Note: Descriptions are shown in the official language in which they were submitted.
CA 02518352 2005-09-06
WO 2005/022120 PCT/US2004/007138
PROCESS FOR PRODUCING NANOCRYSTALS AND NANOCRYSTALS
PRODUCED THEREBY
BACKGROUND OF THE INVENTION
Field of the Invention
[0001] The present invention relates to the field of semiconductor
nanocrystals
and to a process for preparing same.
Description of Background Art
[0002] Nanocrystals have gained a great deal of attention for their
interesting
and novel properties in electrical, chemical, optical and other applications.
Such nanomaterials have a wide variety of expected and actual applications,
including use as semiconductors for nanoscale electronics, optoelectronic
applications in emissive devices, e.g., nanolasers, LEDs, etc., photovoltaics,
and sensor applications, e.g., as nanoChemFETS.
(0003] While commercial applications of the molecular, physical, chemical
and optical properties of nanocrystals are beginning to be realized;
commercially viable processes for the production of a wide variety of
nanocrystals have been limited. Both the starting materials used and the
conditions under which the nanocrystals are grown are commercially
prohibitive. The chemical reaction used to produce nanocrystals involves
nanocrystal nucleation and growth. Lack of control over the nucleation event
and growth phase in synthetic process has prevented the production of a wide
variety of nanocrystal types.
[0004] Nanocrystals of semiconductors are traditionally formed by the fast
injection of pyrophoric precursors into hot coordinating solvents. U.S. Patent
No. 6,225,198 B 1 to Alivisatos et al., the full disclosure of which is hereby
incorporated by reference in its entirety for all purposes, discloses a
process for
the formation of rod-shaped II-VI semiconductor nanocrystals. In the disclosed
CA 02518352 2005-09-06
WO 2005/022120 PCT/US2004/007138
-2-
method, a cold solution (-10 °C ) of a Group II metal and Group VI
element is
injected into a binary surfactant mixture heated to temperatures around 360
°C
to initiate nanocrystal nucleation, which reduces the reaction temperature to
around 300 °C. The nanocrystals are grown at temperatures about 50-70
°C
lower than the nucleation temperature. A variation in temperature drop of as
little as 5 °C leads to different growth rates and different size,
shape and
structure nanocrystals can result.
[0005] Published U.S. Patent Application No. 20020066401 to Peng et al., the
full disclosure of which is hereby incorporated by reference in its entirety
for
all purposes, discloses a method of synthesizing colloidal nanocrystals, in
which a Group II metal compound is combined with a coordinating solvent
and heated to temperatures around 360 °C. A cold solution of a Group VI
element is injected to initiate nucleation, which reduces the reaction
temperature to around 300 °C. The nanocrystals are grown at
temperatures
about 50-70 °C lower than the nucleation temperature. A variation in
temperature drop of as little as 5 °C leads to different growth rats
and different
size, shape and structure nanocrystals can result.
[0006] Accordingly, it would be desirable to have a process of producing
nanocrystals that is commercially viable, offering greater control,
predictability
and reproducibility, as well as a process that is amenable to the production
of a
wide variety of semiconductor nanocrystal shapes and types.
SUMMARY OF THE INVENTION
[0007] The present invention relates to processes for producing nanocrystals.
An embodiment comprises: contacting a metal precursor with a mixture
comprising a coordinating solvent to form a first precursor mixture; heating
the first precursor mixture to a first temperature; contacting the first
precursor
mixture with a second precursor mixture comprising one of a Group V and
Group VI compound to form a reaction mixture at a second temperature; and
CA 02518352 2005-09-06
WO 2005/022120 PCT/US2004/007138
-3-
heating the reaction mixture at a third temperature to grow nanocrystals;
whereby the second temperature is no more than about 15 °C lower than
the
first temperature. Alternatively, the second temperature is no more than about
°C, 7 °C, 5 °C, 3 °C or 1 °C lower than the
first temperature.
[0008] A further embodiment of the present invention comprises: contacting a
metal precursor with a mixture comprising a coordinating solvent and a metal
catalyst to form a first precursor mixture; heating the first precursor
mixture to
a first temperature; contacting the first precursor mixture with a second
precursor mixture comprising one of a Group V and Group VI compound to
form a reaction mixture at a second temperature; and heating the reaction
mixture at a third temperature to grow nanocrystals; whereby the second
temperature is no more than about 15 °C lower than the first
temperature.
Alternatively, the second temperature is no more than about 10 °C, 7
°C, 5 °C,
3 °C or l °C lower than the first temperature.
[0009] Another embodiment of the present invention relates to a composition
of rod-shaped III-V nanocrystals having at least about 50% hexagonal crystal
structure and an aspect ratio of at least about 4:1. Alternatively, the
composition of rod-shaped III-V nanocrystals has at least about 70%, 80%,
90% or 95% hexagonal crystal structure and an aspect ratio of at least about
4:1.
[0010] Additional features and advantages of the invention will be set forth
in
the description that follows, and in part will be apparent from the
description,
or may be learned by practice of the invention. The advantages of the
invention will be realized and attained by the structure and particularly
pointed
out in the written description and claims hereof as well as the appended
drawings.
[0011] It is to be understood that both the foregoing general description and
the following detailed description are exemplary and explanatory and are
intended to provide further explanation of the invention as claimed.
CA 02518352 2005-09-06
WO 2005/022120 PCT/US2004/007138
-4-
BRIEF DESCRIPTION OF THE FIGURES
[0012] The accompanying drawings, which are included to illustrate
exemplary embodiments of the invention and axe incorporated in and
constitute a part of this specification, illustrate embodiments of the
invention
and together with the description serve to explain the principles of the
invention. In the drawings:
[0013] FIG. 1 is a flow chart depicting the traditional process of producing
nanocrystals.
[0014] FIG. 2(a)-(b) are flowcharts depicting the preparation of the first
precursor mixture in accordance with the presentinvention.
[0015] FIG. 3(a)-(b) are flowcharts depicting the preparation of the second
precursor mixture in accordance with the present invention.
[0016] FIG. 4 is a flow chart depicting the process of producing nanocrystals
in accordance with the invention.
[0017] FIG. 5 depicts a pyramid-shaped II-VI or III-V type nanocrystal 500
having cubic crystal structure with four faces 502-508.
[0018] FIG. 6 depicts a tetrapod-shaped II-VI or III-V type namocrystal 600
having cubic crystal structure in the center pyramid region 500 and hexagonal
crystal structure in the four arms 602-608.
[0019] FIG. 7 depicts a rod-shaped II-VI or III-V type nanocrystal 700.
[0020] FIG. 8a is a Transmission Electron Microscope (TEM) micrograph of
rod-shaped CdSe nanocrystals, produced in accordance with the present
invention.
(0021] FIG. 8b shows an X-ray diffraction (XRD) pattern taken of the rod-
shaped CdSe nanocrystals. The x-axis is in degrees 29 and x-ray source is Cu-
Ira radiation.
[0022] FIG. 9 is a series of three TEM micrographs showing the production of
tetrapod-shaped CdSe nanocrystals in accordance with the present invention.
CA 02518352 2005-09-06
WO 2005/022120 PCT/US2004/007138
-5-
[0023] FIG. 10 shows the XRD patterns for samples of tetrapods with
different arm lengths taken from four different nanocrystal syntheses.
[0024] The present invention will now be described with reference to the
accompanying drawings. In the drawings, like reference numbers indicate
identical or functionally similar elements. Additionally, the left-most
digits)
of a reference number identifies the drawing in which the reference number
first appears.
DETAILED DESCRIPTION OF THE INVENTION
[0025] Reference will now be made in detail to the embodiments of the
present invention, examples of which are illustrated in the accompanying
drawings.
[0026] Many variables contribute to the shape of the produced nanocrystal.
For example, the variables include the reaction mixture temperature, the
concentration of precursor compounds, the molar ratio of the precursor
compounds and the concentration and type of surfactant and coordinating
solvent. The inventors have discovered that a minimum, reproducible and
predictable temperature change between the first and second temperatures
affords maximum control and reproducibility in nanocrystal synthesis. This
control has allowed for the production of a wide range of nanocrystal types
and
shapes, including shaped nanocrystal types that were not possible using
previous processes known in the art.
[0027] FIG. 1 illustrates the traditional process of producing CdSe
nanocrystals. The process comprises mixing, 106, a surfactant, 102, and a
phosphine oxide, 104, and heating, 108, the mixture to produce a precursor
mixture, 110. The process further comprises contacting, 118, simultaneously, a
cadmium salt, 116, cooled below room temperature, and a selenium-phosphine
complex, 114, also cooled below room temperature, to form a reaction
mixture,120, at a second temperature. The second temperature is at least about
CA 02518352 2005-09-06
WO 2005/022120 PCT/US2004/007138
-6-
30 °C to about 70 °G lower than the first temperature. The term
"about"
includes the specified number ~5%. For example, "about 400 °C" includes
380-420 °C. The reaction mixture is further processed by heating, 122,
the
reaction mixture to form nanocrystals and isolating, 124, the nanocrystals, to
produce a nanocrystal composition, 126.
[0028] The present invention comprises a process for producing nanocrystals
of II-VI or III-V semiconductors, which offers control over the nanocrystal
nucleation event and growth phase, and in turn the shape and size of the
nanocrystal, by minimizing the temperature change between the first and
second temperatures. Examples of II-VI or III-V semiconductor nanocrystals
made according to the present invention include: any combination of an
element from Group II, such as Zn, Cd and Hg, with any element from Group
VI, such as S, Se, Te, Po, of the Periodic Table; and any combination of an
element from Group III, such as B, Al, Ga, In, and Tl, with any element from
Group V, such as N, P, As, Sb and Bi, of the Periodic Table. Specific
examples include, but are not limited to ZnS, ZnSe, ZnTe, CdS, CdSe, CdTe,
GaN, GaP, GaAs, InN, InP and InAs nanocrystals. The present invention also
allows for control over the resulting shape and size of the nanocrystals.
Examples of shapes that are made according to the invention include, but are
not limited to, spheres, rods, arrowheads, teardrops and tetrapods.
(0029] Nanocrystals made in accordance with the present invention will
optionally be subjected to further processing. For example, surface chemistry
modifications are optionally made to the nanocrystals of the present
invention.
Examples of surface modification include, but are not limited to, the addition
of coating layers and the addition of shells over the nanocrystals of the
present
invention. The coating layers and/or shells can be any material, for example,
semiconductor materials, or the like. Such processing steps are known to one
of skill in the art, see, for example, U.S. Patent Nos. 6,207,229 B1 and
6,322,901 B1, the full disclosures of which are hereby incorporated by
reference in their entirety for all purposes.
CA 02518352 2005-09-06
WO 2005/022120 PCT/US2004/007138
(0030] FIG. 2a illustrates one embodiment of the present invention,
comprising first contacting a metal precursor, 200, with a mixture comprising
a coordinating solvent, 202, to form a first precursor mixture, 204. The metal
precursor can be any metal compound that comprises an element from Group
II or Group III of the periodic table, such as a metal oxide, metal salt or
organometallic complex. Metal oxides for use in the present invention include
oxides of the elements Zn, Cd, Hg, B, Al, Ga, In and Tl. Examples of metal
oxides include but are not limited to CdO, ZnO, A1203 and Ina03. Metal salts
for use in the present invention include salts of the elements Zn, Cd, Hg, B,
Al,
Ga, In and Tl. Examples of metal salts include, but are not limited to, metal
halides, metal carboxylates, metal carbonates, metal sulfates and metal
phosphates, such ZnFa, ZnClz, ZnBr2, ZnI~, Zn(acetate)Z, ZnS04, CdF2, CdCl2,
CdBr2, CdI2, Cd(acetate)Z, Cd(OH)2, Cd(N03)2, Cd(BF4)2, CdS04, CdC03,
AlF3, A1C13, AlBr3, AlI3, Al(OH)Z(COzCH3), AlNH4(S04)2, Al(OH)3,
Al(NO3)3, Al(C104)3, A1P04, A12(SO4)3, GaF3, GaCl3, GaBr3, GaI3, Ga(N03)3,
Ga(C104)3, Ga2(SO4)3W'3~ ~C13, InBr3, InI3, In(NO3)3, In(C1O4)3 and
In(acetate)3. Organometallic complexes for use in the present invention
include any organometallic complex of the elements Zn, Cd, Hg, B, Al, Ga, In
and Tl. Examples of organometallic complexes include, but are not limited to,
complexes between Group II or Group III elements and alkyl, haloalkyl,
alkenyl, alkynyl, aryl, alkoxyl, alkenoxyl and aryloxyl groups. Specific
examples of organometallic complexes include, but are not limited to,
dialkylzinc, diallcylcadmium, dialkylmercury, trialkylaluminum,
trialkylgallium and trialkylindium, including Zn(CH3)2, Zn(CH2CH3)a,
Cd(CH3)2, Cd(CHaCH3)Z, Hg(CH3)2, Hg(CH~CH3)z, Al(CH3)3, Al(CH2CH3)3,
Ga(CH3)3 Ga(CH2CH3)3, In(CH3)3 and In(CH2CH3)3.
[0031] Coordinating solvents for use in the present invention include solvents
that can coordinate to metals and have boiling points greater than 150
°C.
Preferably, the solvent has a decomposition temperature above 300
°C.
Examples of coordinating solvents for use in the present invention include
CA 02518352 2005-09-06
WO 2005/022120 PCT/US2004/007138
_g_
those with the formula X=Y(R)3 wherein X is selected from the group
consisting of O and S, or alternatively, X does not exist; Y is selected from
the
group consisting of N and P; and each R is selected from the group consisting
of alkyl and haloalkyl. If Y is N, then X does not exist. It is understood by
one
of skill in the art that the nitrogen atom, N, is not pentavalent under
conditions
of present invention, and therefore, X cannot exist if N is Y and N is bonded
to
three R groups. Allcyl is used herein to refer to any branched or unbranched
saturated hydrocarbon chain with 4 to 40 carbon atoms. Haloalkyl is used
herein to refer to alkyl chains substituted by any number of halogen atoms
such as Cl, F, Br and I. Examples include perfluorooctyl (-C8F1~) and
pentadecafluorooctyl (-CH2CF15). Examples of coordinating solvents include
but are not limited to trioctylamine, trihexylphosphine, trihexylphosphine
oxide, trioctylphosphine, trioctylphosphine oxide, tridecylphosphine,
tridecylphosphine oxide, tridodecylphosphine, tridodecylphosphine oxide,
tritetradecylphosphine, tritetradecylphosphine oxide, trihexadecylphosphine,
trihexadecylphosphine oxide, and trioctadecylphosphine,
trioctadecylphosphine oxide.
[0032] Refernng back to FIG. 2a, the mixture comprising a coordinating
solvent, 202, optionally further comprises a surfactant. Surfactant is used
herein to refer to any molecule that interacts dynamically with the surface of
a
II-VI or III-V semiconductor nanocrystal. A surfactant is understood to act
dynamically with a nanocrystal surface if the surfactant is capable of
removing
and/or adding molecules to the nanocrystal, or alternatively, if the
surfactant is
capable of adhering, adsorbing or binding to the nanocrystal surface. The
surfactants include alkylcarboxcylic acids, alkylamines, alkylamine oxides,
sulphonates, sulphonic acids, phosphonates, phosphonic acids, phosphinic
acids, phosphine oxides and polymers thereof. Examples include
hexylphosphonic acid, octylphosphonic acid, decylphosphonic acid,
dodecylphosphonic acid and phosphonate esters and polymers of the
CA 02518352 2005-09-06
WO 2005/022120 PCT/US2004/007138
_9_
phosphoric acids, including dimers, trimers, tetramers, pentamers, hexamers,
heptamers, etc. of the phosphoric acid.
[0033] In another embodiment of the present invention, as shown in FIG. 2a,
the first precursor mixture, 204, further comprises a metal catalyst, 250. The
metal catalyst facilitates nanocrystal nucleation and/or growth. Metal
catalysts
for use in the present invention include, but are not limited to, colloidal
metal
nanoparticles. Metal nanoparticles for use in the invention include any metal
nanoparticles that facilitate the anisotropic growth of II-VI or III-V
semiconductor nanocrystals, for example, gold. Other metals for use in the
present invention include any of the transition metals from the Periodic
Table,
including, but not limited to, copper, silver, nickel, palladium, platinum,
cobalt, rhodium, iridium, iron, ruthenium, osmium, manganese, chromium,
molybdenum, tungsten, vanadium, niobium, tantalum, titanium, zirconium and
hafnium. The metal nanoparticles can be any shape, preferably spheres, and
have sizes in the range of about 1 to about 50 nanometers.
[0034] Refernng to FIG. 2a, the first precursor mixture, 204, is heated, 206,
to
a first temperature, which is sufficient enough to initiate nanocrystal
synthesis.
For example, the first temperature is about 200 to 500 °C.
Alternatively, the
first temperature is about 250 to 450 °C. Alternatively, the first
temperature is
about 290 to 400 °C. The first precursor mixture heated to a first
temperature,
208, is used in the nanocrystal synthesis immediately, or alternatively, there
is
a time delay, 210, wherein the first precursor mixture is held for a period of
time at the first temperature before using it further. The period of time is
in the
range of about 5 minutes to about 12 hours. Alternatively, the first precursor
mixture heated to a first temperature, 208, is cooled, 212, to a temperature
of
about 0 °C to about 100 °C and then optionally heated, 214, to
form the first
precursor mixture at the first temperature, 208.
(0035] FIG. 2b illustrates another embodiment of the present invention.
Contacting the metal precursor, 200, with a surfactant, 216, and optionally a
coordinating solvent, 217, forms the first precursor mixture, 204, further
CA 02518352 2005-09-06
WO 2005/022120 PCT/US2004/007138
-10-
comprising a metal precursor complex, 226. For example, a surfactant such as
hexylphosphonic acid and a metal precursor such as Cd0 react to form a Cd-
hexylphosphonic acid precursor complex.
[0036] The first precursor mixture, 204, is optionally heated, 218. The metal
precursor complex, 226, is further optionally isolated, 220, by cooling the
metal precursor mixture andlor adding a solvent, for example methanol,
capable of precipitating the metal precursor complex. Optionally, the process
comprises the steps of purifying, 222, and drying, 224, the metal precursor
complex, 226. Contacting the metal precursor complex, 226, with a
coordinating solvent, 217, and optionally a surfactant, 216, and optionally a
metal catalyst, 250, forms the first precursor mixture, 204. Heating, 228, the
first precursor mixture, 204, to a first temperature forms the first precursor
mixture at a first temperature, 208.
[0037] FIG. 3a illustrates another embodiment of the present invention. A
second precursor mixture, 302, comprises one of a Group V and Group VI
compound, 304. Group V compound is used herein to refer to any compound
that comprises a Group V element of the Periodic Table. Elements from Group
V of the Periodic Table include N, P, As, Sb and Bi. Examples of Group V
compounds include, but are not limited to, N(TMS)3, P(TMS)3, As(TMS)3,
Sb(TMS)3 and Bi(TMS)3, wherein TMS refers to the trimethylsilyl group -
Si(CH3)3; N(CH3)3, N(CH2CH3)3, P(CH3)3, P(CHaCH3)3, As(CH3)3,
As(CH2CH3)3, Sb(CH3)3, Sb(CHZCH3)3, Bi(CH3)3 and Bi(CH2CH3)3. Group
VI compound is used herein to refer to any compound that comprises a Group
VI element of the Periodic Table. Elements from Group VI of the Periodic
Table include O, S, Se, Te and Po. Examples of Group VI compounds include,
but are not limited to, elemental chalcogens such as S, Se, Te and Po.
[0038] Referring back to FIG. 3a, the second precursor mixture, 302,
optionally further comprises a coordinating solvent, 217, for example, a
trialkylphosphine oxide such as trioctylphosphine oxide or
tritetradecylphosphine oxide. Optionally, the second precursor mixture further
CA 02518352 2005-09-06
WO 2005/022120 PCT/US2004/007138
-11-
comprises a surfactant, 216, for example, hexylphosphonic acid. Alternatively,
the second precursor mixture, 302, comprises no coordinating solvent, 217, or
surfactant, 216. Preferably, the second precursor mixture, 302, comprises
about 70-100% Group V or VI precursor compound. Preferably, the amount of
coordinating solvent, 217, and optional surfactant, 216, used is such that
when
the second precursor mixture, 302, contacts the first precursor mixture heated
to a first temperature, 208, and forms a reaction mixture at a second
temperature, as described below, the second temperature is not more than
about 15 °C lower than the first temperature.
[0039] Referring back to FIG. 3a, the second precursor mixture, 302, is
optionally heated, 310, to form a second precursor mixture at a temperature
about 25 to 400 °C, 312. Preferably, the second precursor mixture, 302,
is
heated to a temperature such that when it contacts the first precursor mixture
heated to a first temperature, 208, and forms a reaction mixture at a second
temperature, as described below, the second temperature is not more than
about 15 °C lower than the first temperature.
[0040] FIG. 3b illustrates a further embodiment of the present invention. A
fractional amount of second precursor mixture, 302, is optionally diluted with
surfactant, 216, and coordinating solvent, 217, to form a diluted second
precursor mixture, 318. The diluted second precursor mixture, 318, comprises
a different concentration of Group V or Group VI compound, 304, than the
second precursor mixture, 302. The diluted second precursor mixture, 318,
however, has the same volume as the second precursor mixture, 302. Thus, the
present invention allows for changing the molar ratio between the two
elements in II-VI and III-V type semiconductor nanocrystals, without losing
control, predictability and reproducibility over the nanocrystal synthesis.
This
process of dilution can be repeated any number of times. Varying the
fractional
amounts of second precursor mixture, 302, produces any number of diluted
second precursor mixtures, all having varying concentrations of Group V or
Group VI compound, 304, but all having constant volume. Optionally, heating,
CA 02518352 2005-09-06
WO 2005/022120 PCT/US2004/007138
-12-
316, the diluted second precursor mixture, 318, forms a diluted second
precursor mixture at a temperature about 25 to 400 °C, 320.
[0041] Alternatively, the molar ratio between the two elements in II-VI and
III-V type semiconductor nanocrystals is varied by changing the amount and/or
concentration of metal precursor, used in the first precursor mixture. Or
alternatively, the amount and/or concentration of metal precursor used and the
concentration of the Group V or Group VI compound used in the second
precursor mixture are both varied.
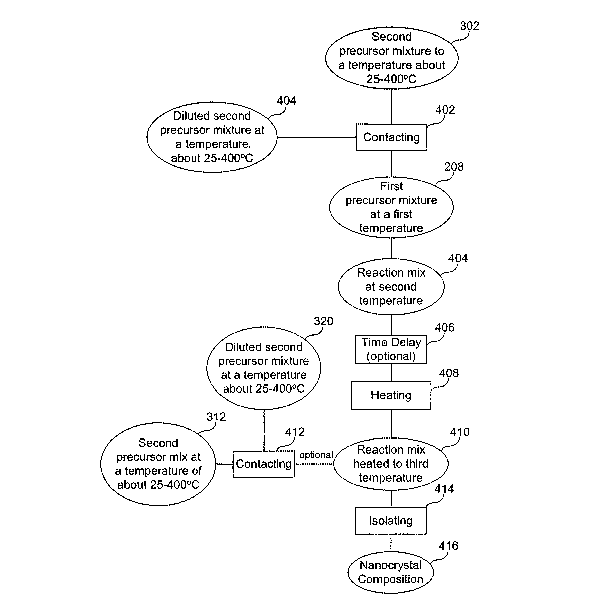
[0042] FIG. 4 illustrates a further embodiment of the present invention.
Contacting, 402, the first precursor mixture, 208, with a second precursor
mixture, 312, or optionally, a diluted second precursor mixture, 320, forms a
reaction mixture at a second temperature, 404. The contacting, 402, can be
performed by any means known to one of ordinary skill in the art. For
example, the second precursor mixture is rapidly injected into the first
precursor mixture. The second temperature is no more than about 15 °C
lower
than the first temperature, alternatively, the second temperature is no more
than about 10 °C, 7 °C, 5 °C, 3 °C, or 1 °C
lower than the first temperature.
Alternatively, there is no temperature change, meaning the first and second
temperatures are equal. After the contacting, there is an optional time delay,
406, wherein the reaction mixture is held at the second temperature for a
period of time. This period of time is about 10 seconds to about 10 minutes.
The process further comprises heating, 408, the reaction mixture, 404, at a
third temperature to form a reaction mixture at a third temperature, 410, and
to
grow nanocrystals. The third temperature can be any temperature that allows
for the controlled growth of nanocrystals in a predetermined and defined
crystal structure. For example, the nanocrystals are grown at a temperature of
about 100 to about 450 °C. The reaction mixture is heated at the third
temperature for a period of time to grow the nanocrystals. The length of time
for the heating, 408, is in the range of about one minute to about one hour.
CA 02518352 2005-09-06
WO 2005/022120 PCT/US2004/007138
-13-
[0043] In another embodiment of the present invention, the process shown in
FIG. 4, further comprises contacting, 412, the reaction mixture heated to a
third temperature, 410, with additional second precursor mixture, 312, or
optionally, diluted second precursor mixture, 320. The additional second
precursor mixture can be added all at once. Alternatively, the additional
second precursor mixture or diluted second precursor mixture can be added in
a series of additions. Alternatively, the additional second precursor mixture
or
diluted second precursor mixture can be added slowly and constantly over the
course of the heating, 408.
[0044] The process shown in FIG. 4, further comprises isolating, 414, the
nanocrystal composition, 416, from the reaction mixture. The isolating can be
performed by any method known to one of ordinary skill in the art. One
example of isolating comprises cooling the reaction mixture to room
temperature, adding a sufficient amount of polar solvent, such as methanol,
isopropanol or acetone and collecting the nanocrystals by any method such as
filtration or centrifugation. The nanocrystals can be separated by size and
shape. Preferably, the nanocrystals have a narrow size and shape distribution
and require no size or shape separation. The nanocrystals in composition 416
vary not more than about 20% in size. Alternatively, the nanocrystals in
composition 416 vary not more than about 15%, 10% or 5% in size. Not more
than about 20% of the nanocrystals in composition 416 have varying shape.
Alternatively, not more than about 15%, 10% or 5% of the nanocrystals in
composition 416 have varying shape.
[0045] Nanocrystals can be separated according to size and shape by any
method known to one of skill in the art. For example, the nanocrystals can be
separated according to size by passing a composition of nanocrystals through
filters having progressively smaller pores. Filters can have pore sizes in the
range of about 100 nm to about 10 ~,m. Alternatively, the nanocrystals can be
separated using shape selective precipitation. The addition of a different
CA 02518352 2005-09-06
WO 2005/022120 PCT/US2004/007138
-14-
polarity solvent to a solution of nanocrystals precipitates less soluble
nanocrystals, while the shapes that are more soluble remain in solution.
[0046] Controlling the temperature change between the first and second
temperatures allows for precise control over the temperature of the reaction
mixture, and thus precise control over the crystal structure in which the
nanocrystals will nucleate and grow. The controlled temperature change also
allows for a wider range of suitable first temperatures because the second
temperature remains sufficiently high to grow the nanocrystals in the desired
crystal structure after the reaction mixture is formed. In addition, the wider
range of first temperatures allows for the use of a wider variety of reagents,
coordinating solvents and surfactants, thus reducing manufacturing costs.
[0047] Producing II-VI type nanocrystals in an anisotropic shape depends on
nucleating and/or growing the material in a particular crystal structure.
Anisotropic is used herein to mean nanocrystals having properties that differ
according to the direction of measurement. For example, anisotropic rod-
shaped nanocrystals have anisotropic aspect ratios of about 2:1 to about 10:1
or greater. An aspect ratio of 2:1 for a rod-shaped nanocrystal means the
length
of the rod is 2 times the width of the rod. An example is shown in FIG. 5,
wherein rod-shaped nanocrystal, 500, has length, 504, and width, 502. Aspect
ratio can be measured by any method known to one of skill in the art, for
example, High Resolution or Low Resolution Transmission Electron
Microscopy (HRTEM or TEM, respectively), scanning electron microscopy
(SEM) or atomic force microscopy (AFM).
[0048] Crystal structure is used herein to mean the geometric arrangement of
the points in space at which the atoms of the nanocrystal occur. As a specific
example of crystal structure, CdSe, like other II-VI nanocrystals, forms
hexagonal and cubic crystal structures. FIG. 6 depicts a nanocrystal, 600, in
a
pyramid-shape, resulting from nucleation and/or growth in the cubic crystal
structure, with four faces, 602, 604, 606, and 608. A higher temperature is
required to nucleate CdSe and other II-VI nanocrystals in the hexagonal
crystal
CA 02518352 2005-09-06
WO 2005/022120 PCT/US2004/007138
-15-
structure than the cubic crystal structure. Likewise, it also requires a
higher
temperature to grow CdSe and other nanocrystals in the hexagonal crystal
structure than in the cubic crystal structure. Nucleation and growth of CdSe
and other II-VI and ffI-V nanocrystals in the hexagonal crystal structure
leads
to anisotropic rod-shaped nanocrystals. The crystal structure of the
nanocrystal
can be determined by any process known to one of skill in the art and
includes,
but is not limited to, X-ray crystallography, transmission electron microscopy
(TEM), scanning electron microscopy (SEM) and solid state nuclear magnetic
resonance (SSNMR). Preferably, X-ray crystallography or TEM is used.
[0049) A further embodiment of the present invention comprises a process for
producing anisotropic rod-shaped II-VI and III-V nanocrystals. The process
comprises contacting a first precursor mixture heated to a first temperature,
with a second precursor mixture to form a reaction mixture at a second
temperature. The first and second temperatures are sufficiently high to
nucleate and/or grow II-VI or III-V nanocrystals in the hexagonal crystal
structure. The reaction mixture is then heated to a third temperature, which
is
also sufficiently high to grow the II-VI or III-V nanocrystals in the
hexagonal
crystal structure. The second temperature is no more than about 15 °C
lower
than the first temperature, alternatively, the second temperature is no more
than about 10 °C, 7 °C, 5 °C, 3 °C, or 1 °C
lower than the first temperature.
Alternatively, there is no temperature change, meaning the first and second
temperatures are equal. For no extended period of time, therefore, does the
temperature for nucleation or growth drop below that which is required to
nucleate and/or grow the II-VI or III-V nanocrystals in the hexagonal crystal
structure. The phrase "extended period of time" is used herein to mean a
period of time on the same order as the time required for the growth of
nanocrystals. For example, if nanocrystals are grown for 10 minutes, then an
extended period of time would be in the range of about one minute to about 10
minutes.
CA 02518352 2005-09-06
WO 2005/022120 PCT/US2004/007138
-16-
[0050] In yet a further embodiment, the present invention comprises a process
for producing tetrapod-shaped II-VI and III-V nanocrystals. The process
comprises contacting a first precursor mixture, which is heated to a first
temperature, with a second precursor mixture to form a reaction mixture at a
second temperature. The first and second temperatures are sufficiently high to
nucleate II-VI and III-V nanocrystals in the cubic crystal structure. The
reaction mixture is then heated to a third temperature, which is sufficiently
high to grow the II-VI and III-V nanocrystals in the hexagonal crystal
structure. The second temperature is no more than about 15 °C lower
than the
first temperature, alternatively, the second temperature is no more than about
10. °C, 7 °C, 5 °C, 3 °C, or 1 °C lower
than the first temperature.
Alternatively, there is no temperature change, meaning the first and second
temperatures are equal. For no extended period of time, therefore, does the
temperature for nucleation drop below that which is required to nucleate the
II-
VI and III-V nanocrystals in the cubic crystal structure. And at no time,
therefore, does the temperature for growth drop below that which is required
to
grow the II-VI and III-V nanocrystals in the hexagonal crystal structure. As
shown in FIG. 7, this process results in a tetrapod-shaped nanocrystal, 700,
having 4 rod-shaped anus, 702, 704, 706, and 708 (each having hexagonal
crystal structure) extending from each corresponding face, 602, 604, 606, and
608 of the center of the nanocrystal, 600 (each having cubic crystal
structure).
[0051] FIG. 8a is a Transmission Electron Microscope (TEM) micrograph of
rod-shaped CdSe nanocrystals, produced in accordance with the present
invention. The micrograph shows the nanocrystals have rod shape with
uniform length and aspect ratio. FIG. 8b shows an X-ray diffraction (XRD)
pattern taken of the rod-shaped CdSe nanocrystals. The XRD pattern shows
the nanocrystals are formed in the hexagonal crystal structure.
[0052] FIG. 9 is a series of three TEM micrographs showing the production of
tetrapod-shaped nanocrystals over time in accordance with the present
invention. The series of micrographs shows the precise control the process of
CA 02518352 2005-09-06
WO 2005/022120 PCT/US2004/007138
-17-
the present invention offers over the length of the arms on each tetrapod. The
reaction progresses as shown from top to bottom and shows increasing length
of the tetrapod arms. FIG. 10 shows the XRD patterns for samples of
tetrapods taken over time during the nanocrystal synthesis. The bottom XRD
patterns show that early on in the process, the nanocrystals have a larger
portion of the cubic crystal structure present. As the process progresses with
time, each XRD pattern (moving up on the graph) shows an increasing amount
of hexagonal crystal structure in the nanocrystals, corresponding to the
growth
of arms on the tetrapods. This data confirms the nanocrystals nucleate in the
cubic crystal structure, forming the pyramid shaped center, but the
nanocrystals grow in the hexagonal crystal structure to produce the rod-shaped
arms of the tetrapods.
[0053] Another embodiment of the present invention comprises a process for
producing rod-shaped III-V nanocrystals with at least about 50% hexagonal
crystal structure and aspect ratio of at least about 4:1. Alternatively, the
rod-
shaped III-V nanocrystals have at least about 60%, 70%, 80%, 90% or 95%
hexagonal crystal structure and aspect ratio of at least about 4:1. The
process
comprises contacting a metal precursor comprising a Group III element of the
Periodic Table, with a mixture comprising a coordinating solvent, and a metal
catalyst to form a first precursor mixture. The process further comprises
heating the first precursor mixture to a first temperature. The first
temperature
is sufficiently high to nucleate and/or grow III-V nanocrystals in the
hexagonal
crystal structure. The process further comprises contacting the first
precursor
mixture with a second precursor mixture comprising a Group V compound to
form a reaction mixture at a second temperature, and heating the reaction
mixture at a third temperature to grow nanocrystals. The second temperature is
no more than about 15 oC lower than the first temperature, and at no time does
the temperature drop below that which is required to grow the III-V
nanocrystals in the hexagonal crystal structure. This process of employing a
CA 02518352 2005-09-06
WO 2005/022120 PCT/US2004/007138
-18-
metal catalyst and a minimum temperature change is especially useful for the
isotropic growth of rod-shaped III-V nanocrystals.
[0054] Another embodiment of the present invention, therefore, relates to a
composition of rod-shaped ILI-V nanocrystals having at least about 50%
hexagonal crystal structure and an aspect ratio of at least about 4:1.
Alternatively, the composition of rod-shaped III-V nanocrystals have at least
about 60%, 70%, 80%, 90% or 95% hexagonal crystal structure and aspect
ratio of at least about 4:1. It is preferable to produce rod-shaped
nanocrystals
having no cubic crystal structure, because the areas having cubic crystal
structure act as stacking faults such that the shape of the nanocrystal is not
a
straight rod but a zigzag-shaped rod. This zigzag shape can adversely affect
the
optical and electronic properties of the nanocrystal. The percentage of
crystal
structure for a particular nanocrystal can be determined by any method known
to those of ordinary skill in the art. For example, measuring the amount of
the
nanocrystal in one crystal structure to the total amount of the nanocrystal,
or by
measuring the ratio of crystal structure in the produced nanocrystal to that
of a
nanocrystal pure in one crystal structure determines the percentage of crystal
structure. X-ray diffraction patterns of nanocrystals pure in crystal
structure are
known to those of ordinary skill in the art and can be made, for example,
theoretically, ih silico or experimentally.
[0055] The nanocrystals of the present invention have useful optical and
electronic properties that can be applied in a variety of devices. Examples of
devices include, but are not limited to electrooptic devices, such as white
light
sources, light emitting diodes (LED), photorefractive devices, RF filters,
such
as those for optical data storage, communication and photovoltaic devices,
such as those for solar energy conversion.
[0056] In a device, the nanocrystals are deposited on a substrate, for
example,
an electrode, or sandwiched between two or more substrates. Substrates for use
in the present invention include, but are not limited to silicon and other
inorganic semiconductors, for example, ZnO, Ti02 and In203-SnO2 (ITO);
CA 02518352 2005-09-06
WO 2005/022120 PCT/US2004/007138
-19-
polymers such as semiconductive polymers, for example,
polyphenylenevinylene; and glass, such as ITO-coated glass. Methods for
applying the nanocrystals to a substrate surface are well known to those of
ordinary skill in the art. For example, the nanocrystals are applied from
solution via spin coating.
[0057] The nanocrystals can be deposited neat or as a mixture comprising the
nanocrystals. The mixture further comprises materials that include, but are
not
limited to electrooptical and semiconductive organic and inorganic molecules
and polymers. Specific examples of molecules and polymers include, but are
not limited to amines, such as triarylamines and polymers or dendrimers
thereof; inorganic semiconductors, such as GaAs, InP and Ti02; polyarylenes,
such as polythiophene, polypyrrole, polyphenylene, and polyfluorene, and
polyarylvinylenes, such as polyphenylenevinylene and polythienylvinylene.
[0058] Nanocrystals are deposited as a single layer or as multilayers. A layer
comprises only one type of nanocrystal, for example, II-VI rods.
Alternatively,
a layer comprises two or more different types of nanocrystals. For example, a
layer comprises two, three, four, five, six, seven, eight, nine, ten, etc.
different
types of nanocrystals. As a non-limiting example of a layer comprising three
different types of nanocrystals, a layer comprises II-VI rods, II-VI tetrapods
and III-V rods. When nanocrystals are deposited in multilayers, each layer
comprises the same type of nanocrystal. Alternatively, when nanocrystals are
deposited in multilayers, each layer comprises a different type of
nanocrystal.
Layer thickness is about 10 nm to about 1000 ~.m. Preferably, the layer
thickness is about 50 ~,m to about 100 ~,m. Layer thickness can be measured by
any method known to one of ordinary skill in the art, for example, atomic
force
microscopy (AFM) or scanning electron microscopy (SEM).
[0059] The nanocrystals are oriented on the electrode surface in one
direction.
Alternatively, the nanocrystals are randomly oriented. The nanocrystals are
oriented by any method known to those of shill in the art. For example, the
CA 02518352 2005-09-06
WO 2005/022120 PCT/US2004/007138
-20-
nanocrystals are oriented under an applied electrical, optical or magnetic
field,
or the nanocrystals are oriented mechanically by fluid flow orientation.
(0060] The following examples are illustrative, but not limiting, of the
method
and compositions of the present invention. Other suitable modifications and
adaptations of the variety of conditions and parameters normally encountered
in nanocrystal synthesis and which are obvious to those skilled in the art are
within the spirit and scope of the invention.
EXAMPLE 1
[0061] High quality CdSe rods were prepared by admixing about 0.74g
octadecylphosphonic acid (ODPA), about 3.23g of trioctylphosphine oxide
(TOPO) and about 0.095g of Cd0 into a 3-neck flask. The flask was degassed
and about l.Slg of trioctylphosphine (TOP) was added to form a first
precursor mixture. In a separate flask, a selenium precursor mixture (Se:TOP)
was prepared with about 10% selenium by weight. About O.llg Se:TOP
mixture was added to about 0.41g of TOP for a total weight of about 0.52g.
The first precursor mixture was heated to about 320 oC. The new selenium
precursor mixture with additional TOP was injected into the heated first
precursor mixture to nucleate CdSe nanocrystals and form the reaction
mixture. The temperature of the reaction mixture dropped to about 315
°C
upon inj ection. The reaction mixture was heated at about 315 °C for
about 15
minutes to produce high quality wurzite CdSe rods.
EXAMPLE 2
[0062] High quality CdTe tetrapods were prepared by admixing about 0.40g
octadecylphosphonic acid (ODPA), about 3.638 of trioctylphosphine oxide
(TOPO) and about O.OSOg of Cd0 into a 3-neck flask. The flask was degassed
by heating under vacuum and about l.SOg of trioctylphosphine (TOP) was
added to form a first precursor mixture. In a separate flask, a tellurium
CA 02518352 2005-09-06
WO 2005/022120 PCT/US2004/007138
-21-
precursor mixture (Te:TOP) was prepared with about 10% tellurium by
weight. About 0.16g Te:TOP mixture was added to about 0.39g of TOP for a
total weight of about 0.55g. The first precursor mixture was heated to about
320 °C. The new tellurium precursor mixture with additional TOP was
injected into the heated first precursor mixture to nucleate CdTe nanocrystals
and form the reaction mixture. The temperature of the reaction mixture
dropped to about 315 °C upon injection. The reaction mixture was heated
at
about 315 °C for about 15 minutes to produce high quality CdTe
tetrapods.
[0063] It will be understood by those skilled in the art that various changes
in
form and details may be made therein without departing from the spirit and
scope of the present invention as defined in the appended claims. Thus, the
breadth and scope of the present invention should not be limited by any of the
above-described exemplary embodiments, but should be defined only in
accordance with the following claims and their equivalents.