Note: Descriptions are shown in the official language in which they were submitted.
CA 02522031 2005-10-03
METHOD FOR TREATMENT OF OIL SANDS TAILINGS
WITH LIME OR WITH LIME AND CARBON DIOXIDE
FIELD OF THE INVENTION
The present invention is directed to methods for the treatment of oil sands
tailings to
achieve non-segregating consolidation of the solids in the tailings, and to
recover the release
water with acceptable chemical properties for its use in the bitumen
extraction process.
BACKGROUND OF THE INVENTION
Over 700,000 barrels of synthetic crude oil are produced daily by commercial
plants
engaged in surface mining of Athabasca oil sands in Northern Alberta. At these
plants,
bitumen is extracted from oil sands using caustic and non-caustic water slurry-
based
extraction processes. An average sample of surface-mineable Athabasca oil sand
ore
contains about 11 % bitumen (by weight), 3-6% water, 12% fines (less than 44
microns) and
72% sand.
Water slurry-based extraction processes use large volumes of water: the
production of
each cubic meter of bitumen produced require about 7 to 9 cubic meters of
water, of which
about 70% is recycled from the surface zone of the tailings pond. In this
process, about 0.3
cubic meters of water per ton of oil sands feed is withdrawn from the
Athabasca River.
Commercial plants operating in the Athabasca oil sands must comply with
regulatory
requirements which stipulate no discharge of process-affected water. As a
result of this "zero
discharge" policy, waste water containment ponds are formed and waste water
(including
drainage and other sources) and tailings are discharged into these ponds.
During the extraction of bitumen from the oil sands by water slurry-based
extraction
processes, a coarse tailings stream is produced in the form of a slurry,
containing about 55%
solids (by weight), of which about 82% is sand, about 17% is fines smaller
than 44 microns
in diameter, and about I% is unrecovered bitumen.
-1-
CA 02522031 2012-09-07
In early tailings disposal practice, the oil sands tailings discharged from
the extraction
plant were hydraulically transported and deposited in the tailings ponds. In
this process, the
coarse sand particles segregate quickly and form a beach. The remaining fine
tails of 6 to 10
percent weight accumulate in the tailings ponds. Fine tails settle quickly,
forming a
suspension of 20 percent weight solids content and over a few years to 30
percent weight
solids (85 percent water by volume) with a stable slurry structure, which is
called mature fine
tails (also called MFT). Mature fine tails remain in a fluid state for
centuries because of their
very slow consolidation rate. It is predicted that if the conventional
tailings disposal practice
is continued, the accumulated volume of mature fine tails will increase from
the current level
of 325 million cubic meters to over 1 billion cubic meters by the year 2020.
Accumulation of
large volumes of mature fine tails with a very stable fluid structure creates
environmental
concerns and a long-term environmental liability.
To eliminate formation of the mature fine tails, or to reduce the inventory of
the
existing mature fine tails, a method was developed to produce a nonsegregating
tailings mix
(also called consolidated tailings or composite tailings or CT) for the
disposal of the oil sands
tailings. In this method, the coarse tailings are passed through cyclones, the
sand-rich cyclone
underflow (typically having at least about 90% sand) is blended with the
mature fine tails
(comprising about 30-33% solids, which in turn comprise about 98% fines) to
produce a
nonsegregating mix for final disposal. In this process, chemical additives
such as gypsum
(CaSO4) are used to prevent segregation. Also in this process, the fines-rich
cyclone overflow
(with about 20-30% solids, comprising about 50% fines) is discharged to a
tailings pond. The
fines in the cyclone overflow stream also form the mature fine tailings, which
is a concern for
long-term operations.
As low-temperature or low-energy bitumen extraction processes became adopted
by
commercial plants, it became desirable to achieve fast recovery of the process
water from the
tailings stream to save thermal energy. As a result, the cyclone overflow
stream was
thickened in thickeners, with the help of preferably polymeric flocculent.
Disposal of
thickener underflow and cyclone underflow in an environmentally acceptable
manner remains
the challenge for the oil sands industry.
-2-
CA 02522031 2012-09-07
SUMMARY OF THE INVENTION
The present invention is based on the treatment of oil sands tailings,
produced from a
process involving water slurry-based extraction of bitumen from oil sand, with
lime
(Ca(OH)2) or with lime and carbon dioxide (C02). By the treatment of oil sands
tailings with
Ca(OH)2 or with Ca(OH)2 and C02, the oil sands tailings become a non-
segregating mix; i.e.,
the sand and the fines sediment and consolidate together. Also by the
treatment of oil sands
tailings with Ca(OH)2 or with Ca(OH)2 and CO2, the release water recovered
from the settled
and consolidated tailings would have acceptable water chemistry
characteristics in terms of
reduced concentrations of calcium ions (Ca2+) , magnesium ions (Mg2+), sodium
ions (Na'),
and chloride ions (Cl-).
When the oil sand tailings are treated with Ca(OH)2, the following ion
exchange
reaction takes place between the clay and the tailings pore water, which
results in flocculation
of the clay particles, mainly (but not necessarily only) by the following
reaction:
Ca(OH)2+2Clay-Na-->(Clay)2Ca+2NaOH (1)
resulting in increase in the viscosity of the fines-water suspension, and the
consequent
formation of the yield stress which holds the sand particles in the fines-
water suspension
matrix and prevents the segregation of the sand particles. Treatment of oil
sands tailings with
Ca(OH)2 also reduces the activity of the asphaltic acids in the aqueous
solution because of
the formation of the insoluble calcium salts of the asphaltic acids, which
results in an
increase in surface and interfacial tension of the water, which promotes the
clay flocculation.
Both excess Ca(OH)2 and sodium hydroxide (NaOH) produced by the reaction
expressed in Equation (1) act as a base. As a result, the suspension and the
release water pH
are kept above 7 (pH being expressed as the minus of the logarithm of the
hydrogen ions
concentration [H+]; i.e., pH = -log [H+], a scale to express the acidity of
the matter).
When the oil sand tailings are treated with Ca(OH)2, the bicarbonate hardness
caused
mainly by the water-soluble calcium bicarbonate (Ca(HCO3)2 of the tailings is
also reduced,
by converting Ca(HCO3)2) into water-insoluble calcium carbonate (CaCO3) by the
following
reactions:
-3-
CA 02522031 2012-09-07
Ca(HCO3)2 +Ca(OH)2 -+ 2CaCO3 + 2H20 (2)
Ca(HCO3)2 +2NaOH --- CaCO3 +Na2CO3 + 2H20 (3)
As an example, Ca2+ ions concentration in the release water recovered from the
untreated tailings was at about 60 mg/L (milligram per liter), while Ca 2+
ions concentration
in the release water recovered from the tailings treated with Ca(OH)2 was at
about 30 mg/L.
Sodium carbonate (Na2CO3) produced by the chemical reaction expressed by
Equation (3)
also promotes the basic nature of the suspension and the release water; for
example, it helps
the pH to be greater than 7 by the hydrolysis reaction:
Na2CO3 + H2 0 -* NaHCO3 + Na + + OH- (4)
When oil sands tailings are treated with Ca(OH)2, the release water recovered
from the
tailings contains the excess Ca(OH)2, NaOH, and Na2CO3 as a result of the
chemical reactions
expressed in Equations (1) and (3). If the release water is blended with the
make-up water (e.g.,
river water, recycle water) or with any water with bicarbonate hardness for
its use in the
extraction process, the chemical species Ca(OH)2, NaOH, and Na2CO3 contained
in the release
water reduces the bicarbonate hardness by the chemical reactions expressed in
Equation (2),
Equation (3) as well as by the following chemical reactions:
Ca(HCO3 )2 + Na2CO3 -> CaCO3 + 2NaOH + 2CO2 (5)
by which the water soluble Ca(HCO3)2 is converted to water insoluble CaCO3.
Also, because
of the nature of the species produced by the chemical reactions expressed by
Equations (1), (3),
(4), and (5), the pH of the blended water would be basic; i.e., the pH of the
blended water would
be greater than 7. The pH of the blended water would be determined, however,
by the dosage
of Ca(OH)2 treatment, the solids content, the fines content or fines-to-sand
ratio (SFP), or more
specifically the clay content of the tailings and the exposure of the water
(release water, recycle
water or blended water, or the tailings) to the atmospheric C02, which could
diffuse into the
aqueous phase and acts as acid and reduces the pH by a reaction such as (but
not limited to) the
following:
-4-
CA 02522031 2005-10-03
Ca(OH)2 + CO2 -* CaCO3 + H2O (6)
When Ca(OH)2 is used to treat oil sands tailings, the blended water (obtained
from the
blend of the release water with the make-up water, for its use in the
extraction process) would
have a pH higher than 7. Since the pH of the blended water is greater than 7,
the bitumen
extraction process would work better since the asphaltic acids contained in
bitumen would be
water soluble and would act as surfactants. Further, it would decrease surface
tension and
interfacial tension of the water, thus promoting clay dispersion and bitumen
extraction
efficiency. As a result, conditions favoring higher extraction efficiency by
better dispersing
the clays in the extraction process, however, would produce a tailings stream
with difficult
properties to handle since the fines and clay particles in the tailings would
be more attractive
to the water. This difficulty, however, could be overcome by treating the oil
sands tailings
with Ca(OH)2 in the first place.
If needed, the excess amount of Ca(OH)2 added into the oil sands tailings
could be reduced by the controlled injection of CO2 into tailings after
treating the tailings
with Ca(OH)2 or by controlled injection of CO2 into the release water. When
CO2 is injected
in a controlled manner (i.e., by keeping the final pH of the tailings
suspension or the release
water preferably in the range of 11.0 < pH < 11.6), the Ca 2+ content in the
aqueous media
could be reduced to at about 30 mg/L range without causing any change in the
segregating
property of the tailings, by precipitating Ca(OH)2 in the form of CaCO3 by the
chemical
reaction expressed in Equation (6).
Uncontrolled or excessive injection of CO2 into the tailings after treating it
with
Ca(OH)2 or into the release water may cause the reduction of the pH in the
range where
water-soluble calcium bicarbonate would be formed by the following reaction:
CaCO3 +CO2 +H2O -> Ca(HCO3)2 (7)
which causes an increase in the Ca 2+ ions concentration in the release water
which could
harm the bitumen extraction efficiency if the release water is recycled to the
extraction plant.
Also, the chemical reaction expressed in Equation (7) would cause segregation
of the
tailings.
-5-
CA 02522031 2012-09-07
It has been observed experimentally by the inventor that the injection of CO2
into
tailings appears to help flotation of the residual bitumen in the form of a
water-in-oil
emulsion.
Formation of the fresh CaCO3 crystals in the tailings as a result of the
chemical
reactions expressed in Equations (2), (3), (5), and (6) would result in the
adsorption of the
ultra fines (i.e., clay particles smaller than 0.2 m size) on the surface of
newly formed
CaCO3 crystal and precipitate them together with it, which results in
reduction of the gel
formation property of the tailings, since the ultra fines content of the
tailings is partly
responsible for the gel formation strength of the oil sands tailings. Also,
the formation of the
fresh CaCO3 crystals in the tailings would result in the adsorption of Na' and
Cl- ions, on the
surface of newly-formed CaCO3 crystals and precipitate them together with it,
which results
in the reduction of the Na' and Cl" ions in the release water recovered from
the tailings. As
an example, Na' ions concentration in the release water recovered from the
untreated tailings
was at about 130 mg/L, while Na' ions concentration in the release water
recovered from the
tailings treated with Ca(OH)2 was at about 115 mg/L.
Similarly, formation of the fresh CaCO3 crystals in the release water or any
kind of
blended water obtained by blending the release water with the make-up water,
as a result of
the chemical reactions expressed in Equations (2), (3), (5), and (6), would
result in the
adsorption of Na' and Cl- ions, and even the organic compounds responsible for
the
coloration of the water, on the surface of newly-formed CaCO3 crystal surface
and
precipitated together with it, which results in the reduction of the Na' and
Cl- ions, and even
in the reduction of the coloration of the water.
BRIEF DESCRIPTION OF THE DRAWINGS
Embodiments of the invention will now be described with reference to the
accompanying figures, in which numerical references denote like parts, and in
which:
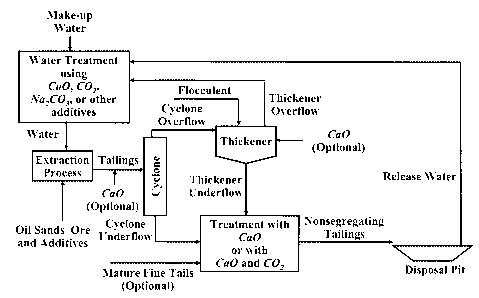
FIGURE 1 is a schematic flow chart of a method of treating oil sands tailings
in accordance with a first embodiment of the present invention.
-6-
CA 02522031 2012-09-07
FIGURE 2 is is a schematic flow chart of a method of treating oil sands
tailings in accordance with a second embodiment of the invention.
DESCRIPTION OF THE PREFERRED EMBODIMENT
Experiments performed by the inventor have indicated that treatment of oil
sands
tailings with Ca(OH)2 improves the nonsegregating, settling and consolidation
characteristics
of the oil sands tailings. It is known that oil sands tailings have the
tendency to become a
nonsegregating mix as the solids content increases and as the fines content
(i.e., fraction of
the solids smaller than 325 mesh or 44 micron size) increases. Addition of
Ca2+ ions into the
oil sands tailings improves the nonsegregation behavior of the tailings; for
example, it pushes
the segregation boundary towards a lower solids content region.
It is important that segregation boundary lines (i.e., the lines of solids
contents for
various fines contents or SFRs, lower than which the tailings behave
segregatingly, and
higher than which the tailings behave non-segregatingly) for the tailings,
both without any
chemical additive and with different chemical additives, are parallel to the
fixed fines-water
ratio lines (the fines-water ratio being defined as the ratio of the
fines/(fines + water) on mass
basis), which is the indication that rheological characteristics of the fines-
water suspension
control the segregation behavior of the tailings. Addition of chemicals such
as Ca(OH)2 into
the oil sands tailings changes the rheology of the fines-water suspension;
i.e., forms sufficient
yield stress and prevents segregation.
It is known that asphaltic acids present in oil sands bitumen become water
soluble
when the oil sands ore water suspension pH is kept slightly above 7. These
asphaltic acids
are partly aromatic in nature and contain oxygen functional groups of
phenolic, carboxylic
and sulphonic types. When the asphaltic acids become water soluble, they act
as surfactants
and reduce the surface and interfacial tension of the water (aqueous media);
as a result, they
act as clay dispersants and promote the liberation of bitumen, thus enhancing
the bitumen
recovery efficiency of the extraction process. The conditions favoring clay
dispersion in
extraction process also favor production of oil sands tailings with difficult
settling and
consolidation characteristics.
-7-
CA 02522031 2012-09-07
As stated earlier, presence of water-soluble asphaltic acids in the tailings
promote the
clay dispersion. Addition of small quantities of Ca2+ ions into the tailings
reduces the
activity of asphaltic acids in the aqueous phase. This results in an increase
in surface and
interfacial tension, which promotes the clay (and fines) flocculation, in
addition to the
mechanism of clay bonding by Ca2+ ions as explained by the chemical reaction
expressed in
Equation (1). Therefore, addition of a small quantity of Ca2+ ions into the
tailings
suspension by treating the tailings with Ca(OH)2 would promote flocculation of
clay and
fines, and would promote the nonsegregating property of the tailings. Also,
addition of
small quantity of CaO, as small as 0.4 g/L CaO, into oil sands tailings
increases the pH,
which results in the precipitation of the Mg2+ ions in the form of Mg(OH)2,
therefore
resulting in the production of a release water with Mg2+ concentration less
than 1.8 mg/L.
The Mg 2+ concentration of the release water produced from oil sands tailings
without
Ca(OH)2 treatment was about 24 mg/L.
Treatment of the tailings with Ca(OH)2 provides additional advantages as
discussed
in the "Summary of the Invention" section of the present patent application.
Treatment of oil
sands tailings with Ca(OH)2 reduces bicarbonate hardness in the tailings by
converting
Ca(HCO3)2 to CaCO3. Also, Ca 2+ ions in the release water caused by excessive
dosage of
Ca(OH)2 could be reduced by controlled injection of CO2 into tailings or by
controlled
injection of CO2 into the release water. Furthermore, the release water could
be treated, if
needed, with soda ash (Na2C03) which precipitates excess Ca(OH)2 in form CaCO3
by the
following reaction:
Ca(OH)2 + Na2CO3 -_> CaCO3 + 2NaOH (8)
Production of caustic soda (NaOH) by the ion exchange reactions between the
clay
and Ca(OH)2 as explained by Equation (1) and by treating the release water
containing
excess Ca(OH)2 with Na2CO3 as explained by Equation (8) are additional
advantages of
using Ca(OH)2 for the treatment of oil sands tailings, because a valuable
chemical NaOH,
which costs about $1,000 per ton, could be produced from CaO time (when CaO is
dissolved
in water it forms Ca(OH)2 by the chemical reaction, CaO+H20---~Ca(OH)2), which
costs
about $50 per ton and Na2CO3 which costs about $100 per ton.
-8-
CA 02522031 2012-09-07
Precipitation of excess Ca 2, ions by the controlled injection of CO2 into oil
sands
tailings after its treatment with Ca(OH)2 provides another advantage. The
ultra fine clay
particles, cations and anions like Na' and Cl" and organic molecules causing
color in the
water are adsorbed on the freshly formed surfaces of the CaCO3 crystals and
precipitated
together with it, which results in faster water release rate and lower Na' ion
concentration in
the release water. Reduction of Na' in the release water would be another
reason to use
Ca(OH)2 for the treatment of oils ands tailings, since the accumulation of Na'
in the recycle
water over the years would have a detrimental effect on the extraction
efficiency.
Based on the experimental evidence observed by the author of the present
patent
application, which are explained in "Summary of the Application" and
"Description of the
Preferred Embodiment" sections, oil sands tailings could be treated with CaO
or with CaO
and CO2 to improve its settling, consolidation and segregation characteristics
and to produce
a release water with acceptable water chemistry properties for its use in the
extraction
process, in the followings methods or their minor modifications could be used:
First Embodiment of the Method
Figure 1 schematically illustrates a method of treating oil sands fines in
accordance
with a first embodiment of the invention. This method is suitable when the oil
sands tailings
discharged from the extraction plant are not of sufficiently high solids and
fines contents to
form a nonsegregating tailings even after treated with Ca(OH)2.
In this case, oil sands tailings are passed through cyclones, producing
cyclone
underflow and cyclone overflow streams. Depending on the plant operating
conditions,
cyclone underflow may be at about above 60% solids with 3% fines (i.e., 3% of
the solids
smaller than 44 micron size) and cyclone overflow could be at about above 15
percent solids
with 50% fines (i.e., 50% of the solids smaller than 44 micron size). Cyclone
overflow will
be thickened in a thickener with the help of preferably polymeric flocculent
to about over
40% solids content (fines content of the thickened tailings would be the same
provided that
all of the fines are flocculated and settled, and that the thickener overflow
would not contain
any fines).
-9-
CA 02522031 2005-10-03
Then, the cyclone underflow and the thickener underflow will be blended at
proper
proportions and treated with Ca(OH)2 to produce a nonsegregating mix for its
final disposal.
It is also possible to add existing mature fine tails (MFT, which is of about
33% solids and
98% fines, accumulated over the years as a result of the disposal of the hot
water extraction
process tailings) into the blend of cyclone underflow and thickener underflow,
to improve the
non-segregating characteristic of the tailings mix (although this may result
in the reduction of
settling and consolidation rates).
The dosage of Ca(OH)2 treatment could be as low as, but not limited to, the
equivalent of 400 g/m3 (grams per cubic meter of tailings) of CaO, which would
be the
sufficient Ca(OH)2 dosage to produce a nonsegregating mix with acceptable
settling and
consolidation properties and to produce release water with acceptable water
chemistry
properties for its recycle to the extraction plant. The minimum dosage of
Ca(OH)2 required
in this process would be a function of the solids content, fines content (or
sand-to-fines ratio,
SFR, the ratio of the masses of sand to fines), and the extent of clay
dispersion. The release
water produced in accordance with this first embodiment of the method will
contain reduced
amounts of Cat+, Mgt+, Na+ and Cl" ions and lesser amount of ultra fines than
the original
tailings before the Ca(OH)2 treatment.
The Ca(OH)2 used for the treatment of the tailings would reduce the
bicarbonate
hardness of the tailings pore water. The remaining excess amount of the
Ca(OH)2 and the
chemicals produced by the chemical reactions as a result of the Ca(OH)2
treatment present in
this release water could be used to reduce the bicarbonate hardness of the
make-up water
(which could be river water, recycle water, or water from any other suitable
source). The
thickener overflow, which is basically the process water recovered during the
thickening of
the cyclone overflow, could also be blended in any suitable proportions with
the release
water. A final water conditioning plant for the blended water (i.e., blend of
the release water
with fresh river water, thickener overflow, or water from another suitable
source), by using
CaO, CO2, Na2CO3 or another conventionally used water conditioning chemical,
could be
considered as an option.
-10-
CA 02522031 2012-09-07
As indicated in Figure 1, the oil sands tailings optionally may also be
treated with
CaO lime (or Ca(OH)2) depending on the needs of the plant operation,
preferably but not
limited to (i) by adding CaO into the tailings before the cyclones, (ii) by
adding CaO after
blending the Thickener Underflow and Cyclone Underflow and Mature Fines
Tailings (MFT)
as optional, (iii) by adding CaO into the tailings before the cyclone, in the
thickener, and
after blending the Thickener Underflow and Cyclone Underflow and Mature Fines
Tailings
(MFT) as optional, or by any combination of these steps. Addition of CaO into
the tailings
before the cyclone or in the thickener provides a process option for the
thickening process in
the thickener due to the combined effects of polymeric flocculent and calcium
ions (Ca2+) on
the flocculation of clay size particles.
Second Embodiment of the Method
Figure 2 schematically illustrates a method of treating oil sands fines in
accordance
with a second embodiment of the invention. This method is suitable when the
oil sands
tailings discharged from the extraction plant are of sufficiently high solids
and fines contents
to form a non-segregating tailings after being treated with Ca(OH)2.
In this case, the tailings treatment plant would not need to have cyclones and
thickeners. Oil sands tailings treated with Ca(OH)2 would be of nonsegregating
and of
sufficiently fast-settling and consolidation properties. It is also possible
to add existing
mature fine tails (MFT, which is of about 33% solids and 98% fines,
accumulated over the
years as a result of the disposal of the hot water extraction process
tailings) into the tailings
discharged from the extraction plant, which would improve the nonsegregating
characteristic
of the tailings mix (although this may result in the reduction of settling and
consolidation
rates). The release water produced in accordance with this second embodiment
of the
method will contain reduced amounts of Ca2+, Mgt+, Na' and Cl- ions and lesser
amount of
ultra fines than the original tailings before the Ca(OH)2 treatment. The
Ca(OH)2 used for the
treatment of the tailings would reduce the bicarbonate hardness of the
tailings pore water.
The remaining excess amount of the Ca(OH)2 and the chemicals produced by the
chemical
reactions as a result of the Ca(OH)2 treatment present in this release water
could be used to
reduce the bicarbonate hardness of the make-up water which could be the river
water, the
-11-
CA 02522031 2005-10-03
recycle water or water from any other suitable source. A final water
conditioning plant for
the blended water (i.e., blend of the release water with fresh river water,
thickener overflow,
or water from another suitable source) by using CaO, CO2, Na2CO3 or another
conventionally used water conditioning chemical could be considered as an
option.
Experimental studies performed by the inventor have indicated that the methods
described herein could be used for the oil sands tailings produced by caustic
or non-caustic,
hot or cold water slurry-based extraction processes. The dosage of Ca(OH)2
treatment would
be a function of the solids content and the sand-to-fines ratio of the
tailings, the extent of clay
dispersion in the extraction process (which is strongly dependent upon the pH
of the
extraction process), and the composition of the make-up water to be blended
with the release
water produced from the tailings treated with Ca(OH)2.
Persons skilled in the field of the invention will appreciate that the present
invention
provides a variety of advantages and benefits, including the following:
1. Production of substantially non-segregating tailings with acceptable
settling,
consolidation and water release rate properties, with simultaneous production
of
release water with acceptable water chemistry characteristics for use in the
bitumen extraction process.
2. Production of release water with lower concentrations of Cat+, Mgt+, Na+,
C1
ions, ultra fines, and color-making organic species concentrations.
3. Release water with excess Ca(OH)2 and other chemicals produced by treating
the
oil sands tailings with Ca(OH)2 may be used for the conditioning of the make-
up
water.
4. Existing inventories of mature fine tails (MFT) may be reduced by adding
the
MFT into the blend of cyclone underflow and thickener underflow, or into the
whole tailings discharged from the extraction plant, depending on the
composition
of the tailings produced by the extraction process.
-12-
CA 02522031 2012-09-07
5. Cost-effective management of oil sands tailings produced by any kind of
water
slurry-based bitumen extraction process.
6. The process of the invention is simple to use in commercial environments
and
uses low-cost chemicals for the management of oil sands tailings.
7. The process can be smoothly integrated into existing bitumen extraction and
oil
sands tailings management processes.
In this patent document, the word "comprising" is used in its non-limiting
sense to
mean that items following that word are included, but items not specifically
mentioned are
not excluded. A reference to an element by the indefinite article "a" does not
exclude the
possibility that more than one of the element is present, unless the context
clearly requires
that there be one and only one such element.
-13-