Note: Descriptions are shown in the official language in which they were submitted.
CA 02517056 2005-08-24
TITLE
Ni-Cr-Co ALLOY FOR ADVANCED GAS TURBINE ENGINES
FIELD OF THE INVENTION
This invention relates to wroughtable high strength alloys for use at elevated
temperatures. In particular, it is related to alloys which possess sufficient
creep strength, thermal
stability, and resistance to strain age cracking to allow for fabrication and
service in gas turbine
transition ducts and other gas turbine components.
BACKGROUND OF THE INVENTION
To meet the demand for increased operating efficiency, gas turbine engine
designers
would like to employ higher and higher operating temperatures. However, the
ability to increase
operating temperatures is often limited by material properties. One
application with such a
limitation is gas turbine transition ducts. Transition ducts are often welded
components made of
sheet or thin plate material and thus need to be weldable as well as
wroughtable. Often gamma-
prime strengthened alloys are used in transition ducts due to their high-
strength at elevated
temperatures. However, current commercially available wrought gamma-prime
strengthened
alloys either do not have the strength or stability to be used at the very
high temperatures
demanded by advanced gas turbine design concepts, or can present difficulties
during
fabrication. 1n particular, one such fabrication difficulty is the
susceptibility of many wrought
CA 02517056 2005-08-24
gamma-prime strengthened alloys to strain age cracking. The problem of strain
age cracking
will be described in more detail later in this document.
Wrought gamma-prime strengthened alloys are often based on the nickel-chromium-
cobalt system, although other base systems are also used. These alloys will
typically have
aluminum and titanium additions which are responsible for the formation of the
gamma-prime
phase, Ni3(AI,Ti). Other gamma-prime forming elements, such as niobium and/or
tantalum, can
also be employed. An age-hardening heat treatment is used to develop the gamma-
prime phase
into the alloy microstructure. This heat treatment is normally given to the
alloy when it is in the
annealed condition. The presence of gamma-prime phase leads to a considerable
strengthening
of the alloy over a broad temperature range. Other elemental additions rnay
include
molybdenum or tungsten for solid solution strengthening, carbon for carbide
formation, and
boron for improved high temperature ductility.
Strain age cracking is a problem which limits the weldability of many gamma-
prime
strengthened alloys. This phenomenon typically occurs when a welded part is
subjected to a
high temperature for the first time after the welding operation. Often this is
during the post-weld
annealing treatment given to most welded gamma-prime alloy fabrications. The
cracking occurs
as a result of the formation of the gamma-prime phase during the heat up to
the annealing
temperature. The formation of the strengthening gamma-prime phase in
conjunction with the
low ductility many of these alloys possess at intermediate temperatures, as
well as the
mechanical restraint typically imposed by the welding operation will often
lead to cracking. The
problem of strain age cracking can limit alloys to be used up to only a
certain thickness since
greater material thickness leads to greater mechanical restraint.
2
CA 02517056 2005-08-24
Several types of tests to evaluate the susceptibility of an alloy to strain
age cracking have
been developed. These include the circular patch test, the restrained plate
test, and various
dynamic thermal-mechanical tests. One test which can be used to evaluate the
susceptibility of
an alloy to strain age cracking is the controlled heating rate tensile (CHRT)
test developed in the
1960's. Recent testing at Haynes International has found the CHRT test to
successfully rank the
susceptibility of several commercial alloys in an order consistent with field
experience. In the
CHRT test, a sheet tensile sample is heated from a low temperature up to the
test temperature at
a constant rate (a rate of 25°F to 30°F per minute was used in
the tests run at Haynes
International). Once reaching the test temperature the sample is pulled to
fracture at a constant
engineering strain rate. The test sample starts in the annealed (not age-
hardened) condition, so
that the gamma-prime phase is precipitating during the heat-up stage as would
be the case in a
welded component being subjected to a post-weld heat treatment. The percent
elongation to
fracture in the test sample is taken as a measure of susceptibility to strain
age cracking (lower
elongation values suggesting greater susceptibility to strain age cracking).
The elongation in the
CHRT is a function of test temperature and normally will exhibit a minimum at
a particular
temperature. The temperature at which this occurs is around 1500°F for
many wrought gamma-
prime strengthened alloys.
Good strength and thermal stability at the high temperatures demanded by
advanced gas
turbine concepts are two properties lacking in many current commercially
available wrought
gamma-prime strengthened alloys. High temperature strength has long been
evaluated with the
use of creep-rupture tests, where samples are isothermally subjected to a
constant load until the
sample fractures. The time to fracture, or rupture life, is then used as a
measure of the alloy
3
CA 02517056 2005-08-24
strength at that temperature. Thermal stability is a measure of whether the
alloy microstructure
remains relatively unaffected during a thermal exposure. Many high-temperature
alloys can
form brittle intermetallic or carbide phases during thermal exposure. The
presence of these
phases can dramatically reduce the room-temperature ductility of the material.
This loss of
ductility can be effectively measured using a standard tensile test.
Many wrought gamma-prime strengthened alloys are available in sheet form today
in
today's marketplace. The Rene-41 or R-41 alloy (U.S. Patent No. 2,945,758) was
developed by
General Electric in the 1950's for use in turbine engines. It has excellent
creep strength, but is
limited by poor thermal stability and resistance to strain age cracking. A
similar General Electric
alloy, M-252 alloy (U.S. Patent No. 2,747,993), was also developed in the
1950's. Although
currently available only in bar form, the composition would easily lend itself
to sheet
manufacture. The M-252 alloy has good creep strength and resistance to strain
age cracking, but
like R-41 alloy is limited by poor thermal stability. The Pratt & Whitney
developed alloy known
commercially as WASPALOY alloy (apparently having no U.S. patent coverage) is
another
gamma-prime strengthened alloy intended for use in turbine engines and
available in sheet form.
However, this alloy has marginal creep strength above 1500°F, marginal
thermal stability, and
has fairly poor resistance to strain age cracking. The alloy commercially
known as 263 alloy
(U.S. Patent 3,222,165) was developed in the late 1950's and introduced in
1960 by Rolls-Royce
Limited. This alloy has excellent thermal stability and resistance to strain
age cracking, but has
very poor creep strength at temperatures greater than 1500°F. The PK-33
alloy (U.S. Patent No.
3,248,213) was developed by the International Nickel Company and introduced in
1961. This
alloy has good thermal stability and creep strength, but is limited by a poor
resistance to strain
4
CA 02517056 2005-08-24
age cracking. As suggested by these examples, no currently commercially
available alloys are
available which possess the unique combination of three key properties: good
creep strength and
good thermal stability in the 1600 to 1700°F temperature range as well
as good resistance to
strain age cracking.
SUMMARY OF THE INVENTION
The principal objective of this invention is to provide new wrought age-
hardenable
nickel-chromium-cobalt based alloys which are suitable for use in high
temperature gas turbine
transition ducts and other gas turbine components possessing a combination of
three specific key
properties, namely resistance to strain age cracking, good thermal stability,
and good creep-
rupture strength.
It has been found that this objective can be reached with an alloy containing
a certain
range of chromium and cobalt, a certain range of molybdenum and possibly
tungsten, and a
certain range of aluminum, titanium and possibly niobium, with a balance of
nickel and various
minor elements and impurities.
Specifically, the preferred ranges are 17 to 22 wt.% chromium, 8 to 15 wt.%
cobalt, 4.0
to 9.5 wt.% molybdenum, up to 7.0 wt.% tungsten, 1.28 to 1.65 wt.% aluminum,
1.50 to 2.30
wt.% titanium, up to 0.80 wt.% niobium, up to 3 wt.% iron, 0.01 to 0.2 wt.%
carbon, and up to
0.015 wt.% boron, with a balance of nickel and impurities.
CA 02517056 2005-08-24
DESCRIPTION OF THE FIGURES
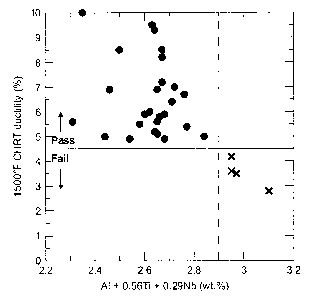
Figure 1 is a graph of the ductility of the studied wrought age-hardenable
nickel-
chromium-cobalt based alloys in a controlled heating rate tensile test at
1500°F.
Figure 2 is a graph of the ductility of the studied wrought age-hardenable
nickel-
chromium-cobalt based alloys in a standard tensile test at room temperature.
DESCRIPTION OF THE PREFERRED EMBODIMENT
The wrought age-hardenable nickel-chromium-cobalt based alloys described here
have
sufficient creep strength, thermal stability, and resistance to strain age
cracking to allow for
service in sheet or plate form in gas turbine transition ducts as well as in
other product forms and
other demanding gas turbine applications. This combination of critical
properties is achieved
through control of several critical elements each with certain functions. The
presence of gamma-
prime forming elements such as aluminum, titanium, and niobium contribute
significantly to the
high creep-rupture strength through the formation of the gamma-prime phase
during the age-
hardening process. However, the combined amount of aluminum, titanium, and
niobium must
be carefully controlled to allow for good resistance to strain age cracking.
Molybdenum and
possibly tungsten are added to provide additional creep-rupture strength
through solid solution
strengthening. Again, however, the total combined molybdenum and tungsten
concentration
must be carefully controlled, in this case to ensure sufficient thermal
stability of the alloy.
Based on the projected requirements for the next generation of gas turbine
transition
ducts, gamma-prime strengthened alloys have significant potential. Three of
the more critical
properties are creep strength, weldability (i.e. strain age cracking
resistance), and thermal
6
CA 02517056 2005-08-24
stability. However, producing a gamma-prime strengthened alloy which excels in
all three of
these properties is not straightforward and no commercially available alloy
was found which
possessed all three properties to a sufficient degree.
I tested 26 experimental and 5 commercial alloys whose compositions are set
forth in
Table 1. The experimental alloys have been labeled A through Z. The commercial
alloys were
HAYNES R-41 alloy, HAYNES WASPALOY alloy, HAYNES 263 alloy, M-252 alloy, and
IVIMONIC PK-33 alloy. The alloys (including both the experimental and the
commercial alloys
had a Cr content which ranged from 17.5 to 21.3 wt.%, as well as a cobalt
content ranging from
8.3 to 19.6 wt.%. The aluminum content ranged from 0.49 to 1.89 wt.%, the
titanium content
from 1.53 to 3.12 wt.%, and the niobium content ranged from nil to 0.79 wt.%.
The
molybdenum content ranged from 3.2 to 10.5 wt.% and the tungsten ranged from
nil up to 8.3
wt.%. Intentional minor element additions carbon and boron ranged from 0.034
to 0.163 wt.%
and from nil to 0.008 wt.%, respectively. Iron ranged from nil to 3.6 wt.%.
All testing of the alloys was performed on sheet material of 0.047" to 0.065"
thickness.
The experimental alloys were vacuum induction melted, and then electro-slag
remelted, at a heat
size of 50 1b. The ingots so produced were soaked at 2150°F and then
forged and rolled with
starting temperatures of 2150°F. The sheet thickness after hot rolling
was 0.085". The sheets
were annealed at 2150°F for 15 minutes and water quenched. The sheets
were then cold rolled
to 0.060" thickness. The cold rolled sheets were annealed at temperatures
between 2050 and
2175°F as necessary to produce a fully recrystallized, equiaxed grain
structure with an ASTM
gain size between 4 and 5. Finally, the sheet material was given an age-
hardening heat
treatment of 1475°F for 8 hours to produce the gamma-prime phase. The
commercial alloys
CA 02517056 2005-08-24
HAYNES R-41 alloy, HAYNES WASPALOY alloy, HAYNES 263 alloy, and NIMONIC PK-
33 alloy were obtained in sheet form in the mill annealed condition. Since no
commercially
available M-252 alloy sheet could be found, a 50 1b. heat was produced for
evaluation using the
same method as described above for the experimental alloys. All five of the
commercial alloys
were given post-anneal age-hardening heat treatments according accepted
standards. These heat
treatments are reported in Table 2.
To evaluate the three properties identified above as important (strain age
cracking
resistance, thermal stability, and creep strength) three different tests were
employed on each of
the alloys. The first test was the controlled heating rate tensile test
(CHRT). The results of the
CHRT testing are given in Table 3. The critical property in this test is the
tensile ductility, as
measured by a measurement of the elongation to failure. Alloys with a greater
ductility in this
test are expected to have greater resistance to strain age cracking. The
objective of the present
study was to have a ductility of 4.5% or greater. Of the experimental alloys,
only alloy W failed
to meet this requirement. For the commercial alloys, M-252 alloy and 263 alloy
met the
requirement, while PK-33 alloy, WASPALOY alloy, and R-41 alloy did not. It was
found that
the performance of a given alloy in the CHRT test could be correlated to the
amount of the
gamma-prime forming elements in the alloy using the following equation (where
the elemental
compositions are in wt.%):
Al+0.56Ti+0.29Nb<2.9 (1)
The values of the left hand side of Eq. (1) for all of the alloys in this
study axe given in
Table 1. All of the alloys which passed the CHRT test were found to obey Eq.
(1). Furthermore,
all of the alloys which did not obey Eq. (1) did not pass the CHRT test
requirement, that is, they
CA 02517056 2005-08-24
were found to have a 1500°F CHRT ductility less than 4.5%. This
relationship is shown more
clearly in Fig. l, where the 1500°F CHRT ductility is plotted against
the value of the left hand
side of Eq. (1) for all of the alloys in the study. All testing was performed
on samples in the
annealed condition. The tensile ductility (measured as the percent elongation
to failure) is
plotted as a function of the compositional variable A1 + 0.56Ti + 0.29Nb
(where the elemental
compositions are in wt.%). A line is drawn on the figure corresponding to a
tensile ductility of
4.5%. All alloys plotted above this line (symbol: filled circles) were
considered to have passed
the controlled heating rate tensile test, while alloys plotted below the line
(symbol: x-marks)
were considered to have failed. A dashed vertical line is drawn at a value of
2.9 wt.% for the
compositional variable, A1 + 0.56Ti + 0.29Nb. All alloys with a value greater
than 2.9 were
found to fail the controlled heating rate tensile test.
9
CA 02517056 2005-08-24
TABLE 1
AlloyNi Cr Co W Mo Ti A1 Nb C B Fe Mo+0.52WAI+0.56Ti+p.29Cb
A BAL 19.110.77.0 5.5 1.911.53< 0.0790.003<0.19.1 2.60
0.05
B BAL 19.510.95.4 4.3 2.071.510.020.0970.006<0.17.1 2.68
C BAL 19.210.86.3 5.1 2.201.60< 0.0950.006<0.18.4 2.84
0.05
D BAL 19.010.75.9 6.3 1.711.570.630.0900.005<0.19.3 2.71
E BAL I9.210.76.8 5.3 1.591.510.790.0850.003<0.18.8 2.63
F BAL 19.410.95.9 4.5 2.051.47< 0.0860.005<0.17.6 2.62
0.05
G BAL 19.110.76.3 5.1 2.031.40< 0.0970.002<0.18.4 2.54
0.05
H BAL 19.310.86.1 4.6 I 1.63< 0.0880.0030.27.8 2.67
.85 0.05
I BAL 19.310.76.1 4.7 1.891.29< 0.0750.0040.27.9 2.35
0.05
J BAL 19.210.76.1 4.6 2.281.30< 0.0740.0030.27.8 2.58
0.05
K BAL 19.210.76.2 4.8 2.071.60< 0.0800.0030.28.0 2.77
0.05
L BAL 19.210.86.0 4.8 2.081.480.020.0880.005<0.17.9 2.65
M BAL 19.310.76.1 4.6 1.971.39< 0.0810.0032.67.8 2.50
0.05
N BAL 21.38.3 6.0 4.7 2.131.45< 0.0730.0040.27.8 2.65
0.05
O BAL 17.514.26.1 4.7 2.111.47< 0.0770.0040.27.9 2.66
0.05
P BAL 19.410.76.2 4.6 1.981.52< 0.0340.0060.27.8 2.64
0.05
Q BAL 19.210.72.7 6.2 2.011.54< 0.0560.0060.27.6 2.68
0.05
R BAL 19.910.1< 7.2 2.051.50< 0.0580.0060.77.2 2.65
0.1 0.05
S BAL 20.29.6 < 8.3 2.121.48< 0.0620.0070.78.3 2.67
0.1 0.05
T BAL 18.910.1< 9.3 2.071.56< 0.0660.0060.79.3 2.72
0.1 0.05
U BAL 18.710.58.3 6.3 1.801.43< 0.0890.0020.110.6 2.44
0.05
V BAL 19.610.90.1 9.9 2.211.330.650.0940.004<0.19.9 2.76
W BAL 19.410.95.4 4.3 2.301.66< 0.0960.006<0.17.1 2.95
0.05
X BAL 18.810.37.6 6.0 1.531.390.720.089< <0.19.9 2.46
0.002
Y BAL 19.210.64.1 3.2 2,131.45< 0.0800.0040.25.3 2.65
0.05
Z BAL 19.210.80.1 10.52.101.46< 0.0770.0040.210.5 2.64
0.05
M-252BAL 18.99.7 < 10.02.301.010.040.1630.0050.210.0 2.31
0.1
PK-33BAL 18.813.1--- 7.2 1.901.89-- 0.0480.0030.77.2 2,95
263 BAL 20.519.6< 5.9 2.160.49< 0.0600.0020.45.9 1.61
0.1 0.05
WASP BAL 19.113.3< 4.3 2.921.450.050.0800.0081.04.3 2.97
0.1
R-41 BAL 19.110.9< 9.7 3.121.48< 0.0900.0083.69.7 3.10
0.1 0.05
CA 02517056 2005-08-24
TABLE 2
Allo Heat Treatments*
Ex erimental alloys 1475F/8hr./AC
A-Z
R-41 alto 2050F/30min./AC + 1650F/4hr./AC
WASPALOY alloy 1825F/2hr./AC + 1550F/4hr./AC + 1400F/l6hr./AC
263 alloy 1472F/8hr./AC
M-252 allo 1400F/l5hr./AC
PK-33 alloy 1562F/4hr./AC
* All heat treatments performed after an annealing heat treatment.
AC = air cool
TABLE 3
Allo 1500F CHRT Ductilit (% Elon
.)
A 5.9
B 4.9
C 5.0
D 6.4
E 9.5
F 6.0
G 4.9
H 8.5
I 10.0
J 5.5
K 5.4
L 5.7
M 8.5
N 5.6
O 5.8
P 5.2
Q 5.9
R 6.9
S 8.2
T 7.0
U 5.0
V 6.7
W 4.2
X 6.9
Y 5.1
Z 9.3
R-41 alloy 2.8
WASPALOY alloy3.5
263 alloy 22.9
11
CA 02517056 2005-08-24
M-2_52 alto 5.6
PK-33 alloy 3.6
To evaluate the thermal stability of the alloys, their room temperature
tensile ductility
was determined after a long term thermal exposure. After performing the age-
hardening heat
treatments given in Table 2, samples from all of the experimental and
commercial alloys were
given a thermal exposure of 1600°F/1000 hrs./AC. A room temperature
tensile test was
performed on the thermally exposed samples and the results are given in Table
4. Ductility
greater than 20% was considered acceptable. Using this guideline, the
experimental alloys U, V,
X, and Z were found to fail along with the commercial alloys M-252 alloy,
WASPALOY alloy,
and R-41 alloy. It was found that control of the elements molybdenum and
tungsten was critical
to develop a thermally stable alloy. The following relationship was found
(where the elemental
compositions are in wt.%):
Mo + 0.52W < 9.5 (2)
The values of the left hand side of Eq. (2) for all of the alloys in this
study are given in
Table I . All of the alloys which did not obey Eq. (2) were found to not have
sufficient thermal
stability, that is, their room temperature tensile ductility after a 1000 hour
thermal exposure at
1600°F was found to be less than 20%. One alloy (WASPALOY alloy) was
found to satisfy Eq.
(2), but to have poor thermal stability. However, this alloy did not satisfy
Eq. (1) and therefore
is not suitable for the target application. From this example, it is clear
that to ensure thermal
stability for this class of alloys, it is necessary to control the amount of
aluminum, titanium, and
niobium as well as the molybdenum and tungsten. The usefulness of Eq. (2)
becomes quite clear
when considering Fig. 2, where the ductility of the thermally exposed samples
is plotted against
I2
CA 02517056 2005-08-24
the value of the left hand side of Eq. (2) for all of the alloys in the study.
Only alloys which
satisfy the relationship Al + 0.56Ti + 0.29Nb < 2.9 (where the elemental
compositions are in
wt.%) are plotted in the graph. All testing was performed on samples given an
age-hardening
heat treatment followed by a thermal exposure of 1600°F for 1000 hours.
In the graph, the
tensile ductility (measured as the percent elongation to failure) is plotted
as a function of the
compositional variable Mo + 0.52W (where the elemental compositions are in
wt.%). A line is
drawn on the figure corresponding to a tensile ductility of 20%. All alloys
plotted above this
line (symbol: filled circles) were considered to have passed the thermal
stability test, while
alloys plotted below the line (symbol: x-marks) were considered to have
failed. A dashed
vertical line is drawn at a value of 9.5 wt.% for the compositional variable,
Mo + 0.52W. All
alloys with a value greater than 9.5 were found to fail the thermal stability
test.
TABLE 4
Allo Ductilafter 1600F/1000 hrs./AC %
Elon .
A 27.8
B 29.2
C 28.8
D 22.2
E 24.3
F 29.5
G 26.3
H 29.3
I 34.3
J 30.8
K 28.3
L 30.2
M 32.1
N 23.5
O 32.5
P 32.8
Q 29.4
R 34.5
S 33.6
13
CA 02517056 2005-08-24
T 29.9
U 10.4
V 9.2
W 27.3
X 19.0
Y 33.6
Z 18.0
R-41 alloy 2.6
WASPALOY 12.8
263 alloy 40.9
M-252 alloy10.1
PK-33 alloy- - 26.2 -
The third key property for the target application is creep strength. The creep-
rupture
strength of the alloys was measured at 1700°F with a load of 7 ksi. A
rupture life of greater than
300 hours was the established goal. The results for the experimental and
commercial alloys are
shown in Table 5. All of the experimental alloys were found to pass the goal,
with the exception
of alloys V, Y, and Z. The commercial alloys all passed with the exception of
263 alloy and
WASPALOY alloy. Of the total of five alloys which failed the creep-rupture
goal, three of them
(alloys V and Z, as well as WASPALOY alloy) did not satisfy one or both of
Eqs. (1) and (2)
and were thermally unstable. Thermal instability can be a negative influence
on creep strength.
The other two alloys which did not meet the creep strength goal (alloy Y and
263 alloy) both had
a relatively low total content of the solid solution strengthening elements
molybdenum and
tungsten. Additionally, the 263 alloy had a low total content of the gamma-
prime forming
elements aluminum, titanium, and niobium. To ensure adequate levels of both
the solid solution
strengthening elements and the gamma-prime forming elements, the Eqs. (1) and
(2) were
modified respectfully as (where the elemental compositions are in wt.%):
2.2 < Al + 0.56Ti + 0.29Nb < 2.9 (3)
and
14
CA 02517056 2005-08-24
6.5<Mo+0.52W<9.5 (4)
Of the 31 total experimental and commercial alloys tested in this study, 20
were found to
pass all three key property tests, i.e. the CHRT test, the thermal exposure
test, and the creep-
rupture test. All 20 of the acceptable alloys (experimental alloys A through
T) had compositions
which satisfied both Eqs. (3) and (4). The 11 alloys which were deemed
unacceptable (which
included experimental alloys U through Z and all five of the commercial
alloys) had
compositions which failed to satisfy one or both of Eqs. (3) and (4). From
Table 1 it can be seen
that the acceptable alloys contained in weight percent 17.5 to 21.3 chromium,
8.3 to 14.2 cobalt,
4.3 to 9.3 molybdenum, up to 7.0 tungsten, 1.29 to 1.63 aluminum, 1.59 to 2.28
titanium, up to
0.79 niobium, 0.034 to 0.097 carbon, 0.002 to 0.007 boron and up to 2.6 iron.
For the reasons
explained below, alloys containing these elements within the following ranges
and meeting Eqs.
(3) and (4) should provide the desired properties: 17 to 22 chromium, 8 to 15
cobalt, 4.0 to 9.5
molybdenum, up to 7.0 tungsten, 1.28 to 1.65 aluminum, 1.50 to 2.30 titanium,
up to 0.80
niobium, 0.01 to 0.2 carbon and up to 0.015 boron with the balance being
nickel plus impurities.
The alloy may also contain tantalum, up to 1.5 wt. %, manganese, up to 1.5 wt.
%, silicon, up to
0.5 wt. %, and one or more of magnesium, calcium, hafnium, zirconium, yttrium,
cerium and
lanthanum. Each of these seven elements may be present up to O.OS wt. %. The
acceptable
alloys had a range of values for Al + 0.56 Ti + 0.29 Nb of from 2.35 to 2.84
and a range for Mo
+ 0.52 W of from 7.1 to 9.3.
TABLE 5
Alloy Rupture Life (hours)
A 304
B 560
C 48I
CA 02517056 2005-08-24
D 375
E 346
F 522
G 584
H 764
I 410
J 767
K 560
L 522
M 581
N 401
O 403
P 664
Q 419
R 328
S 641
T 506
U 384
V 284
W 463
X 339
Y 271
Z 283
R-41 alloy 618
WASPALOY 243
263 alloy 139
252 alloy 392
M-
_ ~ 412
PK-33 alloy
The presence of chromium (Cr) in alloys used in high temperature environments
provides
for necessary oxidation and hot corrosion resistance. In general, the higher
the Cr content the
better the oxidation resistance, however, too much Cr can lead to thermal
instability in the alloy.
For the alloys of this invention, it was found that the chromium level should
be between about
17 to 22 wt.%.
Cobalt (Co) is a common element in many wrought gamma-prime strengthened
alloys.
Cobalt decreases the solubility of aluminum and titanium in nickel at lower
temperatures
16
CA 02517056 2005-08-24
allowing for a greater gamma-prime content for a given level of aluminum and
titanium. It was
found that Co levels of about 8 to 15 wt.% are acceptable for the alloys of
this invention.
As mentioned previously, aluminum (Al), titanium (Ti), and niobium (Nb)
contribute to
the creep-strength of the alloys of this invention through the formation of
the strengthening
gamma-prime phase upon an age-hardening heat treatment. The combined total of
these
elements is limited by Eq. (3) above. In terms of the individual elements, it
was found that Al
could range from 1.28 to 1.65 wt.%, Ti could range from 1.50 to 2.30 wt.%, and
Nb could range
from nil to 0.80 wt.%.
As mentioned previously, molybdenum (Mo) and tungsten (W) contribute to the
creep-
rupture strength of the alloys of this invention through solid solution
strengthening. The
combined total of these elements is limited by Eq. (4) above. In terms of the
individual
elements, it was found that Mo could range from about 4.0 to 9.5 wt.%, while W
could range
from nil to about 7.0 wt.%.
Carbon (C) is a necessary component and contributes to creep-strength of the
alloys of
this invention through formation of carbides. Carbides are also necessary for
proper grain size
control. Carbon should be present in the amount of about 0.01 to 0.2 wt.%.
Iron (Fe) is not required, but typically will be present. The presence of Fe
allows
economic use of revert materials, most of which contain residual amounts of
Fe. An acceptable,
Fe-free alloy might be possible using new furnace linings and high purity
charge materials. The
presented data indicate that levels up to at least about 3 wt.% are
acceptable.
17
CA 02517056 2005-08-24 --
Boron (B) is normally added to wrought gamma-prime strengthened alloys in
small
amounts to improve elevated temperature ductility. Too much boron may Lead to
weldability
problems. The preferred range is up to about 0.01 S wt.%.
Tantalum (Ta) is a gamma-prime forming element in this class of alloys. It is
expected
that tantalum could be partially substituted for aluminum, titanium, or
niobium at levels up to
about 1.5 wt.%.
Manganese (Mn) is often added to nickel based alloys to help control problems
arising
from the presence of sulfur impurities. It is expected that Mn could be added
to alloys of this
invention to levels of at least 1.5 wt.%.
Silicon (Si) can be present as an impurity and is sometimes intentionally
added for
increased environmental resistance. It is expected that Si could be added to
alloys of this
invention to levels of at least 0.5 wt.%.
Copper (Cu) can be present as an impurity originating either from the use of
revert
materials or during the melting and processing of the alloy itself. It is
expected that Cu could be
present in amounts up to at least 0.5 wt.%.
The use of magnesium (Mg) and calcium (Ca) is often employed during primary
melting
of nickel base alloys. It is expected that levels of these elements up to
about 0.05 wt.% could be
present in alloys of this invention.
Often, small amounts of certain elements are added to nickel based alloys to
provide
increased environmental resistance. These elements include, but are not
necessarily limited to
lanthanum (La), cerium (Ce), yttrium (Y), zirconium (Zr), and hafnium (HfJ. It
is expected that
18
CA 02517056 2005-08-24
amounts of each of these elements up to about 0.05 wt.% could be present in
alloys of this
invention.
Even though the samples tested were limited to wrought sheet, the alloys
should exhibit
comparable properties in other wrought forms (such as plates, bars, tubes,
pipes, forgings, and
wires) and in cast, spray-formed, or powder metallurgy forms, namely, powder,
compacted
powder and sintered compacted powder. Consequently, the present invention
encompasses all
forms of the alloy composition.
The combined properties of good thermal stability, resistance to strain age
cracking and
good creep rupture strength exhibited by this alloy make it particularly
useful for fabrication into
gas turbine engine components and particularly useful for transition ducts in
these engines. Such
components and engines containing these components can be operated at higher
temperatures
without failure and should have a longer service life than those components
and engines
currently available.
Although I have disclosed certain preferred embodiments of the alloy, it
should be
distinctly understood that the present invention is not limited thereto, but
may be variously
embodied within the scope of the following claims.
19