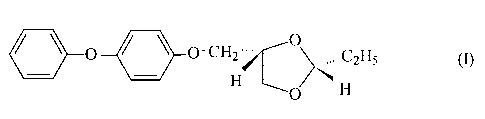Note: Descriptions are shown in the official language in which they were submitted.
CA 02510796 1992-02-21
30584-107D
-1-
Methyldioxolan
This is a divisional application of Canadian
Patent Application No. 2,061,630, filed February 21, 1992.
The present invention relates to the novel 2R,4S-
2-ethyl-4-[(4-phenoxyphenoxy)methyl]-dioxolan, to processes
for the preparation thereof, to pesticides comprising that
compound, and to the use thereof in the control of pests of
the division Arthropoda, especially of insects and
representatives of the order Acarina. The invention relates
also to novel intermediates which have been developed for
the preparation of the novel compound.
The subject matter of this divisional application
is directed to novel methyldioxolan compounds, pesticidal
compositions comprising the compounds, methods of
controlling insects using the compounds, processes for
producing the compounds, and intermediates useful in the
processes.
The subject matter of the parent was restricted to
a catalyst useful in producing the compounds. However, it
should be understood that the expression "the invention" and
the like, when used herein, encompasses the subject matter
of both the divisional and this parent application.
The 2R,4S-2-ethyl-4-[(4-
phenoxyphenoxy)methyl]dioxolan according to the invention
corresponds to formula I
O
~ O_S R 2 5 C H
(J-o
(I)
_ H
0 H
CA 02510796 1992-02-21
30584-107D
-1a-
In the literature, the enantiomeric mixture of 2-
ethyl-4-[(4-phenoxyphenoxy)methyl]-dioxolan is known, for
example, from DE-OS 2 655 910. The enantiomeric mixture is
distinguished by the fact that it can be used successfully
in the control of pests of the division Arthropoda,
especially of the class Insecta and of the order Acarina.
In spite of the good activity, the properties of the known
isomeric mixture were not always completely satisfactory
against all undesired pests when used as a pesticide. In
particular, phytotoxic effects are occasionally observed in
the treated crops of useful plants in unfavourable weather
conditions and in the case of unintentional overdosing, for
example when spray strips overlap as a result of wind drift
or inaccurate strip spraying. There is therefore a
continued need for a pest control composition having
improved properties.
Surprisingly, this need can largely be satisfied
by the use of the individual isomer of formula I proposed
according to the invention, since it has been found that, as
compared with the enantiomeric mixture, the 2R,4S-isomer
according to the invention not only has improved, greater
activity against the undesired pests, but also,
surprisingly, is better tolerated by the treated plants
than is the enantiomeric mixture. Increased activity and
reduced phytotoxicity result in a wider safety margin for
the user, which means that the
CA 02510796 1992-02-21
30584-107D
-2-
amount of active ingredient applied may be increased if necessary, for example
in order to
control effectively even pests that are difficult to control, without the
attendant risk of
damage to the treated useful plants.
According to the invention, therefore, 2R,4S-2-ethyl-4-[(4-
phenoxyphenoxy)methyl]-
dioxolan is proposed as a composition for pest control, especially for
controlling insects
and representatives of the order Acarina.
In principle, it is possible to obtain the compound of formula I according to
the invention
from the previously known enantiomeric mixture by separation methods suitable
for
enantiomers. Examples of such methods are physical methods, such as fractional
crystal-
lisation or chromatography, if desired also on chiral stationary phases, and
also derivati-
sation with defined optically active auxiliary substances and separation of
the resulting
enantiomer pairs by the mentioned separation methods. The pure optical
antipodes are
then obtained from such isolated enantiomer derivatives by removal of the
auxiliary
substance. In practice, however, it is in most cases advantageous to prepare
the desired
individual isomer by specific stereoselective synthesis.
By means of such specific synthesis methods, the compound of formula I is
obtained, for
example, either by
a) dehydrobrominating the bromoethyldioxolan of formula II
0 ~ ~ O-CH2,....... O
- ~ }- HCH3 (II)
p Br
in the presence of tert-butanol and potassium tert-butoxide, and hydrogenating
the
resulting ketene acetal of formula III
0 0 O-CH2,.........-= O \=
~ CH-CH3
H J 3 ( )
O/
with hydrogen in the presence of a palladium/calcium carbonate catalyst, or
CA 02510796 2006-10-18
30584-107D
-3-
b) reacting the diol of formula IV
0 o-CH2 ............. OH
(IV)
H OH
in the presence of the stereoselective catalyst of formula V
CH3
H3C I CF -SO
~ ( 3 3%
CH3 (V)
H3C P P
Rh
H3C-CN NC-CH3
NC-CH3
with a propionaldehyde acetal of formula VI
RIC2R5 0
>- (VI)
0
wherein R is C1-C4alkyl, preferably methyl or ethyl.
The above two processes are preferably carried out in suitable inert organic
solvents. For
process a) there are suitable especially alcohols, such as methanol, ethanol,
isopropanol or,
very especially, tert-butanol. For process b) there come into consideration
aromatic
hydrocarbons, such as toluene, xylene, mesitylene, benzene or Tetralin,Mor
halogenated
hydrocarbons, such as methylene chloride, chloroform, trichloroethane or
tetrachloro-
ethane.
CA 02510796 1992-02-21
30584-107D
-4-
Process variants a) and b) yield the product of formula I with a very high
optical purity.
Enantiomers having undesired orientation of the substituents on the two
asymmetric
carbon atoms of the dioxolan ring are formed in only very small amounts.
Typical degrees
of isomeric purity are 94 % in process a) and at least 90 % in process b).
In a typical reaction, process a) is carried out in such a manner that the
bromoethyl-
dioxolan of formula II is suspended together with two equivalents of potassium
tert-
butoxide in dry tert-butanol and heated at a temperature of from +80 C to +100
C for
approximately 4 hours. After cooling, this solution contains the ketene acetal
of
formula III. In order to avoid losses of that compound by hydrolysis and
decomposition
during the isolation, the resulting reaction product is reacted, without a
further purification
step, with hydrogen gas in an autoclave in the presence of a 2 % to 15 %,
preferably 5 %,
palladium-on-calcium carbonate catalyst. Typical reaction conditions for this
hydrogenation step are from 80 bar to 150 bar at temperatures of from +15 C to
+30 C.
Working up yields the product of formula I in the 2R,4S-configuration with a
purity of
94 %. 6 % of the resulting reaction product has the 2R,4R-configuration.
In its typical form, process b) is carried out in such a manner that the diol
of formula IV
and the propionaldehyde acetal of formula VI are introduced in equimolar
amounts into a
suitable solvent, and the catalyst of formula V is added in an amount of
approximately
from 0.01 to 0.0001, preferably 0.0005, molar equivalents. At a reaction
temperature of
from +20 C to +30 C, the reaction times until the starting materials have
reacted
completely are up to 48 hours. Working up yields the product of formula I with
a degree
of purity of over 90 % of the 2R,4S-isomer. The proportion of the 2R,4R-isomer
which is
likewise formed is less than 10 %.
The starting materials and intermediates of formulae II, III and IV have been
developed
specifically for the synthesis of the compound of formula I. The present
invention
therefore relates also to them. The intermediates of formula VI are known and
are avail-
able commercially. The acetalisation catalyst of formula V is also novel and
was
developed specifically for synthesis process b).
The compound of formula II is obtained in an acetalisation reaction, by
reacting the diol of
formula IV in the presence of an acid catalyst with an a-bromopropionaldehyde
acetal of
formula VII
CA 02510796 1992-02-21
30584-107D
-5-
R-O
)-- CHBr-CH3 (VII)
R-O/
wherein R is Ci-C4alkyl, preferably methyl or ethyl.
The reaction (IV + VII - II) is carried out under the conditions customary for
an
acetalisation reaction. Preferably, an inert organic solvent is selected, such
as an aromatic
hydrocarbon, for example toluene or xylene, and the reactants are heated in
equimolar
amounts in the presence of an acid catalyst until the starting materials have
reacted
completely, at a temperature corresponding to the boiling point of the
reaction mixture.
Suitable acid catalysts are both strong inorganic acids, such as HCl, H2SO4
and H3P04,
and also organic acids, such as F3C-COOH, F3C-S03-H and H3C-C6H4-SO3H, and
acid
ion exchangers.
If desired, the intermediate of formula III may be isolated from the reaction
mixture after
the first process step of process variant a), by working up the reaction
mixture after the
elimination of HBr with the exclusion of water.
The diol of formula IV is advantageously obtainable by the following two
processes.
According to a first process, the diol of formula IV is obtained by reacting 4-
phenoxy-
phenol of formula VIII
0 0 \ / OH (VIII)
in the presence of a base, such as potassium tert-butoxide, in a polar
solvent, such as
dimethylsulfoxide, with 4R-2,2-dimethyl-4-tosyloxymethyldioxolan of formula IX
H3C / \ S02 O CH2,,,,,,.,,~~~~~~ O
/< (IX)
H O
and subjecting the resulting 4-phenoxyphenylmethyl ether of forniula X
CA 02510796 1992-02-21
30584-107D
-6-
/ \ / \ o-cH2......... S
H /'<
-
(X)
0
to hydrolysis in the presence of an acid catalyst.
The hydrolysis reaction (X - IV) is preferably carried out in a solvent, such
as methanol,
ethanol, isopropanol or water. The reaction temperatures in both reaction
steps (VIII + IX
- X and X - IV) are generally from +10 C to +30 C. Inorganic and organic acids
may
be used as the acid catalyst. Examples are HCI, H2SO4, H3PO4, CF3SO3H and
F3C-COOH. Acid ion exchangers may also be used as acid catalysts. Both
reaction steps
generally produce yields of from 90 to 95 % of the theoretical yield. The
optical purity of
the product of formula IV is greater than 95 %.
In a second process, the diol of formula IV is obtained by reacting racemic
glycerol with a
camphorsulfonamide of formula XI
(XI)
CH2
I
S02-N(C3H7-i)2
in the presence of an acid catalyst, isolating from the resulting reaction
mixture the
crystallising individual diastereoisomer of formula XII
.,,,.......
HO CH2 ~~
S (XII),
H
o CH2-S02 N(C3H7-i)2
converting the latter into the mesylate of formula XIII
CA 02510796 1992-02-21
30584-107D
-7-
H3C-S02-O-CH2 ~~~~,....... R O
(XIII)
O CH2-S02-N(C3H7-i)2
in the presence of a base, by means of methanesulfonic acid chloride,
transetherifying the
intermediate of formula XIII in the presence of a base with 4-phenoxyphenol of
formula VIII, and hydrolysing the resulting adduct of formula XIV
a O 0 O-CH2....... S O
H (XIV)
O
CH2-S02 N(C3H7-i)2
in the presence of an acid.
The first reaction step of this process for the preparation of the diol of
formula IV is
carried out under conditions customary for an acetalisation reaction (XI -
XII). For
example, this condensation step can be carried out in the presence of acids
such as
p-toluenesulfonic acid, camphor-l0-sulfonic-acid or acid ion exchanger resin,
in an
anhydrous solvent, for example toluene or benzene, azeotropic distillation.
The 4S
individual diastereoisomer of formula XII can readily be separated from the
mixture of the
resulting acetals and identified on account of the fact that it crystallises
from the diastereo-
isomeric mixture. It has a melting point of from 98 to 100 C. The third
reaction step
(XIII - XIV) takes place under the customary conditions of an SN reaction in
the
presence of a base. The use of potassium carbonate as the base has proved
especially
suitable in the present process step. In the fourth reaction step (XIV - IV),
the hydrolysis
of the acetal body is carried out under customary conditions in the presence
of an acid,
such as HCI, H2SO4 or H3P04, in water or an aqueous reaction medium of
methanol,
ethanol or isopropanol. The diol of formula IV is obtained in this process
variant with an
optical purity of 98 % and a melting point of 88.4 C to 89.5 C. The optical
rotation is
RT
[ a] D =-6.34 . In addition to the diol of formula IV, the camphorsulfonamide
of
formula XI is recovered in the last step of the reaction sequence, so that it
can be used
CA 02510796 1992-02-21
30584-107D
-8-
again when the reaction to prepare the compound IV is repeated.
The staning materials of formulae VII, VIII, IX and XI are known and are
available
commercially. The intermediates of formulae X and XIV are novel. They were
developed specifically for the synthesis of the compound of formula I, and the
present
invention therefore relates also to them.
The novel stereoselective catalyst of formula V was likewise developed
specifically for
the synthesis of the compound of fornmula I. That stereoselective
acetalisation catalyst is
obtained by reacting 3 equivalents of methylphenylphosphine lithium of formula
XV
aP - Li (~l)
Hwith trichloroneopentane of formula XVI
H3C-C(CH2Cl)3 (XVI),
converting the resulting triphosphine of formula XVII
CH3
F CL P P P \ / \ (XVII),
H3C / CH3
H3C
by means of bicyclo[2.2.1]hepta-2,5-diene rhodium(I) chloride dimer of formula
XVIII
CA 02510796 1992-02-21
30584-107D
-9-
-===--Rh-CI
CI- Rh- - - (XVIII)
and silver trifluoromethanesulfonate (F3C-S02-O-Ag), into a complex of formula
XIX
CH3
H3C I
F3C-SOP
H3C P P CH3 (XIX)
h
and treating that pre-product in acetonitrile with trifluoromethanesulfonic
acid.
The starting materials of formulae XV, XVI and XVIII for the preparation of
the
catalyst V are known in the literature. The intermediates of formulae XVII and
XIX are
novel and were developed specifically for the synthesis of the novel catalyst
of formula V.
It has now been found that the compound of formula I according to the
invention is a
valuable active ingredient in pest control while being well tolerated by warm-
blooded
animals, fish and plants. The compound according to the invention can be used
especially
against insects and arachnids which occur on useful plants and omamentals in
agriculture,
especially in cotton, vegetable and fruit crops, in forestry, in the
protection of stored goods
and material stocks, and also in the hygiene sector, especially on domestic
animals and
productive livestock. It is effective against all or individual development
stages of
normally sensitive and also resistant species. Its action may manifest itself
in the death of
CA 02510796 1992-02-21
30584-107D
-10-
the pests immediately or only at a later date, for example at moulting, or in
reduced ovi-
position and/or a reduced hatching rate. The above-mentioned pests include:
of the order Lepidoptera, for example
Acleris spp., Adoxophyes spp., Aegeria spp., Agrotis spp., Alabama
argillaceae, Amylois
spp., Anticarsia gemmatalis, Archips spp., Argyrotaenia spp., Autographa spp.,
Busseola
fusca, Cadra cautella, Carposina nipponensis, Chilo spp., Choristoneura spp.,
Clysia
ambiguella, Cnaphalocrocis spp., Cnephasia spp., Cochylis spp., Coleophora
spp.,
Crocidolomia binotalis, Cryptophlebia leucotreta, Cydia spp., Diatraea spp.,
Diparopsis
castanea, Earias spp., Ephestia spp., Eucosma spp., Eupoecilia ambiguella,
Euproctis spp.,
Euxoa spp., Grapholita spp., Hedya nubiferana, Heliothis spp., Hellula
undalis, Hyphantria
cunea, Keiferia lycopersicella, Leucoptera scitella, Lithocollethis spp.,
Lobesia botrana,
Lymantria spp., Lyonetia spp., Malacosoma spp., Mamestra brassicae, Manduca
sexta,
Operophtera spp., Ostrinia nubilalis, Pammene spp., Pandemis spp., Panolis
flammea,
Pectinophora gossypiella, Phthorimaea operculella, Pieris rapae, Pieris spp.,
Plutella
xylostella, Prays spp., Scirpophaga spp., Sesamia spp., Sparganothis spp.,
Spodoptera spp.,
Synanthedon spp., Thaumetopoea spp., Tortrix spp., Trichoplusia ni and
Yponomeuta
spp.;
of the order Coleoptera, for example
Agriotes spp., Anthonomus spp., Atomaria linearis, Chaetocnema tibialis,
Cosmopolites
spp., Curculio spp., Dermestes spp., Diabrotica spp., Epilachna spp., Eremnus
spp.,
Leptinotarsa decemlineata, Lissorhoptrus spp., Melolontha spp., Orycaephilus
spp.,
Otiorhynchus spp., Phlyctinus spp., Popillia spp., Psylliodes spp.,
Rhizopertha spp.,
Scarabeidae, Sitophilus spp., Sitotroga spp., Tenebrio spp., Tribolium spp.
and
Trogoderma spp.;
of the order Orthoptera, for example
Blatta spp., Blattella spp., Gryllotalpa spp., Leucophaea maderae, Locusta
spp.,
Periplaneta spp. and Schistocerca spp.;
of the order Isoptera, for example
Reticulitermes spp.;
of the order Psocoptera, for example
Liposcelis spp.;
of the order Anoplura, for example
Haematopinus spp., Linognathus spp., Pediculus spp., Pemphigus spp. and
Phylloxera
spp.;
of the order Mallophaga, for example
Damalinea spp. and Trichodectes spp.;
CA 02510796 1992-02-21
30584-107D
-11-
of the order Thysanoptera, for example
Frankliniella spp., Hercinothrips spp., Taeniothrips spp., Thrips palmi,
Thrips tabaci and
Scirtothrips aurantii;
of the order Heteroptera, for example
Cimex spp., Distantiella theobroma, Dysdercus spp., Euchistus spp., Eurygaster
spp.,
Leptocorisa spp., Nezara spp., Piesma spp., Rhodnius spp., Sahlbergella
singularis,
Scotinophara spp. and Triatoma spp.;
of the order Homoptera, for example
Aleurothrixus floccosus, Aleyrodes brassicae, Aonidiella spp., Aphididae,
Aphis spp.,
Aspidiotus spp., Bemisia tabaci, Ceroplaster spp., Chrysomphalus aonidium,
Chrysomphalus dictyospermi, Coccus hesperidum, Empoasca spp., Eriosoma
larigerum,
Erythroneura spp., Gascardia spp., Laodelphax spp., Lecanium corni,
Lepidosaphes spp.,
Macrosiphus spp., Myzus spp., Nephotettix spp., Nilaparvata spp., Paratoria
spp.,
Pemphigus spp., Planococcus spp., Pseudaulacaspis spp., Pseudococcus spp.,
Psylla spp.,
Pulvinaria aethiopica, Quadraspidiotus spp., Rhopalosiphum spp., Saissetia
spp.,
Scaphoideus spp., Schizaphis spp., Sitobion spp., Trialeurodes vaporariorum,
Trioza
erytreae and Unaspis citri;
of the order Hymenoptera, for example
Acromyrmex, Atta spp., Cephus spp., Diprion spp., Diprionidae, Gilpinia
polytoma,
Hoplocampa spp., Lasius spp., Monomorium pharaonis, Neodiprion spp.,
Solenopsis spp.
and Vespa spp.;
of the order Diptera, for example
Aedes spp., Antherigona soccata, Bibio honulanus, Calliphora erythrocephala,
Ceratitis
spp., Chrysomyia spp., Culex spp., Cuterebra spp., Dacus spp., Drosophila
melanogaster,
Fannia spp., Gastrophilus spp., Glossina spp., Hypoderma spp., Hyppobosca
spp.,
Liriomyza spp., Lucilia spp., Melanagromyza spp., Musca spp., Oestrus spp.,
Orseolia
spp., Oscinella frit, Pegomyia hyoscyami, Phorbia spp., Rhagoletis pomonella,
Sciara spp.,
Stomoxys spp., Tabanus spp., Tannia spp. and Tipula spp.;
of the order Siphonaptera, for example
Ceratophyllus spp., Xenopsylla cheopis;
of the order Acarina, for example
Acarus siro, Aceria sheldoni, Aculus schlechtendali, Amblyomma spp., Argas
spp.,
Boophilus spp., Brevipalpus spp., Bryobia praetiosa, Calipitrimerus spp.,
Chorioptes spp.,
Dermanyssus gallinae, Eotetranychus carpini, Eriophyes spp., Hyalomma spp.,
Ixodes
spp., Olygonychus pratensis, Ornithodoros spp., Panonychus spp.,
Phyllocoptruta oleivora,
Polyphagotarsonemus latus, Psoroptes spp., Rhipicephalus spp., Rhizoglyphus
spp.,
CA 02510796 1992-02-21
30584-107D
-12-
Sarcoptes spp., Tarsonemus spp. and Tetranychus spp.; and
of the order Thysanura, for example
Lepisma saccharina.
The compound of formula I is suitable especially for controlling pests in
fruit and citrus
crops. In particular, scale-insects, such as Aonidiella aurantii, Saissetia
olea, Lepido-
saphes beckii, Quadraspidiotus perniciousus, Planococcus citri, Unaspis citri,
Ceroplastes
floridensis, Ceroplastes sinensis, Parlatoria pergandei and Lepidosaphes ulmi,
and fruit
pests, such as Adoxophyes orana, Cydia pomonella, Psylla piricola, Leucoptera
scitella
and Lobesia botrana, are controlled effectively. Good activity is also
observed against the
rice pest Nilaparvata lugens and against ticks, such as Boophilus microplus.
The good pesticidal activity of the compound of formula I according to the
invention
corresponds to a mortality of at least 50-60 % of the mentioned pests.
The activity of the compound of the invention and of the compositions
comprising it can
be substantially broadened and adapted to prevailing circumstances by the
addition of
other insecticides and/or acaricides. Examples of suitable additives include
represen-
tatives of the following classes of compounds: organophosphorus compounds,
nitro-
phenols and derivatives thereof, formamidines, ureas, carbamates, pyrethroids,
chlorinated
hydrocarbons, and Bacillus thuringiensis preparations.
The compound of formula I is used in unmodified form or, preferably, together
with the
adjuvants conventionally employed in formulation technology, and can therefore
be
formulated in known manner e.g. into emulsifiable concentrates, directly
sprayable or
dilutable solutions, dilute emulsions, wettable powders, soluble powders,
dusts, granules,
and also encapsulations in polymer substances. As with the nature of the
compositions,
the methods of application, such as spraying, atomising, dusting, scattering
or pouring, are
chosen in accordance with the intended objectives and the prevailing
circumstances. The
compound of formula I is also suitable for use in the treatment of seeds. It
is possible both
to treat or dress the seeds with the active ingredient or with a formulation
comprising the
active ingredient before sowing, and to apply the active ingredient to the
furrow at the
time of sowing.
The formulations, i.e. the compositions, preparations or mixtures comprising
the
compound (active ingredient) of formula I, or combinations of that compound
with other
CA 02510796 1992-02-21
30584-107D
-13-
insecticides or acaricides, and, where appropriate, a solid or liquid
adjuvant, are prepared
in known manner, e.g. by homogeneously mixing and/or grinding the active
ingredients
with extenders, e.g. solvents, solid carriers and, where appropriate, surface-
active
compounds (surfactants).
Suitable solvents are: aromatic hydrocarbons, preferably the C8 to C12
fractions of
alkylbenzenes such as xylene mixtures or alkylated naphthalenes, aliphatic or
cyclo-
aliphatic hydrocarbons such as cyclohexane, paraffins or
tetrahydronaphthalene, alcohols
such as ethanol, propanol or butanol, and glycols and their ethers and esters
such as
propylene glycol, dipropylene glycol ether, ethylene glycol, ethylene glycol
monomethyl
or monoethyl ether, ketones such as cyclohexanone, isophorone or diacetone
alcohol,
strongly polar solvents such as N-methyl-2-pyrrolidone, dimethyl sulfoxide or
dimethyl-
formamide, or water, vegetable oils such as rape oil, castor oil, coconut oil
or soybean oil;
and, where appropriate, also silicone oils.
The solid carriers used, e.g. for dusts and dispersible powders, are normally
natural
mineral fillers such as calcite, talcum, kaolin, montmorillonite or
attapulgite. In order to
improve the physical properties it is also possible to add highly dispersed
silicic acid or
highly dispersed absorbent polymers. Suitable granulated adsorptive carriers
are porous
types, for example pumice, broken brick, sepiolite or bentonite; and suitable
nonsorbent
carriers are calcite or sand. In addition, a great number of granulated
materials of
inorganic or organic nature can be used, e.g. especially dolomite or
pulverised plant
residues.
Depending on the nature of the compound of formula I to be formulated, or of
the
combinations of that compound with other insecticides or acaricides, suitable
surface-active compounds are non-ionic, cationic andJor anionic surfactants
having good
emulsifying, dispersing and wetting properties. The term "surfactants" will
also be
understood as comprising mixtures of surfactants.
Both so-called water-soluble soaps and also water-soluble synthetic surface-
active
compounds are suitable anionic surfactants.
Suitable soaps are the alkali metal salts, alkaline earth metal salts or
unsubstituted or
substituted ammonium salts of higher fatty acids (Clo-C22), e.g. the sodium or
potassium
salts of oleic or stearic acid, or of natural fatty acid mixtures which can be
obtained e.g.
CA 02510796 1992-02-21
30584-107D
-14-
from coconut oil or tall oil. Mention may also be made of fatty acid
methyltaurin salts.
More frequently, however, so-called synthetic surfactants are used, especially
fatty
sulfonates, fatty sulfates, sulfonated benzimidazole derivatives or
alkylarylsulfonates.
The fatty sulfonates or sulfates are usually in the form of alkali metal
salts, alkaline earth
metal salts or unsubstituted or substituted ammonium salts and generally
contain a
Cg-C22alkyl radical, which also includes the alkyl moiety of acyl radicals,
e.g. the sodium
or calcium salt of lignosulfonic acid, of dodecylsulfate or of a mixture of
fatty alcohol
sulfates obtained from natural fatty acids. These compounds also comprise the
salts of
sulfated and sulfonated fatty alcohol/ethylene oxide adducts. The sulfonated
benz-
imidazole derivatives preferably contain 2 sulfonic acid groups and one fatty
acid radical
containing approximately 8 to 22 carbon atoms. Examples of alkylarylsulfonates
are the
sodium, calcium or triethanolamine salts of dodecylbenzenesulfonic acid,
dibutylnaphthalenesulfonic acid, or of a condensate of naphthalenesulfonic
acid and
formaldehyde. Also suitable are corresponding phosphates, e.g. salts of the
phosphoric
acid ester of an adduct of p-nonylphenol with 4 to 14 mol of ethylene oxide,
or phospho-
lipids.
Non-ionic surfactants are preferably polyglycol ether derivatives of aliphatic
or
cycloaliphatic alcohols, or saturated or unsaturated fatty acids and
alkylphenols, said
derivatives containing 3 to 30 glycol ether groups and 8 to 20 carbon atoms in
the
(aliphatic) hydrocarbon moiety and 6 to 18 carbon atoms in the alkyl moiety of
the
alkylphenols. Further suitable non-ionic surfactants are the water-soluble
adducts of
polyethylene oxide with polypropylene glycol, ethylenediaminopolypropylene
glycol and
alkylpolypropylene glycol containing 1 to 10 carbon atoms in the alkyl chain,
which
adducts contain 20 to 250 ethylene glycol ether groups and 10 to 100 propylene
glycol
ether groups. These compounds usually contain 1 to 5 ethylene glycol units per
propylene
glycol unit.
Representative examples of non-ionic surfactants are
nonylphenolpolyethoxyethanols,
castor oil polyglycol ethers, polypropylene/polyethylene oxide adducts,
tributylphenoxy-
polyethoxyethanol, polyethylene glycol and octylphenoxypolyethoxyethanol.
Fatty acid
esters of polyoxyethylene sorbitan, e.g. polyoxyethylene sorbitan trioleate,
are also
suitable non-ionic surfactants.
CA 02510796 1992-02-21
30584-107D
-15-
Cationic surfactants are preferably quaternary ammonium salts which contain,
as
N-substituent, at least one C8-C22alkyl radical and, as funher substituents,
unsubstituted or
halogenated lower alkyl, benzyl or hydroxy-lower alkyl radicals. The salts are
preferably
in the form of halides, methyl sulfates or ethyl sulfates, e.g.
stearyltrimethylammonium
chloride or benzyldi (2-chloroethyl)ethyl ammonium bromide.
The surfactants customarily employed in fomnulation technology are described,
for
example, in.the following publications:
"McCutcheon's Detergents and Emulsifiers Annual", MC Publishing Corp., Glen
Rock,
NJ, USA, 1988,
H. Stache, "Tensid-Taschenbuch", 2nd edition, C. Hanser Verlag, Munich, Vienna
1981,
M. and J. Ash, "Encyclopedia of Surfactants", Vol. I-III, Chemical Publishing
Co., New
York, 1980-1981.
The pesticidal compositions usually comprise 0.1 to 99 %, preferably 0.1 to 95
%, of a
compound of formula I or combinations of that compound with other insecticides
or
acaricides, 1 to 99.9 % of a solid or liquid adjuvant, and 0 to 25 %,
preferably 0.1 to 25 %,
of a surfactant. Whereas commercial products will preferably be formulated as
concen-
trates, the end user will normally employ dilute formulations which have
considerably
lower active ingredient concentrations. Typical application concentrations are
from 0.1 to
1000 ppm, preferably from 0.1 to 500 ppm. The rates of application per hectare
are
generally from 1 to 1000 g of active ingredient per hectare, preferably from
25 to
500 g/ha.
Preferred formulations have especially the following composition (throughout,
percen-
tages are by weight):
Emulsifiable concentrates:
active ingredient: 1 to 90 %, preferably 5 to 20 %
surface-active agent: I to 30 %, preferably 10 to 20 %
liquid carrier: 5 to 94 %, preferably 70 to 85 %
Dusts:
active ingredient: 0.1 to 10 %, preferably 0.1 to 1%
CA 02510796 1992-02-21
30584-107D
-16-
solid carrier: 99.9 to 90 %, preferably 99.9 to 99 %
Suspension concentrates:
active ingredient: 5 to 75 %, preferably 10 to 50 %
water. 94 to 24 %, preferably 88 to 30 %
surface-active agent: 1 to 40 %, preferably 2 to 30 %
Wettable powders:
active ingredient: 0.5 to 90 %, preferably 1 to 80 %
surface-active agent: 0.5 to 20 %, preferably 1 to 15 %
solid-carrier: 5 to 95 %, preferably 15 to 90 %
Granules:
active ingredient: 0.5 to 30 %, preferably 3 to 15 %
solid carrier: 99.5 to 70 %, preferably 97 to 85 %
The compositions may aiso comprise further auxiliaries such as stabilisers,
for example
vegetable oils or epoxidised vegetable oils (epoxidised coconut oil, rape oil
or soybean
oil), antifoams, for example silicone oil, preservatives, viscosity
regulators, binders, tacld-
fiers as well as fertilisers or other active ingredients for obtaining special
effects.
The following Examples serve to illustrate the invention, but do not limit the
invention.
Example P1: 2R,4S-2-Ethvl-4-[(4-phenoxvphenoxy)methvlldioxolan
0 O-CH2..- O C H
7 ~_,..N' 2 5
H
O H
a) 2RS,4S-2-( I -bromoethyl)-4-[(4-phenoxyphenoxy)methyl)dioxolan
S \
H~.~__. /
}-- CHBr-CH3
167 g of 2R-2,3-dihydroxy-1-(4-phenoxyphenoxy)propane, 162.5 g of 2-
bromopropion-
aldehydediethyl acetal and 167 g of acidic ion exchanger resin (DOWEX 50W.8,
16-40 mesh, H(D form) are mixed with 1.3 litres of toluene and heated under t-
eflux for
5.5 hours. During that time, 700 m1 of an azeotrope of and toluene arti
distilled off
*'_'=ade-ma=k
CA 02510796 1992-02-21
30584-107D
-17-
from the mixture. After cooling to room temperature, the ion exchanger resin
is filtered
off and the filtrate is concentrated by evaporation. The residue is
chromatographed over
1.2 kg of silica gel using an eluant mixture of hexane/ethyl acetate (5:1).
223.3 g of
2RS,4S-2-(1-bromoethyl)-4-[(4-phenoxyphenoxy)methyl]dioxolan are obtained in
the
fornl of a slightly yellowish oil, nD20: 1.5691.
b) 4S-2-Ethylidene-4-[(4-phenoxyphenoxy)methyl]dioxolan
O
O & O-CH2,........... S
~ CH-CH3
H
A suspension of 50 g of 2RS,4S-2-(1-bromoethyl)-4=[(4-phenoxyphenoxy)methyl]-
dioxolan, 29.65 g of freshly sublimed potassium tert-butoxide and 500 ml of
tert-butanol
freshly distilled over CaO is stirred at a temperature of +95 C for 3.5 hours
under an argon
atmosphere. After cooling to +25 C, that mixture is used directly in the
following
reaction step.
c) The reaction mixture obtained under Example Pl b) is rinsed with a further
300 ml of
tert-butanol in an autoclave, with air-tight sealing. After the addition of
31.4 g of 5 %
dried palladium-on-calcium carbonate catalyst, hydrogen gas is introduced
under pressure
up to a pressure of 120 bar. The reactor is kept at +30 C for 38 hours. The
reaction
mixture is then diluted with 150 ml of ethanol and filtered over diatomaceous
earth. The
filtrate is concentrated by evaporation, taken up in ethyl acetate, washed
with water, dried
over sodium sulfate and concentrated by evaporation. The resulting 38.3 g of
crude
product are purified by chromatography over 500 g of silica gel using a
hexane/diethyl
ether mixture (9:1) as eluant, yielding 22.8 g of the product having an
optical purity of
94 % of the 2R,4S-enantiomer, and a further fraction of 8.55 g of the product
having a
purity of less than 90 %.
Example P2: 2R-2,3-Dihydroxy-l-(4-phenoxyphenoxy)propane
CA 02510796 1992-02-21
30584-107D
=I8-
R OH
0 0 0 0-CH2 ..................... C
~
H
CH2-OH
a) 4S-2-Spiro[(l0-diisopropylaminosulfonyl)-2-bomane]-4-hydroxymethyldioxolan
HO-CH2 ........ S 0
H
O CH2-S02-N(C3H-ri)2
4.8 g of 10-diisopropylaminosulfonylcamphor, 1.6 ml of glycerol and 230 mg of
p-toluenesulfonic acid monohydrate are taken up in 90 ml of benzene. The
suspension is
boiled for 37 hours using a water separator. If a thin-layer chromatogram
indicates an
incomplete reaction, a further 1.6 ml of glycerol and 234 mg of p-
toluenesulfonic acid are
added, and the mixture is boiled for a further 23 hours using the water
separator.
Concentration of the reaction mixture by evaporation and chromatography of the
residue
over silica gel using a hexane%thyl acetate mixture (4:1) yield, in addition
to 1.63 g of a
diastereoisomeric mixture, 1.45 g of the diastereoisomerically pure 4S-2-
spiroj(10-diiso-
propylaminosulfonyl)-2-bornane]-4-hydroxymethyldioxolan in crystalline form
with a
melting point of 98-100 C. The absolute configuration of the spiro-carbon atom
was not
determined.
b) 4R-2-Spiro[(10-diisopropylaminosulfonyl)-2-bornane]-4-
methylsulfonyloxymethyl-
dioxolan
H3C-S02-0-CH2 ~1"...... ~ O
H CH2-S02-N(C3H7-i)2
O
CA 02510796 1992-02-21
30584-107D
-19-
To a solution of 482.5 mg of 4S-2-spiro[(10-diisopropylaminosulfonyl)-2-
bornane]-4-
hydroxymethyldioxolan in 10 ml of methylene chloride there are added at 0 C
first 270 41
of triethylamine and then 120 ~.1 (1.54 mmol) of methanesulfonyl chloride.
After stirring
at room temperature for 5 hours, the slightly yellowish reaction solution is
poured onto
20 ml of 1N aqueous hydrochloric acid and diluted with 40 ml of methylene
chloride. The
organic phase is extracted twice with 20 ml of 1N hydrochloric acid each time.
All the
aqueous phases are washed with 2 x 50 ml of methylene chloride. The combined
organic
phases are dried over sodium sulfate and concentrated. Drying in vacuo yields
580 mg of
diastereoisomerically pure 4R-2-spiro[(10-diisopropylaminosulfonyl)-2-bornane]-
4-
methylsulfonyloxymethyldioxolan in the form of a colourless oil which
crystallises when
left to stand; m.p. 60.5-63.5 C.
c) 4S-2-Spiro[(10-diisopropylaminosulfonyl)-2-bornane]-4-(4-
phenoxyphenoxymethyl)-
dioxolan
O O-CH2,.... S 0
...
H >4
O CH2-S02-N(C3H7-i)2
1.5 g of 4R-2-spiro[(10-diisopropylaminosulfonyl)-2-
bornane]methylsulfonyloxymethyl-
dioxolan, 720 mg of 4-phenoxyphenol and 970 mg of anhydrous potassium
carbonate are
taken up in 10 ml of dimethyl sulfoxide, and the mixture is then stirred at
100 C for
20 hours. The reaction mixture is then poured onto water and extracted three
times with
ether. The ethereal phases are washed with water and brine, combined, dried
over
magnesium sulfate and concentrated. Chromatography of the oily residue over
silica gel
using a hexane/ethyl acetate mixture (8:1) yields 1.44 g of 4S-2-spiro[(10-
diisopropyl-
aminosulfonyl)-2-bornane]-4-(4-phenoxyphenoxymethyl)dioxolan in the form of a
colourless oil.
d) 2.04 g of 4S-2-spiro[(10-diisopropylaminosulfonyl)-2-bornane]-4-(4-
phenoxyphenoxy-
methyl)dioxolan are dissolved in 25 ml of methanol, and 10 ml of 4N aqueous
hydro-
chloric acid are added at room temperature. The suspension, which immediately
turns
milky, is stirred at +50 C for 16 hours. Then the reaction mixture is diluted
with a 9:1
mixture of methanol and water and extracted three times with cyclohexane. The
cyclo-
hexane phases are washed twice with 75 ml of a 9:1 mixture of methanol and
water,
CA 02510796 1992-02-21
30584-107D
-20-
combined, dried over magnesium sulfate and concentrated. 920 mg of a
colourless oil are
obtained as residue. The methanol/water phases are combined and concentrated
completely by evaporation. The residue is taken up in water and extracted
three times
with ether. The ethereal phases are washed with brine, combined, dried over
magnesium
sulfate and concentrated. In this manner, a further 2.1 g of colourless oil
are obtained as
residue. The two oily residues are combined (tota13.02 g) and stirred into
boiling hexane.
With vigorous stirring, the mixture is then slowly cooled to 0 C and the
crystallisate that
forms is filtered off. Drying in vacuo yields 797 mg of optically pure (more
than 98 % ee)
(-)-rotatory 2R-2,3-dihydroxy-1-(4-phenoxyphenoxy)propane, m.p. 88.4-89.5 C,
N
[a15s9=-6.34
By concentrating the mother liquor completely by evaporation, 1.08 g of the 10-
diiso-
propylaminosulfonylcamphor that was used are recovered in the form of an oil.
Example P3: 2R,4S-2-Ethyl-4-[(4-phenoxyphenoxy)methylldioxolan
a) a,a',a"-tris(methylphenylphosphino)neopentane
CH3
O p P\ Q
H3C / / P CH3
H3C
400 ml of a solution of 12 g of inethylphenylphosphine in degassed
tetrahydrofuran are
cooled to -78 C in a one-litre three-necked flask. 66.2 ml of butyllithium are
added drop-
wise within a period of 20 minutes, and the mixture is stirred at -78 C for
one hour. After
warming to room temperature, 5.62 g (0.032 mol) of a,a',a"-trichloroneopentane
are
added dropwise within a period of 35 minutes. The mixture is then heated under
reflux for
2 hours. 250 ml of degassed ether and 50 ml of degassed water are added to the
solution,
and the organic phase is separated off and dried over magnesium sulfate.
Removal of the
solvent by evaporation using a water-jet vacuum yields 12.14 g of a,a',a"-
tris(methyl-
phenylphosphino)neopentane as crude product in the form of a light-yellow oil.
CA 02510796 1992-02-21
30584-107D
-21-
Purification is effected by stirring the crude product, under reflux, with 250-
300 ml of
degassed methanol until the solution becomes clear light-yellow. It is cooled
to -78 C and
the methanol solution is drawn off from the frozen oil, yielding the
analytically pure
product in the form of a clear oil, yield 8.77 g.
Elemental analysis calculated for C26H33P3 (438.47):
C: 71.22; H:7.59; P:21.19
found C: 71.28; H:7.58; P:21.45
1H-NMR (200 MHz, CDC13): 7.59-7.26 (m, Ph),
2.30-1.78 (m, 2J (P,H) = 3.7Hz, PCH2)
1.34-1.23 (3xd,2 (P,H) = 3,7 Hz, PCH3,
RRS/SSR, RSR/SRS, SRR/RSS)
1.16 (d, 2J(P,H) = 3.7Hz, PCH3, RRR/SSS)
1.03 (s, CH3, RRR/SSS)
1.01 (s, CH3, RRS/SSR, RSR/SRS, RSS/SRR)
31P-NMR (200 MHz, CDC13): -44.89 (S, RRR/SSS)
-45.08 (s, RRS/SSR, RSR/SRS, RSS/SRR).
13C-NMR (200 MHz, CDC13): 141.6 (d, 1J(P,C) = 11.9Hz, PC1)
132.3 (d, 2J (P,C) = 19.9Hz, PC2), 128.5 (d, 3J (P,C) = 6Hz, PC3),
128.3 (s, PC4), 46.0 (d.t, 1J (P,C) = 14Hz, 4J (P,C) = 8Hz, PCH2),
38.4 (q, 3J (P,C) = 12.6Hz, CH3), 29.1 (d, 2J P,C) = 9Hz, C),
14.6 d, 1J (P,C) = 12Hz, PCHg).
b) a,a',a"-Tris(methylphenylphosphino)-neopentane-norbonanediene-rhodium(I)-
trifluoromethanesulfonate complex
CA 02510796 1992-02-21
30584-107D
-22-
O
CH3
~
H3C P
F3C-SO ~
CH3
H3C P P /
\Rh,
990 mg of a,a',a"-tris(methylphenylphosphino)neopentane in 20 ml of degassed
acetone
are introduced into a 250 ml two-necked flask and cooled to -78 C. 521 mg of
dimeric
rhodium(I)-norbonadiene-chloro complex and 581 mg of silver
trifluoromethanesulfonate
(Ag-O-SO2-CF3) are dissolved in 100 ml of degassed acetone, and the mixture is
stirred
for 20 minutes. At -78 C, the latter solution is added to the phosphine
solution over a
paper filter. The mixture is warmed to room temperature and the solvent is
removed by
evaporation. The crude yield of the resulting a,a',a"-
tris(methylphenylphosphino)-
neopentane-norbonadiene-rhodium(I)-trifluoromethanesulfonate complex in the
form of a
deep yellow powder is 1.74 g.
Recrystallisation is effected by dissolving the crude product in 23 ml of
acetone and
adding 18 ml of ether, which is added to the stirred solution carefully over a
period of
15 minutes. After a further 15 minutes, the solution becomes slightly cloudy
and, for the
purpose of crystallisation, is cooled to 0 C for 18 hours. The resulting
orange powder is
filtered off and washed with pentane. Yield: 360 mg of analytically pure
product.
The mother liquor is concentrated to about 20 ml of acetone, and then pentane
is added
until precipitation occurs; the precipitate is filtered off and washed with
pentane. In this
manner, a further 1.06 g of product are obtained.
Elemental analysis calculated for C3A.H41O3F3P3SRh (782.58);
C: 52.18 H: 5.28
found C: 51.68 H:5.32
31P-NMR (101 MHz, CDC13): 14.2 (d.t,1J(Rh,P) = 112Hz
CA 02510796 1992-02-21
30584-107D
-23-
2J(P,P) = 46Hz)
-3.7 (d.d.d,IJ(Rh,P) = 113Hz
2J(PS,PR) = 46Hz
2J(PS,PR) = 31Hz)
1H-NMR (200 MHz,CDCl3): 8.2-6.6(m,Ph), 3.7(m, = CH),
3.4(m,CH), 3.1(m,CH2),
2.5-2.0(3xd,1 J(P,H) =14Hz,P-CH3),
1.8-1.0(m,1J(H,H) = 7Hz
2J(Rh,H) = 140Hz,P-CH2)
1.9-1.7(g,4J(P,H) = lOHz,PCH3)
IR (RbI-Pressing): 3054 (m), 2987 (m), 2919 (m), 1433 (m)
1272 (s), 1221 (m), 1148 (s), 1097 (m)
1029 (s), 899 (s), 880 (s), 741 (m)
696 (m), 636 (m), 492 (m), 460 (m)
c) a,a',a"-Tris(methylphenylphosphino)-neopentane-tris(acetonitrile)-
rhodium(III)-tris-
trifluoromethanesulfonate complex
3p
CH3
H3C i (CF SO3~)
~ 3 3
H3C P 1KO
H3C-CN NC-CH3
NC-CH3
100 mg of the complex obtained according to Example P3c) are dissolved in 2 ml
of
degassed acetonitrile in a 10 ml flask, and the solution is saturated with
hydrogen gas for
minutes. 126 l of trifluoromethanesulfonic acid are added by means of a micro-
CA 02510796 1992-02-21
30584-107D
-24-
syringe, whereupon the orange solution turns yellow. After stirring for 15
minutes, the
a,a',a"-tris (methylphenylphosphino)-neopentane-tris(acetonitrile)-
rhodium(III)-tris-
trifluoromethanesulfonate complex is precipitated from the solution by means
of degassed
ether. The white precipitate is removed by centrifugation and washed with
ether and
pentane. Yield: 132 mg of analytically pure catalyst of formula V.
Elemental analysis calculated for C35H42N3O9FgP3S3Rh (1111.73):
C: 37.81 H: 3.81 N: 3.78
found C: 35.85 H: 3.72 N: 2.69
d) 26 g of 2RS-2,3-dihydroxy-1-(4-phenoxyphenoxy)propane and 14.5 g of propion-
aldehydediethyl acetal are dissolved in 30 ml of toluene. 55 mg of the
acetalisation
catalyst prepared according to Example 3Pc) are added to that mixture, which
is stirred at
room temperature for 50 hours, yielding 29 g of pure 2R,4S-2-ethyl-4-[(4-
phenoxy-
phenoxy)methyl]dioxolan having an optical purity greater than 90 %, in the
form of a
colourless oily liquid having a refractive index of nD20: 1.5459.
Example P4: 2R-2,3-Dihydroxy-l-(4-phenoxyphenoxy)propane
a) 4S-2,2-Dimethyl-4-[(4-phenoxyphenoxy)methyl]dioxolan
I S O
H x
O
A total of 25.3 g of potassium tert-butoxide are added in portions at room
temperature,
with stirring and under a nitrogen atmosphere, and with slight external
cooling, to a
solution of 38.1 g of 4-phenoxyphenol in 350 ml of anhydrous
dimethylsulfoxide. Then,
within a period of one hour and at room temperature, a solution of 64.4 g of D-
a,(3-iso-
propylideneglycerol y-tosylate in 30 ml of dimethylsulfoxide is added dropwise
to the
resulting green solution. The mixture is stirred at room temperature for 65
hours and is
then poured onto 500 ml of ice-water and extracted repeatedly with diethyl
ether. The
combined ethereal phases are washed repeatedly with water and saturated sodium
chloride
solution, dried over sodium sulfate, and the ether is removed by distillation,
yielding 4S-
2,2-dimethyl-4-[(4-phenoxyphenoxy)methyl]-1,3-dioxolan in the form of a light-
yellow
CA 02510796 1992-02-21
30584-107D
-25-
oil which solidifies in crystalline form after some time, [(X]D20 =+5.5
(CHC13)/purity ee
-99 %, m.p. 36.5-37 C.
b) 60 g of acidic ion exchange resin (DOWEX 50 W 8 H(I) form, 16-40 mesh) are
added to
a solution of 63.9 g of 4S-2,2-dimethyl-4-[(4-phenoxyphenoxy)methyl]-1,3-
dioxolan in
500 ml of pure methanol, and the mixture is stirred vigorously at room
temperature for
48 hours. The methanol solution is then filtered off from the ion exchanger,
the exchange
resin is washed repeatedly with methanol, and the solvent is removed from the
combined
methanol solutions by distillation in vacuo. The crude product is dissolved in
150 ml of
ethyl acetate with the application of heat, filtered while hot through a glass
suction filter, a
total of 125 ml of n-hexane are added, and the mixture is made to crystallise
by cooling.
The resulting colourless crystals of 2R-2,3-dihydroxy-l-(4-
phenoxyphenoxy)propane have
a melting point of 88.6-89.5 C, purity: ee -99 %, [a]D20: -6.7 0.8 (ethanol).
Formulation Examples (throughout, percentages are by weight)
Example Fl: Wettable powders a) b) c)
compound I 25 % 50 % 75 %
sodium lignosulfonate 5 % 5 % -
sodium laurylsulfate 3 % - 5 %
sodium diisobutylnaphthalene-
sulfonate - 6 % 10 %
octylphenol polyethylene
glycol ether (7-8 mol of
ethylene oxide) - 2 % -
highly dispersed silicic acid 5 % 10 % 10 %
kaolin 62 % 27 % -
The active ingredient or active ingredient combination is mixed with the
adjuvants and the
mixture is thoroughly ground in a suitable mill, affording wettable powders
which can be
diluted with water to give suspensions of the desired concentration.
Example F2: Emulsifiable concentrate
compound 1 10%
octylphenol polyethylene glycol
ether (4-5 mol of ethylene oxide) 3%
calcium dodecylbenzenesulfonate 3 %
CA 02510796 1992-02-21
30584-107D
-26-
castor oil polyethylene glycol ether
(36 mol of ethylene oxide) 4 %
cyclohexanone 30 %
xylene mixture 50 %
Emulsions of any desired concentration can be produced from this concentrate
by dilution
with water.
Example F3: Dusts a) b)
compound 1 5 % 8 %
talcum 95 % -
kaolin - 92 %
Ready-for-use dusts are obtained by mixing the carrier with the active
ingredient and
grinding the mixture in a suitable mill.
Example F4: Extruder granules
compound 1 10%
sodium lignosulfonate 2 %
carboxymethylcellulose I %
kaolin 87 %
The active ingredient or active ingredient combination is mixed and ground
with the
adjuvants, and the mixture is moistened with water. The mixture is extruded,
granulated
and then dried in a stream of air.
Example F5: Coated granules
compound I 3 %
polyethylene glycol (mol. wt. 200) 3%
kaolin 94 %
The finely ground active ingredient or active ingredient combination is
uniformly applied,
in a mixer, to the kaolin moistened with polyethylene glycol. Non-dusty coated
granules
are obtained in this manner.
Example F6: Susi?ension concentrate
CA 02510796 1992-02-21
30584-107D
-27-
compound I 40 %
ethylene glycol 10 %
nonylphenol po]yethylene glycol
ether (15 mol of ethylene oxide) 6 %
sodium lignosulfonate 10 %
carboxymethvlcellulose I %
silicone oil in the form of a 75 %
aqueous emulsion 1 %
water 32 %n
The finely ground active ingredient or active ingred.ient combination is
intimately mixed
with the adjuvants, giving a suspension concentrate from which suspensions of
any
desired concentration can be obtained by dilution with water.
Biological Examples
Example B 1: Action against Nilaparvata lugens
Rice plants are sprayed with an aqueous emulsion comprising 400 ppm of the
compound
of formula I. After the spray coating has dried, the rice plants are populated
with cicada
larvae in the 2nd and 3rd stages. Evaluation is made 21 days later. The
percentage
reduction in the population (% activity) is determined by comparing the number
of survi-
ving cicadas on the treated plants with that on untreated plants.
The compound of formula I exhibits good activity against Nilaparvata lugens in
this test.
Example B2: Action against Boophilus microplus
Adult female ticks which are replete with blood are affixed to a PVC plate and
covered
with a cotton wool swab. For treatment, 10 ml of an aqueous test solution
comprising
125 ppm of the compound of formula I are poured over the test insects. The
cotton wool
swab is then removed and the ticks are incubated for 4 weeks until oviposition
has taken
place. The action against Boophilus micropIus manifests itself either as
mortality or
sterility in the females, or as ovidical action in the eggs.
The compound of formula I exhibits good activity against Boophilus microplus
in this test.
Example B3: Ovicidal action against Adoxophyes reticulana
CA 02510796 1992-02-21
30584-107D
- 28 -
Egg deposits of Adoxophyes reticulana on filter paper are immersed for a short
time in an
aqueous acetone test solution comprising 400 ppm of the compound of formula I.
After
the test solution has dried, the eggs are incubated in petri dishes. After 6
days, the
percentage of eggs which have hatched is evaluated in comparison with
untreated controls
(% reduction in hatching rate).
The compound of formula I exhibits good activity against Adoxophyes reticulana
in this
test.
Example B4: Ovicidal action against Lobesia botrana
Egg deposits of Lobesia botrana on filter paper are immersed for a short time
in an
aqueous acetone test solution comprising 400 ppm of the compound of formula I.
After
the test solution has dried, the eggs are incubated in petri dishes. After 6
days, the
percentage of eggs which have hatched is evaluated in comparison with
untreated controls
(% reduction in hatching rate).
The compound of formula I exhibits good activity against Lobesia botrana in
this test.
Example B5: Action aaainst Aonidiella aurantii
Potato tubers are populated with crawlers of Aonidiella aurantii (red citrus
scale). After
about 2 weeks, the potatoes are immersed in an aqueous emulsion or suspension
comprising the test compound in a concentration of 400 ppm. After the treated
potato
tubers have dried, they are incubated in a plastics container. Evaluation is
made 10-12
weeks later by comparing the survival rate of the crawlers of the first
subsequent
generation of the treated scale population with that of untreated controls.
The compound of formula I exhibits good activity against Aonidiella aurantii
in this test.
In particular, the compound of formula I remains 100 % effective at a
concentration of
0.1 ppm, whereas with the previously known enantiomeric mixture having the
same
structure, such complete activity was achieved only at 0.75 ppm.
Example B6: Ovicidal action against Heliothis virescens
Egg deposits of Heliothis virescens on filter paper are immersed for a short
time in an
aqueous acetone test solution comprising 400 ppm of the compound of formula I.
After
the test solution has dried, the eggs are incubated in petri dishes. After 6
days, the
percentage of eggs which have hatched is evaluated in comparison with
untreated controls
CA 02510796 1992-02-21
30584-107D
-29-
(% reduction in hatching rate).
The compound of formula I exhibits good activity against Heliothis virescens
in this test.
In particular, this compound exhibits 90 % activity at only 200 ppm, whereas
the
previously known enantiomeric mixture is ineffective at 400 ppm.
Example B7: Phytotoxicity test
Cotton plants in the 4-leaf stage are sprayed with aqueous suspensions of the
test
compounds in concentrations of 2000, 1000, 500 and 250 ppm. After the spray
coating
has dried, the treated plants are cultivated in a greenhouse. After 7 days,
the test is
evaluated by assessing the damage in percent to the treated plants in
comparison with that
to untreated control plants.
Result: Test plant cotton
Evaluation 7 days after application
Phytotoxicity in %
Concentration Compound of Previously known enantiome 'c
ppm formula I mixture of the same structur
2000 15 25
1000 5 15
500 < 2.5 5
250 < 2.5 0
In this test, the compound of formula I exhibits markedly reduced damage to
the treated
cultivated plants as compared with the previously known enantiomeric mixture.
The same
degrees of damage were obtained with the previously known enantiomeric mixture
at half
the active ingredient concentration of the individual isomer of formula I
according to the
invention.