Note: Descriptions are shown in the official language in which they were submitted.
CA 02509546 2001-03-27
METHOD FOR TREATING DRY EYE
FIELD OF THE INVENTION
This invention relates to a method for treating dry eye. In particular,
solutions
comprising a cationic cellulosic polymer have been found useful for
alleviating the
symptoms of dry eye.
BACKGROUND
Dry eye, also known generically as keratoconjunctivitis sicca and dyslacrima,
is a
common ophthalmological disorder affecting millions of people. A patient with
dry eye
may experience bunting, a feeling of dryness and persistent irritation. In
severe cases,
dry eye can seriously impair a person's vision and hence handicap the sufferer
in
activities such as driving. Certain diseases such as Sjogren's disease
manifest dry eye
symptoms. Also, as people age, the lacrimal ducts in the eye may produce less
moisture,
resulting in eyes that become dry, inflamed, itchy and gritty.
Although it appears that dry eye may result from a variety of underlying,
unrelated pathogenic causes, all presentations of the condition share a common
effect,
namely the breakdown of the pre-ocular tear film, which commonly results in
dehydration of the exposed outer surface and hence the symptoms descn'bed
above.
A number of approaches exist for the treatment of dry eye. One common
approach has been to supplement the ocular tear film using artificial tears
instilled
throughout the day. Examples of the tear substitute approach include the use
of buffered,
isotonic saline solutions and aqueous solutions containing water-soluble
polymers that
render the solutions more viscous and thus less easily shed by the eye by the
washing
action of the tear fluid. See, for example, U.S. Patent No. 5,209,927 to
Gressel, et al.;
U.S. Patent No. 5,294,607 to Glonek, et'al.; and U.S. Patent No. 4,409,205 to
Shively.
Although these approaches have met with some success in some cases,
significant
challenges in the treatment of dry eye nevertheless remain. Problems include
the fact
that the use of tear substitutes, while temporarily effective, generally
require repeated
application over the course of a patient's waking hours, not uncommonly ten to
twenty
times over the course of a day. Such an approach is not only inconvenient and
time
1
CA 02509546 2001-03-27
consuming, but not very effective in preventing at least the initiation of dry-
eye
symptoms. Although increasing the viscosity of the dry-eye product may extend
the
product's duration in the eye to a limited extent, still further increases in
duration would
be highly desirable.
U.S. Patent No. 5,645,827 to Marlin, et al. discloses the use of compositions
comprising a cationic polysaccharide in combination with an anionic
therapeutic agent,
for example, hyaluronic acid or its salt, which is a lmown demulcent for the
treatment of
dry eye. Marlin, et al. believe that the anionic therapeutic agent is
electrostatically
bonded to the cationic polysaccharide which in tum is substantive to the
mucosal surface.
Substantivity is characterized by an increase of the cationic polysaccharide
on the
mucosal surface and can be measured through the use of an ocular fluorometer.
European Application 088770 A1 to Marlin et al. discloses cationic cellulose
polymers to
deliver cationic therapeutic agents, especially for the treatment of glaucoma.
U.S. Patent Nos. 4,436,730 and 5,401,327 to Ellis, et al. disclose the use of
cationic cellulosic derivatives in contact-lens treating solutions, including
the
combination of a cationic cellulose polymer and an ethoxylated glucose such as
glucam.
In column 4, lines 42-57, the latter patent states that the combination of a
cationic
cellulose material with a PEO component such as glucam is particularly
advantageous
for the reason that the cationic component complexes with the PEO component
and the
complex more strongly absorbs on the lens surface. The cationic cellulose
polymer and
entangled PEO is believed to reach into the aqueous phase to provide
cushioning and
protein resistance.
German Application DE 3440352 teaches a treatment for dry eye that contains an
acrylate-based dry eye gel, containing from 0.2 to 1.0 parts acrylate polymer
such as
Carbopol), 0.15 to 3 parts base (such as NaOITJ, and remainder water; and a
dry eye
solution containing 0.1 to 1 part by weight water-soluble cationic cellulose
derivatives
(such as various UCARE~ Polymer JR), 0.5 parts by weight of an isotonic agent
and
remainder water.
In view of the above, it would be desirable to provide an eye-drop solution
that
will better alleviate the symptoms of dry eye and that is safe, convenient and
economical
to use. In particular, it would be highly des>I-able to develop a product
having
2
CA 02509546 2001-03-27
significantly greater duration of efficacy, in order to significantly decrease
the number of
times that the product may need to be administered to the eye, over the course
of a day,
in order to effectively treat the symptoms of dry eye.
SUMMARY OF THE INVENTION
The present invention is directed to a method of treating dry eye employing a
solution comprising an effective amount of a cationic cellulose for
alleviating the
symptoms of dry eye. Such solutions are effective in the absence of hyaluronic
acid and
other anionic therapeutic agents. The invention is also directed to a method
of using the
foregoing composition to treat the symptoms of dry eye. In one preferred
embodiment,
the method employs a solution having low ionic strength. In another preferred
embodiment, the method employs a solution having relatively low concentrations
of
mono- or di-saccharides. In a particularly preferred embodiment, the solution
of the
invention is essentially free of chloride ions and essentially free of mono-
or di-
saccharides.
BRIEF DESCRIPTION OF THE DRAWINGS
The objects, features and advantages of the various embodiments of the present
invention will become more readily apparent from the following detailed
description
together with the following drawings.
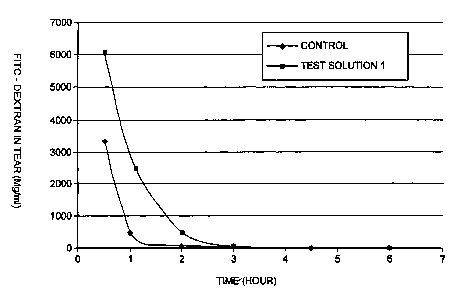
FIG. 1 shows the results from the tests of Example 2 below, involving the
measurement of the concentration of FITC-Dextran in a solution according to
the present
invention versus the concentration of FITC-Dextran in a control solution over
a period of
six hours in rabbit eyes.
FIG. 2 shows the results from the tests of Example 2 below, involving
measurement of the concentration of FITC-Dextran in a second solution
according to the
present invention versus the concentration of FTTC-Dextran in a control
solution over a
period of six hours in rabbit eyes.
FIGS. 3 and 4 show the results for the tests of Example 3 below, involving
measurement of the increase in non-invasive break up time (NIBUT) of tear film
over a
3
CA 02509546 2001-03-27
period of instillation of a solution according to present invention over
thirty (30) minutes
in rabbit eyes.
DETAILED DESCRIPTION OF THE INVENTION
The present invention is directed to a method of treating the symptoms of dry
eye
employing an ophthalmic solution that can be applied in the form of drops and
which
comprises a cationic cellulosic polymer that exhl'bits prolonged duration in
the eye. A
wide variety of cationic cellulosic materials may be used in the practice of
this invention.
Specific examples include cellulosic polymers containing N,N-
dimethylaminoethyl
groups (either protonated or quaternized) and cellulosic polymers containing
N,N-
dimethylamino-2-hydroxylpropyl groups (either protonated or quaternized).
Cationic
cellulosic polymers are commercially available or can be prepared by methods
known in
the art. As an example, quaternary nitrogen-containing ethoxylated glucosides
can be
prepared by reacting hydroxyethyl cellulose with a trimethylammonium-
substituted
epoxide. Various preferred cationic cellulosic polymers are commercially
available, for
example water-soluble polymers available under the CTFA (Cosmetic, Toiletry,
and
Fragrance Association) designation Polyquaternium-10. Such polymers are
commercially available under the tradename UCARE~ Polymer from Amerchol Corp.,
Edison, NJ, USA. These polymers contain quaternized N,N-dimethylamino groups
along the cellulosic polymer chain.
The cationic cellulosic component may be employed in the compositions at about
0.01 to about ten (10) weight percent of the composition, preferably at about
0.05 to
about five (5) weight percent, with about 0.1 to about one (1) weight percent
being
especially preferred. Suitable cationic cellulosic materials have the
following formula:
R2 R3
Wherein R, Rz and R3 are selected from H, derivatives of C,-CZO carboxylic
acid, CI-CZo
alkyl groups, Cl to C3 monohydric and dihydric allcanols, hydroxyethyl groups,
4
CA 02509546 2001-03-27
hydroxypropyl groups, ethylene oxide groups, propylene oxide groups, phenyl
groups,
"Z" groups and combinations thereof. At Ieast one of Rl, RZ,and R3 is a Z
group.
The nature of the "Z" groups is:
R~ H
Z=X R"-N+-~CH2-~--f-CH~CHZ-~
R.../
where:
R', R" and R"' can be H, CH3, C2H5, CH2CH20H and
CHZ ~ HCH20H
OH
x=0-5, y=0-4, and z=0-5
X' = Cf, Br, I; HSO,,; CH3S04, HZP04, N03
Optionally, one or more additional polymeric or non-polymeric demulcents may
be combined with the above-named ingredients. Demulcents are known to provide
wetting, moisturizing and/or lubricating effects, resulting in increased
comfort.
Polymeric demulcents can also act as a water-soluble viscosity builder.
Included among
the water-soluble viscosity builders are the non-ionic cellulosic polymers
like methyl
cellulose, hydroxyethyl cellulose, hydroxypropyl cellulose, and carboxymethyl
cellulosc,
poly(N-vinylpyrrolidone), poly(vinylalcohol) and the like. Such viscosity
builders or
demulcents may be employed in a total amount ranging from about 0.01 to about
5.0
weight percent or less. Suitably, the viscosity of the final formulation is 10
cps to 50 cps.
Comfort agents such as glycerin or propylene glycol can also be added.
In contrast to the prior art, the solutions of the present invention are
effective in
the absence of conventional anionic therapeutic agents for the treatment of
dry eye. Prior
art anionic therapeutic agents include glycosaminoglycans such as hyaluronic
acid,
hylan, hylaluronan, heparin, heparan sulfate, chondroitin sulfate, keratin
sulfate and
dermatan sulfate. Various glycosaminoglycans absent from the present
composition are
3
CA 02509546 2001-03-27
listed in U.S. Patent No. 5,358,706, hereby incorporated by reference.
Hyaluronic acid is
an anionic biopolymer that has been identified as useful in the treatment of
the symptoms
of dry eye. Synthetic anionic polymers for the treatment of dry eye also
include
carboxy-vinyl polymers known as Carbopol,~ commercially available from B.F.
Goodrich, as described in U.S. Patent No. 5,209,927 to Gressel, et al.
Thus, it has been found that the cationic polysaccharides are, in themselves,
effective for the treatment of dry eye. Without wishing to be bound by theory,
it may be
that the polymers, after binding to the mucosal tissue of the eye, in turn
promote the
mucin in the eye, either by supplementing the mucin and/or by helping to bind
and
maintain mucin on the surface of the eye. Mucins are proteins, which are
heavily
glycosylated with glucosamine-based moieties. Mucins have been shown to be
secreted
by vesicles and discharged on the surface of the conjunctiva) epithelium of
the eye. See
for example, Greiner, et al., "Mucus Secretory Vesicles in Conjunctiva)
Epithelial Cells
of Wearers of Contact Lenses," Archives of Ophthalmology, Vol. 98, pages 1843-
1846
(1980). Mucins provide lubrication and additionally attract and hold moisture
and
sebaceous material for lubrication.
The present composition may also contain a disinfecting amount of a
preservative
or an antimicrobial agent. A particularly preferred preservative is sorbic
acid (0.15%).
Antimicrobial agents are defined as organic chemicals that derive their
antimicrobial
activity through a chemical or physiochemical interaction with the microbial
organisms.
For example, biguanides include the free bases or salts of alexidine,
chlorhexidine,
hexamethylene biguanides and their polymers, and combinations of the
foregoing. The
salts of alexidine and chlorhexidine can be either organic or inorganic and
are typically
gluconates, nitrates, acetates, phosphates, sulfates, halides and the like.
The preferred
biguanide is the hexamethylene biguanide commercially available from Zeneca,
Wilmington, DE under the trademark CosmocilTM CQ. Generally, the hexamethylene
biguanide polymers, also referred to as polyaminopropyl biguanide (PAPB), have
molecular weights of up to about 100,000.
If used in the subject solution, the antimicrobial agent should be used in an
amount which will at least partially reduce the microorganism population in
the
formulations employed. Preferably, a disinfecting amount is that which will
reduce the
6
CA 02509546 2001-03-27
microbial burden by two log orders in four hours and more preferably by one
log order in
one hour. Most preferably, a disinfecting amount is an amount which will
eliminate the
microbial burden on a contact lens when used in regimen for the recommended
soaking
time (FDA Chemical Disinfection Efficacy Test-July, 1985 Contact Lens Solution
Draft
Guidelines). Typically, such agents are present in concentrations ranging from
about
O.OOOOI to about 0.5% (w/v), and more preferably, from about 0.00003 to about
0.05%
(w/v).
The aqueous solutions empkoyed in this invention may contain, in addition to
the
active ingredients described above, one or more other components that are
commonly
present in ophthalmic solutions, for example, buffers, stabilizers, tonicity
agents and the
like, which aid in making ophthalmic compositions more comfortable to the
user. The
aqueous solutions of the present invention are typically adjusted with
tonicity agents to
approximate the tonicity of normal lacrimal fluids which is equivalent to a
0.9% solution
of sodium chloride or 2.8°/ of glycerol solution. The solutions are
made substantially
isotonic with physiological saline used alone or in combination; otherwise, if
simply
blended with sterile water aid made hypotonic or made hypertonic, the lenses
will lose
their desirable optical parameters. Correspondingly, excess salt or other
tonicity agents
may result in the formation of a hypertonic solution that will cause stinging
and eye
irritation. An osmolality of about 225 to 400 mOsm/kg is preferred, more
preferably 280
to 320 mOsm/kg.
In a preferred embodiment, the ionic strenght of the present solutions is
relatively
low. In this preferred embodiment of the invention, low ionic strength has
surprisingly
been found to be associated with improved performance. The solutions
preferably
contain less than'4 mM calcium chloride, and is more preferably essentially
free of
calcium chloride. The solutions preferably contain less than 150 mM sodium
chloride,
more preferably less than 75 mM sodium chloride. The solutions preferably
contain less
than 15 n~IVI potassium chloride, more preferably less than 6 mM potassium
chloride. In
one preferred embodiment, the chloride ion concentration of the solution is
less than 300
mM, more preferably less than 100 mM.
The solutions of the present invention preferably contain less than 300
mOsm/lg
equivalents of a mono- or di-saccharide, more preferably less than 100
mOsm/lcg of a
7
CA 02509546 2001-03-27
mono- or a di-saccharide, and most preferably contain essentially no mono- or
di-
saccharide. Maintaining the concentrations of mono- and di-saccharide below
the
specified limits has surprisingly been found to be associated with improved
performance.
In an alternate emobidment, the solutions of the present invention may contain
one or more ionic or non-ionic surfactants and may be used as cleaning and/or
conditioning solutions for hydrogel or RGP (rigid-gas-permeable) contact
lenses.
Effective amounts of surfactant are preferably in the range of from about
0.01% to about
5% by weight. In a preferred embodiment of the surfactant-containing solutions
of the
invention, the surfactant is a neutral or non-ionic surfactant.
The pH of the present solutions used to treat dry eye should be maintained
within
the range of 5.0 to 8.0, more preferably about 6.0 to 8.O,.most preferably
about 6.5 to 7.8;
suitable buffers may be added, such as borate, citrate, bicarbonate, TRIS and
various
mixed phosphate buffers (including combinations of l~Ta2HP04, NaHZP04 and
KH2P04)
and mixtures thereof. Borate buffers are preferred, particularly for enhancing
the
efficacy of PAPB. Generally, buffers will be used in amounts ranging from
about 0.05 to
2.5 percent by weight, and preferably, from 0.1 to 1.5 percent.
In addition to buffering agents, in some instances it may be desirable to
include
sequestering agents in the present solutions in order to bind metal ions,
which might
otherwise react with the lens aud/or protein deposits and collect on the lens.
Ethylene-
diaminetetraacetic acid (EDTA) and its salts (disodium) are preferred
examples. They
are usually added in amounts ranging from about 0:01 to about 0.2 weight
percent.
The solutions employed in the present invention can be prepared by a variety
of
techniques. One method employs two-phase compounding procedures. In the first
phase, about 30 percent of the distilled water is used to dissolve the
cationic cellulosic
polymer by mixing for about 30 minutes at around 50° C. The first-phase
solution is
then autoclaved at about 120° C for 30 minutes. In a second phase,
alkali metal
chlorides, sequestering agents, preservatives and buffering agents are then
dissolved in
about 60 percent of the distilled water under agitation, followed by the
balance of
distilled water. The second-phase solution can then be sterilely added into
the first-phase
solution by forcing it through an 0.22 micron filter by means of pressure,
followed by
packaging in sterilized plastic containers.
8
CA 02509546 2001-03-27
A.s indicated above, the present invention is useful for treating dry eye, or,
more
specifically, its symptoms. For that purpose, compositions for use in the
present
invention may be sold in a wide range of small-volume containers from 1 to 30
ml in
size. Such containers can be made from HDPE (high density polyethylene), LDPE
(low
density polyethylene), polypropylene, polyethylene terepthalate) and the like.
Flexible
bottles having conventional eye-drop dispensing tops are especially suitable
for use with
the present invention.
The above-described solutions, iri accordance with the present invention, may
be
used by instilling, for example, about one (1) or three (3) drops in the
affected eyes) as
needed, for the temporary relief of burning and irritation due to dryness in
the eye and for
use as a protectant against further irritation, or to relieve dryness to the
eye. '
The following specific experiments and examples demonstrate the compositions
and methods of the present invention. However, it is to be understood that
these
examples are for illustrative purposes only and do not purport to be wholly
definitive as
to conditions and scope. All percentages are by weight of the solution, unless
indicated
otherwise.
9
CA 02509546 2001-03-27
EXAMPLE 1
An aqueous solution for use in treating the symptoms of dry eye according to
the
present invention, by means of eyedrops of the solution administered to the
eye, is
prepared with the following ingredients in water:
TABLE 1
Ingredient mg/g % w/w
UCAR.E~ Polymer JR 30m 5.00 0.50
Benzalkonium Chloride, 50% 0.22 0.022
Boric Acid 10.00 1.00
Sodium Borate 1.10 0.11
-_ _
Glycerin 10.00 1.00
The formulation is prepared in bulk as follows. In a 316-grade stainless-steel-
jacketed pressure kettle equipped with agitation, distilled water is added in
the amount of
about 800 g and heated to 60° to 70°C, preferably 65°C.
Under agitation the following
batch quantities of the following ingredients are added, wherein after one
ingredient is
dissolved or hydrated, the next is added: Polymer JR, Boric Acid, Sodium
Borate,
Benzalkonium Chloride, Glycerin. Agitation is maintained throughout the entire
processing of the batch. Upon dissolution of these components, the batch is
charged with
purified water to 98 percent of the final weight. The solution is mixed for a
minimum of
thirty(30) minutes to ensure complete dissolution. If necessary, the pH is
adjusted to 6.5
to 7.4 at 25°C with 2.5 N I~TaOH or 1N HCl and measured at 6.8. The
osmolality is
measured at 300 mOsm/Kg. The solution may be sterilized by autoclave at 121-
124°C
for thirty (30) to forty-five (45) minutes and then immediately cooled to
40°C, after
which a sufficient quantity of cool purified water may be added to obtain the
final
weight. For best product clarity, the finished solution should be aseptically
passed
through a sterile 40-50 micrometer polishing filter. For use in the above
process, suitable
polishing filters include Pall RigimeshT"" RR 40 micrometer and Filterite
DynalloyT"" 30
micrometer PSP 12-l OSL-M7 filters.
CA 02509546 2001-03-27
EXAMPLE 2
This example illustrates the effective duration in the eye of solutions
according to
the present invention as measured using FITC-dextran (also referred to as.
"FD's. This
material, having a molecular weight of 40,000, is commercially available from
the Sigma
Chemical Company (MO, USA). The control solution was five (5) percent FD
dissolved
in distilled, de-ionized water.
Test solution 1 contained five (5~ percent FITC-dextran with the combination
of
UCARE~ Polymer JR 30M (0.5 percent) and Carbowax~ Sentry~ Polyethylene Glycol
20M (0.5 percent). Test Solution 2 contained five (~ percent FITC-dextran with
UCARE~ Polymer JR 30M (0.5 percent) only. The two solutions were tested in
triplicate in male New Zealand rabbits (weighing 1.5 to 2 l~logranas). The
test solution
was administered into the right eye and the control solution was administered
into the left
eye of the rabbit. The solutions (25mg) were instilled in the lower cul-de-sac
of each
rabbit eye. All solutions were administered with a micropipettor. The eyes
were held
closed for thirty (30) seconds after instillation.
Tear fluid was collected at 0.5,1, 2, 3, 4.5 and 6 hours using 2 ~1 disposable
glass
capillaries. The upper eyelid of the rabbit eye was held up, and the capillary
tube was
placed in the upper cul-de-sac. The tear was collected by gently drawing the
end of the
tube between the globe of the eye and the eyelid for about one minute. During
sampling,
contact between the capillary tip and any visible gel lumps was avoided. The
fluid in the
capillaries was flushed into tubes containing one ml of water, and the
resulting samples
were stored at 4°C until analysis of the FD content. The concentration
of FD in the
samples was determined using a spectrofluorophotometer. The excitation
wavelength
was 491 nm, and the enussion wavelength was 513 nm. A standard curve was
obtained
using solutions containing 0.05, 0.1, 0.2, 0.4, 0.6 and 1.0 pg/ml of FD.
Because the
volume of tears in each rabbit eye is different, the concentration of FD in
one ml of teat
was calculated The FD concentrations versus time curves were plotted.
A non-parametric statistical test (Wilcoxon rank sum test) was used to compare
the FD concentrations in tears, obtained following administration of the test
solution and
the control.
lI
CA 02509546 2001-03-27
FD concentrations in the tear fluid obtained following administration of the
test
solution 2 were higher than those obtained using the control solution
(a=0.05), which
was maintained for three (3) hours. For the test solution 1, the resulting FD
concentrations, although not statistically significant due to the limited
sample size, were
also higher than those of the control solution up to two (2) hours. The
results for the
concentration of FITC-Dextran in tears (mg/ml) over time (n 3) are shown in
Table 2
below for the average. Figures 1 and 2 show a comparison to the control for
the test
solutions 1 and 2, respectively, in tear over six (r7 hours.
TABLE 2
Test SolutionTime FITC-Dextran VVilco~on Rank
Concentration
(Flour) Control Test Sum Test a=0.0
1 0.5 3322 6089 no difference
1 409 2426 no difference
2 83 541 no difference
3 17 28 no difference
4.5 4 21 no difference
6 48 4 no difference
2 0.5 1793 9000 Test > Control
1 161 6272 Test > Control
2 164 3431 Test > Control
3 47 870 Test > Control
4.5 20 12 no difference
6 31 99 no difference
EXAMPLE 3
This example .illustrates the effectiveness of the present invention to
stabilize the
tear film and decrease the symptoms of dry eye in an animal model. New Zealand
white
rabbits had the nictitating membrane and the accessory and main lacrimal
glands
surgically removed from their right eye. As a result, the tear film stability
was
significantly reduced compared to that of the left contralateral control eye.
The non-
invasive break up. time (IvTIBUT) was then measured in minutes for each eye.
After
baseline measurements were taken in the right eye, one (1) drop of the test
solution was
12
CA 02509546 2001-03-27
administered and the N1BUT was measured immediately and at 5, 15 and 30
minutes
after instillation. Five (5) solutions were tested. The first was a borate-
buffered saline
(BBS); the second was a composition containing 0.5% Polymer JR and 1% Glycerin
in
the BBS; and the third was a combination of 1% Propylene Glycol and 0.3%
Glycerin in
the BBS. The compositions of the fourth through eighth solutions are shown
below in
Table 3. The effectiveness of each solution is represented in terms of percent
of increase
in NIBITT over baseline is shown in Tables 4 through 7, below.
The results were also illustrated in Figures 3 and 4. Initially, both
solutions 2 and
3 were very effective while solution 1 (saline control) had little effect.
Solution 2's
effect was significantly longer lasting than the solution 3's; in fact, at 30
minutes after
instillation, the solution 2 still restored the tear filin stability by 113.8%
compared to
20.1% for the solution 3. Solution 4, which was free of added sodium chloride,
potassium chloride and calcium chloride, restored tear film stability by 154%
at 30
minutes after instillation.
TABLE 3
Inb edientsConcentration SolutionSolution Solution Solution Solut
% w/w 4 5 6 7 pon
Pol er JR 0.2 X X X X ' K
PVP 1 X X X ' K
_
Gl cerin 1 X X X X
_
Propylene 1 X X
Gl col
_
Dextrose I 2.5% 4: 7%
Monoh drate
_
Sodium 0.267 X
Chloride
_
Potassium 0.132 X
Chloride
Calcium 0.0294 (2~ X X : K
Chloride
drb drate
_
Other common
in edients
in Formulations
A-E: 0.3%
Boric Acid,
0.035%
Sodium
Borate
and 0.9
m PHN IB,
13
CA 02509546 2001-03-27
TABLE 4
Test Solution011'Iin 5 Min 15 Min 30 Min
1 (Control) 7.9 31.8 -13.4 -14.1
2 248.7 149.7 120.6 113.8
3 115.7 39.1 15.9 20.1
TABLE 5
Test Solution5 Ilfin 10 llZin 15 Min 30 Min
4 274.9 214.4 194 154
195.1 155.7 135.4 86
TABLE 6
Time Solution Solution Solution Solution Solution
#4 #5 #6 #7 #8
min Mean Mean Mean Mean Mean
5 274.9 195.1 163.2 129.5 125.9
214.4 155.7 123.5 73.4 119.1
194.0 135.4 112.2 12.5 88.0
30 154.0 86.0 58.1 73.5
60 142.2 94.1 15.9 60.3
90 91.1 43.7
120 63.2 43.1 27.1
180 23.3
270 3 6.7
14
CA 02509546 2001-03-27
TABLE 7
Solution
#8
Time Mean
min.)
125.9
119.1
88.0
30 73.5
60 _
60.3
120 27.1
180 23.3
Solution
#4
Time Mean
min.
S 274.9
10 214.4
15 194.0
30 154.0
60 142.2
90 91.1
120 63.2
270 36.7-
Many other modifications and variations of the present invention are possible
in
light of the teachings herein. It is therefore understood that, within the
scope of the
claims, the present invention can be practiced other than as herein
specifically described.
1~