Note: Descriptions are shown in the official language in which they were submitted.
CA 02506780 2001-06-19
WO 02/065.11 PCT/CA01/0087.1
PRODUCTION OF ZINC OXIDE FROM ACID SOLUBLE ORE USING
PRECIPITATION METHOD
BACKGROUND OF THE INVENTION
Field of the Invention
The present invention relates to method and apparatus for the extraction and
recovery of zinc from zino-bearing materials (solutions or solids) through a
process carried
out in a sulphuric acid media by leaching the zinc followed by precipitation
of zinc as
oxide and upgrading zinc content in precipitates by flotation or granulometric
sizing.
Description of the Related Art
Acid soluble zinc ores (zinc oxide, zinc carbonate, zinc silicate, etc.) have
been
used in the production of zinc metal since the very beginnings of the
industry. The
leaching of theses ores and purification of impurities associated with these
ores are well
established. Wood, in a paper published in the Journal of Metals, Vol. 29,
page 7,
incorporated herein by reference, describes a process for the
hydrometallurgical treatment
of zinc silicate. U.S. Patent No. 3,954,937, incorporated herein by reference,
provide a
process which utilizes a series of leaching tanks in which the acidity is
progressively
increased over a period of at least three hours, in such a quantity that the
final acidity of the
solution will be 1.5 to 1 S gn, while carefully maintaining the temperature at
70° to 90°C,
thereby inducing the lixiviation of the material and simultaneously the
reprecipitation of
silica in a crystalline form which can be eliminated by filtration. U.S.
Patent No.
3,656,941, incorporated herein by reference, describes a process for the
recovery of metal
values from a siliceous zinc ore whereby the ore is treated with sulphuric
acid in a
CA 02506780 2001-06-19
WO 02/065.11 PCT/CA0110087~
2
continuous manner in stirred tanks. U.S. Patent No. 5,585,079, incorporated
herein by
reference, describes a method for recovering zinc from a zinc oxide bearing
material
containing silicates. According to this method, the leaching of zinc compounds
is carried
out in circumstances where the silicates remain undissolved and consequently
do not cause
filtering problems. The silicate bearing residue is treated in a Waelz
process.
Although, there are many processes that can provide a method to dissolve zinc
from .
the acid soluble zinc ore and precipitate impurities such as iron and silica
to make filtrate
filterable, none of the above-mentioned techniques addresses a process to
recover zinc
from zinc bearing solutions. Most of the above-mentioned processes use
conventional
electrowinning process to recover zinc from zinc sulphate solutions.
Electrowinning is
well known technology, however, it requires high capital cost. Therefore, it
is only
suitable technology if the metal value is significant enough to build an
electrowinning
plant or, otherwise, a recovery process has to be located at and integrated
with present zinc
refineries.
Another problem with the conventional electrowinning process is that the zinc
concentration level must be higher than a certain level to operate. Therefore,
if the zinc
concentration in the final filtrate of the leaching operation is not high
enough, the zinc
concentration in the ~Itrate of leached acid soluble zinc materials has to be
upgraded.
Zinc in the solution can be conc~trated by several known methods, such as
solvent
extraction or ion exchange. However, those processes have several drawbacks,
such as
limitation of extraction capacity, organic substance contamination and high
capital cost. In
addition, these approaches still require a conventional electrowinning process
to recover ,
zinc from zinc bearing solution.
Another approach to recover zinc from the zinc bearing solution is to
precipitate
CA 02506780 2001-06-19
WO 02/065x1 PCT/CAOI/0087.~
zinc from the solution itself. The precipitated zinc now can be easily sent to
the zinc
refineryor other facilities which requires such materials. Zinc can be
precipitated from
zinc sulphate solution as zinc oxide, zinc hydroxide, or basic zinc sulphate
by adding
alkaline. Also, zinc can be precipitated as zinc sulphide by adding sulphide
or as zinc
carbonate by adding carbon dioxide. However, the operation of the process for
producing
zinc sulphide or zinc carbonate is expensive. Also, producing zinc hydroxide
causes
severe filtering problems and producing basic zinc sulphate results in high
transportation
cost since the grade of zinc in basic zinc sulphate is very low. Producing
zinc oxide is the
most suitable approach, but unless you are using sodium hydroxide, which is
very
expensive reagent, the zinc oxide will be contaminated with a large amount of
gypsum that
is co-precipitated during zinc oxide precipitation.
Therefore, it is desirable to develop a process for producing zinc oxide from
zinc
sulphate solution that is originated from acid soluble zinc materials.
SUMMARY OF THE INVENTION
It is an object of the invention to provide an apparatus for producing zinc
oxide
firom an acid soluble zino-containing material. The apparatus comprises a
leaching unit for
leaching the acid soluble zinc-containing material with sulphuric acid, a
first precipitating
unit for precipitating iron, silica and other metals (Al, As, Sb, etc.) from
the leach solution
using calcium oxide (or Ca(OH~, MgO, Zn0), and removing, copper, cadmium,
nickel
and cobalt from the leach solution by cementation with zinc dust. A second
precipitating
unit is used to precipitate zinc oxide from the leach solution using calcium
oxide (or
Ca(OH)Z). Then the precipitated zinc oxide is separated from calcium sulphate
by flotation
with cationic collectors such as dodecylamine hydrochloride, and dodecylamine
sulphate,
CA 02506780 2001-06-19
WO 02/06~.t1 PCT/CA01/0087.1
4
andlor potassium amyl xanthate as a collector. This separation of zinc oxide
from calciut»
sulphate also can be achieved by granulometric sizing. In addition, a
combination of the
two techniques (sizing and flotation) can be used for separating of zinc oxide
from calcium
sulphate, In addition, the tailing of these steps can be recycled to the
leaching step to
recover zinc value.
A further object of the present invention is to provide a process for
producing zinc
oxide from an acid soluble zinc-containing material. The process comprises the
steps of
leaching the acid soluble zinc material with sulphuric acid, precipitating
iron, silica and
other metals (Al, As, Sb, etc.) from the leach solution using calcium oxide
(or Ca(OH)2,
MgO, Zn0), cementing copper, cadmium, nickel and cobalt in the Ieach solution
with zinc
dust, and precipitating zinc oxide from the leach solution using calcium oxide
(or
Ca(OH)2). Then the precipitated zinc oxide is separated from calcium sulphate
by flotation
with cationic collectors such as dodecylamine hydrochloride, and dodecylamine
sulphate,
andlor potassium amyl xanthate. The separation of zinc oxide from calcium
sulphate can
also be achieved by granulometric sizing. In addition, a combination of the
two techniques
(granulometric sizing and flotation) can be used for separating zinc oxide
from calcium
sulphate. The tailing of these steps can also be recycled to the leaching step
to recover zinc
value.
Still another object of the present invention is to provide a zinc oxide
precipitate
thaf is substantially free of basic zinc sulphate by leaching an acid soluble
zinc-containing
material with sulphuric acid. Iron, silica and other metals {Al, As, Sb, etc.)
are precipitated
from the hch solution u~ing calcium oxide (or Ca(OH~, MgO, Zn0), and copper, ,
cadmium, nickel and cobalt are cemented from the leach solution using zinc
dust. Zinc
oxide is precipitated from the leach solution using calcium oxide.
CA 02506780 2001-06-19
WO 02/0651 PCT/CA01/0087.1
A further object of the present invention is to provide a process for
recovering
copper and cadmium from an acid soluble zino-containing material. The process
comprises the steps of leaching the acid soluble zinc material with sulphuric
acid,
precipitating iron, silica and other metals (Al, As, Sb, etc.) from the leach
solution using
calcium oxide (or Ca(OH)2, MgO, Zn0) and recovering copper and cadmium from
the
leach solution by cementing with zinc dust.
Still another object of the present invention is to provide a process for
separating
zinc oxide from gypsum, which precipitates during zinc oxide precipitation.
The process
comprisesd the steps of leaching the acid soluble zinc-containing material
with sulphuric
acid, precipitating iron, silica and other metals (AL, As, Sb, etc.) from the
leach solution
using calcium oxide (or Ca(OH~, MgO, Zn0), and cementing copper, cadmium,
nickel
and cobalt frorri the leach solution using zinc dust. The precipitated zinc
oxide is separated
from co-precipitated gypsum by flotation with cationic collectors such as
dodecylamine
hydrochloride, and dodecylamine sulphate, and/or potassium amyl xanthate as a
collector.
The separation of zinc oxide from calcium sulphate can also be achieved by
granulometric
sizing. In addition, a combination of the two techniques (granulometric sizing
and
flotation) can be used for separating zinc oxide from calcium sulphate. The
tailing of
the flotation step can also be recycled to the leaching step to recover zinc
value.
The invention is described in more detail below with reference to the
accompanying figure.
BRIEF DESCRIPTION OF THE DRAWIrIGS
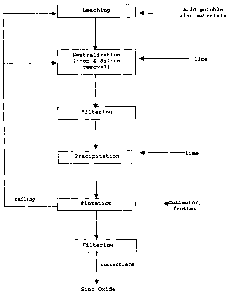
Figure 1 is a flowchart illustrating the preferred process for producing zinc
oxide
from an acid soluble zinc material using the invented process.
CA 02506780 2001-06-19
wo ozio6:;.n pcT~cAOnoog~.~
Figure 2 is a flowchart illustrating the preferred process for producing zinc
oxide
from an acid soluble zinc material using the invented process including the
granulometric
sizing stage.
Figure 3 is a schematic block diagram depicting the apparatus according to the
present invention.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
1. Introduction
The present invention relates to method and apparatus for the extraction and
recovery of zinc from zino-bearing materials (solutions or solids) through a
process carried
out in a sulphuric acid media by leaching the zinc followed by precipitation
of zinc as
oxide and upgrading the zinc content in precipitates by flotation or
granulometric sizing.
This upgrading of zinc also can be achieved by using a granulometric sizing
technique.
This process also allows for the recovery of other metals, such as iron,
copper, nickel,
cobalt, lead, silver, cadmium, etc. The present invention further relates to a
process for
producing zinc oxide from an acid soluble zinc material, an apparatus for
performing the
process, and processes for recovering iron, copper, and cadmium from an acid
soluble zinc
material. More particularly, the invention relates to a pmcess of producing
zinc oxide from
such an acid soluble zinc material by, preferably, leaching the acid soluble
zinc material
with sulphuric acid, followed by precipitation of iron, silica and other
metals (AI, As, Sb,
etc.) from the leach solution using calcium oxide (or Ca(OH)2, MgO, Zn0), and
cementation of copper, cadmium and cobalt using zinc dust. Zinc oxide may then
be
precipitated from the leach solution using calcium oxide as a mixture of zinc
oxide and
calcium sulphate. The zinc grade in the mixture of zinc oxide and calcium
sulphate may
CA 02506780 2001-06-19
WO 02/065.11 PCT/CA01/0087.1
be improved by flotation andlor granulometric sizing techniques. The novelty
of this
invention is precipitating zinc oxide from zinc sulphate solution and
upgrading zinc
content by flotation and/or granulometric sizing techniques.
By the phrase "acid soluble zinc materials" as used in this specification is
meant
primarily a zinc silicate ore but it is also intended to include zinc oxide
ore, zinc carbonate
ore and a zinc materials containing substantial quantities of soluble zinc in
sulphuric acid
The presently preferred process of the present invention is comprised of a
series of
steps, as will be described in detail below.
2. Leaching and Neutralization
According to a preferred embodiment of the present invention there is provided
a
process for the recovery of metal values from an acid soluble ore, where the
process is
conducted in a continuous concurrent manner. A process comprises a first
stage, effected
in one or more vessels, in which the said ore is leached with aqueous
sulphuric acid to an
end-point pH in the range 1.0 to 4.0, preferably in the range 1.5 to 2.5, to
obtain a leach
liquor or pulp containing dissolved zinc values and other impurities including
silica and
iron. A second stage, effected in one or more vessels, in which the pH of the
liquor or pulp
from the first stage is raised, for example by addition of neutralising agent,
to a pH in the
range 3 to 6, preferably in the range 3.5 to 4.5, such that substantially the
whole or a major
part of the silica. content of said leach liquor or pulp is converted into a
form in which it is
readily separated by conventional techniques.
Both process stages may be operated successfully at temperatures up to the
boiling
points of the solutions at atmospheric pressure, and operating temperatures
between
ambient temperature and about 95°C are therefore envisaged. The aqueous
sulphuric acid
CA 02506780 2001-06-19
WO 02/065.11 PCT/CA01/0087.!
s
necessary for the treatment of the acid soluble ores. Spent electrolyte from a
conventional
process for the electrowinning of zinc, or such spent electrolyte fortified to
compensate for
acid losses, may conveniently be used as a leaching agent. Residence times of
the liquor or
pulp in each of the process stages may lie in the range 30 minutes to 10
hours, with a
preferred residence time in the first stage of 1 - 4 hours, and a preferred
residence time in
the second stage of 1 to 6 hours.
The pulp leaving from the second stage contains suspended silica and iron
hydroxide in particulate form, and will be subjected to solid liquid
separation by using
thickening process or conventional filtering technology. In some circumstance
it may be
desirable to re-circulate portions of pulp or thickened pulp from the second
stage to first or
second stages of the process.
The filtrate from this step is sent to cementation.
3. Zinc oxide precipitation
At a third stage, the pH of the liquor from solid-liquid separation process
right after
second stage is raised, for example by the addition of neutralising agent such
as lime, to a
pH in the range of 5 to 14, preferably in the range of 8.5 to 9.5 such that
substantially the
whole of metal values including zinc is precipitated as oxide forms and the
whole or a
major part of lime is converted to gypsum. The third stage may be operated
successfully at
temperature up to boiling points of the solution at atrnospheric pressure,
preferably in the
range of 60 to 95°C. The pulp leaving the third stage is sent to the
flotation stage with or
without solid-liquid separation. In some circumstance, it may be desirable to
re-circulate
portions of pulp or thickened pulp from the third stage to very beginning or
in the process
of third stages of the process.
CA 02506780 2001-06-19
WO 02/0651 PCT/CA01/0087a
9
In some circumstances, it may be desirable to purify the liquor before third
stage.
1n this case, zinc dust may be added into the solution with or without other
reagent such as
copper sulphate, arsenic trioxide and/or antimony. The preferable condition
will be pH in
the range of 3 to 6, preferably in the range of 4.0 to 5.0, and temperature in
the range of 60
to 95°C. The residence time of this process maybe in the range of 1 to
4 hours.
The use of Ca0 as the neutralizing reagent results, theoretically, in the
following
reactions:
ZnS04 + HZO ~ Zn0 + H2S04 (hydrolysis) (1)
H2S04 + Ca0 -~ Ca S04 + H20 (neutralization) {2)
ZnS04 + Ca0 ~ Zn0 + CaS04 (overall) (3)
This series of reactions represents a conventional neutralization process with
the objective
of generating a Zn0 product of high purity.
4. Flotation
The next stage is where the pulp from the third stage or the re-pulped cake
from the
solid-liquid separation is subjected to the flotation step. Flotation is a
process for
separating finely ground minerals from their associated gangue. This process
is usually
used to separate one solid from another by using the affinity of air bubbles
to solids. In this
stage, zinc oxide including metal oxides or hydroxides is recovered by
flotation
concentrate and gypsum is recovered as tailing. Cationic collectors, such as
dodecylamine
hydrochloride, are used as flotation agents. Potassium amyl xanthate or
dodecylamine
sulphate may be used for this purpose. Any frother could be used, but a
Dowfroth 250 was
found useful. The flotation procxss may be operated at ambient temperature and
also
successfully at temperature up to 90°C and the pulp from the third
stage may be used
CA 02506780 2001-06-19
WO 02/06i.t1 PCT/CA01/0087~
without any heating or cooling stages. The gypsum from the flotation step can
be recycled
to either of the leaching or neutralization steps.
The Zn0 product obtained according to the present invention could be
introduced
in an entirely independent circuit in a zinc refinery, or it could be
introduced in an existing
plant circuit for increasing plant capacity through full operation of a
cellhouse. The Zn0
product can be dissolved very easily in spent electrolyte at room temperature
and the
dissolved zinc can be recovered by electrowinning method.
5. Granulometric Sizing
This operation may be used in conjunction with, or as an alternative to,
flotation.
The sizing may be performed by any method, either individually or in
combination.. For
example, sizing may be performed by using one or more of the following
techniques:
classifier, elutriation, settling, screening, tables, and cyclones. A
classifier is a device for
subjecting comminuted ore to the action of water either in such a way that a
division of the
ore particle is made into two or more products according to relative settling
powers.
Cyclones are devices primarily used for separation of solids from fluids.
Cyclones oppose
centrifugal forces collinear to fluid drag, substantially at right angles to a
rapid carrying
current. Since such separation depends on relative particle size and specific
gravity, it can
be used for separation of solids from each other.
From the zinc oxide precipitation step, the zinc oxide in the precipitated
slurry can
be separated from gypsum by a screening technology that uses appropriate
sieves. In this
stage, the precipitated slurry is wet-screened with a series of sieves and the
undersized
materials are collected for the final product. The oversized materials are
collected and
CA 02506780 2001-06-19
WO 02/065.11 PCT/CA01/0087.t
11
recycled to the leaching or neutralization step. Also, the oversized materials
can be
leached with sulphuric acid to dissolve zinc. Then, the zinc bearing solution
can be
recycled to the leaching, neutralization or precipitation stage to recover
zinc. The leached
residue can be washed and recovered as pure gypsum. The undersized materials
also can
be treated further by flotation or granulometric operation, as described in
the previous
sections, to increase zinc grade.
Fig. 3 depicts an apparatus of the present invention. Leaching Tank 2 is used
to
leach an acid soluble zinc-containing material with sulphuric acid. After
separation in the
Solid-liquid Separation Tank 4, the leach solution is then sent to
Precipitating Tank 6
where the precipitation is performed. After the precipitate solution is sent
to Flotation Cell
8 to separate the precipitated zinc oxide from gypsum, which is co-
precipitated during the
precipitation process. It is, however, to be understood that the apparatus is
not limited to
the disclosed embodiment. Persons with skill in the art will know that many
different
apparatuses can be constructed with equivalent arrangements and still be
within the spirit
and scope of the appended claims. For example, the precipitation means can
take place in
one or more tanks (vessels). In addition, the means to convey the material
from each of
these tanks are numerous and well known in the art.
Ezamples
The present invention will now be described with reference to the following
examples. It should be noted that although a complete process described in
here is
applicable for any leaching solutions which bear zinc, the following examples
will focus
on the precipitation of zinc oxide from leaching solutions originated from the
zinc oxide
CA 02506780 2001-06-19
WO 02/065.11 PCT/CA01/0087a
is
ores.
Example 1
A zinc oxide ore containing 31.4% total zinc, 5.06% silica and 2.49 % iron was
treated by two-stage continuous process in accordance with the invention using
sulphuric
acid solution. Leaching (first stage) and silica removal (second stage) tests
were conducted
in 4.0-L reactor equipped with baffle. The pH of the pulp was maintained by
adding
sulphuric acid at 2.0 and leaching temperature was controlled at SO°C
for 4 hours. The
agitation speed was set at 750 rpm. The second stage was performed in the same
reactor
without previous solid-liquid separation using Ca(OH~ for neutralisation to pH
4.0 for 3
hours. The agitation speed was set at 900 rpm. After neutralisation, the
slurry was filtered
and the filtrate was saved for the precipitation test.
The zinc extraction was 90% and the acid consumption was 2.52 tlt of zinc
extracted. The Ca(OH)2 consumption for neutralisation was 0.18 t/t of zinc
extracted. The
final filtrate contained 47.1 g/1 zinc, and 119 mg/1 silica.
Zinc precipitation (third stage) test was conducted in 2.0-L reactor equipped
with baffle.
The pH was monitored and controlled at 9.5 with a pH meter. Precipitation
temperature
was controlled at 90 °C and the agitation speed at 750 rpm. The tests
were conducted in a
continuous mode for three retention times of 30 minutes, for total test
duration of 90
minutes. The test consisted in neutralizing a ZnS04 solution from the second
stage with
Ca(OH)2. The zinc sulphate solution at 47.1 g/L of Zn was pumped continuously
at 50.0
ml/min into a 2.0-L reactor. The zinc solution was neutralized to pH 9.5 with
a calcium
hydroxide slurry at 20%. During the test, 5000 ml of ZnS04 at 47.1 g/1 of Zn
was
neutralized with 1455 ml of lime slurry at 20% producing 923 g of precipitate.
Results
CA 02506780 2001-06-19
WO 021065.11 PCT/CA01/0087.1
13
showed a complete removal of Zn (more than 99.9%) from the ZnS04 solution with
a
residual Zn concentration in the filtrate of 1.6 mg/L. The solids produced
were
approximately 25% Zn, this Zn content indicated production of good quality Zn0
and
CaS04.2H20 since Zn content of pure mixture of Zn0 and CaS04.2H20 i~' 26%. In
addition, analysis of the solids by X-Ray Diffraction confirmed the presence
of hexagonal
Zincite (Zn0) and monoclinic Gypsum (CaS04.2Hz0) as major phases.
Stoichiometric
calculation based on equation (3) showed that only 4% excess of Ca(OH)z was
used to
precipitate Zn content of the total volume of ZnSOa solution treated. In
addition,
calculated mass of Zn0 + CaS04.2H20 produced according to equation (3) and
based on
the Zn treated would be 954 g or 103% of the mass of precipitate measured.
The solid from third stage {zinc precipitation) was mixed with filtrate of the
precipitation test and mechanically stirred to obtain a slurry. The weight of
the solid taken
on dry basis was 166.0 g. The flotation test.was conducted in a 1 liter
laboratory Denver
flotation cell at 1204 rpm. The collector solution of the required, DDA
(dodecylamine
hydrochloride, C,zH23NHz~HC1), was mixed with flotation pulp. The volume of
collector
added was 3 mg followed by adding 0.05 ml of frother, Dowfroth 250C. The
concentrate
was collected for 3 minutes and filter, dried and weighed. Result showed 77.5%
of zinc
was recovered and the grade of concentrate was 41.2% zinc. The amount of zinc
in the
tailing was found to be 10.8%, however, this zinc can be recycled to the first
stage for
recovery.
Example 2
From the same solid of the third stage of Example 1, the slurry was screened
with
400 mesh sieve. The undersized material is 48% Zn and the zinc recovery was
92%. The
CA 02506780 2001-06-19
WO 02/0651 PCT/CA01/0087.1
14
oversized material is 22.7% Ca and 3.8% Zn. The same sample was screened with
635
mesh sieve. The undersized material was 56.5% Zn and the zinc recovery was
87.4%.
While the present invention has been described for what are presently
considered
the preferred embodiments, the invention is not so limited. To the contrary,
the invention
is intended to cover various modifications and equivalent arrangements
included within the
spirit and scope of the appended claims. The scope of the following claims is
to be
accorded the broadest interpretation so as to encompass all such modifications
and
equivalent structures and functions