Note: Descriptions are shown in the official language in which they were submitted.
CA 02504153 2010-08-31
1
WO 20041041788 PCTIEP20031011515
Pyrimidine-4,6-dicarboxylic acid diamides for selectively inhibiting
collagenases
The invention relates to novel pyrimidine-4,6-dicarboxylic acid diamides
and to their use for selectively Inhibiting collagenase (MMP 13). The
pyrimidine-4,6-dicarboxylic acid diamides can therefore be used for treating
degenerative joint diseases.
It is known that pyrimidine-4,6-dicarboxylic acid diamides and 2,4-
substituted pyridine N-oxides Inhibit the enzymes proline hydroxylase and
lysine hydroxylase and thereby bring about an inhibition of collagen
biosynthesis by exerting an influence on the collagen-specific hydroxylation
reaction (EP 0418797; EP 0463592). This inhibition of collagen
biosynthesis results in the formation of a nonfunctional, under-hydroxylated
collagen molecule which the cells can only release into the extracellular
space in small quantity. In addition, the under-hydroxylated collagen cannot
be incorporated into the collagen matrix and is very readily degraded
proteolytically. As a consequence of these effects, the overall quantity of
collagen which is deposited extracellularly decreases. It is known from
patent applications WO 02/064571 and WO 02/064080 that certain
pyridine-2,4-dicarboxylic acid diamides and pyrimidine-4,6-dicarboxylic acid
diamides can be allosteric Inhibitors of MMP 13.
in diseases such as osteoarthritis and rheumatism, destruction of the joint
takes place, with this destruction being caused, in particular, by the
proteolytic breakdown of collagen due to collagenases. Collagenases
belong to the metalloproteinase (MP) or matrix metalloproteinase (MMP)
superfamily. Under physiological conditions, MMPs cleave collagen,
laminin, proteoglycans, elastin or gelatin and therefore play an important
role in bone and connective tissue. A large number of different inhibitors of
the MMPs and/or collagenases have been disclosed (EP 0 606 046; WO
94128889). Known MMP inhibitors frequently suffer from the disadvantage,
of lacking the specificity involved in inhibiting only one class of MMPs. As a
result, most MMP inhibitors inhibit several MMPs simultaneously because
the structure of the catalytic domain in the MMPs is similar. As a
consequence, the Inhibitors have the undesirable property of acting on
many enzymes, including those which have a vital function (Massova I., et
at, The FASEB Journal (1998)12, 1075-1095).
CA 02504153 2005-04-28
2
In an endeavor to find effective compounds for treating connective tissue
diseases, it has now been found that the compounds which are employed
in accordance with the invention are powerful inhibitors of matrix
metalloproteinase 13 while essentially having no effect on MMPs 3 and 8.
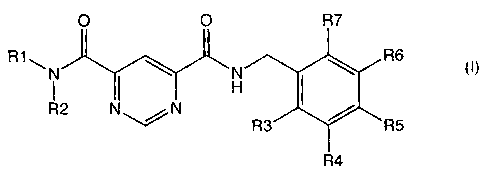
The invention therefore relates to a compound of the formula I
O O R7
R6
R1,,N H {
R2\%N #R3 R5
R4
and/or all the stereoisomeric forms of the compound of the formula I and/or
mixtures of these forms in any ratio, and/or a physiologically tolerated salt
of the compound of the formula I, where
for the case a)
R1 is hydrogen atom or -(C1-C6)-alkyl,
R2 is -(C1-C6)-alkyl, where alkyl is substituted, once, twice or three
times, by
1. -(C1-C6)-alkyl-O-(C6-C14)-aryl,
2. -(Co-C6)-alkyl-N(R8)-C(O)-O-(C1-C6)-alkyl, in which R8 is
i) hydrogen atom or
ii) -(C1-C6)-alkyl,
3. -(C(O)-N(R9)-(R10), in which R9 and R10 are identical or
different and are, independently of each other,
i) hydrogen atom or
ii) -(C1-C6)-alkyl, or
R9 and R10 form, together with the nitrogen atom to which
they are bonded, a 5-, 6- or 7-membered saturated ring,
where a heteroatom from the series oxygen, sulfur and
nitrogen can also replace one or two further carbon atoms
and, in the case of nitrogen, the nitrogen atoms can,
independently of each other, be unsubstituted or substituted
by (C1-C6)-alkyl,
4. -(C6-C14)-aryl, in which aryl is substituted, once, twice or
three times, independently of each other, by
CA 02504153 2005-04-28
3
4.1) -CH2-C(O)-O-R8, in which R8 has the
abovementioned meaning,
4.2) -(C0-C6)-alkyl-C(O)-N(R9)-(R10), in which R9 and
R10 have the abovementioned meaning,
4.3) -(C0-C6)-alkyl-C(O)-NH-CN,
4.4) -O-(C0-C6)-alkyl-C(O)-N(R9)-(R10), in which R9 and
R10 have the abovementioned meaning,
4.5) -S(O)y-(C1-C6)-alkyl-C(O)-0-R8, in which R8 has the
abovementioned meaning and y is 1 or 2,
4.6) -S(O)Z-(C1-C6)-alkyl-C(O)-N(R9)-(R10), in which R9
and R10 have the abovementioned meaning and z is
0, 1 or 2,
4.7) -(CO-C6)-alkyl-C(O)-N(R8)-(C0-C6)-alkyl-N(R9)-
(R10), in which R8, R9 and R10 have the
abovementioned meaning,
4.8) -C(O)-N(R8)-(C0-C6)-alkyl-Het, where R8 has the
abovementioned meaning and Het is a saturated or
unsaturated monocyclic or bicyclic, 3- to 10-
membered heterocyclic ring system which contains
1, 2 or 3 identical or different ring heteroatoms from
the series nitrogen, oxygen and sulfur and is
unsubstituted or substituted, once, twice or three
times, independently of each other, by
a) halogen,
b) cyano,
c) nitro
d) hydroxyl,
e) amino,
f) -C(O)-O-(C1-C6)-alkyl,
g) -C(O)-OH,
h) -(C1-C6)-alkyl, where alkyl is unsubstituted or
substituted, once, twice or three times, by
halogen,
i) -O-(C1-CO-alkyl, where alkyl is unsubstituted or
substituted, once, twice or three times, by
halogen,
4.9) -C(O)-N(R8)-(C0-C6)-alkyl-(C6-C14)-aryl, where aryl
is unsubstituted or substituted, once, twice or three
CA 02504153 2005-04-28
4
times, independently of each other, by the
abovementioned radicals a) to i),
4.10) -CH2-N(R9)-(R10), in which R9 and R10 have the
abovementioned meaning,
4.11) -(CH2)y-N(R8)-C(O)-(C1-C6)-alkyl in which alkyl is
unsubstituted or substituted, once, twice or three
times, independently of each other, by the
abovementioned radicals a) to i) and y is 1 or 2,
4.12) -(CH2)X-N(R8)-C(O)-(Co-C6)-alkyl-(C6-C14)-aryl in
which aryl is unsubstituted or substituted, once, twice
or three times, independently of each other, by the
abovementioned radicals a) to i) and x is 0, 1, 2, 3 or
4,
4.13) -(CH2)X-N(R8)-C(O)-(Co-C6)-alkyl-Het in which Het is
unsubstituted or substituted, once, twice or three
times, independently of each other, by the
abovementioned radicals a) to i), and x is 0, 1, 2, 3 or
4,
4.14) -(CH2)X-N(R8)-C(O)-O-(C1-C6)-alkyl in which alkyl is
unsubstituted or substituted, once, twice or three
times, independently of each other, by the
abovementioned radicals a) to i) and x is 0, 1, 2, 3 or
4,
4.15) -(CH2)X-N(R8)-C(O)-O-(CO-C6)-alkyl-(C6-C14)-aryl in
which aryl is unsubstituted or substituted, once, twice
or three times, independently of each other, by the
abovementioned radicals a) to i), and x is 0, 1, 2, 3 or
4,
4.16) -(CH2)X-N(R8)-C(O)-O-(CO-C6)-alkyl-Het in which
Het is unsubstituted or substituted, once, twice or
three times, independently of each other, by the
abovementioned radicals a) to i), and x is 0, 1, 2, 3 or
4,
4.17) -(CH2)X-N(R8)-C(O)-N(R11)-R12 in which R8 and x
have the abovementioned meaning and R11 and
R12 are identical or different and are, independently
of each other,
4.17.1) hydrogen atom,
4.17.2) -(C1-C6)-alkyl,
CA 02504153 2005-04-28
4.17.3) -(Co-C6)-alkyl-(C6-C14)-aryl in which aryl
is unsubstituted or substituted, once,
twice or three times, independently of
each other, by the abovementioned
5 radicals a) to i),
4.17.4) -(Co-C6)-alkyl-Het in which Het is
unsubstituted or substituted, once, twice
or three times, independently of each
other, by the abovementioned radicals a)
to i),
4.17.5) -C(O)-(C1-C6)-alkyl,
4.17.6) -C(O)-(Co-C6)-alkyl-(C6-C14)-aryl,
4.17.7) -C(O)-(Co-C6)-alkyl-Het,
4.17.8) -S02-(C1-C6)-alkyl,
4.17.9) -S02-(Co-C6)-alkyl-(C6-C14)-aryl,
4.17.10) -S02-(Co-C6)-alkyl-Het
4.18) -(CH2)X-N(R8)-S(0)2-(Co-C6)-alkyl-(C6-C14)-aryl in
which aryl is unsubstituted or substituted, once, twice
or three times, independently of each other, by the
abovementioned radicals a) to i) and x and R8 have
the abovementioned meaning,
4.19) -(CH2)X-N(R8)-S(O)2-(Co-C6)-alkyl-Het, in which Het
is unsubstituted or substituted, once, twice or three
times, independently of each other, by the
abovementioned radicals a) to i), and x and R8 have
the abovementioned meaning,
4.20) -(CH2)X-N(R8)-S(0)2-N(R8)-(C1-C6)-alkyl in which
alkyl is unsubstituted or substituted, once, twice or
three times, independently of each other, by the
abovementioned radicals a) to i) and x and R8 have
the abovementioned meaning, independently of each
other,
4.21) -(CH2)X-N(R8)-S(O)2-N(R8)-(Co-C6)-alkyl-(C6-C14)-
aryl in which aryl is unsubstituted or substituted,
once, twice or three times, independently of each
other, by the abovementioned radicals a) to i) and x
CA 02504153 2005-04-28
6
and R8 have the abovementioned meaning,
independently of each other,
4.22) -(CH2)x-N(R8)-S(0)2-N(R8)-(Co-C6)-alkyl-Het in
which Het is unsubstituted or substituted, once, twice
or three times, independently of each other, by the
abovementioned radicals a) to i) and x and R8,
independently of each other, have the
abovementioned meaning,
4.23) -(CH2)X-N(R8)-C(O)-N(R8)-SO2-R13, where x and
R8, independently of each other, have the
abovementioned meaning and R13 is -(C1-C6)-alkyl
or -(Co-C6)-alkyl-(C6-C14)-aryl,
4.24) -S(0)2-N(R8)-(Co-C6)-alkyl-(C6-C14)-aryl in which
aryl is unsubstituted or substituted, once, twice or
three times, independently of each other, by the
abovementioned radicals a) to i) and R8 has the
abovementioned meaning,
4.25) S(O)2-N(R8)-(Co-C6)-alkyl-Het in which Het is
unsubstituted or substituted, once, twice or three
times, independently of each other, by the
abovementioned radicals a) to i) and R8 has the
abovementioned meaning,
4.26) -S(O)2-N(R8)-(C1-C6)-alkyl in which alkyl is
unsubstituted or substituted, once, twice or three
times, independently of each other, by the
abovementioned radicals a) to i) and R8 has the
abovementioned meaning,
4.27) -S(O)2-(Co-C6)-alkyl-(C6-C14)-aryl in which aryl is
unsubstituted or substituted, once, twice or three
times, independently of each other, by the
abovementioned radicals a) to i),
4.28) -S(0)2-(Co-C6)-alkyl-Het in which Het is
unsubstituted or substituted, once, twice or three
times, independently of each other, by the
abovementioned radicals a) to i),
4.29) -0-Het in which Het is unsubstituted or substituted,
once, twice or three times, independently of each
other, by the abovementioned radicals a) to i), or
CA 02504153 2005-04-28
7
4.30) -Het in which Het is unsubstituted or substituted,
once, twice or three times, independently of each
other, by the abovementioned radicals a) to i), or
4.31) -phenyl where the phenyl ring is unsubstituted or
substituted, once, twice or three times, by
4.31.1) halogen,
4.31.2) -(C1-C6)-alkyl,
4.31.3) -O-(Cl-C6)-alkyl,
4.31.4) -S(O)2-R16 where R16 is (C1-C6)-
alkyl or -NH2,
5. -C(O)-N(R8)-(Co-C6)-alkyl-(C6-C14)-aryl in which aryl is
unsubstituted or substituted, once, twice or three times,
independently of each other, by the abovementioned radicals
4.1) to 4.31) or 4.8) a) to 4.8) i) and R8 has the
abovementioned meaning, or
6. -C(O)-N(R8)-(C0-C6)-alkyl-Het in which Het has the
abovementioned meaning and is unsubstituted or
substituted, once, twice or three times, independently of
each other, by the abovementioned radicals 4.1) to 4.31) or
4.8) a) to 4.8) i) and R8 has the abovementioned meaning,
or
7. -NH-(C6-C14)-aryl in which aryl is unsubstituted or
substituted, once, twice or three times, independently of
each other, by the abovementioned radicals 4.1) to 4.30) or
4.8) a) to 4.8) i), or
8. -NH-Het in which Het has the abovementioned meaning and
is unsubstituted or substituted, once, twice or three times,
independently of each other, by the abovementioned radicals
4.1) to 4.31) or 4.8) a) to 4.8) i),
R3, R4, R5, R6 and R7 are identical or different and are, independently of
each other,
1. hydrogen atom,
2. halogen,
3. -(C1-C6)-alkyl in which alkyl is unsubstituted or substituted, once,
twice or three times, by halogen,
4. -O-(C1-C6)-alkyl in which alkyl is unsubstituted or substituted,
once, twice or three times, by halogen, or
CA 02504153 2005-04-28
8
5. -S-(C1-C6)-alkyl, or
R4 and R5 or R5 and R6 form, together with the carbon atoms to which
they are in each case bonded, independently of each other, a 5- or 6-
membered ring which is aromatic or saturated and contains zero, one or
two heteroatoms from the series oxygen, nitrogen or sulfur, where the ring
is unsubstituted or is substituted, at one or at several carbon atoms, once
or twice, by halogen and the other radicals R3, R6 and R7 or R3, R4 and
R7 have the abovementioned meaning of 1. to 5.
or for the case b)
R1 is hydrogen atom or -(C1-C6)-alkyl,
R2 is -(C1-C6)-alkyl, where alkyl is substituted, once, twice or three times,
by
1. -C(O)-O-R8', in which R8' is
1.1) hydrogen atom or
1.2) -(C1-C6)-alkyl,
2. -(C1-C6)-alkyl-O-R8', in which R8' has the abovementioned
meaning,
3. -(C6-C14)-aryl in which aryl is substituted, once, twice or
three times, independently of each other, by
3.1) -(C2-C6)-alkyl-C(O)-O-R8' in which R8' has the
abovementioned meaning,
3.2) -O-(C1-C6)-alkyl-C(O)-O-R8' in which R8' has the
abovementioned meaning,
3.3) -N(R14)-(R15) in which R14 and R15 form, together
with the nitrogen atom to which they are bonded, a
5-, 6- or 7-membered saturated ring, where a
heteroatom from the series oxygen, sulfur and
nitrogen can also replace one or two further carbon
atoms and, in the case of nitrogen, the nitrogen
atoms can, independently of each other, be
unsubstituted or substituted by (C1-C6)-alkyl,
3.4) -(CH2)k-N(R9')-(RlO') in which k is 2, 3, 4 or 5 and
R9' and R10' are identical or different and are,
independently of each other,
3.4.1) hydrogen or
CA 02504153 2005-04-28
9
3.4.2) -(C1-C6)-alkyl, or
R9' and RIO' form, together with the nitrogen atom to
which they are bonded, a 5-, 6- or 7-membered
saturated ring, where a heteroatom from the series
oxygen, sulfur and nitrogen can also replace one or
two further carbon atoms and, in the case of
nitrogen, the nitrogen atoms can, independently of
each other, be unsubstituted or substituted by
(C1-C6)-alkyl,
3.5) -O-(C2-C6)-alkyl-N(R9')-R10', where R9' and R10'
have the abovementioned meaning,
3.6) -N(R8')-C(O)-(C1-C6)-alkyl in which alkyl is
unsubstituted or substituted, once, twice or three
times, by
3.6.1) halogen,
3.6.2) cyano,
3.6.3) nitro
3.6.4) hydroxyl,
3.6.5) amino,
3.6.6) -C(0)-0-(C1-C6)-alkyl, or
3.6.7) -C(O)-OH, and R8' has the above-
mentioned meaning,
3.7) -phenyl, where the phenyl ring is unsubstituted or
substituted, once, twice or three times, by
3.7.1) halogen,
3.7.2) -(C1-CO-alkyl,
3.7.3) -O-(C1-C6)-alkyl,
3.7.4) -S(O)2-R16', where R16' is (C1-C6)-alkyl
or -NH2,
4. Het, where Het is a saturated or unsaturated monocyclic or
bicyclic, 3- to 10-membered heterocyclic ring system which
contains 1, 2 or 3 identical or different ring heteroatoms from
the series nitrogen, oxygen and sulfur and is unsubstituted or
substituted, once, twice or three times, by
4.1) halogen,
4.2) cyano,
4.3) nitro,
CA 02504153 2005-04-28
4.4) hydroxyl,
4.5) amino,
4.6) -C(O)-O(C1-C6)-alkyl,
4.7) -C(O)-OH,
5 4.8) -(C1-C6)-alkyl, where alkyl is unsubstituted or
substituted, once, twice or three times, by halogen,
4.9) -O-(C1-C6)-alkyl, where alkyl is unsubstituted or
substituted, once, twice or three times, by halogen,
4.10) pyridyl, or
10 4.11) phenyl, where phenyl is unsubstituted or substituted,
once or more than once and independently of each
other, by a radical from the series halogen, -(C1-C6)-
alkoxy and -(C1-C6)-alkyl, and
R4 and R5 or R5 and R6 form, together with the carbon atoms to
which they are in each case bonded, independently of each
other, a 5- or 6-membered ring which is saturated and contains
one or two heteroatoms from the series oxygen, nitrogen or
sulfur, where the ring is unsubstituted or substituted, at one or at
several carbon atoms, once or twice, by halogen, and the other
radicals R3, R6 and R7 or R3, R4 and R7 are hydrogen, with the
proviso that the unsubstituted benzo[1,3]dioxole ring is excluded.
Michael Murray showed that compounds which contain an unsubstituted
benzo[1,3]dioxole ring as a radical inhibit the cytochrome P450 liver
enzymes (Michael Murray, Current Drug Metabolism 2000, 67-84). Said
radical is held to be responsible for these significant toxicological effects.
For this reason, it has been excluded in the compounds of the formula I.
The invention also relates to a compound of the formula I where, for the
case a)
R1 is hydrogen atom or -(C1-C6)-alkyl,
R2 is -(C1-C6)-alkyl, where alkyl is substituted, once, twice or three
times, by
1. -(C1-C6)-alkyl-O-(C6-C14)-aryl,
2. -(Co-C6)-alkyl-N(R8)-C(O)-O-(C1-C6)-alkyl, in which R8 is
i) hydrogen atom
ii) -(C1-C6)-alkyl, in which alkyl is unsubstituted or
substituted, once, twice or three times, independently
CA 02504153 2005-04-28
11
of each other, by -NH2, -CN, -OH, -C(O)-OH, -
C(O)-0-(C1-C6)-alkyl, -C(O)-NH-OH, NO2 or halogen, or
iii) OH,
3. -C(O)-N(R9)-(R10), in which R9 and R10 are identical or
different and are, independently of each other,
i) hydrogen atom or
ii) -(C1-C6)-alkyl, or
R9 and R10 form, together with the nitrogen atom to
which they are bonded, a 5-, 6- or 7-membered
saturated ring where a heteroatom from the series
oxygen, sulfur and nitrogen can also replace one or
two further carbon atoms and, in the case of
nitrogen, the nitrogen atoms can, independently of
each other, be unsubstituted or substituted by
(Cl-C6)-alkyl,
4. phenyl, in which phenyl is substituted, once, twice or three
times, independently of each other, by
4.1) -(Co-C6)-alkyl-C(O)-O-R8, in which R8 has the
abovementioned meaning,
4.2) -(Co-C6)-alkyl-C(O)-N(R9)-(R10), in which R9 and
R10 have the abovementioned meaning,
4.3) -(Co-C6)-alkyl-C(O)-NH-CN,
4.4) -(C0-C6)-alkyl-C(O)-(C0-C6)-alkyl-Het, where Het is
a radical from the group: azepine, azetidine,
aziridine, benzimidazole, benzofuran,
benzo[1,4]dioxin, 1,3-benzodioxole, 4H-
benzo[1,4]oxazine, benzoxazole, benzothiazole,
benzothiophene, quinazoline, quinoline, quinoxaline,
chroman, cinnoline, 1,2-diazepine, 1,3-diazepine,
1,4-diazepine, 1,4-dioxin, dioxole, furan, imidazole,
indazole, indole, isoquinoline, isochroman, isoindole,
isothiazole, isoxazole, morpholine, 1,2-oxazine, 1,3-
oxazine, 1,4-oxazine, oxazole, oxiran, piperazine,
piperidine, phthalazine, pyran, pyrazine, pyrazole,
pyridazine, pyridine, pyrimidine, pyridoimidazole,
pyridopyridine, pyridopyrimidine, pyrrole, pyrrolidine,
tetrazole, 1,2-thiazine, 1,3-thiazine, 1,4-thiazine,
thiazole, thiomorpholine, thiophene, thiopyran, 1,2,3-
triazine, 1,3,5-triazine, 1,2,4-triazine, 1,2,3-triazole or
CA 02504153 2005-04-28
12
1,2,4-triazole, and in which Het is unsubstituted or
substituted, once, twice or three times, independently
of each other, by
a) halogen,
b) cyano,
c) nitro,
d) hydroxyl,
e) amino,
f) -C(O)-O-(C1-C6)-alkyl,
g) -C(O)-OH,
h) -(C1-C6)-alkyl, where alkyl is unsubstituted or
substituted, once, twice or three times, by
halogen,
i) -O-(C1-C6)-alkyl, where alkyl is unsubstituted or
substituted, once, twice or three times, by
halogen, or
-N(R9)-(R10),
1) =0,
k) -Het, in which Het is defined as above,
I) -(C2-C6)-alkenyl, where alkenyl is unsubstituted
or substituted, once, twice or three times, by
halogen, or
-N(R9)-(R10), or
m) -(C2-C6)-alkynyl, where alkynyl is unsubstituted
or substituted, once, twice or three times, by
halogen or -N(R9)-(R10),
4.5) -(CO-C6)-alkyl-C(O)-(CO-C6)-alkyl-OH,
4.6) -O-(CO-C6)-alkyl-C(O)-N(R9)-(R10), in which R9 and
R10 have the abovementioned meaning,
4.7) -(CO-C6)-alkyl-C(O)-N(R8)-(CO-C6)-alkyl-N(R9)-
(R10), in which R8, R9 and R10 have the
abovementioned meaning,
4.8) -(CO-C4)-alkyl-N(R8)-3(0)2-(CO-C6)-alkyl-Het, in
which Het is defined as above and is unsubstituted or
substituted, once, twice or three times, independently
of each other, by the abovementioned radicals a) to
m) and R8 has the abovementioned meaning,
4.9) -(CO-C4)-alkyl-S(O)2-(CO-C6)-alkyl-(C6-C14)-phenyl,
in which phenyl is unsubstituted or substituted, once,
CA 02504153 2005-04-28
13
twice or three times, independently of each other, by
the abovementioned radicals a) to m),
4.10) -(CO-C6)-alkyl-C(O)-N(R8)-(CO-C6)-alkyl-Het, where
R8 has the abovementioned meaning and Het has
the abovementioned meaning and is unsubstituted or
substituted, once, twice or three times, independently
of each other, by the abovementioned radicals a) to
m),
4.11) -(CO-C6)-alkyl-C(O)-N(R8)-(CO-C6)-alkyl-(C6-C14)-
phenyl, where phenyl is unsubstituted or substituted,
once, twice or three times, independently of each
other, by the abovementioned radicals a) to m),
4.12) -(CO-C6)-alkyl-N(R9)-(R10), in which R9 and R10
have the abovementioned meaning,
4.13) -(CH2)y-N(R8)-C(O)-(C1-C6)-alkyl, in which alkyl is
unsubstituted or substituted, once, twice or three
times, independently of each other, by the
abovementioned radicals a) to m) and y is 1 or 2,
4.14) -(CO-C4)-alkyl-N(R8)-C(O)-(CO-C6)-alkyl-(C6-C14)-
phenyl, in which phenyl is unsubstituted or
substituted, once, twice or three times, independently
of each other, by the abovementioned radicals a) to
m),
4.15) -(CO-C4)-alkyl-N(R8)-C(O)-(CO-C6)-alkyl-Het, in
which Het is unsubstituted or substituted, once, twice
or three times, independently of each other, by the
abovementioned radicals a) to m),
4.16) -(CO-C4)-alkyl-N(R8)-C(O)-O-(C1-C6)-alkyl, in which
alkyl is unsubstituted or substituted, once, twice or
three times, independently of each other, by the
abovementioned radicals a) to m),
4.17) -(CO-C4)-alkyl-N(R8)-C(O)-O-(C1-C6)-alkenyl, in
which alkenyl is unsubstituted or substituted, once,
twice or three times, independently of each other, by
the abovementioned radicals a) to m),
4.18) -(CO-C4)-alkyl-N(R8)-C(O)-O-(C1-C6)-alkynyl, in
which alkynyl is unsubstituted or substituted, once,
twice or three times, independently of each other, by
the abovementioned radicals a) to m),
CA 02504153 2005-04-28
14
4.19) -(CO-C4)-alkyl-N(R8)-C(O)-O-(CO-C6)-alkyl-
(C6-C14)-phenyl, in which phenyl is unsubstituted or
substituted, once, twice or three times, independently
of each other, by the abovementioned radicals a) to
m),
4.20) -(CO-C4)-alkyl-N(R8)-C(O)-O-(CO-C6)-alkyl-Het, in
which Het is defined as above and is unsubstituted or
substituted, once, twice or three times, independently
of each other, by the abovementioned radicals a) to
m),
4.21) -(CO-C4)-alkyl-N(R8)-C(O)-(CO-C6)-alkyl-N(R11)-
R12, in which R8 has the abovementioned meaning
and R11 and R12 are identical or different and are,
independently of each other,
4.21.1) a hydrogen atom,
4.21.2) -(C1-C6)-alkyl,
4.21.3) -(CO-C6)-alkyl-(C6-C14)-phenyl, in
which phenyl is unsubstituted or
substituted, once, twice or three
times, independently of each other, by
the abovementioned radicals a) to m),
4.21.4) -(CO-C6)-alkyl-Het, in which Het is
defined as above and is unsubstituted
or substituted, once, twice or three
times, independently of each other, by
the abovementioned radicals a) to m),
4.21.5) -C(O)-(C1-C6)-alkyl, in which alkyl is
unsubstituted or substituted, once,
twice or three times, independently of
each other, by the abovementioned
radicals a) to m),
4.21.6) -C(O)-(C3-C6)-cycloalkyl, in which
cycloalkyl is unsubstituted or
substituted, once, twice or three
times, independently of each other, by
the abovementioned radicals a) to m),
4.21.7) -C(O)-(CO-C6)-alkyl-(C6-C14)-phenyl,
in which phenyl is unsubstituted or
substituted, once, twice or three
CA 02504153 2005-04-28
times, independently of each other, by
the abovementioned radicals a) to m),
4.21.8) -C(O)-(CO-C6)-alkyl-Het, in which Het
is defined as above and is
5 unsubstituted or substituted, once,
twice or three times, independently of
each other, by the abovementioned
radicals a) to m),
4.21.9) -S02-(CO-C6)-alkyl, in which alkyl is
10 unsubstituted or substituted, once,
twice or three times, independently of
each other, by the abovementioned
radicals a) to m),
4.21.10) -NH-S02-(CO-C6)-alkyl, in which alkyl
15 is unsubstituted or substituted, once,
twice or three times, independently of
each other, by the abovementioned
radicals a) to m),
4.21.11) -S02-(CO-C6)-alkyl-(C6-C1 4)-phenyl-
(CO-C6)-alkyl, in which phenyl is
unsubstituted or substituted, once,
twice or three times, independently of
each other, by the abovementioned
radicals a) to m),
4.21.12) -S02-(CO-C6)-alkyl-Het, in which Het
is defined as above and is
unsubstituted or substituted, once,
twice or three times, independently of
each other, by the abovementioned
radicals a) to m),
4.22) -O-(CO-C6)-alkyl-Het, in which Het is defined as
above and is unsubstituted or substituted, once,
twice or three times, independently of each other, by
the abovementioned radicals a) to m), or
4.23) -(CO-C4)-alkyl-Het, in which Het is defined as above
and is unsubstituted or substituted, once, twice or
three times, independently of each other, by the
abovementioned radicals a) to m),
CA 02504153 2005-04-28
16
5. -C(O)-N(R8)-(Co-C6)-alkyl-phenyl, in which phenyl is
unsubstituted or substituted, once, twice or three times,
independently of each other, by the abovementioned radicals
4.1) to 4.23) or 4.4) a) to 4.4) m) and R8 has the
abovementioned meaning, or
6. -C(O)-N(R8)-(Co-C6)-alkyl-Het, in which Het is azepine,
azetidine, aziridine, benzimidazole, benzofuran,
benzo[1,4]dioxin, 1,3-benzodioxole, 4H-benzo[1,4]oxazine,
benzoxazole, benzothiazole, benzothiophene, quinazoline,
quinoline, quinoxaline, chroman, cinnoline, 1,2-diazepine,
1,3-diazepine, 1,4-diazepine, 1,4-dioxin, dioxole, furan,
imidazole, indazole, indole, isoquinoline, isochroman,
isoindole, isothiazole, isoxazole, morpholine, 1,2-oxazine,
1,3-oxazine, 1,4-oxazine, oxazole, oxirane, piperazine,
piperidine, phthalazine, pyran, pyrazine, pyrazole,
pyridazine, pyridine, pyrimidine, pyridoimidazole,
pyridopyridine, pyridopyrimidine, pyrrole, pyrrolidine,
tetrazole, 1,2-thiazine, 1,3-thiazine, 1,4-thiazine, thiazole,
thiomorpholine, thiophene, thiopyran, 1,2,3-triazine, 1,3,5-
triazine, 1,2,4-triazine, 1,2,3-triazole or 1,2,4-triazole, and
Het is unsubstituted or substituted, once, twice or three
times, independently of each other, by the abovementioned
radicals 4.1) to 4.4) or 4.4) a) to 4.4) m) and R8 has the
abovementioned meaning,
R3, R4, R5, R6 and R7 are identical or different and are, independently of
each other,
1. hydrogen atom,
2. halogen,
3. -(C1-C6)-alkyl, in which alkyl is unsubstituted or substituted,
once, twice or three times, by halogen, or
4. -O-(C1-C6)-alkyl, in which alkyl is unsubstituted or
substituted, once, twice or three times, by halogen, or
R4 and R5 or R5 and R6 form, together with the carbon atoms to which
they are in each case bonded, independently of each other, a dioxane,
dioxole, dihydrofuran or furan ring, where the ring is unsubstituted or
substituted, at one or at several carbon atoms, once or twice, by halogen
CA 02504153 2005-04-28
17
and the other radicals R3, R6 and R7 or R3, R4 and R7 have the
abovementioned meaning of 1. to 4,
or for the case b)
R1 is hydrogen atom or -(C1-C4)-alkyl,
R2 is -(C1-C4)-alkyl, where alkyl is substituted, once, twice or three times,
by
1. -C(O)-O-R8', in which R8' is
1.1) hydrogen atom or
1.2) -(C1-C4)-alkyl,
2. -(C1-C4)-alkyl-O-R8', in which R8' has the abovementioned
meaning,
3. phenyl, in which phenyl is substituted, once, twice or three
times, independently of each other, by
3.1) -(C2-C4)-alkyl-C(O)-O-R8', in which R8' has the
abovementioned meaning,
3.2) -O-(C1-C4)-alkyl-C(O)-O-R8', in which R8' has the
abovementioned meaning,
3.3) -N(R14)-(R15) in which R14 and R15 form, together
with the nitrogen atom to which they are bonded, a
radical which can be derived from pyrrolidine,
piperidine, pyrazolidine, pyrazine, tetrazine,
imidazolidine, piperazine, isoxazolidine, morpholine,
isothiazolidine or thiomorpholine, and, in the case of
nitrogen, the nitrogen atoms can, independently of
each other, be unsubstituted or substituted by
(C 1-C4)-alkyl,
3.4) -(CH2)k-N(R9')-(RlO') in which k is 2, 3, 4 or 5 and
R9' and R10' are identical or different and are,
independently of each other,
3.4.1) hydrogen atom or
3.4.2) -(C1-C6)-alkyl, or
R9' and R10' form, together with the nitrogen atom to
which they are bonded, a radical which can be
derived from pyrrolidine, piperidine, pyrazolidine,
pyrazine, tetrazine, imidazolidine, piperazine,
isoxazolidine, morpholine, isothiazolidine or
thiomorpholine, and, in the case of nitrogen, the
CA 02504153 2005-04-28
18
nitrogen atoms can, independently of each other, be
unsubstituted or substituted by (C1-C4)-alkyl,
3.5) -O-(C2-C6)-alkyl-N(R9')-R10', where R9' and R10'
have the abovementioned meaning,
3.6) -N(R8')-C(O)-(Cl-C6)-alkyl, in which alkyl is
unsubstituted or substituted, once, twice or three
times, by
3.6.1) halogen,
3.6.2) cyano,
3.6.3) nitro
3.6.4) hydroxyl,
3.6.5) amino,
3.6.7) -C(O)-O-(C1-C6)-alkyl, or
3.6.8) -C(O)-OH, and R8' has the abovementioned
meaning,
3.7) -phenyl, where the phenyl ring is unsubstituted or
substituted, once, twice or three times, by
3.7.1) halogen,
3.7.2) -(C1-C6)-alkyl,
3.7.3) -O-(C1-C6)-alkyl, or
3.7.4) -S(O)2-R16', where R16' is (C1-C6)-alkyl or
-N H2,
4. Het, where Het is azepine, azetidine, aziridine,
benzimidazole, benzofuran, benzo[1,4]dioxin,
1,3-benzodioxole, 4H-benzo[1,4]oxazine, benzoxazole,
benzothiazole, benzothiophene, quinazoline, quinoline,
quinoxaline, chroman, cinnoline, 1,2-diazepine, 1,3-
diazepine, 1,4-diazepine, 1,4-dioxin, dioxole, furan,
imidazole, indazole, indole, isoquinoline, isochroman,
isoindole, isothiazole, isoxazole, morpholine, 1,2-oxazine,
1,3-oxazine, 1,4-oxazine, oxazole, oxirane, piperazine,
piperidine, phthalazine, pyran, pyrazine, pyrazole,
pyridazine, pyridine, pyrimidine, pyridoimidazole,
pyridopyridine, pyridopyrimidine, pyrrole, pyrrolidine,
tetrazole, 1,2-thiazine, 1,3-thiazine, 1,4-thiazine, thiazole,
thiomorpholine, thiophene, thiopyran, 1,2,3-triazine,
1,3,5-triazine, 1,2,4-triazine, 1,2,3-triazole or 1,2,4-triazole,
CA 02504153 2005-04-28
19
and Het is unsubstituted or substituted, once, twice or three
times, independently of each other, by
4.1) halogen,
4.2) cyano,
4.3) nitro,
4.4) hydroxyl,
4.5) amino,
4.6) -C(O)-O(C1-C6)-alkyl,
4.7) -C(O)-OH,
4.8) -(C1-C6)-alkyl, where alkyl is unsubstituted or
substituted, once, twice or three times, by halogen,
4.9) -O-(Cl-C6)-alkyl, where alkyl is unsubstituted or
substituted, once, twice or three times, by halogen,
4.10) pyridyl, or
4.11) phenyl, where phenyl is unsubstituted or substituted,
once, twice or three times, independently of each
other, by a radical from the series halogen, -(C1-C6)-
alkoxy and -(C1-C6)-alkyl, and
R4 and R5 or R5 and R6 form, together with the phenyl ring and
the carbon atoms to which they are in each case bonded,
independently of each other, a ring system from the series
benzo[1,4]dioxane, 2,3-dihydrobenzofuran and 2,2-
difluorobenzo[1,3]dioxole, and the other radicals R3, R6 and R7
or R3, R4 and R7 are hydrogen atom.
The invention also relates to a compound of the formula I where, for the
case a),
R1 is hydrogen atom,
R2 is -(C1-C3)-alkyl, where alkyl is substituted by
1. phenyl, in which phenyl is substituted, once, twice or three
times, independently of each other, by
1.1) -CH2-C(O)-O-R8, in which R8 is hydrogen, methyl,
ethyl, propyl or butyl,
1.2) -(Co-C6)-alkyl-C(O)-N(R9)-(R10), in which R9 and
R10 are hydrogen atom, methyl, ethyl, propyl or
butyl, or
R9 and R10 form, together with the nitrogen atom to
which they are bonded, a radical which can be
CA 02504153 2005-04-28
derived from pyrrolidine, piperidine, pyrazolidine,
pyrazine, tetrazine, imidazolidine, piperazine,
isoxazolidine, morpholine, isothiazolidine or
thiomorpholine, and, in the case of nitrogen, the
5 nitrogen atoms can, independently of each other, be
unsubstituted or substituted by (C1-C4)-alkyl,
1.3) -(Co-C4)-alkyl-C(O)-NH-CN,
1.4) -O-(Co-C6)-alkyl-C(O)-N(R9)-(R10), in which R9 and
R10 have the meaning mentioned above under 1.2),
10 1.5) -(Co-C6)-alkyl-C(O)-N(R8)-(Co-C6)-alkyl-N(R9)-
(R10), in which R8, R9 and R10 have the
abovementioned meaning,
1.6) -C(O)-N(R8)-(Co-C2)-alkyl-Het, where R8 has the
abovementioned meaning and Het is azepine,
15 azetidine, aziridine, benzimidazole, benzofuran,
benzo[1,4]dioxin, 1,3-benzodioxole, 4H-benzo[1,4]-
oxazine, benzoxazole, benzothiazole,
benzothiophene, quinazoline, quinoline, quinoxaline,
chroman, cinnoline, 1,2-diazepine, 1,3-diazepine,
20 1,4-diazepine, 1,4-dioxin, dioxole, furan, imidazole,
indazole, indole, isoquinoline, isochroman, isoindole,
isothiazole, isoxazole, morpholine, 1,2-oxazine,
1,3-oxazine, 1,4-oxazine, oxazole, oxirane,
piperazine, piperidine, phthalazine, pyran, pyrazine,
pyrazole, pyridazine, pyridine, pyrimidine,
pyridoimidazole, pyridopyridine, pyridopyrimidine,
pyrrole, pyrrolidine, tetrazole, 1,2-thiazine,
1,3-thiazine, 1,4-thiazine, thiazole, thiomorpholine,
thiophene, thiopyran, 1,2,3-triazine, 1,3,5-triazine,
1,2,4-triazine, 1,2,3-triazole or 1,2,4-triazole and Het
is unsubstituted or substituted, once, twice or three
times, independently of each other, by
a) halogen
b) cyano,
c) nitro,
d) hydroxyl,
e) amino,
f) -C(O)-O-(C1-C4)-alkyl,
g) -C(O)-OH,
CA 02504153 2005-04-28
21
h) -(C1-C4)-alkyl, where alkyl is unsubstituted or
substituted, once, twice or three times, by
halogen,
i) -O-(C1-C4)-alkyl, where alkyl is unsubstituted or
substituted, once, twice or three times, by
halogen, or
1.7) -C(O)-N(R8)-(Co-C4)-alkyl-phenyl, where phenyl is
unsubstituted or substituted, once, twice or three
times, independently of each other, by the
abovementioned radicals a) to i),
1.8) -CH2-N(R9)-(R10), in which R9 and R10 have the
abovementioned meaning,
1.9) -(CH2)y-N(R8)-C(O)-(C1-C4)-alkyl in which alkyl is
unsubstituted or substituted, once, twice or three
times, independently of each other, by the
abovementioned radicals a) to i), and y is 1 or 2,
1.10) -(CH2)X-N(R8)-C(O)-(Co-C2)-alkyl-phenyl, in which
phenyl is unsubstituted or substituted, once, twice or
three times, independently of each other, by the
abovementioned radicals a) to i), and x is 0, 1 or 2,
1.11) -(CH2)X-N(R8)-C(O)-(Co-C2)-alkyl-Het, in which Het
is unsubstituted or substituted, once, twice or three
times, independently of each other, by the
abovementioned radicals a) to i), and x is 0, 1 or 2,
1.12) -(CH2)X-N(R8)-C(O)-O-(Ci-C4)-alkyl, in which alkyl is
unsubstituted or substituted, once, twice or three
times, independently of each other, by the
abovementioned radicals a) to i), and x is 0, 1 or 2,
1.13) -(CH2)X-N(R8)-C(O)-O-(Co-C4)-alkyl-phenyl, in which
phenyl is unsubstituted or substituted, once, twice or
three times, independently of each other, by the
abovementioned radicals a) to i), and x is 0, 1 or 2,
1.14) -(CH2)X-N(R8)-C(O)-O-(Co-C4)-alkyl-Het in which
Het is unsubstituted or substituted, once, twice or
three times, independently of each other, by the
abovementioned radicals a) to i), and x is 0, 1 or 2,
1.15) -(CH2)X-N(R8)-C(O)-N(R11)-R12, in which R8 and x
have the abovementioned meaning and R11 and
CA 02504153 2005-04-28
22
R12 are identical or different and are, independently
of each other,
1.15.1) hydrogen atom,
1.15.2) methyl, ethyl, propyl or butyl,
1.15.3) -(Co-C2)-alkyl-phenyl, in which phenyl is
unsubstituted or substituted, once, twice
or three times, independently of each
other, by the abovementioned radicals a)
to i),
1.15.4) -(Co-C2)-alkyl-Het, in which Het is
unsubstituted or substituted, once, twice
or three times, independently of each
other, by the abovementioned radicals a)
to i),
1.15.5) -C(O)-(C1-C4)-alkyl,
1.15.6) -C(O)-(Co-C2)-alkyl-phenyl,
1.15.7) -C(O)-(Co-C2)-alkyl-Het,
1.15.8) -S02-(C1-C4)-alkyl,
1.15.9) -S02-(CO-C4)-alkyl-phenyl, or
1.15.10) -S02-(Co-C2)-alkyl-Het,
R3, R4, R5, R6 and R7 are identical or different and are, independently of
each other,
1. hydrogen atom,
2. halogen,
3. -(C1-C6)-alkyl, in which alkyl is unsubstituted or substituted,
once, twice or three times, by halogen,
4. -O-(C1-C6)-alkyl in which alkyl is unsubstituted or substituted,
once, twice or three times, by halogen, or
R4 and R5 or R5 and R6 form, together with the carbon atoms to which
they are bonded, independently of each other, a dioxane, dioxole,
dihydrofuran or furan ring and the other radicals R3, R6 and R7 or R3,
R4 and R7 have the abovementioned meaning of 1. to 4.,
or, for the case b),
R1 is hydrogen atom,
CA 02504153 2005-04-28
23
R2 is -(C1-C2)-alkyl, where alkyl is substituted, once, twice or three times,
by
1. -C(O)-O-R8', in which R8' is
1.1) hydrogen atom or
1.2)-(C1-C2)-alkyl,
2. phenyl, in which phenyl is substituted, once, twice or three times,
independently of each other, by
2.1)-O-(C2-C4)-alkyl-N(R9')-RlO', where R9' and R10' are,
independently of each other, hydrogen atom, methyl or ethyl,
or R9' and R10' form, together with the nitrogen atom to
which they are bonded, a radical which can be derived from
pyrrolidine, piperidine, piperazine, morpholine or
thiomorpholine, and, in the case of piperazine, the second
nitrogen atom can be substituted by methyl or ethyl,
2.2)-O-(C1-C2)-alkyl-C(O)-O-R8', in which R8' is, independently
of each other, hydrogen atom, methyl or ethyl, or
2.3)-N(R14)-(R15) in which R14 and R15 form, together with the
nitrogen atom to which they are bonded, a radical which can
be derived from pyrrolidine, piperidine, pyrazolidine,
pyrazine, tetrazine, imidazolidine, piperazine, isoxazolidine,
morpholine, isothiazolidine or thiomorpholine, and, in the
case of nitrogen, the nitrogen atoms can, independently of
each other, be unsubstituted or substituted by methyl or
ethyl,
2.4)-(CH2)k-N(R9')-(RlO') in which k is 2, 3 or 4 and R9' and
R10' are identical or different and are, independently of each
other, hydrogen atom, methyl or ethyl, or
R9' and R10' form, together with the nitrogen atom to which
they are bonded, a radical which can be derived from
pyrrolidine, piperidine, piperazine, morpholine or
thiomorpholine, and, in the case of piperazine, the second
nitrogen atom can be substituted by methyl or ethyl, and
R4 and R5 or R5 and R6 form, together with the phenyl ring and the carbon
atoms to which they are in each case bonded, independently of each other,
a ring system from the series benzo[1,4]dioxane, 2,3-dihydrobenzofuran
and 2,2-difluorobenzo[1,3]dioxole, and the other radicals R3, R6 and R7 or
R3, R4 and R7 are hydrogen atom.
The invention also relates to a compound of the formula I such as
CA 02504153 2005-04-28
24
pyrimidine-4,6-carboxylic acid 4-(3-methoxybenzylamide) 6-(4-propyl-
carbamoylbenzylamide),
pyrimidine-4,6-carboxylic acid 4-(4-isopropylcarbamoylbenzylamide)
6-(3-methoxybenzylamide),
[4-({[6-(3-methoxybenzylcarbamoyl)pyrimidine-4-carbonyl]amino}methyl)-
phenyl]carboxyamino isopropyl ester,
pyrimidine-4,6-carboxylic acid 4-(3-methoxybenzylamide) 6-[(2-phenoxy-
ethyl)amide],
(5-{[6-(3-methoxybenzylcarbamoyl)pyrimidine-4-carbonyl]amino}pentyl)-
carboxyamino methyl ester,
pyrimidine-4,6-carboxylic acid 4-[4-(2-dimethylaminoethylcarbamoyl)-
benzylamide] 6-(3-methoxybenzylamide),
pyrimidine-4,6-carboxylic acid 4-[(2,3-dihydrobenzo[1,4]dioxin-6-ylmethyl)-
amide] 6-[4-(2-dimethylaminoethylcarbamoyl)benzylamide],
pyrimidine-4,6-carboxylic acid 4-(3-chloro-4-fluorobenzylamide)
6-[4-(2-dimethylaminoethylcarbamoyl)benzylamide],
pyrimidine-4,6-carboxylic acid 4-dimethylcarbamoylmethylamide
6-(3-methoxybenzylamide),
[4-({[6-(3-aminobenzylcarbamoyl)pyrimidine-4-carbonyl]amino}methyl)-
phenyl]carboxyamino tert-butyl ester,
pyrimidine-4,6-dicarboxylic acid 4-(3-chlorobenzylamide) 6-(4-fluoro-
3-methylbenzylamide),
pyrimidine-4,6-dicarboxylic acid 4-[(2-chloropyridin-4-ylmethyl)amide]
6-(4-fluoro-3-methylbenzylamide),
pyrimidine-4,6-dicarboxylic acid 4-benzylamide 6-(4-fluoro-3-methylbenzyl-
amide),
pyrimidine-4,6-dicarboxylic acid 4-(4-fluoro-3-methylbenzylamide)
6-[(pyridin-4-ylmethyl)amide],
pyrimidine-4,6-dicarboxylic acid 4-(4-fluoro-3-methylbenzylamide)
6-(pyridin-3-ylmethyl)amide],
pyrimidine-4,6-carboxylic acid 4-(4-fluoro-3-methylbenzylamide) 6-$4-
[2-(4-methylpiperazin-1-yl)-2-oxoethyl]benzylamide},
pyrimidine-4,6-carboxylic acid 4-(4-fluoro-3-methylbenzylamide)
6-[4(2-morpholin-4-yl-2-oxoethoxy)benzylamide],
pyrimidine-4,6-carboxylic acid 4-(4-diethylcarbamoylmethoxybenzylamide)
6-(4-fluoro-3-methylbenzylamide),
pyrimidine-4,6-carboxylic acid 4-(4-fluoro-3-methylbenzylamide)
6-[4-(isopropylcarbamoylmethyl)benzylamide],
CA 02504153 2005-04-28
pyrimidine-4,6-carboxylic acid 4-(4-fluoro-3-methylbenzylamide)
6-{4-[(2-morpholin-4-ylethylcarbamoyl)methyl]benzylamide},
pyrimidine-4,6-carboxylic acid 4-(4-diethylcarbamoylmethylbenzylamide)
6-(4-fluoro-3-methylbenzylamide),
5 pyrimidine-4,6-carboxylic acid 4-(4-fluoro-3-methylbenzylamide)
6-[4-(2-morpholin-4-yl-2-oxoethyl)benzylamide],
pyrimidine-4,6-carboxylic acid 4-(4-fluoro-3-methylbenzylamide)
6-[4-(isopropylcarbamoylmethoxy)benzy[amide],
pyrimidine-4,6-dicarboxylic acid 4-(3-methoxybenzylamide) 6-[(pyridin-
10 3-ylmethyl)amide],
pyrimidine-4,6-carboxylic acid 4-(3-methoxybenzylamide) 6-({[(pyridin-
4-ylmethyi)carbamoyl]methyl}amide),
pyrimidine-4,6-carboxylic acid 4-({[(2-chloropyridin-4-ylmethyl)carbamoyl]-
methyl}amide) 6-(3-methoxybenzylamide),
15 pyrimidine-4,6-carboxylic acid 4-(3-chloro-4-fluorobenzylamide)
6-({[(2-chloropyridin-4-ylmethyl)carbamoyl]methyl}amide),
[4-({[6-(3-methoxybenzylcarbamoyl)pyrimidine-4-carbonyl]amino}methyl)-
phenyl]carboxyamino isobutyl ester,
[4-({[6-(3-methoxybenzylcarbamoyl)pyrimidine-4-carbonyl]amino}methyl)-
20 phenyl]carboxyamino ethyl ester,
[4-({[6-(3-methoxybenzylcarbamoyl)pyrimidine-4-carbonyl]amino}methyl)-
phenyl]carboxyamino allyl ester,
pyrimidine-4,6-carboxylic acid 4-(3-chloro-4-fluorobenzylamide)
6-[4-(1-methylpiperidin-3-yloxy)benzylamide],
25 pyrimidine-4,6-carboxylic acid 4-(3-chloro-4-fluorobenzylamide)
6-({[(pyridin-3-ylmethyl)carbamoyl]methyl}amide),
pyrimidine-4,6-carboxylic acid 4-(3-methoxybenzylamide)
6-[4-(2-morpholin-4-ylethylcarbamoyl)benzylamide],
pyrimidine-4,6-carboxylic acid 4-(3-methoxybenzylamide) 6-[4-(2-pyrrolidin-
1 -yl-ethylcarbamoyl)benzylamide],
pyrimidine-4,6-carboxylic acid 4-[(2,3-dihydrobenzo[1,4]dioxin-6-ylmethyl)-
amide] 6-[(2'-sulfamoylbiphenyl-2-ylmethyl)amide];
pyrimidine-4,6-carboxylic acid 4-[(2,3-dihydrobe nzofuran-5-ylmethyl)amide]
6-[(thiophen-2-ylmethyl)amide],
pyrimidine-4,6-carboxylic acid 4-[(2,3-dihydrobenzofuran-5-ylmethyl)amide)
6-[(5-methylfuran-2-ylmethyl)amide],
pyrimidine-4,6-carboxylic acid 4-[(2,3-dihydrobe nzofuran-5-ylmethyl)amide]
6-[(5-methylfuran-2-yl methyl)amide],
CA 02504153 2005-04-28
26
pyrimidine-4,6-carboxylic acid 4-[(2,3-dihydrobenzofuran-5-ylmethyl)amide]
6-[(5-pyridin-2-ylthiophen-2-ylmethyl)amide],
pyrimidine-4,6-carboxylic acid 4-[(2,3-dihydrobe nzofuran-5-ylmethyl)amide]
6-[(pyridin-3-ylmethyl)amide];
pyrimidine-4,6-carboxylic acid 4-[(2,3-dihydrobenzo[1,4]dioxin-6-ylmethyl)-
amide] 6-[(pyridin-3-ylmethyl)amide],
pyrimidine-4,6-carboxylic acid 4-[(2,3-dihydrobenzo[1,4]dioxin-6-ylmethyl)-
amide] 6-[(5-methylfuran-2-ylmethyl)amidej,
pyrimidine-4,6-carboxylic acid 4-[(2,3-dihydrobenzo[1,4]dioxin-6-ylmethyl)-
amide] 6-[(thiophen-2-ylmethyl)amide];
pyrimidine-4,6-carboxylic acid 4-[(2,3-dihydrobe nzofuran-5-ylmethyl)amide]
6-[(5-methyl isoxazol-3-ylmethyl)amidej,
pyrimidine-4,6-carboxylic acid 4-[(2,3-dihydrobenzofuran-5-ylmethyl)amide]
6-[(1-methyl-1 H-pyrazol-4-ylmethyl)amide],
pyrimidine-4,6-carboxylic acid 4-[(2,3-dihydrobenzofuran-5-ylmethyl)amide]
6-[(2,5-dimethylfuran-3-ylmethyl)amide];
pyrimidine-4,6-carboxylic acid 4-[(6-aminopyridin-3-ylmethyl)amide]
6-[(2,3-dihydrobenzofuran-5-ylmethyl)amide];
pyrimidine-4,6-carboxylic acid 4-[(2,3-dihydrobenzofuran-5-ylmethyl)amide]
6-[(1-methyl-1 H-pyrrol-2-ylmethyl)amide],
pyrimidine-4,6-carboxylic acid 4-[(1 H-benzoimidazol-2-ylmethyl)amide]
6-[(2,3-dihydrobe nzofuran-5-ylmethyl)amide],
pyrimidine-4,6-carboxylic acid 4-[(2,3-dihydrobenzofuran-5-ylmethyl)amide]
6-[(pyrazin-2-ylmethyl)amide],
pyrimidine-4,6-carboxylic acid 4-[(2,2-difluorobenzo[1,3]dioxol-5-ylmethyl)-
amide] 6-[(pyridin-4-ylmethyl)amide],
({6-[(2,3-dihydrobenzo[1,4]dioxin-6-ylmethyl)carbamoyl]pyrimidine-
4-carbonyl}amino)acetic acid methyl ester,
pyrimidine-4,6-carboxylic acid 4-[(2,3-dihydrobe nzofuran-5-ylmethyl) amid e]
6-[(2-methyl-1 H-imidazol-4-ylmethyl)amide],
pyrimidine-4,6-carboxylic acid 4-[(2,3-dihydrobenzofuran-5-ylmethyl)amide]
6-[(2-pyridin-2-ylethyl)amide],
pyrimidine-4,6-carboxylic acid 4-[(2,3-dihydrobenzofuran-5-ylmethyl)amide]
6-{[3-(4-fluorophenyl)-1 H-pyrazol-4-ylmethyl]amide};
pyrimidine-4,6-carboxylic acid 4-[(2,3-dihydrobenzo[1,4]dioxin-6-ylmethyl)-
amide] 6-[4-(3-dimethylaminopropoxy)benzylamide],
pyrimidine-4,6-carboxylic acid 4-[(2,3-dihydrobenzo[1,4]dioxin-6-ylmethyl)-
amide] 6-[4-(2-dimethylaminoethoxy)benzylamide],
CA 02504153 2005-04-28
27
pyrimidine-4,6-carboxylic acid 4-[(2,3-dihydrobenzo[1,4]dioxin-6-ylmethyl)-
amide] 6-[3-(2-dimethylaminoethoxy)benzylamide],
pyrimidine-4,6-carboxylic acid 4-[(2,3-dihydrobenzofuran-5-ylmethyl)amide]
6-[(pyrid in-4-ylmethyl )amide],
pyrimidine-4,6-dicarboxylic acid 4-(3-chloro-4-fluorobenzylamide)
6-(4-[3'-methylsulfonyl]ureidobenzylamide),
pyrimidine-4,6-dicarboxylic acid 4-(3-methoxybenzylamide) 6-[4-(4-oxo-
piperidine-1-carbonyl)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-(4-fluoro-3-methylbenzylamide)
6-[4-(4-oxopiperidine-1-carbonyl)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-[(2,3-dihydrobenzofuran-5-ylmethyl)-
amide] 6-[4-(4-oxopiperidine-l-carbonyl)benzyI amid el,
pyrimidine-4,6-dicarboxylic acid 4-[4-(4-hydroxypiperidine-1 -carbonyl)-
benzylamide] 6-(3-methoxybenzylamide),
pyrimidine-4,6-dicarboxylic acid 4-[(2 ,3-dihydrobenzofuran-5-yI methyl)-
amide] 6-[4-(4-hydroxypiperidine-1-carbonyl)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-(4-fluoro-3-methylbenzylamide)
6-[4-(4-hydroxypiperidine-1-carbonyl)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-(4-fluoro-3-methylbenzylamide)
6-[4-(thiomorpholine-4-carbonyl)benzy[amide],
pyrimidine-4,6-dicarboxylic acid 4-(3-methoxybenzylamide)
6-[4-(thiomorpholine-4-carbonyl)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-[(2,3-dihydrobe nzofuran-5-ylmethyl)-
amide] 6-[4-(thiomorpholine-4-carbonyl)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-(3-methoxybenzylamide) 6-[4-(3-oxo-
piperazine-1 -carbonyl)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-[(2,3-dihydrobenzofuran-5-ylmethyl)-
amide] 6-[4-(3-oxopiperazine-l-carbonyl)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-(4-fluoro-3-methylbenzylamide)
6-[4-(3-oxopiperazine-1 -carbonyl)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-[(2,3-dihydrobenzofuran-5-ylmethyl)-
amide] 6-[4-(2-hydroxyethylcarbamoyl)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-(4-fluoro-3-methylbenzylamide)
6-[4-(2-hydroxyethylcarbamoyl )benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-(4-fluoro-3-methylbenzylamide)
6-{4-[(pyrid in-4-ylmethyl )carbamoyl]benzylamide},
pyrimidine-4,6-dicarboxylic acid 4-(4-cyanocarbamoylbenzylamide)
6-(4-fl uoro-3-methylbenzylamide),
CA 02504153 2005-04-28
28
pyrimidine-4,6-dicarboxylic acid 4-(3-methoxybenzylamide)
6-[4-(3-morpholin-4-ylpropylcarbamoyl)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-[(2,3-dihydrobenzofuran-5-ylmethyl)-
amide] 6-[4-(3-morpholin-4-yl-propylcarbamoyl)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-(4-fluoro-3-methylbenzylamide)
6-[4-(4-methylpiperazine-1-carbonyl)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-[(2,3-dihydrobenzofuran-5-yimethyl)-
amide] 6-{4-[(pyridin-4-ylmethyl)carbamoyl]benzylamide},
pyrimidine-4,6-dicarboxylic acid 4-(4-fluoro-3-methylbenzylamide)
6-(4-[3'-methylsulfonyl]ureidobenzylamide),
pyrimidine-4,6-dicarboxylic acid 4-[(2,3-dihydrobenzofuran-5-ylmethyl)-
amide] 6-(4-[3-methylsulfonyl]ureidobenzylamide),
pyrimidine-4,6-dicarboxylic acid 4-(4-N-cyanocarbamoylbenzylamide)
6-[(2,3-dihydrobenzofuran-5-ylmethyl)amide],
pyrimidine-4,6-dicarboxylic acid 4-(4-N-cyanocarbamoylbenzylamide)
6-(3-methoxybenzylamide ),
pyrimidine-4,6-dicarboxylic acid 4-(4-fluoro-3-methylbenzylamide)
6-[4-(morpholine-4-carbonyi)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-(3-methoxybenzylamide) 6-(3-
[3'-methylsulfonyl]ureidobenzylamide),
pyrimidine-4,6-dicarboxylic acid 4-(4-hydroxycarbamoylbenzylamide)
6-(3-methoxybenzylamide),
pyrimidine-4,6-dicarboxylic acid 4-[(2,3-dihydrobe nzofuran-5-ylmethyl)-
amide] 6-[4-(hydroxycarbamoylmethylcarbamoyi)benzylamide],
pyrimidine-4,6-dicarboxylic acid 44(2 ,3-dihydrobenzofuran-5-ylmethyl )-
amide] 6-[4-(1-methylpiperidin-3-yloxy)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-(4-fluoro-3-methylbenzylamide)
6-[4-(2-piperazin-1-ylethylcarbamoyl)benzy[amide],
pyrimidine-4,6-dicarboxylic acid 4-(4-fluoro-3-methylbenzylamide)
6-(4-hydroxycarbamoylbenzylamide),
pyrimidine-4,6-dicarboxylic acid 4-[(2,3-dihydrobe nzofuran-5-ylmethyl)-
amide] 6-(4-hydroxycarbamoylbenzylamide),
pyrimidine-4,6-dicarboxylic acid 4-(4-fluoro-3-methylbenzylamide)
6-[4-(1-methylpiperidin-3-yloxy)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-(4-tert-butylcarbamoylbenzylamide)
6-(3-methoxybenzylamide),
pyrimidine-4,6-dicarboxylic acid 4-(3-methoxybenzylamide) 6-{4-[methyl-
(1-methylpiperidin-4-yl)carbamoyl]benzylamide},
CA 02504153 2005-04-28
29
{4-[({6-[(2,3-dihydrobenzofuran-5-ylmethyl)carbamoyl]pyrimidin-
4-carbonyl}amino)methyl]benzoylamino}acetic acid,
pyrimidine-4,6-dicarboxylic acid 4-(4-fluoro-3-methylbenzylamide)
6-[4-(2-pyrrolidin-1-yl-ethylcarbamoyl)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-{4-[4-(2-d imethylaminoethyl)piperazine-
1-carbonyl]benzylamide} 6-(3-methoxybenzylamide),
pyrimidine-4,6-dicarboxylic acid 4-(3-methoxybenzylamide)
6-(4-[3'-methyl sulfonyl]ureidobenzylamid e),
pyrimidine-4,6-dicarboxylic acid 4-(3-methoxybenzylamide)
6-[3-(2-morpholin-4-ylethylcarbamoyl)benzylamide],
[4-({[6-(4-fluoro-3-methylbenzylcarbamoyl )pyrimid in-4-carbonyl]amino}-
methyl)benzoylamino]acetic acid,
pyrimidine-4,6-dicarboxylic acid 4-(3-methoxybenzylamide)
6-[4-(2-piperazin-1-ylacetylamino)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-(4-fluoro-3-methylbenzylamide)
6-[4-(2-morpholin-4-yl-ethylcarbamoyl)benzylamide],
[4-({[6-(4-fluoro-3-methylbenzylcarbamoyl)pyrimidin-4-carbonyi]amino}-
methyl)benzoylamino]acetic acid methyl ester,
pyrimidine-4,6-dicarboxylic acid 4-(3-methoxybenzylamide)
6-[3-(morpholine-4-carbonyl)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-(3-methoxybenzylamide) 6-{4-[(piperidin-
4-ylmethyl)carbamoyl]benzylamide},
pyrimidine-4,6-dicarboxylic acid 4-(3-methoxybenzylamide) 6-[4-(piperidin-
4-ylcarbamoyl)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-(4-fluoro-3-methylbenzylamid e)
6-[4-(piperidin-4-ylcarbamoyl)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-(4-fluoro-3-methylbenzylamide)
6-{4-[methyl-(1-methyl pipe ridin-4-yl)carbamoyl]benzylamide},
pyrimidine-4,6-dicarboxylic acid 4-(4-fluoro-3-methylbenzylamide)
6-[(4-methyl-3,4-dihydro-2H-benzo[1,4]oxazin-7-ylmethyl)amide],
pyrimidine-4,6-dicarboxylic acid 4-(4-fluoro-3-methylbenzylamide)
6-{4-[(piperidin-4-ylmethyl)carbamoyl]benzylamide},
pyrimidine-4,6-dicarboxylic acid 4-(3-methoxybenzylamide) 6-[4-(4-methyl-
piperazine-1-carbonyl)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-(3-methoxybenzylamide) 6-[4-(4-pyridin-
4-ylpiperazine-1-carbonyl)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-(3-methoxybenzylamide)
6-[4-(2-morpholin-4-ylacetylamino)benzylamide],
CA 02504153 2005-04-28
pyrimidine-4,6-dicarboxylic acid 4-[(2,3-dihydrobenzofuran-5-ylmethyl)-
amide] 6-[4-(morpholine-4-carbonyl)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-(3-methoxybenzylamide)
6-(4-[p-toluenesulfonyl]ureidobenzylamide),
5 pyrimidine-4,6-dicarboxylic acid 4-[(2,3-dihydrobe nzofuran-5-ylmethyl)-
amide] 6-[4-(4-m ethyl piperazine-1-carbonyl)benzylamid e],
pyrimidine-4,6-dicarboxylic acid 4-[(2,3-dihydrobenzofuran-5-ylmethyl)-
amide] 6-[4-(2-pyrrolidin-1-yl-ethylcarbamoyl)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-(3-methoxybenzylamide)
10 6-(4-[3'-phenylsulfonyl]ureidobenzylamide),
pyrimidine-4,6-dicarboxylic acid 4-[(2,3-dihydrobenzofuran-5-ylmethyl)-
amide] 6-[4-(2-morpholin-4-yl-ethylcarbamoyl)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-[(2,3-dihydrobenzofuran-5-ylmethyl)-
amide] 6-[4-(2-pyrrolidin-1-ylethoxy)benzylamide],
15 pyrimidine-4,6-dicarboxylic acid 4-[4-(3-cyclohexanecarbonylureido)-
benzylamide] 6-(3-methoxybenzylamide),
pyrimidine-4,6-dicarboxylic acid 4-(3-methoxybenzylamide)
6-{4-[3-(pyridine-3-carbonyl)ureido]benzylamide},
pyrimidine-4,6-dicarboxylic acid 4-[4-(3-isobutyrylureido)benzylamide]
20 6-(3-methoxybenzylamide),
pyrimidine-4,6-dicarboxylic acid 4-(3-methoxybenzylamide)
6-[4-(2-pyrrolidin-1 -ylacetylamino)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-[(4-chlorothiophen-2-ylmethyl)amide]
6-[(2,3-dihydrobenzofuran-5-ylmethyl)amide],
25 pyrimidine-4,6-dicarboxylic acid 4-(3-methoxybenzylamide) 6-{4-[2-(2-oxo-
pyrrolidin-1-yl)acetylamino]benzylamide},
pyrimidine-4,6-dicarboxylic acid 4-[(2,3-dihydrobenzofuran-5-ylmethyl)-
amide] 6-[(thiophen-3-ylmethyl)amide],
pyrimidine-4,6-dicarboxylic acid 4-[(2,3-dihydrobenzofuran-5-ylmethyl)-
30 amide] 6-[(3-methylthiophen-2-ylmethyl)amide],
pyrimidine-4,6-dicarboxylic acid 4-[(2, 3-d ihydrobenzofuran-5-ylmethyl)-
amide] 6-[(5-methylthiophen-2-ylmethyl)amide],
pyrimidine-4,6-dicarboxylic acid 4-[4-(2-dimethylaminoacetylamino)-
benzylamide] 6-(3-methoxybenzylamide),
pyrimidine-4,6-dicarboxylic acid 4-[(2,3-dihydrobenzofuran-5-ylmethyl)-
amide] 6-[4-(2-morpholin-4-ylethoxy)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-[4-(3-cyclohexylureido)benzylamide]
6-(3-methoxybenzylamide),
CA 02504153 2005-04-28
31
pyrimidine-4,6-dicarboxylic acid 4-{4-[3-(2,6-dichloropyridin-4-yl)ureido]-
benzylamide} 6-(3-methoxybenzylamide),
pyrimidine-4,6-dicarboxylic acid 4-[4-(3-tert-butylureido)benzylamide]
6-(3-methoxybenzylamide),
[4-({[6-(3-methoxybenzylcarbamoyl)pyrimidine-4-carbonyl]amino}methyl)-
phenyl]carboxyamino but-2-ynyl ester,
pyrimidine-4,6-dicarboxylic acid 4-(4-ethanesulfonylaminobenzylamide)
6-(3-methoxybenzylamide),
pyrimidine-4,6-dicarboxylic acid 4-(3-methoxybenzylamide)
6-[4-(thiophene-2-sulfonylamino)benzylamid e],
pyrimidine-4,6-dicarboxylic acid 4-(3-methoxybenzylamide)
6-[4-(2,2,2-trifluoroethanesulfonylamino)benzylamide],
[4-({[6-(3-methoxybenzylcarbamoyl)pyrimidin-4-carbonyl]amino}methyl)-
phenyl]carboxyamino methyl ester,
[4-({[6-(3-methoxybenzylcarbamoyl)pyrimidine-4-carbonyl]amino)methyl)-
phenyl]carboxyamino prop-2-ynyl ester,
[4-({[6-(3-methoxybenzylcarbamoyl)pyrimidin-4-carbonyl]amino}methyl)-
phenyl]carboxyamino 2-methoxyethyl ester,
[4-({[6-(3-methoxybenzylcarbamoyl )pyrimidine-4-carbonyl]amino}methyl )-
phenyl]carboxyamino 4-fluorophenyl ester,
pyrimidine-4,6-dicarboxylic acid 4-[4-(3-benzoylureido)benzylamide]
6-(3-methoxybenzylamide),
[3-({[6-(3-methoxybenzylcarbamoyl)pyrimidine-4-carbonyl]amino}methyl)-
phenyl]carboxyamino but-2-ynyl ester,
[3-({[6-(3-methoxybenzylcarbamoyl)pyrimidine-4-carbonyl]amino}methyl)-
phenyl]carboxyamino prop-2-ynyl ester,
[3-({[6-(3-methoxybenzylcarbamoyl)pyrimidine-4-carbonyl]amino}methyl )-
phenyl]carboxyamino isopropyl ester,
pyrimidine-4,6-dicarboxylic acid 4-(3-chloro-4-fluorobenzylamide)
6-[4-(2-pyrrolidin-1-ylethylcarbamoyl)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-(3-methoxybenzylamide)
6-[4-(morpholine-4-carbonyl)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-(3-methoxybenzylamide) 6-{4-[(pyridin-
4-ylmethyl)carbamoyl]benzylamide},
pyrimidine-4,6-dicarboxylic acid 4-(3-chloro-4-fluorobenzylamide)
6-(4-diethylcarbamoylbenzylamide),
pyrimidine-4,6-dicarboxylic acid 4-(3-chloro-4-fluorobenzylamide)
6-[4-(morpholine-4-carbonyl)benzylamide],
CA 02504153 2005-04-28
32
pyrimidine-4,6-dicarboxylic acid 4-(3-chloro-4-fluorobenzylamide)
6-[4-(2-morpholin-4-ylethylcarbamoyl)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-{4-[2-(2,6-dimethyl pipe ridin-1-yl)-2-oxo-
ethyl]benzylamide} 6-(4-fluoro-3-methylbenzylamide),
pyrimidine-4,6-dicarboxylic acid 4-(3-methoxybenzylamide) 6-[4-(1-methyl-
piperidin-3-yloxy)benzylamide],
pyrimidine-4,6-dicarboxylic acid 4-(4-diethylcarbamoylbenzylamide)
6-(3-methoxybenzylamide),
pyrimidine-4,6-dicarboxylic acid 4-[(2-chloropyridin-4-ylmethyl)amide]
6-[(2,3-dihydrobenzofuran-5-ylmethyl)amide]
pyrimidine-4,6-dicarboxylic acid 4-(3-chloro-4-fluorobenzylamide)
6-(4-methanesulfonylaminobenzylamide), or
pyrimidine-4,6-dicarboxylic acid 4-(4-methanesulfonylbenzylamide)
6-(3-methoxybenzylamide).
The invention also relates to the use of a compound of the formula I,
O O R7
R1~N I N R6
R2 N\%N R3 R5
R4
and/or all the stereoisomeric forms of the compound of the formula I and/or
mixtures of these forms in any ratio, and/or a physiologically tolerated salt
of the compound of the formula I, for producing a pharmaceutical for the
prophylaxes and therapy of diseases in whose course an increase in the
activity of matrix metalloproteinase 13 is involved.
The term "halogen" is understood as meaning fluorine, chlorine, bromine or
iodine.
The term "alkyl" is understood as meaning, in the widest possible sense,
hydrocarbon radicals whose carbon chain is straight-chain or branched or
which are composed of cyclic hydrocarbon groups or of combinations of
linear and cyclic groups. For example, linear and branched hydrocarbon
radicals can be methyl, ethyl, propyl, i-propyl, butyl, tert-butyl, pentyl or
hexyl, while cyclic groups can be cyclopropyl, cyclobutyl, cyclopentyl or
cyclohexyl and a combination of linear and cyclic radicals can be
cyclopropylmethyl, cyclobutylmethyl or cyclopentylmethyl. However, alkyl
CA 02504153 2005-04-28
33
can also be singly or multiply unsaturated, such as (C2-C6)-alkenyl, e.g.
ethylene, propylene, butene, methylpropene, isobutylene, 1,3-butadiene or
1,3-pentadiene, or (C2-C6)-alkynyl, e.g. acetylene, propylyne, butyne,
2-methyl-3-hexyne, 1,4-pentadiyne or 2-hexen-4-yne. The term "-(Co-C6)-
alkyl" is understood as meaning hydrocarbon radicals whose carbon chain
is straight-chain or branched and contains from 1 to 6 carbon atoms, for
example methyl, ethyl, propyl, i-propyl, butyl, tert-butyl, pentyl or hexyl.
"-Co-alkyl" is a covalent bond.
The tem "-(C6-C14)-aryl" is understood as meaning aromatic carbon
radicals having from 6 to 14 carbon atoms in the ring. Examples of
-(C6-C14)-aryl radicals are phenyl, naphthyl, for example 1-naphthyl and
2-naphthyl, biphenylyl, for example 2-biphenylyl, 3-biphenylyl and
4-biphenylyl, anthryl and fluorenyl. Biphenylyl radicals, naphthyl radicals
and, in particular, phenyl radicals are preferred aryl radicals.
The term "R4 and R5 or R5 and R6 form, together with the carbon atoms to
which they are in each case bonded, independently of each other, a 5- or
6-membered ring which is aromatic or saturated and contains zero, one or
two heteroatoms from the series oxygen, nitrogen or sulfur" is understood
as meaning ring systems which can be derived from dioxole, pyrrole,
pyrrolidine, pyridine, piperidine, dioxane, tetrahydropyridine, pyrazole,
imidazole, pyrazoline, imidazoline, pyrazolidine, imidazolidine, pyridazine,
pyrimidine, pyrazine, piperazine, pyran, furan, dihydrofuran,
tetrahydrofuran, oxazole, isoxazole, 2-isoxazoline, isoxazolidine,
morpholine, oxothiolane, thiopyran, thiazole, isothiazole, 2-isothiazoline,
isothiazolidine or thiomorpholine.
The term "Het" is understood as meaning a saturated or unsaturated
monocyclic or bicyclic, 3- to 10-membered heterocyclic ring system which
contains 1, 2 or 3 identical or different ring heteroatoms from the series
nitrogen, oxygen and sulfur. In the underlying monocyclic or bicyclic
heterocyclic ring system, Het contains 3, 4, 5, 6, 7, 8, 9, or 10 ring atoms.
The monocyclic ring system can be a 3-, 4-, 5-, 6- or 7-membered ring. In
the bicyclic Het, two rings can be linked to each other, with it being
possible
for one of the rings to be a 5-membered or 6-membered heterocyclic ring
and the other to be a 5- or 6-membered heterocyclic or carbocyclic ring. A
bicyclic Het group can be composed, for example, of 8, 9 or 10 ring atoms.
Het comprises saturated heterocyclic ring systems which do not possess
any double bond in the rings and also unsaturated heterocyclic ring
systems, including monounsaturated and polyunsaturated heterocyclic ring
systems, which possess one or more double bonds and form a stable ring
CA 02504153 2005-04-28
34
system. Unsaturated rings can be partially unsaturated or form an aromatic
system. The Het group contains identical or different heteroatoms from the
series nitrogen, oxygen and sulfur. Examples of heterocycles from which
the Het group can be derived are acridinyl, azocinyl, benzimidazolyl,
benzofuranyl, benzothiofuranyl, benzothiophenyl, benzoxazolyl,
benzthiazolyl, benztriazolyl, benztetrazolyl, benzisoxazolyl,
benzisothiazolyl, benzimidazalinyl, carbazolyl, 4aH-carbazolyl, carbolinyl,
chromanyl, chromenyl, cinnolinyl, decahydroquinolinyl, 2H-6H-1,5,2-
dithiazinyl, dihydrofuro[2,3-b]tetrahydrofuran, furanyl, furazanyl,
imidazolidinyl, imidazolinyl, imidazolyl, 1 H-indazolyl, indolinyl,
indolizinyl,
indolyl, 3H-indolyl, isobenzofuranyl, isochromanyl, isoindazolyl,
isoindolinyl,
isoindolyl, isoquinolinyl (benzimidazolyl), isothiazolyl, isoxazolyl,
morpholinyl, naphthyridinyl, octahydroisoquinolinyl, oxadiazolyl, 1,2,3-
oxadiazolyl, 1,2,4-oxadiazolyl, 1,2,5-oxadiazolyl, 1,3,4-oxadiazolyl,
oxazolidinyl, oxazolyl, oxazolidinyl, pyrimidinyl, phenanthridinyl,
phenanthrolinyl, phenazinyl, phenothiazinyl, phenoxathiinyl, phenoxazinyl,
phthalazinyl, piperazinyl, piperidinyl, pteridinyl, purynyl, pyranyl,
pyrazinyl,
pyroazolidinyl, pyrazolinyl, pyrazolyl, pyridazinyl, pryidooxazole,
pyridoimidazole, pyridothiazole, pyridinyl, pyridyl, pyrimidinyl,
pyrrolidinyl,
pyrrolinyl, 2H-pyrrolyl, pyrrolyl, quinazolinyl, quinolinyl, 4H-quinolizinyl,
quinoxalinyl, quinuclidinyl, tetrahydrofuranyl, tetrahydroisoquinolinyl,
tetrahydroquinolinyl, 6H-1,2,5-thiadazinyl, 1,2,3-thiadiazolyl, 1,2,4-
thiadiazolyl, 1,2,5-thiadiazolyl, 1,3,4-thiadiazolyl and xanthenyl.
Preference is given to azepine, azetidine, aziridine, benzimidazole,
benzofuran, benzo[1,4]dioxin, 1,3-benzodioxole, 4H-benzo[1,4]oxazine,
benzoxazole, benzothiazole, benzothiophene, quinazoline, quinoline,
quinoxaline, chroman, cinnoline, 1,2-diazepine, 1,3-diazepine,
1,4-diazepine, 1,4-dioxin, dioxole, furan, imidazole, indazole, indole,
isoquinoline, isochroman, isoindole, isothiazole, isoxazole, morpholine,
1,2-oxazine, 1,3-oxazine, 1,4-oxazine, oxazole, oxirane, piperazine,
piperidine, phthalazine, pyran, pyrazine, pyrazole, pyridazine, pyridine,
pyrimidine, pyridoimidazole, pyridopyridine, pyridopyrimidine, pyrrole,
pyrrolidine, tetrazole, 1,2-thiazine, 1,3-thiazine, 1,4-thiazine, thiazole,
thiomorpholine, thiophene, thiopyran, 1,2,3-triazine, 1,3,5-triazine,
1,2,4-triazine, 1,2,3-triazole or 1,2,4-triazole etc. and also to ring systems
which result from the listed heterocycles by the latter being linked to, or
fused with, a carbocyclic ring, for example benzo fused, cyclopenta fused,
cyclohexa fused or cyclohepta fused derivatives of these heterocycles.
Suitable nitrogen heterocycles can also be present as N-oxides or as
CA 02504153 2005-04-28
quaternary salts in which a suitable nitrogen atom is alkylated with (Ci-C4)-
alkyl radicals.
The Het groups can be unsubstituted or substituted in accordance with the
listed definitions.
5 The term "R9 and R10 or R14 and R15 form, together with the nitrogen
atom to which they are bonded, a 5-, 6- or 7-membered saturated ring,
where a heteroatom from the series oxygen, sulfur and nitrogen can also
replace one or two further carbon atoms" is understood as meaning
radicals which can be derived from imidazolidine, isothiazolidine,
10 isoxazolidine, morpholine, piperazine, piperidine, pyrazine, pyrazolidine,
pyrrolidine, tetrazine or thiomorpholine.
The compounds of the formula I can be prepared, for example, by reacting
a compound of the formula II
0
II
NI
(11)
C~-Y
N II
0
a) with a compound of the formula Illa or Illb
R4 R3
R1~
H Ilia R5 NH2 Illb
R2
R6 R7
where R1, R2, R3, R4, R5, R6 and R7 have the meanings given in
formula I and Y is halogen, hydroxyl or C1-C4-alkoxy or, together
with the carbonyl group, forms an active ester or a mixed anhydride,
with a compound of the formula I being formed and the reaction
products being converted, where appropriate, into their
physiologically tolerated salts, or
b) reacting a compound of the formula II with a compound of the
formula Illa or Illb to give a compound of the formula IVa or lVb
CA 02504153 2005-04-28
36
R3 0 0
O 0
R4 Y
\
NZi RI Y ! H
f
I NA/N R5 # R7 \/N
R2
R6
(IVa) (IVb)
where R1 to R7 have the meanings given in formula I and Y is
halogen, hydroxyl or C1-C4-alkoxy or, together with the carbonyl
group, forms an active ester or a mixed anhydride, and purifying the
compound of the formula IVa or IVb, where appropriate, and then
converting it, with a compound of the formula Ilia or Ilib, into a
compound of the formula I.
The preparation of compounds according to formula I, and the preparation
of the starting substances which are required for this purpose, insofar as
the substances are not commercially available, is described in more detail
below.
The compounds according to the invention are most readily prepared by
mixing the two components, i.e. the pyrimidine derivative according to
formula (II) and the amine according to formula Ilia or Illb, in equimolar
quantities and reacting them, at temperatures of between -30 C and
150 C, preferably at from 20 C to 100 C, to give compound of the formula
IVa or IVb and then reacting the compounds of the formula Na or IVb, in an
analogous manner, with up to an equimolar quantity of amine according to
formula Illb or Ilia. The end of the reaction can be determined, for example,
by means of thin layer chromatography or HPLC-MS. A variant of this
method comprises carrying out the reaction in a suitable solvent, such as
diethyl ether, dimethoxyethane or tetrahydrofuran, chlorinated
hydrocarbons, such as methylene chloride, chloroform, trichloroethylene or
tetrachloroethylene, benzene or toluene, or else polar solvents such as
dimethylformamide, acetone or dimethyl sulfoxide. In this case, the reaction
temperatures are between room temperature and the boiling point of the
solvent, with temperatures in the range from room temperature to 130 C
being particularly preferred.
CA 02504153 2005-04-28
37
The reaction can also take place by way of a mixed anhydride, such as
ethyl chloroformate, or by way of an active ester, such as paranitrophenyl
ester (Y = CICH2-COO or N02-C6H4-O). Appropriate methods are known
and described in the literature.
A compound of the formula 11 or a compound of the formula IVa or lVb can
also react with an amine of the formula Ilia or Illb if Y is OH and the
corresponding carboxylic acid is activated in situ using customary coupling
reagents. Examples of these coupling reagents are carbodiimides, such as
dicyclohexylcarbodiimide (DCC) or diisopropylcarbodiimide (DCI), or N,N'-
carbonyldiazoles, such as N,N'-carbonyldiimidazole, or a uronium salt,
such as O-((cyano(ethoxycarbonyl)methylene)amino)-1,1,3,3-tetramethyl-
uronium tetrafluoroborate (TOTU) or O-(7-azabenzotriazol-1-yl)-1,1,3,3-
tetramethyluronium hexafluorophosphate (HATU). Appropriate methods are
known.
If amines of the formula Ilia or Illb are not commercially available, they can
be prepared from appropriate commercially available starting compounds
using methods known from the literature. Examples of suitable starting
compounds for amines are nitriles, nitro compounds, carboxamides,
carboxylic acid esters, carboxylic acids, aldehydes and bromides. Nitriles,
nitro compounds and carboxamides can be reduced to amines using
known methods. Carboxylic acids and carboxylic acid esters can be
converted into the carboxamides. Aldehydes can be converted directly into
the amines by way of reductive amination using NH4Ac/NaBH4, or else
initially converted into the oximes using hydroxylamine and then converted
into the amines by reduction.
Where appropriate, the reaction can also take place in the presence of
bases. Examples of suitable additional bases are carbonates or hydrogen
carbonates, such as sodium carbonate or potassium carbonate or sodium
hydrogen carbonate or potassium hydrogen carbonate, or tertiary amines,
such as triethylamine, tributylamine or ethyl diisopropylamine, or
heterocyclic amines, such as N-alkylmorpholine, pyridine or quinoline, or
dialkylanilines.
Where appropriate, the products, in particular the compound of the formula
IVa or lVb, can be worked up, for example, by extraction or
chromatography, e.g. through silica gel. The isolated product can be
CA 02504153 2005-04-28
38
recrystallized and, where appropriate, reacted with a suitable acid to give a
physiologically tolerated salt. Examples of suitable acids are:
mineral acids, such as hydrochloric acid and hydrobromic acid and also
sulfuric acid, phosphoric acid, nitric acid and perchloric acid, or organic
acids, such as formic acid, acetic acid, propionic acid, succinic acid,
glycolic acid, lactic acid, malic acid, tartaric acid, citric acid, maleic
acid,
fumaric acid, phenylacetic acid, benzoic acid, methanesulfonic acid,
toluenesulfonic acid, oxalic acid, 4-aminobenzoic acid, naphthalene-
1,5-disulfonic acid or ascorbic acid.
Insofar as they are not commercially available, the starting compounds of
the formula Illa or Ilib can be synthesized readily (e.g. Organikum,
Organisch Chemisches Grundpraktikum [Organicum, basic practical course
in organic chemistry], 15th Edition, VEB Deutscher Verlag der
Wissenschaften, 1976; an overview of the different possibilities can be
found on p. 822 in the methods index).
The starting compounds of the formula (II) are obtained, for example, by
using methods which are known from the literature to convert pyrimidine-
4,6-dicarboxylic acid into the corresponding pyrimidine-4,6-dicarbonyl
halide, preferably dicarbonyl chloride, preferably in the presence of a
catalyst such as dimethylformamide. This acid halide can then, for
example, be reacted either with a suitable alcohol, for example
paranitrobenzyl alcohol, to give the corresponding active ester or else with
lower alcohols, such as methanol or ethanol, to give the corresponding
esters. The pyrimidine-4,6-dicarboxylic acid can also initially be converted,
in the added presence of a suitable carboxylic acid or of a carboxylic acid
ester such as ethyl chloroformate, into a mixed anhydride, which is then
reacted with the amines of the compound of the formulae Illa or Illb and
IVa or lVb to give the products according to the invention. An appropriate
method is likewise described in the literature.
The pyrimidine-4,6-dicarboxylic acid is prepared using methods known
from the literature, for example by oxidizing 4,6-dimethylpyrimidine which,
for its part, can be obtained, for example, by the catalytic hydrogenation of
commercially available 2-mercapto-4,6-dimethylpyrimidine.
Insofar as compounds of the formula I permit diastereoisomeric or
enantiomeric forms and accrue as their mixtures in the synthesis which is
CA 02504153 2005-04-28
39
selected, separation into the pure stereoisomers is achieved either by
chromatography on an optionally chiral support material or, provided the
racemic compound of the formula I is capable of salt formation, by
fractionally crystallizing the diastereomeric salts which are formed using an
optically active base or acid as auxiliary substance. Examples of suitable
chiral stationary phases for the thin-layer or column chromatographic
separation of enantiomers are modified silica gel supports (what are termed
Pirkle phases) and also high molecular weight carbohydrates, such as
triacetyl cellulose. Following appropriate derivatization, which is known to
the skilled person, gas-chromatographic methods on chiral stationary
phases can also be used for analytical purposes. In order to separate the
enantiomers of the racemic carboxylic acids, the diastereomeric salts,
which differ in solubility, are formed using an optically active, as a rule
commercially available, base such as (-)-nicotine, (+)- and (-)-
phenylethylamine, quinine bases, L-lysine or L- and D-arginine, the more
sparingly soluble component is isolated as a solid, the more readily soluble
diastereomer is separated out from the mother liquor, and the pure
enantiomers are isolated from the diastereomer salts which have been
obtained in this way. The racemic compounds of the formula I which
contain a basic group, such as amino group, can, in what is in principle the
same manner, be converted into the pure enantiomers using optically
active acids, such as (+)-camphor-10-sulfonic acid, D- and L-tartaric acid,
D- and L-lactic acid and (+) and (-)-mandelic acid. Chiral compounds which
contain alcohol or amine functions can also be converted into the
corresponding esters or amides using appropriately activated or optionally
N-protected enantiomerically pure amino acids or, conversely, chiral
carboxylic acids can be converted into the amides using carboxyl-protected
enatiomerically pure amino acids or into the corresponding chiral esters
using enantiomerically pure hydroxyl carboxylic acids such as lactic acid.
The chirality of the amino acid or alcohol radical which has been introduced
in enantiomerically pure form can then be used for separating the isomers
by the diastereomers, which are now present, being separated by means of
crystallization or chromatography on suitable stationary phases and, after
that, using suitable methods to once again eliminate the entrained chiral
molecule moiety.
Acidic or basic products of the compound of the formula I may be present
in the form of their salts or in free form. Preference is given to
pharmacologically tolerated salts, e.g. alkali metal salts or alkaline earth
CA 02504153 2005-04-28
metal salts or hydrochlorides, hydrobromides, sulfates, hemisulfates, all
possible phosphates and also salts of the amino acids, natural bases or
carboxylic acids.
5 Physiologically tolerated salts are prepared in a manner known per se from
compounds of the formula I, including their stereoisomeric forms, which are
capable of salt formation. The carboxylic acids form stable alkali metal
salts, alkaline earth metal salts or optionally substituted ammonium salts
with basic reagents such as hydroxides, carbonates, hydrogen carbonates,
10 alkoxides and ammonia or organic bases, for example trimethylamine,
triethylamine, ethanolamine or triethanolamine, or else basic amino acids,
for example lysine, ornithine or arginine. Insofar as the compounds of the
formula I possess basic groups, stable acid addition salts can also be
prepared using strong acids. Both inorganic and organic acids, such as
15 hydrochloric acid, hydrobromic acid, sulfuric acid, phosphoric acid,
methanesulfonic acid, benzenesulfonic acid, p-toluenesulfonic acid,
4-bromobenzenesulfonic acid, cyclohexylamidosulfonic acid,
trifluoromethylsulfonic acid, acetic acid, oxalic acid, tartaric acid,
succinic
acid and trifluoroacetic acid are suitable for this purpose.
As a result of their pharmacological properties, the compounds of the
formula I are suitable for the prophylaxis and therapy of all those diseases
in whose course an increase in the activity of matrix meta Iloproteinase 13 is
involved.
These diseases include degenerative joint diseases, such as
osteoarthroses, spondyloses or cartilage loss following joint trauma or a
relatively long period of joint immobilization following meniscus injuries or
patella injuries or ligament rupture. They also include diseases of the
connective tissue such as collagenoses, periodontal diseases, wound
healing disturbances and chronic diseases of the locomotory apparatus,
such as inflammatory, immunologically determined or metabolism-
determined, acute and chronic arthritides, arthropathies, myalgias and
disturbances of bone metabolism or cancer diseases such as breast
cancer.
The pharmaceuticals according to the invention can be administered by
subcutaneous, intraarticular, intraperitoneal or intravenous injection.
CA 02504153 2005-04-28
41
Intraarticular injection is preferred. Rectal, oral, inhalative or transdermal
administration is also possible.
The invention also relates to a process for producing a pharmaceutical,
wherein at least one compound of the formula I is brought, together with a
pharmaceutically suitable and physiologically tolerated carrier and, where
appropriate, other suitable active compounds, additives or auxiliary
substances, into a suitable form for administration.
The compounds of the formula I are mixed with the additives, such as
carrier substances, stabilizers or inert diluents, which are suitable for the
purpose and brought into suitable administration forms, such as tablets,
sugar-coated tablets, hard gelatine capsules, aqueous, alcoholic or oily
suspensions or aqueous or oily solutions, using the customary methods.
The examples of inert carrier substances which can be used are gum
Arabic, magnesium oxide, magnesium carbonate, potassium phosphate,
lactose, glucose or starch, in particular corn starch. In this connection, the
preparation can be effected either as dry granules or as wet granules. The
examples of suitable oily carrier substances or solvents are vegetable or
animal oils, such as sunflower oil or cod liver oil.
For subcutaneous, intraarticular, intraperitoneal or intravenous
administration, the active compounds are, if desired, brought into solution,
suspension or emulsion using the substances which are suitable for this
purpose, such as solubilizers, emulsifiers or other auxiliary substances.
Examples of suitable solvents are physiological sodium chloride solution or
alcohols, e.g. ethanol, propanol or glycerol, and, in addition, sugar
solutions, such as solutions of glucose or mannitol, or else a mixture which
is composed of the different solvents mentioned.
Customary adjuvants, such as carrier substances, disintegrants, binding
agents, coating agents, swelling agents, glidants or lubricants, flavorings,
sweeteners and solubilizers are also used. Auxiliary substances which are
frequently employed and which may be mentioned are magnesium
carbonate, titanium dioxide, lactose, mannitol and other sugars, talc, milk
protein, gelatine, starch, cellulose and its derivatives, animal and vegetable
oils, such as cod liver oil, sunflower oil, peanut oil or sesame seed oil,
polyethylene glycol and solvents such as sterile water and monohydric or
polyhydric alcohols, such as glycerol.
CA 02504153 2005-04-28
42
The compounds of the formula I are preferably prepared as pharmaceutical
preparations and administered in dosage units, with each unit containing a
defined dose of the compound of the formula I as the active constituent.
For this purpose, the compounds of the formula I can be administered
orally in doses of from 0.01 mg/kg/day to 25.0 mg/kg/day, preferably from
0.01 mg/kg/day to 5.0 mg/kg/day, or parenterally in doses of from
0.001 mg/kg/day to 5 mg/kg/day, preferably of from 0.001 mg/kg/day to
2.5 mg/kg/day. The dosage can also be increased in severe cases.
However, smaller doses are also adequate in many cases. These figures
relate to the treatment of an adult.
The invention is explained in more detail below with the aid of examples.
Example 1:
Ethyl [4-({[6-(4-fluoro-3-methylbenzylcarbamoyl)pyrimidine-4-carbonyl]-
amino}methyl)phenyl]acetate
N3c\
0 0
CH3
F
N N
0 0
a) Methyl 6-(4-fluoro-3-methylbenzylcarbamoyl)pyrimdine-4-carboxy-
late
8.81 g (0.045 mol) of dimethyl pyrimidine-4,6-dicarboxylate were dissolved
in 200 ml of DMF, after which 6.25 g (0.045 mol) of 4-fluoro-3-
methylbenzylamine were added and the mixture was stirred at 60 C for 48
hours (h). The solvent was removed in vacuo and the residue was taken up
in ethyl acetate. The organic phase was washed with a saturated solution
of sodium hydrogen carbonate and 0.5 N HCI and then dried (MgSO4).
After filtering, and evaporating the solvent in vacuo, the residue was stirred
up in isopropanol. This resulted in 8.75 g of product, which was subjected
to further reaction without any further purification.
=CA 02504153 2010-08-31
43
b) 6-(4-Fluoro-3-methylbenzylcarbamoyl)pyrimdine-4-carboxylic acid
8.75 g (0.02 mol) of methyl 6-(4-fluoro-3-methylbenzylcarbamoyl)-
pyrimidine-4-carboxylate (70%) were taken up in 150 ml of ethanol, after
which 1.89 g (0.022 moll) of NaOH in 6 ml of water were added. After 3
hours (h) at room temperature, the solvent was removed under reduced
pressure and water was added to the residue; the solution was then
brought to pH < 2 with conc. HC1. The precipitate was filtered off with
suction and dried. This resulted in 5.5 g (94%) of 6-(4-fluoro-3-
methylbenzylcarbamoyl)pyrimidine-4-carboxylic acid. MS (ES+): m/e =
289.09
c) Ethyl (4-aminomethylphenyl)acetate
0.5 g (2.6 mmol) of ethyl (4-cyanophenyl)acetate was dissolved in 70 ml of
ethanolic ammonia solution and hydrogenated, at room temperature and
under standard pressure, over RaneyTM nickel. After 45 minutes, the mixture
was filtered and evaporated. This resulted in 0.42 g (82%) of ethyl (4-
aminomethylphenyi)acetate. MS (ES`): We = 194.11
d) Ethyl [4-(([6-(4-fluoro-3-methylbenzylcarbamoyl)pyrimidine-4-carbon-
y1jamino)methyl)phenyl)acetate
1.3 g (4.5 mmot) of 6-(4-fluoro-3-methylbenzylcarbamoyl)pyrimidine-4-
carboxylic acid and 1.042 g (5.4 mmot) of ethyl (4-aminomethyl-
phenyl)acetate were dissolved in 30 ml of DMF, after which 1.02 g
(4.9 mmol) of dicyclohexylcarbodiimide and 0.607 g (4.5 mmol) of
hydroxybenzotriazole were added at 5 C. The mixture was stirred for 5
hours (h) and filtered with suction. The solvent was removed in vacuo and
the residue was taken up in ethyl acetate; this solution was washed with a
saturated aqueous solution of NaHCO3. The organic phase was dried
(MgSO4), filtered and evaporated under reduced pressure. This resulted In
2.66 g of product, which was further purified by means of preparative
HPLC. MS (ES+): m/e = 464.19
Example 2:
(4-({[6-(4-Fluoro-3-methylbenzylcarbamoyl)pyrimidine-4-carbonyljamino}-
methyl)phenyljacetic acid
CA 02504153 2005-04-28
44
HO 0
CH,
F
J___NI'/\N
N /
O O
2.4 g (5.2 mmol) of ethyl [4-({[6-(4-fluoro-3-methyl benzylcarbamoyl)-
pyrimidine-4-carbonyl]amino}methyl)phenyl]acetate were taken up in
150 ml of water, after which 10 ml of water and 0.227 g (5.7 mmol) of
NaOH were added. After 5 days of stirring at room temperature, the solvent
was removed under reduced pressure and the residue was stirred up with
ethanol and filtered off. This resulted in 1.51 g (67%) of [4-({[6-(4-fluoro-3-
methylbenzylcarbamoyl)pyrimidine-4-carbonyl]amino}methyl)phenyl]acetic
acid. MS (ES+): m/e = 436.15
Example 3
Pyrimidine-4,6-dicarboxylic acid 4-(4-diethylcarbamoylbenzylamide)
6-(3-methoxybenzylamide)
CH 0
HC ) I N N N I O.CH3
3
O O
a) Synthesizing 6-(3-methoxybenzylcarbamoyl)pyrimdine-4-carboxylic
acid
26 g (88 mmol) of methyl 6-(3-methoxybenzylcarbamoyl)pyrimidine-4-
carboxylate (prepared by reacting dimethyl pyrimidine-4,6-dicarboxylate
with 3-methoxybenzylamine) were dissolved in 100 ml of tetrahydrofuran,
after which 104 ml (1.2 equivalents) of a 1 molar aqueous solution of
lithium hydroxide were added and the reaction mixture was then stirred at
room temperature for 18 hours.
The majority of the solvent employed was then distilled off under reduced
pressure and insoluble by-products were filtered off from the residue; the
filtrate was then acidified with a 20% aqueous solution of citric acid. In
connection with this, 6-(3-methoxybenzylcarbamoyl)pyrimidine-4-carboxylic
CA 02504153 2005-04-28
acid crystallized out in the form of pale yellow crystals, which were filtered
off.
This resulted in 19 g (66.2 mmol) of 6-(3-methoxybenzylcarbamoyl)-
5 pyrimidine-4-carboxylic acid (yield 75% of theory; MS (ES+): m/e = 287.8)
b) Methyl 4-({[6-(3-methoxybenzylcarbamoyl)pyrimdine-4-carbonyl]-
amino}methyl)benzoate
10 4.3 g of 6-(3-methoxybenzylcarbamoyl)pyrimidine-4-carboxylic acid
(15 mmol) from a) were dissolved in 50 ml of absolute
N,N-dimethylformamide, after which 3.3 g (16.5 mmol) of methyl 4-(amino-
methyl)benzoate hydrochloride, 5.4 g (16.5 mmol) of O-[(cyanoethoxy-
carbonylmethylene)amino]-N,N,N',N'-tetramethyluronium tetrafluoroborate
15 (TOTU) and 4.6 ml of triethylamine (33 mmol) were added consecutively, at
0 C and while stirring. The reaction mixture was stirred at 0 C for 1 hour
and then stirred at room temperature for 12 hours.
For the working up, the solvent was distilled off under reduced pressure
20 and the residue was taken up in 100 ml of dichloromethane. The organic
phase was washed with 100 ml of a saturated aqueous solution of sodium
hydrogen carbonate and then washed three times with in each case 100 ml
of water. After the organic phase had been dried with Na2SO4, the solvent
was distilled off under reduced pressure. The oily residue was triturated
25 with a little diethyl ether, during which colorless crystals crystallized
out.
After the reaction product had been filtered off and washed with n-pentane,
6.6 g of methyl 4-({[6-(3-methoxybenzylcarbamoyl)pyrimidine-4-
carbonyl]amino}methyl)benzoate (pale yellow crystals) were obtained.
According to LC-MS analysis, the purity of the reaction product is 88% (MS
30 (ES+): m/e = 435.2).
c) 4-({[6-(3-Methoxybenzylcarbamoyl)pyrimidine-4-carbonyl]amino}-
methyl)benzoic acid
6.6 g of the methyl ester prepared in b) were dissolved in 100 ml of
35 tetrahydrofuran, after which 36 ml (2.4 equivalents) of a 1 molar solution
of
lithium hydroxide were added and the reaction mixture was then stirred for
4 hours while refluxing the solvent.
CA 02504153 2005-04-28
46
After that, the solvent was distilled off under reduced pressure. After 50 ml
of water had been added, the mixture was filtered through Celite filter aid
and the filtrate was acidified with 2 n aqueous hydrochloric acid. The
reaction product precipitated out on acidification and was filtered off.
This resulted in 3.05 g of 4-({[6-(3-methoxybenzylcarbamoyl)pyrimidine-4-
carbonyl]amino}methyl)benzoic acid, pale yellow crystals [yield 48% of
theory; MS (ES+): m/e = 421.31]
d) Pyrimidine-4,6-dicarboxylic acid 4-(4-diethylcarbamoylbenzylamide)
6-(3-methoxybenzylamide)
420 mg of 4-({[6-(3-methoxybenzylcarbamoyl)pyrimdine-4-carbonyl]amino}-
methyl)benzoic acid from c) were dissolved in 5 ml of absolute
N,N-dimethylformamide, after which 115 l of diethylamine, 361 mg of
O-[(cyanoethoxycarbonylmethylene)amino]-N,N,N',N'-tetramethyluronium
tetrafluoroborate (TOTU) and 153 l of triethylamine were added
consecutively, at 0 C and while stirring, and the reaction mixture was
stirred at 0 C for 1 hour and then at room temperature for 12 hours.
For the working up, the solvent was distilled off under reduced pressure
and the residue was taken up in 100 ml of dichloromethane. The organic
phase was washed with 30 ml of a saturated aqueous solution of sodium
hydrogen carbonate and then washed three times with in each case 30 ml
of water. After the organic phase had been dried with Na2SO4, the solvent
was distilled off under reduced pressure. The oily residue was purified by
column chromatography on silica gel (40-63 ) using ethyl
acetate/n-heptane, mixing ratio 2:1, as the mobile phase. After the solvent
had been distilled off, an oily residue was obtained, with this residue slowly
crystallizing after a little diethyl ether had been added.
This resulted in 270 mg of pyrimidine-4,6-dicarboxylic acid 4-(4-diethyl-
carbamoylbenzylamide) 6-(3-methoxybenzylamide), colorless crystals
(yield 57% of theory [MS (ES'): m/e = 476.40]).
Example 62
Pyrimidine-4,6-dicarboxylic acid 4-[(2,3-dihydrobenzofuran-5-ylmethyl)-
amide] 6-[(pyridin-4-ylmethyl)amide]
CA 02504153 2005-04-28
47
N / IAN / O
\ N
N \
Y_I
O O
a) Synthesizing 6-[(pyridin-4-ylmethyl)carbamoyl]pyrimidine-4-carboxylic
acid
9.7 g (35.7 mmol) of methyl 6-[(pyridin-4-ylmethyl)carbamoyl]pyrimidine-4-
carboxylate (prepared by reacting dimethyl pyrimidine-4,6-dicarboxylate
with pyridin-4-ylmethylamine) were dissolved in 80 ml of tetrahydrofuran
and 40 ml of water, after which 40 ml of a 1 molar aqueous solution of
NaOH were added and the reaction mixture was subsequently stirred at
room temperature for 2 hours.
For the working-up, the reaction mixture was concentrated down, on a
rotary evaporator and under reduced pressure, to half the original volume.
The residue was then acidified with 22 ml of an aqueous 2 N solution of
hydrochloric acid and the reaction mixture was concentrated down to
dryness on the rotary evaporator.
This resulted in 12.2 g of a colorless solid product, which was further
reacted directly as described in 62 b).
b) Pyrimidin-4,6-dicarboxylic acid 4-[(2,3-dihydrobenzofuran-5-
ylmethyl)amide] 6-[(pyridin-4-ylmethyl)amide]
12.2 g of the compound prepared under 62a) were dissolved in 150 ml of
absolute DMF, after which 6.63 g (35.7 mmol) of 5-aminomethyl-2,3-
dihydrobenzofuran hydrochloride, 11.7 g (35.7 mmol) of O-[(cyano-
ethoxycarbonylmethylene)amino]-N,N,N',N'-tetramethyluronium tetrafluoro-
borate (TOTU) and 20 ml of triethylamine were added consecutively while
the mixture was being stirred at 0 C. After the addition had been
completed, the reaction mixture was stirred at 0 C for 1 hour and at room
temperature for 4 hours.
For the working-up, the solvent was distilled off under reduced pressure
and the residue was taken up in 200 ml of dichloromethane. The organic
phase was then washed twice with a saturated aqueous solution of sodium
CA 02504153 2005-04-28
48
hydrogen carbonate and once with water and then dried with sodium
sulfate; the solvent was then removed under reduced pressure on a rotary
evaporator. The reaction product, which accrued as an oily residue,
crystallized out in the form of pink crystals after a little diethyl ether had
been added. In order to purify it further, it was recrystallized twice from in
each case 200 ml of isopropanol.
This resulted in 10g (25.6 mmol) of pyrimidine-4,6-dicarboxylic acid
4-[(2,3-dihydrobenzofuran-5-ylmethyl)amide] 6-[(pyridin-4-ylmethyl)amide]
(yield 72 % of theory, based on both reaction steps; MS (ES+): 390.08)
'H-NMR (400 MHz, d6-DMSO): 6 = 3.13 (t, J = 8.6 Hz,2H), 4.42 (d, J = 6.4
Hz, 2H), 4.48 (t, J = 8.6 Hz, 2 H), 4.54 (d, J = 6.4 Hz, 2 H), 6.69 (d, J = 8
Hz, 1 H), 7.07 (m, 1 H), 7.22 (m, 1 H), 7.31 (m, 2 H), 8.46 (m, 1 H), 8.50 (m
2 H), 9.47 (m, 1 H), 9.58 (t, J = 6.4 Hz, 1 H), 9.80 (t, J = 6.4 Hz, 1 H)
Example 117
Pyrimidine-4,6-dicarboxylic acid 4-[(2,3-dihydrobenzofuran-5-ylmethyl)-
amide] 6-[4-(morpholine-4-carbonyl)benzylamide]
0
rN NON O
N N
OJ
O O
a) Synthesizing 6-[(2,3-dihydrobenzofuran-5-ylmethyl)carbamoyl]-
pyrimidine-4-carboxylic acid
16.1 g (51 mmol) of methyl 6-[(2,3-dihydrobenzofuran-5-ylmethyl)-
carbamoyl]pyrimidine-4-carboxylate (prepared by reacting dimethyl
pyrimidin-4,6-dicarboxylate with 5-aminomethyl-2,3-dihydrobenzofuran)
were dissolved in 150 ml of tetrahydrofuran, after which 62 ml of a 1 molar
aqueous solution of LiOH were added and the reaction mixture was then
stirred at room temperature for 2 hours.
For the working-up, the reaction mixture was concentrated under reduced
pressure on a rotary evaporator. The crude product was then taken up in
100 ml of water and, after active charcoal had been added, this solution
was filtered through a Celite clarifying layer. The resulting mother liquor
was then acidified by adding a 2 N solution of aqueous HCl, in connection
CA 02504153 2005-04-28
49
with which the reaction product slowly precipitated out in the form of
colorless crystals.
After the reaction product had been filtered off and dried, 8.3 g (27 mmol)
of a colorless solid product were obtained, with this product then being
further reacted directly to give 117b; yield 53% of theory.
MS (ES+): 300.1
b) Synthesizing methyl 4-[({6-[(2,3-dihydrobenzofuran-5-ylmethyl)-
carbamoyl]pyrimidine-4-carbonyl}amino)methyl]benzoate
4.7 g (15.7 mmol) of the compound prepared under a) were dissolved in
30 ml of absolute DMF, after which 3.5 g (17.3 mmol) of methyl 4-amino-
methylbenzoate, 5.7 g (17.3 mmol) of O-[(cyanoethoxycarbonylmethylene)-
amino]-N,N,N',N'-tetramethyluronium tetrafluoroborate (TOTU) and 4.8 ml
of triethylamine were added consecutively while the mixture was being
stirred at 0 C. After the addition had been completed, the reaction mixture
was stirred at 0 C for 1 hour and at room temperature for 8 hours.
For the working-up, the solvent was distilled off under reduced pressure
and the residue was taken up in 100 ml of dichloromethane. The organic
phase was then washed twice with a saturated aqueous solution of sodium
hydrogen carbonate and once with water and then dried with sodium
sulfate; the solvent was then removed under reduced pressure on a rotary
evaporator. The reaction product, which accrued as an oily residue,
crystallized in the form of pale yellow crystals after a little diethyl ether
had
been added.
This resulted in 6.8 (15.2 mmol) of methyl 4-[({6-[(2,3-dihydrobenzofuran-5-
ylmethyl)carbamoyl]pyrimidine-4-carbonyl}amino)methyl] benzoate, yield
97% of theory.
MS (ES+): 447.1
c) 4-[({6-[(2,3-dihydrobenzofuran-5-ylmethyl)carbamoyl]pyrimidine-4-
carbonyl}amino)methyl]benzoic acid
6.28 g (14 mmol) of methyl 4-[({6-[(2,3-dihydrobenzofuran-5-ylmethyl)-
carbamoyl]pyrimidine-4-carbonyl}amino)methyl]benzoate (see 117b)
CA 02504153 2005-04-28
were suspended in 150 ml of tetrahydrofuran and 70 ml of water, after
which 16.9 ml of a 1 N aqueous solution of NaOH were added and the
reaction mixture was then stirred at room temperature for 24 hours.
5 For the working-up, the reaction mixture was concentrated down, on a
rotary evaporator and under reduced pressure, to a volume of approx.
50 ml, after which 100 ml of ice water were added. The mixture was then
acidified with a 2N aqueous solution of HCI, in connection with which the
reaction product precipitated out in the form of pale yellow crystals.
After the reaction product had been filtered off, washed with a little water
and dried, 5.4 g (12.5 mmol) of a colorless solid product were obtained,
with this product being further reacted directly to give 117d; yield: 89% of
theory.
MS (ES+): 433.2
1H-NMR (400 MHz, d6-DMSO): b = 3.13 (t, J = 8.7 Hz, 2 H), 4.43 (d, J = 6.1
Hz, 2 H), 4.48 (t, J = 8.7 Hz, 2 H), 4.59 (d, J = 6.3 Hz, 2 H), 6.69 (d, J =
8.1
Hz, 1 H), 7.07 (m, 1 H), 7.22 (m, 1 H), 7.44 (m, 2 H), 7.88 (m, 1 H), 7.90
(m, 1 H), 8.47 (d, J 1.5 Hz, 1 H), 9.46 (d, J = 1.3 Hz, 1 H), 9.56 (t, J = 6.3
Hz, 1 H), 9.76 (t, J = 6.3 Hz, 1 H), 12.90 (br s, 1 H)
d) Pyrimidine-4,6-dicarboxylic acid 4-[(2,3-dihydrobenzofuran-5-ylmethyl)-
amide] 6-[4-(morpholine-4-carbonyl)benzylamide]
432 mg (1 mmol) of the compound prepared under c) were dissolved in
5 ml of absolute DMF after which 96 pl (1.1 mmol) of morpholine, 361 mg
(1.1 mmol) of O-[(cyanoethoxycarbonylmethylene)amino]-N,N,N',N'-
tetramethyluronium tetrafluoroborate (TOTU) and 155 pl of triethylamine
were added consecutively while the mixture was being stirred at 0 C. After
the addition had been completed, the reaction mixture was stirred at 0 C
for 1 hour and at room temperature for 8 hours.
For the working-up, the solvent was distilled off under reduced pressure
and the residue was taken up in 30 ml of dichloromethane. The organic
phase was then washed twice with a saturated aqueous solution of sodium
hydrogen carbonate and once with water and dried with sodium sulfate; the
solvent was then removed under reduced pressure on a rotary evaporator.
The crude product, which accrued as an oily residue, was purified by
CA 02504153 2005-04-28
51
means of chromatography on silica gel (40-63 p; mobile phase: ethyl
acetate/methanol = 20/1). After the mobile phase had been removed by
distilling under reduced pressure, an oily reaction product was obtained,
with this reaction product crystallizing out in the form of colorless crystals
after diethyl ether had been added.
340 mg (068 mmol) of colorless crystals were obtained, yield 68% of
theory.
MS (ES+): 502.27
1H-NMR (500 MHz, d6-DMSO): 6 = 2.60 (m, 2 H), 3.4 - 3.6 (br m, 8 H),
4.42(d,J=6.5Hz,2H),4.48(t,J=8.6Hz,2H),4.56(d,J=6.5Hz,2H),
6.68 (d, J = 8.3 Hz, 1 H), 7.07 (d, J = 6.5 Hz, 1 H), 7.22 (s, 1 H), 7.88 (m,
4
H), 8.46 (m, 1 H), 9.46 (m, 1 H), 9.57 (t, J = 6.5 Hz, 1 H), 9.75 (t, J = 6.5
Hz, 1 H)
The following compounds were prepared in a comparable manner.
Table 1:
Example Structure MS
(ESI+)
4 462.36
0 0
5 462.37
H H
H
\ N / N \
0 0
6 478.34
Y II H
0 0
CA 02504153 2005-04-28
52
7 407.36
\ I N I/ N \ I /
p~~ 0
0 0
8 430.2
NON /
N N N
0 0 0
9 0 0 491.38
\ H \ I \
O N
NH
519.36
o 0
I \ H ~\ H
/ N
O
NH
11 0 0 513.31
a
H I --~ I
O / N\/ N F
NH
N"r
12 O N/~ N / 372.19
N I
N I /
O
O O
CA 02504153 2005-04-28
53
13 477.41
H
\ /OyN1
/xIf yl-~Iy ' N
O O
14 470.20
F I
N N 0
N yl:-~y N H
O O
15 471.29
F
H N.;_-N 0
I / \ ~ H II
O O
16 436.22
F I
H N-;~ I N 0
H
0 O
17 437.17
F N~~N 0
H ",,~ H
O O
18 437.17
N
y F HN/N 0
I N
H
0 0
CA 02504153 2005-04-28
54
19 N 519.48
F N
N N
l i N N) O
'_.J-',
O O
20 522.41
F( N N O N
N N~~ O
O 0
21 O 508.44
F N^N I ON
N N)
O 0
22 478.44
F N N\/
N ~ N O
O O
23 COD 549.50
N
J
F N i I N
s N N O
O O
24 492.45
F N'N N~-
~ - N N O -r~ O O
25 (J 506.45
F NON N
.~ N N N N j O
0 0
CA 02504153 2005-04-28
26 494.32
O N
F \ j N/~N N
N
O
27 0 N N 435.38 ~,, N r~ N \N N O
N O O
28 0 N N 435.38
AN N
N\~ N JZJLO
O O
29 CI 0 NN 469.37
)N N
N \ N O
O O
30 0 N^N a F 491.31
CI )N i N
N CI
N 0 0
31 O N 492.26
o N N
O N ~ N C O-
O 0
32 OXN N---- N 464.20
O Nor N
O
O 0
33 O .f N, N^ N ~ 476.23
0 N N
O
0 0
34 ~O NN F 512.38
N N
N CI
0 0
CA 02504153 2005-04-28
56
35 'OI N N F 457.29
NJ~N i N CI
O O
N
36 N 0
I N'' N 533.30
N N N I O-
O 0
37 CN -,,- N O N N 517.27
N WA,~ N O
O O
38 O
N
O I/ N \ p
g/O O O
//
560.20
39 N'N
S N \ I N
0\/ 00
395.30
40 NN
H3C O`
0 0
393.27
41 CH3
0
N/ 1
, 0 W- \- / 0
N I / N \
0 0
486.15
CA 02504153 2005-04-28
57
42
N /
/ S
N NN N 0
O O
472.12
43 0
/ II N/\N
N
N I / \
O O
390.38
44
I~\N O
N
O o 406.16
45 0
N N O
H3C 0 0 0 409.16
46 N^N O
N /
S
Y~y
0 0 411.17
47 H3c
0'N NN 0
N I / N
O O
394.04
48 H3C
N\ I N I' N O
/ N \
O O
393.28
CA 02504153 2005-04-28
58
49 H3C
O NN 0
CH3 N N
0 0 407.10
50 HzN i
N~\ N O
N Q / N
O O
405.31
51 N-CH3
N I / N \
o 0 392.09
52 Q
N
N-i) I~~N / f O
N / N \ I
o o 429.12
53 N
CN N"'ZZ:~'N O
N I / N \
o o 391.05
54 0 0
F O
Fx N N~ \ N + \
N
428.14
0 N/~N
H3C\ II N N
O O
0 0 387.18
56 N
H3C-- -'
N N/Z~N / O
N I N \
0 0 393.24
CA 02504153 2005-04-28
59
57
NON
N N
O 0
404.36
58 F
N
N--~N
N I / N ~
O 0
473.30
59
N \ I /
\ I N I / O
O O
506.26
60 \N/~/O / N^N / O1
N I / N
O
0 0 492.39
61
/N~/~O \ I N I/ N \ I O
O O
492.31
62 N N N
\ I N I^ N / I O
O
390.08
63 H H
~I / N/\N F
O O \ I H/ H
O 0
535.06
CA 02504153 2005-04-28
64 0
~~\/ ref \ N /
Hyk~y H \ I Cr"
N
O
0 0 502.19
0
~J - F
%/ ~ N/\ N YH
0 0 503.9
66 0
%~ N/-N O
0
0 0 514.15
67 0
\ I H i N \ I /
Fq a
0 0 504.14
68 0
N-\ N
\ I H / H J~
HO
0 0 516.19
69 0
H
HCr'o
0 0 506.16
0
F
/ N^ N /
H
0 0 508.16
71 0
H
N \ I
0 0 506.09
72 0
(~ \ N N/\ N \
0 0 518.1
CA 02504153 2005-04-28
61
73 0
..\/ \ I N N 0/
0 0
503.14
74 0
O / N^N H 0
Y----ly 0 0 515.14
75 0
fN H F
H I
0 0 0 505.13
75a 0
N'--- N O
\ I H I/ H \ I
0 0 476.14
76 0
/'- N F
H I H ~ ` H \
0 0 466.12
77 0
\ H ~" F
0 0 513.05
78 0
\ \ I
W^N H \ F
H
0 0 464.12
79 0
I N
H
0 0
547.16
80 o
\ I H I / H "W
N/\ N / O
0 0
559.18
CA 02504153 2005-04-28
62
81 0
J~ F
0 0 505.13
82 0
H H H
N/\ N
0 0 523.15
83 0\ S/N N / N
N
0 0 N -r~ N,
0 0 515.12
84 o H H
N
O O N
O 0
525.28
85 0
N\
H H N H
\ / N \
O 0
457.21
86 0
N
O 0
445.13
87 0
N N
\/ \ I N N \ I F
O 0
492.39
88 0 0 ~ N
II I H H
H
0 0
513.28
CA 02504153 2005-04-28
63
89 o
N/~N
H H K`\y//~
O O
436.18
90 H 0
HD-" N N 0
H / H
O N N~ \
O O
505.23
91 \ ( \ NI-' N / I O
0 0
502.25
92 HN( 0
N N Hy, N /
O O
534.21
93 0
HD- / N^ N F
H
\ I NyI /
O O
438.18
94 0
HD~,
H
a
yC)
O O
448.15
95 \ / I i 'N I F
\ a a \
0 0
492.23
CA 02504153 2005-04-28
64
96 0
H
7\ / N-`~-, N
~Y~
0 0
476.27
97 \ 0
N\II II N
0 0
531.31
98 0
H N^ N / 0
0 N,?~N
o o
490.26
gg
<DN o
H \ I N I/ N \ i
0 0
519.28
100 0
JN
N N I/ \ I O
0 0
560.31
101 H H
O\s- N\ /N / ~N
A0 0 H ` H
0 0
513.25
102 H \ I H ,,~ N
N N
O O
533.23
CA 02504153 2005-04-28
103 0
N/\N
F
H N ~/~ N1
O H\ I
O O
480.29
104 ^^/ H
HN,_,, IOI Y/y N \ I O/
O O
518.25
105 Io o
O 0
535.25
106 0
/ ^N
I / N
O H \ N Yly ,
O O
494.18
107 0~ \ ( ~N
N N
O O O
490.25
108 0
N
H H H
H \ I / 0
O O
517.28
109 Ha 0
/ N
/
H `~
\ I N\ I~NV\
O O
503.28
110 HNa
N N\F
\ I
H H I ,Y H 0 0
505.23
CA 02504153 2005-04-28
66
0
rN N
rT
0 0
533.26
112 F / 1 N
N
NH N O
0
450.31
113 0
N^ N F
HN N I / H \
O 0
519.27
114 0 0
/ I H I\ H I\
~N \ N,,/ N
0 0
503.24
115 0 0
I / I H Y\ rI \
N \ Nom/ N /
0
566.25
116 H
N HH /\
0 \ I N i /N N \ I /
0 0
519.25
117 dLOU 0
H
N
0 0
502.27
118 /
H
\ I S/N\/H / rI N /
~ 'moo H II ~/~~(/H
O \ N\~I II N \ I 0/
0 0
589.25
CA 02504153 2005-04-28
67
119 0
I~ N \ I N__-_ N
\Ykjyj
N
O O
515.36
120 0N/~N / 0 O O
529.29
121 H N H
0 y \ IH
N Ii ' N N \ O/
O 0
~OJ~ tJ
575.23
122 Ol~ 0
N^ N O
~
H N k/ N
O O
545.24
123 Nr~N 0
O 0
502.2
125
OY H H
N tJ ' Y'-(IH N
O 0 N / N ( O/
O 0
545.25
126
H H /~
N Z 0 Ny N I y~, ' N \ D/ 0 O 0
540.25
127 { q~ /
O O
0 O
505.25
CA 02504153 2005-04-28
68
128
N
Lr o
N
FN"( H
N N \ I o/
0 0
503.35
129 s o
0 0
429.13
130 0 H
/
tN'-f "- / N/\ N H
H
\ I / \ I
0 0
517.32
131 s N~\ N o
o 0
395.23
132 N^N
o 0
409.27
133 /~
N N
0 0
409.17
134 H /
N N
0 N H
/ \ I c
0 0
477.3
CA 02504153 2005-04-28
69
135 p
IN
o 0
518.34
136 H H
aNyN,, tJ N
yk/ H \ I p/
O O
517.49
H H
137
a N \/I N-\ N
H
N/ O I/ \ I O/
O O O
580.35
138 *11Y N
N-' --N O N / H
O O
491.34
139
ay o
H \ I N N
O O
488.22
140
o1 Sao
H \ I I /~N N
N,1~~/ \
1 O
O O
484.35
141
o, s o
N { i / N \
\~I O
O o
538.15
CA 02504153 2005-04-28
142 F
F F
O~ _O
\I p N- N a I 0
0 0
538.34
143 oY
H ^~ /
N
N
0 0
450.34
144
OY 0
H
0 0
474.41
145
0Y0
HJ\\j~ N^ N
y H ' ~Iy H
0 0
494.45
146 F
0 `I~ \ o
\ I N I / N \
0 0
530.43
147
N---I N
OY N
H
0 0
539.23
CA 02504153 2005-04-28
71
148 O / N^ N
N
488.41
149 0 / -~~ N /
YI-Ily H 0O O
474.41
150 I N
O
O O
478.41
150a
/ N/\ N F
H
\ I a~~p \ I a
0 0
539.4
151 0
N-'N 0"0, /
1 0,
0 0
490.43
152 0
V~N
H
0 0
511.41
153 0
J \I " F
N\ I I ~N
0 0
498.36
154 0
F
N
O O
512.37
CA 02504153 2005-04-28
72
155 r,,~ o
Y N I F
H
0 0
555.4
156 0
F
532.46
157 0\1 , \ I N NI f ~ N
0 0
490.35
158 0
H H
N I / \ O/
0 0
476.4
159
N I N---' N
N H
0 0
424.21
160 0 H F
N\N
/ ~ HH
O \ I N ~ / \
O 0
492.12
161
\ I "' n I /
\\I I
0 0
455.1
CA 02504153 2010-08-31
73
Pharmacological examples
Determining the enzyme activity of the catalytic domain of human
collagenase 3 (MMP-13).
This protein is obtained as an inactive proenzyme from INVITEK, Berlin
(Catalogue No. 30 100 803). Activating the proenzyme:
2 parts by volume of proenzyme are incubated with I part by volume of
APMA solution at 37 C for 1.5 hours. The APMA solution is prepared from
a 10 mmol/L solution of p-aminophenylmercuric acetate in 0.1 mmol/L
NaOH by diluting with 3 parts by volume of tris/HCi buffer, pH 7.5 (see
below). The pH is adjusted to between 7.0 and 7.5 by adding I mmol/L
HCI. After the enzyme has been activated, it is diluted to a concentration of
1.67 pg/ml using the tris/HCI buffer.
In order to measure the enzyme activity, 10 L of enzyme solution are
incubated for 15 minutes with 10 pL of a 3% (vlv) buffered solution of
dimethyl sulfoxide (reaction 1). In order to measure the enzyme inhibitor
activity, 10 NL of enzyme solution are incubated with 10 L of a 3% (v/v)
buffered solution of dimethyl sulfoxide containing the enzyme inhibitor
(reaction 2).
In the case of both reaction 1 and reaction 2, the enzyme reaction is
monitored by fluorescence spectroscopy (328 nm (extinction)/393 nm
(emission)) after adding 10 gL of a 3% (vlv) aqueous solution of dimethyl
sulfoxide containing 0.75 mmol of the substrate/L.
The enzyme activity is depicted as Increase in extinction/minute.
The effect of the inhibitor is calculated as a percentage inhibition using the
following formula:
% inhibition = 100 - [(increase in extinction/minute in reaction 2)/(increase
in extinction/minute in reaction 1) x 100].
The lC5O, i.e. the inhibitor concentration which is required for inhibiting
the
enzyme activity by 50%, is determined graphically by plotting the
percentage inhibitions at different inhibitor concentrations.
The buffer solution contains 0.05% Brij" (Sigma, Delsenhofen, Germany)
and
0.1 mol of tris/HCI/L, 0.1 mot of NaCUL and 0.01 mol of CaC12/L (pH = 7.5).
The enzyme solution contains 1.67 g of the enzyme domain/mL.
CA 02504153 2005-04-28
74
The substrate solution contains 0.75 mmol/L of the fluorogenic substrate
(7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-3-(2',4'-dinitrophenyl)-L-
2,3-diaminopropionyl-Ala-Arg-NH2 (Bachem, Heidelberg, Germany).
Table 2 below shows the results.
Table 2:
Ex- IC50 Ex- IC50 Ex- IC50 Ex- IC50
ample MMP13 ample MMP13 ample MMP13 ample MMP13
(nM) (nM) (nM) (nM)
3 4 73 25 96 50 126 2.3
4 13 74 30 98 20 127 3.3
6 20 75 70 99 50 128 60
36 10 75a 34 101 2 129 22
39 30 76 23 105 23 135 20
44 200 78 3 106 40 136 20
62 20 82 30 109 50 137 40
63 9 83 5 112 45 138 50
64 10 84 3.2 114 50 139 10
65 15 85 3.5 115 20 144 9
66 10 86 2.5 117 8 147 3
67 15 87 24 118 2.4 150 80
68 22 88 33 119 35 151 9
69 32 89 18 121 30 153 15
70 24 93 20 122 43 154 22
71 9 94 20 125 4 158 4
72 10
Determining the enzyme activity of the catalytic domain of human
neutrophil collagenase (MMP-8) and of human Stromelysin (MMP-3).
The [lacuna] enzymes human neutrophil collagenase and human
Stromelysin, prepared as active catalytic domains, were carried out as
described in Weithmann et al Inflamm Res, 46 (1997), pages 246-252. The
measurement of the enzyme activity, and the determination of the inhibitory
CA 02504153 2005-04-28
effect of inhibitors on the enzyme activity, were also carried out as
described in that publication.
When determining human neutrophil collagenase and human Stromelysin,
5 the compounds described in the above examples in each case had IC50
values of more than 100 000 nM. These compounds are therefore virtually
without activity as regards inhibiting MMP 3 and 8.