Note: Descriptions are shown in the official language in which they were submitted.
CA 02512227 2005-07-15
ENERGY RECLAIMING PROCESS
FIELD OF THE INVENTION
The invention relates to gaseous sources from which to reclaim energy using a
pressurized direct contact heat exchanger. In particular, those sources
containing a
condensable vapor While the main applications involve water as the condensable
vapor,
the process is applicable to other vapors, e.g. those in the chemical and
petroleum
industries where various organic solvents are used.
The reclaimed energy can be in the form of a hot fluid, process steam and/or
electricity. It has particular application to: a pressure combustion furnace
and to DOE's
Clean Coal Technology; the combustion of wet fuels (biomass, peat); pulp
&paper;
electrolysis of alumina or water; wet oxidation, thermal depolymerization ,
enhanced oil
recovery (and sequestering of carbon dioxide), phytotechnology,
If the source is not already under pressure, the invention converts it to a
higher pressure.
DESCRIPTION OF THE PRIOR ART
Present processes release large volumes of gas into the atmosphere, resulting
in a
loss of energy, especially the latent heat of any condensable vapor, resulting
in low
thermal efficiencies.
While various direct contact heat exchange systems have been proposed to
recover
this energy, all of them operate at close to atmospheric pressure and recover
mainly the
sensible heat and the temperature of the recovered fluids are near or below
the boiling
point of the fluid at the recovered pressure.
US Patents 3,920,505 and 4,079,585 are previous disclosures relating to the
recovery of waste sulfite liquors using a pressurized heat exchange process.
CA 02512227 2005-07-15
SUMMARY OF THE INVENTION
The basis embodiment of the invention comprises:
(a) providing a source from which to reclaim any additional energy from
pressurized
gases, continuously being produced within and/or emanating from the source,
and if
necessary, converting the source to a higher pressure, so that pressurized
gases are
produced;
(b) continuously bringing the pressurized gases into intimate contact with a
cooler
liquid, in a pressurized direct-contact heat exchanger, a vertical vessel
consisting of
various sections, including a hot well, where the gases will enter at the
bottom, flow
counter-current to a flow of the cooler liquid and where any condensable vapor
will
condense and the gases will become drier, and leave at the top where the
cooler liquid
enters, said exchanger being capable of being divided into several areas /
sections; the
first area being where any evaporative and heating property of the gases could
be used to
dry materials, a second area where part of the condensing and heating property
of any
vapor in the gas will be utilized to heat the cooler liquid to the highest
temperature it
could have when in equilibrium with the gases at the given pressure, and
thereby cool the
gases; as well as allow heated liquid and condensed vapor to collect in the
hot well within
the area, while still maintaining the highest possible hot well temperature,
and
continuously removing liquid from the hot well as reclaimed energy for further
use or
alternatively, continuously removing the liquid in the hot well and sending it
to a flash
chamber to produce vapor and the cooler flashed liquor reintroduced into this
second area
to cool further gases; and the third area is where the gas and liquid will
continue to
CA 02512227 2005-07-15
progressively exchange heat content and supply heated liquid to the hot well,
until the gas
approaches the temperature of the coot liquid entering at the top.
(c) continuously replenishing the cool liquid entering at the top of the
exchanger
(d) continuously removing the cooled gases from the top of the exchanger as
reclaimed energy for further use.
Other embodiments are listed below
BRIEF DESCRIPTION OF THE DRAWINGS.
To avoid complexity, valuing and other obvious operations are not always
shown,
or labeled e.g. exhaust steam from steam turbines could go to a condenser; the
gas
compressor in Fig. 3 and elsewhere could be connected directly to the turbine
expander,
along with an electric motor. An ''o" indicates a pump; particulate removers
would be
installed when they are required, etc.
The following drawings are schematic representations of the various
embodiments
/ applications of the present invention:
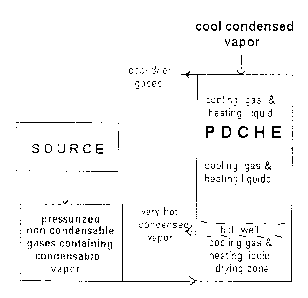
Figure 1 illustrates the main embodiment described above The figure shows two
"cooling gas and heating liquid "areas, as the liquid in the hot well can
alternatively be
sent to a flash chamber and the cooler flashed liquor reintroduced into the
second area to
cool further gases, see Figure IB.
Figure 1 A illustrates schematically how Carson's Fluidized Spray Tower can be
incorporated in the present invention.
-3-
CA 02512227 2005-07-15
Figure 1 B illustrates an embodiment where the liquid in the hot well is sent
to a
flash chamber / evaporator to produce steam and the cooler flashed liquor
reintroduced
into the second area to cool further gases.
Figure 1 C illustrates an embodiment where the hot water from the hot well is
sent
through a pressurized indirect contact heat exchanger, heated by the hot
gases, to produce
high temperature, high pressure steam, for use as process steam and / or to
generate
electricity using high efficiency steam turbines.
Figure 1D illustrates an embodiment where the steam from the flash chamber is
sent through a pressurized indirect contact heat exchanger, heated by the hot
gases, to
produce superheated steam.
Figure 2 illustrates an embodiment where a known process (Source) is adapted
to
produce the gases required for the embodiment shown in Figure 1
Figure 3 illustrates an embodiment where the gases from a known process
(Source)
are passed through a gas compressor to produce the pressurized hot gases
required for the
embodiment shown in Figure 1.
Figure 4 illustrates an embodiment where the liquid from the hot well is
heated to a
higher temperature indirectly before flashing it in the flash evaporator. The
indirect heater
could be located within the Source.
Figure 5 illustrates an embodiment where the pressurized gas-steam mixture is
heated prior to going to the pressurized direct contact heat exchanger.
Figure 6 illustrates an embodiment where the non-condensable gas content is in
the
low range and the gases are further pressurized by using a high pressure pump
which
-4-
CA 02512227 2005-07-15
condenses more of the water vapor prior to going to a secondary pressurized
direct
contact exchanger.
Figure 7 illustrates an embodiment where combustible material is combusted
under
the earth or sea and the gases processed above the site in the pressurized
direct contact
exchanger.
Figure 8 illustrates an embodiment where gaseous material under the earth or
sea
can be brought above and processed in the pressurized direct contact
exchanger.
Figure 9 illustrates an embodiment where a number of the embodiments are
involved in an overall process, applicable to the Pulp & Paper Industry.
Figure 10 illustrates an embodiment where the electrolysis of water under
pressure
supplies oxygen to a pressure combustion furnace and illustrating a further
symbiotic
relationship with the invention. Combining it with that of the embodiment of
Figure 9
would illustrate a further symbiotic relationship, in that the Paper Machine
Dryers could
also contribute further oxygen, present in the air and steam, to the
combustion step.
Figure 11 illustrates an embodiment where a pressurized direct contact
exchanger
is combined with a pressurized indirect heat contact exchanger, (which could
be located
within the Source), to generate high pressure high temperature steam, in order
to take
advantage of the higher efficiency of high pressure, high temperature steam
turbines.
Figurel2 illustrates an embodiment where greenhouse gases, such as carbon
dioxide, are produced which can be recycled through its use to accelerate
biomass
growth. In this embodiment a pressurized direct contact exchanger and
pressurized
combustion is combined with pressurized electrolysis of water to generate
pressurized
oxygen for the combustion, and hydrogen as a by-product, as well as produce
-s-
CA 02512227 2005-07-15
substantially pure carbon dioxide in the flue / exit gases, when the fuel is
essentially
carbon.
Figure 13 illustrates an embodiment where by operating a fuel cell at elevated
pressures and temperature and passing the hot gases through the pressurized
direct contact
exchanger, the efficiency of the cell is increased,
Figure 14 illustrates an embodiment where energy is reclaimed from a process
involving the electrolysis of alumina
Figure 15 illustrates an embodiment where energy is reclaimed from a process
involving the electrolysis of water.
Figure 16 illustrates an embodiment where energy is reclaimed from a process
involving the electrolysis of steam using the Cerametec Process and combined
with other
embodiments illustrated above. The steam from the flash evaporator could be
processed
as illustrated in Figure 1 C.
Figure 17 illustrates an embodiment where the electrolysis of water or steam
is
combined with other processes and embodiments and the results used in various
applications e.g. oil enhancement, phytotechnology .
Figures 18 & 19 illustrate an embodiment where various substances are
processed
in a pressure reactor and the reacting materials are handled in two different
ways to
produce steam.
Figure 20 illustrates an embodiment where thermal depolymerization is carried
out.
Figures 21 & 22 illustrate embodiments where gases existing at lower pressures
can produce hot fluids (which in the case of water can produce high pressure
steam and /
or electricity).
-6-
CA 02512227 2005-07-15
Figure 23 illustrates an embodiment where further energy can be reclaimed in
pressure combustion projects in the Clean Coal Technology Program sponsored by
the US
Department of Energy.
Figure 24 illustrates an embodiment where further energy can be reclaimed in
gasification projects in the Clean Coal Technology Program sponsored by the US
Department of Energy.
Figures 25 and 26 illustrate an embodiment where the invention can be applied
to
the recovery of bitumen (i.e. oil) from Oil Sands, including the recovery of
energy and
water.
Figure 27 illustrates an embodiment where the invention can be applied to a
new
paper technology referred to as Impulse Drying.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The following embodiments are process sequences that provide a wide range of
choice to fit a wide variety of circumstances, applications and available
technologies.
Because of the wide range of process variables involved and technologies to
choose from
it is nearly impossible to describe in any detail how a particular embodiment
is carried
out. For example, while many of the embodiments below will use water as the
condensable vapor it will be understood that wherever feasible there
embodiments can be
used for other condensable vapors, such as the many organic solvents used in
the
chemical industry. In most cases computer simulation will be required to
balance the
various variables such as the rate of recirculation of the hot well liquid
through the flash
chamber; the cool liquid supply; the excess liquid removal, which can be done
at the
appropriate location: etc.
CA 02512227 2005-07-15
The embodiments as illustrated and described is such as to obtain maximum
thermal efficiency, noting that, the higher the pressure and the lower the
temperature of
the gas leaving the pressurized direct contact heat exchanger, the lower the
vapor content
of the exit gases and the higher the thermal efficiency. Embodiments involving
lower
pressures are also being included, see Figures 21 & 22.
Referring to the accompanying drawings, the symbols used have the following
meaning
G Generator for electricity GT Gas Turbine
TC Turbine Compressor TE Turbine Expander
PR Particulate Remover M Motor electric
ST Steam Turbine C Condenser
P Pump PM Paper Machine
PDCHE Pressurized Direct Contact Heat Note: TC also represents
Exchanger
PICHE a rotary blower
Pressurized
Indirect
Contact
Heat
Exchanger
Referring to the drawings in greater detail.
Figure 1 shows the basic embodiment described above.
The gases can contain two components of heat, sensible heat and latent heat.
If
there is little or no condensable vapor in the gases, if will essentially be
all sensible heat
and the cooler liquid will extract heat and become hotter. If there is
condensable vapor the
cooler liquid will condense the vapor and the resulting heat will be absorbed
by the cooler
liquid and become hotter
Examples of further use for the condensed vapor in the hot well are numerous
and
well known in the trades in which a particular condensed vapor is involved,
and which
will also depend on the temperature of the condensed vapor in the hot well,
which is
determined by the pressure of the hot gases and the vapor pressure of the
condensed
liquid.
_g_
CA 02512227 2005-07-15
For example, if the condensed vapor is water and the pressure of the gases in
the
heat exchanger is 200 psia the temperature in the hot well will be somewhat
below 195 C
(382 F) depending on the efficiency of the heat exchange, Similarly, further
use of the
gases will depend on the type of non condensable gas involved and the dryness
of the gas
will depend on the pressure and temperature of the exiting gases, i.e. Henry's
Law of
Partial Pressures. For example, using the following equation for gas saturated
with water
vapor at t°F:
lb.mols H20 / lb.mols dry gas M = vapor pressure of water at t°F /
total pressure
- 1 - vapor pressure water at t°F / total pressure
we find that at 100°F & 250 psia M=0.0038 which is way below that of a
normal ambient
condition, so if this temperature was attainable for the exiting flue gases,
it would greatly
improve the overall thermal efficiency, especially if the air being fed into
the turbine
compressor had a high water vapor content. Even at 160°F & 250 psia M =
0.0193 & at
200 psia M = 0.0243 & at 150 psia M = 0.0326, all within a normal ambient
range.
These higher pressures are required when higher hot well temperature are
desired
and / or when the invention is used in connection with a flash evaporator /
chamber to
produce fairly high steam pressures and to concentrate effluents. The lower
end of
pressure spectrum, e.g, in the range of that produced by a rotary blower, can
be used to
reclaim the energy as illustrated below in Figures 21 & 22.
The cool dry pressurized gases are source of energy for the production of
electricity using a turbo-expander connected to a generator.
Where water is the condensed liquid in the hot well it could obviously be used
to
heat large living and business complexes especially in remote places. Further
use for the
cool gases and water in the hot well are described in the various embodiments
below.
-9-
CA 02512227 2005-07-15
While the various areas or zones of the pressurized direct contact exchanger
are some
times shown in one chamber, they could be located in separate chambers or
sections Here
the hot well is shown near the top of lower zone so as to illustrate that the
area below it
could be used to dry solid materials. Normally it would be near the bottom.
Various technologies are available in determining how the chambers are
constructed and the best type of heat exchanger to use, while maintaining
maximum heat
exchange and minimum pressure drop, e.g. the Field gas scrubber; bubble
columns;
packed towers; turbo-gas absorber; cascades; collecting the cooler liquid at
any point in
the pressurized exchanger and recycling it in the exchanger until its
temperature
approaches that of the gas; etc. While the cooling liquid introduced into
various areas is
shown as entering at one point, depending on the mixing technology used, it
could be
introduced at various points in each area or section. To increase the dwell
time of contact
between the gas and the cooler liquid, a portion of the descending liquid may
be
withdrawn from the top section and re-circulated back through the gas This
procedure
may be repeated at any place in the exchanger where it seems appropriate. The
top
location could be the best place to remove any liquid in order to maintain a
water balance
as its heat content would be the least.
A particular heat exchange process used for gases at atmospheric pressure is
the
"Fluidized Spray Tower" technology, recently developed by William D. Carson
and
disclosed in US Patent Application 20030015809). The disclosure of which is
hereby
incorporated by reference, as embodiments of that Process, designed to recover
heat from
non-condensable gases containing a condensable vapor, are directly pertinent
to this
-10-
CA 02512227 2005-07-15
invention, designed to recover heat from gases at pressures and temperatures
greatly
higher than presently attempted.
As illustrated schematically in FIGS lA, using water as the condensable vapor,
the
pressurized hot gases enter at the lower end of the First Tower and if
necessary can be
scrubber clean of solid material and leave with the waste condensate; Water
(heated) in
the Second Tower enters the First Tower to be further heated and accumulate in
the
''reservoir"' i.e. hot well; the still hot gases from the First Tower are
introduced into the
Second Tower to be further cooled and dried by the very cool water entering
the Second
Tower. For gases at lower temperatures, one Tower would suffice, possibly
using the
single chamber embodiment, and for very high temperatures possibly more than
two
Towers may be necessary.
The whole chamber or any one of the separate chambers could be located within
the confines of the Source depending on the process producing the hot gases
and other
factors. Further elaboration is given in various embodiments below.
Existing high pressure process sources include: (a) pressurized combustion
projects
in the Clean Coal Technology Program sponsored by the US Department of Energy,
where pressures up towards 250 psia are reached using combustion furnaces
developed by
such fines as Foster Wheeler, ABB (now Alstom Power), & Babcock & Wilcox; (b)
high
pressure char oxidation; processing of wood in digesters; etc.
In Figure 2, the Source involves a known process which does not provide the
pressurized gases required, but can be adapted to perform at a substantially
elevated
pressure and, if feasible, higher temperature as was done above for coal.
Examples:
CA 02512227 2005-07-15
Combustion / incineration of materials that produce water vapor, e.g. wet
combustibles.
While some emphasis is on biomass fuels, the process could have application to
the
combustion of (a) solid / liquid fossils fuels; (b) fuels intermediate between
the two i.e.
lignite (brown coal), peat, etc, where the high moisture content is a
deterrent to their use;
(c) Diverse fuels, such as Tire Derived Fuel (TDF), and various sludges, etc.
(2) Diverse processes such the smelting of ores; wet oxidation; chemical,
electrochemical,
metallurgical processes (blast furnaces), and intermediary operations such as:
drying;
stripping, extraction; boiling and the like.
In Figure 3, there is shown an arrangement wherein the increase in pressure of
the
source process cannot be carried out, then the gases from the source process
are turbo-
compressed to the desired pressure, with the temperature increased by the
compression.
For example in the drying of pulp or paper, enormous quantities of air and
steam are
expelled to the atmosphere, here the air-steam mixture could be turbo-
compressed and
their heat content recovered in the pressurized exchanger. See embodiments
below.
It is also possible, to collect other non-condensable gases containing water
vapor
(which are outside of the source) and turbo-compressing them to a pressure
sufficient to
introduce them into the source process. For example, in the above paragraph
the air-steam
mixtures could be turbo-compressed and introduced into a combustion furnace.
Other
such mixtures include naturally occurring ones such as fog banks, low clouds,
mists,
steam eruptions from the earth, etc.
In most of the following embodiments, water will be the "condensed vapor" used
in the examples. Embodiments involving the flash evaporator will generally
also be used
along with the use of low pressure steam turbines but it is understood that
wherever there
-t2-
CA 02512227 2005-07-15
is need to increase the thermal efficiency of the turbines the above
embodiments shown in
Figures C & D can be used.
The following embodiment involves expanding the alternative use of the hot
well
liquid of the main embodiment as follows:
continuously removing heated liquid from the hot well and flash evaporating it
in
a flash chamber at a pressure lower than the pressure corresponding to the
equilibrium or
hot well temperature to thereby ( 1 ) convert some of the water in the liquid
into steam and
(2) cool the liquid to a temperature corresponding to the pressure of the
flashed steam and
allow it to collect in a sump in the flash chamber, continuously removing
cooled liquid
from the flash chamber and re-introducing it to the direct contact heat
exchange section;
at a point in the second area where the gas in the area is at about the same
temperature as
that of the liquid in the sump, so as to cool further gases, and where the gas
and cooled
liquid will progressively exchange heat content, until the gas as it cools
approaches the
temperature of the liquid from the flash chamber; continuously removing the
flashed
steam from the flash chamber for further use;
This further embodiment is illustrated in FIG 1B, and examples of further use
for
the flashed steam and cool gases are also shown, namely, as process steam
and/or as a
source of energy for the production of electricity using steam turbines
connected to a
generator for the flashed steam; and as a source of energy for the production
of electricity
using a turbo-expander connected to a generator for the cool gases.
As mentioned above the temperature of the water in the hot well will depend on
the
pressure in the exchanger and correspondingly this will determine the pressure
of the
steam from the flash chamber. As mentioned, at 200 psia the temperature in the
hot well
-13-
CA 02512227 2005-07-15
will be somewhat below 195 C (382 F) so this could produce steam pressures in
a range
somewhere below 200 psia depending on the flashing potential used and several
other
factors, including the enthalpy of the gases,
It should be noted that the pressurized direct contact heat exchanger in
combination with a flash chamber / evaporator can concentrate cool effluents
containing
solids, used to cool the gases, which if combustible can be burnt in a
combustion furnace.
Examples are given below Another feature of this combination, is that when the
efficiency of lower pressure steam turbines has been significantly increased,
it will not be
necessary to use the embodiments illustrated in Figures 1C & 1D, and so avoid
the high
cost and maintenance of pressurized indirect contact heat exchangers
Figure 1 C illustrates how the hot well water can be upgraded, especially
where the
temperature of the hot gases is high enough, as it would be, for example, when
the gases
come from a high pressure combustion furnace. This means that the hot water
can now be
turned into high temperature, high pressure steam, and used in high efficiency
steam
turbines to produce electricity, by passing it through a pressurized indirect
heat exchanger
i.e.boiler. In Figure 1C it is shown separately but can be located within the
source e.g. a
pressure combustion furnace. Alternatively, if the Source is not pressurized,
the hot well
water can be upgraded by passing it through a conventional atmospheric boiler.
Figure ID illustrates how the medium to low pressure steam from the flash
chamber can be upgraded in order to improve the efficiency of the lower
pressure steam
turbines, should their efficiency not be high enough. Here the lower pressure
steam is
superheated by passing it through a pressurized indirect contact heat
exchanger, before
passing through the lower pressure steam turbines. The pressurized indirect
heat
-14-
CA 02512227 2005-07-15
exchanger i.e. a super-heater is shown separately but can be located within
the source e.g.
a combustion furnace. Similarly, as mentioned above the low pressure steam can
be
upgraded by passing it through a conventional atmospheric boiler.
Figures 9 & 10 illustrate how the overall efficiency of the process can be
upgraded
by passing the pressurized hot gases through a gas turbine connected to a
generator to
produce electricity, before they are sent to the pressurized direct contact
heat exchanger.
In this case, the pressure of the gases from the turbine should still be high
enough to
operate the exchanger satisfactorily. The gases from the turbines could also
go to a
pressurized indirect contact heat exchanger before going the direct contact
exchanger, as
illustrated in Figures 1C & 1D.
Which of the above embodiments is chosen could depend on which is less
expensive approach.
Figure 4 illustrates an arrangement where the liquid from the hot well is
heated
indirectly to a higher temperature to thereby increase the steam pressure in
the flash
evaporator. For example, by passing the liquid through a tube bank within the
source
process, should it be capable of heating the liquid.
Figure 5 illustrates an embodiment where the pressurized gases are further
heated
prior to going to a pressurized direct contact heat exchanger, For example, by
burning oil
or gas in the mixture, where it will consume any remaining oxygen or to which
additional
oxygen may be added, one can also heat the cool gases leaving the pressurized
direct
contact heat exchanger prior to them entering the turbine expander. For
example, by
burning oil or gas in the mixture, or by combining the operations of the
expander and
compressor and introducing inter-stage cooling and heating, as mentioned in
one
-15-
CA 02512227 2005-07-15
embodiment below. This may be necessary to avoid water condensing or freezing
in the
turbine expander, if the pressure is very high and the temperature low.
As previously mentioned, a further arrangement is where, if the pressure and
temperature of the hot gases from the source process are high enough, after
removing any
particulates, they are passed through a gas turbine connected to a generator
to produce
electricity, before being sent to the pressurized direct contact heat
exchanger.
This is particularly advantageous for a combustion process where high gas
temperatures
are achievable as illustrated in Figure 9 & 10. It could be important to dry
any wet fuels
prior to combustion so as to obtain a maximum temperature. The drying could be
done
using the gases after leaving the gas turbine as shown in Figure 9.
Oxygen, if required in any of the embodiments, is supplied by a source under a
pressure greater than the pressure required for the source of the pressurized
hot gases This
makes the process more efficient by eliminating the need for a turbine
compressor. The
electrolysis of water or steam is one such source, where it is more efficient
at the higher
pressures, with pressurized hydrogen as a valuable by-product This is
illustrated in
Figure 10 and expanded below. Alternatively, the oxygen may be supplied in
bulk or by
air liquefaction with nitrogen as a by-product.
By using cool liquids, containing dissolved or suspended materials as the
cooling
liquid, the liquid can be concentrated by the recycling of the liquid through
the
pressurized direct contact heat exchanger and flash evaporator. Once the
concentration of
the materials in the circulating liquor reaches the desired level, a portion
can be removed
at a rate that will prevent further concentration.
-16-
CA 02512227 2005-07-15
If appropriate, the liquid may be used in the source process, e.g. where that
process
is one of combustion and the material in the liquid is combustible. This is
illustrated in
Figures 9 & 10. (see below) Other such liquids are effluents from many other
mills, as
well as from sewage treatment plants.
Other examples would be (a) the desalination of salt water, the liquor would
provide a source of salt and the condensed steam a source of salt-free water
suitable for
irrigation; (b) concentration of dilute sugar sources, i.e. cane, beet and
maple sugars,
where any residues or forest biomass can be combusted under pressure to
produce the hot
gases; water associated with oil from the wells (producer water) when
separated from the
oil can serve as the cool liquid and when concentrated can be added to the oil
and burnt
and the noncombustible pollutants removed in the ash for proper disposal; etc.
It is also possible that the first area of step (b) in the embodiment of FIG
l, is used
to dry materials. Here all or a portion of the hot gases would be introduced
into a chamber
containing the material to be dried, and the drying done in a number of ways,
such as
flash drying, a fluidized bed, rotary tumble drier, etc, and the dry or
partially dried
material removed through a screw press or decompression chambers, etc or sent
directly
to the Source. Various bio-masses, such as peat, lignite, bark, leaves,
branches, roots, and
many other materials considered as waste can thus be dried or partially dried.
The gases
after being so used and before the saturation temperature has been reached,
would be sent
to the rest of the pressurized direct contact heat exchanger. If the dried
material is still
considered waste and is combustible and the source process is one of
combustion then it
can be sent there and consumed. This is illustrated in Figures 9 & 10.
CA 02512227 2005-07-15
Undesirable solids and/or gases present in the hot gases can be removed in the
heat exchanger by maintaining the circulating liquid alkaline for acidic gases
and acidic
for alkaline gases. The substances so formed can then be concentrated and
removed from
the flash evaporator (see above).
This could allow greater use of fossil fuels containing a high sulphur
content. If the
solids / gases are very soluble in the water, they could be put through a
scrubbing
chamber prior to the pressurized direct contact heat exchanger, were a minimum
of liquid
could reduce their concentration.
Illustrated in Figure 6 is where the non-condensable gas content is in the low
range. Here the pressurized hot gases are sent to a primary pressurized direct
contact heat
exchanger and processed through the first and second areas of the main
embodiment, then
they are removed from the exchanger at a temperature close to that of the
temperature of
the flashed liquid in the evaporator and fed to the suction side of the pump
which is
removing the flashed liquid from the flash evaporator, which is capable of
pressurizing
this removed mixture to a pressure which will condense most of the steam in
this removed
gas mixture, this pressurized liquid and gas mixture is then sent to a
secondary
pressurized direct contact heat exchanger where the liquid and gases separate
at a
temperature corresponding to that of the pump pressure, the separated liquid
in the
secondary pressurized direct contact heat exchanger is sent to the top of the
primary
pressurized direct contact heat exchanger at a point where the removed gases
exit, the heat
content of the separated gases containing a low amount of steam can then be
recovered as
desired e.g. in a turbine expander connected to a generator, etc.
-18-
CA 02512227 2005-07-15
In certain applications, it is desirable to minimize the presence of the non-
condensables in the source process, e.g. in the pressurized thermomechanical
pulping of
wood chips, by presteaming the chips prior to their entering the refiner.
If the steam from the flash evaporator is unsuitable for a particular use, or
cannot
be cleaned by conventional means, it is passed through a reboiler for further
use.
As illustrated in Figure 7, where the source process is a combustion process
carried
out under the earth or sea under pressure, where there is combustible
material, where the
combustion is supported by a pressurized gas containing oxygen and controlled
by water
piped to the combustion site from above the site. The pressurized hot gases
would be
piped to a pressurized direct contact heat exchanger above the site and
processed utilizing
any of the other embodiments that will give the desired result
Illustrated in Figure 8 is an embodiment where the source process is carried
out
below the earth or sea under pressure, where there is recoverable material,
and where the
process is activated by high pressure steam, preferably superheated steam,
which allows
the material to flow to a pressurized direct contact heat exchanger above the
site and
processed as for any of the other embodiments.
As illustrated, high pressure super-heated steam could flow down an insulated
pipe to
melt the methane hydrate ice and allow it and steam to flow up another pipe to
the
pressurized direct contact heat exchanger above the site to be dried as in
Figure 1.
Alternatively, the two pipes could consist of concentric inner and outer
pipes, with
the steam flowing down the inner pipe to melt the hydrate, which will flow up
the outer
concentric pipe which is wide enough to trap the methane and in which the
pressure is
less than that of the liberated methane. Some of the methane could be used in
a
-t9-
CA 02512227 2005-07-15
conventional boiler to produce the steam and the water supplied from the hot
well. The
end product would be a pressurized, substantially dry methane gas.
This could also be applicable to number of fossil fuels, e.g. unmineable,
gassy coal
beds containing methane; wells of natural gases, volatile oils, etc after the
wells have been
somewhat depleted; where the steam will act as a sweep gas.
Figure 9 illustrates how a number of the above embodiments can function within
the one process, with particular application to the Pulp and Paper Industry
where it forms
a somewhat symbiotic relationship.
A collector receives air-steam emissions from the paper and pulp mill,
especially
those from the drier section of the paper machines (other sources not
indicated include
those from thermomechanical pulping processes). This air-steam mixture,
monitored for
the correct amount of air required for combustion, is passed through a turbine
compressor
where it is compressed to a pressure high enough for the process to generate a
steam
pressure suitable for the dryers of the papermachine, as well as operate a gas
turbine e.g.
250 psia. and higher. The compressed air-steam mixture goes to the pressure
combustion
furnace where combustible wet fuels are burnt to produce hot flue gases.
Auxiliary fuel,
oil or gas, can be added to the hot gases and burnt to maintain uniform
combustion and an
optimum temperature for the gas turbine. (see above)
These hot gases are passed through a particulate remover and a gas turbine and
then through a first section or area of the pressurized direct contact heat
exchanger, a
drier, which dries biomass material, e.g. forest waste and bark including,
liquid
concentrate from the flash evaporator, to a moisture content amenable to
combustion in
the pressure combustion furnace. From the drier the flue gases pass to the
main second
-20-
CA 02512227 2005-07-15
section or area of the pressurized direct contact heat exchanger a scrubber,
where they
come into intimate contact with a liquid concentrate, containing dissolved and
suspended
solids from paper & pulp effluents. In applications where only an effluent
concentrate is
to be combusted or the wet fuels are dry enough to combust, the drier would be
omitted
and the flue gases would pass directly to the pressurized direct contact heat
exchanger.
The above concentrate would be generated in the initial start-up of the
process as the
dilute effluent is concentrated in the flash evaporator.
By continuously removing the heated concentrate and evaporating it in the
flash
evaporator at a pressure lower than that corresponding to the equilibrium or
hot well
temperature, so as to (a) convert some of the water in the concentrate into
steam, (b)
further concentrate the liquid, and (c) cool the concentrate to a temperature
lower than the
hot well temperature, and then returning the cooled concentrate from the flash
evaporator
to be reheated in the pressurized direct contact heat exchanger; and removing
the steam
from the flash evaporator, much of the heat content of the flue gases is
converted into
process steam.
The saturated flue gases from the main pressurized direct contact heat
exchanger,
after they are cooled to approximately the temperature of the liquid
concentrate from the
evaporator, are passed through the last section or area of the pressurized
direct contact
heat exchanger to come into intimate contact with cool dilute effluent to
further cool the
flue gases and preheat the effluent;
Thus depending on the temperature of the entering effluent and the efficiency
of
the pressurized direct contact heat exchanger heater, if the pressure of the
flue gases is
around 250 psia the water content in the flue gases could be approximately
0.10 lbs per lb
-21 -
CA 02512227 2005-07-15
of dry flue gas, which is that of the water content of most ambient air, and
the thermal
efficiency of the process could approach 90% depending on other factors.
Then by continuously removing some of the heated concentrate and adding the
required preheated dilute effluent, the proper liquid concentration and
balance in the
system can be maintained.
The cooled flue gases from the pressurized direct contact heat exchanger
heater are
passed through a turbine expander to recover some of remaining enthalpy, which
is used
to compress the air-steam mixture. If necessary they can be put through a
particulate
remover before going through the turbine expander. Any make-up power for the
compression can be supplied by a motor or, while not shown in the drawing, the
cooled
flue gases can be passed through a combustion chamber in which oil or gas can
be burnt
to heat the gases to the required temperature before they pass through a
turbine expander.
(See the above embodiment) Any excess power can used to generate electrical
energy by
arranging for the motor to also act as a generator.
To remove any acidic gases from the flue gases, alkaline substances can be
added
to the liquor circulating in the pressurized direct contact heat exchanger. By
a proper
choice of substances these will reappear in the ash being removed from the
furnace, a
portion of which may then be extracted using hot dilute effluent and returned
to the
pressurized direct contact heat exchanger.
The rest of the drawing illustrates how the water from effluents and the steam
in
the emissions from the paper and pulp mill is recycled back to mill. The steam
from the
flash evaporator if necessary is passed through a particular remover or a
reboiler and then
sent back to the paper machine dryers, or some used in the pulp mill. Any
excess steam
-22-
CA 02512227 2005-07-15
can be used to generate electrical energy using condensing steam turbines. The
condensate from the dryers is used as clean make-up water at the wet end of
the paper
machine. This water reappears again in the white waters from the wet end which
are sent
to a fiber recovery system, from which they appear in the effluents from that
system and
are sent to the effluent collector, where they join effluents from the pulp
mill. Condensate
from the steam turbines can be used similarly in the paper & pulp mill where
it will return
via the effluents from the mill. To increase the efficiency of the steam
turbines the steam
from the evaporator can be processed as illustrated in Figure 1D.
Figure I O further illustrates how flexible the invention is and that it can
even enter
into further symbiotic relationships with other processes. One such process is
the
electrolysis of water under pressure (mentioned in the embodiment above)
Electrical
energy required for the electrolysis is supplied directly by any generator
adapted to
produce the direct current, as converting alternating current to direct
current is inefficient.
If the pressurized hydrogen, so produced, is not also used in the source
process e.g. where
carbon monoxide is produced and this is combined with the hydrogen to form
methanol, it
becomes a very valuable by-product. If the electrolysis unit is located where
further
oxygen is required e.g .for pulping and bleaching, this may be a further
advantage.
Depending on the choice of material being burnt the exit gas will be fairly
pure carbon
dioxide, another by-product of the process, which has a wide use e.g. for
urea, methanol,
enhanced oil recovery, refrigeration , ete.
In a further embodiment energy can be removed from the pressurized direct
contact heat exchanger for various purposes, and the resulting cooled liquid
returned to
the pressurized direct contact heat exchanger to be reheated. For example, a
primary flash
- 23 -
CA 02512227 2005-07-15
evaporator produces steam at the highest possible pressure level, the flashed
liquid from
the primary is then flashed in a secondary flash evaporator to produce steam
at a lower
level, if desired this sequence could be continued or, at any stage, the
flashed liquid could
be used to indirectly heat other media e.g. hot water heating of a building,
with the final
cooler liquid returned to the pressurized direct contact heat exchanger for re-
heating.
Similarly, by subdividing the hot well liquid and liquid after flashing and
using several
independent circulating systems, the rates of circulation, which may depend on
the rate of
steam production, are not inflexibly tied in with rates and methods of cooling
the
combustion hot gases.
In an embodiment the cooled gases from the top of zone are cooled further, in
order to reclaim further latent heat, by bringing them into indirect contact
with the cooler
gases between expansion stages in the gas expander. This is an example of how
inter-
stage-cooling and inter-stage-heating could be practiced in a counter-current
or parallel
arrangement.
One can be combine various embodiments wherein the electricity produced is one
of direct current which is then fed directly to the electrolysis of water,
thereby increasing
the efficiency of the overall process. This can also apply to any electricity
produced in
steps (e) & (g). Similarly, in the case of the electrolysis of steam, the
process can supply
the direct current as well as the steam as illustrated in Figure 16.
In some arrangements, advantages of other operations can be made use of in the
pressurized direct contact heat exchanger process. For example, transportation
of
materials by pipeline can often be less expensive than that by land or air.
Thus, after the
appropriate comminution of the material and its suspension in water, it can be
pumped to
-24-
CA 02512227 2005-07-15
the primary site, where the wetted material is not a problem and the excess
water can be
used to cool the gases in pressurized direct contact heat exchanger and any
dissolved /
suspended material in the water concentrated in the flash evaporator. This
could be very
useful for pressure combustion processes, where the combustible material (e.g.
coal, peat,
and various biomasses) can be transported to the combustion site by pipeline.
Figure 11 illustrates how the pressurized direct contact heat exchanger is
combined with a pressurized indirect contact heat exchanger, by generating
high pressure
steam in order to take advantage of the higher efficiency of high pressure,
high
temperature steam turbines. While the amount of energy extracted by the
pressurized
indirect contact heat exchanger will vary depending on the application, a
maximum
amount would require that enough energy be left in the hot gases in order to
operate the
pressurized direct contact heat exchanger so the latent energy of the water
vapor in the
gases can be extracted in the flash evaporator.
While the pressurized indirect contact heat exchanger is shown as a separate
chamber outside of the source, it could be located within the confines of the
source
depending on the process producing the hot pressurized gases. Where the source
is a
combustion process, the pressurized indirect contact heat exchanger could
consist of tube
banks located within the combustion chamber.
A pressurized indirect contact heat exchanger can be introduced into any one
of the
above embodiments depending on the desired outcome.
In certain circumstances it may be possible to maximize the thermal efficiency
further by combining both gas and steam turbine technologies with the
pressurized direct
contact heat exchanger Process, by extracting some of the energy first in a
gas turbine,
- 25 -
CA 02512227 2005-07-15
then further energy in a pressurized indirect contact heat exchanger using
high pressure
steam turbines (as shown in the above) and finally the remaining energy in a
pressurized
direct contact heat exchanger using the steam generated there either as
process and / or in
lower pressure steam turbines. Where the generation of electrical energy is
the prime
objective, this embodiment could offer the highest thermal efficiency. This
could be the
case for generation of electricity from coal, especially high sulphur coils.
(See
embodiment above)
Another application involves coal bed methane and the sequestering of carbon
dioxide, where unmineable, gassy coal beds are swept with pressurized gases
containing
carbon dioxide which releases the methane and traps the carbon dioxide. The
gases
containing carbon dioxide are also effective in increasing oil recovery, by
reducing its
viscosity and providing a driving force towards the wells The addition of
water / steam
improves the sweep efficiency and the water can be recovered in the
pressurized direct
contact heat exchanger.
In these applications, by using the already pressurized gases from the
pressurized
direct contact heat exchanger the cost of the pressurization of the gases is
avoided.
In this technology, while one objective is the removal of the polluting carbon
dioxide, in
other situations nitrogen is also used to sweep the methane from the coal, so
how this
application is used could depend on the proportion of carbon dioxide and
nitrogen in the
gases from the pressurized direct contact heat exchanger as well as the use of
the end
product of this application, which will be pressurized gases containing
methane, e.g. this
methane can be used to further heat the hot gases as described above.
-26-
CA 02512227 2005-07-15
The present invention also has application to processes which produce gases
which
on combustion yield hot pressurized non-condensable gases containing water
vapor. The
following is an example: a pressurized fluidized-bed gasifier transforms coal
into a coal
gas containing hydrogen and methane (and carbon monoxide), which after
suitable
cleaning is combusted with a gas turbine to produce electricity, the hot gases
containing
water vapor exit the turbine at a pressure sufficient to operate the
pressurized direct
contact heat exchanger and produce low pressure steam as well as operate a
pressurized
indirect contact heat exchanger which can supply high pressure steam to the
gasifier, as
illustrated in an embodiment above, Whether or not the pressurized indirect
contact heat
exchanger produces steam for high pressure steam turbines is a separate
consideration. In
present systems, the hot gases from the turbine are sent to a conventional
heat recovery
steam generator, so that the energy in the water vapor is lost to the
atmosphere.
Figure 12 illustrates a way to reduce greenhouse gases, where a pressurized
direct
contact heat exchanger and pressurized combustion is combined with pressurized
electrolysis of water to generate pressurized oxygen for the combustion, and
hydrogen as
a by-product. This produces substantially pure carbon dioxide in the flue /
exit gases,
which is used to accelerate biomass growth in a confined or enclosed space
(e.g. an
inflated plastic covering, see "solar tower" below). Low pressure steam from
the flash
evaporator can be to heat the enclosed space. Part of the carbon dioxide can
also be
combined with ammonia to make compounds such as urea, which can also be used
to
accelerate biomass growth as urea.
By creating a false ceiling below the canopy or covering over the enclosed
space,
the oxygen and water vapour, generated by the biomass , being lighter than the
carbon
-27-
CA 02512227 2005-07-15
dioxide, can be segregated and removed and used in the pressurized direct
contact process
(and the carbon dioxide recycled to the enclosed space or "greenhouse ").
Some or all of the biomass can be used for combustion / human consumption and
any waste from the latter use can be recycled through the combustion cycle.
If air liquefaction is used in place of or in addition to water electrolysis
to produce
the pressurized oxygen then the nitrogen from the liquefaction can be used
along with the
hydrogen (in case of the latter) to produce ammonia with can then be used to
produce the
urea.
A further symbiotic situation is where the above is combined with
EnviroMission's
(Australian firm) "solar tower" (a vertical wind farm) where a chimney,
connected to and
surrounded by a shallow, circular, acrylic greenhouse, (7 km in diameter) will
provide
sufficient draft for the hot air generated by the greenhouse, to power turbo-
generators to
produce electricity.
A special embodiment is as follows: A fuel cell takes in hydrogen and a gas .
containing oxygen and generates electricity and expels hot gases laden with
water vapour.
By operating the fuel cell at elevated pressures and passing the hot gases
through the
pressurized direct contact heat exchanger the efficiency of the cell is
increased' If the
gases are not hot enough, pressurized combustible gases / oil can be burnt
within the
gases to increase their temperature and consume any remaining oxygen or they
can be
heated by any of the methods described above. Figure 13 illustrates this.
Possibly combining this with pressurized water electrolysis other efficiencies
might develop. See below. If only hydrogen & oxygen are used, any residual
hydrogen &
oxygen could also be recycled back to the fuel cells, rather than put through
a turbine
-2s-
CA 02512227 2005-07-15
expander to produce electricity. If air is used in place of the oxygen the
energy in the
residual pressurized nitrogen would be recovered in the turbine expander.
Other embodiments involve electrochemical processes where the "overvoltage",
etc generates heat, which is generally dissipated, thereby decreasing the
efficiency of the
process.
One such embodiment involving electrolysis is illustrated in Figure 14, where
the
electrochemical process is that of the electrolysis of alumina and the hot non
condensable
gas is mainly carbon monoxide, and where the carbon monoxide content of the
gas can be
increased by combining the process with the pressurized direct contact heat
exchanger as
well as taking advantage of the high solubility of the carbon dioxide in water
and the
corresponding very low solubility of the carbon monoxide. Here the gas is sent
to
Solution Chamber where cool water absorbs the carbon dioxide, which when sent
to a Gas
Separator under atmospheric pressure or a slight vacuum, releases the carbon
dioxide and
is returned to the solution chamber to absorb more carbon dioxide. The energy
of the
carbon monoxide enriched gas is recovered by combustion in a Heat Recovery
Steam
Generator and steam generated used for process or to produce electricity using
steam
turbines
It should be noted that the proportion of carbon monoxide in the hot gas
depends
on the alumina content in the hot bath. By carefully controlling this content
(e.g. keeping
track of the cell voltage) this proportion can be kept to a maximum and the
carbon dioxide
to a minimum and the carbon monoxide bleed off.
-29-
CA 02512227 2005-07-15
A further embodiment involving electrolysis is that of the electrolysis of
water,
which was mentioned above in a general way in a symbiotic association with
other
processes.
In particular it relates to those hydrogen-oxygen generators that operate at
relatively high pressures, e.g. high pressure water electrolysis presently
allow the
generation of hydrogen at pressures up to 5 MPa. (750 psi). One such unit
under
development / available is made by GHW (Gesellschaft fur
Hochleistungswasserelektroly
seure). Generally these operate at normal temperatures, however by a similar
choice of
material, these can be made to operate at fairly high temperatures at was done
in the
Cerametec process mentioned below.
Figure 15 illustrates how a pressurized high temperature oxygen-hydrogen
generator can be combined with the pressurized direct contact heat exchanger.
In previous
embodiments the pressurized direct contact heat process was generally involved
with a
source having a single stream of hot pressurized non-condensable gases
containing water
vapor. Since in the present embodiment there are two streams, they are
represented side
by side.
Where necessary present oxygen-hydrogen generators are cooled to keep the
temperature below 100 C (e.g. 65-60 C) mainly to avoid the formation of too
much water
vapor. In the present embodiment, since the temperature is much higher, a fair
amount of
steam with pass along with the gases, as illustrated in Figure 15
Most of the rest of Figure 15 has been explained and described in more detail
in
many of the previous embodiments and need not be repeated here. Since normally
nearly
pure water is used to replenish that used up in the electrolysis, water here
is taken from
-30-
CA 02512227 2005-07-15
steam turbine condensate. Some of the generated steam is used to help preheat
this water,
prior to being pumped to the generator, with the possible additional use of a
jet pump. To
further heat this water to the operating temperature of the cell and to heat
the electrolyte at
start-up, as well as help keep an even temperature in the cell, an alternating
current could
be superimposed on the direct current or used within a separate circuit.
Figure 15 shows the use of a single flash chamber for both gases, however, if
there
is too much cross contamination of the gases, each should have its own flash
chamber.
Also to reduce a loss of gas with the steam from the flash chamber, an inert
substance can
be dissolved in the re-circulating liquid to reduce the solubility of each gas
in the liquid.
When the above embodiment is combined with pressure combustion (see
embodiment above) only one stream of gas would be involved as illustrated in
Figure 10
i.e. that of hydrogen, as the pressurized oxygen from the generator would pass
directly to
the pressure combustion furnace. Similarly, the pressurized oxygen could be
used in
anyone of the many other oxidation processes involving oxygen e.g. as
mentioned in an
embodiment above: in the pulp & paper industry for pulping and bleaching and
in the
manufacture of sulfuric acid by the contact process, where the higher pressure
and
temperature could be of benefit.
It should be noted that the above embodiment, Figure 12, leads (i) to a nearly
pure
source of pressurized carbon dioxide which can be more readily used
commercially or
disposed of than the present gases emanating from the various power combustion
plants
all over the country, e.g. biomass growth and oil enhancement Figure 17 (a)
and (ii) to
easily attained higher combustion pressures (by using high pressure oxygen)
thereby
allowing for greater use of the higher efficiency gas turbine technology.
-31 -
CA 02512227 2005-07-15
Figure 16 illustrates how the present invention can improve the recently
developed
Cerametic process (mentioned above) for the high temperature electrolysis of
steam.
Besides improving the efficiency of the process it also shows how the high
pressure, high
temperature steam that is needed for the generator can be generated in the
Combustion
Furnace.
Further examples of symbiosis are illustrated in Figure 17, where the process
illustrated in Figure 15 or Figure 16 can be located in various locations.
(a) Here the process in Figure 17 is located at a depleted oil source where
the oil
could used to fuel the high pressure combustion and the pressurized carbon
dioxide could
serve as a working fluid in enhanced oil recovery (Figure 17 (a). In addition,
the (i)
carbon dioxide would be sequestered (ii) hydrogen would serve as a means of
storing
electricity for use in fuel cells; (iii) which in turn be used to decrease
pollution arising
from other activities producing carbon dioxide. Being pressurized the plant
would be very
compact and could be moved from one depleted oil well to another,
(b) A further example is Phytotechnology ( Figure 17 (b)) which was mentioned
above, where carbon dioxide is supplied as a nutrient for accelerated growth
of biomass
crops (Figure 12) as well as use up the C02. The biomass is produced in a
closed-
atmosphere, controlled-environment that provides complete control of an
enriched C02
atmosphere from 1000 to 3000 PPM. The Phytotechnology process enhances the
plant
photosynthesis to achieve higher rates of C02 conversion into biomass,
including
BIOFUEL (and food, etc) and mass-cell-culture and algae culture for energy.
Normally
the process is carried out at normal pressure, however if done at higher
pressures the large
amount of water vapor produced could be sent directly along with the biomass
to the
-32-
CA 02512227 2005-07-15
furnace and its energy recovered. Presumably the EnviroMission firm, mentioned
above
in connection with Figure 12 uses higher pressures. Using oxygen for
combustion, the
water content of the biomass could be quite high and still burn.
A further embodiment involves the general processing of substances in a
reactor
under high pressure as illustrated in Figure 18.The configuration of the
equipment will
depend on the process used. If the heat developed is time dependent, then to
insure that
the hottest part of the aqueous medium is located where the medium leaves the
reactor
with a minimum of mixing, various reactor shapes and baffles can be used e.g.
an
elongated baffled vertical chamber. While the make-up water could come from
the
condensed steam, hotter water would of course be preferable. By regulating its
use the
concentration of the reactants in the circulating aqueous medium can be
increased /
controlled.
Any gas produced in the reactor can be separated from the aqueous medium in a
special separator chamber, as shown in Figurel8, where the separated gas and
steam goes
to the pressurized direct contact heat exchanger, with the hot well water
being returned to
the gas separator, and the hot aqueous medium to a flash evaporator where it
can be
concentrated and returned to the reactor. Such an arrangement is necessary to
avoid
excessive gas being released in the flash chamber, which could lower the
efficiency of a
condensing steam turbine. Energy in the gas and steam is recovered in a
turbine expander.
Alternatively, it may be used to heat the make-up water and reactants. To
maintain a
sealing level of liquid in the separator, a portion of the degassed medium can
be
recirculated back to the separator (with the proper controls).
-33-
CA 02512227 2005-07-15
An example of a reactor process is that of wet oxidation (combustion), where
substantial steam is present with the gas that is produced, and the heat
content of the gas
and steam is recovered more efficiently, by passing the cool make-up water
(e.g.
condensed steam) through the pressurized heat exchanger. A dry cool gas is
also
produced, the energy of which is recovered in a turbine expander.
Alternatively, if the gas is pressurized carbon dioxide (a) it could be used
for oil
enhancement as shown in Figure 17 (a); or where after de-pressurizing in the
expander, it
can be used in the production of biofuel as shown in Figure 17 (b).
Reactants include compressed air or pressurized oxygen and any oxidizable
material, including inorganics, with a COD. Examples are: (a) Caustic streams:
refinery
spent caustic and soda pulping liquor; (b) Dangerous, obnoxious and toxic
substances:
effluents containing cyanide, phenols, etc. (c) Waste biological sludges.
Figure 19 illustrates where the gas separator can be eliminated by sending the
hot
aqueous medium directly to the pressurized direct contact heat exchanger
Which of the embodiment in Figures 18 & 19 is used will depend on the nature
of
the wet oxidation.
The embodiment illustrated in Figure 20 could be used in various pressurized
thermal depolymerization reactions involving two Reactors. Here the medium
from the
First Reactor goes to the first Gas Separator and the liquid from the first
Gas Separator
goes to a Fraction Separator and the top fraction goes to a Second Reactor,
and the
medium from that Reactor goes to a second Gas Separator, with the hot gases
from there
joining the gases from the first Gas Separator on their way to the pressurized
heat
exchanger, and the liquid from the second Gas Separator going to Conventional
-34-
CA 02512227 2005-07-15
Distillation Column to yield the required Products, the hot gases from which,
if
pressurized, could join those going to the pressurized heat exchanger. the
bottom fraction
in the Fraction Separator is returned to the first Reactor for further
processing.
The number of reactors will depend on the substances being depolymerization
The following embodiment illustrated in Figure 21, covers the situation where
lower hot well temperatures are produced and a flash evaporator / chamber is
not
required. Here lower pressures are used, i.e. higher than that which are used
presently, and
are pressurized using a rotary blower (see below for attainable pressures) and
sent to the
pressurized direct contact heat exchanger to reclaim the energy as described
above.
Figure 22 illustrates where the condensable vapor is water. The pressure
chosen
depends on the temperature desired for the water in the hot well, which
depends on the
vapor pressure of the water being used to cool the gases, as well as the
pressures
obtainable using rotary blowers, which are less expensive than turbine
compressors.
For example, a pressure of about 30 psia ( I Spsi) corresponds to a hot well
water
temperature of about 120 C (250 F) and thermal efficiency would depend on the
temperature of the cooled gases. The pressurized gases can be passed through a
turbine
expander connected to the rotary blower.
Here the hot water could be sent to a boiler (possibly located in the Source)
to
produce very high pressure, high temperature steam for process or for
generating
electricity using highly efficient steam turbines. If cool enough the steam
condensate
could be recycled back to the pressurized heat exchanger. The hot water could
of course
be used for other purposes. In terms of Carson's Fluidized Spray Tower
illustrated in
Figure IA, one Tower or chamber should suffice for this embodiment.
-35-
CA 02512227 2005-07-15
The present invention could have particular application to existing high
pressure
combustion projects in the Clean Coal Technology Program sponsored by the US
Department of Energy, (mentioned above).
(a) In various projects, a water paste of coal and limestone and compressed
air are fed
to pressurized circulating fluidized-bed combustor where combustion takes
place at a
pressure of about 200 psig, the hot flue gas pass through equipment to remove
the
particulates, etc, then through a gas turbine and the heat in the gas from the
turbine is
recovered in a conventional steam generator, in which case the latent heat of
any water
vapor in the final flue gas is lost.
Figure 23 illustrates how the present invention can increase the thermal
efficiency
of that process. Here the water paste of coal and limestone and compressed air
are fed to
pressurized circulating fluidized-bed combustor where combustion takes place
at a
pressure of about 200 psig, the hot flue gas pass through equipment to remove
the
particulates, etc, then through a gas turbine and some of the heat in the gas
from the
turbine is recovered in a pressurized indirect contact heat exchanger (i.e a
boiler), to
generate very high pressure and high temperature steam with which the generate
electricity using high efficiency stream turbines using the hot well water
from the
pressurized direct heat exchanger, the pressurized hot gases from the boiler
go to the
pressurized direct contact heat exchanger to reclaim essentially all the
remaining energy
in the gases. as described above in various embodiments.
Various details are left out since they vary from one type of process to the
other.
Since the limestone removes about 95% of the sulfur and the ash content in the
hot gas is
low, the hot well water should be suitable to produce the high pressure high
temperature
-36-
CA 02512227 2005-07-15
steam for the steam turbines. Here (Figure 23) the pressurized indirect
contact heat
exchanger (boiler) is shown after the gas turbine, while in Figure 24 it is
shown before,
whichever is selected may depend of various factors. The pressure of the gases
leaving the
turbine should be high enough so as to reclaim the latent heat in the gasses
in the heat
exchanger. If desired the turbine gas could be left out to simplify and reduce
the cost of
the process.
(b) In another series of projects, a pressurized gasifer is supplied with
steam, oxygen,
and a water paste of coal and limestone to produce a fuel gas rich in hydrogen
and carbon
monoxide, which is cleaned and used to fire a gas turbine. Again it appears
that the latent
heat of any water vapor in the final flue gas is lost, which could be high
since hydrogen is
one of fuel gas components.
Figure 24 illustrates how the present invention can increase the thermal
efficiency
of that process. The process is essentially the same as described above for
Figure 23,
except the fuel gas goes to a pressure combustion furnace, containing a
pressurized
indirect contact heat exchanger (i.e a boiler), where the hot well water is
used to generate
the high pressure steam before the gases go through the gas turbine. The
pressure of the
gases leaving the turbine should be high enough so as to reclaim the latent
heat in the
gasses in the heat exchanger.
As a further example of symbiosis, a high pressure electrolysis of water plant
(see
Figure 15) could be located nearby to supply the requires pressurized oxygen.
The gas
turbine could be left out and the hot gases from the fuel gas combuster could
go directly
to the pressurized exchanger. In some processes air is used in place of
oxygen.
CA 02512227 2005-07-15
In another embodiment, Figure 25 illustrates how the invention can be applied
to
the recovery of bitumen (i.e. oil) from Oil Sands, including the recovery of
energy and
water.
Here the process is concerned with the present technique of using high
pressure
steam to lower the viscosity of the bitumen in the sands so that it will flow
towards a well
which will raise it above the ground. In the present embodiment care is taken
to collect as
much steam and gas as possible emanting from the oil well and compress it to
the same
gas pressure as that for the gases coming from a pressurized combustion
furnace, which
will also contain water vapor. Both gases are combined and processed through
the
pressurized direct contact heat exchanger to recover the heat energy in the
gases as well as
produce very hot well water which is used to make the high pressure steam in a
boiler in
the pressurized furnace.
This high pressure steam can also be used to produce electricity with which to
operate the system, using high efficiency condensing steam turbines. The
condensate can
then be used to cool the gases in the heat exchanger. Where it is introduced
could depend
on its temperature. As indicated the coolest water available is introduced at
the top of the
heat exchanger and its temperature will determine the thermal efficiency of
the process.
Depending on the type of fuel used to fire the furnace, it may be necessary to
clean
the gases before they go to the heat exchanger so as to ensure that hot well
water is
suitable for the production of the high pressure steam. The gas cleaning
technology is well
known and used in the Clean Coal Program sponsored by the US Department of
Energy.
To that end it might be desirable to mix limestone with the fuel so as trap
any sulfur
compound in the fuel so that they will exit with the ash and not the gas
steam. The
-38-
CA 02512227 2005-07-15
pressure in the furnace will depend on the temperature that is desired for the
hot well
water, as well as the degree of thermal efficiency desired, as was explained
above.
Figure 26 illustrates how the hot well water can be used in the present
technique of
using hot water to separate the bitumen from the Oil Sands. The water fraction
can then
be cleaned and sent back to the heat exchanger at the proper location,
depending on its
temperature, to cool further gases.
The present invention can also be used to break the bitumen down into various
fractions using the any of the above embodiments, one of which is illustrated
in Figure 20.
As an alternative to the above the gas and steam from the oil well can be
processed
as described in Figures 21 & 22.
Other embodiments involving lower pressures are situations where drying (and
boiling) is involved e.g. web drying. Here the drying could be accelerated by
subjecting
the web to a mild vacuum using either the suction side of rotary blower or a
vacuum
pump and the steam and any entrained air could go a pressurized direct contact
heat
exchanger, such as the fluidized spray tower mentioned above, where some of
the steam
would be condensed using the water in the hot well of the second heat
exchanger as
cooling water. The water in the hot well can be sent to a high pressure boiler
to produce
high pressure steam.
The remaining steam containing low amounts of air can then to connected to the
suction side of a high pressure water pump being fed cooler water e.g the
condensate
from the steam turbines and then sent to a pressurized direct contact heat
exchanger as
disclosed above in connection with Figure 6 , where more steam will condense
and the
-39-
CA 02512227 2005-07-15
energy in the pressurized air can be recovered in turbine expander, which can
be used to
power the rotary blower or vacuum pump.
This embodiment can be very effectively used with a new paper technology
referred to a Impulse Drying where a very hot roll is heated by either very
hot steam or a
gas flame or magnetic induction This is illustrated in Figure 27, where a
vacuum chamber
encloses the hot roll. Details of how the roll is heated and how the webs are
introduced
and removed from the Chamber are not shown as they are well understood in the
trade.
By insulating the Vacuum Chamber not will the heat content of the gases
involved be
reclaimed but also that from the heating process used for the roll. In this
latter aspect,
magnetic induction is recommended especially that involving a new type of roll
called
"Optimized Heated Roll" presently being marketed by Comaintel Inc.
While alternatively a low pressure chamber could be used, the above low vacuum
chamber would seem more advantageous.
It should be noted to avoid cleaning gases using expensive equipment one can
by
using an indirect heat exchanger use the unclean hot well water to heat the
condensate
from the steam turbines and send this now clean hot water to the boiler to
make high
pressure steam.
The preceding description of the invention is merely exemplary and is not
intended
to limit the scope of the present invention in any way thereof.
-40-