Note: Descriptions are shown in the official language in which they were submitted.
CA 02509257 2005-06-08
WO 2004/111319 PCT/US2003/039200
TITLE OF THE INVENTION
SACRIFICIAL TEMPLATE METHOD OF FABRICATING A NANOTUBE
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims priority from U.S. provisional application
serial
number 60/461,346 filed on April 8, 2003, incorporated herein by reference in
its entirety.
[0002] This application claims priority from U.S. provisional application
serial
number 60/454,038 filed on March 11, 2003, incorporated herein by reference
in its entirety.
[0003] This application claims priority from U.S. provisional application
serial
number 60/432,104 filed on December 9, 2002, incorporated herein by
reference in its entirety.
STATEMENT REGARDING FEDERALLY SPONSORED RESEARCH
OR DEVELOPMENT
[0004] This invention was made with Government support under Contract No.
DE-AC03-76SF00098, awarded by the Department of Energy and Grant No.
DMR-0092086, awarded by the National Science Foundation. The
2o Government has certain rights in this invention.
INCORPORATION-BY-REFERENCE OF MATERIAL
SUBMITTED ON A COMPACT DISC
[0005] Not Applicable
BACKGROUND OF THE INVENTION
1. Field of the Invention
[0006] This invention pertains generally to fabricating nanotubes, and more
particularly to a method of fabricating a nanotube over a sacrificial nanowire
3o template.
2. Description of Related Art
[0007] Since the discovery of carbon nanotubes (see lijima, S., Helical
-1-
CA 02509257 2005-06-08
WO 2004/111319 PCT/US2003/039200
microtubules of graphitic carbon, Nature, 354, 56 (1991 ), incorporated herein
by reference), there have been significant research efforts devoted to
nanoscale tubular forms of various solids (see Tenne, R. & Zettl, A. K.,
Nanotubes from inorganic materials, Top. Appl. Phys. 80, 81-112 (2001);
Tenne, R., Inorganic nanoclusters with fluorine-like structure and nanotubes,
Prig. Inure. Chem. 50, 269-315 (2001 ); Partake, G. R., Cromlech, F. & Nester,
R., Oxidic nanotubes and nanorods - Anisotropic modules for a future
nanotechnology, Angew. Chem. Int. Ed. 41, 2446-2461 (2002); Martin, G. R.,
Nanomaterials-a membrane-based synthetic approach, Science, 266, 1961-
~0 65 (1994); Ajayan, P. M. et al., Carbon nanotubes as removable templates
for
metal-oxide nanocomposites and nanostructures, Nature, 375, 564-566
(1996); Yang S. M, et al., Formation of hollow helicoids in mesoporous silica:
Supramolecuiar Origami, Adv. Mater. 11, 1427-30 (1999); Kondo, Y. &
Takanayagi, K., Synthesis and characterization of helical multi-shell gold
~5 nanowires, Science, 289, 606-608 (2000); Li Y. et al., Bismuth nanotubes,
J.
Am. Chem. Soc. 123, 9904-05 (2001 ); and Wu, Y. & Yang, P., Melting and
welding semiconductor nanowires in nanotubes, Adv. Mater. 13, 520-523
(2001 ), the above references being incorporated herein by reference).
(0008] The formation of tubular nanostructures generally requires a layered or
2o anisotropic crystal structure (see Tenne, R. & Zettl, A. K., Nanotubes from
inorganic materials, Top. Appl. Phys. 80, 81-112 (2001); Tenne, R., Inorganic
nanoclusters with fluorine-like structure and nanotubes, Prig. Inure. Chem.
50,
269-315 (2001 ); Partake, G. R., Cromlech, F. & Nester, R., Oxidic nanotubes
and nanorods - Anisotropic modules for a future nanotechnology, Angetnr.
25 Chem. Int. Ed. 41, 2446-2461 (2002), the preceding references incorporated
herein by reference).
(0009] There are reports of nanotube formation of solids lacking layered
crystal structures, such as silica, alumina, silicon and metals through
templating of carbon nanotubes and porous membranes or thin film rolling
so Schmidt, O. G. & Eberl, K., Thin solid films roll up into nanotubes,
Nature,
410, 168 (2001 ), incorporated herein by reference).
-2-
CA 02509257 2005-06-08
WO 2004/111319 PCT/US2003/039200
[0010] The nanotubes produced by the above methods, however, are either
amorphous, polycrystalline, or they exist only in ultra-high vacuum
environments.
[0011] The significance of hollow inorganic nanotubes is being recognized and
they have wide applicability in bioanalysis and catalysis (see Lee, S. B.;
Mitcell, D. T.; Trofin, L.; Nevanen, T. K.; Soderlund, H.; Martin, C. R.
Science
2002, 296, 2198, incorporated herein by reference). Among these hollow
nanotubes silica nanotubes are of special interest because of their
hydrophilic
nature, colloidal suspension formation, and surface functionalization
accessibility for both inner and outer walls. These modified silica nanotubes
and nanotube membranes for example have applicability for bioseparation
and biocatalysis (see Mitchell, D. T.; Lee, S. B.; Trofin, L.; Li, N. C.;
Nevanen,
T. K.; Soderlund, H.; Martin, C. R. J. Am. Chem. Soc. 2002, 124, 11864,
incorporated herein by reference).
15 [0012] Recently, bright visible photoluminescence from sol-gel template
synthesized silica nanotubes was observed by Zhang et al. (see Zhang, M.;
Ciocan, E.; Bando, Y.; Wada, K.; Cheng, L. L.; Pirouz, P. Appl. Phys. Lett.
2002, 80, 491; incorporated herein by reference). In addition, the study of
the
physical and chemical nature of molecules or ions confined within the
2o inorganic nanotubes is of great current interest.
[0013] Silica nanotubes have been synthesized typically within the pores of
porous alumina membrane templates using the sol-gel coating technique (see
Martin, C. R. Chem. Mater. 1996, 8, 1739, incorporated herein by reference).
Alumina templates can be dissolved to liberate single silica nanotubes.
2s These nanotubes prepared at low temperature have porous walls and are
relatively fragile. Once the templates are removed, the silica nanotubes will
generally bundle up and become less oriented. The same applies to the silica
nanotubes prepared at low temperature using other templates (see Obare, S.
O.; Jana, N. R.; Murphy, C. J. Nano Lett. 2001, 1, 601; Jung, J. H.; Shinkai,
3o S.; Shimizu, T. Nano Lett. 2002, 2, 17; Yin, Y. D.; Lu, Y.; Sun, Y. G.;
Xia, Y. N.
Nano Lett. 2002, 2, 427, incorporated herein by reference).
[0014] Accordingly, the growth of single-crystalline semiconductor nanotubes
-3-
CA 02509257 2005-06-08
WO 2004/111319 PCT/US2003/039200
provides a number of advantages for nanoscale electronics, optoelectronics,
and biochemical sensing applications. The present invention satisfies those
needs, as well as others, and overcomes the deficiencies of previously
developed nanoscale growth methods.
BRIEF SUMMARY OF THE INVENTION
[0015] The present invention comprises single and multiple layer nanotube
structures and methods of fabrication. In the case of multilayered nanotube
structures, the interface between cylindrical layers of the nanotubes in the
structure may form insulated or non-insulated device junctions, or provide
other material properties. In addition, longitudinal portions (segments) of
the
nanotube may be processed differently to yield longitudinal junctions along a
given nanotube.
[0016] A key aspect of the present invention is that the nanotubes and
composite structures are formed over a sacrificial template which is
preferably
a nanowire core. The general fabrication process of the present invention
involves creating a core (nanotube template), over which a sheath is formed.
Numerous methods may be utilized for creating both the core and forming one
or more sheaths. It will be appreciated that the core and sheath sections may
be formed from a variety of materials.
20 [0017] The core, for example, may be selected from material comprising ZnO,
Si, GaN, Ge, Ag, group II - VI materials, group I I I - V materials, elemental
group IV materials (i.e. Si, Ge), and metals. The groupings are considered to
describe material groups as shown on a periodic table of the elements.
[0018] While the sheath, for example, may be selected from the materials
25 consisting of gallium nitride (GaN), silicon oxide (Si02), group II - VI
materials,
group III - V materials, elemental group IV (i.e. Si, Ge), metals, oxides of
the
above materials, and polymers. It should also be appreciated that the sheath
material may be doped as desired (i.e. during formation) to alter the
characteristics of the base material.
so [0019] It will be appreciated, therefore, that the invention generally
comprises
a method for fabricating a nanotube, comprising (a) forming a nanowire
template; (b) depositing a sheath over the nanowire template; and (c)
-4-
CA 02509257 2005-06-08
WO 2004/111319 PCT/US2003/039200
removing the nanowire template. Two embodiments of the method will now
be described, one for forming GaN nanotubes over a zinc oxide nanowire
template, and one for Si02 nanotubes over a Si nanowire template. In
general, nanotubes according to the present invention may be formed utilizing
a casting process, an etching process, or combinations thereof. By way of
example, an epitaxial casting process will be described first for producing
GaN
nanotubes. Then, an oxidation and etching process, such as for producing
silicon oxide (Si02) nanotubes, will be described.
[0020] In one embodiment of a nanotube fabrication process according to the
o present invention, an "epitaxial casting" approach is utilized for the
synthesis
of single-crystalline nanotubes, such as technologically important gallium
(III)
nitride (GaN) nanotubes with inner diameters of approximately 30 nm to 200
nm and wall thicknesses of approximately 5 nm to 50 nm. Nanowires, such
as within a nanowire array, were used as templates for the epitaxial
~5 overgrowth of thin GaN layers in a chemical vapor deposition system. By way
of example the nanowire fiemplates can be fabricated from hexagonal zinc (II)
oxide (Zn0) material over which the GaN nanotube is grown. The template
material is subsequently removed, preferably by a simple thermal reduction
and evaporation step, resulting in ordered arrays of GaN nanotubes on the
2o substrates. Arrays of the Zn0 nanowires were grown on substrates, such as
sapphire wafers, using a vapor deposition process. The same approach to
synthesizing nanotubes detailed herein may operate for the majority of group
III nitrides.
[0021] In another embodiment, nanotubes are formed of silicon oxide (Si02) in
25 an oxidation process and the nanowire cores removed in an etching process.
The nanotube cores (templates) are created from silicon (Si) nanowires, with
a cap (i.e. Au), such as fabricated using thermal oxidation and etching. The
process comprises thermal oxidation of the Si nanowire arrays which results in
arrays of thin Si nanowires sheathed by a thick layer of silicon oxide (Si02).
3o This oxidized nanowire array is then selectively etched, such as with xenon
fluorine (XeF2) to remove the silicon nanowire cores, leaving an array of
ordered silicon dioxide nanotubes with controllable inner diameters. The inner
-5-
CA 02509257 2005-06-08
WO 2004/111319 PCT/US2003/039200
diameters are controlled by the initial diameters of the silicon nanowires and
the thermal oxidation process. The inner tube diameter of the nanotubes may
be controlled in the range of from approximately 10 nm to 200 nm. It is
contemplated that, with further refinements of the oxidation and etching
process, nanotubes having an inside diameter of less than 5 nm can be
produced in this manner.
(0022] A number of aspects of the invention are addressed herein, including
but not limited to the following.
[0023] An aspect of the invention is the formation of nanotube structures.
[0024] An aspect of the invention is the formation of single-crystalline
nanotube structures.
[0025] Another aspect of the invention is forming nanotubes of gallium nitride
(GaN).
[0026] Another aspect of the invention is forming silica nanotubes (Si02).
[0027] Another aspect of the invention is forming a nanowire to utilize as a
template for forming the nanotube.
[0028] Another aspect of the invention is utilizing a zinc oxide (Zn0)
nanowire
as a template for forming the nanotube.
[0029] Another aspect of the invention is utilizing a silicon (Si) nanowire as
a
2o template for forming the nanotube.
[0030] Another aspect of the invention is utilizing an epitaxial casting
process
to form a sheath of over the nanowire, such as GaN over a Zn0 nanowire.
[0031] Another aspect of the invention is utilizing an oxidation and etching
process to form a sheath of material over the nanowire, such as Si02 over a
Si nanowire.
[0032] Another aspect of the invention is the formation of multiple sheath
layers over a sacrificial template (core).
[0033] Another aspect of the invention is the formation of multiple sheath
layers over a sacrificial core.
so [0034] Another aspect of the invention is the formation of sheath layers in
longitudinal segments along the length of a sacrificial core.
[0035] A still further aspect of the invention are methods for forming single-
-6-
CA 02509257 2005-06-08
WO 2004/111319 PCT/US2003/039200
crystalline nanotubes which may be utilized in electronic devices, nanofluidic
devices, or combinations thereof.
[0036] Further aspects of the invention will be brought out in the following
portions of the specification, wherein the detailed description is for the
purpose of fully disclosing preferred embodiments of the invention without
placing limitations thereon.
BRIEF DESCRIPTION OF THE SEVERAL VIEWS OF THE DRAWINGS)
[0037] The invention will be more fully understood by reference to the
following drawings which are for illustrative purposes only:
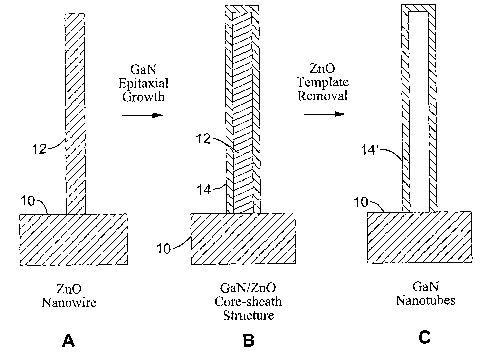
[0038] FIG. 1A-1C are cross section views of the epitaxial casting process for
fabricating nanotubes according to an embodiment of the present invention,
showing GaN nanotubes formed over ZnO nanowires.
[0039] FIG. 2A is an image of a nanowire template array according to an
aspect of the present invention fabricated from ZnO, showing in an inset the
cross-sections of the nanowire array.
[0040] FIG. 2B is an image of a nanotube array formed over the sacrificial
nanowire array of FIG. 2A according to an aspect of the present invention
fabricated from GaN, shown in an inset is the fractured interface between the
GaN nanotubes and the substrates.
[0041] FIG. 3 is a plot of diffraction of the GaN nanotube array of FIG. 2B
according to an aspect of the present invention, showing nanotube
composition.
[0042] FIG. 4A-4C are images of the nanotubes of FIG. 2B according to an
aspect of the present invention, showing the relative uniformity in diameter
and wall thickness.
[0043] FIG. 4D is a high resolution image of the exterior wall structure in a
GaN nanotube of FIG. 2B according to an aspect of the present invention.
[0044] FIG. 4E is a high resolution image of the interior wall structure in a
GaN
nanotube of FIG. 2B according to an aspect of the present invention, shown
3o with inset of electron diffraction pattern taken on the nanotube along the
[110]
zone axis.
-7-
CA 02509257 2005-06-08
WO 2004/111319 PCT/US2003/039200
[0045] FIG. 5 is a plot of nanotube composition across the nanotube profile
according to an aspect of the present invention, as probed by energy
dispersive X-ray spectroscopy.
[0046] FIG. 6 is an image of nanotubes formed according to an embodiment
of the present invention and shown end-on.
[0047] FIG. 7 is an image of a single-crystalline GaN nanotube fabricated
according to an embodiment of the present invention and showing its smooth
features.
[0048] FIG. 8 is a plot of the electron energy loss spectrum collected on the
GaN nanotube of FIG. 7.
[0049] FIG. 9A is an image of an array of nanotubes fabricated according to
an embodiment of the present invention, and shown with the nanowire
template partially removed.
[0050] FIG. 9B is an image of a nanotube fabricated according to an
embodiment of the present invention with nanowire template partially
removed, showing insets of electron diffraction patterns recorded on the core-
sheath and the pure tube region along the [110] zone axis.
[0051] FIG. 10 is a plot of line profiles for the core-sheath of a nanotube at
the
upper arrow position in FIG. 9B, and showing Ga and Zn signals.
20 [0052] FIG. 11 is a plot of line profiles for the core-sheath of a nanotube
at the
lower arrow position in FIG. 9B, and showing Ga and Zn signals.
[0053] FIG. 12 is a plot of photoluminescence spectra collected on a GaN
nanotube according to an aspect of the present invention, showing spectra
from both a thin-walled and thick-walled nanotube.
25 [0054] FIG. 13 is a plot of temperature dependence curves of a single GaN
nanotube according to an aspect of the present invention.
[0055] FIG. 14A-14G are steps in forming Si02 nanotubes according to an
embodiment of the present invention, shown with parylene deposition stages
during etching.
30 [0056] FIG. 15A-15D are images of silicon nanotube array formation
according
to aspects of the present invention, shown including detail views on insets of
_$_
CA 02509257 2005-06-08
WO 2004/111319 PCT/US2003/039200
FIG. 15B-15D.
[0057] FIG. 16A-16B are images of silica nanotubes according to aspects of
the present invention.
[0058] FIG. 17 is a cross-section of a multilayer nanotube according to an
aspect of the present invention and shown with a gallium nitride sheath
sandwiched between insulating aluminum nitride layers.
[0059] FIG. 18 is a cross-section of a multilayer nanotube according to an
aspect of the present invention and shown with a P-doped sheath over an N-
doped sheath which surrounds the sacrificial core.
[0060] FIG. 19 is a cross-section of a multilayer nanotube according to an
aspect of the present invention and shown with an N-doped sheath over a P-
doped sheath which surrounds the sacrificial core.
[0061] FIG. 20 is a perspective view of a sacrificial core covered with a
solid
sheath and having two longitudinal nanotube segments according to an
aspect of the present invention.
[0062] FIG. 21 is a perspective view of a sacrificial core covered with
multiple
sheaths and having multiple longitudinal nanotube segments according to an
aspect of the present invention.
[0063] FIG. 22 is a cross-section of fabricating a nanotubular device
according
2o to an aspect of the present invention, shown comprising a hollow core NPN
transistor.
DETAILED DESCRIPTION OF THE INVENTION
[0064] In accordance with the present invention, a nanotube is formed by
creating at least one sheath layer around a nanowire template. The nanowire
2s template functions as a sacrificial core which is later removed to
establish the
central opening through the nanotube. Once the sacrificial core is removed,
the nanotube can be used in any conventional manner.
[0065] By way of example, and not of limitation, two embodiments of a method
of fabricating nanotubes using a sacrificial core in accordance with the
present
3o invention will be described. It will be appreciated, however, that the
invention
contemplates any method in which a sacrificial core is used as a template for
nanotube fabrication. In a first embodiment, a layer of material such as
_g_
CA 02509257 2005-06-08
WO 2004/111319 PCT/US2003/039200
gallium nitride (GaN) is epitaxially grown on the exterior of a nanowire core,
such as zinc oxide (Zn0), followed by removal of the nanowire core. In a
second embodiment, a nanowire core such as silicon (Si) is oxidized to form
an Si02 sheath layer, and then the nanowire core is removed to leave the
oxide sheath.
[0066] Epitaxial Casting Method
[0067] FIG. 1A through FIG. 1 C illustrate the general steps in what we refer
to
as an "epitaxial casting" approach. FIG. 1A depicts a substrate 10 upon which
a nanowire 12 is being formed, preferably a single-crystalline nanowire. FIG.
1 B depicts depositing a preferably single-crystalline sheath 14 over nanowire
12. FIG. 1 C depicts removing the nanowire template (core) 12 thereby
forming a nanotube 14'.
[0068] In one embodiment, the nanowires 12, such as pre-fabricated
hexagonal-shaped single-crystalline nanowires (preferably Zn0) are employed
as templates for tubular deposition of a material, such as GaN. Since Zn0
and GaN both have wurtzite crystal structures and have similar lattice
constants (ZnO: a=3.249 A, c=5.207 A; GaN: a=3.189 A, c= 5.185 ~), GaN
can grow epitaxially on the side {110) planes of these ZnO nanocylinders and
form a thin GaN layer that is single-crystalline in nature. It will be
appreciated
2o that many combinations of materials have sufficiently similar crystalline
structures and lattice constants to allow epitaxial growth of the sheath
material
on the nanowire material.
[0069] Once the Zn0 nanocylinders are coated with a thin GaN sheath 14
(FIG. 1B), the template 12 (FIG. 1A) is subsequently removed, such as by
2s thermal processes, leaving a GaN nanotube 14'. By way of example and not
of limitation, two possible mechanisms for the removal of Zn0 templates can
be employed.
[0070] In one approach, Zn0 is chemically etched by ammonia (NH3) at high
temperature (see Hamdani F. et al., Effect of buffer layer and substrate
so surface polarity on the growth by molecular beam epitaxy of GaN on ZnO,
Appl. Phys. Lett. 71, 3111-13 (1997), incorporated herein by reference).
Prolonged heating of samples after GaN coating in ammonia (NH3) readily
-10-
CA 02509257 2005-06-08
WO 2004/111319 PCT/US2003/039200
yields pure GaN nanotubes (FIG. 1 C).
[0071] Another approach is to utilize a thermal reduction process at high
temperatures (e.g. 600 °C in hydrogen gas, H2). The single-crystalline
wurtzite GaN nanotubes here differ fundamentally from theoretically simulated
GaN nanotubes, where a metastable graphitic GaN structure was proposed
(see Lee S. M., Stability and electronic structure of GaN nanotubes from
density-functional calculations, et al. Phys. Rev. B, 60, 7788-7791 (1999),
incorporated herein by reference).
[0072] Example 1
[0073] The nanowire cores employed in the present invention can be formed
in any conventional manner. For example, arrays of zinc oxide (Zn0)
nanowires were grown on a substrate material, such as (110) sapphire wafers,
preferably using a vapor deposition process (see Huang M. et al., Room-
temperature ultraviolet nanowire nanolasers, Science, 292, 1897-99 (2001 ),
incorporated herein by reference). These Zn0 nanowire arrays were placed
inside a reaction tube (i.e. MOCVD reaction tube) for GaN chemical vapor
deposition. Trimethylgallium and ammonia were used as precursors and fed
into the system with argon or nitrogen carrier gas. The deposition
temperature was set at 600 °C to 700 °C.
[0074] After the GaN deposition, the samples were treated in a hydrogen
atmosphere at elevated temperature, such as 600 °C with 10% H2 in
argon,
for removing the Zn0 nanowire templates. It should be appreciated that other
methods and materials may be utilized (although in some instances less
preferably) for forming the nanowires, covering the nanowires with the
nanotube material, and for sacrificially removing the nanowire material (in
select applications only a portion of the nanowire material need be removed
according to the present invention).
[0075] FIG. 2A shows a scanning electron microscopy (SEM) image of the
starting Zn0 nanowire array templates, which were found to have uniform
so lengths, such as in the range of from 2-5 pm, and each having a uniform
diameter with diameters within the array of nanowires ranging from 30-200
nm. The nanowires are well-facetted as seen in the inset of FIG. 2A with
-11-
CA 02509257 2005-06-08
WO 2004/111319 PCT/US2003/039200
hexagonal cross-sections, exhibiting {110} planes on the sides. After the GaN
deposition and template removal to form the nanotubes, the color of the
sample had shifted from white to yellowish or darker.
[0076] FIG. 2B is an example image illustrating that the morphology of the
initial nanowire arrays was maintained in the nanotubes, except for the
increase in the diameters of the resulting nanostructures. The nanostructures
appear less facetted compared with the original Zn0 nanowire templates.
Compositional analysis on the final product shows only a relatively minor Zn
signal.
[0077] FIG. 3 illustrates the result of X-ray diffraction (XRD) on the sample
which shows only (001) diffraction peaks of the wurtzite GaN structure
indicative of excellent epitaxy/texturing for the GaN coating.
[0078] FIG. 4A through FIG. 4C depict images of dispersing the GaN
nanotubes sample in FIG. 2B onto a transmission electron microscopy (TEM)
grid for further structural analysis. It was found that the majority of the
nanostructures exhibit tubular structures with uniform wall thicknesses, which
can be generally seen from FIG. 4A. These nanotubes were found to have
inner diameters ranging from 30 nm to 200 nm, similar to the Zn0 nanowire
arrays, and wall thicknesses between 5 nm to 50 nm.
[0079] It was found that the majority of the nanotubes have only one end
open, however, tubes with both ends open were also observed. These
observations are consistent with the SEM studies, where round-shaped and
less-facetted ends are observed after the GaN coating, as depicted in FIG.
2B. It was concluded that the open nanotube ends are originally located at
the GaN and substrate interface, which were fractured open during TEM
sample preparation. Indeed we have frequently observed these open ends on
the substrate surface together with the corresponding nanotubes, an example
of which is shown in the inset of FIG. 2B. TEM studies also indicate that the
inner cross-section of the nanotubes remains pseudo-hexagonal after
so template removal.
[0080] Significantly, electron diffraction (ED) taken on these GaN nanotubes
indicates that these tubes are single-crystalline. Returning to FIG. 4E, the
-12-
CA 02509257 2005-06-08
WO 2004/111319 PCT/US2003/039200
inset shows one ED pattern taken along the [110] zone axis. It can be readily
seen that the nanotube is oriented along the c-axis of the wurtzite GaN
structure. This is consistent with the XRD data where only (001 ) peaks were
observed. Along the tube axis, a lattice spacing of 0.51 nm for (001 ) planes
of
s the wurtzite structure can be readily resolved on high resolution TEM images
of both the tube surface FIG. 4D and the inside of the tubes FIG. 4E.
[0081] FIG. 5 illustrates compositional line profile probed by energy
dispersive
X-ray spectroscopy (EDX) showing well-correlated gallium and nitrogen
signals across the tube walls which are indicators of stoichiometric GaN
1o formation during the deposition. This is also clearly reflected in the
electron
energy loss spectra (EELS) recorded on these nanotubes, as shown in FIG. 8,
where strong nitrogen signals were observed. It should be noted that the
interfacial diffusion between the GaN layer and the Zn0 nanowire templates
result in a small amount of Zn or O incorporation within the GaN tube wall.
15 [0082] FIG. 6 is a transmission electron microscopy image of an end-on view
of several GaN nanotubes. At least two important features can be seen in the
image: (1 ) the inner cross-section of the tubes is pseudo-hexagonal, (2)
nanotubes are connected at their base with a porous GaN layer, which is
believed to be the primary pathway for the escape of zinc and oxygen species
2o during thermallchemical etching.
[0083] FIG. 7 is a transmission electron microscopy image of a single-
crystalline GaN nanotube showing its very smooth internal and external
surface.
[0084] FIG. 8 is a plot of nitrogen K-edge electron energy loss spectrum
25 collected on the GaN nanotube of FIG. 7.
[0085] Taken together, it will be appreciated that high-density arrays of
single-
crystalline nanotubes can be successfully prepared, such as described for
GaN nanotubes fabricated on sapphire substrates. It is important to point out
that the GaN nanotube formation process described herein is a marked
so departure from previous work on inorganic nanotubes (see lijima, S.,
Helical
microtubules of graphitic carbon, Nature, 354, 56 (1991 ); Tenne, R. & Zettl,
A.
-13-
CA 02509257 2005-06-08
WO 2004/111319 PCT/US2003/039200
K., Nanotubes from inorganic materials, Top. Appl. Phys. 80, 81-112 (2001 );
Tenne, R., Inorganic nanoclusters with fluorine-like structure and nanotubes,
Prig. Inure. Chem. 50, 269-315 (2001 ); Partake, G. R., Cromlech, F. & Nester,
R., Oxidic nanotubes and nanorods - Anisotropic modules for a future
s nanotechnology, Angevv. Chem. Int. Ed. 41, 2446-2461 (2002); Martin, C. R.,
Nanomaterials-a membrane-based synthetic approach, Science, 266, 1961-
65 (1994); Ajayan, P. M. et al., Carbon nanotubes as removable templates for
metal-oxide nanocomposites and nanostructures , Nature, 375, 564-566
(1996), these being incorporated herein by reference).
[0086] Previous studies on inorganic nanotubes have been directed toward
materials with layered structures (e.g. VOX, MoS2, NiCI~, BN). For those
studies on materials that do not have structural anisotropy, (in porous
alumina) templating approaches (see Caruso, R. A & Antonietti, M. Sol-Gel
nanocoating: an approach to the preparation of structured materials, Chem.
Mater. 13, 3272-3282 (2001 ), incorporated herein by reference) are generally
used, which result in predominantly amorphous or polycrystalline tubes. The
distinction between amorphous or polycrystalline tubes and the beneficial
single crystal tubes shown as being preferably fabricated according to the
present invention will be readily recognized by one of ordinary skill in the
art.
[0087] FIG. 9A, 9B and FIG. 10, FIG. 11 illustrate details of removing the
nanowire template within the single-crystalline nanotube. The "epitaxial
casting" mechanism described by the invention has been confirmed with TEM
studies. In FIG. 9A arrays of GaN nanotubes are shown with their Zn0
nanowire templates partially removed. It should be noted that at the bottom of
these nanotubes a thin layer of porous GaN film exists. In addition, residues
of Zn0 nanowire templates remain in the upper portion of the sealed GaN
nanotubes. These two observations suggest that the zinc and oxygen species
(generated during the thermal chemical etching process) escape from the
GaN nanotubes primarily through the underneath porous GaN layer (as shown
so in FIG. 6).
[0088] In FIG. 9B a detailed view of a nanotube with a partially removed
-14-
CA 02509257 2005-06-08
WO 2004/111319 PCT/US2003/039200
template is shown at the boundary between the filled (upper arrow) and empty
portions (lower arrow) of the nanotube. Electron diffraction shown on the
insets of FIG. 9B for the filled and unfilled portions of the nanotube depict
an
identical set of diffraction patterns for both the tube and the core-sheath
region, indicating the wurtzite GaN growth is epitaxial.
[0089] The core-sheath nanostructure can be considered as a seamless
single domain of a wurtzite GaN/Zn0 structure type. Furthermore,
comparison of EDX line profiles across the GaN nanotube (aligned at lower
arrow) shown in FIG. 11 and the ZnO-GaN core-sheath structure, aligned at
1o the upper arrow, and shown in FIG. 10 unambiguously support the growth
mechanism of GaN nanotubes on the Zn0 nanowire templates. Once the
Zn0 nanocylinder is removed, single-crystalline tubes of GaN result. The
formation of these single-crystalline GaN nanotubes as taught herein accords
a number of benefits over the use of polycrystalline nanotubes (see Li, J. Y.
et
al. Synthesis of GaN nanotubes, J. Mater. Sci. Lett. 20, 1987-1988 (2001 ),
incorporated herein by reference), in particular in view of the fact that
these
polycrystalline nanotubes are generally subject to having an irregular shape.
It is also interesting to note that microscale tubes of Zn0 have been prepared
in solution through a preferential chemical dissolution process (see
2o Vayssieres, L., Keis, K., Hagfeldt, A. & Lindquist, S. Three-dimensional
array
of highly oriented crystalline Zn0 microtubes, Chem. Mater. 13, 4395-4398
(2007 ), incorporated herein by reference).
(0090] Importantly, the electrical and optical characteristics of these single-
crystalline GaN nanotubes are comparable to those of high-quality GaN
epilayers grown on Zn0 substrates (see Hamdani F. et al., Microstructure and
optical properties of epitaxial GaN on Zn0 (0001 ) grown by reactive molecular
beam epitaxy, J. Appl. Phys. 83, 983-990 (1998), incorporated herein by
reference) as well as those of GaN nanowires (see Huang, Y., Duan, X., Cui,
Y. & Lieber, C. M. Gallium nitride nanowire nanodevices, Nano. Letfi. 2, 101-
so 104 (2002); Kim, J. et al. Electrical transport properties of individual
gallium
nitride nanowires synthesized by chemical vapor deposition, Appi. Phys. Left.
-15-
CA 02509257 2005-06-08
WO 2004/111319 PCT/US2003/039200
80, 3548-3550 (2002), incorporated herein by reference).
[0091] FIG. 12 depicts a low temperature photoluminescence (PI-) spectra plot
of the as produced nanotubes measured using fourth harmonic output of a
YAG laser (266 nm) as an excitation source. If should be noted that no
midgap yellow emission was observed. The band edge emission was
observed in these nanotube samples between 375 nm and 360 nm, with the
thinner tubes emitting at shorter wavelengths. This slight blue shift of the
emission (see Hamdani F. et al., Microstructure and optical properties of
epitaxial GaN on Zn0 (0001 ) grown by reactive molecular beam epitaxy, J.
1o Appl. Phys. 83, 983-990 (1998), incorporated herein by reference) could be
attributed to the quantum confinement effect since some of the nanotubes
have walls as thin as 5 nm, which is smaller than the exciton Bohr radius of
GaN.
[0092] Referring to the figure, photoluminescence spectra was collected on
15 the GaN nanotubes at 10 K. The samples were excited by 266 nm line of a
pulsed Nd:YAG laser (i.e. Spectra PhysicsT"~). The photoluminescence signal
was transmitted to a 0.3 meter imaging monochromator by an optical fiber,
detected by an intensified CCD working under gate mode. Only band edge
emission was observed, with the spectra depicted on the left corresponding to
2o the spectra collected on thin-walled (< 10nm) GaN nanotubes, while the
spectra depicted on the right corresponds to the spectra collected from thick-
walled (> 10nm) GaN nanotubes, respectively. It should be appreciated that
the emission spectra for the thin tubes is relatively broad due to the broad
distribution of tube wall thicknesses for the tested sample.
25 [0093] FIG. 13 depicts an example of electron transport measurements which
indicate the resistances of these nanotubes are on the order of 10 MSZ at
room temperature and increases with decreasing temperature, similar to those
of high quality GaN nanowires. Referring to the figure, temperature
dependence I-V curves of a single GaN nanotube are shown. The electrodes
30 (20 nm titanium, Ti and 80 nm gold, Au) for the electrical measurements
were
fabricated using e-beam lithography and thermal evaporation, although other
techniques may be utilized. To form a stable contact, a rapid thermal
-16-
CA 02509257 2005-06-08
WO 2004/111319 PCT/US2003/039200
annealing step was perFormed at 450 °C for about thirty seconds,
although
any convenient means of contact formation may be utilized.
[0094] The successful preparation of single-crystalline GaN nanocapillaries
utilizing the present epitaxial casting process is indicative of the ability
to
prepare nanotubes/nanocapillaries, in particular single-crystalline
nanotubes/nanocapillaries, of inorganic solids having non-layered crystal
structures. It is anticipated that this new class of semiconductor
nanotubes/nanocapillaries can be utilized in a number of beneficial technical
applications in the fields of nanoscale electronics, optoelectronics, and
1o chemistry in addition to use with fluidic systems. The present invention
provides robust semiconductor nanotubes, having uniform inner diameter, and
inner walls that can be readily functionalized, while both ends of the
nanotubes can be made accessible for fluid flow applications.
[0095] Oxidation and Etching-Method
1s [0096] Referring now to FIG. 14A through FIG. 14G, a second method of
fabricating nanotubes using a sacrificial template according to the present
invention is illustrated. We refer to this method as "oxidation and etching"
since this method forms robust nanotube arrays by translating vertical
nanowire arrays into oxide nanotube arrays. In one embodiment, nanotube
2o cores (templates) are formed from silicon (Si) nanowires, with a metal cap
(i.e.
Au), such as commonly fabricated using thermal oxidation and etching. Next,
the Si nanowire arrays are thermally oxidized which results in arrays of thin
Si
nanowires sheathed by a thick layer of silicon oxide (Si02). This oxidized
nanowire array is then selectively etched, such as with xenon fluorine (XeF2)
25 to remove the silicon nanowire cores, leaving an array of ordered silicon
dioxide nanotubes with controllable inner diameters. The inner diameters are
controlled by the initial diameters of the silicon nanowires and the thermal
oxidation process. The inner tube diameter of the nanotubes may be in the
range of from approximately 10 nm to 200 nm.
so [0097] It should be appreciated that single nanotubes or random samples can
be formed as an alternative to forming the nanotubes in an array. Other
nanotube compositions can also be fabricated in this manner as well,
-17-
CA 02509257 2005-06-08
WO 2004/111319 PCT/US2003/039200
including, but not limited to, GaO, In0 and other oxides and insulating
materials. The following describes implementation details of an embodiment
of the present fabrication process.
[0098] Example 2
s [0099] FIG. 14A illustrates silicon nanowire arrays which were prepared
using
chemical vapor deposition (CVD) epitaxial growth employing silicon
tetrachloride (SiCl4, Aldrich, 99.99%) as the silicon source. Hydrogen (10%
balanced by argon) was used to reduce SiCl4 at high temperature (900-950
°C). Gold (Au) thin film was coated on Si (111 ) substrates 30 to
initiate the
1o growth of silicon nanowires 32 via the vapor-liquid-solid growth mechanism.
The gold remains as caps 34 on the Si nanowires. This approach to growing
Si nanowires was developed and is utilized in our lab for the synthesis of
vertical Si/SiGe superlattice nanowire arrays (see Wu, Y.; Fan, R.; Yang, P.
D.
Nano Lett. 2002, 2, 83; Wu, Y.; Yan, H.; Huang, M.; Messer, B.; Song, J.;
15 Yang, P. Chem.-Eur. J. 2002, 8, 1260; incorporated herein by reference).
The
silicon nanowire array samples were heated, such as loaded into a tube
furnace and heated at 800 - 1000 °C for one hour under the continuous
flow
of pure oxygen (02).
[00100] FIG. 14B depicts the nanowires 32 after being uniformly oxidized to
2o provide Si02 sheaths 36 with continuous silicon cores inside. During
oxidation, the nanowire tips 34 are preferably oxidized to provide an oxide
cap
34' on each verfiical wire for preventing the selective etching of silicon
cores.
Therefore, the first step after thermal oxidation is to selectively remove the
Si02 caps 34' from the Si/Si02 core-sheath nanowires.
25 [00101] FIG. 14C illustrates a preferred mode of removing the Si02 caps. A
polymer 38 is deposited to fill in the space between the nanowires such that
the Si02 sidewall 36 is protected by the matrix polymer as an etch-resistant
material. In the present example, parylene dimer (di-para-xylylene, fCH2-Ph-
CH2~2 ) was thermally evaporated at 160 °C, dissociated at about 650
°C and
so deposited onto the Si/Si02 core-sheath nanowire array sample for
approximately five (5) hours to yield a continuous coating of parylene (poly-
para-xylylene, fCH2-Ph-CH2~n ) polymer. This parylene deposition is
-18-
CA 02509257 2005-06-08
WO 2004/111319 PCT/US2003/039200
conformal, starting from thin layer coating on the surface of nanowires and
then filling all the interval space between nanowires. This process leads to a
highly conformal wrapping of the nanowires without pinholes or cracks.
[00102] FIG. 14D illustrates the core-sheath array subsequent to oxygen
plasma etching of the surface of the polymer fill 38, such as the parylene in
order to expose the tips of the Si/Si02 nanowires.
[00103] FIG. 14E depicts the core-sheath array after immersion in a buffered
hydrofluoric acid solution for about two (2) minutes to selectively remove the
Si02 caps 34' and expose the silicon cores 32.
[00104] FIG. 14F illustrates the sheath array after the silicon nanowire cores
32
were removed by an etchant, such as XeF2 etchant gas. It will be noted that
although some material has been removed, a layer of etch-resistant material
38' still protects the bulk of the nanotube walls. Etching is preferably
performed by loading the core-sheath array into a XeF2 etching chamber, with
a chamber temperature for example being adjusted to 40 °C. After
purging
and flushing with nitrogen, the XeF2 vapor was introduced together with
nitrogen gas, N2 (XeF2:N2 = 4:5) to conduct etching for thirty (30) seconds at
total pressure of about nine Torr. The chamber was then evacuated and
flushed with nitrogen and etching carried out for a second cycle. In the
2o present embodiment eight cycles were carried out to achieve complete
etching of the silicon cores.
[00105] According to the above process silica nanotube arrays were obtained
which are embedded in the parylene membrane 38, wherein the continuous
pores run through the entire polymer film.
2s [00106] FIG. 14G depicts a resulting nanotube array 36' after the parylene
matrix was etched away, such as using high-power oxygen plasma treatment
for thirty (30) minutes to yield a vertically oriented, robust silica nanotube
matrix attached to substrate 30.
[00107] Example 3
30 [00108] FIG. 15A-15D are images of nanotube formation according to the
invention, registered as scanning electron micrographs (SEM). A silicon
nanowire array is shown in FIG. 15A, with the Si nanowires vertically
-19-
CA 02509257 2005-06-08
WO 2004/111319 PCT/US2003/039200
orientated to form a substantially perfect array. Typical sizes of the silicon
nanowires are 50-200 nm, and the length is around 8 pm. On the top of each
nanowire can be seen a bright gold tip indicative of the vapor-liquid-solid
growth (see Wu, Y.; Yan, H.; Huang, M.; Messer, B.; Song, J.; Yang, P.
Chem.-Euro. J. 2002, 8, 1260, incorporated herein by reference).
[00109] FIG. 15B illustrates the nanotubes after parylene deposition, Si02 cap
removal, and the etching of the silicon cores, wherein a silica nanotube array
embedded in parylene membrane is formed. The pores can be readily seen
on the polymer surface. The bright spots on the image corresponding to the
gold nanoparticle tips, which nearly take the shape of half spheres. The
membrane has a relatively flat surface. The inset within FIG. 15B depicts high
magnification of two silica nanotubes embedded in the parylene membrane,
clearly showing the hollow pores with silica walls.
[00110] FIG. 15C and FIG. 15D are perspective and top views, respectively, of
15 the nanotube array after oxygen (02) plasma etching of parylene wherein a
free-standing silica nanotube array is obtained. As can be seen, the
nanotubes are well aligned and retain the vertical orientation of the starting
silicon nanowire templates. The inset of FIG. 15C shows a zoom view of the
nanotubes in a high magnification SEM image showing clearly the morphology
20 of the vertical nanotube array. The images reveal that the Si nanowires are
vertically oriented in an array, with uniform diameters along their length
ranging from approximately 50 nm to 200 nm, with lengths of up to
approximately 8 pm, and an average length of about 5 pm. The average
diameter of the resulting silica nanotubes exceeds that of the template
silicon
2s nanowires, as a result of the structural expansion caused by thermal
oxidation. The inset of FIG. 15D is a detailed top view from which the
hexagonal shape of the tube is visible. The scale bars on FIG. 15A, 15B, 15C
are 10 pm, 7 pm, and 10 pm respectively. The silica walls of the nanotubes
were found to exhibit a well-defined hexagonal shape indicative of the <111 >
30 orientation of the original Si nanowires and the anisotropic in-plane
etching
rates.
[00111] FIG. 16A and 16B are transmission electron microscopy (TEM) images
-20-
CA 02509257 2005-06-08
WO 2004/111319 PCT/US2003/039200
which further illustrate the high-quality of the silica nanotube formation. In
FIG. 16A the uniform inner diameter is shown, which generally persists along
the entire length of the nanotube. The pore sizes for the nanotubes range
from about 10 nm to 200 nm, with smooth inner and outer walls.
s [00112] Nanotube thickness was found to be around 70 nm for a 1000 °C
thermal treatment, despite the range of pore sizes for the nanotubes. This
result is considered reasonable because the oxidation layer thickness is
expected to be the same for the nanowires under a constant thermal
treatment condition since the thermal oxidation of the silicon is a self-
limiting
1o process. The self-limitation of the process can be taken advantage of for
controlling tube size and wall thickness by adjusting the characteristics of
the
thermal treatment process, such as the treatment temperature.
[00113] As an example of how nanotube characteristics can be controlled, a
sample oxidized at 900 °C has a typical wall thickness of around 55-65
nm,
15 while a temperature of about 300 °C yields a wall thickness of
around 30-35
nm. The nanotube shown in FIG. 16B has a pore size of approximately 20
nm, however as can be seen, it still is uniform and has a smooth inner wall.
Occasionally branched nanotubes were produced, it should be appreciated
that these nanotubes will provide benefits for select nanofluidic and
electronic
2o applications.
[00114] This multiuse approach of making silica nanotube array templates from
silicon nanowire arrays is a well-controlled process capable of controlling
the
pore size and the array height, while the resultant nanotubes can be readily
subjected to differenfi surface modification on inner and outer walls. The
25 respective surface modification of inner and outer walls can be important
in
applications such as bioseparation and smart molecule transport. In addition,
the walls of these nanotubes are formed from pinhole-free condensed thermal
oxide, which can be advantageous in terms of its mechanical robustness and
fluidic stability.
so [00115] Consequently, this new class of semiconductor nanotubes represented
by the present invention is mechanically robust, electrically and optically
active. Therefore, these nanotubes could offer additional opportunities for
-21-
CA 02509257 2005-06-08
WO 2004/111319 PCT/US2003/039200
further fundamental research as well as technological applications in
nanocapillary electrophoresis, nanofluidic biochemical sensing, nanoscale
electronics and optoelectronics (see Schoening, M & Poghossian, A. Recent
advances in biologically sensitive field-effect transistors (BioFETs),
Analyst,
127, 1137-1151 (2002), incorporated herein by reference). It should be
appreciated that the successful preparation of single-crystalline GaN
nanotubes using this "epitaxial casting" approach suggests that it is
generally
possible to prepare single-crystalline nanotubes of inorganic solids that have
non-layered crystal structures (see Lauhon, L. J., Gudiksen, M. S., Wang, D.
& Lieber, C. M. Epitaxial core-shell and core-multishell nanowire
heterostructures. Nature, 420, 57-61 (2002); and He, R., Law, M., Fan, R.,
Kim, F. & Yang, P. Functional bimorph composite nanotapes, Nano. Lett. 2,
1109-1112 (2002), the preceding incorporated herein by reference).
[00116] It should also be appreciated that the techniques described herein may
~5 be further extended by forming multiple sheath layers. Each of these sheath
layers may comprise different materials, different doping constituents or
levels. Still further, longitudinal portions (segments) of the nanotube may be
differentially processed to yield different properties between segments of the
nanotube structure, or multilayer nanotube structure. The following
2o nanotubular structure are provided by way of example and not by way of
limitation.
[00117] FIG. 17 depicts a multilayer nanotube 50 comprising a sacrificial Zn0
nanowire 12 (prior to removal) over which a gallium nitride (GaN) sheath 54 is
held between two sheaths 52, 56 of aluminum nitride (AIN). It will be
25 appreciated that the sacrificial nanowire may be removed at any time after
at
least the first sheath layer has been deposited over the nanowire, and it
could
be removed subsequent to depositing the last sheath layer.
(00118] FIG. 18 and FIG. 19 depict forming sheaths of alternately doped
material 60. FIG. 18 showing P-doped GaN 62 over a sacrificial core 12 (prior
3o to removal), such as ZnO, and N-doped GaN material 64 over the P-doped
material. Similarly, FIG. 19 illustrates the converse of FIG. 18 with P-doped
material 74 over N-doped material 72 which sheaths core 12 (prior to its
-22-
CA 02509257 2005-06-08
WO 2004/111319 PCT/US2003/039200
removal). It should be appreciated that from the present methods numerous
circuits may be fabricated, including diodes, light emitters, light detectors,
electron transport devices (i.e. bipolar transistors, FETs, insulated gate
FETs,
and so forth) and combinations thereof. Connection to device layers can be
s provided from the core, or external circumferential connections, while
connections may also be embedded into the material layers. The above
process methodology may be continued for producing any desired number of
nested sheaths within a given nanotube.
[00199] FIG. 20 and FIG. 21 depict forming segmented nanotube sheaths by
the present invention, wherein the different segments are formed from
different materials, different dopants, different levels of doping, or
combinations thereof. These sheaths may be fabricated segment-by-segment
in any convenient manner, such as utilizing conventional masking techniques.
[00120] In FIG. 20 a nanotube 80 is depicted having two segments of different
~s sheath material 84, 86 disposed longitudinally over a sacrificial core 82.
FIG.
21 depicts a nanotube 90 formed from three or more longitudinal segments of
different material, differently doped material, or material that is otherwise
configured to provide different properties. Furthermore, the nanotube is
shown having at least two sheaths of material.
20 [00121] A core 92 is shown prior to removal, with an upper-inner sheath 94,
an
upper-outer sheath 96, a middle-inner sheath 98, a middle-outer sheafih 7 00,
a lower-inner sheath 102, and a lower-outer sheath 104. It should be
recognized that any desired number of sheath layers may be deposited and
that nanotube may be fabricated with any number of longitudinal segments. It
25 should also be appreciated that insulators and electrical connections on
the
sheath layers may be formed as portions of different sheath segments.
Furthermore, the removed core of the nanotube may be utilized as a fluid via,
or lined with material, such as metal, to form another layer (i.e. conductive
contact layer).
30 [00122] FIG. 22 illustrates by way of example a cross-section of a nested
sheath of layers 110 forming a bipolar transistor. A hollow 12' represents
from
where the sacrificial nanowire core was removed. The interior of hollow 12' is
-23-
CA 02509257 2005-06-08
WO 2004/111319 PCT/US2003/039200
shown lined as a metallic contact 112. Three sheaths are shown in the figure.
A P-doped semiconducting inner sheath 114 is shown. Separated middle
sheaths of N-doped semiconductor 116, 118, are depicted between which a
central insulating ring 120 is shown surrounding inner sheath 114. Finally a
conductive outer sheath is shown with upper conductor 122 and lower
conductor 124 separated by insulating sheath segment 126. It will be
appreciated that the simple example depicts a form of bipolar NPN transistor
along the nanotube length, having exterior emitter contact 122 and collector
contact 124 and a base contact 112 lining hollow core 12'. The thickness of
the layers may be varied to achieve desired electrical properties, or to
enhance rigidity such as provided by the external sheath segments 122, 124,
126.
[00123] The transistor is provided by way of example and a wide assortment of
devices may be fabricated according to the techniques of the present
~s invention. It should be appreciated that various material and electrical
properties may be achieved utilizing the methods of the present invention.
Furthermore, various electronic devices, such as diodes, light emitting
diodes,
lasers, transistors, field effect transistors, and so forth may be produced in
accord with the teachings of the present invention.
20 [00124] As can be seen, therefore, the present invention comprises a method
of fabricating nanotubes by forming a sheath over a sacrificial core, and then
removing the core. Two general methods were described: (i) epitaxial casting
and (ii) oxidation and etching. Furthermore; examples of specific nanotube
structures were described, such as a GaN nanotube (over a Zn0 sheath)
25 using the epitaxial casting method and a S102 nanotube (over a Si sheath)
using the oxidation and etching method. However, other materials can be
used including, without limitation. GaN, Ge, Ag, group II-VI, III-V, elemental
group !V (e.g., Si, Ge), and metals as core materials, and further, including
without limitation, group II-VI, II-V, elemental group IV, metals, oxides of
the
so above, and polymers as sheath materials. Note also that all of the sheaths
can be doped during formation.
[00125] Although the description above contains many details, these should not
-24-
CA 02509257 2005-06-08
WO 2004/111319 PCT/US2003/039200
be construed as limiting the scope of the invention but as merely providing
illustrations of some of the presently preferred embodiments of this
invention.
Therefore, it will be appreciated that the scope of the present invention
fully
encompasses other embodiments which may become obvious to those skilled
in the art, and that the scope of the present invention is accordingly to be
limited by nothing other than the appended claims, in which reference to an
element in the singular is not intended to mean "one and only one" unless
explicitly so stated, but rather "one or more." All structural, chemical, and
functional equivalents to the elements of the above-described preferred
~o embodiment that are known to those of ordinary skill in the art are
expressly
incorporated herein by reference and are intended to be encompassed by the
present claims. Moreover, it is not necessary for a device or method to
address each and every problem sought to be solved by the present invention,
for it to be encompassed by the present claims. Furthermore, no element,
~5 component, or method step in the present disclosure is intended to be
dedicated to the public regardless of whether the element, component, or
method step is explicitly recited in the claims. No claim element herein is to
be construed under the provisions of 35 U.S.C. 112, sixth paragraph, unless
the element is expressly recited using the phrase "means for."
-25-