Note: Descriptions are shown in the official language in which they were submitted.
CA 02521029 2001-03-28
PROCESS FOR THE PREPARATION OF
1,5-IMINO,-1,5,6-TRIDEOXY HEXITOL
This application is a divisional of Canadian
Application No. 2,362,836, filed March 28, 2001.
BACKGROUND OF THE INVENTION
(1) Field of the Invention
The present invention relates to a process for the
preparation of 1,5-imino,-1,5-6-trideoxy hexitol which
comprises reacting methyl-2,3,4,6-tetra-0-acetyl-5-
hexulosonic acid oxime with hydrogen and a hydrogenation
catalyst to form a 1, 5, 6-triacetoxy acid lactam and reducing
and deacetylating the lactam with a reducing agent to form
the 1,5-imino hexitol.
(2) Description of Related Art
Over the last three decades there has been a
continued interest in natural and synthetic imino-sugars
because of their high potency as glycosidase inhibitors ((a)
Grabner, R. W., et al., U.S. Patent No. 5,695,969; (b)
Boshagen, H., et al., U.S. Patent No. 4,940,705; (c)
Shilvock, J. P., et al., Tetrahedron Lett., 37 8569-8572
(1996)1 (d) Rajanikanth, D. B., et al., J. Chem. Soc.
CA 02521029 2001-03-28
-2-
Perkiw Trans_ I 2151-2152 (1992); (e) Hussain, A., et
al., Tetrahedron, 49 2123-2130 (1993); (f) Defoin, A.,
et al., Tetrahedron Lett. 34 4327-4330) (1997); (g)
Defoin, A., et al., Tetrahedron 53 13769-13782 (1997);
(h) Defoin, A., et al., Tetrahedron Lett. 35 5653-5656
(1994); (i) Fleet, G.W.J., et al., Tetrahedron lett. 29
2871-2874 (1988); (j) Fleet, G.W.J., et al., Tetrahedron
45 327-336 (1989); (k) Takahashi, S., et al. Chem. Lett.
21-24 (1992); (1) Takahashi, S., et al., J. Chem. Soc.,
Perkin Trans. I, 607-612 (1997); (m) Hendry, D., et al.,
Tetrahedron Lett. 28 4597-4600 (1987); (n) Hendry, D.,
et al., Tetrahedron Lett. 28 4601-4604 (1987); (o)
Straub, A., et al., J. Org. Chem. 55 3926-3932 (1990);
Delinck, D.L., et al., Tetrahedron Lett. 31 3093-3096
(1990); (r) Look, G. C., et al., Acc. Chem. Res. 26 182-
190 (1993); (s) Kajimoto, T., et al., J. Am. Chem. Soc.
113 6678-6680 (1991)). Glycosidases catalyze the
hydrolysis of glycosidic linkages and are the key
enzymes in the degradation of complex carbohydrates.
One of their main metabolic roles is the conversion of
complex non-absorbable carbohydrates into absorbable
mono- or oligosaccharides (Truscheit, E., et al., Angew.
Chem. Int. Ed. Engl. 20 744-761 (1981)). The rapid
action of these enzymes can lead, however, to
undesirable elevations in blood glucose in diabetes.
Iminosugars have been shown to act as glycosidase
inhibitors and to retard and regulate the intestinal
carbohydrate digestion. They are therefore excellent
drug candidates for diabetes therapy (Liu, P.S., U.S.
Patent No. 4,634,765 (1987)). An even more exciting
CA 02521029 2001-03-28
-3-
potential use of iminosugars is in the treatment of
cancer and viral diseases (Rohrschneider, L. R., et al.,
U.S. Patent No. 4,837,237 (1989)). It has been shown
that modification of oligosaccharide structures may
alter metastatic capacity of cancer cells and 1,5-
diimino-1,5-dideoxyglucitol (deoxynojirimycin) (1)
(Tsuruoka, T., et al., U.S. Patent No. 5,250,545 (1993))
swainsonine (2) (Dennis, J. W., Cancer Res. 46 5131-5136
(1986)) and castanospermine (3) (Humphries, M. J., et
al., Cancer Res. 46 5215-5222 (1986)) (Figure 1.) can
markedly inhibit metastasis of cancer cells. They
might, therefore, be used for the effective treatment of
cancer.
OH OH
HO NH H HO.., ..OH
HO N ...OH ... H
OH OH
OH
9 3
N-Butyl-deoxynojirimyciin shows excellent activity
against herpes virus (Jacob, G. S., et al., U.S. Patent
No. 4,957,926 (1990)) whilst having low cyto-toxicity
and no inhibitory effect on the growth of normal cells.
The greatest prospect for the use of iminosugars as
drugs is probably for the treatment of AIDS.
Glycosidase inhibitors prevent the processing of N-
linked complex oligosaccharides. This results in the
disruption of the synthesis of viral coat glycoproteins
CA 02521029 2001-03-28
-4-
such as the critical one called gp120. This supposedly
leads to the loss of recognition by the CD-4 receptor of
the target cell with concomitant reduction of syncytia
formation resulting in the reduction of virus
infectivity and the inhibition of viral replication
(Walker, B. D., et al., Proc. Natl. Acad. Sci. USA 84
8120-8124 (1987); Karpas, A., et al., Proc. Natl. Acad.
Sci. USA 85 9229-9233 (1988); Fleet, G.W.J., et al.,
FEBS Lett. 237 128-132 (1988)). Clinical trials have
been launched for N-Butyl-deoxynojirimycin
(Rohrschneider, L. R., U.S. Patent No. 5,643,888
(1997)). The iminosugars that have been the most
investigated are deoxynojirimycin ((a) Schroder, T., et
al., U.S. Patent No. 4,806,650 (1989); (b) Koebernick,
W., U.S. Patent No. 4,611,058 (1986); (c) Anzeveno,
P.B., et al. U.S. Patent No. 5,227,479 (1993); (d)
Anzeveno, U.S. Patent No. 4,908,439 (1990); (e) Tsuda,
Y., et al., Heterocycles, 27 63-66 (1988); (f) Inouye,
S., et al., Tetrahedron 23 2125-2144 (1968); (g)
Vasella, A_, et al., Helv. Chim. Acta 65 1134-1144
(1982); Ikota, N., et al., Heterocycles 46 637-643
(1997); (i) Paulsen, H., et al., Chem. Ber 100 802-815
(1967); (j) Rudge, A.J., et al., Angew. Chem. Int. Ed.
Engl. 33 2320-2322 (1994); (k) Behling, J., et al.,
Synth. Commun. 21 1383-1386 (1991); (1) Kinast, G., et
al., Angew. Chem. Int. Ed. Engl. 20 805-806 (1981); (m)
Pederson, R. L., et al., Tetrahedron Lett. 29 4645-4648
(1988); (n) Osten, C.H., et al., J. Am. Chem. Soc. 111
3924-3927 (1989)) and its N-alkyl analogues (Grabner, R.
W., et al., U.S. Patent No. 5,610,039 (1997); U.S.
CA 02521029 2001-03-28
-5-
Patent No. 4,806,650; U.S. Patent No. 4,611,058; U.S. Patent
No. 4,940,705).
The chemical synthesis of nojirimycin derivatives
are generally too involved and not suitable for commercial
applications. The chemo-microbiological method patented by
Grabner (U. S. Patent Nos. 5,695,969; 5,610,039)) provides
an elegant method for transforming a sugar into its
imino-derivative by reductive animation of a 5-keto aldose
obtained by bacterial oxidation of glucose. The method is
in particular however, not applicable to the D-galacto
derivatives of the present invention.
Other related patents are: U.S. Patent Nos.
5,227,479, 5,250,545, 5,695,969, 4,957,926, 4,908,439 and
4,634,765.
SUMMARY OF INVENTION
The present invention relates to a process for the
preparation of 1,5-imino,-1,5-6-trideoxy hexitol as a
product which comprises:
(a) reacting methyl-2, 3,4, 6-tetra-O-acetyl
-5-hexulosonic acid oxime with hydrogen and a hydrogenation
catalyst at a temperature between about 20 and 80°C and a
pressure between about 200 and 400 psi in an acidic solvent
to form a 1,5,6-triacetoxy acid lactam;
(b) reducing and deacetylating the lactam with a
reducing agent to form the 1,5-imino hexitol.
The present invention also relates to methyl-
2,3,4,6-tetra-O-acetyl-L-xylo-5-hexulosonic acid hydrazide
oxime.
BRIEF DESCRIPTION OF DRAWINGS
Figure 1 is a drawing showing the schematic
CA 02521029 2001-03-28
-6-
reactions of Examples 1 and 2. The numbers are for the
structures of the compounds of these Examples.
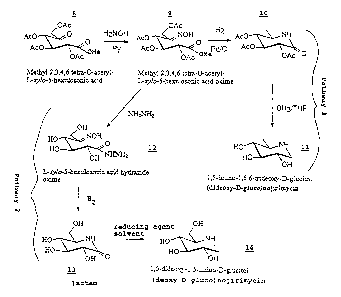
Figure 2 is a drawing showing the schematic
reactions of the reactions of Examples 3 to 6. The numbers
are for structures of the compounds of these Examples.
Figure 3 is a drawing showing the reactions where
an oxime group is replaced with an imino alkyl group.
Figure 4 is a drawing showing the reaction of the
hexitol with an aldehyde to produce an alkyl group on the
nitrogen.
DESCRIPTION OF PREFERRED EMBODIMENTS
A related invention relates to methyl-2,3,4,
6-tetra-O-acetyl-L-arabino-5-hexulosonic acid oxime 5
(Figure 1) as an intermediate for the synthesis of D-dideoxy
galacto nojirimycins 7. The present invention provides a
method for the preparation of 1,5-imino-1,5-dideoxy and
1,5,6-triteoxy alditols with the D-galacto configurations
starting from ~i-glactosides via hexulosonic acid oximes
which have not been reported before now. The procedure is
especially valuable because of its high stereoselectivity
and straightforwardness. The key steps are the reduction of
the oxime derivatives to the lactams which is then further
reduced to the target compounds. The C6 position can be
deoxygenated during the reduction if it bears an acetoxy
group. The trideoxy imino sugars are then produced.
Deacetylation prior to oxime reduction gives the dideoxy
compounds.
A related invention provides a simple access to
D-galactonojirimycins from the new oxime intermediate methyl
2,3,4,6-tetra-O-acetyl-L-arabino-5-hexulosonic
CA 02521029 2001-03-28
_7_
acid oxime 5. The method also allows access to the 5-
amino-5-deoxy-D-galacturonic acid b-lactams. This also
is not known before now although the gluco-isomer has
been made_by the oxidation of nojirimycin (Kajimoto, T.,
et al., J. Am. Chem. Soc. 113 6187-6196 (1991)). In
this method, the ketoaldonic acid methyl ester is
converted to the previously unreported oxime which is
then reduced to the amine which cyclizes to give the
lactam. The lactam is reduced to the imino sugar by
borane or a metal hydride reagent. (Scheme 1). Despite
the formation of both the cis- and traps oximes, no L
derivatives are formed Reduction of the peracetylated
oxime leads to deoxygenation of the 6 position to give
the tri-deoxydiiminoalditol (dideoxy-D-galacto
nojirimycin 4).
EXAMPLE 1
Methyl-2,3,4,6-tetra-O-acetyl-L-arabino-5-
hexulosonic acid oxime. 5 The ketoaldonic acid 4 (7g,
18.61 mmol) was dissolved in pyridine (16 ml) and the
solution cooled to OoC. Hydroxylamine hydrochloride (2
g, 28.77 mmol) was then added and the solution stirred
at O~C for 15 minutes and then for another 2 hours at
room temperature. The mixture was poured onto ice and
water and then extracted three times with chloroform.
The combined chloroform layers were subsequently washed
with water, dried with Na2S09 and then evaporated.
Crystallization from hot ethanol gave white crystals of
the oxime (85~) as a mixture of cis-traps isomers: 1H
NMR (CDC13) ~ isomer 1:1.98 (s, 3 H, OAc), 2.01 (s, 3 H,
CA 02521029 2001-03-28
_g_
OAc), 2.08 (s, 3 H, OAc), 2.15 (s, 3 H, OAc), 3.70 (s,
3H, OCH3) , 9.82 (d, 1H, J6a,sb 14. 6 Hz, H6-a) , 5. 11 (d,
1H, H6-b) , 5. 35 (d, 1H, J3~4 1. 9 Hz, H-4 ) , 5. 68 (d, 1H,
J3,2 9. 0, Hz, H-2) , 5.84 (dd, IH, H-3) ; 13C NMR (CDC13)
S b 20.2, 20.3, 20.4, 20.5, 52.6, 56.4, 68.7, 69.2, 69.6,
149.9, 167.5, 168.9, 169.3, 170.0, 170.3.
EXAMPLE 2
1,5-imino-1,5,6-trideoxy-D-galactito (dideoxy-
D-galacto)nojirimycin. 7 This was prepared from the
oxime 5 (7.4 g, 18.92 mmol) by reduction with hydrogen
on palladium in acetic acid. The intermediate amino
ester was cyclized to form a lactam 6 that was then
reduced by borane. Flash column chromatography using a
chloroform-methanol (6:1) mixture gave (dideoxy-D-
galacto)nojirimycin 7 (1.5 g, 300): [a]23D+27.Oo (c 1.3,
CHC13) , lit. + 49.0 (c 1, CHC13) [20] ; 1H NMR (D20) b
1.21 (d, 3H, J5~6 6.6 Hz, H-6) , 2.73 (t, 1H, Jla,le-Jla,2
11. 9 Hz, H-la) , 3. 30 (dd, 1H, Jle,2 5. 4 Hz, H-le) , 3. 37
(m, 1H, H-5 ) , 3 . 50 (dd, 1H, J2, 3 9 . 6 Hz, J3.4 3 . 1 Hz, H-
3), 3.90 (d, 1H, JQ_5 3.1 Hz, H-4), 3.91 (ddd, 1H, H-2);
i3C NMR (D20) ~ 14.2, 46.1, 55.0, 64.4, 69.9, 73.1.
Methyl-2,3,4-6-tetra-O-acetyl-D-xylo-5
hexulosonic acid oximes are intermediates for the
preparation of di and tri-deoxynojirimycins_ The
present invention provides a general method for the
preparation of 1,5-imino-1,5-6,trideoxy alditols with
the D-gluco configurations starting from the previously
unreported methyl-2,3,4,6-tetra-O-acetyl-D-xylo-5-
hexulosonic acid oxime 9 (Figure 2). The key steps are
CA 02521029 2001-03-28
-9-
the selective reduction of the oxime derivatives to
lactams which are further reduced to the target
compounds. The C6 position can be deoxygenated during
the reduction if it bears an acetoxy group. The
trideoxy imino sugars are then produced. Deacetylation
prior to oxime reduction gives the dideoxy compounds.
The present invention provides a simple access
to D-gluco nojirimycins from the new oxime intermediate
Methyl-2,3,4,6-tetra-O-acetyl-L-arabino-S-hexulosonic
acid oxime. The method also allows access to the 5
amino-5-deoxy-D-glucuronic acid b-lactams. This also
is known from the oxidation of nojirimycin (Kajimoto,
T., et al., J. Am. Chem. Soc. 113 6187-6196 (1991)). It
is an excellent glycosidase inhibitor at concentrations
100 times lower than most of the other inhibitors tested
(Kajimoto, T., et al., J. Am. Chem. Soc. 113 6187-6196
( 1991 ) ) . In the method we describe here the ketoaldonic
acid methyl ester is converted to the previously
unreported oxime which is then reduced to the amine
which cyclizes to give the lactam. The lactam is
reduced to the imino sugar by borane or a metal hydride
reagent. (Pathway 1). Despite the formation of both the
cis- and trans oximes, no L-derivatives are formed.
Reduction of the peracetylated oxime leads to
deoxygenation of the 6 position to give the tri-
deoxydiiminoalditol (dideoxy-D-gluco-nojirimycin) 11.
Access to the 6-hydroxy derivatives was readily achieved
by deacetylating the oxime with hydrazine prior to
reduction. The deacetylation yielded the acyl hydrazide
in quantitative yield (Pathway 2).
CA 02521029 2001-03-28
-10-
EXAMPLE 3
Methyl-2,3,4,6-tetra-0-acetyl-D-xylo-5-
hexulosonic acid oxime. 9 The ketone 8 (7 g, 18.61
mmol) was dissolved in pyridine (16 ml) and the solution
cooled to OoC. Hydroxylamine hydrochloride (2 g, 28.77
mmol) was then added and the solution stirred at O~C for
minutes and then for another 2 hours at room
temperature. The mixture was poured onto ice and water
and then extracted three times with chloroform. The
10 combined chloroform layers were subsequently washed with
water, dried with Na2S09 and then evaporated.
Crystallization from hot ethanol gave white crystals of
the oxime 9 (6.9 g, 950) as a 3:2 mixture of cis-trans
isomers: Isomer l: 1H NMR (CDC13) b 1.93 (s, 3 H, OAc),
15 1 . 94 (s, 3 H, OAc) , 2. 00 (s, 3 H, OAc) , 2.01 (s, 3 H,
OAc) , 3.56 (s, 3 H, OCH3) , 4.36 (d, 1H, J6a,sb 12. 4 Hz,
H6-a) , 4. 72 (d, 1H, H6-b) , 4. 99 (d, 1H, J3,9 2. 6 Hz, H-
4) , 5.72 (dd, 1H, J3~2 7. 8 Hz, H-3) , 6.28 (d, 1H, H-2) ;
13C NMR (CDC13) b 20.5, 20.4, 52.8, 61.3, 66.1, 69.5,
69.8, 149.9, 167.3, 169.4, 169.5, 170.1; HRMS (M+H+)
calcd. 392.1193, found 392.1198. Isomer 2: mp=121-
122oC; 1H NMR (CDC13) b 1.88 (s, 3 H, OAc) , 1.89 (s, 3
H, OAc) , 1. 98 (s, 3 H, OAc) , 2.00 (s, 3 H, OAc) , 3. 56
(s, 3H, OCH3) , 4 .82 (s, 2H, H-6) , 5. 16 (d, 1H, J3,9 2. 6
Hz, H-4 ) , 5 . 62 (d, 1H, J3, 2 8 . 5, H-2 ) , 5. 78 (dd, 1H, H-
3)~; 13C NMR (CDC13) b 20. 5, 20. 4, 52 _ 8, 61. 3, 66. 1,
69.5, 69.8, 149.9, 167.3, 169.4, 169.5, 170.1.
EXAMPLE 4
Tri-O-acetyl-S-amino-5,6-dideoxy-D-gluconic
CA 02521029 2001-03-28
-11-
acid lactam. ZO A solution of oxime 9 (6.9, g, 17.69
mmol) in glacial acetic acid (275 ml), containing 10~
Pd/C (2.76 g) was hydrogenated in a Parr reactor under
a HZ pressure of 300-400 psi for 40 hours at 55~C. The
S reaction mixture was filtered through celite and washed
with ethanol. The solvent was rotary-evaporated and the
lactam 10 (5 g, 1000 was obtained as a light yellow
syrup: [oc] Z3 D+70. Oo (c 1. 56, CHC13) ; iH NMR (CDC13) b
1.11 (d, 3H, J5,6 6.3 Hz, H-6), 1.94 (s, 3 H, OAc), 1.98
(s, 3 H, OAc), 2.00 (s, 3 H, OAc), 3.51 (m, 1H, J4,5
9.7, Hz, H-5) , 4 . 94 (t, 1H, J3,4 9.7 Hz, H-3) , 4 . 96 (d,
1H, H-2), 5.40 (t, 1H, H-4); 13C NMR (CDC13) b 18.0,
20.3, 20.3, 48.7, 70.6, 70.9, 71.4, 166.7, 169.4, 169.6,
169.8; HRMS (M+H+) calcd. 288.1083, found 288.1089.
EXAMPLE 5
1,S-imino-1,5,6-trideoxy-D-glucitol 11 1M
BH3/THF (50 ml, SO mmol) was added under N2 to a
solution of lactam 10 (5 g, 17.41 mmol) in THF (33 ml).
The mixture was stirred at room temperature for 1.5
hours and then refluxed for another 1.5 hour. After
cooling to room temperature 9o methanolic HCl (40 ml)
was carefully added and the resulting solution was
refluxed for 30 minutes. The THF was removed by rotary
evaporation and the reaction mixture was dissolved
repeatedly in methanol, followed by evaporation to
remove borates. Water was added to the dry crude
product 10 and the solution was passed through an anion
exchange resin (Amberlite IR-45 OH-form) and then dried
on the rotary evaporator. To remove the last traces of
*Trade-mark
CA 02521029 2001-03-28
-12-
borates, a solution of 1M NaOH (15 mol) and methanol (6
ml) were added to the crude product and the mixture was
stirred overnight at room temperature. The methanol was
evaporated and the aqueous solution was lyophilized. A
S methanolic HC1 solution was added, which precipitated
NaCl while the methanolic solution was dried, to give
the product 10 (2.43 g, 95%): [a]23D+15.50 (c 1.88,
H20), lit. +13.~ (c 1.0, HZO) [18]; 1H NMR (DZO) b 1.25
(d, 3H, J5,6 6. 3 Hz, H-6) , 2.77 (dd, 1H, Jla,le 12.4 Hz,
Jia,2 11. 7 Hz, H-la) , 3. 02 (dd, IH, JQ,S 10.0 Hz, H-5) ,
3. 23 (dd, 1H, J3, q (dd, 1H, J3,4 9. 0 Hz, H-4 ) , 3 . 33 (dd,
1H, Jle,2 5. 1 Hz, H-le) , 3.31 (dd, 1H, J2,3 9.2 Hz, H-3) ,
3. 63 (ddd, 1H, H-2) ; 13C NMR (D20) b 17.5, 49.5, 55.2,
71.4, 76.7, 79Ø
EXAMPLE 6
Tetra-O-acetyl-5-amino-5-deoxy-gluconic acid
lactam. 13 The acetylated oxime 9 (1.5 g, 3.84 mmol)
was deacetylated with concomitant conversion to the acyl
hydrazide by treatment with anhydrous hydrazine (0.75
ml, 23.89 mmol) in methanol (15 ml) at room temperature
for 2 hours. Evaporation of the solvent gave the crude
acid hydrazide 12: 1H NMR (DZO) b 4.18 (1H, dd, J=4.6
Hz, J--7.0 Hz) 4.51 (1H, d, J--6.5 Hz), 4.43 (1H, d,
2S J=14.9 Hz), 9.53 (1H, d, J--14.8 Hz), 5.18 (1H, d, J=4.6
Hz); 13C NMR (D20) b 61.1, 69.1, 73.4, 73.5, 160.7,
173.9. This hydrazide 12 was hydrogenated in glacial
acetic acid with 10°s, Pd/C (0.4 g) at 50~C and 300 psi
pressure of H2 for 2 days. After filtration through
celite, the solution was dried on the rotary evaporator
*Trade-mark
CA 02521029 2001-03-28
-13-
and the crude product acetylated with acetic anhydride
(15 ml) and pyridine (15 ml) for 5 hours at room
temperature . The mixture was poured into cold water and
extracted with chloroform. The chloroform layer was
dried with Na2S09. Evaporation of the solvent gave
crude product 13 (1.47 g), which was subjected to flash
chromatography on silica (eluent hexane-acetone = 2:1)
to give the perahydroxy lactam 13 (0.5 g) C-5 epimer:
mp=177-178oC; [a]23D+88.6 (c 1.11, CHC13), lit.+104a (c
1.73, CHC13)[17]; 1H NMR (CDC13) b 2.03 (s, 3H, OAc),
2. 06 (s, 3 H, OAc) , 2.08 (s, 3 H, OAc) , 2. 10 (s, 3 H,
OAc) , 3.75 (ddd, 1H, Jq,s 9.7 Hz, J5,6a2. 9 Hz, J5,6b 6.5
Hz, H-5) , 3. 96 (dd, 1H, J6a,6b 11. 7 Hz, H6-b) , 4 .22 (dd,
1H, H-6a ) , S . 06 (d, 1H, J3, Z 9 . 5 Hz, H-2 ) , S . 20 ( t, 1H,
J3,4 9.5 Hz, H-3), 5.53 (dd, 1H, H-4), 6.48 (s, 1H, s,
NH) ; 13C NMR (CDC13) b 20. 5, 20. S, 20. S, 20. 6, 52. 4,
62.7, 67.2, 70.4, 70.5, 166.2, 169.4, 169.6, 170.0,
170.4 HRMS (M+H+) calcd. 346.1060, found 346.1143.
Epimer: [a)23D+3.1~ (c 1.81, CHC13); 1H NMR (CDC13) 1.98
(s, 3 H, OAC), 1.99 (s, 3 H, (OAC), 2.00 (2, 3 H, OAC),
2.02 (s, 3 H, OAC), 3.88 (1H, m, H-5), 4.04 (dd, 1H,
J6a, sb 11. 4 Hz, J5, 6b 6. 3 Hz, H6-b) , 4 . 18 (dd, 1H, J5, 6a
3. 9 Hz, H-6a) , 5. 15 (dd, 1H, JQ,S 9.5 Hz, J3,q 7. 5 Hz, H-
4 ) , 5 . 15 (d, 1H, J2, 3 7 . 5 Hz, H-2 ) , 5 . 39 ( t, 1H, H-3 ) ,
7.27 (1H, s, broad, NH); 13C NMR (CDC13) b 20.2, 20.3,
20.4, 50.0, 62.0, 68.0, 69.8, 70.0, 166.7, 169.3, 169.7,
170.3, 170.6. The lactam 13 was converted to the 1,5-
diamino-1,5-dideoxy-D-glucitol (deoxy-D-
gluco)nojirimycin 14 as in Example 5.
It will be appreciated that the imino group
CA 02521029 2001-03-28
-I4-
can contain a lower alkyl group containing 1 to 6 carbon
atoms rather than hydrogen. The oxime group in compound
would then be an imino alkyl group, preferably where
alkyl contains 1 to 8 carbon atoms. The reactions are
S shown in Figure 3. The hydrogen on the hexitol can be
replaced with an alkyl group by reaction with an alkyl
aldehyde and a reducing agent as shown in Figure 4.
It is intended that the foregoing description
be only illustrative of the present invention and that
the present invention be limited only by the hereinafter
appended claims.