Note: Descriptions are shown in the official language in which they were submitted.
CA 02518969 2010-05-28
1
BLOOD TREATMENT DEVICE AND METHOD WITH SELECTIVE SOLUTE
EXTRACTION
Field of the invention
The object of the present invention is a device and a method for the treatment
of
blood with selective extraction of solutes.
The object of this patent application is the filtration of blood to
selectively separate
and extract dissolved substances of chosen molecular size by means of
extracorporeal
systems designed for the, separation of substances.
Such systems are used for the treatment of blood containing solutes with
different
molecular weights. Such substances are, for example, urea, of molecular weight
60
daltons, phosphate (96-97 daltons), creatinine (113 daltons), vitamin B12 (1
355 daltons),
inulin (5 200 daltons), beta 2-microglobulin (12 000 daltons), and albumin (58
000
daltons).
Are hereafter termed 'small-sized molecules' molecules of molecular mass less
than
about 2 000 daltons, 'medium-sized molecules' molecules of molecular mass
between
2 000 and 50 000 daltons, and 'large-sized molecules' molecules of molecular
mass
greater than 50 000 daltons (for example, proteins).
State of the art
It is known from W096/28198 a two-stage size separation circuit based on the
available filtration technology and dialysis hardware for the treatment of
acute renal
failure, which is said to be capable of offering high single-pass elimination
of the
free virus (and boasting a time constant of approx. 10 to 15 minutes for the
systemic clearance): such a circuit shows a closed recirculation sub-circuit
(see for
example figure 2).
CA 02518969 2010-05-28
1a
At the same time, the circuit depicted in W096/28198 shows a line,
referenced by number 17, which is an inlet line (allowing the liquid to enter
into the
treatment unit 13): therefore, such a line is not a "discharge line" of the
treatment
unit.
Such systems are often systems with extracorporeal membranes for the
separation of solutes of molecular weight lower than that of albumin, applied
to the
treatment of renal insufficiency.
Improvements have always been sought in particular to ameliorate clearance,
reduce treatment time and to make such systems simpler and less costly. We
remind that
clearance of a solute is the amount of that solute in a given volume of
treated blood..
CA 02518969 2011-02-02
2
In the field of dialysis, the first membranes used were highly permeable to
small
solutes of molecular size up to 200 daltons. The clearance of small solutes
depends on
the permeability and diffusion capacity of the membrane used.
The lack of permeability of the first membranes for certain medium-sized
solutes in
the vitamin B12 range (1 355 daltons) was blamed for the occurrence of
multiple uraemic
neuropathies.
To improve the clearance of medium-sized molecules, a first response was to
add
to the diffusion flow through the membrane a convection flow using high flow
membranes
with a molecular size cut-off value of 40 000 daltons. The cut-off value of a
membrane is
defined as the molecular size for which no more than 10 % of the solute
travels through
the membrane.
However, problems met in embodying this response include difficulty in
controlling
the ultrafiltration rate obtained by the convection flow, and the high loss of
useful plasma
constituents such as hormones, vitamins and amino acids.
A second response for the improvement of the clearance of medium-sized
molecules was haemo-filtration, a purely convective method for the elimination
of solutes
by the membrane. However, this method extracts a large amount of liquid,
therefore
requiring a compensatory pre- and (or) post-dilution with sterile liquid, and
a membrane
that is highly permeable to solutes of molecular size up to 40 000 daltons.
However, in a
purely convective mode, the clearance depends on the mode of dilution (pre- or
post-
dilution), the blood flow rate and the infusate flow rate. With conventional
haemo-filtration,
the clearance of small-sized molecules is poorer than that obtained in
haemodialysis
mode. The clearance in haemo-filtration mode could reach that of haemodialysis
if the
infusate flow rate, the blood flow and the membrane area were increased.
However, this
is impractical, increases treatment cost and results in loss of amino acids
and hormones.
In addition, the blood flow rate is limited, in particular in patients with
poor blood access.
Concerning the clearance of small-sized molecules, when it was discovered that
this clearance was limited in haemo-filtration mode, the two processes of
haemo-filtration
and haemodialysis were combined. This simultaneous method was known as haemo-
diafiltration. However, problems that arise include difficulty in precisely
controlling the
haemo-filtration flow, high loss of hormones and amino acids, the complexity
of the
CA 02518969 2011-02-02
3
system, the large quantities of sterile liquid and dialysate necessary, and
consequently
the high cost of the treatment.
Thus the use of a single filter working in different operating modes still
failed to
solve the particular problems of loss of molecules in a certain size range,
and of high
treatment cost.
A proposal was then made by Drs J.C. Kingswood and F.D.Thompson of a
continuous haemo-filtration with no re-injection liquid: the treatment of the
ultra-filtrate was
performed by a second membrane also working in spontaneous ultrafiltration.
Figure 1
represents the dialysis set-up derived from this proposal.
The procedure is to treat a first ultra-filtrate, obtained from a first hollow
fibre
membrane, by sending it through a second hollow fibre membrane in
ultrafiltration mode.
A first ultrafiltration is performed through a first high-flow membrane
impermeable to
molecules having mass larger than 10 000 daltons. The apertures in the second
membrane are smaller than those in the first.
As shown in Figure 1, at the outlet from the first membrane the unfiltered
liquid,
mainly containing large-sized molecules, is sent to the patient for re-
injection. The first
ultra-filtrate containing small- and medium-sized molecules is filtered
through the second
membrane. The liquid not filtered by the second membrane, mainly containing
medium-
sized molecules, is collected in a waste bag. The second ultra-filtrate,
mainly containing
small-sized molecules, is re-injected in post-dilution via the patient's
venous line.
This saves consuming excessive amounts of sterile liquid in post-injection,
and
allows re-injection in the patient of a liquid containing few medium-sized
molecules.
Even so, a high loss of nutrients, amino acids, glucose and vitamins occurs,
and
the clearance, of small ions such as potassium is poor.
Accordingly, another dialysis device was designed. It was considered that the
uraemic molecules that had to be removed were of molecular mass less than 200
daltons
and those between 10 000 and 40 000 daltons.
This consideration gave rise to a device composed of three filters, depicted
in
Figure 2.
CA 02518969 2011-02-02
4
A first filter has a cut-off value of about 40 000 daltons. The blood flows
through
this first filter to yield a first filtrate containing small-sized and medium-
sized molecules,
i.e., molecules of molecular mass less than 40 000 daltons. The solutes of
mass between
000 and 40 000 daltons are then eliminated by ultrafiltration through the
second filter,
which has a cut-off value below 10 000 daltons. The second filtrate is then
treated by
haemo-filtration with a membrane with a cut-off value of about 200 daltons.
Thus the
purified filtrate, containing solutes between 200 and 10 000 daltons, is
returned for post-
infusion to the patient, who also receives the molecules of molecular mass
greater than
40 000 daltons.
10 However, the clearance of all the solutes depends on the ultrafiltration
rate in filter
1, which cannot exceed 30 % of the blood flow, a value that is low compared
with that
attained in conventional haemodialysis. This raises operating costs.
Lastly, Patent US 6,193,681 describes an apparatus to treat septicaemia in the
blood, depicted here in Figure 3. The blood flows first through a UV
irradiation device and
then through a blood concentrator before re-injection in the patient. A
secondary circuit is
connected to a second outlet from the blood concentrator from which the fluid
flows out
through a filter followed by a membrane module and a dilution source, and is
then injected
upstream of the blood concentrator.
There is in addition an analogous problem with plasmapheresis. Therapeutic
exchange plasmapheresis is carried out on patients whose plasma contains one
or more
harmful or toxic substances.
These solutes are eliminated from the plasma by the same principle as the
elimination of solutes from blood, one difference being the greater molecular
mass of the
solutes to be extracted from the plasma.
Thus recurrent problems have been encountered in the design of the devices in
prior art, namely:
- High consumption of perfusion liquid,
- High losses of nutrients, amino acids, glucose and vitamins,
- Poor clearance of solutes,
- High cost of devices comprising several filters and pumps.
CA 02518969 2010-05-28
The problem addressed in this patent application is how to achieve selective
elimination of molecules in one or more molecular mass range(s) with good
clearance, yet
consume very small amounts of sterile liquid.
For example, for patients in a state of septicaemia, many medium-sized
molecules
have to be eliminated, while still maintaining satisfactory elimination of
small-sized
molecules. Septicaemia is characterised by abundant repeated release of
specific
pathogenic germs from an initial point of infection.
An auxiliary problem is optimally adapting such a system for long-term therapy
carried out in an intensive care environment without a risk of filter
clogging. Such an
adaptation can be achieved by judicious choice of mode of operation of the
various filters,
of use and appropriate positioning of means to regulate flow rate, of
controlled flow rates
and of hydraulic design of the lines.
Description of the invention
According to the present invention, there is provided a device for the
extracorporeal treatment of blood comprising:
- at least one exchanger (1) having a semi-permeable membrane (6) dividing
said exchanger into a first chamber (7) and a second chamber (8), at least one
first
inlet (2) for blood to be treated in fluid communication with the first
chamber (7), a
first fluid outlet (4) in fluid communication with the first chamber (7) and a
second
fluid outlet (5) in fluid communication with the second chamber (8) of the
exchanger
(1),
- an input line (10) for blood to be treated connected to the first inlet (2)
of the
exchanger (1),
- a blood output line (11) connected to the first outlet (4) of the exchanger
(1),
- at least one treatment unit (21) comprising a semi-permeable membrane
(26), dividing the treatment unit (21) into a first chamber (27) and a second
chamber (28), at least one first fluid inlet (22) in fluid communication with
the
CA 02518969 2010-05-28
6
second chamber (28), and at least one first fluid outlet (24) in fluid
communication
with the first chamber (27) of the treatment unit (21),
- the second outlet (5) of the exchanger (1) being in fluid communication with
the first inlet (22) of the treatment unit (21),
- the first outlet (24) of the treatment unit (21) being in fluid
communication
with the input line (10),
wherein
- the treatment unit (21) includes a second fluid outlet (25) in fluid
communication with the second chamber (28) of the treatment unit (21),
- the second outlet (25) of the treatment unit (21) is in fluid communication
with a first waste liquid discharge line (30) having a first end and a second
end, the
first end of said first waste liquid discharge line being connected with the
second
outlet (25) of the treatment unit (21) and the second end of said first waste
liquid
discharge being connected to a drain or to a first waste liquid container
(31).
Preferably, in order to solve the problem outlined above, the invention
provides for an extracorporeal blood treatment device comprising at least one
exchanger 1 equipped with at least one first inlet 2 for the blood to be
treated, a first
fluid outlet 4 and a second fluid outlet 5, an input line 10 for blood to be
treated
connected to the first inlet 2 of the exchanger 1, a blood output line (or
venous line)
11 connected to the first outlet 4 of the exchanger 1, at least one treatment
unit 21
comprising at least one first fluid inlet 22 and at least one first fluid
outlet 24, the
second outlet 5 of the exchanger 1 being in fluid communication with the first
inlet
22 of the treatment unit 21, characterized the first outlet 24 from the
treatment unit
21 is in fluid communication with the input line 10.
Preferably, the invention also concerns an extracorporeal blood treatment
method to be implemented by means of the extracorporeal blood treatment device
comprising an exchanger 1 to which are connected a blood input line 10 and a
CA 02518969 2010-05-28
6a
blood output line 11 and a treatment unit 21, which method comprises the
following
steps: blood is sent through input line 10 connected to exchanger 1, filtered
first in
the exchanger 1 to produce a first filtrate, which is filtered at least a
second time by
the treatment unit 21 to produce a second filtrate, which is sent through the
input
line 10 for pre-dilution of blood to be treated, and the blood is sent from
the outlet
from exchanger 1 to the output line 11.
Other advantages and characteristic of the invention will be inferred from the
following description.
Brief description of the drawings
The description refers to the appended drawings, where:
Figure 1 shows the state of the art concerning the use of two filters with
different cut-off
values and with post-dilution re-injection.
Figure 2 shows the state of the art concerning the use of three filters with
different cut-off
values and with post-dilution re-injection.
Figure 3 shows the state of the art of Patent US 6,193,681.
Figures 4 to 9 are schematic diagrams of the device for the treatment of
physiological fluid according to the invention, together with various
embodiments.
Figures 10 and 11 show the estimated results in terms of clearance according
to
the molecular size of the solutes for two configurations of the device
according to
the invention.
Detailed description
Figure 4 shows the principle of the invention in diagrammatic form: blood
inflow through
an input line, its arrival in the exchanger and its outflow from the exchanger
through an
output line, together with the treatment of the first filtrate by a treatment
unit and the
injection of the liquid leaving the treatment unit in pre-dilution in the
arterial line. We can
define this concept as a "cascade" of filtration steps with re-injection of
the final filtrate in
pre-dilution; in detail: a first filtrate is a second time filtrated, and the
second filtrate is then
injected at the inlet of the first filter, or in "pre-dilution".
CA 02518969 2010-05-28
6b
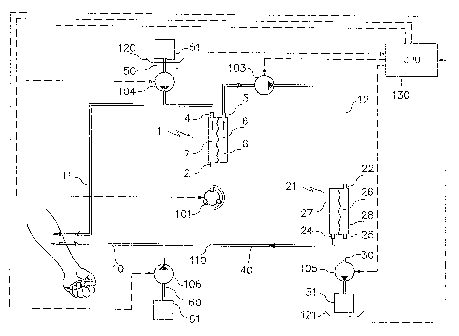
Figure 5 shows the extracorporeal blood treatment device of the invention
consisting of an
exchanger 1 comprising a first inlet.2 for the blood to be treated, a first
fluid outlet 4 and a
second fluid outlet 5, an input line 10 for the blood to be treated, or
arterial line, connected
to the first inlet 2 of the exchanger 1, a blood output line, or venous line,
11 connected to
the first outlet 4 of the exchanger 1. A treatment unit 21 comprises a first
fluid inlet 22, and
a first fluid outlet 24, the second fluid outlet 25 of the exchanger 1 is in
fluid
communication with the first inlet 22 of the treatment unit 21, and the first
outlet 24 of the
treatment unit 21 is in fluid communication with the input line 10
The fluid communication between the first inlet 22 of the treatment unit 21
and the second
outlet 5 of the exchanger 1 is made by a duct 12.
CA 02518969 2011-02-02
7
The exchanger 1 can be equipped with a semi-permeable membrane 6 that divides
it into
a first chamber 7 and a second chamber 8. The first inlet 2 of the exchanger
is in fluid
communication with the first chamber 7 of the exchanger, the first outlet 4 of
the
exchanger is in fluid communication with the first chamber 7 of the exchanger,
and the
second outlet 5 of the exchanger is in fluid communication with the second
chamber 8 of
the exchanger.
The blood input line 10, termed the 'arterial line', connected to the first
inlet 2 of the
exchanger 1, the blood output line 11 termed the 'venous line', connected to
the first
outlet 4 of the exchanger and the first chamber 7 of the exchanger form part
of an
extracorporeal blood treatment circuit.
In one embodiment shown in Figure 6, the exchanger 1 can include a second
inlet 3 in
fluid communication with the second chamber 8 and in fluid communication with
a fist
source of dialysis liquid 9. In this mode of operation the blood and the
dialysis liquid flow
in opposite directions in each of the two chambers.
Figure 5 shows the treatment unit 21 equipped with a semi-permeable membrane
26 that
divides it into a first chamber 27 and a second chamber 28.
The treatment unit 21 can advantageously have a second fluid outlet 25.
Thus the first outlet 24 of the treatment unit 21 is in fluid communication
with the first
chamber 27 of the treatment unit 21 and the second outlet 25 of the treatment
unit 21 is in
fluid communication with the second chamber 28 of the treatment unit 21.
Alternatively, the first inlet 22 of the treatment unit 21 can be in fluid
communication with
either the second chamber 28 of the treatment unit 21, or with the first
chamber 27 of the
treatment unit 21,
The second outlet 25 of the treatment unit 21 is in fluid communication with a
first waste
liquid discharge line 30, which discharge line 30 can connect the second
outlet 25 of the
treatment unit 21 to a drain or to a first waste liquid container 31.
CA 02518969 2011-02-02
8
The treatment unit 21 can also have a second inlet 23, which second inlet 23
is in fluid
communication with the second chamber 28 and with a second source of dialysis
liquid
29. In this operating mode of the treatment unit, shown in Figure 7, the
dialysis liquid
flows in the opposite direction to the physiological liquid arriving via the
first inlet 22.
The exchanger 1 and the treatment unit 21 have different characteristics. The
membrane
6 of the exchanger 1 can be a high flow membrane, and the membrane 26 of the
treatment unit 21 can be a low flow membrane.
A low-flow membrane has low water permeability. The ultrafiltration
coefficient is between
2 and 10 milh,mmHg,m2. A high-flow membrane has a much higher hydraulic
permeability. The ultrafiltration coefficient is 20 to 50mL/h,mmHg,m2.
The exchanger or the treatment unit may comprise a hollow fiber membrane
(called also
capillary filter) or a plate fiber membrane, this means with membrane sheets.
Thus the permeability to molecules of the membrane 6 in the exchanger 1 is
greater than
the permeability to molecules of the membrane 26 in the treatment unit 21, at
least above
a certain molecular mass.
More particularly, we can define a ratio or a difference between the two cut
off values of
the first membrane and the second membrane. Thus, we can define ratio of the
cut-off
value of the first membrane to the cut off value of the second membrane less
than or
equal to three. In another way, we can define the difference in cut-off value
between the
first membrane and the second membrane is between 20 000 and 30 000 daltons.
The
cut-off value of the first membrane might be less than or equal to 40 000
daltons, and the
cut-off value of the second membrane might be less than or equal to 10 000
daltons. In
one embodiment the cut-off value of the first membrane is approximately 40 000
daltons
and the cut-off value of the second membrane is approximately 10 000 daltons.
To re-infuse water to the patient being treated, it is possible to connect to
the output line
11 a post-dilution line 50 connected to a first source of sterile liquid 51
and (or) to the
input line 10 a pre-dilution line 60 connected to a second source of sterile
liquid 61.
A duct 40 makes a fluid connection between the first outlet 24 of the
treatment unit 21 and
the first inlet 2 of the exchanger 1.
CA 02518969 2010-05-28
9
The pre-dilution line 60 can be connected directly to said second duct 40 or
directly to
input line 10.
The different sources of sterile liquid 51, 61 can be bags of sterile liquid
and (or) can be
obtained by on-line preparation of sterile liquid from water drawn from a main
supply
system.
In the application of this invention to the special case of plasmapheresis,
shown in
Figures 9 and 10, the exchanger is a plasma filter. The plasma filter has a
cut-off value
between one million and five million daltons.
In this application, the exchanger or the treatment unit may comprise a hollow
fiber
membrane (also called capillary filter) or a plate membrane, this means with
membrane
sheets.
Thus the treatment unit 21 includes a unit able to fixate at least one given
substance. This
unit can be an adsorption cartridge, or a reactor, for example an
electropheresis cell.
The treatment unit can be equipped with a semi-permeable membrane 26 that
divides it
into a first chamber 27 with a first outlet 24 and a second chamber 28 with a
first inlet 22
and a second outlet 25. The second outlet is connected to a discharge line.
The treatment
unit can have a cut-off value less than or equal 250 000 daltons.
The cut off value can be less than or equal to 200000 dalton.
The treatment unit can have a cut-off value such that 100% of the albumin
molecules
(with 58000 daltons as molecular weigh) can pass through the membrane.
Figure 9 shows a device comprising means to act on at least certain molecules
70.
These means are connected to the first tube 12 between the second outlet 5 of
the
exchanger 1 and the first inlet 22 of the treatment unit 21. These means to
act on at
least certain molecules 70 can be a reactor, an adsorber or a radiation-based
CA 02518969 2010-05-28
9a
device, for example an electropheresis, enzyme reaction, radiation, or
ultraviolet
irradiation device. The plasma filter can then have pores of one micron. The
treatment unit can have a cut-off value less than or equal to 90 000 daltons,
letting
proteins through to the patient's blood.
CA 02518969 2011-02-02
Another feature of the invention is that it adds a third means of filtration
to eliminate a
further molecular mass range, shown in Figure 8. The device can comprise at
least one
auxiliary exchanger 81 with a membrane 86 that separates it in a first chamber
87 that is
in fluid communication with a first inlet 82 and a first outlet 84 and in a
second chamber
88 in fluid communication with at least one second outlet 85. The cut-off
value of such an
auxiliary exchanger will be less than the cut-off values of the other two
membranes (6,
26).
The first inlet 82 of the auxiliary exchanger 81 is in fluid communication
with the second
outlet 24 of the treatment unit 21, and one of the two outlets 84 or 85 of the
auxiliary
10 exchanger 81 is in fluid communication with the first inlet 2 of the
exchanger 1.
A second waste liquid discharge line 90 connects the other outlet 84, 85 of
the auxiliary
exchanger 81 to a drain, which drain can be a second waste liquid container
91.
Figure 8 shows the auxiliary exchanger operating in dialysis mode: the
auxiliary
exchanger 81 has a second inlet 83 in fluid communication with the second
chamber 88
of the auxiliary exchanger 81 and in fluid communication with a third source
of dialysis
liquid 89, the first outlet 84 of the auxiliary exchanger 81 being in fluid
communication with
the first inlet 82 of the exchanger 1, the second outlet 85 of the auxiliary
exchanger 81
being in fluid communication with a drain 91 via a second waste liquid
discharge line 90.
The choice of the three membranes will be made very precisely according to the
patient
and the treatment required according to the molecular mass ranges that are to
be
eliminated or retained. The first membrane 6 is appropriate for molecules of
high
molecular mass (preferentially in haemo-filtration mode), the second membrane
26 is
appropriate for molecules of mid-range molecular mass (preferentially in haemo-
filtration
mode), and the third membrane 86 is appropriate for molecules of low molecular
mass,
i.e., preferentially in dialysis mode. The auxiliary exchanger 81 can still
operate in
ultrafiltration mode if necessary. The choice of operating mode allows the
treatment to be
tailored to patient needs and to obtain optimal running with minimal clogging.
Concerning the regulation of the various fluid flow rates, first means for
regulating active
liquid flow rate 101 is placed on the input line 10 connected to the first
inlet 2 of the
exchanger 1. Alternatively, the first means to regulate flow rate (101) can be
placed
CA 02518969 2011-02-02
11
exactly between the first inlet 2 of the exchanger 1 and the connection point
110
connecting the input line to the duct or upstream of the connecting point 110
connecting
the input line 10 to the second duct 40.
In the first alternative, the pressure drop in the second duct 40 requires a
lower positive
pressure in the first duct 12 to reach the desired trans-membrane pressure
(TMP) of the
membrane 26.
Also, in the first alternative, it is not necessary to fit a pump on the
second duct 40: a
single pump 101 suffices for the second duct 40 and the arterial line 11.
In the second alternative, second means for regulating active liquid flow rate
102 is placed
an the second duct 40 connecting the first outlet 24 of the treatment unit 21
to the first
inlet 2 of the exchanger 1.
Third means for regulating active liquid flow rate 103 is placed on the first
duct 12
connecting the second outlet 5 of the exchanger 1 to one of the inlets 22 or
23 of the
treatment unit 21.
Also, fourth means for regulating active liquid flow rate 104 can be connected
to the post-
dilution line 50.
Fifth means for regulating active liquid flow rate 105 can be connected to the
waste liquid
discharge line 30 connecting the second outlet 25 of the treatment unit 21 to
a drain 31.
In the configuration with at least three means of regulating flow rate 101 on
the input line,
2 0 102 on the second duct 40 and 105 on the discharge line 30, special care
must be taken
to make sure the different flow rates set are compatible.
Sixth means for regulating active liquid flow rate 106 can be connected to the
pre-dilution
line 60.
The means for regulating flow rate 101, 102, 103, 104 and 105 can be pumps and
(or)
valves. In particular, the means for regulating flow rate on the discharge
line 30, or on the
post-dilution line 50 or the pre-dilution line 60 will be valves.
In a specific embodiment, the first post-dilution sterile liquid source 51 is
a bag of sterile
liquid and the first waste liquid container 31 connected to the discharge line
leaving the
treatment unit is a bag of waste liquid. The device comprises a first balance
120 to weigh
30 the bag of sterile liquid 51 and a second balance 121 to weigh the bag of
waste liquid 31.
Alternatively, a single balance 120-121 can weigh the bag of sterile liquid 51
together with
the bag of waste liquid 31.
CA 02518969 2011-02-02
12
In this case, a calculation and control unit 130 will receive the signals
emitted by at least
one balance 120-121 and will control the means for regulating liquid flow rate
101, 102,
103, 104 and 105.
The calculation and control unit periodically calculates the real flow rate or
a
parameter that is a function of the real flow rate, for example from the
weight and the time
interval between each two measurements. It will compare the real flow rate
measured to
the desired flow rate and will be able to control one or more means for
regulating active
liquid flow rate (101, 102, 103, 104, or 105).
Thus the quantities of sterile liquid and waste liquid, or their difference,
can be
monitored and controlled during the treatment. Knowing these weight
quantities, the
control unit can' obtain a desired quantity of sterile liquid solution and
waste liquid.
The hydric equilibrium can be well controlled in this way.
The device described above is applicable to plasmapheresis.
The invention also concerns a method for the extracorporeal treatment of blood
to be
implemented on a device for the extracorporeal treatment of blood comprising
an
exchanger 1 to which are connected a blood input line 10 and a blood output
line 11, and
a treatment unit 21, which method comprises the following steps:
- Sending blood through the input line 10 connected to the first inlet of the
exchanger 1,
- Carrying out a first filtration of the blood in the exchanger 1, producing a
first
filtrate passing through the second outlet of the exchanger,
- Filtering the first filtrate at least a second time in the treatment unit
21, producing a
second filtrate,
- Sending the second filtrate exiting the first outlet of the treatment unit
to the input
line 10 to effect a pre-dilution of the blood to be treated,
- Sending the blood out from the first outlet of the exchanger to the output
line 11
In particular, the method will include a second filtration carried out through
a semi-
permeable membrane 26 in a treatment unit 21 divided into a first chamber 27
and a
second chamber 28, yielding the second filtrate and sending the non-filtered
liquid to the
drain line 30.
CA 02518969 2010-05-28
13
Another feature of the method is that the first filtration is carried out
through a semi-
permeable membrane 6 that divides the exchanger 1 into a first chamber 7 and a
second
chamber 8.
Another feature of the method is that the membrane 26 of the treatment unit
filters
molecules of molecular mass less than the molecular mass of the molecules
filtered from
the membrane 16 of the exchanger.
Another feature of the invention is that the method includes a step in which a
sterile liquid
is perfused in the blood output line 11 of the exchanger.
Another feature of the invention is that the method includes a step in which a
sterile liquid
is perfused in the blood input line 10 of the exchanger.
Another feature of the invention is that the method uses an exchanger membrane
16 with
a cut-off value less than 40 000 dalton.
Another feature of the invention is that the method uses an exchanger membrane
16 with
a cut-off value less than 10 000 dalton.
Another feature of the invention is that the treatment carried out is a
plasmapheresis and
the treatment unit fixates at least one certain given substance.
Another feature of the invention is that the exchanger membrane 16 has a cut-
off value
between one million and five million dalton.
Another feature of the invention is that the treatment unit membrane 16 has a
cut-off
value less than 250 000 dalton.
Simulations have been performed using filters with different cut-off values.
Figures 10 and 11 show the estimated results in terms of clearance as a
function of
CA 02518969 2010-05-28
13a
the molecular mass of solutes for two configurations of the device according
to the
invention. Figure 11 shows a first configuration using an exchanger with a cut-
off
value equal to 40 000 daltons, and a treatment unit using an exchanger with a
cut-
off value equal to 10 000 daltons. The clearance (curve 1) for molecules of
about
11 000 daltons is very good, while
CA 02518969 2011-02-02
14
the clearance of small molecules is kept constant relative to an operating
device equipped
with a single filter (curve 2).
Figure 12 shows a second configuration for plasmapheresis using an exchanger
with a
cut-off value equal to 1 000 000 dalton, and a treatment unit using an
exchanger with a
cut-off value equal to 250 000 dalton. The clearance (curve 1') for molecules
of about
300 000 dalton is very good, while the clearance of medium-sized molecules is
kept
constant relative to an operating device equipped with a single filter (curve
2').
The invention offers numerous advantages. It allows:
- A three- to fourfold increase in the clearance of medium-sized molecules (or
1 large-sized molecules in plasmapheresis) relative to a standard long-term
treatment, with no increase in the quantity of exchange liquid and with no
change in the standard clearance of small-sized molecules (small- and medium-
sized in plasmapheresis),
- Large savings in sterile liquid, and therefore lower operating costs,
- Sufficient elimination of medium-sized molecules,
- Retention of trace elements and nutrients, which are returned to the
patient,
- High volume filtration.
In particular, in the configuration illustrated in Figure 5, many other
advantages are
offered. Minimal means of regulating flow rate are required: a peristaltic
pump 101 on the
20 arterial line and a pump 103 on the duct 40 are sufficient to operate the
device.
Also, the positioning of the means for regulating flow rate is well conceived:
there is not
necessarily any need for a pump on the second duct 40, although one can still
be fitted,
and the means of regulating flow rate 103 need not be very powerful. This
permits long-
term operation for intensive care while avoiding heavy pore clogging of the
various
membranes.
Lastly, application of this operating scheme is envisaged for another mode of
extracorporeal blood treatment, namely plasmapheresis. Plasmapheresis
operation is
optimal when the membranes are carefully chosen and used.