Note: Descriptions are shown in the official language in which they were submitted.
CA 02521776 2002-05-21
CRYSTALLINE FORMS OF TR-(R*,R*)]-2-(4-FLUOROPHENYL)-BETA,DELTA- DIHYDROXY-5-(1-
METHYLETHYL)-3-PHENYL-4-[PHENLYAMINO) CARBONYL]-iH-PYRROLE-1- HEPTANOIC ACID
CALCIUM SALT (2:1) (ATORVASTATIi~
This is a divisional application of Canadian patent application CA 2,450,111
filed on
May 21, 2002 arising out of a limitation of claims of CA 2,450,111 on the
direction of the
Commissioner of Patents pursuant to subsection 36(2.1) of the Patent Act.
FIELD OF THE INVENTION
The present invention relates to novel crystalline forms of atorvastatin which
is
known by the chemical name [R-(R*,R*)]-2-(4-fluorophenyl)-~i,8-dihydroxy-5-(1-
methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-1H-pyrrole-1-heptanoic acid
hemi
calcium salt useful as pharmaceutical agents, to methods for their production
and isolation, to
pharmaceutical compositions which include these compounds and a
pharmaceutically
acceptable carrier, as well as methods of using such compositions to treat
subjects, including
human subjects, suffering from hyperlipidemia, hypercholesterolemia,
osteoporosis, and
Alzheimer's disease.
BACKGROUND OF THE INVENTION
The conversion of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) to
mevalonate is an early and rate-limiting step in the cholesterol biosynthetic
pathway. This
step is catalyzed by the enzyme HMG-CoA reductase. Statins inhibit HMG-CoA
reductase
from catalyzing this conversion. As such, statins are collectively potent
lipid lowering agents.
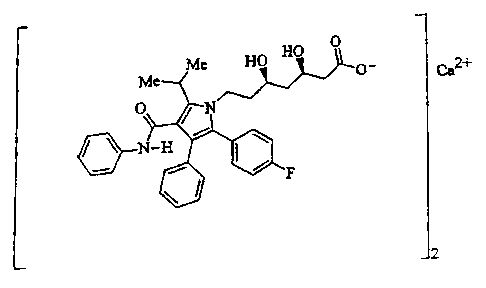
Atorvastatin calcium, disclosed in United States Patent No. 5,273,995, is
currently
sold as Lipitor~ having the chemical name [R-(R*,R*)]-2-(4-fluorophenyl)-(3,s-
dihydroxy-S-
(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-1H-pyrrole-1-heptanoic acid
calcium
salt (2:1 ) trihydrate and the formula
, _ ~ ~2-,-
CA 02521776 2002-05-21
2
Atorvastatin calcium is a selective, competitive inhibitor of HMG-CoA
reductase. As such,
atorvastatin calcium is a potent lipid lowering compound and is thus useful as
a
hypolipidemic and/or hypocholesterolemic agent.
United States Patent Number 4,681,893 discloses certain traps-6-[2-(3- or 4-
carboxamido-substituted-pyrrol-1-yl)alkyl]-4-hydroxy-pyran-2-ones including
traps-(~)-5-(4-
fluorophenyl)-2-(1-methylethyl)-N, 4-diphenyl-1-[(2-tetrahydro-4-hydroxy-6-oxo-
2H-pyran-
2-yl)ethyl]-1 H-pyrrole-3-carboxamide.
United States Patent Number 5,273,995 discloses the enantiomer having the R
form of
the ring-opened acid of traps-5-(4-fluorophenyl)-2-(1-methylethyl)-N, 4-
diphenyl-1-[(2-
tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)ethyl]-1H-pyrrole-3-carboxamide, ie,
[R-
(R*,R*)]-2-(4-fluorophenyl)-(3,8-dihydroxy-5-( 1-methylethyl)-3-phenyl-4-
[(phenylamino)-
carbonyl]-1H-pyrrole-1-heptanoic acid which is atorvastatin.
United States Patent Numbers 5,003,080; 5,097,045; 5,103,024; 5,124,482;
5,149,837; 5,155,251; 5,216,174; 5,245,047; 5,248,793; 5,280,126; 5,397,792;
5,342,952;
5,298,627; 5,446,054; 5,470,981; 5,489,690; 5,489,691; 5,510,488; 5,998,633;
and 6,087,511
disclose various processes and key intermediates for preparing amorphous
atorvastatin.
Amorphous atorvastatin has unsuitable filtration and drying characteristics
for large-scale
production and must be protected from heat, light, oxygen, and moisture.
Crystalline forms of atorvastatin calcium are disclosed in United States
Patent
Numbers 5,969,156 and 6,121,461.
International Published Patent Application Number WO 01/36384 allegedly
discloses
a polymorphic form of atorvastatin calcium.
Stable oral formulations of atorvastatin calcium are disclosed in United
States Patent
Numbers 5,686,104 and 6,126,971.
Atorvastatin is prepared as its calcium salt, ie, [R-(R*,R*)]-2-(4-
fluorophenyl)-(3,8-
dihydroxy-5-( 1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-1 H-pyrrole-1-
heptanoic
acid calcium salt (2:1). The calcium salt is desirable since it enables
atorvastatin to be
conveniently formulated in, for example, tablets, capsules, lozenges, powders,
and the like for
oral administration. Additionally, there is a need to produce atorvastatin in
a pure and
CA 02521776 2002-05-21
3
crystalline form to enable formulations to meet exacting pharmaceutical
requirements and
specifications.
Furthermore, the process by which atorvastatin is produced needs to be one
which is
amenable to large-scale production. Additionally, it is desirable that the
product should be in
a form that is readily filterable and easily dried. Finally, it is
economically desirable that the
product be stable for extended periods of time without the need for
specialized storage
conditions.
We have now surprisingly and unexpectedly found novel crystalline forms of
atorvastatin. Thus, the present invention provides atorvastatin in new
crystalline forms
designated Forms V, VI, VII, VIII, IX, X, XI, XII, XIII, XIV, XV, XVI, XVIT,
XVIII, and
XIX. The new crystalline forms of atorvastatin are purer, more stable, or have
advantageous
manufacturing properties than the amorphous product.
SUMMARY OF THE INVENTION
Accordingly, the present invention is directed to crystalline Form V
atorvastatin and
hydrates thereof characterized by the following X-ray powder diffraction
pattern expressed in
terms of the 26 and relative intensities with a relative intensity of >10%
measured on a
Shimadzu~ diffractometer with CuKa radiation:
28 Relative Intensity
(> 10%)a
4.9 (broad) 9
6.0 15
7.0 100
8.0 (broad) 20
8.6 57
9.9 22
16.6 42
19.0 27
21.1 35
a Relative intensity of 4.9 (broad) 20 is 9.
CA 02521776 2002-05-21
4
Additionally, the following X-ray powder diffraction pattern of crystalline
Form V
atorvastatin expressed in terms of the 28 values was measured on an IneITM
(capillary)
diffractometer:
28
5.0
6.1
7.5
8.4 (broad)
8.7 (broad)
9.9
16.7
19.0
21.2
Further, the present invention is directed to crystalline Form V atorvastatin
and
hydrates thereof characterized by the following solid-state'3C nuclear
magnetic resonance
(ssNMR) spectrum wherein chemical shift is expressed in parts per million:
Assignment Chemical Shift
C 12 or C25 185.7
C I 2 or C25 176.8
C16 166.9
Aromatic Carbons 138.7
C2-C5, C 13-C 18, 136.3
C 19-C24, C27-C32
133.0
128.4
122.0
117.0
116.3
C8, C 10 68.0
Metl~lene Carbons 43.1
C6, C7, C9, C 11
C33 25.6
C34 19.9
CA 02521776 2002-05-21
Additionally, the present invention is directed to crystalline Form V
atorvastatin and
hydrates thereof characterized by the following Raman spectrum having peaks
expressed in
cm':
3062
1652
1604
1528
1478
1440
1413
1397
1368
1158
1034
1001
825
245
224
130
In a preferred embodiment of the first aspect of the invention, crystalline
Form V
5 atorvastatin is a trihydrate.
In a second aspect, the present invention is directed to crystalline Form VI
atorvastatin and hydrates thereof characterized by the following X-ray powder
diffraction
pattern expressed in terms of the 28 and relative intensities with a relative
intensity of >10%
measured on a Shimadzu~ diffractometer with CuKa radiation:
CA 02521776 2002-05-21
6
28 Relative Intensity
(> l0a/o)a
7.2 11
8.3 77
11.0 20
12.4 11
13.8 9
16.8 14
18.5 100
19.7 (broad) 22
20.9 14
25.0 (broad) 15
a Relative intensity of 13.8 (broad) 28 is 9.
Additionally, the following X-ray powder diffraction pattern of crystalline
Form VI
atorvastatin expressed in terms of the 28 values was measured on an IneITM
(capillary)
diffractometer:
28
7.3
8.5
11.2
12.7
14.0
17.1 (broad)
18.7
19.9
21.1 (broad)
25.2 (broad)
Further, the present invention is directed to crystalline Form VI atorvastatin
and
hydrates thereof characterized by the following solid-state'~C nuclear
magnetic resonance
spectrum wherein chemical shift is expressed in parts per million:
CA 02521776 2002-05-21
7
Assignment Chemical Shift
C 12 or C25 176.5
C 16 or C 12 168.2
or C25
C 16 or C 12 163.1
or C25
C 16 or C 12 159.8
or C25
Aromatic Carbons 136.8
C2-C5, C 13-C 18, 127.8
C19-C24, C27-C32
122.3
118.8
113.7
C8, C 10 88.2
C8, C10 79.3
70.5
Methylene Carbons 43.3
C6, C7, C9, C 11 36.9
31.9
C33, C34 25.9
C33, C34 22.5
In a third aspect, the present invention is directed to crystalline Form VII
atorvastatin
and hydrates thereof characterized by the following X-ray powder diffraction
pattern
expressed in terms of the 20 and relative intensities with a relative
intensity of >10%
measured on a Shimadzu~ diffractometer with CuKa radiation:
CA 02521776 2002-05-21
8
28 Relative Intensity
(> 10%)
8.6 76
10.2 70
12.4 (broad) 12
12.8 (broad) 15
17.6 20
18.3 (broad) 43
19.3 100
22.2 (broad) 14
23.4 (broad) 23
23.8 (broad) 26
25.5 (broad) 16
Additionally, the following X-ray powder diffraction pattern of crystalline
Form VII
atorvastatin expressed in terms of the 28 values was measured on an IneITM
(capillary)
diffractometer:
28
8.7
10.2
12.4
12.9
17.6
18.4
19.4
22.2
23.5
23.9
25.6
CA 02521776 2002-05-21
9
Further, the present invention is directed to crystalline Form VII
atorvastatin and
hydrates thereof characterized by the following solid-state'3C nuclear
magnetic resonance
spectrum wherein chemical shift is expressed in parts per million:
Assignment Chemical Shift
C I2 or C25 186.5
C 12 or C25 183.3
C 12 or C25 176.8
C 16 166.5
159.2
Aromatic Carbons 137.6
C2-C5, C 13-C 128.3
18,
C 19-C24, C27-C32
122.3
119.2
C8, C10 74.5
C8, C10 70.3
C8, C10 68.3
C8, C 10 66.2
Meth~lene Carbons 43.5
C6, C7, C9, C 11 40.3
C33, C34 26.3
C33, C34 24.9
C33, C34 20.2
Additionally, the present invention is directed to crystalline Form VII
atorvastatin and
hydrates thereof characterized by the following Raman spectrum having peaks
expressed in
cm ~
CA 02521776 2002-05-21
Raman Spectrum
3060
2927
1649
1603
1524
1476
1412
1397
1368
1159
1034
998
824
114
In a preferred embodiment of the third aspect of the invention, crystalline
Form VII
atorvastatin is a sesquihydrate.
5 In a fourth aspect, the present invention is directed to crystalline Form
VIII
atorvastatin and hydrates thereof characterized by the following X-ray powder
diffraction
pattern expressed in terms of the 28 and relative intensities with a relative
intensity of >10%
measured on a Shimadzu " diffractometer with CuKo, radiation:
CA 02521776 2002-05-21
11
28 Relative Intensity
(> 10%ae
7.5 61
9.2 29
10.0 16
12.1 10
12.8 6
13.8 4
15.1 13
16.7 (broad) 64
18.6 (broad) 100
20.3 (broad) 79
21.2 24
21.9 30
22.4 19
25.8 33
26.5 20
27.4 (broad) 38
30.5 20
a Relative intensity of 12.8 2A is 6 and 13.8 28 is 4.
Additionally, the following X-ray powder diffraction pattern of crystalline
Form VIII
atorvastatin expressed in terms of the 28 values was measured on an InelTM
(capillary)
diffractometer:
2A
7.5
9.3
10.1
12.2
12.8
CA 02521776 2002-05-21
12
28
13.8
15.1
16.6-16.9
18.5-18.9
20.2-20.6
21.3
22.0
22.5
25.9
26.5
27.4 (broad)
30.6
Further, the present invention is directed to crystalline Form VIII
atorvastatin and
hydrates thereof characterized by the following solid-state'3C nuclear
magnetic resonance
spectrum wherein chemical shift is expressed in parts per million:
Assignment Chemical Shift
C 12 or C25 186.1
C 12 or C25 179.5
C16 167.9
C16 161.0
Aromatic Carbons 139.4
C2-C5, C 13-C 132.9
18,
C 19-C24, C27-C32
128.7
124.7
121.8
116.6
C8, C 10 67.0
S
CA 02521776 2002-05-21
13
Assignment Chemical Shift
Methylene Carbons 43.3
C6, C7, C9, C 11
C33, C34 26.7
C33, C34 24.7
C33, C34 20.9
C33, C34 20.1
Additionally, the present invention is directed to crystalline Form VIII
atorvastatin
and hydrates thereof characterized by the following Raman spectrum having
peaks expressed
in cm~l:
Raman Spectrum
3065
2923
1658
1603
1531
1510
1481
1413
997
121
In a preferred embodiment of the fourth aspect of the invention, crystalline
Form VIII
atorvastatin is a dihydrate.
In a fifth aspect, the present invention is directed to crystalline Form IX
atorvastatin
and hydrates thereof characterized by the following X-ray powder diffraction
pattern
expressed in terms of the 28 and relative intensities with a relative
intensity of >10%
measured on a Shimadzu° diffractometer with CuKQ radiation:
CA 02521776 2002-05-21
14
28 Relative Intensity
(>10Io)
8.8 50
9.4 (broad) 32
11.2-11.7 (broad)26
16.7 59
17.5 (broad) 33
19.3 (broad) 55
21.4 (broad) 100
22.4 (broad) 33
23.2 (broad) 63
29.0 (broad) 15
30.0 11
Additionally, the following X-ray powder diffraction pattern of crystalline
Form IX
atorvastatin expressed in terms of the 28 values was measured on an IneITM
(capillary)
diffractometer:
28
9.0
9.4
10.0-10.5 (broad)
11.8-12.0 (broad)
16.9
17.5 (broad)
19.4 (broad)
21.6 (broad)
22.6 (broad)
23.2 (broad)
29.4 (broad)
In a sixth aspect, the present invention is directed to crystalline Form X
atorvastatin
and hydrates thereof characterized by the following X-ray powder diffraction
pattern
CA 02521776 2002-05-21
expressed in terms of the 28 and relative intensities with a relative
intensity of >10%
measured on a Shimadzu~ diffractometer with CuKa radiation:
28 Relative Intensity
(>10%)
4.7 35
5.2 24
5.8 11
6.9 13
7.9 53
9.2 56
9.5 50
10.3 (broad) 13
1 I .8 20
16.1 13
16.9 39
19.1 100
19.8 71
21.4 49
22.3 (broad) 36
23.7 (broad) 37
24.4 15
28.7 31
5 Additionally, the following X-ray powder diffraction pattern of crystalline
Form X
atorvastatin expressed in terms of the 28 values was measured on an IneITM
(capillary)
diffractometer:
CA 02521776 2002-05-21
16
28
4.7
5.2
5.8
6.9
7.9
9.2
9.6
10.2-10.4
11.9
16.2
16.9
19.1
19.9
21.5
22.3-22.6
23.7-24.0 (broad)
24.5
28.8
Further, the present invention is directed to crystalline Form X atorvastatin
and
hydrates thereof characterized by the following solid-state ~3C nuclear
magnetic resonance
spectrum wherein chemical shift is expressed in parts per million:
Assignment Chemical Shift
C 12 or C25 187.0
C 12 or C25 179.5
C16 165.5
C16 159.4
CA 02521776 2002-05-21
17
Assignment Chemical Shift
Aromatic Carbons137.9
C2-C5, C 13-C 134.8
18,
C 19-C24, C27-C32129.4
127.9
123.2
119.9
C8, C10 71.1
Methylene Carbons43.7
C6, C7, C9, C 40.9
11
C33 26.4
25.3
C34 20.3
18.3
Additionally, the present invention is directed crystalline Form X
atorvastatin and
hydrates thereof characterized by the following Raman spectrum having peaks
expressed in
cm':
Raman Spectrum
3062
2911
1650
1603
1525
1478
1411
1369
1240
1158
1034
999
CA 02521776 2002-05-21
18
Raman Spectrum
824
116
In a preferred embodiment of the sixth aspect of the invention, crystalline
Form X
atorvastatin is a trihydrate.
In a seventh aspect, the present invention is directed to crystalline Form XI
atorvastatin and hydrates thereof characterized by the following X-ray powder
diffraction
pattern expressed in terms of the 28 and relative intensities with a relative
intensity of >10%
measured on a Shimadzu° diffractometer with CuKa radiation:
28 Relative Intensity
(>10%)
10.8 (broad) 58
12.0 12
13.5 11
16.5 52
17.6-18.0 (broad)35
19.7 82
22.3 100
23.2 26
24.4 28
25.8 17
26.5 30
27.3 31
28.7 19
29.5 12
30.9 (broad) 17
32.8 (broad) 11
33.6 (broad) 15
36.0 (broad) 15
38.5 (broad) 14
CA 02521776 2002-05-21
19
In an eighth aspect, the present invention is directed to crystalline Form XII
atorvastatin and hydrates thereof characterized by the following X-ray powder
diffraction
pattern expressed in terms of the 28 and relative intensities with a relative
intensity of >10%
measured on a Shimadzu° diffractometer with CuK~ radiation:
26 Relative Intensity
(>10%)a
5.4 11
7.7 24
8.0 25
8.6 42
8.9 25
9.9 36
10.4 (broad) 24
12.5 18
13.9 (broad) 9
16.2 10
17.8 70
19.4 100
20.8 51
21.7 13
22.4-22.6 (broad)18
24.3 19
25.5 24
26.2 11
27.1 8
° Relative intensity of 13.9 (broad) 28 is 9 and
27.1 2B is 8.
Additionally, the following X-ray powder diffraction pattern of crystalline
Form XII
atorvastatin expressed in terms of the 28 values was measured on an IneITM
(capillary)
diffractometer:
CA 02521776 2002-05-21
28
5.4
7.7
8.1
8.6
8.9
10.0
10.5
12.6
14.0 (broad)
16.2
17.9
19.4
20.9
21.8
22.5-22.8 (broad)
24.4
25.6
26.4
27.2
Additionally, the present invention is directed crystalline Form XII
atorvastatin and
hydrates thereof characterized by the following Raman spectrum having peaks
expressed in
5 cm ~
Raman Spectrum
3064
2973
292b
1652
1603
1527
CA 02521776 2002-05-21
21
Raman Spectrum
1470
1410
1367
1240
1159
1034
1002
823
In a ninth aspect, the present invention is directed to crystalline Form XIII
atorvastatin
and hydrates thereof characterized by the following X-ray powder diffraction
pattern
expressed in terms of the 28 and relative intensities with a relative
intensity of >10%
measured on a Shimadzu° diffractometer with CuKa radiation:
28 Relative Intensity
(>10%)
8.4 100
8.9 82
15.7 (broad) 45
16.4 (broad) 46
17.6 (broad) 57
18.1 (broad) 62
19.7 (broad) 58
20.8 (broad) 91
23.8 (broad) 57
In a tenth aspect, the present invention is directed to crystalline Form XIV
atorvastatin
and hydrates thereof characterized by the following X-ray powder diffraction
pattern
CA 02521776 2002-05-21
22
expressed in terms of the 28 and relative intensities with a relative
intensity of >10%
measured on a Bruker D5000 diffractometer with CuKa radiation:
26 Relative Intensity
(> 10%)
5.4 41
6.7 31
7.7 100
8.1 35
9.0 65
16.5 (broad) 15
17.6 (broad) 17
18.0-18.7 (broad)21
19.5 (broad) 18
In an eleventh aspect, the present invention is directed to crystalline Form
XV
atorvastatin and hydrates thereof characterized by the following X-ray powder
diffraction
pattern expressed in terms of the 28 and relative intensities with a relative
intensity of >10%
measured on a Bruker° D5000 diffractometer with CuKa radiation:
28 Relative Intensity
(>I O%)
5.7 26
6.1 ZI
6.8 18
7.5 39
8.1 39
8.5 42
9.5 33
10.5 (broad) 18
19.1-19.6 (broad)32
CA 02521776 2002-05-21
23
In a twelfth aspect, the present invention is directed to crystalline Form XVI
atorvastatin and hydrates thereof characterized by the following X-ray powder
diffraction
pattern expressed in terms of the 28 and relative intensities with a relative
intensity of >10%
measured on a Bruker~ DS000 diffractometer with CuKa radiation:
28 Relative Intensity
(> 10%)
5.2 37
6.4 34
7.5 100
8.7 79
10.5 (broad) 19
12.0 (broad) 10
I2.7 (broad) 17
16.7 26
18.3 (broad) 27
I9.5 23
20.1-20.4 (broad)37
21.2-21.9 (broad)32
22.9-23.3 (broad)38
24.4-25.0 {broad)35
Additionally, the following X-ray powder diffraction pattern of crystalline
Form XVI
atorvastatin expressed in terms of the 28 values was measured on a
Shimadzu° diffractometer
with CuKa radiation:
26
7.6
8.8
10.2
12.5
CA 02521776 2002-05-21
24
28
16.8
18.2
19.3
20.5
23.0
24.8
In addition, the following X-ray powder diffraction pattern of crystalline
Form XVI
atorvastatin expressed in terms of the 26 values was measured on an IneITM
(capillary)
diffractometer:
2A
5.1
6.2
7.3
8.7
10.2 (broad)
12.0 (broad)
12.7 (broad)
16.7
18.0 (broad)
19.5 (broad)
20.0-20.5 (broad)
21.5-21.6 (broad)
22.9-23.3 (broad)
24.0-25.0 (broad)
In a thirteenth aspect, the present invention is directed to crystalline Form
XVII
atorvastatin and hydrates thereof characterized by the following X-ray powder
diffraction
pattern expressed in terms of the 28 and relative intensities with a relative
intensity of >10%
measured on a Bruker° D5000 diffractometer with CuKQ radiation:
CA 02521776 2002-05-21
28 Relative Intensity
(>10%)
5.0 27
6.1 33
7.3 100
7.9 30
8.5 29
9.1 22
10.0 45
12.1 (broad) 24
14.8 17
16.0-16.5 (broad)20
17.5 (broad) 28
19.0 (broad) 46
19.5 65
20.2 (broad) 47
21.3 64
21.6 55
22.0 45
In a fourteenth aspect, the present invention is directed to crystalline Form
XVIII
atorvastatin and hydrates thereof characterized by the following X-ray powder
diffraction
5 pattern expressed in terms of the 28 and relative intensities with a
relative intensity of >10%
measured on a Broker ° D5000 diffractometer with CuI~ radiation:
CA 02521776 2002-05-21
26
2B Relative Intensity
(>10%)
8.0 100
9.2 (broad) 52
9.7 (broad) 40
12.1 24
16.6 (broad) 48
18.5 67
Additionally, the following X-ray powder diffraction pattern of crystalline
Form
XVIII atorvastatin expressed in terms of the 28 values was measured on a
Shimadzu~
diffractometer with CaKa radiation:
28
7.7
9.3
9.9
12.2
16.8
18.5
In addition, the following x-ray powder diffraction pattern of crystalline
Form XVIII
atorvastatin expressed in terms of the 28 values was measured on an IneITM
(capillary)
diffractometer:
7.9
9.2 (broad)
9.8 (broad)
12.2 (broad)
16.7 (broad)
18.5
CA 02521776 2002-05-21
27
In a fifteenth aspect, the present invention is directed to crystalline Form
XIX
atorvastatin and hydrates thereof characterized by the following X-ray powder
diffraction
pattern expressed in terms of the 2B and relative intensities with a relative
intensity of >10%
measured on a Bruker~ D5000 diffractometer with CuKa radiation:
28 Relative Intensity
(>10%)
5.2 32
6.3 28
7.0 I 00
8.6 74
10.5 34
11.6 (broad) 26
12.7 (broad) 35
14.0 15
16.7 (broad) 30
I 8.9 86
20.8 94
23.6 (broad) 38
25.5 (broad) 32
In a further aspect of the present invention, there is provided crystalline
Forms V to
XIX atorvastatin or hydrates thereof characterized by X-ray powder diffraction
containing
such 28 values measured using CuKaradiation or by solid state'3C nuclear
magnetic
resonance having such chemical shifts expressed in parts per million or by
Raman
spectroscopy having such peaks, or characterized by combinations of such
values obtained
from two or more of such analytical techniques, as are sufficient to
distinguish each such
Form from other different crystalline forms.
A yet further aspect of the present invention is each of crystalline Forms V
to XIX
atorvastatin or a hydrate thereof having the diffractogram or spectrum, as the
case may be, of
the corresponding graph within Graphs 1 to 35 of this description.
CA 02521776 2002-05-21
2$
As inhibitors of HMB-CoA reductase, the novel crystalline forms of
atorvastatin are
useful hypolipidemic and hypocholesterolemic agents as well as agents in the
treatment of
osteoporosis and Alzheimer's disease.
A further embodiment of the present invention is a pharmaceutical composition
comprising the above-noted crystalline Form V, VI, VII, VIII, IX, X, XI, XII,
XIII, XIV, XV,
XVI, XVII, XVIII, or XIX atorvastatin or a hydrate thereof and at least one
pharmaceutically
acceptable excipient, diluent or carrier.
In a yet further aspect, there is provided a pharmaceutical atorvastatin
composition
prepared using the above-noted crystalline Forms V to XIX atorvastatin or
hydrates thereof.
A yet further embodiment of the present invention comprises the use of the
above-
noted crystalline Form V, VI, VII, VIII, IX, X, XI, XII, XIII, XIV, XV, XVI,
XVII, XVIII, or
XIX, atorvastatin or a hydrate thereof in the treatment of hyperlipidemia,
hypercholesterolemia, osteoporosis or Alzheimer's disease.
In a still further aspect, there is provided herein the use of the above-noted
crystalline
Forms V to XIX or hydrates thereof in the manufacture of a medicament for use
in the
treatment of hyperlipidemia, hypercholesterolemia, osteoporosis or Alzheimer's
disease.
Yet further, the present invention comprises the use of the above-noted
crystalline
Form V, VI, VII, VIII, IX, X, XI, XII, XIII, XIV, XV, XVI, XVII, XVIII, or XIX
atorvastatin
or a hydrate thereof in the preparation of a medicament containing
atorvastatin or a hydrate
thereof. Also comprised in the present invention is such use where the
crystalline form of
atorvastatin or hydrate thereof contained in the medicament corresponds to the
crystalline
form used in the preparation of such medicament.
The present invention is directed also to methods for production of Form V,
Form VI,
Form VII, Form VIII, Form IX, Form X, Form XI, Form XII, Form XIII, Form XIV,
Form
XV, Form XVI, Form XVII, Form XVIII, or Form XIX atorvastatin.
The present invention is further directed to amorphous atorvastatin for use in
the
preparation of crystalline Form V, Form VI, Form VII, Form VIII, Form IX, Form
XI, Form
XIV, Form XV, Form XVI, Form XVII, and Form XIX atorvastatin or hydrates
thereof, to
amorphous atorvastatin seeded with crystalline Form VII atorvastatin or a
hydrate thereof for
CA 02521776 2002-05-21
29
use in the preparation of crystalline Form VIII atorvastatin or a hydrate
thereof, and to
amorphous atorvastatin seeded with crystalline Form IX atorvastatin or a
hydrate thereof for
use in the preparation of crystalline Form IX atorvastatin or a hydrate
thereof. Yet further,
the present invention is directed to crystalline Form I atorvastatin or a
hydrate thereof for use
in the preparation of crystalline Form V and Form XIII atorvastatin or
hydrates thereof.
Finally the present invention is directed to crystalline Form XVI atorvastatin
or a hydrate
thereof for use in the preparation of crystalline Form XVIII atorvastatin or a
hydrate thereof.
BRIEF DESCRIPTION OF THE DRAWINGS
The invention is further described by the following nonlimiting examples which
refer
to the accompanying Figures 1 to 35, short particulars of which are given
below. These
thirty-five diffractograms or spectra, as the case may be, are reproduced as
corresponding
Graphs 1 to 35 below in this description.
F~_' lure 1
Diffractogram of Form V atorvastatin carried out on Shimadzu° XRD-
6000
diffractometer.
Fi au re 2
Diffractogram of Form VI atorvastatin carried out on Shimadzu° XRD-
6000
diffractometer.
Fi u~ re 3
Diffractogram of Form VII atorvastatin carried out on Shimadzu°
XRD-6000
diffractometer.
Fi_ '_ ug re 4
Diffractogram of Form VIII atorvastatin carried out on Shimadzu°
XRD-6000
diffractometer.
Fi- u~ re 5
Diffractogram of Form IX atorvastatin carried out on Shimadzu° XRD-
6000
diffractometer.
CA 02521776 2002-05-21
Fi ore 6
Diffractogram of Form X atorvastatin carried out on Shimadzu ° XRD-
6000
diffractometer.
Fi ore 7
5 Diffractogram of Form XI atorvastatin carried out on Shimadzu° XRD-
6000
diffractometer.
Fy u~ re 8
Diffractogram of Form XII atorvastatin carried out on Shimadzu°
XRD-6000
diffractometer.
10 Fy ug re 9
Diffractogram of Form XIII atorvastatin carried out on Shimadzu°
XRD-6000
diffractometer.
Fi u,~re 10
Diffractogram of Form XIV atorvastatin carried out on Broker ° D
5000
15 diffractometer.
Fi-u~ re 11
Diffractogram of Form XV atorvastatin carried out on Bnuker° D 5000
diffractometer.
Figure 12
Diffractogram of Form XVI atorvastatin carried out on Bruker° D
5000
20 diffractometer.
Fi u~ ry e13
Diffractogram of Form XVII atorvastatin carried out on Bruker° D
5000
diffractometer.
Fi u-~ re 14
25 Diffractogram of Form XVIII atorvastatin carried out on Bruker° D
5000
diffractometer.
CA 02521776 2002-05-21
31
Fi urn a 15
Diffractogram of Form XIX atorvastatin carried out on Bruker° D
5000
diffractometer.
Fi ug. re 16
Diffractogram of Form V atorvastatin carried out on IneITM XRG-3000
diffractometer.
Fig-ure 17
Diffractogram of Form VI atorvastatin carried out on IneITM XRG-3000
diffractometer.
Fi ug-re 18
Diffractogram of Form VII atorvastatin carried out on IneITM XRG-3000
diffractometer.
Fi.ure 19
Diffractogram of Form VIII atorvastatin carried out on IneITM XRG-3000
diffractometer.
Fi a re 20
Diffractogram of Form IX atorvastatin carried out on IneITM XRG-3000
diffractometer.
Figure 21
Diffractogram of Form X atorvastatin carried out on IneITM XRG-3000
diffractometer.
Figure 22
Diffractogram of Form XII atorvastatin carried out on InelTM XRG-3000
diffractometer.
Figure 23
Diffractogram of Form XVI atorvastatin carried out on IneITM XRG-3000
diffractometer.
Figure 24
Diffractogram of Form XVIII atorvastatin carried out on IneITM XRG-3000
diffractometer.
CA 02521776 2002-05-21
32
F~ure 25
Solid-state'3C nuclear magnetic resonance spectrum with spinning side bands
identified by an asterisk of Form V atorvastatin.
Figure 26
Solid-state'3C nuclear magnetic resonance spectrum with spinning side bands
identified by an asterisk of Form VI atorvastatin.
Fi uQ re 27
Solid-state'3C nuclear magnetic resonance spectrum with spinning side bands
identified by an asterisk of Form VII atorvastatin.
Figure 28
Solid-state'3C nuclear magnetic resonance spectrum with spinning side bands
identified by an asterisk of Form VIII atorvastatin.
Fi -urn a 29
Solid-state ~ ~C nuclear magnetic resonance spectrum of Form X atorvastatin.
Figure 30
Raman spectrum of Form V.
Figure 31
Raman spectrum of Form VI.
F~ure 32
Raman spectrum of Form VII.
Fi ugLre 33
Raman spectrum of Form VIII.
F~ure 34
Raman spectrum of Form X.
Figure 35
Raman spectrum of Form XII.
DETAILED DESCRIPTION OF THE INVENTION
Crystalline Form V, Form VI, Form VII, Form VIII, Form IX, Form X, Form XI,
Form XII, Form XIII, Form XIV, Form XV, Form XVI, Form XVII,
CA 02521776 2002-05-21
33
Form XVIII, and Form XIX atorvastatin may be characterized by their X- ray
powder
diffraction patterns, by their solid state nuclear magnetic resonance spectra
(NMR), and/or
their Raman spectra.
X-RAY POWDER DIFFRACTION
Forms V, VI, VII, VIII, IX, X, XI, XII, XIII, XIV, XV, XVI, XVII, XVIII, and
XIX
Forms V, VI, VII, VIII, IX, X, XI, XII, XIII, XIV, XV, XVI, XVII, XVIII, or
XIX
atorvastatin were characterized by their X-ray powder diffraction pattern.
Thus, the X-ray
diffraction patterns of Forms V, VI, VII, VIII, IX, X, XI, XII, or Form XIII
atorvastatin were
carried out on a Shimadzu° XRD-6000 X-ray powder diffractometer using
CuKa radiation.
The instrument is equipped with a fine-focus X-ray tube. The tube voltage and
amperage
were set at 40 kV and 40 mA, respectively. The divergence and scattering slits
were set at 1 °,
and the receiving slit was set at 0.15 mm. Diffracted radiation was detected
by a NaI
scintillation detector. A theta-two theta continuous scan at 3°/min
(0.4 sec/0.02° step) from
2.5 to 40 °28 was used. A silicon standard was analyzed each day to
check the instrument
alignment. The X-ray diffraction patterns of Forms XIV, XV, XVI, XVII, XVIII,
and XIX
were carried out on a Bruker° D5000 diffractometer using copper
radiation, fixed slits ( 1.0,
1.0, 0.6 mm), and a KevexTM solid state detector. Data was collected from 3.0
to 40.0
degrees in 28 using a step size of 0.04 degrees and a step time of 1.0
seconds. It should be
noted that Bruker° Instruments purchased Siemens°; thus, a
Bruker° D 5000 instrument is
essentially the same as a Siemens° D 5000.
The X-ray diffraction patterns of Forms V, VI, VII, VIII, IX, X, XII, XVI, and
XVIII
were also carried out on an IneITM diffractometer. X-ray diffraction analyses
were carried out
on an IneITM XRG-3000 diffractometer, equipped with a Curved Position
Sensitive (CPS)
detector with a 28 range of 120 degrees. Real time data were collected using
CuKq radiation
starting at approximately 4 °28 at a resolution of 0.03 °28. The
tube voltage and amperage
were set to 40 kV and 30 mA, respectively. Samples were prepared for analysis
by packing
them into thin-walled glass capillaries. Each capillary was mounted onto a
goniometer head
that is motorized to permit spinning of the capillary during data acquisition.
Instrument
calibration was performed daily using a silicon reference standard. The IneITM
diffractograms
CA 02521776 2002-05-21
34
for the available forms are shown in the figures without baseline subtraction.
Calculating the
intensities from these diffractograms is within the skill of the art and
involves using baseline
subtraction to account for background scattering (e.g., scattering from the
capillary).
To perform an X-ray powder diffraction measurement on a Shimadzu° or
Broker
instrument like the ones used for measurements reported herein, the sample is
typically
placed into a holder which has a cavity. The sample powder is pressed by a
glass slide or
equivalent to ensure a random surface and proper sample height. The sample
holder is then
placed into the instrument (Shimadzu° or Broker°). The source of
the X-ray beam is
positioned over the sample, initially at a small angle relative to the plane
of the holder, and
moved through an arc that continuously increases the angle between the
incident beam and
the plane of the holder. Measurement differences associated with such X-ray
powder analyses
result from a variety of factors including: (a) errors in sample preparation
(e.g., sample
height), (b) instrument errors (e.g., flat sample errors), (c) calibration
errors, (d) operator
errors (including those errors present when determining the peak locations),
and (e) preferred
orientation. Calibration errors and sample height errors often result in a
shift of all the peaks
in the same direction and by the same amount. Small differences in sample
height on a flat
holder lead to large displacements in XRPD peak positions. A systematic study
showed that,
using a Shimadzu° XRD-6000 in the typical Bragb Brentano configuration,
sample height
differences of 1 mm led to peak shifts as high as 1 °29(Chen, et al.,
J. Pharmaceutical and
Biomedical Analysis, 2001;26:63). These shifts can be identified from the X-
ray
diffractogram and can be eliminated by compensating for the shift (applying a
systematic
correction factor to all peak position values) or recalibrating the
instrument. In contrast, the
Inel instrument used herein places the sample in a capillary which is
positioned at the center
of the instrument. This minimizes sample height errors (a) and preferred
orientation (e).
Since, when using capillaries, the sample height is not established manually,
the peak
locations from the IneITM measurements are typically more accurate than those
from the
Shimadzu° or the Bruker° instrument. As mentioned above, it is
possible to rectify
measurements from the various machines by applying a systematic correction
factor to bring
the peak positions into agreement. In general, this correction factor will
bring the peak
positions from the Shimadzu° and Broker into agreement with the IneITM
and will be in the
range of 0 to 0.2 °29.
CA 02521776 2002-05-21
Table 1 lists the 2B and relative intensities of all lines in the sample with
a relative
intensity of >10% for crystalline Forms V-XIX atorvastatin. The numbers listed
in this table
are rounded numbers.
CA 02521776 2002-05-21
~
7G~a~ o
N N N M N ~ O O
d O~~
O N
w
~t # N
d v:O;N 00
~ O vDQ\pv C
r
v7r 000000O~
cd
p
...
, .Ns m
~ay o 0
ai n o0N ~ N ~ N O v000t~O ~ 'O
~'~
(y~ V:~ - ~ M 00 N N M c~
O
4.
u.
O
00 M
0oO v:~n~Ot~ M N d oo~ ~~ ~_..~
m O N ~ ~ ~ ~C
N , ~ .- N N N N N N
_, r
O O
_ w
> ~ w s w
y o c
E ~ ~nd'~ M M ~O O M O M O~ O
~
s.. ~ fx..~ M N .--~ v~in ~n~ N ~
.
O
w
O
~, ~.
M 00 m
m I~N 00Q~O~N v:p ..:y p Os N
N d ~!1V O l~O~ O~ pp
W
O
r- N y b
> .~s
g o
o .a
O N vOO~M ~ O M M ~ ~
'~~D
.
U ~C~, ~ M N ~ M Vi c
.
M N
~
W -~ 00
~ fL ~ N
M ~ y ~ .w~ a~.x~ .x
'
N ~ h M d ~ N O O
in by
N N N
00O~ N
r~ C
j y y O
> .~~ w
. '
cC iCy O
O
N N ~ O W O ~ d Ovd O
O O~
~ CWC ~ON ~ ~Od Vr l~N M
-.
m
N
3 ~ 0000 ~ t~~Oc:N Os
d
_
O v~N O ~ ~ ~ ~ ~ 00O N
N
N l~ ~
r
y V
O > in~
'
w
U ~ ~ O
~ _ O 0 O N m O r: O d M ~O~O
4-. f'L y l~1~~ ~ N d ~ ~ N N ~ W
_ O
O
Q U iFj: iF -1~iE W%
N d o0v0M crN ~ oov:
v. y.
O ~ m ~ O N N ~ oo Q~cVM M v~
N pp r. N N N N
O ~ v
O~
.
cC ~ y ~ d
O b
x '~ ~ d o O d
C C t~O --~~ d N
cG ~ ~ N ~ O
v o w
c u.
c o d o000 ~nn o.o w
O N <'~' ~ r v0 a0O~O
y.. N r pp~ , ~ N N
C
i/u O y v y
y ~ m 6~ .
C
C yr fC~ O O O
O O (~N N l~v1 >
N V7N d N M
y O
u. W
~ O
c O OvO O ~OcT
C
E"~ N d W0t~00a0Os N
CA 02521776 2002-05-21
a~
T
..
~ p~ ~ 00O~N -.
G'
O
. 00
rC
O
N
w
.
f L N
~00 l~fVM V:N .~
~ON N ~ N N N N
N O
N
O
N
>
.L
o
~
D1N ~ ~ V
.y ~
C
O
,-, .- t.
n .-
X c~
~
E
'- M
0
u. .~~.~ ~ Y
m ~'r?ovoov?o ~n
N ~~ O cVM v000
NN
M
M M M M
O O
w
~ 'O
~
"~ v~ -~ ~Ot~~ ~ C
.cC ra M M cr;
O
o CG
~
v
w ~ ,
L
O GL ~ m
+ O 00~ M l~~ l
-
N ~N N
O N N
C 'U
>
t ~ c~
.i
E-.i n ~O
~~
~
O
n
~
M x N
L O
N ~p N
L'
M .r
' n b0 O
N
C
v.
> O
w
>.
y ~ MO ooO
C
O
MN N N
7 CL' m
~
~
. N
~
3
ON NN N
C
O O
w
cv O~
~
O
V= N .._
'i a~
- y n
_ ~ ~c
m
O i..
_
u. b
cd
p ~w O
C
~ 0 01
_
~ N
~ O
.c
_ 7 _
.Y a
~
cG ~ N O
~ ~n ~ rC.. w
/~
x
~p O
C
c~ 'b m T
...
L N ;
G
tn ~
> C
.
7 ~ .
~
.ccS
~
O
C ~ ~
.>
. L CO
O
m
N ~
r
CA 02521776 2002-05-21
00
. o0 ~ d d ~Dv~ O d 00N
S vD
G N N ,_,l~M N M ~ M O~ M M
p M 00 m
y
X x
,~
n
N
o .~~. -~
N M O v0~'~OI~O ~ ~~ ~ V:
N W J l~~ ~ - ~ a O N N O
.
m
N
a> b
~ c~
~
='inO O
~
~ O ,Nr,d N d ~D
~
x '' ~.
n
0. o,
.
~
E
.x .~~ ~ 'n
'~ s ~ ~ >C
~ o o; ; ~
_ E
o c
u.
~'S ~ ~ O a~N m d t~ OOO VOv;I~d V%~ ~d
p t M M N N d N ~- NN d ~Od V
~ N M ~O %
d
v~ L ,_, c0
E ; m
;
O ~._,00 v0v-.~O'nN v0 00
I1. O .~ M O~Vi~ M O
p N V;vO I~(~oOO~~ N ~ p~ ~ ~ ~N NN
C
c~ c0
E'~ N
y
~
_ r d O OvQ'O Iw0 t~ Mf~ N o0W
c NM M M M
O
% G M M ~ ~ .-..-.--N N N
~
y c 7 a~ ~ 00
', j~
w.. w C~
,
C
"
k w
OO M
M ~ a> E O
y by v. ~ OsM
ca o -~-*-'~,~~ ~;O M v: ..~
G. LL N d ~ ~ ~ O ~ M N N N N
m v;v0 (~00O N N ~ 00 ~.- N O~d
N
O N N
N N
O~
> 00OvO~N M 00N . C
in ~
~
O
cC N N - M M d M M
~
x as
7 ~ o0
n
3 x
~ '
' >
ti.m ,~,~ op~;r-.v~v;,~;_ a
O N v~~O Vrf~0000OvO
o ' E w
os
> O
S
O ~ .-. v 'r~;~ N
cC ~ M ~ ; ~V
O M
x~
~s--
0
o ~ ~
_
d t~ t~~ O mJ~ V%
U' ~ d
m
N v;v0 I~00O WO~ O'O~ r~C
'
~_i
,~
O
~C
w
C O ~' O N v W 1~N 00-rt~
~ D y
cC'D ~y~ O OO d d v v;a,~n ~'
'' ~ ,
n
a~ ~ . ~ C
a .
~, i E ~C
.d
= O o t -~~c~c-x.x-.x- c
LL d O~ t~d v0- t~0000
~
O'_' ~ 0000 ~p~ 00U O N N
--n - ~ >
x ._
O
O
c~c aF
CL
a
H
CA 02521776 2002-05-21
39
While a number of crystalline forms of atorvastatin are known, each form can
be
identified and distinguished from the other crystalline forms by either a
combination of lines
or a pattern that is different from the X-ray powder diffraction of the other
forms.
For example, Table 2 lists combinations of 28 peaks for Forms V to XIX
atorvastatin,
i.e., a set of X-ray diffraction lines that are unique to each form. Forms I
to IV atorvastatin
disclosed in United States Patent Numbers 5,969,156 and 6,121,461 are included
for
comparison. Table 3 lists further combinations of 28 peaks fox Forms V to XIX
atorvastatin
that are unique to each form.
CA 02521776 2002-05-21
o\ o\N ~n
N
v ~ ~ a' ov , r',
w ,Y
a.
m
N
~,
".
~ ~ x ~
00~ ~ ~ N ~ O N ~O O
~ o w
w E a, ~
O
w
E
0
U
~r
O jE ~ ~ t~.
>
,nN tw o M ~ w
pv y 0 00O :b
N '~(~ r' O ~ ~ M ~O ~,
O
, .~c y s ' p
, .
w ~ ~ot' ~ o a N N
O
w
w.
a~
x
.x. ~ 3
> N ~ M w.
~p oo M O, Cq
y ~ 00O N ~ 00 - y E 3F
Q, O r p ~ O
>
w
m x N ~ ~ ~ N N b
~
E ~i~o ~ ~ ':o,
'~
w ~
c
C
O
O
> U
O v~
N M 3
0
~ 0 ~,
O ~ ~' SC
U w > ~ ...
x a, x
~ ~ o
o
o a- E ~ ~ o
o
o
o, >
x
x >
0 o p o,
0 0 ~ ~ ~ ~ ~ o
w
w
~. o
> w
~
1~N l~d; ~ cue,
v7 t~a\ ~ 'C
O
w O
O
r~
n
~_
0000 O
"'" d O~N w
M 'O
E ~ O~
~ N
O
w
O
.7
t O vJOs
O~
y"'" ~ ~ 00 0000
t~ O~
-. l ~ ~
O
0 a\ - O w .
p o N ~
r
w l' U
Ov
~'
3
~n
>C~-
~, t~M o
M ~ ~ p ~ ~ ,~ a,N b
O ~
z
p p p N vC"r O ~ ~ N 'n
~ 0
O w c
w
O
ca
~-
w
n,
CA 02521776 2002-05-21
~t os t~
0
w
x
r o ~o o,
E w t~ 00 00 00 0
0
w
x
x
o yo ~
O x N M O
w
0
w
w
m
N
p ~ N 00 N V
s. ~ ~ Ov N
p w r..
X O N
N
D, D\
O
w
0
~
;
x
_
~.
Qv N
w
_
x > o ~
O
O
4.
w
c~ '
w_
N OGl
'~~ ~c
O V
a
,
,
..,
w .~
o
r.', c
.-. D
p
x
~
~
N v N
?
~
~ n: O
d
~
N ~
- 0 w GG
0
0
O N N ~ 0 s
~ c~
O
O
C
O
'c7
L
x r, r., 00 Cd
'
N M ~ ~'. E vW0 v0 F
E. t~ 00 ~ O~ O N
3
v
..,
3
x
0
o
~
> .- ~ ~
x >
5C
o 0 0 .~ os ~ ~ ~
00 0, E u~ 'o w ~ E
~ E
~
,
,
~
~
w
w w
CA 02521776 2002-05-21
42
SOLID STATE NUCLEAR MAGNETIC RESONANCE (NMR)
Methodolo~y
Solid-state'3C NMR spectra were obtained at 270 or 360 MHz TecmagTM
instruments. High-power proton decoupling and cross-polarization with magic-
angle
S spinning at approximately 4.7 and 4.2 kHz or 4.6 and 4.0 kHz were used for
68 MHz ('3C
frequency) data acquisition, 4.9 and 4.4 kHz were used for 91 MHz ('3C
frequency) data
acquisition. The magic angle was adjusted using the Br signal of KBr by
detecting the side
bands. A sample was packed into a 7 nun DotyTM rotor and used for each
experiment. The
chemical shifts were referenced externally to adamantine except for Form X
where the
chemical shifts are arbitrary.
Table 4 shows the solid-state NMR spectrum for crystalline Forms V, VI, VII,
VIII,
and X atorvastatin.
. 31 34 33 39 .
/ p OH OH O
26 5 1 6
29 ~ 27 N 25 / N 8 10 12 O
g 9 2 ~ Ca 2+
3 ".'
lg 13 19
23 ~ 18
16
21
F
2
CA 02521776 2002-05-21
43
Table 4. Chemical Shifts for Forms V, VI, VII, VIII, and X Atorvastatin
Chemical Shift
V VI VII VIII X
185.7 186.5 186.1 187.0
183.3 179.5
176.8 176.5 176.8 179.5
166.9 168.2 166.5 167.9 165.5
163.1 161.0
159.8 159.2 159.4
138.7 136.8 137.6 139.4 137.9
136.3 132.9 134.8
133.0
129.4
128.4 127.8 128.3 128.7 127.9
124.7 123.2
122.0 122.3 122.3
121.8
118.8 119.2 119.9
I 17.0
116.3 116.6
113.7
88.2 74.5
79.3
70.5 70.3 71.1
68.0 68.3 67.0
66.2
43. I 43.3 43.5 43.3 43.7
40.3
36.9 40.9
CA 02521776 2002-05-21
44
Table 4. Chemical Shifts for Forms V, VI, VII, VIII and X Atorvastatin (cont)
Chemical Shift
V VI VII VIII X
25.6 25.9 26.3 26.7 26.4
24.9 24.7 25.3
22.5 20.2 20.9 20.3
I 9.9 20.1
18.3
Forms V, VI, VII, VIII, and X: Relative peak intensity over 20 are shown here
(4.5, 4.6. 4.7,
or 4.9 kHz CPMAS). Spectra were obtained using two different magic-angle
spinning rates
to determine spinning sidebands.
Form X: Relative peak intensity over 20 are shown here (5.0 kHz CPMAS).
Table 5 shows unique solid-state NMR peaks for Forms V, VI, VII, VIII and X
atorvastatin, ie, peaks within ~1.0 ppm. Forms T to IV atorvastatin are
included for
comparison.
CA 02521776 2002-05-21
M
O
3~
~ M
O
r~
r~
1/7 M 00
O
w
w
x
z
M
b
w
0
~ t~ o0 0
V~ ~p M
~C O ~ ~ .M-i
w
c
0
a'
M
' r'
o -
0
w
w
s~ ~ ~ M ~p M
O r...,'-'
w
0 0 0
M .-~ o
0
w
a\
N
CA 02521776 2002-05-21
46
RAMAN SPECTROSCOPY
Methodoloøy
The Raman spectrum was obtained on a Raman accessory interfaced to a Nicolet
Magna 860 FourierTM transform infrared spectrometer. The accessory utilizes an
excitation
wavelength of 1064 nm and approximately 0.45 W of neodymium-doped yttrium
aluminum
garnet (Nd:YAG) laser power. The spectrum represents 64 or 128 co-added scans
acquired at
4 cm' resolution. The sample was prepared for analysis by placing a portion of
a 5-mm
diameter glass tube and positioning this tube in the spectrometer. The
spectrometer was
calibrated (wavelength) with sulfur and cyclohexane at the time of use.
Table 6 shows the Raman spectra for Forms V, VI, VII, VIII, X, and XII
atorvastatin.
CA 02521776 2002-05-21
47
Table 6. Raman Peak Listing for Forms V, VI, VII, VIII, X and XII Atorvastatin
Form V Form VI Form VII Form VIII Form X Form XII
3062 3058 3060 3065 3062 3064
2973
2935 2927 2923 2911 2926
1652 1651 1649 1658 1650 1652
1604 1603 1603 1603 1603 1603
1528 1556 1524 1531 1525 1527
1525 1510
1481
1478 1478 1476 1478 1470
1440
1413 1412 1412 1413 1411 1410
1397 1397
1368 1368 1369 1367
1240 1240
1158 1157 1159 1158 1159
1034 1034 1034 1034
1001 997 998 997 999 1002
825 824 824 823
245
224
130
114 121 116
Relative peak intensity over 20 are shown.
Table 7 lists unique Raman peaks for Forms V, VI, VII, VIII, X, and XII
atorvastatin,
ie, only one other form has a peak with ~4 cm'. In the case of Forms VI and X,
it is unique
combination of peaks. Forms I to IV atorvastatin are included for comparison.
CA 02521776 2002-05-21
'C I M
IN
N ~ N
s.~. O ~
O M N
~ .~ M ,_,
"'~ ~ ~ N
Vl~f~,-,
O
w
x
td
N ~
N
O
w
x
a-
o ~
~, M N r.
~-,
x O
'
, w
b
c~f
O
r~
M
~ M
O
w
r~
t.
O
w
~ M ~ N
.--iM
~ N
O
w
H
00 O O ~ l~ N ~ cxC
M ~O
O 00 N 00
N .~ C
w
r.
-, O
M ~
O ~O O
~ M
w
0
U
Q)
O Ov N
w d~
M
jc
CA 02521776 2002-05-21
49
As noted above, one aspect of the present invention is crystalline Forms of
atorvastatin or
hydrates thereof characterized by combinations of values determined from two
or more of X-ray
powder diffraction, solid state'3C nuclear magnetic resonance and Raman
spectroscopy. Table 8
sets out such combinations of values for crystalline Forms V, VI, VII, VIII, X
and XII
atorvastatm.
CA 02521776 2002-05-21
,U
V~ '~ +
+ + O
~'~ ~ ~ N
per.,~ G'
LL
+ M + +
0
~c + + + + +
o M
~:
U
M 0
U
U + ~ U c4-~,
~+ ~ v~ ~ O
N O
, .~ .~ ry ~
~ O
+ + M + +
00 ~ C
a x
x
~ pip00 0~0
~ U
Ow
~ N
U C/~C1,
U cC
O
H
I~+ ~~ + U ~C) O c~
U
~
'' pp - M M m .~ _
U p ~ ~~ ~ N U
,~''~,~ O U N U V U
,_~ .G
+ + + + N O O
~ ~- O p.,
O M v0 ~~n,Os av ~ .~
(s o0 00 l~ l~ ~0 N
X
p, O ~ N
r~
Z G1. Vin.A. .U N
, ~ ~ ~
00 M 'a_"'~ O
W a' ..-. b
o oMOo~po, ~ U v
+ .~ 'n. r. ~, 3 ~ o,
+ o 0
,~P~C00 '_' ~ ~ ~ ~ ~ c
+ c~ 'O
+ M + o +
0 o o
0
O N
f~ l~ 00 t~ th c~ ~i ci
~ ~ x x
CA 02521776 2002-05-21
51
Crystalline Forms V to XIX atorvastatin of the present invention may exist in
anhydrous
forms as well as hydrated and solvated forms. In general, the hydrated forms
are equivalent to
unhydrated forms and are intended to be encompassed within the scope of the
present invention.
Crystalline Form XIV contains about 6 mol of water. Preferably, Form XIV
contains 6 mol of
S water. Crystalline Forms V, X, and XV atorvastatin contain about 3 mol of
water. Preferably,
Forms V, X, and XV atorvastatin contain 3 mol of water.
Crystalline Form VII contains about 1.5 mol of water. Preferably, Form VII
atorvastatin
contains 1.5 mol of water. Crystalline Form VIII contains about 2 mol of
water. Preferably, Form
VIII atorvastatin contains 2 mol of water.
Crystalline Forms XVI-XIX may exist as a solvate.
Crystalline forms of atorvastatin of the present invention, regardless of the
extent of
hydration and/or solvation having equivalent x-ray powder diffractograms,
ssNMR, or Raman
spectra are within the scope of the present invention.
Also intended to be included within the scope of the invention are solvates of
any of
crystalline Forms V to XIX whose unsolvated forms have the x-ray powder
diffraction, ssNMR
or Raman spectroscopy values described herein.
Crystalline forms, in general, can have advantageous properties. A polymorph,
solvate, or
hydrate is defined by its crystal structure and properties. The crystal
structure can be obtained
from X-ray data or approximated from other data. The properties are determined
by testing. The
chemical formula and chemical structure does not describe or suggest the
crystal structure of any
particular polymorphic or crystalline hydrate form. One cannot ascertain any
particular crystalline
form from the chemical formula, nor does the chemical formula tell one how to
identify any
particular crystalline solid form or describe its properties. Whereas a
chemical compound can
exist in three states-solid, solution, and gas-crystalline solid forms exist
only in the solid state.
Once a chemical compound is dissolved or melted, the crystalline solid form is
destroyed and no
longer exists (Wells J.L, Aulton M.E. Pharmaceutics. The 'Science of Dosage
Form Design.
Reformulatiov, Aulton M.E. ed., Churchill Livingstone, 1988;13:237).
The new crystalline forms of atorvastatin described herein have advantageous
properties.
Form VII has good chemical stability, which is comparable to Form I (disclosed
in United States
Patent Number 5,969,156).
Since noncrystalline forms of atorvastatin are not chemically stable, this is
a significant
advantage, which would translate into enhanced shelf life and longer
expiration dating. Form
CA 02521776 2002-05-21
52
VII can be prepared from acetone/water, whereas Form I is prepared from the
more toxic
methanol/water system. Form VII is the sesquihydrate and contains less water,
meaning that a
unit weight of Form VII contains more atorvastatin molecules, meaning it is of
higher
potency.
The ability of a material to form good tablets at commercial scale depends
upon a
variety of drug physical properties, such as the Tableting Indices described
in Hiestand H.
and Smith D., Indices of Tableting Performance, Powder Technology, I984;~8:145-
159.
These indices may be used to identify forms of atorvastatin calcium which have
superior
tableting performance. One such index is the Brittle Fracture Index (BFI),
which reflects
brittleness, and ranges from 0 (good - low brittleness) to 1 (poor - high
brittleness). For
example, Form VII has a BFI value 0.09, while Form I has a BFI value 0.81.
Thus, Form VII
is less brittle than Form I. This lower brittleness indicates greater ease of
manufacture of
tablets.
Form VIII also has less water than Form I (dihydrate vs trihydrate) and thus a
gram of
Form VIII contains more atorvastatin molecules.
Form X is advantageous in that it can be prepared from the less toxic
isopropanol
(IPA):water system, thus avoiding the more toxic methanol:water system.
Form XII has the highest melting point (210.6). Since high melting point
correlates
with stability at high temperature, this means this form is most stable at
temperatures near the
melting point. High melting forms can be advantageous when process methods
involving
high temperatures are used. Form XII is also prepwed from the less toxic
tetrahydrofuran
(THF) water system.
Form XIV is prepared using the less toxic THF/water system.
The present invention provides a process for the preparation of crystalline
Forms V to
XIX atorvastatin which comprises crystallizing atorvastatin from a solution in
solvents under
conditions which yield crystalline Forms V to XIX atorvastatin.
The precise conditions under which crystalline Forms V to XIX atorvastatin are
formed may be empirically determined, and it is only possible to give a number
of methods
which have been found to be suitable in practice.
CA 02521776 2002-05-21
53
The compounds of the present invention can be prepared and administered in a
wide
variety of oral and parenteral dosage forms. Thus, the compounds of the
present invention
can be administered by injection, that is, intravenously, intramuscularly,
intracutaneously,
subcutaneously, intraduodenally, or intraperitoneally. Also, the compounds of
the present
invention can be administered by inhalation, for example, intranasally.
Additionally, the
compounds of the present invention can be administered transdermally. It will
be obvious to
those skilled in the art that the following dosage forms may comprise as the
active
component, either compounds or a corresponding pharmaceutically acceptable
salt of a
compound of the present invention.
For preparing pharmaceutical compositions from the compounds of the present
invention, pharmaceutically acceptable carriers can be either solid or liquid.
Solid form
preparations include powders, tablets, pills, capsules, cachets,
suppositories, and dispersible
granules. A solid carrier can be one or more substances which may also act as
diluents,
flavoring agents, solubilizers, lubricants, suspending agents, binders,
preservatives, tablet
disintegrating agents, or an encapsulating material.
In powders, the carrier is a finely divided solid which is in a mixture with
the finely
divided active component.
In tablets, the active component is mixed with the carrier having the
necessary
binding properties in suitable proportions and compacted in the shape and size
desired.
The powders and tablets preferably contain from two or ten to about seventy
percent
of the active compound. Suitable carriers are magnesium carbonate, magnesium
stearate, talc,
sugar, lactose, pectin, dextrin, starch, gelatin, tragacanth, methylcellulose,
sodium
carboxymethylcellulose, a low melting wax, cocoa butter, and the like. The
term
"preparation" is intended to include the formulation of the active compound
with
encapsulating material as a carrier providing a capsule in which the active
component, with
or without other carriers,
is surrounded by a carrier, which is thus in association with it. Similarly,
cachets and
lozenges are included. Tablets, powders, capsules, pills, cachets, and
lozenges can be used as
solid dosage forms suitable for oral administration.
CA 02521776 2002-05-21
54
For preparing suppositories, a low melting wax, such as a mixture of fatty
acid
glycerides or cocoa butter, is first melted and the active component is
dispersed
homogeneously therein, as by stirring. The molten homogenous mixture is then
poured into
convenient sized molds, allowed to cool, and thereby to solidify.
Liquid form preparations include solutions, suspensions, retention enemas, and
emulsions, for example water or water propylene glycol solutions. For
parenteral injection,
liquid preparations can be formulated in solution in aqueous polyethylene
glycol solution.
Aqueous solutions suitable for oral use can be prepared by dissolving the
active
component in water and adding suitable colorants, flavors, stabilizing, and
thickening agents
as desired.
Aqueous suspensions suitable for oral use can be made by dispersing the finely
divided active component in water with viscous material, such as natural or
synthetic gums,
resins, methylcellulose, sodium carboxymethylcellulose, and other well-known
suspending
agents.
Also included are solid form preparations which are intended to be converted,
shortly
before use, to liquid form preparations for oral administration. Such liquid
forms include
solutions, suspensions, and emulsions. These preparations may contain, in
addition to the
active component, colorants, flavors, stabilizers, buffers, artificial and
natural sweeteners,
dispersants, thickeners, solubilizing agents, and the like.
The pharmaceutical preparation is preferably in unit dosage form. In such
form, the
preparation is subdivided into unit doses containing appropriate quantities of
the active
component. The unit dosage form can be a packaged preparation, the package
containing
discrete quantities of preparation, such as packeted tablets, capsules, and
powders in vials or
ampoules. Also, the unit dosage form can be a capsule, tablet, cachet, or
lozenge itself, or it
can be the appropriate number of any of these in packaged form.
The quantity of active component in a unit dose preparation may be varied or
adjusted
from 0.5 mg to 100 mg, preferably 2.5 mg to 80 mg according to the particular
application
and the potency of the active component. The composition can, if desired, also
contain other
compatible therapeutic agents.
CA 02521776 2002-05-21
In therapeutic use as hypolipidemic andlor hypocholesterolemic agents and
agents to
treat osteoporosis and Alzheimer's disease, the crystalline Forms V to XIX
atorvastatin
utilized in the pharmaceutical method of this invention are administered at
the initial dosage
of about 2.5 mg to about 80 mg daily. A daily dose range of about 2.5 mg to
about 20 mg is
5 preferred. The dosages, however, may be varied depending upon the
requirements of the
patient, the severity of the condition being treated, and the compound being
employed.
Determination of the proper dosage for a particular situation is within the
skill of the art.
Generally, treatment is initiated with smaller dosages which are less than the
optimum dose
of the compound. Thereafter, the dosage is increased by small increments until
the optimum
10 effect under the circumstance is reached. For convenience, the total daily
dosage may be
divided and administered in portions during the day if desired.
The following nonlimiting examples illustrate the inventors' preferred methods
for
preparing the compounds of the invention.
EXAMPLE 1
15 [R-(R*,R*)]-2-(4-Fluorophenyl)-(3,8-dihydroxy-5-(1-methylethyl)-3-phenyl-4-
[(phenylamino)carbonyl]-1H-pyrrole-1-heptanoic acid hemi calcium salt (Forms V-
XIX
atorvastatin)
Form V Atorvastatin
Method A
20 Amorphous atorvastatin calcium (United States Patent Number 5,273,995) was
slurried in a mixture of acetonitrile/water (9:1 ) to afford crystalline Form
V atorvastatin.
Method B
Crystalline Form I atorvastatin calcium (United States Patent Number
5,969,156) was
slurried in a mixture of acetonitrile/water (9:1) at 60°C overnight,
filtered, and air dried to
25 afford crystalline Form V atorvastatin.
Method C
Amorphous atorvastatin calcium (United States Patent Number 5,273,995) was
stressed under vapors of acetonitrile/water (9:1 ) to afford crystalline Form
V atorvastatin.
CA 02521776 2002-05-21
56
Method D
Acetonitrile was added to a solution of amorphous atorvastatin calcium (United
States
Patent Number 5,273,995) in tetrahydrofuran/water (9:1) and cooled to afford
crystalline
Form V atorvastatin.
Method E
Acetonitrile was added to a solution of amorphous atorvastatin calcium (United
States
Patent Number 5,273,995) in dimethylformamide/water and fast evaporation
affords
crystalline Form V atorvastatin.
Method F
Amorphous atorvastatin calcium (United States Patent Number 5,273,995)
diffused in
a vapor of acetonitrile/water (9:1 ) to afford crystalline Form V
atorvastatin.
Crystalline Form V atorvastatin, mp 171.4°C, trihydrate
Karl Fischer 4.88% (3 mol of water).
Form VI Atorvastatin
Method A
Amorphous atorvastatin calcium (United States Patent Number 5,273,995) was
placed
into a vapor jar containing dimethylformamide/water (9:1) for 20 days to
afford crystalline
Form VI atorvastatin.
Method B
Fast evaporation of a dimethylformamide/water solution of amorphous
atorvastatin
calcium (United States Patent Number 5,273,995) afforded crystalline Form VI
atorvastatin.
Method C
Fast evaporation of a dimethylformamide/water (saturated) solution of
amorphous
atorvastatin calcium (United States Patent Number 5,273,995) seeded with
crystalline Form
VI afforded crystalline Form VI atorvastatin.
Crystalline Form VI atorvastatin, mp 145.9°C.
CA 02521776 2002-05-21
57
Form VII Atorvastatin
Method A
A solution of amorphous atorvastatin calcium (United States Patent Number
5,273,995) in acetone/water (1:1) (5.8 mg/mL) was stirred overnight. A solid
formed which
was filtered to afford crystalline Form VII atorvastatin.
Method B
A solution of amorphous atorvastatin calcium (United States Patent Number
5,273,995) in acetone/water (1:1) was evaporated at 50°C to afford
crystalline Form VII
atorvastatin.
Method C
A saturated solution of amorphous atorvastatin calcium (United States Patent
Number
5,273,995) in acetone/water (1:1) was seeded with crystalline Form VII
atorvastatin to afford
crystalline Form VII atorvastatin.
Method D
Fast evaporation of a saturated solution of amorphous atorvastatin calcium
(United
States Patent Number 5,273,995) in acetone/water (1:1) was seeded with
crystalline Form VII
to afford crystalline Form VII atorvastatin.
Crystalline Form VII atorvastatin, mp 195.9°C, I.5 hydrate
Karl Fischer 2.34°Io ( 1.5 mol of water).
Form VIII Atorvastatin
Method A
A solution of amorphous atorvastatin calcium (United States Patent Number
5,273,995) in dimethylformamide/water (saturated) (9:1), was seeded with
crystalline Form
VII and evaporated to afford crystalline Form VIII atorvastatin.
Method B
Fast evaporation of a solution of amorphous atorvastatin calcium (United
States
Patent Number 5,273,995) in dimethylformamide/water (9:1) affords crystalline
Form VIII
atorvastatm.
CA 02521776 2002-05-21
58
Crystalline Form VIII atorvastatin, mp 151 °C, dehydrate
Karl Fischer 2.98% (2 mol of water).
Form IX Atorvastatin
Method A
A solution of amorphous atorvastatin calcium (United States Patent Number
5,273,995) in acetone/water (6:4) (3.4 m~mL) was evaporated on a rotary
evaporator to
afford crystalline Form IX atorvastatin.
Method B
A solution of amorphous atorvastatin calcium (United States Patent Number
5,273,995) in acetone/water (6:4) was filtered, seeded with crystalline Form
IX evaporated on
a rotary evaporator to afford crystalline Form IX atorvastatin.
Method C
A solution of amorphous atorvastatin calcium (United States Patent Number
5,273,995) in acetone/water (6:4) was stirred for 0.5 hours, filtered,
evaporated on rotary
evaporator to concentrate the solution, and dried in a vacuum oven to afford
crystalline Form
IX atorvastatin.
Form X Atorvastatin
Method A
A slurry of amorphous atorvastatin calcium (United States Patent Number
5,273,995)
in isopropanol/water (9:1 ) was stirred for a few days, filtered, and air
dried to afford
crystalline Form X atorvastatin.
Method B
A slurry of amorphous atorvastatin calcium (United States Patent Number
5,273,995)
in isopropanol/water (9:1 ) was stirred for 5 days, filtered, and air dried to
afford crystalline
Form X atorvastatin.
Method C
A saturated solution of amorphous atorvastatin calcium (United States Patent
Number
5,273,995) in isopropanol/water (9:1) was stirred for 2 days, filtered, and
air dried to afford
CA 02521776 2002-05-21
59
crystalline Form X atorvastatin.
Crystalline Form X atorvastatin, mp 180.1 °C, trihydrate
Karl Fischer 5.5% (3.5 mol of water).
Form XI Atorvastatin
A solution of amorphous atorvastatin calcium (United States Patent Number
5,273,995) in acetonitrile/water (9:1) was filtered and allowed to evaporate
slowly to afford
crystalline Form XI atorvastatin.
Form XII Atorvastatin
Crystalline Form I atorvastatin calcium (United States Patent Number
5,969,156) was
slurried in tetrahydrofuran/water (2:8) at 90°C for 5 days, filtered,
and air dried to afford
crystalline Form XII atorvastatin.
Crystalline Form XII atorvastatin, mp 210.6°C.
Form XIII Atorvastatin
Crystalline Form I atorvastatin calcium (United States Patent Number 5,969, I
56) was
added to 10 mL 2:8 water:methanol to leave a layer of solid on the bottom of a
vial. The
slurry was heated to about 70°C for 5 days. The supernatant was
removed, and the solid air
dried to afford crystalline Form XIII atorvastatin.
Form XIV Atorvastatin
Amorphous atorvastatin calcium (United States Patent Number 5,273,995), 1 g,
was
slurried for 3 weeks in 45 mL of isopropyl alcohol/5 mL of water (9:1) at
ambient
temperature. The mixture was filtered to afford crystalline Form XIV
atorvastatin after drying
at ambient temperature.
Differential scanning calorimetry (DSC) indicates a low desolvation event at
about
60°C (peak) followed by a melt at about 150°C. Combustion
analysis indicates that the
compound is a hexahydrate. Thermographic infrared spectroscopy (TG-IR) shows
the
compound contains water. Karl Fischer shows the compound contains 5.8% water.
Form XV Atorvastatin
Amorphous atorvastatin calcium (United States Patent Number 5,273,995), 1 g,
was
slurried for 3 weeks in 45 mL acetonitrile/5 mL of water (9:1) at ambient
temperature. The
mixture was filtered to afford crystalline Form XV atorvastatin after drying
at ambient
CA 02521776 2002-05-21
temperature. DSC indicates a low desolvation event at about 78°C (peak)
followed by a melt
at about 165°C. Combustion analysis indicates that the compound is a
trihydrate. TG-1R
shows the compound contains water.
Form XVI Atorvastatin
5 Amorphous atorvastatin calcium (United States Patent Number 5,273,995), 1 g,
was
slurried for about 1 day in 9:1 acetonitrile/water at room temperature. The
mixture was
filtered to afford crystalline Form XVI atorvastatin after drying at ambient
temperature. DSC
indicates a broad endotherm at peak temperature of 72°C and an
endotherm with onset
temperature of 164°C. The weight loss profile by thermographic analysis
(TGA) indicates a
10 total weight loss of about 7°lo at 30°C to 160°C.
Combustion analysis indicates that TGA and
Karl Fischer analysis (shows 7.1 °Io water) indicates the compound
is a
tetrahydrate/acetonitrile solvate.
Form XVII Atorvastatin
Amorphous atorvastatin calcium (United States Patent Number 5,273,995), 0.5 g,
was
15 slurried for about 2 days in 5 mL of 9:1 dimethylformamide (DMF)/water
containing 25 mL
of acetonitrile at room temperature. The mixture was filtered to afford
crystalline Form XVII
atorvastatin after drying at ambient temperature. DSC showed multiple broad
endotherms
indicating the compound was a solvate.
Form XVIII Atorvastatin
20 Crystalline Form XVI atorvastatin, 0.5 g, was dried for about 1 day at room
temperature to afford crystalline Form XVIII atorvastatin. DSC showed a broad
endotherm at
low temperature indicating the compound was a solvate. Karl Fischer analysis
showed the
compound contained 4.4% water.
Form XIX Atorvastatin
25 Amorphous atorvastatin calcium (United States Patent I\TUmber 5,273,995),
0.4 g, was
slurried for about 7 days in 4 mL methyl ethyl ketone at room temperature. The
mixture was
filtered to afford crystalline Form XIX atorvastatin after drying at ambient
temperature. DSC
indicated a low desolvation event at about 50°C (peak) followed by a
melt at about 125°C.
TGA analysis indicates that the compound is a solvate that desolvates at low
temperature.
CA 02521776 2002-05-21
61
1 /35
a4
o
t--
N
M~
O
M
N
O
N
O
GRAPH 1
O O
O O
O
O
CA 02521776 2002-05-21
62
2/35
a~o
a~
o~
s
N
u7
M t
H
O
M
N
O
N
O
GRAPH 2
O O O O
O O O
O O O
m N ~--
CA 02521776 2002-05-21
63
3/35
a~o
.a
o ~-
~r
s
N
N
M~
H
O
M
N
O
N
r-
O
r
u7
GRAPH 3
O O O
O m~
r
CA 02521776 2002-05-21
64
4/35
a4
-a
o~
s
N
cC
N
Ms
O
M
N
O
N
c-
O
GRAPH 4
V
O O O O
O r- r.
<IMG>
CA 02521776 2002-05-21
66
6/35
a~
-a
o"
s
H
N
u7
M t
H
O
M
N
O
N
O
GRAPH 6
O O O O
O p O
O N
M
CA 02521776 2002-05-21
67
7/3 5
a~
a
0
a
s
H
N
u7 N
M~
H
O
M
N
O
N
O
r-
GRAPH 7
O O O
O O
O O
N '-
<IMG>
<IMG>
CA 02521776 2002-05-21
10/3 S
0
M
O
M
u7 cd
N V
N
O
N
N
O
GRAPH 1 ~
p O O O O O O O O O O O O O
p O O O O O O O O O O O O
c~ N .- O d1 CO fw
<IMG>
<IMG>
<IMG>
CA 02521776 2002-05-21
74
14/35
0
0
M
a
U
O
N ,~,
N
s
h-
N
O
M
GRAPH 14
O O O O O O O O O O O O O O V v
O O O O O O O O O O O O O O O
Ln ~ M N ~ O 01 00 IW ~ in d' M N
r- ~ r- c- r r-
<IMG>
CA 02521776 2002-05-21
76
16/35
+~
.N
C
N
C
0
d'
M
O
M
N
O
N N
t
N
M
O
GRAPH 16
p O
O O O O
O
d- M N r'
CA 02521776 2002-05-21
17/3 5
0
M
O
M
N
cat
O
N
H
N
O
.N
C
N
+r
C
GRAPH 17
O O
O O
p O O
M N
CA 02521776 2002-05-21
7g
18/3 5
.N
N
+r
0
d'
M
0
M
N
ccS
O
N
N
O
GRAPH 18
O
O O O O
O
N '- '--
CA 02521776 2002-05-21
79
19/35
0
d'
M
O
M
N
cd
O
N -_
H
N
r-
.N
C
N
GRAPH 19
O O
O 00 p O
O O
O O
<IMG>
CA 02521776 2002-05-21
81
21 /3 5
0
~r
M
O
M
N
cd
O N
N
N
O
O O O O
O ~ O
N
+T
C
C
GRAPH 21
CA 02521776 2002-05-21
82
22/3 5
0
d'
M
O
M
N
c~
+r
O N
N
N
r-
O
u7
+~
.N
N
GRAPH 22
O O ~ O
p O O
O O
N N c- r-
CA 02521776 2002-05-21
83
23/35
0
d'
m
0
m
N
cd
O
N
N
O
+~
N
C
GRAPH 23
O O O O
O I~ O
N ~ '-
CA 02521776 2002-05-21
84
24/3 5
0
M
O
M
N
ca
+r
O
N
N
O
C
+~
GRAPH 24
Op O O
O O O O
N r
<IMG>
<IMG>
<IMG>
<IMG>
CA 02521776 2002-05-21
89
29/35
E
a
0
0
0
0
0
0
0
N
O
N
O
O
M
O
O ~ ~ O O
O O O O O ~ O
M N
GRAPH 29
CA 02521776 2002-05-21
30/35
E
t
c
E
n
0
0
0
M
O
O
M
O
O
O
d'
C .N
cd
E a~
GRAPH 30
M N ~- a ~ CJ IW ~ I~ Wit' M
CA 02521776 2002-05-21
91
31 /35
n
r-
r
U
+~
4-
C
O t~
O
O
N
O
O
N
O
O
O
M
O
O
M
cd 'n
c
GRAPH 31
m N ~ o ~ co Iw ~ u7 d~ cw N ,--
r- r r r
CA 02521776 2002-05-21
92
3 2/3 5
n
E
v
w
c
E
0
0
~.r~ ,
N
O
O
O
M
O
O
M
O
O
O
d'
C =~
cd ~'
GRAPH 32
O d~ N
r. ~ r- r- O O O O
CA 02521776 2002-05-21
93
33/35
n
U
y s
N
C
fb
f~
O
N
O
O
O
M
O
O
M
O
O
O
d'
C N
c~
33
GRAPH
tn d- M N ~-
CA 02521776 2002-05-21
94
34/35
n
0
0
U
S
C
cd
O
O
O
O
N
O
O
O
M
O
O
M
C
C~
GRAPH 34
O ~n O tn O tn O m O ~n
In d' d- M M N N '- ~-- O
CA 02521776 2002-05-21
95
35/35
0
0
0
E
O U
O '~'_
N -~
N
C
(d
f~
O
O
O
M
C ~N
cd
C
GRAPH 3~
O ~ ~ N O O ~ Q cV
.- r- r- r- O O O O