Note: Descriptions are shown in the official language in which they were submitted.
CA 02508483 1999-12-22
CORNEAL IMPLANT AND
METHOD OF MANUFACTURE
This application is a divisional application of co-pending application
serial number 2,356,297 filed June 21, 2001.
The field of this invention relates to prosthetic implants designed to be
implanted in the cornea for modifying the cornea curvature and altering the
corneal refractive power for correcting myopia, hyperopia, astigmatism, and
presbyopia, and, in addition, to such implants formed of a micro-porous
hydrogel
material.
BACKGROUND OF THE INVENTION
It is well known that anomalies in the shape of the eye can be the cause of
visual disorders. Normal vision occurs when light that passes through and is
refracted by the cornea, the lens, and other portions of the eye, and
converges at or
near the retina. Myopia or near-sightedness occurs when the light converges at
a
point before it reaches the retina and, conversely, hyperopia or far-
sightedness
occurs when the light converges a point beyond the retina. Other abnormal
conditions include astigmatism where the outer surface of the cornea is
irregular in
shape and effects the ability of light to be refracted by the cornea. In
addition, in
patients who are older, a condition called presbyopia occurs in which there is
a
diminished power of accommodation of the natural lens resulting from the loss
of
elasticity of the lens, typically becoming significant after the age of 45.
Corrections for these conditions through the use of implants within the
body of the cornea have been suggested. Various designs for such implants
CA 02508483 1999-12-22
include solid and split-ring shaped, circular flexible body members and other
types of ring-shaped devices that are adjustable. These implants are inserted
within the body of the cornea for changing the shape of the cornea, thereby
altering the its refractive power.
These types of prostheses typically are implanted by first making a tunnel
and/or pocket within the cornea which leaves the Bowman's membrane intact and
hence does not relieve the inherent natural tension of the membrane.
In the case of hyperopia, the corneal curvature must be steepened, and in
the correction of myopia, it must be flattened. The correction of astigmatism
can
be done by flattening or steepening various portions of the cornea to correct
the
irregular shape of the outer surface. Bi-focal implants can be used to correct
for
presbyopia.
It has been recognized that desirable materials for these types of prostheses
include various types of hydrogels. Hydrogels are considered desirable because
they are hydrophilic in nature and have the ability to transmitting fluid
through the
material. It has been accepted that this transmission of fluid also operates
to
transmit nutrients from the distal surface of the implant to the proximal
surface for
providing proper nourishment to the tissue in the outer portion of the cornea.
However, while hydrogel lenses do operate to provide fluid transfer
through the materials, it has been found that nutrient transfer is problematic
because of the nature of fluid transfer from cell-to-cell within the material.
Nutrients do not pass through the hydrogel material with the same level of
efficacy as water. Without the proper transfer of nutrients, tissue in the
outer
portion of the cornea will die causing further deterioration in a patient's
eyesight.
Thus, there is believed to be ~a demonstrated need for a material for corneal
implants that will allow for the efficacious transmission of nutrients from
the inner
surface of a corneal implant to the outer surface, so that tissue in the outer
portion
of the cornea is properly nourished. There is also a need for a more effective
corneal implant for solving the problems discussed above.
2
CA 02508483 1999-12-22
DESCRIPTION OF THE PRIOR ART
Summary of the Invention
The present invention is directed to a corneal implant formed of a
biocompatible, permeable, micro-porous hydrogel with a refractive index
substantially similar to the refractive index of the cornea. The device, when
placed under a lamellar dissection made in the cornea (such as a corneal
flap), to
relieve tension of Bowman's membrane, alters the outer surface of the cornea
to
correct the refractive error of the eye. By relieving the pressure and
subsequent
implantation of the device, the pressure points which typically are generated
in
present corneal surgeries are eliminated, and hence reduced risk to patients
of
extrusion of implants.
The implant is preferably generally circular in shape and is of a size
greater than the size of the pupil in normal or bright light, and can
specifically be
used to correct hyperopia, myopia, astigmatism, and/or presbyopia. Due to the
complete non-elastic nature of the corneal tissue, it is necessary to place
the
implant in the cornea with Bowman's membrane compromised, such as through a
corneal lamellar dissection, to prevent extrusion of the implant from the
cornea
over the lifetime of the implant. Extrusion is undesirable because it tends to
cause
clinical complications and product failure.
Preferably, for the correction of hyperopia, the implant is formed into a
meniscus-shaped disc with its anterior surface radius smaller (steeper) than
the
posterior surface radius, and with negligible edge thickness. This design
results in
a device that has a thickness or dimension between the anterior and posterior
surfaces along the central axis greater than at its periphery. When such an
implant
is placed under the corneal flap, the optical zone of the cornea is steepened
and a
positive optical power addition is achieved.
For the correction of myopia, the implant is shaped into a meniscus lens
with an anterior surface curvature that is flatter than the posterior surface.
When
CA 02508483 1999-12-22
the implant is placed concentrically on the stromal bed the curvature of the
anterior surface of the cornea in the optic zone is flattened to the extent
appropriate to achieve the desired refractive correction.
For astigmatic eyes, implants are fabricated with a cylindrical addition
along one of the axes. This device can be oval or elliptical in shape, with a
longer
axis either in the direction of cylindrical power addition or perpendicular to
it.
The implant preferably has a pair of markers such as, for example,
protrusions,
indentations or other types of visual indicators, in the direction of the
cylindrical
axis to easily mark and identify this direction. This indexing assists the
surgeon in
the proper placement of the implant under the flap with the correct
orientation
during surgery to correct astigmatism in any axis.
For simple or compound presbyopia, the implant is made by modifying the
radius of curvature in the central 1.5-3mm, thereby forming a mufti-focal
outer
corneal surface where the central portion of the cornea achieves an added plus
power for close-up work. The base of an implant designed for compound
presbyopia can have a design to alter the cornea to achieve any desired
correction
for the myopic, hyperopic, or astigmatic eye.
The material from which any one or more of these implants are made is
preferably a clear, permeably, microporous hydrogel with a water content
greater
than 40% up to approximately 90%. The refractive index should be substantially
identical to the refractive index of corneal tissue. The permeability of the
material
is effected through a network of irregular passageways such as to permit
adequate
nutrient and fluid transfer to prevent tissue necrosis, but which are small
enough
to act as a barrier against the tissue ingrowth from one side of the implant
to
another. This helps the transmembrane tissue viability while continuing to
make
the implant removable and exchangeable.
The refractive index of the implant material should be in the range of 1.36-
1.39, which is substantially similar to that of the cornea (1.376). This
4
CA 02508483 1999-12-22
substantially similar refractive index prevents optical aberrations due to
edge
effects at the cornea-implant interface.
The microporous hydrogel material can be foamed from at least one (and
preferably more) hydrophilic monomer, which is polymerized and cross-linked
with at least one mufti- or di-olefinic cross-linking agent.
The implants described above can be placed in the cornea by making a
substantially circular lamellar flap using any commercially available
microkeratome. When the flap is formed, a hinge is preferably left to
facilitate
proper alignment of the dissected corneal tissue after the implant is placed
on the
exposed cornea.
BRIEF DESCRIPTION OF THE DRAWINGS
A better understanding of the invention can be obtained from the detailed
description of exemplary embodiments set forth below, when considered in
conjunction with the appended drawings, in which:
Fig. 1 is a schematic illustration of a horizontal section of a human eye;
Fig. 2 is a schematic illustration of an eye system showing adjustment of
the cornea to steepen the corneal slope to con ect for hyperopia;
Fig. 3 is a schematic illustration of an eye system showing adjustment of
the cornea to flatten the corneal slope to correct for myopia;
Figs. 4a and 4b are sectional and plan views of a solid corneal implant for
correcting hyperopia;
Figs. Sa and Sb are sectional and plan views of a solid corneal implant for
correcting myopia;
Figs. 6a and 6b are sectional and plan views of ring-shaped corneal
implant for correcting myopia;
Figs. 7a and 7b are schematic representations of a lamellar dissectomy,
with Fig. 7b showing in particular the portion of the dissected cornea being
connected through a hinge to the intact cornea;
5
CA 02508483 1999-12-22
Fig. 8 is a schematic representations of a cornea in which an implant has
been implanted for a hyperopic correction;
Figs. 9 and 10 are schematic representations of a cornea in which solid and
ring-shaped implants, respectively, have been implanted lamellar for a myopic
correction;
Figs. l la, l lb, and l lc are plan and sectional views of an implant useful
for correcting astigmatism where two axes have different diopter powers;
Figs. 12a, 12b, and 12c are plan and sectional views of an second implant
for correcting astigmatism where the implant is elliptical in shape;
Fig. 13 is a plan view of an implant with a pair of tabs used to identify an
axis for astigmatic correction;
Fig. 14 is a plan view of a second implant for astigmatic correction where
indentations are used instead of tabs;
Figs. 15 and 16 are schematic representations showing implants with tabs
orientated along the astigmatic axis for correcting astigmatism;
Fig. 17 is a sectional view of a corneal implant shaped,to correct for
compound presbyopia with an additional power in the center of an implant for
correcting hyperopia;
Fig. 18 is a sectional view of another corneal implant shaped to correct for
compound presbyopia with additional power in the center of an implant for
correcting myopia;
Fig. 19 is a sectional view of a corneal implant with additional power in
the center for correcting simple presbyopia;
Fig. 20a is a schematic representation of a corneal implant for an
astigmatic correction with a central power add for correcting presbyopia,
showing
in particular a pair of tabs for proper alignment of the lens;
Fig. 20b is a schematic representation of a another corneal implant with a
center power add for non-astigmatic correction, which shows in particular a
steep
transition between the central add and the remainder of the implant; and
6
CA 02508483 1999-12-22
Figs. 21a and 21b are schematic representations showing the use of a
lamellar dissection for implanting a lens of the type shown in Fig. 20b.
DETAILED DESCRIPTION OF EXEMPLARY EMBODIMENTS
Referring first to Fig. 1 of the drawings, a schematic representation of the
globe of the eye 10 is shown, which resembles a sphere with an anterior bulged
spherical portion 12 that represents the cornea. The eye 10 is made up of
three
concentric coverings that enclose the various transparent media through which
light must pass before reaching the light sensitive retina 14.
The outer-most covering is a fibrous protective portion that includes a
posterior layer which is white and opaque, called the sclera 16, which is
sometimes referred to as the white of the eye where it is visible from the
front.
The anterior 1/6th of this outer layer is the transparent cornea 12.
A middle covering is mainly vascular and nutritive in function and is made
up of the choroid 18, the ciliary 20 and the iris 22. The choroid generally
functions to maintain the retina. The ciliary muscle 21 is involved in
suspending
the lens 24 and accommodating the lens. The iris 22 is the most anterior
portion
of the middle covering of the eye and is arranged in a frontal plane. The iris
is a
thin circular disc corresponding to the diaphragm of a camera, and is
perforated
near its center by a circular aperture called the pupil 26. The size of the
pupil
varies to regulate the amount of light that reaches the retina 14. It
contracts also to
accommodate, which serves to sharpen the focus by diminishing spherical
aberrations. The iris 22 divides the space between the comes 12 and the lens
24
into an anterior chamber 28 and posterior chamber 30.
The inner-most covering is the retina 14, consisting of nerve elements
which form the true receptive portion for visual impressions that are
transmitted to
the brain. The vitreous 32 is a transparent gelatinous mass which fills the
7
CA 02508483 1999-12-22
posterior 4/Sths the globe 10. The vitreous supports the ciliary body 20 and
the
retina 14.
Referring to Fig. 2 of the drawings, the globe of an eye 10 is shown as
having a cornea 12 with a normal curvature represented by a solid line 34. For
people with normal vision, when parallel rays of light 3G pass through the
corneal
surface 34, they are refracted by the corneal surfaces to converge eventually
near
the retina 14 (Fig. 1 ). The diagram of Fig. 2 discounts, for the purposes of
this
discussion, the refractive effect of the lens or other portions of the eye.
However,
as depicted in Fig. 2, when the eye is hyperopic the rays of light 3G are
refracted
to converge at a point 38 behind the retina.
If the outer surface of the cornea 12 is caused to steepen, as shown by
dotted lines 40, such as through the implantation of a corneal implant of an
appropriate shape as discussed below, the rays of light 3G are refracted from
the
steeper surface at a greater angle as shown by dotted lines 42, causing the
light to
focus at a shorter distance, such as directly on the retina 14.
Fig. 3 shows a similar eye system to that of Fig. 2 except that the normal
corneal curvature causes the light rays 3G to focus at a point 44 in the
vitreous
which is short of the retinal surface. This is typical of a myopic eye. If the
cornea
is flattened as shown by dotted lines 4G through the use of a properly-shaped
corneal implant, light rays 36 will be refracted at a smaller angle and
converge at a
more distant point such as directly on the retina 14 as shown by dotted lines
48.
A hyperopic eye of the type shown in Fig. 2 can be corrected by
implanting an implant SO having a shape as shown in Figs. 4a, 4b. The implant
50
is in the shape of a meniscus lens with an outer surface 52 that has a radius
of
curvature that is smaller than the radius of curvature of the inner surface
54.
When a lens of this type is implanted using the method discussed below, it
will
cause the outer surface of the cornea to become steeper in shape as shown by
reference numeral 40 in Fig. 2, correcting the patient's vision so that light
entering
the eye will converge on the retina as shown by the dotted lines 42 in Fig. 2.
s
CA 02508483 1999-12-22
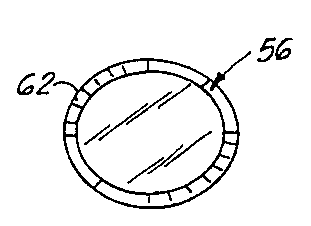
On the other hand, in order to cure myopia, an implant 56 having the shape
shown in Figs. Sa, Sb, can be used where an outer surface 58 is flatter or
formed
with a larger radius than that of the inner surface 60 which is formed with a
radius
of curvature substantially identical to that of the corneal stroma bed
generated by
the lamellar dissection described below. The implant 56 has a transition zone
62
formed between the outer and inner surfaces 58, 60, which is outside of the
optical
zone. In this way, the curvature of the outer surface of the cornea, as shown
in
Fig. 3, is flattened to an extent appropriate to achieve the proper refractive
correction desired so that light entering the eye will converge on the retina
as
shown in Fig. 3.
Alternatively, instead of using a solid implant as shown in Figs. Sa, Sb, for
correcting myopia, a ring 64 of the type shown in Figs. 6a, 6b could be used.
This
ring has substantially the same effect as the implant shown in Figs.Sa, Sb, by
flattening the outer surface of the cornea shown in Fig. 3. The ring 64 has a
center
opening 66 that is preferably larger than the optical zone so as not to cause
spherical aberrations in light entering the eye.
Implants of the type shown in Figs. 4, 5 and 6 can be implanted in the
cornea using a lamellar dissectomy shown schematically in Figs. 7a, 7b. In
this
procedure, a keratome (not shown) is used in a known way to cut a portion of
the
outer surface of the cornea 12 along dotted lines 68 as shown in Fig. 7a. This
type
of cut is used to form a corneal flap 70 shown in Fig. 7b, which remains
attached
to the cornea 12 through what is called a hinge 72. The hinge 72 is useful for
allowing the flap 70 to be replaced with the same orientation as before the
cut.
As is also known in the art, the flap is cut deeply enough to dissect the
Bowman's membrane portion of the cornea, such as in keratome surgery or for
subsequent removal of the tissue by laser or surgical removal. A corneal flap
of
100 to 200 microns, typically 160 to 180 microns, will be made to eliminate
the
Bowman's membrane tension. This reduces the possibility of extrusion of the
implants due to pressure generated within the cornea caused by the addition of
the
9
CA 02508483 1999-12-22
implant. Implants of the type shown in Figs. 4, S and 6 are shown implanted in
corneas in Figs. 8, 9 and 10, respectively, after the flap has been replaced
in its
normal position. These figures show the corrected shape for the outer surface
of
the cornea as a result of implants of the shapes described.
Implants can also be formed with a cylindrical addition in one axis of the
lens in order to correct for astigmatism, as shown in the implants in Figs. 11-
16.
Such implants can be oval or elliptical in shape, which the longer axis either
in the
direction of cylindrical power addition or perpendicular to it. For example,
the
implant can be circular as shown in Fig. 11 a where the implant 72 has axes
identified as x, y. In the case of a circular implant 72, the axes of the
implant have
different diopter powers as shown in Figs. 1 lb and 1 lbc, which are cross-
sectional
views of the implant 72 along the x and y axes, respectively. The different
thicknesses of the lenses in Figs. l lb and l lc illustrate the different
diopter
powers along these axes.
Alternatively, as shown in Fig. Sa, an astigmatic implant 74 can be oval or
elliptical in shape. The implant 74 also has axes x, y. As shown in the cross-
sectional views of the implant 74 in Figs. 12b, 12c, along those two axes,
respectively, the implant has different diopter powers as shown by the
different
thicknesses in the figures.
Because implants of the type identified by reference numeral 72, 74 are
relatively small and transparent, it is difficult for the surgeon to maintain
proper
orientation along the x and y axes. In order to assist the surgeon, tabs 76a,
76b or
indentations 78a, 78b are used to identify one or the other of the axis of the
implant to maintain proper alignment during implantation. This is shown in
Figs.
15, 16 where, for example, indentations 76a, 76b, are aligned with axis x
which
has been determined as the proper axis for alignment in order to effect the
astigmatic correction. Alternatively, other types of markers could be used
such as
visual indicators such as markings on or in the implants outside of the
optical
zone.
CA 02508483 1999-12-22
Referring to Figs. 17-21, implants with presbyopic corrections are shown.
In Fig. 17, an compound implant 80 is shown, which is appropriate for
hyperopic
correction, which has an additional power section 82 in the center. As shown,
the
implant 82 has anterior and posterior curvatures similar to those in Figs. 4a,
4b, in
order to correct for hyperopia. In Fig. 18, a central power add 84 is formed
on
another compound implant 86, which has a base shape similar to the one shown
in
Figs. Sa, Sb, and is appropriate for a myopic correction. In Fig. 19, a
central
power portion 88 is added to an simple planal implant 90 which has outer and
inner surfaces of equal radii, which does not add any correction other than
the
central power.
The central power add portions 82, 84, and 88 arc preferably within the
range of 1.5-3mm in diameter, most preferably 2111111, and which provide a
multi-
focal outer corneal surface where the central portion of the cornea achieves
an
added plus power for close-up work. In addition to the based device having no
correction, or corrections for hyperopia or myopia, the base device can have a
simple spherical correction for astigmatism as shown in Fig. 20a, where a
central
power add 92 is added to an implant 94 similar to the one shown in Fig.l 1 a,
which
also includes tabs 76a, 76b.
As shown in Fig. 20b in order to enhance the acuity of a presbyopic
implant, a transition zone 96 can be formed around the central power add 98
for
implant 100. This transition zone 96 is a sharp zone change in power from
central
added power to peripheral base power and is anchored over a radial distance
0.5 to
0.2 mm start to from the end of the central zone,
Implantation of the device shown in Fig. 20b, is illustrated in Figs. 21 a,
21b, where a flap 102 formed through a lamellar dissectomy is shown pulled
back
in Fig. 21 a so that the implant 100 can be positioned, and then replaced as
shown
in Fig. 21 b for the presbyopic correction. As shown, the formation of a sharp
transition 96 on the implant 100 provides a well defined central power after
implantation is complete.
11
CA 02508483 1999-12-22
The implants described above are preferably formed of a microporous
hydrogel material in order to provide for the eff cacious transmission of
nutrients
from the inner to the outer surface of the implants. The hydrogels also
preferably
have micropores in the form of irregular passageways, which are small enough
to
screen against tissue ingrowth, but large enough to allow for nutrients to be
transmitted. These microporous hydrogels are different from non-microporous
hydrogels because they allow fluid containing nutrients to be transmitted
between
the cells that make up the material, not from cell-to-cell such as in normal
hydrogel materials. Hydrogels of this type can be formed from at least one,
and
preferably more, hydrophillic monomer which is polymerized and cross-linked
with at least one mufti-or di-olefinic cross-linking agent.
An important aspect of the materials of the present invention is that the
microporous hydrogel have micropores in the hydrogel. Such micropores should
in general have a diameter ranging from SO Angstroms to 10 microns, more
particularly ranging from 50 Angstroms to 1 micron. A microporous hydrogel in
accordance with the present invention can be made from any of the following
methods.
Hydrogels can be synthesized as a zero gel by ultraviolet or thermal curing
of hydrophillic monomers and low levels of cross-linking agents such as
diacrylates and other UV or thermal initiators. These lightly cross-linked
hydrogels are then machined into appropriate physical dimensions and hydrated
in
water at elevated temperatures. Upon complete hydration, hydrogel prosthesis
are
flash-frozen to temperatures below negative 40°c, and then gradually
warmed to a
temperature of negative 20°c to negative 10°c and maintained at
the same
temperature for some time, typically 12 to 48 hours, in order to grow ice
crystals
to larger dimensions to generate the porous structure via expanding ice
crystals.
The frozen and annealed hydrogel is then quickly thawed to yield the
microporous
hydrogel device. Alternatively, the hydrated hydrogel device can be
lyophilized
and rehydrated to yield a microporous hydrogel.
12
CA 02508483 1999-12-22
Still further, the mieroporous hydrogel can also be made by starting with a
known formulation of monomers which can yield a desired cross-linked hydrogel,
dissolving in said monomer mixture a low molecular weight polymer as a filler
which is soluble in said mixture and then polymerizing the mixture. Resulted
S polymer is converted into the required device shape and then extracted with
an
appropriate solvent to extract out the filled polymer and the result in a
matrix
hydrated to yield a microporous device.
Still further and alternatively, microporous hydrogels can also be made by
any of the above methods with the modification of adding an adequate amount of
solvent or water to give a pre-swollen finished hydrogel, which can then be
purified by extraction. Such formulation can be directly cast molded in a
desired
configuration and do not require subsequent machining processes for
converting.
13