Note: Descriptions are shown in the official language in which they were submitted.
CA 02520736 2005-09-28
WO 2004/088768 PCT/EP2004/003424
METHOD FOR OPERATING A MEMBRANE ELECTROCHEMICAL GENERATOR
BACKGRQUND ~F THE INVENTION
Fuel cells are known as devices for the direct conversion of the chemical
energy of
a fuel (such as pure hydrogen or hydrogen mixtures obtained by hydrocarbon
steam reforming) to electric energy without involving a combustion step as
instead
occurs in the conventional generators based on the combustion-compressed
vapour production-turbine expansion cycle or on the cycle comprising gas
turbines. The absence of such combustion step sets the fuel cells free from
the
constraints of the known Carrot's principle and imparts them an intrinsically
higher
energy efficiency than the conventional generators.
Fuel cells can be divided into families depending on the operating
temperature,
Which is ~0-g~~C f~r the pr~t~n-e~~chanc~..e membrane apes (c~mm~nty
identified
as PEIFC), 'i ~0-~~0~C for the $ypes rnal~ing use off' ph~sph~ric acid as the
ele~rof~~e (P~aFC), ~~~-d'~0~C for the ~pe~ ire c;~,~icf~ the eUec'dr~I~~e
~on~ist~ ~fi
mixture of molten carbonates (MCFC) and X00-1000°C for those in which
the
electrolyte is a solid state ion-conducing oxide (SOFC).
here~~ eacfn 'e'~pe of fuel cells I-~~~ its rnerit~ in torm~ of energc~
e~'wcienc~,~,
promptness in following the power demand transients, sort-up rapidity,
maintenance in conditions of zero power generation, it's a matter of fact that
the
presence of liquid aggressive electrolytes (phosphoric acid, molten
carbonates) or
the high temperatures (600-1000°C) impose the use of sophisticated
construction
materials and particular measures in the design and operation, particularly as
concerns the remarleable thermomechanical solicitations. For all these
reasons,
PAFC, MCFC and SPFC-type fuel cells are normally considered to be practically
destined to the construction of big units with power exceeding 1 megawatt,
such
as for example distributed electric power plants, wherein the operation is
subjected
to transients of moderate entity and wherein some form of supervision by
specialised personnel is available. Conversely, membrane fuel cells are
substantially solid-state devices, hence free of the problems associated to
CA 02520736 2005-09-28
WO 2004/088768 PCT/EP2004/003424
2
corrosivity, as is the case with phosphoric acid and molten carbonates, to
safety,
since in the case of liquid electrolytes the separation of the fuel from the
air
reactant is ensured just by the capillary forces holding the electrolyte
inside the
pores of an inert matrix, and finally to the temperature as seen for the
conductive
solid oxides. Furthermore membrane fuel cells, being devices characterised by
an
extremely reduced electrode and membrane mass, have a particular capabilifiy
of
following steep power demands and of reaching the nominal power generation in
very low times even starting from a zero generation condition. This set of
characteristics makes fuel cells very attractive for their use in the
automotive and
also in the stationary field for small power applications, as is the case,
very
interesting from a commercial standpoint, of systems directed to be installed
in
private houses, hotels, hospitals.
Next to the positive characteristics illustrated, membrane fuel cells present
hov~ever als~ some incon~~enien~s: among these, particularly relevant us the
need
of having to maintain the proton-exchange membranes, ~~sh~se c~nducti~i'cy is
a
f~arrcti~n oar the ~,~af~:er ~ntenr~, in a fully hydrated state.
IVlembranes are usually 20 to 100 micron thick films consisting of a polymer
on
whose backbone groups with an acidic function, usually sulphonic groups, are
ins~r~~d. The p~lyrn~r must b~ resistant to the highly aggressive acti~n or
pero~aidic and radicalic comp~unds generated as intermediate products of
reaction
of air. For this reason, the polymers currently used for the production of
membranes presently available on the market as commercial or experimental
products (best known suppliers: ~uPont/tJSA, Asahi GIassIJapan, Asahi
ICasei/Japan, Gore/Japan and Solvay Solexis/ltaly) are invariably
perfluorinated
polymers characterised by high chemical inertia, even though a remarkable
research activity is directed to the development of non-fluorinated polymers,
for
instance having an aromatic structure, whose long term chemical inertia and
whose mass production scale economics have still to be demonstrated.
The acidic functions, and in particular the sulphonic groups, must be
dissociated:
the resulting free electric charge determines in fact a particular space
orientation
of the polymer chains with formation of reticular channels along which proton
CA 02520736 2005-09-28
WO 2004/088768 PCT/EP2004/003424
3
migration takes place. The dissociation, which is thus a required passage for
channel formation, occurs only in the presence of certain water amounts,
resulting
in the need of keeping the membrane hydrated. The hydration condition is the
outcome of a delicate equilibrium between water of reaction and water
extracted
from the gases flowing through the fuel cells during operation, in particular
from
the air and fuel respectively fed on the cathode side and on the anode side.
The
water extraction may become dangerously high for the membrane hydration when
the fuel cell is operated at near ambient pressure, hence with particularly
elevated
gas volumetric flow-rates. This situation is especially critical on the air
side, since
in order to maintain a sufficient oxygen partial pressure also in proximity of
the cell
outlet it is mandatory to operate with flow-rates substantially higher than
the
theoretical stoichiometric value, indicatively two-fold. On the hydrogen side
the
problem is less serious, since when operating with pure hydrogen the flow-
rates
must be practically c~rrespondent to the theoretical values, aside from an
excess
oi~ about ~ ~d~ to guarantee the ~~~ithdra~~al ~f inerEs ~~~hich rnay
otherwise
~cc~ar~ulat~: it is cl~~r thet such m~dest p~ara~~ fl~~~,~-r~t~ ~~~r~cts
ne~iigib6e
amounts of water. The framework doesn't change significantly even when the
fuel
cell anode side is directly supplied with gas coming from the steam refom~ing
unit
~,~hich rney c~ntain, depending ~n the ~p~rati~~ c~ns~iti~ns, ~I~ t~ ~~~4~
h~pdrogen
and which has a volumetric f'iov~-rate indicatively equivalent up to 40-~~~~~
of the
volumetric flow-rate of air. The capability of withdrawing water is further
reduced
due to the fact that the gas coming from the steam reforming unit is normally
saturated with water vapour.
In the case of air, on the contrary, the water withdrawal capacity is high due
both
to the stoichiometric excess employed, resulting in a remarkable volumetric
flow-
rate particularly for the case of near-ambient pressure operation, and to the
reduced moisture content present in the ambient air intake of fans or
compressors
used for the cell feeding. To correct this unfavourable situation, the prior
art
discloses various devices directed to saturate the air feed with water vapour
at
temperatures close to the fuel cell working temperature. The basic idea is in
fact to
eliminate the water-extracting capacity of air along the whole fuel cell
crossing,
CA 02520736 2005-09-28
WO 2004/088768 PCT/EP2004/003424
during which the air temperature rises anyway to values close to those of the
cell.
The saturation of air at temperatures close to cell temperature can be
obviously
achieved allowing the air to bubble in adequate external saturators consisting
of
vessels containing demineralised water kept at the desired temperature, for
instance by thermal exchange with the water circulating inside the fuel cell:
this
procedure has the drawback of operating at an average temperature sensibly
lower than that of the cell, as a consequence of the temperature differences
required to reduce the thermal exchange surfaces. To carry out the saturation
at
temperatures close to that of the fuel cell it would then be necessary to
resort to
additional thermal energy sources with a consequent decrease of the energy
efficiency of the overall system. The device moreover requires level control
instrumentation, pumps for feeding water, purge flow-rate control to prevent
the
build up of impurities inevitably present, albeit in traces, in the water to
be
evaporated, coming from the condensation of vapour contained in the exhaust
gases of the system bef~re their release t~ the 2~tm~sphere, all ~f this
inv~Iving
n~n negligibly additi~nal c~sts. ~ similar pr~~dur~ is clairn~d in ~1~
~,~~~,~~~,
wherein the air feed is added with atomised liquid water and the mixture so
obtained is made progress across a heat exchanger whereto the required thermal
~n~rg~y f~r ~~ap~rating the ~,~,rater is pr~vid~d. This al~~i~ pr~s~nts the
s~~m~
inconveniences discussed for the previous case.
In U~ 6,066,408 a humid~cation method is disclosed comprising humidification
cells intercalated to the fuel cells of a stack: the humidification cells
thereby work
practically as cooling cell wherein the cooling is ensured by the evaporation
of the
water required to saturate the incoming air-flow. The humidification
temperature
results higher than that relative to the above discussed external saturators,
although somehow lower than the fuel cell temperature since a certain thermal
difference is in any case required to maintain an adequate heat exchange rate.
With this type of device it is no more possible to employ additional heat
sources to
increase the humid~cation temperature until making it practically coincide
with the
cell temperature. The efficiency of the device is associated to the thermal
level
established in the stack, decreasing as the temperature decreases. The device
is
CA 02520736 2005-09-28
WO 2004/088768 PCT/EP2004/003424
thus practically inactive during the start-up of the stack and during the
periods of
low power generation when the temperature is significantly lower than that of
nominal operation.
In US 2001/0015501 the use of a unit generally defined as enthalpic unit is
disclosed. This unit consists of a vessel subdivided into two compartments by
a
selective water-permeable membrane: the two compartments are supplied
respectively with air at ambient temperature to be delivered to the stack and
with
water vapour-saturated hot exhaust air from the stack outlet. A heat and water
exchange from the exhaust air to the air to be delivered to the stack, which
is
thereby heated and humidified, takes place through the membrane: also in this
case, however, the final temperature of fibs air to be delivered to the stack
is
certainly lower than the fuel cell working temperature. This type of air-feed
conditioning is acceptable in the case of pressurised operation, where the air
volumetric fl~c~-rate is substantially reduced and may have a temperature
ab~ve
ambient under the efl'ecr~ ~f cornpressi~n, while being arguably and
relatively less
reliable i°or near-ambient pre~~~are ~perati~n. A device functi~ning in
a similar ~~ay
is disclosed in ~E 199 15 549 wherein the water and heat transfer does not
take
place through a selective membrane, but rather employing a rotating drum
subdivided int~ sect~rs ~m~hose internal e,~~alls are pr~via3ed ~~,~ith a ~Irn
~f
hygr~scopic material, for instance a lithium salt. The rotation of the drum
puts each
sector subsequently in communication first with the exhaust air that transfers
its
water content to the hygroscopic material and heat to the support structur~,
and
then with the low-humidity air feed which heats up and extracts water from the
hygroscopic material. This device suffers as well of the limitations mentioned
for
US 2001/0015501.
In US 5,441,521 it is proposed to ensure a certain humidification and
temperature
level through the recycle of the exhaust air to the air-feed fan or
compressor: in
this case, imagining that the exhaust air is saturated with water vapour, the
humidity resulting from the overall air stream is a function of the ratio
between the
recycle and the environment air intake ("fresh" air) flow-rates. Since this
ratio
cannot be too large, in order to contain the fan or compressor size and the
CA 02520736 2005-09-28
WO 2004/088768 PCT/EP2004/003424
relevant energy consumption within reasonable limits, the overall air humidity
content results to be not completely satisfactory, exactly as previously seen
for the
other kinds of devices. Furthermore the recycle of the oxygen-depleted exhaust
air
implies the oxygen average partial pressure inside the fuel cells being lower
than
the one characteristic of the recycle free operation. This may entail some
performance lessening.
To obviate to the above mentioned drawbacks and to meet the objective of
ensuring a safe and complete membrane hydration, US 6,406,807 discloses a
direct water injection inside the fuel cells: the evaporation effectively
withdraws the
heat generated during operation and at the same time generates the vapour
partial
pressure required to maintain a correct membrane hydration. This method still
presents a remarkable critical point as that the water amounts have to be
careful
calibrated with respect to the power generation to prevent the two opposite
risks of
h~sdration Ions (injection of insufficient amounts of ~~~ater) and of flooding
of the
porous eltr~des (injection of excessive amounts of ~,~ater): all this requires
injecti~n p~arnps, ~n,~at~r distributors inside the fuel cells and relevant
c~ntr~Is, ~~hich
besides implying additional costs introduce problems of functioning
reliability as
well.
~a further gay t~ fag the pr~blem of rnembrane dehydrati~n is discussed in US
200~0088~~4: in this case, besides the humidi~cation carried ~ut ~,~ith one ~f
the
above described processes, the electrode in contact with the membrane is
provided with reduced porosity in the air inlet region, which experience has
shown
being the most exposed to the risks of excessive water evaporation. In this
way,
water diffusion in the vapour or even in the liquid phase results the more
hindered
the lower is the residual porosity and consequently the water-to-air transfer
also
results slowed down, with a better preservation of membrane hydration. This
procedure entails two serious inconveniences, one associated to the
simultaneous
restraint of the oxygen diffusion velocity accompanied by a performance loss,
the
other associated to the more complex electrode structure hardly in accordance
with the low cost mass production requirements.
OBJECTS OF THE INVENTION
CA 02520736 2005-09-28
WO 2004/088768 PCT/EP2004/003424
It is an object of the present invention to overcome the limitations relative
to the
prior art.
In a first aspect of the invention the stable operation of membrane fuel
cells, single
or arranged in stacks, is obtained by cooling the non-humidified air feed to
an
adequate temperature.
In a second aspect of the invention the cooling of the non-humidified air feed
is
obtained by means of a suitable unit consisting of a fan or compressor
followed by
a heat exchanger wherein heat is extracted making use of ambient air as the
coolant, followed in its tum by an expander wherein the air pressure is
reduced
down to the level required for achieving the desired thermal level.
In a third aspect of the invention the expander is connected to the fan or
compressor so as to achieve a partial recovery of energy.
DESCRIPTION OF THE INVENTION
'These aspects, together with other features ~f the device of the invention
and of
the functi~ning there~f are discussed in detail in the f~11~~~ing secti~n ~f
toy ~,~,~ith
the relc~v~nf figures, listed hereafter:
- Figure ~ : cell voltage (indicating the energy conversion efficiency) and
power
as functions of the current density for a fuel cell fed with non pre-
humidified
co~led air acc~rding to the in~enti~n.
- Figure ~: comparison ~f the data of figure ~ pith those relatiese to a fuel
cell fed
with pre-humidified warm air according to the indications of the prior art.
- Figure 3: maximum allowable exhaust air outlet temperatures as a function of
the working pressure for a fuel cell fed with non pre-humidified cooled air
according to the invention.
- Figure 4: stoichiometric factor as a function of the maximum exhaust air
outlet
temperature and of the working pressure.
- Figure 5: executive layout of the device of the invention.
- Figure 6: compression work multiplier as a function of ambient air
temperature
and of the working pressure.
During the operation of a fuel cell, single or arranged as a multiplicity of
cells in a
stack, the region of membrane most exposed to the risk of dehydration is the
one
CA 02520736 2005-09-28
WO 2004/088768 PCT/EP2004/003424
immediately adjacent to the inlet of gases, and in particular to the air inlet
to which
future reference will be made hereafter. This region, in fact, is subject to a
quick
evaporation of the product water generated by the reaction between the air
itself,
the proton migrating across the membrane and the electrons flowing through the
external circuit. If the rate of evaporation is higher than that of water
formation the
membrane undergoes a progressive dehydration hindering the proton migration
with a consequent conductivity and performance loss. It must be noted that if
the
dehydration or scarce hydration situation persists in time, the polymer
structure is
subject to a slow process of structural reorganisation making the conductivity
loss
irreversible. Moreover, in conditions of deep dehydration, the mechanical
characteristics of the membrane and in particular the plastic reserve thereof
steeply decay, with an intolerable increase of the damage frequency in form of
porosities and micro-fractures especially localised in the regions of higher
mechanical strain, such as for instance the edges and the opti~nal
irregularities ~f
the electrode surface.
T~ pr~~~nt these pr~kalems, the pri~r ~~ is substantially directed either t~
ensure
the air feed humidification, which however, being not complete for the above
mentioned reasons, softens the risks of dehydration without eliminating the
same,
~r t~ e~r~p6oy electrodes pr~via~ea~ e~~,~ith red~ace~9 p~rosiire the gas
inlet regi~n,
capable of hindering the diffusion of the product ~~~ater thus bettcer
preserr~sing fhe
membrane hydration, but at the cost of a performance loss and of non
negligible
complications in the production phase.
The inventors, on the basis of a wide testing, have surprisingly found that it
is
possible to make a fuel cell operate in a stable fashion in a wide range of
current
density as required by the practical applications, with voltages
representative of
good energy conversion efficiencies, with stoichiometric factors equalling
those of
common use in the prior art (indicatively comprised between 1.2 and 3) also at
near-ambient pressure and with no pre-humidification of the air feed, if the
air feed
is pre-cooled below 35°C and preferably below 30°C, with an
optimum value
around 252°C. By stoichiometric factor, as known to the experts of the
field, it is
intended the ratio between motes of fed reactant and moles of reactant
required by
CA 02520736 2005-09-28
WO 2004/088768 PCT/EP2004/003424
9
the reaction stoichiometry.
The behaviour of a stack fed with non pre-humidified air cooled down to
26°C
according to the invention and formed by ten fuel cells with 225 cm2 active
area,
equipped with commercial electrodes provided with 30% platinum supported on
Vulcan XC-72 supplied by E-TEK DivisionlDe Nora North America and with Nafion
115 DuPont proton-exchange membranes in a former version, and 40 micron thick
Gore membranes in a latter version (totally equivalent behaviour) with cooling
cells
crossed by demineralised water intercalated thereto, is shown in figure 1;
curve
(100) indicates the cell voltage trend (left hand ordinate axis) and curve
(200)
indicates the concurrent power trend (right-hand axis). The data were obtained
operating at low pressure (1.5 bar) and with st~ichiometric factor limited to
2;
nevertheless it may be observed how a voltage of 0.7 V (representative of an
energy conversion efficiency of about 50% considered as a lower acceptable
limit)
can be obtained at a current densifi~ of 4 IIm~ corresponding to a specific
p~~ver
~f 2.3 I~W/m~. This value ~f specific p~~,~~er alloe~~~s c~r~taining the
dirnensi~ns ~f
ccmrnercial sfacl~s ~~~ithin ec~nomilly interesting limits.
Figure 2 is a comparison of the perfomnances of the stack in figure 1 with
those or
a stack also working at 1.5 bar absolute but with the known parameters of the
curren"~ techn~I~gy, in pardicular e,~ith air ~°eed pre-h~ar~idi~ed at
3~°C and
st~ichiometric factor of 2.5: the voltage and p~~~Per curves are respectively
indicated ' h (101) and (201). Surprisingly, the boo peeformances show only
marginal differences.
Without wishing the present invention to be bound to any particular theory, it
may
be assumed fihat if the air feed temperature is decreased to the specified
values,
the reduced water vapour tensions in the air inlet region substantially reduce
the
local evaporation rate and the amount of water that can be evaporated. Of
course
the air, moving across the fuel cells, progressively increases its temperature
with a
simultaneous increase of the water vapour tensions and with a progressive
evaporation: however such evaporation results distributed along the cell
active
area. In other words, the merit of the present invention might be distributing
the
water evaporation avoiding the hazardous concentration thereof in limited
areas as
CA 02520736 2005-09-28
WO 2004/088768 PCT/EP2004/003424
-10
occurs in the fuel cells operated according to the indications of the prior
art.
Figure 3 shows the result of a series of tests carried out adjusting the
cooling
water temperature andlor flow-rate so as to vary the outlet exhaust air
temperature
up to the limit where a performance loss began to be noticed, likely
associated to
the onset of membrane dehydration. The tests were carried out at 25°C
with a
stoichiometric factor of 2. From the diagram it can be observed that the
maximum
temperature of the outlet exhaust air (ordinate value) compatible with a
stable
operation in time using the indicated stoichiometric factor is a function of
the
working pressure (abscissa value): if the limiting factor is effectively the
onset of
membrane dehydration the result of figure 3 is then unsurprising since lower
air
volumetric flow-rates correspond to higher pressures and hence, all other
conditions being equal, the capacity of withdrawing vapour decreases, which
permits increasing the outlet temperature. Wishing to limit the fuel cell
working
pressure t~ 1-1.5 bar absolute, it can be concluded that the temperature
and/or
flo~~~-rate ~f the ~~ling mater (or in the more general case ~f the c~~lant
fluid)
r~iust be regulated s~ as t~ guarantee a ma~simum exhaust air ~utlet
temperat~ar
of 60-~'0°C. The ambient or near ambient pressure operation is
particularly
interesting under the system reliability standpoint, considering the fact that
the
empl~yed machines, particularly the air feed fan, and the seals, particularly
the
stacl~ perimetrical gasl~ets vahich have a very high overall linear
development,
result not too critical.
In figure 4 the variation of the stoichiometric factor (indicated as f,;~, on
the ordinate
axis) is reported as a function of exhaust air outlet maximum temperature
(value
on the abscissa) and of the working pressure. In particular, curve (301) is
relative
to an absolute pressure of 1.1 bar, (302) to 1.2 bar, (303) to 1.3 bar, (304)
to 1.4
bar, (305) to 1.5 bar, (306) to 2 bar. It can be noticed that for a given
exhaust air
maximum outlet temperature the stoichiometric factor increases as the working
pressure increases. In fact, as already said, when the working pressure
increases
the volumetric flow rate, and thus the water-withdrawing capacity of air
flowing
inside the fuel cells is decreased: it follows that increasing the
stoichiometric
factor, and consequently the air flow-rate, is allowed provided the critical
condition
CA 02520736 2005-09-28
WO 2004/088768 PCT/EP2004/003424
1't
corresponding to a water withdrawal inducing membrane dehydration is not
exceeded. For example with the maximum air outlet temperature fiixed at
65°C the
allowed stoichiometric factor results 1.6 at 1.1 bar absolute working pressure
and
2.3 at 1.5 bar absolute. It is convenient to recall that to a higher
stoichiometric
factor corresponds a lower oxygen depletion of air crossing the fuel cells
with a
consequent performance improvement.
As regards the cooling water, it is necessary to maintain the flow-rate
thereof
within reasonable limits in order to contain the energy consumption for the
circulation within acceptable values: this condition practically results in a
temperature variation between fuel cell inlet and outlet of about 10-
15°C (with inlet
temperatures of about 45-60°C), corresponding t~ flow-rates around 25-
40
litrelhourlm2. In any case the cooling water outlet temperature results
substantially
coincident with that of exhaust air.
The facfi that stable operati~n is possible with a non-humid~ied air feed,
pr~vided
the same is pre-co~led, is ~r~ainly surprising and p~sitive under the
operati~n
simplici~ standp~int, h~~,~~~e~er ire is clear that the pr~lalern ~~' h~~,~,~
t~ effect such pre-
cooling has to be posed, considering that in many applications typical of
stacks
there is no coolant available at suitable temperature. In fact, in the vast
majority of
cases the t~asE~ of c~~ling systerr~rs c~rr~g~rised ~f fuel III stags is
a~eputed t~ the
ambient air, which in summertime and particularly in some ge~graphic areas
rnay
reach values of 40-45°C. Assuming that a temperature difference of at
least 10°C
is required between cooling air and fluid to be cooled to limit the sire of
the
thermal exchange equipment, one concludes that the minimum temperature that
the fluid to be cooled may reach is around 50-55°C. This limit is
acceptable for the
cooling water which, as seen above, may have a temperature of 45-60°C
at the
fuel cell inlet. Nothing can be done of course as regards the air feed which
must
be pre-cooled to at least 35°C, and preferably at least 30°C,
with an optimum
value of about 25°C.
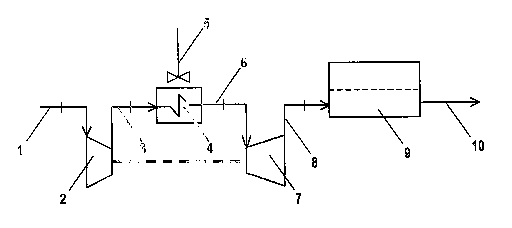
Figure 5 represents a scheme of realisation of a conditioning device capable
of
taking the air feed to the above temperature levels: (1) indicates the air
feed
aspiration tubing, (2) the fan whose purpose is to ensure the required flow-
rate
CA 02520736 2005-09-28
WO 2004/088768 PCT/EP2004/003424
with a modest overpressure, indicatively from 1.1 to 1.5 bar absolute (the
described scheme is applicable, although with lesser advantages, to systems
operating under pressures of 2-5 bar absolute, in which case (2) is a suitable
compressor), (3) the delivery tubing of air exiting the fan (or compressor),
(4) a
heat exchanger directed to cool down the air warming up in the fan (or
compressor) (2) using ambient air (5), (6) is the air feed delivery tubing to
the
expander (7), for instance a rotating expander, in which the air pressure is
partially
reduced with production of mechanical work, that is transmitted to the fan
(2), and
with temperature decrease, (8) the delivery tubing to the fuel cell stack (9),
and
(10) the exhaust air outlet tubing.
The temperature level that can be reached in the expander is a function of the
pressure in (8) to pressure in (6) ratio: for a given pressure in (8) (stack
operating
pressure) the temperature level attainable by expansion is only a function of
pressure in (6) (practically the fan or c~mpressor (~) ~utlet pressure) and of
the
reen~perature in (0), in its turn a func$i~n s~f the arn6ient air
terrnperature, ~~~hich in
~h~ m~s'~ unf~~~urable c~nditi~ns (design c~ndi$i~ns) is ~ssaarned t~ be a~-
~5°C. In
principle, very low temperatures can be achieved, even below 0°C, but
this would
require excessive pressures in (6) with unacceptable energy consumption.
~~~~re~~~r, ~~, pr~~iously discussed, inter~stine~ r~~ul~;s ~r~ alre~d~
~bt~in~a~ ~n,~ith
ternperatures of about ~5°C. using this ~salue as the ~ptirs~al c~oling
level, the
following data are obtained in the case of a 9 klnl stack consisting of 100
fuel cells,
each of 225 cm2 active area, and 100 cooling cells intercalated thereto fed
with
demineralised water:
- pressure at the fan (2) aspiration: ambient pressure
- temperature at the fan (2) aspiration: 45°C max (worst case)
- pressure at the fan (2) delivery: 1.3 bar absolute
- temperature at the fan (2) delivery: 78°C
- temperature downstream the heat exchanger (4): 51 °C
- pressure after expansion, coincident with stack (9) operating pressure: 1.05
bar
absolute
- air temperature after expansion: 25°C
CA 02520736 2005-09-28
WO 2004/088768 PCT/EP2004/003424
- power absorbed by the fan (2):1.4 kW
- power generated by the expander (7) and transmitted to the fan (2): 0.7 kW
- net power required by the device: 0.7 kW (8% of stack power)
It may be noticed that the heat exchanger (4) represents no critical component
for
the system design, as it simply has to cool down the air-feed flow from a
temperature at the fan outlet which in the worst of cases is lower than
80°C, to a
temperature of delivery to the expander around 50°C (typically
50~3°C): this may
be easily accomplished making use of a second ambient air flow as the cooling
fluid, considering also the modest thermal content of the non-humidified air
feed.
Figure 6 indicates how, wishing to maintain the temperature of the air-feed to
the
stack at 25°C, the compression work is characterised by a multiplying
factor
(indicated as c~ on the ordinate axis) which is a function of ambient
temperature
(on the abscissa) and of the stack working pressure: curve (401 ) is relative
to an
absolute v~orlaing pressure ~f 1.05 bar, curve (40~) t~ 1.1 bar, curve (408)
t~ 1.5
bar, curve (404) to ~.~ bar. 'The data in the fegure sh~~~ that the device is
p~r~icuhrl~ c~nv~ni~nt ~t I~~~,~ v~alue~ ~f st~cC~ G~,~~rC~ing prc~ss~ar~.
Although the present invention was described making reference to a
particularly
preferred embodiment, the experts of the field will reckon that several
changes
end rr~-ce~di~~:i~ns r~~ay b~ m~d~ ~,~~ri~:hout dep~~ing~ fr~rn the spirit
therf, s~acl~
changes and modifications being intended as enc~rnpassed in the domain wherein
protection is claimed, as comprised in the scope of the invention.