Note: Descriptions are shown in the official language in which they were submitted.
CA 02511183 2005-06-20
WO 2004/057068 PCT/EP2003/014786
APPARATUS AND METHOD FOR STORING PROTEINS
Field of the Invention
The invention relates to an apparatus and a method for the storage of
proteins.
Prior Art
One of the problems associated with the long-term storage of proteins is that
they lose
their biological properties over time as the molecule is degraded. Prior art
methods of
storing proteins have been developed to overcome this problem.
For example, Japanese Patent Application JP-A-63-092671 (I~anebo) teaches a
method
for the storage of proteins in which fibroin or collagen is dissolved in an
aqueous solution.
A hydrolysing enzyme is added to the solution followed by a chelating agent.
The pH of
the mixture is adjusted in the range 4.5 to 7.5 and the solution filtered
through a filter
having pores of around 1 ~.m or less. Finally the solution is dried to give a
polymer with an
average degree of polymerisation in the range of 200-600.
It would be desirable, however, to be able to store the protein in its natural
state and
without enzyme treatment or treatment with chelating agents.
In nature, several methods are known for the storage of proteins. Natural
silks are fme,
lustrous filaments produced by the silkworm Bombyx mori and other invertebrate
species
from a stored protein dope or feedstock. The silks offer advantages compared
with the
synthetic polymers currently used for the manufacture of materials and their
properties
seem to be substantially unaffected by long-term storage of the protein dope
within the
organism's gland. For example, the tensile strength and toughness of the
dragline silks of
certain spiders can exceed that of I~evlarTM fibre, the toughest and strongest
man-made
fibre. Spider dragline silks also possess high thermal stability. Many silks
are also
biodegradable and do not persist in the environment. They are recyclable and
are
produced by a highly efficient low pressure and low temperature process using
only water
as a solvent. The natural spinning process is remarkable in that an aqueous
solution of
CA 02511183 2005-06-20
WO 2004/057068 PCT/EP2003/014786
protein is converted into a tough and highly insoluble material. The process
in spiders and
silkworms produce silk threads has been outlined in an article "Liquid
crystalline spinning
of spider silk" by Vollrath and Knight, Nature, vol. 410, 29 March 2001, pages
541-8. The
authors note that lessons are to be learnt from the manner in which spiders
and silkworms
store their protein dope molecules and extrude them into strong threads. The
article
reviews the evidence that the natural spinning mechanism in spiders involves
an addition
of hydrogen ions and potassium ions and the removal of sodium ions as the
spinning dope
passes down the spinning duct.
In an article by Knight and Vollrath "Biological Liquid Crystal Elastomers",
Trans. Phil.
R. Soc. B. 357, 155-163 (2002), the authors point out that the assembly of
spidroin and
fibroin proteins to form tough threads depends on the fact that the chief
structural proteins
of silks, fibroins in silkworms and spidroins in spiders, are amphiphilic
repetitive block
copolymers of the type ABAB, where A represents a hydrophobic block and B a
less
hydrophobic one.
Spidroin and fibroin are found in two states: The first state is a safe
storage state in which
the extremely long protein chains are thought to be folded into short, rather
compact rod-
shaped molecules. The fibroin or spidroin proteins in this first state have a
predominantly
random coil and/or helical secondary structure. The second state is a solid
state with a
predominantly beta crystalline secondary structure. This second state is a
nanofibrillar
composite, containing a high packing fraction of very long nanofibrils
approximately 5
nm in diameter. The nanofibrils are oriented substantially parallel to the
long axis of the
tough thread and are thought to contain all or most of the beta crystallites.
The beta
crystallites have a width of about Snm and are arranged substantially parallel
to the long
axis of the nanofibrils. Small quantities of a less crystalline and more
disordered material
are thought to form the matrix between the nanofibrils.
The first, storage state is metastable. Its conversion to the second
nanofibrillar state is
thought to involve both an aggregation of the molecules and a change in
conformation of
the secondary structure. A change in conformation is thought to occur in the
most
hydrophobic of the block types of the repetitive block co-polymer which
transform from a
'random coil' / alpha helical to the beta crystalline structure. At least five
factors are
thought to be involved in the aggregation and conformation transition which
forms tough
2,
CA 02511183 2005-06-20
WO 2004/057068 PCT/EP2003/014786
threads in vivo: a reduction of the pH; the addition of potassium ions to the
protein; the
removal of sodium ions from the protein; the loss of water and the application
of
mechanical strain to the forming threads. In addition the conversion may be
promoted by
the secretion of polyols and surfactants. Fine longitudinal ridges with low
surface energy
in the final part of the spinning duct lining in spiders may help to promote
the conversion
of the protein dope to a solid thread. Once short nanofibrils have formed
these may act as
seeds initiating further aggregation and conformation change of the protein,
thus
enhancing the overall rate of the transition.
If solutions of the first storage state of fibroin or spidroin are left
untreated they transform
spontaneously into the insoluble beta crystalline state thus prematurely
forming insoluble
flocs or gels that cannot be extruded or spun into useful threads. The
transformation can
be remarkably rapid, usually taking 1-3 days for completion. This is a problem
when
extruding or spinning threads from silk proteins. Bacterial proteolysis may
play a part in
this transformation in vitro by cutting proteins into shorter peptides that
change in
conformation or crystallise more readily. Alternatively proteolysis may
promote the
transformation by removing protective domains which inhibit aggregation or
conformation change. Transformation of a native fibroin or spidroin solution
to the
insoluble form can also be promoted by seeding with material already
transformed into
the beta state. Freezing or mechanically shearing native solutions will also
result in
transformation to the beta crystalline state. Regenerated fibroin and spidroin
solutions
prepared by dissolving silk threads in a chaotropic agent such as lithium
bromide, lithium
thiocyanate, sodium thiocyanate, calcium chloride, calcium nitrate also
gradually undergo
an analogous formation of a floc or gel when the chaotropic agent is removed
by dialysis.
This again presents a problem when seeking to spin or extrude materials from
regenerated
silks.
The first storage state is found in the posterior and middle divisions of the
gland in
silkwornis and in the analogous A-zone in spiders. In these regions of the
respective
glands the protein is stored at remarkably high concentrations (20-40% w/v).
In spiders,
the protein is thought to be stored as a highly viscous liquid crystalline sol
that persists
through the first, second and most of third limb of the silk gland's duct. In
silkworms, the
protein is stored as a gel within the posterior and middle division of the
silk gland in
newly moulted final instar silkworms, but is transformed into a sol in the
duct (anterior
3~
CA 02511183 2005-06-20
WO 2004/057068 PCT/EP2003/014786
part of the anterior division) shortly before the protein is spun. Thereafter
the
transformation from gel to sol propagates backwards down the secretory pathway
to
enable the stored material in the middle and posterior divisions to flow
forward down so
that it can be drawn down to a filament in the anterior (distal part) of the
duct...
Summary of the Invention
The object of the invention is to provide an apparatus and method for the
storage of
proteins.
These and other objects of the invention are solved by providing an apparatus
for the
storage of a protein comprising a first compartment for storing the protein
and a second
compartment for storing an alkaline buffer. In the preferred embodiment of the
invention,
the second compartment contains an alkaline buffer containing calcium
chloride. The
second compartment is in fluid (i.e. liquid or gaseous) communication with the
first
compartment. The protein stored in the first compartment is therefore in an
alkaline
condition containing calcium ions under which conditions it is considerably
more stable
than untreated spidroin or fibroin solutions removed directly from the
organisms' glands
or prepared from by dissolving spider or silkworm silk in chaotropic agents.
The
decomposition or premature formation of the beta-sheet form formation of the
beta state
of the protein is thereby greatly retarded.
In one embodiment of the invention, the alkaline buffer is selected from the
group of
alkalis consisting of ammonia, ammonium acetate, ammonium formate, ammonium
citrate, Tris/HCI, HEPES, PIPES, sodium carbonate, potassium carbonate, sodium
phosphate, potassium phosphate or a mixture of these.
In an embodiment, sodium azide is added to the protein in addition to the
alkaline buffer.
Alternatively, phenyl thiourea, sodium cyanide or potassium cyanide might be
added.
Also in an embodiment, 100-700 mM of calcium ions are added to the alkaline
buffer,
preferably as chloride. Under these circumstances, the alkaline buffer may not
contain
carbonate or phosphate ions.
4:
CA 02511183 2005-06-20
WO 2004/057068 PCT/EP2003/014786
When calcium ions are added to the alkaline buffer, they cause concentrated
solution of
silk proteins or their analogues to gel. The gel produced in this way is
considerably more
stable than the sol state solutions of silk proteins. The gel state induced in
this way can be
converted back to a sol state before extruding, spinning or otherwise forming
the silk. This
can be achieved by dialysis against a dialysis solution containing 20-100 mM
ethylenediamine tetraacetic acid (EDTA) solution adjusted to pH 7.0-7.~ with
ammonia or
sodium hydroxide solution. This treatment removes calcium ions. Alternatively,
calcium
ions can be removed by dialysis against ion-free water. In either case
polyethylene glycol
or another water soluble polymer can be added to the dialsyant to maintain or
increase the
protein concentration by reverse dialysis.
In the preferred embodiment at least part of the inner surface of the walls of
the first
storage compartment are formed from or coated with a material with low surface
energy
such as polytetrafluoroethylene (PTFE), silane, nylon, polyethylene or
polycarbonate
which also helps to prevent premature formation of the beta sheet form of the
protein.
In the apparatus the alkaline buffer is in either a gaseous form or a
solution. If it is used in
a gaseous form it can be applied directly to the surface or surfaces of the
protein solution
or can be allowed to diffuse into it through a porous or semi-permeable
surface. If the
alkaline buffer is used in solution it can be separated from the protein by a
porous or
semipermeable membrane or a separate flow of buffer solution can be applied to
the flow
of protein solution and subsequently lead away from the surface of the protein
solution. If
the alkaline buffer is in a gaseous form it can diffuse directly into the
protein solution to
render it alkaline. If the alkaline buffer is separated from the protein by a
porous or
semipermeable membrane, then both buffer and calcium ions it may contain can
diffuse
through the said membrane into the protein solution.
In a further embodiment of the invention, the protein is mixed directly with
an alkaline
solution as this helps keep the protein in a sol state. Calcium ions can be
added directly to
the alkaline solution.
In one preferential embodiment of the invention, sodium azide is added to a
buffer with a
pH greater than 7.4. to give a final concentration in excess of 0.0001 M.
5~
CA 02511183 2005-06-20
WO 2004/057068 PCT/EP2003/014786
The stored protein can be either a natural protein obtained, for example, by
the dissection
of an animal, or recombinant protein obtained by genetically engineering or a
regenerated
silk solution prepared by dissolving silkworm or silk fibres in a chaotropic
agent that is
subsequently removed by dialysis, or a mixture of the aforementioned proteins
or protein
analogues. The invention has been found to be useful in the storage of fibroin
or spidroin
proteins or homologues thereof or regenerated solutions of fibroin and/or
spidroin.
More generally, the proteins stored are repetitive amphiphilic block co-
polymeric proteins
or protein analogues both containing charged groups and which are prepared by
chemical
synthesis or genetic engineering
The object of the invention is also solved by providing a method for the
storage of a
protein comprising a first step of placing the protein in a first storage
compartment. In a
second step, the protein is exposed to an alkaline buffer (preferably
containing calcium
ions and sodium azide) for a period of time. In a third step, the protein is
maintained in an
alkaline environment in the first storage compartment.
This provides a long-term storage solution for silk proteins or their
analogues and
regenerated silk solutions.
Description of the Drawings
Fig. 1 shows a schematic diagram of a first embodiment of an apparatus
suitable for
the storage of proteins.
Fig. 2 shows a schematic diagram of a second embodiment of an apparatus
suitable
for the storage of proteins.
Fig. 3 shows the results of effect of alkaline buffer and sodium azide on
storage times
for concentrated native fibroin solutions.
6,
CA 02511183 2005-06-20
WO 2004/057068 PCT/EP2003/014786
Detailed Description of the Invention
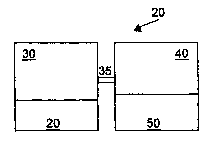
Fig. 1 is a schematic diagram illustrating a first embodiment of an apparatus
10 suitable
for storage of a protein 20. The apparatus 10 has a protein storage
compartment 30 in
which the protein 20 is placed. The protein storage compartment 30 is
connected by
means of a pipe or tube 35 to an alkali storage compartment 40. The alkali
storage
compartment 40 stores an alkaline solution 50. The protein storage compartment
30 has
preferably in part or substantially all inner walls which are made from or
coated with a
material with a low surface energy such as polytetrafluoroethylene (PTFE),
polyethylene
or polycarbonate.
The protein 20 in the protein storage compartment 30 can be a natural protein
that is
obtained, for example, by the dissection of an animal. Examples of such
natural proteins
include, but are not limited to, spidroin protein obtained from the major
ampullate gland
of spiders of the genus Nephila or fibroin protein obtained from Brombyx mori
or other
species of silkworm. The invention is also applicable to homologues of these
proteins or
recombinant proteins obtained by genetic engineering. The invention is further
applicable
to regenerated silk solutions prepared by dissolving silks in solutions
containing
chaotropic agents. More generally, it is thought that the invention is
applicable to the
storage of any proteins or protein analogues that are repetitive amphiphilic
block co-
polymers and which contain charged groups, although these materials are not
limiting of
the invention.
In the alkali storage compartment 40 several different types of alkaline
solution can be
used. For example, the alkali can be ammonia/acetic acid, ammonium acetate,
ammonium/formic acid, or ammonium fornlate. These buffers are volatile and
create in
the protein storage compartment 30 an alkaline atmosphere. Tris/HCI, HEPES or
PIPES
can be used instead but these buffers are not volatile. In one embodiment of
the invention,
the alkaline buffer is selected from the group of alkalis consisting of
ammonia,
ammonium acetate, ammonium formate and ammonium citrate buffer. Potassium
phosphate and potassium carbonate may also be suitable. In a preferred
embodiment of
the invention, the alkaline buffer contains 100-700 mM of calcium ions,
preferably added
in the fornl of calcium chloride.
7
CA 02511183 2005-06-20
WO 2004/057068 PCT/EP2003/014786
In the preferred embodiment sodium azide is also added to the protein 20 in
the first
storage compartment in addition to the alkaline buffer. In an alternative
embodiment of
the invention, phenyl thiourea, sodium cyanide or potassium cyanide is added
to the
protein 20.
In another embodiment of the invention, shown in Fig. 2, the protein storage
compartment
30 is separated from the alkali storage compartment 40 by means of a semi-
permeable or
porous membrane 60. The semi-permeable or porous membrane 60 allows the
passage of
ions to change the pH of the protein 20 stored in the protein storage
compartment 30. In
the event that a semi-permeable membrane is used, polyethylene glycol can be
added to
the alkaline buffer solution of up to 70% w/v to remove water from the protein
solution in
the protein storage compartment by reverse dialysis. Under these
circumstances, the
molecular weight of the polyethylene glycol used must be above the molecular
weight
cut-off of the semipermeable membrane. Other polymers can be used in this way
provided
that they are water soluble and are of sufficient size to prevent them from
passing through
the dialysis membrane.
Thus the protein 20 can be prevented from premature coagulation by treatment
in the first
compartment 30 for a period of time as short as one minute but preferably for
periods of at
least 20 minutes. This period of time depends on the quantity of the protein
20, its initial
pH value, the temperature, the surface area of the protein 20 exposed to the
alkaline
buffer, the distance through which the alkaline buffer is required to diffuse
to reach all of
the protein 20 and the buffering capacities of the protein 20 and of the
alkaline buffers.
In a preferred embodiment of the invention, the protein 20 is mixed with an
alkaline
buffer solution such as ammonium acetate or ammonium formate having a pH
higher than
7.4 and a concentration equal to or greater than O.1M. In the preferred
embodiment of the
invention, the alkaline buffer solution contains 100 to 700 mM of calcium ions
and in
excess of 0.0001 M sodium azide.
The suitability of different alkaline solutions for promoting the stability of
the protein 20
was assessed in two ways: First, small drops of concentrated protein solutions
were
dialysed against different alkaline buffer solutions for time periods of up to
four weeks
and the gel or sol state of the protein solution determined at intervals.
Typical results are
8,
CA 02511183 2005-06-20
WO 2004/057068 PCT/EP2003/014786
shown in Fig 3. Secondly, the validity of this approach was confirmed by
attempting to
spin fibres from small volumes of protein solution after storing them for
different lengths
of time in contact with alkaline buffers. In this second approach, a
biomimetic spinning
device of the general type described in PCT application No WO-A-01/38614 was
used,
the teachings of which are incorporated herein by reference. Tests with the
biomimetic
spinning device have shown that the protein 20 stored in contact with an
atmosphere
saturated with vapour derived from 1M ammonium hydroxide solution can still be
spun to
make a thread, fibre or filament after one week. The length of time for
storage can be
increased if sodium azide is added to the protein 20.
The apparatus and method can not only be used for storing natural and
recombinant
proteins, it may also be used to store regenerated solutions of fibroin and
spidroin both of
which have been prepared by dissolving silks made from this proteins in an
appropriate
solution containing a chaotropic (hydrogen bond breaking) agent. One example
of such a
chaotropic agent is a 50:50 v/v mixture of saturated lithium bromide and
absolute ethanol.
Example 1: Extension of Storage Time
In the following example, the storage time is extended for protein solutions
comprising
silk worm protein obtained from the silk glands of Bombyx mori, regenerated
Bombyx
mori fibroin solution or concentrated spidroin solution obtained from the
major ampullate
glands of Nephila spiders.
In a first step the protein solution was transferred to a dialysis bag (MWCO 5-
8 lcDa) and
concentrated by reverse dialysis against a solution containing 20% w/v PEG (MW
1 S-20
kDa) and 0.1 mM ammonium acetate puffer of pH 7.8 for five hours at
4°C.
The protein is gelled by dialysis against a solution containing 500 mM calcium
chloride
solution and 0.1 mM ammonium acetate buffer at pH 7.8 for one hour at
4°C. Sodium
azide can be added to the dialysant to a final concentration of 0.001 mM to
prevent
bacterial growth.
The resulting gel can be stored at 4°C for at least four weeks.
9;
CA 02511183 2005-06-20
WO 2004/057068 PCT/EP2003/014786
The resulting gel can be converted back to a sol by dialysis against distilled
water or
aqueous 100 mM ethylene diamine tetracetic acid solution prior to extrusion or
otherwise
forming the object.
Example 2: Demonstration that fibroin is stored as a gel prior to spinning in
a Bombyx
mori silkworm.
The state of the fibroin in the posterior, middle and posterior part
(glandular) of the
anterior division of silk gland of the Bombyx mori silkworm was assessed by
dissection
under a binocular microscope at different stages in the silkworm's
development. Glands
rapidly removed from silkworms were transferred to silkworm Ringer solution
(pH 7.8)
for observation. The material in the lumen of the gland appears to be
initially present as a
sol at all stages up until the anal instar a few days before cocoon spinning
commences
whereafter it is stored as a gel up to and during the initial stage of cocoon
spinning. By
cutting the duct (anterior part of the anterior division) across and watching
whether the
fibroin dope would flow out, it was demonstrated that the fibroin was present
as a sol in
this division immediately prior to and during spinning. Once spinning
commences, sol
formation appears to propagate progressively backwards through the silk dope
as the size
of the silk gland diminished during spinning. This demonstrates that gel
formation was
essential for safe storage of the silk and sol formation essential for the
flow of silk dope
down the duct for spinning.
Example 3: Effect of the addition and removal of calcium ions on the sol/gel
state of the
stored native fibroin
Solutions of fibroin dope were obtained by diluting the pooled contents of the
middle
division of the gland with 1 ml of 100 mM ammonium acetate buffer containing
10 mM
sodium azide and 100 mM EDTA adjusted to pH 7.8 with concentrated ammonia
solution
or acetic acid. These solutions could be rapidly gelled by addition of lvolume
of 1 M
calcium chloride to 1 volume of the fibroin dope solution or by dialysis
against 500 nM
calcium chloride aqueous solutions. The protein could be returned to the sol
state by
dialysis against distilled water or 100 mM ammonium acetate buffer (pH 7.8) or
more
rapidly by dialysis against 100 mM ammonium acetate buffer (pH 7.8) containing
500
mM EDTA. This suggests that the sol /gel transition can be induced by the
addition of
10~'
CA 02511183 2005-06-20
WO 2004/057068 PCT/EP2003/014786
calcium ions and the gel can be caused to revert to the sol state by removing
the calcium
ions again. The calcium-induced transition appeared to be reversible after
storage for days
and indeed weeks in the gel condition.
Example 4: Effect of calcium ion addition or removal on the storage of native
ftbroin
solutions.
A concentrated fibroin solution was obtained and gelled by the addition of 1 M
calcium
chloride as in described in Example 3. The length of time in which the gel
could be stored
stably in a state that could be turned into a sol by removal of calcium ions
was tested. To
do this samples of the gel that had been at 4° C for different lengths
of time were taken
and immediately dialysed against 100 mM ammonium acetate buffer (pH 7.8)
containing
500 mM EDTA. These observations indicated that the calcium-fibroin gel could
be safely
transformed into a sol after storage for at least four weeks. In contrast the
sol formed in
the absence of calcium ions could only be stored for 5-8 days at 4°C
before it formed a
polymerised material which could not be converted to a sol even by the
addition of further
EDTA. Similar results were obtained using regenerated silk solution prepared
by
dissolving degummed silk in 9.6 M lithium bromide and dialysing the resultant
solution in
a low molecular weight cut of dialysis bag (Spectropor 10 KDa) against a
solution
containing 100 mM lithium, 20~/°w/v polyethylene glycol (nominal
molecular weight
l5KDa) . The gel prepared by adding calcium ions as above to this solution was
however
considerably less stiff than that obtained by gelling native fibroin.
~,1