Note: Descriptions are shown in the official language in which they were submitted.
CA 02505976 2005-05-12
WO 2004/052820 PCT/US2003/038305
-1-
PROCESS FOR THE PURIFICATION OF A CRUDE CARBOXYLIC ACID
SLURRY
FIELD OF~ INVENTION
The present invention relates to a process for the purification of a
crude carboxylic acid slurry. More specifically, the present invention relates
to a process for the purification of a crude carboxylic acid slurry by
utilizing
a solid-liquid displacement zone between a primary oxidation zone and a
staged oxidation zone.
BACKGROUND OF THE INVENTION:
Terephthalic acid is commercially produced by oxidation of
paraxylene in the presence of a catalyst, such as, for example, Co, Mn, Br
and a solvent. Terephthalic acid used in the production of polyester fibers,
films, and resins must be further treated to remove impurities present due to
the oxidation of para-xylene. Typical commercial process produce,a crude
terephthalic acid then dissolve the solid crude terephthalic acid in water at
high temperatures and pressures, hydrogenate the resultant solution, cool
and crystallize the terephthalic acid product out of solution, and separate
the solid terephthalic product from the liquid as discussed in U.S. Patent
No. 3,584,039 herein incorporated by reference.
CA 02505976 2005-05-12
WO 2004/052820 PCT/US2003/038305
-2-
A number of processes for producing the purified terephthalic acid
solid have been developed and are commercially available. Usually, the
purified terephthalic acid solid is produced in a multi-step process wherein a
crude terephthalic acid is produced. The crude terephthalic acid does not
have sufficient quality for direct use as starting material in commercial
polyethylene terephthalate(PET). Instead, the crude terephthalic acid is
usually refined to purified terephthalic acid solid.
Liquid phase oxidation of p-xylene produces crude terephthaiic acid.
The crude terephthalic acid is dissolved in water and hydrogenated for the
purpose of converting 4-carboxybenzaldehyde to p-toluic acid, which is a
more water-soluble derivative, and for the purpose of converting
characteristically yellow compounds to colorless derivatives. Significant 4-
carboxybenzaldehyde and p-toluic acid in the firial purified terephthalic acid
product is particularly detrimental to polymerization processes as they may
act as chain terminators during the condensation reaction between
terephthalic acid and ethylene glycol in the production of PET. Typical
purified terephthalic acid contains on a weight basis less than 250 parts per
million (ppm) 4-carboxybenzaldehyde and less than 150 ppm p-toluic acid.
The crude terephthalic acid typically contains on a weight basis from
about 800 to 7,000 parts per million (ppm) 4-carboxybenzaldehyde and
about 200 to 1,500 ppm p-toluic acid as the main impurities. The crude
terephthalic acid also contains lesser amounts, about 20-200 ppm range, of
aromatic compounds having the structures derived from benzil, fluorenone,
CA 02505976 2005-05-12
WO 2004/052820 PCT/US2003/038305
-3-
and/or anthraquinone, which are characteristically yellow compounds as
impurities resulting from coupling side reactions occurring during oxidation
of p-xylene
Such a purification process typically comprises adding water to the
crude terephthalic acid to form a crude terephthalic acid slurry, which is
heated to dissolve the crude terephthalic acid. The crude terephthalic acid
solution is then passed to a reactor zone in which the solution is contacted
with hydrogen in the presence of a heterogeneous catalyst at temperatures
of about 200° to about 375° G. This reduction step converts the
various
color causing compounds present in the crude terephthalic acid to colorless
derivatives. The principal impurity, 4-carboxybenzaldehyde, is converted to
p-toluic acid.
Typical crude terephthalic acid contains excessive amounts of both
4-carboxybenzaldehyde and p-toluic acid on aweight basis. Therefore, to
achieve less than 250 ppmv~i 4-carboxybenzafdehyde and less than 150
ppmw p-toluic acid in the purified terephthalic acid requires mechanisms for
purifying the crude terephthalic acid and removing the contaminants.
!n many processes, colored impurities are hydrogenated to colorless
derivatives and leave the process with the terephthalic acid solid product
and waste water streams. However, one embodiment of this invention
provides an attractive process to produce a purified carboxylic acid slurry by
utilizing a solid-liquid displacement zone comprising a solid-liquid separator
CA 02505976 2005-05-12
WO 2004/052820 PCT/US2003/038305
-4-
after oxidation of a crude carboxylic acid slurry product and prior to final
filtration and drying without the use of an hydrogenation step.
SUMMARY OF THE INVENTION
In one embodiment of the invention, a process to produce the
purified carboxylic acid product is provided without the use of hydrogenation
of the terephthalic acid or a process separating impurities from oxidation
19 solvent as disclosed in U.S. patent #3,584,039.
In another embodiment of this invention, a process to produce a
slurry product is provided. The process comprises removing impurities .from
a crude carboxylic acid slurry in a solid-liquid displacement zone to form a
slurry product.
In another embodiment of this invention, a process to produce a
purified carboxylic acid product is provided. The process comprises:
(a) removing impurities from a crude carboxylic acid slurry in a
solid-liquid displacement zone to form a slurry product;
(b) oxidizing the slurry product in a staged oxidation zone to form
a staged oxidation product;
(c) crystallizing the staged oxidation product in a crystallization
zone to forma crystallized product.
CA 02505976 2005-05-12
WO 2004/052820 PCT/US2003/038305
-5-
In another embodiment of this invention, a process to produce a
purified carboxylic acid slurry is provided. The process comprises:
(a) ~ removing in a solid-liquid displacement zone impurities from a
crude carboxylic acid slurry to form a slurry product; wherein the crude
carboxylic acid siurry comprises terephthalic acid, catalyst, acetic acid, and
impurities that is withdrawn at a temperature between about 140°C and
about 170°C from the oxidation of paraxylene in a primary oxidation
zone;
(b) oxidizing the slurry product in a staged oxidation zone to form
a staged oxidation product; wherein the oxidizing is conducted at a
temperature between about 190°C to about 2~0 °C; and wherein the
oxidizing is at a higher temperature in the staged oxidation zone than in the
primary oxidation zone;
(c) crystallizing the staged oxidation product in a crystallization
zone to form a crystallized product;
(d) cooling the crystallized product in a cooling zone to form a
cooled purified carboxylic acid slurry; and
(e) filtering and optiorially drying the cooled purified carboxylic
slurry in a filtration and drying zone to remove a portion of the solvent from
the cooled carboxylic acid slurry to produce the purified carboxylic acid
product.
In yet another embodiment of this invention, a process to produce a
purified carboxylic acid product is. provided. The process comprises:
CA 02505976 2005-05-12
WO 2004/052820 PCT/US2003/038305
-6-
(a) oxidizing an aromatic feed stock in a primary oxidation zone to
form a crude carboxylic acid slurry; wherein the crude carboxylic acid slurry
comprises terephthalic acid; wherein the oxidizing is conducted at a
temperature between about 120°C to about 190 °C;
(b) removing in a solid-liquid displacement zone impurities from a
crude carboxylic acid slurry to form a slurry product; wherein the crude
carboxylic acid slurry comprises terephthalic acid, catalyst, acetic acid, and
impurities that is withdrawn at a temperature between about 140°C and
about 170°C from the oxidation of paraxylene in a primary oxidation
zone;
(c) oxidizing the slurry product in a staged oxidation zone to form
a staged oxidation product; wherein the oxidizing is conducted at a
temperature between about 190°C to about 280 °C; and wherein the
oxidizing is at a higher temperature in the staged oxidation zone than in the
primary oxidation zone;
(d) crystallizing the staged oxidation product in a crystallization
zone to form a crystallized product;
(e) cooling the crystallized product in a cooling zone to form a
cooled purified carboxylic acid slurry; and
(f) filtering and optionally drying the cooled purified carboxylic
slurry in a filtration and drying zone to remove a portion of the solvent from
the cooled carboxylic acid slurry to produce the purified carboxylic acid
product.
CA 02505976 2005-05-12
WO 2004/052820 PCT/US2003/038305
These objects, and other objects, will become more apparent to
others with ordinary skill in the art after reading this disclosure.
BRIEF DESCRIPTION OF THE DRAWINGS
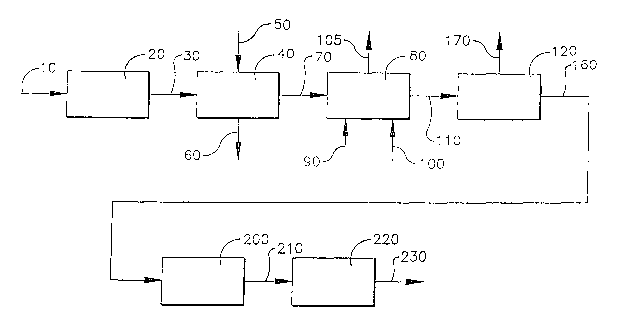
Figure 1 is a schematic of the inventive process for the oxidative
purification of carboxylic acid wherein a solid-liquid displacement zone 40 is
utilized between the primary oxidation zone 20 and the staged oxidation
zone 80.
DETAILED DESCRIPTION OF THE INVENTION
The present invention provides a process for the purification of a
1 ~ crude carboxylic acid slurry 30. The process comprises the steps of
displacing a mother liquor from the crude carboxylic acid slurry in a solid-
liquid displacement zone 40 to form a slurry product 70.
Crude terephthalic acid is conventionally made via the liquid phase
air oxidation of paraxylene in the presence of a suitable oxidation catalyst.
Suitable catalysts comprises at least one selected from, but are not limited
to, cobalt, bromine and manganese compounds, which are soluble in the
selected solvent. Suitable solvents include, but are not limited to, aliphatic
mono-carboxylic acids, preferably containing 2 to 6 carbon atoms, or
CA 02505976 2005-05-12
WO 2004/052820 PCT/US2003/038305
_g_
benzoic acid and mixtures thereof and mixtures of these compounds with
water. Preferably the solvent is acetic acid mixed with water, in a ratio of
about 5:1 to about 25:1, preferably befiveen about 8:1 and about 20:1.
Throughout the specification acetic acid will be referred to. as the solvent.
However, it should be appreciated that other suitable solvents, such as
those disclosed previously, may also be utilized. Patents disclosing the
production of terephthalic acid such as U.S patent #4,158,738 and
#3,996,271 are hereby incorporated by reference.
In an embodiment of this invention, a process to produce slurry
product 70 is provided in Figure 1. The process comprises removing
impurities from a crude carboxylic acid slurry 30 in.a solid-liquid
displacement zone 40 to form a slurry. product 70; wherein the slurry
product 70 is formed without a hydrogenation step..'
The solid-liquid displacement zone 40, impurities, crude carboxylic
acid slurry 30, and slurry product 70 are all described subsequently in this
disclosure.
In another embodiment of this invention a process to produce a
purified carboxylic acid product 230 is provided in Figure 1. The process
comprises:
Step (a) comprises removing impurities from a crude carboxylic acid
slurry 30 in an solid-liquid displacement zone 40 to form a slurry. product
70;
A crude carboxylic acid slurry 30 comprises at least one carboxylic
acid, catalyst, at least one solvent, and impurities is withdrawn via like 30.
CA 02505976 2005-05-12
WO 2004/052820 PCT/US2003/038305
-9-
The impurities typically comprise at least one or more of the following
compounds: 4-carboxybenzaldehyde(4-CBA), trimellitic acid(TMA), and 2,6-
dicarboxyfluorenone(2,6-DCF). The solvent typically comprises acetic acid,
but can be any solvent that has been previously mentioned.
The crude carboxylic acid slurry 30 is produced by oxidizing in a
primary oxidation zone 20 an aromatic feed stock 10. In one embodiment,
the aromatic feedstock comprises paraxylene. The primary oxidation zone
20 comprises at least one oxidation reactor, and the crude carboxylic acid
slurry 30 comprises at least one carboxylic acid. The oxidation reactor can
be operated at temperatures between about 120°C to about 200°C,
preferably about 140°C to about 170°C. Typically the aromatic
feed stock
10 is paraxylene and the carboxylic acid is terephthalic acid. In one
embodiment of the invention the primary oxidation gone comprises a bubble
column.
Therefore, when terephthalic acid is utilized, the crude carboxylic
acid slurry 30 would be referred to as crude terephthalic acid slurry and the
purified carboxylic acid product 230 would be referred to as a purified
terephthalic acid product.
Carboxylic acids include aromatic carboxylic acids produced via
controlled oxidation of an organic substrate. Such aromatic carboxylic acids
include compounds with at least one carboxylic acid group attached to a
carbon atom that is part of an aromatic ring, preferably having at least 6
carbon atoms, even more preferably having only carbon atoms. Suitable
CA 02505976 2005-05-12
WO 2004/052820 PCT/US2003/038305
-10-
examples of such aromatic rings include, but are not limited to, benzene,
biphenyl, terphenyl, naphthalene, and other carbon-based fused aromatic
rings. Examples of suitable carboxylic acids include, but are not limited to,
terephthalic acid, benzoic acid, p-toluic, isophthalic acid, trimellitic acid,
naphthalene dicarboxylic acid, and 2,5-diphenyl-terephthalic acid. Each of
the embodiments of this invention can be practiced wherein there is a
substantial absence of terephthalic acid and isophthalic acid in the crude
carboxylic acid slurry. When the term substantial absence is used it means
less than 5% by weight.
Crude terephthalic acid slurry is conventionally synthesized via the
liquid phase oxidation of paraxylene in the presence of suitable oxidation
catalyst. Suitable catalysts include, but are not limited to, cobalt,
manganese and bromine compounds, which are soluble in the selected
solvent. In one embodiment of the invention the catalyst comprises cobalt,
bromine and manganese. The cobalt and manganese combined can be in
concentrations of about 150 ppm to about 3200 ppm by weight in the crude
carboxylic acid slurry. The bromine can be in concentrations of about 10
ppm to about 5000 ppm by weight in the crude carboxylic acid slurry.
Preferably, the cobalt and manganese combined can be in concentrations
of about 1050 ppm to about 2700 ppm by weight in the crude carboxylic
acid slurry. The bromine can be in concentrations of about 1000 ppm to
about 2500 ppm by weight in the crude carboxylic acid slurry.
CA 02505976 2005-05-12
WO 2004/052820 PCT/US2003/038305
-11-
The crude carboxylic acid slurry in conduit 30 is fed to a solid-liquid
displacement zone 40 capable of removing a portion of the liquid contained
in the crude carboxylic acid slurry 30 to produce the slurry product in
conduit 70. A portion means at least 5~/o by weight of the liquid is removed.
The removal of a portion of the liquid to produce a slurry product in conduit
70 can be accomplished by any means known in the art. Typically, the
solid-liquid displacement zone 40 comprises a solid-liquid separator that is
selected from the group consisting of a decanter centrifuge, rotary disk
centrifuge, belt filter, rotary vacuum filter, and the like. The crude
carboxylic
acid slurry in conduit 30 is fed to the solid-liquid displacement zone 40
comprising a solid-liquid separator. The solid-liquid separator is operated at
temperatures between about 50°C to about 200°C, preferably
140°C to
about 170°C. The solid-liquid separator is operated at pressures
between
about 30 psig to about 200 psig. The solid-liquid separator in the solid-
liquid displacement zone 40 'may be operated in continuous or batch mode,
although it will be appreciated that for commercial processes, the
continuous mode is preferred.
The impurities are displaced from the solid-liquid displacement zone
40 in a mother liquor and withdrawn via line 60. In one embodiment of the
invention, additional solvent is~fed to~the solid-liquid displacement zone 40
via line 50 to reslurry the crude carboxylic acid slurry 30 and form a slurry
product 70. The mother liquor 60 is withdrawn from solid-liquid
displacement zone 40 via line 60 and comprises a solvent, typically acetic
CA 02505976 2005-05-12
WO 2004/052820 PCT/US2003/038305
-12-
acid, catalyst, and bromine compounds. The mother liquor in line 60 may
either be sent to a process for separating impurities from oxidation solvent
via lines not shown or recycled to the catalyst system via lines not shown.
One technique for impurity removal from the mother liquor 60 commonly
used in the chemical processing industry is to draw out or "purge" some
portion of the recycle stream. Typically, the purge stream is simply disposed
of or, if economically justified, subjected to various treatments to remove
undesired impurities while recovering valuable components. Examples of
impurity removal processes include U.S. Patent # 4,939,297 and U.S.
Patent 4,356,319, herein incorporated by reference.
Step (b) comprises oxidizing the slurry product 70 in a staged
oxidation zone 80 to form a staged oxidation product 110.
In one embodiment of the invention, the slurry product 70 is
withdrawn via line 70 to a staged oxidation zone 80 where it is heated to
between about 190°C to about 280°C and preferably between about
200°C
to about 250°C and further oxidized with air fed by line 100 to produce
a
staged oxidation product 110.
The staged oxidation zone 80 comprises at least one staged
oxidation reactor vessel. The slurry product 70 is fed to the staged
oxidation zone 80. The term "staged"means that the oxidation occurs in
both the primary oxidation zone 20 discussed previously as well as in the
staged oxidation zone 80. For example, the staged oxidation zone 80 can
comprise staged oxidation reactor vessels in series.
CA 02505976 2005-05-12
WO 2004/052820 PCT/US2003/038305
-13-
When the carboxylic acid is terephthalic acid, the staged oxidation
zone 80 comprises an oxidation reactor that is heated to between about
190°C to 'about 280°C, preferably between about 200°C to
about 250°C,
and most preferably between 205 °C to 225°C and further oxidized
with air
or a source of molecular oxygen fed by line 100 to produce a staged
oxidation product 110. Generally, oxidation in the staged oxidation zone 80
is at a higher temperature than the oxidation in the primary oxidation zone
20 to enhance the impurity removal. The staged oxidation zone 80 can be
heated directly with solvent vapor, or steam via conduit 90 or indirectly by
any means known in the art. Purification in the staged oxidation zone takes
place by a mechanism involving recrystallization or crystal growth and
oxidation of impurities.
Additional air or molecular oxygen may be fed via conduit 100 to the
staged oxidation zone 80 in an amount necessary to oxidize a substantial
portion of the partially oxidized products such as 4-carboxybenzaldehyde
(4-CBA) in the crude carboxylic acid slurry 30 or slurry product 70 to the
corresponding carboxylic acid.. Generally, at least 70% by weight of the 4-
CBA is converted to terephthafic acid in the staged oxidation zone 80.
Preferably, at least 80% by weight of the 4-CBA is converted to terephthalic
acid in the staged oxidation zone 80. Significant concentrations of 4-
carboxybenzaldehyde and p-toluic acid in the terephthalic acid product are
particularly detrimental to polymerization processes as they may act as
chain terminators during the condensation reaction between terephthalic
CA 02505976 2005-05-12
WO 2004/052820 PCT/US2003/038305
-14-
acid and ethylene glycol in the production of polyethylene
terephthalate(PET). Typical terephthalic acid product contains on a weight
basis less than about 250 parts per million (ppm) 4-carboxybenzaldehyde
and less than about 150 ppm p-toluic acid
Impurities in the crude carboxylic acid slurry 30 or slurry product 70
go into solution as the terephthalic acid particles are dissolved and re-
crystallized in staged oxidation zone 80. OfFgas from the staged oxidation
zone 80 is withdrawn via line 105 and fed to a recovery system where the
solvent is removed from the offgas comprising volatile organic compounds
(VOCs). VOCs including methyl bromide may be treated, for example by
incineration in a catalytic oxidation unit. The staged oxidation product 110
from the staged oxidation zone 80 is withdrawn via line 110.
Step (c) comprises crystallizing the staged oxidation product 110 in a
crystallization zone 120 to form a crystallized product 160. Generally, the
crystallization zone 120 comprises at least one crystallizer. Vapor product
from the crystallization zone can be condensed in at least one condenser
and returned to the crystallization zone. Optionally, the liquid from the
condenser or vapor product from the crystallization zone can be recycled,
or it can be withdrawn or sent to an energy recovery device. In addition, the
crystallizer offgas is removed ,via line 170 and can be routed to a recovery
system where the solvent is removed and crystallizer ofFgas comprising
VOCs may be~treated, for example by incineration in a catalytic oxidation
unit.
CA 02505976 2005-05-12
WO 2004/052820 PCT/US2003/038305
-15-
When the carboxylic acid is terephthalic acid, the staged oxidation
product 110 from the staged oxidation zone 80 is withdrawn via line 110
and fed to~a crystallization zone 120 comprising at least one crystallizer
where it is cooled to a temperature between about 110°C to about
190°C to
form a crystallized product 160, preferably to a temperature between about
140°C to about 180°C, most preferably about 150°C to
about 170°C.
The crystallized product 160 from the crystallization zone 120 is
withdrawn via line 160. Typically, the crystallized product 160 is then fed
directly to a vessel and cooled to form a cooled purified carboxylic acid
slurry 210. When the carboxylic acid is terephthalic acid, the cooled
crystallized purified carboxylic acid slurry 210 is cooled in a vessel to
typically a temperature of approximately 90° C or less before being
introduced into a process for recovering the terephthalic acid as a dry
powder or wet cake.
Step (d) comprises cooling the crystallized product in a cooling zone
200 to form a cooled purified carboxylic acid slurry 210.
The crystallized product 160 is withdrawn from the crystallization
zone 120 via line 160. The crystallized product 160 is fed to a cooling zone
200 and cooled to less than about 90 °C to form the cooled purified
carboxylic acid slurry 210. The cooling of the purified carboxylic acid slurry
can be accomplished by any means known in the art, typically the cooling
zone 200 comprises a flash tank..
CA 02505976 2005-05-12
WO 2004/052820 PCT/US2003/038305
-16-
Step (e) comprises filtering and optionally drying the cooled purified
carboxylic acid slurry 210 in a filtration and drying zone 220 to remove a
portion of the solvent from the cooled purified carboxylic acid slurry 210 to
produce the purified carboxylic acid product 230.
The cooled, purified carboxylic acid slurry 210 is withdrawn from
cooling zone 200 and fed to a filtration and drying zone 220. A portion of
the solvent and remaining catalyst and impurities is separated, and the
purred carboxylic acid product is withdrawn via line 230.
The filtration and drying zone 220 comprises a filter suitable for
recovering the solid carboxylic acid and a dryer. The filtration can be
accomplished by any means known in the art. For~.example, a rotary
vacuum filter can be used for the filtration to produce a filtration cake. The
filtration cake goes through an initial solvent removal step, is then rinsed
with acid wash to remove residual catalyst, and then solvent removed again
before being sent to the dryers. The drying of the filter cake can be
accomplished by any means known in the art that's capable of evaporating
at least 10% of the volatiles remaining in the filter cake to produce the
carboxylic acid product. For example, a Single Shaft Porcupine~ Processor
dryer can be used.
The purified carboxylic acid product 230 has a b* less than about
4.5. Preferably, the b* color of the purified carboxylic acid product 230 is
less than about 3.5. Most preferably, the b* color in purified carboxylic acid
product 230 is less than about 3. The b* color is one of the three-color
CA 02505976 2005-05-12
WO 2004/052820 PCT/US2003/038305
-17-
attributes measured on a spectroscopic reflectance-based instrument. The
color can be measured by any device known in the art. A Hunter Ultrascan
XE instrurrient in reflectance mode is typically the measuring device.
Positive readings signify the degree of yellow (or absorbance of blue), while
negative readings signify the degree~of blue (or absorbance of yellow).
It should be appreciated that the process zones previously described
can be utilized in any other logical order to produce the purified carboxylic
acid product. It should also be appreciated that when the process zones are
reordered that the process conditions may change.
In another embodiment of this invention each embodiment can
optionally include an additional step comprising decolorizing the carboxylic
acid or an esterified carboxylic acid via hydrogenation.
The decolorizing of the purified carboxylic acid slurry or an esterified
carboxylic acid can be accomplished by any means known in the art and is
not limited to hydrogenation: However, for example in one embodiment of
the invention, the decolorizing can be accomplished by reacting a
carboxylic acid that has undergone esterification treatment , for example
with ethylene glycol, with molecular hydrogen in the presence of a
hydrogenation catalyst in a reactor zone to produce a decolorized
carboxylic acid solution or a decolorized ester product. For the reactor
zone, there are no special limitations in the form or construction thereof,
subject to an arrangement that allows supply of hydrogen to effect intimate
contact of the carboxylic acid or ester product with the catalyst in the
CA 02505976 2005-05-12
WO 2004/052820 PCT/US2003/038305
-18-
reactor zone. Typically, the hydrogenation catalyst is usually a single
Group Vlll metal or combination of Group Vlll metals. Preferably, the
catalyst is ~~selected from a group consisting of palladium, ruthenium,
rhodium and combination thereof. The reactor zone comprises a
hydrogenation reactor that operates at a temperature and pressure
sufficient to hydrogenate a portion of the characteristically yellow
compounds to colorless derivatives
CA 02505976 2005-05-12
WO 2004/052820 PCT/US2003/038305
-19-
EXAMPLES
This invention can be further illustrated by the following example of
preferred embodiments thereof, although it will be understood that these
examples are included merely for purposes of illustration and are not
intended to limit the scope of the invention unless otherwise specifically
indicated.
Example
Paraxylene was oxidized at 160°C utilizing a Co, Mn, Br catalyst
system to produce a crude terephthalic acid slurry having 30-35% solids.
The crude terephthalic acid slurry was crystallized and purified using the
process shown in Figure 1. with the omission of a hydrogenation step and
the crystallized product from the crystallization zone 120 was transferred
directly to flash tank. The product was removed after filtration and drying
and analyzed for 4-carboxybenzaldehyde(4-CBA), trimellitic acid(TMA), and
2,6-dicarboxyfluorenone(2,6-DCF), percent transmittance and b*. The b* is
one of the three-color attributes measured on a spectroscopic reflectance-
based instrument. A Hunter Ultrascan XE instru.ment is. typically the
measuring device. Positive readings signify the degree of yellow (or
absorbance of blue), while negative readings signify the degree of blue (or
absorbance of yellow).
The concentrations of 4-CBA, TMA, 2,6-DCF in the terephthalic acid
were analyzed via liquid chromatography. To determine the percent
CA 02505976 2005-05-12
WO 2004/052820 PCT/US2003/038305
-20-
transmittance, a 10% solution of terephthalic acid product in 2M KOH was
measured using a UV visible spectrometer at 340nm. The b* of the
terephthalic acid was measured using a reflectance color method at 340nm.
The results are shown in Table 1.
Ex. # 4-CBA TMA~ 2,6-DCF %T b*
(ppm) (ppm) (ppm)
1 103 51 10 89 4.1
The amount of 4-CBA present in the purified terephthalic acid
product produced by the process of the present invention decreased
significantly from typical levels found in the crude carboxylic acid slurry.
The
typical levels weren't measured during this trial but these levels were known
to those skilled in the art to be about what has been previously disclosed
wherein the crude carboxylic acid slurry comprising terephthalic acid,
typically contains on a weight basis from about 800 to 7,000 parts per
million (ppm) 4-carboxybenzaldehyde. The % transmittance of the purified
terephthalic acid product has a direct influence on the color of the
polyethylene terephthalate (PET) produced. Desirable PTA (purified
terephthalic acid) is white (which is referred to as having low color). Higher
transmittance indicates less color in the PTA. The degree of
improvement in all the measured categories is particularly surprising given
CA 02505976 2005-05-12
WO 2004/052820 PCT/US2003/038305
-21-
the simplicity of the centrifugation in the solid-liquid separation zone and
that no hydrogenation step was performed. In the past, comparable purity
levels have been achieved typically by utilization of a hydrogenation plant
which includes numerous steps and pieces of equipment, and significant
capital investment.
The invention has been described in detail with particular reference
to preferred embodiments thereof, but it will be understood 'that variations
and modifications can be effected within the spirit and scope of the
invention