Note: Descriptions are shown in the official language in which they were submitted.
CA 02503388 2010-09-22
IMPROVED ENDOPROSTHESES AND METHODS OF MANUFACTURE
RELATED APPLICATIONS
This application is claims the benefit of Provisional U.S. Patent Application
Serial
No. 60/426,737, filed November 15, 2002, and U.S. Patent Application Serial
No.
10/342,622, filed January 15, 2003, now issued as U.S. Patent No. 6,877,266.
FIELD OF THE INVENTION
The invention herein relates generally to medical devices and the manufacture
thereof,
and more particularly to improved endoprostheses for use in the treatment of
strictures in
lumens of the body.
BACKGROUND OF THE INVENTION
Ischemic heart disease is the major cause of death in industrialized
countries.
Ischemic heart disease, which often results in myocardial infarction, is a
consequence of
coronary atherosclerosis. Atherosclerosis is a complex chronic inflammatory
disease and
involves focal accumulation of lipids and inflammatory cells, smooth muscle
cell
proliferation and migration, and the synthesis of extracellular matrix. Nature
1993;362:801-
809. These complex cellular processes result in the formation of atheromatous
plaque, which
consists of a lipid-rich core covered with a collagen-rich fibrous cap,
varying widely in
thickness. Further, plaque disruption is associated with varying degrees of
internal
hemorrhage and luminal thrombosis because the lipid core and exposed collagen
are
thrombogenic. JAm Coll Cardiol. 1994;23:1562-1569 Acute coronary syndrome
usually
occurs as a consequence of such disruption or ulceration of a so called
"vulnerable plaque".
Arterioscler Thromb Vasc Biol. Volume 22, No. 6, June 2002, p. 1002.
In addition to coronary bypass surgery, a current treatment strategy to
alleviate
vascular occlusion includes percutaneous transluminal coronary angioplasty,
expanding the
internal lumen of the coronary artery with a balloon. Roughly 800,000
angioplasty
procedures are performed in the U.S. each year (Arteriosclerosis, Thrombosis,
and Vascular
Biology Volume 22, No. 6, June 2002, p. 884). However, 30% to 50% of
angioplasty
CA 02503388 2005-04-21
WO 2004/045450 PCT/US2003/035951
angioplasty patients soon develop significant restenosis, a narrowing of the
artery through
migration and growth of smooth muscle cells.
In response to the significant restenosis rate following angioplasty,
percutaneously
placed endoprostheses have been extensively developed to maintain fluid flow
through a
diseased coronary artery.. Such endoprostheses, or stents, which have been
traditionally
fabricated using metal alloys, include self-expanding or balloon-expanded
devices that are
"tracked" through the vasculature and deployed proximate one or more lesions.
Stents
considerably enhance the long-term benefits of angioplasty, but 10% to 50% of
patients
receiving stents still develop restenosis. (JAm Coll Cardiol. 2002; 39:183-
193.
Consequently, a significant portion of the relevant patient population
undergoes continued
monitoring and, in many cases, additional treatment.
Continued improvements in stent technology aim at producing easily tracked,
easily
visualized and readily deployed stents, which exhibit the requisite radial
strength without
sacrificing a small delivery profile and sufficient flexibility to traverse
the diseased human
vasculature. Further, numerous therapies directed to the cellular mechanisms
of
accumulation of inflammatory cells, smooth muscle cell proliferation and
migration show
tremendous promise for the successful long-term treatment of ischemic heart
disease.
Consequently, advances in coupling delivery of such therapies to the
mechanical support of
vascular endoprostheses, delivered proximate the site of disease, offer great
hope to the
numerous individuals suffering heart disease.
While advances in the understanding of ischemic heart disease as a complex
chronic
inflammatory process take place, traditional diagnostic techniques such as
coronary
angiography yield to next generation imaging modalities. In fact, coronary
angiography may
not be at all useful in identifying inflamed atherosclerotic plaques that are
prone to
producing clinical events. Imaging based upon temperature differences, for
example, are
undergoing examination for use in detecting coronary disease. Magnetic
resonance imaging
(MRI) is currently emerging as the state of the art diagnostic arterial
imaging, enhancing the
detection, diagnosis and monitoring of the formation of vulnerable plaques.
Transluminal
intervention guided by MRI is expected to follow. However, metals produce
distortion and
artifacts in MR images, rendering use of the traditionally metallic stents in
coronary, biliary,
esophageal, ureteral, and other body lumens incompatible with the use of MRI.
Consequently, an emerging clinical need for interventional devices that are
compatible with and complementary to new imaging modalities is evident.
Further, devices
2
CA 02503388 2005-04-21
WO 2004/045450 PCT/US2003/035951
that exhibit improved trackability to previously undetectable disease within
remote regions
of the body, especially the coronary vasculature are needed. And finally,
devices that both
exhibit improved mechanical support and are readily compatible with adjunct
therapies in
order to lower or eliminate the incidence of restenosis are needed.
SUMMARY OF THE INVENTION
Improved endoprostheses and methods of manufacture are herein provided. An
endoprosthesis according to the invention may comprise a woven or braided,
substantially
tubular structure, wherein said endoprosthesis further comprises a delivery
configuration
and a deployed configuration. Said endoprosthesis comprises one or more means
for
maintaining the endoprosthesis in the deployed configuration. The
endoprosthesis may
further be comprised of erodible materials that are compatible with magnetic
resonance
imaging. An endoprosthesis according to the invention may comprise a
therapeutic agent or
a coating that comprises a therapeutic agent.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a plan view of the distal end of a conventional balloon catheter
having a
stent according to the invention mounted thereon.
FIG. 2 shows the embodiment of FIG. 1 in its deployed configuration.
FIG. 3 illustrates detail area A of Fig. 2.
FIGS. 4A shows an example of fibers that may be employed to fabricate the
embodiment of FIGS. 1-3.
FIG. 5 is a plan view of the distal end of a conventional delivery catheter
having an
alternative embodiment according to the invention mounted thereon.
FIG. 6 shows the embodiment of FIG. 5 in its deployed configuration.
FIG. 7 illustrates detail area B of Fig. 6.
FIG. 8A and 8B show examples of fibers that may be employed to fabricate an
embodiment according to the invention.
FIG. 9 depicts another embodiment according to the invention in its deployed
configuration.
FIG. 10 shows detail area C of FIG. 9.
3
CA 02503388 2005-04-21
WO 2004/045450 PCT/US2003/035951
FIGS. IIA and 11B illustrate a component of an alternative embodiment
according
to the invention.
FIG. 12A is a plan view of yet another embodiment according to the invention
in its
deployed configuration.
FIG. 12B shows an end view of the embodiment illustrated in FIG. 12A.
FIG. 13A is a plan view of an alternative embodiment according to the
invention.
FIG. 13B is an end view of the embodiment of FIG. 13A.
FIGS. 14A-14B illustrate a plan view of an embodiment according to the
invention.
FIGS. 14C-14E illustrate alternative embodiments of the locking regions of
endoprostheses according to the invention.
FIG. 15 is a plan view of yet another embodiment according to the invention in
its
expanded configuration.
DETAILED DESCRIPTION OF THE ENVENTION
Although the invention herein is not limited as such, some embodiments of the
invention comprise materials that are erodible. "Erodible" refers to the
ability of a material
to maintain its structural integrity for a desired period of time, and
thereafter gradually
undergo any of numerous processes whereby the material substantially loses
tensile strength
and mass. Examples of such processes comprise hydrolysis, enzymatic and non-
enzymatic
degradation, oxidation, enzymatically-assisted oxidation, and others, thus
including
bioresorption, dissolution, and mechanical degradation upon interaction with a
physiological
environment into components that the patient's tissue can absorb, metabolize,
respire, and/or
excrete. Polymer chains are cleaved by hydrolysis and are eliminated from the
body
through the Krebs cycle, primarily as carbon dioxide and in urine. "Erodible"
and
"degradable" are intended to be used interchangeably herein.
The term "endoprosthesis" refers to any prosthetic device placed within a body
lumen or duct to in order to therapeutically treat the body lumen or duct,
including but
limited to the objective of restoring or enhancing flow of fluids through a
body lumen or
duct.
A "self-expanding" endoprosthesis has the ability to revert readily from a
reduced
profile configuration to a larger profile configuration in the absence of a
restraint upon the
device that maintains the device in the reduced profile configuration.
4
CA 02503388 2005-04-21
WO 2004/045450 PCT/US2003/035951
"Balloon expandable" refers to a device that comprises a reduced profile
configuration and an expanded profile configuration, and undergoes a
transition from the
reduced configuration to the expanded configuration via the outward radial
force of a
balloon expanded by any suitable inflation medium.
The term "balloon assisted" refers to a self-expanding device the final
deployment of
which is facilitated by an expanded balloon.
The term "fiber" refers to any generally elongate member fabricated from any
suitable material, whether polymeric, metal or metal alloy, natural or
synthetic.
The phrase "points of intersection", when used in relation to fiber(s), refers
to any
point at which a portion of a fiber or two or more fibers cross, overlap,
wrap, pass
tangentially, pass through one another, or come near to or in actual contact
with one another.
As used herein, a device is "implanted" if it is placed within the body to
remain for
any length of time following the conclusion of the procedure to place the
device within the
body.
As used herein, the term "braid" refers to any braid or mesh or similar woven
structure produced from between 1 and several hundred longitudinal and/or
transverse
elongate elements woven, braided, knitted, helically wound, or intertwined any
manner, at
angles between 0 and 180 degrees and usually between 45 and 105 degrees,
depending upon
the overall geometry and dimensions desired.
Unless specified, suitable means of attachment may include by thermal melt
bond,
chemical bond, adhesive, sintering, welding, or any means known in the art.
"Shape memory" refers to the ability of a material to undergo structural phase
transformation such that the material may define a first configuration under
particular
physical and/or chemical conditions, and to revert to an alternate
configuration upon a
change in those conditions. Shape memory materials may be metal alloys
including but not
limited to nickel titanium, or may be polymeric. A polymer is a shape memory
polymer if
the original shape of the polymer is recovered by heating it above a shape
recovering
temperature (defined as the transition temperature of a soft segment) even if
the original
molded shape of the polymer is destroyed mechanically at a lower temperature
than the
shape recovering temperature, or if the memorized shape is recoverable by
application of
another stimulus. Such other stimulus may include but is not limited to pH,
salinity,
hydration, and others. Some embodiments according to the invention may
comprise one or
more polymers having a structure that assumes a first configuration, a second
configuration,
5
CA 02503388 2005-04-21
WO 2004/045450 PCT/US2003/035951
and a hydrophilic polymer of sufficient rigidity coated upon at least a
portion of the
structure when the device is in the second configuration. Upon placement of
the device in
an aqueous environment and consequent hydration of the hydrophilic polymer,
the polymer
structure reverts to the first configuration.
As used herein, the term "segment" refers to a block or sequence of polymer
forming
part of the shape memory polymer. The terms hard segment and soft segment are
relative
terms, relating to the transition temperature of the segments. Generally
speaking, hard
segments have a higher glass transition temperature than soft segments, but
there are
exceptions. Natural polymer segments or polymers include but are not limited
to proteins
such as casein, gelatin, gluten, zein, modified zein, serum albumin, and
collagen, and
polysaccharides such as alginate, chitin, celluloses, dextrans, pullulane, and
polyhyaluronic
acid; poly(3-hydroxyalkanoate)s, especially poly(.beta.-hydroxybutyrate),
poly(3-
hydroxyoctanoate) and poly(3-hydroxyfatty acids).
Representative natural erodible polymer segments or polymers include
polysaccharides such as alginate, dextran, cellulose, collagen, and chemical
derivatives
thereof (substitutions, additions of chemical groups, for example, alkyl,
alkylene,
hydroxylations, oxidations, and other modifications routinely made by those
skilled in the
art), and proteins such as albumin, zein and copolymers and blends thereof,
alone or in
combination with synthetic polymers.
Suitable synthetic polymer blocks include polyphosphazenes, poly(vinyl
alcohols),
polyamides, polyester amides, poly(amino acid)s, synthetic poly(amino acids),
polyanhydrides, polycarbonates, polyacrylates, polyalkylenes, polyacrylamides,
polyalkylene glycols, polyalkylene oxides, polyalkylene terephthalates,
polyortho esters,
polyvinyl ethers, polyvinyl esters, polyvinyl halides, polyvinylpyrrolidone,
polyesters,
polylactides, polyglycolides, polysiloxanes, polyurethanes and copolymers
thereof.
Examples of suitable polyacrylates include poly(methyl methacrylate),
poly(ethyl
methacrylate), poly(butyl methacrylate), poly(isobutyl methacrylate),
poly(hexyl
methacrylate), poly(isodecyl methacrylate), poly(lauryl methacrylate),
poly(phenyl
methacrylate), poly(methyl acrylate), poly(isopropyl acrylate), poly(isobutyl
acrylate) and
poly(octadecyl acrylate).
Synthetically modified natural polymers include cellulose derivatives such as
alkyl
celluloses, hydroxyalkyl celluloses, cellulose ethers, cellulose esters,
nitrocelluloses, and
chitosan. Examples of suitable cellulose derivatives include methyl cellulose,
ethyl
6
CA 02503388 2005-04-21
WO 2004/045450 PCT/US2003/035951
cellulose, hydroxypropyl cellulose, hydroxypropyl methyl cellulose,
hydroxybutyl methyl
cellulose, cellulose acetate, cellulose propionate, cellulose acetate
butyrate, cellulose acetate
phthalate, arboxymethyl cellulose, cellulose triacetate and cellulose sulfate
sodium salt.
These are collectively referred to herein as "celluloses".
Examples of synthetic degradable polymer segments or polymers include
polyhydroxy acids, such as polylactides, polyglycolides and copolymers
thereof;
poly(ethylene terephthalate); poly(hydroxybutyric acid); poly(hydroxyvaleric
acid);
poly[lactide-co-(.epsilon.-caprolactone)]; poly[glycolide-co-(.epsilon.-
caprolactone)];
polycarbonates, poly(pseudo amino acids); poly(amino acids);
poly(hydroxyalkanoate)s;
polyanhydrides; polyortho esters; and blends and copolymers thereof.
For those embodiments comprising a shape memory polymer, the degree of
crystallinity of the polymer or polymeric block(s) is between 3 and 80%, more
often
between 3 and 65%. The tensile modulus of the polymers below the transition
temperature
is typically between 50 MPa and 2 GPa (gigapascals), whereas the tensile
modulus of the
polymers above the transition temperature is typically between 1 and 500 MPa.
Most often,
the ratio of elastic modulus above and below the transition temperature is 20
or more.
The melting point and glass transition temperature of the hard segment are
generally
at least 10 degrees C., and preferably 20 degrees C., higher than the
transition temperature
of the soft segment. The transition temperature of the hard segment is
preferably between -
60 and 270 degrees C., and more often between 30 and 150 degrees C. The ratio
by weight
of the hard segment to soft segments is between about 5:95 and 95:5, and most
often
between 20:80 and 80:20. The shape memory polymers contain at least one
physical
crosslink (physical interaction of the hard segment) or contain covalent
crosslinks instead of
a hard segment. The shape memory polymers can also be interpenetrating
networks or semi-
interpenetrating networks.
Rapidly erodible polymers such as poly(lactide-co-glycolide)s, polyanhydrides,
and
polyorthoesters, which have carboxylic groups exposed on the external surface
as the
smooth surface of the polymer erodes, can also be used. In addition, polymers
containing
labile bonds, such as polyanhydrides and polyesters, are well known for their
hydrolytic
reactivity. Their hydrolytic degradation rates can generally be altered by
simple changes in
the polymer backbone and their sequence structure.
Examples of suitable hydrophilic polymers include but are not limited to
poly(ethylene oxide), polyvinyl pyrrolidone, polyvinyl alcohol, poly(ethylene
glycol),
7
CA 02503388 2010-09-22
polyacrylamide poly(hydroxy alkyl methacrylates), poly(hydroxy ethyl
methacrylate),
hydrophilic polyurethanes, HYPANTM, oriented HYPANTM, poly(hydroxy ethyl
acrylate),
hydroxy ethyl cellulose, hydroxy propyl cellulose, methoxylated pectin gels,
agar, starches,
modified starches, alginates, hydroxy ethyl carbohydrates and mixtures and
copolymers
thereof.
Hydrogels can be formed from polyethylene glycol, polyethylene oxide,
polyvinyl
alcohol, polyvinyl pyrrolidone, polyacrylates, poly (ethylene terephthalate),
poly(vinyl
acetate), and copolymers and blends thereof. Several polymeric segments, for
example,
acrylic acid, are elastomeric only when the polymer is hydrated and hydrogels
are formed.
Other polymeric segments, for example, methacrylic acid, are crystalline and
capable of
melting even when the polymers are not hydrated. Either type of polymeric
block can be
used, depending on the desired application and conditions of use.
Curable materials include any material capable of being able to transform from
a
fluent or soft material to a harder material, by cross-linking,
polymerization, or other suitable
process. Materials may be cured over time, thermally, chemically, or by
exposure to
radiation. For those materials that are cured by exposure to radiation, many
types of radiation
may be used, depending upon the material. Wavelengths in the spectral range of
about 100-
1300 nm may be used. The material should absorb light within a wavelength
range that is not
readily absorbed by tissue, blood elements, physiological fluids, or water.
Ultraviolet
radiation having a wavelength ranging from about 100-400 nm may be used, as
well as
visible, infrared and thermal radiation. The following materials are examples
of curable
materials: urethanes, polyurethane oligomer mixtures, acrylate monomers,
aliphatic urethane
acrylate oligomers, acrylamides, UV curable epoxies, photopolymerized
polyanhydrides and
other UV curable monomers and polymers. Alternatively, the curable material
can be a
material capable of being chemically cured, such as silicone based compounds
which
undergo room temperature vulcanization.
Some embodiments according to the invention comprise materials that are cured
in a
desired pattern. Such materials may be cured by any of the foregoing means.
Further, for
those materials that are photocurable, such a pattern may be created by
coating the material in
a negative image of the desired pattern with a masking material using standard
photoresist
technology. Absorption of both direct and incident radiation is thereby
prevented in the
masked regions, curing the device in the desired pattern. A variety of
biocompatibly eroding
coating materials may be used for such "masking", including but not limited to
8
CA 02503388 2005-04-21
WO 2004/045450 PCT/US2003/035951
gold, magnesium, aluminum, silver, copper, platinum, inconel, chrome, titanium
indium,
indium tin oxide. Projection optical photolithography systems that utilize the
vacuum
ultraviolet wavelengths of light below 240 nm provide benefits in terms of
achieving
smaller feature dimensions. Such systems that utilize ultraviolet wavelengths
in the 193 nm
region or 157 nm wavelength region have the potential of improving precision
masking
devices having smaller feature sizes.
Though not limited thereto, some embodiments according to the invention have
been
surface treated to comprise one or more therapeutic substances that will elute
from the
structure of prosthesis independently or as the material comprising the stent
erodes.
Alternatively, therapeutic substances may be incorporated into the materials
that comprise
the endoprosthesis. According to the invention, such surface treatment and/or
incorporation
of therapeutic substances may be performed utilizing one or more of numerous
processes
that utilize carbon dioxide fluid, e.g., carbon dioxide in a liquid or
supercritical state.
A supercritical fluid is a substance above its critical temperature and
critical pressure
(or "critical point"). Compressing a gas normally causes a phase separation
and the
appearance of a separate liquid phase. However, all gases have a critical
temperature above
which the gas cannot be liquefied by increasing pressure, and a critical
pressure or pressure
which is necessary to liquefy the gas at the critical temperature. For
example, carbon
dioxide in its supercritical state exists as a form of matter in which its
liquid and gaseous
states are indistinguishable from one another. For carbon dioxide, the
critical temperature is
about 31 degrees C (88 degrees D) and the critical pressure is about 73
atmospheres or about
1070 psi.
The term "supercritical carbon dioxide" as used herein refers to carbon
dioxide at a
temperature greater than about 31 degrees C and a pressure greater than about
1070 psi.
Liquid carbon dioxide may be obtained at temperatures of from about -15
degrees C to
about -55 degrees C and pressures of from about 77 psi to about 335 psi. One
or more
solvents and blends thereof may optionally be included in the carbon dioxide.
Illustrative
solvents include, but are not limited to, tetraflouroisopropanol, chloroform,
tetrahydrofuran,
cyclohexane, and methylene chloride. Such solvents are typically included in
an amount, by
weight, of up to about 20%.
In general, carbon dioxide may be used to effectively lower the glass
transition
temperature of a polymeric material to facilitate the infusion of
pharmacological agent(s)
into the polymeric material. Such agents include but are not limited to
hydrophobic agents,
9
CA 02503388 2005-04-21
WO 2004/045450 PCT/US2003/035951
hydrophilic agents and agents in particulate form. For example, following
fabrication, an
endoprosthesis and a hydrophobic pharmacological agent may be immersed in
supercritical
carbon dioxide. The supercritical carbon dioxide "plasticizes" the polymeric
material, that
is, it allows the polymeric material to soften at a lower temperature, and
facilitates the
infusion of the pharmacological agent into the polymeric endoprosthesis or
polymeric
coating of a stent at a temperature that is less likely to alter and/or damage
the
pharmacological agent.
As an additional example, an endoprosthesis and a hydrophilic pharmacological
agent can be immersed in water with an overlying carbon dioxide "blanket". The
hydrophilic pharmacological agent enters solution in the water, and the carbon
dioxide
"plasticizes" the polymeric material, as described above, and thereby
facilitates the infusion
of the pharmacological agent into a polymeric endoprosthesis or a polymeric
coating of an
endoprosthesis.
As yet another example, carbon dioxide may be used to "tackify", or render
more
adherent a polymeric endoprosthesis or a polymeric coating on an
endoprosthesis to
facilitate the application of a pharmacological agent thereto in a dry,
micronized form. A
membrane- forming polymer, selected for its ability to allow the diffusion of
the
pharmacological agent therethrough, may then be applied in a layer over the
endoprosthesis.
Following curing by suitable means, a membrane that permits diffusion of the
pharmacological agent over a predetermined time period forms.
In alternative embodiments of the present invention, at least one monomer or
comonomer can be solubilized in carbon dioxide and copolymerized with a
fluoromonomer.
Any suitable monomers or comonomers can be employed, including, but not
limited to,
acrylate, methacrylate, acrylamide, methacrylamide, styrenics, ethylene, and
vinyl ether
monomers. The copolymerization of the present invention may be carried out
under
temperature and pressure conditions similar to those given above.
Objectives of therapeutics substances incorporated into materials forming or
coating
an endoprosthesis according to the invention include reducing the adhesion and
aggregation
of platelets at the site of arterial injury, block the expression of growth
factors and their
receptors; develop competitive antagonists of growth factors, interfere with
the receptor
signaling in the responsive cell, promote an inhibitor of smooth muscle
proliferation.
Anitplatelets, anticoagulants, antineoplastics, antifibrins, enzymes and
enzyme inhibitors,
CA 02503388 2005-04-21
WO 2004/045450 PCT/US2003/035951
antimitotics, antimetabolites, anti-inflammatories, antithrombins,
antiproliferatives,
antibiotics, and others may be suitable.
Details of the invention can be better understood from the following
descriptions of
specific embodiments according to the invention. As an example, in FIG. 1,
distal end 3 of
standard delivery catheter 1 is shown, bearing endoprosthesis 10. Although an
endoprosthesis according to the invention may be self-expanding,
endoprosthesis 10
mounted on distal end 3 is balloon-expandable. Accordingly, endoprosthesis 10
is deployed
via delivery catheter 1, which comprises balloon 5 at distal end 3.
Endoprosthesis 10, which
may be fabricated from any of the foregoing conventional or shape memory
materials
including metal alloys, polymers, or other suitable materials selected for
molecular weight,
chemical composition and other properties, manufactured to achieve any desired
geometries
and processed according to any of the foregoing descriptions, is "crimped"
down upon
balloon 5 into its low-profile delivery configuration. Endoprosthesis 10 can
then be tracked
to a lesion site within a lumen of the body where endoprosthesis 10 can be
deployed, In
order to deploy endoprosthesis 10, balloon 5 is inflated via inflation medium
through
catheter 1. The outward radial force of expanding balloon 5 expands
endoprosthesis 10 to
its deployed configuration.
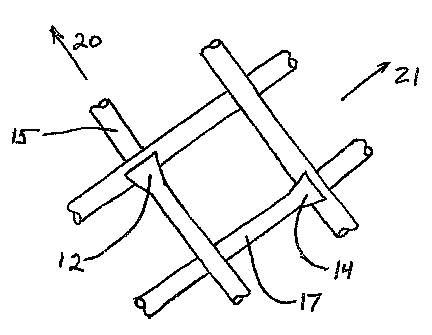
FIG. 2 illustrates endoprosthesis 10 in its deployed configuration, following
removal
of catheter 1. Accordingly, endoprosthesis 10 is at its deployed diameter,
which may be
between 0.5 mm and 4.0 mm, depending upon the size of the vessel of the
patient (not
pictured). Endoprosthesis 10 comprises between one and fifty fibers 15 and 17,
which may
be homogenous or composite, fabricated from one or more different materials.
Endoprosthesis 10 may alternatively comprise additional fibers. Fibers 15 and
17 are
braided in any suitable manner as discussed above to intersect one another at
one or more
points and to form a generally tubular structure.
Locking elements 12 protrude from fibers 15 in a first direction 20 at an
angle
between 1 and 90 degrees, and most suitably at an angle between 10 and 45
degrees.
Locking elements 12 are spaced apart from one another at a distance of between
1.0 mm and
5.0 mm, and most often at a distance of 1.0 mm and 3.0 mm, and can operate
singly, in
pairs, or in groups. Similarly, locking elements 14 protrude from fibers 17 in
a second
direction 21, perpendicular to first direction 20, and are spaced apart from
one another at a
distance corresponding to the desired dimensions of stent 10. Locking elements
12 are
oriented such that when endoprosthesis 10 is undergoing expansion, fibers 15
pass over
11
CA 02503388 2005-04-21
WO 2004/045450 PCT/US2003/035951
locking elements 12 in a first direction 20 until endoprosthesis 10 is
expanded to a desired
diameter. Similarly, fibers 17 pass over locking elements 14 in a second
direction 21, until
stent 10 is expanded to a desired diameter. Fibers 15 and 17 cannot pass over
locking
elements 14 and 17 in a reverse direction. Consequently, when stent 10 has
reached a
desired diameter, locking elements 12 and 14 engage fibers 15 and 17
respectively where
fibers 15 and 17 intersect one another. Locking elements 12 and 14 thereafter
prevent fibers
and 17 from sliding past one another, thereby maintaining the position of
fibers 15 and
17 with respect to one another. Consequently, endoprosthesis 10 is prevented
from
returning to a smaller diameter, thereby enabling endoprosthesis 10 to exert a
continual
10 outward radial force upon the walls of the vessel or duct of a patient in
order to enhance or
restore the flow of fluids therethrough.
FIG. 3 depicts endoprosthesis 10 of FIG. 2 in greater detail at area A.
Although
alternative arrangements are possible, pairs of locking elements 12 protrude
from fibers 15
in a first direction as depicted by arrow 20, engaging fibers 17 where they
intersect with
15 fibers 15 and exerting a force in direction of arrow 20. Similarly,
although they may also,
for example, alternatively act solely, pairs of locking elements 14 protrude
from fibers 17 in
a second direction 21. Locking elements 14 exert a force on fibers 17 in a
second direction
21, perpendicular to direction 20, as depicted by arrow 21. The positions of
fibers 15 and 17
with respect to one another are thereby maintained, and endoprosthesis 10 is
able to
maintain its treatment diameter and exert an outward radial force upon the
walls of the
narrowed vessel, in order that fluid flow through the lumen is enhanced or
restored.
As shown in FIG. 4A, fiber 15 comprises one or more locking elements 12, which
may be arranged solely, in pairs, or in any number of alternative suitable
arrangements.
Locking elements 12 may be affixed to fiber 15 in any number of suitable
manners known
in the art including but not limited to affixing by adhesives, welding, melt
attaching, or
others, or may be bump coextruded with fiber 15. Locking elements 12 may be
fabricated
of the same material as fiber 15, or may be chosen from a group of materials
that exhibits
greater rigidity than that of fiber 15. Endoprosthesis 10 may alternatively
further comprise
one or more therapeutic agents for elution in situ.
Turning now to FIG. 5, another embodiment according to the invention is
disclosed.
Distal end 33 of exemplary delivery catheter 31 is shown. Although an
endoprosthesis
according to the invention may in the alternative be balloon expandable,
endoprosthesis 30
mounted on distal end 33 is self-expanding. Accordingly, endoprosthesis 30 is
crimped
12
CA 02503388 2005-04-21
WO 2004/045450 PCT/US2003/035951
down to its low-profile delivery configuration for tracking through the
patient's vasculature,
and maintained in the low-profile configuration via sheath 34. When distal end
33 is
positioned proximate a lesion to be treated (not shown), sheath 34 is
withdrawn, allowing
endoprosthesis 30 to returned to its larger diameter, deployed configuration.
Endoprosthesis
30 may be fabricated from any number of suitable shape memory materials,
including
polymeric materials and metal alloys discussed above, chosen for desired
chemical
properties, molecular weight, and other characteristics, and processed to
achieve
sterilization, desired geometries and in vivo lifetime.
FIG. 6 depicts endoprosthesis 30 in its deployed configuration. Similar to the
embodiment discussed in relation to FIGS 1-4 above, endoprosthesis 30 is
braided to form a
generally tubular structure with fibers 35 and 37 intersecting at one or more
points of
intersection 39. Fibers 35 and 37, which may be homogenous or composite, and
may be
fabricated of the same or different materials, intersect one another at angles
of between 25
and 105 degrees. When endoprosthesis 30 has expanded to its desired deployment
diameter,
fibers 35 and 37 "nest" with one another at points of intersection 39, thereby
locking
endoprosthesis 30 in its deployed configuration. Fibers 35 and 37 are
permitted to nest with
one another via notches 40, which are between 0.25 mm and 1.0 mm, spaced apart
from one
another at a distance of between 1.0 mm and 5.0 mm, depending upon the desired
deployment dimensions.
Notches 40 can be better seen in FIGS. 7 and 8. For example, FIG. 7 depicts a
portion of endoprosthesis 30 in greater detail at area B, illustrating fibers
35 and 37 in their
nested configuration. FIGS. 8 A and 8B depict alternative examples of fibers
that may be
used to fabricate an endoprosthesis according to the invention. Fiber 35,
shown in isolation
in FIG. 8A, comprises notch 40. Similarly, fiber 41 of FIG. 8B, having an
alternative
configuration, comprises notches 44. Other configurations may be suitable.
When stent 30 is in its delivery configuration, notches 40 and 42 are
disengaged
from fibers 35 and 37. Upon deployment, as the shape memory properties of the
materials
used to fabricate endoprosthesis 30 cause endoprosthesis 30 to return to its
deployed
configuration, stent 30 exhibits an outward radial force. Further, fibers 35
and 37 spring to
"nest" within notches 40 and 42 at points of intersection 39, thereby locking
the stent 30
more reliably into the deployed configuration and resisting pressure exerted
by the vessel to
return to a smaller diameter.
13
CA 02503388 2005-04-21
WO 2004/045450 PCT/US2003/035951
Although not limited thereto, endoprosthesis 30 could be fabricated overall
from or
coated with one or curable materials, or comprise one or more curable
materials at points of
intersection 39. Ultraviolet light is delivered within a device and points of
intersection are
"welded" together in the expanded and locked position. Following curing of
such curable
materials, the stability of the "nesting" function of notches 40 and 42 may be
enhanced. In
yet another alternative embodiment, endoprosthesis 30 could be fabricated from
one or more
curable materials and cured in a pattern utilizing photolithographic technique
as discussed
above, to enhance curing at notches 40 and 42. Further, endoprosthesis 30
could
alternatively be processed to comprise a therapeutic incorporated into the
materials
comprising endoprothesis 30 or coated on its surface utilizing any of the
technologies
discussed above.
Yet another embodiment according to the invention can be more clearly
described in
relation to FIGS. 9 and 10. Similar to embodiments according to the invention
discussed in
relation to FIGS. 1-8, endoprosthesis 50, shown in FIG. 9 in its deployed
configuration, also
has a low-profile delivery configuration. Endoprosthesis 50 may be self-
expanding,
balloon expandable, or balloon-assisted. Endoprosthesis 50 comprises fibers 52
and 54,
which may be fabricated in any of the number of possible manners and from any
of the
number of possible materials as the fibers discussed above, are woven at
angles to one
another to form a generally tubular structure. Fibers 52 comprise bead-like
"male" elements
58 and fibers 54 comprise "female" elements 56, which are configured to allow
male
elements 58 to pass through in one direction only. Upon expansion of stent 50
by
appropriate means, male elements 58 pass through female elements 56, and
cannot pass
back through in the reverse direction. Fibers 52 are thereby "locked" in
relation to one
another, and endoprosthesis 50 is consequently "locked" in its deployed
configuration once
expanded to its desired diameter. Female elements 56 and male elements 58 may
alternatively comprise curable materials and/or endoprosthesis 50 may be cured
in a pattern
to enhance the stability of stent 50 following deployment.
In FIGS. 11A and 11B, an alternative configuration of the invention as set
forth in
FIGS. 9-10 is illustrated. In FIG. 11A, male element 55 and female element 57
are
illustrated prior to mating. Male element 55 is configured as a barb-like
structure, and
female element 57 is configured as a cup-like structure. In FIG. 11B, male
element 55 has
moved in direction of arrow 59, and has been irreversibly received within
female element
57. Male element 55 cannot be pulled back through female element 57 in the
direction
14
CA 02503388 2005-04-21
WO 2004/045450 PCT/US2003/035951
opposite that represented by arrow 59. It should be emphasized, however, that
the foregoing
are merely examples, and that male and female elements may be configured in
any of a
number of suitable configurations for irreversible coupling.
Turning now to an altogether alternative embodiment, endoprosthesis 60 is
shown in
FIG. 12. endoprosthesis 60 comprises a braided fiber structure similar to the
embodiments
illustrated above. Endoprosthesis 60 further comprises one or more axial
members 64,
which extend substantially the length of endoprosthesis 60. In the embodiment
of FIG.
12A, endoprosthesis 60 comprises three axial members 64, spaced approximately
120
degrees from one another. Axial member 64 may be fabricated from any number of
elastomeric or shape memory materials. Axial member 64 may be affixed to
endoprosthesis 60 in any suitable manner known in the art including but not
limited to the
use of a suitable adhesive, chemically attached, melt bonded, or curable in
situ, etc. FIG
12B depicts an end view of the embodiment of FIG. 12. An example of the
possible spacing
of axial members 64 can be seen.
Axial members 64 exert a foreshortening force on endoprosthesis 60 in the
direction
of arrows 65 and 66. Such foreshortening force acts to prevent endoprosthesis
60 from
elongating, thereby preventing a decrease in the diameter of endoprosthesis
60. Axial
members 64 thereby act to "lock" endoprosthesis 60 at the desired deployed
diameter.
Although not limited thereto, axial members 64 and/or endoprosthesis 60, when
in a reduced
profile configuration, may be coated with a hydrophilic polymer in order to
maintain
endoprosthesis 60 in the reduced profile configuration. Upon exposure to
physiological
fluids, such hydrophilic polymer would erode, allowing axial member 64, and
consequently
endoprosthesis 60, to return to a larger profile, deployment diameter.
FIG. 13A illustrates an embodiment similar to that discussed in relation to
FIGS.
12A and 12B. In FIG. 13A, endoprosthesis 70 is shown in its deployed
configuration.
Endoprosthesis 70 comprises one or more axial element 74 affixed by any
suitable means at
or near distal terminus 75 of stent 70. Axial element 74 further comprises
male elements 76
and female element 78 at or near proximal terminus 73 of stent 70. Upon
deployment of
endoprosthesis 70, axial element 74 is "tightened" to exert a foreshortening
force upon
endoprosthesis 70. Male elements 76, which can be of any number of suitable
configurations, are pulled irreversibly through female element 74 in direction
of arrow 77.
Male elements 76 cannot pass in the opposite direction. Similar to the
foregoing
embodiments discussed, endoprosthesis 70 and axial element 74 may be
fabricated using
CA 02503388 2005-04-21
WO 2004/045450 PCT/US2003/035951
any of the aforementioned materials according to any of the aforementioned
processes.
FIG. 13B shows an end view of the embodiment discussed in relation to FIG.
13A.
FIGS. 14A and 14B illustrate an alternative embodiment according to the
invention.
Endoprosthesis 80 is similar to the embodiments described in relation to FIGS.
1-13 to the
extent that it comprises a braided, generally tubular structure fabricated
from two or more of
any number of suitable materials. Further, following deployment,
endoprosthesis 80
comprises one or more locking regions 86 at one or more fiber points of
intersection 84.
Locking regions 86 may alternatively be defined by numerous other
configurations. In the
embodiment of FIG. 14A-B, locking regions 86 comprise a chemical bond between
fibers
82 and 83.
More specifically, endoprosthesis 80 comprises alginate fibers 82 and calcium
fibers
83. Calcium fibers 83 are coated with one or more of any number of suitable
hydrophilic
coatings 85. Upon deployment of endoprosthesis 80 within an aqueous
environment,
hydrophilic coating 85 dissolves, leaving calcium fibers 83 exposed and in
contact with
alginate fibers 82 at one or more, and typically numerous, fiber points of
intersection 84.
Upon contact between alginate fibers 82 with calcium fibers 83, a chemical
reaction
between the materials produces a material that cures at body temperature.
Locking regions
86 are thereby formed, as shown in FIG. 14B. Endoprosthesis 80 could
alternatively be
fabricated from materials curable by other means, including photocurable
materials, and
potentially cured according to a desired pattern using photolithographic
technique as set
forth in more detail above.
FIGS. 14C-E represent different embodiments according to the invention that
also
comprise one or more locking regions at or near fiber crossing points. In the
embodiment of
FIG. 14C, one or more fiber crossing points 91 comprise thermocouple 93
composed of any
suitable material. Once an endoprosthesis comprising thermocouple 93 achieves
its
deployed configuration, inductive heating may be employed to join fibers 90
and 94 at
points of intersection 91, thereby locking such an endoprosthesis in its
deployed
configuration. Alternatively, a radiofrequency signal may be employed to heat
thermocouple 93 in order to weld or otherwise join fibers at or near points of
intersection.
FIG. 14D-E illustrate an alternative embodiment of a locking region of an
endoprosthesis before and after deployment. In FIG 14D, prior to deployment,
fiber 97
crosses fiber 98 at angle 99. Locking element 95 is disposed at or near point
of intersection
96. Following deployment by self expansion or other means, fiber 97 then
crosses fiber 98
16
CA 02503388 2005-04-21
WO 2004/045450 PCT/US2003/035951
at angle 100, causing locking element 95 to engage, thereby locking fibers 97
and 98 at or
near angle 100, and consequently an endoprosthesis comprising locking element
95 to
remain in the deployed configuration.
Any of the foregoing embodiments may further comprise a therapeutic agent to
be
eluted independently or as the endoprosthesis erodes. As a first step in
preparing any of the
foregoing endoprostheses, a suitable polymer in supercritical carbon dioxide
solution may
be admixed with a hydrophobic therapeutic agent. As a result, the hydrophobic
therapeutic
agent is incorporated into the polymer. Alternatively, an embodiment according
to the
invention may comprise an outer layer 120, shown in FIG. 15, into which a
hydrophilic
therapeutic agent has been incorporated. As described above, following
fabrication,
endoprosthesis 117, formed from any of the aforementioned materials, has been
immersed
in a solution of polymer, water and hydrophilic therapeutic agent, underlying
a "blanket" of
supercritical carbon dioxide. The carbon dioxide renders the polymer more
receptive to the
incorporation of therapeutic agent. The polymer comprising the therapeutic
agent forms
layer 120 on the surface of endoprosthesis 117.
Endoprosthesis 117 further comprises end cap 118, formed of a shape memory
material, and disposed at or near one or more ends 119. End cap 118 exerts an
outward
radial force serves to maintain endoprosthesis 117 in its deployed
configuration.
While particular forms of the invention have been illustrated and described
above,
the foregoing descriptions are intended as examples, and to one skilled in the
art will it will
be apparent that various modifications can be made without departing from the
spirit and
scope of the invention.
17