatively short
period of
time. This test is known to be useful, but suffers from certain drawbacks. Of
note, the
need to perform numerous steps in a timed sequence is problematic. Yet
further, the
relatively long time period required to complete the test is undesirable.
The second technology is embodied by "card" type diagnostic devices. These
devices
include a plurality of membranes. In use, the card is dipped in a specimen jar
of urine,
to bring the membranes in contact with the urine, and a predetermined period
of time is
allowed to elapse. The presence of drugs of abuse is indicated by the
appearance/disappearance of markings on the membranes. The results are stable
for
about 30 minutes. This technology is known to be useful, but also suffers from
certain
-1 -
CA 02499516 2005-03-07
drawbacks. Of note, this technology requires the use of a relatively large
sample in the
urine cup. It is known to avoid the use of a urine cup, and to transfer urine
to the
membranes by pipette. However, this technique is very cumbersome, since the
required sample volume for each membrane needs to be added slowly over several
steps, to make sure there is no sample overflow.
SUMMARY OF THE INVENTION
An improved card-type diagnostics device for chemical screening forms one
aspect of
the invention. The device is of the type having a housing defining one or more
membrane-receiving receptacles, and a membrane for and fitted in each of said
one or
more membrane-receiving receptacles, the membrane being porous to permit the
flow
of liquid therethrough and adapted, when loaded with liquid, to present visual
indicia as
to the presence in said liquid of a chemical associated with the membrane. The
improvement comprises a reservoir defined in the housing and in fluid
communication
with each of said one or more membrane-receiving receptacles such that, when
the
housing is operatively orientated, the deposit of a predetermined volume of
fluid in the
reservoir will result in the flow of liquid from the reservoir to said each
receptacle, to
load the membrane fitted in said each' receptacle.
Preferably, the housing defines, for each receptacle, a fluid channel
extending between
the reservoir and said each receptacle.
Preferably, the housing also defines, for each receptacle, an air vent adapted
to permit
the escape of air from the membrane fitted in said each receptacle as said
membrane is
loaded with liquid.
The invention permits a sample to be tested for the presence of a chemical in
a method
which consumes only a small volume of the sample, which requires only a single
pipette
step, and which provides visual indicia as to the presence of the chemical
which are
stable for a relatively long period of time.
_2_
CA 02499516 2005-03-07
Other advantages, features and characteristics of the present invention, as
well as
methods of operation and functions of the related elements of the structure,
and the
combination of parts and ecanomies of manufacture, will become more apparent
upon
consideration of the following detailed description and the appended claims
with
reference to the accompanying drawings, the latter being briefly described
hereinafter.
BRIEF DESCRIPTION OF THE DRAWINGS
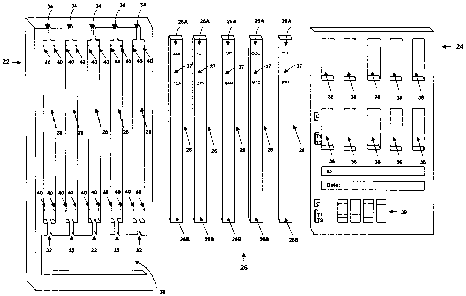
FIGURE 1 is a front, left side perspective view of a diagnostics device
according to the
preferred embodiment of the present invention;
FIGURE 2 is an exploded perspective view of the structure of FIGURE 1;
FIGURE 3 is a front plan view of a portion of the structure of FIGURE 1; and
FIGURE 4 is a front plan view of another portion of the structure of FIGURE 1.
DETAILED DESCRIPTION
With general reference to FIGURE 1, a first preferred embodiment of the
present invention, a diagnostics device, is illustrated, and is designated by
the general
reference numeral 20.
As best seen in the exploded view of FIGURE 2, the device includes a housing
22,24
and a plurality of membranes 26.
The housing 22,24 includes a back portion 22 and a front portion 24.
The back portion 22 defines one or more, more specifically, five, membrane-
receiving
receptacles 28. The back portion 22 further defines a reservoir 30, a
plurality of fluid
-3-
CA 02499516 2005-03-07
channels 32 and a plurality of air vents 34. The fluid channels 32 are
provided one for
each receptacle 28, each extending between the reservoir 30 and the receptacle
28 for
which it is provided, such that the reservoir 30 is in fluid communication,
via the fluid
channels 32, with the receptacles 28. The air vents 34 extend from the
receptacles 28
to the periphery of the housing 22,24. Back portion 22 is preferably injection-
moulded
out of plastic.
The front portion 24 defines a plurality of first viewing ports 36 and a
plurality of second
viewing ports 38 and is preferably constructed out a plastic stamping, the
stamping
having an adhesive on its rear surface (not shown) which is covered with a
release film
(not shown).
The membranes 26 are provided one for each receptacle 28. Each membrane 26
includes an upper portion 26A and a lower portion 26B. The upper portion 26A
of each
membrane includes a notation 37 as to the types) of drug or other chemical
with which
it is associated. The lower portion 26B is of a conventional construction,
more
specifically, is a porous material adapted to permit the flow of liquid
therethrough, and is
adapted, when loaded with liquid, to present visual indicia as to the presence
in said
liquid of the chemicals) associated with the membrane 26. The membranes 26
shown
are each adapted to detect the presence of two drugs of abuse, specifically,
AMP/TCA,
THC/OXY, OPI/BAR, COC/MTD and PCP/PPX.
To produce the device shown in FIGURE 1, each membrane 26 is fitted into a
respective receptacle 28, with the upper portion 26A disposed towards the air
vent 34
and the lower portion 26B disposed towards the reservoir 30. Notches 40 are
provided
in the receptacles 28 to hold the membranes 26 in position in this step. As
well, the
release paper is peeled from the front portion 24, to expose the adhesive.
Thereafter,
the front portion 24 is stacked upon and adhesively secured to the back
portion 22, with
each membrane-receiving receptacle 28 in communication with a respective first
viewing port 36 and a respective second viewing port 38, such that the indicia
37 on the
membrane 26 is visible through the second viewing port 38.
-4-
CA 02499516 2005-03-07
In use, the housing 22,24 is operatively orientated with the front portion 24
presenting
upwardly and the back portion 22 presenting downwardly. Thereafter, a
predetermined
volume of fluid is deposited in the reservoir 30 by pipette or the like. This
liquid flows
from the reservoir 30 to said each receptacle 28, to load the membrane 26
fitted in said
each receptacle 28. The air vents 34 permit the escape of air from the
membrane 26
fitted in said each receptacle 28 as said membrane 26 is loaded with liquid.
After a
predetermined period of time has elapsed, visual indicia appear on each of the
membranes 26 in a conventional manner, to indicate the presence of the drug or
other
chemicals) with which said each membrane 26 is associated. The indicia (not
shown)
are visible through the first viewing port 36. A table 39 is provided on the
front portion
24, to assist the user in reading the results. The device 20 can also be used
in the
manner of a conventional card-type device, that is, dipped into a specimen
jar, if
desired. This may be advantageous if the use of a large-volume specimen is not
problematic.
Persons of ordinary skill will immediately recognize the advantage obtained by
the
device. The device permits a sample to be tested for the presence of a
chemical in a
method which consumes only a small volume of the sample, which requires only a
single pipette step, and which provides visual indicia as to the presence of
the chemical
which are stable for a relatively long period of time. Another advantage
associated with
the device is the ease by which it can be customized to meet the needs of
various
users: in the assembly step, membranes adapted to test for the presence of any
desired compounds can be inserted into the receptacles to meet the wishes of
the user.
Whereas but a single preferred embodiment is illustrated herein, it will be
understood
that various changes can be made. For example, whereas the device of the
preferred
embodiment holds five membranes, the housing could readily be adapted to hold
other
numbers of membranes, for example, two membranes. Also, whereas the membranes
illustrated are of the type adapted to test for two drugs, this need not be
the case;
membranes adapted to test for less or more than two drugs could readily be
substituted.
-5-
CA 02499516 2005-03-07
Yet further, whereas the membranes shown are adapted to test for various
specific
drugs, namely, AMP/TCA, THC/OXY, OPI/BAR, COCIMTD and PCP/PPX, membranes
adapted for testing other possible combinations of these drugs and other drugs
can
readily be substituted therefar. Various changes in size and shape could also
be made
without departing from the spirit or scope of the invention. Accordingly, it
should be
understood that the scope of the invention is limited only by the claims
appended
hereto, purposively construed.
-6-