Note: Descriptions are shown in the official language in which they were submitted.
CA 02504303 2009-02-10
1
DESCRIPTION
PROCESS FOR PRODUCING KRAFT PULP
TECHNICAL FIELD
The present invention relates to a process for
producing kraft pulp.
BACKGROUND ART
As well known in the art, the process for producing
kraft pulp basically comprises: (A) a cooking step of
treating raw chips with a cooking liquor containing sodium
hydroxide and sodium sulfide as main components to convert
the chips into pulp; (B) a pulp washing step of washing
the resultant pulp, and separating and recovering a black
solution containing sodium carbonate and sodium sulfate,
which are obtained from the cooking liquor; (C) a pulp
bleaching step of treating the pulp with a bleaching agent
in the presence of alkali; (D) a black solution
concentrating step of concentrating the black solution
separated and recovered in the pulp washing step (B); (E)
a black solution combustion step of burning the
concentrated black solution to reduce sodium sulfate into
sodium sulfide and further recovering sodium sulfate and
sodium carbonate from a combustion exhaust gas by a dust
CA 02504303 2009-02-10
2
collector; and (F) a causticization step of treating a
green solution as an aqueous solution of a smelted product
recovered in the combustion step with calcium oxide to
convert sodium carbonate contained in the green solution
into sodium hydroxide, thereby obtaining a white solution,
wherein the white solution recovered in the
causticization step (F) is recycled to the cooking step
(A).
In the above-described process for producing kraft
pulp, chemicals used therein have been recovered in a
closed system to enhance a recovery percentage thereof
more and more. More specifically, sodium carbonate or
sodium sulfate has also been recovered from ashes captured
and recovered from a combustion exhaust gas generated in
the combustion step (E) by the dust collector.
Meanwhile, in the case where valuable substances are
recovered from the captured ashes as described above, it
is required to remove impurities turned from raw wood
materials, for example, potassium components in order to
prevent accumulation of these impurities in the recovered
valuable substances.
As a method of removing common salt (sodium
chloride) and potassium salts from ashes captured in a
sodium-recovering boiler, there has been proposed the
method of adjusting a pH value of a water slurry
CA 02504303 2005-04-28
3
containing the captured ashes to not more than 10 by
adding sulfuric acid thereto and further adjusting a
temperature of the water slurry to not less than 20 C;
keeping the water slurry under the above condition for a
predetermined period of time to dissolve common salt and
potassium salts contained in the captured ashes in water;
cooling the resultant water slurry to a temperature less
than 20 C to precipitate solids; re-dissolving the solids
in the black solution before being concentrated; and
returning the resultant black solution to an upstream side
of a concentrator for the black solution, thereby
recovering the solids (Japanese Patent Application Laid-
Open (KOKAI) No. 9-29201(1997)).
However, the above conventional method has the
following problems: (1) a loss of valuable substances is
large since a large amount of these substances are
contained in an effluent discharged out of the system; (2)
a large amount of dilute sulfuric acid should be used for
enhancing a sodium recovery percentage; (3) increased
costs are required for operating an ice maker for
producing ice used in a precipitation tank; (4) running
costs are disadvantageously large owing to these problems;
and (5) equipments used tend to suffer from abrasion and
deterioration due to the slurry.
Under these circumstances, in order to overcome the
CA 02504303 2005-04-28
4
above problems, the present inventors have proposed as the
method of treating ashes captured in a cooking chemical
recovery step, such a method of treating an aqueous
solution of the captured ashes with an ion exchange resin
to recover and reuse a fraction containing a large amount
of sulfate ions and carbonate ions (Japanese Patent
Application Laid-Open (KOKAI) Nos. 2002-138381, 2002-
138382 and 2002-146691).
In the above Japanese Patent Applications, there are
described a step of adsorbing and removing potassium ions
using Na-type strong acid ion exchange resins, a step of
adsorbing and removing polyvalent metal ions using chelate
resins, and a step of separating chlorine ions using
ampholytic ion exchange resins. Further, in these
Japanese Patent Applications, as a method of regenerating
the Na-type strong acid ion exchange resins and chelate
resins which need for regeneration of chemicals, there are
described the method of flowing an acid (for example, an
aqueous hydrochloric acid solution) through the resins and
then flowing an aqueous sodium hydroxide solution or an
aqueous sodium chloride solution through the resultant
resins, and the method of flowing an acid (for example, an
aqueous hydrochloric acid solution) through the resins and
then flowing an aqueous sodium hydroxide solution through
the resultant resins.
CA 02504303 2005-04-28
An object of the present invention is to provide an
industrially useful process for producing kraft pulp,
which is capable of recovering chemicals in a closed
system, in particular, preventing, accumulation of
potassium impurities, and effectively utilizing the
chemicals used in the process.
BRIEF DESCRIPTION OF THE DRAWING
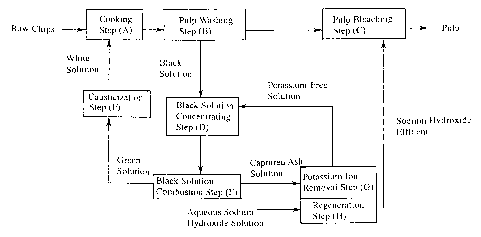
Fig. 1 is a flow diagram showing an example of the
process for producing kraft pulp according to the present
invention. Fig. 2 shows an effluent curve obtained in the
potassium ion removal step in Example 1.
DISCLOSURE OF THE INVENTION
As a result of the present inventors' earnest study,
the following knowledge has been obtained. That is, in
the bleaching step of treating the pulp with a bleaching
agent in the presence of alkali, as the alkali, there is
usually used a high-purity sodium hydroxide. Meanwhile,
in the case where the Na-type cation exchange resins are
regenerated by the method of flowing an aqueous sodium
hydroxide solution therethrough, a sodium hydroxide
effluent containing a large amount of potassium ions is
by-produced. Such a sodium hydroxide effluent can be used
in the bleaching step with substantially same effect as
CA 02504303 2009-02-10
6
obtained by the high-purity sodium hydroxide.
The present invention has been attained on the basis
of this finding. Namely, in the present invention, as a
method of regenerating the Na-type cation exchange resin,
there is selected the regeneration method using an aqueous
sodium hydroxide solution, and further the sodium
hydroxide effluent obtained from the regeneration step is
suitably utilized.
To accomplish the aim, in a first aspect of the
present invention, there is provided a process for
producing kraft pulp, which comprises (A) a cooking step
of treating raw chips with a cooking liquor containing
sodium hydroxide and sodium sulfide as main components to
convert the chips into pulp; (B) a pulp washing step of
washing the resultant pulp, and separating and recovering
a black solution containing sodium carbonate and sodium
sulfate, which are obtained from the cooking liquor,
therefrom; (C) a pulp bleaching step of treating the pulp
with a bleaching agent in the presence of alkali; (D) a
black solution concentrating step of concentrating the
black solution separated and recovered in the pulp washing
step (B); (E) a black solution combustion step of burning
the concentrated black solution to reduce the sodium
sulfate into sodium sulfide and further capturing and
recovering ashes containing sodium sulfate and sodium
CA 02504303 2009-02-10
7
carbonate from a combustion exhaust gas generated therein
by a dust collector; and (F) a causticization step of
treating a green solution as an aqueous solution of a
smelted product recovered from the combustion step with
calcium oxide to reduce sodium carbonate contained in the
green solution to sodium hydroxide, thereby obtaining a
white solution, the white solution recovered in the
causticization step (F) being recycled to the cooking
step (A);
the process further comprising:
(G) a potassium ion removal step of flowing an
aqueous solution containing the ashes captured and
recovered from the combustion exhaust gas generated in the
black solution combustion step (E) by the dust collector
through a packed bed filled with a Na-type cation exchange
resin to adsorb and remove potassium ions contained in the
aqueous solution; and
(H) a regeneration step of treating the cation
exchange resin used in the potassium ion removal step (G)
with an aqueous sodium hydroxide solution to regenerate
the cation exchange resin,
wherein a fraction recovered from the potassium ion
removal step (G), which contains a large amount of sodium
sulfate and sodium carbonate, is recycled to the black
solution concentrating step (D), and a sodium hydroxide
CA 02504303 2009-02-10
8
effluent recovered from the regeneration step (H) is
recycled to the bleaching step (C).
In another aspect, the invention provides a process for
producing kraft pulp, which comprises:
(A) a cooking step of treating raw chips with a cooking liquor
containing sodium hydroxide and sodium sulfide as main
components to convert the chips into pulp; (B) a pulp washing
step of washing the resultant pulp, and separating and
recovering a black solution containing sodium carbonate and
sodium sulfate, which are obtained from the cooking liquor,;
(C) a pulp bleaching step of treating the pulp with a bleaching
agent in the presence of alkali; (D) a black solution
concentrating step of concentrating the black solution
separated and recovered in the pulp washing step (B); (E) a
black solution combustion step of burning the concentrated
black solution to reduce the sodium sulfate into sodium sulfide
and further recovering ashes containing sodium sulfate and
sodium carbonate from a combustion exhaust gas generated
therein by a dust collector; and (F) a causticization step of
treating a green solution as an aqueous solution of a smelted
product recovered from the combustion step with calcium oxide
to reduce sodium carbonate contained in the green solution to
sodium hydroxide, thereby obtaining a white solution, said
white solution recovered in the causticization step (F) being
recycled to the cooking step (A);
CA 02504303 2009-02-10
8a
said process further comprising:
(G) a potassium ion removal step of flowing an aqueous
solution containing the ashes captured and recovered from the
combustion exhaust gas generated in the black solution
combustion step (E) by the dust collector through a packed bed
filled with a Na-type cation exchange resin to adsorb and
remove potassium ions contained in the aqueous solution; and
(H) a regeneration step of treating the cation exchange
resin used in the potassium ion removal step (G) with an
aqueous sodium hydroxide solution to regenerate the cation
exchange resin,
wherein a fraction recovered from the potassium ion
removal step (G), which contains a large amount of sodium
sulfate and sodium carbonate, is recycled to the black
solution concentrating step (D), and a sodium hydroxide
effluent recovered from the regeneration step (H) is recycled
to the bleaching step (C).
Next, the present invention is described in detail
with reference to the accompanying drawings.
The process for producing kraft pulp according to
the present invention basically comprises (A) a cooking
step, (B) a pulp washing step, (C) a pulp bleaching step,
(D) a black solution concentrating step, (E) a black
solution combustion step and (F) a causticization step.
These steps may be conducted under known conditions.
CA 02504303 2009-02-10
8b
In the cooking step (A), raw chips are treated with
a cooking liquor containing sodium hydroxide and sodium
sulfide as main components in a digester to convert the
chips into pulp. The treatment of the raw chips may be
conducted under high-temperature and high-pressure
conditions, at a temperature of usually 150 to 160 C under
a pressure of usually 8.5 to 9.5 Kg/cmzG.
In the pulp washing step (B), the pulp obtained in
the step (A) is washed with a washing solution, and the
resultant black solution (i.e., the resultant washing
solution containing the pulp cooking liquor) containing
sodium carbonate and sodium sulfate, which are obtained from
the cooking liquor, is separated and recovered from the
pulp. The black solution contains organic substances
contained in the chips in addition to the chemical
CA 02504303 2005-04-28
9
components. The solid content in the black solution is in
the range of usually 15 to 25% by weight.
In the pulp bleaching step (C), the pulp is treated
with a bleaching agent in the presence of alkali. In this
step, the pulp is treated with not only a single chemical
but also the following various chemicals. For example,
there may be used a method (1) in which bleaching with
chlorine or chlorine dioxide, alkali extraction, bleaching
with hypochlorite and bleaching with chlorine dioxide are
successively conducted; a method (2) in which bleaching
with chlorine or chlorine dioxide, alkali extraction,
bleaching with hypochlorite, alkali extraction and
bleaching with chlorine dioxide are successively conducted,
as well as methods in which a part of the above-mentioned
these steps are omitted. The alkali extraction stage
finishes a lignin removal step subsequent to steps
composed of the cooking step, the bleaching step with
oxygen and a chlorination step. In the alkali extraction
stage, sodium hydroxide may be mainly used as the alkali.
Further, in order to enhance the lignin removal efficiency,
the alkali extraction stage may be conducted using
hydrogen peroxide (Ep) or hypochlorite (EH) in combination
with the alkali, and further using oxygen in combination
therewith (Eop, EoH). Subsequent to the alkali extraction
stage, for the purpose of removing residual high-condensed
CA 02504303 2005-04-28
lignin, bleaching with hypochlorite and chlorine dioxide
may be conducted, thereby obtaining bleached pulp stably
exhibiting a high brightness. Recently, from
environmental viewpoints, there has been adopted a
chlorine-free bleaching (ECF) method using no chlorine nor
hypochlorite.
In the black solution concentrating step (D), the
black solution having a solid concentration of 15 to 25%
by weight, which is discharged from the pulp washing step
(B) is concentrated by an evaporator until the solution
reaches a combustible concentration, usually until
reaching a solid concentration of about 70 to 80% by
weight.
In the black solution combustion step (E), the
concentrated black solution is burned to reduce sodium
sulfate into sodium sulfide. More specifically, the black
solution is injected into a boiler to form a deposit
called char having a temperature of about 1000 C at a
bottom thereof, and the char is burned therein to recover
a smelted product (chemical components) melted at about
800 C from the bottom of the boiler. In addition, in the
black solution combustion step (E), ashes containing
sodium sulfate and sodium carbonate are captured and
recovered from the resultant combustion exhaust gas by a
dust collector disposed in a flue of the boiler.
CA 02504303 2009-02-10
11
in the causticization step (F), a green solution as
an aqueous solution of the smelted product recovered from
the combustion step is treated with calcium oxide to
convert sodium carbonate contained in the green solution
into sodium hydroxide, thereby obtaining a white solution.
Meanwhile, the calcium oxide is converted into lime sludge.
Also, although not shown in Fig. 1, the causticization
step (F) may further include a step of removing sludge
from a milk solution (emulsion) containing the lime sludge
to obtain a purified white solution, a step of burning the
thus separated and removed lime sludge in a kiln to covert
the sludge into calcium oxide again, or the like. The
white solution converted in the causticization step (F)
is reused as a cooking liquor in the cooking step (A).
In the process for producing kraft pulp according to
the present invention, there are further provided (G) a
potassium ion removal step of flowing an aqueous solution
containing the ashes captured and recovered from the
combustion exhaust gas generated in the black solution
combustion step (E) by the dust collector, through a
packed bed filled with a Na-type cation exchange resin to
adsorb and remove potassium ions contained in the aqueous
solution; and (H) a regeneration step of treating the
cation exchange resin used in the potassium ion removal
step (G) with an aqueous sodium hydroxide solution to
CA 02504303 2005-04-28
12
regenerate the cation exchange resin.
In the potassium ion removal step (G), in the case
where the ashes captured by a dry electric dust collector
is dissolved in water, a warm water may be used in the
consideration of a solubility of the captured ashes. On
the other hand, in the case where a wet electric dust
collector (mist Cottrell precipitator) is used for
capturing the ashes, there may be used an ash-recovering
solution obtained from a wet scrubber fitted thereto. The
amount of water used for dissolving the captured ashes is
usually 3 to 10 times (by weight) that of the captured
ashes.
As the Na-type cation exchange resin, there may be
suitably used, for example, "DIAION (registered trademark)
UBK550" produced by Mitsubishi Kagaku Co., Ltd. In order
to form the packed column filled with the cation exchange
resin, there may be used ordinary ion exchange columns.
In addition, the fluid may be flowed through the packed
column at a space velocity (SV) of usually 1 to 10 hr-1 at
a temperature of usually 20 to 80 C. When the fluid is
continuously flowed under the above conditions, leakage of
potassium ions from the column is soon initiated. At that
time, the flowing of the fluid through the column is
stopped.
In the regeneration step (H), the aqueous sodium
CA 02504303 2005-04-28
13
hydroxide solution is flowed through the cation exchange
resin to desorb the potassium ion adsorbed in the cation
exchange resin therefrom. More specifically, in the
previous potassium ion removal step (G), the supply of the
aqueous solution containing the captured ashes is stopped
upon initiation of the leakage of potassium ions from the
packed column. In order to replace the aqueous solution
containing the captured ashes which still remain in the
ion exchange column, with water, an inside of the ion
exchange column is washed with water. Successively, in
order to regenerate the cation exchange resin, the aqueous
sodium hydroxide solution is fed thereto. At this time,
the concentration of the aqueous sodium hydroxide solution
used is in the range of usually 2 to 20%. Upon the
regeneration, the space velocity of the aqueous sodium
hydroxide solution flowed through the cation exchange
resin is in the range of usually 1 to 10 hr-1, and the
temperature thereof is in the range of usually 20 to 80 C.
By continuously conducting the regeneration procedure, the
potassium ions adsorbed in the cation exchange resin is
desorbed therefrom, so that the cation exchange resin is
regenerated into the Na-type cation exchange resin. When
the above procedure is repeated, it is possible to
continuously remove potassium ions from the aqueous
solution containing the captured ashes.
CA 02504303 2005-04-28
14
Further, in the present invention, the fraction
containing a large amount of sodium sulfate and sodium
carbonate (potassium-free solution) is circulated to the
black solution concentrating step (D), whereby a recovery
percentage of the valuable components (sodium sulfate and
sodium carbonate) can be enhanced.
Further, in the present invention, the sodium
hydroxide effluent recovered in the above regeneration
step (H) is reused in the previous bleaching step (C).
More specifically, the sodium hydroxide effluent is
supplied to the alkali extraction stage of the bleaching
step (C). This enables effective use of the sodium
hydroxide effluent discharged from the regeneration step.
Besides, the use of the sodium hydroxide effluent in the
same amount as conventionally can provide the same
bleaching effect as conventionally attained. Therefore,
according to the present invention, the supply of an
additional amount of sodium hydroxide from an outside of
the system is not required.
Meanwhile, in the present invention, there may be
further provided, if required, one or both of a step for
adsorptive removal of polyvalent metal ions with chelate
resins and a step for separation of chlorine ions with
ampholytic ion exchange resins (both not shown). In this
case, the respective steps are preferably successively
CA 02504303 2005-04-28
conducted in the order of the step for adsorptive removal
of polyvalent metal ions, the step for separation of
chlorine ions, and the potassium ion removal step (G).
PREFERRED EMBODIMENT FOR CARRYING OUT THE INVENTION
Thus, the present invention is carried out as
described above. The essential parts of the process
according to the present invention, namely the potassium
ion removal step (G) and the regeneration step (H) as well
as the bleaching step (C), are described in more detail
below by referring to the Examples, but the Examples are
only illustrative and, therefore, not intended to limit
the scope of the present invention.
Example 1:
In the same process for producing kraft pulp as
shown in Fig. 1, ashes captured and recovered from a
combustion exhaust gas discharged from a recovery boiler
used in the combustion step (E) by a dust collector were
dissolved in pure water at 60 C to obtain a 30 W/V %
solution, and then the resultant solution was filtered
through a 0.45 pm-mesh membrane filter, thereby obtaining
a filtrate having a composition shown in Table 1.
CA 02504303 2005-04-28
16
Table 1
pH 10.5
Na+ 78 [g/L]
K+ 23.6 [g/L]
C1- 13.7 [g/L]
SO42- 149 [g/L]
C032- 27.3 [g/L]
2000 mL of the raw solution (filtrate) maintained at
60 C was flowed through a glass column having an inner
diameter of 30 mm which was packed with 500 mL of a cation
exchange resin "DIAION UBK550 (registered trademark)"
produced by Mitsubishi Kagaku Co., Ltd., at a space
velocity of 2 hr-1 to adsorb potassium ions contained in
the solution on the resin. Further, 1000 mL of pure water
was flowed through the glass column at a space velocity of
2 hr-1 to wash the resin.
Successively, 1500 mL of a 4 W/V % sodium hydroxide
aqueous solution was flowed through the glass column at a
space velocity of 2 hr-1 to desorb potassium ions adsorbed
on the cation exchange resin therefrom. Further, 1000 mL
of pure water was flowed through the glass column at a
space velocity of 2 hr-1 to wash the resin. At this time,
CA 02504303 2005-04-28
17
there was obtained an effluent curve (showing the change
in concentrations of potassium ions and sodium ions in an
effluent discharged from the column) as shown in Fig. 2.
The fraction A of the effluent as shown in Fig. 2 was
recovered and subjected to pulp bleaching treatment. The
composition of the obtained alkali effluent is shown in
Table 2 below.
Table 2
Na+ 30 [g/L]
K+ 5 [ g/L ]
Next, a pulp bleaching test was conducted using the
above effluent obtained after regenerating the cation
exchange resin (Experiments 1 to 3). In the bleaching
step, chlorine dioxide bleaching (D1), alkali extraction
(Eop: treatment with sodium hydroxide, oxygen and sodium
hypochlorite) and chlorine dioxide bleaching (D2) were
successively carried out in this order. The addition of
the alkali (regeneration effluent) was conducted while
controlling the pH value at an outlet of the alkali
extraction stage to 11. Bleaching efficiencies and pulp
qualities at addition rates of alkali (regeneration
effluent) of 1.29% (Experiment 1), 1.03% (Experiment 2)
and 1.11% (Experiment 3) are shown in Tables 3 and 4.
CA 02504303 2005-04-28
18
Comparative Example 1:
The same procedure for pulp bleaching test as
defined in Example 1 was conducted except that the
regeneration effluent of the cation exchange resin was
replaced with sodium hydroxide as currently used, namely
fresh sodium hydroxide before being used for regeneration
of the cation exchange resin, thereby performing the pulp
bleaching test (Experiments 4 to 6). The results are
shown in Tables 3 and 4.
The measuring methods and definitions of the
respective properties shown in Tables 3 and 4 are as
follows.
(1) Brightness:
The brightness is an index of whiteness of pulp or
papers, and was measured as brightness by Hunter using a
Digital Hunter brightness meter manufactured by Toyo Seiki
Co., Ltd. (according to JIS P8123).
(2) Kappa number:
The Kappa number is one of indices showing a cooking
degree or a delignification degree of pulp, and was
expressed by mL of a 1/1ON potassium permanganate which
was consumed per 1 g of absolute dry pulp (according to
TAPPI T236).
(3) Viscosity:
CA 02504303 2005-04-28
19
The viscosity is an index of an average
polymerization degree of cellulose from which pulping
degree and deterioration degree upon bleaching can be
relatively determined (according to TAPPI T230).
(4) A brightness:
The A brightness represents the difference in
brightness by Hunter between before and after
discoloration of pulp, and was measured using a Digital
Hunter brightness meter manufactured by Toyo Seiki Co.,
Ltd.
(5) Ab value:
The Ab value represents the difference in hue b
value between before and after discoloration of pulp, and
was measured using a color difference meter "Spectrometer
SE2000" manufactured by Nippon Denshoku Kogyo Co., Ltd.
(6) AE value:
The AE value was calculated from the differences in
hue L, a and b values measured before and after
discoloration of pulp according to the following formula:
AE value = V((AL)2 + (Aa)2 + (Ab)2)
(7) PC value:
The PC value is one of methods of expressing a
discoloration degree, and was calculated from brightness
values measured before and after heat-aging test at 105 C
according to the following formula:
CA 02504303 2005-04-28
PC value = 100 x {(K/S) - (KO/SO)}
wherein K and KO represent light absorption coefficients
after and before discoloration of pulp, respectively; and
S and SO represent light scattering coefficients after and
before discoloration of pulp, respectively.
Table 3
Eop stage
Rate of
addition of Brightness Kappa number
alkali (%)
Example 1
Experiment 1 1.29 71.9 2.9
Experiment 2 1.03 72.2 2.7
Experiment 3 1.11 71.8 2.8
Average 1.14 72.0 2.8
Comparative
Example 1
Experiment 4 1.30 71.5 2.9
Experiment 5 1.19 72.2 2.8
Experiment 6 1.37 72.0 2.8
Average 1.29 71.9 2.8
CA 02504303 2005-04-28
21
Table 4
D2 stage
Brightness Viscosity
Example 1
Experiment 1 85.9 21
Experiment 2 86.3 20
Experiment 3 86.0 21
Average 86.1 21
Comparative
Example 1
Experiment 4 85.8 20
Experiment 5 86.2 20
Experiment 6 86.1 19
Average 86.0 20
CA 02504303 2005-04-28
22
Table 4 (continued)
D2 stage
Accelerated heat discoloration degree
A brightness Ab value AE value PC value
Example 1
Experiment 1 -1.6 1.1 1.17 0.31
Experiment 2 -1.4 1.2 1.20 0.26
Experiment 3 -1.6 1.1 1.18 0.30
Average -1.5 1.1 1.18 0.29
Comparative
Example 1
Experiment 4 -1.6 1.0 1.18 0.31
Experiment 5 -1.5 1.1 1.17 0.28
Experiment 6 -1.6 1.1 1.19 0.30
Average -1.6 1.1 1.18 0.29
As shown in Tables 3 and 4, from the comparison
between the current alkali extraction treatment in which
the alkali extraction was conducted using fresh sodium
hydroxide (Comparative Example) and the alkali extraction
of the present invention in which the regeneration
effluent of the cation exchange resin was used as alkali
(Example 1), it was confirmed that both the treatments
CA 02504303 2005-04-28
23
exhibited substantially no difference in pulp brightness
upon the alkali extraction (Eop) stage and chlorine
dioxide bleaching (D2) stage as well as results of
accelerated heat discoloration test for hue (PC value, 0
brightness, Ab value and AE value) from each other, and
further both the treatments also exhibited substantially
no difference in pulp viscosity values upon the chlorine
dioxide (D2) stage from each other. As a result, it was
confirmed that the regeneration effluent of the cation
exchange resin was recycled to the alkali extraction stage
and reused therein without problems such as deterioration
in quality of the obtained pulp. In addition, it was
confirmed that the percentage of addition of alkali to
pulp required for controlling the pH value at the outlet
of the alkali extraction stage to 11, was 1.29% in the
case of the current alkali extraction treatment
(Comparative Example 1), whereas the same rate was reduced
to 1.14% in the case of the alkali treatment of the
present invention using the regeneration effluent (Example
1). That is, it was confirmed that the alkali extraction
method of the present invention was substantially
identical in quality of the obtained pulp and bleaching
property to those using the conventional methods.
CA 02504303 2005-04-28
24
INDUSTRIAL APPLICABILITY
As described above, according to the present
invention, there can be provided the process for producing
kraft pulp which is capable of recovering chemicals in a
closed system; preventing, in particular, accumulation of
potassium impurities; and effectively utilizing chemicals
used in the process. Therefore, the present invention
exhibits a remarkable industrial value.