Note: Descriptions are shown in the official language in which they were submitted.
CA 02520798 2008-01-22
PROCESS FOR THE ELECTROLYSIS OF
ALUMINIUMSULFIDE
The invention relates to a process for the electrolysis of A12S3, using a bath
of
molten salt, preferably a bath of molten chloride salt, in which A12S3 is
dissolved.
The most commonly used method for the production of aluminitun from
aluminium ore is the Hall-H6roult process.
Primary aluminum production via electrolysis in the Hall-Heroult process
consumes about 13-15 MWh of electrical energy per tonne of aluininum. The
anodes
are consumed during the process and have to be changed periodically. In
addition, the
Hall-Heroult process gives rise to green house emissions such as CF4 and C2F6,
which
should be removed from the off-gas in compliance with environmental
legislation.
In au alternative process, aluminium oxide is converted in a sulfidation step
into
aluminium sulfide A12S3 by a reaction with carbonsulfide CS2. A more detailed
description of the alternative process, also referred to as Compact Aluminium
Production Process or CAPP or more general as sulfide process, is given in
patent
application WO/00/37691. The aluminum metal can be extracted from A12S3 by
electrolysis, producing sulfur gas at the anode, preferably a graphite anode.
The
sulfur gas will be collected and recycled to produce CS2, which is used in the
sulfidation step, which is of particular advantage in combination with the
CAPP-
process. The simplified reactions (assuming no complexions) of the
electrolysis
process are:
cathode: A13+ + 3e" - Al (1.)
anode: 2 S2' --> S2 (g) + 4 e' (2.)
Overall: A12S3 -> 2 Al + 1.5 S2 (g) (3.)
By-products of the Hall-Heroult process such as fluoride off-gases as well as
spent pot linings will not be produced, since the electrolyte is basically
composed of
chlorides.
CA 02520798 2005-09-28
WO 2004/088000 2 PCT/EP2004/003625
Figure 1 shows the decomposition potential of various aluminium compounds to
produce aluminium by electrolysis and shows immediately that the electrolysis
of A12S3
is very advantageous with regard to energy consumption, i.e. it has the lowest
decomposition potential. The first bar is a theoretical value for comparison.
The second
bar represents a process wherein A1203 is converted into A1C13 which is
decomposed.
The fourth bar represents the alternative sulfide process and the third bar
represents the
actual Hall-Heroult process. The tlieoretical value of the decomposition
potential is
determined by:
0
E AG , whereas
nF
E = the decomposition potential
(4.)
OG = the Gibbs free energy
n = the valency of the ion (3 for aluminium)
F = Faraday's constant
A problem with the sulfide process is the low current density that can be
achieved in the known molten chloride bath.
The eutectic composition of a MgC12-NaCl-KCI mixture (50-30-20 mole %) has
been proposed previously as an appropriate electrolyte for the electrolyses of
A12S3 (see
N.Q.Minh, R.O. Loutfy, N.P.Yao, "The Electrolysis of A12S3 in A1C13-MgC12-NaCl-
KCl Melts", J.Appl. Electrochem, Vol 12, 1982, 653-658; R.O. Loutfy, N.Q.
Ming, C.
Hsu, N.P. Yao, "Potential Energy Savings in the Production of Aluminium:
Aluminium
Sulfide Route", Chemical Metallurgy - A Tribute To Carl Wagner, Proc. Of Symp.
on
Metallurgical Thermodynamics and Electrochemistry at the 110th AIME annual
meeting, N.A. Gokcen, Ed., Chicago, Febr. 1981, The Metallurgical Society of
AIME,
New York, 1981; N.Q. Mingh, R.O. Loutfy, N.P. Yao, "The Electrochemical
Behaviour of A12S3 in Molten MgC12-NaCl-KC1 Eutectic", J. Electroanal. Chem.
Vol.
131, 198, 229-242).
It was tliought that the limiting factor in the achievable current density in
the
bath of molten chloride salt is the solubility of A12S3. The solubility was
enhanced by
the use of MgC12 which was thought to increase the solubility according to the
reaction.
CA 02520798 2005-09-28
WO 2004/088000 3 PCT/EP2004/003625
MgCla + A12S3 --> 2A1SC1 + MgS (s) (5.)
A limiting current density of 0,3 A/cm2 at the saturation solubility of A12S3
(;Z~ 3
wt %) and of 0,2 A/cm2 in the MgC12-NaCl-KCl eutectic composition containing
about
2 wt % A12S3 was measured.
The current efficiency, i.e. the percentage of the current that is actually
used for
the electrolysis was determined to be about 80 % at a current destiny of 0,2
A/cm2, a
cell potential of about 1,5 V and an interelectrode gap between anode and
cathode of 3
cm.
It is reported in literature that electrochemical studies of the electrolysis
of
A12S3 in a chloride melt showed that the reduction of Al-ions at a graphite
electrode is a
diffusion controlled process and proceeds via a reversible, 3-electron charge
transfer.
The oxidation of S-ions in the chloride electrolyte should be a reversible
diffusion
controlled process proceeding via a mechanism based on two steps:
S2- -~ S + 2e (electrochemical 2-electron process) (6.)
S + S> S2 (dimerization of sulfur atoms to S2) (7.)
The normal current density at which the Hall-Heroult process is carried out is
about 0,8 A/cm2. The achievable current density in the electrolysis of A12S3
in a
eutectic MgC12-NaC1-KCl bath is about 0,3 A/cm2. This means that the cell
area, when
applying the sulfide process should be about three times larger then required
for the
Hall-Heroult process. This makes the sulfide process not an attractive
alternative
despite the drawbacks associated with the Hall-Heroult process.
Because the limiting current densities found are too low to compete with the
Hall-Heroult process, where about 0.8 A=cm 2 is employed, the addition of
fluxes to the
melt was considered to increase the solubility of A12S3 and to increase the
activity of
both Al and S in the melt.
It is known that increasing the acidity of the bath of molten salt has a
beneficial
effect on the solubility of A12S3. The addition of A1C13 results in a more
acidic melt and
should favour the solubility. However, due to the high vapour pressure of
A1C13
(boiling point 447 C) additions to the molten salt melt are limited because
of
CA 02520798 2005-09-28
WO 2004/088000 4 PCT/EP2004/003625
volatilization. The addition of 5 - 10 wt % A12S3 increases the solubility of
A1C13 up to
a maximum of 5- 7 wt % according to the following reaction.
A1C13 + A12S3 --)' 3A1SC1
The addition of A1C13 allows current densities of up to 2 A/cm2, but the use
of
A1C13 is not a viable alternative. Even though the eutectic temperature of a
MgC12-
NaCI-KCI mixture is relatively low, the high vapour pressure of A1C13 causes a
considerable amount of A1C13 to volatise.
In prior publications A1C13 was used to enhance the electrowinning process.
Since A1C13 is readily volatilized from the melt and has to be separated from
sulfur
downstreain to recycle it to the electrowinning process, it was discarded as
being
impractical.
It is asi object of the present invention to provide a process for the
electrolysis of
A12S3 which allows a high current density, preferably coinparable with or
higher than
the current density achieved in the Hall-Heroult process.
It is further object to the present invention to provide a process for the
electrolysis of A12S3 which allows a high current density without the use of
A1C13.
It is another object of the invention to provide a process for the
electrolysis of
A12S3 in which virtually all of the sulfur can be recycled to form new A12S3
from A1203.
These and further objects are reached in a process for the electrolysis of
A12S3
using a bath of molten salt, preferably a bath of molten chloride salt, in
which A12S3 is
dissolved which is characterised in that measures are talcen to improve the
electrical
conductivity of the bath, so as to enable an increase in the current density
in the batli.
Different from what is suggested in the prior art, the present inventors have
found that the solubility of A12S3 in a bath of molten salt having an
appropriate
composition is not the limiting factor in the achievable current density. The
cell
potential of an electrolysis cell is built up of thermodynamic, kinetic
(activation
potential and mass transfer limitations) and ohmic contributions. The
inventors have
taken a different approach and found a nearly linear relationship between
current
density and cell potential which indicates that the electrolysis process, at
least above a
CA 02520798 2005-09-28
WO 2004/088000 5 PCT/EP2004/003625
minimum concentration of dissolved A12S3, is not diffusion controlled, but has
ohmic
limitations. Then, as observed by the present inventors, an increased
solubility of A12S3
does not result in a substantial enhancement of the cell performance. The
relationship
between cell potential and current density is nearly linear, which means that
this
relation is determined by an ohmic relation. Consequently the allowable
current density
can be increased by improving the electrical conductivity of the bath.
Preferably the conductivity is improved in an embodiment of the invention in
which the measures comprise adding an additive to the bath.
The additives are selected so as to increase the overall electrical
conductivity in
the bath of molten salt. As an additional effect the additives may increase
the activity of
both aluminium and sulfur and also the solubility of A12S3. As described above
A1C13 is
not a preferred addition.
A preferred embodiment of the process according to the invention is
characterised in that the additive comprises, preferably mainly consists of a
fluoride
coinpound.
This embodiment is based on the insight that the amount of fluoride has a
positive effect on the electrolysis process resulting from a higher activity
of A1Fn '-
than A1S+ species. Also, as complexing of aluminium with fluor is favoured
over
complexing of aluininium with sulfur, the concentration of sulfur ions is
higher when
fluoride is added, favouring the anodic reaction.
A further preferred embodiment of the process according to the invention is
characterised in that the fluoride compound is cryolite.
It has been found that addition of cryolite shows a larger improvement of the
conductivity than the addition of other fluorides such as NaF, although the
specific
conductivity of NaF is much higher.
Another advantages of adding cryolite, is that cryolite has a high melting
point
(1012 C and therefore inuch higher than the boiling point of A1C13) and
volatilization
of cryolite at the normal operating temperature of the electrolysis cell is
assumed to be
negligible.
CA 02520798 2005-09-28
WO 2004/088000 6 PCT/EP2004/003625
It can be argued that adding fluoride is not desirable, since this results in
fluoride emissions. However, the required amount of cryolite is relatively
small and
operating temperatures are only about 700 C, compared to about 950 C for the
conventional Hall-Heroult process. Thus the vapor pressure of fluorides will
be very
low. The anode effect can be avoided, because sulfur reacts at the anode. As
non-
consumable anodes can be used, the electrowinning can be carried out in a
closed
system, providing improved off-gas capturing.
Another embodiment of the process according to the inventors is characterised
in that the concentration of the cryolite is in the range of 5 - 30 wt %,
preferably 7 - 15
wt %, more preferably about 10 wt %. Test have shown that relatively low
concentrations of cryolite are sufficient to obtain the desired increase in
conductivity
with an optimum concentration of about 10 wt %.
From the relationship between current density and cell potential in an
electrolysis cell containing a bath of molten salt, and from the effect of
cryolite and
NaF addition, it was concluded that the beneficial effect of the addition of
fluoride
containing additives cannot solely be ascribed to the increased specific
conductivity of
the melt.
It was concluded that the process according to the invention is also improved
in
a embodiment which is characterised in that the measures comprise enhancing
the
effective area of an anode extending into the bath by reducing the amount
and/or size of
gas bubbles covering the anode.
The following observations have been made that justify the conclusion that the
beneficial effect of the addition of fluoride containing fluxes camiot solely
be ascribed
to the increased specific conductivity of the melt:
= The slope of the current density vs. cell potential relationship increases
by almost a
factor 3 on the addition of cryolite to the MgCl2-NaCl-KCl eutectic, which is
much
more than can be expected from the increased conductivity.
= The addition of 10 wt.% cryolite shows a larger iinprovement of the apparent
conductivity than the addition of NaF, although the specific conductivity of
NaF is
much higher.
CA 02520798 2005-09-28
WO 2004/088000 7 PCT/EP2004/003625
= There seems to be an optimum amount of fluoride, or fluoride to aluminium
ratio,
in the electrolyte.
The explanation proposed is that a significant portion of the olunic drop is
not
related to the bath of molten salt itself but due to the gas bubbles at the
anode, since
they have virtually zero conductivity and reduce the available anode surface.
In
literature it has been shown that the main contribution to the cell potential
is due to the
anodic reaction. It has been deterinined previously for chlorine evolution in
a chloride
melt that the apparent conductivity was only about 40% of the specific
conductivity of
the electrolyte, due to gas bubbles. Chlorine bubbles have the tendency to
grow and
stick to the anode and the overpotential could be interpreted as an ohmic
potential drop
in a surface layer at the anode. The saine reasoning may apply as regards the
evolution
of sulfur gas in a chloride melt.
It is expected that the quantity of sulfur gas formed at the anode is not
changed
by the addition of cryolite, but that gas bubbles adhere less strongly or are
easier
removed from the anode, so that a layer of gas bubbles is less dense.
Therefore, a hypotliesis can be postulated that on the addition of fluoride, a
complex ion is formed, changing interfacial tension at the anode, resulting in
different
characteristics of the sulfuric bubble layer at the anode surface area,
significantly
reducing the ohmic drop as well as the energy consumption.
A preferred embodiment of the process according to the invention is
characterised in that the bath of molten salt mainly comprises alkali metal
chlorides,
preferably KCl and NaCI.
From the prior art it is known to use a bath of molten chloride salts
comprising
NaCI, KCl and MgC12. In particular the last compound is added to increase the
solubility of A12S3 since the solubility in a bath of molten NaCI and KCl is
negligible.
However the present inventors have realised that addition of suitable
additives like
cryolite increases the solubility of A12S3 in the bath of molten alkali metal
chlorides to a
level at which the solubility is no longer the limiting factor in the
electrolysis process
but the conductivity. This has opened the way to a simple and more
environmentally
friendly bath.
CA 02520798 2005-09-28
WO 2004/088000 8 PCT/EP2004/003625
A particular advantageous embodiment of the process according to the
invention is characterised in that the bath of molten metal is substantially
free of earth
alkaline chlorides.
It was found that a sufficient solubility in combination with a high
conductivity
can also be obtained in a bath of molten salt which is substantially free of
earth alkaline
chlorides, in particular free of MgC12, in particular when the above mentioned
fluoride
additions are made. This is of particular interest since earth alkalines like
Mg react with
the sulfur in the bath and form solid MgS hereby consuming sulfur. Basically
the
sulfide process in the form of CAPP process does not consume sulfur, since all
sulfer
can be recycled.
Through the creation of MgS a considerable amount of sulfur is removed from
the sulfur recycle loop, wliich makes supply of sulfur necessary at extra
costs.
Moreover, formation of MgS will lead to a substantial waste stream of
environmentally
unfriendly material, which needs to be recycled. Finally, formation of MgS
will impede
cell operation, and make regular cleaning of the cell necessary, which
conflicts with the
closed cell concept and leads to poor working conditions. Similar problems are
to be
expected in case other earth alkaline chlorides, such as CaC12, are used.
Therefore,
preferably, the bath of molten salt is substantially free of earth alkaline
chlorides.
In a further preferred embodiment of the process according to the invention
the
electrolysis is carried out at a bath temperature of between 600 C and 850 C,
preferably between 700 C and 800 C.
In the known process MgC12 is added to the bath of molten salt to increase the
solubility of A1203 and to lower the melting temperature of the bath so that
A1C13 can
be added to increase solubility.
By deleting MgC12 and adding cryolite, the melting temperature of the bath is
increased, but that is acceptable since the melting point of cryolite is much
higher than
the proposed batli temperatures. Further it has to be recognised that the
melting
temperature of the NaCl-KCI eutect is still substantially lower than proposed
bath
temperature.
CA 02520798 2005-09-28
WO 2004/088000 9 PCT/EP2004/003625
A still further preferred embodiment of the process according to the
invention,
is characterised in that the electrolysis is carried out in a multi-polar
electrolysis cell.
Because of operation with non-consuinable anodes, the interelectrode gap can
be reduced and kept constant and a multi-polar cell operation is possible,
wliich will
increase productivity, reduce energy consumption and reduce capital costs
The invention will now be further explained and elucidated with reference to
the
drawing in which
Fig. 1 shows the decomposition potential of aluminium compounds to produce
alumimium by electrolysis.
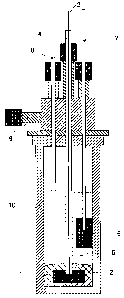
Fig. 2 shows a schematic view of an experimental electrolysis cell.
Fig. 3 shows a plot of cathodic current density as a function of the cell
potential
for the electrolysis of aluminium from A12S3 in a MgC12-NaCl-KCI electrolyte
of 50-
30-20 mole / at 725 C using cryolite as an additive (flux).
Fig. 4 shows a plot of cathodic current density as a functions of the cell
potential for the electrolysis of aluminium from 4 wt % A12S3 in a MgC12-NaCl-
KCI
electrolyte of 50-30-20 mole % at 725 C using different amounts of cryolite
as an
additive (flux).
Fig. 5 shows a plot of the cathodic current density as a function of the cell
potential for the electrolysis of aluminium from 4 wt % A12S3 in a MgC12-NaCl-
KCI
electrolyte of 50-30-20 mole % of 725 C using NaF as an additive (flux).
Fig. 1 has been described above.
The electrolysis of aluminum from aluminuin sulfide is carried out in a two
electrode system. A schematic view of the experimental cell is depicted in
Figure 2.
The cathode is a pool of molten aluminum (1) (effective area 8.1 cm2), which
is
polarized by a graphite block (2) connected by a rod of stainless steel (3)
shielded by a
quartz tube (4). The anode is constructed of a graphite block (5) of 1 cm2, 5
cm high,
which is immersed 2 cm into the electrolyte and is connected by a stainless
steel rod (7)
. The interelectrode gap is 2 cm. The anode acts as the reference electrode,
thus the cell
CA 02520798 2005-09-28
WO 2004/088000 10 PCT/EP2004/003625
potential is measured during the electrolysis. The electrochemical cell is
constructed of
sintered A1203 (Alsint) walls (10). The melt is protected by an inert Ar
atmosphere.
Argon is introduced through inlet (8) and leaves the cell through outlet (9).
The cell is
externally heated by a 2100W cylindrical furnace equipped with heating
elements (not
shown). The maxiinum operating temperature is 1400 C. The temperature is
measured
and controlled by type S thermocouples and a control unit (not shown).
The potential is measured with a potentiostat/galvanostat, which was used in
combination with a current booster, to enable a high current throughput (20 A
range).
The electrochemical measurement system is fully computer controlled.
Tests as described below were carried out with a MgC12-NaC1-KCI mixture, but
the results also apply to a NaCI-KCI mixture.
All chemicals were stored and handled in a glove box, having an argon
atmosphere (< 1 ppm H20, 02). M, NaCl and NaF were of pro analysis quality.
Anhydrous MgC12 (98 %), A12S3 (98 %) and Na3A1F6 (98 %) were commercially
obtained from a supplier. The chloride electrolyte mixture was composed and
put in a
container in the glove box. This container was then taken out of the glove box
and
heated to 450 C while purging HCl gas through the solids and subsequently
through
the melt to remove all water and oxides. After cooling the container was put
back into
the glove box. The A12S3 and additives were added at room temperature in the
glove
box. The electrolysis cell was assembled in the glove box, closed, then
transported to
the furnace wlzere an Ar flow prevented contact with air. An overview of the
salt
mixtures used for the experimental program discussed in this paper is given in
the table
below.
CA 02520798 2005-09-28
WO 2004/088000 11 PCT/EP2004/003625
Overview of Experimental Program of the electrolysis of
Al from A12S3 in a MgC12-NaCl-KCl Electrolyte of 50-30-
20 mole% at 725 C.
A12S3 Amount of fCux
Exp. Flur
YO) (w t, ;-,-'o) A 4 - -
B 4 - -
C 4 Na3A1F6 10
D 10 Na3A1F6 10
E 4 Na3A1F6 5
F 4 Na3A1F6 15
G 4 Na3A1F6 20
H 4 Na3A1F6 30
K 4 NaF 10
L 4 NaF 30
Figure 3 shows the major improvement of the electrolysis performance, because
of the addition of Na3A1F6. When adding 10 wt.% of Na3A1F6, the electrolyte
con7position changes to a quaternary mixture of 48-29-19-4 mole% of MgC12-NaCl-
KCl-Na3A1F6. The current density is more than 3 times larger at a given cell
potential.
By linear extrapolation it has been determined that E = 0.98 V, which equals
the
theoretical decomposition potential. This is another indication, that the
process is
ohmically limited rather than diffusion controlled. The Nernst equation
indicates that
the activity of A12S3 in a melt with cryolite addition approaches unity.
Further increase
of the A12S3 concentration in the melt did not produce a significant effect
(compare
experiments C and D).
Figure 4 depicts a graph showing the influence of the ainount of cryolite
added
to the melt. There seems to be an optimum at about 10 wt.% cryolite addition.
Experimental worlc has been carried out in order to investigate whether the
positive influence of cryolite on the performance of the electrowinning was
caused by
CA 02520798 2005-09-28
WO 2004/088000 12 PCT/EP2004/003625
the amount fluoride added, or by increasing the amount of Al-ions in the
electrolyte.
Therefore, NaF was used as a fluxing agent. The addition of 10 wt.% NaF
results in a
melt composition of 42-25-17-16 mole% MgC12-NaC1-KCl-NaF composition. On an
elemental basis, the amount of F in the electrolyte is comparable to the
cryolite melt,
i.e. 6.4 and 7.6 mole% F respectively. Figure 5 depicts the results of those
experiments.
Increasing the amount of NaF to 30 wt.% shows a deteriorating cell
performance,
which again is in agreement with the results of cryolite additions.
The linear current density-cell potential relationships observed from Figure 3
to
Figure 5 indicate that the electrolysis process with these high concentrations
of
dissolved A12S3 is no longer diffusion controlled, but shows ohmic
limitations. Then, an
increased solubility of A12S3 would not result in a substantial enhancement of
the cell
perforinance. This is supported by the experimental results. It is assumed
that the
addition of Na3AlF6 to the melt has a positive effect on the solubility of
A12S3.
Therefore, the amount of A12S3 added to the quaternary mixture was increased
from 4%
to 10% (Exp. C and D in Figure 3). However, this did not iinprove the cell
performance
significantly, indicating that diffusion is not the rate limiting step with
these relatively
high concentrations of A1ZS3 employed.
At first sight, it can be argued that because of the ohmic control of the
process,
increasing the conductivity of the melt should result in an increased current
density.
Since cryolite has a higher conductivity than the chloride eutectic, a better
performance
is at least to some extent the result of the increased conductivity. When
adding 10 wt.%
of Na3A1F6, the electrolyte composition changes to a quaternary mixture of 48-
29-19-4
mole% of MgC12-NaCl-KCl-Na3AlF6, which can have significantly different
properties.
However, the slope of the linear relationship increased by almost a factor 3
when
cryolite was added, which cannot be attributed to the increased conductivity
of the melt
only. Furthermore, although the specific conductivity of NaF is the highest of
all
components, i.e. 4.2 SZ-1=cm 1 at 725 C, the effect of adding NaF is less
pronounced.
As cryolite and NaF additions produce similar effects, it can be argued that
the
amount of fluoride contributes to the positive effect on the electrolysis
process,
resulting from a higher activity of A1Fõ'_ than A1S+ species. Also, when
complexing of
Al with F is favoured over complexing with S, the concentration of S-ions is
higher
when fluoride is added, favoring the anodic reaction.
CA 02520798 2005-09-28
WO 2004/088000 13 PCT/EP2004/003625
As described earlier, the major benefit of fluoride additions was found to be
an
increase in conductivity of the melt, which is most favourably explained in
terms of a
reduced coverage of the anode by a sulfur gas bubbles layer.