Note: Descriptions are shown in the official language in which they were submitted.
CA 02520486 2005-09-23
WO 2004/085711 PCT/NL2003/000234
1
Apparatus for carrying out an electrolytic process on a
halogenide compound.
The present invention relates to an apparatus for carrying
out an electrolytic process on a halogenide compound, in which apparatus
several electrolytic cells are electrically connected in series, which
electrolytic cells each comprise a cell element, provided with underlying
supply pipes for supplying electrolyte and with collecting discharge
pipes disposed near the upper side thereof for discharging electrolyte
and the gases formed during the electrolytic process, a cathode
compartment including a cathode and an anode compartment including an
anode, and a diaphragm or semi-permeable membrane, in which the
electrolytic cells have been pressed together between two end plates with
a certain bias, so that each anode compartment and each cathode
compartment is constructed as one unit together with the supply pipes and
the collecting discharge pipes.
From US patent No 5,064,514 there is known an arrangement
for the preparation of chloric acid from hypochlorous acid, which
arrangement comprises only one mono-cell construction. The arrangement
that is known from said document does not comprise a bipolar electrode or
intermediate plate, therefore. The cooling system used with said
arrangement consists of two cooling plates arranged adjacently to the
anode and cathode backplates, which plates have a hollowed out or grooved
area which is open on the side adjacent to the anode and the cathode, but
which is closed and solid at the surface of the cooling plate on the side
adjacent to the backplates. This grooved area will permit the circulation
of a coolant to control the heat generated during the electrolytic
process. As a result of the construction used therein, the coolant is in
direct electrical contact with the electrodes
From US patent No 5,082,543 there is known an electrolytic
cell of the filter press type for the production of peroxy and
CA 02520486 2005-09-23
WO 2004/085711 PCT/NL2003/000234
2
perhalogenate compounds. The cell that is known. from said document is of
the semi-filter press type, because each cell is separately electrically
connected. Consequently, a bipolar electrode or intermediate plate is not
used. The electrodes that are used, which are completely made of a metal,
are double-walled, between which parallel walls a coolant is pumped. In
practice, full submersion in coolant of this cell is not possible within
reason because of the great number of electrical connections. As a result
of the construction of the double-walled electrodes, the coolant that is
passed therethrough is under an electrode voltage.
From German Offenlegungsschrift No 199 10 639 there is
known a reactor for generating ozone; said document does not provide any
information as regards the electrolytic cell that is used, however.
The apparatus referred to in the introduction is known per
se from NL 8303210, in which chlorine gas intended for chlorinating
water, such as swimming pool water, drinking water or waste water, is
prepared by means of an electrolytic process. Said Dutch laid-open
document shows an electrolytic cell built up of two anode compartments
and two cathode compartments arranged in alternating relationship.
Disposed between a first anode compartment and a first cathode
compartment is a membrane made from a material which is suitable for this
purpose, which membrane is permeable to cations and impermeable to
anions. A similar modular cell unit is formed by the second anode
compartment, the second cathode compartment and the cation-permeable
membrane disposed therebetween. The modular cell units are arranged
adjacently to each other, with the interposition of a liquid-impermeable
gasket or insulator. End plates are arranged at the ends of the structure
of cell units, through which plates tie rods or other suitable fixing
means are'passed, which tie rods also extend through the cell units so as
to keep the entire structure together in this way. Each cell element is
provided with a collecting pipe, also called degasser, in which the gas
formed during the electrolytic process separates from the electrolyte.
CA 02520486 2005-09-23
WO 2004/085711 PCT/NL2003/000234
3
One drawback of the apparatus as discussed above is the
fact that the temperature of the series-connected electrolytic cells can
rise to undesirable levels. For chemical and mechanical reasons, it is
thus desirable in practice to have a possibility of influencing the
temperature. In practice, so-called heat exchangers are used for this
purpose, which heat exchangers are installed outside the cell block,
however, which means that the temperature is influenced externally. Such
external influencing cannot prevent the electrolytic cells in particular
from exhibiting an impermissible thermal deviation in the centre of the
cell package, however. Thus it is desirable to provide an apparatus by
means of which thermal influencing can be effected mainly at the position
where the thermal deviation is greatest.
The object of the present invention is thus to provide an
apparatus for carrying out an electrolytic process on a halogenide
compound, which apparatus provides a possibility of internal thermal
influencing at the position at which the thermal deviation originates,
thus ensuring internal thermal stability.
Another object of the present invention is to provide an
apparatus for carrying out an electrolytic process on a halogenide
compound, which apparatus collects the corrosive fluids and gases that
may have formed due to leakage.
According to the present invention, the invention as
referred to in the introduction is characterized in that the assembly of
end plates and electrolytic cells is present in a container which
contains a liquid, heat-transferring medium, with an electrically non--
conducting cell partition being present between the cathode and the
anode, which cell partition, in addition to supply pipes and collecting
discharge pipes corresponding to the cell element, comprises one or more
through channels for the passage therethrough of the heat-transferring
medium that is present in the container, which channels have been formed
in the cell partition in such a manner that the heat-transferring medium
CA 02520486 2008-07-23
4
that is present in the channels is not under an electric voltage, and that no
liquid
contact takes place between the electrolyte that is present in the
electrolytic
cells and the heat-transferring medium that is present in the container,
outside
the electrolytic cells.
Preferably, according to the present invention, the complete cell
package, including the two end plates, is thus placed in a heat- transferring
medium, for example water, in which the heat-transferring medium in fact
performs two functions, viz. the function of a cooling medium, both internally
in
the through channels that are present in the cell partition and externally in
the
container outside the electrolytic cells, and the function of a medium that
catches any leakage. Because a significant part of the electric energy that is
supplied for the electrolytic process is collected in the heat-transferring
medium
as a result of the cooling action effected in the present invention, energy
recovery becomes feasible, as a result of which an energy saving is achieved.
In principle, the through channels that are used in the present
invention may have any conceivable sectional shape, for example circular,
rectangular, trapezoidal and the like. The present invention is in particular
used
in environments where gaseous halogenides are wanted, for example for use as
a disinfectant for swimming pools or drinking water.
In one preferred embodiment, each combination of cathode and
anode is separated by the present cell partition, so that a cooling function
will at
all times be performed at the position where heat is being generated.
Preferably,
a bipolar electrode is used.
In a special embodiment it is desirable for the heat-transferring
medium that is present in the container to be passed through a through
channels in a forced manner, which can be effected, for example, by placing
one
or more pumps.
The heat-transferring medium that is present in the
CA 02520486 2005-09-23
WO 2004/085711 PCT/NL2003/000234
container can also be utilised for regulating the temperature in the
electrolytic cell package and thus the temperature of the electrolytic
process, for example by varying the temperature of the medium and/or the
rate of circulation, for example by means of forced circulation, using
5 one or more pumps. Because the complete electrolysis unit is submersed in
the heat-transferring medium, the risk of gases or electrolytes leaking
out is also prevented.
A special embodiment of the present invention is
characterized in that a reversing element is disposed adjacently to the
electrolytic cell package, which reversing element is provided with
underlying supply pipes for supplying electrolyte to the adjacent
electrolytic cell package, and furthermore with collecting discharge
pipes disposed near the upper side thereof for discharging electrolyte
and the gases formed during the electrolytic process in the adjacent
electrolytic cell package, for effecting the return of electrolyte from
the collecting discharge pipes to the supply pipes, which reversing
element is provided with one or more through channels for the passage
therethrough of the heat-transferring medium, which channels are so
configured that no liquid contact takes place between the electrolyte
that is present in the electrolytic cells and the heat transferring
medium that is present in the container, outside the electrolytic cells.
In a special embodiment, the electrically non-conducting
cell partition is provided with means for electrically interconnecting
the various adjacent electrodes without any exchange of electrolyte
between the two electrolytic cells via said connection or electrolytic
corrosion between the various electrode metals taking place.
In addition to that it is possible to lead the depleted
electrolytes to be discharged after the electrolytic process through the
heat transferring medium present in the container via a pipe, so that the
thermal energy contained in the electrolytes is transferred to the heat-
transferring medium.
CA 02520486 2005-09-23
WO 2004/085711 PCT/NL2003/000234
6
The present invention will be explained hereinafter with
reference to a number of drawings, which drawings must not be construed
as constituting a limitation of the invention, however.
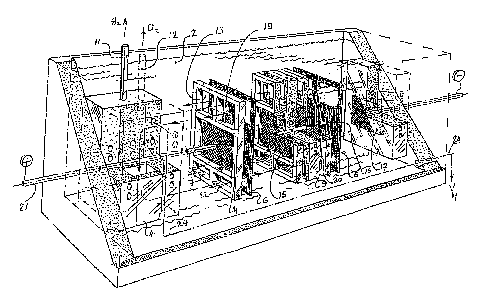
Figure 1 is a perspective view of the present apparatus.
Figure 2 is a schematic cross sectional view of the
apparatus of Figure 1.
Figure 3 is a schematic representation of the present cell
partition.
Figure 4 is a schematic representation of the cell
partition of Figure 3.
According to Figure 1, two electrolytic cells of the filter
press type, which are electrically connected in series, are present in a
container 1 which contains a heat-transferring medium 2, for example
water; for the sake of simplicity, the elements for supplying
electrolyte, for example HC1, are not shown in the figure. It should be
understood that the present invention is by no means restricted to such a
number. The anode 14 is separated from the cathode 15 by a semi-permeable
membrane 6. Cathode 15 is separated from anode 16 by means of a cell
partition 9, and anode 16 is in turn separated from cathode 17 by the
semi-permeable membrane 6. The electrolyte that passes through the
electrolytic cells via collecting discharge pipes 13, 19 and supply pipes
7 and 22 for supplying electrolyte is subjected to an electrolytic
process at the anode, during which process chlorine gas is formed, for
example, which chlorine gas ends up in collecting discharge pipes 19 via
the anode compartment and subsequently exits the apparatus via gas
discharge pipe 12. Hydrogen gas is formed at the cathode 15, 17 as a
result of the electrolytic process, which hydrogen gas rises from the
cathode compartment and collects in the collecting discharge pipe 13, in
which collecting discharge pipes 13 a separation between electrolyte and
hydrogen gas takes place. From the collecting discharge pipes 13, the
electrolyte, which is still hot, can subsequently be discharged from the
CA 02520486 2005-09-23
WO 2004/085711 PCT/NL2003/000234
7
apparatus via the pipe 24, which pipe 24 is led through the medium to so
as to effect a transfer of energy. Finally, the hydrogen gas that has
formed in the electrolysis apparatus is discharged via the pipe 11. In
order to effect a proper flow of electrolyte in the present apparatus, it
is preferred to use a reversing element 4 which is provided with
underlying supply pipes for supplying electrolyte to the adjacent
electrolytic cell package, and which is further provided with collecting
discharge pipes disposed near the upper side thereof for discharging
electrolyte and the gases formed during the electrolytic process in the
adjacent electrolysis package, for effecting the return of electrolyte
from the collecting discharge pipes to the supply pipes. In order to be
able to control the temperature of the electrolyte that is present in the
reversing element, the reversing element is provided with one or more
through channels (not shown) for the passage therethrough of the heat
transferring medium 2, which channels are so configured that no liquid
contact takes place between the electrolyte that is present in the
electrolytic cells and the heat-transferring medium that is present in
the container, outside the electrolytic cells.
Figure 2 is a schematic side elevation of the electrolysis
apparatus of Figure 1. In Figure 2 the flow of electrolyte within the
electrolytic cell package is indicated by means of arrows, from which it
is apparent that the reversing element 4 arranges for the liquid from the
collecting discharge pipes 13, 19 to be returned to the supply pipes 7
and 22 for supplying electrolyte to the electrolytic cells in question.
Figure 3 is a cut-away view of an embodiment of the present
cell partition 9, in which the through channels 20 are schematically
indicated. The through channels 20 arrange for a temperature regulation
to be carried out at the position at which the development of heat mainly
takes place, viz. on the surface of the electrodes, in particular by
passing heat-transferring medium through the channels 20. The cell
parti ti on 9 that is shown in Figure 3 may be formed of two symmetrical
CA 02520486 2005-09-23
WO 2004/085711 PCT/NL2003/000234
8
halves, in one of which (or in each of which) through channels 20 have
been milled out, after which the two halves have been assembled to form
one unit comprising the through channels 20. By thus disposing the
channels slightly at an angle, said channels will readily fill with the
heat-transferring medium upon submersion of the whole of end plates and
the electrolytic cells in the heat-transferring medium.
Figure 4 shows the cell partition 9, with an anode 15
disposed on one side and a cathode 16 disposed on the other side thereof.
The anode 15 and the cathode 16 are electrically interconnected via
connection 21, 23 consisting of two different metals, which connection
21, 23 is so designed that no exchange of electrolyte between the two
electrolytic cells can take place via said connection 21, 23. It will be
apparent from Figure 4 that any cooling that may be desired will take
place at the position where the development of heat mainly takes place,
viz. close to anode 15 and cathode 16, in particular by passing the heat
transferring medium 2 through the cell partition 9 via one or more
through channels 20.