Note: Descriptions are shown in the official language in which they were submitted.
CA 02504159 2005-04-14
TITLE: METHOD OF REMOVING MERCURY FROM MERCURY
CONTAMINATED MATERIALS
FIELD OF THE INVENTION
[0001 ] The invention relates to a method of reducing the levels of mercury in
mercury
contaminated materials. Particularly, the invention relates to the use of
microwave energy to
reduce the levels of mercury in fly ash and sorbent materials.
BACKGROUND OF THE INVENTION
[0002] Mercury is a known contaminant present in the combustion gas stream
from
industrial incinerators and boilers such as those used in the burning of coal
for
stream/electricity generation or those used in the municipal solid waste
treatment for steam
generation or waste removal. The high environmental toxicity of mercury is
well established,
therefore, mercury scrubbing has become a necessary (albeit expensive)
component of flue
gas treatment.
[0003] Mercury scrubbing from flue gas streams may be accomplished by several
methods which vary in complexity, cost and effectiveness. These methods
include sorbent
(carbon or alkaline) filtering, oxidation, chloridation and others.
[0004] Carbon sorbent filtering is well known in the art and utilizes the
known sorbent
characteristics of fme carbon, particularly activated carbon. Several carbon
sorbent
techniques have been disclosed and practiced. For example, US Patent No.
6,558,454 to
Chang et al. teaches the injection of raw carbonaceous material into a mercury
contaminated
gas stream at a temperature sufficient to activate the carbonaceous material
into an effective
adsorbant. US Patent No. 6,521,021 to Pennline et al. discloses a method of
recirculating
semi-combusted coal which has been converted to a stream of thermally
activated carbon
sorbent and which is then reintroduced into the primary combustion chamber. US
Patent
Nos. 6,103,205 and 6,322,613 both to Wojtowicz et al. disclose a method of
producing a
carbon sorbent through the pyrolysis of a carbonaceous feedstock such as scrap
tires,
including a means of regenerating the sorbent through hot-gas vaporization and
the
1
CA 02504159 2005-04-14
production of a highly concentrated mercury rich gas stream which must be
subsequently
treated.
[0005] US Patent No. 5,787,823 to Knowles teaches the use of fly ash, which is
an
automatic byproduct of coal combustion, as a sorbent material owing to its
natural filtration
properties, namely, small particle size and large surface to mass ratio.
Knowles does not
discuss the possible effects of carbon (unburned fuel) in the fly ash nor does
he discuss the
separate roles played by the sorbing fly ash particles and the sorbing carbon
particles. US
Patent No. 5,672,323 to Bhat et al. teaches the injection of activated carbon
as a flue gas
treatment for mercury removal.
[0006] US Patent No. 6,372,187 to Madden et al. discloses the use of alkaline
sorbents,
such as limestone, followed by particulate filtration, as a means of removing
mercury from
flue gas streams.
[0007] All sorbent techniques result in a mercury rich particulate which is
captured in
some form of baghouse or other similar means for separating particulate from
the flue gas
stream prior to release to atmosphere. Inevitably, this mercury rich
particulate complex will
include mercury, sorbent material and some residual fly ash which may have
escaped earlier
stages of fly ash removal. Ultimately, this particulate complex must be
disposed either in
whole as for example by cementation, burial, etc. or by further processing the
material either
to reduce its volume or to regenerate the sorbent. In the case of sorbent
regeneration, the
high cost of sorbent replacement may be avoided or partially offset, and in
the case of volume
reduction, the mercury is further concentrated to yield a mercury-sorbent
volume which is
substantially reduced, thus allowing for a more efficient containment or
disposal. From an
environmental viewpoint, ideally all the mercury originally present in the
coal fuel should
end up being collected in molecular or elemental form which should be easily
manageable.
[0008] Considering the mercury-sorbent mixture to be a separate material
requiring
treatment leads one to consider means for removing mercury from the mixture.
One such
means is through pyrolysis of the mercury by heating the material to the
mercury
vaporization point, followed by a more efficient mercury removal technique
than that which
produced the mercury-sorbent material in the first instance. This is similar,
in many aspects,
2
CA 02504159 2005-04-14
to the problem of removing mercury from mercury contaminated soils and
industrial
materials.
[0009] For example, US Patent No. 6,268,590 to Gale et al. discloses a method
for
retorting mercury from dry, granular materials using an electrically heated
kiln through which
the material is screw transported. A condenser is used to remove the mercury
vapor from the
exhaust gas stream. Gale claims an advantage over earlier methods disclosed in
US Patent
No. 5,569,154 to Navetta and in US Patent No. 1,599,372 to Reed, in that his
process is
continuous and of practical size and complexity.
(0010) US Patent No. 6,024,931 to Hanulik discloses a rotary tubular kiln in
which the
material passes countercurrent to a combustion flame. US Patent Nos. 5,891,216
and
5,989,486 both to Washburn et al. teach a batch retorting method including the
use of a
stirring mechanism to assist in liberating the evaporated mercury vapor. US
Patent No.
5,782,188 to Evans et al. discloses a rotary kiln which is operated as a
pyrolytic incinerator in
the absence of air, from which the combustible gas stream is condensed into
the various
product streams. US Patent No. 5,632,863 to Meador discloses a pyrolysis
method by which
used batteries may then be processed. US Patent No. 5,567,223 to Lindgren et
al. describes a
process whereby mercury contaminated material is heated within a furnace in
the presence of
selenium to form mercury selenide in a hot gas stream, thus leaving the
decontaminated
material for further use.
[0011 ] While each of these methods satisfies the functional need of providing
a process
for reducing the content of mercury in mercury contaminated materials, there
remains a need
for more efficient and economic methods. In addition, in certain cases such as
in the
treatment of fly ash for example, it is often desired to also reduce the level
of carbon in the
material. Thus, there is a need for a method that can allow for the
simultaneous reduction of
both mercury and carbon contents of a mercury contaminated material.
[0012] It is therefore an object of the invention to provide an improved
method of
reducing mercury from mercury contaminated materials. The method uses
microwave
energy.
3
CA 02504159 2005-04-14
[0013] It is also an object of the invention to provide a method allowing for
a
simultaneous reduction of mercury and carbon contents in mercury contaminated
materials
using microwave energy.
[0014] It is still an object of the invention to provide the use of a bubbling
fluidized bed
reactor vessel in the process according to the invention.
[0015] It is still an object of the invention to provide the use of a host bed
material in
the process according to the invention.
SUMMARY OF THE INVENTION
[0016] The present invention discloses a process and means whereby microwave
energy is used to pyrolyse mercury from a mercury contaminated solid mixture
consisting of
fly ash and sorbent material. By means of this process, the mercury is
produced as a hot
vapour in a gas stream which is subsequently condensed, leaving the solid
residue, namely
the fly ash or the sorbent material available for reuse or clean disposal.
[0017] The process of the invention includes the use of microwave energy to
provide
the heat necessary for mercury vaporization without the need for any flame or
combustion
gases. The means for such a process, for example a metallic fluidized bed
vessel into which
the mercury contaminated material is continuously fed and removed and into
which
microwave energy is introduced, is a compact and efficient equipment which has
certain
advantages over other retorts and pyrolysers.
[0018] The basic mechanism for microwave heating in this instance is a
combination of
dielectric and ohmic heating whereby both electrical displacement and
conduction currents
are utilized to convert the electromagnetic energy directly into heat within
the material. The
efficiency of this energy conversion is dependent upon the dielectric
properties of the
material to be treated. In this instance, both the fly ash and sorbent
materials contain
significant receptor elements, principally carbon, which may be rapidly heated
in a controlled
manner. The mercury evaporates when the temperature is raised to about
357°C (boiling
point of mercury) at normal atmospheric pressure.
4
CA 02504159 2005-04-14
[0019] The use of a bubbling fluidized bed reactor vessel provides several
practical
advantages, including: self containment of the microwave energy, natural
material agitation
to assist flushing of mercury vapour, continuous material flow into and out of
the vessel, and
natural segregation of the solid and gas streams.
[0020] In accordance with a first aspect, the invention provides a method of
reducing
mercury level in a mercury contaminated material comprising placing the
mercury
contaminated material in a microwave reactor, providing a stream of gas in the
microwave
reactor, the stream causing agitation of the mercury contaminated material,
and exposing the
mercury contaminated material to microwave radiation so as to raise the
temperature to at
least 357°C, producing a vapour phase which contains mercury and a
treated material.
[0021 j In accordance with a second aspect, the invention provides a method of
reducing mercury level in a mercury contaminated material comprising placing a
carbon-free
material in a microwave reactor, placing the mercury contaminated material in
the microwave
reactor, providing a stream of gas in the microwave reactor, the stream
causing agitation of
the mercury contaminated material and the carbon-free material so as to form a
mixture, and
exposing the mercury contaminated material to microwave radiation so as to
raise the
temperature to at least 357°C, producing a vapour phase which contains
mercury and a
treated material.
[0022] In accordance with a third aspect, the invention provides a method of
reducing
mercury and carbon levels in a mercury contaminated material comprising
placing the
mercury contaminated material in a microwave reactor, providing a stream of
gas in the
microwave reactor, the stream causing agitation of the mercury contaminated
material, and
exposing the mercury contaminated material to microwave radiation so as to
raise the
temperature to at least 600°C, producing a vapour phase which contains
mercury and a
treated material.
[0023] In accordance with a fourth aspect, the invention provides a method of
reducing
mercury and carbon levels in a mercury contaminated material comprising
placing a carbon-
free material in a microwave reactor, placing the mercury contaminated
material in the
microwave reactor, providing a stream of gas in the microwave reactor, the
stream causing
S
CA 02504159 2005-04-14
agitation of the mercury contaminated material and the carbon-free material so
as to form a
mixture, and exposing the mercury contaminated material to microwave radiation
so as to
raise the temperature to at least 600°C, producing a vapour phase which
contains mercury
and a treated material.
[0024] In a preferred embodiment of the first and third aspects, the method
can further
comprise the steps of removing the vapour phase from the reactor, terminating
exposure of
microwave radiation, removing the treated material from the reactor, and
introducing fresh
mercury contaminated material in the reactor. Also, in a preferred embodiment
of the second
and fourth aspects of the invention, the method can further comprise the steps
of removing
the vapour phase from the reactor, terminating exposure of microwave
radiation, removing
the treated material from the reactor, introducing fresh carbon-free material
in the reactor, and
introducing fresh mercury contaminated material in the reactor.
[0025] More preferably, the above steps may be continuous and optionally, the
method
may further comprise the step of introducing the vapour phase in a filtration
device such as a
cyclonic separator. The method according to the invention may also comprise
the further
step of trapping the vapour phase which contains mercury into a container.
[0026] The microwave reactor used in the method of the invention is preferably
a
fluidized bed reactor vessel, and the microwave radiation may have a frequency
of between
about 300 MHz and about 30 GHz. Preferably, the frequency can be within the
Industrial,
Scientific and Medical (ISM) bands of approximately 915 MHz and 2450 MHz. The
microwave radiation power level and process duration time sufficient to
produce a specific
energy can be between about 2 kW-h/t and about 20 kW-h/t.
[0027] The ratio of mercury contaminated material to carbon-free material in
the above
second and fourth aspects of the invention can be between about 25/75 and
about 75/25.
Preferably, this ratio is about 50/50. The mercury contaminated material may
have a mercury
content of up to SO% by weight, and the material treated according to the
method of the
invention can have a mercury content of less than about 10 ppb. Preferably,
the mercury
content of the treated material is less than 5 ppb. The carbon content of the
mercury
contaminated material can be up to 60% by weight.
6
CA 02504159 2005-04-14
[0028] In accordance with a sixth aspect, there is provided an apparatus which
is
specially adopted to carry out the method according to the invention.
[0029] The method of reducing the mercury content of mercury contaminated
materials
according to the invention uses microwave energy, it is efficient, economic
and versatile.
BRIEF DESCRIPTION OF THE DRAWINGS
(0030] These and other advantages of the invention will become apparent upon
reading
the following detailed description and upon refernng to the drawings in which:
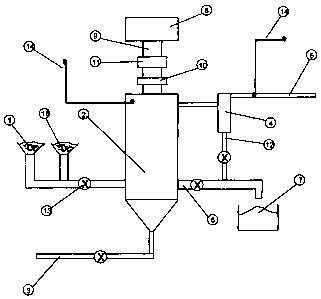
(0031 ] FIGURE 1 is schematic representation of an apparatus for carrying out
an
embodiment of the method according to the invention.
[0032] While the invention has been described in conjunction with the
illustrated
embodiment, it will be understood that it is not intended to limit the
invention to such
embodiment. On the contrary, it is intended to cover all alternatives,
modifications and
equivalents as may be included within the spirit and scope of the invention as
defined by the
appended claims.
DETAILED DESCRIPTION OF THE INVENTION
[0033] Referring to Fig. 1 which illustrates a preferred embodiment of the
present
invention, an input feedstream ( 1 ) of mercury contaminated material is
introduced in a
continuous fashion into a microwave reactor vessel (2) which is operated as a
bubbling
fluidized bed. The operation of the bubbling fluidized bed is well known in
the art. Integral
to this preferred embodiment is the use of a dual composition fluidized bed
consisting of a
host bed material in addition to the mercury contaminated material. The host
bed material is
selected as an essentially carbon-free mineral which is inert to the process
at hand and which
can withstand the operating conditions of the process without chemical or
mechanical effect.
The host bed material is further described as having a size distribution and
density which are
sufficiently greater than that of the sorbent material such that the sorbent
material is more
highly fluidized within the reactor vessel. Notwithstanding this, the dynamic
action of the
7
CA 02504159 2005-04-14
host bed material is such that the host bed material and the sorbent material
feedstream form
a single, integral fluidized bed medium within the reactor vessel. In addition
to its size and
density properties described above, the host bed material is selected to be a
microwave
receptive material such that it can be directly heated by microwave energy
irrespective of the
properties of the other material within the bed. It has been found that the
use of this dual
composition fluidized bed, in the proportion of at least 50% by weight host
material, allows
the process to be operated at a significantly higher temperature without
causing fusing or
clinkering of the carbon rich sorbent material, hence leading to a higher unit
throughput.
[0034] The feed material (1) within the reactor vessel (2) forms a fluidized
bed by
means of a gas stream (3) which is fed into the base of the reactor through a
system of
nozzles or closely spaced apertures in a solid plate, thereby effectively
suspending the
material in the reactor vessel. This aspect of fluidized bed operation is
determined by the gas
velocity needed to effectively suspend the material and is well known to those
practicing in
the art. The fluidizing gas passes through the reactor vessel and exits
through a filtration
device (4), such as a cyclonic separator, which removes all or most of the
entrained fme
particulate from the gas stream. The gas stream (5), now essentially
particulate free, is
available for further treatment such as mercury removal.
[0035] Material being fed into the reactor vessel is continuously removed, for
example
by means of an overflow discharge pipe (6), and is collected in a hopper (7)
or other suitable
container for further treatment or use.
[0036] Attached to the reactor vessel is a means by which a microwave
generator (8) is
connected, usually a waveguide (9), in which a microwave transparent barrier (
10) is installed
to effectively isolate the reactor vessel atmosphere from the waveguide.
[0037] Microwave energy is supplied to the reactor vessel, which is
constructed of a
suitable metallic material so as to effectively contain the microwave fields
introduced therein.
When in contact with the fluidized material within the reactor vessel, a
substantial portion of
the microwave energy is converted into heat, thereby raising the fluidized bed
temperature.
The efficiency of coupling of the microwave energy into the fluidized bed
material is
controlled by means of a tuning device (11) installed in the waveguide. Such
tuning devices
8
CA 02504159 2005-04-14
may be electronically controlled to continuously optimize the power transfer.
When the
fluidized bed temperature reaches the boiling point of mercury, or
approximately 357°C at
normal atmospheric pressure, the mercury passes into the vapor phase and is
carned out of
the vessel in the fluidizing gas stream. The fluidizing gas may be ambient air
if one wishes to
combust the bed material or the gas may be selected to be inert (for example
nitrogen) with
respect to mercury and carbon, hence the heating process within the reactor
vessel cannot
combust the sorbent material.
[0038] The hot gas stream which exits the reactor vessel passes through a
cyclonic
separator as described above. Since the gas temperature is maintained above
the boiling
point of mercury, the mercury vapor is carried on to the gas discharge (5)
where it is
condensed or otherwise filtered for recovery.
[0039] Disentrained particulate (12) from the cyclonic separator is combined
with the
other discharge solids (7).
[0040] As is known in the fluidized bed art, various valves (13) are employed
in the
material streams into and out of the reactor vessel (and cyclonic separator)
in order to prevent
gas leakage.
[0041 ] In order to monitor and control the heating process, various
instrumentation ( 14)
are installed in the apparatus. Temperature probes are installed at various
positions within
the fluidized bed and all feed and discharge lines, including the gas inlet
and outlet lines. Gas
pressure and product monitors are installed in all gas lines. Material flow
through the reactor
vessel is measured either through flow meters or by mass measurements. The
system so
instrumented may be operated manually or automatically to maintain the system
operation
within a set minimum-maximum boundary.
[0042] Incorporation of the host bed material is by means of a separate
feedstream (15)
which is merged with the contaminated material feedstream and controlled to
offset the host
bed material loss through the reactor. If desired, the host bed material may
be separated from
the reconstituted sorbent material (for example by flotation or gravity
separation) and
recirculated to the input hopper for reuse.
9
CA 02504159 2005-04-14
[0043] In accordance with a preferred embodiment of the invention, when an
inert gas
is used for fluidization, mercury contaminated material may be effectively
purged of mercury
without combustion, thereby allowing the sorbent material to be reused. The
mercury thus
released may, as described above, be effectively captured. This method of
retorting mercury
has distinct advantages over other means of heating, owing mainly to the
efficiency and
speed of heat generation using microwave energy.
Example 1
[0044] The apparatus as schematically represented in Figure 1 was set up to
process a
quantity of coal combustion fly ash which was known to contain mercury. A
microwave
frequency of 915 MHz was used. The fluidizing gas was ambient air.
[0045] The feedstock fly ash was processed at a temperature of approximately
820°C.
Throughout the test period of approximately 400 minutes duration, the material
was passed
through the reactor vessel at a rate of approximately 6 Ibs per minute.
[0046] The mercury content of the feedstock was measured to be 79 parts per
billion
(ppb). The unburned carbon content, characterized as the LOI (Loss On
Ignition), was
measured to be 8.5%.
[0047] Samples of processed ash were taken periodically through the experiment
and
the mercury content was measured. The results obtained are shown in Table 1
below. The
LOI of the treated material was 1.5%.
10
CA 02504159 2005-04-14
Table 1 Mercury Measurements
s' ~ ~ ~a - ~,.. ~ :a E ""~ ..
~ 3.. a 3a;.,3~a
v~9y x ~~~8 f g ~,$
''~,~1~ 7~ 3~,a~r ~ ~. .~?-;3:.In
a ~ y~.. 3, ~_ ,a ~
e~,~rs9~~'i~.. ~'
~ ~ ~! ;: ~
a,,. , '~.
~ . :~
,
P
0 79 79
167 22 23
197 11 1
392 8 2
419 7 3
(0048] It can be seen that, once the process has reached steady state
operation with
respect to mercury evolution, the mercury content of the discharge material
(product and
cyclone discharge) has been substantially reduced compared to its initial
value. One may
reasonably expect that the mercury level may be further reduced by slowing the
feed rate,
thus increasing the average residence time of the material within the reactor.
Nevertheless,
the effectiveness of the process in reducing mercury concentration is evident.
Example 2
[0049] The apparatus as schematically represented in Figure 1 was set up to
process a
quantity of coal combustion fly ash which was known to contain mercury. A
microwave
frequency of 915 MHz was used. The fluidizing gas was ambient air.
[0050] The feedstock fly ash was processed at a temperature of approximately
820°C.
Throughout the test period of approximately 500 minutes duration, the material
was passed
through the reactor vessel at a rate of approximately 6 lbs per minute.
[0051 ] The mercury content of the feedstock was measured to be 33 parts per
billion
(ppb). The unburned carbon content, characterized as the LOI (Loss On
Ignition), was
measured to be 17.5%.
11
CA 02504159 2005-04-14
[0052] Samples of processed ash were taken periodically through the experiment
and
the mercury content was measured. The results obtained are shown in Table 2
below. The
LOI of the treated material was 0.4%.
Table 2 Mercury Measurements
3 Z3 '~% 9 .'a;
.
~
~ g ~'F '' ' ~zK ~3 ~ P
~~~I 39 ~g ~ ~~~~~ ,
~
~ 1l t" ~ ~ .:~,R~,v
a >~ R " 18 ~c~m, mss
,.n 8 sh .a..~a -~."5>n~
,F . ~. iz~~t
1"s
0 33 33
185 25 15
481 3 7
Example 3
[0053] The apparatus as schematically represented in Figure 1 was set up to
process a
quantity of coal combustion fly ash which was known to contain mercury. A
microwave
frequency of 915 MHz was used. The fluidizing gas was ambient air.
[0054] The feedstock fly ash was processed at a temperature of approximately
820°C.
Throughout the test period of approximately 400 minutes duration, the material
was passed
through the reactor vessel at a rate of approximately 6 lbs per minute.
[0055] The mercury content of the feedstock was measured to be 142 parts per
billion
(ppb). The unburned carbon content, characterized as the LOI (Loss On
Ignition), was
measured to be 4.5%.
[0056] Samples of processed ash were taken periodically through the experiment
and
the mercury content was measured. The results obtained are shown in Table 3
below. The
final LOI was 0.3%.
12
CA 02504159 2005-04-14
Table 3 Mercury Measurements
~~~ ~ ' ' ~
-s3 ~ ..~~'.:r, ~
a ~ .W~:.~"~ ~~ ~' ~mvn~ ~'~.~,~~~~
' ' ' " ~ ~ 9
s a ~,.,,
;e
r
0 142 142
187 8 7
230 8 1
300 2 1
337 8 1
[0057] It is evident from the foregoing Examples that the process disclosed
herein is
effective in reducing mercury concentrations irrespective of the initial
mercury content of the
material or its LOI.
[0058] Although the examples cited herein were conducted at a microwave
frequency
of 915 MHz, being one of the readily available electromagnetic ISM
(Industrial, Scientific,
Medical) bands for unlicensed operation, it is within the scope of this
present invention that
any frequency generally within the microwave region (300 MHz - 30 GHz) may be
used, the
principal effect being in the dimensions of the resonant reactor vessel in a
manner which is
well understood by one practicing in the field of microwave.
[0059] In the examples cited above, the fluidizing gas was ambient air since
the
processing operation was directed primarily to the combustion of the unburned
carbon in the
ash as well as the volatilization of the mercury. In a manner directly
analogous to the above
examples, as has been practiced by the present inventor, one may substitute an
inert gas,
nitrogen for example, for ambient air, with the result that the material
effectively heats (due
to microwave absorption) although without any combustion.
[0060] It is also noted that, while the above examples use an operating
temperature of
approximately 820°C for the purpose of combusting unburned carbon from
the ash, it is only
necessary to achieve a temperature of 357°C in order to vaporize
mercury (at normal
13
CA 02504159 2005-04-14
atmospheric pressure), hence the process according to the invention may be
operated at any
temperature at or above 357°C (at normal atmospheric pressure) provided
that one does not
exceed the temperature at which the ash constituents will significantly fuse
and agglomerate;
such a condition will be known to one practicing in the art of metallurgical
processing of
minerals and ores.
[0061] While the cited examples utilize a reactor vessel which operates on the
known
principles of a bubbling fluidized bed, it is within the scope of this present
invention that one
may utilize other vessel designs which can be adapted for use as microwave
containment
vessels. This includes, but is not limited to, rotary kilns, vibrating drums,
multimode
cavities; transport fluidized beds, packed tubes and conveyorized cavities.
[0062] Thus it is apparent that there has been provided in accordance with the
invention a method of reducing the mercury content of mercury contaminated
materials using
microwave energy, that fully satisfy the needs and advantages set forth above.
While the
invention has been described in conjunction with the illustrated embodiment,
it is evident that
many alternatives, modifications and variations will be apparent to those
skilled in the art in
light of the foregoing description. Accordingly, it is intended to embrace all
such
alternatives, modifications and variations as fall within the spirit and broad
scope of the
invention.
14