Note: Descriptions are shown in the official language in which they were submitted.
CA 02519736 2005-09-13
METHOD FOR EXTRACTING AND UPGRADING OF HEAVY AND SEMI-HEAVY
OILS AND BITUMENS
FIELD OF THE INVENTION
[0001] Selective extraction of components from a raw
feedstock with a supercritical fluid - in effect, a
fractionation of the feed - is well known and at present
widely used in commercial production of pharmaceuticals,
perfumes and spices as well as in the manufacture of prepared
foodstuffs such as caffeine-free coffee. The extractor fluids
deployed in these operations are usually supercritical carbon
dioxide or propane.
[0002] More recently substantial R & D has centered on the
use of ~~supercritical water~~ for generating from coal, oil
shales and oil sands relatively low-molecular-weight oils or
oil precursors that are amenable to conventional upgrading or
refining techniques.
[0003] We have found that, like heavier fossil
hydrocarbons, heavy oils can also be upgraded to refinable
crude oils by interaction with supercritical water. But the
extent to which the average molecular size, and hence the
viscosity of these feedstocks, is reduced is critically
dependent on operating conditions, and these in turn, are
directly governed by the chemical reactions that accompany
processing.
[0004] This invention has to do with a novel method of
processing heavier fossil hydrocarbons or heavy oils utilizing
CA 02519736 2005-09-13
nominally supercritical water to obtain lower viscosity
hydrocarbons with notably less coke.
BACKGROUND OF THE INVENTION
[0005] It is known to use supercritical water in processes
which attempt to upgrade complex hydrocarbons, notably bitumen
and heavy oils. Various processes are noted below, but each
has drawbacks, described below, at least some of which this
invention overcomes.
PRIOR ART
[0006] Brons (US 5,695,632) deals with removal of sulfur
and other organically bound heteroatoms and metals from heavy
oil. The heavy oil is contacted with aqueous sodium hydroxide
and subsequently water (and optionally hydrogen) at
temperatures in the range 380°C-450°C, to produce sodium
sulfide, which is subsequently removed from the mixture.
Reaction times are about 5 minutes to 3 hours. When hydrogen
is added to the system, pressures range from 50-700 psi;
otherwise, pressure is not defined. The teaching of the use
of water at temperatures which may be near to supercritical to
upgrade heavy oil by removal of sulfur and metals is of some
interest.
[0007] Brons (US 5,695,632) is limited to removal of
undesirable components (namely organically bound sulfur,
heteroatoms and metals) from a heavy oil feedstock. The Brons
invention does not deal with the upgrading of heavy oil to
unrefined crude oil quality, especially with regard to
2
CA 02519736 2005-09-13
favorable changes in viscosity and density. Moreover, sodium
sulfide is corrosive and difficult to handle. Handling of
hydrogen at high pressures and temperatures is also difficult.
There are therefore limits to the usefulness of Brons~s (US
5,695,632) invention as disclosed.
[0008] Brons (US 5,635,056) is similar to Brons (US
5,695,632) in that it deals with removal of a class of
organically-bound sulfur and metals from heavy oil. This
patent specifies a different class of such components.
Operating conditions and methodologies are similar to those
specified in Brons (US 5,695,632). Again, water is supplied
together with a transition metal in an intermediate step to
modify the end-stage. The disclosure notes, as an aside, that
the asphaltene content, density and viscosity may also be
reduced using the water-with-transitional-metal process.
Brons (US 5,635,056) does not provide for any specific
pressure range, and emphasizes removal of undesirable
components.
[0009] As in Brons (US 5,695,632), the handling of sodium
sulfide and hydrogen is difficult.
[0010] These two Brons patents (US 5,635,056 and US
5,695,632) rely fundamentally on mixing and reaction of heavy
oil with aqueous sodium sulfide, and both suffer the
difficulty of having to deal with corrosive sodium sulfide or
the difficulty of obtaining hydrogen and the danger of
handling high pressure and high temperature hydrogen.
3
CA 02519736 2005-09-13
[0011] Siskin (US 5,611,915) deals with removal of
heteroatoms from high asphaltene materials (such as from heavy
oil production) and coal, to favorably lower molecular
weights. The patent deals with use of supercritical water in
the presence of CO at ~ 500 psi-2700 psi, with water
temperatures in the range of 400°C to 600°C. The teaching of
the use of supercritical water together with CO is of some
interest.
[0012] This patent (Siskin (US 5,611,915)) relies
fundamentally on addition of CO, at high temperatures (400°C-
600°C). No provision is made for any convenient apparatus
design for mixing and processing the reactants. This patent
teaches away from Berkowitz (CA 2,000,251), which it cites for
use of CO to extract liquids from tar sands, by stressing only
N and S removal. Siskin X915 in fact is limited in its scope
by the prior Berkowitz patent application (CA 2,000,251) which
already covers all of the subject-matter in Siskin, except
that Berkowitz (CA 2,000,251) did not specifically mention N
or S removal. Siskin is problematic in requiring high
temperatures and the addition of CO, while not providing for
any convenient process methodology. Siskin~s contribution to
the art in the X915 patent is limited to removal of N and S
using a prior piece of art, namely Berkowitz~s prior published
Canadian application (CA 2,000,251).
[0013] Siskin (US 5,338,443) deals with upgrading organic
materials such as coal and oil shale, using water at sub-
critical temperatures (200°C-374.4°C) in the presence of an
4
CA 02519736 2005-09-13
acid catalyst. The patent explicitly emphasizes upgrading of
coal and oil shale, and does not deal with tar/oil sands.
Treatment times are 5 minutes to 1 week (with preference for
30 minutes-3 hours). A key requirement of this process is
that for each contacting temperature, the corresponding
pressure is the autogenous pressure, i.e., the pressure is
kept higher than the critical one in order to maintain the
water in liquid form, apparently in a closed reactor. Siskin
(US 5,338,443) is problematic in that it relies on addition of
an acid catalyst in addition to the water, thus the process
involves the expense and complexity of acquiring, stockpiling,
handling and balancing catalyst. Moreover, the pressure
corresponding to each temperature is high (e. g., Siskin
requires a pressure of about 3199.6 psi at the critical
temperature of 374.4°C), necessitating expensive and dangerous
processing equipment and techniques for its commercial
operation; the invention as described does not specify
maintaining the contacting water in liquid or supercritical
form. There are problems with high temperature, high
pressures, and the required use of a catalyst. Additionally,
there are unanswered questions with respect to the form of the
water during the reaction cycles, and there is a lack of
specificity in the nature of the reactor required for the
process described, although the maintenance of autogenous
pressures leads to batch or closed-system apparati.
[0014] Coenen (US 4,485,003) deals with processing coal to
make a hydrocarbon liquid using supercritical water at 380°C-
CA 02519736 2005-09-13
600°C in a high pressure reactor. Required pressures range
from about 3800 psi to about 6500 psi, and the process also
requires addition of hydrogen and a sodium or potassium salt
as a catalyst to the coal. Contact times are 10-120 minutes.
The teaching of the use of supercritical water to upgrade a
fossil fuel to hydrocarbon liquid is of some interest;
however, Coenen (US 4,485,003) is problematic in that it
requires the addition of expensive hydrogen and uses corrosive
and difficult to handle salts as a necessary catalyst. It also
deals with very high pressures, and somewhat lengthy process
times.
[0015] de Bruijn (CA 2,103,508) discloses the use of a
water-gas-shift (WGS) in a continuous process to thermally
rearrange liquid oil molecules and thus reduce viscosity and
density. T he aim is to produce an oil/water emulsion with a
sufficiently low viscosity and density to allow transport of
the emulsion via pipeline. The process requires contact with
CO or synthesis gas, together with a bifunctional catalyst
(such as production fines), at temperatures in the range
250°C-460°C and pressures in the range 100-10,000 psi, and
reactor residence times of 3 minutes to 10 hours. de Bruijn
(CA 2,103,508) is problematic in that it relies on addition of
a catalyst (together with CO or synthesis gas, and water).
Moreover, de Bruijn emphasizes production of oil/water
emulsion rather than cracking of the constituent oil
molecules, and does not provide for a lowered viscosity
hydrocarbon reaction product, but rather an emulsion requiring
6
CA 02519736 2005-09-13
further decomposition by additional processing steps to
demulsify the reaction product and further separate the water
and oil into useful components. Very high operating pressure
and temperature conditions are also required.
[0016] Gregoli (US 4,818,370) uses a continuous reaction to
upgrade heavy oil by injecting brine at supercritical
conditions. The aim is to lower the API gravity (density) and
viscosity of the hydrocarbon feedstock, as well as to reduce
the sulfur, nitrogen and heavy metal content. "Brine" refers,
in Gregoli, to captured or connate water from the formation.
Specified operating temperatures and pressures are about
376°C-482°C and 3400-4000 psi, respectively, while reactor
residence times range from 15 minutes to 6 hours. Gregoli (US
4,818,370) relies on relatively long reactor residence times
and very high pressure and temperature ranges for operation.
In particular, both the pressure arid residence time ranges
are high, causing some process delay and complexity to
required equipment. Gregoli contemplates that the continuous
reaction be accomplished in situ in a production well, by
introduction of heated brine and withdrawal of reaction
products after a designed dwell-time in situ at desired
pressures and temperatures which are quite high. The teaching
leads to the use of connate water with included or dissolved
minerals, thus contemplating a catalyst-like added feature to
the near supercritical brine. Connate water may vary
significantly from production well to production well in its
composition (chemicals in addition to the water), and in situ
7
CA 02519736 2005-09-13
conditions may be difficult to maintain and expensive and
difficult to control or predict.
(0017] Enomoto (CA 2,220,800) cites as an essential element
the injection of water/steam into a well, and the return of
mixed oil and water/steam, prior to treatment in a reactor
system. The processing thus cannot begin except at the
production well-site, and is thus constrained in the location
of at least some of its apparatus, and by definition uses at
least two reaction chambers (the well and a reactor system),
and perhaps requires more. Enomoto (CA 2,220,800)
contemplates either heavy oil premixed with water, preferably
underground (in an oil reservoir or well), and then
heating/pressurizing of the mixture; high-temperature water is
then added to the system. There are a great number of
individual steps and stages to the processes disclosed.
Because Enomoto considers an in situ system, pressure and
temperature ranges are not well defined nor well controlled.
In broad terms, they range from 71-1420 psi and 20°C-350°C,
respectively, and thus near supercriticality of the water used
is not important for the entire reaction process as specified.
[0018] For the portion of the disclosure dealing
specifically with the use of supercritical water in the
upgrading process, Enomoto prefers a temperature range of
300°C-500°C in a very high pressure range, most preferably of
2840-7100 psi. Enomoto discusses an in situ system with
several steps, but actually discloses tests performed in a
batch mode (i.e., in a closed, and not continuous, system of
8
CA 02519736 2005-09-13
autoclaves). The test data disclosed uses high operating
conditions of 430°C, a high pressure 6390 psi, and reaction
times of 5, 15, 30 minutes (actually the in-system dwell time
is longer by an unspecified amount of time, because this is
the time described for reaction AFTER REACHING the target
temperature by heating in the autoclave over an unspecified
preparation time). The Enomoto disclosure may not be
workable, discloses a system and process using a number of
different reaction chambers, pre-mixes and then heats the
hydrocarbon and water, and deals with high pressures, high
temperatures, and long in-system dwell times.
[0019] Furthermore, Enomoto (CA 2,220,800) specifies a
system in which water from the reactor system is removed in a
phase separator while at high temperature, thus requiring the
treatment and handling of high temperature water and
hydrocarbons, which may also be problematic, dangerous and
complex, requiring specialized techniques and equipment.
[0020] Brons (US 5,316,659) deals with upgrading of bitumen
asphaltenes obtained from oil sands. The method involves
separating solid asphaltene materials from whole bitumen that
is recovered from tar sands. Solvent de-asphalting of the
whole bitumen is achieved using a C3-C5 aliphatic hydrocarbon
solvent such as propane or butane. The precipitated
asphaltenes are then contacted with water at temperatures of
300°C-425°C but at no particular pressure and for no
particular reaction time, in order to produce material with a
lower average molecular weight. Examples mention reactions in
9
CA 02519736 2005-09-13
an autoclave, with reactions at 350°C and 400°C over 2 hours.
Brons (US 5,316,659) requires a key addition of a de-
asphalting solvent to separate asphaltenes from the whole
bitumen, and then uses heated water to treat only the
resulting asphaltenes. Thus, there are required two separate
reaction stages, involving quite different reactions (solvent
de-asphalting of the whole bitumen and then upgrading of the
resulting asphaltenes). The reaction time is quite lengthy,
and the process appears to be done in batches.
[0021] Brons (US 5,326,456) is identical to Brons (US
5,316,659), except that it specifies the addition of a soluble
carbonate salt, and possibly a transition metal oxide, to the
water. These additions further improve the quality of the
product. Otherwise, the two disclosures share the same
shortcomings.
[0022] Paspek (US 5,096,567) deals with a process of
upgrading heavy hydrocarbons. The method of this invention
features production of an oil/water emulsion to permit
pipeline transfer of the heavy hydrocarbons, together with a
method to process the emulsified oil feedstock to obtain light
hydrocarbon products. The method first requires as an
essential element the premixing of the oil feedstock and an
immiscible solvent (predominantly water) to form. an emulsion
with specified oil droplet sizes. While the claims indicate
that use only of water as the immiscible solvent is
sufficient, it is known that heavy oils will not typically
form an emulsion with water (and certainly not in the small
CA 02519736 2005-09-13
range of droplet sizes indicated in the patent) without the
addition of some surfactant or other such component. Thus, it
will be inferred and understood that Paspek (US 5,096,567)
requires the addition of some surfactant or other similar
material, or rely upon some other unspecified process step in
order to work as otherwise described.
[0023] Other parts of the Paspek (US 5,096,567) patent
advocate the addition of emulsifying materials such as short-
chained alcohols, salts, or other catalysts such as ruthenium
carbonyl. The addition of one or more of these catalysts is
key, but adds expense, complexity and the need for other
materials to the processes involved. The emulsion is
subsequently heated in a reactor system and the lighter
hydrocarbons are separated. Paspek (US 5,096,567) mentions
reaction temperatures in the range 350- 1000°C, but preferably
in the range 450°C-500°C. Reaction pressures are not
specified, but the embodiment teaches pressures in the range
of 3000-5000 psi. It can therefore be appreciated that high
temperatures, high pressures and complex additives are
concerns with the Paspek (US 5,096,567) invention.
Furthermore, Paspek teaches a reaction time of 30 minutes,
which means that the reaction process described will involve a
lengthy processing time. It is noted that the suggestion for
use of an immiscible solvent mixed or replaced by short-
chained alcohols or other emulsifying materials as a preferred
embodiment teaches away from use only of water as the
immiscible solvent, and in particular away from the use of
11
CA 02519736 2005-09-13
supercritical water as a satisfactory solvent on its own, thus
introducing the need, in the preferred embodiment, of
additives and more complex processes.
[0024] Murthy (US 4,446,012) deals with upgrading of heavy
hydrocarbons into light hydrocarbons by contacting the
feedstock with water at temperatures in the range of 380°C-
480°C (most preferably between 430°C-460°C) and at
pressures
in the range of 725-2175 psi. An essential element of the
patent is use of two reaction zones - the first to heat the
hydrocarbon and water simultaneously to produce a uniform
mixture, and the second in which the temperature and pressure
are maintained for some time while the uniform mixture is
separated into a residue and a vapor phase comprised of a
mixture of light hydrocarbons, gas and water. The residue is
removed from this second zone and the light hydrocarbon is
then recovered from the remaining materials in a phase
separation vessel. Thus, the system requires at least two
separate zones with separate characters in its reactions.
[0025] Another critical feature of this patent is that the
specified range of temperature and pressure is maintained in
both the first and the second zones. Separation of the
hydrocarbon, gas and water mixture occurs only subsequently,
after the residue is first removed. Residence times in the
continuous flow system range from a few minutes to 20 minutes.
Murthy (US 4,446,012) is unique in its essential requirement
of two separate reaction zones, in its maintenance of high
pressures and temperatures in both zones, and in its method to
12
CA 02519736 2005-09-13
separate and recover a light hydrocarbon phase. Also, the
hydrocarbon and water are first mixed and only then heated,
apparently to provide a uniformity of the mixture. Murthy
requires, in addition to the two separate zones of different
character (and thus complex control and sensing mechanisms in
the processing apparatus), high temperatures for its
processes, and deals with the removal of light and vaporous
hydrocarbons as part of the processing stages, thus
introducing some further complexity in materials handling and
concerns with safe handling of pressurized hydrocarbon vapors
at high temperatures.
RELATED PUBLICATIONS
[0026] The present application is based in part on and
involves improvements over published Canadian applications
2,208,046; 2,242,774; 2,252,218; and 2,316,084, all
incorporated by reference to the extent consistent with the
present disclosure.
SUMMARY OF THE INVENTION
[0027] Supercritical water is fluid water brought by a
combination of heat and pressure to the point at which, as a
near vapor, it combines properties of a gas and a liquid.
[0028] Unlike supercritical propane or carbon dioxide,
supercritical, near-supercritical, and nominally-supercritical
water (hereinafter °supercritical water~~ or SCW) exists only
at temperatures of 250°C-450°C or more and at such
temperatures, high molecular weight hydrocarbons are prone to
13
CA 02519736 2005-09-13
thermal decomposition. Such degradation, synonymous with
cracking, tends to increase with time at reaction temperatures
and as a rule entails two net reaction sets, one generating
gas and another yielding high molecular weight carbonaceous
products loosely termed coke.
[0029] As is apparent from the background information
above, there are numerous disadvantages to processes and
process equipment used in the prior art to upgrade high
molecular weight hydrocarbons such as heavy and semi-heavy
oils, hydrocarbons recovered from tar sands and oil shales,
coals, coal liquids, oil sand, bitumens, shale oils, oil
precursors and other bitumens (all of which are referred to
below as "high molecular weight hydrocarbons"). We note that
hydrocarbons recovered using conventional Steam Assisted
Gravity Drainage (SAGD) production processes for heavy oil
production may contain some water, which is not deleterious to
the processes of this invention; thus hydrocarbons with water
from SAGD recovery processes are included amongst the
potential feedstocks for the process of this invention.
[0030] It is apparent, as well, that the term "upgrading",
when used in the description of this invention and in the
claims, means both upgrading of heavy and semi-heavy oils to
unrefined crude oil quality in aspects of viscosity, density,
and/or molecular weight, as well as possible reduction in
sulfur, nitrogen and/or metal concentrations, but also means
extraction of acceptable oils and oil precursors from oil sand
bitumens, coals, coal liquids, oil shales, shale oils, and
14
CA 02519736 2005-09-13
other bitumens as referenced above, possibly pretreated,
"acceptable oils and oil precursors" being defined as
hydrocarbons suitable for conventional transport and
processing/refining.
[0031] In particular, problems with the prior art processes
and equipment arise where complex multi-reactor or multi-step
devices or processes are used, additives such as connate water
or catalysts are required, coke by-products or caustic or
dangerous chemicals are produced, or other problems as
identified above are encountered.
[0032] In the presence of nominally supercritical water, we
find that these processes are also accompanied by thermally-
driven hydrolysis of the general form:
R-R'+H20 ~ R-H +R'-OH
[0033] This is, however, reversible because -C-OH is
inherently unstable under reaction conditions, and thus
represents a transient process. Maximizing the hydrolyzed
reaction product and concurrently inhibiting extreme thermal
cracking, which yields gas and coke by random radical
recombinations, therefore requires an empirically established
compromise between reaction temperature, pressure and the in-
reactor residence time of [R-H], [R'-OH] and other species
sufficiently degraded to be 'soluble' in SCW. While it is
therefore desirable to minimize the in-reactor residence time
for both maximizing production rate and minimizing coke
formation, it has been found that for practical reasons in-
CA 02519736 2005-09-13
reactor residence times of less than 25 seconds are often
inadequate to accomplish the objectives of the present
invention.
[0034] These considerations, confirmed by data from an
extensive series of laboratory tests, lead us to the
conclusion that a simple stirred pressure-reactor precludes
optimal hydrocarbon upgrading with supercritical water. The
water used in making supercritical water for use in the
present invention can be, but is not limited to, tap water,
distilled water, de-ionized water, river water, lake water,
ground water, and the like, and/or can comprise or consist of
water retrieved from the cooling system and/or the collection
vessel, and any such water used may contain small amounts of
accompanying salts and/or minerals.
[0035] It is an object of the present invention to obviate
or mitigate at least one disadvantage of previous processes or
process apparatus.
[0036] With respect to extraction and upgrading of oil from
coals, coal liquids, oil shales, shale oils, and other similar
sources of bitumens, prior art (Berkowitz and Calderon, 1987,
1990; Ogunsola and Berkowitz, 1995) has demonstrated that oil
products can be extracted by exposing these feedstocks to hot
water, and/or steam, and/or SCW. Exposure of crushed coal
and/or oil shale material to SCW in the flow-through system of
the current invention acts in the same manner to extract the
16
CA 02519736 2005-09-13
oil, at which point upgrading (in terms of reducing viscosity
and density) occurs as described herein.
[0037] A far more efficient system offers itself by use of
a process and with an apparatus comprising an appropriately
designed and scaled flow-through reactor in accordance with
the following:
1. The apparatus of the invention is a flow-
through reactor for upgrading high molecular weight
hydrocarbons, the reactor comprises:
a. a single reaction chamber for maintenance
of continuously introduced materials at operating temperatures
between in the range of 250 to 300°C and as high as 450°C, or
even slightly more, and at operating pressures between 500 and
3000 psi, preferably 1000 to 3000 psi, more preferably 1000 to
2000 psi, still more preferably 1000 to 1500 psi, or in some
cases alternatively 800-1500 psi, more preferably 900-1200
psi, while the materials are mixed and held inside the chamber
for a desired amount of time;
b. a port for introducing water, including
SCW , into the chamber under pressure in a continuous manner;
c. optionally and preferably, a preheater for
the high molecular weight hydrocarbons which, if in the form
of coal, shale or other bitumen sources, can have been
subjected to pretreatment, e.g. by crushing into small
particles, to facilitate their injection into the reactor
system, and mixed in a slurry with water and/or other liquid
hydrocarbons;
17
CA 02519736 2005-09-13
d. a port for introducing high molecular
weight hydrocarbons into the chamber under pressure in a
continuous manner, for example fed by a mechanical conveyor
belt or train car system, or injected in a slurry of water
and/or other liquid hydrocarbons;
e. an exit port to permit reaction products
to leave the chamber under pressure in a continuous manner;
and
f. optionally, a port for introduction of
pressurized CO or nitrogen, or optionally other gases, e.g.
inert or inactive gases.
2. The process involves a flow-through reactor for
upgrading high molecular weight hydrocarbons, the reactor
having a single reaction chamber being held at pressures
desirably in the range of about 500-3000 psi and temperatures
in the range 250°C-300°C to about 450°C while water and
the
hydrocarbons to be upgraded are introduced into the chamber,
and then mixed, being held in the chamber for a predefined
period of reaction time and thereafter the products of the
resulting reaction are permitted to leave the chamber, all on
a continuous basis during operation.
[0038] Other aspects and features of the present invention
will become apparent to those ordinarily skilled in the art
upon review of the following description of specific
embodiments of the invention in conjunction with the
accompanying figures.
18
CA 02519736 2005-09-13
BRIEF DESCRIPTION OF THE DRAWINGS
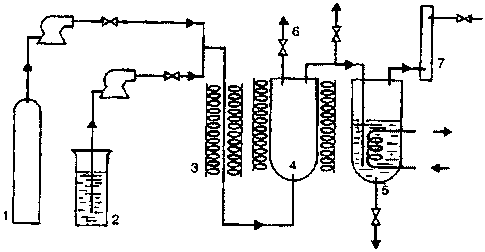
[0039] Figure 1 shows a general flow-diagram charting the
interrelationship of pieces of equipment in one embodiment.
[0040] Figures 2-5 are flow diagrams of improved
embodiments, with Figure 2 showing a most preferred embodiment
when the oil feedstock is already hot and entering the system
directly from SAGD production well, or from a preheater in
which the oil has been pre-heated to roughly 60-90°C prior to
injection into the main reactor. The parameters shown in
Figure 2 are exemplary only, not intended to be limiting.
DETAILED DESCRIPTION
[0041] The principal components of a suitable reactor of
this type are exemplified in the attached diagram (Figure 1).
The numbering in that schematic diagram represents
1. optionally, high-pressure nitrogen or CO - the
latter for enhancement of oil quality (see below);
2. water reservoir;
3. preheater in which the SCW is formed;
4. stirred reactor;
5. pressure letdown vessel;
6. sampling or gas release valve; and
7. activated carbon trap (or other gas collector).
The inlet to the reactor for the hydrocarbon feedstock is not
shown, but is desirably between the preheater 3 and the
reactor 4 or directly into the reactor 4.
19
CA 02519736 2005-09-13
[0042] In such a system, supercritical water, generated by
pumping water from the reservoir 2 through the preheater 3, is
injected into the reactor 4 at rates similar to those at which
it and its entrained hydrocarbon load is withdrawn into the
pressure letdown vessel 5 in order to maintain desired
operating pressures in the reactor. The reaction can be
followed by periodically sampling the exiting stream through a
release valve 6, and uncondensed vapors as well as gaseous
reaction products are captured as required in an appropriately
cooled trap 7. Oils carried into the pressure letdown vessel
are recovered by holding its pressure and/or temperature
regime sufficiently below that of the reactor to allow the
oils to fall out from then-sub-critical water, draining the
oils, and substantially freeing them from uncondensed water by
phase-separation.
[0043] The inclusion of a source of high-pressure carbon
monoxide in the schematic reflects our finding that co-
introduction of CO can in some instances - notably when the
feedstock is predominantly aromatic - improve the quality of
the product oil by increasing the proportion of aliphatics at
the expense of aromatics and (hetero-atom bearing) polar
compounds. Table 1 illustrates this with data for an Alberta
bitumen and also show that pressures above 15-17 MPa, roughly
2200-2400 psi, can prove counterproductive.
CA 02519736 2005-09-13
Table 1
1 2 3 4
Feed 36 11 37 16
Reacted with H20 at 400C/14 .0MPa 30 19 39 12
400C/17 .9MPa 24 24 40 12
400C/24 .5MPa 28 27 43 2
Reacted with H20+CO at 400C/14 .0MPa 74 5 19 2
400C/17 .9MPa 72 5 21 2
400C/24 .5MPa 66 5 27 2
1. Aliphatics; 2. Aromatics; 3. Polar Compounds;
4. Asphaltenes
H20/CO mole ratios in these runs ranged from 1.05 and 1.30
to 2.20
[0044] The reference to "hetero-atoms" means that the
feedstock may contain sulfur, nitrogen and/or metals. By
reducing the proportion of polar compounds from the feedstock,
this process, "by definition", has the advantage of also
removing sulfur, nitrogen and/or metals, when such hetero-
atoms are present in the feedstock.
[0045] We have provisionally ascribed the intervention of
CO to generation of active hydrogen by
CO + H20 ~ C02 + H2
or to an ionic reaction path of the form
H20 ~ H+ + OH-; CO + OH- ~ HC02-; HC02 + H20 ~ H2C02 +
OH ; H2CO2 ~ H2 + COZ
[0046] As indicated above, the operating parameters are
important. In particular, the sweep rate equivalent to in-
21
CA 02519736 2005-09-13
reactor residence time should not exceed 10 minutes, and more
preferably should not exceed about 60 seconds, but should
exceed 25 seconds, and more preferably should be at least 28
seconds. For practical operation, the in-reactor residence
time should more preferably be at least 35 seconds, and even
more preferably at least 45 seconds. In the special case of
operating temperatures below 300°C, e.g. 250-299°C, more
preferably 250-295°C, the in-reactor residence time can be
reduced to less than 25 seconds, i.e. any sweep rate below 10
minutes and preferably below 60 seconds.
[0047] The injection ratios of water to high molecular
weight hydrocarbons feedstock material into the continuous
flow-through reactor, as well as the preferred particle
diameter of such a feedstock material when it is in solid
form, such as crushed coal or crushed oil shale, can be
adjusted according to the desired operating conditions, the
nature of the feedstock material, the design of the flow-
through reactor, and the chemical composition of the reaction
products. While not constraining ourselves by any particular
application and/or theory, the injection volume ratio of water
to feedstock material may be varied in preferred embodiments
from about 10:1 to about 1:10, and our tests reveal a
preferred ratio of about 1:1 to about 1:5. When the feedstock
material is a solid, it may be desirable to add a wetting
agent, such as sodium silicate or other alkaline material, to
aid the extraction of the oil from the oil sand, coal or oil
shale.
22
CA 02519736 2005-09-13
[0048] An important improvement according to the present
invention is the provision of a cooling system/heat exchange
as shown in Figures 2-5. As the hydrocarbon/water product
exits the main reactor, it is desirably cooled prior to
entering the collection vessel from a temperature as low as
250 or 300°C up to about 450°C. In a preferred embodiment,
the outlet tube from the main reactor is coiled and placed in
one or more tanks or tubing sleeves of cooling water. This of
course will heat the cooling water which, as shown in Figure
2, is fed counter current to the product flow. The resultant
warm water is then returned, e.g. pumped, to the steam
generator as shown, or to a water preheating unit prior to
injection into the oil-water reactor, and/or into a steam
generating facility for SAGD injection. This reduces energy
requirements for heating water.
[0049] An advantage to this approach is that the reactor
outflow products can be cooled even to as low as room
temperature, making the product easy to work with and reducing
demands on the type of phase separator (oil, water, gas)
required. In addition, the partially heated water from the
heat exchanger fed to the steam generator or the preheating
unit is "clean".
[0050] Another improvement involves treatment of the
process water separated from the upgraded oil. As shown in
Figure 2, such process water is desirably sent to a filtering
unit for removing contaminants which have been separated from
the crude oil, such contaminants including sulfur- and/or
23
CA 02519736 2005-09-13
nitrogen-containing compounds and metal complexes, among other
contaminants. Thus, rather than discarding this dirty process
water, it is subjected to filtering in the filtering unit,
thus producing "clean" water which is then sent to the water
preheating unit prior to injection into the oil-water reactor,
and/or to the cooling system described above, and/or to a
steam generation facility for SAGD.
[0051] Shown below in Table 2 are results achieved
according to the present invention.
[0052] Table 2 shows upgrading of the raw hydrocarbon in
terms of reduction in the relative resin and asphaltene
component contents and concurrent increases in the relative
contents of saturated and aromatic hydrocarbons. TLC/FID
analyses of eight different treatments (in addition to
analysis of the raw heavy oil), are presented. All samples
were collected after in-reactor residence times of ~30 seconds
(except for one experiment with a ~8 minute residence time).
Operating parameters (i.e., temperature in °C and pressure in
psi) for the main reactor are given for each treatment.
[0053] Most notable is the reduction in asphaltene content,
which in some cases decreases to less than 2%; resin contents
were reduced in some cases to less than 50% of their initial
fraction. These reductions were compensated by increases
mostly in the aromatic hydrocarbon content and to a lesser
extent to a rise in the saturated hydrocarbons. Best results
were achieved at high temperature and pressure combinations,
24
CA 02519736 2005-09-13
but even at a pressure of 1000 psi a substantial reduction in
asphaltene content was measured. Longer in-reactor residence
times and the addition of CO (last two lines of table) to the
reactor did not change significantly the resulting hydrocarbon
composition.
Table 2
Changes in Hydrocarbon Composition
Treatment Saturates Aromatics Resins AsphaltenesComments
(%) (%) (%) (%)
Raw heavy oil 29 46 14 11
1000 psi; 300C 27 51 12 10 Experiment
1
1000 psi; 300C 28 52 11 9 Experiment
2
1000 psi; 300C 26 58 7 9 residence
time -8 min
1000 psi; 375C 15 67 12 6
1000 psi; 450C 18 71 10 1 Experiment
1
1000 psi; 450C 26 65 8 1 Experiment
2
2000 psi; 375C 38 53 7 2
2000 psi; 450C 33 59 6 1
3000 psi; 450C 17 76 6 0
1000 psi; 300C, 25 58 9 8
CO
1000 psi; 450C, 31 56 8 4
CO*
* Case 1000 psi.450°C.NoCO produced considerable amounts of
heavy coke material as well as low viscosity liquid. The
values shown here are for the low viscosity liquid
[0054] Table 3 demonstrates the effect of the present
method on the physical properties of the resulting hydrocarbon
(i.e., density and viscosity), as well as on the contents of
other elements (sulfur, nickel and vanadium). Significant
reductions in both viscosity and density are clear. Moreover,
analyses of sulfur content, as well as nickel and vanadium
CA 02519736 2005-09-13
concentrations, demonstrate that the present method forces
undesirable heteroatoms from the hydrocarbon feedstock.
Table 3
Reduction in Viscosity, Density, Sulfur Content, and
Nickel/Vanadium Concentrations
Treatment Viscosity Density Sulfur Ni V
(23C)
(cSt) (% wt.) (ppm) (ppm)
(API)
Raw heavy oil 9075.55 0.99 12 3.46 53.72 97.18
1000 psi; 300C 675.05 0.91 24 3.44 39.16 84.70
1000 psi; 375C 9.03 0.94 19 2.03 7.37 3.68
2000 psi; 450C 2.90
1000 psi; 300C 1.78
1000 psi; 450C 0.78
[0055] While Table 2 suggests that 1000 psi /300°C and 1000
psi / 375° treatments to provide limited changes in
composition, Table 3 indicates that these treatments had the
greatest effect on density and viscosity of the resulting
hydrocarbon. In repeated experiments, the 1000 psi / 300°C
and 1000 psi /375°C treatments consistently yielded
hydrocarbons of ~~uniformly low viscosity" with little coke
production.
[0056] It should be emphasized that the treatments
presented here -- as well as similar ones -- should and can be
optimized once target output parameters are prescribed.
[0057] Figure 5 describes a non-limiting embodiment of the
present invention. The numbering in that schematic
represents:
26
CA 02519736 2005-09-13
1. water reservoir;
2. water pump;
3. water preheater for SCW formation;
4. flow-through reactor;
5. hydrocarbon feedstock reservoir;
6. hydrocarbon feedstock pump;
7. hydrocarbon feedstock preheater (optional but
preferred) ;
8. cooling system;
9. pressure release valve;
10. collection vessel;
11, activated carbon trap or other gas collector
(optional); and
12. high-pressure carbon monoxide, nitrogen or other
gas source (optional).
[0058] In such a system, SCW generated by pumping water
from the reservoir (1) by a pump (2) to the preheater (3), is
injected into the flow-through reactor (4). At the same time,
oil feedstock material (e. g. heavy or semi-heavy oil, coal
liquids, shale oils, or a slurry of oil sand bitumen, crushed
coal, or crushed oil shale), is pumped from the reservoir (5)
by a pump (6) to the (optional) preheater (7), and injected
into the flow-through reactor (4). The rates at which the oil
feedstock and the water are injected are variable, and
selected to allow in-reactor residence times (in reactor (4))
of a few seconds up to 10 minutes, preferably at least 28
seconds and no more than about 60 seconds. The injected SCW
27
CA 02519736 2005-09-13
together with its entrained hydrocarbon load flows through a
cooling system (8), and through a pressure release valve (9)
into a pressure letdown vessel (10).
[0059] The reaction and quality of the output product can
be followed by periodically sampling the exiting stream in the
collection vessel (10) itself, and uncondensed vapors as well
as gaseous reaction products are captured (if required) in an
appropriately cooled trap (11). Oils carried into the
pressure letdown vessel are recovered by holding its pressure
and/or temperature regime sufficiently below that of the
reactor to allow the oils and any other reaction products to
fall out from the then subcritical water, draining them and
substantially freeing them from condensed water by phase
separation.
[0060] Temperature and pressure gauges are attached to each
of preheaters (3, 7) and to the flow-through reactor (4), to
permit monitoring and control of the process. Each preheater
(3, 7) and the flow-through reactor (4) contain heating
elements to control liquid temperatures.
[0061] A preferred, but non-binding embodiment of the
system is our use of a single (pressure-letdown) collection
vessel, at the outlet of the flow-through reactor cell, in
which product material is condensed, collected and passively
separated. Additional collection vessels can be added in
series to condense and/or capture any fugitive gases and other
light hydrocarbon materials.
28
CA 02519736 2005-09-13
[0062] In some cases, it is beneficial to co-inject carbon
monoxide or other gases into the system. This can be achieved
through direct injection into the flow-through reactor (4),
using a high-pressure source of CO or other gas (12) or
through prior mixing with either the water in reservoir (1)
and/or preheater (3), and/or through prior mixing with either
the hydrocarbon feedstock in reservoir (5) and/or preheater
(7). The inclusion of a source of high-pressure carbon
monoxide reflects our finding that co-introduction of CO can
in some instances - notably when the feedstock is
predominantly aromatic - improve the quality of the product
oil by increasing the proportion of aliphatics at the expense
of aromatics and (hetero-atom bearing) polar compounds. Use
of nitrogen, for example, can assist in maintaining a constant
in-reactor pressure.
[0063] A preferred, but non-binding embodiment of the
system, is inclusion of a cooling system, at the outlet of the
flow-through reactor (4): this system can consist of coiled
tubing emplaced in cooling water tanks, to condense product
material prior to product material collection in a vessel at
(near) ambient (atmospheric) pressure and temperature
conditions. A preferred but non-binding embodiment involves
recycling water through these cooling tanks, with the
partially heated water subsequently being fed into the water
preheater (3), and/or into a steam generation facility for
underground (SAGD) injection, to reduce energy requirements
for heating water (i.e., increase the economic viability).
29
CA 02519736 2005-09-13
[0064] The foregoing description of the specific
embodiments will so fully reveal the general nature of the
invention that others can, by applying current knowledge,
readily modify and/or adapt for various applications such
specific embodiments without undue experimentation and without
departing from the generic concept, and, therefore, such
adaptations and modifications should and are intended to be
comprehended within the meaning and range of equivalents of
the disclosed embodiments. It is to be understood that the
phraseology or terminology employed herein is for the purpose
of description and not of limitation. The means, materials,
and steps for carrying out various disclosed functions may
take a variety of alternative forms without departing from the
invention.
[0065] Thus the expressions "means to..." and "means
for...", or any method step language, as may be found in the
specification above and/or in the claims below, followed by a
functional statement, are intended to define and cover
whatever structural, physical, chemical or electrical element
or structure, or whatever method step, which may now or in the
future exist which carries out the recited function, whether
or not precisely equivalent to the embodiment or embodiments
disclosed in the specification above, i.e., other means or
steps for carrying out the same functions can be used; and it
is intended that such expressions be given their broadest
interpretation.
CA 02519736 2005-09-13
REFERENCES
Berkowitz and Calderon, "On "Partial" Coal Conversion by
Extraction with Supercrtical H20", Fuel Processing
Technology 16:245-256 (1987)
Berkowitz and Calderon, "Extraction of Oil Sand Bitumens with
Supercritical Water", Fuel Processing Technology 25:33-44
(1990)
Ogunsola and Berkowitz, "Extraction of Oil shales with sub-
and near-critical water", Fuel Processing Technology
45:95-107 (1995)
31