Note: Descriptions are shown in the official language in which they were submitted.
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
EXPRESS MAIL LABEL NO.
METHOD AND APPARATUS FOR REGULATING CHARGING OF
ELECTROCHEMICAL CELLS
CROSS-REFERENCE TO RELATED APPLICATIONS
This application claims priority to provisional USSN 60/421,624 filed
October 25, 2002, the disclosure of which is hereby incorporated by reference
as if
set forth in its entirety herein.
STATEMENT REGARDING FEDERALLY
SPONSORED RESEARCH OR DEVELOPMENT
BACKGROUND OF THE INVENTION
[0001] The present invention relates generally to niclcel rechargeable cells,
such as
niclcel metal hydride (NiMH) cells, and more specifically to a method and
apparatus
for automatically reversibly terminating a cell charging process. Tlus
invention may
also be employed in nicl~el cachnium (NiCd) cells.
[0002] For greater convenience and portability, many modern electrical
appliances
and consumer products may be operated to draw electric current from batteries
of
standard size and electrical performance. For convenience and economy, various
rechargeable batteries have been developed, such as nicl~el metal hydride
cells and
the like.
[0003] Metal hydride cell technology provides excellent high-rate performance
at
reasonable cost when compared to nicl~el cachnium and lithium ion technology.
Moreover, metal hydride cells have about a 50% higher volumetric energy
density
than NiCd cells and about equal to lithium ion cells. The internal chemistry
of metal
hydride rechargeable cells has an impact on the ability to charge such cells.
Issues
affecting the ability to charge nicl~el rechargeable cells arise as a result
of the
internal chemistry of such cells. When a niclcel rechargeable cell approaches
a full
charge state, oxygen is generated at the positive electrode as follows:
40H- -~ OZ (gas) + 2H20 + 4e
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
[0004] The oxygen gas diffuses across a gas-permeable separator to the
negative
electrode where it is recombined into cadmium hydroxide or water as follows:
1/202 (gas) + H20 + Cd ~ Cd(OH)Z + Heat @ Cadmium negative electrode
1/202 (gas) + HZ ~ Hz0 + Heat @ Hydride negative electrode
[0005] When recharging such cells, it is important to ascertain when the cell
has
become fully charged. For example, if a cell were to become overcharged for an
extended period of time, the pressure buildup within the cell could cause the
cell to
fail as well as electrolyte to leaf, thereby further subjecting the charger to
potential
damage.
[0006] Metal hydride rechargeable cells are typically recharged by applying a
constant current rather than constant voltage to the cells. In this scheme,
cell voltage
increases gradually until the cell approaches full charge whereupon the cell
voltage
peaks. As the cells reach the overcharge state, the released heat causes the
cell
temperature to increase dramatically, which in turn causes the cell voltage to
decrease. Cell pressure also rises dramatically during overcharge as oxygen
gas is
generated in quantities larger than the cell can recombine. Unfortunately, it
is
lmown that the rate of pressure change is several orders of magnitude faster
than the
rate of voltage or temperature change. Thus, conventional constant current
charge
interruption methods cannot support a very fast charge rate without risking
internal
pressure buildup, rupture, and electrolyte lealcage. For this reason, metal
hydride
cells may be provided with safety vents.
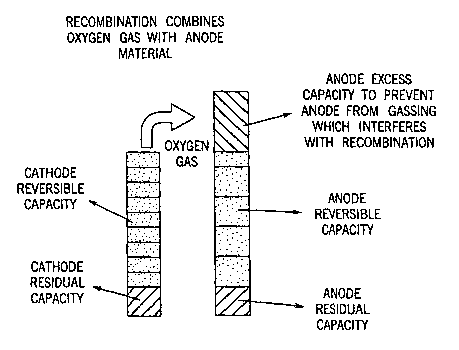
[0007] One common way to reduce pressure buildup at the full-charge state is
to
provide a negative electrode having an excess capacity of greater by 40-50%
more
than the positive electrode, a gas-permeable separator, and limited
electrolyte to
accommodate effective diffusion of gasses. This avoids the production of
hydrogen
gas at the negative electrode while permitting the oxygen to recombine with
the
negative electrode material. When a cell reaches full charge, oxygen gas
continues
to be produced at the positive electrode, but hydrogen is not produced from
the
negative electrode. If hydrogen were produced, the cell could rupture from
excess
pressure. The oxygen recombination reaction therefore controls the cell
pressure, as
is illustrated in Fig. 1. The oxygen gas then crosses the separator and reacts
with the
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
negative electrode material. Detrimental aspects of this arrangement include
reduced cell capacity and corresponding shorter cell cycle life due to
degradation of
the negative electrode from overcharge with oxidation and heat.
[0008] It is important to stop charging a cell or plurality of cells when a
full charge
state is reached to avoid possible cell rupture or leakage due to the
increasing
internal gas pressure. Conventional metal,hydride rechargeable cells camlot
themselves signal a suitable charge termination point. One must instead rely
upon
expensive and sophisticated detection circuitry in an associated charger
device to
determine when charging should end. Charge termination is typically determined
by
the detection circuitry based on (1) peak cell voltage, (2) peak cell
temperature
(TCO), (3) duration of charging time, (4) -dV, and (5) dT/dt. Each known
method
for terminating a constant current charge has disadvantages. For example, time-
based termination can be unreliable except at very low charge rates because
the cell
can become overcharged before termination.
[0009] Charge termination based on peak voltage can be unreliable at the end
of the
charging period because an over-voltage condition can exist before
termination.
Termination based on a voltage decline (-dV) is necessarily associated with
oxygen
recombination and the accompanying detrimental temperature rise. In practice,
this
means that voltage detection must be accurate and fast. Unless the ambient
temperature is steady, it can be difficult to accurately measure a change in
voltage.
Moreover, when the charge rate is slower than 0.3 C, the voltage drop
measurement
is too small to be detected accur ately. By definition, a charge rate of 1 C
draws in
one hour of constant charge a current substantially equal (e.g., within 80%)
to the
rated discharge capacity of the electrochemical cell or battery. Termination
based
only on peals temperature is also easily affected by ambient temperature
changes.
[0010] Termination based upon the rate of change in temperature over time
(dT/dt)
is somewhat more reliable than detecting an absolute temperature change
because it
is less subject to effects caused by ambient temperature change and because
there is
less negative effect on cycle life, but it is still based on heat which is
detrimental to
cell performance and cycle life. This is because temperature increases faster,
and, in
fact, precedes, the drop in voltage. Accordingly, there is somewhat less risk
of
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
rupture and leakage than in the other methods noted above. This makes it the
most
common charge termination method in use today.
[0011] Others in the art have sought pressure-based mechanisms for breaking
the
connection between the electrode and the cell terminal when pressure exceeds a
predetermined level. For example, U.S. Patent No. 5,026,615 discloses a
pressure-
sensitive switch in an end cap assembly that comprises a conductive spring
member,
a nonconductive fulcnun member and a moveable conductive member. The
conductive spring member is in electrical cormection with a terminal on one
end and
with the moveable conductive member on the other end. The moveable conductive
member is in turn in electrical comlection with an electrode. As the interial
cell
pressure increases, the moveable conductive member exerts force on the spring
member, which pivots on the nonconductive fulcrum member and discomiects from
the terminal. This patent therefore requires a first and second contact, one
of which
being movable with respect to the other and rotatable about a fulcrmn in order
to
pivot with respect to the other contact. This arrangement requires more
essential
pants than necessary, and fuuther requires that the assembly be constructed
with tight
tolerances, thereby increasing complexity as well as the cost of production.
[0012] Other examples of these technologies include US Patent Nmnbers
5,747,187,
5,405,715, 5,741,606, 5,609,972, 6,018,286, 6,078,244, and 6,069,551, all of
which
are incorporated herein by reference as if set forth in their entirety. Some
such
mechanisms prevent a pressure-induced rupture of the cell but in doing so
permanently disable the cell. In other cases, reversible switch devices
prevent cell
rupture, but do not detect an early charge termination state to avoid heat
build up
and to ensure superior cell performance and cycle life.
[0013] With constant voltage charge, on the other hand, the charging cmTent is
high
at the beginning of the charge, when the cell can accept higher currents, and
then
decreases to lower levels as the cell approaches full charge. When constant
voltage
charging, the above-noted signals for the end of a constant current charge
process
are not useful because as the cell approaches the full charge state, the cell
voltage is
constant and the cell temperature is leveling. Like a constant cmTent charge
approach, charging time cannot be used for the constant voltage chaxge when
the
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
charge rate is higher than 0.3C due to run away of pressure that can damage
the cell
and the charger. As a result of these shortcomings it has been difficult to
identify an
effective termination signaling means and constant voltage charging for metal
hydroxide cells has therefore been generally considered to be impractical.
[0014] With alternating current charge, the charging current may be modulated
at a
defined frequency or combination of frequencies to produce a net positive
current
that enables the cell to become charged. An alternating current charge can
provide a
fast charge with less pressl~re buildup and lower temperature increase than
constant
current or constant voltage charge. However, when using an alternating current
charge, the above-noted signals for the end of a constant current charge
process are
not useful because as the cell approaches the full charge state, changes in
the cell
voltage are difficult to detect above the voltage response to the applied
alternating
current. As a result it has been difficult to identify an effective
termination
signaling means and alternating current charging for metal hydroxide cells has
also
therefore been generally considered to be impractical. It should be
appreciated that
an alternating current charge is used throughout the present disclosure to
mean a
varying current that produces a net positive charge, such as a modulated
alternating
current. For example, an alternating current may be half wave rectified or
full-wave
rectified to produce a series of current pulses, or an alternating current may
be offset
by a desired DC current.
[0015] Published Australian patent application number 199926971 A1 discloses a
method for fast charging a nickel metal hydride battery in an implant by
transcutaneous transmission of electric power from an external power-
transmission
part to a power-receiving part in the implant. The patent application
considers the
desirability of an initial rapid lugh-cwTent charge phase when the internal
cell
resistance is low, followed by a second lower-current, constant cell voltage
charge
phase to ensure that the cell is charged only with as much energy as the
electrochemical state allows, without excess gassing or heating of the cell.
Harmful
effects on the battery are precluded while, at the same time, the charging
rate
remains lugh. hi the method disclosed therein, a first of two charging phases
includes the step of allowing a relatively high constant charging current to
flow to
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
the power receiving part while the cell voltage rises until it reaches a
predetermined
limiting charging voltage. In the second charging phase, the charging current
is
lower than the current level at the end of the first phase while the cell
voltage is Dept
at least approximately at the predetermined constant voltage value. In the
Australian
patent application, the second charge phase ends when an associated micro-
electrouc controller determines that the rate of change of the charging cmTent
over
time does not reach a predetermined slope. This cumbersome two-step constant
current/constant voltage approach is typical of prior approaches in the art.
[0016] In summary, as the metal hydride rechargeable cell reaches its fully
charged
state, oxygen is evolved from the positive electrode, thereby increasing the
internal
cell pressure acid driving the exothennic oxygen recombination reaction. At a
very
high constant current charge rate, usually less than one hour, charge current
is still
very high at the end of charge. This results in severe heating of the cell and
shortened cycle life. The available methods of terminating constant current
charge
are not very reliable when cell temperature is high. In addition, cell heating
is
detrimental and it is desirable to terminate the charge before significant
cell heating
at the stage where damaging pressure begins to rise within the cell.
[0017] What is therefore needed is a method and apparatus for more accurately
determining the charge termination point for a cell that is fully rechargeable
under
constant voltage, constant current, and altercating current/voltage charging.
[0018] What would be desirable is a reversible regulating switch that is
responsive
to a stimulus for determining a charge termination point that is less complex
and less
destructive than those currently available.
[0019] What is also desirable is a more cost-efficient and reliable charge
termination
detection apparatus thail that currently achieved, and that is compatible with
conventional rechargeable batteries.
BRIEF SUMMARY OF THE INVENTION
[0020] In one aspect the invention provides an axially extending rechargeable
electrochemical cell including an outer can that defines an internal cavity
with an
open end, a positive and negative electrode disposed in the internal cavity,
and a
terminal end cap enclosing the open end. The cell has an end cap assembly that
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
includes a flexible member formed from a material having a heat deflection
temperature greater than 100 C at 264 PSI and a tensile strength greater than
75Mpa.
The flexible member extends radially inwardly from the can and flexes from a
first
position towards a second position in response to internal cell pressure. The
end cap
assembly further includes a first conductive element in electrical
communication
with the terminal end cap. The end cap assembly also includes a second
conductive
element in electrical communication with the positive electrode, and in
removable
electrical communication with the first conductive element. The second
conductive
element is in mechanical communication with the flexible member. The first and
second conductive elements are removed from electrical communication when the
flexible member flexes towards the second position in response to an internal
pressure exceeding a predetermined threshold.
[0021] The foregoing and other aspects of the invention will appear from the
following description. In the description, reference is made to the
accompanying
drawings which form a part hereof, and in which there is shown by way of
illustration, and not limitation, a preferred embodiment of the invention.
Sllch
embodiment does not necessarily represent the full scope of the invention,
however,
acid reference must therefore be made to the claims herein for interpreting
the scope
of the invention.
BRIEF DESCRIPTION OF THE SEVERAL VIEWS OF THE DRAWINGS
[0022] Fig. 1 is a schematic illustration of the oxygen recombination reaction
controlling cell pressure;
[0023] Fig. 2A is a cross-sectional view of an end cap assembly containing a
pressure-responsive switch and a pressure-release vent constructed in
accordance
with a preferred embodiment of the invention, illustrated in a low pressure
position;
[0024] Fig. 2B is a cross-sectional view of the end cap assembly illustrated
in Fig.
2A in a high pressure position;
[0025] Fig. 3 is a cross-sectional isometric view of an end cap assembly
containing
a pressure-responsive switch and a pressure-release vent constructed in
accordance
with an alternate embodiment of the invention, depicted in a low pressure
position;
[0026] Fig. 4 is a cross-sectional elevation view of the end cap assembly of
Fig. 3;
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
8
[0027] Fig. 5 depicts an exploded view of the components of the end cap
assembly
of Fig. 3;
[0028] Fig. 6A is a sectional side elevation view of the positive terminal of
a cell
incorporating a switch constructed in accordance with an alternate embodiment
of
the invention;
[0029] Fig. 6B is a view similar to Fig. 6A, but constructed in accordance
with an
alternate embodiment of the invention.
[0030] Fig. 7 is a sectional side elevation view of the positive terminal of a
cell
incorporating a switch constructed in accordance with an alternate embodiment
of
the invention;
[0031] Fig. 8 is a graph plotting capacity (Ah) vs. dP (psig) for a nicl~el
metal
hydride cell during alternating current and constant current charge;
[0032] Fig. 9 is a graph plotting capacity (Ah) vs. ~P (psig) for a nicl~el
metal
hydride cell during alternating current and constant voltage charge;
[0033] Fig. 10 is a graph plotting internal cell pressure (psig) vs. time
(min) for a
plurality of cells constructed in accordance with the preferred embodiment;
[0034] Fig. 11 is a graph plotting pressure, temperature, and voltage vs. time
(min)
for a cell during charging using a constant current charge, and subsequent
discharging;
[0035] Fig. 12 is a graph plotting internal pressure (psig) vs. time (min) for
various
cycles during charging using a constant current charge, and subsequent
discharging;
[0036] Fig. 13 is a graph plotting the pressure rise for the cell illustrated
in Fig. 12
during charging;
[0037] Fig. 14 is a graph plotting pressure fall for the cell illustrated in
Fig. 12
during discharging;
[0038] Fig. 15 is a graph plotting pressure and temperature vs. time for cells
at
different cycles under a constant current charge;
[0039] Fig. 16 is a graph plotting pressure vs. time for a plurality of cells
at different
cycles under a constant current charge;
[0040] Fig. 17 is a graph plotting pressure, temperature, and current vs. time
for
plurality of cells under a constant voltage charge.
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
[0041] Fig. 18 is a graph plotting and comparing internal pressure vs. applied
charge
capacity during constant cmTent charging versus constant voltage charging;
[0042] Fig. 19 is a graph illustrating and comparing the cunent profile of two
cells
during charging under constant voltage versus constant current.
[0043] Fig. 20 is a graph plotting and comparing cell temperature vs. capacity
for
two cells charged under constant current versus constant voltage,
respectively;
[0044] Fig. 21 is a graph plotting and comparing the voltage profile vs. time
for the
two cells illustrated in Fig. 20;
[0045] Fig. 22 is a graph plotting and comparing temperature and capacity vs.
time
during charging under varying constant voltages
[0046] Fig. 23 is a sectional side elevation view of an end cap assembly
containing a
pressure-responsive switch and a pressure-release vent constructed in
accordance
with an alternate embodiment of the invention, illustrated in a low pressure
position;
[0047] Fig. 24 is a sectional side elevation view of an end cap assembly
containing a
pressure-responsive switch and a pressure-release vent constructed in
accordance
with another alternate embodiment of the invention, illustrated in a low
pressure
position;
[0048] Fig. 25 is a sectional side elevation view of an end cap assembly
containing a
pressure-responsive switch and a pressure-release vent constructed in
accordance
with yet another alternate embodiment of the invention, illustrated in a low
pressure
position;
[0049] Fig. 26A is a schematic view of a battery pack constructed in
accordance
with one embodiment of the present invention;
[0050] Fig. 26B is a schematic view of a battery pacl~ constructed in
accordance
with an alternate embodiment of the present invention;
[0051] Fig. 26C is a schematic view of a battery paclc constructed in
accordance
with another alternate embodiment of the present invention;
[0052] Fig. 27 is a graph illustrating the charge and discharge capacity for
battery
packs having matched and mismatched cells;
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
[0053] Fig. 28A is a graph illustrating %elongation at break vs. tensile
strength for
polymers usable in rechargeable cells in accordance with a preferred
embodiment of
the present invention;
[0054] Fig. 28B is a graph illustrating heat deflection temperature vs.
tensile
strength for polymers usable in rechargeable cells in accordance with a
preferred
embodiment of the present invention;
[0055] Fig. 29 is a graph illustrating charge capacity vs. charge time for
rechargeable NiMH cells having a reduced active volume in accordance with an
alternate embodiment of the present invention;
[0056] Fig. 30 is a chant comparing characteristics of a NiMH size AA cell
constructed in accordance with the embodiment described with reference to Fig.
29
compared to supercapacitors having similar volume;
[0057] Figs. 31A-B illustrate an assembly of a battery pack constructed in
accordance with one embodiment of the present invention;
[0058] Figs. 32A-B illustrate an assembly of a battery pacl~ constmcted in
accorda~lce with an alternate embodiment of the present invention; and
[0059] Figs. 33A-C illustrate various embodiments that produce cell electrodes
with
reduced electrode volumes.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
[0060] Referring now to Fig. 2A, an axially extending cell includes a can 12
having
closed end (not shown) and an open end 13 disposed opposite the open end and
axially downstream therefrom. A cap assembly 10 includes a positive terminal
end
cap 18 that is secured in the open end of the negative cam 12 to provide
closure to
the cell. In particular, the end cap assembly 10 and the open end of the can
12 are
adapted in size and shape such that the end cap assembly 10 is sealingly
accommodated in the open end by crimping the negative can 12 during assembly
of
a cylindrical rechargeable metal hydride cell. The closed end of the can is
conventional and is not shown.
[0061] A positive (e.g., niclcel hydroxide) electrode 14 is in removable
electrical
connection with the positive terminal cap 18, as will become more apparent
from the
description below. The cell further contains a negative electrode 21 (e.g.,
hydride
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
11
electrode) that is in electrical connection with the can 12, and an allcaline
electrolyte
(e.g., potassium hydroxide) alone or in combination with other alkali metal
hydroxides. The electrodes are disposed in an internal cavity 15, and are
separated
by a separator 16. A cell comprising the can 12 and the end cap assembly 10 of
the
invention can further comprise conventional positive 14 and negative 21 wound
electrodes in its interior, although the relative size of these electrodes can
be
adjusted to meet the physical and electrical specifications of the cell.
[0062] The positive terminal cap 18 has a nubbin 20 that is sized and shaped
to
provide a positive terminal to the cell having a pressure-responsive switch 11
constructed in accordance with the present invention. The pressure-responsive
switch 11 comprises a flexible non-conductive mono-stable grommet 22 adapted
in
size and shape to fit securely in the open end 13. Grommet includes a radially
outer
seal 25, an inner hub 27, and an ann 29 that extends substantially radially
and
connects the seal to the hub. It should be appreciated that arm 29 extends
radially
tluoughout the cell and, accordingly, the terms "arm" and "disc" are to be
used
interchangeably throughout tlus disclosure. Grommet 22 further includes has a
centrally disposed opening 19 extending axially through the hub 27 in which is
seated a conductive spool-shaped comlector 24 having a pair of oppositely
disposed
radially extending outer flanges 23. The space between the outer surface of
grommet 22 a.nd inner surface of terminal end cap 18 defines a cavity 17 in
the end
cap assembly 10.
[0063] Connector 24 is securely fixed in the opening 19 of grormnet 22 such
that the
conductive comiector moves in concert with the grommet. A first annular
conductive contact 26, which is a metal washer in accordance with the
illustrated
embodiment, surrounds the hub of connector 24 and has an upper surface in
electrical contact with the upper flange 23. A second armular conductive
contact 28
(which can also be a metal washer) surrounds the grommet and is positioned
axially
upstream and adjacent the first contact 26. The first and second contacts 26,
28 are
circular plates in Fig. 2A but they can be provided in other shapes, as
illustrated, for
example, in Figs. 3-5. Contact 28 has an upper surface 29 that is in
electrical
connection with the terminal cap, and in removable mechanical (and therefore
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
12
electrical) connection with the bottom surface of the first contact 26, as
will become
more apparent from the description below.
[0064] The grommet 22 can be formed of any sufficiently flexible,
nonconductive
inert material that does not adversely impact the cell chemistry. Suitable
materials
include but are not limited to polypropylene, polyolefin, and nylon, including
glass
filled nylon and other glass filled polymers, as will be described in more
detail
below.
[0065] The outer seal 25 of grommet 22 includes an upwardly and radially
inwardly
extending peripheral lip 38 that is shaped and sized to form a tight seal with
the open
end of the can to provide a barrier between the interior and the exterior of
the cell.
The lip 38 also partially defines a cavity in the outer seal 25 in which the
outer end
of terminal end cap 18 and second contact 28 are disposed. The lip 38 presents
a
radially outer convex surface to permit the can 12 to be crimped over the
grommet
22 during assembly of the cell. When the axially downstream end of can 12 is
crimped over the grormnet 22 during assembly, a tight seal is provided between
the
grommet 22, second contact 28, and terminal end cap 18 to isolate the interior
of the
cell from the ambient enviromnent. An optional sealant such as asphalt or tar
can
also be employed between the end cap assembly 10 and the can 12 to strengthen
the
seal.
[0066] A flexible conductive tab 30 electrically comlects the conductive
connector
24 to the positive electrode 14 in the interior of the cell. The conductive
connector
24 can be an eyelet or rivet that is secured in the central opening 19 by
crimping at
its ends to provide flanges 23 that secure the hub 27 of gronmnet 22 and the
first
contact 26. The conductive connector 24 is in electrical and physical contact
with
the first contact 26 thereby helping to secure the conductive connector 24
into
position.
[0067] Fig. 2A illustrates the end cap assembly in a low pressure state, such
that the
grommet 22 is in its stable position. In tlus low pressure state, the positive
electrodes 14 are in electrical comlection with the positive terminal cap 18
via the
conductive tab 30, comzector 24, first contact 26, and second contact 28.
Accordingly, the cell may be charged by introducing a recharging current or
voltage
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
13
to the cell. Advantageously, when internal pressure within the cell
accumulates
beyond a predetermined threshold, the grommet 22 flexes (reversibly) axially
downstream along the direction of arrow A to bias the pressure-responsive from
the
first position illustrated in Fig. 2A to a second position illustrated in Fig.
2B. It
should be appreciated that the predetermined threshold may depend on the
intended
type of charge being used (e.g. constant current, constant voltage, etc. ..),
and may
be determined by the material selected for the grommet, and thicl~ness and
flexibility
of the ann 29.
[0068] Referring now to Fig. 2B, when the internal pressure within the cell
exceeds
the predetermined threshold sufficient to flex the grommet 22, the hub 27 is
translated axially dovnmstream, thereby also translating the first contact
axially
downstream with respect from the second contact 28, and removing the
electrical
coimection therebetween. As a result, an electrical connection at the nubbin
20 will
not transfer to the electrodes 14 within the cell, and further charging is
prevented
until the averpressure situation subsides.
[0069] Optionally, an insulating oveipressure stop 32 can also be provided in
an
interior cavity defined by the nubbin 20. The overpressure stop 32 can also be
used
to pre-load the contact pressure as desired~and can limit motion of the
conductive
connector 24 in the direction of the nubbin 20 when internal cell pressure is
high. A
stop washer 34 can also optionally be disposed between the second contact 28
and
terminal end cap 18 to restrain the movement of the second contact when the
grommet 22 flexes, thereby further insuring that the electrical connection
will be
severed between the two contacts during a high pressure state.
[0070] It should be appreciated that a plurality of cells could be installed
in a battery
pacl~ and comlected in series within a charger that is configured to supply a
constant
voltage or constant cunent charge to the cell. So long as at least one of the
cells
includes a pressure responsive switch in accordance with the invention
(assuming
pressure accumulates similarly within each cell), charging will terminate once
the
pressure within that cell activates the switch to remove electrical
communication
between the end cap 18 and electrode 14. Alternatively, each cell could
include the
switch such that the charging of all cells vc~ould terminate once one of the
cells
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
14
reaches a maximum permissible internal pressure. Alternatively, the cells
could be
comlected in parallel to a charging source, in which case each cell would
include a
pressure responsive switch in accordance with the present invention.
[0071] Figs. 2A-B also illustrate an optional a safety system for venting
excess
pressure (gas) from the cell when in an overpressure condition. In particular,
the
conductive connector 24 can define a centrally disposed pressure release
chamzel 36
extending axially there through. Accordingly, gas produced at the electrodes
is able
to flow axially downstream from the cell interior 15 and through channel 36 to
end
cap interior 17. The end cap 18 also defines one or more outlets 35 extending
there-
through to enable the gas to flow from the end cap assembly 10 to the outside
environment. The outlet can be secured against undesired leakage with a seal
(not
shown) adapted in tensile strength to yield at a pre-selected pressure level
to release
gas from the cell. The seal can be reversible or irreversible.
[0072] Alternatively, outlets) 35 may always be open to the environment, in
which
case a reversible airtight seal to the interior of the cell is maintained by
blocking the
pressure release chamlel 36. In particular, the oveipressure stop 32 can also
function
as a overpressure release control if it is formed of a suitably deformable
plastic
material such as nibber for sealing pressure release channel 36 and outlets)
35 (if
not open to the environment). h1 addition to the defonnable material shown,
other
structures for releaseably blocking the pressure release channel include,
without
limitation, a plug or a spring. When the internal cell pressure rises to a
sufficiently
high level, the block is urged away from charnel 36 and from outlets) 35 to
define a
pressure release path from the cell interior to the outside enviromnent. The
pressure
at which the vent system releases the cell internal pressure depends on how
much
internal pressure a battery can withstand; the plastic material of the
overpressure
stop 32 is selected to respond to a pressure at which venting is desired, but
to remain
securely in place at lower pressures. Generally spealcing, for a metal hydride
rechargeable cell, the safety vent system responds to cell internal pressures
between
the pressure required to activate the switch and the presswe required to de-
crimp the
cell, for example greater than 400 psig and less than 2000 psig depending on
the cell
size. For instance, a size AAA cell can be configured to vent at a pressure
between
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
15 .
1400 and 2000 psig, while a size AA, C, and Sub C cell can be configured to
vent at
a pressure between 400 and 1200 psig.
[0073] The opening and closing of the pressure release path through channel 36
and
outlets) 35 can be reversible but may also be made irreversible by employing a
blocl~ made of materials that do not revert to a shape or size or position
that can
effectively blocl~ the pressure release path after a first pressure rise. It
will be
appreciated that blocl~s other than those disclosed herein can be employed in
both
reversible and irreversible vent systems, as will be described in more detail
below.
[0074] Referring now to Fig. 3, one example of an end cap assembly having an
irreversible vent is illustrated, in which life elements to those illustrated
in Figs. 2A
and 2B are identified by the same reference numerals. Fig. 5 illustrates these
elements prior to being assembled into the can 12.
[0075] In accordance with this embodiment, the first contact 26 is not flat,
but rather
includes a flat central portion and foL~r arms, each arnz having a distal
portion and a
transition portion that connects the distal and central portions, which are
not
coplanar with each other. The central portion is in electrical contact with
the
conductive connector 24 and the second contact 28. The second contact 28 is
electrically connected to end cap 18. Each distal portion of contact 26 is
electrically
isolated from the end cap 18 by an electrical isolator 40 that is disposed
therebetween and aligned with the distal portion of contact 26.
[0076] When internal pressure builds up within the cell, grommet 22 flexes,
thereby
removing,contact 26 from electrical communication with washer 28. The
electrical
comiection between terminal end cap 18 and the electrodes is also thereby
removed.
Insulator 40 limits the permissible axial movement of contact 26, and further
prevents electrical connnunication between the distal ends of contact 26 and
the end
cap 18. The first contact 26 thus responds well in concert with the grommet 22
to
changes in the internal cell pressure, and is well-suited to urging reversion
of the
switch to the low pressure position when internal pressure subsides.
[0077] The venting system of Figs. 3-5 is also configured somewhat differently
than
that of Fig. 2 in that the pressure release chamlel is plugged with an
adhesively- or
frictionally-engaged fmstoconical plug 42 adapted to be irreversibly expelled
from
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
16
the channel at lugh internal cell pressures, for example between 400 and 1200
for a
size AA, C, or sub C cell, and between 400 and 2000 psig for a size AAA cell.
Referring to Fig. 4, the insulator 40 may extend radially from terminal end
cap 18 to
plug 42.
[0078] During operation, when the electrical coimection is brol~en between
electrical
contacts 26 and 28, current flow drops to zero. This zero current flow can be
detected by conventional charger circuitry (not shown) and can be interpreted
as a
signal that the cell is fully charged. The charger circuitry can then signal
the end of
charge condition. These circuits are considered to be conventional. More
importantly, only complete current flow drop needs to be detected, rather than
any
more subtle change in pressure, voltage, temperature or rate of current flow
as is
typical in conventional metal hydride recharging systems.
[0079] The internal cell pressure at which the pressure-responsive switch is
biased
from the low pressure position to the high pressure position (the "biasing
pressure")
can vary according to the size and shape of the battery, the charging rate and
other
charging conditions such as ambient temperature. For example, when the
negative
electrode of a battery has a much higher capacity than the positive electrode
of the
battery, the cell internal pressure at a low overcharge rate may be stabilized
at a
relatively low level such as 30-50 prig. Similarly, the higher the charge
rate, the
higher the cell internal pressure will be when a battery approaches the full
charge
state or reaches a~1 overcharge state. Thus, when a switch is built for a
battery
having a much higher capacity at the negative electrode and/or when the
battery will
be charged at a very low rate, the biasing pressure of the pressure-responsive
switch
should be low enough to ensure that charge can be stopped when the battery
reaches
a full charge or overcharge state. On the contrary, when a switch is used in a
battery
that has similar negative electrode and positive electrode capacities, or when
the
battery will be charged at a high rate, the biasing pressure can be set at any
level that
satisfies battery safety concerns since there is no question that the cell
internal
pressure can reach the biasing pressure. .
[0080] Preferably, however, a presslue-responsive switch should have a switch
pressure that is close to the internal pressure when the cell reaches the full
charge
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
17
state, to prevent problems such as overheating. One of ordinary shill in the
art
snows how to determine cell internal pressure at the point of full charge or
overcharge. Generally spearing, for a fast niclcel metal hydride rechargeable
cell, a
pressure-responsive switch may have a biasing pressure of between about 50
psig
and 500 psig. It is preferable that the switch pressure is less than the
venting
pressure, for example between 100 and 400 psig. In particular, it is
preferable that
the switch pressure is between 150 and 300 psig for a size AA, C, and sub C
cell,
a~zd between 250 and 400 psig for a size AAA cell.
[0081] Referring now to Fig. 6A, a reversible pressure responsive switch 100
constructed in accordance with an alternate embodiment of the invention is
disposed
within a positive terminal cap 102 at the open end of a niclcel rechargeable
cell 104.
The cell 104 may be conventional apart from the cap and its electrical
connection to
the cell electrodes. Cells made according to the present invention may
comprise
wound positive 106 and negative 108 electrodes in its interior, wherein the
negative
electrode (such as a hydride electrode) is in electrical connection with a can
110
having an.open end and a closed end, and wherein the positive (e.g., niclcel
hydroxide) electrode is in electrical connection with the positive terminal
cap 102
that is secured in the open end of the negative can 110. The cell contains an
electrolyte, typically potassium hydroxide:
[0082] The open end of the cell 104 includes a cap assembly 112 constructed in
accordance with the preferred embodiment, and disposed in the open end of the
can
110. The open end of the negative can 110 is shaped to sealingly accommodate
the
cap assembly 112 in the open end during manufacture. The closed end of the
cell
can is not depicted but is conventional. The cap assembly 112 includes the
positive
terminal cap 102 and a pressure-responsive switch 100 constnicted in
accordance
with the present invention.
[0083] The pressure-responsive switch 100 comprises a grommet 114 that
provides
both a flexible seal and main spring, and has a centrally disposed conductive
comzector.116, or "rivet" or "pin," extending axially there-through. The
gronmnet
114 may be formed of any material that does not negatively interact with the
chemistry of the cell but which is sufficiently flexible to move in response
to a
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
18
pressure increase to bias the switch of the invention, as described above. The
grommet 114 further includes an outwardly and upwardly extending lip 115 that
is
shaped and sized to form a tight seal with the open end of the can 110 to
separate the
interior of the cell from the exterior. The lip creates a radially inwardly
facing void
117 that is occupied by end cap assembly components, as will be described in
more
detail below. In the illustrated embodiment, the lip 115 has a convex outer
surface
to accommodate a concave inner surface of the can 110 that allows the can to
be
crimped into position durlllg cell assembly. An optional sealant such as
asphalt or tar
can also be employed between the cap assembly 112 and the can 110 to further
seal
the open end.
[0084] Toward the interior of the cell, a conductive tab 118 electrically
connects the
central conductive pin 116 to the positive electrode 106. Toward the exterior
of the
cell, the central pin 11 G is also in electrical contact with a contact ring
120 which
also serves to secure the central pin into its position. Contact ring 120 is a
washer
that surrounds the central pin 116 and, along with contact plate 122, is
disposed in
an internal cavity 126 that is defined by the positive terminal cap 102 and
the
flexible grommet 114. Contact ring 120 is thllS 111 cOllStallt electrical
CO1n111L1111Cat1011
with the central pin 116. Secured in the void 117 are a circular conductive
contact
plate 122 and the positive terminal cap 102 having a nubbin 124 sized and
shaped to
provide a standard positive terminal for the cell 104. The contact plate 122
is thus in
electrical connection with both of the aforementioned positive end cap 102 and
the
contact ring 120 when the cell 104 is in the low-pressure state illustrated in
Fig. 6A.
Accordingly, the nubbin 124 is in electrical communication with the electrode
106
via end cap 102, contact plate 122, contact ring 120, central conductor 116,
and tab
118.
[0085] In operation, the grommet 114 flexes outwardly in response to high
internal
cell pressure. When the internal cell pressure is sufficiently great to cause
the
grommet 114 to flex, the central pin 116 is urged toward the over-pressure
stop 128,
thereby biasing contact plate 120 axially away from contact plate 122 (not
shown).
The electrical comlection between contact ring 120 and the contact plate 122
terminates, thereby terminating the electrical communication between the
nubbin
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
19
124 and electrode 106. Further charging is thus prevented. Advantageously, the
switch 100 is reversible, in that the connection between contact ring 120 and
contact
plate is reestablished once the overpressure situation subsides. Also provided
on an
Timer surface of the positive terminal cap nubbin 124 in the cap assembly 112
cavity
is a non-conductive over-pressure stop 128 which can also be used to pre-load
the
contact pressure as desired.
[0086] As described above, once the overpressure situation exists within the
cell
104, the electrical contact is brol~en between contacts 120 and 122, current
flow
within the cell 104 drops to zero. This zero current flow can be detected by
conventional charger circuitry and can be interpreted as a signal that the
cell is fully
charged. The charger circuitry can then signal the charge termination. These
circuits are considered to be conventional. As was noted above, the rise in
pressure,
which follows gassing in the cell, precedes the damaging temperature rise that
shortens cell cycle life.
[0087] Grommet 114 further includes a grormnet arm having a neclced-down
section
121 that is designed to fail when the internal cell pressure reaches a venting
pressure
that is greater than the pressure required to flex the grommet outwardly as
described
above. Once section 121 fails, pressurized cell contents are able to exit the
cell via
an apeuture 123 extending through positive terminal cap 102.
[0088] Referring now to Fig. 7, a reversible pressure-responsive switch 150 is
illustrated in accordance with an alternate embodiment of the invention. In
particular, cell 154 comprises a negative can 152 having an open end that is
shaped
to accommodate and seal the cap assembly 172 in the open end during
manufacture.
The remainder of the cell can is conventional. The cap assembly 172 includes
the
positive terminal cap 156 having a nubbin 157 that is sized and shaped to
provide a
positive terminal to the cell.
[0089] The regulating switch 150 illustrated in Fig. 7 includes a flexible
grommet
158 adapted in size and shape to fit securely in the open end and having a
central
opening 119 there through. A conductive connector 160 is securely fixed in the
central opening such that the conductive connector moves in concert with the
flexible grommet 158. A first conductive contact 162 surrounds the connector
160
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
and is in constant electrical communication therewith. A second conductive
contact
164 extends radially inwardly from the radially outer wall of gronnnet 158
such that
at least a portion of its upper surface is axially aligned and in severable
contact with
the lower surface of contact 162.
[0090] Grommet 158 includes grormnet arm, of the type described above, having
a
neclc-down portion 159 that is operable to fail at a predetermined pressure
greater
than the pressure necessary to open the switch, but less than the pressure
required to
de-crimp the cell 154. A stop 166 is disposed axially downstream from contact
162,
and limits the axial displacement of the grommet 158. Stop 166 is a bent
annulus
whose outer periphery includes a pair of locations that contact terminal cap
156.
The remaining outer periphery of stop 166 enables pressurized cell content to
flow
into channel 176 when the necked-down section of grommet ann 159 fails. An
insulating layer 168 is disposed between contact 162 and the stop 166.
Accordingly,
the stop 166 does not form part of the electrical circuit.
[0091] The grommet 158 may be formed of any sufficiently flexible,
nonconductive
inert material described herein that does not adversely impact the cell
chemistry.
Depending on the configuration of the switch elements, the switch 150 may be
responsive to pressure, temperature, or both, as will become more apparent
from the
description below.
[0092] The terminal cap 156 and the flexible grommet 158 define a cavity 170
within the cap assembly 172 in which the first and second contacts 162 and
164, and
stop 166 are provided. While the first and second contacts 162 and 164 are
circular
washers plates as illustrated in Fig. 7, they may be provided in other shapes
and
sizes, as described above. The second contact 164 includes three protuusions
174
proximal its radially inner edge that extend axially towards the first contact
162 and
are radially spaced 120° from each other. When the internal pressure is
less than a
predeterrriined threshold, determined in large part by the flexibility of
grommet 158,
the protnisions 174 are in connection with the lower surface of the first
contact 162,
thereby completing the electrical circuit and pemnitting the cell to be
charged.
[0093] Toward the interior of the cell, a conductive tab (not shown)
electrically
connects the central conductive pin 160 to the positive electrode in the
mamler
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
21
described above. The hub of grommet 158 further serves to secure the central
pin
160 in its proper position. Secured in the peripheral lip of the grommet 158
are the
circular conductive contact plate 164 and positive terminal cap 156. The
contact
plate 164 is in electrical connection with both of the aforementioned positive
end
cap and the contact ring 162, although the latter comlection is disconnected
when the
high temperature or pressure condition exists.
[0094] As described above, the end cap assembly 172 can also coyprise a system
for venting pressure from the cell. When the assembly comprises a vent system,
the
conductive connector 160 can define there through a pressure release channel
for gas
to flow from the cell interior on a first side of the flexible grommet 158
into the end
cap assembly 172 on the second side similarly described in Figure 3 and Figure
4.
The battery end cap 156 also defines one or more outlets 176 extending
therethrough
for gas to flow from the end cap assembly 172 to the outside enviromnent. The
vent
mechanism can be reversible or irreversible. If the described vent system is
not
employed, other vent means can be provided.
(0095] In operation, the grommet 158, flexes (reversibly) axially downstream
towards the positive end cap 156 and against the spring force of stop 166 in
response
to high internal cell pressure. The regulating switch 150 is thus biased from
the
closed position (illustrated in Fig. 7) to an open position (not shown), in
which the
central pin 160 moves axially downstream in concert with the grommet 158.
Accordingly, the first electrical contact 162 becomes displaced from the
second
contact 164 and free from protnision 174. The electrical contact between the
contact
ring 162 and the contact plate 164 is thus brol~en, and further charging is
prevented,
until the overpressure situation subsides and the grommet returns to the
position
illustrated in Fig. 7, and the electrical connection between contacts 162 and
164 is
reestablished.
[0096] The stop 166 illustrated in Fig. 7 may further be manufactured from a
temperature-responsive material that changes shape when a predefined
temperature
is attained. hi this way, a stop can be fashioned to reversibly deflect or
defoizn at a
certain internal cell temperature, thereby reducing or removing the preload
force on
the central pin and reducing the pressure required to break electrical contact
between
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
22
the contact ring and the contact plate. In this way, a potentially harmful
temperature
rise is prevented, even if no overpressure condition exists within the cell.
In
operation, when the cell reaches a predefined temperature, the stop 166 can
r eversibly deflect or deform and pull the conductive connector 162 away from
the
contact plate 164, thus breaking electrical contact between the contact ring
and the
contact plate. Alternatively, the stop 166 can be comiected to the conductive
corrector or central pin 160 and the terminal cap 156.
[0097] While any temperature-responsive material can be used, the stop is
preferably formed from a bimetal composed of two layers of metals or alloys or
other materials with different coefficients of thermal expansion. One layer
has a
lugher thermal expansion and the other layer has a lower thermal expansion.
This
causes the bimetal to deflect or deform in response to temperature in a way
that can
be defined by the choice of metals or alloys used in each layer.
Alterlatively, a
shape memory material can be used to form the temperature-responsive stop 166,
such as a nickel-titanium alloy.
[0098] The temperature-responsive stop 166 can additionally operate as a
pressure-
responsive stop. Shape memory materials include alloys of Nickel-Titanium,
Copper-Zinc-Ahuninum, or Copper-Aluminum-Nickel. These materials are pre-
forned to the concave disc shape 166 as shown to act as the spring and to
apply a
pre-determined amount force that will hold the conductive contact 162 and
contact
plate 164 together for electrical continuity. These materials have the ability
to
deform and flatten out when heated to a pre-determined temperature or become
flatten out also when interial pressure reaches a pre-determined value. It has
been
found that the most desirable temperature range for these materials to work
with
nickel-metal hydride or nickel-cadmium cells is between 70 deg C and 100 deg
C.
[0099] It should be further appreciated that the stops illustrated in
accordance with
any of the previous embodiments may also be constructed to be responsive to
temperature and/or pressl~re.
[00100] As described above, the charger may conclude that charging has
teminated based on a zero cLUrent flow within the cell, or when charging time
has
reached a pre-determined value. The charger may then either discontinue the
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
23
charge, or it could continue charging, in which case the pressure responsive
switch
will continue to open and close. The charging would therefore continue until a
timer
reaches a termination point at a pre-set value. This charging mode can be
particularly advantageous when chargil~g at a rate faster than 30 minutes,
where
pressure increases significantly when the cell is approaching a fully charge
state, and
the on-off of current provided by the pressure switching mechanism will
continue to
top up the charge to the maximuun charge state. If the cell is being charged
under
constant voltage, constant current or alternating current at a very charge
fast rate
(charge termination within 30 minutes or less) the cell may be only charged to
approximately 70-90%, as it is known that internal cell pressure increases
ahead of a
full cell charge during charging. The present inventors have determined that a
constant voltage charge is more advantageous than a constant current or
alternating
when achieving a very fast charge rate (charge termination in 30 minutes or
less),
because charge current continues to decrease toward the end of charge with
constant
voltage, and as the result, pressure and temperature are not rising as quicl~
in
comparison to charging with a constant current. For example, up to 85-90% of
charge can be achieved with constant voltage before the opening of the switch
in
comparison to 80-85% with alternating cmTent and 65-70% with constant current.
In some instances, the fast charging accomplished using the switch presented
in
accordance with the present invention offsets the disadvantage associated with
the
partial charging of the cell.
[00101] In other instances, it may be desirable to sacrifice time to ensure
that
the cell has become fully charged. In this instance, once the charger detects
a zero-
current, it waits until the internal pressure within the cell subsides and
then measures
the OCV for the cell (a pressure release vent would be particularly
advantageous in
such cells~to minimize the cell depresswization time). Based on the OCV, the
charger may determine whether the cell has been fully charged.
[00102] For example, it is l~~own that a fully charged metal hydride cell will
have an OCV of 1.42 V. Accordingly, if the OCV of the cell that is being
charged
has exceeded a predetermined threshold of 1.42- 1.48V, the charger would
detemnine that the cell is fully charged. Otherwise, the charger will conclude
that
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
24
the cell has not yet been fully charged. Accordingly, once pressure within the
cell
has dissipated such that the electrical connection between contacts is
established, the
charger will again subj ect the cell to the alternating or constant current
charge until
the internal pressure within the cell breaks the electrical connection. This
iterative
process may continue until the cell reaches a predetermined OCV or a
predetermined number of iterations, at which point the charger will provide an
appropriate message to the user, for example by illuminating an indicator.
Alternatively, the user could select a charge termination (e.g., 80%
capacity), at
which point the charger would calculate the corresponding OCV and terminate
charging when the cell has reached the user-selected charge termination
threshold.
[00103] This process would be more desirable when using constant current or
alterlating cwTent charging, as pressure is known to build up significantly
before
the cell is fully charged. If a constant voltage charge is applied to the
cell, it would
be expected that the cell would be substantially frilly charged after the
first iteration,
thereby allowing the charger to detect a zero current and indicate that the
cell is fully
charged. While the zero current flow method described above could also be used
in
combination with constant current and altercating current charging, the cell
may not
be fully charged when the first iteration ternzinates.
[00104] One advantage of the reversible switches illustrated and described in
accordance with the present invention is that detection of charge termination
is not
dependent on oxygen recombination. Therefore, there is no longer any need to
provide excess negative electrode capacity. Oxygen at the positive electrode
and
hydrogen at the negative electrode can be evolved. Both gasses contribute to
the
pressure. h1 this case, the negative electrode capacity can be made equal to
the
positive electrode capacity, for a net increase in cell capacity. When
charging
current stops, oxygen recombines with hydrogen to fore water: 1 /202 + HZ -~
H20.
[00105] Another advantage is that a non gas-permeable separator may be
used. Tlus eliminates the needs for having open flow channels within the
separator
for the gas to be recombined with negative electrode, which had contributed to
separator dry out and limited cell cycle life. With the pressure-responsive
switch of
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
the invention, additional electrolyte can fill in the channels. Therefore,
cycle life and
discharge efficiency would be increased.
[00106] Another advantage is that sophisticated analytical circuitry is not
employed for detecting an end-of charge condition, thereby reducing the cost
of an
associated charger device.
[00107] Another advantage is that charging cam proceed at a faster rate than
in
prior cells.. For example, a rechargeable metal hydride battery according to
the
invention can be charged in 45 minutes or less, preferably in 30 minutes or
less, and
most preferably in 20 minutes or less, even 10 to 15 minutes for a NiMH 1.3 Ah
AA
cell, whereas conventional cells require about 1 hour or more to charge
(1.2C). The
charging rate can be accelerated because the invention eliminates the concerns
about
ovetpressure and high temperature conditions at the end of charging. In this
regard,
fast charging may be achieved at rate less than an hour.
[00108] A~zother advantage is that a cell of the present invention can have a
higher capacity than a conventional rechargeable metal hydride battery. This
is
because a cell constricted in accordance with the present invention can have a
greater balance of negative electrode material to positive electrode material.
Unlilce
prior art cells, in which the negative electrode has an excess capacity of
greater by
40-50% more than the positive electrode, a cell of the present invention can
have a
ratio of ayywhere between .9:1-1.5:1 by weight of negative electrode material
to
positive electrode material in accordance with the preferred embodiment.
[00109] Another advantage is that a gas impermeable separator may be
implemented, which may be manufactured thimler and denser than the prior art,
leaving more room for electrolyte within the cell. Cycle life is thereby
increased, as
is discharge efficiency.
[00110] In particular, oxygen at the positive electrode and hydrogen at the
negative electrode can be evolved during charging. Both gasses contribute to
the
pressure. In this case, the negative electrode capacity can be made equal to
the
positive electrode capacity, for a net increase in cell capacity. When
charging
current stops, oxygen recombines with hydrogen to form water: 1/202 + HZ -~
H20.
Because, in such an embodiment, the separator may be gas impermeable, the
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
26
limitation on electrolyte filling for preventing the separator to be totally
saturated in
prior art cells is eliminated.
[00111] Furthermore, whereas the positive electrode of prior art rechargeable
metal hydride cells typically comprise type ABS alloys, it also possible to
employ
the higher-capacity AB2 alloys that have traditionally been disfavored in such
cells
because of overpressure concerns.
[00112] The present invention further includes a method of charging a cell or
a plurality of cells that contain the pressure-responsive switch of the
present
invention. The method comprises the steps of connecting the cells) to a power
source, such as a dedicated charger, charging the cells) until the cell
internal
pressure reaches a predetermined level whereupon the switch is biased to the
high-
pressure position and the charging circuit is interrupted. When the charging
circuit
is interrupted, the drop in charging current to zero can be manually or
automatically
noted. A charger used to charge the battery can include circuitry for
detecting zero
charging current or a timer set to a pre-determined value for terminating, and
an
indicator for displaying that the charge has terminated. Alterlatively, as
described
above, the charger could undergo a plurality of charging iterations to provide
a full
charge to the cell.
[00113] While any type of method may be used to charge a cell incorporating
a reversible switch in accordance with the present invention, a constant
voltage
charging method is preferred, since the current is allowed to seelc its omn
decreasing
level as charging proceeds without concert that the cell will be subject to
overcharging or overpressure. With constant applied voltage charge method, as
the
cell voltage increases during charge, the cmTent is automatically reduced
toward the
end of charge. Accordingly, the charging current is high at the beginning of
charging when the cell's charge acceptance is high, and tapers to a lower
charge
current toward end of charge when the cell's charge acceptance is reduced. No
expensive and complicated charging control is necessary. The current flowing
into
the cell is regulated by the cell internal resistance and the cell's own state
of charge.
When the cell reaches full charge, the increasing internal pressure will
activate the
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
27
pressure switch to interrupt charging. Accordingly, when the charger indicates
that
the charging has terminated, the cell will be at or near full charge.
[00114] Advantageously, strings of cells in parallel can be charged with the
same voltage source. Multiple cells in series may also be charged together in
accordance with the present invention by receiving the charging voltage that
is equal
to the open circuit voltage of the cell plus the over-voltage caused by cell
internal
resistance and the predisposed resistance of the circuit. Advantageously, with
constant voltage charge, an even faster charge rate than that of constant
current
charge can be reached due to the ability to increase the charging current at
the
beginning of the charge when the cell can accept higher currents.
[00115] It should be appreciated, however, that the present invention is
equally applicable to constant current and alternating current charges. As
described
above, it is lcnown that the pressure inside metal hydride cells rises rapidly
when cell
charging is essentially complete. As was noted above, the rise in pressure,
which
follows gassing in the cell, precedes the damaging temperature rise that may
shorten
cell cycle life. Thus it is desired to terminate charging when the pressure
begins to
rise and prior to onset of a destmctive oveipressure condition.
EXAMPLES
[00116] For a nicl~el metal hydride cell to be charged in 15 minutes or less,
the preferred constant charging voltage is about 1.6V to 1.65V for a AA cell
with
30-40 mOlnn internal resistance determined by voltage difference between cell
OCV
cell voltage at 6 seconds interval at 10 amperes current. For cell with lower
internal
resistance (C-size cells, for example, having internal resistance of 10-20
mOhrns),
charging voltage lower than 1.GV but higher than 1.SV can be applied. The
inventors have determined empirically that constant voltage charging is
preferred
when the ambient temperature is above freezing while constant current charging
is
preferred when the ambient temperature is below freezing.
[00117] ~ Commercial AA and AAA nicl~el metal hydride cells containing a
pressure-responsive switch in the end cap assembly were fully charged in 15 to
30
minutes and charging was terminated when the pressure-responsive switch was
biased into the high pressure condition. The pressure signal was consistent
and
reproducible even with extended cycling. Constant voltage charging method was
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
28
shown to be more favorable when ambient temperature is above freezing.
Constant
current method is more effective when ambient temperature is below freezing.
The
slope of pressure rise and fall of AA NiMH consumer cells remained relatively
constant during the course of cycling. The current-tapering effect when using
constant voltage resulted in a lower pressure rise over time for the cell to
become
fully charged. The drop in current also produced lower temperature rise for
the
same charging period. Charging was demonstrated to be faster at higher
voltages,
although a higher cell temperature was also noted under such conditions.
[00118] As described above, it is known that the pressure inside metal hydride
cells rises rapidly when cell charging is essentially complete. In particular,
the rise
in pressure, which follows gassing in the cell, precedes the damaging
temperature
rise that shortens cell cycle life. Thus it is desired to charge the cells in
a manner
that reduces the possibility of a destwctive overpressure or overheating
condition.
[00119] A constant current charging method or a constant voltage charging
method or a combination method, for example, constant current followed by
constant voltage, can be employed in accordance with the present invention. An
alternating current charging method can be preferred, since the current is
modulated,
thus reducing the chance of overcharging, overpressure or overheating. No
expensive and complicated charging control electronic circuitry is necessary.
[00120] The nature of the alternating current or voltage waveform is
typically,
but not exclusively, sinusoidal. Full or half wave rectification may be
applied to the
alternating current or voltage wavefonn.
[00121] Fig. 8 illustrates the cell pressure and temperature for a 1600 mAh
nicl~el metal hydride cell charged using an alternating cuurent derived from
common
60 Hz line power that was full wave rectified to yield a 120 Hz alternating
current
frequency. The change in cell pressure a~ld temperature are lower at the end
of
charge compared with a constant, or direct, cu vent charge.
[00122] Fig. 9 shows the cell pressure and temperature for a 1600 mAh nickel
metal hydride cell charged using an alternating current as in Fig. 8. The
change in
cell pressure and temperature are lower at the end of charge compared with a
constant, or direct, voltage charge.
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
29
[00123] The examples illustrated herein utilize a full wave rectified current
derived from common 60 Hz line power. Other embodiments encompassed by the
present disclosure include full wave rectified alternating voltage or half
wave
rectified sinusoidal alternating current or voltage. Another embodiment is an
alternating current or voltage charge of any frequency. Another embodiment is
an
alterlating current or voltage comprised of any waveforn, including square
wave,
triangle wave (or sawtooth wave), or any arbitrary waveform or combination of
waveforms. Another embodiment is the combination of rectified and unrectified
alternating current or voltage composed of any frequency or combination of
frequencies, or any waveforn or combination of waveforms. Advantageously, any
of these charging methods may be utilized by a cell having a pressure-
responsive
switch as described above. .
[00124] Referring now to Fig. 10, cell internal pressure vs. time is
illustrated
for a group of four 1600 mAh Nickel Metal hydride cells being charged with a
constant voltage at 1.65V. The internal pressure rises to 300 psig as the
cells reach
full charge in 12 minutes. The pressure returns to the initial state following
discharge of the cells. This demonstrates that the internal pressure of Nickel
Metal
Hydride cell rises and falls in a predictable manner, which can be used as a
reliable
signal to terminate charging of a high rate. Groups of cells can thus be
charged and
discharged reliably when pressure is used as a charge termination signal.
[00125] Referring now to Fig. 11, typical charging and discharging
characteristics of a 1300 mAh NiMH cell were measured under a constant current
charge of 3A followed by a 1A discharge to 1 V. The pressure, temperature, and
voltage were measured, and plotted vs. time. This illustrates that pressure is
a much
stronger signal for charge termination than temperature and voltage. Pressure
rises
at much faster rate than temperature and voltage, therefore pressure is a more
suitable signal than temperature and voltage for charge termination.
[00126] Referring now to Fig. 12-21, the slope of pressure rise and fall
remained relatively constant during the course of cycling in comparison to the
voltage illustrated in Fig. 15. Tlus further indicates the reliability of
pressure as an
indicia for the charge termination point of a cell.
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
[00127] Referring to Fig. 16, three 1600 mAh Nicl~el Metal hydride cells
were subjected to a 3.7A constant current charge and discharge for 150 cycles.
The
internal pressure of the cells was shown at cycle 1, and at cycle 150, and
plotted vs.
time. This further illustrated that pressure signal is reproducible with cycle
life a~ld
different cell size and capacity.
[0012] RefeiTing to Fig. 17, two even smaller 550 mAh Niclcel metal hydride
cells were connected in series and charged with a constant voltage charge
source at
1.65 V per cell. The internal pressure, temperature, a~ld Amperage were
measured
and plotted vs. time.
[00129] Fig. 18 illustrates internal cell pressure as a function of capacity
for a
first cell charged under a constant current at 6A, and a second cell charged
under
constant voltage at 1.65V. Fig. 19 illustrates cell current as a function of
capacity
for the first and second cells. Fig. 20 illustrates internal cell temperature
as a
function of capacity for the first and second cells. Fig. 21 illustrates cell
voltage as a
function of capacity for the first a~ld second cells. As illustrated, one
significant
advantage of constant voltage over constant current is the ability of charging
current
to taper towards then end of the charge as cell voltage rises closer to the
applied
voltage. The tapering effect results in a lower pressure rise and lower
temperature
rise at end of charge, thereby allowing the cell to become more fully charged.
The
drop in current also produces a net lower temperature rises for the same
charging
period.
[00130] RefeiTing now to Fig. 22; cell temperature and charge input capacity
are plotted as a function of time for two cells charged under two different
voltage
conditions. It may be observed that a lugher charge voltage produces a higher
charge current for a cell having the same internal resistance. Accordingly,
charging
is quiclcer. at higher voltage, but the cell is also hotter at higher charge
voltage. This
figure further illustrates that at higher charge voltages, the cell reaches
higher charge
state sooner. This also shows that as the pressure activated switch opens in
case of
the higher charge voltage cell, cell temperature drops as the result of switch
on-off
condition. Cell continues to accept charge at this state but at lower
temperature
order intermittent current condition provided by the pressure switch. Tlus is
an
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
31
advantage for having a pressure switch as a meals for regulating end of charge
condition.
[00131] As described above, it is preferable in accordance with one
embodiment of the invention to provide a constant voltage charge less than or
equal
to approximately 1.6-1.65 V during fast charging, though the present invention
contemplates that any constant voltage charge between 1.2 and 2 V is
contemplated
by the present invention.
[00132] For instance, the present invention recognizes that, in some
instances,
it may be desirable to initiate a controlled voltage charge at a level greater
than 1.65
V. While 1.65 V is used as an example, it should be appreciated that this
level can
be any voltage level between 1.2 and 2.0 V, including those levels that fall
in the
range of 1.2 and 2.0 V in .OS V increments. It should be appreciated that
these
benclmnarlcs are approximate, and include voltage deviations of +/- .OS V from
the
benclnnarl~. In accordance with this embodiment, the constant voltage is
stepped
down as a function of one or more measurable variables such as duration of
charge,
charge current, cell temperature, cell resistance, or cell voltage.
Advantageously,
the cells are exposed to a greater initial charge and, as the cell nears the
charge
termination point that will open the pressure responsive switch, the cell is
receiving
a charge equal to, or less than, 1.65 volts.
[00133] For instance, one embodinient envisions charging the cell initially at
1.75 volts (only as an example), and decreasing the charge voltage by a
predetermined amount in response to increases in cell temperature. The voltage
decreases can be continuous, and not time dependent, thus producing variable
voltage having a negatively sloped voltage curve. The curve can be constant or
linear, depending on the rate of cell temperature change. Alternatively, the
decreases can tale place after a predetermined period of time, thereby
producing a
plurality of stepped constant voltages, wherein subsequent steps are of a less
charge
voltage than the previous step. In accordance with the preferred embodiment,
the
voltage decreases anywhere between .5% and 5 % (preferably between 2% and 4%)
for each degree C of cell temperature W crease, which can be obtained using a
theimistor placed proximal the cell. Once the applied charge voltage is equal
to a
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
32
predetermined base voltage, for example 1.65, the charge voltage will maintain
constant until either an excessive temperature (e.g. approximately 50 C) is
measured, or a predetermined length of time expires (for example less than 15
minutes), it being appreciated that the pressure responsive switch will also
regulate
the cell charging.
[00134] Accordingly, the cell will become fully charged during the charge
cycle. It should be appreciated that, while 1.65 V is used as a benchmark in
accordance with the above example, it should be appreciated that the benchmark
can
be any voltage level between 1.2 and 2.0 V, including those levels that fall
in the
range of 1.2 and 2.0 V in .05 V increments.
[00135] Referring now to Fig. 23, an axially extending cell constructed in
accordance with another alternate embodiment of the invention includes a can
312
having closed end (not shown) and an open end 313 disposed opposite the open
end
and axially downstream therefrom. A cap assembly 310 includes a positive
terminal
end cap 318 that is secured in the open end of the negative can 312 to provide
closure to the cell. In particular, the end cap assembly 310 and the open end
of the
can 312 are adapted in size and shape such that the end cap assembly 310 is
sealingly accoimnodated in the open end by crimping the negative can 312
during
assembly of a cylindrical rechargeable metal hydride cell. The closed end of
the can
is conventional and is not shown.
[00136] ~ A positive (e.g., niclcel hydroxide) electrode 314 is in removable
electrical comlection with the positive terninal cap 318, as will become more
apparent from the description below. The cell further contains a negative
electrode
321 (e.g., hydride electrode) that is in electrical connection with the can
312, and an
alkaline electrolyte (e.g., potassium hydroxide) alone or in combination with
other
alkali metal hydroxides. The electrodes are disposed in an internal cavity
341, and
are separated by a separator 316. A cell comprising the can 312 and the end
cap
assembly 310 of the invention can further comprise conventional positive 314
and
negative 321 wound electrodes in its interior, although the relative size of
these
elechodes can be adjusted to meet the physical and electrical specifications
of the
cell.
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
33
[00137] The positive terminal cap 318 has a nubbin 320 that is sized and
shaped to provide a positive terminal to the cell having a pressure-responsive
switch
311 constructed in accordance with the present invention. The pressure-
responsive
switch 311 comprises a flexible non-conductive mono-stable member in the form
of
grommet 322 adapted in size and shape to fit securely in the open end 313.
Grommet 322 includes a radially outer seal 325, an inner hub 327, and an arm
329
that extends substantially radially and connects the seal to the hub. Grommet
322
fiu-ther includes a centrally disposed opening 315 extending axially through
the hub
327 in which is seated a conductive connector in the form of eyelet 324 having
a
pair of oppositely disposed radially extending outer flanges 323. The space
between
the outer surface of grommet 322 and inner surface of terminal end cap 318
defines
a cavity 317 in the end cap assembly 310. Ann 329 extends radially through the
cell, thereby reducing the volume of cavity 317 compared to cells whose am
extends radially and axially towards the negative end. The internal volume
available
for active cell components of cell 310 is also therefore increased to
correspondingly
increase the cell capacity. In accordance with this embodiment, the distance
between the upper surface of the nubbin 320 to the lower surface of the
grommet
322 is approximately 3.8 mm, thereby leaving the remaining cell height for the
electrodes.
[00138] Connector 324 is securely fixed in the opening of grommet 322 such
that the conductive comlector moves in concert with the grormnet. A first
annular
conductive contact 326, which is a metal washer in accordance with the
illustrated
embodiment, surrounds the hub of connector 324 and has an upper surface in
electrical contact with the upper flange 323. A second annular conductive
contact
328 (which can also be a metal washer) sLUTOUnds the grommet and is positioned
axially upstream and adjacent the first contact 326. The first and second
contacts
326, 328 are cylindrical plates in Fig. 23 but they can be provided in other
shapes, as
described above. A spring member 334 is disposed between the upper surface of
grommet arm 29 and the lower surface of contact 328 so as to bias contact 328
outwardly such that upper surface 351 of contact 328 is in electrical
correction with
the terminal cap 318, and in removable mechanical (and therefore electrical)
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
34
connection with the bottom surface of the first contact 326, as will become
more
apparent from the description below. Spring member 334 is preferably
nonconductive.
(00139] As described above, grommet 322 can be formed of any sufficiently
flexible, nonconductive ineut material that does not adversely impact the cell
chemistry. '
[00140] The outer seal 325 of grormnet 322 includes an upwardly and radially
inwardly extending peripheral lip 338 that is shaped and sized to form a tight
seal
with the open end of the can to provide a barrier between the interior and the
exterior of the cell. The lip 338 also partially defines a cavity in the outer
seal 325
in which the outer end of terminal end cap 318 and second contact 328 are
disposed.
The lip 338 presents a radially outer convex surface to permit the can 312 to
be
crimped over the grommet 322 during assembly of the cell. When the axially
downstream end of can 312 is crimped over the grommet 322 during assembly, a
tight seal is provided between the grommet 322, second contact 328, and
terminal
end cap 318 to isolate the interior of the cell from the ambient enviromnent.
An
optional sealant such as asphalt or tar can also be employed between the end
cap
assembly 310 and the can 312 to strengthen the seal.
(00141] A flexible conductive tab 330 electrically connects the conductive
comlector 324 to the positive electrode 314 in the interior of the cell. The
conductive corrector 324 can be an eyelet or rivet that is secured in the
central
opening by crimping at its ends to provide flanges 323 that secure the hub 327
of
grommet 322 and the first contact 326. The conductive connector 324 is in
electrical and physical contact with the first contact 326 thereby helping to
secure
the conductive corrector 324 into position.
[00142] Fig. 23 illustrates the end cap assembly in a low pressure state, such
that the grommet 322 is in its stable position. In this low pressure state,
the positive
electrodes 314 are in electrical connection with the positive terminal cap 318
via the
conductive tab 330, connector 324, first contact 326, and second contact 328.
Accordingly, the cell may be charged by introducing a recharging current or
voltage
to the cell. Advantageously, when internal pressure witlun the cell
accumulates
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
beyond a predetermined threshold, the grommet 322 flexes (reversibly) axially
downstream along the direction of arrow B to bias the pressure-responsive from
the
first closed position illustrated in Fig. 23 to a second open position. It
should be
appreciated that the predetermined threshold may depend on the intended type
of
charge being used (e.g. constant current, constant voltage, etc. ..), and may
be
determined by the material selected for the grommet, and thicl~ness and
flexibility of
the arm 329.
[00143] When the internal pressure within the cell exceeds the predetermined
threshold sufficient to flex the grommet 322, the hub 327 is translated
axially
downstream, thereby also translating the first contact 326 axially downstream
with
respect from the second contact 328, and removing the electrical connection
therebetween. As a result, an electrical connection at the nubbin 320 will not
transfer to the electrodes 314 within the cell, a~ld further charging is
prevented until
the overpressure situation subsides.
[00144] Fig. 23 also illustrates an optional safety system for venting excess
pressure (gas) from the cell when in an overpressure condition. In particular,
the
conductive comzector 324 can define a centrally disposed pressure release
chamlel
343 extending axially there through. A plug 345, preferably made of a rubber
or
other suitably compliant material, is disposed in channel 343 and provides a
seal to
prevent pressurized gas from flowing through the chamzel 343. Accordingly, as
gas
is produced at the electrodes, pressure accumulates within the cell interior
341.
Once the pressure reaches a predetermined maximum threshold, plug is biased
axially downstream along the direction of.Arrow B and into end cap interior
317.
As the plug 345 will not reseal cha~mel 343, the venting mechanism illustrated
in
Fig. 23 is irreversible. The end cap 318 defines one or more outlets 355
extending
there-tluough to enable the gas to flow from the end cap assembly 310 to the
outside
environment. The outlet 355 can be secured against undesired leal~age with a
seal
(not shown) adapted in tensile strength to yield at a pre-selected pressure
level to
release gas from the cell. The seal can be reversible or irreversible.
Alternatively,
as illustrated, outlets) 355 may always be open to the environment, in which
case an
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
36
airtight seal to the interior of the cell is maintained by blocl~iilg the
pressure release
channel 343 during normal operation.
[00145] Referring now to Fig. 24, cell 310 is illustrated having pressure
responsive switch 311 as illustrated in Fig. 23, but with a different venting
stnicture.
In particular, plug 345 includes a neck 353 that extends axially through
channel 343,
and defines an internal axially extending channel 359. A transverse ann 357 is
disposed at the axially outer end of plug 345, and provides a seal to chamiel
to
prevent gas from escaping into chamber 317 during normal operation. If the
internal
cell pressure reaches a predetermined threshold, however, arm 357 will
rupture,
thereby enabling the pressurized gas to exit the cell via chaimel 359 and
aperture
355. Because arm 357 ruptures during operation, the venting apparatus is
irreversible.
[00146] Referring now to Fig. 25, cell 310 is illustrated having pressure
responsive switch 311 as illustrated in Fig's. 23 and 24, but with a different
venting
structure. In particular, plug 345 includes a seal member 360 that is disposed
within
channel 343 and prevents pressurized gas from flowing into chamber 317. Seal
member 360 is connected via axially extending am 362 to a base plate 364 that
abuts the imzer surface of nubbin 320. Accordingly, when the interlal pressure
reaches a predetermined threshold to displace grommet 322 to open the
electrical
contact between members 326 and 328 as described above, seal member 360 is
displaced axially upstream with respect to gronnnet 322 and eyelet 324. Once
seal
member 360 is clear of the lower surface of eyelet 324, pressurized gas is
able to
flow through channel 343 and exit the cell via aperture 355. If the vent plug
base
plate 364 merely abuts the nubbin 320, but is not attached to nubbin 320, the
plug
will collapse within the cell during venting, thereby rendering the plug
unusable for
future use. However, base plate 364 may alternatively adhere to the inner
surface of
nubbin 320, in which case the stmctural integrity of plug 345 would be
maintained
dwing venting, thereby rendering plug 345 reversible.
[00147] The present invention recognizes that high voltages (generally witlun
the range of 1.2 and 2 V for AAA, AA, C, axzd C cells) and currents (generally
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
37
within the range of 4 and 15 Amps for size AAA and AA cells ) are typically
required when fast charging secondary size AA and AAA cells.
(00148] The result is that the cell is charged at a rate anywhere between 2C
and SOC, depending on the cell size. For instance, using the present
invention, a
2000 mAh AA cell can be charged at 3-SC, and preferably 4C, and an 800 mAh
AAA cell can be charged at 5-7C, and preferably 6C. It should be appreciated
that
size C and D cells that include a pressure switch constructed in accordance
with aaly
of the embodiments described herein can be charged using proportionately
higher
charge currents than AA and AAA cells to generate comparable C rates. For
example, a 3500 mAh C size cell can be charged at a C rate between 3-SC, and
preferably 4C 11S111g cmTents between 10 and 30 A.
[00149] Fast charging produces heat within the cell, thereby increasing cell
temperature during charging. Excessive temperatures have been found to damage
conventional cell components. Accordingly, the development of larger cells
that can
be fast charged has been limited by the temperatures that the cells can
withstand.
Many conventional high power applications would benefit from larger
rechargeable
cells, such as sub C size cells that can be used in, for example, power tools
and the
lilce.
[00150] Several battery systems are currently competing for dominance in
electric vehicles, including lead acid, nicl~el cadmium (NiCd), lithium ion,
zinc air
and nicl~el metal hydride (NiMH). To be acceptable to the driving public, it
is
desirable to minimize the time required to charge the batteries, perhaps no
more time
than is required to fuel existing vehicles with gasoline. This is an important
challenge that has historically limited the acceptance of an electric vehicle
battery
system.
[00151] As described above, an electrochemical cell, especially NiMH,
including a pressure switch that limits overcharge can be charged at constant
voltage. The combination of the pressure switch and the constant voltage
method of
charging permits the cell to be charged at ligh rates. Tlis decreases the time
required to charge a cell, which is a large advantage for a variety of
applications and
devices.
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
38
[00152] For examples large cells including an in-cell charge control
mechanism (i.e., pressure responsive switch of a type described herein) can be
used
in electric vehicle or hybrid electric vehicle batteries. Without limiting the
scope of
the present invention, batteries comprised of cells with in-cell charge
control can
range in sizes greater than 5 cm in length, height, and width, which are
typical of
those being developed commercially. It is, nonetheless, desirable to increase
a cell's
tolerance of elevated temperatures regardless of its size.
[00153] One embodiment of the present invention recognizes that judicious
selection of cell component materials reduces or eliminates the detrimental
effects of
fast charging. Materials capable of providing functionality at high
temperatures
enable the cells to be charged at higher rates. Furthermore, it is desirable
to design
current carrying components of the cell to minimize internal cell resistance,
as the
heat produced by a cell during a high rate charge increases as the cell
resistance
increases. It is therefore desirable to provide low-resistance and heat-stable
materials for fast charging. For example, in pressure responsive switches of
the type
described above, it may be desirable for the gronnnet, plug, insulator,
pressure stop,
and any other nonconductive components that are exposed to elevated
temperatures
during fast charging to comprise a thermally stable material. Otherwise, the
components may fail during operation. It has been determined that certain
properties of polymer materials allow the cell to function at h lgh
temperatures. In a
highly preferred embodiment, a polymer having a "dry as molded" tensile
strength
greater than 75MPa, a percent elongation at breal~ less than or equal to 50%,
aazd a
heat deflection temperature at 263 psi greater than or equal to 100 degrees
Celsius
offer sufficient functionality at the elevated temperatures lil~ely to be
experienced
during fast charging.
[00154] For example, Fig. 28A plots percent elongation at brealc as a function
of tensile strength, and Fig. 28B plots heat deflection temperature as a
function of
tensile strength. Figs. 28A and 28B illustrate that glass filled polyamides,
such as
glass filled nylon 6,6, glass filed nylon 6,12, and glass filed
polyphthalamide, and
aromatic polyamides such as non-glass filled polyphthalamide, as well as other
polymers having a tensile strength greater than 75Mpa and an elongation at
breal~ of
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
39
less than 50% satisfy the above-mentioned characteristics and are highly
preferred
polymers for use in nonconductive cell components that will be exposed to
elevated
temperatures when fast charging. The glass content in the polyamide materials
can
range from 1% to 50% by mass, and more preferably between 5% and 12% by mass.
It has also been found that polymers having a heat deflection temperature
greater
than 120 C, or more preferably greater than 200 C at 264 PSI are suitable for
the
above-described cell components. W some cases, it may be further desirable for
the
separator of the cell to be thermally stable, such as by using a
polypropylene, or
blended, or surface modified, or modified polypropylene or other such heat
stable
materials.
[00155] As discussed above, reducing cell resistance is desirable to limit the
temperature increase during charging. It has been determined that highly
conductive
nonferrous alloy materials could be used for the current carrying metal
components
of a secondary electrochemical cell to lower the cell resistance. For
instance, copper
alloys such as beryllimn-copper can be used, along with alternative metals
having
high thernal and electrical conductivity, including but not limited to silver
plated
electrical contacts or gold plated or Niclcel contacts, in addition to nickel-
plated steel
contacts. It should be appreciated that cells include current-carrying
components
that are also exposed to alkaline electrolyte, such as conductive tab 30 and
rivet 24
of Figs. 2A and 2B, conductive pin 116 and conductive tab 118 of Fig. 6A, and
comiector 160 of Fig. 7. It is desirable that these components, in addition to
being
highly conductive, also be chemically resistant to caustic solutions. Nickel
or
Nickel alloys have been found to produce desirable results due to their high
thermal
and electrical conductivity and low cost.
[00156] Reduced resistance of current carrying components, or other
components in direct contact with the current carrying components may be
achieved
by providing thicker electrode tabs, thereby reducing component resistance to
electric current flow and also increasing heat transfer. For instance,
referring to Fig.
6B, a size Sub C, F, C, or D (essentially any cell whose diameter is greater
than 15
mm), can benefit from a thicker electrode tab 118, wluch is between 5/1000 and
20/1000 inch as illustrated. It should be further appreciated that a 188 can
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
alternatively extend upwardly from each electrode 108 and contact rivet 116.
Furthermore, a conductive disc 125 can connect the bottom of each electrode
106 to
the outer cam 110 in order to increase the conductivity of the current
collector as is
commonly practiced in the art. The accommodation of high charge rates within
the
cell while reducing the heat generated within the cell being charged further
decreases the internal cell pressure. The reduction in pressure and heat
increases
length of time that the cell can be charged before the switch opens while
reducing
any chemical degradation of the cell's electrochemical capacity due to
extended
exposLUe to high temperatures.
[00157] It should be appreciated that the present invention is equally
applicable all NiMH cells, including larger sized cells (e.g., size AAA, AA,
and sub
C) along with small format (e.g., NiMH) cells, for example button cells, coin
cells
and smaller cylindrical cells, such as N and AAAA size cells, and prismatic
cells. It
is intended that small size cells include those cells having volumes less than
3 cm3.
One having ordinary shill in the art will appreciate that the embodiments
discussed
above in accordance with the present invention could be implemented in both
larger
sized NiMH cells and smaller sized NiMH cells. For instance, a pressure
responsive
switch in accordance with any embodiment described herein can be installed in
both
small format and large format NiMH cells. This increases the cell's
usefulness,
especially in applications of wireless devices such as GSM phones, PDAs,
hearing
aids, and headsets where fast charges (voltage levels between 1.2 and 2 V and
current levels between 4 and 15 A) are especially desirable, as small format
cells can
be charged within a few minutes using the fast charging method in combination
with
any of the above-described pressure switches.
[00158] The present invention further recognizes that the safety and
performance of conventional battery paclcs are maximized by first carefully
matched
the capacity of each cell in the pack to avoid overcharging or overdischarging
at
least once cell in the battery pack. Those skilled in the art recognize that
overdischarging a cell in a battery pack can cause the positive and negative
electrodes to reverse, and become the negative and positive electrodes,
respectively.
Accordingly, one embodiment of the present invention contemplates a plurality
of
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
41
secondary cells (including NiMH cells), at least one of which containing a
pressure
responsive switch, that protects against overcharge and overdischarge of
individual
cells in a battery string and fiirther eliminates the requirement of carefully
matching
cells and enables a battery pacl~ to be charged in only a matter of minutes. A
battery
pacle is defined broadly herein as a plurality of cells electrically connected
to
produce a voltage and/or a current output greater than the output of one of
the cells.
The pack can be configured to provide a standard size battery (for example,
when a
phlrality of AA or AAA size cells are comzected to provide a size C or D
battery), or
the pack can be configured to provide a current and/or voltage output greater
than
standard size batteries, such as battery pacl~s that are commonly used to
operate cell
phones, digital cameras, camcorders, power tools such as cliills and screw
drivers,
personal digital assistants or portable computers.
[00159] It should be appreciated that a plurality of cells could be installed
in a
battery pacl~ and connected in series within a charger that is configured to
supply a
constant voltage or constant current charge to the cell. In particular,
referring now
to Figs. 26A-26C, various examples of such battery paclcs 370 include a
plurality of
cells 372 arranged in one or snore strings, wherein each cell may contain a
pressure
responsive switch, in accordance with any of the aforementioned embodiments,
depending on the type of connection between the cells and strings. It should
be
appreciated that the battery pack can provide a large battery of the type
suitable for
electric vehicle or hybrid electric vehicle batteries and the life, or
alternatively can
comprise a plurality of smaller cells (e.g., size AA or AAA) that, in
combination,
provide a size C or D cell.
[00160] Fig. 26A illustrates a battery pacl~ 370 having a string 371 of cells
372 that are connected to a charger circuit 374 in series, Sllch that the
termination of
charging by the opening of the switch contacts in any one of the cells will
terminate
charging of each cell in the series. This embodiment contemplates that a
pressure
responsive switch can be installed in all cells of the series. Accordingly,
when the
cell having the lowest Charglllg capacity terminates charging, current will
cease to
flow tluough all cells in the string 371. As the cells remain in the charger
after the
initial charge termination, the switch in the most charged cell will
iteratively close
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
42
and open at a duty cycle, thereby permitting a charge to flow intermittently
through
all cells in the pack. The mismatched cells having a greater charging capacity
will
thus accept the intermittent charge (either constant current or constant
voltage
charge). The charging capacity of mismatched cells will decrease at a rate
faster
than that of the fully (or almost fully) charged cell(s), thereby enabling the
discharge
capacity of the mismatched cells) to recover relative to the fully charged
cell(s).
Advantageously, the present invention overcomes the need to carefully match
cells
in a given battery pacl~.
[00161] . In accordance with an alterlative embodiment, the string 371 can
include one cell having a relatively low charging capacity. Because the charge
capacity of that cell in the stl-ing 371 will not decrease to a level less
than the other
cells during normal operation (as all cells will be exposed to the same charge
current
and will also be discharged at the same rate), only that cell will contain a
pressure
responsive switch. Installing the switch in the cell having the lowest charge
capacity
thus ensures that none of the cells will become overcharged~during operation
so long
as the charger A) senses the open circuit condition caused by the cell whose
switch
has opened, and B) terninates the charge to prevent 1) overcharging of those
cells
that do not include a pressure switch, and 2) charging those cells without a
pressure
switch to a level greater than the cell with the pressure switch.
Alternatively, more
than one (but less than all) of the cells in the string 371 can include a
pressure
switch. This embodiment recoguzes that cost and resources will be conserved by
providing~a string of cells, wherein not every cell requires a pressure
switch.
[00162] Referring now to Fig. 26B, a battery pack 370 includes a string 371
of cells 372 that are connected to the charger circuit 374 in parallel to
increase the
discharge current of the battery pacl~. Because the cells 372 are connected in
parallel, a disconnection in the charging circuit of one cell will not
discontinue the
charge to all cells, but rather will increase the charging current available
to the cells
372 whose switches have not opened. A pressure responsive switch can be
installed
in each cell, if desired, to prevent cell overcharge.
[00163] As illustrated in Fig. 31A, the battery pack 370 illustrated in Fig.
26B
can be fabricated by providing a plurality of cells 372 (four in this
embodiment).
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
43
The positive terminals 376 and negative ends 378 of cells 372 are aligned. A
conductive disc 380 is provided having a plurality of apertures 382 extending
therethrough configured to receive the positive terminals 376. A pair of
conductive
circular discs 384 are provided and corrected (preferably welded) to the
positive
and negative ends of cells 372. The cells are then encased in a battery paclc
housing
such that the positive and negative ends of cells 372 are electrically
cormected to the
respective terninal ends of the housing, which can be configured as a D or C
size
cell. The housing can alternatively be configured to provide any alternative
cell that
would benefit from the inclusion of a plurality of AAA or AA size cells.
[00164] Fig. 26C contemplates that a battery paclc 370 could further include
more than one string 371 and 373 of cells 372 connected in series, wherein
each
string 371 is connected in parallel. In this embodiment, the pressure switch
disposed
in any given individual cell 372 of string 371 will cease charging for all
cells in that
string. However, because strings 371 and 373 are connected in parallel, cells
372 in
the remaining string 373 will continue charging until the pressure responsive
switch
in one of the cells of string 373 is actuated. It should be further
appreciated that any
number of strings may be connected, depending on the desired discharge
capacity of
the battery paclc 370. It should thus be appreciated that a pressure
responsive switch
cam be installed in only one cell of each string, as described above, or
alternatively
in each cell of one or more strings.
[00165] The fabrication of battery pacl~ 370 illustrated in Fig. 26C is
illustrated in Figs. 32A-B. In particular, the first string 371 of cells 372
is provided
with the positive terminals 376 aligned. The second string 373 is provided
with the
negative terminals 378 aligned with the positive terminals of the first string
371. A
pair of conductive tabs 388 is provided. One tab 388 connects the positive
terminal
ends 376 of the first string 371, while the other tab corrects the negative
terminal
ends 378 of the second string 373. The tabs 388 thus connect cells of each
string in
parallel. The strings 371 and 373 are connected in series via conductive disc
384
that is corrected (preferably welded) to the ends of cells 372 opposite tabs
388. An
insulating member 390 fits over disc 384 to prevent the disc from being
electrically
comzected to any external members. While disc 390 includes four apertures 392
that
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
44
would match the positive terminal ends of four cells (to accommodate the cell
terminal ends 376), though only a pair of apertures 392 need to be formed in
disc
390 in accordance with this embodiment to correspond with the terminal ends
376 of
string 373. Electrical connection to the cells 372 is thus provided only via
tabs 388.
Cells 372 are then inserted into a battery housing of desirable size.
[00166] The ability for mismatched. cells in a given string to recover their
capacity after only a few charge-discharge cycles depends upon the length of
time
that the cells are left in the charger after the switch of the first cell
begins to
iteratively open and close. For example, referring to Fig. 27, two matched
cells are
connected in series during cycles 1-8, and the charge and discharge capacity
of the
battery remains relatively constant. At cycle 9, a pair of mismatched cells
(one of
which having only a 25% charging capacity) is connected in series. When the
cells
are charged, one cell has a greater charge capacity than the other. However,
during
the charging cycle, if the cells remain in the charger past the time when the
lower
charge capacity cell switch begins iterating, the higher charge capacity cell
will
become charged at a higher rate relative to the iterating cell due to the
recombination
reaction (See Fig. 1) present in the iterating cell when the switch of the
iterating cell
is closed, thereby allowing current to flow through all connected cells.
Because the
remaining cells that are not fully charged are not yet undergoing a
recombination
reaction, they will continue to charge even as the iterating cell undergoes
the
recombination reaction. This trend will continue for a number of cycles (5
cycles
total in accordance with the illustrated embodiment) until the capacities of
the two
cells become equalized. Of course, the number of cycles is dependent upon the
length of time that a charge is applied to the cells after the first cell
begins to iterate.
[00167] It is well lmown that the discharge capacity of cells corrected in
parallel will reach equilibrium during discharge, as a higher current output
is
produced by the cell having the higher discharge capacity. The pressure
responsive
switch of the present invention also enables a plurality of mismatched cells
connected in series to become matched over a period of time.
[00168] In accordance with an alternate embodiment of the invention, it is
recognized that a user may prefer a shorter charging time, even if this
results in a
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
slightly reduced cell capacity during use. While the industry trend is to
constantly
strive to increase the capacity of the cells, the present embodiment
recognizes that it
may be desirable to reduce the capacity of the rechargeable cells, for
instance by
manufacturing electrodes of shorter lengths, or lesser tlucknesses, or with
filler
materials that are inert (defined herein as being non-reactive to cell
components or
chemicals), thereby reducing the volume of active cell components, as is
described
with reference to Figs. 33A-C. It is anticipated that between 20 and 40% of
the
active cell components, including anode and cathode, can reduce the time
necessary
to charge the cell, and further can increase cell efficiency, as will now be
described.
[00169] For instance, referring to Fig. 33A, a layer 127 of inert material is
inserted into electrode 106 such that electrode material is disposed on either
side of
layer 127. This increases the overall thiclrness of electrode 106, causing the
thiclmess of electrode 108 to be reduced. Alternatively, a layer 127 of inert
material
can be inserted into both electrodes 106 and 108 as illustrated in Fig. 33B.
Alternatively still, inert material 127 can be intermixed within either
electrode
(electrode 106 as illustrated in Fig. 33C), and an inert layer 127 can be
inserted into
the other electrode 108. Alternatively, the thickness of the other electrode
can be
reduced. Alternatively still, the inert material can be intermixed with both
electrodes 106 and 108. Alternatively still, a combination of reducing
electrode
thickness, inserting an inert layer, and intermixing inert material in either
or both
electrodes can reduce the active material. The embodiments described above
with
reference to Figs. 33A-C maintain the axial length (and hence the contact
surface
area) while decreasing the volume of active material. Because cell efficiency
is
determined by the ratio of surface contact area per vohune of active cell
materials,
the embodiments illustrated in Figs. 33A-C, and their equivalents, increase
cell
efficiency.
[00170] Alternatively, the length of the electrodes can be reduced. While this
would decrease the surface contact area (hence not increasing cell
efficiency), the
decrease in electrode length would result in a reduced length of time
necessary to
charge the cell.
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
46
[00171] In particular, the reduction of active volume in rechargeable cells
(e.g., to achieve a discharge capacity of 700-1600 mAh for size AA cells, and
200-
650 mAh for size AAA cells) has been found to decrease the charge time to only
a
few minutes when fast-charged (as described above) at constant voltage for
cells
with a pressure responsive switch constructed in accordance with any of the
embodiments described herein.
[00172] Such charge times render a NiMH rechargeable cell more
competitive with the fast charge time of supercapacitors, while preserving the
advantages inherent to a battery. For example, Fig. 29 illustrates charge
capacity as
a function of charge time. The charge capacity accepted by a size AA NiMH cell
having a pressure responsive switch in accordance with any of the above-
described
embodiments is charged to 800 mAh after only 5 minutes of charging, and lAh
after
only 7 minutes of charging. One benefit of a NiMH cell is its relatively flat
discharge voltage, while supercapacitors exhibit a steeply sloping discharge
voltage
curve.
[00173] Other advantages of a NiMH AA cell are illustrated in Fig. 30. Since
supercapacitors are not offered commercially in AA sizes, Fig. 30 sets forth
comparisons between a NiMH AA cell and a plurality of supercapacitors of
similar
volume (the NiMH cell and supercapacitors are herein collectively referred to
as
"cells" for the purposes of clarity and convenience). In particular, the
volume of the
cells is illustrated, along with the rated capacitance of each super capacitor
measured
in Farads. The nominal electromotive force (E) is measured in volts (V) for
all cells,
as is the discharge time in minutes. The charge capacity delivered is measured
in
Ampere-hours (Ah) for each cell, as is the energy delivered which is measured
in
Watt-hours (Wh). Finally, the cells are compared on the basis of energy
density,
measL~red in Wh per liter, and the internal resistance of each cell is
measured in
milli-olnns (mW). It can be observed that the energy density of the NiMH
battery is
orders of magnitude greater than the energy density of the supercapacitors
while the
internal resistance is similar to the supercapacitors, thereby enabling the
NiMH
battery to have a higher discharge rate.
CA 02503505 2005-04-22
WO 2004/038831 PCT/US2003/034363
47
[00174] The above description has been that of the preferred embodiment of
the present invention, and it will occur to those having ordinary slcill in
the art that
many modifications may be made without departing from the spirit and scope of
the
invention. In order to apprise the public of the various embodiments that may
fall in
the scope of the present invention, the following claims are made.