Note: Descriptions are shown in the official language in which they were submitted.
CA 02516988 2005-08-24
WO 2004/075974 PCT/US2004/005057
SPLANCHNIC NERVE STIMiJLATION FOR TREATMENT OF OBESITY
Baclc~round of the hlvention
Field of the Invention
The invention relates to nerve stimulation for the treatment of medical
conditions.
Description of the Related Art
Obesity is an epidemic in the U.S. with a prevalence of about 20 percent.
Annual
U.S. healthcare costs associated with obesity are estimated to exceed $200
billion dollars.
Obesity is defined as a body mass index (BMI) that exceeds 30 kg/m2. Normal
BMI is 18.5-
25 kg/m2, and overweight persons have BMIs of 25-30. Obesity is classified
into three
groups: moderate (C1a55 1), severe (Class II), and very severe (Class III).
Patients with
BMIs that exceed 30 are at risk for significant comorbidities such as
diabetes, heart and
kidney disease, dyslipidemia, hypertension, sleep apnea, and orthopedic
problems.
Obesity results from an imbalance between food intake and energy expenditure
such
that there is a net increase in fat reserves. Excessive food intake, reduced
energy
expenditure, or both may cause this imbalance. Appetite and satiety, which
control food
intake, are partly controlled in the brain by the hypothalamus. Energy
expenditw-e 1S also
partly controlled by the hypothalamus. The hypothalamus regulates the
autonomic nervous
system of which there are two branches, the sympathetic and the
parasympathetic. The
sympathetic nervous system generally prepares the body for action by
increasing heart rate,
blood pressure, and metabolism. The parasympathetic system prepares the body
for rest by
lowering heart rate, lowering blood pressure, and stimulating digestion.
Destruction of the
lateral hypothalamus results in hunger suppression, reduced food intake,
weight loss, and
increased sympathetic activity. W contrast, destruction of the ventromedial
nucleus of the
hypothalamus results in suppression of satiety, excessive food intake, weight
gain, and
decreased s5nnpathetic activity. The splanchnic nerves carry sympathetic
neurons that
supply, or innervate, the organs of digestion and adrenal glands, and the
vagus nerve carries
parasympathetic neurons that innervate the digestive system and are involved
in the feeding
and weight gain response to hypothalamic destruction.
Experimental and observational evidence suggests that there is a reciprocal
relationship between food intake and sympathetic nervous system activity.
Increased
sympathetic activity reduces food intake and reduced sympathetic activity
increases food
-1-
CA 02516988 2005-08-24
WO 2004/075974 PCT/US2004/005057
intake. Certain peptides (e.g. neuropeptide Y, galanin) are known to increase
food intake
while decreasing sympathetic activity. Others such as cholecystolcinin,
leptin, enterostatin,
reduce food intalce and increase sympathetic activity. In addition, dnigs such
as nicotine,
ephedrine, caffeine, subitramine, dexfenfluramine, increase s~nnpathetic
activity and reduce
food intake.
Ghrelin is another peptide that is secreted by the stomach that is associated
with
hunger. Peak plasma levels occur just prior to mealtime, and ghrelin levels
are increased
after weight loss. Sympathetic activity can suppress ghrelin secretion. PYY is
a homnone
released from the intestine that plays a role in satiety. PYY levels increase
after meal
ingestion. Sympathetic activity can increase PYY plasma levels.
Appetite is stimulated by various psychosocial factors, but is also stimulated
by low
blood glucose levels. Cells in the hypothalamus that are sensitive to glucose
levels are
thought to play a role in hunger stimulation. Sympathetic activity increases
plasma glucose
levels. Satiety is promoted by distention of the stomach and delayed gastric
emptying.
Sympathetic activity reduces gastric and duodenal motility, causes gastric
distention, and
can increase pyloric sphincter, which can result in distention and delayed
gastric emptying.
The sympathetic nervous system plays a role in energy expenditure and obesity.
Genetically inherited obesity in rodents is characterized by decreased
sympathetic activity
to adipose tissue and other peripheral organs. Catecholamines and coutisol,
which are
released by the sympathetic nervous system, cause a dose-dependent increase in
resting
energy expenditure. W humans, there is a reported negative correlation between
body fat
and plasma catecholamine levels. Overfeeding or underfeeding lean human
subjects has a
significant effect on energy expenditure and sympathetic nervous system
activation. For
example, weight loss in obese subjects is associated with a compensatory
decrease in
energy expenditure, which promotes the regain of previously lost weight. Drugs
that
activate the sympathetic nervous system, such as ephedrine, caffeine and
nicotine, are
kIlOWll to increase energy expenditure. Smokers are k110W11 to have lower body
fat stores
and increased energy expenditure.
The sympathetic nervous system also plays an important role in regulating
energy
substrates for increased expenditure, such as fat and carbohydrate. Glycogen
and fat
metabolism are increased by sympathetic activation and are needed to support
increased
energy expenditure.
_2_
CA 02516988 2005-08-24
WO 2004/075974 PCT/US2004/005057
Animal research involving acute electrical activation of the splanchnic nerves
under
general anesthesia causes a variety of physiologic changes. Electrical
activation of a single
splanchnic nerve in dogs and cows causes a frequency dependent increase in
catecholamine,
dopamine, and cortisol secretion. Plasma levels can be achieved that cause
increased energy
expenditure. In adrenalectomized anesthetized pigs, cows, and dogs, acute
single
splanchnic nerve activation causes increased blood glucose and reduction in
glycogen liver
stores. In dogs, single splanchnic nerve electrical activation causes
increased pyloric
sphincter tone and decrease duodenal motility. Sympathetic and splanchnic
nerve activation
can cause suppression of insulin and leptin hormone secretion.
First line therapy for obesity is behavior modification involving reduced food
intake
and increased exercise. However, these measures often fail and behavioral
treatment is
supplemented with pharmacologic treatment using the phannacologic agents noted
above to
reduce appetite and increase energy expenditure. Other pharmacologic agents
that can cause
these affects include dopamine and dopamine analogs, acetylcholine and
cholinesterase
inhibitors. Pharmacologic therapy is typically delivered orally and results in
systemic side
effects S11C11 a5 tachycardia, sweating, and hypertension. In addition,
t~lerance can develop
such that the response to the drug reduces even at higher d~ses.
More radical forms of therapy involve surgery. In general, these procedures
reduce
the size of the stomach and/or rer~ute the intestinal system to avoid the
stomach.
Representative procedures are gastric bypass surgery and gastric banding.
These
procedures can be very effective in treating obesity, but they are highly
invasive, require
significant lifestyle changes, and can have severe complications.
Experimental forms of treatment for obesity involve electrical stimulation of
the
stomach (gastric pacing) and the vagus nerve (parasympathetic system). These
therapies use
a pulse generator to stimulate electrically the stomach or vagus nerve via
implanted
electrodes. The intent of these therapies is to reduce food intake through the
promotion of
satiety and or reduction of appetite, and neither of these therapies is
believed to affect
energy expenditure. U.S. Patent No. 5,423,872 to Cigaina describes a putative
method for
treating eating disorders by electrically pacing the stomach. U.S. Patent No.
5,263,480 to
Werniclce discloses a putative method for treating obesity by electrically
activating the
vagus nerve. Neither of these therapies increases energy expenditure.
-3-
CA 02516988 2005-08-24
WO 2004/075974 PCT/US2004/005057
Summary of the Invention
The invention includes a method for treating obesity or other disorders by
electrically activating the sympathetic nervous system with a wireless
electrode inductively
coupled with a radiofrequency field. Obesity caal be treated by activating the
efferent
sympathetic nervous system, thereby increasing energy expenditure and reducing
food
intake. Stimulation is accomplished using a radiofrequency pulse generator and
electrodes
implanted near, or attached to, various areas of the sympathetic nervous
system, such as the
sympathetic chain ganglia, the splanclmic nerves (greater, lesser, least), or
the peripheral
ganglia (e.g., celiac, mesenteric). Preferably, the obesity therapy will
employ electrical
activation of the sympathetic nervous system that innervates the digestive
system, adrenals,
and abdominal adipose tissue, such as the splanchnic nerves or celiac ganglia.
Afferent
stimulation can also be accomplished to provide central nervous system
satiety. Afferent
stimulation can occur by a reflex arc secondary to efferent stimulation.
Preferably, both
afferent and efferent stimulation can be achieved.
This method of obesity treatment may reduce food intake by a variety of
mechanisms, including, for example, general increased sympathetic system
activation and
increasing plasma glucose levels upon activation. Satiety may be produced
through direct
effects on the pylorus and duodenum that cause reduced peristalsis, stomach
distention,
and/or delayed stomach emptying. In addition, reducing ghrelin secretion
and/or increasing
P Y i'~ secr etion may reduce food intake. The method can also cause weight
loss by reducing
food absorption, presumably through a reduction in secretion of digestive
enzymes and
fluids and changes in gastrointestinal motility. We have noted an increased
stool output,
increased PYY concentrations (relative to food intake), and decreased ghrelin
concentrations (relative to food intake) as a result of splanchnic nerve
stimulation according
to the stimulation parameters disclosed herein.
This method of obesity treatment may also increase energy expenditure by
causing
catecholamine, cortisol, and dopamine release from the adrenal glands. The
therapy can be
titrated to the release of these hormones. Fat and carbohydrate metabolism,
which are also
increased by s5nnpathetic nerve activation, will accompany the increased
energy
expenditure. Other hornonal effects induced by this therapy may include
reduced insulin
secretion. Alternatively, this method may be used to normalize catecholamine
levels,
which are reduced with weight gain.
-4-
CA 02516988 2005-08-24
WO 2004/075974 PCT/US2004/005057
Electrical sympathetic activation for treating obesity is preferably
accomplished
without causing a rise in mean arterial blood pressure (MAP). This can be
achieved by
using an appropriate stimulation pattern with a relatively short signal-on
time (or "on
period") followed by an equal or longer signal-off time (or "off period").
During activation
therapy, a sinusoidal-lilce fluctuation in the MAP can occur with am average
MAP that is
within safe limits. Alternatively, an alpha sympathetic receptor bloclcer,
such as prazosin,
can be used to blunt the increase in MAP.
Electrical sympathetic activation can be titrated to the plasma level of
catecholamines achieved during therapy. This would allow the therapy to be
monitored and
safe levels of increased energy expenditure to be achieved. The therapy can
also be titrated
to plasma ghrelin levels or PYY levels.
Electrical modulation (inhibition or activation) of the sympathetic nerves can
also
be used to treat other eating disorders such as anorexia or bulimia. For
example, inhibition
of the sympathetic nerves can be useful in treating anorexia. Electrical
modulation of the
synpathetic nerves may also be used to treat gastrointestinal diseases such as
peptic ulcers,
esophageal reflex, gastroparesis, and irritable bowel. For example,
stimulation of the
splanchnic nerves that imiervate the large intestine may reduce the symptoms
of irritable
bowel syndrome, characterized by diarrhea. Pain may also be treated by
electric nerve
modulation of the sympathetic nervous system, as certain pain neurons are
carried in the
Sy111pathetl~ nel'~eS. This therapy may also be used to treat type II
diabetes. These
conditions can require varying degrees of iWibiti~n or stimulation.
Some embodiments include a method for treating a medical Condition, the method
comprising electrically activating a splanchnic nerve in a mammal according to
a
stimulation pattern configured to result in net weight loss in the mammal;
wherein the
stimulation pattern comprises a stimulation intensity, an on time, and an off
time; and
wherein the stimulation pattern is configured such that the ratio of the on
time to the off
time is about 0.75 or less.
In some embodiments the stimulation pattern is configured such that the ratio
of the
on time to the off time is about 0.5 or less, and in some embodiments, about
0.3 or less.
In some embodiments the stimulation pattern is configured such that the on
time is
about two minutes or less. In some embodiments the stimulation pattern is
configured such
that the on time is about one minute or less. W some embodiments the
stimulation pattern
-5-
CA 02516988 2005-08-24
WO 2004/075974 PCT/US2004/005057
is configured such that the on time is about one minute or less and the off
time is about one
minute or more.
In some embodiments the stimulation pattern is configured such that the on
time is
greater than about 15 seconds. In some embodiments the stimulation pattern is
configured
such that the on time is greater than about 30 seconds.
Some embodiments further comprise varying the stimulation intensity over time,
such as by increasing the stimulation intensity over time, sometimes daily.
Some embodiments further comprise creating a unidirectional action potential
in the
splanchnic nerve. This can involve creating an anodal blocp in the splanclmic
nerve.
Some embodiments include a method for treating a medical condition, the method
comprising electrically activating a splanchrlic nerve in a mammal according
to a
stimulation pattern for a first time period; wherein the stimulation pattern
comprises a
stimulation intensity and is configured to result in net weight loss in the
mammal during the
first time period; and reducing or ceasing the electrical activation of the
splanchnic nerve
for a second time period, such that the mammal loses net weight during the
second time
period.
In solve embodiments the first time period is between about 2 weeps and about
15
weeps. In some embodiments the first time period is between about 6 weeks and
about 12
weelcs. In some embodiments the second time period is between about 1 weelc
and about 6
weeps. In solve embodiments the second time period is between about 2 weeks
and about 4.
weeps.
In solve embodiments the electrically activating the splanchnic nerve
comprises
delivering a stimulation intensity to the splanchnic nerve that is
approximately equal to the
stimulation intensity required to produce skeletal muscle twitching in the
mammal. In
some embodiments the stimulation intensity to the splanchrlic nerve is at
least about two
times the Stllnlllatloll lntellSlty required to produce speletal muscle
twitching in the
mammal. In solve embodiments the stimulation intensity to the splanclmic nerve
is at least
about five times the stimulation intensity required to produce speletal muscle
twitching in
the mammal. In some embodiments the stimulation intensity to the splanchnic
nerve is at
least about eight times the stimulation intensity required to produce
slceletal muscle
twitching in the mammal.
-6-
CA 02516988 2005-08-24
WO 2004/075974 PCT/US2004/005057
Some embodiments include a method for treating a medical condition, the method
comprising electrically activating a splanchnic nerve in a mammal according to
a
stimulation pattern for a first time period within a period of about 24 hours,
said stimulation
pattern comprising a stimulation intensity and being configured to result in
net weight loss
in the marmnal; and ceasing the electrical activation of the a splanclmic
nerve for a second
time period within the period of about 24 hours.
Some embodiments further comprise repeating the steps of electrically
activating
and ceasing the electrical activation. W some embodiments the first time
period plus the
second time period equals about 24 hours.
Some embodiments include a method for treating a medical condition, the method
comprising electrically activating a splanclmic nerve in a mammal according to
a
stimulation pattern configured to result in net weight loss in the mammal;
wherein the
stimulation pattern comprises a stimulation intensity and a frequency; and
wherein the
frequency is about 15 Hz or greater, to minimize skeletal muscle twitching.
In some embodiments the frequency is about 20 Hz or greater. In some
embodiments the frequency is about 30 Hz or greater.
W some embodiments the stimulation intensity is at least about 5 times the
stimulation intensity required to produce slceletal muscle twitching in the
mammal. In
some embodiments the stimulation intensity is at least about 10 times the
stimulation
intensity required to produce skeletal muscle twitching in the mammal, and the
frequency is
about 20 Hz or greater.
Some embodiments include a method for producing weight loss, the method
comprising electrically activating a splanchnic nerve in a mammal according to
a
stimulation pattern comprising a stimulation intensity and a frequency; and
the stimulation
pattern is configured to decrease absorption of food from the gastrointestinal
tract, resulting
in increased stool output in the marninal.
In some embodiments the frequency is about 15 Hz or greater, about 20 Hz or
greater, and/or about 30 Hz or greater.
In some embodiments the stimulation intensity is at least about 5 times the
stimulation intensity required to produce skeletal muscle twitching in the
mammal.
CA 02516988 2005-08-24
WO 2004/075974 PCT/US2004/005057
In some embodiments the stimulation intensity is at least about 10 times the
stimulation intensity required to produce skeletal muscle twitching in the
mammal, and the
frequency is about 20 Hz or greater.
Some embodiments include a method for treating a medical condition, the method
comprising placing an electrode in proximity to a splanchnic nerve in a
maxmnal above the
diaphragm; and electrically activating the splanchnic nerve.
Some embodiments further comprise placing the electrode in contact with the
splanclnuc nerve. W some embodiments the electrode is helical or has a cuff,
and further
comprising attaching the electrode to the splanchnic nerve.
hz some embodiments the placing is transcutaneous (that is, percutaneous). In
some
embodiments the placing is into a blood vessel of the mammal. In some
embodiments the
blood vessel is an azygous vein.
Some embodiments further comprise electrically activating the electrode and
observing the patient for skeletal muscle twitching to assess placement of the
electrode near
the splanchnic nerve.
Some embodiments include a method for treating a medical condition, the method
comprising placing an electrode into a blood vessel of a mannnal, in proximity
to a
splanchnic nerve of the mammal; and electrically activating the splanchnic
nerve via the
electrode. In some embodiments the blood vessel is an azygous vein. hz some
embodiments the electrically activating is according to a stimulation pattern
configured to
result in net weight loss in the mammal.
Some embodiments include a method for treating a medical condition, the method
comprising electrically activating a splanclmic nerve in a mammal according to
a
stimulation pattern configured to result in net weight loss in the mammal;
wherein the
stimulation pattern comprises an on time; and wherein the on time is adjusted
based on a
blood pressure of the mammal.
Some embodiments include a method for treating a medical condition, the method
comprising electrically activating a splanchnic nerve in a mammal according to
a
stimulation pattern configured to result in net weight loss in the mammal;
wherein the
stimulation pattern comprises an on time; and wherein the on time is adjusted
based on a
plasma PYY concentration and/or a plasma ghrelin concentration in the mammal..
_g_
CA 02516988 2005-08-24
WO 2004/075974 PCT/US2004/005057
Some embodiments include a method for treating a medical condition, the method
comprising electrically activating a splanchnic nerve in a marmnal according
to a
stimulation pattern, wherein the stimulation pattern comprises a current
amplitude; wherein
the current amplitude is adjusted based on slceletal muscle twitching in the
marmnal.
Some embodiments include a method for treating a medical condition, the method
comprising electrically activating a splanchnic nerve in a mammal according to
a
stimulation pattern, wherein the stimulation pattern comprises a current
amplitude and a
pulse width; wherein the current amplitude is increased to a first level at
which slceletal
muscle twitching begins to occur in the mammal; Keeping the current amplitude
at or near
the first level until the skeletal muscle twitching decreases or ceases.
Some embodiments further comprise further increasing the current amplitude as
habituation to the slceletal muscle twitching occurs. Some embodiments further
comprise
further increasing the current amplitude to a second level at which skeletal
muscle
twitching begins to recur, the second level being greater than the first
level.
Some embodiments include a method for treating a medical condition, the method
comprising electrically activating a splanchnic nerve in a mammal according to
a
stimulation pattern, wherein the stimulation pattern comprises a current
amplitude and a
pulse width; wherein the current amplitude is increased to a first level at
which skeletal
muscle twitching begins to occur in the mammal; increasing the pulse width
while lceeping
the current amplitude at about the first level or below the first level.
Some embodiments include a method for treating a medical condition, the method
comprising electrically activating a splanchnic nerve in a mammal according to
a
stimulation pattern, wherein the stimulation pattern comprises a current
amplitude; wherein
the current amplitude is increased to a first level at which skeletal muscle
twitching begins
to occur in the mammal; and sensing the muscle twitching with a sensor in
electrical
communication with the electrode.
In some embodiments the sensor is electrical. In some embodiments the sensor
is
mechanical.
Some embodiments further comprise further increasing the current amplitude as
habituation to the slceletal muscle twitching implanting the sensor near the
abdominal wall
to sense abdominal muscle twitching.
-9-
CA 02516988 2005-08-24
WO 2004/075974 PCT/US2004/005057
Some embodiments include a device for treating a medical condition, the device
comprising an electrode configured to stimulate electrically a splanchnic
nerve in a
mammal; a generator configured to deliver an electrical signal to the
electrode; and a
sensor in electrical communication with the generator, the sensor configured
to sense
muscle twitching; wherein the device is programmed to stimulate electrically
the splanchnic
nerve according to a stimulation pattern, wherein the stimulation pattern
comprises a
current amplitude and a pulse width; wherein the device is further programmed
to increase
the current amplitude to a first level at which skeletal muscle twitching
begins to occur, and
temporarily hold the current amplitude at or near the first level until the
skeletal muscle
twitching decreases or ceases.
In some embodiments the device is further programmed to increase the pulse
width
while keeping the current amplitude at or near the first level. In some
embodiments the
device is further programmed to increase the current amplitude as habituation
to the muscle
twitching occurs. In some embodiments the device is further programmed to
increase the
current amplitude to a second level at which skeletal muscle twitching begins
to recur, the
second level being greater than the first level.
In some embodiments the device is compatible with magnetic resonance imaging.
In some embodiments the device comprises a nanomagnetic material.
Some embodiments include a method for treating a medical condition, the method
comprising electrically activating a splanchnic nea oje in a mammal according
to a.
stimulation pattern that is configured to result in net weight loss in the
mammal without
causing a substantial rise in a blood pressure of the mammal.
Some embodiments include a method for treating a medical condition, the method
comprising electrically activating a splanchnic nerve in a mammal according to
a
stimulation pattern that is configured to result in net weight loss in the
mammal without
causing prolonged skeletal muscle twitching in the mammal. Avoiding prolonged
slceletal
muscle twitching, in this context, refers to the fact that as soon as the
stimulation threshold
for muscle twitching is reached in this method (as the stimulation intensity
is increased),
current amplitude (or an analogous parameter, such as voltage) is held at or
below this level
until habituation to muscle twitching is reached by the animal. At that point,
the current
amplitude can then be increased until muscle twitching recurs at a higher
stimulation
-10-
CA 02516988 2005-08-24
WO 2004/075974 PCT/US2004/005057
intensity. Then the process is repeated, as a "ramp up" protocol, while
minimizing slceletal
muscle twitching.
The invention will be best understood from the attached drawings and the
following
description, in which similar reference characters refer to similar parts.
Brief Description of the Drawings
Figure 1 is a diagram of the efferent autonomic nervous system.
Figure 2 is a diagram of sympathetic nervous system anatomy.
Figure 3 is an elevation view of the splanchnic nerves and celiac ganglia.
Figure 4 is a schematic of an exemplary stimulation pattern.
Figure 5 is a schematic of an exemplary pulse generator.
Figure 6 is a diagram of an exemplary catheter-type lead and electrode
assembly.
Figure 7 is a graph of known plasma catecholamine levels in various
physiologic
and pathologic states.
Figures 8a, 8b, and 8c are exemplary graphs of the effect of splanclmic nerve
stimulation on catecholamine release rates, epinephrine levels, and energy
expenditure,
respectively.
Figure 9 is a graph of lmown plasma ghrelin levels over a daily cycle, for
various
subj ects.
Figure 10 is a section view of an exemplary instrument placement, for
implantation
?0 of an electrode assembly.
Figures l la and 11b are graphs of electrical signal waveforms.
Figure 12 is a schematic lateral view of an electrode assembly.
Figure 13 shows a rolling seven-day average of animal weight.
Figure 14 shows plasma ghrelin levels before and after splanchnic nerve
stimulation.
Detailed Description of Exemplary Embodiments
The human nervous system is a complex network of nerve cells, or neurons,
found
centrally in the brain and spinal cord and peripherally in the various nerves
of the body.
Neurons have a cell body, dendrites and an axon. A nerve is a group of neurons
that serve a
particular part of the body. Nerves can contain several hundred neurons to
several hundred
thousand neurons. Nerves often contain both afferent and efferent neurons.
Afferent
neurons carry signals baclc to the central nervous system and efferent neurons
carry signals
-11-
CA 02516988 2005-08-24
WO 2004/075974 PCT/US2004/005057
to the periphery. A group of neuronal cell bodies in one location is lcnown as
a ganglion.
Electrical signals are conducted via neurons and nerves. Neurons release
neurotransmitters
at synapses (corrections) with other nerves to allow continuation and
modulation of the
electrical signal. In the periphery, synaptic transmission often occurs at
ganglia.
The electrical signal of a neuron is lmown as an action potential. Action
potentials
are initiated when a voltage potential across the cell membrane exceeds a
certain threshold.
This action potential is then propagated down the length of the neuron. The
action potential
of a nerve is complex and represents the sum of action potentials of the
individual neurons
in it.
Neurons can be myelinated and umnyelinated, of large axonal diameter and small
axonal diameter. In general, the speed of action potential conduction
increases with
myelination and with neuron axonal diameter. Accordingly, neurons are
classified into type
A, E and C neurons based on myelination, axon diameter, and axon conduction
velocity. In
terms of axon diameter and conduction velocity, A is greater than E which is
greater than
C.
The autonomic nervous system is a subsystem of the human nervous system that
controls involuntary actions of the Sln~~t11 11111SC1eS (blood vessels and
digestive system),
the heart, and glands, as shown in Figure 1. The autonomic nervous system is
divided into
the sympathetic and parasympathetic systems. The sympathetic nervous system
generally
prepares the body for action by increasing heart rate, increasing blood
pressure, and
increasing metabolism. The parasympathetic system prepares the body for rest
by lowering
heart rate, lowering blood pressure, and stimulating digestion.
The hypothalamus controls the sympathetic nervous system via descending
neurons
in the ventral hom of the spinal cord, as shown in Figure 2. These neurons
synapse with
preganglionic sympathetic neurons that exit the spinal cord and form the white
communicating ramus. The preganglionic neuron will either synapse in the
paraspinous
ganglia chain or pass through these ganglia and synapse in a peripheral, or
collateral,
ganglion such as the celiac or mesenteric. After synapsing in a particular
ganglion, a
postsynaptic neuron continues on to innervate the organs of the body (heart,
intestines,
liver, pancreas, etc.) or to innervate the adipose tissue and glands of the
periphery and skin.
Preganglionic neurons of the sympathetic system can be both small-diameter
unmyelinated
-12-
CA 02516988 2005-08-24
WO 2004/075974 PCT/US2004/005057
fibers (type C-lilce) and small-diameter myelinated fibers (type B-like).
Postganglionic
neurons are typically unmyelinated type C neurons.
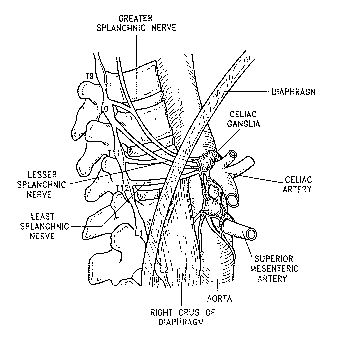
Several large sympathetic nerves and ganglia are formed by the neurons of the
sympathetic nervous system as shoran in Figure 3. The greater splanchnic nerve
(GSN) is
formed by efferent sympathetic neurons exiting the spinal cord from thoracic
vertebral
segment numbers 4 or 5 (T4 or TS) through thoracic vertebral segment numbers 9
or 10 or
11 (T9, T10, or T11). The lesser splanclmic (lesser SN) nerve is formed by
preganglionic
fibers sympathetic efferent fibers from T10 to T12 and the least splanchnic
nerve (least SN)
is formed by fibers from T12. The GSN is typically present bilaterally in
animals, including
humans, with the other splanchnic nerves having a more variable pattern,
present
unilaterally or bilaterally and sometimes being absent. The splanchnic nerves
run along the
anterior-lateral aspect of the vertebral bodies and pass out of the thorax and
enter the
abdomen through the crux of the diaphragm. The nerves run in proximity to the
azygous
veins. Qnce in the abdomen, neurons of the GSN synapse with postganglionic
neurons
primarily in celiac ganglia. Some neurons of the GSN pass through the celiac
ganglia and
synapse on in the adrenal medulla. Neurons of the lesser SN and least SN
synapse wlth
post-ganglionic neurons in the mesenteric ganglia.
Postganglionic neurons, arising from the celiac ganglia that synapse with the
GSN,
innervate primarily the upper digestive system, including the stomach,
pylorus, duodenum,
pancreas, and liver. In addition, blood vessels and adipose tissue of the
abdomen are
innervated by neurons arising from the celiac ganglia/greater splanchnic
nerve.
Postganglionic neurons of the mesenteric ganglia, supplied by preganglionic
neurons of the
lesser and least splanchnic nerve, innervate primarily the lower intestine,
colon, rectum,
lcid~eys, bladder, and sexual organs, and the blood vessels that supply these
organs and
tissues.
W the treatment of obesity, a preferred embodiment involves electrical
activation of
the greater splanchnic nerve of the sympathetic nervous system. Preferably
unilateral
activation would be utilized, although bilateral activation can also be
utilized. The celiac
ganglia can also be activated, as well as the sympathetic chain or ventral
spinal roots.
Electrical nerve modulation (nerve activation or inhibition) is accomplished
by
applying an energy signal (pulse) at a certain frequency to the neurons of a
nerve (nerve
stimulation). The energy pulse causes depolarization of neurons within the
nerve above the
-13-
CA 02516988 2005-08-24
WO 2004/075974 PCT/US2004/005057
activation threshold resulting in an action potential. The energy applied is a
function of the
current (or voltage) amplitude and pulse width or duration. Activation or
inhibition can be a
function of the frequency, with low frequencies on the order of 1 to 50 Hz
resulting in
activation and high frequencies greater than 100 Hz resulting in inhibition.
Inhibition can
also be accomplished by continuous energy delivery resulting in sustained
depolarization.
Different neuronal types may respond to different frequencies and energies
with activation
or inhibition.
Each neuronal type (i.e., type A, B, or C neurons) has a characteristic pulse
amplitude-duration profile (energy pulse signal or stimulation intensity) that
leads to
activation. The stimulation intensity can be described as the product of the
current
amplitude and the pulse width. Myelinated neurons (types A and B) can be
stimulated with
relatively low current amplitudes, on the order of 0.1 to 5.0 milliamperes,
and short pulse
widths, on the order of 50 to 200 microseconds. Umnyelinated type C fibers
typically
require longer pulse widths on the order of 300 to 1,000 microseconds and
higher current
amplitudes. Thus, in one embodiment, the stimulation intensity for efferent
activation
would be in the range of about 0.005-5.0 mAmp-mSec).
The greater splanchnic nerve also contains type A fibers. These fibers can be
afferent and sense the position or state (contracted versus relaxed) of the
stomach or
duodenum. Stimulation of A fibers may produce a sensation of satiety by
transmitting
signals to the hypothalamus. They can also participate in a reflex arc that
affects the state of
the stomach. Activation of both A and B fibers can be accomplished because
stnnulation
parameters that activate efferent B fibers will also activate afferent A
fibers. Activation of
type C fibers may cause both afferent an efferent effects, and may cause
changes in appetite
and satiety via central or peripheral nervous system mechanisms.
Various stimulation patterns, ranging from continuous to intermittent, can be
utilized. With intermittent stimulation, energy is delivered for a period of
time at a certain
fr equency during the signal-on time as shown in Figure 4. The signal-on time
is followed
by a period of time with no energy delivery, referred to as signal-off time.
The ratio of the
on time to the off time is referred to as the duty cycle and it can in some
embodiments
range from about 1% to about 100%. Peripheral nerve stimulation is commonly
conducted
at nearly a continuous, or 100%, duty cycle. However, an optimal duty cycle
for splanchnic
nerve stimulation to treat obesity may be less than 75% in some embodiments,
lees than
-14-
CA 02516988 2005-08-24
WO 2004/075974 PCT/US2004/005057
50% in some embodiments, or even less than 30% in further embodiments. This
may
reduce problems associated with muscle twitching as well as reduce the chance
for blood
pressure or heart rate elevations. The on time may also be important for
splanchnic nerve
stimulation in the treatment of obesity. Because some of the desired effects
involve the
release of hormones, on times sufficiently long enough to allow plasma levels
to rise are
impol-tant. Also gastrointestinal effects on motility and digestive secretions
tale time to
reach a maximal effect. Thus, an on time of approximately 15 seconds, and
sometimes
greater than 30 seconds, may be optimal.
Superimposed on the duty cycle and signal parameters (frequency, on-time,
mAmp,
and pulse width) are treatment parameters. Therapy may be delivered at
different intervals
during the day or weele, or continuously. Continuous treatment may prevent
binge eating
during the off therapy time. Intermittent treatment may prevent the
development of
tolerance to the therapy. Optimal intermittent therapy may be, for example, 18
hours on
and 6 hours off, 12 hours on and 12 hours off, 3 days on and 1 day off, 3
weels on and one
weal off or a another combination of daily or weelcly cycling. Alternatively,
treatment can
be delivered at a higher interval rate, say, about every three hours, for
shorter durations,
such as about 2-30 m11111teS. The treatment duration and frequency can be
tailored to
achieve the desired result. The treatment duration can last for as little as a
few minutes to
as long as several hours. Also, splanchnic nerve activation to treat obesity
can be delivered
at daily intervals, coinciding with meal times. Treatment duration during
mealtime may, in
some embodiments, last from 1-3 hours and start just prior to the meal or as
much as an
hour before.
Efferent modulation of the GSN can be used to control gastric
distention/contraction
and peristalsis. Gastric distention or relaxation and reduced peristalsis can
produce satiety
or reduced appetite for the treatment of obesity. These effects can be caused
by activating
efferent B or C fibers at moderate to high intensities (1.0-5.0 milliAmp
current amplitude
and 0.150-1.0 milliseconds pulse width) and higher frequencies (10-20 Hz).
Gastric
distention can also be produced via a reflex arc involving the afferent A
fibers. Activation
of A fibers may cause a central nervous system mediated reduction in appetite
or early
satiety. These fibers can be activated at the lower range of stimulation
intensity (0.05-0.150
mSec pulse width and 0.1-1.0 mAmp current amplitude) and higher range of
frequencies
given above. Contraction of the stomach can also reduce appetite or cause
satiety.
-15-
CA 02516988 2005-08-24
WO 2004/075974 PCT/US2004/005057
Contraction can be caused by activation of C fibers in the GSN. Activation of
C fibers may
also play a role in centrally mediated effects. Activation of these fibers is
accomplished at
higher stimulation intensities (5-10 X those of B and A fibers) and lower
frequencies
(</=10 Hz).
Electrical activation of the splanclmic nerve can also cause muscle twitching
of the
abdominal and intercostal muscles. Stimulation at higher frequencies (>15 Hz)
reduces the
muscle activity, and muscle twitching is least evident or completely
habituates at higher
frequencies (20-30 Hz). During stimulation at 20 or 30 Hz, a short contraction
of the
muscles is observed followed by relaxation, such that there is no additional
muscle
contraction for the remainder of the stimulation. This can be due to
inhibitory neurons that
are activated with temporal summation.
The muscle-twitching phenomenon can also be used to help guide the stimulation
intensity used for the therapy. ~nce the threshold of muscle twitching is
reached, activation
of at least the A fibers has occurred. Increasing the current amplitude beyond
the threshold
increases the severity of the muscle contraction and can increase discomfort.
Delivering the
therapy at about the threshold for muscle twitching, and not substantially
higher, helps
ensure that the comfort of the patient is maintained, particularly at higher
frequencies.
Once this threshold is reached the pulse width can be increased 1.5 to 2.5
times longer,
thereby increasing the total charge delivered to the nerve, without
significantly increasing
the severity of the muscle twitching. By increasing the pulse width at the
current, activation
of B-fibers is better ensured. Hence, with an electrode placed in close
contact with the
nerve, a pulse width between 0.100 and 0.150 msec and a frequency of 1 Hz, the
current
amplitude can be increased until the threshold of twitching is observed
(activation of A
fibers). This will lilcely occur between 0.25 and 2.5 m Amps of current,
depending on how
close the electrode is to the nerve. It should be noted that patient comfort
can be achieved
at current amplitudes slightly higher than the muscle ttvitch threshold, or
that effective
therapy can be delivered at current amplitudes slightly below the muscle
twitch threshold,
particularly at longer pulse widths.
Habituation to the muscle twitching also occurs, such that the muscle
twitching
disappears after a certain time period. This allows the stimulation intensity
to be increased
to as much as lOX or greater the threshold of muscle twitching. This can be
done without
causing discomfort and ensures activation of the C fibers. It was previously
thought that
-16-
CA 02516988 2005-08-24
WO 2004/075974 PCT/US2004/005057
high stimulation intensities would result in the perception of pain, but this
does not appear
to be seen in experimental settings. The stimulation intensity of the muscle
twitch
threshold can also be used to guide therapy in this instance, because the
twitch threshold
may vary from patient to patient depending on the nerve and contact of the
electrode with
the nerve. Once the threshold of muscle twitching is determined the
stimulation intensity
(current X pulse width) can be increased to 5X or greater than lOX the
threshold.
Habituation occurs by stimulating at the threshold for up to 24 hours.
Increasing the stimulation intensity after habituation at one level occurs can
bring
baclc the muscle activity and require another period of habituation at the new
level. Thus,
the stimulation intensity can be increased in a stepwise manner, allowing
habituation to
occur at each step until the desired intensity is achieved at 5-10 X the
original threshold.
This is important if intermittent treatment frequency is used, as the
habituation process up
to the desired stimulation intensity would have to occur after each interval
when the device
is off. Preferably, the device is programmed to allow a prolonged ramp up of
intensity
over several hours to days, allowing habituation to occur at each level. This
is not the same
as the rapid rise in current amplitude that occurs at the beginning of each on
time during
stimulation. This may be built or programmed directly into the pulse generator
or
controlled/programmed by the physician, who can take into account patient
variability of
habituation time.
Alternatively, the device can sense muscle twitching. One way to do this is to
implant the unplantable pulse generator (IPG) over the muscles that are
activated. The IPG
can then electrically or mechanically sense the twitching and increase the
stimulation
intensity as habituation occurs.
Efferent electrical activation of the splanclnnc nerve can cause an increase
in blood
pressure, for example, the mean arterial blood pressure (MAP), above a
baseline value. A
drop in MAP below the baseline can fo11ow this increase. Because a sustained
increase in
MAP is undesirable, the stimulation pattern can be designed to prevent an
increase in MAP.
One strategy would be to have a relatively short signal-on time followed by a
signal-off
time of an equal or longer period. This would allow the MAP to drop back to or
below the
baseline. The subsequent signal-on time would then raise the MAP, but it can
start from a
lower baseline. In this manner a sinusoidal-lilce profile of the MAP can be
set up during
therapy delivery that would keep the average MAP within safe limits.
-17-
CA 02516988 2005-08-24
WO 2004/075974 PCT/US2004/005057
During stimulation the MAP may rise at a rate of 0.1-1.0 nnnHg/sec depending
on
frequency, with higher frequencies causing a more rapid rise. An acceptable
transient rise
in MAP would be about 10-20% of a patient's baseline. Assuming a normal MAP of
90
imnHg, a rise of 9-18 mm Hg over baseline would be acceptable during
stimulation. Thus a
stimulation on time of approximately 9-54 seconds is acceptable. The off time
would be
greater than the on time or greater than approximately 60 seconds. Habituation
may also
occur with the blood pressure changes. This may allow the on time to be
increased beyond
60 seconds, after habituation has occurred.
In one embodiment a strategy for treating obesity using splanchnic nerve
stimulation
is to stimulate A fibers. The pulse width can be set to 0.05-0.15 mSec and the
current can
be increased (0.1-0.75 mAmp) until the threshold of muscle twitching is
reached. Other
parameters include a frequency of 20-30 Hz and an on time of less than 60
seconds with a
duty cycle of 20-50%. Once habituation to the rise in MAP occurred the on time
can be
increased to greater than 60 seconds.
In another embodiment, a strategy for treating obesity by electrical
activation of the
splanclmic nerve involves stimulating the B and A fibers. This strategy
involves
stimulating the nerve at intensities 2-3X the muscle twitch threshold prior to
any
habituation. The pulse width can preferably be set to a range of about 0.150
mSec to 0.250
mSec with the pulse current increased (allowing appropriate habituation to
occur) to
achieve the desired level above the original muscle twitch threshold.
representative
parameters can be the following:
Current amplitude 0.75- 2.Om Amps,
Pulse width 0.150-0.250 m Seconds,
Frequency 10-20 Hz,
On-time <60 seconds,
Off time > 60 seconds.
These parameters result in gastric relaxation and reduced peristalsis causing
early
satiety and activation of distention receptors in the stomach that would send
satiety signals
baclc to the central nervous system in a reflex manner. Because the effect of
gastric
relaxation is sustained beyond the stimulation period, the off time can be 0.5
to 2.0 times
longer than the on time. This would reduce MAP rise. Once habituation to the
MAP rise
-18-
CA 02516988 2005-08-24
WO 2004/075974 PCT/US2004/005057
occurs, the on-time can be increased to greater than about 60 seconds, but the
duty cycle
should in some embodiments remain less than about 50%.
Sometimes it may be desirable to activate all fiber types (A, B and C) of the
splanchnic nerve. This can be done by increasing the stimulation intensity to
levels 8-12X
the muscle twitch threshold prior to habituation. The pulse width can
preferably be set to a
level of 0.250 mSec or greater. Representative parameters can be these:
Current amplitude >2.0 mAmp
Pulse width >0.250 mSec
Frequency 10-20 Hz
On-time <60 seconds
Off time >60 seconds
Similarly, the on time can be reduced to a longer period, beeping the duty
cycle
between 10 and 50%, once habituation occurred in this parameter.
It should be noted that the current amplitude will vary depending on the type
of
electrode used. A helical electrode that has intimate contact with the nerve
will have a
lower amplitude than a cylindrical electrode that may reside millimeters away
from the
nerve. W general, the current amplitude used to cause stimulation is
proportional to
1/(radial distance from nerve)'. The pulse width can remain constant or can be
increased to
compensate for the greater distance. The stimulation intensity would be
adjusted to activate
the afferent/efferent B or C fibers depending on the electrodes used. Using
the muscle-
twitching threshold prior to habituation can help guide therapy, given the
variability of
contact/distance between the nerve and electrode.
We have found that weight loss induced by electrical activation of the
splanchnic
nerve can be optimized by providing intermittent therapy, or intervals of
electrical
stimulation followed by intervals of no stimulation. Our data show that after
an interval of
stimulation, weight loss can be accelerated by turning the stimulation off.
This is directly
counter to the notion that termination of therapy would result in a rebound
phenomenon of
increased food intal~e and weight gain. These data also indicate that a
dynamic, or
changing, stimulation intensity (e.g., increasing or decreasing daily)
produces a more
pronounced weight loss than stimulation at a constant intensity. Given these
two findings,
two dosing strategies are described below.
-19-
CA 02516988 2005-08-24
WO 2004/075974 PCT/US2004/005057
These treatment algorithms are derived from studies involving canines. The
muscle
twitch threshold using a helical electrode is determined after adequate
healing time post
implant has elapsed (2-6 weeks). This threshold may range from about 0.125
mAinp-mSec
to about 0.5 mAmp-mSec. The stimulation intensity is increased daily over 1-2
weelcs,
allowing some or complete habituation to muscle twitching to occur between
successive
increases, until an intensity of 8-lOX the muscle twitch tlueshold is achieved
(1.0-5.0
mAmp-sec). During this period, a rapid decline in body weight and food intake
is
observed. After the initial weight loss period, a transition period is
observed over 1-4 weeks
in which some lost weight may be regained. Subsequently, a sustained, gradual
reduction
in weight and food intake occurs during a prolonged a stimulation phase of 4-8
weeks.
After this period of sustained weight loss, the stimulation may be terminated,
which is
again followed by a steep decline in weight and food intake, similar to the
initial
stimulation intensity ramping phase. The post-stimulation weight and food
decline may last
for 1-4 weeks, after which the treatment algorithm can be repeated to create a
therapy cycle,
or intermittent treatment interval, that results in sustained weight loss. The
duty cycle
during this intermittent therapy may range from 20-50% with stimulation on-
times of up to
15-60 seconds. This intermittent therapy not only optimizes the weight loss,
but also
extends the battery life of the implanted device.
h1 another intermittent therapy treatment algorithm embodiment, therapy
cycling
occurs during a 24-hour laeriod. In this algoritlmn, the stimulation intensity
is maintained at
1X-3X the muscle twitch threshold for a 12-18 hour period. Alternatively, the
stimulation
intensity can be increased gradually (e.g., each hour) during the first
stimulation interval.
The stimulation is subsequently terminated for 6-12 hours. Alternatively, the
stimulation
intensity can be gradually decreased during the second interval back to the
muscle twitch
tlueshold level. DLIe to this sustained or accelerating effect that occurs
even after cessation
of stimulation, the rislc of binge eating and weight gain during the off
period or declining
stimulation intensity period is minimized.
Alternatively, an alpha-sympathetic receptor bloclcer, such as prazosin, can
be used
to blunt the rise in MAP. Alpha-Mockers are commonly available
antihypertensive
medications. The rise in MAP seen with splanchnic nerve stimulation is the
result of alpha
receptor activation, which mediates arterial constriction. Because the affects
of this therapy
on reduced food intalce and energy expenditure are related to beta-sympathetic
receptor
-20-
CA 02516988 2005-08-24
WO 2004/075974 PCT/US2004/005057
activity, addition of the alpha-bloclcer would not likely alter the
therapeutic weight loss
benefits.
In one embodiment a helical electrode design with platinum iridium ribbon
electrodes is used. The electrodes encircle all or a substantial portion of
the nerve. A
balanced charge biphasic pulse is be delivered to the electrodes, resulting in
a bidirectional
action potential to activate both efferent and afferent neurons. However,
utilizing a
wavefonn that is asymmetrical between the positive and negative phase
deflections can
create a unidirectional action potential, resulting in anodal block without
incidental afferent
fiber activation. Thus, whereas a typical biphasic waveform has equal positive
and
negative phase deflections (Figure 11a), the anodal bloclcing waveform would
have a short
and tall positive deflection followed by a long shallow negative deflection
(Figure llb).
The amperage X time for each deflection would be equal thereby achieves a
charge balance.
Charge balance is a consideration for avoiding nerve damage.
Alternatively, a quadripolar electrode assembly can be used. ~ne pair of
electrode
placed distally on the nerve would be used to produce efferent nerve
activation. The second
proximal pair would be used to bloclc the afferent A fiber conduction. The
bloclcing
electrode pair can have asymmetric electrode surface areas, with the cathode
surface area
being greater than the aazode (described by Petruska, patent 5,755,750)
(Figure 12). Because
of the large surface area at the cathode, the charge density would be
insufficient to cause
activation. The small surface area at the anode would cause hypeupolarization,
particularly
in the A fibers, and thereby block afferent conduction. Signals can be sent to
four
electrodes, timed such that when the efferent activation pair created a bi-
directional action
potential, the blocking pair would be active as the afferent potential
traveled up the nerve.
Alternatively, the bloclcing pair can be activated continuously during the
treatment period.
A tripolar electrode can also be used to get activation of a select fiber size
bilaterally
or to get unilateral activation. To get bi-directional activation of B fibers
and anodal
blocking of A fibers, a tripolar electrode with the cathode flanked proximally
and distally
by anodes would be used. Unidirectional activation would be achieved by moving
the
cathode closer to the proximal electrode and delivering differential current
ratios to the
anodes.
Pulse generation for electrical nerve modulation is accomplished using a pulse
generator. Pulse generators can use microprocessors and other standard
electrical
-21-
CA 02516988 2005-08-24
WO 2004/075974 PCT/US2004/005057
components. A pulse generator for this embodiment can generate a pulse, or
energy signal,
at frequencies ranging from approximately 0.5 Hz to approximately 300 Hz, a
pulse width
from approximately 10 to approximately 1,000 microseconds, and a constant
current of
between approximately 0.1 milliamperes to approximately 20 milliamperes. The
pulse
generator can be capable of producing a ramped, or sloped, rise in the current
amplitude.
The preferred pulse generator can communicate with an external programmer and
or
monitor. Passwords, handshakes and parity checks are employed for data
integrity. The
pulse generator can be battery operated or operated by an external
radiofrequency device.
Because the pulse generator, associated components, and battery can be
implanted, they are,
in some embodiments, preferably encased in an epoxy-titanium shell.
A schematic of the implantable pulse generator (IPG) is shown in Figure 5.
Components are housed in the epoxy-titanium shell. The battery supplies power
to the logic
and control unit. A voltage regulator controls the battery output. The logic
and control unit
control the stimulus output and allow for prograrmning of the various
parameters such as
pulse width, amplitude, and frequency. In addition, the stimulation pattern
and treatment
parameters can be programmed at the logic and control unit. A crystal
oscillator provides
timing signals for the pulse and for the logic and control unit. An anterma is
used for
receiving communications from an external programmer and for status checking
the device.
The programmer would allow the physician to program the required stimulation
intensity
increase to allow for muscle and Ie~IAP habituation for a given patient and
depending on the
treatment frequency. Alternatively, the IPG can be programmed to increase the
stimulation
intensity at a set rate, such as 0.1 mAmp each hour at a pulse width of 0.25-
0.5 mSec. The
output section can include a radio transmitter to inductively couple with the
wireless
electrode to apply the energy pulse to the nerve. The reed switch allows
manual activation
using an external magnet. Devices powered by an external radiofrequency device
would
limit the components of the pulse generator to primarily a receiving coil or
antenna.
Alternatively, an external pulse generator can inductively couple via radio
waves directly
with a wireless electrode implanted near the nerve.
The IPG is coupled to a lead (where used) and an electrode. The lead (where
used)
is a bindle of electrically conducting wires insulated from the surroundings
by a non
electrically conducting coating. The wires of the lead correct the Il'G to the
stimulation
electrodes, which transfers the energy pulse to the nerve. A single wire can
connect the IPG
-22-
CA 02516988 2005-08-24
WO 2004/075974 PCT/US2004/005057
to the electrode, or a wire bundle can connect the IPG to the electrode. Wire
bundles may or
may not be braided. Wire bundles are preferred because they increase
reliability and
durability. Alternatively, a helical wire assembly can be utilized to improve
durability with
flexion and extension of the lead.
The electrodes are preferably platinum or platinum-iridium ribbons or rings as
shown in Figure 6. The electrodes are capable of electrically coupling with
the surrounding
tissue and nerve. The electrodes can encircle a catheter-life lead assembly.
The distal
electrode can form a rounded cap at the end to create a bullet nose shape.
Preferably, this
electrode serves as the cathode. A lead of this type can contain 2 to 4 ring
electrodes
spaced anywhere from 2.0 to 5.0 mm apart with each ring electrode being
approximately
1.0 to approximately 10.0 mm in width. Catheter lead electrode assemblies may
have an
outer diameter of approximately 0.5 mm to approximately 1.5 nun to facilitate
percutaneous placement using an introducer.
Alternatively a helical or cuff electrode is used, as are lmown to those of
slcill in the
art. A helical or cuff electrode can prevent migration of the lead away from
the nerve.
Helical electrodes may be optimal in some settings because they may reduce the
chance of
nerve injury and ischemia.
The generator may be implanted subcutaneously, intra-abdominally, or
intrathoracically, and/or in any location that is appropriate as is lmown to
those of slcill in
the art.
Alternatively, a wireless system can be employed by having an electrode that
inductively couples to an external radiofrequency field. A wireless system
would avoid
problems such as lead fracture and migration, found in wire-based systems. It
would also
simplify the implant procedure, by allowing simple injection of the wireless
electrode in
proximity to the splanclmic nerve, and avoiding the need for lead anchoring,
tunneling, and
subcutaneous pulse generator implantation.
A wireless electrode would contain a coil/capacitor that would receive a
radiofrequency signal. The radiofrequency signal would be generated by a
device that
would create an electromagnetic field sufficient to power the electrode. It
would also
provide the desired stimulation parameters (frequency, pulse width, current
amplitude,
signal on/off time, etc.) The radiofrequency signal generator can be worn
externally or
implanted subcutaneously. The electrode would also have metallic elements for
electrically
-23-
CA 02516988 2005-08-24
WO 2004/075974 PCT/US2004/005057
coupling to the tissue or splanchnic nerve. The metallic elements can be made
of platinum
or platinum-iridium. Alternatively, the wireless electrode can have a battery
that would be
charged by an radiofreqency field that would then provide stimulation during
intervals
without an radiofrequency field.
Bipolar stimulation of a nerve can be accomplished with multiple electrode
assemblies with one electrode serving as the positive node and the other
serving as a
negative node. In this manner nerve activation can be directed primarily in
one direction
(unilateral), such as efferently, or away from the central nervous system.
Alternatively, a
nerve cuff electrode can be employed. Helical cuff electrodes as described in
U.S. Patent
No. 5,251,634 to Weinberg are preferred. Cuff assemblies can similarly have
multiple
electrodes and direct and cause unilateral nerve activation.
Unipolar stimulation can also be performed. As used herein, unipolar
stimulation
means using a single electrode on the lead, while the metallic shell of the
IfG, or another
external portion of the IPG, functions as a second electrode, remote from the
first electrode.
This type of unipolar stimulation can be more suitable for splanclmic nerve
stimulation than
the bipolar stimulation method, particularly if the electrode is to be placed
percutaneously
under fluoroscopic visualization. With fluoroscopically observed percutaneous
placement,
it may not be possible to place the electrodes adjacent the nerve, which can
be preferred for
bipolar stimulation. With unipolar stimulation, a larger energy field is
created in order to
couple electrically the electrode on the lead with the remote external poution
of the IPG, and
the generation of this larger energy field can result in activation of the
nerve even in the
absence of close proximity between the single lead electrode and the tlerve.
This allows
successful nerve stimulation with the single electrode placed in "general
proximity" to the
nerve, meaning that there can be significantly greater separation between the
electrode and
the nerve than the "close proximity" used for bipolar stimulation. The
magnitude of the
allowable separation between the electrode and the nerve will necessarily
depend upon the
actual magnitude of the energy field that the operator generates with the lead
electrode in
order to couple with the remote electrode.
In multiple electrode lead assemblies, some of the electrodes can be used for
sensing nerve activity. This sensed nerve activity can serve as a signal to
commence
stimulation therapy. For example, afferent action potentials in the splanchnic
nerve, created
due to the commencement of feeding, can be sensed and used to activate the IPG
to begin
-24-
CA 02516988 2005-08-24
WO 2004/075974 PCT/US2004/005057
stimulation of the efferent neurons of the splanchnic nerve. Appropriate
circuitry and logic
for receiving and filtering the sensed signal would be used in the IPG.
Because branches of the splanchnic nerve directly innervate the adrenal
medulla,
electrical activation of the splanchnic nerve results in the release of
catecholamines
(epinephrine and norepinephrine) into the blood stream. In addition, dopamine
and cortisol,
which also raise energy expenditure, can be released. Catecholamines can
increase energy
expenditure by about 15% to 20%. By comparison, subitramine, a pharmacologic
agent
used to treat obesity, increases energy expenditure by approximately only 3%
to 5%.
Human resting venous blood levels of norepinephrine and epinephrine are
approximately 25 picograms (pg)hnilliliter (ml) and 300 pg/ml, respectively,
as shown in
Figure 7. Detectable physiologic changes such as increased heart rate occur at
norepinephrine levels of approximately 1,500 pg/ml and epinephrine levels of
approximately 50 pg/ml. Venous blood levels of norepinephrine can reach as
high 2,000
pg/ml during heavy exercise, and levels of epinephrine can reach as high as
400 to 600
pg/ml during heavy exercise. Mild exercise produces norepinephrine and
epmephrme levels
of approximately 500 pg/ml and 100 pg/ml, respectively. It can be desirable to
maintain
catecholalnine levels somewhere between mild and heavy exercise during
electrical
sympathetic activation treatment for obesity.
In anesthetized animals, electrical stimulation of the splanchnic nerve has
shown to
raise blood catecholamine levels in a frequency dependent manner in the range
of about 1
H~ to about 20 H~, such that rates of catecholalnine release/production of 0.3
to 4.0 p.g/min
can be achieved. These rates are sufficient to raise plasma concentrations of
epinephrine to
as high as 400 to 600 pg/ml, which in turn can result in increased energy
expenditure from
10% to 20% as shown in Figure ~. During stimulation, the ratio of epinephrine
to
norepinephrine is 65% to 35%. ~ne can change the ratio by stimulating at
higher
frequencies. In some embodiments this is desired to alter the energy
expenditure and/or
prevent a rise in MAP.
Energy expenditure in humans ranges from approximately 1.5 lccal/min to 2.5
lccal/min. A 15% increase in this energy expenditure in a person with a 2.0
lccal/min energy
expenditure would increase expenditure by 0.3 lccal/min. Depending on
treatment
parameters, this can result in an additional 100 to 250 local of daily
expenditure and 36,000
-25-
CA 02516988 2005-08-24
WO 2004/075974 PCT/US2004/005057
to 91,000 kcal of yearly expenditure. One pound of fat is 3500 kcal, yielding
an annual
weight loss of 10 to 26 pounds.
W creased energy expenditure would is fueled by fat and carbohydrate
metabolism.
Postganglionic branches of the splanchnic nerve innervate the liver and fat
deposits of the
abdomen. Activation of the splanchnic nerve can result in fat metabolism and
the liberation
of fatty acids, as well as glycogen breakdown and the release of glucose from
the liver. Fat
metabolism coupled with increased energy expenditure can result in a net
reduction in fat
reserves.
In some embodiments, it may be desirable to titrate obesity therapy to plasma
ghrelin levels. In humans, venous blood ghrelin levels range from
approximately 250 pg/ml
to greater than 700 pg/ml as shown in Figure 9. Ghrelin levels rise and fall
during the day
with peak levels typically occurring just before meals. Ghrelin surges are
believed to
stimulate appetite and lead to feeding. Surges in ghrelin may be as high as
1.5-2.0 times
that of basal levels. The total ghrelin production in a 24-hour period is
believed to be
related to the energy state of the patient. Dieting that results in a state of
energy deficit is
associated with a higher total ghrelin level in a 24-hour period. Splanchnic
nerve
stimulation has been shov~m to elnninate or substantially reduce ghrelin
surges or spikes. In
a canine model, ghrelin levels prior to splanchnic nerve stimulation showed a
midday surge
of almost 2.0 times basal levels. After one week of stimulation at 20 FIz, on-
time of
approximately 60 seconds, off time of approximately 120 seconds, and a peals
current
intensity of ~~ the muscle twitch threshold, this midday surge was almost
eliminated
(Figure 14). In addition, it increased the total ghrelin production in a 24-
hour period,
reflecting an energy-deficient state (baseline area under the curve = 64.1 X
104, stimulation
area under the curve = 104.1 X 104). Splanclmic nerve activation, in the
treatment of
obesity, can be titrated to reduce gluelin surges and attain the desired
energy deficit state for
optimal weight loss. Reductions in food intake comparable to the increases in
energy
expenditure (i.e. 100 to 250 lccal/day) can yield a total daily kcal reduction
of 200 to 500
per day, and 20 to 50 pounds of weight loss per year.
W anesthetized animals, electrical activation of the splanclmic nerve has also
been
shown to decrease insulin secretion. In obesity, insulin levels are often
elevated, and insulin
resistant diabetes (Type II) is common. Down-regulation of insulin secretion
by splanchnic
nerve activation may help correct insulin resistant diabetes.
-26-
CA 02516988 2005-08-24
WO 2004/075974 PCT/US2004/005057
Implantation of the lead/electrode assembly for activation of the greater
splanchnic
nerve is preferably accomplished percutaneously using an introduces as shown
in Figure 10.
The introduces can be a hollow needle-life device that would be placed
posteriorly through
the skin between the ribs para-midline at the T9-T12 level of the thoracic
spinal column. A
posterior placement with the patient prone is preferred to allow bilateral
electrode
placement at the splanchnic nerves, if desired. Placement of the needle can be
guided using
fluoroscopy, ultrasound, or CT scarming. Proximity to the splanchnic nerve by
the
introduces can be sensed by providing energy pulses to the introduces
electrically to activate
the nerve while monitoring for a rise in MAP or muscle twitching. All but the
tip of the
introduces can be electrically isolated so as to focus the energy delivered to
the tip of the
introduces. The lower the current amplitude used to cause a rise in the MAP or
muscle
twitch, the closer the introduces tip would be to the nerve. Preferably, the
introduces tip
serves as the cathode for stimulation. Alternatively, a stimulation endoscope
can be placed
into the stomach of the patient for electrical stimulation of the stomach. The
evolved
potentials created in the stomach can be sensed in the splanchnic nerve by the
introduces.
To avoid damage to the spinal nerves, the introduces can sense evoked
potentials created by
electrically activating peripheral sensory nerves. Alternatively, evoked
potentials can be
created in the lower intercostal nerves or upper abdominal nerves and sensed
in the
splaalclmic. ~nce the introduces was in proximity to the nerve, a catheter
type lead electrode
assembly would be inserted through the introduces and adjacent to the nerve.
Alternatively,
a wireless, radiofrequency battery charged, electrode can be advanced through
the
introduces to reside alongside the nerve. W either case, stunulating the nerve
and
monitoring for a rise in MAP or muscle twitch can be used to confirm electrode
placement.
Once the electrode was in place the current amplitude would be increased at a
pulse
width of 50 to 500 .sec and a frequency of 1 Hz, until the threshold for
muscle ttuitching
was reached. The current amplitude caal be set slightly above or slightly
below this muscle
twitch threshold. After identifying the desired current amplitude the pulse
width can be
increased by as much as 2.5 times and the frequency increased up to 40 Hz for
therapeutic
stimulation. The lead (where used) and the IPG would be implanted
subcutaneously in the
patient's baclc or side. The lead would be appropriately secured to avoid
dislodgement. The
lesser and least splanchnic nerves can also be activated to some degree by
lead/electrode
placement according to the above procedure, due to their proximity to the
splanchnic nerve.
_27_
CA 02516988 2005-08-24
WO 2004/075974 PCT/US2004/005057
Percutaneous placement of the lead electrode assembly can be enhanced using
direct
or video assisted visualization. An optical port can be incorporated into the
introducer. A
channel can allow the electrode lead assembly to be inserted and positioned,
once the nerve
was visualized. Alternatively, a percuta.neous endoscope can be inserted into
the chest
cavity for viewing advancement of the introducer to the nerve. The parietal
lung pleura are
relatively clear, and the nerves and introducer can be seen running along the
vertebral
bodies. With the patient prone, the lungs are pulled forward by gravity
creating a space for
the endoscope and for viewing. This can avoid the need for single lung
ventilation. If
desired, one lung can be collapsed to provide space for viewing. This is a
common and safe
procedure performed using a bifurcated endotracheal tube. The endoscope can
also be
placed laterally, and positive C~Z pressure can be used to push down the
diaphragm,
thereby creating a space for viewing and avoiding hmg collapse.
Alternatively, stimulation electrodes can be placed along the sympathetic
chain
ganglia from approximately vertebra T4 to T11. This implantation can be
accomplished in a
1 ~ similar percutaneous manner as above. This would create a more general
activation of the
Sympat11et1C neYi,OlIS System, though it would include activation of the
neurons that
comprise the splanchnic nerves.
Alternatively, the lead/electrode assembly can be placed intra-abdominally on
the
portion of the splanchnic nerve that resides retroperitoneally on the
abdominal aorta just
prior to synapsing in the celiac ganglia. Access to the nerve in this region
can be
accomplished laparoscopically, using typical laparoscopic techniques, or via
open
laparotomy. A cuff electrode can be used to encircle the nerve unilaterally or
bilaterally.
The lead can be anchored to the crus of the diaphragm. A cuff or patch
electrode can also
be attached to the celiac ganglia unilaterally or bilaterally. Similar
activation of the
splanclmic branches of the sympathetic nervous system would occur as
implanting the lead
electrode assembly in the thoracic region.
An alternative lead/electrode placement would be a transvascular approach. Due
to
the proximity of the splanchnic nerves to the azygous veins shown in Figure
10, and in
particular the right splanchnic nerve and right azygous vein, modulation can
be
accomplished by positioning a lead/electrode assembly in this vessel. Access
to the venous
system and azygous vein can occur via the subclavian vein using standard
techniques. The
electrode/lead assembly can be mounted on a catheter. A guidewire can be used
to position
-28-
CA 02516988 2005-08-24
WO 2004/075974 PCT/US2004/005057
the catheter in the azygous vein. The lead/electrode assembly would include an
expandable
member, such as a stent. The electrodes would be attached to the stmt, and
using balloon
dilation of the expandable member, can be pressed against the vessel wall so
that energy
delivery can be transferred to the nerve. The expandable member would allow
fixation of
the electrode lead assembly in the vessel. The lPG and remaining lead outside
of the
vasculature would be implanted subcutaneously in a manner similar to a heart
pacemalcer.
h1 some embodiments, the apparatus for nerve stimulation can be shielded or
otherwise made compatible with magnetic resonance imaging (MRI) devices, such
that the
apparatus is less susceptible to the following effects during exposure to
magnetic fields: (a)
current induction and its resultant heat effects and potential malfunction of
electronics in
the apparatus, and (b) movement of the apparatus due to Lorentz forces. This
type of
magnetic shielding can be accomplished by, for example, using materials for
the generator
and/or electrode that are nanomagnetic or utilize carbon composite coatings.
Such
techniques are described in U.S. Patent Nos. 6,506,972 and 6,673,999, and U.S.
Patent
Application No. 2002/0183796, published December 5, 20029 U.S. Patent
Application No.
2003/0195570, published ~ctober 16, 20039 and U.S. Patent Application No.
2002/0147470, published ~ctober 10, 2002. The entireties of all of these
references are
hereby incorporated by reference.
For pwposes of summarizing the invention, certain aspects, advantages, and
novel
features of the invention have been described herein. It is to be understood
that not
necessarily all such advantages may be achieved in accordance with any
particular
embodiment of the invention. Thus, the invention may be embodied or carried
out in a
manner that achieves or optimizes one advantage or group of advantages as
taught herein
without necessarily achieving other advantages as may be taught or suggested
herein.
While certain aspects and embodiments of the invention have been described,
these
have been presented by way of example only, and are not intended to limit the
scope of the
invention. Indeed, the novel methods and systems described herein may be
embodied in a
variety of other forms without departing from the spirit thereof. The
accompanying claims
and their equivalents are intended to cover such forms or modifications as
would fall within
the scope and spirit of the invention.
-29-