Note: Descriptions are shown in the official language in which they were submitted.
CA 02509845 2005-06-13
WO 2004/054646 PCT/EP2003/014424
UNIT DOSE DRY POWDER INHALER
FIELD OF THE INVENTION
[0001] The field of the invention is dry powder
inhalers.
BACKGROUND OF THE INVENTION
[0002] Inhalers are used to deliver drugs into a.
patient's lungs. Typically, an inhaler contains or provides a
mixture of drug particles and air or propellant gas. The
mixture is delivered via the patient inhaling from a
mouthpiece on the inhaler with the air carrying the drug
particles into the patient's lungs.
[0003] In dry powder inhalers, the drug particles, in
the form of a fine dry powder, are entrained into an airflow,
and inhaled by the patient, for treatment for various
conditions, for example, bronchial asthma. Drugs delivered
via a dry powder inhaler can be used to treat many
conditions, including those unrelated to lung conditions, via
the systemic absorption of the drug into the bloodstream, via
the lung.
[0004] For effective dose delivery using a dry powder
inhaler, the powder particles must first be dispersed to form
a powder/air aerosol. Various techniques for forming powder
aerosols have been proposed. One advantageous technique is
described in U.S. Patent No. 6,427, 688 and International
Application No. PCT/US01/03248.
[0005] While certain drugs, such as asthma drugs, may
be taken several times daily, other drugs, including certain
peptides or proteins, are typically taken less frequently.
Due to the delay in using these types of drugs after they are
removed from their packaging, providing a large number of
doses within a single package is not desirable, as some doses
may become unusable due to exposure to the environment. In
addition, many drugs are susceptible to a short shelf life
CA 02509845 2005-06-13
WO 2004/054646 PCT/EP2003/014424
2
when removed from a foil storage pouch or other sealed
container, even under nominal environmental conditions.
These types of drugs must be used almost immediately after
being exposed to the environment. Various other drugs are
also most often taken only in a single or a few dose. These
may include vaccines, antidotes, pain reducers, anti venoms,
as well as many others. Unit dose inhalers are also useful
for single dose treatments, non-chronic applications,
controlled or very expensive drugs where large quantities of
drug would not be acceptable, or for drugs where overdose or
abuse would have serious consequences. Unit dose inhalers may
also be advantageous for children where providing them with a
single dose only avoids the potential for overdosing.
(0006] Inhalation provides several advantages over
other delivery techniques such as .oral delivery via the mouth
or intravenous delivery using a syringe. Inhalation is fast,
patient friendly, non-invasive, and can provide rapid
absorption into the body. While unit dose inhalers have been
proposed in the past, they have met with only varying degrees
of success due to performance or other factors. Accordingly,
there is a need for an improved unit or single dose inhaler
for efficiently providing a prepackaged single dose of a
powdered drug.
BRIEF, STATEMENT OF THE INVENTION
(000?] In. a first aspect, a unit dose dry powder
inhaler has a dispersion chamber, optionally including one or
more beads. A sealed blister or dose container is supported
adjacent to the dispersion chamber. A mouthpiece cover, is
removable from a mouthpiece, with movement of the mouthpiece
cover causing the blister to tear or shear open. An air flow
path extends past, through or under the blister and into the
dispersion chamber. The blister remains sealed until the
inhaler is ready for use. The blister is then automatically
opened when the mouthpiece cover is removed from the
CA 02509845 2005-06-13
WO 2004/054646 PCT/EP2003/014424
3
mouthpiece. Pharmaceutical dry powder is released from the
blister and entrained in air flow through the inhaler, when
the user inhales on the mouthpiece. The powder is dispersed
in air within the, dispersion chamber and forms an aerosol
inhaled by the user.
(0008 In a second aspect, the blister or dose
container is formed with a first or top layer and a second or
bottom layer. A blister post is attached to at least a
portion of the bottom layer. As the mouthpiece cover moves to
an open position, the blister post moves away from the
blister, shearing out the bottom layer, releasing the dry
powder, and forming an air flow path through or by the
blister. Hold up of powder within the blister is reduced,
thereby more reliably providing a full unit dose to the user.
(0009 In a third and separate aspect, a blister is
adhered over an air inlet in a chamber front or top section.
A chamber back or bottom section has a top or first plate
attached to the chamber front, and a bottom or second piece,
including the blister post. In the closed position, a dust
cap covers a mouthpiece on the chamber front section. As the
dust cap is removed from the mouthpiece, the blister post is
moved away from the chamber front section arid the blister,
tearing or shearing out the bottom layer of the blister, and
releasing the powder contents of the blister for inhalation.
The dust cap may optionally be omitted, with the mouthpiece
covered by a separate rigid or flexible material cover, or by
providing the inhaler within a pouch, overwrap or package. In
this case, the blister or container is opened via movement of
the bottom plate or other surface supporting the blister
post.
(OOlOa Other objects, features, and advantages will
appear from the following detailed description.
(0011a The invention resides as well in
subcombinations of the features and steps shown and
described.
CA 02509845 2005-06-13
WO 2004/054646 PCT/EP2003/014424
4
(0012] It is an object of the invention to provide an
improved unit dose dry powder inhaler.
BRIEF DESCRIPTION OF THE DRA~TINGS
(0013] In the drawings, wherein the same reference
number indicates the same element, in each of the views:
(0014] Fig. 1 is a top perspective view of the
present unit dose dry powder inhaler, in the closed or
storage position.
(0015] Fig. 2 is a bottom perspective view of the
inhaler shown in Fig. 1..
(0016] Fig. 3 is a top perspective view of the dust
cap or mouthpiece cover shown in Fig. 1, before assembly.
(0017] Fig. 4 is a bottom or rear perspective view of
the chamber back section shown in Figs. 1 and 2, with the
back chamber assembly shown in an unfolded or unassembled
condition, for purpose of illustration.
[0018] Fig. 5 is a top or front view of the chamber
back section shown in Fig. 4.
(0019] Fig. 6 is a top perspective view showing the
first and second plates forming the chamber back section
being moved together for manufacture of the inhaler.
(0020] Fig. 7 is a top perspective view showing the
chamber back section of Figs. 4-6 in a fully pre-assembled
component.
(0021] Fig. 8 is a top perspective view of the
chamber front section.
(0022] Fig. 9 is a bottom or rear perspective view of
the chamber front section shown in Fig. 8.
(0023] Fig. 10 is a top perspective view of the
chamber back section shown in Figs. 4-7 joined to or
assembled with the chamber front section shown in Figs. 8 and
9, to form a chamber assembly.
CA 02509845 2005-06-13
WO 2004/054646 PCT/EP2003/014424
[0024] Fig. 11 is a top perspective view similar to
Fig. 10, and showing adhesive applied to illustrate the
manufacturing process.
10025] Fig. 12 is a top perspective view showing a
blister adhered to the assembly of Fig. 11.
10026] ' Fig. 13 is a top perspective view of the fully
assembled unit dose dry powder inhaler, in the open position,
with the dust cap of Fig. 3 attached to the dispersion
chamber assembly shown in Fig. 12.
[0027] Fig. 14 is a bottom and side perspective view
of the inhaler shown in Fig. 13.
10028] Fig. 15 is a section view taken along line 15-
of Fig. 1, and showing the inhaler in the closed position.
10029] Fig. 16 is a section view similar to Fig. 15,
and showing the inhaler in the open position.
10030] Fig. 17 is an enlarged detail view of the
blister and inhaler components shown in Fig. 16.
DETAILED DESCRIPTION OF THE DRAWINGS
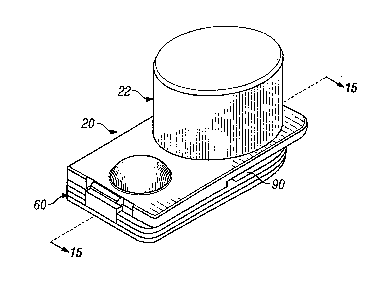
[0031] Turning now to the drawings, as shown in Figs.
1, 2, 13 and 14, a unit dose inhaler 20 includes a back
section 60 attached to a front section 90 which is covered by
a dust cap 22. Figs. 1 and 2 show the inhaler 20 in the
closed or storage position. The inhaler 20 as shown in Fig. 2
may optionally be enclosed within a sealed pouch, overwrapper
or container 26. Hence, while preferred, the dust cap 22
itself is not essential.
10032] Fig. 3 shows the dust cap 22 as a separate
component, before assembly into the inhaler of Figs. 1 and 2.
As shown in Fig. 3, the dust cap 22 includes a generally
cylindrical cap section 32 formed in a base plate 34. A
shield plate 44 is connected to the base plate 34 by a flex
joint or hinge 40. An actuating tab or tooth 50 extends into
a hinge slot 42 in the shield plate 44 at the flex or hinge
CA 02509845 2005-06-13
WO 2004/054646 PCT/EP2003/014424
6
joint 40. A blister shield dome 46 is formed in the shield
plate 44 between the hinge slot 42 and an edge cut-out 48.
The shield dome 46 protrudes upwardly in the opposite
direction of cap section 32, as shown in Fig. 3, which shows
the bottom surface of the dust cap 22 in its unassembled
position. A clearance hole 38 is located in the base plate
34, so that when the shield plate 44 is folded over against
the base plate 34, as shown in Fig. 1, the blister shield
dome 46 is aligned within and protrudes through the clearance
hole 38. A lift tab 36 on the base plate 34,provides a finger
grasping surface, for actuating the inhaler from the position
shown in Fig. 1, to the position shown in Fig. 14.
[0033] The dust cap 22, as well as the front section
90 and back section 60, are preferably made from molded
plastics materials, with all features molded in.
[0034] Turning now to Figs. 4, 5, 6, and 7, the back
section 60 includes a shear plate 62 attached to a chamber
plate 64 by a flex joint or hinge 66. As shown in Figs. 4 and
5, a plate tab 68 and blister post 70 extend downwardly from
the shear plate 62. A chamber clearance hole 72 is provided
in the shear'~plate 62 adjacent to the hinge 66. The chamber
plate 64 has a notch 80 to provide clearance for the plate
tab 68. Similarly, a blister post clearance hole 78 is
provided in the chamber plate 64 to provide clearance for the
blister post 70, when the shear plate 62 and chamber plate 64
are folded together to form the back section 60.
[0035] Referring still to Figs. 4 and 5, a rear
chamber wall 74 is formed in the chamber plate 64 adjacent to
the hinge 66. A rear air inlet slot or passageway 76 in the
chamber plate 64 extends from the tab slot 80, over the
blister post clearance hole 78 and into the chamber rear wall
74,~as best shown in Fig. 5. The back section 60 is assembled
by folding the shear plate 62 and chamber plate 64 together,
as shown in Figs. 6 and 7. The plate tab 68 moves into the
tab slot 80. The blister post 70 extends up through the
CA 02509845 2005-06-13
WO 2004/054646 PCT/EP2003/014424
7
blister post clearance hole 78. The chamber clearance hole 72
provides clearance for the rearward or downwardly projecting
chamber rear wall 74.
[0036] As shown in Fig. 7, as preassembled, the shear
plate 62 is substantially flush against the chamber plate 64.
The plate tab 68 and slot 80 are dimensioned to provide a
snap fit.
[0037] Turning to Figs. 8 and 9, the front section 90
has a generally cylindrical mouthpiece 96 and chamber tube 98
extending upwardly and perpendicularly from a blister plate
92. A blister opening 94 is provided in the blister plate 92,
to provide clearance for a blister. A blister plate tab slot
102 is provided at the top end of the blister plate 92, and
is adapted to provide clearance for, and a snap fit with, the
plate tap 68. A shield plate step 100 extends across the
blister plate 92, to facilitate assembly of the components of
the inhaler 20.
[0038] Referring to Fig. 9, a curved or domed front
chamber wall 106 is formed in the blister plate 92,
concentric with the chamber tube 98. A front air inlet 104
extends from the blister plate tab slot 102 over the blister
opening 94 and into the front chamber wall 106, similar to
the Complimentary features 76, 78, and 74 in the back section
60.
[0039] Referring to Fig. 10, the assembled back
section 60, as shown in Fig. 7, is attached to the front
section 90, via adhesives, sonic welding, snap fit, ~etc. As
shown in Fig. 10, the back section 60 assembled with the
front section 90, together form a dispersion or chamber
assembly 88. The top surface of the chamber plate 64 is
attached to the bottom surface of the blister plate 92. The
chamber back wall 74 in the chamber plate 64 aligns and comes
together with the chamber front wall 106 of the front section
90. The rear air inlet 80 on the chamber plate 64 aligns and
comes together with the front air inlet 104 in the blister
CA 02509845 2005-06-13
WO 2004/054646 PCT/EP2003/014424
8
plate 92, connecting into the dispersion chamber 108 formed
by the back and front chamber walls 74 and 106. The blister
post 70 extends up through the blister opening 94 in the
blister plate 92. The top surface of the blister post 70 is
substantially level with the top surface of the blister plate
92. The annular clearance or spacing between the blister post
70 and the blister plate 92 surrounding it ranges from 1-10,
2-6, or 3-5 mm. This spacing allows the blister post to tear
or shear out the bottom of the blister, without causing the
blister to crush in or extensively deform.
[0040] As shown in Figs. 11 and 12, an adhesive is
applied to the blister plate 92 around the blister opening
94. The adhesive 110 is also applied to the top surface of
the blister post 70. A blister 120 is then adhered to the top
surface of the blister plate 92, with the dome or
hemispherical section of the blister extending upwardly (in
the same direction as the mouthpiece 96). The blister 120
contains a dry pharmaceutical powder 126 sealed between a
base layer 122 and a. shear layer 124. These layers are
typically metal foil and may include other layers as well.
Various blisters may be used as described, for example, in
U.S. Patent No. 4,778,054, or International Patent
Publication WO 01/72605.
[0041] Referring to Fig. 13, the dustcap 22 is then
assembled to the chamber assembly 88. Specifically, the
shield plate 44 is placed over the blister 120, with the
outer edges of the shield plate 44 in contact with the
adhesive 110. This step is performed with the shield plate 44
folded over at the flex joint 40, so that the shield plate~44
is in contact with the base plate 34. With the completion of
this step, the inhaler 20 is fully assembled, as shown in
Figs. 1 and 2. The inhaler 20 may then be placed into a
sealed envelope or overwrap 26.
[0042] The dispersion chamber 108 formed by bringing
together the chamber back wall 74 of the back section 60 and
CA 02509845 2005-06-13
WO 2004/054646 PCT/EP2003/014424
9
the chamber front wall 106 of the front section 90 has an
inner rim 114 at the bottom end of the chamber tube 98 as
shown in Fig. 15. One or more beads 112 are captive within
the dispersion chamber 108, as described in International
Patent Publication WO 01/56640. The rear air inlet 76 and the
front air inlet 104 together form a chamber inlet 116,
extending from the top end of the inhaler 20 (at the slots 80
and 102), extending over (and/or around) the blister post 70
and into the dispersion chamber 108. One or more sheath air
openings 115 may optionally be provided through the
mouthpiece 96.
[0043] In use, as shown in Figs. 1, 2, and 15, the
dust cap 22 is in place over the mouthpiece 96. The
pharmaceutical dry powder 126 is sealed within the blister
120. The blister 120 is enclosed on top by the shield dome 46
and by the shear plate 62 below, to better avoid physical
damage to the blister 120 during storage. and handling. The
beads 112 are placed within the dispersion chamber 108 before
the back section 60 and front section 90 are joined together.
The inner rim 114 prevents the beads 112 from moving out of
the dispersion chamber 108 through the Chamber tube 98.
[0044] The user lifts up on the lift tab 36, pivoting
the base plate 34 from the closed position shown in Fig. 15,
to the open position shown in Fig. 16. This removes the cap
section 32, if used, from the mouthpiece 96.
[0045] Referring to Figs. 15, 16, and 17, as the base
plate 34 is pivoted to the open position, the tab tooth 50
pushes down on the plate tab 68, with a cam-like movement.
This drives the shear plate 62 downwardly. The blister post
70 moves down with the shear plate 62. As the shear layer 124
or bottom layer of the blister 120 is adhered to the blister
post 70, the movement of the blister post 70 shears or tears
out the shear layer 124 of the blister 120. As this occurs,
some of the powder 126 in the blister 120 will move or fall
out of the blister 120 into the air inlet or flow path 116.
CA 02509845 2005-06-13
WO 2004/054646 PCT/EP2003/014424
Some of the powder 126 remains on top of the now separated
shear layer 124' shown in Fig. 17.
L0046~ The user then places the mouthpiece 96 in the
mouth and inhales. Air is drawn through the inlet 116
carrying the powder 126 into the chamber 108. The air inlet
116 extends into the chamber 108 on a chord or tangentially.
The air drawn through the chamber 108 causes the beads 112 to
circulate rapidly around in the chamber. This bead movement
disperses and mixes the powder 126 with air. The air and
powder then move out of the chamber 108 through the chamber
tube 98, for inhalation by the user. Sheath or bleed air, if
used, flows in through the openings 115 and moves generally
parallel to the cylindrical side-walls of the mouthpiece 96.
The sheath air helps to reduce deposition of powder particles
in the mouth and throat allowing more of the particles to
reach the lung.
[0047 While the inhaler 20 may be used in any
orientation, the top end, at the plate tab 68, is preferably
to the side, or above the mouthpiece 96, for better movement
of powder 126 into the chamber 108. After use, the inhaler 20
may be collected for recycling, or it may be discarded.