Note: Descriptions are shown in the official language in which they were submitted.
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
METHOD FOR MAKING CHEMICALLY CROSS-LINKED CELLULOSIC
FIBER IN THE SHEET FORM
FIELD OF THE INVENTION
[0001] The present invention is directed to a method of making chemically
cross-
Iinked cellulosie fiber in the sheet form and to the product resulting from
the
process.
DESCRIPTION OF RELATED ART
[0002] Absorbent articles intended for personal care, such as adult
incontinent pads,
feminine care products, and infant diapers typically are comprised of at least
a top sheet, a back sheet, an absorbent core disposed between the top sheet
and back sheet, and an optional acquisition layer disposed between the top
sheet and the absorbent core. The acquisition layer comprised of, for
example, acquisition fibers, usually is incorporated in the absorbent articles
to provide better distribution of liquid, increase the rate of liquid
absorption,
and reduce gel blocking. A wide variety of acquisition fibers are known in
the art. Included among these are synthetic fibers, a composite of cellulosic
fibers and synthetic fibers, and cross-linked cellulosic fibers. Cross-linked
cellulose fiber is preferred because it is abundant, it is biodegradable, and
it
is relatively inexpensive.
[0003] Cross-linked cellulose fibers and processes for making them have been
described in the literature for many years (see, for example G. C.Tesoro,
Cross-Linking of Cellulose, in Handbook of Fiber Science and technology, Vol.
II,
M. Lewis and S. B. Sello eds. pp 1-46, Mercel Decker, New York (1993)). The
cross-linked cellulose fibers are typically made by reacting cellulose with
polyfunctional agents that are capable of reacting with the hydroxyl groups
of the anhydroglucose repeating units of the cellulose either in the same
chain, or in neighboring chains simultaneously. Cross-linked cellulose fibers
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
generally are characterized by their high absorbent capacity, and their high
resiliency in the wet and dry states.
[0004] Cellulosic fibers typically are cross-linked in fluff form. Processes
for
making cross-linked fiber in the fluff form comprise dipping swollen or non-
swollen fiber in an aqueous solution of cross-linking agent, catalyst, arid
softener. The fiber so treated, usually is then cross-linked by heating it at
elevated temperatures in the swollen state as described in U.S. Patent No.
3,241,553, or in the collapsed state after defiberizing it as described in
U.S.
Patent No. 3,224,926, and European Patent No. 0,427,361 B1, the disclosures
of each of which are incorporated by reference herein in their entirety.
[0005] The art has proposed many solutions to overcome some of the problems of
cross-linking fiber i11 sheet form. One alleged solution to this problem is to
minimize the contact between fibers in the dry state. For example, Graef et
al. in U.S. Pat. 5,399,240, the disclosure of which is incorporated herein by
reference in its entirety, describe a method of treating fiber in the sheet
form
with a cross-linking agent and a de-bonder. Fiber while in the sheet form is
then cured at elevated temperatures. The de-bonder tends to interfere with
the hydrogen bonding between fibers and thus mininLzes the contact
between fibers. As a result, fiber is produced with a relatively low content
of
knots and nits. In addition, the long aliphatic chains tend to reduce the
fibers° absorbency and acquisition rate, thus rendering the fibers
unsuitable
for applications where high rate of absorbency and fast acquisition are
important, such as in absorbent articles.
[0006] l3ernardin et al. in U.S. Patent 3,434,918 disclose a method of
treating fiber in
sheet form with a cross-linking agent and a catalyst. The treated sheet then
is wet-aged to render the cross-linking agent insoluble. The wet-aged fibers
are re-dispersed before curing and mixed with untreated fiber, and then
sheeted and cured. Other documents describing methods of treating fiber in
sheet form include, for example, U.S. Patent Nos. 4,204,054; 3,844,880; and
2
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
3,700,549 (the disclosures of which are incorporated by reference herein in
their entirety).
[0007] The above-described approaches complicate the process of cross-linking
fiber in sheet form, and they render the process time consuming, and costly.
[0008] In previous work (U.S. patent application entitled: "Chemically Cross-
Linked Cellulosic Fiber and Method of Making the Same, filed on June 11,
2002, attorney docket number 60892.000002, and serial No. 09/832,634,
entitled "Cross-Linked Pulp and Method of Making Same, filed April 10,
2001 it was shown that mercerized fiber can be successfully cross-linked in
sheet form. The produced cross-linked fiber showed similar or better
performance characteristics than conventional individualized cross-linked
cellulose fibers. Also, the fiber showed less discoloration and reduced
amounts of knots and nits compared to conventional individualized cross-
linked fiber.
[0009] Fiber mercerization, which is a treatment of fiber with an aqueous
solution of
sodium hydroxide (caustic), is one of the earliest known modifications of
fiber. It was invented 150 years ago by John Mercer (see British Patent 1369,
1850). The process generally is used in the textile industry to improve cotton
fabric's tensile strength, dyeability, and luster (see, for example, R.
Freytag,
J.-J. bonze, Chemical Processing of Fibers and Fabrics, Fundamental and
Applications, Part A, in Handbook of Fiber Science and Technology Vol. I M.
Lewis and S. B. Sello eds. pp.1-46, Mercell Decker, New York (1983)).
[0010] In addition to the above advantages, mercerization adds to fibers
several
other properties. Among these are: (1) mercerized fibers have high a-
cellulose content, since caustic removes residuals such as lignin and
hemicellulose from fiber leftover from pulping and bleaching processes; (2)
mercerized fibers have a round, circular shape (rather than the flat, ribbon-
like shape of conventional fibers) that reduces the contact a~.ld weakens the
hydrogen-bonding between fibers in the sheet form; and (3) mercerization
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
converts cellulose chains from their native structure form, cellulose I, to a
more thermodynamically-stable and less crystalline form, cellulose II. The
cellulosic chains in cellulose II are found to have an anti-parallel
orientation
rather than parallel orientation as in cellulose I (see, for example, R. H.
Atalla,
Comprehensive Natural products Chemistry, Carbohydrates And Their
Derivatives Including Tannins, Cellulose, and Related Lignins Vol. III, D.
Barton
and K. Nakanishi eds. pp 529-598, Elsevier Science, Ltd., Oxford, U.IC.
(1999)).
[0011] The description herein of certain advantages and disadvantages of known
cross-linked cellulosic fibers, and methods of their preparation, is not
intended to limit the scope of the present invention. Indeed, the present
invention may include some or all of the methods and chemical reagents
described above without suffering from the same disadvantages.
SUMMARY OF THE INVENTION
[0012] One feature of an embodiment of the present invention provides cross-
linked
fibers with enhanced bullring characteristics, porosity and rate of
acquisition.
An additional aspect of the present invention is to provide cross-linked
cellulosic fiber having long shelf-life and high stability. Further, another
aspect of an embodiment of the present invention provides fibers useful i11
an acquisition layer and/or in an absorbent core of absorbent products.
Various aspects of the present invention also provide absorbent articles
comprising the cross-linked fiber of the present invention.
[0013] In accordance with these and other aspects and features of embodiments
of
the invention, there is provided a cross-linked sheet of a blend of fibers,
and
a method for cross-linking cellulose fibers in sheet form. In one aspect of
the
invention, the cellulose fibers are a blend of mercerized fibers and
conventional fibers that are cross-linked. In another aspect of the invention,
the cross-linked fibers formed in accordance with the present invention can
4
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
be easily defiberized without serious fiber breakage and with low knot-
content and low nit-content. It will be appreciated, however, that knots and
nits are advantageous for some applications, and accordingly, the present
invention is not in any way limited to producing cross-linked cellulosic
fibers substantially free of knots.
[0014] In accordance with the method, a wet laid sheet of a blend of
mercerized
fibers and cellulose fibers are formed, and then treated a cross-linking agent
to form a sheet impregnated with the cross-linking agent. The cross-linking
agent then is dried and cured to form infra-fiber cross-links.
[0015] These and other objects, features, and advantages of the present
invention
will appear more fully from the following detailed description of the
preferred embodiments of the invention, and the attached drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
[0016] The drawings show electron microscope photographs of representative
cross-linked fibers of the present invention.
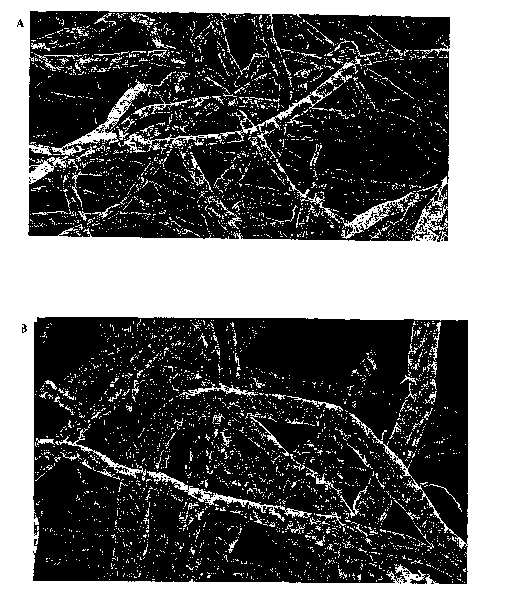
[0017] Figures 1a and 1b are photographs at 100X and 200X magnifications,
respectively, of blends of fibers obtained as shown in Example 2 from
Rayfloc~-J-LD (southern pine Kraft pulp commercially available from
Rayonier Performance Fibers Division, Jesup, GA) and mercerized fibers in
about 1:1 weight ratio using glyoxylic acid (2%) cross-linking agent.
[0018] Figures 2a and 2b are photographs at 100X and 200X magnification,
respectively, of a blend of fibers of the present invention prepared from
Rayfloc~-J-LD and mercerized fibers in 1:1 ratio and cross-linked in
accordance with Example 3.
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
[0019] As used herein, the terms "absorbent garment,' "absorbent article" or
simply "article" or "garment" refer to mechanisms that absorb and contain
body fluids and other body exudates. More specifically, these terms refer to
garments that are placed against or in proximity to the body of a wearer to
absorb and contain the various exudates discharged from the body. A non-
exhaustive list of examples of absorbent garments includes diapers, diaper
covers, disposable diapers, training pants, feminine hygiene products and
adult incontinence products. Such garments may be intended to be
discarded or partially discarded after a single use ("disposable'°
garments).
Such garments may comprise essentially a single inseparable structure
("unitary" garments), or they may comprise replaceable inserts or other
interchangeable parts.
[0020] The present invention may be used with all of the foregoing classes of
absorbent garments, without limitation, whether disposable or otherwise.
Some of the embodiments described herein provide, as an exemplary
structure, a diaper for an infant, however this is not intended to limit the
claimed invention. The invention will be understood to encompass, without
limitation, all classes and types of absorbent garments, including those
described herein.
[0021] The term "component" can refer, but is not limited, to designated
selected
regions, such as edges, corners, sides or the like; structural members, such
as
elastic strips, absorbent pads, stretchable layers or panels, layers of
material,
or the like.
[0022] Throughout this description, the term "disposed°' and the
expressions
"disposed on," "disposed above," "disposed below," "disposing on,"
"disposed in," "disposed between' and variations thereof are intended to
mean that one element can be integral with another element, or that one
element can be a separate structure bonded to or placed with or placed near
6
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
another element. Thus, a component that is "disposed ori' an element of the
absorbent garment can be formed or applied directly or indirectly to a
surface of the element, formed or applied between layers of a multiple layer
element, formed or applied to a substrate that is placed with or near the
element, formed or applied within a layer of the element or another
substrate, or other variations or combinations thereof.
[0023] Throughout this description, the terms "top sheet" and "back sheet"
denote
the relationship of these materials or layers with respect to the absorbent
core. It is understood that additional layers may be present between the
absorbent core and the top sheet and back sheet, and that additional layers
and other materials may be present on the side opposite the absorbent core
from either the top sheet or the back sheet.
[0024] Throughout this description, the expressions "upper layer,' "lower
layer,"
"above' and "below," which refer to the various components included in the
absorbent material are used to describe the spatial relationship between the
respective components. The upper layer or component "above' the other
component need not always remain vertically above the core or component,
and the lower layer or component "below" the other component need not
always remain vertically below the core or component. Other configurations
are contemplated within the context of the present invention.
[0025] Throughout this description, the term "impregnated" insofar as it
relates to a
cross-linking agent impregnated in a fiber, denotes an intimate mixture of
cross-linking agents and cellulosic fiber, whereby the cross-linking agent
may be adhered to the fibers, adsorbed on the surface of the fibers, or linked
via chemical, hydrogen or other bonding (e.g., Van der Waals forces) to the
fibers. Impregnated in the context of the present invention does not
necessarily mean that the cross-linking agent is physically disposed beneath
the surface of the fibers.
7
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
[0026] The present invention concerns chemically cross-linked blends of fibers
that
are useful in absorbent articles, and in particular, that are useful in
forming
acquisition layers or absorbent cores in the absorbent article. The particular
construction of the absorbent article is not critical to the present
invention,
and any absorbent article can benefit from this invention. Suitable absorbent
garments are described, for example, in U.S. Patent Nos. 5,281,207, and
6,068,620, the disclosures of each of which are incorporated by reference
herein in their entirety including their respective drawings. Those skilled in
the art will be capable of utilizing the chemically cross-linked cellulosic
fibers of the present invention in absorbent garments, cores, acquisition
layers, and the like, using the guidelines provided herein.
[0027] Cross-linking of fibers in fluff form is believed to improve the
physical and
chemical properties of the fibers in many ways, such as improving the
stiffness, increasing resiliency (iii the dry and wet state), increasing the
absorbency, reducing wrinkling, and improving shrinkage resistance.
Unfortunately, it has been found that such cross-linking, if carried out on a
fiber in sheet form, may create problems in the fiber which render it
unsuitable for many applications. These problems include severe fiber
breakage and increased amounts of knots and nits (hard fiber clumps).
These problems are attributed to the inter-fiber (fiber-to-fiber) cross-
linking
that occurs between fibers in close contact during the curing process.
Usually, fibers get into close contact in the dry state due to (a) mechanical
entanglement; (b) hydrogen bonding between fibers; and (c) pulping and
bleaching residuals such as lignin and hexnicellulose. As a result, when
fibers treated with a cross-linking agent are heated for curing, fibers in
close
contact tend to form inter-fiber cross-links rather than infra-fiber cross-
links
(chain-to-chain within the single fiber).
[0028] Thus, there is a need for a simple, relatively inexpensive process for
cross-
linking fiber in sheet form that will provide cross-linked fibers with low
liquid retention, enhanced rates of acquisition, and reduced amounts of
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
knots and nits. In another embodiment, the present invention is directed to a
method of making cross-linked fibers in sheet form. The method preferably
comprises treating cellulose fibers in sheet or roll form with an aqueous
solution of a polyfunctional cross-linking agent, followed by drying and
curing at sufficient temperature for adequate time to accelerate the formation
of covalent bonding between hydroxyl groups of cellulose fibers and
functional groups of the cross-linking agent.
[0029] The method of an embodiment of the invention preferably comprises
reacting a blend of fibers in sheet form with one or more reagents selected
from organic molecules having carboxylic acid, an aldehyde and carboxylic
acid (e.g., an acid aldehyde), or epoxy functional groups. In one embodiment
the method of the present invention provides cross-linked fibers i11 sheet
form that can be readily defiberized with low knot-content and without
significant fiber breakage. In another embodiment the method of the present
invention provides cross-linked fibers that are characterized by an enhanced
acquisition rate, resiliency, and absorbency under load. Moreover, cross-
linked fibers of the present invention display a reduction in centrifuge
retention capacity which makes the fiber especially suited for use in
acquisition, distribution and acquisition-distribution layers in absorbent
articles intended for fluid management.
[0030] In one aspect of the present invention, the fiber in sheet form
comprises a
blend of mercerized fibers and conventional fibers. Throughout this
description, the expression "conventional fibers' denotes cellulose fibers of
diverse origins, especially those primarily derived from wood pulp.
Suitable wood pulp can be obtained from any of the chemical processes
known by those of ordinary skill in the art such as Kraft, and sulfite
processes. Preferred fibers are those obtained from various soft wood pulp
such as Southern pine, White pine, Caribbean pine, Western hemlock,
various spruces, (e.g. Sitka Spruce), Douglas fir or mixtures and
combinations thereof. Fibers obtained from hardwood pulp sources, such as
9
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
gum, maple, oak, eucalyptus, poplar, beech, and aspen, or mixtures and
combinations thereof can also be used in the present invention. Other
cellulose fibers derived form cotton linter, bagasse, kemp, flax, and grass
may also be used in the present invention. The fiber can be comprised of a
mixture of two or more of the foregoing cellulose pulp products.
Particularly preferred "conventional" fibers for use in forming the cross-
linked fibers of the present invention are those derived from wood pulp
prepared by Kraft and sulfite-pulping processes.
[0031] The fibers of the present invention preferably have a high surface
purity of
cellulose, but it is not necessarily required that the cellulosic fibers have
a
high cellulose bulls purity. It is preferred that the cellulosic fiber be
cross-
linked in the sheet form, and more preferably, be fiber with "high cellulose
purity." The high cellulose purity refers to the surface purity of the
cellulosic fibers. Throughout this description, the expression "high cellulose
purity" refers to pulp comprising at least about 65%, preferably at least 75%,
and most preferably, at least about 90% a-cellulose.
[0032] The preferred fibers in sheet form that are cross-linked in accordance
with
the present method are blends of conventional cellulose and "mercerized
fibers." Throughout this description, the expression "mercerized fibers'
denotes any of wood pulp fibers or fibers from any source described,
previously treated with an aqueous solution of alkali metal. Fiber
mercerization can be carried out by any method known in the art such as
those described in, for example, Cellulose and Cellulose Derivatives, Vol. V,
Part 1, Ott, Spurlin, and Grafllin, eds., Interscience Publisher (1954). Fiber
mercerization can be performed by mixing pulp in an aqueous solution of
allcali metal (i.e. sodium hydroxide), washing, neutralizing, or washing and
neutralizing, and optionally drying the pulp.
[0033] Reagents suitable for mercerization include but are not limited to,
allcali
metal hydroxides, such as sodium hydroxide, potassium hydroxide, calcium
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
hydroxide, and rubidium hydroxide, lithium hydroxide, and
benzyltrimethylammoruum hydroxide. Sodium hydroxide is a particularly
preferred reagent for use in fiber mercerization in accordance with the
present invention. The pulp of the invention preferably is treated with an
aqueous solution containing from about 8 to about 30% by weight sodium
hydroxide, more preferably from about 12 to about 20%, and most preferably
13% to about 16%. Mercerization may be performed during or after
bleaching, purification, and drying. Preferably mercerization is carried out
during bleaching and or drying processes. After mercerization, the cellulose
fibers preferably contain at least about 80% by weight a,-cellulose,
preferably
at least about 90% by weight, more preferably at least about 95% by weight,
and even more preferably at least about 97% by weight a-cellulose.
[0034] Commercially available caustic extractive pulp (i.e., mercerized pulp")
suitable for use in the present invention includes, for example, Porosanier-J-
HP, available from Rayonier Performance Fibers Division (Jesup, GA), and
Buckeye's HPZ products, available from Buckeye Technologies (Perry, FL).
In one aspect of the present invention, it is preferred that the pulp fibers
be
in sheet or roll form.
[0035] In accordance with the invention, the sheet or roll form of cellulose
preferably is a blend of mercerized fiber and conventional fiber containing
about 10% to about 60% by weight conventional fiber, more preferably from
about 20% to about 60% by weight conventional fiber, and most preferably
from about 30% to about 50% by weight conventional fiber, based on the
total weight of the mixture of fibers.
[0036] In another aspect of the invention, the fiber can be used in wet or dry
state.
It is preferred in the present invention that the cellulosic fibers be
employed
in the dry state.
[0037] Cross-linking agents suitable for use in the present invention are
polyfunctional molecules. As used herein, the expression "polyfunctional
11
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
molecule' refers to a polymeric or monorneric molecule able to form a bridge
between adjacent cellulose chains. Accordingly, any material capable of
reacting with more than one hydroxyl group of cellulose chains can be
suitable for use in the present invention. Particularly suitable cross-linking
agents for use in the present invention are those carrying functional groups
such as, for example, carboxyl, aldehyde and epoxy. Preferably, the cross-
linlcin_g agents include dialdehydes, acid aldehydes, polycarboxylic acids,
and polyepoxides.
[0038] Cross-linking agents preferred for use in the present invention include
acid
aldehydes. As used herein, "acid aldehyde" refers to organic molecules
having carboxylic acid and aldehyde functional groups, such as, for example,
glyoxylic acid and succinic semialdehyde. A particularly preferred acid
aldehyde cross-linking agent is glyoxylic acid.
[0039] Other suitable cross-linking agents include polycarboxylic acids.
Especially
suitable polycarboxylic acids are those having at least two carboxyl groups,
such as,1,2,3,4-butanetetracarbocylic acid,1,2,3-propanetricarboxylic acid,
oxydisuccinic acid, citric acid, itaconic acid, malefic acid, tartaric acid,
glutaric
acid. Particularly preferred polycarboxylic acids are 1,2,3,4-
butanetetracarbocylic acid and citric acid.
[0040] Other suitable polycarboxylic acids include polymeric polycarboxylic
acids
such as those specially prepared from monomers such as, for example,
acrylic acid, vinyl acetate, malefic acid, malefic anhydride, carboxy ethyl
acrylate, itanoic acid, fumaric acid, methacrylic acid, crotonic acid,
aconitic
acid, acrylic acid ester, methacrylic acid ester, acrylic amide, and
methacrylic
amide, butadiene, styrene, or combinations thereof.
[0041] Commercially available examples of these polymers and co-polymers
include polyacrylic acid, polymaleic acid, polyitaconic acid, polyaspartic
acid, polymethacrylic acid, poly(acrylic acid-co-malefic acid),
poly(acrylamide-co-acrylic acid), poly(etheylene-co-acrylic acid), and
12
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
polystyrene-co-malefic acid). Particularly preferred polycarboxylic acids are
polymaleic acid, polyacrylic acid, and a co-polymer of acrylic acid and
malefic acid.
[0042] Other cross-linking agents suitable for use in the present invention
include
polyepoxides, particularly those containing hydrophobic saturated,
unsaturated, branched and un-branched allcyls. Examples of these include
1,4-cyclohexanedimethanol diglycidyl ether, diglycidyl 1,2-
cyclohexanedicrboxylate, N,N-diglycidylaniline, N,N-diglcidyl-4-
glycidyloxyaniline, and diglycidy11,2,3,4-tetrahydrophthalate and glycerol
propoxylate triglycidyl ether. A particularly preferred polyepoxide is 1,4-
cyclohexanedimethanol diglycidyl ether.
[0043] In another aspect of the invention, a mixture or combination of cross-
linking
agents may be used. In another aspect, the present invention provides
chemically cross-linked fibers in sheet form, that are cross-linked with a
blend of cross-linking agents including those described above.
[0044] In one embodiment of the invention, the cross-linking agent may be
applied
to~the cellulose fiber in an aqueous solution. Preferably the aqueous solution
has a pH of from about 1 to about 5, more preferably from about 2 to about 3.
The present inventors have discovered that an aqueous solution of acid
aldehyde cross-linking agent can be used as is without any adjacent or
additional pH control agent.
[0045] In another embodiment of the invention, a water insoluble cross-linking
agent, e.g. polyexpoxide, may be used. When such a water insoluble cross-
linking agent is used, it is preferred to add a minor amount of surfactant
(e.g., a few drops - less than 1% by weight) to emulsify the cross-linking
agent prior to fiber application. The cross-linking agent may then be applied
to the fiber as a dispersion, instead of an aqueous solution.
13
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
[0046] In general, any type of surfactant capable of forming a dispersion with
the
water insoluble cross-linking agent can be used. Suitable surfactants include
nonionic, anionic, or cationic surfactant or mixtures and combinations of
surfactants that are compatible with each other. Preferably the surfactant is
selected from Triton X-100 (Rohm and Haas), Triton X-405 (Rohm and Haas),
sodium lauryl sulfate, and lauryl bromoethyl ammonium chloride,
ethoxylated nonylphenols, polyoxyethylene allcyl ethers, polyoxyethylene
allcyl ethers, and polyoxyethylene fatty acid esters.
[004'l] The cellulosic fiber preferably is treated with an effective amount of
cross-
lirtking agent to achieve the absorbent properties and physical
characteristics
described herein. Generally, the concentration of the cross-linking agent in
aqueous solution is sufficient to provide from about 0.5 to 10.0 weight
percent cross-linking agent on fiber, more preferably from about 1 to 6
weight percent, and most preferably from about 2 to 5 weight percent.
[004] Optionally, the method of forming the cellulosic fiber in accordance
with the
invention includes a catalyst to accelerate the formation of an ester linkage
between the hydroxyl groups of the cellulose and the carboxyl groups of the
polycarboxylic acid and acid aldehyde cross-linking agents. A catalyst also
may be used to accelerate the formation of acetal cross-liy~ks between
hydroxyl groups of cellulose and aldehyde functional groups of acid
aldehyde cross-linking agents. When an acid aldehyde is used as the cross-
linking agent, however, a catalyst is not required. To the extent that a
catalyst is used, any catalyst known in the art that is capable of
accelerating
the formation of an ester cross-link between a hydroxyl group and an acid
group, or capable of accelerating the formation of an acetal cross-link
between a hydroxyl group of cellulose and an aldehyde group could be used
in the present invention. Suitable catalysts for use in the present invention
to
accelerate the formation of ester cross-finks include allcali metal salts of
phosphorous containing acids such as allcali metal hypophosphites, allcali
metal phosphites, allcali metal polyphosphonates, allcali metal phosphates,
14
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
and alkali metal sulfonates. A particularly preferred catalyst of this type is
sodium hypophosphite.
[0049] Suitable catalysts for use in the present invention to accelerate the
formation
of acetal cross-links are Lewis acids consisting of a metal and a halogen,
such
as for example FeCl3, AICI~, and MgCl2.
[0050] A catalyst may also be used to promote the reaction between
polyepoxides
and cellulose hydroxyl groups, to the extent a cross-linking agent containing
polyepoxide groups is used as a cross-linking agent. Any catalyst known in
the art to accelerate the formation of an ether bond or linkage between the
hydroxyl groups of cellulose alid an epoxide group can be used in the
present invention. Preferably, the catalyst is a Lewis acid selected from
aluminum sulfate, magnesium sulfate, and any Lewis acid consisting of a
metal and a halogen, including, for example FeCl3, A1C1~, and MgCl2. The
catalyst can be applied to the fiber as a mixture with the cross-linking
agent,
before the addition of the cross-linkilig agent, or after the addition of
cross-
linking agent to cellulosic fiber. Preferably, the ratio of catalyst to cross-
linkiyig agent is, for example, from about 1:2 to 1:10, more preferably from
about 1:4 to 1:8.
[0051] Optionally, in addition to the cross-linking agent, other finishing
agents such
as water repellent, softening, and wetting agents also can be used to treat
the
cellulosic fiber. Examples of softening agents include fatty alcohols, fatty
acids amides, polyglycol ethers, fatty alcohols sulfonates, and N-stearyl-urea
compounds. Examples of wetting agents include fatty amines, salts of
allcylnaphthalenesulfonic acids, alkali metal salts of dioctyl sulfosuccinate,
and the like.
[0052] Any method of applying the cross-linking agents) to the fiber can be
used in
carrying out the cross-linking method of the invention. Acceptable methods
include, for example, spraying, dipping, impregnation, and the like.
Preferably, the fiber is impregnated with an aqueous solution of cross-
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
linking agent. Impregnation usually creates a uniform distribution of cross-
l~nlcin_g agent on the sheet and provides a better penetration of cross-
linking
agent into the interior part of the fiber.
[0053] In one embodiment of the invention, a sheet including a blend of
mercerized
and conventional fibers in roll form is conveyed through a treatment zone
where a cross-linking agents) is applied on both surfaces by conventional
methods such as spraying, rolling, dipping, knife-coating or other manners
of impregnation. A preferred method of applying the aqueous solution of
the cross-linking agents) to fiber in roll form is by puddle press, size
press,
and blade coater.
[0054] In one embodiment of the present invention, a blend of fibers in sheet
or roll
form after having been treated with a solution of cross-linking agent then
preferably is transported by a conveying device such as a belt or a series of
driven rollers through a two-zone oven for drying and curing.
[0055] Fibers in roll or sheet form after treatment with the cross-linking
agent
preferably are dried and cured in a two stage process, and even more
preferably dried and cured in a one stage process. Such drying and curing
removes water from the fiber, thereupon believed to induce the formation of
an ester and an ether linkage between hydroxyl groups of fiber and cross-
linking agent(s). Curing usually is carried out in a forced draft oven
preferably from about 300 °F to about 450 °F, and more
preferably from
about 320 °F to about 430 °F, and most preferably from about 350
°F to about
420 °F. Curing preferably is carried out for a certain period of time
that
permits complete fiber drying and efficient cross-linking. It is preferred
that
the cellulosic fiber is cured for a period of time ranging from about 5 min to
about 25 min, and more preferably from about 7 min to about 20 min, most
preferably from about 10 min to about 15 min.
[0056] The blend of cellulosic fibers cross-linked in accorda~.lce with the
present
invention can be characterized as having an absorbent capacity within the
16
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
range of about 7.0 g/g to about 12.0 g/g. Preferably, the fibers have an
absorbent capacity of at least 8.0 g/ g, more preferably at least 9.0 g/ g,
even
more preferably at least 10.0 g/ g or higher. The cross-linked cellulosic
fibers
can have a centrifuge retention as determined by the Hanging Cell Test
method within the range of about 0.4 g/ g to about 0.6 g/ g. Preferably, the
fibers have a centrifuge retention of less than about 0.6 g/ g, more
preferably
less than about 0.55 g/ g, and most preferably less than about 0.5 g/ g.
[005'7] The cellulosic fibers cross-linked in accordance with the present
invention
preferably possess characteristics that are desirable in absorbent articles.
For
example, the cross-linked cellulosic fibers preferably have a centrifuge
retention capacity of less than about 0.6 grams of synthetic saline per gram
of
fiber (hereinafter "g/g °). The chemically cross-linked cellulose
fibers also
have desirable properties, such as a free swell of greater than about 10 g/ g,
an absorbent capacity of greater than about 8.0 g/g, an absorbency under
load of greater than about 8.0 g/g, less than about 10% of knots, less than
about 6.5%a of fines, and an acquisition rate upon the third insult (or third
insult strike-through) of less than about 13.0 seconds. The particular
characteristics of the cross-linked cellulosic fibers of the invention are
determined in accordance with the procedures described in more detail in
the examples.
[0058] The centrifuge retention capacity measures the ability of the fiber to
retails
fluid against a centrifugal force. It is preferred that the blend of fibers of
the
invention have a centrifuge retention capacity of less than about 0.6 g/ g,
when cross-linked with any cross-linking agent, more preferably, less than
about 0.55 g/ g, even more preferably less than 0.5 g/ g. The cross-linked
cellulosic fibers of the present invention can have a centrifuge retention
capacity as low as about 0.40 g/g. It also is preferred that the fibers of the
invention have a centrifuge retention capacity of less than about 0.60 g/ g
when cross-linked with an acid aldehyde cross-linking agent, more
17
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
preferably, less than about 0.55 g/ g, even more preferably less than 0.50 g/
g,
and most preferably less than about 0.45 g/ g.
[0059] It is preferred that the fibers of the invention have an absorbent
capacity of
more than about 8.0 g/ g, more preferably, greater than about 8.5 g/ g, even
more preferably greater than about 9.0 g/g, and most preferably greater than
about 10.0 g/ g.
[0060] The absorbency under load measures the ability of the fiber to absorb
fluid
against a restraining or confining force of 0.3 psi over a given period of
time.
It is preferred that the blend of fibers of the invention have an absorbency
under load of greater than about ~.0 g/ g, more preferably, greater than
about 7.5 g/ g, a~.id most preferably, greater than about 8.0 g/ g.
[0061] The third insult strikethrough measures the ability of the fiber to
acquire
fluid, and is measured in terms of seconds. It is preferred that the fibers of
the invention have a third insult strike-through of less than about 15.0
seconds, more preferably, less than about 14 seconds, even more preferably
less than 13 seconds, and most preferably less than about 12 seconds.
[0062] The cross-linked fibers of the present invention preferably have less
than
about 10 % of knots, more preferably less than about 8 % knots, and most
preferably, less than about 5% knots. The cross-lilzked fibers of the present
invention also preferably have less than about 8.0% of fines, preferably less
than about 7.0% fines, and most preferably, less than about 6.0% fines.
[0063] In addition to being more economical, there are several other
advantages for
cross-linking fibers in sheet form in accordance with the present invention.
Fibers cross-linked in sheet form have typically been expected to have an
increased potential for inter-fiber cross-linking which leads to "knots" and
"nits' resulting in poor performance in some applications. For instance,
when a standard purity fluff pulp, Rayfloc-J, is cross-linked in sheet form,
the "knot" content increases substantially, indicating increased deleterious
18
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
inter-fiber bonding. Examination of these "knots" recovered by classification
showed they contained true "nits" (hard fiber bundles). Surprisingly, it was
found that a blend of mercerized pulp and conventional pulp cross-linked in
sheet or roll form actually yields fewer knots and ruts than control pulps
having conventional cellulose purity cross-linked under the same conditions.
Significantly, a blend of fibers in sheet or roll form that were cross-linked
in
accordance with the present invention were found to contain fewer knots
than commercial individualized cross-linked cellulose fibers, like those
produced by the Weyerhaeuser Company, commonly referred to as HBA
(for high-bulk additive), and by The Proctor & Gamble Company ("PEG").
[0064] In another aspect of the invention, it also has been discovered that
the
presence of knots to a certain level in the cross-linked fiber of the present
invention enhances the performance of the cross-linked fiber when used as
an acquisition layer in hygiene products. In this instance, the knots present
in the cross-linked fiber are within the range of 2% to 15%, more preferably
from 3 % to 12 %, and most preferably from 5 % to 10 % .
[0065] Another benefit of the present invention is that cellulose fibers cross-
linked
in sheet form in accordance with the present invention enjoy equal
performance characteristics to conventional individualized cross-linked
cellulose fibers, but avoid the processing problems associated with dusty
individualized cross-linked fibers.
[0066] Scanning electron microscope (S360 Leica Cambridge Ltd., Cambridge,
England) photographs illustrated in Figures 1A and 1B represent cross-
linked fibers of the present invention obtained by cross-linking a blend of
conventional fiber (Rayfloc~-J-LD) and Porosanier, in 1:1 ratio by weight,
using glyoxylic acid (2%) as the cross-linking agent. The photographs were
taken at 100X magnification for Figure 1A, and 200X magnification for Figure
1B.
19
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
[006'7] Scanning Electron Microscope (SEM) photographs illustrated in Figures
2A
and 2B represent cross-linked fibers of the present invention obtained by
cross-linking a blend of conventional fiber (Rayfloc~-J-LD) and Porosanier, in
1:1 ratio by weight, using DP-60 (5%) as the cross-linking agent. The
photographs were taken at 100X magnification for Figure 2A, and 200X
magnification for Figure 2B.
[0068] As shown in these figures, the cross-linked fibers of the present
invention are
twisted and curled. The photographs show a mixture of round, circular-
shaped fibers and flat, ribbon-like fibers. The round, circular-shaped fibers
represent the mercerized fibers while the flat, ribbon-like fibers represent
the
conventional fiber Rayfloc~-J-LD.
[0069] Cross-linked cellulosic fibers prepared in accordance with the present
invention can be utilized, for example, as a bullring material in the
manufacture of high bully specialty fiber applications that require good
absorbency and porosity. The cross-linked fibers can be used, for example,
in non-woven, fluff absorbent applications. The fibers can be used
independently, or preferably can be incorporated with other cellulosic fibers
to form blends using conventional techniques, such as air laying. In an
airlaid process, the cross-linked fibers of the present invention, either
alone
or blended with other fibers, are blown onto a forming screen. A wet laid
process may also be used, combining the cross-linked fibers of the invention
with other cellulosic fibers to form sheets or webs of blends.
[0070] The cross-linked fibers of the present invention can be incorporated
into
various absorbent articles intended for body waste management such as
adult incontinent pads, fem~~;ne care products, and infant diapers. The
cross-linked fibers can be used in an acquisition layer in the absorbent
articles. The cross-linked fibers also can be utilized in the absorbent core
of
the absorbent articles. Towels, wipes and other absorbent products such as
filters also may be made with the cross-linked fibers of the present
invention.
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
Accordingly, an additional feature of the present invention is to provide an
absorbent core and an absorbent article that includes the chemically cross-
linked fibers of the present invention.
[0071] As known in the art, absorbent cores typically are prepared using fluff
pulp
to wick the liquid, and an absorbent polymer (oftentimes a superabsorbent
polymer ("SAP")) to store liquid. As noted previously, the cross-linked
blend of fibers of the present invention have high resiliency and high free
swell capacity. Furthermore, the blend of cross-linked fibers are highly
porous. Accordingly, the cross-linked fibers of the present invention can be
used in combination with the SAP to make an absorbent composite (or core)
having improved porosity, bulls, resiliency, wicking, softness, absorbent
capacity, absorbency under load, low third insult strikethrough, low
centrifuge retention capacity, and the like. The absorbent composite could
be used as an absorbent core in absorbent articles intended for body waste
management.
[0072] It is preferred in the present invention that the cross-linked fibers
be present
in the absorbent composite in an amount ranging from about 10% to about
80% by weight, based on the total weight of the composite. More preferably,
the cross-linked fibers are present in an amount ranging from about 20% to
about 60% by weight. A mixture of cellulosic fibers and cross-linked fibers
of the present invention along with the SAP may also be used to make the
absorbent composite. Preferably, the cross-linked fibers of the present
invention are present in the mixture in an amount ranging from about 1 % to
about 70% by weight, based on the total weight of the fiber mixture, and
more preferably present in an amount from about 10% to about 40% by
weight. Suitable additional conventional cellulosic fibers include any of the
wood fibers mentioned previously, cold caustic treated fibers, conventional
fibers, mercerized fibers and mixtures and combinations thereof.
21
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
[0073] Any suitable superabsorbent polymer, or other absorbent material, can
be
used, to form the absorbent composite. The SAP can be in the form of, for
example, fiber, flakes, or granules capable of absorbing several times its
weight of saline (0.9% solution of NaCl in water) and/or blood. The SAP
also preferably is capable of retaining the liquid when it is subjected to
load.
Non-limiting examples of superabsorbent polymers applicable for use in the
present invention include any SAP presently available on the market,
including, but not limited to, polyacrylate polymers, starch graft copolymers,
cellulose graft copolymers, and cross-linked carboxymethylcellulose
derivatives, and mixtures and combinations thereof.
[0074] An absorbent composite made i11 accordance with the present invention
preferably contains superabsorbent polymer in an amount from about 20%
to about 60% by weight, based on the total weight of the composite, and
more preferably from about 30% to about 60% by weight. The SAP may be
distributed throughout an absorbent composite within the voids in the cross-
linked fiber or the mixture of cross-linked fibers and cellulosic fibers. In
another embodiment, the SAP is attached to the fiber via a binding agent that
includes, for example, a material capable of cross-linking the SAP to the
fiber
via hydrogen bonding (see, for example, U.S. Patent No. 5,614,570, the
disclosure of which is incorporated by reference herein in its entirety).
[0075] A method of making an absorbent composite of the present invention may
include forming a pad comprising cross-linked fibers or a mixture of cross-
linked fibers and cellulosic fibers and incorporating particles of
superabsorbent polymer in the pad. The pad can be wet laid or airlaid,
preferably the pad is airlaid. It also is preferred that the superabsorbent
polymer and cross-linked fibers, or mixture of cross-linked fibers and
cellulosic fibers, are air laid together.
[0076] Absorbent cores containing cross-finked fibers and superabsorbent
polymer
preferably have dry densities ranging from about 0.1 g/cm3 to 0.50 g/cm3
22
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
and more preferably from about 0.2 g/ cm3 to 0.4 g/ cm3. The absorbent core
can be incorporated into a variety of absorbent articles intended for body
waste management, such as diapers, training pants, adult incontinence,
feminine care products, and toweling (e.g. wet and dry wipes).
[0077] To evaluate the various attributes of the present invention, several
tests were
used to characterize the cross-linked fibers' performance improvements
resulting from the presently described method.
[0078] The invention will be illustrated but not limited by the following
examples.
[0079] In the examples, all percentages are by weight and all temperatures ill
degrees Celsius, unless otherwise noted. Also, when referring to pulp
weight, the measurement includes equilibrium moisture content. When
subjected to testing, all fiber contains about 5% to 7% moisture.
Examples
[0080] The following test methods were used to measure and determine various
physical characteristics of the W ventive cross-linked cellulosic fibers.
Test Methods
The Absorbency Test Method
[0081] The absorbency test method was used to determine the absorbency under
load, absorbent capacity, and centrifuge retention capacity of the cross-
linked fibers of the present invention. The absorbency test was carried out in
a one inch inside diameter plastic cylinder having a 100-mesh metal screen
adhering to the cylinder bottom "cell," containing a plastic spacer disk
having a 0.995 inch diameter and a weight of about 4.4 g. In this test, the
weight of the cell containing the spacer disk was determined to the nearest
0.0001 g, and then the spacer was removed from the cylinder and about 0.35
g of cross-linked fibers having a moisture content within the range of from
about 4% to about 8% by weight were air-laid into the cylinder. The spacer
23
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
disk then was inserted back into the cylinder on the fiber, and the cylinder
group was weighed to the nearest 0.0001 g. The fiber in the cell was
compressed with a load of 4 psi for 60 seconds, the load then was removed
and the fiber pad was allowed to equilibrate for 60 seconds. The pad
thickness was measured, and the result was used to calculate the dry bulls of
the cross-linked fiber.
[0082] A load of 0.3 psi then was applied to the fiber pad by placing a 100 g
weight
on the top of the spacer disk, and the pad was allowed to equilibrate for 60
seconds, after which the pad thickness was measured. The cell and its
contents then were hanged in a Petri dish containing a sufficient amount of
saline solution (0.9% by weight saline) to touch the bottom of the cell. The
cell was allowed to stand in the Petri dish for 10 minutes, and then it was
removed and hung in another empty Petri dish and allowed to drip for 30
seconds. While the pad still was under the load, its thickness was measured.
The 100 g weight then was removed and the weight of the cell and contents
was determined. The weight of the saline solution absorbed per gram fiber
then was determined and expressed as the absorbency under load (g/ g).
[0083] The absorbent capacity of the cross-linked fiber was determined in the
same
manner as the test used to determine absorbency under load above, except
that this experiment was carried out under a load of 0.01 psi. The results are
used to determine the weight of the saline solution absorbed per gram fiber
and expressed as the absorbent capacity (g/g).
[0084] The cell from the absorbent capacity experiment then was centrifuged
for 3
min at 1400 rpm (Centrifuge Model HN, International Equipment Co.,
Needham HTS, USA), and weighed. The results obtained were used to
calculate the weight of saline solution retained per gram fiber, and expressed
as the centrifuge retention capacity (g/g).
24
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
Fiber QualitX
[0085] Fiber quality evaluations were carried out on an Op Test Fiber Quality
Analyzer (Op Test Equipment Inc., Waterloo, Ontario, Canada) and Fluff
Fiberization Measuring Instruments (Model 9010, Johnson Manufacturing,
Inc., Appleton, WI, USA).
[0086] Op Test Fiber Quality Analyzer is an optical instrument that has the
capability of measuring the average fiber length, kiilk, curl, and fines
content.
[0087] Fluff Fiberization Measuring Instrument is used to measure nits and
fine
contents of fiber. In this instrument, a sample of fiber in fluff form was
continuously dispersed in vi air stream. During dispersion, loose fibers
passed through a 16 mesh screen (1.18 mm) and then through a 42 mesh
(0.36 mm) screen. Pulp bundles (knots) which remained in the dispersion
chamber and those that were trapped on the 42-mesh screen were removed
and weighed. The former are called "knots" and the latter "accepts." The
combined weight of these two was subtracted from the original weight to
determine the weight of fibers that passed through the 0.36 mm screen.
These fibers were referred to as "fines."
Example 1
[0088] This example illustrates a method for making mercerized fibers and a
method for forming handsheets from a blend of mercerized and Rayfloc°-J-
LD fibers.
[0089] Fiber mercerization was carried out as follows: A sample of
Rayfloc°-J-LD
(never dried) was obtained as a 33.7% solid wet lap from the Rayonier mill at
Jesup, Georgia. Rayfloc°-J-LD is an untreated southern pine Kraft
pulp
commercially available from Rayonier Performance Fibers Division, Jesup,
GA. A 70.0 g (dry weight base) sample of Rayfloc°-J-LD was treated
with an
aqueous solution of 16% (w/w) sodium hydroxide at room temperature at a
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
consistency of about 3.5%. The mixture was agitated for about 10 min, then
excess NaUH was removed by suction filtration or centrifuge. Tlle resulting
mercerized pulp then was washed with excess water, neutralized to a pH of
6.4 with acetic acid solution (0.01 M) at a consistency of about 3.5%, and
optionally sheeted and dried.
[0090] Handsheets of blends of fibers then were formed by thoroughly agitating
a
slurry of Rayfloc~-J-LD and mercerized fiber in water at consistency of about
3% for about 10 min. The blend of fibers then was formed into sheets on a
standard 12X12 inch laboratory sheet mold. The wet sheets were optionally
dried. The formed sheets had approximately the same basis weight (762
gsm) and had a density in the range of 3.2 to 4.6 g/ cm3.
[0091] The sheets prepared in accordance with this example 1 then were cross-
linked as described in the following examples.
Example 2
[0092] This example illustrates a method for cross-linking a blend of fibers
in the
sheet form prepared in the manner described above in example 1. In order
to determine the effect of increasing the amount of conventional fiber on
absorbent properties of fibers cross-linked in the sheet form, sheets with
various proportions of Rayfloc~-J-LD and mercerized fibers were prepared
as described in example 1 and used in this example. Sheets were soaked in a
bath of 2% solution of glyoxylic acid (obtained as 50% solution in water
commercially available from Clarinet Corporation, Charlotte, NC) for about
1 to 2 min and then pressed to a pick-up that affords about 2% of glyoxylic
acid on fiber. Sheets then were dried and cured in one step process at
190°C
for 15 min.
[0093] The absorbent capacity, absorbency under load, centrifuge retention,
knots
and fine contents of the cross-linked sheets were determined. The results are
summarized below in Table 1.
26
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
Table 1
Fiber AbsorbencyAbsorbent~eritrifugeFines Ifnots
Blend.
Under Capacity.Retention( % and
Load )
' (0.3 psi)~g/g) (g/g) nits
' :. , . (l
. .: )
. .:
Rayfloc~-J='Mercerized:
'
L;D.(%) er (%)..... ;:: ,. ,..
:... Fili
0 100 9.8 8.3 0.46
30 70 10.3 8.6 0.43 7.2 0.47
50 50 10.0 8.7 0.46
Example 3
[0094] This example illustrates a method for cross-linking a blend of fibers
in sheet
form using DP-60 as a cross-linking agent (Belclene° DP-60 is a mixture
of
polymaleic acid terpolymer with the malefic acid monomeric unit
predominating (molecular weight of about 1000) and citric acid
commercially available from BioLab Industrial Water Additives Division).
Sheets with various proportions of Rayfloc~-J-LD and mercerized fibers were
prepared as described above in Example 1, and then were soaked in a bath of
DP-60 solution (5%) for about 1 to 2 min and then pressed to a pick-up that
affords about 5 % of DP-60 on fibers. Sheets were then dried and cured in a
one step process at 190 °C for about 15 min.
[0095] The absorbent capacity, absorbency under load, centrifuge retention,
knots
and fine contents of cross-linleed sheets were determined. The results are
summarized in Table 2.
27
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
Table 2
Fibex.Blend' ,A.bsoxbency.Absoxbexit:.:.Centxiuge:.'Kn.ots'Pixies
~LTnder .' Capacity:Retentionand (%)
Load (gsg) (g/g) rifts
(0:3 psi).,.
Rayfloc~-Meireerized(g~ g) ~o/
. . :' ' )
J:LD %) ;. : . :
%) .:
0 100 9.1 10.8 0.52 1.7 5.7
30 70 9.0 11.0 0.52 0.3 4.7
40 60 9.4 11.0 0.52 2.6 4.7
50 50 9.6 11.0 0.51 4.4 4.7
60 40 8.4 10.0 0.50 15.5 4.8
[0096] The results of examples 2 and 3 demonstrate that conventional fibers
(for
example Rayfloc~-J-LD) can be used in an amount of up to about 50% in a
blend with mercerized fibers to produce cross-linked fiber in the sheet form
with properties similar to those obtained using mercerized fiber alone. As
shown in Table 2, the presence of Rayfloc~-J-LD in amounts up to about 50%
showed little or no effect on the absorbent properties of cross-linked fibers
and revealed a slight increase in the amount of knots and nits.
Example 4
[0097] This example illustrates the effect of curing temperature on the
absorbent
properties of representative cross-linked fibers formed in accordance with
the present invention.
[0098] Sheets were formed as described above in example 1 using a blend of
Porosanier-J-HP 70% by weight (mercerized fiber available from Rayonier
Performance Fibers Division (Jesup, GA)) and Rayfloc~-J-LD 30% by weight.
Cross-linking was carried out using glyoxylic acid in the presence and in the
absence of a catalyst. Sheets were soaked in a bath of an aqueous solution of
glyoxylic acid (2%) and magnesium chloride hexahydrate (0.25%) for about 1
to 2 min and then pressed to a pick-up that affords about 2% of glyoxylic
acid and 0.25% of magnesium chloride hexahydrate on fiber.
28
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
[0099] Sheets after treatment were dried in a 50°C oven to about 5 to
7% moisture
contents then cured at various temperatures for about 5 min. The above
experiment was repeated without a catalyst.
[00100] The absorbent capacity, absorbency under load, centrifuge retention,
knots
and fine contents of the cross-linked fibers, and sheet density were
determined. The results are summarized below in Table 3.
Table 3
Cure, ' Absorbency ::Absorbent ' Cenfirifuge
TemperatureUnder Ca R
Load aci/ etentian~
0.3 ) /
si /
C . . No.. With No With _ With
.. ~ _
No
Catal Catal acatalCatal catalystcatal
st st st st st
130 8.6 7.5 10.0 8.7 0.63 0.52
150 8.7 6.8 10.0 8.4 0.57 0.45
1901 8.~ 6.7 9.9 8.0 0.54 0.46
190 8.5 7.4 10.2 8.6 0.43 0.40
1. Curing time in this experiment was about 2 min.
[00101] The results summarized in Table 3 demonstrate that cure temperature
has
little or no effect on absorbency under load and absorbent capacity of cross-
linked fibers, whereas centrifuge retention capacity decreases with
increasing cure temperature. These results indicate that higher temperatures
are preferred to attain higher degrees of cross-linking. In addition, the
results in Table 3 illustrate the effect of using a catalyst on cross-linking
efficiency. As shown in Table 3, fiber cross-linked in the presence of a
catalyst showed lower centrifuge retention compared to those cross-linked in
the absence of a catalyst, especially when cross-linking is carried out at
temperatures below 190°C. However, at cure temperatures of about
190°C,
the presence or absence of catalyst had little or no effect on cross-linking
efficiency.
29
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
Example 5
[00102] This example illustrates the effect of curing time on absorbent
properties of
representative cross-linked fibers formed in accordance with the present
invention.
[00103] Cross-li~ilcing was carried out as described above in Example 2,
except that,
in this example a catalyst, magnesium chloride hexahydrate (0.25 % ) was
used in addition to the cross-linking agent. The fiber was cured at
150°C for
various curing times. Sheets used in this experiment were formed as
described in Example 1 using Rayfloc~-J-LD and Porosanier-J-HP in a weight
ratio of 1:1. The results are summarized below in Table 5.
Table 5
Cure Time :Absorbency. Absorbent- Centrifuge'
:'
(nz~) . Under Loael :Capacity . Retention.
. . (0 3 .(g/ g), (g~ g) ..
'. .: ; si) ~ ~:: . : .. ' ' : .
.
51 7.3 8.6 0.50
7.3 8.6 0.45
7 6.9 8.3 0.45
7.3 8.6 0.44
1. In this experiment the curing was carried out at 130 °C
[00104] As shown in Table 5, cure times ranging from about 5 to about 10
minutes
had little or no effect on absorbency under load and absorbent capacity.
However, the centrifuge retention decreasing by increasing curing time
indicates that cross-linking efficiency can be increased by increasing the
curing time.
Example 6
[00105] This example illustrates the effect of using various amounts of cross-
linking
agent on absorbent properties of produced fibers.
[00106] Sheets used in this experiment were formed as described above in
example 1
using 70% by weight Porosanier-J-HP and 30% by weight Rayfloc~-J-LD.
Fiber treatment was carried out as shown in Example 2 except that in this
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
example various amounts of cross-linking agents were used. Glyoxylic acid
was used as a cross-linking agent in the presence of a constant amount of the
catalyst magnesium chloride hexahydrate (0.25%). Treated sheets were
dried at 50°C then cured at 150 °C for 5 min. The results are
summarized
below in Table 6.
Table 6
Cross-linkingAbsorbency UnderAbsorbent Centrifuge
. '
agent Load (0.3 psi) Capacity (g/g)Retention
,. ,(g/ g) : .
.
(/) .
.
1.0 7.5 7.8 0.48
2.0 6.8 8.4 0.45
3.0 7.4 8.4 0.44
4.0 7.8 8.5 0.42
Example 7
[0010] This example illustrates the effect of using varying amounts of
catalyst on
the absorbent properties of cross-linked fiber. The sheets used in this
example were prepared as described in example 1 using 70% Porosanier-J-
HP by weight and 30 % Rayfloc~-j-LD by weight.
[0010] Fiber treatment was carried out as described above in Example 2 except
that
in this example, glyoxylic acid (2%) was used as a cross-linking agent in the
presence of various amounts of magnesium chloride hexahydrate. Treated
sheets were dried at 50°C, and then cured at 150°C for 5 min.
[00109] The results are summarized below in Table 7.
31
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
Table 7
Catalyst AbsorbencyUnder::Absorbent -:Centrifuge
-'
MgC12:6I-IzO..Load (0.3.psi) Capacity Retention
~ . (g% g) (g%g) .
'(/Q) :. (g/g)
0.00 8.7 10.0 0.57
0.05 9.4 8.8 0.46
0.10 8.2 8.9 0.46
0.25 6.8 8.4 0.45
0.40 7.5 8.4 0.45
[00110] The results summarized in Table 7 indicate that increasing the amount
of
catalyst showed little or only a slight effect on cross-linking efficiency,
since
as can be seen in Table 7, increasing the amount of catalyst from 0.05 % to
about 0.4% showed minimal changes in fiber absorbent properties.
Example 8
[00111] This example illustrates an airlaid method for forming a
representative
absorbent structure of the present invention.
[00112] An airlaid absorbent core formed in accordance with the present
invention
was prepared using an airlaid apparatus known to experts in the art. Fiber
(Rayfloc~-J-LD) and superabsorbent particles (P-02-055-01 obtained from
BASF), were loaded into the airlaying apparatus. Vacuum then was applied,
fibers and superabsorbent particles traveled through plastic tubing and were
combined through an air vortex into a plastic cylinder having a 100-mesh
metal screen adhering to the cylinder bottom. After fibers and
superabsorbent particles were completely combined, the vacuum was
discontinued and the resulting pad was removed from the cylinder. The pad
was then compressed by a hydraulic press to a pressure of 700 PSI for 3 min.
The pressure was then released, and pad was allowed to equilibrate for 60
seconds. The pad thickness was measured before and after pressing and the
density was calculated. The total weight of the pad is about 3.0 g composed
of 55% by weight superabsorbent particles and 45% by weight fiber.
32
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
Example 9
[00113] This example illustrate the acquisition times of cross-finked fibers
made in
accordance with the present invention.
[00114] The acquisition time was determined by the SART test method. The test
evaluates the performance of cross-linked fibers as an acquisition layer in
absorbent article. The test measures the time required for a dose of saline to
be absorbed completely into the absorbent article. The test is conducted on a
sample of absorbent core made in accordance with the method described in
example ~. The core included about 45 % by weight fiber (Rayfloc~-J-LD) and
55 % by weight superabsorbent particles (P-02-055-01 obtained from BASF)
based on the total weight of the core. The core had a circular shape with a
diameter of 60.0 mm, a density of 0.2, and weighed about 3.0 g (~ 0.1 g).
[00115] In this test a sample of cross-lilZked fibers made in accordance with
the
present invention was airlaid into a 60.0 mm pad. The pad weighed about
0.7 g and was compressed with a load of a 7.6 PSI for 60 seconds before
being tested. The core was placed into the testing cell, which consisted of a
plastic base and a funnel cup. The base used to hold the sample was a
plastic cylinder with an inside diameter of 60.0 mm. The funnel cup was a
plastic cylinder with a star shape hole, the outside diameter of the funnel
cup
was 5~ mm. The funnel cup was placed inside the plastic base on top of the
sample and a load of 0.6 PSI having a donut shape was placed on top of the
funnel cup.
[00116] The cell and contents were placed on a level surface and dosed with
three
successive 9.0 mL insults of saline solution; the time interval between doses
was 20 min. The doses were added with a Master Flex Pump (Cole Parmer
Instrument, Barrington, IL, USA) to the funnel cup, and the time in seconds
required for each dose of saline solution to disappear from the funnel cup
was recorded and expressed as an acquisition time.
33
CA 02518145 2005-09-02
WO 2004/083518 PCT/US2004/007728
[00117] The results are summarized in Table 8.
Table 8
Fibex : Cross-linking % of Cross-bra Insixlt
Agent skin . (sec
,. a ent
Porosanier 21.0
Porosaruer Fiber DP-60 5.0 14.0
(sheet
form)
Blend (Rayfloc DP-60 5.0 12.0
50% and
Porosaruer 50
% )
Blend (Rayfloc Glyoxylic acid 2.0 14.2
50% and
Porosaruer 50%)
Blend (Rayfloc Glyoxlic acid 3.0 10.3
40 % and
Porosaruer 60%)
Porosanier Glyoxylic acid 2.0 10.5
Blend (Rayfloc Glyoxylic acid 2.0 10.2
30% and
Porosaruer 70
% )
[00118] The above examples reveal that crosslinked blends of mercerized fibers
and
conventional cellulose fibers have improved absorbency, absorbency under
load, retention capacity, and acquisition times. The blends of the invention
therefore preferably are useful in absorbent articles as an acquisition layer
and/ or an absorbent core.
[00119] While the invention has been described with reference to particularly
preferred embodiments and examples, those skilled in the art recognize that
various modifications may be made to the invention without departing from
the spirit and scope thereof.
34