Note: Descriptions are shown in the official language in which they were submitted.
RD 29346
CA 02509682 2005-06-10
HIERARCHICAL MATERIALS
BACKGROUND OF THE INVENTION
This invention relates to materials for use at high temperatures. More
particularly,
this invention relates to materials designed for enhanced toughness at high
temperatures. This invention also relates to methods for making such
materials.
Materials with the capability to maintain adequate properties at extremely
high
temperatures are highly sought after for use in several widely varying
applications,
including, for example, space vehicles, turbine equipment for power generation
plants
and aircraft engines, and metal forming and glass blowing equipment. For
instance,
increasing the temperature of the combustion gases used to drive a gas turbine
generally increases the potential efficiency with which the turbine can
generate
power. However, the alloys and protective coatings used to fabricate turbine
components typically operate at or near their temperature limits in state-of
the-art
turbine equipment, and even a modest increase in firing temperature of such a
turbine
would degrade the performance of these materials in any of a number of
properties,
including, for example, strength, oxidation resistance, and creep resistance.
Many ceramic materials easily surpass metals in certain high-temperature
properties,
and therefore offer a potential solution to the limitations of alloys noted
above.
Ceramics in general are stronger and lighter than high temperature alloys, and
resist
environmental attack and creep much more effectively. However, ceramic
materials
have seen relatively little use in many engineering structural components due
to their
low tolerance for damage. Ceramics tend to be brittle and highly susceptible
to rapid
catastrophic failure when overloaded, particularly in situations where the
ceramic
contains mechanical damage in the form of cracks, voids, porosity, or other
discontinuity. Brittle materials like ceramics tend to fail with very little
to no plastic
(permanent) deformation, and the energy required to effect a complete
fracture, a
1
RD 29346
CA 02509682 2005-06-10
quantity often referred to in the art as "toughness," is comparatively low.
Metals and
alloys, on the other hand, generally require a comparatively high amount of
energy
before failing because they exhibit significant amounts of plastic
deformation, which
discourages formation of cracks and voids, blunts existing crack tips, and
otherwise
accommodates damage in a way that forestalls catastrophic failure. Materials
with
high toughness tend to tolerate damage to a much larger extent than brittle
materials,
due to their ability to "absorb" higher amounts of energy before failing. To
be useful,
materials that take advantage of the benefits offered by ceramics must also
possess
some mechanism for enhancing overall toughness and damage tolerance.
One of the most commonly used strategies for achieving the needed balance of
strength with toughness in materials incorporating ceramics is the development
of a
composite material, where multiple materials are combined in a fashion to
optimize
their advantages while minimizing their disadvantages. Several classes of
composite
materials have been developed to exploit ceramics. For example, metal-matrix
composites include a tough, ductile metal, such as an aluminum or nickel
alloy, into
which is included a hard, strong, but brittle ceramic that reinforces the
softer metal.
The incorporation of the ceramic boosts the strength of the composite, while
the
presence of the ductile metal matrix maintains requisite levels of toughness
and
damage tolerance. In metal matrix composites, then, the mechanism used to
absorb
stress and thereby enhance toughness is the plastic deformation of the metal
matrix.
Ceramic matrix composites do not include a tough metal phase in the matrix and
thus
generally employ a different toughening mechanism than metal matrix
composites.
For instance, in fiber reinforced ceramic matrix composites, an interfacial
layer of
material may be engineered to be weaker than the respective materials
comprising the
fiber and the matrix. In such situations strain energy may be absorbed, and
failure
delayed, by the formation and propagation of multiple small cracks along the
fiber
interfaces, by frictional sliding of the fiber within the matrix, and other
alternative
failure modes, instead of the formation and rapid, catastrophic propagation of
one
large crack as is commonly observed in monolithic ceramics. Ceramic matrix
composite materials thus attempt to derive toughening in the absence of
plastic
2
RD 29346
CA 02509682 2005-06-10
deformation through the incorporation of failure mechanisms that allow for a
slower,
more incremental failure.
Although conventional ceramic matrix composites (CMC's) have shown
improvements in toughness and damage tolerance over monolithic ceramic
materials,
issues remain that detract from the ability to fully capitalize on the
benefits offered by
ceramic materials. Composite materials in general are mixtures that generally
perform only as well as the worst performing constituent in the mixture. For
example,
poor oxidation resistance in fiber materials results in poor oxidation
resistance for the
entire composite, because the preferential degradation of the reinforcing
fibers has a
major effect on the properties of the overall material. Clearly, there is a
need for
improved materials with high temperature capability and adequate damage
tolerance
to survive demanding applications.
BRIEF DESCRIPTION
Embodiments of the present invention address this and other needs. One
embodiment
is a material comprising a plurality of structural components. The structural
components are configured in a series of increasing structural component size
classes.
The series has a base unit size class and at least one modular size class, and
a
component of the at least one modular size class comprises a plurality of
components
of the next smaller size class in the series. The structural components of the
base unit
size class comprise at least one bulk phase, and the structural components are
bonded
together at interfaces. Mechanical damage originating within a modular size
class
structural component is energetically favored to propagate in a distributed
fashion
among the plurality of structural components contained within the modular size
class
structural component.
A second embodiment is an article comprising the material described above.
3
RD 29346
CA 02509682 2005-06-10
BRIEF DESCRIPTION OF THE DRAWINGS
These and other features, aspects, and advantages of the present invention
will
become better understood when the following detailed description is read with
reference to the accompanying drawings in which like characters represent like
parts
throughout the drawings, wherein:
Figures 1 and 2 are cross-sectional schematics of exemplary embodiments of the
present invention;
Figure 3 is a cross-sectional schematic of a simulated two-level hierarchical
material
in accordance with certain embodiments of the present invention;
Figure 4 is a cross-sectional schematic of a simulated, one-level hierarchical
material;
and
Figure 5 is a graph depicting load-displacement data generated in a computer
simulated test of the materials depicted in Figures 3 and 4.
DETAILED DESCRIPTION
Refernng to the drawings in general and to Figure 1 in particular, it will be
understood that the illustrations are for the purpose of describing an
exemplary
embodiment of the invention and are not intended to limit the invention
thereto.
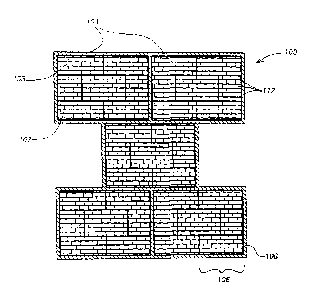
Figure 1 is a schematic representation of a material in accordance with the
present
invention. The material comprises a plurality of structural components 100
configured in a series of increasing structural component size classes. A
structural
component, in accordance with embodiments of the present invention, is a unit
of
structure into which the material of the present invention is organized, and
can be
analogized as the "building blocks" from which the material is made. As used
herein,
"size class" refers to a class of components wherein each component of the
class has a
characteristic length that is within about 25% of a mean characteristic length
for the
4
RD 29346
CA 02509682 2005-06-10
entire class. A characteristic length is any convenient dimension of a
structural
component applied consistently across all size classes to characterize the
structural
components, such as, for example, the diameter of components having circular
cross-
sections, or the length of a longitudinal or transverse leg of a component
having a
rectangular cross section.
The series of structural component size classes has a base unit size class 102
and at
least one modular size class 104. The base unit size class contains structural
components having the smallest characteristic length in the material.
Components of
the base unit size class 102 are thus the fundamental structural components of
the
material. Any of a variety of structures is suitable to serve as a base unit
size class
structural component, including, as illustrative examples, a fiber of the sort
used in
conventional composite lamination processes; a band of deposited material as
is
commonly fabricated in lithography processes; and a self assembled cluster of
molecules of the type used in molecular self assembly processes. The base unit
size
class structural components comprise at least one bulk phase 103. Bulk phase
103, in
some embodiments, comprises at least one of a ceramic, an organic material,
and a
metal. In certain embodiments, bulk phase 103 comprises a ceramic material,
and the
ceramic material comprises at least one of a boride, a nitride, an oxide, a
carbide, a
silicide, a silicate, and mixtures and compounds thereof. Specific examples of
suitable ceramic materials include, but are not limited to, silicon carbide,
titanium
carbide, zirconium carbide, hafnium carbide, molybdenum carbide, tantalum
carbide,
silicon nitride, silicon aluminum oxynitride, aluminum nitride, titanium
nitride,
titanium diboride, molybdenum disilicide, aluminum oxide, and aluminum
silicate.
Components of the at least one modular size class 104 comprise a plurality of
components of the next smaller size class in the series. Examples of suitable
modular
size class structural components include, but are not limited to, a filament
comprising
a plurality of co-extruded fibers; a laminated block of material comprising a
plurality
of the aforementioned filaments; a layer comprising a plurality of bands of
material,
fabricated using photolithography or other patterned deposition process; a
block of
material comprising a plurality of the aforementioned layers; a cylindrical
tube
RD 29346
CA 02509682 2005-06-10
resulting from the spontaneous assembly of self assembled molecular clusters;
and a
filament comprising a plurality of the aforementioned tubes. The material of
the
present invention is thus a multiple-level hierarchical material where
structural
components of a base unit size class are assembled to form larger, modular
structural
components, which, in some embodiments, in turn are assembled to form even
larger
modular structural components, and so on to the largest size class in the
series.
In the exemplary embodiment illustrated in Figure l, the plurality of
structural
components 100 is represented in cross section as a series of brick-like
components,
although it will be appreciated that each "brick" could actually run semi-
infinitely in
the direction perpendicular to the cross-sectional area projection shown, as a
fiber or
band of material would, for example. A typical "brick" of the largest size
class 106
comprises a plurality of "bricks" of the next smaller size class 108, which in
turn
comprises a plurality of "bricks" of the base unit size class 110. This
exemplary
embodiment is thus a three-level hierarchical material, because the structural
components of the material are configured in a series of three increasing
structural
component size classes. In contrast, a typical brick wall may be thought of as
a single
level "hierarchy" because it comprises structural components (bricks) having
only one
size class.
Those skilled in the art will appreciate that in embodiments of the present
invention
there is no theoretical upper limit to the permissible number of size classes
in the
series of progressively increasing structural component size classes, although
practical
limitations imposed by the selected fabrication process may arise. In certain
embodiments, the number of size classes in the series is at least two, and in
particular
embodiments the number of size classes in the series is in the range of from
three to
five. As will be explained below, having more than one size class serves to
significantly slow the spread of damage through the material and forestall
catastrophic
failure, offering the opportunity, usually not available in conventional
ceramic
materials, to detect damage before failure occurs
6
RD 29346
CA 02509682 2005-06-10
The size of the base unit size class structural components is determined by
the degree
of control allowed by the process used to create these components. For
example,
commonly used molecular self assembly techniques are capable of fabricating
structural components, such as molecular clusters, having a mean
characteristic
length on the order of about 10 nanometers, while conventional fiber
manufacturing
methods and lithography techniques are generally limited to a minimum
characteristic
length on the micrometer length scale. On the other hand, the largest size
class of
structural component in a material is limited only by the actual dimensions of
the
component fabricated from the material.
In particular embodiments, such as that illustrated in Figure 1, substantially
all of the
structural components 100, that is, greater than about 80% of the components
100 in a
given sample of material, have a substantially similar shape, although such a
condition is not necessary to the general operability of the material. The
shape of a
structural component 100 is characterized by a cross sectional geometry, such
as, for
instance, the rectangular shape of the components 100 depicted in Figure 1.
The term
"substantially similar shape" herein means the structural components differ in
size but
the general geometric form of a component of one size class does not vary from
that
of a component of a different size class to a degree where one of ordinary
skill in the
art would characterize the geometric forms as different. For example, the
brick-
shaped cross-section of the components as depicted in Figure 1 would be
characterized as substantially similar rectangles by one skilled in the art
even if, for
example, minor departures from precise right angles and some minor rounding of
corners were noted among components of varying size classes. Various cross-
sectional shapes are suitable for use as structural components 100, including,
but not
limited to rectangular cross sections and circular cross sections.
The structural components 100 of all size classes in the series are bonded
together at
interfaces 112, such as, for example, mechanically interlocked interfaces,
chemically
bonded interfaces, and interfaces that use a combination of mechanical
interlocking
and chemical bonding to bond structural components together. The interfaces
112
typically bond the structural components 100 in more than one dimension, such
as, for
7
RD 29346
CA 02509682 2005-06-10
example, interfaces that bond structural components in all three dimensions.
Chemically bonded interfaces comprise at least one interfacial phase, distinct
from the
bulk phase 103, which acts as an adhesive in bonding structural components 100
together. In certain embodiments, the at least one interfacial phase used to
bond
structural components 100 together comprises a material selected from the
group
consisting of a ceramic, a glass ceramic, carbon, and combinations thereof.
Examples
of suitable ceramic materials for the interfacial phase include, but are not
limited to,
hexagonal boron nitride, lanthanum phosphate, aluminum oxide (alumina),
titanium
silicon carbide (Ti3SiC2), silica, zirconia, and mixtures and compounds of any
of the
foregoing materials. The interfacial phase in certain embodiments is the same
throughout the material, regardless of what size class of structural component
is being
bonded. In alternative embodiments, as shown in Figure 2, interfaces 202
bonding
structural components of a first size class 204 comprise a different material
than
interfaces 206 bonding structural components of a second size class 208. It
will be
appreciated that the use of the terms "first" and second" herein are not meant
to refer
to absolute or relative positions of size class in the series of component
size classes,
but are merely used to distinguish one size class from another without
reference to
their place in the series. In particular embodiments, each size class of
structural
components is bonded together by interfaces comprising a material that is
unique to
the interfaces bonding that size class. For example, in a material having
three size
classes of structural components, the base-unit size class components are
bonded
together by interfaces comprising a first material, the modular size class
components
that comprise only these base-unit components (i.e., the next size class in
the series)
are bonded together by interfaces comprising a second material, and the
largest size
class components are bonded together by a third interfacial material, such
that the
first, second , and third interfacial materials are different from each other.
Fabricating an interfacial phase is readily accomplished by any of several
well-known
methods, including, for instance, coating methods and infiltration methods.
Coating
methods are used to encase a structural component with a material desired for
use as
an interfacial phase, and then the coated components are packed together so
that the
8
RD 29346
CA 02509682 2005-06-10
coating material is disposed in the interstices between the packed structural
components. For example, monolithic ceramic fibers are coated with a desired
interfacial material, and then a plurality of coated fibers, still in a green
state (that is,
still containing binders and plasticizers to enable mechanical processing) are
co-
extruded together to form a filament comprising a plurality of base unit
structural
components (the monolithic fibers) bonded together by an interfacial phase
(the
material coated onto the fibers). This filament, which in accordance with
embodiments of the present invention is a modular size class component, is in
turn
coated with a material desired to be a second interfacial phase, and a
plurality of such
coated filaments is co-extruded to form a larger size class filament
comprising a
plurality of structural components (the smaller filaments) bonded together by
an
interfacial phase (the second interfacial phase). In alternative embodiments,
interfacial material is fabricated by selective deposition of desired material
in a
lithography process, sometimes preceded by a selective etch process to create
an
interfacial region to receive the interfacial material. Alternatively, in
certain
embodiments, interfacial material is disposed in desired locations by
infiltrating a
porous network running through the material, as where, for example, the bulk
phase is
a mesoporous ceramic oxide and the interfacial material is infiltrated via
vacuum
infiltration into the mesoporous network of the oxide. Coating methods
include, but
are not limited to, chemical vapor deposition, physical vapor deposition,
application
by spraying or dipping, sol-gel processing, and the like.
Mechanically interlocked interfaces, on the other hand, do not rely on the
presence of
an interfacial phase to achieve bonding of structural components, but instead
rely on
mechanical interactions between the surfaces of the structural components to
create
and maintain the bond. In certain embodiments, mechanically interlocked
interfaces
are designed to have rough features, known as asperities, that accommodate
inelastic
deformation through a sequence of sliding and interlocking events. The
sequential
sliding and interlocking at the interface, subject to transverse constraint by
the bulk of
the material, results in residual displacements accompanied by strain
hardening. The
sliding process along the interface relaxes stress concentrations and hence
retards the
9
RD 29346
CA 02509682 2005-06-10
formation of a dominant crack, while the strain hardening behavior allows
multiple
sites along neighboring interfaces to be activated and to participate in the
process. In
these embodiments, the amplitude and wavelength of the asperities along the
interface
are designed to be large enough to allow formation of multiple sites but not
large
enough to give rise to a local interlocking event of sufficient magnitude to
cause stress
concentration and failure of the material. For example, it is known in the art
that when
the interfacial sliding occurs under a friction coefficient of 0.01-0.1, the
asperities
have to be about 20-50 nm in amplitude and separated by a wavelength of about
50-
200 nm in order to achieve a proper balance of the factors described above.
Examples
of such interlocking interfaces can be found in the structure of nacre, also
known as
mother-of pearl. Compacted (but not sintered) powders and co-extruded fibers
(without interfacial phase additions) are examples of structural components
bonded by
mechanically interlocked interfaces in engineered materials.
The combination of hierarchical structure and specific bulk phase and
interfacial
phase materials selection creates in the material of the present invention an
advantageous condition in which mechanical damage, such as, for example,
cracks,
voids, porosity, and the like, originating within a modular size class
structural
component, is energetically favored, that is, requires less work, to propagate
in a
distributed fashion among the plurality of structural components contained
within the
modular size class structural component. Certain embodiments of the present
invention achieve this distributed failure mode by manipulating the properties
of the
interfaces 112. Referring to Figure 2, in some embodiments a toughness of the
interfaces 206 bonding together modular size class structural components 208
is
greater than a toughness of the interfaces 202 bonding together the plurality
of
structural components 204 contained within the modular size class structural
components 208. As described above, toughness is a term well understood in the
art
to mean the work, also referred to as energy, required to cause a complete
fracture of
a material. Thus the interfaces that are the easiest to fracture in the
material, the ones
along which damage is most likely to propagate, are the interfaces bonding the
base
unit size class components 204, and the interfaces are designed, often through
RD 29346
CA 02509682 2005-06-10
materials selection, to be progressively tougher as the size class of the
structural
components being bonded together by the interfaces increases. Additionally,
the
toughness of the interfaces 206 bonding the modular size class structural
components
208 together is less than a toughness of the at least one bulk phase 210, so
the
toughest portion of the material, the area least likely to propagate damage,
is the
material comprising the base unit size class components 210. In this way,
damage
212 is energetically favored to travel along the highly convoluted pathway
created by
the interfaces 202 of the smallest size class structural components, rather
than directly
through the material as is common in conventional ceramic materials, resulting
in
more energy being required to effect a complete fracture of the overall
material.
Catastrophic crack propagation is contained through crack blunting and
deflection by
the numerous interfaces between structural components, thereby providing a
more
distributive failure mode that allows the material to retain its integrity
even while
damaged. The material of the present invention thus takes advantage of the
benefits
of ceramic materials but has a significantly enhanced toughness than these
materials
generally exhibit.
The properties, such as the toughness, of the interfaces 112 (Fig. 1) may be
controlled
by manipulating the chemical composition of the interfaces 112, by
manipulating their
physical structure, or a combination of both of these. For instance,
interfaces
comprising boron nitride (BN) are used to bond base unit size class components
together into modular size class components. The modular size class components
are
bonded together at interfaces comprising a mixture of aluminum oxide and BN,
which
is tougher than BN, to form a material that energetically favors damage
propagation
along the BN interfaces, in accordance with embodiments of the present
invention.
Structure of the interfaces also may be manipulated to control interfacial
toughness.
For example, in some embodiments the interfaces 112 comprise material having a
predetermined porosity level. In particular embodiments, the porosity level of
an
interface 112 varies as a function of the size class of the structural
components 100
corresponding to the interface 112. For example, in some embodiments, the
chemically bonded interfaces (i.e., those interfaces that comprise an
interfacial phase)
11
RD 29346
CA 02509682 2005-06-10
comprise sintered material. Those skilled in the art will appreciate that by
controlling
sintering parameters, such as, for example, sintering temperature, time, and
starting
material, the porosity of the material being sintered, and hence that of the
interfaces in
this embodiment, can be controlled to a desirable level. Where coating or
infiltration
processes are used to dispose a material to be used as interfacial phase onto
a
structural component, the processing parameters are manipulated according to
known
relationships to control porosity levels to achieve the desired result. In
general the
higher the interfacial porosity, the weaker and less tough the interface is,
because
there is less material at the interface to create bonding sites.
Other embodiments of the present invention include an article comprising the
material
of the present invention, as that material is described herein. In particular
embodiments, the article comprises a component of a gas turbine assembly,
including,
but not limited to, turbine blades, vanes, shrouds, and combustor components.
Materials and articles in accordance with embodiments of the present invention
are
fabricated using any of a variety of techniques that are known to those
skilled in the
art, including, but not limited to, self assembly techniques, conventional
lamination
techniques, and lithographic techniques. Molecular self assembly techniques
are
"bottom-up" approaches to fabrication, in which chemical precursors having a
strong
polar character align themselves into predictable, periodic structures
assembled in
accordance with electrostatic interactions among the molecules of the
precursors.
Depending on the choice of precursors, this assembly may be spontaneous (due
to
naturally occurring interactions), or it may be stimulated by the application
of external
electric, magnetic, or other field, a technique known as "guided self
assembly."
Certain techniques use a combination of spontaneous and guided self assembly
to
stimulate the assembly of structures into useful three-dimensional geometries,
such as,
for example, fibers, sheets, and spheres. Multiple phase structures consistent
with
embodiments of the present invention, such as where the material comprises at
least
one bulk phase and at least one interfacial phase, may be obtained by, as a
non-
limiting example, assembling the bulk phase into a mesoporous "framework" and
then
infiltrating the pores with the interfacial phase as described above.
Interfacial phase
12
RD 29346
CA 02509682 2005-06-10
material may also be coated as previously described onto the surface of
particles or
assemblies comprising the at least one bulk phase using any of a number of
suitable
techniques well known in the art. At various steps along the processing route,
those
skilled in the art will appreciate that various conversion steps may be used
to convert
precursors into intermediate or final compositions. Conversion steps may
include
exposure to heat, other chemical compounds, electromagnetic radiation, and
other
external influences suitable to effect a chemical composition change. As a
result of
the molecular self assembly process, three-dimensional hierarchies of material
are
built up from molecular precursors, and can then be formed into the desired
shape by
any of several suitable processes, including, but not limited to, extrusion,
injection
molding, and the like.
Lamination techniques for processing composite materials are well known in the
art,
and are suitable for use in fabricating materials of the present invention. As
a non-
limiting example, monolithic ceramic fibers or rods of any of a wide variety
of cross
sectional shapes, having first interfacial material disposed in the
interstices between
the fibers (such as, for example, by coating the fibers or rods with the
interfacial
material prior to extruding) are co-extruded to form filaments, where each
filament
contains a plurality of fibers bonded together by the first interfacial
material. These
filaments then are coated with a layer of a second interfacial material that
serves as
the interfacial material binding filaments together when the filaments are
laminated
together, co-extruded, or otherwise processed to form the next level of
structural
component in the series. This second interfacial material is selected to have
properties, such as toughness, in keeping with certain embodiments of the
invention
described above, to promote the distribution of damage among the structural
components contained within the filaments (e.g., along the paths defined by
the first
interfacial material).
Photolithography and other lithographic techniques are another class of well-
known
fabrication techniques suitable for use in manufacturing materials of the
present
invention. These methods use combinations of targeted etching and selective
material
deposition to form a desired pattern on a substrate. The pattern may be two or
three
13
RD 29346
CA 02509682 2005-06-10
dimensional, and can be built up to a desired thickness by repetition of the
pattern a
requisite number of times. Appropriate use of known selective deposition and
etching
techniques enables the fabrication of the various structural components and
corresponding interfaces. For example, a layer of bulk phase material is
deposited on
a substrate, and then selectively etched to form a series of closely spaced
stripes of
bulk phase. The stripes are the base-unit size class structural component in
the
material being fabricated. A first interfacial material is then selectively
deposited in
the interstices between the stripes. This layer of bulk phase bonded together
with first
interfacial phase is a modular size class structural component. The layer is
then
coated with a second interfacial material having a higher toughness than the
first
interfacial phase but a lower toughness than that of the bulk phase, and then
another
Layer of bulk phase/first interfacial material is deposited on top of the
second
interfacial material as in the prior layer. This pattern may be repeated to
form a
hierarchical material of a desired thickness.
EXAMPLE
The following example is presented to further describe and explain embodiments
of
the present invention and is not to be understood as limiting the scope of the
invention
in any way.
Referring to Figure 3, a computer simulation was designed to model the
behavior of a
material S00 in accordance with embodiments of the present invention. The
structural
components of the base unit size class 502 were rectangular bricks in cross-
section,
and these bricks 502 were organized into rectangular modular size class
structural
components 504 such that each modular size component 504 comprised five
courses
of six bricks 502 per course. The first interfacial material 508 bonding the
bricks 502
together was modeled to have a toughness that was a factor of 0.1 times that
of the
second interfacial material 510 bonding the modular size class components 504
together, and a factor of 0.01 times that of the bricks 502 themselves. The
material
500 modeled in the simulation consisted of five courses of modular size class
structural components 504, at three components 504 per course. The model
simulated
14
RD 29346
CA 02509682 2005-06-10
a uniaxial stress state 512 applied to the material 500, wherein the boundary
of the
model material at one end was fixed and a constant displacement was applied to
the
opposite end, incrementally increased in steps of a fixed displacement
quantity. The
response load at this opposite end was calculated at each step, and the
stepwise
increase in displacement was carried out until the model indicated failure of
the
material. For comparison, referring to Figure 4, a second simulated material
600 was
modeled, where the second material 600 consisted only of bricks 602 and an
interfacial material 604 bonding the bricks together. The boundary conditions
were
the same for this comparison material 600, except this material 600 lacked the
presence of modular size class structural components 504 that are present in
the
material of the present invention. Thus the second simulated material 600
modeled a
single-level "hierarchy" and the first material 500 modeled a two-level
material
hierarchy.
The simulation results clearly indicated the superiority of the material of
the present
invention over that of a single-level hierarchical material. Figure 5 depicts
load-
displacement data for the two-level hierarchical material 500 (Fig. 3),
labeled as curve
A, and for the single-level material 600 (Fig. 4), labeled as curve B. Curve A
clearly
shows higher strength (relative height of curve) and toughness (area under
curve) than
curve B, demonstrating superior mechanical properties attributable to the
hierarchical
structure and materials selection of the material of the present invention.
While various embodiments are described herein, it will be appreciated from
the
specification that various combinations of elements, variations, equivalents,
or
improvements therein may be made by those skilled in the art, and are still
within the
scope of the invention as defined in the appended claims.