Note: Descriptions are shown in the official language in which they were submitted.
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 315
NOTE : Pour les tomes additionels, veuillez contacter 1e Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 315
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME
NOTE POUR LE TOME / VOLUME NOTE:
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Protected Monomers
CROSS-REFERENCE TO RELATED APPLICATIONS
This application claims the benefit of United States Provisional Application
No.: 60/463,
772, filed on April 17, 2003; United States Provisional Application No.:
60/465,802, filed on
s April 25, 2003; United States Provisional Application No.: 60/493,986, filed
on August 8, 2003;
United States Provisional Application No.: 60/494,597, filed on August 11,
2003; United States
Provisional Application No.: 60/506,341, filed on September 26, 2003; United
States
Provisional Application No.: 601518,453, filed on November 7, 2003; United
States Provisional
Application No.: 60/469,612, filed on May 9, 2003; United States Provisional
Application No.:
60/510,246, filed on October 9, 2003; United States Provisional Application
No.: 60/510,318,
filed on October 10, 2003; United States Provisional Application No.:
60/465,665, filed on April
25, 2003; International Application No.: [PCT/LTS04/07070], filed on March 8,
2004;
International Application No.: [PCT/LTS04/XXXXX], filed on April 5, 2004;
International
Application No.: [PCT/LTS04/XXXXX], filed on April 9, 2004; and International
Application
~ 5 No.: [PCT/US04/XXXXX], filed on even date herewith. The contents of all of
these prior
applications are hereby incorporated by reference in their entireties.
TECHNICAL FIELD
This invention relates to protected monomers for the synthesis of iRNA agents.
BACKGROUND
2o Organic molecules containing two or more identical or similar functional
groups (e.g.,
FGa, FGa~, and FGa~~) are ubiquitous chemical species. Exposure of such a
molecule to a
particular reagent can be expected to produce products in which most or all of
FGa, FGa~, and
FGa~~ have reacted with the reagent, especially if the functional groups are
located in similar
steric or electronic environments on the molecule. When reaction at only one
particular
25 functional group (e.g., FGa) is desired, it is necessary to block
selectively the remaining
functional groups (e.g., FGa~ and FGa") with e.g., a "protecting group." A
protecting group is a
moiety that is temporarily attached to a potentially reactive site so as to
prevent it from reacting:
Once the reaction is complete at FGa, FGa' and FGa" can be "deprotected," or
restored to their
original chemical form.
3o One example where the protecting group strategy has been utilized to
provide functional
group reaction selectivity is in the synthesis of oligoribonucleotides from
individual
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
ribonucleotide monomer units. There are several chemically similar sites on
the ribonucleoside
monomers, e.g. the 2'-, 3'- and 5'- hydroxyl (OH) groups. However, the monomer
subunits
must be attached in a site-specific manner during RNA synthesis. Specifically,
the 5'hydroxyl of
one nucleoside or nucleotide chain is coupled to the 3' phosphate of a second
nucleoside or
nucleotide chain. This requires functionalizing a site either on the growing
chain or on the
incoming base for attachment of the incoming monomer building block to the
growing chain. To
prevent the incoming monomer from attaching at the wrong site, the wrong sites
must be blocked
while the correct site is left open to react. This requires the use of the
protecting group strategy.
The protecting group must be stable during said reactions and yet must
eventually be removed to
1 o yield the original site. Additionally, the synthesis of oligonucleotides
requires several sites to be
protected and particular sites must be deprotected while others remain
protected. These
protecting groups, together as a set, are termed orthogonal protecting groups.
These approaches have been widely used in the synthesis of DNA molecules.
Phosphoramidite chemistry, so named for a functional group on the monomer
building blocks,
has seen wide use in the synthesis of polynucleotides, see, e.g., U.S.
4,415,732. The
phosphoramidite functional group allows for monomer-by-monomer synthesis in a
relatively
efficient manner. Synthesis is often performed on a solid phase, see, e.g.,
Caxuthers et al. in U.S.
4,45,066. In this approach the growing DNA chain can be attached to an
insoluble support via
a linker, e.g., a long organic linker, which allows the growing DNA chain to
be solubilized in the
2o solvent in which the support is placed. Phosphoramidite chemistry combined
with solid phase
synthesis has helped make directed synthesis of DNA widely available.
Solid phase phosphoramidite oligonucleotide synthesis methods typically use a
dimethoxytrityl,protecting group for the 5' hydroxyl of nucleosides. The 3'
hydroxyl position is
protected with a phosphoramidite functionality. Synthesis generally proceeds
from the 3' to the
5' of the ribose or deoxyribose sugax component of the phosphoramidite
nucleoside. The 5' end
of the growing chain is reacted with and coupled to the 3' phosphoramidite of
the incoming base
to form a phosphite triester intermediate. To insure that only one monomer is
added in a round
of synthesis the 5' hydroxyl of the newly added base is protected by a
dimethoxytrityl group.
Any unreacted 5' hydroxyls are "capped" off to stop the synthesis of this
chain, which would be
one base short at the end of synthesis. Iodine oxidation is used after each
coupling reaction to
yield a more stable phosphotriester intermediate. Oxidation prevents the
relatively unstable
phosphite triester linkage from undergoing cleavage under the acidic
conditions of subsequent
synthesis steps. In order to add another monomer the 5' dimethoxytrityl
protecting group of the
newly added base must be removed or deprotected,-e.g., by reaction with acidic
solution to yield
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
a free 5' hydroxyl group which can be coupled to the next protected nucleoside
phosphoramidite.
This process is repeated until the desired sequence is synthesized.
The use of the dimethoxytrityl group further prevents the use of other acid
labile
protecting groups. This is important for RNA synthesis because another
hydroxyl group at the 2'
position must be protected. Thus, the synthesis of RNA presents additional
problems, e.g., the
need for a suitable 2' protecting group. Incorporation of a dimethoxytrityl
protecting group
strategy at the 5' position therefore prevents the successful use of acid
labile groups for 2'
protection during RNA synthesis. U.S. 5,889,136, has described protection
strategies for use
where the 2' position in ribonucleotides must be protected.
SUMMARY
This invention relates to protected monomers for the synthesis of iRNA agents,
methods
of synthesis, and uses thereof.
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
In one aspect, this invention relates to protected monomers having a formula
(I):
5'
X
XS..
I 5",
(I)
wherein,
B is selected from the group consisting of
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
R1 R2 Rs
\N/ R1o-N R$
N / N R \N R11\ R12
N
3~ ~ I
R N N~ R6 ~~ y. X N
R15 ~ ~ ~18
R16
14 / N
R '
N i N R1~
R13 'nI~'~
p NH
R19 R20 24 25
R \ ~ ~R
\N R21 N N
' '
R23 26
R HN N
X i 1 R22 ~' ~ ~ns~r
i
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
anthracenyl, pyrenyl,
R53
R54
R46 R51
...Q ...Q
..
Q C~ Qn, \ 52 ~ Qm \ ~ R55
' \ R \
N
C~ ~ R48 ~ . ~ R56 .
I
ao
R6 R6~ ___
'Z , , and
R61 R57 R64 37
R72 ... ". Rss
R71
6
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
X2 is an ortho ester protecting group, hydrogen, ethers, alkyl ethers, esters,
halogens,
protected amines, or protected hydroxyl moieties;
X3 is -O-P(OR2~)N(R28)2 or -O-L-R29;
XS~, XS~~, XS~~~ include at least one alkoxy or siloxy substituent;
Rl is hydrogen or C1-C4 alkyl;
R2 is hydrogen, C1-C4 alkyl, or C2-C6 alkenyl optionally substituted with
hydroxy, or
C(O)NHR ;
R3 is hydrogen, halo, C1-C4 alkyl, C1-C4 thioalkoxy, NH2, NHRb, or
NRbR°;
R4 when taken together with R4~ forms oxo, or R4 when taken together with RS
forms a
double bond between the carbon and nitrogen atoms to which they are attached;
R4~ when taken together with R4 forms oxo, or is O-;
RS is hydrogen, C1-C4 alkyl, or when taken together with R4 forms a double
bond
~ 5 between the carbon and nitrogen atoms to which they are attached;
R6 is hydrogen, halo, NH2, NHRb, or NRbR~;
R~ is an unshared electron pair, or Cl-C~ alkyl;
R8 when taken together with R9 forms a double bond between the carbon and
nitrogen
atoms to which they are attached, or R$ when taken together with Rl l forms a
double bond
2o between the carbon and nitrogen atoms to which they are attached;
R9 is hydrogen, C1-C4 alkyl, or when taken together with Rg forms a double
bond
between the carbon and nitrogen atoms to which they are attached;
RI° is hydrogen or is absent;
Rl1 is hydrogen, Cl-C4 alkyl, or when taken together with R$ forms a double
bond
25 between the carbon and nitrogen atoms to which they are attached;
Rlz is hydrogen, formyl, or C1-C4 alkyl optionally substituted with hydroxy or
protected
hydroxy;
R13 and R14 are each independently hydrogen or C1-C4 alkyl;
Rls is hydrogen, Cl-C4 alkyl, or (CHa)nCH(Rd)CH(NHRe)(COORg);
so Ri6 is hydrogen or C1-C4 alkyl;
Rl' is halo, NH2, NHRb, or NRbR°;
Rl8 is cyano, C(=NH)NHa, or CH2NH(Rh);
Rl~ is hydrogen, or C1-C4 alkyl;
R2° is:
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
(i) hydrogen;
(ii) hydroxy or protected hydroxy;
(iii) C1-C4 alkoxy optionally substituted with COORf; or
(iv) C1-C4 alkyl optionally substituted with hydroxy andlor COOR ; NH2, NHRm,
or
CONH2;
R21 is hydrogen, or when taken together with R23 forms a double bond between
the
carbon atoms to which they are attached;
R22 is hydrogen;
R23 is hydrogen, or when taken together with R21 forms a double bond between
the
carbon atoms to which they are attached;
R24 and Ras are each, independently, hydrogen or C1-C4 alkyl;
Ra6 is (CH2)"CH(Rd)CH(NHRe)(COORg);
R2~ is Cl-C6 alkyl optionally substituted with cyano, or C2-C6 alkenyl;
Ra8 is Cl-Cio alkyl;
~ 5 Rz9 is a liquid or solid phase support reagent;
Q is N or CR44;
Q' is N or CR4s;
Q" is N or CR4~;
Q"' is N or CR49;
2o Q'° 1S N Or CRso;
Rte. is hydrogen, halo, hydroxy, nitro, protected hydroxy, NHZ, NHRb, or
NRbR°, Cl-C6
alkyl, C6-Clo aryl, C6-Clo heteroaryl, C3-C8 heterocyclyl, a ligand, a
tethered ligand, or when
taken together with R45 forms -OCH20-;
R45 is hydrogen, halo, hydroxy, nitro, protected hydroxy, NH2, NHRb, or NRbR~,
C1-C6
25 alkyl, C6-Clo aryl, C6-Clo heteroaryl, C3-C8 heterocyclyl, a ligand, a
tethered ligand, or when
taken together with R44 or R46 forms -OCH20-;
R46 is hydrogen, halo, hydroxy, nitro, protected hydroxy, NH2, NHRb, or NRUR~,
Ci-C6
alkyl, C6-Clo aryl, C6-Clo heteroaryl, C3-C8 heterocyclyl, a ligand, a
tethered ligand, or when
taken together with R45 or R4' forms -OCH20-;
3o R4~ is hydrogen, halo, hydroxy, nitro, protected hydroxy, NHZ, NHRb, or
NRbR°, Cl-Cs
alkyl, C6-Clo aryl, Cg-Clo heteroaryl, C3-C8 heterocyclyl, a ligand, a
tethered ligand, or when
taken together with R46 or R48 forms -OCH20-;
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
R4~ is hydrogen, halo, hydroxy, vitro, protected hydroxy, NH2, NHRb, or
NRbR°, Cl-C6
alkyl, C6-Clo aryl, C6-Clo heteroaxyl, C3-C$ heterocyclyl, a ligand, a
tethered ligand, or when
taken together with R47 forms -OCH20-;
R49 R50 R51 R52 R53 R54 R57 R58 R59 R60 R61 R62 R63 R64 R65 R66 R67 R68 R69
> > > > > > > > > > > > > > > > > >
R7°, R71, and R72 are each independently selected from hydrogen, halo,
hydroxy, vitro, protected
hydroxy, NH2, NHRb, or NRbR°, C1-C6 alkyl, C2-C6 alkynyl, C6-Clo aryl,
C6-Clo heteroaryl, C3_
C8 heterocyclyl, NC(O)R17, or NC(O)R°;
R55 is hydrogen, halo, hydroxy, vitro, protected hydroxy, NH2, NHRb, or NRbR~,
C1_C6
alkyl, C2-C6 alkynyl, C6-Clo aryl, C6-Clo heteroaryl, C3-C8 heterocyclyl,
NC(O)R17, or NC(O)R°,
or when taken together with R56 forms a fused aromatic ring which may be
optionally
substituted;
R56 is hydrogen, halo, liydroxy, vitro, protected hydroxy, NH2, NHRb, or
NRbR°, Cl_C6
alkyl, Ca-C6 alkynyl, C6-Clo aryl, C6-Clo heteroaryl, C3-C$ heterocyclyl,
NC(O)R17, or NC(O)R°,
or when taken together with R55 forms a fused aromatic ring which may be
optionally
~5 substituted;
X is O, S, or Se;
YisOorS;
L is -C(O)(CH2)gC(O)-, or -C(O)(CH2)qS-;
Provided that Rl, R2, and R3 cannot all be hydrogen; further provided that
when RS is
2o hydrogen, R6 cannot be NH2, NH(protecting group), or NH(iBu); further
provided that when Rla
is hydrogen and R8 and Rl1 together form a double bond between the carbon and
nitrogen atoms
to which they are attached, R9 and Rl° cannot both be hydrogen; further
provided that when X
and Y are O, Rl9 is hydrogen, and R21 and R23 together form a double bond
between the carbon
atoms to which they are attached, R2° cannot be hydrogen or CH3;
25 ~ Ra is glycinyl, threonyl, or norvalyl, each of which may optionally be
partially or fully
protected;
Rb is C1-C6 alkyl or a nitrogen protecting group;
R~ is Cl-C6 alkyl;
Ra is hydrogen, hydroxy, protected hydroxy, or OOH;
3o Re is hydrogen, a nitrogen protecting group, or COORg;
Rf is hydrogen, or C1-C6 alkyl;
Rg is C1-Clo alkyl;
9
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
R'' is hydrogen, or
R;
R' and Rj when taken together forms a double bond between the carbon atoms to
which
they are attached, or R' and Rj when taken together form -O- between the
carbon atoms to which
they are attached;
Rk and R' are each, independently, hydrogen, a hydroxyl protecting group,a
sugar, or a
fully or partially protected sugar;
Rm is Cl-C4 alkyl optionally substituted with COOH;
R° is alkyl optionally substituted with halo, hydroxy, nitro, protected
hydroxy, NH2,
1o NHRb, orNRbR°, C1-C6 alkyl, CZ-C6 alkynyl, C6-Clo aryl, C6-Clo
heteroaryl, C3-C8 heterocyclyl,
NC(O)Ri~, or NC(O)R°;
n is 1-4; and
q is 0-4.
to
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
In another aspect, this invention relates to protected monomers having a
formula (I):
X5,
..
(I)
wherein,
B is selected from the group consisting of
11
X3 Xc.
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
R1 R2 Rg
\ N / R4 R4,
R1o
N / N R \N N R11\ R12
\ '
> >
3~
R N N R6 N N X,
R15 ~ r,18
R1E
R14 ~ w ~ ~ ,
N il .; R1~
R13 ~r~'
p NH
R1 g R24 R25
\ ~ /
\N N N N
and
26
R H
p~ ~ N N
i
X2 is an ortho ester protecting group, hydrogen, ethers, alkyl ethers, esters,
halogens,
protected amines, or protected hydroxyl moieties;
X3 is -O-P(OR2~)N(RZ8)2 or -O-L-R29;
XS~, XS~~, XS'" include at least one alkoxy or siloxy substituent;
Rl is hydrogen or C1-C4 alkyl;
RZ is hydrogen, C1-C4 alkyl, or CZ-C6 alkenyl optionally substituted with
hydroxy, or
C(O)NHRa;
R3 is hydrogen, C1-C4 alkyl, or C1-C4 thioalkoxy, NHa, NHRb, or NRbR~;
R4 when taken together with R4~ forms oxo, or R4 when taken together with RS
forms a
double bond between the carbon and nitrogen atoms to which they are attached;
12
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
R4~ when taken together with R4 forms oxo, or is O-;
RS is hydrogen, C1-C4 alkyl, or when taken together with R4 forms a double
bond
betyveen the carbon and nitrogen atoms to which they are attached;
R6 is hydrogen, NH2, NHRb, or NRbR°;
R' is an unshared electron pair, or Cl-C4 alkyl;
R$ when taken together with R9 forms a double bond between the carbon and
nitrogen
atoms to which they are attached, or R$ when taken together with R11 forms a
double bond
between the carbon and nitrogen atoms to which they are attached;
R9 is hydrogen, C1-C4 alkyl, or when taken together with R8 forms a double
bond
between the carbon and nitrogen atoms to which they are attached;
RI° is hydrogen or is absent; -
Rl l is hydrogen, Cl-C4 allcyl, or when taken together with Rg forms a double
bond
between the carbon and nitrogen atoms to which they are attached;
RIZ is hydrogen, formyl, or Cl-C4 alkyl optionally substituted with hydroxy or
protected
hydroxy;
R13 and R14 are each independently hydrogen or C1-C4 alkyl;
R15 is hydrogen, C1-C4 alkyl, or (CHZ)"CH(Ra)CH(NHRe)(COORg);
R16 is hydrogen or C1-C4 alkyl;
Ri~ is halo, NH2, NHRb, or NRbR°;
2o Rl8 is cyano, C(=NH)NH2, or CH2NH(Rh);
Rl9 is hydrogen, or Cl-C4 alkyl;
R2° is:
(i) hydrogen;
(ii) hydroxy or protected hydroxy;
(iii) C1-C4 alkoxy optionally substituted with COOR ; or
(iv) C1-C4 alkyl optionally substituted with hydroxy and/or COOR ; NH2, NHRm,
or
CONH2;
RZ1 is hydrogen, or when taken together with R23 forms a double bond between
the
carbon atoms to which they are attached;
3o R22 is hydrogen;
R23 is hydrogen, or when taken together with R21 forms a double bond between
the
carbon atoms to which they are attached;
R24 and R25 are each, independently, hydrogen or Cl-C4 alkyl;
R26 is (CHa)"CH(Rd)CH(NHRe)(COORg);
13
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
R2' is Cl-C6 alkyl optionally substituted with cyano, or C2-C6 alkenyl;
RZ$ is CI-Cl° alkyl;
R29 is a liquid or solid support reagent;
X is O, S, or Se;
YisOorS;
L is -C(O)(CHZ)qC(O)-, or -C(O)(CH2)qS-;
Provided that Rl, R2, and R3 cannot all be hydrogen; further provided that
when RS is
hydrogen, R6 cannot be NH2 or NH(iBu); further provided that when Rl2 is
hydrogen and R8 and
Rl l together form a double bond between the carbon and nitrogen atoms to
which they are
1 o attached, R9 and Rl° cannot both be hydrogen; further provided that
when X and Y are O, R19 is
hydrogen, and Rai and R23 together form a double bond between the carbon atoms
to which they
are attached, R2° cannot be hydrogen or CH3;
Ra is glycyl, threonyl, or norvalyl, each of which is optionally partially or
fully protected;
Rb is Cl-C6 alkyl or a nitrogen protecting group;
~ 5 R~ is Cl-C6 alkyl;
Ra is hydrogen, hydroxy, protected hydroxy, or OOH;
Re is hydrogen, a nitrogen protecting group, or COORg;
Rf is hydrogen, or Cl-C6 alkyl;
Rg is Cl-Ci° alkyl;
2o Rh is hydrogen, or
Rk
Ri
R~
.,
R' and Rj when taken together forms a double bond between the carbon atoms to
which
they are attached, or R' and Rj when taken together form -O- between the
carbon atoms to which
they are attached;
25 Rk and RI are each, independently, hydrogen, a hydroxyl protecting group, a
sugar, or a ;
Rm is Cl-C4 alkyl optionally substituted with COOH;
n is 1-4; and
14
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
q is 0-4.
In a further aspect, this invention relates to protected monomers having a
formula (I):
5'
X
X5" ~~ 0
X5",
(I)
wherein,
B is selected from the group consisting of
anthracenyl, pyrenyl,
R53
Rq.g R51
...Q
..
Q Q ~~~~ t55
~ \ ~ R52
Q / . N
i
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
R62 O
R61 R63 ___
and
R6~ R57 R64
R~
R71
16
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
XZ is an ortho ester protecting group, hydrogen, ethers, alkyl ethers, esters,
halogens,
protected amines, or protected hydroxyl moieties;
X3 1S -O-P(OR2~)N(R28)2 or -O-L-R29;
Xs~, Xs~~, Xs~~~ include at least one alkoxy or siloxy substituent;
Rl~ is halo, NH2, NHRb, or NRbR~;
RZ~ is Cl-C6 alkyl optionally substituted with cyano, or C2-C6 alkenyl;
RZ$ is C1-Clo alkyl;
R29 1S a liquid or solid phase support reagent;
Q is N or CR44;
Q' is N or CR4s;
Q" is N or CR4~;
Q"' is N or CR49;
Q'~ is N or CRso;
R'~ is hydrogen, halo, hydroxy, vitro, protected hydroxy, NHZ, NHRb, or
NRbR°, Cl-C6
alkyl, C6-Clo aryl, C6-Clo heteroaryl, C3-C$ heterocyclyl, a ligand, a
tethered ligand, or when
taken together with R4s forms -OCH2O-;
R4s is hydrogen, halo, hydroxy, vitro, protected hydroxy, NH2, NHRb, or
NRbR°, Cl-C6
alkyl, C6-Clo aryl, Cg-Clo heteroaryl, C3-C$ heterocyclyl, a ligand, a
tethered ligand, or when
2o taken together with R44 or R46 forms -OCH20-;
R46 is hydrogen, halo, hydroxy, vitro, protected hydroxy, NH2, NHRb, or
NRbR°, Cl-C6
alkyl, C6-Clo aryl, C6-Clo heteroaryl, C3-C8 heterocyclyl, a ligand, a
tethered ligand, or when
taken together with R4s or R4~ forms -OCH20-;
R4' is hydrogen, halo, hydroxy, vitro, protected hydroxy, NH2, NHRb, or
NRbR°, Cl-C6
alkyl, C6-Clo aryl, C6-Clo heteroaryl, C3-C8 heterocyclyl, a ligand, a
tethered ligand, or when
taken together with R46 or R48 forms -OCH2O-;
R48 is hydrogen, halo, hydroxy, vitro, protected hydroxy, NH2, NHRb, or
NRbR°, Cl-C6
alkyl, C6-Clo aryl, C6-Clo heteroaryl, C3-C$ heterocyclyl, a ligand, a
tethered ligand, or when
taken together with R4' forms -OCH20-;
so R49 Rso Rsi Rsa Rs3 Rs4 Rs~ Rss Rs9 R6o Rsi R6z Rs3 Rs4 Rss R66 Rs~ Rsa R69
> > > > > > > > > > > > > > > > > >
R'°, R'1, and R'2 are each independently selected from hydrogen, halo,
hydroxy, vitro, protected
hydroxy, NH2, NHRb, or NRbR~, C1-C6 alkyl, C2-C6 alkynyl, C6-Clo aryl, C6-Clo
heteroaryl, C3_
C$ heterocyclyl, NC(O)Rl~, or NC(O)R°;
17
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
R55 is hydrogen, halo, hydroxy, vitro, protected hydroxy, NH2, NHRv, or
NRbR°, Cl-C6
alkyl, Ca-C6 alkynyl, C6-Clo aryl, C6-Clo heteroaryl, C3-C8 heterocyclyl,
NC(O)Rl', or NC(O)R°,
or when taken together with R56 forms a fused aromatic ring which may be
optionally
substituted;
R56 is hydrogen, halo, hydroxy, vitro, protected hydroxy, NHZ, NHRb, or
NRbR°, C1-C6
alkyl, Cz-C6 alkynyl, C6-Clo aryl, C6-Clo heteroaryl, C3-C8 heterocyclyl,
NC(O)Rl', or NC(O)R°,
or when taken together with R55 forms a fused aromatic ring which may be
optionally
substituted;
L is -C(O)(CHZ)qC(O)-, or -C(O)(CH2)aS-;
Rb is Ci-C6 alkyl or a nitrogen protecting group;
R° is C1-C6 alkyl;
R° is alkyl optionally substituted with halo, hydroxy, vitro, protected
hydroxy, NH2,
NHRb, or NRbR°, Cl-C6 alkyl, C2-C6 alkynyl, C6-Clo aryl, C6-Clo
heteroaryl, C3-C$ heterocyclyl,
NC(O)RI', or NC(O)R°;
n is 1-4; and
q is 0-4.
1s
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Embodiments can include one or more of the following features.
B can be:
R1\N/R2
N
Rs . . ~ ,
B can be:
R4 R4' R7
5
R\ N
N
Rs \N N ,
B can be:
R9
R1o N R$
R11 R12
\ N
~/ ~N
19
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
B can be:
R15
14 / N
R
N~ N/
R13
B can be:
O R1$
R1o
~N
17 ~ N
R N
B can be:
Y
R2o
R1s
~N F
F
X N RZa
20
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
B can be:
O
R24 R25
~N N~
O
B can be:
NH
N
R26HN ~N
I
i
B can be:
R46
(~
'R4s
21
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
B can be:
R51
...Q
R52
N
B can be:
R53
R54
...Q
R55
N
56 '
B can be:
R61
R5~
22
Rs2 O
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
B can be:
R63 m Q ivQ _
\ \ ~?
R64 ~ ~ R67
R66
B can be:
R72 ... Q iv Q Rgg
\ \
F27~ / / ,
B can be anthracenyl.
B can be pyrenyl.
R28 can be isopropyl.
' XS~, Xs~~, and XS'~~ can be any combination of the following formula:
23
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
O ' - ' ~ ' H3C ~- '
v
O
i
// ' '
AP
j H3 / O-~_ SO_~_
H3C-Si~O~ ~ , Si '
H3
3 3
O'
0
and O O
3
Xs~and XS" can be siloxy and XS'~~ can be cycloalkoxy.
The orthoester group can have formula (III):
O
R32O ~OR31
(III)
24
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
R31 and R32 Can be the same or different and can be any combination of the
following
formulae:
~~CI , CI , ~~OCH3 , \ \ ,
-, C ''~I
CN O
,
CI
-\ \ c1 , ~ \ ,
\'
c1 ~ cN
R33 R34
R35
O \ R36 ,
I
R33 O
R33
R38 ,
R3a
R33
or
R3a
wherein R33, R34~ R3s~ R36~ ~d Rs~ is a compatible ligand, or hydrogen, or
halogen, alkyl,
or cyano substituent, and R38 is compatible ligand.
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
The orthoester can be:
O
H3C O O CH3
O O
O O
R29 can be a fluoride-stable polystyrene based solid support or PEG.
X2 can be -OC[OCH2CH20C(O)CH3]2; R2~ can be CH3; RZ$ can be (CH3)ZCH-; XS' and
XS" are trimethylsiloxy; XS"' can be cyclododecyloxy; and B can be:
1 2
R \N/R
N
R3 . ~ \
X2 can be -OC[OCH2CH20C(O)CH3]Z; R2~ can be CH3; R28 can be (CH3)2CH-; XS' and
XS" can be trimethylsiloxy; XS"' can be cyclododecyloxy; and B can be:
R4 R4' R7
R \ N
N
Rs \N N
v
26
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
X2 can be -OC[OCH2CH20C(O)CH3]Z; RZ~ can be CH3; R28 can be (CH3)ZCH-; XS' and
XS" can be trimethylsiloxy; XS"' can be cyclododecyloxy; and B can be:
R9
R1o N R$
R11 R12
~N
X~ ~N
X2 can be -OC[OCHZCH20C(O)CH3]2; R2' can be CH3; R28 can be (CH3)ZCH-; XS' and
XS" can be trimethylsiloxy; XS "' can be cyclododecyloxy; and B can be:
Ar O
N
R1~
N ~N
R13
X2 can be -OC[OCHaCH20C(O)CH3]2; RZ' can be CH3; Ra8 can be (CH3)2CH-; XS' and
1 o XS" can be trimethylsiloxy; XS"' can be cyclododecyloxy; and B can be:
s
R16
~N
R1~ ,
IV
27
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
X2 can be -OC[OCH2CHaOC(O)CH3]2; R2' can be CH3; Ra$ can be (CH3)ZCH-; XS' and
XS" can be trimethylsiloxy; XS "' can be cyclododecyloxy; and B can be:
Y
R2o
R19
\ N R21
R2s
N R22
X2 can be -OC[OCHZCH20C(O)CH3]2; RZ~ can be CH3; R28 can be (CH3)2CH-; XS' and
XS" can be trimethylsiloxy; XS"' can be cyclododecyloxy; and B can be:
O
24 25
R \N N/R
O
X2 can be -OC[OCH2CH20C(O)CH3]2; RZ~ can be CH3; R28 can be (CH3)2CH-; XS' and
XS" can be trimethylsiloxy; XS "' can be cyclododecyloxy; and B can be:
NH
N
R26HN ~N
I
i
28
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
X2 can be -OC[OCH2CH20C(O)CH3]Z; RZ~ can be CH3; R28 can be (CH3)2CH-; XS' and
XS" can be trimethylsiloxy; XS"' can be cyclododecyloxy; and B can be:
Q, \ Q"
I
X2 can be -OC[OCHaCH20C(O)CH3]2; R2~ can be CH3; R28 can be (CH3)2CH-; XS' and
XS" can be trimethylsiloxy; XS"' can be cyclododecyloxy; and B can be:
R51
Q,~~~
\ R5a
N
X2 can be -OC[OCH2CH20C(O)CH3]2; RZ~ can be CH3; R2$ can be (CH3)2CH-; XS' and
XS" can be trimethylsiloxy; XS"' can be cyclododecyloxy; and B can be:
R53
mQ
iv'' \ R55
Q
N \
29
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
X2 can be -OC[OCH2CHaOC(O)CH3]2; RZ~ can be CH3; R28 can be (CH3)~CH-; XS' and
XS" can be trimethylsiloxy; XS"' can be cyclododecyloxy; and B can be:
R61
~ N~
Rso i ~ ~ ~R57.
R~~ R
X2 can be -OC[OCH2CH20C(O)CH3]2; R2~ can be CH3; R2$ can be (CH3)2CH-; XS' and
XS" can be trimethylsiloxy; XS"' can be cyclododecyloxy; and B can be:
R63 ", ~ iv
R64 ~ , ~ ~ ~ R67
X2 can be -OC[OCH2CH20C(O)CH3]2; RZ' can be CH3; R28 can be (CH3)aCH-; XS' and
1 o XS" can be trimethylsiloxy; XS"' can be cyclododecyloxy; and B can be:
R72 m Q iv (~ R68
R71
R70 ~ 69
R62
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
X2 can be -OC[OCHaCH20C(O)CH3]2; R2~ can be CH3; R28 can be (CH3)2CH-; XS' and
XS" can be trimethylsiloxy; XS"' can be cyclododecyloxy; and B can be
anthracenyl.
X2 can be -OC[OCH2CHZOC(O)CH3]2; R2~ can be CH3; R28 can be (CH3)ZCH-; XS' and
XS" can be trimethylsiloxy; XS"' can be cyclododecyloxy; and B can be pyrenyl.
B can be an unusual or universal base that can be selected from: 2-
aminoadeninyl, 2-
methyladeninyl, N6-methyladeninyl, 2-methylthio-N6-methyladeninyl, N6-
isopentenyladeninyl,
2-methylthio-N6-isopentenyladeninyl, N6-(cis-hydroxyisopentenyl)adeninyl, 2-
methylthio-N6-
(cis-hydroxyisopentenyl) adeninyl, N6-glycinylcarbamoyladeninyl, N6-
threonylcarbamoyladeninyl, 2-methylthio-N6-threonyl carbamoyladeninyl, N6-
methyl-N6-
1o threonylcarbamoyladeninyl, N6-hydroxynorvalylcarbamoyladeninyl, 2-
methylthio-N6-
hydroxynorvalyl carbamoyladeninyl, N6,N6-dimethyladeninyl, 3-methylcytosinyl,
5-
methylcytosinyl, 2-thiocytosinyl, 5-formylcytosinyl, N4-methylcytosinyl, 5-
hydroxymethylcytosinyl, 1-methylguaninyl, N2-methylguaninyl, 7-methylguaninyl,
N2,N2-
dimethylguaninyl,
NH
COOH
l
H2N N N
H I
31
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
NHCOOCH3 NHCOOCH3 NHCOOCH3
H3COOC H3COOC OH H3COOC OOH
O O O
H3C N~N N) , H3C N~ N~ ~ H3C / -~ > >
"~.n;, N "~,;,~. N N
CH3 CH3 CH3
NH'
H
O H3C O
HsC~N~ O H3C~N
N> N N ,~ ~ N~N
,J.N- CH3 ' CH3
CH3
O O HO HO
HN N H3C~N N HO I HO
\N N,., ~N N , ~ O NH ~ O NH
HN ~ HN
H2N~N ~, H2N~N fuel",
HO HO
beta-galactosyl0 O beta-mannosyl0 O
HN ,
O NH O NH ~ O NH2
HN ~ HN ~ HN
H2N~N N H2N~N N H2N~N N",
1
32
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
N2,7-dimethylguaninyl, N2,N2,7-trimethylguaninyl, 1-methylguaninyl, 7-cyano-7-
deazaguaninyl, 7-aminomethyl-7-deazaguaninyl, pseudouracilyl, dihydrouracilyl,
5-
methyluracilyl, 1-methylpseudouracilyl, 2-thiouracilyl, 4-thiouracilyl, 5-
methyl-2-thiouracilyl, 3-
(3-amino-3-carboxypropyl)uracilyl, 5-hydroxyuracilyl, 5-methoxyuracilyl,
uracilyl 5-oxyacetic
acid, uracilyl 5-oxyacetic acid methyl ester,
5-(carboxyhydroxymethyl)uracilyl, 5-(carboxyhydroxymethyl)uracilyl methyl
ester,
5-methoxycarbonylmethyluracilyl, 5-methoxycarbonylmethyl-2-thiouracilyl,
5-aminomethyl-2-thiouracilyl, 5-methylaminomethyluracilyl, 5-methylaminomethyl-
2-
thiouracilyl, 5-methylaminomethyl-2-selenouracilyl, 5-carbamoylmethyluracilyl,
5-carboxymethylaminomethyluracilyl, 5-carboxymethylaminomethyl-2-thiouracilyl,
3-methyluracilyl, 1-methyl-3-(3-amino-3-carboxypropyl) pseudouracilyl, 5-
carboxymethyluracilyl, 5-methyldihydrouracilyl, 3-methylpseudouracilyl,
F CH3 NHz NH2
l 02N
H3C ~ ~ N w N N/\ N I
I ~ I y ~ ~ , I
F , N , ~ ~ N ,
i ~ ~"""
CH3 O CH3 O p ~ CH3
N~ ' I \ N~ ' I \ N~ ~ I \ N~ H3C
H C ~ / / / CH3
3
NO2 \ \ O OI I W y' ~ O
I ~ ~ ~~ ~ BuHN~N ~ ~ N. _ ' I i ~ '
N N N N~ H ~ 02N
H N \
33
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
C CH3
H3C CH3 ~ ~ W w ~ I W W
~ ' ~ ~
I \ \ N
N N , I / ~ ~ ~ CH3
'f'f CHs
~ z''~~ - N-
( , , , and ~ ~ \ /
1' N
CH3
X2 can be -OC[OCHZCHZOC(O)CH3]2; R2' can be CH3; R2$ can be (CH3)2CH-; XS' and
XS" can be trimethylsiloxy; XS"' can be cyclododecyloxy; and B can be selected
from any of
the unusual or universal bases described above.
X2 can be fluoro.
B can be:
F
H3
F
B can be substituted or unsubstituted (e.g., having one or more fluoro groups)
aryl
attached to a tethered or untethered ligand.
34
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
In one aspect, this invention relates to this invention relates to a protected
monomer
having a formula (II):
X5,
0
5...
(II)
wherein
B is selected from the group selected from:
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
1 2 R9
R \N/R R4 R4, R~ R1°-N R$
/ N R \ N R11 R12
N \ N \ \N
> >
6
R3 N ~ R N ~ X N
v v
R15 ~ ~ R18
N N R1 \ N
R14
N i N R1~ WN N
R13
p NH
R19 R24 R25
\ ~ /
\N N N
R2sHN N
i
36
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
anthracenyl, pyrenyl,
R53
R54
R46 R51
mQ mQ
iv iv~~ \ R55
' ~ ~ R52 '
Q ~ . N . N ~ .
\ R48 ~ ~ R56
i
R61 R63 ", ~ ivQ
\ \
and
R60 R57 R64 ~ ~ R67
R72 ... Q iv Q R68
\ \
R71
R70 ~ 69
37
R62
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
X2 is an ortho ester protecting group, hydrogen, ethers, alkyl ethers, esters,
halogens,
protected amines, or protected hydroxyl moieties;
3 1S -O-P(OR2')N(R28)2 or -~-L-R29;
XS~, XS~~, XS"~ include at least one alkoxy or siloxy substituent;
G is NR3°, S, or CW2;
Rl is hydrogen or C1-C4 alkyl;
R2 is hydrogen, C1-C4 alkyl, or C2-C6 alkenyl optionally substituted with
hydroxy, or
C(O)NHR~;
R3 is hydrogen, halo, Cl-C4 alkyl, Cl-C4 thioalkoxy, NH2, NHRb, or
NRbR°;
1 o R4 when taken together with R4~ forms oxo, or R4 when taken together with
RS forms a
double bond between the carbon and nitrogen atoms to which they are attached;
R4~ when taken together with R4 forms oxo, or is O-;
RS is hydrogen, Cl-C4 alkyl, or when taken together with R4 forms a double
bond
between the carbon and nitrogen atoms to which they are attached;
15 R6 is hydrogen, halo, NH2, NHRb, or NRbR°;
R' is an unshared electron pair, or Cl-C4 alkyl;
R8 when taken together with R9 forms a double bond between the carbon and
nitrogen
atoms to which they are attached, or R8 when taken together with Rl l forms a
double bond
between the carbon and nitrogen atoms to which they are attached;
2o R9 is hydrogen, C1-C4 alkyl, or when taken together with R8 forms a double
bond
between the carbon and nitrogen atoms to which they are attached;
Rl° is hydrogen or is absent;
Rl 1 is hydrogen, Cl-C4 alkyl, or when taken together with Rg forms a double
bond
between the carbon and nitrogen atoms to which they are attached;
25 Ri2 is hydrogen, formyl, or C1-C4 alkyl optionally substituted with hydroxy
or protected
hydroxy;
R13 and R14 are each independently hydrogen or C1-C4 alkyl;
Rls is hydrogen, C1-C4 alkyl, or (CH2)"CH(Ra)CH(NHRe)(COORg);
RI6 is hydrogen or Cl-C4 alkyl;
3o RI~ is halo, NH2, NHRb, or NRbR°;
Rl8 is cyano, C(=NH)NH2, or CH2NH(Rh);
RI9 is hydrogen, or C1-C4 alkyl;
R2° is:
(i) hydrogen;
38
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
(ii) hydroxy or protected hydroxy;
(iii) C1-C4 alkoxy optionally substituted with COORf; or
(iv) C1-C4 alkyl optionally substituted with hydroxy and/or COOR ; NH2, NHRm,
or
CONHZ;
R21 is hydrogen, or when taken together with R23 forms a double bond between
the
carbon atoms to which they are attached;
Ra2 is hydrogen;
R23 is hydrogen, or when taken together with R21 forms a double bond between
the
carbon atoms to which they are attached;
1 o R24 and RZS are each, independently, hydrogen or C1-C4 alkyl;
R26 is (CH2)"CH(Rd)CH(NHRe)(COORg);
R2~ is Cl-C6 alkyl optionally substituted with cyano, or C~-C6 alkenyl;
R28 is Cl-Cio alkyl;
R29 is a liquid or solid phase support reagent;
~5 R3° is Cl-CZO alkyl, C2-C2o alkenyl, C2-C2o alkynyl; C3-C8
cycloalkyl; C6-Cia aryl; 5-10
membered heteroaryl; C~-C14 aralkyl; -C(O)-(CHZ)S-C(O)-(ligand);
-C(O)-(CHZ)S-C(O)O-(ligand); -C(O)-O-(ligand); -C(O)-(CH2)S NH-;
-C(O)-(CH2)S NH-C(O)-(ligand); -C(O)-(CHZ)S (ligand); -C(O)-NH-(ligand);
-C(O)-(ligand); -(CH2)S C(O)-(ligand); -(CH2)S C(O)O-(ligand); -(CH2)S-
(ligand);
20 -(CHa)S NH-; or -(CH2)S-NH-C(O)-(ligand);
Q is N or CR44;
Q' is N or CR4s;
Q" is N or CR4~;
Q"' is N or CR49;
25 Q'~ is N or CRso;
R44 is hydrogen, halo, hydroxy, vitro, protected hydroxy, NH2, NHRb, or
NRbR°, Cl-C6
alkyl, C6-Clo aryl, C6-Clo heteroaryl, C3-C8 heterocyclyl, a ligand, a
tethered ligand, or when
taken together with R45 forms -OCHZO-;
R45 is hydrogen, halo, hydroxy, vitro, protected hydroxy, NHZ, NHRb, or NRbR~,
C1-C6
3o alkyl, C6-Clo aryl, C6-Clo heteroaryl, C3-C8 heterocyclyl, a ligand, a
tethered ligand, or when
taken together with Rø4 or R46 forms -OCH20-;
R46 is hydrogen, halo, hydroxy, vitro, protected hydroxy, NHa, NHRb, or NRbR~,
C1-C6
alkyl, C6-Clo aryl, C6-Clo heteroaryl, C3-C$ heterocyclyl, a ligand, a
tethered ligand, or when
taken together with R45 or R4~ forms -OCH20-;
39
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
R47 is hydrogen, halo, hydroxy, vitro, protected hydroxy, NH2, NHRb, or
NRbR°, C1-C6
alkyl, C6-Clo aryl, C6-Clo heteroaryl, C3-C8 heterocyclyl, a ligand, a
tethered ligand, or when
taken together with R46 or R48 forms -OCH20-;
R48 is hydrogen, halo, hydroxy, vitro, protected hydroxy, NH2, NHRb, or
NRbR°, Cl-C6
alkyl, C6-Clo aryl, C6-Clo heteroaryl, C3-C8 heterocyclyl, a ligand, a
tethered ligand, or when
taken together with R47 forms -OCH20-;
R49 R50' R51' R52' R53~ R54' R5T R58' R59' R60' R61' R62' R63~ R64' R65' R66'
R6T R68' R69'
R7°, R71, and R72 are each independently selected from hydrogen, halo,
hydroxy, vitro, protected
hydroxy, NHZ, NHRb, or NRbR~, C1-C6 alkyl, CZ-C6 alkynyl, C6-Clo aryl, C6-Clo
heteroaryl, C3_
C8 heterocyclyl, NC(O)R17, or NC(O)R°;
R55 is hydrogen, halo, hydroxy, vitro, protected hydroxy, NH2, NHRb, or
NRbR°, Cl-C6
alkyl, CZ-C6 alkynyl, C6-Clo aryl, C6-Clo heteroaryl, C3-Cg heterocyclyl,
NC(O)R17, or NC(O)R°,
or when taken together with R56 forms a fused aromatic ring which may be
optionally
substituted;
~5 R56 is hydrogen, halo, hydroxy, vitro, protected hydroxy, NHZ, NHRv, or
NRbR°, C1-C6
alkyl, C2-C6 alkynyl, C6-Clo aryl, C6-Clo heteroaryl, C3-C8 heterocyclyl,
NC(O)R17, or NC(O)R°,
or when taken together with R55 forms a fused aromatic ring which may be
optionally
substituted;
X is O, S, or Se;
2o Y is O or S;
L is -C(O)(CH2)qC(O)-, or -C(O)(CH2)qS-;
Ra is glycinyl, threonyl, or norvalyl, each of which may optionally be
partially or fully
protected;
Rb is C1-C6 alkyl or a nitrogen protecting group;
25 R~ is C1-C6 alkyl;
Rd is hydrogen, hydroxy, protected hydroxy, or OOH;
Re is hydrogen, a nitrogen protecting group, or COORg;
Rf is hydrogen, or C1-C6 alkyl;
Rg is Cl-Clo alkyl;
3o Rh is hydrogen, or
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
R;
RN
R~
R
R' and Rj when taken together forms a double bond between the carbon atoms to
which
they are attached, or R' and Rj when taken together form -O- between the
carbon atoms to which
they are attached;
Rk and R' are each, independently, hydrogen, a hydroxyl protecting group, a
sugar, or a
fully or partially protected sugar;
Rm is Cl-C4 alkyl optionally substituted with COOH;
R° is alkyl optionally substituted with halo, hydroxy, vitro, protected
hydroxy, NH2,
NHRb; or NRbR°, C1-C6 alkyl, C2-C6 alkynyl, C6-C1° aryl, C6-
Cl° heteroaryl, C3-C8 heterocyclyl,
NC(O)Rl~, or NC(O)R°;
n is 1-4;
q is 0-4;
s is 0-20.
Embodiments can include one or more of the features described above and can
further
include protected monomers in which Rl, R2, and R3 cannot all be hydrogen;
further provided
that when RS is hydrogen, R6 cannot be NH2 NH(protecting group), or NH(iBu);
further provided
that when Rl2 is hydrogen and R8 and Ri 1 together form a double bond between
the carbon and
nitrogen atoms to which they are attached, R9 and Rl° cannot both be
hydrogen; further provided
that when X and Y are O, R19 is hydrogen, and RZl and R23 together form a
double bond between
2o the carbon atoms to which they are attached, R2° cannot be hydrogen
or CH3; and/or W can be
fluoro.
41
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
In another aspect, this invention relates to a protected monomer having a
first
functionalized hydroxyl group, a second functionalized hydroxyl group, and a
ligand, in which
the first functionalized hydroxyl group, the second functionalized hydroxyl
group, and the ligand
are linked to a carrier. The first functionalized hydroxyl group can have the
formula:
5'
X
XS,.
5".
X
Preferred XS', XS", and XS"' include siloxy and alkoxy or cycloalkoxy. The
second
functionalized hydroxyl group can have the formula:
p
or
L
(R2a~2N / P\~R27
RZ8 is C1-Clo alkyl,_e.g., isopropyl; R27 is C1-C6 alkyl optionally
substituted with cyano, or C2-C6
alkenyl, e.g., methyl, allyl and 2-cyanoethyl. L is a linker and ~ is a liquid
or solid support
reagent.
The ligand can be a targeting group (e.g., a lipid, steroid, vitamin,
carbohydrate,
polyamine, amino acid, peptide, peptide mimetic or cleaving molecule) or the
ligand may be a
diagnostic group (e.g., biotin, a fluorophore, an antibody or an antigen). The
ligand can have a
formula (G)C(=H)NHRn, in which G is -O-, -NH-, or -CHZ-; H is O or NH; and R"
is H, Cl-C6
alkyl, C6-Clo aryl, or CS-C1o heteroaryl. The ligand may also be linked to the
carrier through a
tether. The tether can be -C(O)-(CHZ)S-C(O)-(ligand);
-C(O)-(CH2)S-C(O)O-(ligand); -C(O)-O-(ligand); -C(O)-(CHZ)S NH-;
-C(O)-(CH~)S NH-C(O)-(ligand); -C(O)-(CHa)S (ligand); -C(O)-NH-(ligand);
-C(O)-(ligand); -(CHZ)S C(O)-(ligand); -(CHZ)S-C(O)O-(ligand); -(CH2)S
(ligand);
-(CH2)S-NH-; or -(CHa)S NH-C(O)-(ligand).
The Garner can be a a cyclic moiety and may also contain one or more
heteroatoms (e.g.,
nitogen, oxygen, or sulfur). In some embodiments, the ligand can be attached
to the nitrogen
42
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
atom of the cyclic moiety. Preferably, the monomer contains only two
functionalized hydroxyl
groups.
In some embodiments, the cyclic moiety can be:
N (CH2)u" _ _ _
R39
~R4o
R41
in which
R39 and R4° are each independently hydrogen or when taken together
form oxo;
R41 is hydrogen or -C(R42)(R43~_~CHa~u _____~
R42 and R43 are each independently hydrogen or when taken together form oxo;
1o a is 1 or 2; the wavy line represents a point of attachment for a ligand or
a tethered
ligand; and the dotted lines represent points of attachment for the first and
second functionalized
hydroxyl groups.
43
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
In some embodiments, the cyclic moiety can be:
______ u~H2~~ N
,
,
s
,
in which
a is 1 or 2; the wavy line represents a point of attachment for a ligand or a
tethered
ligand; and the dotted lines represent points of attachment for the first
functionalized hydroxyl
group, the second functionalized hydroxyl group, and an unfunctionalized
hydroxyl group, a
protected hydroxyl group, or hydrogen.
In a further aspect, this invention relates to iRNA agents, which incorporate
one or more
of the monomers described herein. The invention also relates to methods of
using the iRNA
agents.
In one aspect, this invention relates to a method of synthesizing a polymer,
the method
includes: providing a 5' protected first monomer, providing a second monomer,
deprotecting the
5' moiety of the first monomer, arid reacting the 3' moiety of the second
monomer with the
~ 5 deprotected 5' monomer, thereby synthesing a polymer, provided that one of
the monomers is a
monomer as described herein. In preferred embodiments, the monomer as
described herein is
provided as the 3' terminal residue of the polymer or the 5' terminal residue
of the polymer.
In another aspect, this invention relates to a di-, tri, or polymeric molecule
which
comprises a 5' silyl protecting group described herein and a subunit
comprising at least one of
2o the monomers described herein.
In a further aspect, this invention relates to a method of making an iRNA
agent, the
method includes providing a first sequence and a second sequence which can
form a duplex,
which includes at least one monomer added by a method described herein. In
some
embodiments, the first and second sequences are between 15 and 30 nucleotides
in length.
25 In one aspect, this invention relates to a method of modulating expression
of a target
gene, the method includes providing an iRNA agent comprising a monomer which
includes a
monomer described herein or which was incorporated by a method described
herein. In some
embodiments, the iRNA agent can be administered to a subject.
44
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
In another aspect, this invention relates to a pharmaceutical composition
comprising an
iRNA agent which includes a monomer described herein or made by a method
described herein.
The details of one or more embodiments of the invention are set forth in the
accompanying drawings and the description below. Other features and advantages
of the
invention will be apparent from the description and drawings, and from the
claims.
DESCRIPTION OF DRAWINGS
FIG 1 is a reaction scheme showing how the protected monomers can be
incorporated
into the terminal and internal positions of a growing chain of monomers.
FIG 2 is a list of substituents that may be present on silicon in OFGI.
FIG 3 is a list of substituents that may be present on the C2'-orthoester
group.
FIG 4 is a general synthetic scheme for incorporation of RRMS monomers into an
oligonucleotide.
FIG 5 is a list of representative RRMS Garners. Panel 1 shows pyrroline-based
RRMSs;
panel 2 shows 3-hydroxyproline-based RRMSs; panel 3 shows piperidine-based
RRMSs; panel
4 shows morpholine and piperazine-based RRMSs; and panel 5 shows decalin-based
RRMSs.
R1 is succinate or phosphoramidate and R2 is H or a conjugate ligand.
FIG. 6 is a structural representation of base pairing in psuedocomplementary
siRNA2.
FIG. 7 is a schematic representation of dual targeting siRNAs designed to
target the HCV
genome.
2o FIG. 8 is a schematic representation of psuedocomplementary, bifmictional
siRNAs
designed to target the HCV genome.
DETAILED DESCRIPTION
PROTECTED MONOMERS
Definitions
As used herein, the term "halo" or "halogen" refers to any radical of
fluorine, chlorine,
bromine or iodine.
The term "alkyl" refers to a hydrocarbon chain that may be a straight chain or
branched
chain, containing the indicated number of carbon atoms. For example, Cl~Cla
alkyl indicates
so that the group may have from 1 to 12 (inclusive) carbon atoms in it. The
term "haloalkyl" refers
to an alkyl in which one or more hydrogen atoms are replaced by halo, and
includes alkyl
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
moieties in which all hydrogens have been replaced by halo (e.g.,
perfluoroalkyl). Alkyl and
haloalkyl groups may be optionally inserted with O, N, or S. The terms
"arylalkyl" or "aralkyl"
refer to an alkyl moiety in which an allcyl hydrogen atom is replaced by an
aryl group. Aralkyl
includes groups in which more than one hydrogen atom has been replaced by an
aryl group.
Examples of "arylalkyl" or "aralkyl" include benzyl, 9-fluorenyl, benzhydryl,
and trityl groups.
The term "alkenyl" refers to a straight or branched hydrocarbon chain
containing 2-12
carbon atoms and characterized in having one or more double bonds. The sp2
carbon may
optionally be the point of attachment of the alkenyl group to another moiety.
Examples of a
typical alkenyl include, but not limited to, allyl, propenyl, 2-butenyl, 3-
hexenyl and 3-octenyl
1o groups. The term "alkynyl" refers to a straight or branched hydrocarbon
chain containing 2-8
carbon atoms and characterized in having one or more triple bonds. The spa
carbon may
optionally be the point of attachment of the alkynyl group to another moiety.
Some examples of
a typical alkynyl are ethynyl, 2-propynyl, and 3-methylbutynyl, and propargyl.
The terms "alkylamino" and "dialkylamino" refer to NH(alkyl) and NH(alkyl)2
~ 5 radicals respectively. The term "aralkylamino" refers to a NH(aralkyl)
radical. The term
"alkoxy" refers to an -O-alkyl radical, and the terms "cycloalkoxy" and
"aralkoxy" refer to an -
O-cycloalkyl and O-aralkyl radicals respectively. The term "siloxy" refers to
a R3Si0- radical.
The teen "mercapto" refers to an SH radical. The term "thioalkoxy" refers to
an -S-alkyl radical.
The term "alkylene" refers to a divalent alkyl (i.e., -R-), e.g., -CH2-, -
CH2CH2-, and -
2o CHzCH2CH2-. The term "alkylenedioxo" refers to a divalent species of the
structure -O-R-O-,
in which R represents an alkylene.
The term "aryl" refers to an aromatic monocyclic, bicyclic, or tricyclic
hydrocarbon ring
system, wherein any ring atom can be substituted. Examples of aryl moieties
include, but are not
limited to, phenyl, naphthyl, anthracenyl, and pyrenyl.
25 The term "cycloalkyl" as employed herein includes saturated cyclic,
bicyclic, tricyclic,or
polycyclic hydrocarbon groups having 3 to 12 carbons, wherein any ring atom
can be substituted.
The cycloalkyl groups herein described may also contain fused rings. Fused
rings are rings that
share a common carbon-carbon bond or a common carbon atom (e.g., spiro-fused
rings).
Examples of cycloalkyl moieties include, but are not limited to, cyclohexyl,
adamantyl, and
3o norbornyl.
The term "heterocyclyl" refers to a nonaromatic 3-10 membered monocyclic, 8-12
membered bicyclic, or 11-14 membered tricyclic ring system having 1-3
heteroatoms if
monocyclic, 1-6 heteroatoms if bicyclic, or 1-9 heteroatoms if tricyclic, said
heteroatoms
selected from O, N, or S (e.g., carbon atoms and 1-3, 1-6, or 1-9 heteroatoms
of N, O, or S if
46
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
monocyclic, bicyclic, or tricyclic, respectively), wherein any ring atom can
be substituted. The
heterocyclyl groups herein described may also contain fused rings. Fused rings
are rings that
share a common carbon-carbon bond or a common carbon atom (e.g., spiro-fused
rings).
Examples of heterocyclyl include, but are not limited to tetrahydrofuranyl,
tetrahydropyranyl,
piperidinyl, morpholino, pyrrolinyl and pyrrolidinyl.
The term "cycloalkenyl" as employed herein includes partially unsaturated,
nonaromatic,
cyclic, bicyclic, tricyclic,or polycyclic hydrocarbon groups having 5 to 12
carbons, preferably 5
to 8 carbons, wherein any ring atom can be substituted. The cycloalkenyl
groups herein
described may also contain fused rings. Fused rings are rings that share a
common carbon-
carbon bond or a common carbon atom (e.g., spiro-fused rings). Examples of
cycloalkenyl
moieties include, but are not limited to cyclohexenyl, cyclohexadienyl, or
norbornenyl.
The term "heterocycloalkenyl" refers to a partially saturated, nonaromatic 5-
10
membered monocyclic, 8-12 membered bicyclic, or 11-14 membered tricyclic ring
system
having 1-3 heteroatoms if monocyclic, 1-6 heteroatoms if bicyclic, or 1-9
heteroatoms if
~5 tricyclic, said heteroatoms selected from O, N, or S (e.g., carbon atoms
and 1-3, 1-6, or 1-9
heteroatoms of N, O, or S if monocyclic, bicyclic, or tricyclic,
respectively), wherein any ring
atom can be substituted. The heterocycloalkenyl groups herein described may
also contain fused
rings. Fused rings are rings that share a common carbon-carbon bond or a
common carbon atom
(e.g., spiro-fused rings). Examples of heterocycloalkenyl include but are not
limited to
2o tetrahydropyridyl and dihydropyran.
The term "heteroaryl" refers to an aromatic 5-8 membered monocyclic, 8-12
membered
bicyclic, or 11-14 membered tricyclic ring system having 1-3 heteroatoms if
monocyclic, 1-6
heteroatoms if bicyclic, or 1-9 heteroatoms if tricyclic, said heteroatoms
selected from O, N, or S
(e.g., carbon atoms and 1-3, 1-6, or 1-9 heteroatoms of N, O, or S if
monocyclic, bicyclic, or
25 tricyclic, respectively), wherein any ring atom can be substituted. The
heteroaryl groups herein
described may also contain fused rings that share a common carbon-carbon bond.
The term "oxo" refers to an oxygen atom, which forms a carbonyl when attached
to
carbon, an N-oxide when attached to nitrogen, and a sulfoxide or sulfone when
attached to sulfur.
The term "acyl" refers to an alkylcarbonyl, cycloalkylcarbonyl, arylcarbonyl,
3o heterocyclylcarbonyl, or heteroarylcarbonyl substituent, any of which may
be further substituted
by substituents.
The term "substituents" refers to a group "substituted" on an alkyl,
cycloalkyl, alkenyl,
alkynyl, heterocyclyl, heterocycloalkenyl, cycloalkenyl, aryl, or heteroaryl
group at any atom of
that group. Suitable substituents include, without limitation, alkyl, alkenyl,
alkynyl, alkoxy,
47
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
halo, hydroxy, cyano, vitro, amino, S03H, sulfate, phosphate, perfluoroalkyl,
perfluoroalkoxy,
methylenedioxy, ethylenedioxy, carboxyl, oxo, thioxo, imino (alkyl, aryl,
aralkyl), S(O)"alkyl
(where n is 0-2), S(O)" aryl (where n is 0-2), S(O)" heteroaryl (where n is 0-
2), S(O)"
heterocyclyl (where n is 0-2), amine (mono-, di-, alkyl, cycloalkyl, aralkyl,
heteroaralkyl, and
combinations thereof), ester (alkyl, aralkyl, heteroaralkyl), amide (mono-, di-
, alkyl, aralkyl,
heteroaralkyl, and combinations thereof), sulfonamide (mono-, di-, alkyl,
aralkyl, heteroaralkyl,
and combinations thereof), unsubstituted aryl, unsubstituted heteroaryl,
unsubstituted
heterocyclyl, and unsubstituted cycloalkyl. In one aspect, the substituents on
a group are
independently any one single, or any subset of the aforementioned
substituents.
1 o The terms "adeninyl, cytosinyl, guaninyl, thyrninyl, and uracilyl" and the
like refer to
radicals of adenine, cytosine, guanine, thymine, and uracil.
A "protected" moiety refers to a reactive functional group, e.g., a hydroxyl
group or an
amino group, or a class of molecules, e.g., sugars, having one or more
functional groups, in
which the reactivity of the functional group is temporarily blocked by the
presence of an attached
~ 5 protecting group. Protecting groups useful for the monomers and methods
described herein can
be found, e.g., in Greene, T.W., Protective Groups ifa Organic Synthesis (John
Wiley and Sons:
New York), 1981, which is hereby incorporated byreference.
Structure of the Protected Monomers
2o In general, the protected monomer compounds include two differently
functionalized
hydroxyl groups (OFGI and OFG2 below) and a ligand, all three of which are
linked to a Garner
molecule (see A below). As used herein, the term "functionalized hydroxyl
group" means that
the hydroxyl proton has been replaced by another substituent. As shown in
representative
structures B and C, one hydroxyl group (OFGI) on the carrier is functionalized
with a silicon-
25 based protecting group. The other hydroxyl group (OFGZ) can be
functionalized with either (1) a
liquid or solid phase synthesis support reagent (solid circle) directly or
indirectly through a
linker, L, as in B, or (2) a phosphorus-containing moiety, e.g., a
phosphoramidite as in C.
48
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
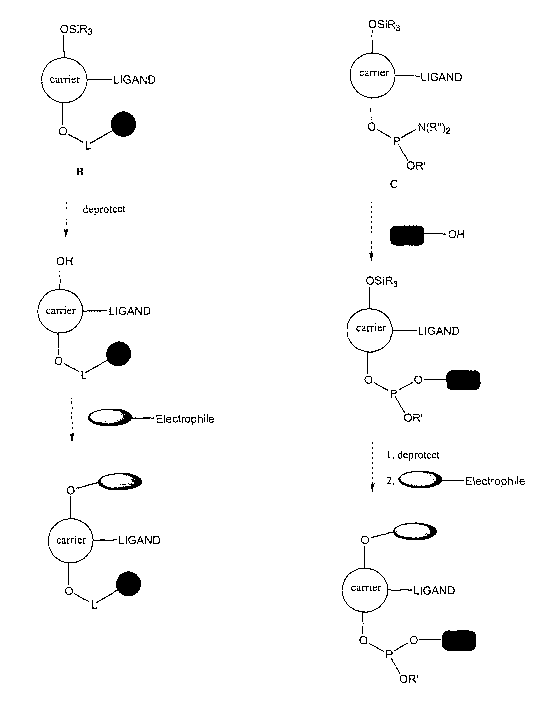
OFG~ OSiR~ OSiR
carrier ~LIGAND [ Garner ~LIGAND LAND
~FGL O~ ~ O~ /N(R")2
L P\
\A
OR'
C
The above combination of substituents allows the monomers to be incorporated
into an
internal or terminal position of a natural or modified oligoribonucleotide, or
a polymeric
molecule comprising any combination of monomer compounds described herein
and/or natural
or modifed ribonucleotides. The monomers described herein can therefore be
used to prepare
iRNA agents. While not wishing to be bound by theory, it is believed that
incorporation of one
or more of the monomers described herein can increase binding affinity of an
iRNA agent to a
target mRNA, increase nuclease resistence, change the geometry of the duplex
form, alter
distribution or target the iRNA agent to a particular part of the body, and
modify the interaction
1o with nucleic acid binding proteins (e.g., during RISC formation and strand
separation).
When the ~FG~ in B includes a linker, e.g., a long organic linker, connected
to a soluble
or insoluble support reagent, solution or solid phase synthesis techniques can
be employed: to
build up a chain of natural and/or modifed ribonucleotides once OFGI is
deprotected and free to
react as a nucleophile with another nucleoside or monomer containing an
electrophilic group
(e.g., an amidite group). Alternatively, a natural or modified ribonucleotide
or
oligoribonucleotide chain can be coupled to monomer C via the amidite group at
OFG2.
Subsequent to this operation, OFGI can be deblocked, and the restored
nucleophilic hydroxyl
group can react with another nucleoside or monomer containing an electrophilic
group (see FIG.
1).
2o OFGI has the general formula D shown below.
49
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
X5,
X
D
Hydroxyl groups, -OH, are nucleophilic groups (i.e., Lewis bases), which react
through the
oxygen with electrophiles (i.e., Lewis acids). Hydroxyl groups in which the
hydrogen has been
replaced with a silicon-based protecting group, i.e. D, are essentially
unreactive as nucleophiles
in displacement reactions. Thus, the silyl-protected hydroxyl group is useful
in preventing e.g.,
homocoupling of compounds exemplified by structure C during oligonucleotide
synthesis. XS',
XS", and XS"' can be selected from substituted or unsubstituted alkyl,
cycloalkyl, aryl, araklyl,
heteroaryl, alkoxy, cycloalkoxy, aralkoxy, aryloxy, heteroaryloxy, or siloxy
(i.e., R3Si0-, the
three "R" groups can be any combination of the above listed groups). XS~,
XS~~, and XS~~~ may all
be the same or different; also contemplated is a combination in which two of
XS~, XS", and XS"'
are identical and the third is different. In certain embodiments XS', XS~~,
and XS~~~ include at least
one alkoxy or siloxy groups and may be any one of the groups listed in FIG. 2,
a preferred
combination includes XS~, XS~~ = trimethylsiloxy and XS'~~ = 1, 3-
(triphenylinethoxy)-2-propoxy or
cyclododecyloxy.
Other preferred combinations of XS~, XS~~, and XS"~ include those that result
in OFGI
groups that meet the deprotection and stability criteria delineated below. The
group is preferably
stable under amidite synthesis conditions, storage conditions, and
oligonucleotide synthesis
conditions. Rapid removal, i.e., less than one minute, of the silyl group from
e.g., a support-
bound oligonucleotide is desirable because it can reduce synthesis times and
thereby reduce
2o exposure timeof the growing oligonucleotide chain to the reagents.
Oligonucleotide synthesis
can be improved if the silyl protecting group is visible during deprotection,
e.g., from the
addition of a chromophore silyl substituent.
Selection of silyl protecting groups can be complicated by the competing
demands of the
essential characteristics of stability and facile removal, and the need to
balance these competitive
goals. Most substituents that increase stability can also increase the
reaction time required for
removal of the silyl group, potentially increasing the level of difficulty in
removal of the group.
The addition of alkoxy and siloxy substituents to OFG1 increases the
susceptibility of the
protecting groups to fluoride cleavage of the silylether bonds. Increasing the
steric bulk of the
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
substituents preserves stability while not decreasing fluoride lability to an
equal extent. An
appropriate balance of substituents on the silyl group makes a silyl ether a
viable nucleoside
protecting group.
Candidate OFGI groups may be tested by exposing a tetrahydrofuran solution of
a
preferred carrier bearing the candidate OFGI group to five molar equivalents
of tetrahydrofuran
at room temperature. The reaction time may be determined by monitoring the
disappearance of
the starting material by thin layer chromatography.
OFG2 may have general formula E or F:
r~
O O
or
( R28)2N / P\O X27
E F
OFGa in structure E is a phosphoramidite group and functions as a site where
the monomer may
be coupled to another monomer described herein or to a natural or modified
ribonucleoside or
oligoribonucleotide chain. For example, a ribonucleotide containing an
unblocked 5'-OH can be
coupled to the monomer via displacement of the N(R28)2 moiety to form a
phosphate ester
linkage beween the monomer and the nucleoside. R2~ can be substituted or
unsubstituted alkyl or
~5 alkenyl. In preferred embodiments, Ray is methyl, allyl or 2-cyanoethyl.
R2$ may a Cl-Clo alkyl
group, preferably it is a branched group containing three or more carbons,
e.g., isopropyl.
OFGZ in F as hydroxyl functionalized with a linker, which in turn contains a
liquid or
solid phase synthesis support reagent at the other linker terminus. The
support reagent can be
any support medium that can support the monomers described herein. The monomer
can be
2o attached to an insoluble support via a linker, L, which allows the monomer
(and the growing
chain) to be solubilized in the solvent in which the support is placed. The
solubilized, yet
immobilized, monomer can react with reagents in the surrounding solvent;
unreacted reagents
and soluble by-products can be readily washed away from the solid support to
which the
monomer or monomer-derived products is attached. Alternatively, the monomer
can be attached
25 to a soluble support moiety, e.g., polyethylene glycol (PEG) and liquid
phase synthesis
techniques can be used to build up the chain. Linker and support medium
selection is within
skill of the art. Generally the linker may be -C(O)(CH2)qC(O)-, or -
C(O)(CHZ)aS-, preferably, it
is oxalyl, succinyl or thioglycolyl. Standard control pore glass solid phase
synthesis supports can
51
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
not be used in conjunction with fluoride labile 5' silyl protecting groups
because the glass is
degraded by fluoride with a significant reduction in the amount of full-length
product. Fluoride-
stable polystyrene based supports or PEG are preferred.
The Garner can be any organic molecule containing attachment points for OFGI,
OFG2,
and the ligand. In certain embodiments, carrier is a cyclic molecule and may
contain
heteroatoms (e.g., O, N or S). E.g., carrier molecules may include aryl (e.g.,
benzene, biphenyl,
etc.), cycloalkyl (e.g., cyclohexane, cis or tYafas decalin, etc.), or
heterocyclyl (piperazine,
pyrrolidine, etc.). Any of the above cyclic systems may include substituents
in addition to OFGI,
OFGZ, and the ligand.
1 o In certain embodiments, the carrier is a nitogenous heterocycle. Exemplary
carriers of
this class include structures G and H. The designation "O" indicates possible
locations for OFGI
and OFGZ. In certain embodiments of G, one of the piperazinyl nitrogens is
substituted with
hydrogen. In the case of structure H, the position left unoccupied by OFGI and
OFG2 can be
substituted by a hydroxyl group, a protected hydroxyl group, or hydrogen. In
both G and H, all
~ 5 positional and stereoisomers are expressly contemplated. Preferred
examples of H include H-p
and H-ss in which "chol" represents a cholesterol radical (e.g., the oxygen
attached to "chol" can
be attached to C-3 of the cholesterol skeleton). The shaded circle in H-ss
represents a liquid or
solid support agent.
52
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
tether/linker-ligand
N
O
N
tether/linker-ligand
G
O
O
~N O-chol
H
X5"-Si-O N
~5~~~
~O- i -N(ipr)~
H-p OCH2CH2CN
O
O
~N O-chol
H
Si O' \ N
Xs.,.
.:
~O-C(O)CH2CH2C(O)NH
H-ss
53
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
In other embodiments, the carrier molecule is an oxygen containing
heterocycle.
Preferably the carrier is a ribose sugar as shown in structure I. In this
embodiment, the protected
monomer is a nucleoside.
5'
X
X5" ~ i O
5..,
X
XJ XG
I
"B" represents an "unusual" nucleobase or a "universal" base.
As used herein, an "unusual" nucleobase can include any one of the following:
2-methyladeninyl,
N6-methyladeunyl,
2-methylthio-N6-methyladeninyl,
N6-isopentenyladeninyl,
2-methylthio-N6-isopentenyladeninyl,
N6-(cis-hydroxyisopentenyl)adeninyl,
2-methylthio-N6-(cis-hydroxyisopentenyl) adeninyl,
N6-glycinylcarb amoyladeninyl,
~ 5 N6-threonylcarbamoyladeninyl,
2-methylthio-N6-threonyl carbamoyladeninyl,
N6-methyl-N6-threonylcarbamoyladeninyl,
N6-hydroxynorvalylcarbamoyladeninyl,
2-methylthio-N6-hydroxynorvalyl carbamoyladeninyl,
2o N6,N6-dimethyladeninyl,
3-methylcytosinyl,
5-methylcytosinyl,
2-thiocytosinyl,
5-formylcytosinyl,
54
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
NH
COOH
W
H2N N N
H "'~"'
N4-methylcytosinyl,
5-hydroxymethylcytosinyl,
1-methylguaninyl,
N2-methylguaninyl,
7-methylguaninyl,
N2,N2-dimethylguaninyl,
55
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
NHCOOCH3 NHCOOCH3 NHCOOCH3
H3COOC H3COOC OH H3COOC OOH
O O O
N N
HsC / ~ y ~ HsC / ~ y ~ HaC / N
N N .,N; N N ..~; N~N
CH3 CH3 CH3
NH2
HOOC OH
O O HsC O
N N HaC~N~N~ HaC~N NJ ,
/ v
H3C , ~ ~ ' N N N ' N~N N
N~N ,N.~ CH3 ~' CH3 ~
CH3
N2,7-dimethylguaninyl,
O O HO HO
HN N H3C~N N HO I HO O
~N~N", ~N ~V", O NH ~ O NH
H N \ H~ \
H2N~N N", H2N \N N,.,
HO HO
beta-galactosyl0 O beta-mannosyl0 O
O NH O NH ~ O HN NH2
HN \ HN \ HN \
H2N~N N H2N~N N H2N~N ,N
56
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
N2,N2,7-trimethylguaninyl,
1-methylguaninyl,
7-cyano-7-deazaguaninyl,
7-aminomethyl-7-deazaguaninyl,
pseudouracilyl,
dihydrouracilyl,
5-methyluracilyl,
1-methylpseudouracilyl,
2-thiouracilyl,
4-thiouracilyl,
2-thiothyminyl
5-methyl-2-thiouracilyl,
3-(3-amino-3-carboxypropyl)uracilyl,
5-hydroxyuracilyl,
5-methoxyuracilyl,
uracilyl 5-oxyacetic acid,
uracilyl 5-oxyacetic acid methyl ester,
5-(carboxyhydroxymethyl)uracilyl,
5-(carboxyhydroxymethyl)uracilyl methyl ester,
5-methoxycarbonylinethyluracilyl,
5-methoxycarbonylmethyl-2-thiouracilyl,
5-aminomethyl-2-thiouracilyl,
5-methylaminomethyluracilyl,
5-methylaminomethyl-2-thiouracilyl,
5-methylaminomethyl-2-selenouracilyl,
5-carbamoylinethyluracilyl,
5-carboxymethylaminomethyluracilyl,
5-carboxymethylaminomethyl-2-thiouracilyl,
3o 3-methyluracilyl,
1-methyl-3-(3-amino-3-carboxypropyl) pseudouracilyl,
5-carboxymethyluracilyl,
5-methyldihydrouracilyl, or
3-methylpseudouracilyl.
57
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
A universal base can form base pairs with each of the natural DNAlRNA bases,
exhibiting relatively little discrimination between them. In general, the
universal bases are non-
hydrogen bonding, hydrophobic, aromatic moieties which can stabilize e.g.,
duplex RNA or
RNA-like molecules, via stacking interactions. A universal base can also
include hydrogen
bonding substituents. As used herein, a "universal base" can include any one
of the following:
F CH3 NH2 NH2
02N
H C ~ ~ N ~N N~N
~~ ° ~ , I \~~~~~ ,
F / N , ~ , N
",;,~ """"
N 02 \ \ O O I \ \ , .~''~ \ O
~ ~ ~~ ~ BuHN~N ~ ~ N. _ ' I i ~ '
N N N N~ H _ ~ 02N
H N \
58
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
CH3 O CH3 O O O CH3
N~ , ~ \ N~ , ~ \ N~ , ~ \ N?~ , HsC
/ / H C / / / / / CH3
3
O CHs
H3C
\ CH3 I \ \ \ N?~ ~ I \ \ ~~ I \ \
/ ' ~ N , ~ / / / / / / '
N ~ CH3
"". ' CH3
\\~~ I\\~~ - N- .
/ / . / / , and \ / \
N
CH3
A universal base can also include an aryl moiety (e.g., phenyl) having a
ligand either
directly attached or indirectly attached, e.g., via a linker or tether, to the
aryl moiety. The aryl
moiety may further include additional substituents, e.g., one or more fluoro
groups.
X2 can include "oxy" or "deoxy" substituents in place of the 2'-OH.
Examples of "oxy"-substituents include alkoxy or aryloxy (OR, e.g., R = H,
alkyl,
cycloallcyl, aryl, aralkyl, heteroaryl, sugar, or protecting group);
polyethyleneglycols (PEG),
O(CH2CH20)"CH2CH20R; "locked" nucleic acids (LNA) in which the 2' hydroxyl is
connected,
1 o e.g., by a methylene bridge, to the 4' carbon of the same ribose sugar; O-
PROTECTED AMINE
(AMINE = NHZ; alkylamino, dialkylamino, heterocyclyl, arylamino, diaryl amino,
heteroaryl
amino, or diheteroaryl amino, ethylene diamine, polyamino) and aminoalkoxy,
O(CHZ)"PROTECTED AMINE, (e.g., AMINE = NH2; alkylamino, dialkylamino,
heterocyclyl,
arylamino, diaryl amino, heteroaryl amino, or diheteroaryl amino, ethylene
diamine, polyamino),
~5 and orthoester. Amine protecting groups can include formyl, amido, benzyl,
allyl, etc.
Preferred orthoesters have the general formula J. The groups R31 and R32 may
be the
same or different and can be any combination of the groups listed in FIG. 3. A
preferred
orthoester is the "ACE" group, shown below as structure K.
59
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
2,
O
~~ R31
J
O
H3C O O CH3
O O
O O
K
"Deoxy" substituents include hydrogen (i.e. deoxyribose sugars, which are
ofparticular
relevance to the overhang portions of partially ds RNA); halo (e.g., fluoro);
protected amino (e.g.
NH2; alkylamino, dialkylamino, heterocyclyl, arylamino, diaryl amino,
heteroaryl amino,
diheteroaryl amino, or amino acid in which all amino are protected); fully
protected polyamino
(e.g., NH(CHZCHZNH)nCH2CH2-AMINE, wherein AMINE = NH2; alkylamino,
dialkylamino,
heterocyclyl, arylamino, diaryl amino, heteroaryl amino,or diheteroaryl amino
and all amino
1 o groups are protected), -NHC(O)R (R = alkyl, cycloalkyl, aryl, aralkyl,
heteroaryl or sugar),
cyano; alkyl-thio-alkyl; thioalkoxy; and alkyl, cycloalkyl, aryl, alkenyl and
alkynyl, which may
be optionally substituted with e.g., a protected amino functionality.
Preferred substitutents are
2'-methoxyethyl, 2'-OCH3, 2'-O-allyl, 2'-C- allyl, and 2'-fluoro.
X3 is as described for OFGa above, and XS', XS~~, and XS~~~ can be selected as
discussed
above.
In another embodiment, the carrier can be a carbocycle, or a sulfur-containing
heterocycle.
Synthesis and Use of the Protected Monomers
A listing of ribonucleosides containing the unusual bases described herein are
described
2o in "The RNA Modification Database" maintained by Pamela F. Crain, Jef
Rozenski and James
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
A. McCloskey; Departments of Medicinal Chemistry and Biochemistry, University
of Utah, Salt
Lake City, UT 84112, USA (RNAmods(c~lib.med.utah.edu )
Carriers G and H can be synthesized by methods described herein and by those
known in
the art.
The protected nucleosides, e.g., compound I, can be employed in solid support
synthesis
of oligonucleotides. The 5' silyl protecting group can be used in conjunction
with acid labile
orthoesters at the 2' position of ribonucleosides to synthesize
oligonucleotides via
phosphoramidite chemistry. Final deprotection conditions are known not to
significantly
degrade RNA products. Functional groups on the unusual and universal bases are
blocked
during oligonucleotide synthesis with protecting groups that are compatible
with the operations
being performed that are described herein . All syntheses can be can be
conducted in any
automated or manual synthesizer on large, medium, or small scale. The
syntheses may also be
carned out in multiple well plates or glass slides.
The 5'-O-silyl group can be removed via exposure to fluoride ions, which can
include any
~5 source of fluoride ion, e.g., those salts containing fluoride ion paired
with inorganic counterions
e.g., cesium fluoride and potassium fluoride or those salts containing
fluoride ion paired with an
organic counterion, e.g., a tetraalkylammonium fluoride. A crown ether
catalyst can be utilized
in combination with the inorganic fluoride in the deprotection reaction.
Preferred fluoride ion
source are tetrabutylammonium fluoride or aminehydrofluorides (e.g., combining
aqueous HF
2o with triethylamine in a dipolax aprotic solvent, e.g., dimethylformamide).
The choice of protecting groups for use on the phosphite triesters and
phosphotriesters
can alter the stability of the triesters towards fluoride. Methyl protection
of the phosphotriester
or phosphitetriester can stabilize the linkage against fluoride ions and
improve process yields.
Since ribonucleosides have a reactive 2' hydroxyl substituent, it can be
desirable to
25 protect the reactive 2' position in RNA with a protecting group that is
compatible with a 5'-O-
silyl protecting group, e.g. one stable to fluoride. Orthoesters meet this
criterion and can be
readily removed in a final acid deprotection step that can result in minimal
RNA degradation.
Tetrazole catalysts can be used in the standard phosphoramidite coupling
reaction.
Preferred catalysts include e.g. tetrazole, S-ethyl-tetrazole, p-
nitrophenyltetrazole.
3o The general process is as follows. Nucleosides axe suitably protected and
functionalized
for use in solid-phase or solution-phase synthesis of RNA oligonucleotides.
The 2'-hydroxyl
group in a ribonucleotide can be modified using a tris orthoester reagent. The
2'-hydroxyl can be
modified to yield a 2'-O-orthoester nucleoside by reacting the ribonucleoside
with the tris
orthoester reagent in the presence of an acidic catalyst, e.g., pyridinium p-
toluene sulfonate.
61
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
This reaction is known to those skilled in the art. The product can then be
subj ected to further
protecting group reactions (e.g., 5'-O-silylation) and functionalizations
(e.g., 3'-O-
phosphitylation) to produce a desired reagent (e.g., nucleoside
phosphoramidite) for
incorporation within an oligonucleotide or polymer by reactions known to those
skilled in the art.
Preferred orthoesters include those comprising ethylene glycol ligands which
are
protected with acyl or ester protecting groups. Specifically, the preferred
acyl group is acetyl.
The nucleoside reagents may then be used by those skilled in the art to
synthesize RNA
oligonucleotides on commercially available synthesizer instruments, e.g. Gene
Assembler Plus
(Pharmacia), 3~OB (Applied Biosystems). Following synthesis (either solution-
phase or solid-
phase) of an oligonucleotide or polymer, the product can be subjected to one
or more reactions
using non-acidic reagents. One of these reactions may be strong basic
conditions, for example,
40% methylamine in water for 10 minutes at SS° C., which will remove
the acyl
protecting groups from the ethylene glycol ligands but leave the orthoester
moiety attached. The
resultant orthoester may be left attached when the polymer or oligonucleotide
is used in
~ 5 subsequent applications, or it may be removed in a final mildly-acidic
reaction, for example, 10
minutes at SS° C. in 50 mM acetic acid, pH 3.0, followed by addition of
equal volume of
150 mM TRIS buffer for 10 minutes at SS° C.
The protected monomer compounds can be separated from a reaction mixture and
further
purified by a method such as column chromatography, high pressure liquid
chromatography, or
2o recrystallization. As can be appreciated by the skilled artisan, further
methods of synthesizing
the compounds of the formulae herein will be evident to those of ordinary
skill in the art.
Additionally, the various synthetic steps may be performed in an alternate
sequence or order to
give the desired compounds. Other synthetic chemistry transformations,
protecting groups (e.g.,
for hydroxyl, amino, etc. present on the bases) and protecting group
methodologies (protection
25 and deprotection) useful in synthesizing the compounds described herein are
known in the art
and include, for example, those such as described in R. Larock, Comprehensive
Organic
Transformations, VCH Publishers (199); T.W. Greene and P.G.M. Wuts, Protective
Groups in
Organic Synthesis, 2d. Ed., John Wiley and Sons (1991); L. Fieser and M.
Fieser, Fieser and
Fieser's Reagents for Organic Synthesis, John Wiley and Sons (1994); and L.
Paquette, ed.,
3o Encyclopedia ofReagents for Ofganic Synthesis, John Wiley and Sons (1995),
and subsequent
editions thereof.
The protected monomer compounds of this invention may contain one or more
asymmetric centers and thus occur as racemates and racemic mixtures, single
enantiomers,
individual diastereomers and diastereomeric mixtures. All such isomeric forms
of these
62
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
compounds are expressly included in the present invention. The compounds
described herein
can also contain linkages (e.g., carbon-carbon bonds, carbon-nitrogen bonds,
e.g., amides) or
substituents that can restrict bond rotation , e.g. restriction resulting from
the presence of a ring
or double bond. Accordingly, all cis/trans, E/Z isomers, and rotational
isomers (rotamers) are
expressly included herein. The compounds of this invention may also be
represented in multiple
tautomeric forms, in such instances, the invention expressly includes all
tautomeric forms of the
compounds described herein (e.g., alkylation of a ring system may result in
alkylation at
multiple sites, the invention expressly includes all such reaction products).
All such isomeric
forms of such compounds axe expressly included in the present invention. All
crystal forms of
the compounds described herein are expressly included in the present
invention.
The monomers and methods described herein can be used to prepare natural or
modified
oligoribonucleotides, or polymeric molecules comprising any combination of
monomer
compounds described herein and/or natural or modified ribonucleotides in which
one or more
subunits contain an unusual or universal base. While not wishing to be bound
by any theory, it is
believed that the incorporation of these bases can optimize binding affinity
of an iRNA agent to a
target mRNA, optimize endonuclease stability, increase the number of hydrogen
bonding
interactions, and create favorable pi-stacking, polarizability and sugar
pucker in the duplex form.
Unusual and universal bases can be incorporated into both the sense and anti-
sense strands of an
iRNA agent. In preferred embodiments, the monomers and methods described
herein can be
2o used to introduce a unusual or universal base-containing subunit into the
3' terminal position of
the natural oligoribonucleotide, modified oligoribonucleotide, or polymer,
and/or the S' terminal
position of natural oligoribonucleotide or modified oligoribonucleotide or
polymer.
Universal. bases are described in "Survey and Summary: The Applications of
Universal
DNA base analogues" Loakes, D., Nucleic Acid Research 2001, 29, 2437, which is
incorporated
by reference in its entirety. Specific examples are described in the
following: Liu, D.; Moran,
S.; Kool, E. T. Chena. Biol.,1997, 4, 919-926; Morales, J. C.; Kool, E. T.
Biochemistry, 2000,
39, 2626-2632; Matray, T, J.; Kool, E. T. J. Am. Claem. Soc., 199, 120, 6191-
6192; Moran, S.
Ren, R. X.-F.; Rumney IV, S.; Kool, E. T. J. Am. Claem. Soc.,1997,119, 2056-
2057; Guckian,
K. M.; Morales, J. C.; Kool, E. T. J. Org. Chena.,1998, 63, 9652-9656; Berger,
M.; Wu. Y.;
3o Ogawa, A. K.; McMinn, D. L.; Schultz, P.G.; Romesberg, F. E. Nucleic Acids
Res., 2000, 28,
2911-2914; Ogawa, A. K.; Wu, Y.; McMinn, D. L.; Liu, J.; Schultz, P. G.;
Romesberg, F. E. J.
Am. Chem. Soc., 2000,122, 3274-3287; Ogawa, A. K.; Wu. Y.; Berger, M.;
Schultz, P. G.;
Romesberg, F. E. J. Am. Claem. Soc., 2000,122, 8803-8804; Tae, E. L.; Wu, Y.;
Xia, G.;
Schultz, P. G.; Romesberg, F. E. J. Ana. Chern. Soc., 2001,123, 7439-7440; Wu,
Y.; Ogawa, A.
63
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
K.; Bergen M.; McMinn, D. L.; Schultz, P. G.; Romesberg, F. E. J. Am. Chem.
Soc., 2000,122,
7621-7632; . McMinn, D. L.; Ogawa. A. K.; Wu, Y.; Liu, J.; Schultz, P. G.;
Romesberg, F. E. J.
Am. Claerra. Soc.,1999,121, 11585-11586; Brotschi, C.; Haberli, A.; Leumann,
C, J. Angew.
Chena. Int. Ed., 2001, 40, 3012-3014; Weizman, H.; Tor, Y. J. Am. Claem. Soc.,
2001,123, 3375-
3376; Lan, T.; McLaughlin, L. W. J. Am. Chem. Soc., 2000, 122, 6512-13.
In certain embodiments the methods and monomers described herein can be used
to
prepare pseudocomplementary double-stranded iRNA agents that contain one or
more interstrand
pairings between unusual bases, e.g., 2-aminoadenine (2-AA) and 2-thiouracil
(2-TU). A
monomer of general structure I having a 2-amino adenine nucleobase can be
incorporated into
1o first strand and a monomer of general structure I having a 2-thiouracil
nucleobase can be
incorporated into a second strand. While not wishing to be bound by any
theory, it is believed
that the ground state of the resultant duplex containing the 2-AA - 2-TU
pairing will be
destabilized relative to the ground state of a duplex containing a 2-AA -
uracil or 2-TU -
adenine pairing. Again, while not wishing to be bound by any theory, it is
believed that this
~ 5 ground state destabilization can facilitate helicase activity, which is
involved in strand separation
of the duplex during processing. 2-aminoadenine and 2-thiouracil-containing
oligonucletide
strands are described in "Oligonucleotides containing 2-aminoadenine and 2-
thiothymine act as
selectively binding complementary agents." Kutyavin, Igor V.; Rhinehart,
Rebecca L.;
Lukhtanov, Eugeny A.; Gorn, Vladimir V.; Meyer, Rich B., Jr.; Gamper, Howard
B., Jr. Epoch
2o Pharmaceuticals Inc., Bothell, WA, USA. Biochemistry (1996), 35(34), 11170-
11176; and
"Double duplex invasion by peptide nucleic acid: a general principle for
sequence-specific
targeting of double-stranded DNA." Lohse, Jesper; Dahl, Otto; Nielsen, Peter
E.. Center for
Biomolecular Recognition, Department of Chemistry, University of Copenhagen,
Copenhagen,
Den. Proceedings of the National Academy of Sciences of tlae United States of
America (1999),
2s 96(21), 11804-11808.
As discussed above, the monomers and methods described herein can be used in
the
preparation of modified RNA molecules. Modified RNA molecules include e.g.
those molecules
containing a chemically or stereochemically modified nucleoside or a
nucleoside surrogate.
Coupling of 5'-hydroxyl groups with phosphoramidites forms phosphite ester
intermediates,
3o which in turn are oxidized e.g., with iodine, to the phosphate diester.
Alternatively, the
phosphites may be treated with e.g., sulfur, selenium, amino, and boron
reagents to form
modified phosphate backbones. Linkages between the monomers described herein
and a
nucleoside or oligonucleotide chain can also be treated with iodine, sulfur,
selenium, amino, and
boron reagents to form unmodified and modified phosphate backbones
respectively. Similarly,
64
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
the monomers described herein may be coupled with nucleosides or
oligonucleotides containing
any of the modifications or nucleoside surrogates described herein.
Li ands
The monomers of the invention can be derivatized with a ligand, as opposed to
a base.
Preferred ligands are moieties, other than naturally occuring bases (A, T, G,
C, and U), that are
coupled, preferrably covalently, to the sugar or carrier moiety of the
monomer. In preferred
embodiments a ligand alters the distribution, targeting or lifetime of an iRNA
agent into which it
is incorporated. In preferred embodiments a ligand provides an enhanced
affinity for a selected
1o target, e.g, molecule, cell or cell type, compartment, e.g., a cellular or
organ compartment, tissue,
organ or region of the body, as, e.g., compared to a species derivatized with
one of the bases A,
G,.T, C, or U. Preferred ligands will not take part in duplex pairing in a
duplexed nucleic acid.
Preferred ligands can improve transport, hybridization, and specificity
properties and may
also improve improve nuclease resistance of the resultant natural or modified
~ 5 oligoribonucleotide, or a polymeric molecule comprising any combination of
monomer
compounds described herein and/or natural or modifed ribonucleotides.
Ligands in general can include therapeutic modifiers, e.g., for enhancing
uptake;
diagnostic compounds or reporter groups e.g., for monitoring distribution;
cross-linking agents;
nuclease-resistance confernng moieties; and natural or unusual nucleobases.
General examples
2o include lipids, vitamins, sugars, proteins, peptides, polyamines, and
peptide mimics.
Ligands can include a naturally occurring substance, such as a protein (e.g.,
human serum
albumin (HSA), low-density lipoprotein (LDL), or globulin); carbohydrate
(e.g., a dextran,
pullulan, chitin, chitosan, inulin, cyclodextrin or hyaluronic acid); or a
lipid. The ligand may
also be a recombinant or synthetic molecule, such as a synthetic polymer,
e.g., a synthetic
25 polyamino acid. Examples of polyamino acids include polyamino acid is a
polylysine (PLL),
poly L-aspartic acid, poly L-glutamic acid, styrene-malefic acid anhydride
copolymer, poly(L-
lactide-co-glycolied) copolymer, divinyl ether-malefic anhydride copolymer, N-
(2-
hydroxypropyl)methacrylamide copolymer (HMPA), polyethylene glycol (PEG),
polyvinyl
alcohol (PVA), polyurethane, poly(2-ethylacryllic acid), N-isopropylacrylamide
polymers, or
3o polyphosphazine. Example of polyamines include: polyethylenimine,
polylysine (PLL),
spermine, spermidine, polyamine, pseudopeptide-polyamine, peptidomimetic
polyamine,
dendrimer polyamine, arginine, amidine, protamine, cationic lipid, cationic
porphyrin,
quarternary salt of a polyamine, or an alpha helical peptide.
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Ligands can also include targeting groups, e.g., a cell or tissue targeting
agent, e.g., a
lectin, glycoprotein, lipid or protein, e.g., an antibody, that binds to a
specified cell type such as a
cancer cell, endothelial cell, bone cell. A targeting group can be a
thyrotropin, melanotropin,
lectin, glycoprotein, surfactant protein A, Mucin carbohydrate, multivalent
lactose, multivalent
galactose, N-acetyl-galactosamine, N-acetyl-gulucosamine multivalent mannose,
multivalent
fucose, glycosylated polyaminoacids, multivalent galactose, transferrin,
bisphosphonate,
polyglutamate, polyaspartate, a lipid, cholesterol, a steroid, bile acid,
folate, vitamin B12, biotin,
or an RGD peptide or RGD peptide mimetic.
Other examples of ligands include dyes, intercalating agents (e.g. acridines),
cross-linkers
(e.g. psoralene, mitomycin C), porphyrins (TPPC4, texaphyrin, Sapphyrin),
polycyclic aromatic
hydrocarbons (e.g., phenazine, dihydrophenazine), artificial endonucleases
(e.g. EDTA),
lipophilic molecules, e.g, cholesterol, cholic acid, adamantane acetic acid, 1-
pyrene butyric acid,
dihydrotestosterone, 1,3-Bis-O(hexadecyl)glycerol, geranyloxyhexyl group,
hexadecylglycerol,
borneol, menthol, 1,3-propanediol, heptadecyl group, palinitic acid, myristic
acid,03-
15 (oleoyl)lithocholic acid, 03-(oleoyl)cholenic acid, dimethoxytrityl, or
phenoxazine)and peptide
conjugates (e.g., antennapedia peptide, Tat peptide), alkylating agents,
phosphate, amino,
mercapto, PEG (e.g., PEG-40K), MPEG, [MPEG]Z, polyamino, alkyl, substituted
alkyl,
radiolabeled markers, enzymes, haptens (e.g. biotin), transport/absorption
facilitators (e.g.,
aspirin, vitamin E, folic acid), synthetic ribonucleases (e.g., imidazole,
bisimidazole, histamine,
2o imidazole clusters, acridine-imidazole conjugates, Eu3+ complexes of
tetraazamacrocycles),
dinitrophenyl, HRP, or AP.
Ligands can be proteins, e.g., glycoproteins, or peptides, e.g., molecules
having a specfic
affinity for a co-ligand, or antibodies e.g., an antibody, that binds to a
specified cell type such as
a cancer cell, endothelial cell, or bone cell. Ligands may also include
hormones and hormone
25 receptors. They can also include non-peptidic species, such as lipids,
lectins, carbohydrates,
vitamins, cofactors, multivalent lactose, multivalent galactose, N-acetyl-
galactosamine, N-acetyl-
gulucosamine multivalent mannose; or multivalent fixcose. The ligand can be,
for example, a
lipopolysaccharid, an activator of p3 ~ MAP kinase, or an activator of NF-KB.
The ligand can be a substance, e.g, a drug, that can increase the uptake of
the iRNA agent
3o into the cell, for example, by disrupting the cell's cytoskeleton, e.g., by
disrupting the cell's
microtubules, microfilaments, and/or intermediate filaments. The drug can be,
for example,
taxon, vincristine, vinblastine, cytochalasin, nocodazole, japlakinolide,
latrunculin A, phalloidin,
swinholide A, indanocine, or myoservin.
66
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
The ligand, e.g., when a drug can increase the uptake of the iRNA agent into
the cell by
activating an inflammatory response, for example. Exemplary ligands that would
have such an
effect include tumor necrosis factor alpha (TNFalpha), interleukin-1 beta, or
gamma interferon.
In one aspect, the ligand is a lipid or lipid-based molecule. Such a lipid or
lipid-based
molecule preferably binds a serum protein, e.g., human serum albumin (HSA). An
HSA binding
ligand allows for distribution of the conjugate to a target tissue, e.g., a
non-kidney target tissue of
the body. Preferably, the target tissue is the liver, preferably parenchyma)
cells of the liver.
Other molecules that can bind HSA can also be used as ligands. For example,
neproxin or
aspirin can be used. A lipid or lipid-based ligand can (a) increase resistance
to degradation of the
conjugate, (b) increase targeting or transport into a target cell or cell
membrane, and/or (c) can be
used to adjust binding to a seru protein, e.g., HSA.
A lipid based ligand can be used to modulate, e.g., control the binding of the
conjugate to
a target tissue. For example, a lipid or lipid-based ligand that binds to HSA
more strongly will
be less likely to be targeted to the kidney and therefore less likey to be
cleared from the body. A
15 lipid or lipid-based ligand that binds to HSA less strongly can be used to
target the conjugate to
the kidney.
In a preferred embodiment, the lipid based ligand binds HSA. Preferably, it
binds HSA
with a sufficient affinity such that the conjugate will be preferably
distributed to a non-kidney
tissue. However, it is preferred that the affinity not be so strong that the
HSA-ligand binding
2o cannot be reversed.
In another preferred embodiment, the lipid based ligand binds HSA weakly or
not at all,
such that the conjugate will be preferably distributed to the kidney. Other
moieties that target to
kidney cells can also be used in place of or in addition to the lipid based
ligand.
In a preferred embodiment, the lipid or lipid based ligand is a
phosphorothioate. In this
25 embodiment, it is preferred that the number of sulfurs on the
phosphorothioate not be so
prevalent that they interfere with binding to a serum protein, e.g., HSA.
In another aspect, the ligand is a moiety, e.g., a vitamin, which is taken up
by a target
cell, e.g., a proliferating cell. These are particularly useful for treating
disorders characterized by
unwanted cell proliferation, e.g., of the malignant or non-malignant type,
e.g., cancer cells.
3o Exemplary vitamins are B vitamin, e.g., folic acid, B12, riboflavin,
biotin, pyridoxal or other
vitamins or nutrients taken up by cancer cells. Also included are HSA 'and low
density
lipoprotein (LDL).
In another aspect, the ligand is a cell-permeation agent, preferably a helical
cell-
permeation agent. Preferably, the agent is amphipathic. An exemplary agent is
a peptide such as
67
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
tat or antennopedia. If the agent is a peptide, it can be modified, including
a pepidylmimetic,
invertomers, non-peptide or pseudo-peptide linkages, and use of D-amino acids.
The helical
agent is preferably an alpha-helical agent, which preferably has a lipophilic
and a lipophobic
phase.
Peptides that target markers enriched in proliferating cells can be used.
E.g., RGD
containing peptides and petomimetics can target cancer cells, in particular
cells that exhibit an
a"(33 integrin. Thus, one could use RGD peptides, cyclic peptides containing
RGD, RGD
peptides that include D-amino acids, as well as synthetic RGD mimics. In
addition to RGD, one
can use other moieties that target the a~ [33 integrin ligand. Generally, such
ligands can be used
to control proliferating cells and angiogeneis. Preferred conjugates of this
type include an iRNA
agent that targets PECAM-1, VEGF, or other cancer gene, e.g., a cancer gene
described herein.
The iRNA agents of the invention are particularly useful when targeted to the
liver. An
iRNA agent can be targeted to the liver by incorporation of a monomore
derivitzed with a ligand
which targets to the liver. For example, a liver-targeting agent can be a
lipophilic moiety.
~5 Preferred lipophilic moieties include lipid, cholesterols, oleyl, retinyl,
or cholesteryl residues (see
Table 1). Other lipophilic moieties that can function as liver-targeting
agents include cholic acid,
adamantane acetic acid, 1-pyrene butyric acid, dihydrotestosterone, 1,3-Bis-
O(hexadecyl)glycerol, geranyloxyhexyl group, hexadecylglycerol, borneol,
menthol, 1,3-
propanediol, heptadecyl group, palinitic acid, myristic acid,03-
(oleoyl)lithocholic acid, O3-
20 (oleoyl)cholenic acid, dimethoxytrityl, or phenoxazine.
An iRNA agent can also be targeted to the liver by association with a low-
density
lipoprotein (LDL), such as lactosylated LDL. Polymeric tamers complexed with
sugar residues
can also function to target iRNA agents to the liver.
A targeting agent that incorporates a sugar, e.g., galactose and/or analogues
thereof, is
25 particularly useful. These agents target, in particular, the parenchyrnal
cells of the liver (see
Table 1). For example, a targeting moiety can include more than one or
preferably two or three
galactose moieties, spaced about 15 angstroms from each other. The targeting
moiety can
alternatively be lactose (e.g., three lactose moieties), which is glucose
coupled to a galactose.
The targeting moiety can also be N-Acetyl-Galactosamine, N-Ac-Glucosamine. A
mannose or
3o mannose-6-phosphate targeting moiety can be used for macrophage targeting.
Conjugation of an iRNA agent with a serum albumin (SA), such as human serum
albumin, can also be used to target the iRNA agent to the liver.
68
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
An iRNA agent can be targeted to a particular cell type in the liver by using
specific
targeting agents, which recognize particular receptors in the liver. Exemplary
targeting moieties
and their associated receptors are presented in Table 1.
s Table 1 Tar~etin~ agents (Li~ands) and their associated receptors
Liver Cells Li~and Receptor
1) Parenchymal Galactose ASGP-R
Cell (PC) (Hepatocytes) (Asiologlycoprotein
receptor)
Gal NAc ASPG-R
(n-acetyl- Gal NAc Receptor
galactosamine)
Lactose
Asialofetuin ASPG-r
2) Sinusoidal Hyaluronan Hyaluronan receptor
Endothelial Cell (SEC)
Procollagen Procollagen receptor
Negatively chargedScavenger receptors
molecules
Mannose Mannose receptors
N-acetyl Scavenger receptors
Glucosamine
Immunoglobulins Fc Receptor
LPS CD14 Receptor
Insulin Receptor mediated
transcytosis
Transferrin Receptor mediated
transcytosis
Albumins Non-specific
Sugar-Albumin
conjugates
Mannose-6- Mannose-6-phosphate
phosphate receptor
69
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
3) Kupffer Cell Mannose Mannose receptors
(KC)
Fucose Fucose receptors
Albumins Non-specific
Mannose-albumin
conj ugates
The selection of ligands is within skill of the art, and useful candidate
ligands can be
identified by routine methods.
Ligands can be can be connected to the carrier via a tether, which may be
selected from -
s C(O)-(CHZ)5-C(Q)-(ligand); -C(O)-(CH2)S C(O)O-(ligand); -C(O)-O-(ligand);
-C(O)-(CH2)S NH-; -C(O)-(CH2)S NH-C(O)-(ligand); -C(O)-(CH2)S (ligand); -C(O)-
NH-(ligand);
-C(O)-(ligand); -(CH2)S C(O)-(ligand); -(CH2)S-C(O)O-(ligand); -(CHZ)S
(ligand); -(CH2)S NH-;
or -(CHZ)S NH-C(O)-(ligand); s can be 0-20, preferably 0-4.
iRNA AGENT STRUCTURE
The monomers described herein can be used to make oligonucleotides which are
useful
as iRNA agents, e.g., RNA molecules, (double-stranded; single-stranded) that
mediate RNAi,
e.g., with respect to an endogenous gene of a subject or to a gene of a
pathogen. In most cases
the iRNA agent will incorporate momomers described herein together with
naturally occuring
~ 5 nucleosides or nucleotides or with other modified nucleosides or
nucleotides. The modified
monomers can be present at any position in the iRNA agent, e.g., at the
terminii or in the middle
region of an iRNA agent or in a duplex region or in an unpaired region. In a
preferred
embodiment iRNA agent can have any architecture, e.g., architecture described
herein. E.g., it
can be incorporated into an iRNA agent having an overhang structure, a hairpin
or other single
2o strand structure or a two-strand structure, as described herein.
An "RNA agent" as used herein, is an unmodified RNA, modified RNA, or
nucleoside
surrogate, all of which are defined herein (see, e.g., the section below
entitled RNA Agents).
While numerous modified RNAs and nucleoside surrogates are described,
preferred examples
include those which have greater resistance to nuclease degradation than do
unmodified RNAs.
25 Preferred examples include those which have a 2' sugar modification, a
modification in a single
strand overhang, preferably a 3' single strand overhang, or, particularly if
single stranded, a 5'
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
modification which includes one or more phosphate groups or one or more
analogs of a
phosphate group.
An "iRNA agent" as used herein, is an RNA agent which can, or which can be
cleaved
into an RNA agent which can, down regulate the expression of a target gene,
preferably an
endogenous or pathogen target RNA. While not wishing to be bound by theory, an
iRNA agent
may act by one or more of a number of mechanisms, including post-
transcriptional cleavage of a
target mRNA sometimes referred to in the art as RNAi, or pre-transcriptional
or pre-translational
mechanisms. An iRNA agent can include a single strand or can include more than
one strands,
e.g., it can be a double stranded iRNA agent. If the iRNA agent is a single
strand it is
particularly preferred that it include a 5' modification which includes one or
more phosphate
groups or one or more analogs of a phosphate group.
The iRNA agent should include a region of sufficient homology to the target
gene, and be
of sufficient length in terms of nucleotides, such that the iRNA agent, or a
fragment thereof, can
mediate down regulation of the target gene. (For ease of exposition the term
nucleotide or
~ 5 ribonucleotide is sometimes used herein in reference to one or more
monomeric subunits of an
RNA agent. It will be understood herein that the usage of the term
"ribonucleotide" or
"nucleotide", herein can, in the case of a modified RNA or nucleotide
surrogate, also refer to a
modified nucleotide, or surrogate replacement moiety at one or more
positions.) Thus, the iRNA
agent is or includes a region which is at least partially, and in some
embodiments fully,
2o complementary to the target RNA. It is not necessary that there be perfect
complementarity
between the iRNA agent and the target, but the correspondence must be
sufficient to enable the
iRNA agent, or a cleavage product thereof, to direct sequence specific
silencing, e.g., by RNAi
cleavage of the target RNA, e.g., mRNA.
Complementarity, or degree of homology with the target strand, is most
critical in the
25 antisense strand. While perfect complementarity, particularly in the
antisense strand, is often
desired some embodiments can include, particularly in the antisense strand,
one or more but
preferably 6, 5, 4, 3, 2, or fewer mismatches (with respect to the target
RNA). The mismatches,
particularly in the antisense strand, are most tolerated in the terminal
regions and if present are
preferably in a terminal region or regions, e.g., within 6, 5, 4, or 3
nucleotides of the 5' andlor 3'
3o terminus. The sense strand need only be sufficiently complementary with the
antisense strand to
maintain the over all double strand character of the molecule.
71
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
As discussed elsewhere herein, an iRNA agent will often be modified or include
nucleoside surrogates in addition to the ribose replacement modification
subunit (RRMS).
Single stranded regions of an iRNA agent will often be modified or include
nucleoside
surrogates, e.g., the unpaired region or regions of a hairpin structure, e.g.,
a region which links
two complementary regions, can have modifications or nucleoside surrogates.
Modification to
stabilize one or more 3'- or 5'-terminus of an iRNA agent, e.g., against
exonucleases, or to favor
the antisense sRNA agent to enter into RISC are also favored. Modifications
can include C3 (or
C6, C7, C12) amino linkers, thiol linkers, carboxyl linkers, non-nucleotidic
spacers (C3, C6, C9,
C12, abasic, triethylene glycol, hexaethylene glycol), special biotin or
fluorescein reagents that
come as phosphoramidites and that have another DMT-protected hydroxyl group,
allowing
multiple couplings during RNA synthesis.
iRNA agents include: molecules that are long enough to trigger the interferon
response
(which can be cleaved by Dicer (Bernstein et al. 2001. Nature, 409:363-366)
and enter a RISC
(RNAi-induced silencing complex)); and, molecules which are sufficiently short
that they do not
~ 5 trigger the interferon response (which molecules can also be cleaved by
Dicer and/or enter a
RISC), e.g., molecules which are of a size which allows entry into a RISC,
e.g., molecules which
resemble Dicer-cleavage products. Molecules that are short enough that they do
not trigger an
interferon response are termed sRNA agents or shorter iRNA agents herein.
"sRNA agent or
shorter iRNA agent" as used herein, refers to an iRNA agent, e.g., a double
stranded RNA agent
20 or single strand agent, that is sufficiently short that it does not induce
a deleterious interferon
response in a human cell, e.g., it has a duplexed region of less than 60 but
preferably less than
50, 40, or 30 nucleotide pairs. The sRNA agent, or a cleavage product thereof,
can down
regulate a target gene, e.g., by inducing RNAi with respect to a target RNA,
preferably an
endogenous or pathogen target RNA.
25 Each strand of an sRNA agent can be equal to or less than 30, 25, 24, 23,
22, 21, or 20
nucleotides in length. The strand is preferably at least 19 nucleotides in
length. For example,
each strand can be between 21 and 25 nucleotides in length. Preferred sRNA
agents have a
duplex region of 17, 18, 19, 29, 21, 22, 23, 24, or 25 nucleotide pairs, and
one or more
overhangs, preferably one or two 3' overhangs, of 2-3 nucleotides.
3o In addition to homology to target RNA and the ability to down regulate a
target gene, an
iRNA agent will preferably have one or more of the following properties:
(1) it will be of the Formula 1, 2, 3, or 4 set out in the RNA Agent section
below;
72
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
(2) if single stranded it will have a 5' modification which includes one or
more
phosphate groups or one or more analogs of a phosphate group;
(3) it will, despite modifications, even to a very large number, or all of the
nucleosides, have an antisense strand that can present bases (or modified
bases) in the proper
three dimensional framework so as to be able to form correct base pairing and
form a duplex
structure with a homologous target RNA which is sufficient to allow down
regulation of the
target, e.g., by cleavage of the target RNA;
(4) it will, despite modifications, even to a very large number, or all of the
nucleosides, still have "RNA-like" properties, i.e., it will possess the
overall structural, chemical
1o and physical properties of an RNA molecule, even though not exclusively, or
even partly, of
ribonucleotide-based content. For example, an iRNA agent cam contain, e.g., a
sense and/or an
antisense strand in which all of the nucleotide sugars contain e.g., 2' fluoro
in place of 2'
hydroxyl. This deoxyribonucleotide-containing agent can still be expected to
exhibit RNA-like
properties. While not wishing to be bound by theory, the electronegative
fluorine prefers an
~ 5 axial orientation when attached to the C2' position of ribose. This
spatial preference of fluorine
can, in turn, force the sugars to adopt a C3>-eyado pucker. This is the same
puckering mode as
observed in RNA molecules and gives rise to the RNA-characteristic A-family-
type helix.
Further,, since fluorine is a good hydrogen bond acceptor, it can participate
in the same hydrogen
bonding interactions with water molecules that are known to stabilize RNA
structures.
20 (Generally, it is preferred that a modified moiety at the 2' sugar position
will be able to enter into
H-bonding which is more characteristic of the OH moiety of a ribonucleotide
than the H moiety
of a deoxyribonucleotide. A preferred iRNA agent will: exhibit a C3.-erado
pucker in all, or at
least 50, 75,80, 85, 90, or 95 % of its sugars; exhibit a C3.-endo pucker in a
sufficient amount of
its sugars that it can give rise to a the RNA-characteristic A-family-type
helix; will have no more
25 than 20, 10, 5, 4, 3, 2, orl sugar which is not a C3.-endo pucker
structure. These limitations are
particularly preferably in the antisense strand;
(5) regardless of the nature of the modification, and even though the RNA
agent
can contain deoxynucleotides or modified deoxynucleotides, particularly in
overhang or other
single strand regions, it is preferred that DNA molecules, or any molecule in
which more than
30 50, 60, or 70 % of the nucleotides in the molecule, or more than 50, 60, or
70 % of the
nucleotides in a duplexed region are deoxyribonucleotides, or modified
deoxyribonucleotides
which are deoxy at the 2' position, are excluded from the definition of RNA
agent.
73
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
A "single strand iRNA agent" as used herein, is an iRNA agent which is made up
of a
single molecule. It may include a duplexed region, formed by intra-strand
pairing, e.g., it may
be, or include, a hairpin or pan-handle structure. Single strand iRNA agents
are preferably
antisense with regard to the target molecule. In preferred embodiments single
strand iRNA
agents axe 5' phosphorylated or include a phosphoryl analog at the 5' prime
terminus. 5'-
phosphate modifications include those which are compatible with RISC mediated
gene silencing.
Suitable modifications include: 5'-monophosphate ((HO)2(O)P-O-5'); 5'-
diphosphate
((HO)2(O)P-O-P(HO)(O)-O-5'); 5'-triphosphate ((HO)2(O)P-O-(HO)(O)P-O-P(HO)(O)-
O-5');
5'-guanosine cap (7-methylated or non-methylated) (7m-G-O-5'-(HO)(O)P-O-
(HO)(O)P-O-
P(HO)(O)-O-5'); 5'-adenosine cap (Appp), and any modified or unmodified
nucleotide cap
structure (N-O-5'-(HO)(O)P-O-(HO)(O)P-O-P(HO)(O)-O-5'); 5'-monothiophosphate
(phosphorothioate; (HO)2(S)P-O-5'); 5'-monodithiophosphate
(phosphorodithioate;
(HO)(HS)(S)P-O-5'), 5'-phosphorothiolate ((HO)2(O)P-S-5'); any additional
combination of
oxygen/sulfur replaced monophosphate, diphosphate and triphosphates (e.g. 5'-
alpha-
~5 thiotriphosphate, 5'-gamma-thiotriphosphate, etc.), 5'-phosphoramidates
((HO)2(O)P-NH-5',
(HO)(NH2)(O)P-O-5'), 5'-alkylphosphonates (R=alkyl=methyl, ethyl, isopropyl,
propyl, etc., e.g.
RP(OH)(O)-O-5'-, (OH)2(O)P-5'-CH2-), 5'-alkyletherphosphonates
(R=alkylether=methoxymethyl (MeOCH2-), ethoxymethyl, etc., e.g. RP(OH)(O)-O-5'-
). (These
modifications can also be used with the antisense strand of a double stranded
iRNA.)
2o A single strand iRNA agent should be sufficiently long that it can enter
the RISC and
participate in RISC mediated cleavage of a target mRNA. A single strand iRNA
agent is at least
14, and more preferably at least 15, 20, 25, 29, 35, 40, or SOnucleotides in
length. It is preferably
less than 200, 100, or 60 nucleotides in length.
Hairpin iRNA agents will have a duplex region equal to or at least 17, 18, 19,
29, 21, 22,
25 23, 24, or 25 nucleotide pairs. The duplex region will preferably be equal
to or less than 200,
100, or 50, in length. Preferred ranges for the duplex region are 15-30, 17 to
23, 19 to 23, and 19
to 21 nucleotides pairs in length. The hairpin will preferably have a single
strand overhang or
terminal unpaired region, preferably the 3', and preferably of the antisense
side of the hairpin.
Preferred overhangs are 2-3 nucleotides in length.
3o A "double stranded (ds) iRNA agent" as used herein, is an iRNA agent which
includes
more than one, and preferably two, strands in which interchain hybridization
can form a region
of duplex structure.
74
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
The antisense strand of a double stranded iRNA agent should be equal to or at
least, 14,
15, 16 17, 18, 19, 25, 29, 40, or 60 nucleotides in length. It should be equal
to or less than 200,
100, or 50, nucleotides in length. Preferred ranges are 17 to 25, 19 to 23,
and 19 to21
nucleotides in length.
The sense strand of a double stranded iRNA agent should be equal to or at
least 14, 15,
16 17, 18, 19, 25, 29, 40, or 60 nucleotides in length. It should be equal to
or less than 200, 100,
or 50, nucleotides in length. Preferred ranges are 17 to 25, 19 to 23, and 19
to21 nucleotides in
length.
The double strand portion of a double stranded iRNA agent should be equal to
or at least,
14, 15, 16 17, 18, 19, 20, 21, 22, 23, 24, 25, 29, 40, or 60 nucleotide pairs
in length. It should be
equal to or less than 200, 100, or 50, nucleotides pairs in length. Preferred
ranges are 15-30, 17
to 23, 19 to 23, and 19 to 21 nucleotides pairs in length.
In many embodiments, the ds iRNA agent is sufficiently large that it can be
cleaved by an
endogenous molecule, e.g., by Dicer, to produce smaller ds iRNA agents, e.g.,
sRNAs agents
It may be desirable to modify one or both of the antisense and sense strands
of a double
strand iRNA agent. In some cases they will have the same modification or the
same class of
modification but in other cases the sense and antisense strand will have
different modifications,
e.g., in some cases it is desirable to modify only the sense strand. It may be
desirable to modify
only the sense strand, e.g., to inactivate it, e.g., the sense strand can be
modified in order to
2o inactivate the sense strand and prevent formation of an active sRNA/protein
or RISC. This can
be accomplished by a modification which prevents 5'-phosphorylation of the
sense strand, e.g.,
by modification with a 5'-O-methyl ribonucleotide (see Nykanen et al., (2001)
ATP requirements
and small interfering RNA structure in the RNA interference pathway. Cell 107,
309-321.)
Other modifications which prevent phosphorylation can also be used, e.g.,
simply substituting
the 5'-OH by H rather than O-Me. Alternatively, a large bulky group may be
added to the 5'-
phosphate turning it into a phosphodiester linkage, though this may be less
desirable as
phosphodiesterases can cleave such a linkage and release a functional sRNA 5'-
end. Antisense
strand modifications include 5' phosphorylation as well as any of the other 5'
modifications
discussed herein, particularly the 5' modifications discussed above in the
section on single
3o stranded iRNA molecules.
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
It is preferred that the sense and antisense strands be chosen such that the
ds iRNA agent
includes a single strand or unpaired region at one or both ends of the
molecule. Thus, a ds iRNA
agent contains sense and antisense strands, preferable paired to contain an
overhang, e.g., one or
two 5' or 3' overhangs but preferably a 3' overhang of 2-3 nucleotides. Most
embodiments
s will have a 3' overhang. Preferred sRNA agents will have single-stranded
overhangs, preferably
3' overhangs, of 1 or preferably 2 or 3 nucleotides in length at each end. The
overhangs can be
the result of one strand being longer than the other, or the result of two
strands of the same length
being staggered. 5' ends are preferably phosphorylated.
Preferred lengths for the duplexed region is between 15 and 30, most
preferably 1 ~, 19,
20, 21, 22, and 23 nucleotides in length, e.g., in the sRNA agent range
discussed above. sRNA
agents can resemble in length and structure the natural Dicer processed
products from long
dsRNAs. Embodiments in which the two strands of the sRNA agent are linked,
e.g., covalently
linked are also included. Hairpin, or other single strand structures which
provide the required
double stranded region, and preferably a 3' overhang are also within the
invention.
15 The isolated iRNA agents described herein, including ds iRNA agents and
sRNA agents
can mediate silencing of a target RNA, e.g., mRNA, e.g., a transcript of a
gene that encodes a
protein. For convenience, such mRNA is also referred to herein as mRNA to be
silenced. Such
a gene is also referred to as a target gene. In general, the RNA to be
silenced is an endogenous
gene or a pathogen gene. In addition, RNAs other than mRNA, e.g., tRNAs, and
viral RNAs,
2o can also be targeted.
As used herein, the phrase "mediates RNAi" refers to the ability to silence,
in a sequence
specific manner, a target RNA. While not wishing to be bound by theory, it is
believed that
silencing uses the RNAi machinery or process and a guide RNA, e.g., an sRNA
agent of 21 to 23
nucleotides.
25 As used herein, "specifically hybridizable" and "complementary" axe terms
which are
used to indicate a sufficient degree of complementarity such that stable and
specific binding
occurs between a compound of the invention and a target RNA molecule. Specific
binding
requires a sufficient degree of complementarity to avoid non-specific binding
of the oligomeric
compound to non-target sequences under conditions in which specific binding is
desired, i.e.,
3o under physiological conditions in the case of in vivo assays or therapeutic
treatment, or in the
case of in vitro assays, under conditions in which the assays are performed.
The non-target
sequences typically differ by at least 5 nucleotides.
76
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
In one embodiment, an iRNA agent is "sufficiently complementary" to a target
RNA,
e.g., a target mRNA, such that the iRNA agent silences production of protein
encoded by the
target mRNA. In another embodiment, the iRNA agent is "exactly complementary"
(excluding
the RRMS containing subunit(s))to a target RNA, e.g., the target RNA and the
iRNA agent
anneal, preferably to form a hybrid made exclusively of Watson-Crick basepairs
in the region of
exact complementarity. A "sufficiently complementary" target RNA can include
an internal
region (e.g., of at least 10 nucleotides) that is exactly complementary to a
target RNA.
Moreover, in some embodiments, the iRNA agent specifically discriminates a
single-nucleotide
difference. In this case, the iRNA agent only mediates RNAi if exact
complementary is found in
1 o the region (e.g., within 7 nucleotides of) the single-nucleotide
difference.
As used herein, the term "oligonucleotide" refers to a nucleic acid molecule
(RNA or
DNA) preferably of length less than 100, 200, 300, or 400 nucleotides.
RNA agents discussed herein include otherwise unmodified RNA as well as RNA
which
have been modified, e.g., to improve efficacy, and polymers of nucleoside
surrogates.
~ 5 Unmodified RNA refers to a molecule in which the components of the nucleic
acid, namely
sugars, bases, and phosphate moieties, are the same or essentially the same as
that which occur in
nature, preferably as occur naturally in the human body. The art has referred
to rare or unusual,
but naturally occurring, RNAs as modified RNAs, see, e.g., Limbach et al.,
(1994) Summary:
the modified nucleosides of RNA, Nucleic Acids Res. 22: 2183-2196. Such rare
or unusual
2o RNAs, often termed modified RNAs (apparently because the are typically the
result of a post
transcriptionally modification) are within the term unmodified RNA, as used
herein. Modified
RNA as used herein refers to a molecule in which one or more of the components
of the nucleic
acid, namely sugars, bases, and phosphate moieties, are different from that
which occur in
nature, preferably different from that which occurs in the human body. While
they are referred
25 to as modified "RNAs," they will of course, because of the modification,
include molecules
which are not RNAs. Nucleoside surrogates are molecules in which the
ribophosphate backbone
is replaced with a non-ribophosphate construct that allows the bases to the
presented in the
correct spatial relationship such that hybridization is substantially similar
to what is seen with a
ribophosphate backbone, e.g., non-charged mimics of the ribophosphate
backbone. Examples of
3o all of the above are discussed herein.
Much of the discussion below refers to single strand molecules. In many
embodiments of
the invention a double stranded iRNA agent, e.g., a partially double stranded
iRNA agent, is
77
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
required or preferred. Thus, it is understood that that double stranded
structures (e.g. where two
separate molecules are contacted to form the double stranded region or where
the double
stranded region is formed by intramolecular pairing (e.g., a hairpin
structure)) made of the single
stranded structures described below are within the invention. Preferred
lengths are described
elsewhere herein.
As nucleic acids are polymers of subunits or monomers, many of the
modifications
described below occur at a position which is repeated within a nucleic acid,
e.g., a modification
of a base, or a phosphate moiety, or the a non-linking O of a phosphate
moiety. In some cases
the modification will occur at all of the subject positions in the nucleic
acid but in many, and
infact in most cases it will not. By way of example, a modification may only
occur at a 3' or 5'
terminal position, may only occur in a terminal regions, e.g. at a position on
a terminal
nucleotide or in the last 2, 3, 4, 5, or 10 nucleotides of a strand. A
modification may occur in a
double strand region, a single strand region, or in both. A modification may
occur only in the
double strand region of an RNA or may only occur in a single strand region of
an RNA. E.g., a
phosphorothioate modification at a non-linking O position may only occur at
one or both termini,
may only occur in a terminal regions, e.g., at a position on a terminal
nucleotide or in the last 2,
3, 4, 5, or 10 nucleotides of a strand, or may occur in double strand and
single strand regions,
particularly at termini. The 5' end or ends can be phosphorylated.
In some embodiments it is particularly preferred, e.g., to enhance stability,
to include
2o particular bases in overhangs, or to include modified nucleotides or
nucleotide surrogates, in
single strand overhangs, e.g., in a 5' or 3' overhang, or in both. E.g., it
can be desirable to
include purine nucleotides in overhangs. In some embodiments all or some of
the bases in a 3'
or 5' overhang will be modified, e.g., with a modification described herein.
Modifications can
include, e.g., the use of modifications at the 2' OH group of the ribose
sugar, e.g., the use of
deoxyribonucleotides, e.g., deoxythymidine, instead of ribonucleotides, and
modifications in the
phosphate group, e.g., phosphothioate modifications. Overhangs need not be
homologous with
the target sequence.
78
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Modifications and nucleotide surrogates are discussed below.
II 5. ~5'~
I w a n (2' OH)
X P Y
Z
ASE
3. H (2' OH)
FORMULA 1
79
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
The scaffold presented above in Formula 1 represents a portion of a
ribonucleic acid.
The basic components are the ribose sugar, the base, the terminal phosphates,
and phosphate
internucleotide linkers. Where the bases are naturally occurring bases, e.g.,
adenine, uracil,
guanine or cytosine, the sugars are the unmodified 2' hydroxyl ribose sugar
(as depicted) and W,
X, Y, and Z are all O, Formula 1 represents a naturally occurring unmodified
oligoribonucleotide.
Unmodified oligoribonucleotides may be less than optimal in some applications,
e.g.,
unmodified oligoribonucleotides can be prone to degradation by e.g., cellular
nucleases.
Nucleases can hydrolyze nucleic acid phosphodiester bonds. However, chemical
modifications
to one or more of the above RNA components can confer improved properties,
and, e.g., can
render oligoribonucleotides more stable to nucleases. Umodified
oligoribonucleotides may also
be less than optimal in terms of offering tethering points for attaching
ligands or other moieties
to an iRNA agent.
Modified nucleic acids and nucleotide surrogates can include one or more of
~ 5 (i) alteration, e.g., replacement, of one or both of the non-linking (X
and Y) phosphate
oxygens and/or of one or more of the linking (W and Z) phosphate oxygens (When
the phosphate
is in the terminal position, one of the positions W or Z will not link the
phosphate to an
additional element in a naturally occurnng ribonucleic acid. However, for
simplicity of
terminology, except where otherwise noted, the W position at the 5' end of a
nucleic acid and the
2o terminal Z position at the 3' end of a nucleic acid, are within the term
"linking phosphate
oxygens" as used herein.);
(ii) alteration, e.g., replacement, of a constituent of the ribose sugar,
e.g., of the 2'
hydroxyl on the ribose sugar, or wholesale replacement of the ribose sugar
with a structure other
than ribose, e.g., as described herein;
25 (iii) wholesale replacement of the phosphate moiety (bracket I) with
"dephospho" linkers;
(iv) modification or replacement of a naturally occurring base;
(v) replacement or modification of the ribose-phosphate backbone (bracket II);
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
(vi) modification of the 3' end or 5' end of the RNA, e.g., removal,
modification or
replacement of a terminal phosphate group or conjugation of a moiety, e.g. a
fluorescently
labeled moiety, to either the 3' or 5' end of RNA.
The terms replacement, modification, alteration, and the like, as used in this
context, do
not imply any process limitation, e.g., modification does not mean that one
must start with a
reference or naturally occurring ribonucleic acid and modify it to produce a
modified ribonucleic
acid bur rather modified simply indicates a difference from a naturally
occurring molecule.
It is understood that the actual electronic structure of some chemical
entities cannot be
adequately represented by only one canonical form (i.e. Lewis structure).
While not wishing to
be bound by theory, the actual structure can instead be some hybrid or
weighted average of two
or more canonical forms, known collectively as resonance forms or structures.
Resonance
structures are not discrete chemical entities and exist only on paper. They
differ from one
another only in the placement or "localization" of the bonding and nonbonding
electrons for a
particular chemical entity. It can be possible for one resonance structure to
contribute to a
~ 5 greater extent to the hybrid than the others. Thus, the written and
graphical descriptions of the
embodiments of the present invention are made in terms of what the art
recognizes as the
predominant resonance form for a particular species. For example, any
phosphoroamidate
(replacement of a nonlinking oxygen with nitrogen) would be represented by X =
O and Y = N
in the above figure.
2o Specific modifications are discussed in more detail below.
The Phosphate Group
The phosphate group is a negatively charged species. The charge is distributed
equally
over the two non-linking oxygen atoms (i. e., X and Y in Formula 1 above).
However, the
phosphate group can be modified by replacing one of the oxygens with a
different substituent.
25 One result of this modification to RNA phosphate backbones can be increased
resistance of the
oligoribonucleotide to nucleolytic breakdown. Thus while not wishing to be
bound by theory, it
can be desirable in some embodiments to introduce alterations which result in
either an
uncharged linker or a charged linker with unsymmetrical charge distribution.
Examples of modified phosphate groups include phosphorothioate,
phosphoroselenates,
3o borano phosphates, borano phosphate esters, hydrogen phosphonates,
phosphoroamidates, alkyl
81
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
or aryl phosphonates and phosphotriesters. Phosphorodithioates have both non-
linking oxygens
replaced by sulfur. Unlike the situation where only one of X or Y is altered,
the phosphorus
center in the phosphorodithioates is achiral which precludes the formation of
oligoribonucleotides diastereomers. Diastereomer formation can result in a
preparation in which
the individual diastereomers exhibit varying resistance to nucleases. Further,
the hybridization
affinity of RNA containing chiral phosphate groups can be lower relative to
the corresponding
unmodified RNA species. Thus, while not wishing to be bound by theory,
modifications to both
X and Y which eliminate the chiral center, e.g. phosphorodithioate formation,
may be desirable
in that they cannot produce diastereomer mixtures. Thus, X can be any one of
S, Se, B, C, H, N,
or OR (R is alkyl or aryl). Thus Y can be any one of S, Se, B, C, H, N, or OR
(R is alkyl or
aryl). Replacement of X and/or Y with sulfur is preferred.
The phosphate linker can also be modified by replacement of a linking oxygen
(i.e., W or
Z in Formula 1) with nitrogen (bridged phosphoroamidates), sulfur (bridged
phosphorothioates)
and carbon (bridged methylenephosphonates). The replacement can occur at a
terminal oxygen
~5 (position W (3') or position Z (5'). Replacement of W with carbon or Z with
nitrogen is
preferred.
Candidate agents can be evaluated for suitability as described below.
The Sugar Group
A modified RNA can include modification of all or some of the sugar groups of
the
2o ribonucleic acid. E.g., the 2' hydroxyl group (OH) can be modified or
replaced with a number of
different "oxy" or "deoxy" substituents. While not being bound by theory,
enhanced stability is
expected since the hydroxyl can no longer be deprotonated to form a 2'
alkoxide ion. The 2'
alkoxide can catalyze degradation by intramolecular nucleophilic attack on the
linker phosphorus
atom. Again, while not wishing to be bound by theory, it can be desirable to
some embodiments
25 to introduce alterations in which alkoxide formation at the 2' position is
not possible.
Examples of "oxy"-2' hydroxyl group modifications include alkoxy or aryloxy
(OR, e.g.,
R = H, alkyl, cycloalkyl, aryl, axalkyl, heteroaryl or sugar);
polyethyleneglycols (PEG),
O(CH2CHa0)"CH2CH20R; "locked" nucleic acids (LNA) in which the 2' hydroxyl is
connected,
e.g., by a methylene bridge, to the 4' carbon of the same ribose sugar; O-
AMINE (AMINE =
3o NH2; alkylamino, dialkylamino, heterocyclyl, arylamino, diaryl amino,
heteroaryl amino, or
82
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
diheteroaryl amino, ethylene diamine, polyamino) and aminoalkoxy,
O(CH2)"AMINE, (e.g.,
AMINE = NHZ; alkylamino, dialkylamino, heterocyclyl, arylamino, diaryl amino,
heteroaryl
amino, or diheteroaryl amino, ethylene diamine, polyamino). It is noteworthy
that
oligonucleotides containing only the methoxyethyl group (MOE), (OCH2CHZOCH3, a
PEG
derivative), exhibit nuclease stabilities comparable to those modified with
the robust
phosphorothioate modification.
"Deoxy" modifications include hydrogen (i.e. deoxyribose sugars, which are
ofparticular
relevance to the overhang portions of partially ds RNA); halo (e.g., fluoro);
amino (e.g. NH2;
alkylamino, dialkylamino, heterocyclyl, arylamino, diaryl amino, heteroaryl
amino, diheteroaryl
amino, or amino acid); NH(CHZCH2NH)nCH2CH2-AMINE (AMINE = NH2; allcylamino,
dialkylamino, heterocyclyl, arylamino, diaryl amino, heteroaryl amino,or
diheteroaryl amino), -
NHC(O)R (R = alkyl, cycloalkyl, aryl, aralkyl, heteroaryl or sugar), cyano;
mercapto; alkyl-thio-
alkyl; thioallcoxy; and alkyl, cycloalkyl, aryl, alkenyl and alkynyl, which
may be optionally
substituted with e.g., an amino functionality. Preferred substitutents are 2'-
methoxyethyl, 2'-
~ 5 OCH3, 2'-O-allyl, 2'-C- allyl, and 2'-fluoro.
The sugar group can also contain one or more carbons that possess the opposite
stereochemical configuration than that of the corresponding carbon in ribose.
Thus, a modified
RNA can include nucleotides containing e.g., arabinose, as the sugar.
Modified RNAs can also include "abasic" sugars, which lack a nucleobase at C-
1'. These
2o abasic sugars can also be further contain modifications at one or more of
the constituent sugar
atoms.
To maximize nuclease resistance, the 2' modifications can be used in
combination with
one or more phosphate linker modifications (e.g., phosphorothioate). The so-
called "chimeric"
oligonucleotides are those that contain two or more different modifications.
2s The modificaton can also entail the wholesale replacement of a ribose
structure with
another entity at one or more sites in the iRNA agent. These modifications are
described in
section entitled Ribose Replacements for RRMSs.
Candidate modifications can be evaluated as described below.
83
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Replacement of the Phosphate Group
The phosphate group can be replaced by non-phosphorus containing connectors
(cf.
Bracket I in Formula 1 above). While not wishing to be bound by theory, it is
believed that since
the charged phosphodiester group is the reaction center in nucleolytic
degradation, its
replacement with neutral structural mimics should impart enhanced nuclease
stability. Again,
while not wishing to be bound by theory, it can be desirable, in some
embodiment, to introduce
alterations in which the charged phosphate group is replaced by a neutral
moiety.
Examples of moieties which can replace the phosphate group include siloxane,
carbonate,
carboxymethyl, carbamate, amide, thioether, ethylene oxide linker, sulfonate,
sulfonamide,
thioformacetal, formacetal, oxime, methyleneimino, methylenemethylimino,
methylenehydrazo,
methylenedimethylhydrazo and methyleneoxymethylimino. Preferred replacements
include the
methylenecaxbonylamino and methylenemethylimino groups.
Candidate modifications can be evaluated as described below.
Replacement of Ribophosphate Backbone
~ 5 Oligonucleotide- mimicking scaffolds can also be constructed wherein the
phosphate
linker and ribose sugar are replaced by nuclease resistant nucleoside or
nucleotide surrogates
(see Bracket II of Formula 1 above). While not wishing to be bound by theory,
it is believed that
the absence of a repetitively charged backbone diminishes binding to proteins
that recognize
polyanions (e.g. nucleases). Again, while not wishing to be bound by theory,
it can be desirable
2o in some embodiment, to introduce alterations in which the bases are
tethered by a neutral
surrogate backbone.
Examples include the mophilino, cyclobutyl, pyrrolidine and peptide nucleic
acid (PNA)
nucleoside surrogates. A preferred surrogate is a PNA surrogate.
Candidate modifications can be evaluated as described below.
25 Terminal Modifications
The 3' and 5' ends of an oligonucleotide can be modified. Such modifications
can be at
the 3' end, 5' end or both ends of the molecule. They can include modification
or replacement of
an entire terminal phosphate or of one or more of the atoms of the phosphate
group. E.g., the 3'
84
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
and 5' ends of an oligonucleotide can be conjugated to other functional
molecular entities such as
labeling moieties, e.g., fluorophores (e.g., pyrene, TAMRA, fluorescein, Cy3
or Cy5 dyes) or
protecting groups (based e.g., on sulfur, silicon, boron or ester). The
functional molecular
entities can be attached to the sugar through a phosphate group and/or a
spacer. The terminal
atom of the spacer can connect to or replace the linking atom of the phosphate
group or the C-3'
or C-5' O, N, S or C group of the sugar. Alternatively, the spacer can connect
to or replace the
terminal atom of a nucleotide surrogate (e.g., PNAs). These spacers or linkers
can include e.g., -
(CH2)ri , -(CH2)"N-, -(CHz)"O-, -(CH2)nS-, O(CH2CH20)"CHZCHZOH (e.g., n = 3 or
6), abasic
sugars, amide, carboxy, amine, oxyamine, oxyimine, thioether, disulfide,
thiourea, sulfonamide,
or morpholino, or biotin and fluorescein reagents. When a spacer/phosphate-
functional
molecular entity-spacerlphosphate array is interposed between two strands of
iRNA agents, this
array can substitute for a hairpin RNA loop in a hairpin-type RNA agent. The
3' end can be an -
OH group. While not wishing to be bound by theory, it is believed that
conjugation of certain
moieties can improve transport, hybridization, and specificity properties.
Again, while not
~ 5 wishing to be bound by theory, it may be desirable to introduce terminal
alterations that improve
nuclease resistance. Other examples of terminal modifications include dyes,
intercalating agents
(e.g. acridines), cross-linkers (e.g. psoralene, mitomycin C), porphyrins
(TPPC4, texaphyrin,
Sapphyrin), polycyclic aromatic hydrocarbons (e.g., phenazine,
dihydrophenazine), artificial
endonucleases (e.g. EDTA), lipophilic carriers (e.g., cholesterol, cholic
acid, adamantane acetic
2o acid, 1-pyrene butyric acid, dihydrotestosterone, 1,3-Bis-
O(hexadecyl)glycerol, geranyloxyhexyl
group, hexadecylglycerol, borneol, menthol, 1,3-propanediol, heptadecyl group,
palmitic acid,
myristic acid,03-(oleoyl)lithocholic acid, 03-(oleoyl)cholenic acid,
dimethoxytrityl, or
phenoxazine)and peptide conjugates (e.g., antennapedia peptide, Tat peptide),
alkylating agents,
phosphate, amino, mercapto, PEG (e.g., PEG-40K), MPEG, [MPEG]2, polyamino,
alkyl,
25 , substituted alkyl, radiolabeled markers, enzymes, haptens (e.g. biotin),
transport/absorption
facilitators (e.g., aspirin, vitamin E, folic acid), synthetic ribonucleases
(e.g., imidazole,
bisimidazole, histamine, imidazole clusters, acridine-imidazole
conjugates,'Eu3+ complexes of
tetraazamacrocycles).
Terminal modifications can be added for a number of reasons, including as
discussed
3o elsewhere herein to modulate activity or to modulate resistance to
degradation. Terminal
modifications useful for modulating activity include modification of the 5'
end with phosphate or
phosphate analogs. E.g., in preferred embodiments iRNA agents, especially
antisense strands,
are 5' phosphorylated or include a phosphoryl analog at the 5' prime terminus.
5'-phosphate
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
modifications include those which are compatible with RISC mediated gene
silencing. Suitable
modifications include: 5'-monophosphate ((HO)2(O)P-O-5'); 5'-diphosphate
((HO)2(O)P-O-
P(HO)(O)-O-5'); 5'-triphosphate ((HO)2(O)P-O-(HO)(O)P-O-P(HO)(O)-O-5'); 5'-
guanosine cap
(7-methylated or non-methylated) (7m-G-O-5'-(HO)(O)P-O-(HO)(O)P-O-P(HO)(O)-O-
5'); 5'-
adenosine cap (Appp), and any modified or unmodified nucleotide cap structure
(N-O-5'-
(HO)(O)P-O-(HO)(O)P-O-P(HO)(O)-O-5'); 5'-monothiophosphate (phosphorothioate;
(HO)2(S)P-O-5'); 5'-monodithiophosphate (phosphorodithioate; (HO)(HS)(S)P-O-
5'), 5'-
phosphorothiolate ((HO)2(O)P-S-5'); any additional combination of oxgen/sulfur
replaced
monophosphate, diphosphate and triphosphates (e.g. 5'-alpha-thiotriphosphate,
5'-gamma-
thiotriphosphate, etc.), 5'-phosphoramidates ((HO)2(O)P-NH-5', (HO)(NH2)(O)P-O-
5'), 5'-
alkylphosphonates (R=alkyl=methyl, ethyl, isopropyl, propyl, etc., e.g.
RP(OH)(O)-O-5'-,
(OH)2(O)P-5'-CH2-), 5'-alkyletherphosphonates (R=alkylether=methoxymethyl
(MeOCH2-),
ethoxymethyl, etc., e.g. RP(OH)(O)-O-5'-).
Terminal modifications can also be useful for monitoring distribution, and in
such cases
~5 the preferred groups to be added include fluorophores, e.g., fluorscein or
an Alexa dye, e.g.,
Alexa 488. Terminal modifications can also be useful for enhancing uptake,
useful
modifications for this include cholesterol. Terminal modifications can also be
useful for cross-
linking an RNA agent to another moiety; modifications useful for this include
mitomycin C.
Candidate modifications can be evaluated as described below.
2o The Bases
Adenine, guanine, cytosine and uracil are the most common bases found in RNA.
These
bases can be modified or replaced to provide RNA's having improved properties.
E.g., nuclease
resistant oligoribonucleotides can be prepared with these bases or with
synthetic and natural
nucleobases (e.g., inosine, thymine, xanthine, hypoxanthine, nubularine,
isoguanisine, or
25 tubercidine) and any one of the above modifications. Alternatively,
substituted or modified
analogs of any of the above bases, e.g., "unusual bases" and "universal bases"
described herein,
can be employed. Examples include without limitation 2-aminoadenine, 6-methyl
and other
alkyl derivatives of adenine and guanine, 2-propyl and other alkyl derivatives
of adenine and
guanine, 5-halouracil and cytosine, 5-propynyl uracil and cytosine, 6-azo
uracil, cytosine and
3o thymine, 5-uracil (pseudouracil), 4-thiouracil, 5-halouracil, 5-(2-
aminopropyl)uracil, S-amino
allyl uracil, 8-halo, amino, thiol, thioalkyl, hydroxyl and other 8-
substituted adenines and
guanines, 5-trifluoromethyl and other 5-substituted uracils and cytosines, 7-
methylguanine, 5-
86
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
substituted pyrimidines, 6-azapyrimidines and N-2, N-6 and O-6 substituted
purines, including 2-
aminopropyladenine, 5-propynyluracil and 5-propynylcytosine, dihydrouracil, 3-
deaza-5-
azacytosine, 2-aminopurine, 5-alkyluracil, 7-alkylguanine, 5-alkyl cytosine,7-
deazaadenine, N6,
N6-dimethyladenine, 2,6-diaminopurine, 5-amino-allyl-uracil, N3-methyluracil,
substituted
1,2,4-triazoles, 2-pyridinone, 5-nitroindole, 3-nitropyrrole, 5-methoxyuracil,
uracil-5-oxyacetic
acid, 5-methoxycarbonylmethyluracil, 5-methyl-2-thiouracil, 5-
methoxycarbonylmethyl-2-
thiouracil, 5-methylaminomethyl-2-thiouracil, 3-(3-amino-
3carboxypropyl)uracil, 3-
methylcytosine, 5-methylcytosine, N4-acetyl cytosine, 2-thiocytosine, N6-
methyladenine, N6-
isopentyladenine, 2-methylthio-N6-isopentenyladenine, N-methylguanines, or O-
alkylated bases.
1o Further purines and pyrimidines include those disclosed in U.S. Pat. No.
3,687,808, those
disclosed in the Concise Encyclopedia Of Polymer Science And Engineering,
pages 858-859,
Kroschwitz, J. L, ed. John Wiley 8i Sons, 1990, and those disclosed by
Englisch et al.,
Angewandte Chemie, International Edition, 1991, 30, 613.
Generally, base changes are less preferred for promoting stability, but they
can be useful
~ 5 for other reasons, e.g., some, e.g., 2,6-diaminopurine and 2 amino purine,
are fluorescent.
Modified bases can reduce target specificity. This should be taken into
consideration in the
design of iRNA agents.
Candidate modifications can be evaluated as described below.
Evaluation of Candidate RNA's
2o One can evaluate a candidate RNA agent, e.g., a modified RNA, for a
selected property
by exposing the agent or modified molecule and a control molecule to the
appropriate conditions
and evaluating for the presence of the selected property. For example,
resistance to a degradent
can be evaluated as follows. A candidate modified RNA (and preferably a
control molecule,
usually the unmodified form) can be exposed to degradative conditions, e.g.,
exposed to a milieu,
25 which includes a degradative agent, e.g., a nuclease. E.g., one can use a
biological sample, e.g.,
one that is similar to a milieu, which might be encountered, in therapeutic
use, e.g., blood or a
cellular fraction, e.g., a cell-free homogenate or disrupted cells. The
candidate and control could
then be evaluated for resistance to degradation by any of a number of
approaches. For example,
the candidate and control could be labeled, preferably prior to exposure,
with, e.g., a radioactive
30 or enzymatic label, or a fluorescent label, such as Cy3 or CyS. Control and
modified RNA's can
be incubated with the degradative agent, and optionally a control, e.g., an
inactivated, e.g., heat
inactivated, degradative agent. A physical parameter, e.g., size, of the
modified and control
87
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
molecules are then determined. They can be determined by a physical method,
e.g., by
polyacrylamide gel electrophoresis or a sizing column, to assess whether the
molecule has
maintained its original length, or assessed functionally. Alternatively,
Northern blot analysis can
be used to assay the length of an unlabeled modified molecule.
A functional assay can also be used to evaluate the candidate agent. A
functional assay
can be applied initially or after an earlier non-functional assay, (e.g.,
assay for resistance to
degradation) to determine if the modification alters the ability of the
molecule to silence gene
expression. For example, a cell, e.g., a mammalian cell, such as a mouse or
human cell, can be
co-transfected with a plasmid expressing a fluorescent protein, e.g., GFP, and
a candidate RNA
agent homologous to the transcript encoding the fluorescent protein (see,
e.g., WO 00/44914).
For example, a modified dsRNA homologous to the GFP mRNA can be assayed for
the ability to
inhibit GFP expression by monitoring for a decrease in cell fluorescence, as
compared to a
control cell, in which the transfection did not include the candidate dsRNA,
e.g., controls with no
agent added and/or controls with a non-modified RNA added. Efficacy of the
candidate agent on
~ 5 gene expression can be assessed by comparing cell fluorescence in the
presence of the modified
and unmodified dsRNA agents.
In an alternative functional assay, a candidate dsRNA agent homologous to an
endogenous mouse gene, preferably a maternally expressed gene, such as c-
rraos, can be injected
into an immature mouse oocyte to assess the ability of the agent to inhibit
gene expression ih
2o vivo (see, e.g., WO 01136646). A phenotype of the oocyte, e.g., the ability
to maintain arrest in
metaphase II, can be monitored as an indicator that the agent is inhibiting
expression. For
example, cleavage of c-mos mRNA by a dsRNA agent would cause the oocyte to
exit metaphase
arrest and initiate parthenogenetic development (Colledge et al. Nature 370:
65-68, 1994;
Hashimoto et al. Nature, 370:68-71, 1994). The effect of the modified agent on
target RNA
25 levels can be verified by Northern blot to assay for a decrease in the
level of target mRNA, or by
Western blot to assay for a decrease in the level of target protein, as
compared to a negative
control. Controls can include cells in which with no agent is added and/or
cells in which a non-
modified RNA is added.
88
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
References
General References
The oligoribonucleotides and oligoribonucleosides used in accordance with this
invention
may be with solid phase synthesis, see for example "Oligonucleotide synthesis,
a practical
approach", Ed. M. J. Gait, IRL Press, 1984; "Oligonucleotides and Analogues, A
Practical
Approach", Ed. F. Eckstein, IRL Press, 1991 (especially Chapter 1, Modern
machine-aided
methods of oligodeoxyribonucleotide synthesis, Chapter 2, Oligoribonucleotide
synthesis,
Chapter 3, 2'-O--Methyloligoribonucleotide- s: synthesis and applications,
Chapter 4,
Phosphorothioate oligonucleotides, Chapter 5, Synthesis of oligonucleotide
phosphorodithioates,
Chapter 6, Synthesis of oligo-2'-deoxyribonucleoside methylphosphonates, and.
Chapter 7,
Oligodeoxynucleotides containing modified bases. Other particularly useful
synthetic
procedures, reagents, blocking groups and reaction conditions are described in
Martin, P., Flelv.
Chim. Acta,1995, 78, 486-504; Beaucage, S. L. and Iyer, R. P.,
Tetrahedron,1992, 48, 2223-
2311 and Beaucage, S. L. and Iyer, R. P., Tetralaedron,1993, 49, 6123-6194, or
references
referred to therein.
Modification described in WO 00/44895, WO01/75164, or WO02/44321 can be used
herein.
The disclosure of all publications, patents, and published patent applications
listed herein
are hereby incorporated by reference.
2o Phosphate Group References
The preparation of phosphinate oligoribonucleotides is described in U.S. Pat.
No.
5,508,270. The preparation of alkyl phosphonate oligoribonucleotides is
described in U.S. Pat.
No. 4,469,863. The preparation of phosphoramidite oligoribonucleotides is
described in U.S.
Pat. No. 5,256,775 or U.S. Pat. No. 5,366,878. The preparation of
phosphotriester
oligoribonucleotides is described in U.S. Pat. No. 5,023,243. The preparation
of borano
phosphate oligoribonucleotide is described in U.S. Pat. Nos. 5,130,302 and
5,177,198. The
preparation of 3'-Deoxy-3'-amino phosphoramidate oligoribonucleotides is
described in U.S. Pat.
No. 5,476,925. 3'-Deoxy-3'-methylenephosphonate oligoribonucleotides is
described in An, H,
et al. J. Org. Chem. 2001, 66, 2789-2801. Preparation of sulfur bridged
nucleotides is described
89
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
in Sproat et al. Nucleosides Nucleotides 1988, 7,651 and Crosstick et al.
Tetrahedron Lett. 1989,
30, 4693.
Sugar Group References
Modifications to the 2' modifications can be found in Verma, S. et al. Annu.
Rev.
Biochem. 1998, 67, 99-134 and all references therein. Specific modifications
to the ribose can be
found in the following references: 2'-fluoro (Kawasaki et. al., J. Med.
Chern.,1993, 36, 831-
841), 2'-MOE (Martin, P. Helv. Chim. Acta 1996, 79, 1930-1938), "LNA" (Wengel,
J. Acc.
Chern. Res. 1999, 32, 301-310).
Replacement of the Phosphate Group References
Methylenemethylimino linked oligoribonucleosides, also identified herein as
MMI linked
oligoribonucleosides, methylenedimethylhydrazo linked oligoribonucleosides,
also identified
herein as MDH linked oligoribonucleosides, and methylenecarbonylamino linked
oligonucleosides, also identified herein as amide-3 linked
oligoribonucleosides, and
methyleneaminocarbonyl linked oligonucleosides, also identified herein as
amide-4 linked
~5 oligoribonucleosides as well as mixed backbone compounds having, as for
instance, alternating
MMI and PO or PS linkages can be prepared as is described in U.S. Pat. Nos.
5,378,825,
5,386,023, 5,489,677 and in published PCT applications PCT/LTS92/04294 and
PCT/US92/04305 (published as WO 92/20822 WO and 92/20823, respectively).
Formacetal and
thioformacetal linked oligoribonucleosides can be prepared as is described in
U.S. Pat. Nos.
20 5,264,562 and 5,264,564. Ethylene oxide linked oligoribonucleosides can be
prepared as is
described in U.S. Pat. No. 5,223,618. Siloxane replacements axe described in
Cormier,J.F. et al.
Nucleic Acids Res. 1988,16, 4583. Carbonate replacements are described in
Tittensor, J.R. J.
Chem. Soc. C 1971, 1933. Carboxymethyl replacements are described in Edge,
M.D. et al. J.
Chem. Soc. Perkin Traras. 1 1972, 1991. Carbamate replacements are described
in Stirchak, E.P.
25 Nucleic Acids Res. 1989, 17, 6129.
Replacement of the Phosphate-Ribose Backbone References
Cyclobutyl sugar surrogate compounds can be prepared as is described in U.S.
Pat. No.
5,359,044. Pyrrolidine sugar surrogate can be prepared as is described in U.S.
Pat. No.
5,519,134. Morpholino sugar surrogates can be prepared as is described in U.S.
Pat. Nos.
30 5,142,047 and 5,235,033, and other related patent disclosures. Peptide
Nucleic Acids (PNAs) are
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
known per se and can be prepared in accordance with any of the various
procedures referred to in
Peptide Nucleic Acids (PNA): Synthesis, Properties and Potential Applications,
Bioorganic ~
Medicinal Chemistry, 1996, 4, 5-23. They may also be prepared in accordance
with U.S. Pat. No.
5,539,083.
Terminal Modification References
Terminal modifications are described in Manoharan, M. et al. Antiserase afad
Nucleic Acid
D~ugDeveloprraent 12, 103-128 (2002) and references therein.
Bases References
N-2 substitued purine nucleoside amidites can be prepared as is described in
U.S. Pat.
1 o No. 5,459,255. 3-Deaza purine nucleoside amidites can be prepared as is
described in U.S. Pat.
No. 5,457,191. 5,6-Substituted pyrimidine nucleoside amidites can be prepared
as is described in
U.S. Pat. No. 5,614,617. 5-Propynyl pyrimidine nucleoside amidites can be
prepared as is
described in U.S. Pat. No. 5,484,908. Additional references can be disclosed
in the above
section on base modifications.
Preferred iRNA A _ ents
Preferred RNA agents have the following structure (see Formula 2 below):
91
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
A~
t~
R7
R~
R~
FORMULA 2
Refernng to Formula 2 above, Rl, R2, and R3 are each, independently, H, (i.e.
abasic
nucleotides), adenine, guanine, cytosine and uracil, inosine, thymine,
xanthine, hypoxanthine,
nubularine, tubercidine, isoguanisine, 2-aminoadenine, 6-methyl and other
alkyl derivatives of
adenine and guanine, 2-propyl and other alkyl derivatives of adenine and
guanine, 5-halouracil
and cytosine, 5-propynyl uracil and cytosine, 6-azo uracil, cytosine and
thymine, 5-uracil
(pseudouracil), 4-thiouracil, 5-halouracil, 5-(2-aminopropyl)uracil, 5-amino
allyl uracil, 8-halo,
1 o amino, thiol, thioalkyl, hydroxyl and other 8-substituted adenines and
guanines, 5-
trifluoromethyl and other 5-substituted uracils and cytosines, 7-
methylguanine, 5-substituted
pyrimidines, 6-azapyrimidines and N-2, N-6 and O-6 substituted purines,
including 2-
aminopropyladenine, 5-propynyluracil and 5-propynylcytosine, dihydrouracil, 3-
deaza-5-
azacytosine, 2-aminopurine, 5-alkyluracil, 7-alkylguanine, 5-alkyl cytosine,7-
deazaadenine, 7-
~ 5 deazaguanine, N6, N6-dimethyladenine, 2,6-diaminopurine, S-amino-allyl-
uracil, N3-
92
'-~ x
A2 Ra
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
methyluracil, substituted 1,2,4-triazoles, 2-pyridinone, 5-nitroindole, 3-
nitropyrrole, 5-
methoxyuracil, uracil-5-oxyacetic acid, 5-methoxycarbonylinethyluracil, 5-
methyl-2-thiouracil,
5-methoxycarbonylmethyl-2-thiouracil, 5-methylaminomethyl-2-thiouracil, 3-(3-
amino-
3carboxypropyl)uracil, 3-methylcytosine, 5-methylcytosine, N4-acetyl cytosine,
2-thiocytosine,
N6-methyladenine, N6-isopentyladenine, 2-methylthio-N6-isopentenyladenine, N-
methylguanines, or O-alkylated bases.
R4, R5, and R6 are each, independently, ORB, O(CH2CH20)mCHzCH20R8; O(CH2)"R9;
O(CHZ)nOR9, H; halo; NHZ; NHRB; N(RB)Z; NH(CH2CH2NH)mCH2CHZNHR9; NHC(O)RB; ;
cyano; mercapto, SRB; alkyl-thin-alkyl; alkyl, aralkyl, cycloalkyl, aryl,
heteroaryl, alkenyl,
alkynyl, each of which may be optionally substituted with halo, hydroxy, oxo,
vitro, haloalkyl,
alkyl, alkaryl, aryl, aralkyl, alkoxy, aryloxy, amino, alkylamino,
dialkylamino, heterocyclyl,
arylamino, diaryl amino, heteroaryl amino, diheteroaryl amino, acylamino,
alkylcarbamoyl,
arylcarbamoyl, aminoalkyl, alkoxycarbonyl, carboxy, hydroxyalkyl,
alkanesulfonyl,
alkanesulfonamido, arenesulfonamido, aralkylsulfonamido, alkylcarbonyl,
acyloxy, cyano, or
~ 5 ureido; or R4, R5, or R6 together combine with R~ to form an [-O-CHZ-]
covalently bound bridge
between the sugar 2' and 4' carbons.
93
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
A1 is:
W1
X1 ~ Y1
1 1 1
X1 Y1 X1 Y1
~ or ~
W1
1 1
or
1 1
X1 Y1
~ X1 P Y1 X1 P Y1
~ 1
1 1 1
; H; OH; OCH3; W1; an abasic nucleotide; or absent;
(a preferred A1 , especially with regard to anti-sense strands, is chosen from
5'-
monophosphate ((HO)Z(O)P-O-5'), 5'-diphosphate ((HO)2(O)P-O-P(HO)(O)-O-5'), 5'-
triphosphate ((HO)Z(O)P-O-(HO)(O)P-O-P(HO)(O)-O-5'), 5'-guanosine cap (7-
methylated or
non-methylated) (7m-G-O-5'-(HO)(O)P-O-(HO)(O)P-O-P(HO)(O)-O-5'), 5'-adenosine
cap
(Appp), and any modified or umnodified nucleotide cap structure (N-O-5'-
(HO)(O)P-O-
(HO)(O)P-O-P(HO)(O)-O-5'), 5'-monothiophosphate (phosphorothioate; (HO)2(S)P-O-
5'), 5'-
monodithiophosphate (phosphorodithioate; (HO)(HS)(S)P-O-5'), 5'-
phosphorothiolate
((HO)Z(O)P-S-5'); any additional combination of oxgen/sulfur replaced
monophosphate,
diphosphate and triphosphates (e.g. 5'-alpha-thiotriphosphate, 5'-gamma-
thiotriphosphate, etc.),
5'-phosphoramidates ((HO)2(O)P-NH-5', (HO)(NHZ)(O)P-O-5'), 5'-
alkylphosphonates
(R=alkyl=methyl, ethyl, isopropyl, propyl, etc., e.g. RP(OH)(O)-O-5'-,
(OH)a(O)P-5'-CH2-), 5'-
alkyletherphosphonates (R=alkylether=methoxymethyl (MeOCH2-), ethoxymethyl,
etc., e.g.
RP(OH)(O)-O-5'-)).
AZ is:
94
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Z2
X2 ~
Y2
~ 2
A3 is:
~ 3
~3 ~ Y3
Z 3
and
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
A4 is:
~ 1
X4 Ya
1 ~ 1
X4 P Y4 or X4 ~ Y4
W 4
1 1
or
~ 1
X Y
4 4 X4 ~ Y4 X4 ~ Y4
Z4 ~4 ~4
; H; Z4; an inverted nucleotide; an abasic nucleotide; or absent.
W1 is OH, (CHZ)nR1°, (CH2)nNHRI°, (CHZ)n ORl°, (CHZ)n
SRl°; O(CHa)nR1°;
O(CH2)"ORl°, O(CH2)"NRl°, O(CH2)"SRl°;
O(CHZ)"SS(CH2)"ORl°, O(CHa)"C(O)ORl°,
NH(CHZ)nRl°; NH(CHZ)nNRI° ;NH(CHZ)nORI°,
NH(CH2)nSRI°; S(CH2)nR1°, S(CHZ)"NRl°,
S(CH2)nORI°, S(CH2)nSRI° O(CH2CH20)n,CH2CH2ORl°;
O(CH2CH20)mCHaCH2NHR1°,
NH(CH~CH2NH)mCH2CH2NHR1°; Q-Rl°, O-Q-Rl° N-Q-Rl°,
S-Q-Rio or -O-. W4 is O, CHZ,
NH, or S.
X1, X2, X3, and X4 are each, independently, O or S.
Y1, Y2, Y3, and Y4 are each, independently, OH, O-, ORB, S, Se, BH3-, H, NHR9,
N(R9)z
alkyl, cycloalkyl, aralkyl, aryl, or heteroaryl, each of which may be
optionally substituted.
~5 Zl, Za, and Z3 are each independently O, CH2, NH, or S. Z4 is OH,
(CHa)nRto~
(CH2)"NHRI°, (CH2)n ORi°, (CH2)" SRl°; O(CH~)"Rl°;
O(CHa)"ORl°, O(CH2)"NRl°,
O(CH2)nSRI°, O(CH2)nSS(CH2)nORI°, O(CHa)nC(O)ORl°;
NH(CH2)nRl°; NH(CH2)nNRlo
;NH(CHZ)"ORl°, NH(CHa)nSRI°; S(CHZ)"Rl°,
S(CHZ)"NRl°, S(CHa)"ORl°, S(CHa)"SRIo
96
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
O(CH2CH2O)",CH2CH2OR1°, O(CH2CH20)",CHZCH2NHR1°,
NH(CH2CH~NH)mCH2CHZNHR1°; Q-Rl°, O-Q-Rl° N-Q-Rlo, S-
Q-Rio.
x is 5-100, chosen to comply with a length for an RNA agent described herein.
R~ is H; or is together combined with R4, R5, or R6 to form an [-O-CH2-]
covalently
bound bridge between the sugar 2' and 4' carbons.
R8 is alkyl, cycloalkyl, aryl, aralkyl, heterocyclyl, heteroaryl, amino acid,
or sugar; R9 is
NH2, alkylamino, dialkylamino, heterocyclyl, arylamino, diaryl amino,
heteroaryl amino,
diheteroaryl amino, or amino acid; and Rl° is H; fluorophore (pyrene,
TAMRA, fluorescein, Cy3
or Cy5 dyes); sulfur, silicon, boron or ester protecting group; intercalating
agents (e.g. acridines),
cross-linkers (e.g. psoralene, mitomycin C), porphyries (TPPC4,texaphyrin,
Sapphyrin),
polycyclic aromatic hydrocarbons (e.g., phenazine, dihydrophenazine),
artificial endonucleases
(e.g. EDTA), lipohilic carriers (cholesterol, cholic acid, adamantane acetic
acid, 1-pyrene butyric
acid, dihydrotestosterone, 1,3-Bis-O(hexadecyl)glycerol, geranyloxyhexyl
group,
hexadecylglycerol, borneol, menthol, 1,3-propanediol, heptadecyl group,
palmitic acid,myristic
15 acid,O3-(oleoyl)lithocholic acid, 03-(oleoyl)cholenic acid,
dimethoxytrityl, or phenoxazine)and
peptide conjugates (e.g., antennapedia peptide, Tat peptide), alkylating
agents, phosphate, amino,
mercapto, PEG (e.g., PEG-40K), MPEG, [MPEG]Z, polyamino; allcyl, cycloalkyl,
aryl, aralkyl,
heteroaryl; radiolabelled markers, enzymes, haptens (e.g. biotin),
transport/absorption facilitators
(e.g., aspirin, vitamin E, folic acid), synthetic ribonucleases (e.g.,
imidazole, bisimidazole,
2o histamine, imidazole clusters, acridine-imidazole conjugates, Eu3+
complexes of
tetraazamacrocycles); or an RNA agent. m is 0-1,000,000, and n is 0-20. Q is a
spacer selected
from the group consisting of abasic sugar, amide, carboxy, oxyamine, oxyimine,
thioether,
disulfide, thiourea, sulfonamide, or morpholino, biotin or fluorescein
reagents.
Preferred RNA agents in which the entire phosphate group has been replaced
have the
25 following structure (see Formula 3 below):
97
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
FORMULA 3
Referring to Formula 3, Al°-A4° is L-G-L; Al° and/or
A4° may be absent, in which L is a
linker, wherein one or both L may be present or absent and is selected from
the group consisting
of CHZ(CHZ)g; N(CH2)g; O(CH2)g; S(CH2)g. G is a functional group selected from
the group
consisting of siloxane, carbonate, caxboxymethyl, carbamate, amide, thioether,
ethylene oxide
linker, sulfonate, sulfonamide, thioformacetal, formacetal, oxime,
methyleneimino,
methylenemethylimino, methylenehydrazo, methylenedimethylhydrazo and
methyleneoxymethylimino.
Rio, RZ°, and R3° are each, independently, H, (i.e. abasic
nucleotides), adenine, guanine,
cytosine and uracil, inosine, thymine, xanthine, hypoxanthine, nubularine,
tubercidine,
98
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
isoguanisine, 2-aminoadenine, 6-methyl and other alkyl derivatives of adenine
and guanine, 2-
propyl and other alkyl derivatives of adenine and guanine, 5-halouracil and
cytosine, 5-propynYl
uracil and cytosine, 6-azo uracil, cytosine and thymine, 5-uracil
(pseudouracil), 4-thiouracil, 5-
halouracil, 5-(2-aminopropyl)uracil, 5-amino allyl uracil, 8-halo, amino,
thiol, thioalkyl,
hydroxyl and other 8-substituted adenines and guanines, 5-trifluoromethyl and
other 5-
substituted uracils and cytosines, 7-methylguanine, 5-substituted pyrimidines,
6-a.zapyrimidines
and N-2, N-6 and O-6 substituted purines, including 2-aminopropyladenine, 5-
propynyluracil
and 5-propynylcytosine, dihydrouracil, 3-deaza-5-azacytosine, 2-aminopurine, 5-
alkyluracil, 7-
alkylguanine, 5-alkyl cytosine,7-deazaadenine, 7-deazaguanine, N6, N6-
dimethyladenine, 2,6-
diaminopurine, 5-amino-allyl-uracil, N3-methYluracil substituted 1,2,4-
triazoles, 2-pyridinone,
5-nitroindole, 3-nitropyrrole, S-methoxyuracil, uracil-5-oxyacetic acid, 5-
methoxycarbonYlmethyluracil, 5-methyl-2-thiouracil, 5-methoxycarbonylmethyl-2-
thiouracil, 5-
methylaminomethyl-2-thiouracil, 3-(3-amino-3carboxypropyl)uracil, 3-
methylcytosine, 5-
methylcytosine, N4-acetyl cytosine, 2-thiocytosine, N6-methyladenine, N6-
isopentyladenine, 2-
~ 5 methylthio-N6-isopentenyladenine, N-methylguanines, or O-alkylated bases.
R4o, Rso, and R6° are each, independently, ORs, O(CH2CHa0)mCH2CHZORB;
O(CH2)"R9;
O(CHZ)"OR9, H; halo; NHZ; NHR8; N(R8)2; NH(CH2CH2NH)mCH2CH2R9; NHC(O)RB;;
cyano;
mercapto, SR'; alkyl-thin-alkyl; alkyl, aralkyl, cycloalkyl, aryl, heteroaryl,
alkenyl, alkynYl, each
of wluch may be optionally substituted with halo, hydroxy, oxo, nitro,
haloalkyl, alkyl, alkaxyl,
2o aryl, aralkyl, alkoxy, aryloxy, amino, alkylamino, dialkylamino,
heterocyclyl, arylamino, diaxyl
amino, heteroaryl amino, diheteroaryl amino, acylamino, alkylcaxbamoyl,
arylcarbamoYl,
aminoalkyl, alkoxycarbonyl, carboxy, hydroxyalkyl, alkanesulfonyl,
alkanesulfonamido, .
arenesulfonamido, aralkylsulfonamido, alkylcarbonyl, acyloxy, cyano, and
ureido groups; or R4o,
Rs°, or R6° together combine with R'° to form an [-O-CH2-
] covalently bound bridge between the
25 sugar 2' and 4' carbons.
x is 5-100 or chosen to comply with a length for an RNA agent described
herein.
R~° is H; or is together combined with R4°, Rs°, or
R6° to form an [-O-CH2-] covalentlY
bound bridge between the sugar 2' and 4' carbons.
R8 is alkyl, cycloalkyl, aryl, axalkyl, heterocyclyl, heteroaryl, amino acid,
or sugar; and
3o R9 is NHa, alkylamino, dialkylamino, heterocyclYl, arylamino, diaryl amino,
heteroaryl amino,
diheteroaryl amino, or amino acid. m is 0-1,000,000, n is 0-20, and g is 0-2.
99
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Preferred nucleoside surrogates have the following structure (see Formula 4
below):
SLRIOO-(M-SLRzoo)X M-SLR3oo
FORMULA 4
S is a nucleoside surrogate selected from the group consisting of mophilino,
cyclobutyl,
pyrrolidine and peptide nucleic acid. L is a linker and is selected from the
group consisting of
CHz(CHz)g; N(CHz)g; O(CHz)g; S(CHz)g; -C(O)(CHz)n or may be absent. M is an
amide bond;
sulfonamide; sulfinate; phosphate group; modified phosphate group as described
herein; or may
1o be absent.
Rioo~ Rzoo~ ~d R3oo ~.e each, independently, H (i. e., abasic nucleotides),
adenine,
guanine, cytosine and uracil, inosine, thymine, xanthine, hypoxanthine,
nubularine, tubercidine,
isoguanisine, 2-aminoadenine, 6-methyl and other alkyl derivatives of adenine
and guanine, 2-
propyl and other alkyl derivatives of adenine and guanine, 5-halouracil and
cytosine, 5-propynyl
~5 uracil and cytosine, 6-azo uracil, cytosine and thymine, 5-uracil
(pseudouracil), 4-thiouracil, 5-
halouracil, 5-(2-aminopropyl)uracil, 5-amino allyl uracil, 8-halo, amino,
thiol, thioalkyl,
hydroxyl and other 8-substituted adenines and guanines, 5-trifluoromethyl and
other 5-
substituted uracils and cytosines, 7-methylguanine, 5-substituted pyrimidines,
6-azapyrimidines
and N-2, N-6 and O-6 substituted purines, including 2-aminopropyladenine, 5-
propynyluracil
2o and 5-propynylcytosine, dihydrouracil, 3-deaza-5-azacytosine, 2-
aminopurine, 5-alkyluracil, 7-
alkylguanine, 5-alkyl cytosine,7-deazaadenine, 7-deazaguanine, N6, N6-
dimethyladenine, 2,6-
diaminopurine, 5-amino-allyl-uracil, N3-methyluracil substituted 1, 2, 4,-
triazoles, 2-
pyridinones, 5-nitroindole, 3-nitropyrrole, 5-methoxyuracil, uracil-5-
oxyacetic acid, 5-
methoxycarbonylmethyluracil, 5-methyl-2-thiouracil, 5-methoxycarbonylmethyl-2-
thiouracil, 5-
25 methylaminomethyl-2-thiouracil, 3-(3-amino-3carboxypropyl)uracil, 3-
methylcytosine, 5-
methylcytosine, N4-acetyl cytosine, 2-thiocytosine, N6-methyladenine, N6-
isopentyladenine, 2-
methylthio-N6-isopentenyladenine, N-methylguanines, or O-alkylated bases.
x is 5-100, or chosen to comply with a length for an RNA agent described
herein; and g is
0-2.
100
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Nuclease resistant monomers
The monomers and methods described herein can be used to prepare an RNA, e.g.,
an
iRNA agent, that also incorporates a nuclease resistant monomer (N12M), such
as those described
herein and those described in copending, co-owned United States Provisional
Application Serial
No. 60/469,612, filed on May 9, 2003, and International Application No.
PCT/LTS04/07070, both
of which are hereby incorporated by reference.
An iRNA agent can include monomers which have been modifed so as to inhibit
degradation, e.g., by nucleases, e.g., endonucleases or exonucleases, found in
the body of a
subj ect. These monomers are referred to herein as NRMs, or nuclease
resistance promoting
monomers or modifications. In many cases these modifications will modulate
other properties of
the iRNA agent as well, e.g., the ability to interact with a protein, e.g., a
transport protein, e.g.,
serum albumin, or a member of the RISC (RNA-induced Silencing Complex), or the
ability of
the first and second sequences to form a duplex with one another or to form a
duplex with
another sequence, e.g., a target molecule.
~ 5 While not wishing to be bound by theory, it is believed that modifications
of the sugar,
base, and/or phosphate backbone in an iRNA agent can enhance endonuclease and
exonuclease
resistance, and can enhance interactions with transporter proteins and one or
more of the
functional components of the RISC complex. Preferred modifications are those
that increase
exonuclease and endonuclease resistance and thus prolong the half life of the
iRNA agent prior
2o to interaction with the RISC complex, but at the same time do not render
the iRNA agent
resistant to endonuclease activity in the RISC complex. Again, while not
wishing to be bound by
any theory, it is believed that placement of the modifications at or near the
3' and/or 5' end of
antisense strands can result in iRNA agents that meet the preferred nuclease
resistance criteria
delineated above. Again, still while not wishing to be bound by any theory, it
is believed that
25 placement of the modifications at e.g., the middle of a sense strand can
result in iRNA agents
that are relatively less likely to undergo off targeting.
Modifications described herein can be incorporated into any double-stranded
RNA and
RNA-like molecule described herein, e.g., an iRNA agent. An iRNA agent may
include a duplex
comprising a hybridized sense and antisense strand, in which the antisense
strand and/or the
3o sense strand may include one or more of the modifications described herein.
The anti sense
strand may include modifications at the 3' end and/or the 5' end and/or at one
or more positions
that occur 1-6 (e.g., 1-S, 1-4, 1-3, 1-2) nucleotides from either end of the
strand. The sense
strand rnay include modifications at the 3' end and/or the 5' end and/or at
any one of the
intervening positions between the two ends of the strand. The iRNA agent may
also include a
101
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
duplex comprising two hybridized antisense strands. The first and/or the
second antisense strand
may include one or more of the modifications described herein. Thus, one
and/or both antisense
strands may include modifications at the 3' end and/or the 5' end andlor at
one or more positions
that occur 1-6 (e.g., 1-5, 1-4, 1-3, 1-2) nucleotides from either end of the
strand. Particular
configurations are discussed below.
Modifications that can be useful for producing iRNA agents that meet the
preferred
nuclease resistance criteria delineated above can include one or more of the
following chemical
and/or stereochemical modifications of the sugar, base, and/or phosphate
backbone:
(i) chiral (SP) thioates. Thus, preferred NRMs include nucleotide dimers with
an enriched
or pure for a particular chiral form of a modified phosphate group containing
a heteroatom at the
nonbridging position, e.g., Sp or Rp, at the position X, where this is the
position normally
occupied by the oxygen. The atom at X can also be S, Se, Nr2, or Br3. When X
is S, enriched or
chirally pure Sp linkage is preferred. Enriched means at least 70, 80, 90, 95,
or 99% of the
preferred form. Such NRMs are discussed in more detail below;
~ 5 (ii) attachment of one or more cationic groups to the sugar, base, andlor
the phosphorus
atom of a phosphate or modified phosphate backbone moiety. Thus, preferred
NRMs include
monomers at the terminal position derivatized at a cationic group. As the 5'
end of an antisense
sequence should have a terminal -OH or phosphate group this NRM is preferably
not used at the
5' end of an anti-sense sequence. The group should be attached at a position
on the base which
2o minimizes interference with H bond formation and hybridization, e.g., away
form the face which
interacts with the complementary base on the other strand, e.g, at the 5'
position of a pyrimidine
or a 7-position of a purine. These are discussed in more detail below;
(iii) nonphosphate linkages at the termini. Thus, preferred NRMs include Non-
phosphate
linkages, e.g., a linkage of 4 atoms which confers greater resistance to
cleavage than does a
25 phosphate bond. Examples include 3' CH2-NCH3-O-CH2-5' and 3' CH2-NH-(O=)-
CH2-5'.;
(iv) 3'-bridging thiophosphates and 5'-bridging thiophosphates. Thus,
preferred NRM's
can included these structures;
(v) L-RNA, 2'-5' linkages, inverted linkages, a-nucleosides. Thus, other
preferred
NRM's include: L nucleosides and dimeric nucleotides derived from L-
nucleosides; 2'-5'
3o phosphate, non-phosphate and modified phosphate linkages (e.g.,
thiophosphates,
phosphoramidates and boronophosphates); dimers having inverted linkages, e.g.,
3'-3' or 5'-5'
linkages; monomers having an alpha linkage at the 1' site on the sugar, e.g.,
the structures
described herein having an alpha linkage;
102
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
(vi) conjugate groups. Thus, preferred NRM's can include e.g., a targeting
moiety or a
conjugated ligand described herein conjugated with the monomer, e.g., through
the sugar , base,
or backbone;
(vi) abasic linkages. Thus, preferred NRM's can include an abasic monomer,
e.g., an
abasic monomer as described herein (e.g., a nucleobaseless monomer); an
aromatic or
heterocyclic or polyheterocyclic aromatic monomer as described herein.; and
(vii) 5'-phosphonates and 5'-phosphate prodrugs. Thus, preferred NRM's include
monomers, preferably at the terminal position, e.g., the 5' position, in which
one or more atoms
of the phosphate group is derivatized with a protecting group, which
protecting group or groups,
are removed as a result of the action of a component in the subject's body,
e.g, a carboxyesterase
or an enzyme present in the subject's body. E.g., a phosphate prodrug in which
a carboxy
esterase cleaves the protected molecule resulting in the production of a
thioate anion which
attacks a carbon adjacent to the O of a phosphate and resulting in the
production of an
unprotected phosphate.
~ 5 One or more different NRM modifications can be introduced into an iRNA
agent or into
a sequence of an iRNA agent. An NRM modification can be used more than once in
a sequence
or in an iRNA agent. As some NRM's interfere with hybridization the total
number
incorporated, should be such that acceptable levels of iRNA agent duplex
formation are
maintained.
2o In some embodiments NRM modifications are introduced into the terminal the
cleavage
site or in the cleavage region of a sequence (a sense strand or sequence)
which does not target a
desired sequence or gene in the subject. This can reduce off target silencing.
Chiral Sp Thioates
25 A modification can include the alteration, e.g., replacement, of one or
both of the non-
linking (X and Y) phosphate oxygens and/or of one or more of the linking (W
and Z) phosphate
oxygens. Formula X below depicts a phosphate moiety linking two sugar/sugar
surrogate-base
moieties, SBl and SB2.
103
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
/SB1
W
X P Y
Z
SB2
FORMULA X
In certain embodiments, one of the non-linking phosphate oxygens in the
phosphate
backbone moiety (X and Y) can be replaced by any one of the following: S, Se,
BR3 (R is
hydrogen, alkyl, aryl, etc.),,C (i.e., an alkyl group, an aryl group, etc.),
H, NR2 (R is hydrogen,
1 o alkyl, aryl, etc.), or OR (R is alkyl or aryl). The phosphorus atom in an
unmodified phosphate
group is achiral. However, replacement of one of the non-linking oxygens with
one of the above
atoms or groups of atoms renders the phosphorus atom chiral; in other words a
phosphorus atom
in a phosphate group modified in this way is a stereogenic center. The
stereogenic phosphorus
atom can possess either the "R" configuration (herein RP) or the "S"
configuration (herein SP).
15 Thus if 60% of a population of stereogenic phosphorus atoms have the RP
configuration, then the
remaining 40% of the population of stereogenic phosphorus atoms have the SP
configuration.
In some embodiments, iRNA agents, having phosphate groups in which a phosphate
non-
linking oxygen has been replaced by another atom or group of atoms, may
contain a population
of stereogenic phosphorus atoms in which at least about 50% of these atoms
(e.g., at least about
20 60% of these atoms, at least about 70% of these atoms, at least about 80%
of these atoms, at least
about 90% of these atoms, at least about 95% of these atoms, at least about
98% of these atoms,
at least about 99% of these atoms) have the SP configuration. Alternatively,
iRNA agents having
phosphate groups in which a phosphate non-linking oxygen has been replaced by
another atom
or group of atoms may contain a population of stereogenic phosphorus atoms in
which at least
25 about 50% of these atoms (e.g., at least about 60% of these atoms, at least
about 70% of these
atoms, at least about 80% of these atoms, at least about 90% of these atoms,
at least about 95%
of these atoms, at least about 98% of these atoms, at least about 99% of these
atoms) have the RP
configuration. In other embodiments, the population of stereogenic phosphorus
atoms may have
104
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
the SP configuration and may be substantially free of stereogenic phosphorus
atoms having the
RP configuration. In still other embodiments, the population of stereogenic
phosphorus atoms
may have the RP configuration and may be substantially free of stereogenic
phosphorus atoms
having the SP configuration. As used herein, the phrase "substantially free of
stereogeni'c
s phosphorus atoms having the RP configuration" means that moieties containing
stereogenic
phosphorus atoms having the RP configuration cannot be detected by
conventional methods
known in the art (chiral HPLC, 1H NMR analysis using chiral shift reagents,
etc.). As used
herein, the phrase "substantially free of stereogenic phosphorus atoms having
the SP
configuration" means that moieties containing stereogenic phosphorus atoms
having the SP
configuration cannot be detected by conventional methods known in the art
(chiral HPLC, 1H
NMR analysis using chiral shift reagents, etc.).
In a preferred embodiment, modified iRNA agents contain a phosphorothioate
group, i.e.,
a phosphate groups in which a phosphate non-linking oxygen has been replaced
by a sulfiar atom.
In an especially preferred embodiment, the population of phosphorothioate
stereogenic
~ 5 phosphorus atoms may have the SP configuration and be substantially free
of stereogenic
phosphorus atoms having the RP configuration.
Phosphorothioates may be incorporated into iRNA agents using dimers e.g.,
formulas X-
1 and X-2. The former can be used to introduce phosphorothioate
105
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
DMTO DMTO
BASE BASE
R2 . R2
S Y S P Y
Z
Z
E BASE
I NC
solid phase reagent ~ P
'\~~0~ ~ N i r
~ p )z
X-1 X-2
at the 3' end of a strand, while the latter can be used to introduce this
modification at the 5' end
s or at a position that occurs e.g., 1, 2, 3, 4, S, or 6 nucleotides from
either end of the strand. In the
above formulas, Y can be 2-cyanoethoxy, W and Z can be O, RZ~ can be, e.g., a
substituent that
can impart the C-3 endo configuration to the sugar (e.g., OH, F, OCH3), DMT is
dimethoxytrityl,
and "BASE" can be a natural, unusual, or a universal base.
X-1 and X-2 can be prepared using chiral reagents or directing groups that can
result in
1 o phosphorothioate-containing dimers having a population of stereogenic
phosphorus atoms
having essentially only the RP configuration (i.e., being substantially free
of the SP configuration)
or only the SP configuration (i.e., being substantially free of the RP
configuration). Alternatively,
dimers can be prepared having a population of stereogenic phosphorus atoms in
which about
50% of the atoms have the RP configuration and about 50% of the atoms have the
SP
~ 5 configuration. Dimers having stereogenic phosphorus atoms with the RP
configuration can be
identified and separated from dimers having stereogenic phosphorus atoms with
the SP
configuration using e.g., enzymatic degradation and/or conventional
chromatography techniques.
106
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Cationic Grouts
Modifications can also include attachment of one or more cationic groups to
the sugar,
base, and/or the phosphorus atom of a phosphate or modified phosphate backbone
moiety. A
c~.tionic group can be attached to any atom capable of substitution on a
natural, unusual or
universal base. A preferred position is one that does not interfere with
hybridization, i.e., does
not interfere with the hydrogen bonding interactions needed for base pairing.
A cationic group
can be attached e.g., through the C2' position of a sugar or analogous
position in a cyclic or
acyclic sugar surrogate. Cationic groups can include e.g., protonated amino
groups, derived
from e.g., O-AMINE (AMINE = NH2; alkylamino, dialkylamino, heterocyclyl,
arylamino, diaryl
1 o amino, heteroaryl amino, or diheteroaryl amino, ethylene diamine,
polyamino); aminoalkoxy,
e.g., O(CH2)"AMINE, (e.g., AMINE = NHZ; alkylamino, dialkylamino,
heterocyclyl, arylamino,
dia 'ryl amino, heteroaryl amino, or diheteroaryl amino, ethylene diamine,
polyamino); amino
(e.g. NH2; alkylamino, dialkylamino, heterocyclyl, arylamino, diaryl amino,
heteroaryl amino,
diheteroaryl amino, or amino acid); or NH(CH2CH2NH)nCHZCHz-AMINE (AMINE = NHZ;
~5 alkylamino, dialkylamino, heterocyclyl, arylamino, diaryl amino, heteroaryl
amino,or
diheteroaryl amino).
Nonphosphate Linkages
Modifications can also include the incorporation of nonphosphate linkages at
the 5'
and/or 3' end of a strand. Examples of nonphosphate linkages which can replace
the phosphate
2o group include methyl phosphonate, hydroxylamino, siloxane, carbonate,
carboxymethyl,
caxbamate, amide, thioether, ethylene oxide linker, sulfonate, sulfonamide,
thioformacetal,
formacetal, oxime, methyleneimino, methylenemethylimino, methylenehydrazo,
methylenedimethylhydrazo and methyleneoxymethylimino. Preferred replacements
include the
methyl phosphonate and hydroxylamino groups.
3'-brid '~,ng thiophosphates and 5'-brid~in~ thiophosphates; locked-RNA, 2'-5'
lika~es,
inverted linkages, a-nucleosides; coniu~at~~ps; abasic linkages; and 5'-
phosphonates and
S'-phosphate prodru~s
Referring to formula X above, modifications can include replacement of one of
the
3o bridging or linking phosphate oxygens in the phosphate backbone moiety (W
and Z). Unlike the
situation where only one of X or Y is altered, the phosphorus center in the
phosphorodithioates is
achiral which precludes the formation of iRNA agents containing a stereogenic
phosphorus
atom.
107
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Modifications can also include linking two sugars via a phosphate or modified
phosphate
group through the 2' position of a first sugar and the 5' position of a second
sugar. Also
contemplated are inverted linkages in which both a first and second sugar are
eached linked
through the respective3' positions. Modified RNA's can also include "abasic"
sugars, which
s lack a nucleobase at C-1'. The sugar group can also contain one or more
carbons that possess the
opposite stereochemical configuration than that of the corresponding carbon in
ribose. Thus, a
modified iRNA agent can include nucleotides containing e.g., arabinose, as the
sugar. In another
subset of this modification, the natural, unusual, or universal base may have
the oc-configuration.
Modifcations can also include L-RNA.
Modifications can also include 5'-phosphonates, e.g., P(O)(O-)Z-X-C5~-sugar
(X= CH2,
CF2, CHF and 5'-phosphate prodrugs, e.g., P(O)[OCH2CH2SC(O)R]2CHZC5~-sugar. In
the
latter case, the prodrug groups may be decomposed via reaction first with
carboxy esterases. The
remaining ethyl thiolate group via intramolecular SN2 displacement can depart
as episulfide to
afford the underivatized phosphate group.
~5 Modification can also include the addition of conjugating groups described
elseqhere
herein, which are prefereably attached to an iRNA agent through any amino
group available for
conjugation.
Nuclease resistant modifications include some which can be placed only at the
terminus
and others which can go at any position. Generally the modifications that can
inhibit
2o hybridization so it is preferably to use them only in terminal regions, and
preferrable to not use
them at the cleavage site or in the cleavage region of an sequence which
targets a subject
sequence or gene.. The can be used anywhere in a sense sequence, provided that
sufficient
hybridization between the two sequences of the iRNA agent is maintained. In
some
embodiments it is desirabable to put the NRM at the cleavage site or in the
cleavage region of a
25 sequence which does not target a subj ect sequence or gene,as it can
minimize off target
silencing.
In addition, an iRNA agent described herein can have an overhang which does
not form a
duplex structure with the other sequence of the iRNA agent-it is an overhang,
but it does
hybridize, either with itself, or with another nucleic acid, other than the
other sequence of the
so iRNA agent.
In most cases, the nuclease-resistance promoting modifications will be
distributed
differently depending on whether the sequence will target a sequence in the
subject (often
referred to as an anti-sense sequence) or will not target a sequence in the
subject (often referred
to as a sense sequence). If a sequence is to target a sequence in the subject,
modifications which
108
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
interfer with or inhibit endonuclease cleavage should not be inserted in the
region which is
subject to RISC mediated cleavage, e.g., the cleavage site or the cleavage
region (As described
in Elbashir et al., 2001, Genes and Dev. 15: 1 ~~, hereby incorporated by
reference, cleavage of
the target occurs about in the middle of a 20 or 21 nt guide RNA, or about 10
or 11 nucleotides
upstream of the first nucleotide which is complementary to the guide sequence.
As used herein
cleavage site refers to the nucleotide on either side of the cleavage site, on
the target or on the
iRNA agent strand which hybridizes to it. Cleavage region means an nucleotide
with l, 2, or 3
nucletides of the cleave site, in either direction.)
Such modifications can be introduced into the terminal regions, e.g., at the
terminal
position or with 2, 3, 4, or 5 positions of the terminus, of a sequence which
targets or a sequence
which does not target a sequence in the subject.
An iRNA agent can have a first and a second strand chosen from the.following:
a first strand which does not target a sequence and which has an NRM
modification at or
within l, 2, 3, 4, 5 , or 6 positions from the 3' end;
a first strand which does not target a sequence and which has an NRM
modification at or
within 1, 2, 3, 4, 5 , or 6 positions from the 5' end;
a first strand which does not target a sequence and which has an NR1VI
modification at or
within 1, 2, 3, 4, 5 , or 6 positions from the 3' end and which has a NRM
modification at or
within l, 2, 3, 4, 5 , or 6 positions from the 5' end;
2o a first strand which does not target a sequence and which has an NRM
modification at the
cleavage site or in the cleavage region;
a first strand which does not target a sequence and which has an NRM
modification at the
cleavage site or in the cleavage region and one or more of an NRM modification
at or within l,
2, 3, 4, 5 , or 6 positions from the 3' end, a NRM modification at or within
1, 2, 3, 4, 5 , or 6
positions from the 5' end, or NRM modifications at or within 1, 2, 3, 4, 5 ,
or 6 positions from
both the 3' and the 5' end; and
a second strand which targets a sequence and which has an NRM modification at
or
within 1, 2, 3, 4, 5 , or 6 positions from the 3' end;
a second strand which targets a sequence and which has an NRM modification at
or
3o within 1, 2, 3, 4, 5 , or 6 positions from the 5' end (5' end NRM
modifications are preferentially
not at the terminus but rather at a position 1, 2, 3, 4, 5 , or 6 away from
the 5' terminus of an
antisense strand);
109
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
a second strand which targets a sequence and which has an NRM modification at
or
within 1, 2, 3, 4, 5 , or 6 positions from the 3' end and which has a NRM
modification at or
within 1, 2, 3, 4, 5 , or 6 positions from the 5' end;
a second strand which targets a sequence and which preferably does not have an
an NRM
modification at the cleavage site or in the cleavage region;
a second strand which targets a sequence and which does not have an NRM
modification
at the cleavage site or in the cleavage region and one or more of an NRM
modification at or
within 1, 2, 3, 4, 5 , or 6 positions from the 3' end, a NRM modification at
or within 1, 2, 3, 4, 5 ,
or 6 positions from the 5' end, or NRM modifications at or within 1, 2, 3, 4,
5 , or 6 positions
from both the 3' and the 5' end(5' end NRM modifications are preferentially
not at the terminus
but rather at a position 1, 2, 3, 4, 5 , or 6 away from the 5' terminus of an
antisense strand).
An iRNA agent can also target two sequences and can have a first and second
strand
chosen from:
a first strand which targets a sequence and which has an NRM modification at
or within
~ 5 1, 2, 3, 4, 5 , or 6 positions from the 3' end;
a first strand which targets a sequence and which has an NRM modification at
or within
1, 2, 3, 4, 5 , or 6 positions from the 5' end (5' end NRM modifications are
preferentially not at
the terminus but rather at a position l, 2, 3, 4, 5 , or 6 away from the 5'
terminus of an antisense
strand);
2o a first strand which targets a sequence and which has an NRM modification
at or within
1, 2, 3, 4, 5 , or 6 positions from the 3' end and which has a NRM
modification at or within l, 2,
3, 4, 5 , or 6 positions from the 5' end;
a first strand which targets a sequence and which preferably does not have an
an NRM
modification at the cleavage site or in the cleavage region;
25 a first strand which targets a sequence and which dose not have an NRM
modification at
the cleavage site or in the cleavage region and one or more of an NRM
modification at or within
1, 2, 3, 4, 5 , or 6 positions from the 3' end, a NRM modification at or
within l, 2, 3, 4, 5 , or 6
positions from the 5' end, or NRM modifications at or within 1, 2, 3, 4, 5 ,
or 6 positions from
both the 3' and the 5' end(5' end NRM modifications are preferentially not at
the terminus but
3o rather at a position 1, 2, 3, 4, S , or 6 away from the 5' terminus of an
antisense strand) and
a second strand which targets a sequence and which has an NRM modification at
or
within 1, 2, 3, 4, 5 , or 6 positions from the 3' end;
a second strand which targets a sequence and which has an NRM modification at
or
within 1, 2, 3, 4, 5 , or 6 positions from the 5' end (5' end NRM
modifications are preferentially
11o
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
not at the terminus but rather at a position l, 2, 3, 4, 5 , or 6 away from
the 5' terminus of an
antisense strand);
a second strand which targets a sequence and which has an NRM modification at
or
within 1, 2, 3, 4, 5 , or 6 positions from the 3' end and which has a NRM
modification at or
within l, 2, 3, 4, 5 , or 6 positions from the 5' end;
a second strand which targets a sequence and which preferably does not have an
an NRM
modification at the cleavage site or in the cleavage region;
a second strand which targets a sequence and which dose not have an NRM
modification
at the cleavage site or in the cleavage region and one or more of an NRM
modification at or
1 o within l, 2, 3, 4, 5 , or 6 positions from the 3' end, a NRM modification
at or within l, 2, 3, 4, 5 ,
or 6 positions from the 5' end, or NRM modifications at or within 1, 2, 3, 4,
5 , or 6 positions
from both the 3' and the 5' end(5' end NRM modifications are preferentially
not at the terminus
but rather at a position 1, 2, 3, 4, 5 , or 6 away from the 5' terminus of an
antisense strand).
~5 Ribose Mimics
The monomers and methods described herein can be used to prepare an RNA, e.g.,
an
iRNA agent, that also incorporates a ribose mimic, such as those described
herein and those
described in copending co-owned United States Provisional Application Serial
No. 60/454,962,
filed on March 13, 2003, and International Application No. PCT/LTS04/07070,
both of which are
2o hereby incorporated by reference.
Thus, an aspect of the invention features an iRNA agent that includes a
secondary
hydroxyl group, which can increase efficacy and/or confer nuclease resistance
to the agent.
Nucleases, e.g., cellular nucleases, can hydrolyze nucleic acid phosphodiester
bonds, resulting in
partial or complete degradation of the nucleic acid. The secondary hydroxy
group confers
25 nuclease resistance to an iRNA agent by rendering the iRNA agent less prone
to nuclease
degradation relative to an iRNA which lacks the modification. While not
wishing to be bound
by theory, it is believed that the presence of a secondary hydroxyl group on
the iRNA agent can
act as a structural mimic of a 3' ribose hydroxyl group, thereby causing it to
be less susceptible
to degradation.
3o The secondary hydroxyl group refers to an "OH" radical that is attached to
a carbon atom
substituted by two other carbons and a hydrogen. The secondary hydroxyl group
that confers
nuclease resistance as described above can be part of any acyclic carbon-
containing group. The
hydroxyl may also be part of any cyclic carbon-containing group, and
preferably one or more of
the following conditions is met (1) there is no ribose moiety between the
hydroxyl group and the
111
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
terminal phosphate group or (2) the hydroxyl group is not on a sugar moiety
which is coupled to
a base.. The hydroxyl group is located at least two bonds (e.g., at least
three bonds away, at least
four bonds away, at least five bonds away, at least six bonds away, at least
seven bonds away, at
least eight bonds away, at least nine bonds away, at least ten bonds away,
etc.) from the terminal
phosphate group phosphorus of the iRNA agent. In preferred embodiments, there
are five
intervening bonds between the terminal phosphate group phosphorus and the
secondary hydroxyl
group.
Preferred iRNA agent delivery modules with five intervening bonds between the
terminal
phosphate group phosphorus and the secondary hydroxyl group have the following
structure (see
formula Y below):
A~W
Y P X
Z
\CH2 R3 R
R /~ ~ ~C~ /NHT
CH n C~
R2 ~ ~ R5
OR7 R6
(Y)
~ 5 Refernng to formula Y, A is an iRNA agent, including any iRNA agent
described herein.
The iRNA agent may be connected directly or indirectly (e.g., through a spacer
or linker) to "W"
of the phosphate group. These spacers or linkers can include e.g., -(CH2)"-, -
(CH2)"N-, -
(CH2)"O-, -(CH2)"S-, O(CHZCH20)"CH~CH20H (e.g., n = 3 or 6), abasic sugars,
amide, carboxy,
amine, oxyamine, oxyimine, thioether, disulfide, thiourea, sulfonamide, or
morpholino, or biotin
2o and fluorescein reagents.
The iRNA agents can have a terminal phosphate group that is unmodified (e.g.,
W, X, Y,
and Z are O) or modified. In a modified phosphate group, W and Z can be
independently NH, O,
or S; and X and Y can be independently S, Se, BH3 , C1-C6 alkyl, C6-Clo aryl,
H, O, O-, alkoxy or
amino (including alkylamino, arylamino, etc.). Preferably, W, X and Z are O
and Y is S.
112
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Rl and R3 are each, independently, hydrogen; or C1-Cloo alkyl, optionally
substituted with
hydroxyl, amino, halo, phosphate or sulfate and/or may be optionally inserted
with N, O, S,
alkenyl or alkynyl.
R2 is hydrogen; C1-Cloo alkyl, optionally substituted with hydroxyl, amino,
halo,
phosphate or sulfate andlor may be optionally inserted with N, O, S, alkenyl
or alkynyl; or, when
n is 1, RZ may be taken together with with R4 or R6 to form a ring of 5-12
atoms.
R4 is hydrogen; C1-Cloo alkyl, optionally substituted with hydroxyl, amino,
halo,
phosphate or sulfate and/or may be optionally inserted with N, O, S, alkenyl
or alkynyl; or, when
n is l, R4 may be taken together with with R2 or RS to form a ring of 5-12
atoms.
RS is hydrogen, C1-Cloo alkyl optionally substituted with hydroxyl, amino,
halo,
phosphate or sulfate and/or may be optionally inserted with N, O, S, alkenyl
or alkynyl; or, when
n is l, RS may be taken together with with R4 to form a ring of 5-12 atoms.
R6 is hydrogen, Cl-Cloo alkyl, optionally substituted with hydroxyl, amino,
halo,
phosphate or sulfate and/or may be optionally inserted with N, O, S, alkenyl
or alkynyl, or, when
~ 5 n is 1, R6 may be taken together with with R2 to form a ring of 6-10
atoms;
R~ is hydrogen, C1-Cloo alkyl, or C(O)(CH2)qC(O)NHR9; T is hydrogen or a
functional
group; n and q are each independently 1-100; Rg is Cl-Clo alkyl or C6-Clo
aryl; and R9 is
hydrogen, C1-C10 alkyl, C6-C10 aryl or a solid support agent.
Preferred embodiments may include one of more of the following subsets of iRNA
agent
2o delivery modules.
In one subset of RNAi agent delivery modules, A can be connected directly or
indirectly
through a terminal 3' or 5' ribose sugar carbon of the RNA agent.
h1 another subset of RNAi agent delivery modules, X, W, and Z are O and Y is
S.
In still yet another subset of RNAi agent delivery modules, n is l, and R2 and
R6 are
25 taken together to form a ring containing six atoms and R4 and RS are taken
together to form a
ring containing six atoms. Preferably, the ring system is a trams-decalin. For
example, the RNAi
agent delivery module of this subset can include a compound of
113
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Formula (Y-1):
A
NHT
O\
BF
sS
The functional group can be, for example, a targeting group (e.g., a steroid
or a
carbohydrate), a reporter group (e.g., a fluorophore), or a label (an
isotopically labelled moiety).
The targeting group can further include protein binding agents, endothelial
cell targeting groups
(e.g., RGD peptides and mimetics), cancer cell targeting groups (e.g., folate
Vitamin B12,
Biotin), bone cell targeting groups (e.g., bisphosphonates, polyglutamates,
polyaspartates),
multivalent mannose (for e.g., macrophage testing), lactose, galactose, N-
acetyl-galactosamine,
monoclonal antibodies, glycoproteins, lectins, melanotropin, or thyrotropin.
As can be appreciated by the skilled artisan, methods of synthesizing the
compounds of
the formulae herein will be evident to those of ordinary skill in the art.The
synthesized
compounds can be separated from a reaction mixture and further purified by a
method such as
~ 5 column chromatography, high pressure liquid chromatography, or
recrystallization.
Additionally, the various synthetic steps may be performed in an alternate
sequence or order to
give the desired compounds. Synthetic chemistry transformations and protecting
group
methodologies (protection and deprotection) useful in synthesizing the
compounds described
herein are known in the art and include, for example, those such as described
in R. Larock,
2o Comprehensive Organic Transforrnations, VCH Publishers (1989); T.W. Greene
and P.GM.
Wuts, Protective Groups irz Organic Synthesis, 2d. Ed., John Wiley and Sons
(1991); L. Fieser
and M. Fieser, Fieser and Fieser's Reagents for Organic Synthesis, John Wiley
and Sons (1994);
and L. Paquette, ed., Encyclopedia of Reagents for Orgarzic Syntlzesis, John
Wiley and Sons
(1995), and subsequent editions thereof.
Ribose Replacement Monomer Subunits
iRNA agents can be modified in a number of ways which can optimize, one or
more
characteristics of the iRNA agent. The monomers and methods described herein
can be used to
114
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
prepare an RNA agent, e.g., an iRNA agent, that includes a ribose replacement
monomer subunit
(RRMS), such as those described herein and those described in one or more of
United States
Provisional Application Serial No. 60/493,986, filed on August 8, 2003, which
is hereby
incorporated by reference; United States Provisional Application Serial No.
601494,597, filed on
August 11, 2003, which is hereby incorporated by reference; United States
Provisional
Application Serial No. 60/506,341, filed on September 26, 2003, which is
hereby incorporated
by reference; United States Provisional Application Serial No. 60/158,453,
filed on November
7, 2003, which is hereby incorporated by reference; and International
Application No.
PCT/LJS04/07070, filed March 8, 2004, which is hereby incorporated by
reference. The
synthetic methods and modifications described in these application can be used
with or
combined with the monomers and methods described herein.
In addition, the monomers and methods described herein can be used to prepare
iRNA
agents having an RRMS and another element described herein. E.g., . The
monomers and
methods described herein can be used to prepare an iRNA agent described
herein, e.g., a
15 palindromic iRNA agent, an iRNA agent having a non canonical pairing, an
iRNA agent which
targets a gene described herein, e.g., a gene active in the kidney, an iRNA
agent having an
architecture or structure described herein, an iRNA associated with an
amphipathic delivery
agent described herein, an iRNA associated with a drug delivery module
described herein, an
iRNA agent administered as described herein, or an iRNA agent formulated as
described herein,
2o which also incorporates a RRMS.
The ribose sugar of one or more ribonucleotide subunits of an iRNA agent can
be
replaced with another moiety, e.g., a non-carbohydrate (preferably cyclic)
Garner. A
ribonucleotide subunit in which the ribose sugar of the subunit has been so
replaced is referred to
herein as a ribose replacement modification subunit (RRMS). A cyclic carrier
may be a
25 carbocyclic ring system, i.e., all ring atoms are carbon atoms, or a
heterocyclic ring system, i.e.,
one or more ring atoms may be a heteroatom, e.g., nitrogen, oxygen, sulfur.
The cyclic carrier
may be a monocyclic ring system, or may contain two or more rings, e.g. fused
rings. The cyclic
carrier may be a fixlly saturated ring system, or it may contain one or more
double bonds.
The carriers further include (i) at least two "backbone attachment points" and
(ii) at least
30 one "tethering attachment point." A "backbone attachment point" as used
herein refers to a
functional group, e.g. a hydroxyl group, or generally, a bond available for,
and that is suitable for
incorporation of the Garner into the backbone, e.g., the phosphate, or
modified phosphate, e.g.,
sulfur containing, backbone, of a ribonucleic acid. A "tethering attachment
point" as used herein
refers to a constituent ring atom of the cyclic carrier, e.g., a carbon atom
or a heteroatom (distinct
115
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
from an atom which provides a backbone attachment point), that connects a
selected moiety.
The moiety can be, e.g., a ligand, e.g., a targeting or delivery moiety, or a
moiety which alters a
physical property, e.g., lipophilicity, of an iRNA agent. Optionally, the
selected moiety is
connected by an intervening tether to the cyclic carrier. Thus, it will
include a functional group,
e.g., an amino group, or generally, provide a bond, that is suitable for
incorporation or tethering
of another chemical entity, e.g., a ligand to the constituent ring.
Incorporation of one or more RRMSs described herein into an RNA agent, e.g.,
an iRNA
agent, particularly when tethered to an appropriate entity, can confer one or
more new properties
to the RNA agent andlor alter, enhance or modulate one or more existing
properties in the RNA
molecule. E.g., it can alter one or more of lipophilicity or nuclease
resistance. Incorporation of
one or more RRMSs described herein into an iRNA agent can, particularly when
the RRMS is
tethered to an appropriate entity, modulate, e.g., increase, binding affinity
of an iRNA agent to a
target mRNA, change the geometry of the duplex form of the iRNA agent, alter
distribution or
target the iRNA agent to a particular part of the body, or modify the
interaction with nucleic acid
~5 binding proteins (e.g., during RISC formation and strand separation).
Accordingly, in one aspect, the invention features, an iRNA agent preferably
comprising
a first strand and a second strand, wherein at least one subunit having a
formula (R-1) is
incorporated into at least one of said strands.
R~ R6
X
R2 R5
R3 / Z
4 Y
R
(R-1)
Referring to formula (R-1), X is N(CO)R~, NR~ or CH2; Y is NRB, O, S,
CR9R1°, or
absent; and Z is CR11Ri2 or absent.
Each of Rl, R2, R3, R4, R9, and Rl° is, independently, H, ORa, ORb,
(CHa)"ORa, or
(CHz)"ORb, provided that at least one of Rl, R2, R3, R4, R9, and Rl° is
ORa or ORb and that at
least one of Rl, R2, R3, R4, R~, and Rl° is (CHZ)nORa, or (CHa)"ORb
(when the RRMS is terminal,
one of Rl, R2, R3, R4, R~, and Rl° will include Ra and one will include
Rb; when the RRMS is
116
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
internal, two of RI, RZ, R3, R4, R9, and Rl° will each include an Rb);
further provided that
preferably ORa may only be present with (CH2)"ORb and (CH2)"ORa may only be
present with
ORb.
Each of R5, R6, Rl l, and Rl2 is, independently, H, Cl-C6 alkyl optionally
substituted with
1-3 R13, or C(O)NHR~; or RS and Rl1 together are C3-C$ cycloalkyl optionally
substituted with
R14
R' is CI-C2° alkyl substituted with NR°Rd; R8 is C1-C6 alkyl;
Ri3 is hydroxy, Cl-C4
alkoxy, or halo; and R14 is NR°R'.
Ra 1S:
A
P B
C
and
Rb is:
A
P O Strand
C
Each of A and C is, independently, O or S.
B is OH, O-, or
O O
O P O P OH
O- O-
R~ is H or C1-C6 alkyl; Ra is H or a ligand; and n is 1-4.
In a preferred embodiment the ribose is replaced with a pyrroline scaffold,
and X is.
N(CO)R~ or NR', Y is CR~RI°, and Z is absent.
117
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
In other preferred embodiments the ribose is replaced with a piperidine
scaffold, and X is
N(CO)R7 or NR', Y is CR9R1°, and Z is CRl iRi2
In other preferred embodiments the ribose is replaced with a piperazine
scaffold, and X is
N(CO)R~ or NR', Y is NRB, and Z is CRllRiz
In other preferred embodiments the ribose is replaced with a morpholino
scaffold, and X
is N(CO)R~ or NR~, Y is O, and Z is CRllRia
In other preferred embodiments the ribose is replaced with a decalin scaffold,
and X
isCH2; Y is CR9R1°; and Z is CR11Ri2; and Rs and Rll together are C6
cycloalkyl.
In other preferred embodiments the ribose is replaced with a decalinlindane
scaffold and ,
1o and X is CH2; Y is CR9R1°; and Z is CRllRlz; and RS and Rll together
are CS cycloalkyl.
In other preferred embodiments, the ribose is replaced with a hydroxyproline
scaffold.
RRMSs described herein may be incorporated into any double-stranded RNA-like
molecule described herein, e.g., an iRNA agent. An iRNA agent may include a
duplex
comprising a hybridized sense and antisense strand, in which the antisense
strand and/or the
15 sense strand may include one or more of the RRIVISs described herein. An
RRMS can be
introduced at one or more points in one or both strands of a double-stranded
iRNA agent. An
RRMS can be placed at or near (within 1, 2, or 3 positions) of the 3' or 5'
end of the sense strand
or at near (within 2 or 3 positions of) the 3' end of the antisense strand. In
some embodiments it
is preferred to not have an RRMS at or near (within l, 2, or 3 positions of)
the 5' end of the
2o antisense strand. An RRMS can be internal, and will preferably be
positioned in regions not
critical for antisense binding to the target.
In an embodiment, an iRNA agent may have an RRMS at (or within 1, 2, or 3
positions
of) the 3' end of the antisense strand. In an embodiment, an iRNA agent may
have an RRMS at
(or within 1, 2, or 3 positions of) the 3' end of the antisense strand and at
(or within l, 2, or 3
25 positions of) the 3' end of the sense strand. In an embodiment, an iRNA
agent may have an
RRMS at (or within 1, 2, or 3 positions of) the 3' end of the antisense strand
and an RRMS at the
5' end of the sense strand, in which both ligands are located at the same end
of the iRNA agent.
In certain embodiments, two ligands are tethered, preferably, one on each
strand and are
hydrophobic moieties. While not wishing to be bound by theory, it is believed
that pairing of the
3o hydrophobic ligands can stabilize the iRNA agent via intermolecular van der
Waals interactions.
In an embodiment, an iRNA agent may have an RRMS at (or within 1, 2, or 3
positions
of) the 3' end of the antisense strand and an RRMS at the 5' end of the sense
strand, in which
both RRMSs may share the same ligand (e.g., cholic acid) via connection of
their individual
118
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
tethers to separate positions on the ligand. A ligand shared between two
proximal RRMSs is
referred to herein as a "hairpin ligand."
In other embodiments, an iRNA agent may have an RRMS at the 3' end of the
sense
strand and an RRMS at an internal position of the sense strand. An iRNA agent
may have an
RRMS at an internal position of the sense strand; or may have an RRMS at an
internal position
of the antisense strand; or may have an RRMS at an internal position of the
sense strand and an
RRMS at an internal position of the antisense strand.
In preferred embodiments the iRNA agent includes a first and second sequences,
which
are preferably two separate molecules as opposed to two sequences located on
the same strand,
1 o have sufficient complementarity to each other to hybridize (and thereby
form a duplex region),
e.g., under physiological conditions, e.g., under physiological conditions but
not in contact with a
helicase or other unwinding enzyme.
It is preferred that the first and second sequences be chosen such that the ds
iRNA agent
includes a single strand or unpaired region at one or both ends of the
molecule. Thus, a ds iRNA
15 agent contains first and second sequences, preferable paired to contain an
overhang, e.g., one or
two 5' or 3' overhangs but preferably a 3' overhang of 2-3 nucleotides. Most
embodiments
will have a 3' overhang. Preferred sRNA agents will have single-stranded
overhangs, preferably
3' overhangs, of 1 or preferably 2 or 3 nucleotides in length at each end. The
overhangs can be
the result of one strand being longer than the other, or the result of two
strands of the same length
2o being staggered. 5' ends are preferably phosphorylated.
An RNA agent, e.g., an iRNA agent, containing a preferred, but nonlimiting
RRMS is
presented as formula (R-2) in FIG. 4. The carrier includes two "backbone
attachment points"
(hydroxyl groups), a "tethering attachment point," and a ligand, which is
connected indirectly to
'the Garner via an intervening tether. The RRMS may be the 5' or 3' terminal
subunit of the RNA
25 molecule, i.e., one of the two "W" groups may be a hydroxyl group, and the
other "W" group
may be a chain of two or more unmodified or modified ribonucleotides.
Alternatively, the
RRMS may occupy an internal position, and both "W" groups may be one or more
unmodified
or modified ribonucleotides. More than one RRMS may be present in a RNA
molecule, e.g., an
iRNA agent.
3o The modified RNA molecule of formula (R-2) can be obtained using
oligonucleotide
synthetic methods known in the art. In a preferred embodiment, the modified
RNA molecule of
formula (II) can be prepared by incorporating one or more of the corresponding
RRMS monomer
compounds (RRMS monomers, see, e.g., A, B, and C in FIG 4) into a growing
sense or
antisense strand, utilizing, e.g., phosphoramidite or H-phosphonate coupling
strategies.
119
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
The RRMS monomers generally include two differently functionalized hydroxyl
groups
(OFGI and OFG2 above), which are linked to the Garner molecule (see A in FIG
4), and a
tethering attachment point. As used herein, the term "functionalized hydroxyl
group" means that
the hydroxyl proton has been replaced by another substituent. As shown in
representative
structures B and C, one hydroxyl group (OFGI) on the carrier is functionalized
with a protecting
group (PG). The other hydroxyl group (OFG2) can be functionalized with either
(1) a liquid or
solid phase synthesis support reagent (solid circle) directly or indirectly
through a linker, L, as in
B, or (2) a phosphorus-containing moiety, e.g., a phosphoramidite as in C. The
tethering
attachment point may be connected to a hydrogen atom, a tether, or a tethered
ligand at the time
that the monomer is incorporated into the growing sense or antisense strand
(see R in Scheme 1).
Thus, the tethered ligand can be, but need not be attached to the monomer at
the time that the
monomer is incorporated into the growing strand. In certain embodiments, the
tether, the ligand
or the tethered ligand may be linked to a "precursor" RRMS after a "precursor"
RRMS monomer
has been incorporated into the strand.
~5 The (OFGI) protecting group maybe selected as desired, e.g., from T.W.
Greene and
P.G.M. Wuts, Protective Groups in Organic Syrathesis, 2d. Ed., John Wiley and
Sons (1991).
The protecting group is preferably stable under amidite synthesis conditions,
storage conditions,
and oligonucleotide synthesis conditions. Hydroxyl groups, -OH, are
nucleophilic groups (i.e.,
Lewis bases), which react through the oxygen with electrophiles (i.e., Lewis
acids). Hydroxyl
2o groups in which the hydrogen has been replaced with a protecting group,
e.g., a triarylmethyl
group or a trialkylsilyl group, are essentially unreactive as nucleophiles in
displacement
reactions. Thus, the protected hydroxyl group is useful in preventing e.g.,
homocoupling of
compounds exemplified by structure C during oligonucleotide synthesis. A
preferred protecting
group is the dimethoxytrityl group.
25 When the OFGZ in B includes a linker, e.g., a long organic linker,
connected to a soluble
or insoluble support reagent, solution or solid phase synthesis techniques can
be employed to
build up a chain of natural and/or modified ribonucleotides once OFGI is
deprotected and free to
react as a nucleophile with another nucleoside or monomer containing an
electrophilic group
(e.g., an amidite group). Alternatively, a natural or modified ribonucleotide
or
30 oligoribonucleotide chain can be coupled to monomer C via an amidite group
or H-phosphonate
group at OFGZ. Subsequent to this operation, OFGI can be deblocked, and the
restored
nucleophilic hydroxyl group can react with another nucleoside or monomer
containing an
electrophilic group (see FIG. 1). R' can be substituted or unsubstituted alkyl
or alkenyl. In
120
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
preferred embodiments, R' is methyl, allyl or 2-cyanoethyl. R" may a C1-
Cl° alkyl group,
preferably it is a branched group containing three or more carbons, e.g.,
isopropyl.
OFGZ in B can be hydroxyl functionalized with a linker, which in turn contains
a liquid
or solid phase synthesis support reagent at the other linker terminus. The
support reagent can be
any support medium that can support the monomers described herein. The monomer
can be
attached to an insoluble support via a linker, L, which allows the monomer
(and the growing
chain) to be solubilized in the solvent in which the support is placed. The
solubilized, yet
immobilized, monomer can react with reagents in the surrounding solvent;
unreacted reagents
and soluble by-products can be readily washed away from the solid support to
which the
monomer or monomer-derived products is attached. Alternatively, the monomer
can be attached
to a soluble support moiety, e.g., polyethylene glycol (PEG) and liquid phase
synthesis
techniques can be used to build up the chain. Linker and support medium
selection is within
skill of the art. Generally the linker may be -C(O)(CH2)qC(O)-, or -
C(O)(CHZ)gS-, preferably, it
is oxalyl, succinyl or thioglycolyl. Standard control pore glass solid phase
synthesis supports can
not be used in conjunction with fluoride labile 5' silyl protecting groups
because the glass is
degraded by fluoride with a significant reduction in the amount of full-length
product. Fluoride-
stable polystyrene based supports or PEG are preferred.
Preferred carriers have the general formula (R-3) provided below. (In that
structure
preferred backbone attachment points can be chosen from Rl or R2; R3 or R4; or
R9 and Rl° if Y
2o is CR9R1° (two positions are chosen to give two backbone attachment
points, e.g., Rl and R4, or
R4 and R9. Preferred tethering attachment points include R'; RS or R6 when X
is CH2. The
carriers are described below as an entity, which can be incorporated into a
strand. Thus, it is
understood that the structures also encompass the situations wherein one (in
the case of a
terminal position) or two (in the case of an internal position) of the
attachment points, e.g., Rl or
RZ; R3 or R4; or R9 or R1° (when Y is CR9R1°), is connected to
the phosphate, or modified
phosphate, e.g., sulfur containing, backbone. E.g., one of the above-named R
groups can be -
CH2-, wherein one bond is connected to the caxrier and one to a backbone atom,
e.g., a linking
oxygen or a central phosphorus atom.)
R~ R6
X
R2 R5
R3 / Z
4 Y
R
121
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
(R-3)
X is N(CO)R~, NR~ or CH2; Y is NRB, O, S, CR9R1°; and Z is CRllRia or
absent.
Each of RI, R2, R3, R4, R9, and Rl° is, independently, H, ORa, or
(CHZ)nORb, provided
that at least two of Rl, RZ, R3, R4, R9, and Rl° are ORa and/or
(CH2)nORb.
Each of R5, R6, RI 1, and R12 is, independently, a ligand, H, Cl-C6 alkyl
optionally
substituted with 1-3 R13, or C(O)NHR~; or RS and Rll together are C3-C8
cycloalkyl optionally
substituted with Ri4
1 o R~ is H, a ligand, or C1-CZ° alkyl substituted with NR°Ra;
R8 is H or C1-C6 alkyl; R13 is
hydroxy, C1-C4 alkoxy, or halo; Rl4 is NR°R~; Rls is C1-C6 alkyl
optionally substituted with
cyano, or CZ-C6 alkenyl; R16 is Cl-Cl° alkyl; and Rl' is a liquid or
solid phase support reagent.
L is -C(O)(CH2)qC(O)-, or -C(O)(CHZ)qS-; Ra is CAr3; Rb is P(O)(O-)H,
P(ORIS)N(R16)2
or L-Rl~; R° is H or Cl-C6 alkyl; and Ra is H or a ligand.
~5 Each Ar is, independently, C6-C1° aryl optionally substituted with
Cl-C4 alkoxy; n is 1-4;
and q is 0-4.
Exemplary carriers include those in which, e.g., X is N(CO)R~ or NR', Y is
CR9R1°, and
Z is absent; or X is N(CO)R~ or NR~, Y is CR9R1°, and Z is CR11R12; or
X is N(CO)R~ or NR~, Y
is NRB, and Z is CR11R1~; or X is N(CO)R~ or NR~, Y is O, and Z is CR11Ri2; or
X is CH2; Y is
20' CR9R1°; Z is CR11R12, and RS and Rll together form C6 cycloalkyl
(H, z = 2), or the indane ring
system, e.g., X is CH2; Y is CR9R1°; Z is CR11Ri2, and Rs and Rll
together form CS cycloalkyl
(H, z =1).
In certain embodiments, the carrier may be based on the pyrroline ring system
or the 3-
hydroxyproline ring system, e.g., X is N(CO)R~ or NR~, Y is CR9R1°, and
Z is absent (D). OFC~1
25 is preferably attached to a primary carbon, e.g., an exocyclic alkylene
122
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
OFG2
CH~OFG~
/Cz
N
LIGAND
D
group, e.g., a methylene group, connected to one of the carbons in the five-
membered ring (-
CH20FG1 in D). OFGZ is preferably attached directly to one of the carbons in
the five-
membered ring (-OFGZ in D). For the pyrroline-based carriers, -CHZOFGI may be
attached to C-
2 and OFG2 may be attached to C-3; or -CHZOFGI may be attached to C-3 and OFGZ
may be
attached to C-4. . In certain embodiments, CHZOFGI and OFG2 may be geminally
substituted to
one of the above-referenced carbons.For the 3-hydroxyproline-based carriers, -
CH20FG1 may be
attached to C-2 and OFGZ may be attached to C-4. The pyrroline- and 3-
hydroxyproline-based
monomers may therefore contain linkages (e.g., carbon-carbon bonds) wherein
bond rotation is
restricted about that particular linkage, e.g. restriction resulting from the
presence of a ring.
Thus, CH20FG1 and OFGa may be cis or traras with respect to one another in any
of the pairings
delineated above Accordingly, all cislta~ahs isomers are expressly included.
The monomers may
also contain one or more asymmetric centers and thus occur as racemates and
racemic mixtures,
single enantiomers, individual diastereomers and diastereomeric mixtures. All
such isomeric
forms of the monomers are expressly included. The tethering attachment point
is preferably
nitrogen.
123
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
In certain embodiments, the Garner may be based on the piperidine ring system
(E), e.g., X is
N(CO)R' or NR~, Y is CR9R1°, and Z is CRl iRia. OFG' is preferably
OFG~
C4/~
Cs
--(CH2)nOFG~
/CZ
N
LIGAND
E
attached to a primary carbon, e.g., an exocyclic alkylene group, e.g., a
methylene group (n=1) or
ethylene group (n=2), connected to one of the carbons in the six-membered ring
[-(CHa)"OFGI in
E). OFG2 is preferably attached directly to one of the carbons in the six-
membered ring (-OFG2
in E). -(CHZ)"OFGI and OFGZ may be disposed in a geminal manner on the ring,
i.e., both
groups may be attached to the same carbon, e.g., at C-2, C-3, or C-4.
Alternatively, -
(CH2)nOFGI and OFG2 may be disposed in a vicinal manner on the ring, i.e.,
both groups may be
attached to adjacent ring carbon atoms, e.g., -(CHZ)"OFGI may be attached to C-
2 and OFG2
may be attached to C-3; -(CHZ)"OFGi may be attached to C-3 and OFGZ may be
attached to C-2;
-(CH2)"OFGI may be attached to C-3 and OFG2 may be attached to C-4; or -
(CH~)nOFGI may be ,-
attached to C-4 and OFGZ may be attached to C-3. The piperidine-based monomers
may
~5 therefore contain linkages (e.g., carbon-carbon bonds) wherein bond
rotation is restricted about
that particular linkage, e.g. restriction resulting from the presence of a
ring. Thus, -(CHZ)nOFGI
and OFGZ may be cis or tans with respect to one another in any of the pairings
delineated
above. Accordingly, all cisltrans isomers are expressly included. The monomers
may also
contain one or more asymmetric centers and thus occur as racemates and racemic
mixtures,
2o single enantiomers, individual diastereomers and diastereomeric mixtures.
All such isomeric
forms of the monomers are expressly included. The tethering attachment point
is preferably
nitrogen.
124
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
In certain embodiments, the carrier may be based on the piperazine ring system
(F), e.g.,
X is N(CO)R~ or NR~, Y is NRB, and Z is CR11R12, or the morpholine ring system
(G), e.g., X is
N(CO)R~ or NR~, Y is O, and Z is CR11R12. OFGI is preferably
R"'
OFG2 OFG2
N./ O./
3
C CH20FG~ \C3 CH20FG~
C Ca
N/ 2 N/
LIGAND LIGAND
F G
attached to a primary carbon, e.g., an exocyclic alkylene group, e.g., a
methylene group,
connected to one of the carbons in the six-membered ring (-CH20FG1 in F or G).
OFGa is
preferably attached directly to one of the carbons in the six-membered rings (-
OFGZ in F or G).
1 o For both F and G, -CH20FG1 may be attached to C-2 and OFG2 may be attached
to C-3; or vice
versa. In certain embodiments, CH2OFG1 and OFG2 may be geminally substituted
to one of the
above-referenced carbons.The piperazine- and morpholine-based monomers may
therefore
contain linkages (e.g., carbon-carbon bonds) wherein bond rotation is
restricted about that
particular linkage, e:g. restriction resulting from the presence of a ring.
Thus, CH20FG1 and
15 OFGa may be cis or traps with respect to one another in any of the pairings
delineated above.
Accordingly, all cisltrans isomers are expressly included. The monomers may
also contain one
or more asymmetric centers and thus occur as racemates and racemic mixtures,
single
enantiomers, individual diastereomers and diastereomeric mixtures. All such
isomeric forms of
the monomers are expressly included. R"' can be, e.g., C1-C6 alkyl, preferably
CH3. The
2o tethering attachment point is preferably nitrogen in both F and G.
In certain embodiments, the carrier may be based on the decalin ring system,
e.g., X is
CH2; Y is CR9R1°; Z is CR11R12, and Rs and Rl l together form C6
cycloalkyl (H, z = 2), or the
indane ring system, e.g., X is CHZ; Y is CR9R1°; Z is CRI IR12, and RS
and Rll together form CS
cycloalkyl (H, z =1). OFGI is preferably attached to a primary carbon,
125
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
OFG2
C~~C~CS~C
,yCH2)nOFG~
C/
2
H
e.g., an exocyclic methylene group (n=1) or ethylene group (n=2) connected to
one of C-2, C-3,
C-4, or C-5 [-(CH2)"OFGI in H]. OFG2 is preferably attached directly to one of
C-2, C-3, C-4,
or C-5 (-OFG2 in H). -(CH2)"OFGI and OFG2 may be disposed in a geminal manner
on the ring,
i.e., both groups may be attached to the same carbon, e.g., at C-2, C-3, C-4,
or C-5.
Alternatively, -(CH2)nOFGI and OFGa may be disposed in a vicinal manner on the
ring, i.e., both
groups may be attached to adjacent ring carbon atoms, e.g., -(CHa)nOFGI may be
attached to C-2
and OFG2 may be attached to C-3; -(CHz)nOFGI may be attached to C-3 and OFG2
may be
attached to C-2; -(CHZ)nOFGI may be attached to C-3 and OFG2 may be attached
to C-4; or -
(CH2)"OFGI may be attached to C-4 and OFGZ may be attached to C-3; -(CHZ)nOFGI
may be
attached to C-4 and OFG2 may be attached to C-5; or -(CH2)"OFGI may be
attached to C-5 and
OFG2 may be attached to C-4. The decalin or indane-based monomers may
therefore contain
linkages (e.g., carbon-carbon bonds) wherein bond rotation is restricted about
that particular
15 linkage, e.g. restriction resulting from the presence of a ring. Thus, -
(CH2)nOFGI and OFGZ may
be cis or traps with respect to one another in any of the pairings delineated
above. Accordingly,
all cisltrans isomers are expressly included. The monomers may also contain
one or more
asymmetric centers and thus occur as racemates and racemic mixtures, single
enantiomers,
individual diastereomers and diastereomeric mixtures. All such isomeric forms
of the monomers
2o are expressly included. In a preferred embodiment, the substituents at C-l
and C-6 are traps
with respect to one another. The tethering attachment point is preferably C-6
or C-7.
Other carriers may include those based on 3-hydroxyproline (~. Thus, -
(CH2)nOFGI and
OFGZ may be cis or traps with respect to one another. Accordingly, all
cisltrans isomers are
expressly included. The monomers may also contain one or more asymmetric
centers
126
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
2GF0(
J
and thus occur as racemates and racemic mixtures, single enantiomers,
individual diastereomers
and diastereomeric mixtures. All such isomeric forms of the monomers are
expressly included.
The tethering attachment point is preferably nitrogen.
Representative carriers are shown in FIG. 5.
In certain embodiments, a moiety, e.g., a ligand may be connected indirectly
to the carrier
via the intermediacy of an intervening tether. Tethers are connected to the
carrier at the tethering
attachment point (TAP) and may include any C1-Cloo carbon-containing moiety,
(e.g. C1-Cps, Cl-
Cso, Cl-Czo~ Cl-Clo, CmC6), preferably having at least one nitrogen atom. In
preferred
embodiments, the nitrogen atom forms part of a terminal amino group on the
tether, which may
serve as a connection point for the ligand. Preferred tethers (underlined)
include TAP=
CH~,N~f-I-; TAP-C O CH~,N~Ii-; or TAP-NR"" CH~,h, in which n is 1-6 and R"" is
C1-
C6 alkyl. and Rd is hydrogen or a ligand. In other embodiments, the nitrogen
may form part of a
terminal oxyamino group, e.g., -ONH2, or hydrazino group, -NHNII2. The tether
may optionally
be substituted, e.g., with hydroxy, alkoxy, perhaloalkyl, and/or optionally
inserted with one or
more additional heteroatoms, e.g., N, O, or S. Preferred tethered ligands may
include, e.g.,
TAP- CH-~,NH(LIGAND),
TAP-C O CH~~LIGAND), or TAP-NR" "(CH~"NH(LIGAND);
2o TAP-(CH~~"ONH(LIGAND), TAP-C O CH )"ONH(LIGAND), or
TAP-NR""(CH~,ONH(LIGAND); TAP-~)"NHNH~ LIGAND ,
TAP-C O CH )"NHNHa LIGAND , or TAP-NR""(CH~"NHNH~ LIGAND .
In other embodiments the tether may include an electrophilic moiety,
preferably at the
terminal position of the tether. Preferred electrophilic moieties include,
e.g., an aldehyde, alkyl
halide, mesylate, tosylate, nosylate, or brosylate, or an activated carboxylic
acid ester, e.g. an
NHS ester, or a pentafluorophenyl ester. Preferred tethers (underlined)
include TAP=
127
LIGAND
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
~CH~ CHO; TAP-C O CH~,CHO; or TAP=NR" "(CH~"CHO, in which n is 1-6 and R"" is
C1-C6 alkyl; or TAP- CH~"C(O)ONHS; TAP-C O CH~"C(O)ONHS; or
TAP-NR""(CH~"C(O)ONHS, in which n is 1-6 and R"" is C1-C6 alkyl;
TAP- CH~"C O OC6F5; TAP-C O CH~"C(O) OC6F5; or TAP-NR""(CH ~"C O OC6F , in
s which n is 1-6 and R"" is C1-C6 alkyl; or -(CH~~,,CH~LG; TAP-C O CH~nCH~LG;
or TAP-
NR" "(CH~"CHzLG, in which n is 1-6 and R"" is CI-C6 alkyl (LG can be a leaving
group,
e.g., halide, mesylate, tosylate, nosylate, brosylate). Tethering can be
carried out by coupling a
nucleophilic group of a ligand, e.g., a tluol or amino group with an
electrophilic group on the
tether.
Tethered Entities
A wide variety of entities can be tethered to an iRNA agent, e.g., to the
earner of an
RRMS. Examples are described below in the context of an RR1VIS but that is
only preferred,
entities can be coupled at other points to an iRNA agent. Preferred entities
are those which
target to the kidney, and also those that specifically target to tissues other
than the kidney.
~ 5 Preferred moieties are ligands, which are coupled, preferably covalently,
either directly or
indirectly via an intervening tether, to the RRMS earner. In preferred
embodiments, the ligand is
attached to the carrier via an intervening tether. As discussed above, the
.ligand or tethered
ligand may be present on the RRMS monomer when the RRMS monomer is
incorporated into
the growing strand. In some embodiments, the ligand may be incorporated into a
"precursor"
2o RRMS after a "precursor" RRMS monomer has been incorporated into the
growing strand. For
example, an RR1VIS monomer having, e.g., an amino-terminated tether (i.e.,
having no associated
ligand), e.g., TAP-(CHa)"NHz may be incorporated into a growing sense or
antisense strand. In a
subsequent operation, i.e., after incorporation of the precursor monomer into
the strand, a ligand
having an electrophilic group, e.g., a pentafluorophenyl ester or aldehyde
group, can
25 ' subsequently be attached to the precursor RRMS by coupling the
electrophilic group of the
ligand with the terminal nucleophilic group of the precursor RRMS tether.
In preferred embodiments, a ligand alters the distribution, targeting or
lifetime of an
iRNA agent into which it is incorporated. In preferred embodiments a ligand
provides an
enhanced affinity for a selected target, e.g, molecule, cell or cell type,
compartment, e.g., a
3o cellular or organ compartment, tissue, organ or region of the body, as,
e.g., compared to a species
absent such a ligand. Preferred ligands will not take part in duplex pairing
in a duplexed nucleic
acid.
Preferred ligands can improve transport, hybridization, and specificity
properties and may
also improve nuclease resistance of the resultant natural or modified
oligoribonucleotide, or a
128
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
polymeric molecule comprising any combination of monomers described herein
and/or natural or
modified ribonucleotides.
Ligands in general can include therapeutic modifiers, e.g., for enhancing
uptake;
diagnostic compounds or reporter groups e.g., for monitoring distribution;
cross-linking agents;
and nuclease-resistance conferring moieties. General examples include lipids,
steroids, vitamins,
sugars, proteins, peptides, polyamines, and peptide mimics.
Ligands can include a naturally occurring substance, such as a protein (e.g.,
human serum
albumin (HSA), low-density lipoprotein (LDL), or globulin); carbohydrate
(e.g., a dextran,
pullulan, chitin, chitosan, inulin, cyclodextrin or hyaluronic acid); or a
lipid. The ligand may
also be a recombinant or synthetic molecule, such as a synthetic polymer,
e.g., a synthetic
polyamino acid. Examples of polyamino acids include polyamino acid is a
polylysine (PLL),
poly L-aspartic acid, poly L-glutamic acid, styrene-malefic acid anhydride
copolymer, poly(L-
lactide-co-glycolied) copolymer, divinyl ether-malefic anhydride copolymer, N-
(2-
hydroxypropyl)methacrylamide copolymer (I~VIPA), polyethylene glycol (PEG),
polyvinyl
~ 5 alcohol (PVA), polyurethane, poly(2-ethylacryllic acid), N-
isopropylacrylamide polymers, or
polyphosphazine. Example of polyamines include: polyethylenimine, polylysine
(PLL),
spermine, spermidine, polyamine, pseudopeptide-polyamine, peptidomimetic
polyamine,
dendrimer polyamine, arginine, amidine, protamine, cationic lipid, cationic
porphyrin,
quaternary salt of a polyamine, or an alpha helical peptide.
2o Ligands can also include targeting groups, e.g., a cell or tissue targeting
agent, e.g., a
lectin, glycoprotein, lipid or protein, e.g., an antibody, that binds to a
specified cell type such as a
kidney cell. A targeting group can be a thyrotropin, melanotropin, lectin,
glycoprotein, surfactant
protein A, Mucin carbohydrate, multivalent lactose, multivalent galactose, N-
acetyl-
galactosamine, N-acetyl-gulucosamine multivalent mannose, multivalent fucose,
glycosylated
25 polyaminoacids, multivalent galactose, transfernn, bisphosphonate,
polyglutamate,
polyaspaxtate, a lipid, cholesterol, a steroid, bile acid, folate, vitamin
B12, biotin, or an RGD
peptide or RGD peptide mimetic.
Other examples of ligands include dyes, intercalating agents (e.g. acridines),
cross-linkers
(e.g. psoralene, mitomycin C), porphyrins (TPPC4, texaphyrin, Sapphyrin),
polycyclic aromatic
3o hydrocarbons (e.g., phenazine, dihydrophenazine), artificial endonucleases
(e.g. EDTA),
lipophilic molecules, e.g, cholesterol, cholic acid, adamantane acetic acid, 1-
pyrene butyric acid,
dihydrotestosterone, 1,3-Bis-O(hexadecyl)glycerol, geranyloxyhexyl group,
hexadecylglycerol,
borneol, menthol, 1,3-propanediol, heptadecyl group, palmitic acid, myristic
acid,03-
(oleoyl)lithocholic acid, 03-(oleoyl)cholenic acid, dimethoxytrityl, or
phenoxazine)and peptide
129
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
conjugates (e.g., antennapedia peptide, Tat peptide), alkylating agents,
phosphate, amino,
mercapto, PEG (e.g., PEG-40I~), MPEG, [MPEG]2, polyamino, alkyl, substituted
alkyl,
radiolabeled markers, enzymes, haptens (e.g. biotin), transport/absorption
facilitators (e.g.,
aspirin, vitamin E, folic acid), synthetic ribonucleases (e.g., imidazole,
bisimidazole, histamine,
imidazole clusters, acridine-imidazole conjugates, Eu3+ complexes of
tetraazamacrocycles),
dinitrophenyl, HRP, or AP.
Ligands can be proteins, e.g., glycoproteins, or peptides, e.g., molecules
having a specific
affinity for a co-ligand, or antibodies e.g., an antibody, that binds to a
specified cell type such as
a cancer cell, endothelial cell, or bone cell. Ligands may also include
hormones and hormone
receptors. They can also include non-peptidic species, such as lipids,
lectins, carbohydrates,
vitamins, cofactors, multivalent lactose, multivalent galactose, N-acetyl-
galactosamine, N-acetyl-
gulucosamine multivalent mannose, or multivalent fucose. The ligand can be,
for example, a
lipopolysaccharide, an activator of p3 8 MAP kinase, or an activator of NF-KB.
The ligand can be a substance, e.g, a drug, which can increase the uptake of
the iRNA
~5 agent into the cell, for example, by disrupting the cell's cytoskeleton,
e.g., by disrupting the cell's
microtubules, microfilaments, and/or intermediate filaments. The drug can be,
for example,
taxon, vincristine, vinblastine, cytochalasin, nocodazole, japlakinolide,
latrunculinA, phalloidin,
swinholide A, indanocine, or myoservin.
The ligand can increase the uptake of the iRNA agent into the cell by
activating an
2o inflammatory response, for example. Exemplary ligands that would have such
an effect include
tumor necrosis factor alpha (TNFalpha), interleukin-1 beta, or gamma
interferon.
In one aspect, the ligand is a lipid or lipid-based molecule. Such a lipid or
lipid-based
molecule preferably, binds a serum protein, e.g., human serum albumin (HSA).
An HSA binding
ligand allows for distribution of the conjugate to a target tissue, e.g., a
non-kidney target tissue of
25 the body: For example, the target tissue can be the liver, including
parenchyma) cells of the liver.
Other molecules that can bind HSA can also be used as ligands. For example,
neproxin or
aspirin can be used. A lipid or lipid-based ligand can (a) increase resistance
to degradation of the
conjugate, (b) increase targeting or transport into a target cell or cell
membrane, and/or (c) can be
used to adjust binding to a serum protein, e.g., HSA.
3o A lipid based ligand can be used to modulate, e.g., control the binding of
the conjugate to
a target tissue. For example, a lipid or lipid-based ligand that binds to HSA
more strongly will
be less likely to be targeted to the kidney and therefore less likely to be
cleared from the body:
A lipid or lipid-based ligand that binds to HSA less strongly can be used to
target the conjugate
to the kidney:
130
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
In a preferred embodiment, the lipid based ligand binds HSA. Preferably, it
binds HSA
with a sufficient affinity such that the conjugate will be preferably
distributed to a non-kidney
tissue. However, it is preferred that the affinity not be so strong that the
HSA-ligand binding
cannot be reversed.
In another preferred embodiment, the lipid based ligand binds HSA weakly or
not at all,
such that the conjugate will be preferably distributed to the kidney Other
moieties that target to
kidney cells can also be used in place of or in addition to the lipid based
ligand.
In another aspect, the ligand is a moiety, e.g., a vitamin, which is taken up
by a target cell,
e.g., a proliferating cell. These axe particularly useful for treating
disorders characterized by
unwanted cell proliferation, e.g., of the malignant or non-malignant type,
e.g., cancer cells.
Exemplary vitamins include vitamin A, E, and I~. Other exemplary vitamins
include are B
vitamin, e.g., folic acid, B12, riboflavin, biotin, pyridoxal or other
vitamins or nutrients taken up
by cancer cells. Also included are HSA and low density lipoprotein (LDL).
In another aspect, the ligand is a cell-permeation agent, preferably a helical
cell-
permeation agent. Preferably, the agent is amphipathic. An exemplary agent is
a peptide such as
tat or antennopedia. If the agent is a peptide, it can be modified, including
a peptidylinimetic,
invertomers, non-peptide or pseudo-peptide linkages, and use of D-amino acids.
The helical
agent is preferably an alpha-helical agent, which preferably has a lipophilic
and a lipophobic
phase.
2o The ligand can be a peptide or peptidornimetic. A peptidomimetic (also
referred to
herein as an oligopeptidomimetic) is a molecule capable of folding into a
defined three-
dimensional structure similar to a natural peptide. The attachment of peptide
and
peptidomimetics to iRNA agents can affect pharmacokinetic distribution of the
iRNA, such as by
enhancing cellular recognition and absorption. The peptide or peptidomimetic
moiety can be
about 5-50 amino acids long, e.g., about 5, 10, 15, 20, 25, 30, 35, 40, 45, or
50 amino acids long
(see Table 2, for example).
131
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Table 2. Exemplary Cell Permeation Peptides
Cell Amino acid Sequence Reference
Permeation
Pe tide
Penetratin RQIKIWFQNRRMKWKK (SEQ ID NO:l) Derossi et al.,
J. Biol.
Chem. 269:10444,
1994
Tat fragmentGRKKRRQRRRPPQC (SEQ m N0:2) Vives et al.,
J. Biol.
(48-60) Chem., 272:16010,
1997
Signal GALFLGWLGAAGSTMGAWSQPKKKRKV Chaloin et al.,
Sequence- (SEQ ID N0:3) Biochem. Biophys.
based peptide Res. Commun.,
243:601, 1998
PVEC LLIILRRRTRKQA_H_A_H_SK (SEQ m N0:4)Elmquist et al.,
Exp.
Cell Res., 269:237,
2001
Transportan GWTLNSAGYLLK1NLKALAALAKKIL Pooga et al.,
FASEB
(SEQ ~ NO:S) J., 12:67, 1998
Amphiphilic KLALKLALKALKAALKLA (SEQ m N0:6) Oehlke et al.,
Mol.
model peptide Ther., 2:339,
2000
~'g9 (SEQ m N0:7) Mitchell et al.,
J.
Pept. Res., 56:318,
2000
Bacterial KFFKFFKFFK (SEQ m NO:B)
cell
wall
permeating
LL-37 LLGDFFRKSKEKIGKEFKRIVQRIKDFLRN
LVPRTES (SEQ m N0:9)
Cecropin SWLSKTAKKLENSAKKRISEGIAIAIQGGP
Pl
R (SEQ m NO:10)
a defensin ACYCRIPACIAGERRYGTCIYQGRLWAFC
C (SEQ m NO:11)
b-defensin DHYNCVSSGGQCLYSACP1FTKIQGTCYR
GKAKCCK (SEQ ID N0:12)
Bactenecin RKCRIVVIRVCR (SEQ ID N0:13)
PR-39 RRRPRPPYLPRPRPPPFFPPRLPPRIPPGFPP
RFPPRFPGKR-NH2 (SEQ ID N0:14)
Indolicidin ILPWKWPWWPWRR-NH2 (SEQ ID NO:15)
A peptide or peptidomimetic can be, for example, a cell permeation peptide,
cationic
peptide, amphipathic peptide, or hydrophobic peptide (e.g., consisting
primarily of Tyr, Trp or
Phe). The peptide moiety can be a dendrimer peptide, constrained peptide or
crosslinked
132
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
peptide. In another alternative, the peptide moiety can include a hydrophobic
membrane
translocation sequence (MTS). An exemplary hydrophobic MTS-containing peptide
is RFGF
having the amino acid sequence AAVALLPAVLLALLAP (SEQ ID N0:16). An RFGF
analogue (e.g., amino acid sequence AALLPVLLAAP (SEQ ID N0:17)) containing a
hydrophobic MTS can also be a targeting moiety. The peptide moiety can be a
"delivery"
peptide, which can carry large polar molecules including peptides,
oligonucleotides, and protein
across cell membranes. For example, sequences from the HIV Tat protein
(GRI~I~RRQRRRPPQ (SEQ ID NO:18)) and the Drosophila Antennapedia protein
(RQIKIWFQNRRMKWI~I~ (SEQ ID N0:19)) have been found to be capable of
functioning as
delivery peptides. A peptide or peptidomimetic can be encoded by a random
sequence of DNA,
such as a peptide identified from a phage-display library, or one-bead-one-
compound (OBOC)
combinatorial library (Lam et al., Nature, 354:82-84, 1991). Preferably the
peptide or
peptidomimetic tethered to an iRNA agent via an incorporated monomer unit is a
cell targeting
peptide such as an arginine-glycine-aspartic acid (RGD)-peptide, or RGD mimic.
A peptide
~ 5 moiety can range in length from about 5 amino acids to about 40 amino
acids. The peptide
moieties can have a structural modification, such as to increase stability or
direct conformational
properties. Any of the structural modifications described below can be
utilized.
An RGD peptide moiety can be used to target a tumor cell, such as an
endothelial tumor
cell or a breast cancer tumor cell (Zitzmann et al., Cancer Res., 62:5139-43,
2002). An RGD
2o peptide can facilitate targeting of an iRNA agent to tumors of a variety of
other tissues, including
the lung, kidney, spleen, or liver (Aoki et al., Cancer Gene Therapy 8:783-
787, 2001).
Preferably, the RGD peptide will facilitate targeting of an iRNA agent to the
kidney. The RGD
peptide can be linear or cyclic, and can be modified, e.g., glycosylated or
methylated to facilitate
targeting to specific tissues. For example, a glycosylated RGD peptide can
deliver an iRNA
25 agent to a tumor cell expressing aw133 (Haubner et al., Jour. Nucl. Med.,
42:326-336, 2001).
Peptides that target markers enriched in proliferating cells can be used.
E.g., RGD
containing peptides and peptidomimetics can target cancer cells, in particular
cells that exhibit an
a~(33 integrin. Thus, one could use RGD peptides, cyclic peptides containing
RGD, RGD
peptides that include D-amino acids, as well as synthetic RGD mimics. In
addition to RGD, one
3o can use other moieties that target the a~ (33 integrin ligand. Generally,
such ligands can be used
to control proliferating cells and angiogeneis. Preferred conjugates of this
type include an iRNA
agent that targets PECAM-1, VEGF, or other cancer gene, e.g., a cancer gene
described herein.
A "cell permeation peptide" is capable of permeating a cell, e.g., a microbial
cell, such as
a bacterial or fungal cell, or a mammalian cell, such as a human cell. A
microbial cell-
133
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
permeating peptide can be, for example, an a helical linear peptide (e.g., LL-
37 or Ceropin Pl), a
disulfide bond-containing peptide (e.g., a -defensin, ~3-defensin or
bactenecin), or a peptide
containing only one or two dominating amino acids (e.g., PR-39 or
indolicidin). A cell
permeation peptide can also include a nuclear localization signal (NLS). For
example, a cell
permeation peptide can be a bipartite amphipathic peptide, such as MPG, which
is derived from
the fusion peptide domain of HIV-1 gp41 and the NLS of SV40 large T antigen
(Simeoni et al.,
Nucl. Acids Res. 31:2717-2724, 2003).
In one embodiment, a targeting peptide tethered to an RRMS can be an
amphipathic a
helical peptide. Exemplary amphipathic a helical peptides include, but are not
limited to,
cecropins, lycotoxins, paradaxins, buforin, CPF, bombinin-like peptide (BLP),
cathelicidins,
ceratotoxins, S. clava peptides, hagfish intestinal antimicrobial peptides
(HFIAPs), magainines,
brevinins-2, dermaseptins, melittins, pleurocidin, H2A peptides, Xenopus
peptides, esculentinis-
1, and caerins. A number of factors will preferably be considered to maintain
the integrity of
helix stability. For example, a maximum number of helix stabilization residues
will be utilized
~ 5 (e.g., leu, ala, or lys), and a minimum number helix destabilization
residues will be utilized (e.g.,
proline, or cyclic monomeric units. The capping residue will be considered
(for example Gly is
an exemplary N-capping residue and/or C-terminal amidation can be used to
provide an extra H-
bond to stabilize the helix. Formation of salt bridges between residues with
opposite charges,
separated by i ~ 3, or i ~ 4 positions can provide stability. For example,
cationic residues such as
20 lysine, arginine, homo-arginine, ornithine or histidine can form salt
bridges with the anionic
residues glutamate or aspartate.
Peptide and petidomimetic ligands include those having naturally occurnng or
modified
peptides, e.g., D or L peptides; a, (3, or y peptides; N-methyl peptides;
azapeptides; peptides
having one or more amide, i.e., peptide, linkages replaced with one or more
urea, thiourea,
25 carbamate, or sulfonyl urea linkages; or cyclic peptides.
Methods for making iRNA agents
The synthesis and purification of oligonucleotide peptide conjugates can be
performed by
established methods. See, for example, Trufert et al., Tetrahedron, 52:3005,
1996; and
Manoharan, "Oligonucleotide Conjugates in Antisense Technology," in Antisense
Drug
3o Technolo~y, ed. S.T. Crooke, Marcel Dekker, Inc., 2001.
In one embodiment of the invention, a peptidomimetic can be modified to create
a
constrained peptide that adopts a distinct and specific preferred
conformation, which can
increase the potency and selectivity of the peptide. For example, the
constrained peptide can be
an azapeptide (Game, Synthesis, 405-413, 1959). An azapeptide is synthesized
by replacing the
134
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
a carbon of an amino acid with a nitrogen atom without changing the structure
of the amino acid
side chain. For example, the azapeptide can be synthesized by using hydrazine
in traditional
peptide synthesis coupling methods, such as by reacting hydrazine with a
"carbonyl donor," e.g.,
phenylchloroformate.
In one embodiment of the invention, a peptide or peptidomimetic (e.g., a
peptide or
peptidomimetic tethered to an RRMS) can be an N-methyl peptide. N-methyl
peptides are
composed of N-methyl amino acids, which provide an additional methyl group in
the peptide
backbone, thereby potentially providing additional means of resistance to
proteolytic cleavage.
N-methyl peptides can by, synthesized by methods known in the art (see, for
example, Lindgren
et al., Trends Pharmacol. Sci. 21:99, 2000; Cell Penetrating Peptides:
Processes and
Applications, Langel, ed., CRC Press, Boca Raton, FL, 2002; Fische et al.,
Bioconjugate. Chem.
12: 825, 2001; Wander et al., J. Am. Chem. Soc., 124:13382, 2002). For
example, an Ant or Tat
peptide can be an N-methyl peptide.
In one embodiment of the invention, a peptide or peptidomimetic (e.g., a
peptide or
peptidomimetic tethered to an RRMS) can be a ~3-peptide. ~i-peptides form
stable secondary
structures such as helices, pleated sheets, turns and hairpins in solutions.
Their cyclic derivatives
can fold into nanotubes in the solid state. (3-peptides are resistant to
degradation by proteolytic
enzymes. ~3-peptides can be synthesized by methods known in the art. For
example, an Ant or
Tat peptide can be a,~-peptide.
2o In one embodiment of the invention, a peptide or peptidomimetic (e.g., a
peptide or
peptidomimetic tethered to an RRMS) can be a oligocarbamate. Oligocarbamate
peptides are
internalized into a cell by a transport pathway facilitated by carbamate
transporters. For
example, an Ant or Tat peptide can be an oligocarbamate.
In one embodiment of the invention, a peptide or peptidomimetic (e.g., a
peptide or
peptidomimetic tethered to an RRMS) can be an oligourea conjugate (or an
oligothiourea
conjugate), in which the amide bond of a peptidomimetic is replaced with a
urea moiety.
Replacement of the amide bond provides increased resistance to degradation by
proteolytic
enzymes, e.g., proteolytic enzymes in the gastrointestinal tract. In one
embodiment, an oligourea
conjugate is tethered to an iRNA agent for use in oral delivery. The backbone
in each repeating
3o unit of an oligourea peptidomimetic can be extended by one carbon atom in
comparison with the
natural amino acid. The single carbon atom extension can increase peptide
stability and
lipophilicity, for example. An oligourea peptide can therefore be advantageous
when an iRNA
agent is directed for passage through a bacterial cell wall, or when an iRNA
agent must traverse
the blood-brain barrier, such as for the treatment of a neurological disorder.
In one embodiment,
135
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
a hydrogen bonding unit is conjugated to the oligourea peptide, such as to
create an increased
affinity with a receptor. For example, an Ant or Tat peptide can be an
oligourea conjugate (or an
oligothiourea conjugate).
The siRNA peptide conjugates of the invention can be affiliated with, e.g.,
tethered to,
RRMSs occurnng at various positions on an iRNA agent. For example, a peptide
can be
terminally conjugated, on either the sense or the antisense strand, or a
peptide can be
bisconjugated (one peptide tethered to each end, one conjugated to the sense
strand, and one
conjugated to the antisense strand). In another option, the peptide can be
internally conjugated,
such as in the loop of a short hairpin iRNA agent. In yet another option, the
peptide can be
affiliated with a complex, such as a peptide-carrier complex.
A peptide-carrier complex consists of at least a Garner molecule, which can
encapsulate
one or more iRNA agents (such as for delivery to a biological system and/or a
cell), and a
peptide moiety tethered to the outside of the carrier molecule, such as for
targeting the carrier
complex to a particular tissue or cell type. A carrier complex can carry
additional targeting
~ 5 molecules on the exterior of the complex, or fusogenic agents to aid in
cell delivery. The one or
more iRNA agents encapsulated within the carrier can be conjugated to
lipophilic molecules,
which can aid in the delivery of the agents to the interior of the carrier.
A Garner molecule or structure can be, for example, a micelle, a liposome
(e.g., a cationic
liposome), a nanoparticle, a microsphere, or a biodegradable polymer. A
peptide moiety can be
2o tethered to the carrier molecule by a variety of linkages, such as a
disulfide linkage, an acid
labile linkage, a peptide-based linkage, an oxyamino linkage or a hydrazine
linkage. 'For
example, a peptide-based linkage can be a GFLG peptide. Certain linkages will
have particular
advantages, and the advantages (or disadvantages) can be considered depending
on the tissue
target or intended use. For example, peptide based linkages are stable in the
blood stream but are
25 susceptible to enzymatic cleavage in the lysosomes.
Tar eg ting
The iRNA agents of the invention are particularly useful when targeted to the
kidney. An
iRNA agent can be targeted to the kidney by incorporation of an RRMS
containing a ligand that
targets the kidney.
3o A targeting agent that incorporates a sugar, e.g., galactose and/or
analogues thereof, can
be useful. These agents target, for example, the parenchymal cells of the
liver. For example, a
targeting moiety can include more than one or preferably two or three
galactose moieties, spaced
about 15 angstroms from each other. The targeting moiety can alternatively be
lactose (e.g.,
three lactose moieties), which is glucose coupled to a galactose. The
targeting moiety can also
136
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
be N-Acetyl-Galactosamine, N-Ac-Glucosamine. A mannose or mannose-6-phosphate
targeting
moiety can be used for macrophage targeting.
Conjugation of an iRNA agent with a serum albumin (SA), such as human serum
albumin, can also be used to target the iRNA agent to a non-kidney tissue,
such as the liver.
An iRNA agent targeted to the kidney by an RRMS targeting moiety described
herein can
target a gene expressed in the kidney.
Ligands on R.RMSs can include folic acid, glucose, cholesterol, cholic acid,
Vitamin E,
Vitamin K, or Vitamin A.
Palindromes
The monomers and methods described herein can be used to prepare an RNA, e.g.,
an
iRNA agent, having a palindrome structure as described herein and those
described in one or
more of United States Provisional Application Serial No. 60/452,682, filed
March 7, 2003;
United States Provisional Application Serial No. 60/462,894, filed April
14,2003; and
International Application No. PCT/LTS04/07070, filed March 8, 2004, all of
which are hereby
~ 5 incorporated by reference. The iRNA agents of the invention can target
more than one RNA
region. For example, an iRNA agent can include a first and second sequence
that are sufficiently
complementary to each other to hybridize. The first sequence can be
complementary to a first
target RNA region and the second sequence can be complementary to a second
target RNA
region. The first and second sequences of the iRNA agent can be on different
RNA strands, and
2o the mismatch between the first and second sequences can be less than 50%,
40%, 30%, 20%,
10%, 5%, or 1%. The first and second sequences of the iRNA agent are on the
same RNA
strand, and in a related embodiment more than 50%, 60%, 70%, 80%, 90%, 95%, or
1% of the
iRNA agent can be in bimolecular form. The first and second sequences of the
iRNA agent can
be fully complementary to each other.
25 The first target RNA region can be encoded by a first gene and the second
target RNA
region can encoded by a second gene, or the first and second target RNA
regions can be different
regions of an RNA from a single gene. The first and second sequences can
differ by at least 1
nucleotide.
The first and second target RNA regions can be on transcripts encoded by first
and
3o second sequence variants, e.g., first and second alleles, of a gene. The
sequence variants can be
mutations, or polymorphisms, for example. The first target RNA region can
include a nucleotide
substitution, insertion, or deletion relative to the second target RNA region,
or the second target
RNA region can a mutant or variant of the first target region.
137
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
The first and second target RNA regions can comprise viral or human RNA
regions. The
first and second target RNA regions can also be on variant transcripts of an
oncogene or include
different mutations of a tumor suppressor gene transcript. In addition, the
first and second target
RNA regions can correspond to hot-spots for genetic variation.
The compositions of the invention can include mixtures of iRNA agent
molecules. For
example, one iRNA agent can contain a first sequence and a second sequence
sufficiently
complementary to each other to hybridize, and in addition the first sequence
is complementary to
a first target RNA region and the second sequence is complementary to a second
target RNA
region. The mixture can also include at least one additional iRNA agent
variety that includes a
1 o third sequence and a fourth sequence sufficiently complementary to each
other to hybridize, and
where the third sequence is complementary to a third target RNA region and the
fourth sequence
is complementary to a fourth target RNA region. In addition, the first or
second sequence can be
sufficiently complementary to the third or fourth sequence to be capable of
hybridizing to each
other. The first and second sequences can be on the same or different RNA
strands, and the third
~ 5 and fourth sequences can be on the same or different RNA strands.
The target RNA regions can be variant sequences of a viral or human RNA, and
in
certain embodiments, at least two of the target RNA regions can be on variant
transcripts of an
oncogene or tumor suppressor gene. The target RNA regions can correspond to
genetic hot-
spots.
2o Methods of making an iRNA agent composition can include obtaining or
providing
information about a region of an RNA of a target gene (e.g., a viral or human
gene, or an
oncogene or tumor suppressor, e.g., p53), where the region has high
variability or mutational
frequency (e.g., in humans). In addition, information about a plurality of RNA
targets within the
region can be obtained, or provided, where each RNA target corresponds to a
different variant or
25 mutant of the gene (e.g., a region including the codon encoding p53 248Q
and/or p53 2495).
The iRNA agent can be constructed such that a first sequence is complementary
to a first of the
plurality of variant RNA targets (e.g., encoding 249Q) and a second sequence
is complementary
to a second of the plurality of variant RNA targets (e.g., encoding 2495), and
the first and second
sequences can be sufficiently complementary to hybridize.
so Sequence analysis, e.g., to identify common mutants in the target gene, can
be used to
identify a region of the target gene that has high variability or mutational
frequency. A region of
the target gene having high variability or mutational frequency can be
identified by obtaining or
providing genotype information about the target gene from a population.
138
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Expression of a target gene can be modulated, e.g., downregulated or silenced,
by
providing an iRNA agent that has a first sequence and a second sequence
sufficiently
complementary to each other to hybridize. In addition, the first sequence can
be complementary
to a first target RNA region and the second sequence can be complementary to a
second target
RNA region.
An iRNA agent can include a first sequence complementary to a first variant
RNA target
region and a second sequence complementary to a second variant RNA target
region. The first
and second variant RNA target regions can correspond to first and second
variants or mutants of
a target gene, e.g., viral gene, tumor suppressor or oncogene. The first and
second variant target
RNA regions can include allelic variants, mutations (e.g., point mutations),
or polymorphisms of
the target gene. The first and second variant RNA target regions can
correspond to genetic hot-
spots.
A plurality of iRNA agents (e.g., a panel or bank) can be provided.
Other than Canonical Watson-Crick Duplex Structures
The monomers and methods described herein can be used to prepare an RNA, e.g.,
an
iRNA agent, having monomers which can form other than a canonical Watson-Crick
pairing
with another monomer, e.g., a monomer on another strand, such as those
described herein and
those described in United States Provisional Application Serial No.
60/465,665, filed April 25,
2003, and International Application No. PCT/LTS04/07070, filed March 8, 2004,
both of which
are hereby incorporated by reference.
The use of "other than canonical Watson-Crick pairing" between monomers of a
duplex
can be used to control, often to promote, melting of all or part of a duplex.
The iRNA agent can
include a monomer at a selected or constrained position that results in a
first level of stability in
the iRNA agent duplex (e.g., between the two separate molecules of a double
stranded iRNA
agent) and a second level of stability in a duplex between a sequence of an
iRNA agent and
another sequence molecule, e.g., a target or off target sequence in a subject.
In some cases the
second duplex has a relatively greater level of stability, e.g., in a duplex
between an anti-sense
sequence of an iRNA agent and a target mRNA. In this case one or more of the
monomers, the
3o position of the monomers in the iRNA agent, and the target sequence
(sometimes referred to
herein as the selection or constraint parameters), are selected such that the
iRNA agent duplex is
has a comparatively lower free energy of association (which while not wishing
to be bound by
mechanism or theory, is believed to contribute to efficacy by promoting
disassociation of the
duplex iRNA agent in the context of the RISC) while the duplex formed between
an anti-sense
139
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
targeting sequence and its target sequence, has a relatively higher free
energy of association
(which while not wishing to be bound by mechanism or theory, is believed to
contribute to
efficacy by promoting association of the anti-sense sequence and the target
RNA).
In other cases the second duplex has a relatively lower level of stability,
e.g., in a duplex
between a sense sequence of an iRNA agent and an off target mRNA. In this case
one or more
of the monomers, the position of the monomers in the iRNA agent, and an off
target sequence,
are selected such that the iRNA agent duplex is has a comparatively higher
free energy of
association while the duplex formed between a sense targeting sequence and its
off target
sequence, has a relatively lower free energy of association (which while not
wishing to be bound
by mechanism or theory, is believed to reduce the level of off target
silencing by contribute to
efficacy by promoting disassociation of the duplex formed by the sense strand
and the off target
sequence). .
Thus, inherent in the structure of the iRNA agent is the property of having a
first stability
for the infra-iRNA agent duplex and a second stability for a duplex formed
between a sequence
from the iRNA agent and another RNA, e.g., a target mRNA. As discussed above,
this can be
accomplished by judicious selection of one or more of the monomers at a
selected or constrained
position, the selection of the position in the duplex to place the selected or
constrained position,
and selection of the sequence of a taxget sequence (e.g., the particular
region of a target gene
which is to be targeted). The iRNA agent sequences which satisfy these
requirements are
2o sometimes referred herein as constrained sequences. Exercise of the
constraint or selection
parameters can e, e.g., by inspection, or by computer assisted methods.
Exercise of the
parameters can result in selection of a target sequence and of particular
monomers to give a
desired result in terms of the stability, or relative stability, of a duplex.
Thus, in another aspect, the invention features, an iRNA agent which includes:
a first
sequence which targets a first target region and a second sequence which
targets a second target
region. The first and second sequences have sufficient complementarity to each
other to
hybridize, e.g., under physiological conditions, e.g., under physiological
conditions but not in
contact with a helicase or other unwinding enzyme. In a duplex region of the
iRNA agent, at a
selected or constrained position, the first target region has a first monomer,
and the second target
3o region has a second monomer. The first and second monomers occupy
complementary or
corresponding positions. One, and preferably both monomers are selected such
that the stability
of the pairing of the monomers contribute to a duplex between the first and
second sequence will
differ form the stability of the pairing between the first or second sequence
with a target
sequence.
140
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Usually, the monomers will be selected (selection of the target sequence may
be required
as well) such that they form a pairing in the iRNA agent duplex which has a
lower free energy of
dissociation, and a lower Tm, than will be possessed by the paring of the
monomer with its
complementary monomer in a duplex between the iRNA agent sequence and a target
RNA
s duplex.
The constraint placed upon the monomers can be applied at a selected site or
at
more than one selected site. By way of example, the constraint can be applied
at more than 1,
but less than 3, 4, 5, 6, or 7 sites in an iRNA agent duplex.
A constrained or selected site can be present at a number of positions in the
iRNA
agent duplex. E.g., a constrained or selected site can be present within 3, 4,
5, or 6 positions
from either end, 3' or 5' of a duplexed sequence. A constrained or selected
site can be present in
the middle of the duplex region, e.g., it can be more than 3, 4, 5, or 6,
positions from the end of a
duplexed region.
In some embodiment the duplex region of the iRNA agent will have, mismatches,
in
~5 addition to the selected or constrained site or sites. Preferably it will
have no more than l, 2, 3,
4, or 5 bases, which do not form canonical Watson-Crick pairs or which do not
hybridize.
Overhangs are discussed in detail elsewhere herein but are preferably about 2
nucleotides in
length. The overhangs can be complementary to the gene sequences being
targeted or can be
other sequence. TT is a preferred overhang sequence. The first and second iRNA
agent
2o sequences can also be joined, e.g., by additional bases to form a hairpin,
or by other non-base
linkers.
The monomers can be selected such that: first and second monomers are
naturally
occurnng ribonuceotides, or modified ribonucleotides having naturally occurnng
bases, and
when occupying complemetary sites either do not pair and have no substantial
level of H-
25 bonding, or form a non canonical Watson-Crick pairing and form a non-
canonical pattern of H
bonding, which usually have a lower free energy of dissociation than seen in a
canonical
Watson-Crick pairing, or otherwise pair to give a free energy of association
which is less than
that of a preselected value or is less, e.g., than that of a canonical
pairing. When one (or both) of
the iRNA agent sequences duplexes with a target, the first (or second) monomer
forms a
3o canonical Watson-Crick pairing with the base in the complemetary position
on the target, or
forms a non canonical Watson-Crick pairing having a higher free energy of
dissociation and a
higher Tm than seen in the paring in the iRNA agent. The classical Watson-
Crick parings are as
follows: A-T, G-C, and A-U. Non-canonical Watson-Crick pairings are known in
the art and
can include, U-U, G-G, G-At,.ans, G-A~;S, and GU.
141
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
The monomer in one or both of the sequences is selected such that, it does not
pair, or
forms a pair with its corresponding monomer in the other sequence which
mininuzes stability
(e.g., the H bonding formed between the monomer at the selected site in the
one sequence and its
monomer at the corresponding site in the other sequence are less stable than
the H bonds formed
by the monomer one (or both) of the sequences with the respective target
sequence. The
monomer is one or both strands is also chosen to promote stability in one or
both of the duplexes
made by a strand and its target sequence. E.g., one or more of the monomers
and the target
sequences are selected such that at the selected or constrained position,
there is are no H bonds
formed, or a non canonical pairing is formed in the iRNA agent duplex, or
otherwise they
otherwise pair to give a free energy of association which is less than that of
a preselected value
or is less, e.g., than that of a canonical pairing, but when one ( or both)
sequences form a duplex
with the respective target, the pairing at the selected or constrained site is
a canonical Watson-
Crick paring.
The inclusion of such a monomers will have one or more of the following
effects: it will
~ 5 destabilize the iRNA agent duplex, it will destabilize interactions
between the sense sequence
and unintended target sequences, sometimes referred to as off target
sequences, and duplex
interactions between the a sequence and the intended target will not be
destabilized.
By way of example:
The monomer at the selected site in the first sequence includes an A (or a
modified base
2o which pairs with T), and the monomer in at the selected position in the
second sequence is
chosen from a monomer which will not pair or which will form a non-canonical
pairing, e.g., G.
These will be useful in applications wherein the target sequence for the first
sequence has a T at
the selected position. In embodiments where both target duplexes axe
stabilized it is useful
wherein the target sequence for the second strand has a monomer which will
form a canonical
25 Watson-Crick pairing with the monomer selected for the selected position in
the second strand.
The monomer at the selected site in the first sequence includes U (or a
modified base
which pairs with A), and the monomer in at the selected position in the second
sequence is
chosen from a monomer which will not pair or which will form a non-canonical
pairing, e.g., U
or G. These will be useful in applications wherein the target sequence for the
first sequence has
3o a T at the selected position. In embodiments where both target duplexes are
stabilized it is useful
wherein the target sequence for the second strand has a monomer which will
form a canonical
Watson-Crick pairing with the monomer selected for the selected position in
the second strand.
The monomer at the selected site in the first sequence includes a G (or a
modified base
which pairs with C), and the monomer in at the selected position in the second
sequence is
142
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
chosen from a monomer which will not pair or which will form a non-canonical
pairing, e.g., G,
A~,S, Atta"S, or U. These will be useful in applications wherein the target
sequence for the first
sequence has a T at the selected position. In embodiments where both target
duplexes are
stabilized it is useful wherein the target sequence for the second strand has
a monomer which
will form a canonical Watson-Crick pairing with the monomer selected for the
selected position
in the second strand.
The monomer at the selected site in the first sequence includes a C (or a
modified base
which pairs with G), and the monomer in at the selected position in the second
sequence is
chosen a monomer which will not pair or which will form a non-canonical
pairing. These will be
1 o useful in applications wherein the target sequence for the first sequence
has a T at the selected
position. In embodiments where both target duplexes are stabilized it is
useful wherein the target
sequence for the second strand has a monomer which will form a canonical
Watson-Crick
pairing with the monomer selected for the selected position in the second
strand.
A non-naturally occurring or modified monomer or monomers can be chosen such
that
~ 5 when a non-naturally occurring or modified monomer occupies a positions at
the selected or
constrained position in an iRNA agent they exhibit a first free energy of
dissociation and when
one (or both) of them pairs with a naturally occurring monomer, the pair
exhibits a second free
energy of dissociation, which is usually higher than that of the pairing of
the first and second
monomers. E.g., when the first and second monomers occupy complementary
positions they
2o either do not pair and have no substantial level of H-bonding, or form a
weaker bond than one of
them would form with a naturally occurring monomer, and reduce the stability
of that duplex, but
when the duplex dissociates at least one of the strands will form a duplex
with a taxget in which
the selected monomer will promote stability, e.g., the monomer will form a
more stable pair with
a naturally occurring monomer in the target sequence than the pairing it
formed in the iRNA
25 agent.
An example of such a pairing is 2-amino A and either of a 2-thio pyrimidine
analog of U
or T.
When placed in complementary positions of the iRNA agent these monomers will
pair
very poorly and will minimize stability. However, a duplex is formed between 2
amino A and
30 ~ the U of a naturally occurring target, or a duplex is between 2-thio U
and the A of a naturally
occurring target or 2-thio T and the A of a naturally occurring target will
have a relatively higher
free energy of dissociation and be more stable. This is shown in the FIG. 6.
The pair shown in FIG. 6 (the 2-amino A and the 2-s U and T) is exemplary. In
another
embodiment, the monomer at the selected position in the sense strand can be a
universal pairing
143
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
moiety. A muversal pairing agent will form some level of H bonding with more
than one and
preferably all other naturally occurring monomers. An examples of a universal
pairing moiety is
a monomer which includes 3-nitro pyrrole. (Examples of other candidate
universal base analogs
can be found in the art, e.g., in Loakes, 2001, NAR 29: 2437-2447, hereby
incorporated by
reference. Examples can also be found in the section on Universal Bases
below.) In these cases
the monomer at the corresponding position of the anti-sense strand can be
chosen for its ability to
form a duplex with the target and can include, e.g., A, U, G, or C.
iRNA agents of the invention can include:
A sense sequence, which preferably does not target a sequence in a subject,
and an anti-
sense sequence, which targets a target gene in a subject. The sense and anti-
sense sequences
have sufficient complementarity to each other to hybridize hybridize, e.g.,
under physiological
conditions, e.g., under physiological conditions but not in contact with a
helicase or other
unwinding enzyme. In a duplex region of the iRNA agent, at a selected or
constrained position,
the monomers are selected such that:
~ 5 The monomer in the sense sequence is selected such that, it does not pair,
or forms a pair
with its corresponding monomer in the anti-sense strand which minimizes
stability (e.g., the H
bonding formed between the monomer at the selected site in the sense strand
and its monomer at
the corresponding site in the anti-sense strand are less stable than the H
bonds formed by the
monomer of the anti-sense sequence and its canonical Watson-Crick partner or,
if the monomer
2o in the anti-sense strand includes a modified base, the natural analog of
the modified base and its
canonical Watson-Crick partner);
The monomer is in the corresponding position in the anti-sense strand is
selected such
that it maximizes the stability of a duplex it forms with the target sequence,
e.g., it forms a
canonical Watson-.Crick paring with the monomer in the corresponding position
on the target
25 stand;
Optionally, the monomer in the sense sequence is selected such that, it does
not pair, or
forms a pair with its corresponding monomer in the anti-sense strand which
minimizes stability
with an off target sequence.
The inclusion of such a monomers will have one or more of the following
effects: it will
3o destabilize the iRNA agent duplex, it will destabilize interactions between
the sense sequence
and unintended target sequences, sometimes referred to as off target
sequences, and duplex
interactions between the anti-sense strand and the intended target will not be
destabilized.
144
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
The constraint placed upon the monomers can be applied at a selected site or
at more than
one selected site. By way of example, the constraint can be applied at more
than 1, but less than
3, 4, 5, 6, or 7 sites in an iRNA agent duplex.
A constrained or selected site can be present at a number of positions in the
iRNA agent
duplex. E.g., a constrained or selected site can be present within 3, 4, 5, or
6 positions from
either end, 3' or 5' of a duplexed sequence. A constrained or selected site
can be present in the
middle of the duplex region, e.g., it can be more than 3, 4, 5, or 6,
positions from the end of a
duplexed region.
In some embodiment the duplex region of the iRNA agent will have, mismatches,
in
addition to the selected or constrained site or sites. Preferably it will have
no more than 1, 2, 3,
4, or 5 bases, which do not form canonical Watson-Crick pairs or which do not
hybridize.
Overhangs are discussed in detail elsewhere herein but are preferably about 2
nucleotides in
length. The overhangs can be complementary to the gene sequences being
targeted or can be
other sequence. TT is a preferred overhang sequence. The first and second iRNA
agent
~5 sequences can also be joined, e.g., by additional bases to form a hairpin,
or by other non-base
linkers.
The monomers can be selected such that: first and second monomers are
naturally
occurring ribonuceotides, or modified ribonucleotides having naturally
occurring bases, and
when occupying complemetary sites either do not pair and have no substantial
level of H-
2o bonding, or form a non canonical Watson-Crick pairing and form a non-
canonical pattern of H
bonding, which usually have a lower free energy of dissociation than seen in a
canonical
Watson-Crick pairing, or otherwise pair to give a free energy of association
which is less than
that of a preselected value or is less, e.g., than that of a canonical
pairing. When one (or both) of
the iRNA agent sequences duplexes with a target, the first (or second) monomer
forms a
25 canonical Watson-Crick pairing with the base in the complemetary position
on the target, or
forms a non canonical Watson-Crick pairing having a higher free energy of
dissociation and a
higher Tm than seen in the paring in the iRNA agent. The classical Watson-
Crick parings are as
follows: A-T, G-C, and A-U. Non-canonical Watson-Crick pairings axe known in
the art and
can include, U-U, G-G, G-Atta"S, G-A~;S, and GU.
3o The monomer in one or both of the sequences is selected such that, it does
not pair, or
forms a pair with its corresponding monomer in the other sequence which
minimizes stability
(e.g., the H bonding formed between the monomer at the selected site in the
one sequence and its
monomer at the corresponding site in the other sequence axe less stable than
the H bonds formed
by the monomer one (or both) of the sequences with the respective target
sequence. The
145
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
monomer is one or both strands is also chosen to promote stability in one or
both of the duplexes
made by a strand and its target sequence. E.g., one or more of the monomers
and the target
sequences are selected such that at the selected or constrained position,
there is are no H bonds
formed, or a non canonical pairing is formed in the iRNA agent duplex, or
otherwise they
s otherwise pair to give a free energy of association which is less than that
of a preselected value
or is less, e.g., than that of a canonical pairing, but when one (or both)
sequences form a duplex
with the respective target, the pairing at the selected or constrained site is
a canonical Watson-
Crick paring.
The inclusion of such a monomers will have one or more of the following
effects: it will
destabilize the iRNA agent duplex, it will destabilize interactions between
the sense sequence
and unintended target sequences, sometimes referred to as off target
sequences, and duplex ~
interactions between the a sequence and the intended target will not be
destabilized.
By way of example:
The monomer at the selected site in the first sequence includes an A (or a
modified base
~5 which pairs with T), and the monomer in at the elected position in the
second sequence is
chosen from a monomer which will not pair or which will form a non-canonical
pairing, e.g., G.
These will be useful in applications wherein the target sequence for the first
sequence has a T at
the selected position. In embodiments where both target duplexes axe
stabilized it is useful
wherein the target sequence for the second strand has a monomer which will
form a canonical
2o Watson-Crick pairing with the monomer selected for the selected position in
the second strand.
The monomer at the selected site in the first sequence includes U (or a
modified base
which pairs with A), and the monomer in at the selected position in the second
sequence is
chosen from a monomer which will not pair or which will form a non-canonical
pairing, e.g., U
or G. These will be useful in applications wherein the target sequence for the
first sequence has
25 a T at the selected position. In embodiments where both target duplexes are
stabilized it is useful
wherein the target sequence for the second strand has a monomer which will
form a canonical
Watson-Crick pairing with the monomer selected for the selected position in
the second strand.
The monomer at the selected site in the first sequence includes a G (or a
modified base
which pairs with C), and the monomer in at the selected position in the second
sequence is
3o chosen from a monomer which will not pair or which will form a non-
canonical pairing, e.g., G,
A~;S, Ana"S, or U. These will be useful in applications wherein the target
sequence for the first
sequence has a T at the selected position. In embodiments where both target
duplexes are
stabilized it is useful wherein the target sequence for the second strand has
a monomer which
146
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
will form a canonical Watson-Crick pairing with the monomer selected for the
selected position
in the second strand.
The monomer at the selected site in the first sequence includes a C (or a
modified base
which pairs with G), and the monomer in at the selected position in the second
sequence is
chosen a monomer which will not pair or which will form a non-canonical
pairing. These will be
useful in applications wherein the target sequence for the first sequence has
a T at the selected
position. In embodiments where both target duplexes are stabilized it is
useful wherein the target
sequence for the second strand has a monomer which will form a canonical
Watson-Crick
pairing with the monomer selected for the selected position in the second
strand.
A non-naturally occurring or modified monomer or monomers can be chosen such
that
when a non-naturally occurring or modified monomer occupies a positions at the
selected or
constrained position in an iRNA agent they exhibit a first free energy of
dissociation and when
one (or both) of them pairs with a naturally occurring monomer, the pair
exhibits a second free
energy of dissociation, which is usually higher than that of the pairing of
the first and second
~ 5 monomers. E.g., when the first and second monomers occupy complementary
positions they
either do not pair and have no substantial level of H-bonding, or form a
weaker bond than one of
them would form with a naturally occurring monomer, and reduce the stability
of that duplex, but
when the duplex dissociates at least one of the strands will form a duplex
with a target in which
the selected monomer will promote stability, e.g., the monomer will form a
more stable pair with
2o a naturally occurnng monomer in the target sequence than the pairing it
formed in the iRNA
agent.
An example of such a pairing is 2-amino A and either of a 2-thio pyrimidine
analog of U
or T.
When placed in complementary positions of the iRNA agent these monomers will
pair
25 very poorly and will minimize stability. However, a duplex is formed
between 2 amino A and
the U of a naturally occurring target, or a duplex is between 2-thio U and the
A of a naturally
occurring target or 2-thio T and the A of a naturally occurring target will
have a relatively higher
free energy of dissociation and be more stable.
The monomer at the selected position in the sense strand can be a universal
pairing
3o moiety. A universal pairing agent will form some level of H bonding with
more than one and
preferably all other naturally occurring monomers. An examples of a universal
pairing moiety is
a monomer which includes 3-nitro pyrrole. (Examples of other candidate
universal base analogs
can be found in the art, e.g., in Loakes, 2001, NAR 29: 2437-2447, hereby
incorporated by
reference. Examples can also be found in the section on Universal Bases
below.) In these cases
147
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
the monomer at the corresponding position of the anti-sense strand can be
chosen for its ability to
form a duplex with the target and can include, e.g., A, U, G, or C.
iRNA agents of the invention can include:
A sense sequence, which preferably does not target a sequence in a subject,
and an anti-
s sense sequence, which targets a target gene in a subject. The sense and anti-
sense sequences
have sufficient complementarity to each other to hybridize hybridize, e.g.,
under physiological
conditions, e.g., under physiological conditions but not in contact with a
helicase or other
unwinding enzyme. In a duplex region of the iRNA agent, at a selected or
constrained position,
the monomers are selected such that:
The monomer in the sense sequence is selected such that, it does not pair, or
forms a pair
with its corresponding monomer in the anti-sense strand which minimizes
stability (e.g., the H
bonding formed between the monomer at the selected site in the sense strand
and its monomer at
the corresponding site in the anti-sense strand are less stable than the H
bonds formed by the
monomer of the anti-sense sequence and its canonical Watson-Crick partner or,
if the monomer
15 in the anti-sense strand includes a modified base, the natural analog of
the modified base and its
canonical Watson-Crick partner);
The monomer is in the corresponding position in the anti-sense strand is
selected such
that it maximizes the stability of a duplex it forms with the target sequence,
e.g., it forms a
canonical Watson-Crick paring with the monomer in the corresponding position
on the target
2o stand;
Optionally, the monomer in the sense sequence is selected such that, it does
not pair, or
forms a pair with its corresponding monomer in the anti-sense strand which
minimizes stability
with an off target sequence.
The inclusion of such a monomers will have one or more of the following
effects: it will
25 destabilize the iRNA agent duplex, it will destabilize interactions between
the sense sequence
and unintended target sequences, sometimes referred to as off target
sequences, and duplex
interactions between the anti-sense strand and the intended target will not be
destabilized.
The constraint placed upon the monomers can be applied at a selected site or
at
more than one selected site. By way of example, the constraint can be applied
at more than 1,
3o but less than 3, 4, 5, 6, or 7 sites in an iRNA agent duplex.
A constrained or selected site can be present at a number of positions in the
iRNA
agent duplex. E.g., a constrained or selected site can be present within 3, 4,
5, or 6 positions
from either end, 3' or 5' of a duplexed sequence. A constrained or selected
site can be present in
148
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
the middle of the duplex region, e.g., it can be more than 3, 4, 5, or 6,
positions from the end of a
duplexed region.
The iRNA agent can be selected to target a broad spectrum of genes, including
any of the genes described herein.
In a preferred embodiment the iRNA agent has an architecture (architecture
refers
to one or more of overall length, length of a duplex region, the presence,
number, location, or
length of overhangs, sing strand versus double strand form) described herein.
E.g., the iRNA agent can be less than 30 nucleotides in length, e.g., 21-23
nucleotides.
Preferably, the iRNA is 21 nucleotides in length and there is a duplex region
of about 19 pairs.
In one embodiment, the iRNA is 21 nucleotides in length, and the duplex region
of the iRNA is
19 nucleotides. In another embodiment, the iRNA is greater than 30 nucleotides
in length.
In some embodiment the duplex region of the iRNA agent will have, mismatches,
in
addition to the selected or constrained site or sites. Preferably it will have
no more than 1, 2, 3,
4, or 5 bases, which do not form canonical Watson-Crick pairs or which do not
hybridize.
~ 5 Overhangs are discussed in detail elsewhere herein but are preferably
about 2 nucleotides in
length. The overhangs can be complementary to the gene sequences being
targeted or can be
other sequence. TT is a preferred overhang sequence. The first and second iRNA
agent
sequences can also be joined, e.g., by additional bases to form a hairpin, or
by other non-base
linkers.
2o One or more selection or constraint parameters can be exercised such that:
monomers at
the selected site in the sense and anti-sense sequences are both naturally
occurring
ribonucleotides, or modified ribonucleotides having naturally occurring bases,
and when
occupying complementary sites in the iRNA agent duplex either do not pair and
have no
substantial level of H-bonding, or form a non-canonical Watson-Crick pairing
and thus form a
25 non-canonical pattern of H bonding, which generally have a lower free
energy of dissociation
than seen in a Watson-Crick pairing, or otherwise pair to give a free energy
of association which
is less than that of a preselected value or is less, e.g., than that of a
canonical pairing. When one,
usually the anti-sense sequence of the iRNA agent sequences forms a duplex
with another
sequence, generally a sequence in the subject, and generally a target
sequence, the monomer
so forms a classic Watson-Crick pairing with the base in the complementary
position on the target,
or forms a non-canonical Watson-Crick pairing having a higher free energy of
dissociation and a
higher Tm than seen in the paring in the iRNA agent. Optionally, when the
other sequence of the
iRNA agent, usually the sense sequences forms a duplex with another sequence,
generally a
sequence in the subj ect, and generally an off target sequence, the monomer
fails to forms a
149
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
canonical Watson-Crick pairing with the base in the complementary position on
the off target
sequence, e.g., it forms or forms a non-canonical Watson-Crick pairing having
a lower free
energy of dissociation and a lower Tm.
By way of example:
s the monomer at the selected site in the anti-sense stand includes an A (or a
modified base
which pairs with T), the corresponding monomer in the target is a T, and the
sense strand is
chosen from a base which will not pair or which will form a noncanonical pair,
e.g., G;
the monomer at the selected site in the anti-sense stand includes a U (or a
modified base
which pairs with A), the corresponding monomer in the target is an A, and the
sense strand is
chosen from a monomer which will not pair or which will form a non-canonical
pairing, e.g., U
or G;
the monomer at the selected site in the anti-sense stand includes a C (or a
modified base
which pairs with G), the corresponding monomer in the target is a G, and the
sense strand is
chosen a monomer which will not pair or which will form a non-canonical
pairing, e.g., G, A~;S,
15 Atrans~ or U; or
the monomer at the selected site in the anti-sense stand includes a G (or a
modified base
which pairs with C), the corresponding monomer in the target is a C, and the
sense strand is
chosen from a monomer which will not pair or which will form a non-canonical
pairing.
In another embodiment a non-naturally occurring or modified monomer or
monomers is
2o chosen such that when it occupies complementary a position in an iRNA agent
they exhibit a
first free energy of dissociation and when one (or both) of them pairs with a
naturally occurnng
monomer, the pair exhibits a second free energy of dissociation, which is
usually higher than that
of the pairing of the first and second monomers. E.g., when the first and
second monomers .
occupy complementary positions they either do not pair and have no substantial
level of H-
25 bonding, or form a weaker bond than one of them would form with a naturally
occurring
monomer, and reduce the stability of that duplex, but when the duplex
dissociates at least one of
the strands will form a duplex with a target in which the selected monomer
will promote
stability, e.g., the monomer will form a more stable pair with a naturally
occurring monomer in
the target sequence than the pairing it formed in the iRNA agent.
3o An example of such a pairing is 2-amino A and either of a 2-thio pyrimidine
analog of U
or T. As is discussed above, when placed in complementary positions of the
iRNA agent these
monomers will pair very poorly and will minimize stability. However, a duplex
is formed
between 2 amino A and the U of a naturally occurring target, or a duplex is
formed between 2-
lso
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
thio U and the A of a naturally occurnng target or 2-thio T and the A of a
naturally occurring
target will have a relatively higher free energy of dissociation and be more
stable.
The monomer at the selected position in the sense strand can be a universal
pairing
moiety. A universal pairing agent will form some level of H bonding with more
than one and
preferably all other naturally occurring monomers. An examples of a universal
pairing moiety is
a monomer which includes 3-vitro pyrrole. Examples of other candidate
universal base analogs
can be found in the art, e.g., in Loakes, 2001, NAR 29: 2437-2447, hereby
incorporated by
reference. In these cases the monomer at the corresponding position of the
anti-sense strand can
be chosen for its ability to form a duplex with the target and can include,
e.g., A, U, G, or C.
In another aspect, the invention features, an iRNA agent which includes:
a sense sequence, which preferably does not target a sequence in a subject,
and an anti-
sense sequence, which targets a plurality of target sequences in a subject,
wherein the targets
differ in sequence at only 1 or a small number, e.g., no more than 5, 4, 3 or
2 positions. The
sense and anti-sense sequences have sufficient complementarity to each other
to hybridize, e.g.,
~ 5 under physiological conditions, e.g., under physiological conditions but
not in contact with a
helicase or other unwinding enzyme. In the sequence of the anti-sense strand
of the iRNA agent
is selected such that at one, some, or all of the positions which correspond
to positions that
differe in sequence between the target sequences, the anti-sense strand will
include a monomer
which will form H-bonds with at least two different target sequences. In a
preferred example the,
2o anti-sense sequence will include a universal or promiscuous monomer, e.g.,
a monomer which
includes 5-vitro pyrrole, 2-amino A, 2-thio U or 2-thio T, or other universal
base referred to
herein.
In a preferred embodiment the iRNA agent targets repeated sequences (which
differ-at
only one or a small number of positions from each other) in a single gene, a
plurality of genes, or
25 a viral genome, e.g., the HCV genome.
An embodiment is illustrated in the FIGs. 7 and 8.
In another aspect, the invention features, determining, e.g., by measurement
or
calculation, the stability of a pairing between monomers at a selected or
constrained positoin in
the iRNA agent duplex, and preferably determining the stability for the
corresponding pairing in
3o a duplex between a sequence form the iRNA agent and another RNA, e.g., a
taret sequence. The
determinations can be compared. An iRNA agent thus analysed can be used in the
devolopement
of a further modified iRNA agent or can be administered to a subject. This
analysis can be
performed successively to refine or desing optimized iRNA agents.
151
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
In another aspect, the invention features, a kit which inlcudes one or more of
the
folowing an iRNA described herein, a sterile container in which the iRNA agent
is discolsed,
and instructions for use.
In another aspect, the invention features, an iRNA agent containing a
constrained
sequence made by a method described herein. The iRNA agent can target one or
more of the
genes referred to herein.
iRNA agents having constrained or selected sites, e.g., as described herein,
can be
used in any way described herein. Accordingly, they iRNA agents having
constrained or
selected sites, e.g., as described herein, can be used to silence a target,
e.g., in any of the methods
1 o described herein and to target any of the genes described herein or to
treat any of the disorders
described herein. iRNA agents having constrained or selected sites, e.g., as
described herein, can
be incorporated into any of the formulations or preparations, e.g.,
pharmaceutical or sterile
preparations described herein. iRNA agents having constrained or selected
sites, e.g., as
described herein, can be administered by any of the routes of administration
described herein.
~ 5 The term "other than canonical Watson-Crick pairing" as used herein,
refers to a pairing
between a first monomer in a first sequence and a second monomer at the
corresponding position
in a second sequence of a duplex in which one or more of the following is
true: (1) there is
essentially no pairing between the two, e.g., there is no significant level of
H bonding between
the monomers or binding between the monomers does not contribute in any
significant way to
2o the stability of the duplex; (2) the monomers are a non-canonical paring of
monomers having a
naturally occurring bases, i.e., they are other than A-T, A-U, or G-C, and
they form monomer-
monomer H bonds, although generally the H bonding pattern formed is less
strong than the
bonds formed by a canonical pairing; or(3) at least one of the monomers
includes a non-naturally
occurring bases and the H bonds formed between the monomers is, preferably
formed is less
25 strong than the bonds formed by a canonical pairing, namely one or more of
A-T, A-U, G-C.
The term "off target" as used herein, refers to as a sequence other than the
sequence to be
silenced.
Universal Bases: "wild-cards" ; shape-based complementarity
Bi-stranded, multisite replication of a base pair between difluorotoluene and
adenine: confirmation by
'inverse' sequencing. Liu, D.; Moran, S.; Kool, E. T. Claern. Biol., 1997, 4,
919-926)
152
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
r L
(Importance of terminal base pair hydrogen-bonding in 3'-end proofreading by
the Klenow fragment of
DNA polymerase I. Morales, J. C.; Kool, E. T. Biochemistry, 2000, 39, 2626-
2632)
(Selective and stable DNA base pairing without hydrogen bonds. Matray, T, J.;
Kool, E. T. J. Am. Chem.
Soc., 1998, 120, 6191-6192)
F
(Difluorotoluene, a nonpolar isostere for thymine, codes specifically and
efficiently for adenine in DNA
replication. Moran, S. Ren, R. X.-F.; Rumney IV, S.; Kool, E. T. J. Am. Chern.
Soc., 1997, 119, 2056-2057)
(Structure and base pairing properties of a replicable nonpolar isostere for
deoxyadenosine. Guckian, K.
M.; Morales, J. C.; Kool, E. T. J. O~g. Clzern., 1998, 63, 9652-9656)
F , CH
H3~ ~ N
HO I / F HO 'N I /
O O
OH F OH
153
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
N02
HO /
N
O
OH
3-nitropyrrole
NOZ
HO N
O
OH
5-nitroindole
I ~ ~~ I
I
N O IJ O N O
MICS PIM 5MICS
(
(Universal bases for hybridization, replication and chain termination. Berger,
M.; Wu. Y.; Ogawa, A. K.;
McMinn, D. L.; Schultz, P.G.; Romesberg, F. E. Nucleic Acids Res., 2000, 28,
2911-2914)
/ \ / \ / \ / / \ /
O .,~,,~, O
TM DM ICS PICS
/ \ , / \
/ \ / \ / / I w / \ / \ /
N N
2MN DMN 7A1 2Np 3MN
(1. Efforts toward the expansion of the genetic alphabet: Information storage
and replication with unnatural
hydrophobic base pairs. Ogawa, A. K.; Wu, Y.; McMinn, D. L.; Liu, J.; Schultz,
P. G.; Romesberg, F. E. J. Arn.
Cherra. Soc., 2000, 122, 3274-3287. 2. Rational design of an unnatural base
pair with increased kinetic
selectivity. Ogawa, A. K.; Wu. Y.; Bergen M.; Schultz, P. G.; Romesberg, F. E.
J. Arn. Chena. Soc., 2000, 122,
8803-8804)
154
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
N
N
lAl
(Efforts toward expansion of the genetic alphabet: replication of DNA with
three base pairs. Tae, E. L.;
Wu, Y.; Xia, G.; Schultz, P. G.; Romesberg, F. E. J. Arra. Chern. Soc.,
2001,123, 7439-7440)
I
N /
~N
HO ' /
O
OH
(1. Efforts toward expansion of the genetic alphabet: Optimization of
interbase hydrophobic interactions.
Wu, Y.; Ogawa, A. K.; Berger, M.; McMinn, D. L.; Schultz, P. G.; Romesberg, F.
E. J. Am. Chem. Soc., 2000, 122,
7621-7632. 2. Efforts toward expansion of genetic alphabet: DNA polyrnerase
recognition of a highly stable, self
pairing hydrophobic base. McMinn, D. L.; Ogawa. A. I~.; Wu, Y.; Liu, J.;
Schultz, P. G.; Romesberg, F. E. J. Am.
Chem. Soc.,1999,121, 11585-11586)
(A stable DNA duplex containing a non-hydrogen-bonding and non-shape
complementary base couple:
Interstrand stacking as the stability determining factor. Brotschi, C.;
Haberli, A.; Leumann, C, J. Angew. Claena. Int.
Ed., 2001, 40, 3012-3014)
(2,2'-Bipyridine Ligandoside: A novel building block for modifying DNA with
infra-duplex metal
complexes. Weizman, H.; Tor, Y. J. Am. Chem. Soc., 2001, 123, 3375-3376)
NHz NH2
N N' \ N
HO I / HO I
O O
OH OH
d2APy d2APm
(Minor groove hydration is critical to the stability of DNA duplexes. Lan, T.;
McLaughlin, L. W. J. Arn.
Claern. Soc., 2000, 122, 6512-13)
155
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
(Effect of the Universal base 3-nitropyrrole on the selectivity of neighboring
natural bases. Oliver, J. S.;
Parker, K. A.; Suggs, J. W. Organic Lett., 2001, 3, 1977-1980. 2. Effect of
the 1-(2'-deoxy-~i-D-ribofuranosyl)-3-
nitropyrrol residue on the stability of DNA duplexes and triplexes. Amosova,
O.; George J.; Fresco, J. R. Nucleic
Acids Res., 1997, 25, 1930-1934. 3. Synthesis, structure and deoxyribonucleic
acid sequencing with a universal
nucleosides: 1-(2'-deoxy-~i-D-ribofuranosyl)-3-nitropyrrole. Bergstrom, D. E.;
Zhang, P.; Toma, P. H.; Andrews, P.
C.; Nichols, R. J. Ana. Chena. Soc., 1995,117, 1201-1209)
OH
H
N-H~~,",~,
HO
"..~. N
\ \ O ' N-H......
HO II ,~~N~ \
/ / N~N~Bu Hp O L~p~~,~~~"I
I I v
~N H H
H Fi N~ p
OH ,N N Nn~.
Bu / / I OH
\ \
(Model studies directed toward a general triplex DNA recognition scheme: a
novel DNA base that binds a
CG base-pair in an organic solvent. Zimmerman, S. C.; Schmitt, P. J. Am. Chem.
Soc., 1995, 117, 10769-10770)
~--o
o
DNA \
O ~ / NO2
O
O~
DNA
(A universal, photoeleavable DNA base: nitropiperonyl 2'-deoxyriboside. J.
Oag. Claem., 2001, 66, 2067-
2071)
156
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
(Recognition of a single guanine bulge by 2-acylamino-1,8-naphthyridine.
Nakatani, K.; Sando, S.; Saito, I.
J. Arn. Cherra. Soc., 2000, 122, 2172-2177. b. Specific binding of 2-amino-1,8-
naphthyridine into single guanine
bulge as evidenced by photooxidation of GC doublet, Nakatani, K.; Sando, S.;
Yoshida, K.; Saito, I. Bioorg. Med.
Claern. Lett., 2001, Il, 335-337)
z
O
Asymmetrical Modifications
The monomers and methods described herein can be used to prepare an RNA, e.g.,
an
iRNA agent, can be asymmetrically modified as described herein, and as
described in
International Application Serial No. PCT/LTS04/07070, filed March 8, 2004,
which is hereby
incorporated by reference.
An asymmetrically modified iRNA agent is one in which a strand has a
modification
which is not present on the other strand. An asymmetrical,modification is a
modification found
15 on one strand but not on the other strand. Any modification, e.g., any
modification described
herein, can be present as an asymmetrical modification. An asymmetrical
modification can
confer any of the desired properties associated with a modification, e.g.,
those properties
discussed herein. E.g., an asymmetrical modification can: confer resistance to
degradation, an
alteration in half life; target the iRNA agent to a particular target, e.g.,
to a particular tissue;
2o modulate, e.g., increase or decrease, the affinity of a strand for its
complement or target
sequence; or hinder or promote modification of a terminal moiety, e.g.,
modification by a kinase
or other enzymes involved in the RISC mechanism pathway. The designation of a
modification
157
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
as having one property does not mean that it has no other property, e.g., a
modification referred
to as one which promotes stabilization might also enhance targeting.
While not wishing to be bound by theory or any particular mechanistic model,
it is
believed that asymmetrical modification allows an iRNA agent to be optimized
in view of the
different or "asymmetrical" functions of the sense and antisense strands. For
example, both
strands can be modified to increase nuclease resistance, however, since some
changes can inhibit
RISC activity, these changes can be chosen for the sense stand . Tn addition,
since some
modifications, e.g., targeting moieties, can add large bulky groups that,
e.g., can interfere with
the cleavage activity of the RISC complex, such modifications are preferably
placed on the sense
strand. Thus, targeting moieties, especially bulky ones (e.g. cholesterol),
are preferentially added
to the sense strand. In one embodiment, an asymmetrical modification in which
a phosphate of
the backbone is substituted with S, e.g., a phosphorothioate modification, is
present in the
antisense strand, and a 2' modification, e.g., 2' OMe is present in the sense
strand. A targeting
moiety can be present at either (or both) the 5' or 3' end of the sense strand
of the iRNA agent. In
~ 5 a preferred example, a P of the backbone is replaced with S in the
antisense strand, 2'OMe is
present in the sense strand, and a targeting moiety is added to either the 5'
or 3' end of the sense
strand of the iRNA agent.
In a preferred embodiment an asymmetrically modified iRNA agent has a
modification
on the sense strand which modification is not found on the antisense strand
and the antisense
2o strand has a modification which is not found on the sense strand.
Each strand can include one or more asymmetrical modifications. By way of
example:
one strand can include a first asymmetrical modification which confers a first
property on the
iRNA agent and the other strand can have a second asymmetrical modification
which confers a
second property on the iRNA. E.g., one strand, e.g., the sense strand can have
a modification
25 which targets the iRNA agent to a tissue, and the other strand, e.g., the
antisense strand, has a
modification which promotes hybridization with the target gene sequence.
In some embodiments both strands can be modified to optimize the same
property, e.g.,
to increase resistance to nucleolytic degradation, but different modifications
are chosen for the
sense and the antisense strands, e.g., because the modifications affect other
properties as well.
3o E.g., since some changes can affect RISC activity these modifications are
chosen for the sense
strand.
In an embodiment one strand has an asymmetrical 2' modification, e.g., a 2'
OMe
modification, and the other strand has an asymmetrical modification of the
phosphate backbone,
e.g., a phosphorothioate modification. So, in one embodiment the antisense
strand has an
158
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
asymmetrical 2' OMe modification and the sense strand has an asymmetrical
phosphorothioate
modification (or vice versa). In a particularly preferred embodiment the RNAi
agent will have
asymmetrical 2'-O alkyl, preferably, 2'-OMe modifications on the sense strand
and
asymmetrical backbone P modification, preferably a phosphothioate modification
in the
antisense strand. There can be one or multiple 2'-OMe modifications, e.g., at
least 2, 3, 4, 5, or
6, of the subunits of the sense strand can be so modified. There can be one or
multiple
phosphorothioate modifications, e.g., at least 2, 3, 4, 5, or 6, of the
subunits of the antisense
strand can be so modified. It is preferable to have an iRNA agent wherein
there are multiple 2'-
OMe modifications on the sense strand and multiple phophorothioate
modifications on the
antisense strand. All of the subunits on one or both strands can be so
modified. A particularly
preferred embodiment of multiple asymmetric modification on both strands has a
duplex region
about 20-21, and preferably 19, subunits in length and one or two 3' overhangs
of about 2
subunits in length.
Asymmetrical modifications are useful for promoting resistance to degradation
by
nucleases, e.g., endonucleases. iRNA agents can include one or more
asymmetrical
modifications which promote resistance to degradation. In preferred
embodiments the
modification on the antisense strand is one which will not interfere with
silencing of the target,
e.g., one which will not interfere with cleavage of the target. Most if not
all sites on a strand are
vulnerable, to some degree, to degradation by endonucleases. One can determine
sites which are
2o relatively vulnerable and insert asymmetrical modifications which inhibit
degradation. It is often
desirable to provide asymmetrical modification of a UA site in an iRNA agent,
and in some
cases it is desirable to provide the UA sequence on both strands with
asymmetrical modification.
Examples of modifications which inhibit endonucleolytic degradation can be
found herein.
Particularly favored modifications include: 2' modification, e.g., provision
of a 2' OMe moiety
on the U, especially on a sense strand; modification of the backbone, e.g.,
with the replacement
of an O with an S, in the phosphate backbone, e.g., the provision of a
phosphorothioate
modification, on the U or the A or both, especially on an antisense strand;
replacement of the U
with a CS amino linker; replacement of the A with a G (sequence changes are
preferred to be
located on the sense strand and not the antisense strand); and modification of
the at the 2', 6', 7',
so or 8' position. Preferred embodiments are those in which one or more of
these modifications are
present on the sense but not the antisense strand, or embodiments where the
antisense strand has
fewer of such modifications.
Asymmetrical modification can be used to inhibit degradation by exonucleases.
Asymmetrical modifications can include those in which only one strand is
modified as well as
159
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
those in which both are modified. In preferred embodiments the modification on
the antisense
strand is one which will not interfere with silencing of the target, e.g., one
which will not
interfere with cleavage of the target. Some embodiments will have an
asymmetrical
modification on the sense strand, e.g., in a 3' overhang, e.g., at the 3'
terminus, and on the
antisense strand, e.g., in a 3' overhang, e.g., at the 3' terminus. If the
modifications introduce
moieties of different size it is preferable that the larger be on the sense
strand. If the
modifications introduce moieties of different charge it is preferable that the
one with greater
charge be on the sense strand.
Examples of modifications which inhibit exonucleolytic degradation can be
found herein.
Particularly favored modifications include: 2' modification, e.g., provision
of a 2' OMe moiety
in a 3' overhang, e.g., at the 3' terminus (3' terminus means at the 3' atom
of the molecule or at
the most 3' moiety, e.g., the most 3' P or 2' position, as indicated by the
context); modification
of the backbone, e.g., with the replacement of a P with an S, e.g., the
provision of a
phosphorothioate modification, or the use of a methylated P in a 3' overhang,
e.g., at the 3'
~5 terminus; combination of a 2' modification, e.g., provision of a 2' O Me
moiety and
modification of the backbone, e.g., with the replacement of a P with an S,
e.g., the provision of a
phosphorothioate modification, or the use of a methylated P, in a 3' overhang,
e.g., at the 3'
terminus; modification with a 3' alkyl; modification with an abasic pyrolidine
in a 3' overhang,
e.g., at the 3' terminus; modification with naproxene, ibuprofen, or other
moieties which inhibit
2o degradation at the 3' terminus. Preferred embodiments are those in which
one or more of these
modifications are present on the sense but not the antisense strand, or
embodiments where the
antisense strand has fewer of such modifications.
Modifications, e.g., those described herein, which affect targeting can be
provided as
asymmetrical modifications. Targeting modifications which can inhibit
silencing, e.g., by
25 inhibiting cleavage of a target, can be provided as asymmetrical
modifications of the sense
strand. A biodistribution altering moiety, e.g., cholesterol, can be provided
in one or more, e.g.,
two, asymmetrical modifications of the sense strand. Targeting modifications
which introduce
moieties having a relatively large molecular weight, e.g., a molecular weight
of more than 400,
500, or 1000 daltons, or which introduce a charged moiety (e.g., having more
than one positive
3o charge or one negative charge) can be placed on the sense strand.
Modifications, e.g., those described herein, which modulate, e.g., increase or
decrease,
the affinity of a strand for its compliment or target, can be provided as
asymmetrical
modifications. These include: 5 methyl U; 5 methyl C; pseudouridine, Loclced
nucleic acids ,2
160
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
thio U and 2-amino-A. In some embodiments one or more of these is provided on
the antisense
strand.
iRNA agents have a defined structure, with a sense strand and an antisense
strand, and in
many cases short single strand overhangs, e.g., of 2 or 3 nucleotides are
present at one or both 3'
ends. Asymmetrical modification can be used to optimize the activity of such a
structure, e.g.,
by being placed selectively within the iRNA. E.g., the end region of the iRNA
agent defined by
the 5' end of the sense strand and the 3' end of the antisense strand is
important for function.
This region can include the terminal 2, 3, or 4 paired nucleotides and any 3'
overhang. In
preferred embodiments asymmetrical modifications which result in one or more
of the following
are used: modifications of the 5' end of the sense strand which inhibit kinase
activation of the
sense strand, including, e.g., attachments of conjugates which target the
molecule or the use
modifications which protect against 5' exonucleolytic degradation; or
modifications of either
strand, but preferably the sense strand, which enhance binding between the
sense and antisense
strand and thereby promote a "tight" structure at this end of the molecule.
~5 The end region of the iRNA agent defined by the 3' end of the sense strand
and the 5'end
of the antisense strand is also important for function. This region can
include the terminal 2, 3,
or 4 paired nucleotides and any 3' overhang. Preferred embodiments include
asymmetrical
modifications of either strand, but preferably the sense strand, which
decrease binding between
the sense and antisense strand and thereby promote an "open" structure at this
end of the
2o molecule. Such modifications include placing conjugates which target the
molecule or
modifications which promote nuclease resistance on the sense strand in this
region. Modification
of the antisense strand which inhibit kinase activation are avoided in
preferred embodiments.
Exemplary modifications for asymmetrical placement in the sense strand include
the
following:
25 (a) backbone modifications, e.g., modification of a backbone P, including
replacement of
P with S, or P substituted with alkyl or allyl, e.g., Me, and dithioates (S-
P=S); these
modifications can be used to promote nuclease resistance;
(b) 2'-O alkyl, e.g., 2'-OMe, 3'-O alkyl, e.g., 3'-OMe (at terminal and/or
internal
positions); these modifications can be used to promote nuclease resistance or
to enhance binding
30 of the sense to the antisense strand, the 3' modifications can be used at
the 5' end of the sense
strand to avoid sense strand activation by RISC;
(c) 2'-5' linkages (with 2'-H, 2'-OH and 2'-OMe and with P=O or P=S) these
modifications can be used to promote nuclease resistance or to inhibit binding
of the sense to the
161
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
antisense strand, or can be used at the 5' end of the sense strand to avoid
sense strand activation
by RISC;
(d) L sugars (e.g., L ribose, L-arabinose with 2'-H, 2'-OH and 2'-OMe); these
modifications can be used to promote nuclease resistance or to inhibit binding
of the sense to the
antisense strand, or can be used at the S' end of the sense strand to avoid
sense strand activation
by RISC;
(e) modified sugars (e.g., locked nucleic acids (LNA's), hexose nucleic acids
(HNA's)
and cyclohexene nucleic acids (CeNA's)); these modifications can be used to
promote nuclease
resistance or to inhibit binding of the sense to the antisense strand, or can
be used at the 5' end of
the sense strand to avoid sense strand activation by RISC;
(fj nucleobase modifications (e.g., C-5 modified pyrimidines, N-2 modified
purines, N-7
modified purines, N-6 modified purines), these modifications can be used to
promote nuclease
resistance or to enhance binding of the sense to the antisense strand;
(g) cationic groups and Zwitterionic groups (preferably at a terminus), these
~ 5 modifications can be used to promote nuclease resistance;
(h) conjugate groups (preferably at terminal positions), e,g., naproxen,
biotin, cholesterol,
ibuprofen, folic acid, peptides, and carbohydrates; these modifications can be
used to promote
nuclease resistance or to target the molecule, or can be used at the 5' end of
the sense strand to
avoid sense strand activation by RISC.
2o Exemplary modifications for asymmetrical placement in the antisense strand
include the
following:
(a) backbone modifications, e.g., modification of a backbone P, including
replacement of
P with S, or P substituted with alkyl or allyl, e.g., Me, and dithioates (S-
P=S);
(b) 2'-O alkyl, e.g., 2'-OMe, (at terminal positions);
25 (c) 2'-5' linkages (with 2'-H, 2'-OH and 2'-OMe) e.g., terminal at the 3'
end); e.g., with
P=O or P=S preferably at the 3'-end, these modifications are preferably
excluded from the 5' end
region as they may interfere with RISC enzyme activity such as kinase
activity;
(d) L sugars (e.g, L ribose, L-arabinose with 2'-H, 2'-OH and 2'-OMe); e.g.,
terminal at
the 3' end; e.g., with P=O or P=S preferably at the 3'-end, these
modifications are preferably
3o excluded from the 5' end region as they may interfere with kinase activity;
(e) modified sugars (e.g., LNA's, HNA's and CeNA's); these modifications are
preferably excluded from the 5' end region as they may contribute to unwanted
enhancements of
paring between the sense and antisense strands, it is often preferred to have
a "loose" structure in
the 5' region, additionally, they may interfere with kinase activity;
162
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
(f) nucleobase modifications (e.g., C-5 modified pyrimidines, N-2 modified
purines, N-7
modified purines, N-6 modified purines);
(g) cationic groups and Zwitterionic groups (preferably at a terminus);
conjugate groups (preferably at terminal positions), e,g., naproxen, biotin,
cholesterol,
ibuprofen, folic acid, peptides, and carbohydrates, but bulky groups or
generally groups which
inhibit RISC activity should are less preferred.
The 5'-OH of the antisense strand should be kept free to promote activity. In
some
preferred embodiments modifications that promote nuclease resistance should be
included at the
3' end, particularly in the 3' overhang.
1o In another aspect, the invention features a method of optimizing, e.g.,
stabilizing, an
iRNA agent. The method includes selecting a sequence having activity,
introducing one or more
asymmetric modifications into the sequence, wherein the introduction of the
asymmetric
modification optimizes a property of the iRNA agent but does not result in a
decrease in activity.
The decrease in activity can be less than a preselected level of decrease. In
~5 preferred embodiments decrease in activity means a decrease of less than~5,
10, 20, 40, or 50
activity, as compared with an otherwise similar iRNA lacking the introduced
modification.
Activity can, e.g., be measured in vivo, or in vitro, with a result in either
being sufficient to
demonstrate the required maintenance of activity.
The optimized property can be any property described herein and in particular
the
2o properties discussed in the section on asymmetrical modifications provided
herein. The
modification can be any asymmetrical modification, e.g., an asymmetric
modification described
in the section on asymmetrical modifications described herein. Particularly
preferred
asymmetric modifications are 2'-O alkyl modifications, e.g., 2'-OMe
modifications, particularly
in the sense sequence, and modifications of a backbone O, particularly
phosphorothioate
25 modifications, in the antisense sequence.
In a preferred embodiment a sense sequence is selected and provided with an
asymmetrical modification, while in other embodiments an antisense sequence is
selected and
provided with an asymmetrical modification. In some embodiments both sense and
antisense
sequences are selected and each provided with one or more asymmetrical
modifications.
3o Multiple asymmetric modifications can be introduced into either or both of
the sense and
antisense sequence. A sequence can have at least 2, 4, 6, 8, or more
modifications and all or
substantially all of the monomers of a sequence can be modified.
Table 3 shows examples having strand I with a selected modification and strand
II with a
selected modification.
163
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Table 3. Exemplary strand I- and strand II-modifications
Strand I Strand II
Nuclease Resistance (e.g., Biodistribution (e.g., P=S)
2'-OMe)
Biodistribution conjugate Protein Binding Functionality
(e.g., Lipophile) (e.g., Naproxen)
Tissue Distribution Functionality' Cell Targeting Functionality
(e.g., Carbohydrates) (e.g., Folate for cancer cells)
Tissue Distribution FunctionalityFusogenic Functionality
(e.g., Kidney Cell Targetingmoieties)(e.g., Polyethylene imines)
Cancer Cell Targeting Fusogenic Functionality
(e.g., RGD peptides and imines)(e.g., peptides)
Nuclease Resistance (e.g., crease in binding Affinity (5-Me-C,
2'-OMe) 5-Me-U, 2-
thio-U, 2-amino-A, G-clamp, LNA)
Tissue Distribution FunctionalitygISC activity improving Functionality
Helical conformation changingTissue Distribution Functionality
Functionalities (P=S; lipophile, carbohydrates)
Z-X-Y Architecture
The monomers and methods described herein can be used to prepare an RNA, e.g.,
an
iRNA agent, having a Z-X-Y architecture or structure such as those described
herein and those
described in copending, co-owned United States Provisional Application Serial
No. 60/510,246,
filed on October 9, 2003, which is hereby incorporated by reference,
copending, co-owned
United States Provisional Application Serial No. 60/510,318, filed on October
10, 2003, which is
1 o hereby incorporated by reference, and copending, co-owned International
Application No.
PCT/LTS04/07070, filed March 8, 2004.
Thus, an iRNA agent can have a first segment, the Z region, a second segment,
the X
region, and optionally a third region, the Y region:
Z-X Y.
164
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
It may be desirable to modify subunits in one or both of Zand/or Y on one hand
and X on
the other hand. In some cases they will have the same modification or the same
class of
modification but it will more often be the case that the modifications made in
Z and/or Y will
differ from those made in X.
The Z region typically includes a terminus of an iRNA agent. The length of the
Z region
can vary, but will typically be from 2-14, more preferably 2-10, subunits in
length. It typically is
single stranded, i.e., it will not base pair with bases of another strand,
though it may in some
embodiments self associate, e.g., to form a loop structure. Such structures
can be formed by the
end of a strand looping back and forming an intrastrand duplex. E.g., 2, 3, 4,
5 or more intra-
strand bases pairs can form, having a looped out or connecting region,
typically of 2 or more
subunits which do not pair. This can occur at one or both ends of a strand. A
typical
embodiment of a Z region is a single strand overhang, e.g., an over hang of
the length described
elsewhere herein. The Z region can thus be or include a 3' or 5' terminal
single strand. It can be
sense or antisense strand but if it is antisense it is preferred that it is a
3- overhang. Typical
15 inter-subunit bonds in the Z region include: P=O; P=S; S-P=S; P-NRZ; and P-
BR2. Chiral P=X,
where X is S, N, or B) inter-subunit bonds can also be present. (These inter-
subunit bonds are
discussed in more detail elsewhere herein.) Other preferred Z region subunit
modifications (also
discussed elsewhere herein) can include: 3'-OR, 3'SR, 2'-OMe, 3'-OMe, and 2'OH
modifications and moieties; alpha configuration bases; and 2' arabino
modifications.
2o The X region will in most cases be duplexed, in the case of a single strand
iRNA agent,
with a corresponding region of the single strand, or in the case of a double
stranded iRNA agent,
with the corresponding region of the other strand. The length of the X region
can vary but will
typically be between 10-45 and more preferably between 15 and 35 subunits.
Particularly
preferred region X's will include 17, 18, 19, 29, 21, 22, 23, 24, or 25
nucleotide pairs, though
25 other suitable lengths are described elsewhere herein and can be used.
Typical X region subunits
include 2'-OH subunits. In typical embodiments phosphate inter-subunit bonds
are preferred
while phophorothioate or non-phosphate bonds are absent. Other modifications
preferred in the
X region include: modifications to improve binding, e.g., nucleobase
modifications; cationic
nucleobase modifications; and C-5 modified pyrimidines, e.g., allylamines.
Some embodiments
3o have 4 or more consecutive 2'0H subunits. While the use of phosphorothioate
is sometimes non
preferred they can be used if they connect less than 4 consecutive 2'OH
subunits.
The Y region will generally conform to the the parameters set out for the Z
regions.
However, the X and Z regions need not be the same, different types and numbers
of
165
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
modifications can be present, and infact, one will usually be a 3' overhang
and one will usually
be a 5' overhang.
In a preferred embodiment the iRNA agent will have a Y and/or Z region each
having
ribonucleosides in which the 2'-OH is substituted, e.g., with 2'-OMe or other
alkyl; and an X
region that includes at least four consecutive ribonucleoside subunits in
which the 2'-OH
remains unsubstituted.
The subunit linkages (the linkages between subunits) of an iRNA agent can be
modified,
e.g., to promote resistance to degradation. Numerous examples of such
modifications are
disclosed herein, one example of which is the phosphorothioate linkage. These
modifications
can be provided bewteen the subunits of any of the regions, Y, X, and Z.
However, it is
preferred that their occureceis minimized and in particular it is preferred
that consecutive
modified linkages be avoided.
In a preferred embodiment the iRNA agent will have a Y axed Z region each
having
ribonucleosides in which the 2'-OH is substituted, e.g., with 2'-OMe; and an X
region that
15 includes at least four consecutive subunits, e.g., ribonucleoside subunits
in which the 2'-OH
remains unsubstituted.
As mentioned above, the subunit linkages of an iRNA agent can be modified,
e.g., to
promote resistance to degradation. These modifications can be provided between
the subunits of
any of the regions, Y, X, and Z. However, it is preferred that they are
minimized and in
2o particular it is preferred that consecutive modified linkages be avoided.
Thus, in a preferred embodiment, not all of the subunit linkages of the iRNA
agent are
modified and more preferably the maximum number of consecutive subunits linked
by other than
a phospodiester bond will be 2, 3, or 4. Particulary preferred iRNA agents
will not have four or
more consecutive subunits, e.g., 2'-hydroxyl ribonucleoside subunits, in which
each subunits is
25 joined by modified linkages - i.e. linkages that have been modified to
stabilize them from
degradation as compared to the phosphodiester linkages that naturally occur in
RNA and DNA.
It is particularly preferred to minimize the occurrence in region X. Thus, in
preferred
embodiments each of the nucleoside subunit linkages in X will be
phosphodiester linkages, or if
subunit linkages in region X are modified, such modifications will be
minimized. E.g., although
3o the Y and/or Z regions can include inter subunit linkages which have been
stabilized against
degradation, such modifications will be minimized in the X region, and in
particular consecutive
modifications will be minimized. Thus, in preferred embodiments the maximum
number of
consecutive subunits linked by other than a phospodiester bond will be 2, 3,
or 4. Particulary
preferred X regions will not have four or more consecutive subunits, e.g., 2'-
hydroxyl
166
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
ribonucleoside subunits, in which each subunits is joined by modified linkages
- i.e. linkages
that have been modified to stabilize them from degradation as compared to the
phosphodiester
linkages that naturally occur in RNA and DNA.
In a preferred embodiment Y and for Z will be free of phosphorothioate
linkages, though
either or both may contain other modifications, e.g., other modifications of
the subunit linkages.
In a preferred embodiment region X, or in some cases, the entire iRNA agent,
has no
more than 3 or no more than 4 subunits having identical 2' moieties.
In a preferred embodiment region X, or in some cases, the entire iRNA agent,
has no
more than 3 or no more than 4 subunits having identical subunit linkages.
In a preferred embodiment one or more phosphorothioate linkages (or other
modifications of the subunit linkage) are present in Y and/or Z, but such
modified linkages do
not connect two adjacent subunits, e.g., nucleosides, having a 2'
modification, e.g., a 2'-O-alkyl
moiety. E.g., any adjacent 2'-O-alkyl moieties in the Y and/or Z, are
connected by a linkage
other than a a phosphorothioate linkage.
~ 5 In a preferred embodiment each of Y and/or Z independently has only one
phosphorothioate linkage between adjacent subunits, e.g., nucleosides, having
a 2' modification,
e.g., 2'-O-alkyl nucleosides. If there is a second set of adjacent subunits,
e.g., nucleosides,
having a 2' modification, e.g., 2'-O-alkyl nucleosides, in Y and/or Z that
second set is connected
by a linkage other than a phosphorothioate linkage, e.g., a modified linkage
other than a
2o phosphorothioate linkage.
In a prefered embodiment each of Y and/orZ independently has more than one
phosphorothioate linkage connecting adjacent pairs of subunits, e.g.,
nucleosides, having a 2'
modification, e.g., 2'-O-alkyl nucleosides, but at least one pair of adjacent
subunits, e.g.,
nucleosides, having a 2' modification, e.g., 2'-O-alkyl nucleosides, are be
connected by a linkage
20 other than a phosphorothioate linkage, e.g., a modified linkage other than
a phosphorothioate
linkage.
In a prefered embodiment one of the above recited limitation on adjacent
subunits in Y
and or Z is combined with a limitation on the subunits in X. E.g., one or more
phosphorothioate
linkages (or other modifications of the subunit linkage) are present in Y
and/or Z, but such
3o modified linkages do not connect two adjacent subunits, e.g., nucleosides,
having a 2'
modification, e.g., a 2'-O-alkyl moiety. E.g., any adjacent 2'-O-alkyl
moieties in the Y and/or Z,
are connected by a linkage other than a a phosporothioate linkage. In
addition, the X region has
no more than 3 or no more than 4 identical subunits, e.g., subunits having
identical 2' moieties or
the X region has no more than 3 or no more than 4 subunits having identical
subunit linkages.
167
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
A Y and/or Z region can include at least one, and preferably 2, 3 or 4 of a
modification
disclosed herein. Such modifications can be chosen, independently, from any
modification
described herein, e.g., from nuclease resistant subunits, subunits with
modified bases, subunits
with modified intersubunit linkages, subunits with modified sugars, and
subunits linked to
s another moiety, e.g., a targeting moiety. In a preferred embodiment more
than 1 of such subunits
can be present but in some emobodiments it is prefered that no more than 1, 2,
3, or 4 of such
modifications occur, or occur consecutively. In a preferred embodiment the
frequency of the
modification will differ between Yand /or Z and X, e.g., the modification will
be present one of
Y and/or Z or X and absent in the other.
An X region can include at least one, and preferably 2, 3 or 4 of a
modification disclosed
herein. Such modifications can be chosen, independently, from any modification
desribed
herein, e.g., from nuclease resistant subunits, subunits with modified bases,
subunits with
modified intersubunit linkages, subunits with modified sugars, and subunits
linked to another
moiety, e.g., a targeting moiety. In a preferred embodiment more than 1 of
such subunits can b
~5 present but in some emobodiments it is prefered that no more than l, 2, 3,
or 4 of such
modifications occur, or occur consecutively.
An RRMS (described elswhere herein) can be introduced at one or more points in
one or
both strands of a double-stranded iRNA agent. An RRMS can be placed in a Y
and/or Z region,
at or near (within 1, 2, or 3 positions) of the 3' or 5' end of the sense
strand or at near (within 2
20 or 3 positions of) the 3' end of the antisense strand. In some embodiments
it is preferred to not
have an RRMS at or near (within 1, 2, or 3 positions of) the 5' end of the
antisense strand. An
RRMS can be positioned in the X region, and will preferably be positioned in
the sense strand or
in an area of the antisense strand not critical for antisense binding to the
target.
25 Differential Modification of Terminal Duplex Stability
In one aspect, the monomers and methods described herein can be used to
prepare an
il2NA agent having differential modification of terminal duplex stability
(DMTDS).
In addition, the monomers and methods described herein can be used to prepare
iRNA
agents having DMTDS and another element described herein. E.g., the monomers
and methods
3o described herein can be used to prepare an iRNA agent described herein,
e.g., a palindromic
iRNA agent, an iRNA agent having a non canonical pairing, an iRNA agent which
targets a gene
described herein, e.g., a gene active in the kidney, an iRNA agent having an
architecture or
structure described herein, an iRNA associated with an amphipathic delivery
agent described
herein, an iRNA associated with a drug delivery module described herein, an
iRNA agent
168
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
administered as described herein, or an iI~NA agent formulated as described
herein, which also
incorporates DMTDS.
iRNA agents can be optimized by increasing the propensity of the duplex to
disassociate
or melt (decreasing the free energy of duplex association), in the region of
the 5' end of the
antisense strand duplex. This can be accomplished, e.g., by the inclusion of
subunits which
increase the propensity of the duplex to disassociate or melt in the region of
the 5' end of the
antisense strand. It can also be accomplished by the attachment of a ligand
that increases the
propensity of the duplex to disassociate of melt in the region of the 5'end .
While not wishing to
be bound by theory, the effect may be due to promoting the effect of an enzyme
such as helicase,
for example, promoting the effect of the enzyme in the proximity of the 5' end
of the antisense
strand.
The inventors have also discovered that iRNA agents can be optimized by
decreasing the
propensity of the duplex to disassociate or melt (increasing the free energy
of duplex
association), in the region of the 3' end of the antisense strand duplex. This
can be
accomplished, e.g., by the inclusion of subunits which decrease the propensity
of the duplex to
disassociate or melt in the region of the 3' end of the antisense strand. It
can also be
accomplished by the attachment of ligand that decreases the propensity of the
duplex to
disassociate of melt in the region of the 5'end.
Modifications which increase the tendency of the 5' end of the duplex to
dissociate can
2o be used alone or in combination with other modifications described herein,
e.g., with
modifications which decrease the tendency of the 3' end of the duplex to
dissociate. Likewise,
modifications which decrease the tendency of the 3' end of the duplex to
dissociate can be used
alone or in combination with other modifications described herein, e.g., with
modifications
which increase the tendency of the 5' end of the duplex to dissociate.
Decreasifag the stability of the AS S' egad of the duplex
Subunit pairs can be ranked on the basis of their propensity to promote
dissociation or
melting (e.g., on the free energy of association or dissociation of a
particular pairing, the simplest
approach is to examine the pairs on an individual pair basis, though next
neighbor or similar
analysis can also be used). In terms of promoting dissociation:
A:U is preferred over G:C;
G:U is preferred over G:C;
I:C is preferred over G:C (I=inosine);
169
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
mismatches, e.g., non-canonical or other than canonical pairings (as described
elsewhere herein) are preferred over canonical (A:T, A:U, G:C) pairings;
pairings which include a universal base are preferred over canonical pairings.
A typical ds iRNA agent can be diagrammed as follows:
S 5' Rl Nl Nz N3 N4 Ns
[N] N_s N_4 N_3 N_z N_l Rz 3'
AS 3' R3 Ni N2 N3 N4 Ns
[N] N_s N_4 N_3 N_z N_1 R4 5'
S:AS Pi Pz P3 P4
Ps [N] P-s P~ P_3 P_2 P_1
5'
S indicates the sense strand; AS indicates antisense strand; R1 indicates an
optional (and
nonpreferred) 5' sense strand overhang; Rz indicates an optional (though
preferred) 3' sense
overhang; R3 indicates an optional (though preferred) 3' antisense sense
overhang; R4 indicates
an optional (and nonpreferred) 5' antisense overhang; N indicates subunits;
[N] indicates that
2o additional subunit pairs may be present; and PX, indicates a paring of
sense NX and antisense NX.
Overhangs are not shown in the P diagram. In some embodiments a 3' AS overhang
corresponds
to region Z, the duplex region corresponds to region X, and the 3' S strand
overhang corresponds
to region Y, as described elsewhere herein. (The diagram is not meant to imply
maximum or
minimum lengths, on which guidance is provided elsewhere herein.)
It is preferred that pairings which decrease the propensity to form a duplex
are used at 1
or more of the positions in the duplex at the 5' end of the AS strand. The
terminal pair (the most
5' pair in terms of the AS strand) is designated as P_l, and the subsequent
pairing positions
(going in the 3' direction in terms of the AS strand) in the duplex are
designated, P_z, P_3, P.~, P-s,
and so on. The preferred region in which to modify to modulate duplex
formation is at P_s
3o through P_l, more preferably P_4 through P_1 , more preferably P_3 through
P_l. Modification at P_
1, is particularly preferred, alone or with modifications) other position(s),
e.g., any of the
positions just identified. It is preferred that at least 1, and more
preferably 2, 3, 4, or 5 of the
pairs of one of the recited regions be chosen independently from the group of
170
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
A:U
G:U
I:C
mismatched pairs, e.g., non-canonical or other than canonical pairings or
pairings
which include a universal base.
In preferred embodiments the change in subunit needed to achieve a pairing
which
promotes dissociation will be made in the sense strand, though in some
embodiments the change
will be made in the antisense strand.
In a preferred embodiment the at least 2, or 3, of the pairs in P_l, through
P_4, are pairs
which promote disociation.
In a preferred embodiment the at least 2, or 3, of the pairs in P_l, through
P_4, are A:U.
In a preferred embodiment the at least 2, or 3, of the pairs in P_l, through
P_4, are G:U.
In a preferred embodiment the at least 2, or 3, of the pairs in P_l, through
P_4, are I:C.
In a preferred embodiment the at least 2, or 3, of the pairs in P_l, through
P_4, are
mismatched pairs, e.g., non-canonical or other than canonical pairings
pairings.
In a preferred embodiment the at least 2, or 3, of the pairs in P_l, through
P_4, are pairings
which include a universal base.
Increasing the stability of the AS 3' end of the duplex
Subunit pairs can be ranked on the basis of their propensity to promote
stability and
2o inhibit dissociation or melting (e.g., on the free energy of association or
dissociation of a
particular pairing, the simplest approach is to examine the pairs on an
individual pair basis,
though next neighbor or similar analysis can also be used). In terms of
promoting duplex
stability:
G:C is preferred over A:U
Watson-Crick matches (A:T, A:U, G:C) are preferred over non-canonical or other
than canonical pairings
analogs that increase stability are preferred over Watson-Crick matches (A:T,
A:U, G:C)
2-amino-A:U is preferred over A:U
2-thio U or 5 Me-thio-U:A are preferred over U:A
G-clamp (an analog of C having 4 hydrogen bonds):G is preferred over C:G
guanadinium-G-clamp:G is preferred over C:G
171
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
psuedo uridine:A is preferred over U:A
sugar modifications, e.g., 2' modifications, e.g., 2'F, ENA, or LNA, which
enhance binding are preferred over non-modified moieties and can be present on
one or both
strands to enhance stability of the duplex. It is preferred that pairings
which increase the
propensity to form a duplex are used at 1 or more of the positions in the
duplex at the 3' end of
the AS strand. The terminal pair (the most 3' pair in terms of the AS strand)
is designated as P1,
and the subsequent pairing positions (going in the 5' direction in terms of
the AS strand) in the
duplex are designated, P2, P3, P4, P$, and so on. The preferred region in
which to modify to
modulate duplex formation is at PS through P1, more preferably P4 through Pl ,
more preferably
1o P3 through P1. Modification at P1, is particularly preferred, alone or with
mdification(s) at other
position(s), e.g.,any of the positions just identified. It is preferred that
at least 1, and more
preferably 2, 3, 4, or 5 of the pairs of the recited regions be chosen
independently from the group
of
G:C
a pair having an analog that increases stability over Watson-Crick matches
(A:T,
A:U, G:C)
2-amino-A:U
2-thio U or 5 Me-thio-U:A
2o G-clamp (an analog of C having 4 hydrogen bonds):G
guanadinium-G-clamp: G
psuedo uridine:A
a pair in which one or both subunits has a sugar modification, e.g., a 2'
modification, e.g., 2'F, ENA, or LNA, which enhance binding.
In a preferred embodiment the at least 2, or 3, of the pairs in P_l, through
P_4, are pairs
which promote duplex stability.
In a preferred embodiment the at least 2, or 3, of the pairs in P1, through
P4, are G:C.
In a preferred embodiment the at least 2, or 3, of the pairs in P1, through
P4, are a pair
so having an analog that increases stability over Watson-Crick matches.
In a preferred embodiment the at least 2, or 3, of the pairs in Pl, through
P4, are 2-amino-
A:U.
In a preferred embodiment the at least 2, or 3, of the pairs in PI, through
P4, are 2-thio U
or 5 Me-thio-U:A.
172
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
In a preferred embodiment the at least 2, or 3, of the pairs in P1, through
P4, are G-
clamp:G.
In a preferred embodiment the at least 2, or 3, of the pairs in P1, through
P4, are
guanidinium-G-clamp:G.
In a preferred embodiment the at least 2, or 3, of the pairs in P1, through
P4, are psuedo
uridine:A.
In a preferred embodiment the at least 2, or 3, of the pairs in P1, through
P4, are a pair in
which one or both subunits has a sugar modification, e.g., a 2' modification,
e.g., 2'F, ENA, or
LNA, which enhances binding.
G-clamps and guanidinium G-clamps are discussed in the following references:
Holmes
and Gait, "The Synthesis of 2'-O-Methyl G-Clamp Containing Oligonucleotides
and Their
Inhibition of the HIV-1 Tat-TAR Interaction," Nucleosides, Nucleotides &
Nucleic Acids,
22:1259-1262, 2003; Hohnes et al., "Steric inhibition of human
immunodeficiency virus type-1
Tat-dependent trans-activation in vitro and in cells by oligonucleotides
containing 2'-O-methyl
~ 5 G-clamp ribonucleoside analogues," Nucleic Acids Research, 31:2759-2768,
2003; Wilds, et al.,
"Structural basis for recognition of guanosine by a synthetic tricyclic
cytosine analogue:
Guanidinium G-clamp," Helvetica Chimica Acta, 86:966-978, 2003; Rajeev, et
al., "High-
Affinity Peptide Nucleic Acid Oligomers Containing Tricyclic Cytosine
Analogues," Organic
Letters, 4:4395-4398, 2002; Ausin, et al., "Synthesis of Amino- and Guanidino-
G-Clamp PNA
2o Monomers," Organic Letters, 4:4073-4075, 2002; Maier et al., "Nuclease
resistance of
oligonucleotides containing the tricyclic cytosine analogues phenoxazine and 9-
(2-
aminoethoxy)-phenoxazine ("G-clamp") and origins of their nuclease resistance
properties,"
Biochemistry, 41:1323-7, 2002; Flanagan, et al., "A cytosine analog that
confers enhanced
potency to antisense oligonucleotides," Proceedings Of The National Academy Of
Sciences Of
25 The United States Of America, 96:3513-8, 1999.
Simultaneously decreasing the stability of the AS 5'end of the duplex and
increasing the
stability of the AS 3' end of the duplex
As is discussed above, an iRNA agent can be modified to both decrease the
stability of
3o the AS 5'end of the duplex and increase the stability of the AS 3' end of
the duplex. This can be
effected by combining one or more of the stability decreasing modifications in
the AS 5' end of
the duplex with one or more of the stability increasing modifications in the
AS 3' end of the
duplex. Accordingly a preferred embodiment includes modification in P_5
through P_l, more
preferably P_4 through P_1 and more preferably P_3 through P_l. Modification
at P_I, is particularly
173
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
preferred, alone or with other position, e.g., the positions just identified.
It is preferred that at
least 1, and more preferably 2, 3, 4, or 5 of the pairs of one of the recited
regions of the AS 5'
end of the duplex region be chosen independently from the group of
s A:U
G:U
I:C
mismatched pairs, e.g., non-canonical or other than canonical pairings which
include a universal base; and
a modification in PS through P1, more preferably P4 through P1 and more
preferably P3
through Pl. Modification at Pl, is particularly preferred, alone or with other
position, e.g., the
positions just identified. It is preferred that at least 1, and more
preferably 2, 3, 4, or 5 of the
pairs of one of the recited regions of the AS 3' end of the duplex region be
chosen independently
from the group of
G:C
a pair having an analog that increases stability over Watson-Crick matches
(A:T,
A:U, G:C)
2-amino-A:U
2-thio U or 5 Me-thio-U:A
G-clamp (an analog of C having 4 hydrogen bonds):G
guanadinium-G-clamp:G
psuedo uridine:A
a pair in which one or both subunits has a sugar modification, e.g., a 2'
modification, e.g., 2'F, ENA, or LNA, which enhance binding.
The invention also includes methods of selecting and making iRNA agents having
DMTDS. E.g., when screening a target sequence for candidate sequences for use
as iRNA
3o agents one can select sequences having a DMTDS property described herein or
one which can be
modified, preferably with as few changes as possible, especially to the
AS strand, to provide a desired level of DMTDS.
174
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
The invention also includes, providing a candidate iRNA agent sequence, and
modifying
at least one P in P_5 through P_1 and/or at least one P in PS through P1 to
provide a DMTDS
iRNA agent.
DMTDS iRNA agents can be used in any method described herein, e.g., to silence
any
gene disclosed herein, to treat any disorder described herein, in any
formulation described herein,
and generally in and/or with the methods and compositions described elsewhere
herein. DMTDS
iRNA agents can incorporate other modifications described herein, e.g., the
attachment of
targeting agents or the inclusion of modifications which enhance stability,
e.g., the inclusion of
nuclease resistant monomers or the inclusion of single strand overhangs (e.g.,
3' AS overhangs
1o and/or 3' S strand overhangs) which self associate to form intrastrand
duplex structure.
Preferably these iRNA agents will have an architecture described herein.
~ther Embodiments
An RNA, e.g., an iRNA agent, can be produced in a cell i~c vivo, e.g., from
exogenous
DNA templates that are delivered into the cell. For example, the DNA templates
can be inserted
into vectors and used as gene therapy vectors. Gene therapy vectors can be
delivered to a subject
by, for example, intravenous injection, local administration (U.S. Pat. No.
5,328,470), or by
stereotactic injection (see, e.g., Chen et al.,1'~oc. lVatl. Acad. Sci. USA
91:3054-3057, 1994).
The pharmaceutical preparation of the gene therapy vector can include the gene
therapy vector in
an acceptable diluent, or can comprise a slow release matrix in which the gene
delivery vehicle is
2o imbedded. The DNA templates, for example, can include two transcription
units, one that
produces a transcript that includes the top strand of an iRNA agent and one
that produces a
transcript that includes the bottom strand of an iRNA agent. When the
templates are transcribed,
the iRNA agent is produced, and processed into sRNA agent fragments that
mediate gene
silencing.
In vivo Delivery
An iRNA agent can be linked, e.g., noncovalently linked to a polymer for the
efficient
delivery of the iRNA agent to a subj ect, e.g., a mammal, such as a human. The
iRNA agent can,
for example, be complexed with cyclodextrin. Cyclodextrins have been used as
delivery
3o vehicles of therapeutic compounds. Cyclodextrins can form inclusion
complexes with drugs that
are able to fit into the hydrophobic cavity of the cyclodextrin. In other
examples, cyclodextrins
form non-covalent associations with other biologically active molecules such
as oligonucleotides
and derivatives thereof. The use of cyclodextrins creates a water-soluble drug
delivery complex,
175
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
that can be modified with targeting or other functional groups. Cyclodextrin
cellular delivery
system for oligonucleotides described in U.S. Pat. No. 5,691,316, which is
hereby incorporated
by reference, are suitable for use in methods of the invention. In this
system, an oligonucleotide
is noncovalently complexed with a cyclodextrin, or the oligonucleotide is
covalently bound to
adamantine which in turn is non-covalently associated with a cyclodextrin.
The delivery molecule can include a linear cyclodextrin copolymer or a linear
oxidized
cyclodextrin copolymer having at least one ligand bound to the cyclodextrin
copolymer.
Delivery systems , as described in U.S. Patent No. 6,509,323, herein
incorporated by reference,
are suitable for use in methods of the invention. An iRNA agent can be bound
to the linear
cyclodextrin copolymer and/or a linear oxidized cyclodextrin copolymer. Either
or both of the
cyclodextrin or oxidized cyclodextrin copolymers can be crosslinked to another
polymer and/or
bound to a ligand.
A composition for iRNA delivery can employ an "inclusion complex," a molecular
compound having the characteristic structure of an adduct. In this structure,
the "host molecule"
spatially encloses at least part of another compound in the delivery vehicle.
The enclosed
compound (the "guest molecule") is situated in the cavity of the host molecule
without affecting
the framework structure of the host. A "host" is preferably cyclodextrin, but
can be any of the
molecules suggested in U.S. Patent Publ. 200310008818, herein incorporated by
reference.
Cyclodextrins can interact with a variety of ionic and molecular species, and
the resulting
2o inclusion compounds belong to the class of "host-guest" complexes. Within
the host-guest
relationship, the binding sites of the host and guest molecules should be
complementary in the
stereoelectronic sense. A composition of the invention can contain at least
one polymer and at
least one therapeutic 'agent, generally in the form of a particulate composite
of the polymer and
therapeutic agent, e.g., the iRNA agent. The iRNA agent can contain one or
more complexing
agents. At least one polymer of the particulate composite can interact with
the complexing agent
in a host-guest or a guest-host interaction to form an inclusion complex
between the polymer and
the complexing agent. The polymer and, more particularly, the complexing agent
can be used to
introduce functionality into the composition. For example, at least one
polymer of the particulate
composite has host functionality and forms an inclusion complex with a
complexing agent
3o having guest functionality. Alternatively, at least one polymer of the
particulate composite has
guest functionality and forms an inclusion complex with a complexing agent
having host
functionality. A polymer of the particulate composite can also contain both
host and guest
functionalities and form inclusion complexes with guest complexing agents and
host complexing
agents. A polymer with functionality can, for example, facilitate cell
targeting and/or cell
176
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
contact (e.g., targeting or contact to a kidney cell), intercellular
trafficking, and/or cell entry and
release.
Upon forming the particulate composite, the iRNA agent may or may not retain
its
biological or therapeutic activity. Upon release from the therapeutic
composition, specifically,
from the polymer of the particulate composite, the activity of the iRNA agent
is restored.
Accordingly, the particulate composite advantageously affords the iRNA agent
protection
against loss of activity due to, for example, degradation and offers enhanced
bioavailability.
Thus, a composition may be used to provide stability, particularly storage or
solution stability, to
an iRNA agent or any active chemical compound. The iRNA agent may be further
modified
with a ligand prior to or after particulate composite or therapeutic
composition formation. The
ligand can provide further functionality. For example, the ligand can be a
targeting moiety.
Physiolo 'cal Effects
The iRNA agents described herein can be designed such that determining
therapeutic
~ 5 toxicity is made easier by the complementarity of the iRNA agent with both
a human and a non-
human animal sequence. By these methods, an iRNA agent can consist of a
sequence that is
fully complementary to a nucleic acid sequence from a human and a nucleic acid
sequence from
at least one non-human animal, e.g., a non-human mammal, such as a rodent,
ruminant or
primate. For example, the non-human mammal can be a mouse, rat, dog, pig,
goat, sheep, cow,
2o monkey, Pan paniscus, Pan troglodytes, Macaca mulatto, or Cynomolgus
monkey. The sequence
of the iRNA agent could be complementary to sequences within homologous genes,
e.g.,
oncogenes or tumor suppressor genes, of the non-human mammal and the human. By
determining the toxicity of the iRNA agent in the non-human mammal, one can
extrapolate the
toxicity of the iRNA agent in a human. For a more strenuous toxicity test, the
iRNA agent can
25 be complementary to a human and more than one, e.g., two or three or more,
non-human
animals.
The methods described herein can be used to correlate any physiological effect
of an
iRNA agent on a human, e.g., any unwanted effect, such as a toxic effect, or
any positive, or
desired effect.
Delivery Module
The monomers and methods described herein can be used to prepare an RNA, e.g.,
an
iRNA agent described herein, that can be used with a drug delivery conjugate
or module, such as
those described herein and those described in copending, co-owned United
States Provisional
177
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Application Serial No. 60/454,265, filed on March 12, 2003, and International
Application Serial
No. PCT/LTS04/07070, filed March 8, 2004, both of which are hereby
incorporated by reference.
The iRNA agents can be complexed to a delivery agent that features a modular
complex. '
The complex can include a Garner agent linked to one or more of (preferably
two or more, more
preferably all three of): (a) a condensing agent (e.g., an agent capable of
attracting, e.g., binding,
a nucleic acid, e.g., through ionic or electrostatic interactions); (b) a
fusogenic agent (e.g., an
agent capable of fusing and/or being transported through a cell membrane,
e.g., an endosome
membrane); and (c) a targeting group, e.g., a cell or tissue targeting agent,
e.g., a lectin,
glycoprotein, lipid or protein, e.g., an antibody, that binds to a specified
cell type such as a
1 o kidney cell.
An iRNA agent, e.g., iRNA agent or sRNA agent described herein, can be linked,
e.g.,
coupled or bound, to the modular complex. The iRNA agent can interact with the
condensing
agent of the complex, and the complex can be used to deliver an iRNA agent to
a cell, e.g., ih
vitr~ or in vivo. For example, the complex can be used to deliver an iRNA
agent to a subject in
~5 need thereof, e.g., to deliver an iRNA agent to a subject having a
disorder, e.g., a disorder
described herein, such as a disease or disorder of the kidney.
The fusogenic agent and the condensing agent can be different agents or the
one and the
same agent. For example, a polyamino chain, e.g., polyethyleneimine (PEI), can
be the
fusogenic and/or the condensing agent.
20 The delivery agent can be a modular complex. For example, the complex can
include a
carrier agent linked to one or more of (preferably two or more, more
preferably all three of):
(a) a condensing agent (e.g., an agent capable of attracting, e.g., binding, a
nucleic acid,
e.g., through ionic interaction),
(b) a fusogenic agent (e.g., an agent capable of fusing and/or being
transported through a
25 cell membrane, e.g., an endosome membrane), and
(c) a targeting group, e.g., a cell or tissue targeting agent, e.g., a lectin,
glycoprotein, lipid
or protein, e.g., an antibody, that binds to a specified cell type such as a
kidney cell. A targeting
group can be a thyrotropin, melanotropin, lectin, glycoprotein, surfactant
protein A, Mucin
carbohydrate, multivalent lactose, multivalent galactose, N-acetyl-
galactosamine, N-acetyl-
3o gulucosamine multivalent mannose, multivalent fucose, glycosylated
polyaminoacids,
multivalent galactose, transferrin, bisphosphonate, polyglutamate,
polyaspartate, a lipid,
cholesterol, a steroid, bile acid, folate, vitamin B 12, biotin, Neproxin, or
an RGD peptide or
RGD peptide mimetic.
i78
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Carrier agents
The Garner agent of a modular complex described herein can be a substrate for
attachment of one or more of: a condensing agent, a fusogenic agent, and a
targeting group. The
Garner agent would preferably lack an endogenous enzymatic activity. The agent
would
preferably be a biological molecule, preferably a macromolecule. Polymeric
biological Garners
are preferred. It would also be preferred that the Garner molecule be
biodegradable..
The carrier agent can be a naturally occurring substance, such as a protein
(e.g., human
serum albumin (HSA), low-density lipoprotein (LDL), or globulin); carbohydrate
(e.g., a
dextran, pullulan, chitin, chitosan, inulin, cyclodextrin or hyaluronic acid);
or lipid. The Garner
molecule can also be a recombinant or synthetic molecule, such as a synthetic
polymer, e.g., a
synthetic polyamino acid. Examples of polyamino acids include polylysine
(PLL),
poly L-aspartic acid, poly L-glutamic acid, styrene-malefic acid anhydride
copolymer, poly(L-
lactide-co-glycolied) copolymer, divinyl ether-malefic anhydride copolymer, N-
(2-
hydroxypropyl)methacrylamide copolymer (IEVIPA), polyethylene glycol (PEG),
polyvinyl
15 alcohol (PVA), polyurethane, poly(2-ethylacryllic acid), N-
isopropylacrylamide polymers, or
polyphosphazine. Other useful carrier molecules can be identified by routine
methods.
A carrier agent can be characterized by one or more of (a) is at least 1 Da in
size; (b) has
at least 5 charged groups, preferably between 5 and 5000 charged groups; (c)
is present in the
complex at a ratio of at least 1:1 carrier agent to fusogenic agent; (d) is
present in the complex at
2o a ratio of at least 1:1 carrier agent to condensing agent; (e) is present
in the complex at a ratio of
at least 1:1 carrier agent to targeting agent.
Fuso~enic age, nts
A fusogenic agent of a modular complex described herein can be an agent that
is
25 responsive to, e.g., changes charge depending on, the pH environment. Upon
encountering the
pH of an endosome, it can cause a physical change, e.g., a change in osmotic
properties which
disrupts or increases the permeability of the endosome membrane. Preferably,
the fusogenic
agent changes charge, e.g., becomes protonated, at pH lower than physiological
range. For
example, the fusogenic agent can become protonated at pH 4.5-6.5. The
fusogenic agent can
3o serve to release the iRNA agent into the cytoplasm of a cell after the
complex is taken up, e.g.,
via endocytosis, by the cell, thereby increasing the cellular concentration of
the iRNA agent in
the cell.
In one embodiment, the fusogenic agent can have a moiety, e.g., an amino
group, which,
when exposed to a specified pH range, will undergo a change, e.g., in charge,
e.g., protonation.
179
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
The change in charge of the fusogenic agent can trigger a change, e.g., an
osmotic change, in a
vesicle, e.g., an endocytic vesicle, e.g., an endosome. For example, the
fusogenic agent, upon
being exposed to the pH environment of an endosome, will cause a solubility or
osmotic change
substantial enough to increase the porosity of (preferably, to rupture) the
endosomal membrane.
The fusogenic agent can be a polymer, preferably a polyamino chain, e.g.,
polyethyleneimine (PEI). The PEI can be linear, branched, synthetic or
natural. The PEI can be,
e.g., alkyl substituted PEI, or lipid substituted PEI.
In other embodiments, the fusogenic agent can be polyhistidine, polyimidazole,
polypyridine, polypropyleneimine, mellitin, or a polyacetal substance, e.g., a
cationic polyacetal.
In some embodiment, the fusogenic agent can have an,alpha helical structure.
The fusogenic
agent can be a membrane disruptive agent, e.g., mellittin.
A fusogenic agent can have one or more of the following characteristics: (a)
is at least
1Da in size; (b) has at least 10 charged groups, preferably between 10 and
5000 charged groups,
more preferably between 50 and 1000 charged groups; (c) is present in the
complex at a ratio of
~ 5 at least 1:1 fusogenic agent to carrier agent; (d) is present in the
complex at a ratio of at least 1:1
fusogenic agent to condensing agent; (e) is present in the complex at a ratio
of at least 1:1
fusogenic agent to targeting agent.
Other suitable fusogenic agents can be tested and identified by a skilled
artisan. The
ability of a compound to respond to, e.g., change charge depending on, the pH
environment can
2o be tested by routine methods, e.g., in a cellular assay. For example, a
test compound is
combined or contacted with a cell, and the cell is allowed to take up the test
compound, e.g:, by
endocytosis. An endosome preparation can then be made from the contacted cells
and the
endosome preparation compared to an endosome preparation from control cells. A
change, e.g.,
a decrease, in the endosome fraction from the contacted cell vs. the control
cell indicates that the
25 test compound can function as a fusogenic agent. Alternatively, the
contacted cell and control
cell can be evaluated, e.g., by microscopy, e.g., by light or electron
microscopy, to determine a
difference in endosome population in the cells. The test compound can be
labeled. In another
type of assay, a modular complex described herein is constructed using one or
more test or
putative fusogenic agents. The modular complex can be constructed using a
labeled nucleic acid
3o instead of the iRNA. The ability of the fusogenic agent to respond to,
e.g., change charge
depending on, the pH environment, once the modular complex is taken up by the
cell, can be
evaluated, e.g., by preparation of an endosome preparation, or by microscopy
techniques, as
described above. A two-step assay can also be performed, wherein a first assay
evaluates the
ability of a test compound alone to respond to, e.g., change charge depending
on, the pH
180
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
environment; and a second assay evaluates the ability of a modular complex
that includes the test
compound to respond to, e.g., change charge depending on, the pH environment.
Condensing went
The condensing agent of a modular complex described herein can interact with
(e.g.,
attracts, holds, or binds to) an iRNA agent and act to (a) condense, e.g.,
reduce the size or charge
of the iRNA agent and/or (b) protect the iRNA agent, e.g., protect the iRNA
agent against
degradation. The condensing agent can include a moiety, e.g., a charged
moiety, that can
interact with a nucleic acid, e.g., an iRNA agent, e.g., by ionic
interactions. The condensing
agent would preferably be a charged polymer, e.g., a polycationic chain. The
condensing agent
can be a polylysine (PLL), spermine, spermidine, polyamine, pseudopeptide-
polyamine,
peptidomimetic polyamine, dendrimer polyamine, arginine, amidine, protamine,
cationic lipid,
cationic porphyrin, quarternary salt of a polyamine, or an alpha helical
peptide.
A condensing agent can have the following characteristics: (a) at least 1Da in
size; (b)
~ 5 has at least 2 charged groups, preferably between 2 and 100 charged
groups; (c) is present in the
complex at a ratio of at least 1:1 condensing agent to carrier agent; (d) is
present in the complex
at a ratio of at least l :l condensing agent to fusogenic agent; (e) is
present in the complex at a
ratio of at least l :l condensing agent to targeting agent.
Other suitable condensing agents can be tested and identified by a skilled
artisan, e.g., by
2o evaluating the ability of a test agent to interact with a nucleic acid,
e.g., an iRNA agent. The
ability of a test agent to interact with a nucleic acid, e.g.; an iRNA agent,
e.g., to condense; or
protect the iRNA agent, can be evaluated by routine techniques. In one assay,
a test agent is
contacted with a nucleic acid; and the size and/or charge of the contacted
nucleic acid is
evaluated by a technique suitable to detect changes in molecular mass andlor
charge. Such
25 techniques include non-denaturing gel electrophoresis, immunological
methods, e.g.,
immunoprecipitation, gel filtration, ionic interaction chromatography, and the
like. A test agent
is identified as a condensing agent if it changes the mass and/or charge
(preferably both) of the
contacted nucleic acid, compared to a control. A two-step assay can also be
performed, wherein
a first assay evaluates the ability of a test compound alone to interact with,
e.g., bind to, e.g.,
3o condense the charge and/or mass of, a nucleic cid; and a second assay
evaluates the ability of a
modular complex that includes the test compound to interact with, e.g., bind
to, e.g., condense
the charge and/or mass of, a nucleic acid.
181
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Amphipathic Delivery Agents
The monomers and methods described herein can be used to prepare an RNA, e.g.,
an
iRNA agent described herein, that can be used with an amphipathic delivery
conjugate or
module, such as those described herein and those described in copending, co-
owned United
States Provisional Application Serial No. 60/455,050, filed on March 13, 2003,
and International
Application Serial No. PCT/LTS04/07070, filed March 8, 2004, which is hereby
incorporated by
reference.
An amphipathic molecule is a molecule having a hydrophobic and a hydrophilic
region.
Such molecules can interact with (e.g., penetrate or disrupt) lipids, e.g., a
lipid bylayer of a cell.
As such, they can serve as delivery agent for an associated (e.g., bound) iRNA
(e.g., an iRNA or
sRNA described herein). A preferred amphipathic molecule to be used in the
compositions
described herein (e.g., the amphipathic iRNA constructs descriebd herein) is a
polymer. The
polymer may have a secondary structure, e.g., a repeating secondary structure.
One example of an amphipathic polymer is an amphipathic polypeptide, e.g., a
15 polypeptide having a secondary structure such that the polypeptide has a
hydrophilic and a
hybrophobic face. The design of amphipathic peptide structures (e.g., alpha-
helical polypeptides)
is routine to one of skill in the art. For example, the following references
provide guidance:
Grell et al. (2001) "Protein design and folding: template trapping of self
assembled helical
bundles" J Pept Sci 7(3):146-51; Chen et al. (2002) "Determination of
stereochemistry stability
2o coefficients of amino acid side-chains in an amphipathic alpha-helix" J
Pept Res 59(1):18-33;
Iwata et al. (1994) "Design and synthesis of amphipathic 3(10)-helical
peptides and their
interactions with phospholipid bilayers and ion channel formation" J Biol Chem
269(7):4928-33;
Cornut et. al. (1994) "The amphipathic alpha-helix concept. Application to the
de novo design of
ideally amphipathic Leu, Lys peptides with hemolytic activity higher than that
of melittin"
25 FEBS Lett 349(1):29-33; Negrete et al. (1998) "Deciphering the structural
code for proteins:
helical propensities in domain classes and statistical multiresidue
information in alpha-helices,"
Protein Sci 7(6):1368-79.
Another example of an amphipathic polymer is a polymer made up of two or more
amphipathic subunits, e.g., two or more subunits containing cyclic moieties
(e.g., a cyclic moiety
3o having one or more hydrophilic groups and one or more hydrophobic groups).
For example, the
subunit may contain a steroid, e.g., cholic acid; or a aromatic moiety. Such
moieties preferably
can exhibit atropisomerism, such that they can form opposing hydrophobic and
hydrophilic faces
when in a polymer structure.
182
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
The ability of a putative amphipathic molecule to interact with a lipid
membrane, e.g., a
cell membrane, can be tested by routine methods, e.g., in a cell free or
cellular assay. For
example, a test compound is combined or contacted with a synthetic lipid
bilayer, a cellular
membrane fraction, or a cell, and the test compound is evaluated for its
ability to interact with,
penetrate or disrupt the lipid bilayer, cell membrane or cell. The test
compound can labeled in
order to detect the interaction with the lipid bilayer, cell membrane or cell.
In another type of
assay, the test compound is linked to a reporter molecule or an iRNA agent
(e.g., an iRNA or
sRNA described herein) and the ability of the reporter molecule or iRNA agent
to penetrate the
lipid bilayer, cell membrane or cell is evaluated. A two-step assay can also
be performed,
wherein a first assay evaluates the ability of a test compound alone to
interact with a lipid
bilayer, cell membrane or cell; and a second assay evaluates the ability of a
construct (e.g., a
construct described herein) that includes the test compound and a reporter or
iRNA agent to
interact with a lipid bilayer, cell membrane or cell.
An amphipathic polymer useful in the compositions described herein has at
least 2,
15 preferably at least 5, more preferably at least 10, 25, 50, 100, 200, 500,
1000, 2000, 50000 or
more subunits (e.g., amino acids or cyclic subunits). A single amphipathic
polymer can be
linked to one or more, e.g., 2, 3, 5, 10 or more iRNA agents (e.g., iRNA or
sRNA agents
described herein). In some embodiments, an amphipathic polymer can contain
both amino acid
and cyclic subunits, e.g., aromatic subunits.
2o The invention features a composition that includes an iRNA agent (e.g., an
iRNA or
sRNA described herein) in association with an amphipathic molecule. Such
compositions may
be referred to herein as "amphipathic iRNA constructs." Such compositions and
constructs are
useful in the delivery or targeting of iRNA agents, e.g., delivery or
targeting of iRNA agents to a
cell. While not wanting to be bound by theory, such compositions and
constructs can increase
25 the porosity of, e.g., can penetrate or disrupt, a lipid (e.g., a lipid
bilayer of a cell), e.g., to allow
entry of the iRNA agent into a cell.
In one aspect, the invention relates to a composition comprising an iRNA agent
(e.g., an
iRNA or sRNA agent described herein) linked to an amphipathic molecule. The
iRNA agent and
the amphipathic molecule may be held in continuous contact with one another by
either covalent
30 or noncovalent linkages.
The amphipathic molecule of the composition or construct is preferably other
than a
phospholipid, e.g., other than a micelle, membrane or membrane fragment.
The amphipathic molecule of the composition or construct is preferably a
polymer. The
polymer may include two or more amphipathic subunits. One or more hydrophilic
groups and
183
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
one or more hydrophobic groups may be present on the polymer. The polymer may
have a
repeating secondary structure as well as a first face and a second face. The
distribution of the
hydrophilic groups and the hydrophobic groups along the repeating secondary
structure can be
such that one face of the polymer is a hydrophilic face and the other face of
the polymer is a
hydrophobic face.
The amphipathic molecule can be a polypeptide, e.g., a polypeptide comprising
an
a-helical conformation as its secondary structure.
In one embodiment, the amphipathic polymer includes one or more subunits
containing
one or more cyclic moiety (e.g., a cyclic moiety having one or more
hydrophilic groups and/or
one or more hydrophobic groups). In one embodiment, the polymer is a polymer
of cyclic
moieties such that the moieties have alternating hydrophobic and hydrophilic
groups. For
example, the subunit may contain a steroid, e.g., cholic acid. In another
example, the subunit
may contain an aromatic moiety. The aromatic moiety may be one that can
exhibit
atropisomerism, e.g., a 2,2'-bis(substituted)-1-1'-binaphthyl or a 2,2'-
bis(substituted) biphenyl.
~ 5 A subunit may include an aromatic moiety of Formula (M):
184
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
The invention features a composition that includes an iRNA agent (e.g., an
iRNA or
sRNA described herein) in association with an amphipathic molecule. Such
compositions may
be referred to herein as "amphipathic iRNA constructs." Such compositions and
constructs are
useful in the delivery or targeting of iRNA agents, e.g., delivery or
targeting of iRNA agents to a
cell. While not wanting to be bound by theory, such compositions and
constructs can increase
the porosity of, e.g., can penetrate or disrupt, a lipid (e.g., a lipid
bilayer of a cell), e.g., to allow
entry of the iRNA agent into a cell.
In one aspect, the invention relates to a composition comprising an iRNA agent
(e.g., an
iRNA or sRNA agent described herein) linked to an amphipathic molecule. The
iRNA agent and
the amphipathic molecule may be held in continuous contact with one another by
either covalent
15 or noncovalent linkages.
The amphipathic molecule of the composition or construct is preferably other
than a
phospholipid, e.g., other than a micelle, membrane or membrane fragment.
The amphipathic molecule of the composition or construct is preferably a
polymer. The
polymer may include two or more amphipathic subunits. One or more hydrophilic
groups and
20 one or more hydrophobic groups may be present on the polymer. The polymer
may have a
repeating secondary structure as well as a first face and a second face. The
distribution of the
hydrophilic groups and the hydrophobic groups along the repeating secondary
structure can be
185
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
such that one face of the polymer is a hydrophilic face and the other face of
the polymer is a
hydrophobic face.
The amphipathic molecule can be a polypeptide, e.g., a polypeptide comprising
an
a-helical conformation as its secondary structure.
In one embodiment, the amphipathic polymer includes one or more subunits
containing
one or more cyclic moiety (e.g., a cyclic moiety having one or more
hydrophilic groups and/or
one or more hydrophobic groups). In one embodiment, the polymer is a polymer
of cyclic
moieties such that the moieties have alternating hydrophobic and hydrophilic
groups. For
example, the subunit may contain a steroid, e.g., cholic acid. hi another
example, the subunit
1 o may contain an aromatic moiety. The aromatic moiety may be one that can
exhibit
atropisomerism, e.g., a 2,2'-bis(substituted)-1-1'-binaphthyl or a 2,2'-
bis(substituted) biphenyl.
A subunit may include an aromatic moiety of Formula (M):
(M)
186
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Referring to Formula M, Rl is C1-Cloo alkyl optionally substituted with aryl,
alkenyl,
alkynyl, alkoxy or halo and/or optionally inserted with O, S, alkenyl or
alkynyl; C1-Cloo
perfluoroalkyl; or ORS.
R2 is hydroxy; vitro; sulfate; phosphate; phosphate ester; sulfonic acid; OR6;
or Cl-Cloo
alkyl optionally substituted with hydroxy, halo, vitro, aryl or alkyl
sulfinyl, aryl or alkyl sulfonyl,
sulfate, sulfonic acid, phosphate, phosphate ester, substituted or
unsubstituted aryl, carboxyl,
carboxylate, amino carbonyl, or alkoxycarbonyl, and/or optionally inserted
with O, NH, S, S(O),
502, alkenyl, or alkynyl.
R3 is hydrogen, or when taken together with R4 froms a fused phenyl ring.
R4 is hydrogen, or when taken together with R3 froms a fused phenyl ring.
RS is Cl-Cloo alkyl optionally substituted with aryl, allcenyl, alkynyl,
alkoxy or halo
and/or optionally inserted with O, S, alkenyl or alkynyl; or Cl-Cloo
perfluoroalkyl; and R6 is C1-
Cioo alkyl optionally substituted with hydroxy, halo, vitro, aryl or alkyl
sulfinyl, aryl or alkyl
~5 sulfonyl, sulfate, sulfonic acid, phosphate, phosphate ester, substituted
or unsubstituted aryl,
carboxyl, carboxylate, amino carbonyl, or alkoxycarbonyl, and/or optionally
inserted with O,
NH, S, S(O), SOZ, alkenyl, or alkynyl.
Increasing cellular uptake of dsRNAs
2o A method of the invention that can include the administration of an iRNA
agent and a
drug that affects the uptake of the iRNA agent into the cell. The drug can be
administered.
before, after, or at the same time that the iRNA agent is administered. The
drug can be
covalently linked to the iRNA agent. The drug can be, for example, a
lipopolysaccharide, an
activator of p3~ MAP kinase, or an activator of NF-KB. The drug can have a
transient effect on
25 the cell.
The drug can increase the uptake of the iRNA agent into the cell, for example,
by
disrupting the cell's cytoskeleton, e.g., by disrupting the cell's
microtubules, microfilaments,
and/or intermediate filaments. The drug can be, for example, taxon,
vincristine, vinblastine,
cytochalasin, nocodazole, japlakinolide, latrunculin A, phalloidin, swinholide
A, indanocine, or
30 myoservln.
The drug can also increase the uptake of the iRNA agent into the cell by
activating an
inflammatory response, for example. Exemplary drug's that would have such an
effect include
tumor necrosis factor alpha (TNFalpha), interleukin-1 beta, or gamma
interferon.
187
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
iRNA coniu~ates
An iRNA agent can be coupled, e.g., covalently coupled, to a second agent. For
example,
an iRNA agent used to treat a particular disorder can be coupled to a second
therapeutic agent,
e.g., an agent other than the iRNA agent. The second therapeutic agent can be
one which is
directed to the treatment of the same disorder. For example, in the case of an
iRNA used to treat
a disorder characterized by unwanted cell proliferation, e.g., cancer, the
iRNA agent can be
coupled to a second agent which has an anti-cancer effect. For example, it can
be coupled to an
agent which stimulates the immune system, e.g., a CpG motif, or more generally
an agent that
activates a toll-like receptor and/or increases the production of gamma
interferon.
iRNA PRODUCTION
An iRNA can be produced, e.g., in bulk, by a variety of methods. Exemplary
methods
include: organic synthesis and RNA cleavage, e.g., in vitro cleavage.
Or~Lanic Synthesis
An iRNA can be made by separately synthesizing each respective strand of a
double-
stranded RNA molecule. The component strands can then be annealed.
A large bioreactor, e.g., the OligoPilot II from Pharmacia Biotec AB (LJppsala
Sweden),
can be used to produce a large amount of a particular RNA strand for a given
iRNA. The
OligoPilotII reactor can efficiently couple a nucleotide using only a 1.5
molar excess of a
2o phosphoramidite nucleotide. To make an RNA strand, ribonucleotides amidites
are used. .
Standard cycles of monomer addition can be used to synthesize the 21 to 23
nucleotide strand for
the iRNA. Typically, the two complementary strands axe produced separately and
then annealed,
e.g., after release from the solid support and deprotection.
Organic synthesis can be used to produce a discrete iRNA species. The
complementary
of the species to a particular target gene can be precisely specified. For
example, the species
may be complementary to a region that includes a polymorphism, e.g., a single
nucleotide
polymorphism. Further the location of the polymorphism can be precisely
defined. In some
embodiments, the polymorphism is located in an internal region, e.g., at least
4, 5, 7, or 9
nucleotides from one or both of the termini.
188
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
dsRNA Cleavage
iRNAs can also be made by cleaving a larger ds iRNA. The cleavage can be
mediated in
vitro or in vivo. For example, to produce iRNAs by cleavage in vitro, the
following method can
be used:
In vitro transcription. dsRNA is produced by transcribing a nucleic acid (DNA)
segment
in both directions. For example, the HiScribeTM RNAi transcription kit (New
England Biolabs)
provides a vector and a method for producing a dsRNA for a nucleic acid
segment that is cloned
into the vector at a position flanked on either side by a T7 promoter.
Separate templates are
generated for T7 transcription of the two complementary strands for the dsRNA.
The templates
are transcribed in vitv~o by addition of T7 RNA polymerise and dsRNA is
produced. Similar
methods using PCR and/or other RNA polymerises (e.g., T3 or SP6 polymerise)
can also be
used. In one embodiment, RNA generated by this method is carefully purified to
remove
endotoxins that may contaminate preparations of the recombinant enzymes.
In vitro cleavage. dsRNA is cleaved in vitro into iRNAs, for example, using a
Dicer or
comparable RNAse III-based activity. For example, the dsRNA can be incubated
in an ira vitro
extract from Drosophila or using purified components, e.g. a purified RNAse or
RISC complex
(RNA-induced silencing complex ). See, e.g., Ketting et al. Genes Dev 2001 Oct
15;15(20):2654-9. and Hammond Scienee 2001 Aug 10;293(5532):1146-50.
dsRNA cleavage generally produces a plurality of iRNA species, each being a
particular
21 to 23 nt fragment of a source dsRNA molecule. For example, iRNAs that
include sequences
complementary to overlapping regions and adj acent regions of a source dsRNA
molecule may be
present.
Regardless of the method of synthesis, the iRNA preparation can be prepared in
a
solution (e.g., an aqueous and/or organic solution) that is appropriate for
formulation. For
example, the iRNA preparation can be precipitated and redissolved in pure
double-distilled
water, and lyophilized. The dried iRNA can then be resuspended in a solution
appropriate for the
intended formulation process.
Synthesis of modified and nucleotide surrogate iRNA agents is discussed below.
FORMULATION
3o The iRNA agents described herein can be formulated for administration to a
subject
For ease of exposition the formulations, compositions and methods in this
section are
discussed largely with regard to unmodified iRNA agents. It should be
understood, however,
189
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
that these formulations, compositions and methods can be practiced with other
iRNA agents, e.g.,
modified iRNA agents, and such practice is within the invention.
A formulated iRNA composition can assume a variety of states. In some
examples, the
composition is at least partially crystalline, uniformly crystalline, and/or
anhydrous (e.g., less
than 80, 50, 30, 20, or 10% water). In another example, the iRNA is in an
aqueous phase, e.g., in
a solution that includes water.
The aqueous phase or the crystalline compositions can, e.g., be incorporated
into a
delivery vehicle, e.g., a liposome (particularly for the aqueous phase) or a
particle (e.g., a
microparticle as can be appropriate for a crystalline composition). Generally,
the iRNA
composition is formulated in a manner that is compatible with the intended
method of
administration (see, below).
In particular embodiments, the composition is prepared by at least one of the
following
methods: spray drying, lyophilization, vacuum drying, evaporation, fluid bed
drying, or a
combination of these techniques; or sonication with a lipid, freeze-drying,
condensation and
~ 5 other self assembly.
A iRNA preparation can be formulated in combination with another agent, e.g.,
another
therapeutic agent or an agent that stabilizes a iRNA, e.g., a protein that
complexes with iRNA to
form an iRNP. Still other agents include chelators, e.g., EDTA (e.g., to
remove divalent rations
such as Mg2+), salts, RNAse inhibitors (e.g., a broad specificity RNAse
inhibitor such as
2o RNAsin) and so forth.
In one embodiment, the iRNA preparation includes another iRNA agent, e.g., a
second
iRNA that can mediated RNAi with respect to a second gene, or with respect to
the same gene.
Still other preparation can include at least 3, 5, ten, twenty, fifty, or a
hundred or more different
iRNA species. Such iRNAs can mediated RNAi with respect to a similar number of
different
25 genes.
In one embodiment, the iRNA preparation includes at least a second therapeutic
agent
(e.g., an agent other than an RNA or a DNA). For example, a iRNA composition
for the
treatment of a viral disease, e.g. HIV, might include a lmown antiviral agent
(e.g., a protease
inhibitor or reverse transcriptase inhibitor). In another example, a iRNA
composition for the
3o treatment of a cancer might further comprise a chemotherapeutic agent.
Exemplary formulations are discussed below:
190
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Liposomes
For ease of exposition the formulations, compositions and methods in this
section are
discussed largely with regard to unmodified iRNA agents. It should be
understood, however,
that these formulations, compositions and methods can be practiced with other
iRNA agents,
e.g., modified iRNA s agents, and such practice is within the invention. An
iRNA agent, e.g., a
double-stranded iRNA agent, or sRNA agent, (e.g., a precursor, e.g., a larger
iRNA agent which
can be processed into a sRNA agent, or a DNA which encodes an iRNA agent,
e.g., a double-
stranded iRNA agent, or sRNA agent, or precursor thereof) preparation can be
formulated for
delivery in a membranous molecular assembly, e.g., a liposome or a micelle. As
used herein, the
1 o term "liposome" refers to a vesicle composed of amphiphilic lipids
arranged in at least one
bilayer, e.g., one bilayer or a plurality of bilayers. Liposomes include
unilamellar and
multilamellar vesicles that have a membrane formed from a lipophilic material
and an aqueous
interior. The aqueous portion contains the iRNA composition. The lipophilic
material isolates
the aqueous interior from an aqueous exterior, which typically does not
include the iRNA
composition, although in some examples, it may. Liposomes are useful for the
transfer and
delivery of active ingredients to the site of action. Because the liposomal
membrane is
structurally similar to biological membranes, when liposomes are applied to a
tissue, the
liposomal bilayer fuses with bilayer of the cellular membranes. As the merging
of the liposome
and cell progresses, the internal aqueous contents that include the iRNA are
delivered into the
2o cell where the iRNA can specifically bind to a target RNA and can mediate
RNAi. In some
cases the liposomes are also specifically targeted, e.g., to direct the iRNA
to particular cell types,
e.g., to cells of the kidney, such as those described herein.
A liposome containing a iRNA can be prepared by a variety of methods.
In one example, the lipid component of a liposome is dissolved in a detergent
so that
micelles are formed with the lipid component. For example, the lipid component
can be an
amphipathic cationic lipid or lipid conjugate. The detergent can have a high
critical micelle
concentration and may be nonionic. Exemplary detergents include cholate,
CHAPS,
octylglucoside, deoxycholate, and lauroyl sarcosine. The iRNA preparation is
then added to the
micelles that include the lipid component. The cationic groups on the lipid
interact with the
3o iRNA and condense around the iRNA to form a liposome. After condensation,
the detergent is
removed, e.g., by dialysis, to yield a liposomal preparation of iRNA.
If necessary a Garner compound that assists in condensation can be added
during the
condensation reaction, e.g., by controlled addition. For example, the carrier
compound can be a
191
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
polymer other than a nucleic acid (e.g., spermine or spermidine). pH can also
adjusted to favor
condensation.
Further description of methods for producing stable polynucleotide delivery
vehicles,
which incorporate a polynucleotide/cationic lipid complex as structural
components of the
delivery vehicle, are described in, e.g., WO 96137194. Liposome formation can
also include one
or more aspects of exemplary methods described in Felgner, P. L. et al.,
Py~oc. Natl. Acad. Sci.,
USA 8:7413-7417, 1987; U.S. Pat. No. 4,897,355; U.S. Pat. No. 5,171,678;
Bangham, et al. M.
Mol. Biol. 23:238, 1965; Olson, et al. Biochim. Biophys. Acta 557:9, 1979;
Szoka, et al. Proc.
Natl. Acad. Sci. 75: 4194, 1978; Mayhew, et al. Bioclaim. Biophys. Acta
775:169, 1984; Kim, et
al. Bioclaim. Biophys. Acta 728:339, 1983; and Fukunaga, et al. Endocrinol.
115:757, 1984.
Commonly used techniques for preparing lipid aggregates of appropriate size
for use as delivery
vehicles include sonication and freeze-thaw plus extrusion (see, e.g., Mayer,
et al. Biochina.
Biophys. Acta 858:161, 1986). Microfluidization can be used when consistently
small (50 to 200
nm) and relatively uniform aggregates are desired (Mayhew, et al. Biochim.
Biophys. Acta
775:169, 1984). These methods are readily adapted to packaging iRNA
preparations into
liposomes.
Liposomes that are pH-sensitive or negatively-charged, entrap nucleic acid
molecules
rather than complex with them. Since both the nucleic acid molecules and the
lipid are similarly
charged, repulsion rather than complex formation occurs. Nevertheless, some
nucleic acid
2o molecules are entrapped within the aqueous interior of these liposomes. pH-
sensitive hiposomes
have been used to deliver DNA encoding the thymidine kinase gene to cell
monohayers in
culture. Expression of the exogenous gene was detected in the target cells
(Zhou et al., .Iou~nal
of Controlled Release, 19, (1992) 269-274).
One major type of liposomah composition includes phospholipids other than
naturahly-
derived phosphatidylcholine. Neutral liposome compositions, for example, can
be formed from
dimyristoyl phosphatidylcholine (DMPC) or dipalmitoyh phosphatidylcholine
(DPPC). .Anionic
liposome compositions generally are formed from dimyristoyl
phosphatidylglycerol, while
anionic fusogenic liposomes are formed primarily from dioleoyl
phosphatidylethanolamine
(DOPE). Another type of liposomal composition is formed from
phosphatidylcholine (PC) such
3o as, for example, soybean PC, and egg PC. Another type is formed from
mixtures of
phospholipid and/or phosphatidylcholine and/or cholesterol.
Examples of other methods to introduce hiposomes into cells in vitro and in
vivo include
U.S. Pat. No. 5,283,185; U.S. Pat. No. 5,171,678; WO 94/00569; WO 93/24640; WO
91/16024;
Felgner, J. Biol. Cherra. 269:2550, 1994; Nabeh, Proc. Natl. Acad. Sci.
90:11307, 1993; Nabel,
192
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Human Gene TlaeY. 3:649, 1992; Gershon, Bioclaem. 32:7143, 1993; and Strauss
EMBO J.
11:417, 1992.
In one embodiment, cationic liposomes are used. Cationic liposomes possess the
advantage of being able to fuse to the cell membrane. Non-cationic liposomes,
although not able
to fuse as efficiently with the plasma membrane, are taken up by macrophages
in vivo and can be
used to deliver iRNAs to macrophages.
Further advantages of liposomes include: liposomes obtained from natural
phospholipids
are biocompatible and biodegradable; liposomes can incorporate a wide range of
water and lipid
soluble drugs; liposomes can protect encapsulated iRNAs in their internal
compartments from
1o metabolism and degradation (Rosoff, in "Pharmaceutical Dosage Forms,"
Lieberman, Rieger and
Banker (Eds.), 1988, volume 1, p. 245). Important considerations in the
preparation of liposome
formulations are the lipid surface chaxge, vesicle size and the aqueous volume
of the liposomes.
A positively charged synthetic cationic lipid, N-[1-(2,3-dioleyloxy)propyl]-
N,N,N-
trimethylammonium chloride (DOTMA) can be used to form small liposomes that
interact
spontaneously with nucleic acid to form lipid-nucleic acid complexes which are
capable of
fusing with the negatively charged lipids of the cell membranes of tissue
culture cells, resulting
in delivery of iRNA (see, e.~., Felgner, P. L. et al., Proc. Natl. Acad. Sci.,
USA 8:7413-7417,
1987 and U.S. Pat. No. 4,897,355 for a description of DOTMA and its use with
DNA).
A DOTMA analogue, 1,2-bis(oleoyloxy)-3-(trimethylammonia)propane (DOTAP) can
be
2o used in combination with a phospholipid to form DNA-complexing vesicles.
LipofectinTM
Bethesda Research Laboratories, Gaithersburg, Md.) is an effective agent for
the delivery of
highly anionic nucleic acids into living tissue culture cells that comprise
positively charged
DOTMA liposomes which interact spontaneously with negatively charged
polynucleotides to
form complexes. When enough positively charged liposomes are used, the net
charge on the
resulting complexes is also positive. Positively charged complexes prepared in
this way
spontaneously attach to negatively charged cell surfaces, fuse with the plasma
membrane, and
efficiently deliver functional nucleic acids into, for example, tissue culture
cells. Another
commercially available cationic lipid, 1,2-bis(oleoyloxy)-3,3-
(trimethylammonia)propane
("DOTAP") (Boehringer Mannheim, Indianapolis, Indiana) differs from DOTMA in
that the
oleoyl moieties are linked by ester, rather than ether linkages.
Other reported cationic lipid compounds include those that have been
conjugated to a
variety of moieties including, for example, carboxyspermine which has been
conjugated to one
of two types of lipids and includes compounds such as 5-carboxyspermylglycine
dioctaoleoylamide ("DOGS") (TransfectamTM, Promega, Madison, Wisconsin) and
193
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
dipalmitoylphosphatidylethanolamine 5-carboxyspermyl-amide ("DPPES") (see,
e.g., U.S. Pat.
No. 5,171,678).
Another cationic lipid conjugate includes derivatization of the lipid with
cholesterol
("DC-Chol") which has been formulated into liposomes in combination with DOPE
(See, Gao,
X. and Huang, L., Biochim. Biophys. Res. Commun. 179:280, 1991).
Lipopolylysine, made by
conjugating polylysine to DOPE, has been reported to be effective for
transfection in the
presence of serum (Zhou, X. et al., Biochim. Biophys. Acta 1065:8, 1991). For
certain cell lines,
these liposomes containing conjugated cationic lipids, are said to exhibit
lower toxicity and
provide more efficient transfection than the DOTMA-containing compositions.
Other
1 o commercially available cationic lipid products include DMRIE and DMRIE-HP
(Vical, La Jolla,
California) and Lipofectamine (DOSPA) (Life Technology, Inc., Gaithersburg,
Maryland).
Other cationic lipids suitable for the delivery of oligonucleotides are
described in WO 98/39359
and WO 96/37194.
Liposomal formulations are particularly suited for topical administration,
liposomes
~ 5 present several advantages over other formulations. Such advantages
include reduced side
effects related to high systemic absorption of the administered drug,
increased accumulation of
the administered drug at the desired target, and the ability to administer
iRNA, into the skin. In
some implementations, liposomes are used for delivering iRNA to epidermal
cells and also to
enhance the penetration of iRNA into dermal tissues, e.g., into skin. For
example, the liposomes
2o can be applied topically. Topical delivery of drugs formulated as liposomes
to the skin has been
documented (see, e.g., Weiner et al., .Iournal of Drug Targeting, 1992, vol.
2,405-410 and du
Plessis et al., Arativiral Research, 18, 1992, 259-265; Mannino, R. J. and
Fould-Fogerite, S.,
Biotechniques 6:682-690, 1988; Itani, T. et al. Gene 56:267-276. 1987;
Nicolau, C. et al. Meth.
Enz. 149:157-176, 1987; Straubinger, R. M. and Papahadjopoulos, D. Meth. Enz.
101:512-527,
25 1983; Wang, C. Y. and Huang, L., Proc. Natl. Acad. Sci. USA 84:7851-7855,
1987).
Non-ionic liposomal systems have also been examined to determine their utility
in the
delivery of drugs to the skin, in particular systems comprising non-ionic
surfactant and
cholesterol. Non-ionic liposomal formulations comprising Novasome I (glyceryl
dilaurate/cholesterol/polyoxyethylene-10-stearyl ether) and Novasome II
(glyceryl distearate/
3o cholesterol/polyoxyethylene-10-stearyl ether) were used to deliver a drug
into the dermis of
mouse skin. Such formulations with iRNA are useful for treating a
dermatological disorder.
Liposomes that include iRNA can be made highly deformable. Such deformability
can
enable the liposomes to penetrate through pore that are smaller than the
average radius of the
liposome. For example, transfersomes are a type of deformable liposomes.
Transferosomes can
194
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
be made by adding surface edge activators, usually surfactants, to a standard
liposomal
composition. Transfersomes that include iRNA can be delivered, for example,
subcutaneously
by infection in order to deliver iRNA to keratinocytes in the skin. In order
to cross intact
mammalian skin, lipid vesicles must pass through a series of fine pores, each
with a diameter less
than 50 nm, under the influence of a suitable transdermal gradient. In
addition, due to the lipid
properties, these transferosornes can be self optimizing (adaptive to the
shape of pores, e.g., in
the skin), self repairing, and can frequently reach their targets without
fragmenting, and often
self loading: The iRNA agents can include an RRMS tethered to a moiety which
improves
association with a liposome.
Surfactants
For ease of exposition the formulations, compositions and methods in this
section are
discussed largely with regard to unmodified iRNA agents. It should be
understood, however,
that these formulations, compositions and methods can be practiced with other
iRNA agents,
e.g., modified iRNA agents, and such practice is within the invention.
Surfactants find wide
~5 application in formulations such as emulsions (including microemulsions)
and liposomes (see
above). iRNA (or a precursor, e.g., a larger dsRNA which can be processed into
a iRNA, or a
DNA which encodes a iRNA or precursor) compositions can include a surfactant.
In one
embodiment, the iRNA is formulated as an emulsion that includes a surfactant.
The most
common way of classifying and ranking the properties of the many different
types of surfactants,
2o both natural and synthetic, is by the use of the hydrophile/lipophile
balance (HLB). The nature
of the hydrophilic group provides the most useful means for categorizing the
different surfactants
used in formulations (Rieger, in "Pharmaceutical Dosage Forms," Marcel Dekker,
Inc.; New
York, NY, 1988, p. 285).
If the surfactant molecule is not ionized, it is classified as a nonionic
surfactant.
25 Nonionic surfactants find wide application in pharmaceutical products and
are usable over a
wide range of pH values. In general their HLB values range from 2 to about 18
depending on
their structure. Nonionic surfactants include nonionic esters such as ethylene
glycol esters,
propylene glycol esters, glyceryl esters, polyglyceryl esters, sorbitan
esters, sucrose esters, and
ethoxylated esters. Nonionic alkanolamides and ethers such as fatty alcohol
ethoxylates,
3o propoxylated alcohols, and ethoxylated/propoxylated block polymers are also
included in this
class. The polyoxyethylene surfactants are the most popular members of the
nonionic surfactant
class.
195
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
If the surfactant molecule carries a negative charge when it is dissolved or
dispersed in
water, the surfactant is classified as anionic. Anionic surfactants include
carboxylates such as
soaps, acyl lactylates, acyl amides of amino acids, esters of sulfuric acid
such as alkyl sulfates
and ethoxylated alkyl sulfates, sulfonates such as alkyl benzene sulfonates,
aryl isethionates,
acyl taurates and sulfosuccinates, and phosphates. The most important members
of the anionic
surfactant class are the alkyl sulfates and the soaps.
If the surfactant molecule carries a positive charge when it is dissolved or
dispersed in
water, the surfactant is classified as cationic. Cationic surfactants include
quaternary ammonium
salts and ethoxylated amines. The quaternary ammonium salts are the most used
members of
1 o this class.
If the surfactant molecule has the ability to carry either a positive or
negative charge, the
surfactant is classified as amphoteric. Amphoteric surfactants include acrylic
acid derivatives,
substituted alkylamides, N-alkylbetaines and phosphatides.
The use of surfactants in drug products, formulations and in emulsions has
been reviewed
(Rieger, in "Pharmaceutical Dosage Forms," Marcel Dekker, Inc., New York, NY,
1988, p. 285).
Micelles and other Membranous Formulations
For ease of exposition the micelles and other formulations, compositions and
methods in
this section are discussed largely with regard to unmodified iRNA agents. It
should be
understood, however, that these micelles and other formulations, compositions
and methods can
2o be practiced with other iRNA agents, e.g., modified iRNA agents, and such
practice is within the
invention. The iRNA agent, e.g., a double-stranded iRNA agent, or sRNA agent,
(e.g., a
precursor, e.g., a larger iRNA agent which can be processed into a sRNA agent,
or a DNA which
encodes an iRNA agent, e.g., a double-stranded iRNA agent, or sRNA agent, or
precursor
thereof)) composition can be provided as a micellar formulation. "Micelles"
are defined herein
as a particular type of molecular assembly in which amphipathic molecules are
arranged in a
spherical structure such that all the hydrophobic portions of the molecules
are directed inward,
leaving the hydrophilic portions in contact with the surrounding aqueous
phase. The converse
arrangement exists if the environment is hydrophobic.
A mixed micellar formulation suitable for delivery through transdermal
membranes may
3o be prepared by mixing an aqueous solution of the iRNA composition, an
alkali metal C8 to C2z
alkyl sulphate, and a micelle forming compounds. Exemplary micelle forming
compounds
include lecithin, hyaluronic acid, pharmaceutically acceptable salts of
hyaluronic acid, glycolic
acid, lactic acid, chamomile extract, cucumber extract, oleic acid, linoleic
acid, linolenic acid,
196
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
monoolein, monooleates, monolaurates, borage oil, evening of pri~ose oil,
menthol, trihydroxy
oxo cholanyl glycine and pharmaceutically acceptable salts thereof, glycerin,
polyglycerin,
lysine, polylysine, triolein, polyoxyethylene ethers and analogues thereof,
polidocanol alkyl
ethers and analogues thereof, chenodeoxycholate, deoxycholate, and mixtures
thereof. The
micelle forming compounds may be added at the same time or after addition of
the alkali metal
alkyl sulphate. Mixed micelles will form with substantially any kind of mixing
of the ingredients
but vigorous mixing is preferred in order to provide smaller size micelles.
In one method a first micellar composition is prepared which contains the iRNA
composition and at least the alkali metal alkyl sulphate. The first micellar
composition is then
mixed with at least three micelle forming compounds to form a mixed micellar
composition. In
another method, the micellar composition is prepared by mixing the iRNA
composition, the
alkali metal alkyl sulphate and at least one of the micelle forming compounds,
followed by
addition of the remaining micelle forming compounds, with vigorous mixing.
Phenol and/or m-cresol may be added to the mixed micellar composition to
stabilize the
formulation and protect against bacterial growth. Alternatively, phenol and/or
m-cresol may be
added with the micelle forming ingredients. An isotonic agent such as glycerin
may also be
added after formation of the mixed micellar composition.
For delivery of the micellar formulation as a spray, the formulation can be
put into an
aerosol dispenser and the dispenser is charged with a propellant. The
propellant, which is under
2o pressure, is in liquid form in the dispenser. The ratios of the ingredients
are adjusted so that the
aqueous and propellant phases become one, i. e. there is one phase. If there
are two phases, it is
necessary to shake the dispenser prior to dispensing a portion of the
contents, e.g. through a
metered valve. The dispensed dose of pharmaceutical agent is propelled from
the metered valve
in a fine spray.
The preferred propellants are hydrogen-containing chlorofluorocarbons,
hydrogen-
containing fluorocarbons, dimethyl ether and diethyl ether. Even more
preferred is HFA 134a
(1,1,1,2 tetrafluoroethane).
The specific concentrations of the essential ingredients can be determined by
relatively
straightforward experimentation. For absorption through the oral cavities, it
is often desirable to
3o increase, e.g. at least double or triple, the dosage for through injection
or administration through
the gastrointestinal tract.
The iRNA agents can include an RRMS tethered to a moiety which improves
association
with a micelle or other membranous formulation.
197
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Parti ~le~
For ease of exposition the particles, formulations, compositions and methods
in this
section are discussed largely with regard to unmodified iRNA agents. It should
be understood,
however, that these particles, formulations, compositions and methods can be
practiced with
other iRNA agents, e.g., modified iRNA agents, and such practice is within the
invention. In
another embodiment, an iRNA agent, e.g., a double-stranded iRNA agent, or sRNA
agent, (e.g.,
a precursor, e.g., a larger iRNA agent which can be processed into a sRNA
agent, or a DNA
which encodes an iRNA agent, e.g., a double-stranded iRNA agent, or sRNA
agent, or precursor
thereof) preparations may be incorporated into a particle, e.g., a
microparticle. Microparticles
1o can be produced by spray-drying, but may also be produced by other methods
including
lyophilization, evaporation, fluid bed drying, vacuum drying, or a combination
of these
techniques. See below for further description.
Sustained-Release Formulations. An iRNA agent, e.g., a double-stranded iRNA
agent,
or sRNA agent, (e.g., a precursor, e.g., a larger iRNA agent which can be
processed into a sRNA
agent, or a DNA which encodes an iRNA agent, e.g., a double-stranded iRNA
agent, or sRNA
agent, or precursor thereof) described herein can be formulated for
controlled, e.g., slow release.
Controlled release can be achieved by disposing the il2NA within a structure
or substance which
impedes its release. E.g., iRNA can be disposed within a porous matrix or in
an erodable matrix,
either of which allow release of the iRNA over a period of time.
2o Polymeric particles, e.g., polymeric in microparticles can be used as a
sustained-release
reservoir of iRNA that is taken up by cells only released from the
microparticle through
biodegradation. The polymeric particles in this embodiment should therefore be
large enough to
preclude phagocytosis (e.g., larger than 10 ~,m and preferably larger than 20
,um). Such particles
can be produced by the same methods to make smaller particles, but with less
vigorous mixing of
the first and second emulsions. That is to say, a lower homogenization speed,
vortex mixing
speed, or sonication setting can be used to obtain particles having a diameter
around 100 ~,m
rather than 10 ~.m. The time of mixing also can be altered.
Larger microparticles can be formulated as a suspension, a powder, or an
implantable
solid, to be delivered by intramusculax, subcutaneous, intradermal,
intravenous, or intraperitoneal
3o injection; via inhalation (intranasal or intrapulmonary); orally; or by
implantation. These
particles are useful for delivery of any iRNA when slow release over a
relatively long term is
desired. The rate of degradation, and consequently of release, varies with the
polymeric
formulation.
198
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Microparticles preferably include pores, voids, hollows, defects or other
interstitial
spaces that allow the fluid suspension medium to freely permeate or perfuse
the particulate
boundary. For example, the perforated microstructures can be used to form
hollow, porous spray
dried microspheres.
Polymeric particles containing iRNA (e.g., a sRNA) can be made using a double
emulsion technique, for instance. First, the polymer is dissolved in an
organic solvent. A
preferred polymer is polylactic-co-glycolic acid (PLGA), with a
lactic/glycolic acid weight ratio
of 65:35, 50:50, or 75:25. Next, a sample of nucleic acid suspended in aqueous
solution is added
to the polymer solution and the two solutions are mixed to form a first
emulsion. The solutions
can be mixed by vortexing or shaking, and in a preferred method, the mixture
can be sonicated.
Most preferable is any method by which the nucleic acid receives the least
amount of damage in
the form of nicking, shearing, or degradation, while still allowing the
formation of an appropriate
emulsion. For example, acceptable results can be obtained with a Vibra-cell
model VC-250
sonicator with a 1/8" microtip probe, at setting #3.
~ 5 Spray-Drying. An iRNA agent, e.g., a double-stranded iRNA agent, or sRNA
agent,
(e.g., a precursor, e.g., a larger iIRNA agent which can be processed into a
sRNA agent, or a DNA
which encodes an iltNA agent, e.g., a double-stranded iIZNA agent, or sltNA
agent, or precursor
thereof)) can be prepared by spray drying. Spray dried ilRNA can be
administered to a subject or
be subjected to further formulation. A pharmaceutical composition of iRNA can
be prepared by
2o spray drying a homogeneous aqueous mixture that includes a iRNA under
conditions sufficient
to provide a dispersible powdered composition, e.g., a pharmaceutical
composition. The material
for spray drying can also include one or more of a pharmaceutically acceptable
excipient, or a
dispersibility-enhancing amount of a physiologically acceptable, water-soluble
protein. The
spray-dried product can be a dispersible powder that includes the iRNA.
25 Spray drying is a process that converts a liquid or slurry material to a
dried particulate
form. Spray.drying can be used to provide powdered material for various
administrative routes
including inhalation. See, for example, M. Sacchetti and M. M. Van Oort in:
Inhalation Aerosols:
Physical and Biological Basis for Therapy, A. J. Hickey, ed. Marcel Dekkar,
New York, 1996.
Spray drying can include atomizing a solution, emulsion, or suspension to form
a fine
3o mist of droplets and drying the droplets. The mist can be projected into a
drying chamber (e.g., a
vessel, tank, tubing, or coil) where it contacts a drying gas. The mist can
include solid or liquid
pore forming agents. The solvent and pore forming agents evaporate from the
droplets into the
drying gas to solidify the droplets, simultaneously forming pores throughout
the solid. The solid
(typically in a powder, particulate form) then is separated from the drying
gas and collected.
199
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Spray drying includes bringing together a highly dispersed liquid, and a
sufficient volume
of air (e.g., hot air) to produce evaporation and drying of the liquid
droplets. The preparation to
be spray dried can be any solution, course suspension, slurry, colloidal
dispersion, or paste that
may be atomized using the selected spray drying apparatus. Typically, the feed
is sprayed into a
current of warm filtered air that evaporates the solvent and conveys the dried
product to a
collector. The spent air is then exhausted with the solvent. Several different
types of apparatus
may be used to provide the desired product. For example, commercial spray
dryers manufactured
by Buchi Ltd. or Niro Core. can effectively produce particles of desired size.
Spray-dried powdered particles can be approximately spherical in shape, nearly
uniform
in size and frequently hollow. There may be some degree of irregularity in
shape depending
upon the incorporated medicament and the spray drying conditions. In many
instances the
dispersion stability of spray-dried microspheres appears to be more effective
if an inflating agent
(or blowing agent) is used in their production. Particularly preferred
embodiments may comprise
an emulsion with an inflating agent as the disperse or continuous phase (the
other phase being
~5 aqueous in nature). An inflating agent is preferably dispersed with a
surfactant solution, using,
for instance, a commercially available microfluidizer at a pressure of about
5000 to 15,000 psi.
This process forms an emulsion, preferably stabilized by an incorporated
surfactant, typically
comprising submicron droplets of water immiscible blowing agent dispersed in
an aqueous
continuous phase. The formation of such dispersions using this and other
techniques are common
20 and well known to those in the art. The blowing agent is preferably a
fluorinated compound (e.g.
perfluorohexane, perfluorooctyl bromide, perfluorodecalin, perfluorobutyl
ethane) which
vaporizes during the spray-drying process, leaving behind generally hollow,
porous
aerodynamically light microspheres. As will be discussed in more detail below,
other suitable
blowing agents include chloroform, freons, and hydrocarbons. Nitrogen gas and
carbon dioxide
25 are also contemplated as a suitable blowing agent.
Although the perforated microstructures are preferably formed using a blowing
agent as
described above, it will be appreciated that, in some instances, no blowing
agent is required and
an aqueous dispersion of the medicament and surfactants) are spray dried
directly. In such cases,
the formulation may be amenable to process conditions (e.g., elevated
temperatures) that
3o generally lead to the formation of hollow, relatively porous
microparticles. Moreover, the
medicament may possess special physicochemical properties (e.g., high
crystallinity, elevated
melting temperature, surface activity, etc.) that make it particularly
suitable for use in such
techniques.
200
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
The perforated microstructures may optionally be associated with, or comprise,
one or
more surfactants. Moreover, miscible surfactants may optionally be combined
with the
suspension medium liquid phase. It will be appreciated by those skilled in the
art that the use of
surfactants may further increase dispersion stability, simplify formulation
procedures or increase
bioavailability upon administration. Of course combinations of surfactants,
including the use of
one or more in the liquid phase and one or more associated with the perforated
microstructures
are contemplated as being within the scope of the invention. By "associated
with or comprise" it
is meant that the structural matrix or perforated microstructure may
incorporate, adsorb, absorb,
be coated with or be formed by the surfactant.
1o Surfactants suitable for use include any compound or composition that aids
in the
formation and maintenance of the stabilized respiratory dispersions by forming
a layer at the
interface between the structural matrix and the suspension medium. The
surfactant may comprise
a single compound or any combination of compounds, such as in the case of co-
surfactants.
Particularly preferred surfactants are substantially insoluble in the
propellant, nonfluorinated, and
selected from the group consisting of saturated and unsaturated lipids,
nonionic detergents,
nonionic block copolymers, ionic surfactants, and combinations of such agents.
It should be
emphasized that, in addition to the aforementioned surfactants, suitable (i.
e. biocompatible)
fluorinated surfactants are compatible with the teachings herein and may be
used to provide the
desired stabilized preparations.
2o Lipids, including phospholipids, from both natural and synthetic sources
may be used in
varying concentrations to form a structural matrix. Generally, compatible
lipids comprise those
that have a gel to liquid crystal phase transition greater than about
40° C. Preferably, the
incorporated lipids are relatively long chain (i. e. C6 -Ca2) saturated lipids
and more preferably
comprise phospholipids. Exemplary phospholipids useful in the disclosed
stabilized preparations
comprise egg phosphatidylcholine, dilauroylphosphatidylcholine,
dioleylphosphatidylcholine,
dipalmitoylphosphatidyl-choline, disteroylphosphatidylcholine, short-chain
phosphatidylcholines, phosphatidylethanolamine,
dioleylphosphatidylethanolamine,
phosphatidylserine, phosphatidylglycerol, phosphatidylinositol, glycolipids,
ganglioside GM1,
sphingomyelin, phosphatidic acid, cardiolipin; lipids bearing polymer chains
such as,
3o polyethylene glycol, chitin, hyaluronic acid, or polyvinylpyrrolidone;
lipids bearing sulfonated
mono-, di-, and polysaccharides; fatty acids such as palmitic acid, stearic
acid, and oleic acid;
cholesterol, cholesterol esters, and cholesterol hemisuccinate. Due to their
excellent
biocompatibility characteristics, phospholipids and combinations of
phospholipids and
poloxamers are particularly suitable for use in the stabilized dispersions
disclosed herein.
201
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Compatible nonionic detergents comprise: sorbitan esters including sorbitan
trioleate
(SpansTM 85), sorbitan sesquioleate, sorbitan monooleate, sorbitan
monolaurate, polyoxyethylene
(20) sorbitan monolaurate, and polyoxyethylene (20) sorbitan monooleate, oleyl
polyoxyethylene
(2) ether, stearyl polyoxyethylene (2) ether, lauryl polyoxyethylene (4)
ether, glycerol esters, and
sucrose esters. Other suitable nonionic detergents can be easily identified
using McCutcheon's
Emulsifiers and Detergents (McPublishing Co., Glen Rock, N.J.). Preferred
block copolymers
include diblock and triblock copolymers of polyoxyethylene and
polyoxypropylene, including
poloxamer 188 (Pluronic® F68), poloxamer 407 (Pluronic® F-127), and
poloxamer
338. Ionic surfactants such as sodium sulfosuccinate, and fatty acid soaps may
also be utilized. In
1o preferred embodiments, the microstructures may comprise oleic acid or its
alkali salt.
In addition to the aforementioned surfactants, cationic surfactants or lipids
are preferred
especially in the case of delivery of an iRNA agent, e.g., a double-stranded
iRNA agent, or
sRNA agent, (e.g., a precursor, e.g., a larger iRNA agent which can be
processed into a sRNA
agent, or a DNA which encodes an iRNA agent, e.g., a double-stranded iRNA
agent, or sRNA
agent, or precursor thereof). Examples of suitable cationic lipids include:
DOTMA, N-[-(2,3-
dioleyloxy)propyl]-N,N,N-trimethylammonium-chloride; DOTAP,1,2-dioleyloxy-3-
(trimethylammonio)propane; and DOTB, 1,2-dioleyl-3-(4'-
trimethylammonio)butanoyl-sn-
glycerol. Polycationic amino acids such as polylysine, and polyarginine are
also contemplated.
For the spraying process, such spraying methods as rotary atomization,
pressure
2o atomization and two-fluid atomization can be used. Examples of the devices
used in these
processes include "Parubisu [phonetic rendering] Mini-Spray GA-32" and
"Parubisu Spray Drier
DL-41 ", manufactured by Yamato Chemical Co., or "Spray Drier CL-8," "Spray
Drier L-8,"
"Spray Drier FL-12'," "Spray Drier FL-16" or "Spray Drier FL-20," manufactured
by Okawara
Kakoki Co., can be used for the method of spraying using rotary-disk atomizer.
While no particular restrictions are placed on the gas used to dry the sprayed
material, it
is recommended to use air, nitrogen gas or an inert gas. The temperature of
the inlet of the gas
used to dry the sprayed materials such that it does not cause heat
deactivation of the sprayed
material. The range of temperatures may vary between about 50°C to
about 200°C, preferably
between about 50°C and 100°C. The temperature of the outlet gas
used to dry the sprayed
3o material, may vary between about 0°C and about 150°C,
preferably between 0°C and 90°C, and
even more preferably between 0°C and 60°C.
The spray drying is done under conditions that result in substantially
amorphous powder
of homogeneous constitution having a particle size that is respirable, a low
moisture content and
flow characteristics that allow for ready aerosolization. Preferably the
particle size of the
202
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
resulting powder is such that more than about 98% of the mass is in particles
having a diameter
of about 10 ,um or less with about 90% of the mass being in particles having a
diameter less than
pm. Alternatively, about 95% of the mass will have particles with a diameter
of less than 10
,um with about 80% of the mass of the particles having a diameter of less than
5 ,um.
5 The dispersible pharmaceutical-based dry powders that include the iRNA
preparation
may optionally be combined with pharmaceutical carriers or excipients which
are suitable for
respiratory and pulmonary administration. Such carriers may serve simply as
bulking agents
when it is desired to reduce the iRNA concentration in the powder which is
being delivered to a
patient, but may also serve to enhance the stability of the iRNA compositions
and to improve the
1 o dispersibility of the powder within a powder dispersion device in order to
provide more efficient
and reproducible delivery of the iRNA and to improve handling characteristics
of the iRNA such
as flowability and consistency to facilitate manufacturing and powder filling.
Such Garner materials may be combined with the drug prior to spray drying, i.
e., by
adding the carrier material to the purified bulk solution. In that way, the
carrier particles will be
~5 formed simultaneously with the drug particles to produce a homogeneous
powder. Alternatively,
the carriers may be separately prepared in a dry powder form and combined with
the dry powder
drug by blending. The powder carriers will usually be crystalline (to avoid
water absorption), but
might in some cases be amorphous or mixtures of crystalline and amorphous. The
size of the
carrier particles may be selected to improve the flowability of the drug
powder, typically being in
2o the range from 25 ,um to 100 ,um. A preferred carrier material is
crystalline lactose having a size
in the above-stated range.
Powders prepared by any of the above methods will be collected from the spray
dryer in a
conventional manner for subsequent use. For use as pharmaceuticals and other
purposes, it will
frequently be desirable to disrupt any agglomerates which may have formed by
screening or
25 other conventional techniques. For pharmaceutical uses, the dry powder
formulations will
usually be measured into a single dose, and the single dose sealed into a
package. Such packages
axe particularly useful for dispersion in dry powder inhalers, as described in
detail below.
Alternatively, the powders may be packaged in multiple-dose containers.
Methods for spray drying hydrophobic and other drugs and components are
described in
3o U.S. Pat. Nos. 5,000,888; 5,026,550; 4,670,419, 4,540,602; and 4,486,435.
Bloch and Speison
(1983) Pharm. Acta Helv 58:14-22 teaches spray drying of hydrochlorothiazide
and
chlorthalidone (lipophilic drugs) and a hydrophilic adjuvant (pentaerythritol)
in azeotropic
solvents of dioxane-water and 2-ethoxyethanol-water. A number of Japanese
Patent application
Abstracts relate to spray drying of hydrophilic-hydrophobic product
combinations, including JP
203
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
806766; JP 7242568; JP 7101884; JP 7101883; JP 71018982; JP 7101881; and JP
4036233.
Other foreign patent publications relevant to spray drying hydrophilic-
hydrophobic product
combinations include FR 2594693; DE 2209477; and WO 88/07870.
s LYOPHILIZATION.
An iRNA agent, e.g., a double-stranded iRNA agent, or sRNA agent, (e.g., a
precursor,
e.g., a larger iRNA agent which can be processed into a sRNA agent, or a DNA
which encodes
an iRNA agent, e.g., a double-stranded iRNA agent, or sRNA agent, or precursor
thereof)
preparation can be made by lyophilization. Lyophilization is a freeze-drying
process in which
1o water is sublimed from the composition after it is frozen. The particular
advantage associated
with the lyophilization process is that biologicals and pharmaceuticals that
are relatively unstable
in an aqueous solution can be dried without elevated temperatures (thereby
eliminating the
adverse thermal effects), and then stored in a dry state where there are few
stability problems.
With respect to the instant invention such techniques are particularly
compatible with the
~ 5 incorporation of nucleic acids in perforated microstructures without
compromising physiological
activity Methods for providing lyophilized particulates are known to those of
skill in the art and
it would clearly not require undue experimentation to provide dispersion
compatible
microstructures in accordance with the teachings herein. Accordingly, to the
extent that
lyophilization processes may be used to provide microstructures having the
desired porosity and
2o size, they are conformance with the teachings herein and are expressly
contemplated as being
within the scope of the instant invention.
Tar eting
For ease of exposition the formulations, compositions and methods in this
section are
discussed largely with regard to unmodified iRNAs. It should be understood,
however, that
25 these formulations, compositions and methods can be practiced with other
iRNA agents, e.g.,
modified iRNA agents, and such practice is within the invention.
In some embodiments, an iRNA agent, e.g., a double-stranded iRNA agent, or
sRNA
agent, (e.g., a precursor, e.g., a larger iRNA agent which can be processed
into a sRNA agent, or
a DNA which encodes an iRNA agent, e.g., a double-stranded iRNA agent, or sRNA
agent, or
3o precursor thereof) is targeted to' a particular cell. For example, a
liposome or particle or other
structure that includes a iRNA can also include a targeting moiety that
recognizes a specific
molecule on a target cell. The targeting moiety can be a molecule with a
specific affinity for a
target cell. Targeting moieties can include antibodies directed against a
protein found on the
204
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
surface of a target cell, or the ligand or a receptor-binding portion of a
ligand for a molecule
found on the surface of a target cell. For example, the targeting moiety can
recognize a cancer-
specific antigen of the kidney (e.g., G250, CA15-3, CA19-9, CEA, or HER2/neu)
or a viral
antigen, thus delivering the iRNA to a cancer cell or a virus-infected cell.
Exemplary targeting
s moieties include antibodies (such as IgM, IgG, IgA, IgD, and the like, or a
functional portions
thereof), ligands for cell surface receptors (e.g., ectodomains thereof).
Table 4 provides a number of antigens which can be used to target an iRNA to a
selected
cell, such as when targeting of the iRNA agent to a tissue other than the
kidney is desired.
Table 4. Targeting Antigens
ANTIGEN Exemplary tumor tissue
CEA (carcinoembryonic antigen) colon, breast, lung
PSA (prostate specific antigen)prostate cancer
CA-125 ovarian cancer
CA 15-3 breast cancer
CA 19-9 breast cancer
HER2/neu breast cancer
a,-feto protein testicular cancer, hepatic cancer
(3-HCG (human chorionic gonadotropin)testicular cancer, choriocarcinoma
MCTC-1 breast cancer
Estrogen receptor breast cancer, uterine cancer
Progesterone receptor breast cancer, uterine cancer
EGFr (epidermal growth factor bladder cancer
receptor)
In one embodiment, the targeting moiety is attached to a liposome. For
example, US
Patent 6,245,427 describes a method for targeting a liposome using a protein
or peptide. In
15 another example, a cationic lipid component of the liposome is derivatized
with a targeting
moiety For example, WO 96/37194 describes converting N-
glutaryldioleoylphosphatidyl
ethanolamine to a N-hydroxysuccinimide activated ester. The product was then
coupled to an
RGD peptide.
GENES AND DISEASES
2o In one aspect, the invention features, a method of treating a subj ect at
risk for or afflicted
with unwanted cell proliferation, e.g., malignant or nonmalignant cell
proliferation. The method
includes:
providing an iRNA agent, e.g., an sRNA or iRNA agent described herein, e.g.,
an iRNA
having a structure described herein, where the iRNA is homologous to and can
silence, e.g., by
25 cleavage, a gene which promotes unwanted cell proliferation;
205
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
administering an iRNA agent, e.g., an sRNA or iRNA agent described herein to a
subject,
preferably a human subj ect,
thereby treating the subject.
In a preferred embodiment the gene is a growth factor or growth factor
receptor gene, a
kinase, e.g., a protein tyrosine, serine or threonine kinase gene, an adaptor
protein gene, a gene
encoding a G protein superfamily molecule, or a gene encoding a transcription
factor.
In a preferred embodiment the iRNA agent silences the PDGF beta gene, and thus
can be
used to treat a subj ect having or at risk for a disorder characterized by
unwanted PDGF beta
expression, e.g., testicular and lung cancers.
1o In another preferred embodiment the iRNA agent silences the Erb-B gene, and
thus can
be used to treat a subject having or at risk for a disorder characterized by
unwanted Erb-B
expression, e.g., breast cancer.
In a preferred embodiment the iRNA agent silences the Src gene, and thus can
be used to
treat a subject having or at risk for a disorder characterized by unwanted Src
expression, e.g.,
15 colon cancers.
In a preferred embodiment the iRNA agent silences the CRIB gene, and thus can
be used
to treat a subject having or at risk for a disorder characterized by unwanted
CRK expression, e.g., .
colon and lung cancers.
In a preferred embodiment the iRNA agent silences the GRB2 gene, and thus can
be used
2o to treat a subject having or at risk for a disorder characterized by
unwanted GRB2 expression,
e.g., squamous cell carcinoma.
In another preferred embodiment the iRNA agent silences the RAS gene, and thus
can be
used to treat a subj ect having or at risk for a disorder characterized by
unwanted RAS
expression, e.g., pancreatic, colon and lung cancers, and chronic leukemia.
25 In another preferred embodiment the iRNA agent silences the MEKK gene, and
thus can
be used to treat a subject having or at risk for a disorder characterized by
unwanted MEKK
expression, e.g., squamous cell carcinoma, melanoma or leukemia.
In another preferred embodiment the iRNA agent silences the JNK gene, and thus
can be
used to treat a subj ect having or at risk for a disorder characterized by
unwanted JNK expression,
3o e.g., pancreatic or breast cancers.
In a preferred embodiment the iRNA agent silences the RAF gene, and thus can
be used
to treat a subject having or at risk for a disorder characterized by unwanted
RAF expression, e.g.,
lung cancer or leukemia.
206
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
In a preferred embodiment the iRNA agent silences the Erkl/2 gene, and thus
can be used
to treat a subject having or at risk for a disorder characterized by unwanted
Erkl/2 expression,
e.g., lung cancer.
In another preferred embodiment the iRNA agent silences the PCNA(p21) gene,
and thus
can be used to treat a subject having or at risk for a disorder characterized
by unwanted PCNA
expression, e.g., lung cancer.
In a preferred embodiment the iRNA agent silences the MYB gene, and thus can
be used
to treat a subject having or at risk for a disorder characterized by unwanted
MYB expression,
e.g., colon cancer or chronic myelogenous leukemia.
In a preferred embodiment the iRNA agent silences the c-MYC gene, and thus can
be
used to treat a subject having or at risk for a disorder characterized by
unwanted c-MYC
expression, e.g., Burkitt's lymphoma or neuroblastoma.
In another preferred embodiment the iRNA agent silences the JUN gene, and thus
can be
used to treat a subject having or at risk for a disorder characterized by
unwanted JUN expression,
~5 e.g., ovarian, prostate or breast cancers.
In another preferred embodiment the iRNA agent silences the FOS gene, and thus
can be
used to treat a subject having or at risk for a disorder characterized by
unwanted FOS expression,.
e.g., skin or prostate cancers.
In a preferred embodiment the iRNA agent silences the BCL-2 gene, and thus can
be
2o used to treat a subject having or at risk for a disorder characterized by
unwanted BCL-2
expression, e.g., lung or prostate cancers or Non-Hodgkin lymphoma.
In a preferred embodiment the iRNA agent silences the Cyclin D gene, and thus
can be
used to treat a subject having or at risk for a disorder characterized by
unwanted Cyclin D
expression, e.g., esophageal and colon cancers.
25 In a preferred embodiment the iRNA agent silences the VEGF gene, and thus
can be used
to treat a subject having or at risk for a disorder characterized by unwanted
VEGF expression,
e.g., esophageal and colon cancers.
In a preferred embodiment the iRNA agent silences the EGFR gene, and thus can
be used
to treat a subject having or at risk for a disorder characterized by unwanted
EGFR expression,
3o e.g., breast cancer.
In another preferred embodiment the iRNA agent silences the Cyclin A gene, and
thus
can be used to treat a subject having or at risk for a disorder characterized
by unwanted Cyclin A
expression, e.g., lung and cervical cancers.
207
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
In another preferred embodiment the iRNA agent silences the Cyclin E gene, and
thus
can be used to treat a subject having or at risk for a disorder characterized
by unwanted Cyclin E
expression, e.g., lung and breast cancers.
In another preferred embodiment the iRNA agent silences the WNT-1 gene, and
thus can
be used to treat a subject having or at risk for a disorder characterized by
unwanted WNT-1
expression, e.g., basal cell carcinoma.
In axlother preferred embodiment the iRNA agent silences the beta-catenin
gene, and thus
can be used to treat a subject having or at risk for a disorder characterized
by unwanted beta-
catenin expression, e.g., adenocarcinoma or hepatocellular carcinoma.
1 o In another preferred embodiment the iRNA agent silences the c-MET gene,
and thus can
be used to treat a subject having or at risk for a disorder characterized by
unwanted c-MET
expression, e.g., hepatocellular carcinoma.
In another preferred embodiment the iRNA agent silences the PKC gene, and thus
can be
used to treat a subject having or at risk for a disorder characterized by
unwanted PKC
expression, e.g., breast cancer.
In a preferred embodiment the iRNA agent silences the NFKE gene, and thus can
be used.
to treat a subject having or at risk for a disorder characterized by unwanted
NFKB expression,
e.g., breast cancer.
In a preferred embodiment the iRNA agent silences the STAT3 gene, and thus can
be
2o used to treat a subject having or at risk for a disorder characterized by
unwanted STAT3
expression, e.g., prostate cancer.
In another preferred embodiment the iRNA agent silences the survivin gene, and
thus can
be used to treat a subject having or at risk for a disorder characterized by
unwanted survivin
expression, e.g., cervical or pancreatic cancers.
In another preferred embodiment the iRNA agent silences the Her2/Neu gene, and
thus
can be used to treat a subject having or at risk for a disorder characterized
by unwanted
Her2/Neu expression, e.g., breast cancer.
In another preferred embodiment the iRNA agent silences the topoisomerase I
gene, and
thus can be used to treat a subj ect having or at risk for a disorder
characterized by unwanted
3o topoisomerase I expression, e.g., ovarian and colon cancers.
In a preferred embodiment the iRNA agent silences the topoisomerase II alpha
gene, and
thus can be used to treat a subject having or at risk for a disorder
characterized by unwanted
topoisomerase II expression, e.g., breast and colon cancers.
208
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
In a preferred embodiment the iRNA agent silences mutations in the p73 gene,
and thus
can be used to treat a subj ect having or at risk for a disorder characterized
by unwanted p73
expression, e.g., colorectal adenocarcinoma.
In a preferred embodiment the iRNA agent silences mutations in the
p21(WAFl/CIP1)
gene, and thus can be used to treat a subject having or at risk for a disorder
characterized by
unwanted p21(WAFl/CIP1) expression, e.g., liver cancer.
In a preferred embodiment the iRNA agent silences mutations in the p27(KIP1)
gene, and
thus can be used to treat a subject having or at risk for a disorder
characterized by unwanted
p27(KIP1) expression, e.g., liver cancer.
1 o In a preferred embodiment the iRNA agent silences mutations in the PPM1D
gene, and
thus can be used to treat a subject having or at risk for a disorder
characterized by unwanted
PPM1D expression, e.g., breast cancer.
In a preferred embodiment the iRNA agent silences mutations in the RAS gene,
and thus
can be used to treat a subject having or at risk for a disorder characterized
by unwanted RAS
expression, e.g., breast cancer.
In another preferred embodiment the iRNA agent silences mutations in the
caveolin I
gene, and thus can be used to treat a subject having or at risk for a disorder
characterized by
unwanted caveolin I expression, e.g., esophageal squamous cell carcinoma.
In another preferred embodiment the iRNA agent silences mutations in the MIB I
gene,
2o and thus can be used to treat a subject having or at risk for a disorder
characterized by unwanted
MIB I expression, e.g., male breast carcinoma (MBC).
In another preferred embodiment the iRNA agent silences mutations in the MTAI
gene,
and thus can be used to treat a subject having or at risk for a disorder
characterized by unwanted
MTAI expression, e.g., ovarian carcinoma.
In another preferred embodiment the iRNA agent silences mutations in the M68
gene,
and thus can be used to treat a subject having or at risk for a disorder
characterized by unwanted
M68 expression, e.g., human adenocarcinomas of the esophagus, stomach, colon,
and rectum.
In preferred embodiments the iRNA agent silences mutations in tumor suppressor
genes,
and thus can be used as a method to promote apoptotic activity in combination
with
3o chemotherapeutics.
In a preferred embodiment the iRNA agent silences mutations in the p53 tumor
suppressor gene, and thus can be used to treat a subject having or at risk for
a disorder
characterized by unwanted p53 expression, e.g., gall bladder, pancreatic and
lung cancers.
209
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
In a preferred embodiment the iRNA agent silences mutations in the p53 family
member
DN-p63, and thus can be used to treat a subject having or at risk for a
disorder characterized by
unwanted DN-p63 expression, e.g., squamous cell carcinoma
In a preferred embodiment the iRNA agent silences mutations in the pRb tumor
suppressor gene, and thus can be used to treat a subject having or at risk for
a disorder
characterized by unwanted pRb expression, e.g., oral squamous cell carcinoma
In a preferred embodiment the iRNA agent silences mutations in the APC1 tumor
suppressor gene, and thus can be used to treat a subject having or at risk for
a disorder
characterized by unwanted APC 1 expression, e.g., colon cancer.
In a preferred embodiment the iRNA agent silences mutations in the BRCAI tumor
suppressor gene, and thus can be used to treat a subject having or at risk for
a disorder
characterized by unwanted BRCAl expression, e.g., breast cancer.
In a preferred embodiment the iRNA agent silences mutations in the PTEN tumor
suppressor gene, and thus can be used to treat a subject having or at risk for
a disorder
~ 5 characterized by unwanted PTEN expression, e.g., hamartomas, gliomas, and
prostate and
endometrial cancers.
In a preferred embodiment the iRNA agent silences MLL fusion genes, e.g., MLL-
AF9,
and thus can be used to treat a subject having or at risk for a disorder
characterized by unwanted
MLL fusion gene expression, e.g., acute leukemias.
2o In another preferred embodiment the iRNA agent silences the BCR/ABL fusion
gene,
and thus can be used to treat a subject having or at risk for a disorder
characterized by unwanted
BCR/ABL fusion gene expression, e.g., acute and chronic leukemias.
In another preferred embodiment the iRNA agent silences the TEL/AMLl fusion
gene,
and thus can be used to treat a subject having or at risk for a disorder
characterized by unwanted
25 TEL/AML1 fusion gene expression, e.g., childhood acute leukemia.
In another preferred embodiment the iRNA agent silences the EWS/FLI1 fusion
gene,
and thus can be used to treat a subject having or at risk for a disorder
characterized by unwanted
EWS/FLI1 fusion gene expression, e.g., Ewing Sarcoma.
In another preferred embodiment the iRNA agent silences the TLS/FUS1 fusion
gene,
so and thus can be used to treat a subject having or at risk for a disorder
characterized by unwanted
TLS/FUS 1 fusion gene expression, e.g., Myxoid liposarcoma.
In another preferred embodiment the iRNA agent silences the PAX3/FKHR fusion
gene,
and thus can be used to treat a subject having or at risk for a disorder
characterized by unwanted
PAX3/FK_H_R_ fusion gene expression, e.g., Myxoid liposarcoma.
210
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
In another preferred embodiment the iRNA agent silences the AML1/ETO fusion
gene,
and thus can be used to treat a subject having or at risk for a disorder
characterized by unwanted
AML1/ETO fusion gene expression, e.g., acute leukemia.
In another aspect, the invention features, a method of treating a subject,
e.g., a human, at
s risk for or afflicted with a disease or disorder that may benefit by
angiogenesis inhibition e.g.,
cancer. The method includes:
providing an iRNA agent, e.g., an iRNA agent having a structure described
herein, which
iRNA agent is homologous to and can silence, e.g., by cleavage, a gene which
mediates
angiogenesis;
administering the iRNA agent to a subject,
thereby treating the subject.
In a preferred embodiment the iRNA agent silences the alpha v-integrin gene,
and thus
can be used to treat a subject having or at risk for a disorder characterized
by unwanted alpha V
integrin, e.g., brain tumors or tumors of epithelial origin.
~ 5 In a preferred embodiment the iRNA agent silences the Flt-1 receptor gene,
and thus can
be used to treat a subject having or at risk for a disorder characterized by
unwanted Flt-1
receptors, eg. Cancer and rheumatoid arthritis.
In a preferred embodiment the iRNA agent silences the tubulin gene, and thus
can be
used to treat a subject having or at risk for a disorder characterized by
unwanted tubulin, eg.
2o Cancer and retinal neovascularization.
In a preferred embodiment the iRNA agent silences the tubulin gene, and thus
can be
used to treat a subject having or at risk for a disorder characterized by
unwanted tubulin, eg.
Cancer and retinal neovascularization.
In another aspect, the invention features a method of treating a subject
infected with a
25 virus or at risk for or afflicted with a disorder or disease associated
with a viral infection. The
method includes:
providing an iRNA agent, e.g., and iRNA agent having a structure described
herein,
which iRNA agent is homologous to and can silence, e.g., by cleavage, a viral
gene of a cellular
gene which mediates viral function, e.g., entry or growth;
3o administering the iRNA agent to a subject, preferably a human subject,
thereby treating the subject.
Thus, the invention provides for a method of treating patients infected by the
Human
Papilloma Virus (HPV) or at risk for or afflicted with a disorder mediated by
HPV, e.g, cervical
211
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
cancer. HPV is linked to 95% of cervical carcinomas and thus an antiviral
therapy is an
attractive method to treat these cancers and other symptoms of viral
infection.
In a preferred embodiment, the expression of a HPV gene is reduced. In another
preferred embodiment, the HPV gene is one of the group of E2, E6, or E7.
In a preferred embodiment the expression of a human gene that is required for
HPV
replication is reduced.
The invention also includes a method of treating patients infected by the
Human
Immunodeficiency Virus (HIV) or at risk for or afflicted with a disorder
mediated by HIV, e.g.,
Acquired Immune Deficiency Syndrome (AIDS).
1 o In a preferred embodiment, the expression of a HIV gene is reduced. In
another preferred
embodiment, the HIV gene is CCRS, Gag, or Rev.
In a preferred embodiment the expression of a human gene that is required for
HIV
replication is reduced. In another preferred embodiment, the gene is CD4 or
Tsg101.
The invention also includes a method for treating patients infected by the
Hepatitis B
~5 Virus (HBV) or at risk for or afflicted with a disorder mediated by HBV,
e.g., cirrhosis and
heptocellular carcinoma.
In a preferred embodiment, the expression of a HBV gene is reduced. In another
preferred embodiment, the targeted HBV gene encodes one of the group of the
tail region of the
HBV core protein, the pre-cregious (pre-c) region, or the cregious (c) region.
In another
2o preferred embodiment, a targeted HBV-RNA sequence is comprised of the
poly(A) tail.
In preferred embodiment the expression of a human gene that is required for
HBV
replication is reduced.
The invention also provides for a method of treating patients infected by the
Hepatitis A
Virus (HAV), or at risk for or afflicted with a disorder mediated by HAV.
25 In a preferred embodiment the expression of a human gene that is required
for HAV
replication is reduced.
The present invention provides for a method of treating patients infected by
the Hepatitis
C Virus (HCV), or at risk for or afflicted with a disorder mediated by HCV,
e.g., cirrhosis
In a preferred embodiment, the expression of a HCV gene is reduced.
3o In another preferred embodiment the expression of a human gene that is
required for
HCV replication is reduced.
The present invention also provides for a method of treating patients infected
by the any
of the group of Hepatitis Viral strains comprising hepatitis D, E, F, G, or H,
or patients at risk for
or afflicted with a disorder mediated by any of these strains of hepatitis.
212
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
In a preferred embodiment, the expression of a Hepatitis, D, E, F, G, or H
gene is
reduced.
In another preferred embodiment the expression of a human gene that is
required for
hepatitis D, E, F, G or H replication is reduced.
Methods of the invention also provide for treating patients infected by the
Respiratory
Syncytial Virus (RSV) or at risk for or afflicted with a disorder mediated by
RSV, e.g, lower
respiratory tract infection in infants and childhood asthma, pneumonia and
other complications,
e.g., in the elderly.
In a preferred embodiment, the expression of a RSV gene is reduced. In another
1o preferred embodiment, the targeted HBV gene encodes one of the group of
genes N, L, or P.
In a preferred embodiment the expression of a human gene that is required for
RSV
replication is reduced.
Methods of the invention provide for treating patients infected by the Herpes
Simplex
Virus (HSV) or at risk for or afflicted with a disorder mediated by HSV, e.g,
genital herpes and
~ 5 cold sores as well as life-threatening or sight-impairing disease mainly
in immunocompromised
patients.
In a preferred embodiment, the expression of a HSV gene is reduced. In another
preferred embodiment, the targeted HSV gene encodes DNA polymerase or the
helicase-
prnnase.
2o In a preferred embodiment the expression of a human gene that is required
for HSV
replication is reduced.
The invention also provides a method for treating patients infected by the
herpes
Cytomegalovirus (CMV) or at risk for or afflicted with a disorder mediated by
CMV, e.g.,
congenital virus infections and morbidity in immunocompromised patients.
25 In a preferred embodiment, the expression of a CMV gene is reduced.
In a preferred embodiment the expression of a human gene that is required for
CMV
replication is reduced.
Methods of the invention also provide for a method of treating patients
infected by the
herpes Epstein Barr Virus (EBV) or at risk for or afflicted with a disorder
mediated by EBV,
3o e.g., NK/T-cell lymphoma, non-Hodgkin lymphoma, and Hodgkin disease.
In a preferred embodiment, the expression of a EBV gene is reduced.
In a preferred embodiment the expression of a human gene that is required for
EBV
replication is reduced.
213
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Methods of the invention also provide for treating patients infected by
Kaposi's Sarcoma-
associated Herpes Virus (KSHV), also called human herpesvirus 8, or patients
at risk for or
afflicted with a disorder mediated by KSHV, e.g., Kaposi's sarcoma,
multicentric Castleman's
disease and AIDS-associated primary effusion lymphoma.
In a preferred embodiment, the expression of a KSHV gene is reduced.
In a preferred embodiment the expression of a human gene that is required for
KSHV
replication is reduced.
The invention also includes a method for treating patients infected by the JC
Virus (JCV)
or a disease or disorder associated with this virus, e.g., progressive
multifocal
leukoencephalopathy (PML).
In a preferred embodiment, the expression of a JCV gene is reduced.
In preferred embodiment the expression of a human gene that is required for
JCV
replication is reduced.
Methods of the invention also provide for treating patients infected by the
myxovirus or
~5 at risk for or afflicted with a disorder mediated by myxovirus, e.g.,
influenza.
In a preferred embodiment, the expression of a myxovirus gene is reduced.
In a preferred embodiment the expression of a human gene that is required for
myxovirus
replication is reduced.
Methods of the invention also provide for treating patients infected by the
rhinovirus or at
2o risk for of afflicted with a disorder mediated by rhinovirus, e.g., the
common cold.
In a preferred embodiment, the expression of a rhinovirus gene is reduced.
In preferred embodiment the expression of a human gene that is required for
rhinovirus
replication is reduced.
Methods of the invention also provide for treating patients infected by the
coronavirus or
25 at risk for of afflicted with a disorder mediated by coronavirus, e.g., the
common cold.
In a preferred embodiment, the expression of a coronavirus gene is reduced.
In preferred embodiment the expression of a human gene that is required for
coronavirus
replication is reduced.
Methods of the invention also provide for treating patients infected by the
flavivirus West
3o Nile or at risk for or afflicted with a disorder mediated by West Nile
Virus.
In a preferred embodiment, the expression of a West Nile Virus gene is
reduced. In
another preferred embodiment, the West Nile Virus gene is one of the group
comprising E, NS3,
or NSS.
214
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
In a preferred embodiment the expression of a human gene that is required for
West Nile
Virus replication is reduced.
Methods of the invention also provide for treating patients infected by the
St. Louis
Encephalitis flavivirus, or at risk for or afflicted with a disease or
disorder associated with this
virus, e.g., viral haemorrhagic fever or neurological disease.
In a preferred embodiment, the expression of a St. Louis Encephalitis gene is
reduced.
In a preferred embodiment the expression of a human gene that is required for
St. Louis
Encephalitis virus replication is reduced.
Methods of the invention also provide for treating patients infected by the
Tick-borne
1 o encephalitis flavivirus, or at risk for or afflicted with a disorder
mediated by Tick-borne
encephalitis virus, e:g., viral haemorrhagic fever and neurological disease.
In a preferred embodiment, the expression of a Tick-borne encephalitis virus
gene is
reduced.
In a preferred embodiment the expression of a human gene that is required for
Tick-
~ 5 borne encephalitis virus replication is reduced.
Methods of the invention also provide for methods of treating patients
infected by the
Murray Valley encephalitis flavivirus, which commonly results in viral
haemorrhagic fever and
neurological disease.
In a preferred embodiment, the expression of a Murray Valley encephalitis
virus gene is
2o reduced.
In a preferred embodiment the expression of a human gene that is required for
Murray
Valley encephalitis virus replication is reduced.
The invention also includes methods for treating patients infected by the
dengue
flavivirus, or a disease or disorder associated with this virus, e.g., dengue
haemorrhagic fever.
25 In a preferred embodiment, the expression of a dengue virus gene is
reduced.
In a preferred embodiment the expression of a human gene that is required for
dengue
virus replication is reduced.
Methods of the invention also provide for treating patients infected by the
Simian Virus
40 (SV40) or at risk for or afflicted with a disorder mediated by SV40, e.g.,
tumorigenesis.
3o In a preferred embodiment, the expression of a SV40 gene is reduced.
In a preferred embodiment the expression of a human gene that is required for
SV40
replication is reduced.
215
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
The invention also includes methods for treating patients infected by the
Human T Cell
Lymphotropic Virus (HTLV), or a disease or disorder associated with this
virus, e.g., leukemia
and myelopathy.
In a preferred embodiment, the expression of a HTLV gene is reduced. In
another
preferred embodiment the HTLV 1 gene is the Tax transcriptional activator.
In a preferred embodiment the expression of a human gene that is required for
HTLV
replication is reduced.
Methods of the invention also provide for treating patients infected by the
Moloney-
Marine Leukemia Virus (Mo-MuLV) or at risk for or afflicted with a disorder
mediated by Mo-
MuLV, e.g., T-cell leukemia.
In a preferred embodiment, the expression of a Mo-MuLV gene is reduced.
In a preferred embodiment the expression of a human gene that is required for
Mo-MuLV
replication is reduced.
Methods of the invention also provide for treating patients infected by the
~5 encephalomyocarditis virus (EMCV) or at risk for or afflicted with a
disorder mediated by
EMCV, e.g. myocarditis. EMCV leads to myocarditis in mice and pigs and is
capable of
infecting human myocardial cells. This virus is therefore a concern for
patients undergoing
xenotransplantation.
In a preferred embodiment, the expression of a EMCV gene is reduced.
2o In a preferred embodiment the expression of a human gene that is required
for EMCV
replication is reduced.
The invention also includes a method for treating patients infected by the
measles virus
(MV) or at risk for or afflicted with a disorder mediated by MV, e.g. measles.
In a preferred embodiment, the expression of a MV gene is reduced.
25 In a preferred embodiment the expression of a human gene that is required
for MV
replication is reduced.
The invention also includes a method for treating patients infected by the
Vericella zoster
virus (VZV) or at risk for or afflicted with a disorder mediated by VZV, e.g.
chicken pox or
shingles (also called zoster).
3o In a preferred embodiment, the expression of a VZV gene is reduced.
In a preferred embodiment the expression of a human gene that is required for
VZV
replication is reduced.
The invention also includes a method for treating patients infected by an
adenovirus or at
risk for or afflicted with a disorder mediated by an adenovirus, e.g.
respiratory tract infection.
216
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
In a preferred embodiment, the expression of an adenovirus gene is reduced.
In a preferred embodiment the expression of a human gene that is required for
adenovirus
replication is reduced.
The invention includes a method for treating patients infected by a yellow
fever virus
(YFV) or at risk for or afflicted with a disorder mediated by a YFV, e.g.
respiratory tract
infection.
In a preferred embodiment, the expression of a YFV gene is reduced. In another
preferred embodiment, the preferred gene is one of a group that includes the
E, NS2A, or NS3
genes.
In a preferred embodiment the expression of a human gene that is required for
YFV
replication is reduced.
Methods of the invention also provide for treating patients infected by the
poliovirus or at
risk for or afflicted with a disorder mediated by poliovirus, e.g., polio.
In a preferred embodiment, the expression of a poliovirus gene is reduced.
~ 5 In a preferred embodiment the expression of a human gene that is required
for poliovirus
replication is reduced.
Methods of the invention also provide for treating patients infected by a
poxvirus or at
risk for or afflicted with a disorder mediated by a poxvirus, e.g., smallpox
In a preferred embodiment, the expression of a poxvirus gene is reduced.
2o In a preferred embodiment the expression of a human gene that is required
for poxvirus
replication is reduced.
In another, aspect the invention features methods of treating a subject
infected with a
pathogen, e.g., a bacterial, amoebic, parasitic, or fungal pathogen. The
method includes:
providing a iRNA agent, e.g., a siRNA having a structure described herein,
where siRNA
25 is homologous to and can silence, e.g., by cleavage of a pathogen gene;
administering the iRNA agent to a subject, prefereably a human subject,
thereby treating the subject.
The target gene can be one involved in growth, cell wall synthesis, protein
synthesis,
transcription, energy metabolism, e.g., the Krebs cycle, or toxin production.
3o Thus, the present invention provides for a method of treating patients
infected by a
plasmodium that causes malaria.
In a preferred embodiment, the expression of a plasmodium gene is reduced. In
another
preferred embodiment, the gene is apical membrane antigen 1 (AMA1).
217
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
In a preferred embodiment the expression of a human gene that is required for
plasmodium replication is reduced.
The invention also includes methods for treating patients infected by the
Mycobacterium
ulcerans, or a disease or disorder associated with this pathogen, e.g. Buruli
ulcers.
In a preferred embodiment, the expression of a Mycobacterium ulcerans gene is
reduced.
In a preferred embodiment the expression of a human gene that is required for
Mycobacterium ulcerans replication is reduced.
The invention also includes methods for treating patients infected by the
Mycobacterium
tuberculosis, or a disease or disorder associated with this pathogen, e.g.
tuberculosis.
1 o In a preferred embodiment, the expression of a Mycobacterium tuberculosis
gene is
reduced.
In a preferred embodiment the expression of a human gene that is required for
Mycobacterium tuberculosis replication is reduced.
The invention also includes methods for treating patients infected by the
Mycobacterium
~ 5 leprae, or a disease or disorder associated with this pathogen, e.g.
leprosy.
In a preferred embodiment, the expression of a Mycobacterium leprae gene is
reduced.
In a preferred embodiment the expression of a human gene that is required for
Mycobacterium leprae replication is reduced.
The invention also includes methods for treating patients infected by the
bacteria
2o Staphylococcus aureus, or a disease or disorder associated with this
pathogen, e.g. infections of
the skin and muscous membranes.
In a preferred embodiment, the expression of a Staphylococcus aureus gene is
reduced.
In a preferred embodiment the expression of a human gene that is required for
Staphylococcus aureus replication is reduced.
25 The invention also includes methods for treating patients infected by the
bacteria
Streptococcus pneumoniae, or a disease or disorder associated with this
pathogen, e.g.
pneumonia or childhood lower respiratory tract infection.
In a preferred embodiment, the expression of a Streptococcus pneumoniae gene
is
reduced.
3o In a preferred embodiment the expression of a human gene that is required
for
Streptococcus pneumoniae replication is reduced.
The invention also includes methods for treating patients infected by the
bacteria
Streptococcus pyogenes, or a disease or disorder associated with this
pathogen, e.g. Strep throat
or Scarlet fever.
218
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
In a preferred embodiment, the expression of a Streptococcus pyogenes gene is
reduced.
In a preferred embodiment the expression of a human gene that is required for
Streptococcus pyogenes replication is reduced.
The invention also includes methods for treating patients infected by the
bacteria
Chlamydia pneumoniae, or a disease or disorder associated with this pathogen,
e.g. pneumonia
or childhood lower respiratory tract infection
In a preferred embodiment, the expression of a Chlamydia pneumoniae gene is
reduced.
In a preferred embodiment the expression of a human gene that is required for
Chlamydia
pneumoniae replication is reduced.
The invention also includes methods for treating patients infected by the
bacteria
Mycoplasma pneumoniae, or a disease or disorder associated with this pathogen,
e.g. pneumonia
or childhood lower respiratory tract infection
In a preferred embodiment, the expression of a Mycoplasma pneumoniae gene is
reduced.
In a preferred embodiment the expression of a human gene that is required for
~ 5 Mycoplasma pneumoniae replication is reduced.
In one aspect, the invention features, a method of treating a subject, e.g., a
human, at risk
for or afflicted with a disease or disorder characterized by an unwanted
immune response, e.g.,
an inflammatory disease or disorder, or an autoimmune disease or disorder. The
method
includes:
2o providing an iRNA agent, e.g., an iRNA agent having a structure described
herein, which
iRNA agent is homologous to and can silence, e.g., by cleavage, a gene which
mediates an
unwanted immune response;
administering the iRNA agent to a subject,
thereby treating the subj ect.
25 In a preferred embodiment the disease or disorder is an ischemia or
reperfusion injury,
e.g., ischemia or reperfusion injury associated with acute myocardial
infarction, unstable angina,
cardiopulmonary bypass, surgical intervention e.g., angioplasty, e.g.,
percutaneous transluminal
coronary angioplasty, the response to a transplantated organ or tissue, e.g.,
transplanted cardiac
or vascular tissue; or thrombolysis.
3o In a preferred embodiment the disease or disorder is restenosis, e.g.,
restenosis associated
with surgical intervention e.g., angioplasty, e.g., percutaneous transluminal
coronary angioplasty.
In a prefered embodiment the disease or disorder is Inflammatory Bowel
Disease, e.g.,
Crohn Disease or Ulcerative Colitis.
219
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
In a prefered embodiment the disease or disorder is inflammation associated
with an
infection or injury.
In a prefered embodiment the disease or disorder is asthma, lupus, multiple
sclerosis,
diabetes, e.g., type II diabetes, arthritis, e.g., rheumatoid or psoriatic.
In particularly preferred embodiments the iRNA agent silences an integrin or
co-ligand
thereof, e.g., VLA4, VCAM, ICAM.
In particularly preferred embodiments the iRNA agent silences a selectin or co-
ligand
thereof, e.g., P-selectin, E-selectin (ELAM), I-selectin, P-selectin
glycoprotein-1 (PSGL-1).
In particularly preferred embodiments the iRNA agent silences a component of
the
complement system, e.g., C3, C5, C3aR, CSaR, C3 convertase, CS convertase.
In particularly preferred embodiments the iRNA agent silences a chemokine or
receptor
thereof, e.g., TNFI, TNFJ, IL,-lI, IL-1J, IL-2, IL-2R, IL-4, IL-4R, IL-5, IL-
6, IL-8, TNFRI,
TNFRII, IgE, SCYAl l, CCR3.
In other embodiments the iRNA agent silences GCSF, Grol, Gro2, Gro3, PF4, MIG,
Pro-
~5 Platelet Basic Protein (PPBP), MIP-lI, MIP-1J, RANTES, MCP-1, MCP-2, MCP-3,
CMBKRl,
CMBKR2, CMBKR3, CMBKRS, AIF-1, I-309.
In one aspect, the invention features, a method of treating a subject, e.g., a
human, at risk- '
for or afflicted with acute pain or chronic pain. The method includes:
providing an iRNA agent, which iRNA is homologous to and can silence, e.g., by
2o cleavage, a gene which mediates the processing of pain;
administering the iRNA to a subject,
thereby treating the subject.
In particularly preferred embodiments the iRNA agent silences a component of
an ion
channel.
25 In particularly preferred embodiments the iRNA agent silences a
neurotransmitter
receptor or ligand.
In one aspect, the invention features, a method of treating a subj ect, e.g.,
a human, at risk
for or afflicted with a neurological disease or disorder. The method includes:
providing an iRNA agent which iRNA is homologous to and can silence, e.g., by
3o cleavage, a gene which mediates a neurological disease or disorder;
administering the iRNA agent to a subject,
thereby treating the subject.
In a prefered embodiment the disease or disorder is Alzheimer's Disease or
Parkinson
Disease.
220
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
In particularly preferred embodiments the iRNA agent silences an amyloid-
family gene,
e.g., APP; a presenilin gene, e.g., PSENl and PSEN2, or I-synuclein.
In a preferred embodiment the disease or disorder is a neurodegenerative
trinucleotide
repeat disorder, e.g., Huntington disease, dentatorubral pallidoluysian
atrophy or a
spinocerebellar ataxia, e.g., SCAl, SCA2, SCA3 (Machado-Joseph disease), SCA7
or SCAB.
In particularly preferred embodiments the iRNA agent silences HD, DRPLA, SCAT,
SCA2, MJD1, CACNLlA4, SCA7, SCAB.
The loss of heterozygosity (LOH) can result in hemizygosity for sequence,
e.g., genes, in
the area of LOH. This can result in a significant genetic difference between
normal and disease-
state cells, e.g., cancer cells, and provides a,useful difference between
normal and disease-state
cells, e.g., cancer cells. This difference can arise because a gene or other
sequence is
heterozygous in euploid cells but is hemizygous in cells having LOH. The
regions of LOH will
often include a gene, the loss of which promotes unwanted proliferation, e.g.,
a tumor suppressor
gene, and other sequences including, e.g., other genes, in some cases a gene
which is essential
~ 5 for normal function, e.g., growth. Methods of the invention rely, in part,
on the specific cleavage
or silencing of one allele of an essential gene with an iRNA agent of the
invention. The iRNA
agent is selected such that it targets the single allele of the essential gene
found in the cells
having LOH but does not silence the other allele, which is present in cells
which do not show
LOH. In essence, it discriminates between the two alleles, preferentially
silencing the selected
2o allele. In essence polyrnorphisms, e.g., SNPs of essential genes that are
affected by LOH, are
used as a target for a disorder characterized by cells having LOH, e.g.,
cancer cells having LOH.
E.g.; one of ordinary skill in the art can identify essential genes which are
in proximity to
tumor suppressor genes, and which are within a LOH region which includes the
tumor
suppressor gene. The gene encoding the large subunit of human RNA polymerase
II, POLR2A,
25 a gene located in close proximity to the tumor suppressor gene p53, is such
a gene. It frequently
occurs within a region of LOH in cancer cells. Other genes that occur within
LOH regions and
are lost in many cancer cell types include the group comprising replication
protein A 70-kDa
subunit, replication protein A 32-kD, ribonucleotide reductase, thymidilate
synthase, TATA
associated factor 2H, ribosomal protein 514, eukaryotic initiation factor SA,
alanyl tRNA
3o synthetase, cysteinyl tRNA synthetase, NaK ATPase, alpha-1 subunit, and
transferrin receptor.
Accordingly, the invention features, a method of treating a disorder
characterized by
LOH, e.g., cancer. The method includes:
optionally, determining the genotype of the allele of a gene in the region of
LOH and
preferably determining the genotype of both alleles of the gene in a normal
cell;
221
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
providing an iRNA agent which preferentially cleaves or silences the allele
found in the
LOH cells;
administerning the iRNA to the subject,
thereby treating the disorder.
The invention also includes a iRNA agent disclosed herein, e.g, an iRNA agent
which
can preferentially silence, e.g., cleave, one allele of a polymorphic gene
In another aspect, the invention provides a method of cleaving or silencing
more than one
gene with an iRNA agent. In these embodiments the iRNA agent is selected so
that it has
sufficient homology to a sequence found in more than one gene. For example,
the sequence
AAGCTGGCCCTGGACATGGAGAT (SEQ ID NO:28) is conserved between mouse lamin B1,
lamin B2, keratin complex 2-gene 1 and lamin AIC. Thus an iRNA agent targeted
to this
sequence would effectively silence the entire collection of genes.
The invention also includes an iRNA agent disclosed herein, which can silence
more
than one gene.
ROUTE OF DELIVERY
For ease of exposition the formulations, compositions and methods in this
section are
discussed largely with regard to unmodified iRNA agents. It should be
understood, however,
that these formulations, compositions and methods can be practiced with other
iRNA agents,
e.g., modified iRNA agents, and such practice is within the invention. A
composition that
2o includes a iRNA can be delivered to a subject by a variety of routes.
Exemplary routes include:
intravenous, topical, rectal, anal, vaginal, nasal, pulmonary, ocular.
The iRNA molecules of the invention can be incorporated into pharmaceutical
compositions suitable for administration. Such compositions typically include
one or more
species of iRNA 'and a pharmaceutically acceptable Garner. As used herein the
language
"pharmaceutically acceptable carrier" is intended to inclufe any and all
solvents, dispersion
media, coatings, antibacterial and antifungal agents, isotonic and absorption
delaying agents, and
the like, compatible with pharmaceutical administration. The use of such media
and agents for
pharmaceutically active substances is well known in the art. Except insofar as
any conventional
media or agent is incompatible with the active compound, use thereof in the
compositions is
3o contemplated. Supplementary active compounds can also be incorporated into
the compositions.
The pharmaceutical compositions of the present invention may be administered
in a
number of ways depending upon whether local or systemic treatment is desired
and upon the
area to be treated. Administration may be topical (including ophthalmic,
vaginal, rectal,
222
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
intranasal, transdermal), oral or parenteral. Parenteral administration
includes intravenous drip,
subcutaneous, intraperitoneal or intramuscular injection, or intrathecal or
intraventricular
administration.
The route and site of administration may be chosen to enhance targeting. For
example, to
target muscle cells, intramuscular injection into the muscles of interest
would be a logical choice.
Lung cells might be targeted by administering the iRNA in aerosol form. The
vascular
endothelial cells could be targeted by coating a balloon catheter with the
iRNA and mechanically
introducing the DNA.
Formulations for topical administration may include transdermal patches,
ointments,
lotions, creams, gels, drops, suppositories, sprays, liquids and powders.
Conventional
pharmaceutical carriers, aqueous, powder or oily bases, thickeners and the
like may be necessary
or desirable. Coated condoms, gloves and the like may also be useful.
Compositions for oral administration include powders or granules, suspensions
or
solutions in water, syrups, elixirs or non-aqueous media, tablets, capsules,
lozenges, or troches.
~5 In the case of tablets, carriers that can be used include lactose, sodium
citrate and salts of
phosphoric acid. Various disintegrants such as starch, and lubricating agents
such as magnesium
stearate, sodium lauryl sulfate and talc, are commonly used in tablets. For
oral administration in
capsule form, useful diluents are lactose and high molecular weight
polyethylene glycols. When
aqueous suspensions are required for oral use, the nucleic acid compositions
can be combined
2o with emulsifying and suspending agents. If desired, certain sweetening
and/or flavoring agents
can be added.
Compositions for intrathecal or intraventricular administration may include
sterile
aqueous solutions which may also contain buffers, diluents and other suitable
additives.
Formulations for parenteral administration may include sterile aqueous
solutions which
25 may also contain buffers, diluents and other suitable additives.
Intraventricular injection may be
facilitated by an intraventricular catheter, for example, attached to a
reservoir. For intravenous
use, the total concentration of solutes should be controlled to render the
preparation isotonic.
For ocular administration, ointments or droppable liquids may be delivered by
ocular
delivery systems known to the art such as applicators or eye droppers. Such
compositions can
3o include mucomimetics such as hyaluronic acid, chondroitin sulfate,
hydroxypropyl
methylcellulose or polyvinyl alcohol), preservatives such as sorbic acid, EDTA
or
benzylchronium chloride, and the usual quantities of diluents and/or carriers.
223
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Topical DeliverX
For ease of exposition the formulations, compositions and methods in this
section are
discussed largely with regard to unmodified iRNA agents. It should be
understood, however,
that these formulations, compositions and methods can be practiced with other
iRNA agents, e.g.,
modified iRNA agents, and such practice is within the invention. In a
preferred embodiment, an
iRNA agent, e.g., a double-stranded iRNA agent, or sRNA agent, (e.g., a
precursor, e.g., a larger
iRNA agent which can be processed into a sRNA agent, or a DNA which encodes an
iRNA
agent, e.g., a double-stranded iRNA agent, or sRNA agent, or precursor
thereof) is delivered to a
subject via topical administration. "Topical administration" refers to the
delivery to a subject by
1 o contacting the formulation directly to a surface of the subj ect. The most
common form of topical
delivery is to the skin, but a composition disclosed herein can also be
directly applied to other
surfaces of the body, e.g., to the eye, a mucous membrane, to surfaces of a
body cavity or to an
internal surface. As mentioned above, the most common topical delivery is to
the skin. The term
encompasses several routes of administration including, but not limited to,
topical and
transdermal. These modes of administration typically include penetration of
the skin's
permeability barrier and efficient delivery to the target tissue or stratum.
Topical administration
can be used as a means to penetrate the epidermis and dermis and ultimately
achieve systemic
delivery of the composition. Topical administration can also be used as a
means to selectively
deliver oligonucleotides to the epidermis or dermis of a subject, or to
specific strata thereof, or to
2o an underlying tissue.
The term "skin," as used herein, refers to the epidermis and/or dermis of an
animal.
Mammalian skin consists of two major, distinct layers. The outer layer of the
skin is called the
epidermis. The epidermis is comprised of the stratum corneum, the stratum
granulosum, the
stratum spinosum, and the stratum basale, with the stratum corneum being at
the surface of the
skin and the stratum basale being the deepest portion of the epidermis. The
epidermis is between
50 pm and 0.2 mm thick, depending on its location on the body
Beneath the epidermis is the dermis, which is significantly thicker than the
epidermis.
The dermis is primarily composed of collagen in the form of fibrous bundles.
The collagenous
bundles provide support for, inter alia, blood vessels, lymph capillaries,
glands, nerve endings
so and immunologically active cells.
One of the major functions of the skin as an organ is to regulate the entry of
substances
into the body. The principal permeability barrier of the skin is provided by
the stratum corneum,
which is formed from many layers of cells in various states of
differentiation. The spaces
224
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
between cells in the stratum corneum is filled with different lipids arranged
in lattice-like
formations that provide seals to further enhance the skins permeability
barner.
The permeability barrier provided by the skin is such that it is largely
impermeable to
molecules having molecular weight greater than about 750 Da. For larger
molecules to cross the
skin's permeability barrier, mechanisms other than normal osmosis must be
used.
Several factors determine the permeability of the skin to administered agents.
These
factors include the characteristics of the treated skin, the characteristics
of the delivery agent,
interactions between both the drug and delivery agent and the drug and skin,
the dosage of the
drug applied, the form of treatment, and the post treatment regimen. To
selectively target the
1o epidermis and dermis, it is sometimes possible to formulate a composition
that comprises one or
more penetration enhancers that will enable penetration of the drug to a
preselected stratum.
Transdermal delivery is a valuable route for the administration of lipid
soluble
therapeutics. The dermis is more permeable than the epidermis and therefore
absorption is much
more rapid through abraded, burned or denuded skin. Inflammation and other
physiologic
~5 conditions that increase blood flow to the skin also enhance transdermal
adsorption. Absorption
via this route may be enhanced by the use of an oily vehicle (inunction) or
through the use of one
or more penetration enhancers. Other effective ways to deliver a composition
disclosed herein
via the transdermal route include hydration of the skin and the use of
controlled release topical
patches. The transdermal route provides a potentially effective means to
deliver a composition
2o disclosed herein for systemic andlor local therapy.
In addition, iontophoresis (transfer of ionic solutes through biological
membranes under
the influence of an electric field) (Lee et al., Critical Reviews in
Therapeutic Drug Carrier
Systems, 1991, p. 163), phonophoresis or sonophoresis (use of ultrasound to
enhance the
absorption of various therapeutic agents across biological membranes, notably
the skin and the
25 cornea) (Lee et al., Critical Reviews in Therapeutic Drug Carner Systems,
1991, p. 166), and
optimization of vehicle characteristics relative to dose position and
retention at the site of
administration (Lee et al., Critical Reviews in Therapeutic Drug Carner
Systems, 1991, p. 168)
may be useful methods for enhancing the transport of topically applied
compositions across skin
and mucosal sites.
so The compositions and methods provided may also be used to examine the
function of
various proteins and genes in vitro in cultured or preserved dermal tissues
and in animals. The
invention can be thus applied to examine the function of any gene. The methods
of the invention
can also be used therapeutically or prophylactically. For example, for the
treatment of animals
that are known or suspected to suffer from diseases such as psoriasis, lichen
planus, toxic
225
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
epidermal necrolysis, ertythema multiforme, basal cell carcinoma, squamous
cell carcinoma,
malignant melanoma, Paget's disease, Kaposi's sarcoma, pulmonary fibrosis,
Lyme disease and
viral, fungal and bacterial infections of the skin.
Pulmonary Delivery
For ease of exposition the formulations, compositions and methods in this
section are
discussed largely with regard to unmodified iRNA agents. It should be
understood, however,
that these formulations, compositions and methods can be practiced with other
iRNA agents, e.g.,
modified iRNA agents, and such practice is within the invention. A composition
that includes an
iRNA agent, e.g., a double-stranded iRNA agent, or sRNA agent, (e.g., a
precursor, e.g., a larger
iRNA agent which can be processed into a sRNA agent, or a DNA which encodes an
iRNA
agent, e.g., a double-stranded iRNA agent, or sRNA agent, or precursor
thereof) can be
administered to a subj ect by pulmonary delivery. Pulmonary delivery
compositions can be
delivered by inhalation by the patient of a dispersion so that the
composition, preferably iRNA,
within the dispersion can reach the lung where it can be readily absorbed
through the alveolar
15 region directly into blood circulation. Pulmonary delivery can be effective
both for systemic
delivery and for localized delivery to treat diseases of the lungs.
Pulmonary delivery can be achieved by different approaches, including the use
of
nebulized, aerosolized, micellular and dry powder-based formulations. Delivery
can be achieved
with liquid nebulizers, aerosol-based inhalers, and dry powder dispersion
devices. Metered-dose
2o devices are preferred. One of the benefits of using an atomizer or inhaler
is that the potential for
contamination is minimized because the devices are self contained. Dry powder
dispersion
devices, for example, deliver drugs that may be readily formulated as dry
powders. A iRNA
composition may be stably stored as lyophilized or spray-dried powders by
itself or in
combination with suitable powder earners. The delivery of a composition for
inhalation can be
25 mediated by a dosing timing element which can include a timer, a dose
counter, time measuring
device, or a time indicator which when incorporated into the device enables
dose tracking,
compliance monitoring, and/or dose triggering to a patient during
administration of the aerosol
medicament.
The term "powder" means a composition that consists of finely dispersed solid
particles
3o that are free flowing and capable of being readily dispersed in an
inhalation device and
subsequently inhaled by a subject so that the particles reach the lungs to
permit penetration into
the alveoli: Thus, the powder is said to be "respirable." Preferably the
average particle size is
less than about 10 ~,m in diameter preferably with a relatively uniform
spheroidal shape
226
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
distribution. More preferably the diameter is less than about 7.5 ~,m and most
preferably less than
about 5.0 Vim. Usually the particle size distribution is between about 0.1 ,um
and about 5 ,um in
diameter, particularly about 0.3 ~,m to about 5 Vim.
The term "dry" means that the composition has a moisture content below about
10% by
weight (% w) water, usually below about 5% w and preferably less it than about
3% w. A dry
composition can be such that the particles are readily dispersible in an
inhalation device to form
an aerosol.
The term "therapeutically effective amount" is the amount present in the
composition that
is needed to provide the desired level of drug in the subject to be treated to
give the anticipated
1 o physiological response.
The term "physiologically effective amount" is that amount delivered to a subj
ect to give
the desired palliative or curative effect.
The term "pharmaceutically acceptable carrier" means that the carrier can be
taken into
the lungs with no significant adverse toxicological effects on the lungs.
~5 The types of pharmaceutical excipients that are useful as carrier include
stabilizers such
as human serum albumin (HSA), bulking agents such as carbohydrates, amino
acids and
polypeptides; pH adjusters or buffers; salts such as sodium chloride; and the
like. These carriers
may be in a crystalline or amorphous form or may be a mixture of the two.
Bulking agents that are particularly valuable include compatible
carbohydrates,
2o polypeptides, amino acids or combinations thereof. Suitable carbohydrates
include
monosaccharides such as galactose, D-mannose, sorbose, and the like;
disaccharides, such as
lactose, trehalose, and the like; cyclodextrins, such as 2-hydroxypropyl-
.beta.-cyclodextrin; and
polysaccharides, such as raffinose, maltodextrins, dextrans, and the like;
alditols, such as
mannitol, xylitol, and the like. A preferred group of carbohydrates includes
lactose, threhalose,
25 raffinose maltodextrins, and mannitol. Suitable polypeptides include
aspartame. Amino acids
include alanine and glycine, with glycine being preferred.
Additives, which are minor components of the composition of this invention,
may be
included for conformational stability during spray drying and for improving
dispersibility of the
powder. These additives include hydrophobic amino acids such as tryptophan,
tyrosine, leucine,
3o phenylalanine, and the like.
Suitable pH adjusters or buffers include organic salts prepared from organic
acids and
bases, such as sodium citrate, sodium ascorbate, and the like; sodium citrate
is preferred.
Pulmonary administration of a micellar iRNA formulation may be achieved
through
metered dose spray devices with propellants such as tetrafluoroethane,
heptafluoroethane,
227
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
dimethylfluoropropane, tetrafluoropropane, butane, isobutane, dimethyl ether
and other non-CFC
and CFC propellants.
Oral or Nasal Delivery
For ease of exposition the formulations, compositions and methods in this
section are
discussed largely with regard to unmodified iRNA agents. It should be
understood, however,
that these formulations, compositions and methods can be practiced with other
iRNA agents,
e.g., modified iRNA agents, and such practice is within the invention. Both
the oral and nasal
membranes offer advantages over other routes of administration. For example,
drugs
administered through these membranes have a rapid onset of action, provide
therapeutic plasma
levels, avoid first pass effect of hepatic metabolism, and avoid exposure of
the drug to the hostile
gastrointestinal (GI) environment. Additional advantages include easy access
to the membrane
sites so that the drug can be applied, localized and removed easily.
In oral delivery, compositions can be targeted to a surface of the oral
cavity, e.g., to
sublingual mucosa which includes the membrane of ventral surface of the tongue
and the floor of
the mouth or the buccal mucosa which constitutes the lining of the cheek. The
sublingual mucosa
is relatively permeable thus giving rapid absorption and acceptable
bioavailability of many
drugs. Further, the sublingual mucosa is convenient, acceptable and easily
accessible.
The ability of molecules to permeate through the oral mucosa appears to be
related to
molecular size, lipid solubility and peptide protein ionization. Small
molecules, less than 1000
2o daltons appear to cross mucosa rapidly. As molecular size increases, the
permeability decreases
rapidly. Lipid soluble compounds are more permeable than non-lipid soluble
molecules.
Maximum absorption occurs when molecules are un-ionized or neutral in
electrical charges.
Therefore charged molecules present the biggest challenges to absorption
through the oral
mucosae.
A pharmaceutical composition of iRNA may also be administered to the buccal
cavity of
a human being by spraying into the cavity, without inhalation, from a metered
dose spray
dispenser, a mixed micellar pharmaceutical formulation as described above and
a propellant. In
one embodiment, the dispenser is first shaken prior to spraying the
pharmaceutical formulation
and propellant into the buccal cavity.
3o Devices
For ease of exposition the devices, formulations, compositions and methods in
this
section are discussed largely with regard to unmodified iRNA agents. It should
be understood,
228
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
however, that these devices, formulations, compositions and methods can be
practiced with other
iRNA agents, e.g., modified iRNA agents, and such practice is within the
invention. An iRNA
agent, e.g., a double-stranded iRNA agent, or sRNA agent, (e.g., a precursor,
e.g., a larger iRNA
agent which can be processed into a sRNA agent, or a DNA which encodes an iRNA
agent, e.g.,
a double-stranded iRNA agent, or sRNA agent, or precursor thereof) can be
disposed on or in a
device, e.g., a device which implanted or otherwise placed in a subject.
Exemplary devices
include devices which axe introduced into the vasculature, e.g., devices
inserted into the lumen of
a vascular tissue, or which devices themselves form a part of the vasculature,
including stems,
catheters, heart valves, and other vascular devices. These devices, e.g.,
catheters or stems, can be
placed in the vasculature of the lung, heart, or leg.
Other devices include non-vascular devices, e.g., devices implanted in the
peritoneum, or
in organ or glandular tissue, e.g., artificial organs. The device can release
a therapeutic substance
in addition to a iRNA, e.g., a device can release insulin.
Other devices include artificial joints, e.g., hip joints, and other
orthopedic implants.
In one embodiment, unit doses or measured doses of a composition that includes
iRNA
are dispensed by an implanted device. The device can include a sensor that
monitors a parameter
within a subject. For example, the device can include pump, e.g., and,
optionally, associated
electronics.
Tissue, e.g., cells or organs, such as the kidney, can be treated with An iRNA
agent, e.g.,
2o a double-stranded iRNA agent, or sRNA agent, (e.g., a precursor, e.g., a
larger iRNA agent
which can be processed into a sRNA agent, or a DNA which encodes an iRNA
agent, e.g., a
double-stranded iRNA agent, or sRNA agent, or precursor thereof) ex vivo and
then
administered or implanted in a subject.
The tissue can be autologous, allogeneic, or xenogeneic tissue. For example,
tissue (e.g.,
kidney) can be treated to reduce graft v. host disease. In other embodiments,
the tissue is
allogeneic and the tissue is treated to treat a disorder characterized by
unwanted gene expression
in that tissue, such as in the kidney In another example, tissue containing
hematopoietic cells,
e.g., bone marrow hematopoietic cells, can be treated to inhibit unwanted cell
proliferation.
Introduction of treated tissue, whether autologous or transplant, can be
combined with
other therapies.
In some implementations, the iRNA treated cells are insulated from other
cells, e.g., by a
semi-permeable porous barrier that prevents the cells from leaving the
implant, but enables
molecules from the body to reach the cells and molecules produced by the cells
to enter the body
In one embodiment, the porous barrier is formed from alginate.
229
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
hl one embodiment, a contraceptive device is coated with or contains an iRNA
agent,
e.g., a double-stranded iRNA agent, or sRNA agent, (e.g., a precursor, e.g., a
larger iRNA agent
which can be processed into a sRNA agent, or a DNA which encodes an iRNA
agent, e.g., a
double-stranded iRNA agent, or sRNA agent, or precursor thereof). Exemplary
devices include
condoms, diaphragms, IIJD (implantable uterine devices, sponges, vaginal
sheaths, and birth
control devices. In one embodiment, the iRNA is chosen to inactive sperm or
egg. In another
embodiment, the iRNA is chosen to be complementary to a viral or pathogen RNA,
e.g., an RNA
of an STD. In some instances, the iRNA composition can include a spermicide.
D~SAGE
In one aspect, the invention features a method of administering an iRNA agent,
e.g., a
double-stranded iRNA agent, or sRNA agent, to a subject (e.g., a human
subject). The method
includes administering a unit dose of the iRNA agent, e.g., a sRNA agent,
e.g., double stranded
sRNA agent that (a) the double-stranded part is 19-25 nucleotides (nt) long,
preferably 21-23 nt,
(b) is complementary to a target RNA (e.g., an endogenous or pathogen target
RNA), and,
15 optionally, (c) includes at least one 3' overhang 1-5 nucleotide long. In
one embodiment, the unit
dose is less than 1.4 mg per kg of bodyweight, or less than 10, 5, 2, l, 0.5,
0.1, 0.05, 0.01, 0.005,
0.001, 0.0005, 0.0001, 0.00005 or 0.00001 mg per kg of bodyweight, and less
than 200 nmole of
RNA agent (e.g. about 4.4 x 1016 copies) per kg of bodyweight, or less than
1500, 750, 300, 150,
75, 15, 7.5, 1.5, 0.75, 0.15, 0.075, 0.015, 0.0075, 0.0015, 0.00075, 0.00015
nmole of RNA agent
2o per kg of bodyweight.
The defined amount can be an amount effective to treat or prevent a disease or
disorder,
e.g., a disease or disorder associated with the target RNA, such as an RNA
present in the kidney.
The unit dose, for example, can be administered by injection (e.g.,
intravenous or intramuscular),
an inhaled dose, or a topical application. Particularly preferred dosages are
less than 2, 1, or 0.1
25 mglkg of body weight.
In a preferred embodiment, the unit dose is administered less frequently than
once a day,
e.g., less than every 2, 4, 8 or 30 days. In another embodiment, the unit dose
is not administered
with a frequency (e.g., not a regular frequency). For example, the unit dose
may be administered
a single time.
3o In one embodiment, the effective dose is administered with other
traditional therapeutic
modalities. In one embodiment, the subject has a viral infection and the
modality is an antiviral
agent other than an iRNA agent, e.g., other than a double-stranded iRNA agent,
or sRNA agent,.
In another embodiment, the subject has atherosclerosis and the effective dose
of an iRNA agent,
230
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
e.g., a double-stranded iRNA agent, or sRNA agent, is administered in
combination with, e.g.,
after surgical intervention, e.g., angioplasty.
In one embodiment, a subject is administered an initial dose and one or more
maintenance doses of an iRNA agent, e.g., a double-stranded iRNA agent, or
sRNA agent, (e.g.,
a precursor, e.g., a larger iRNA agent which can be processed into a sRNA
agent, or a DNA
which encodes an iRNA agent, e.g., a double-stranded iRNA agent, or sRNA
agent, or precursor
thereof). The maintenance dose or doses are generally lower than the initial
dose, e.g., one-half
less of the initial dose. A maintenance regimen can include treating the subj
ect with a dose or
doses ranging from 0.01 ,ug to 1.4 mg/kg of body weight per day, e.g., 10, 1,
0.1, 0.01, 0.001, or
0.00001 mg per kg of bodyweight per day. The maintenance doses are preferably
administered
no more than once every 5, 10, or 30 days. Further, the treatment regimen may
last for a period
of time which will vary depending upon the nature of the particular disease,
its severity and the
overall condition of the patient. In preferred embodiments the dosage may be
delivered no more
than once per day, e.g., no more than once per 24, 36, 48, or more hours,
e.g., no more than once
for every 5 or 8 days. Following treatment, the patient can be monitored for
changes in his
condition and for alleviation of the symptoms of the disease state. The dosage
of the compound
may either be increased in the event the patient does not respond
significantly to current dosage
levels, or the dose may be decreased if an alleviation of the symptoms of the
disease state is
observed, if the disease state has been ablated, or if undesired side-effects
are observed.
2o The effective dose can be administered in a single dose or in two or more
doses, as
desired or considered appropriate under the specific circumstances. If desired
to facilitate
repeated or frequent infusions, implantation of a delivery device, e.g., a
pump, semi-permanent
stmt (e.g., intravenous, intraperitoneal, intracisternal or intracapsular), or
reservoir may be
advisable.
In one embodiment, the iRNA agent pharmaceutical composition includes a
plurality of
iRNA agent species. In another embodiment, the iRNA agent species has
sequences that are
non-overlapping and non-adjacent to another species with respect to a
naturally occurnng target
sequence. In another embodiment, the plurality of iRNA agent species is
specific for different
naturally occurring target genes. In another embodiment, the iRNA agent is
allele specific.
3o In some cases, a patient is treated with a iRNA agent in conjunction with
other
therapeutic modalities. For example, a patient being treated for a kidney
disease, e.g., early stage
renal disease, can be administered an iRNA agent specific for a target gene
known to enhance the
progression of the disease in conjunction with a drug known to inhibit
activity of the target gene
product. For example, a patient who has early stage renal disease can be
treated with an iRNA
231
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
agent that targets an SGLT2 RNA, in conjunction with the small molecule
phlorizin, which is
known to block sodium-glucose cotransport and to subsequently reduce single
nephron
glomerular filtration rate. In another example, a patient being treated for a
cancer of the kidney
can be administered an iRNA agent specific for a target essential for tumor
cell proliferation in
conjunction with a chemotherapy.
Following successful treatment, it may be desirable to have the patient
undergo
maintenance therapy to prevent the recurrence of the disease state, wherein
the compound of the
invention is administered in maintenance doses, ranging from 0.01 ~.g to 100 g
per kg of body
weight (see US 6,107,094).
The concentration of the iRNA agent composition is an amount sufficient to be
effective
in treating or preventing a disorder or to regulate a physiological condition
in humans. The
concentration or amount of iRNA agent administered will depend on the
parameters determined
for the agent and the method of administration, e.g. nasal, buccal, pulmonary.
For example,
nasal formulations tend to require much lower concentrations of some
ingredients in order to
~ 5 avoid irritation or burning of the nasal passages. It is sometimes
desirable to dilute an oral
formulation up to 10-100 times in order to provide a suitable nasal
formulation.
Certain factors may influence the dosage required to effectively treat a
subject, including
but not limited to the severity of the disease or disorder, previous
treatments, the general health
and/or age of the subject, and other diseases present. Moreover, treatment of
a subject with a
2o therapeutically effective amount of an iRNA agent, e.g., a double-stranded
iRNA agent, or sRNA
agent, (e.g., a precursor, e.g., a larger iRNA agent which can be processed
into a sRNA agent, or
a DNA which encodes an iRNA agent, e.g., a double-stranded iRNA agent, or sRNA
agent, or
precursor thereof) can include a single treatment or, preferably, can include
a series of
treatments. It will also be appreciated that the effective dosage of a iRNA
agent such as a sRNA
25 agent used for treatment may increase or decrease over the course of a
particular treatment.
Changes in dosage may result and become apparent from the results of
diagnostic assays as
described herein. For example, the subject can be monitored after
administering a iRNA agent
composition. Based on information from the monitoring, an additional amount of
the iRNA
agent composition can be administered.
3o Dosing is dependent on severity and responsiveness of the disease condition
to be treated,
with the course of treatment lasting from several days to several months, or
until a cure is
effected or a diminution of disease state is achieved. Optimal dosing
schedules can be calculated
from measurements of drug accumulation in the body of the patient. Persons of
ordinaxy skill can
easily determine optimum dosages, dosing methodologies and repetition rates.
Optimum dosages
232
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
may vary depending on the relative potency of individual compounds, and can
generally be
estimated based on ECSOs found to be effective in in vitro and in vivo animal
models. In some
embodiments, the animal models include transgenic animals that express a human
gene, e.g. a
gene that produces a target RNA. The transgenic animal can be deficient for
the corresponding
endogenous RNA. In another embodiment, the composition for testing includes a
iRNA agent
that is complementary, at least in an internal region, to a sequence that is
conserved between the
target RNA in the animal model and the target RNA in a human.
The inventors have discovered that iRNA agents described herein can be
administered to
mammals, particularly large mammals such as nonhuman primates or humans in a
number of
1 o ways.
In one embodiment, the administration of the iRNA agent, e.g., a double-
stranded iRNA
agent, or sRNA agent, composition is parenteral, e.g. intravenous (e.g., as a
bolus or as a
diffusible infusion), intradermal, intraperitoneal, intramuscular,
intrathecal, intraventricular,
intracranial, subcutaneous, transmucosal, buccal, sublingual, endoscopic,
rectal, oral, vaginal,
~5 topical, pulmonary, intranasal, urethral or ocular. Administration can be
provided by the
subject or by another person, e.g., a health care provider. The medication can
be provided in
measured doses or in a dispenser which delivers a metered dose. Selected modes
of delivery are
discussed in more detail below.
The invention provides methods, compositions, and kits, for rectal
administration or
2o delivery of iRNA agents described herein.
Accordingly, an iRNA agent, e.g., a double-stranded iRNA agent, or sRNA agent,
(e.g.,
a precursor, e.g., a larger iRNA agent which can be processed into a sRNA
agent , or a DNA
which encodes a an iRNA agent, e.g., a double-stranded iRNA agent, or sRNA
agent, or
precursor thereof) described herein, e.g., a therapeutically effective amount
of a iRNA agent
25 described herein, e.g., a iRNA agent having a double stranded region of
less than 40, and
preferably less than 30 nucleotides and having one or two 1-3 nucleotide
single strand 3'
overhangs can be administered rectally, e.g., introduced through the rectum
into the lower or
upper colon. This approach is particularly useful in the treatment of,
inflammatory disorders,
disorders characterized by unwanted cell proliferation, e.g., polyps, or colon
cancer.
3o The medication can be delivered to a site in the colon by introducing a
dispensing device,
e.g., a flexible, camera-guided device similar to that used for inspection of
the colon or removal
of polyps, which includes means for delivery of the medication.
The rectal administration of the iRNA agent is by means of an enema. The iRNA
agent
of the enema can be dissolved in a saline or buffered solution. The rectal
administration can also
233
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
by means of a suppository, which can include other ingredients, e.g., an
excipient, e.g., cocoa
butter or hydropropylmethylcellulose.
Any of the iRNA agents described herein can be administered orally, e.g., in
the form of
tablets, capsules, gel capsules, lozenges, troches or liquid syrups. Further,
the composition can
be applied topically to a surface of the oral cavity.
Any of the iRNA agents described herein can be administered buccally. For
example, the
medication can be sprayed into the buccal cavity or applied directly, e.g., in
a liquid, solid, or gel
form to a surface in the buccal cavity. This administration is particularly
desirable for the
treatment of inflammations of the buccal cavity, e.g., the gums or tongue,
e.g., in one
embodiment, the buccal administration is by spraying into the cavity, e.g.,
without inhalation, ,
from a dispenser, e.g., a metered dose spray dispenser that dispenses the
pharmaceutical
composition and a propellant.
Any of the iRNA agents described herein can be administered to ocular tissue.
For
example, the medications can be applied to the surface of the eye or nearby
tissue, e.g., the inside
~5 of the eyelid. They can be applied topically, e.g., by spraying, in drops,
as an eyewash, or an
ointment. Administration can be provided by the subject or by another person,
e.g., a health care
provider. The medication can be provided in measured doses or in a dispenser
which delivers a
metered dose. The medication can also be administered to the interior of the
eye, and can be
introduced by a needle or other delivery device which can introduce it to a
selected area or
2o structure. Ocular treatment is particularly desirable for treating
inflammation of the eye or
nearby tissue.
Any of the iRNA agents described herein can be administered directly to the
skin. For
example, the medication can be applied topically or delivered in a layer of
the skin, e.g:, by the
use of a microneedle or a battery of microneedles which penetrate into the
skin, but preferably
25 not into the underlying muscle tissue. Administration of the iRNA agent
composition can be
topical. Topical applications can, for example, deliver the composition to the
dermis or
epidermis of a subject. Topical administration can be in the form of
transdermal patches,
ointments, lotions, creams, gels, drops, suppositories, sprays, liquids or
powders. A composition
for topical administration can be formulated as a liposome, micelle, emulsion,
or other lipophilic
3o molecular assembly. The transdermal administration can be applied with at
least one penetration
enhancer, such as iontophoresis, phonophoresis, and sonophoresis.
Any of the iRNA agents described herein can be administered to the pulmonary
system.
Pulmonary administration can be achieved by inhalation or by the introduction
of a delivery
device into the pulmonary system, e.g., by introducing a delivery device which
can dispense the
234
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
medication. A preferred method of pulmonary delivery is by inhalation. The
medication can be
provided in a dispenser which delivers the medication, e.g., wet or dry, in a
form sufficiently
small such that it can be inhaled. The device can deliver a metered dose of
medication. The
subject, or another person, can administer the medication.
Pulmonary delivery is effective not only for disorders which directly affect
pulmonary
tissue, but also for disorders which affect other tissue.
iRNA agents can be formulated as a liquid or nonliquid, e.g., a powder,
crystal, or aerosol
for pulmonary delivery.
Any of the iRNA agents described herein can be administered nasally. Nasal
administration can be achieved by introduction of a delivery device into the
nose, e.g., by
introducing a delivery device which can dispense the medication. Methods of
nasal delivery
include spray, aerosol, liquid, e.g., by drops, or by topical administration
to a surface of the nasal
cavity. The medication can be provided in a dispenser with delivery of the
medication, e.g., wet
or dry, in a form sufficiently small such that it can be inhaled. The device
can deliver a metered
dose of medication. The subject, or another person, can administer the
medication.
Nasal delivery is effective not only for disorders which directly affect nasal
tissue, but
also for disorders which affect other tissue
iRNA agents can be formulated as a liquid or nonliquid, e.g., a powder,
crystal, or for
nasal delivery.
2o An iRNA agent can be packaged in a viral natural capsid or in a chemically
or
enzymatically produced artificial capsid or structure derived therefrom.
The dosage of a pharmaceutical composition including a iRNA agent can be
administered
in order to alleviate the symptoms of a disease state, e.g., cancer or a
cardiovascular disease. A
subject can be treated with the pharmaceutical composition by any of the
methods mentioned
above.
Gene expression in a subject can be modulated by administering a
pharmaceutical
composition including an iRNA agent.
A subject can be treated by administering a defined amount of an iRNA agent,
e.g., a
double-stranded iRNA agent, or sRNA agent, (e.g., a precursor, e.g., a larger
iRNA agent which
3o can be processed into a sRNA agent) composition that is in a powdered form,
e.g., a collection of
microparticles, such as crystalline particles. The composition can include a
plurality of iRNA
agents, e.g., specific for one or more different endogenous target RNAs. The
method can include
other features described herein.
235
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
A subject can be treated by administering a defined amount of an iRNA agent
composition that is prepared by a method that includes spray-drying, i. e.
atomizing a liquid
solution, emulsion, or suspension, immediately exposing the droplets to a
drying gas, and
collecting the resulting porous powder particles. The composition can include
a plurality of
iRNA agents, e.g., specific for one or more different endogenous target RNAs.
The method can
include other features described herein.
The iRNA agent, e.g., a double-stranded iRNA agent, or sRNA agent, (e.g., a
precursor,
e.g., a larger iRNA agent which can be processed into a sRNA agent, or a DNA
which encodes
an iRNA agent, e.g., a double-stranded iRNA agent, or sRNA agent, or precursor
thereof), can be
1 o provided in a powdered, crystallized or other finely divided form, with or
without a Garner, e.g.,
a micro- or nano-particle suitable for inhalation or other pulmonary delivery.
This can include
providing an aerosol preparation, e.g., an aerosolized spray-dried
composition. The aerosol
composition can be provided in and/or dispensed by a metered dose delivery
device.
The subj ect can be treated for a condition treatable by inhalation, e.g., by
aerosolizing a
~ 5 spray-dried iRNA agent, e.g., a double-stranded iRNA agent, or sRNA agent,
(e.g., a precursor,
e.g., a larger iRNA agent which can be processed into a sRNA agent, or a DNA
which encodes
an iRNA agent, e.g., a double-stranded iRNA agent, or sRNA agent, or precursor
thereof)
composition and inhaling the aerosolized composition. The iRNA agent can be an
sRNA. The
composition can include a plurality of iRNA agents, e.g., specific for one or
more different
2o endogenous target RNAs. The method can include other features described
herein.
A subject can be treated by, for example, administering a composition
including an
effectiveldefined amount of an iRNA agent, e.g., a double-stranded iRNA agent,
or sRNA agent,
(e.g., a precursor, e.g., a larger iRNA agent which can be processed into a
sRNA agent, or a
DNA which encodes an iRNA agent, e.g., a double-stranded iRNA agent, or sRNA
agent, or
25 precursor thereof), wherein the composition is prepared by a method that
includes spray-drying,
lyophilization, vacuum drying, evaporation, fluid bed drying, or a combination
of these
techniques
In another aspect, the invention features a method that includes: evaluating a
parameter
related to the abundance of a transcript in a cell of a subject; comparing the
evaluated parameter
3o to a reference value; and if the evaluated parameter has a preselected
relationship to the reference
value (e.g., it is greater), administering a iRNA agent (or a precursor, e.g.,
a larger iRNA agent
which can be processed into a sRNA agent, or a DNA which encodes a iRNA agent
or precursor
thereof) to the subject. In one embodiment, the iRNA agent includes a sequence
that is
complementary to the evaluated transcript. For example, the parameter can be a
direct measure
236
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
of transcript levels, a measure of a protein level, a disease or disorder
symptom or
characterization (e.g., rate of cell proliferation and/or tumor mass, viral
load).
In another aspect, the invention features a method that includes:
administering a first
amount of a composition that comprises an iRNA agent, e.g., a double-stranded
iRNA agent, or
sRNA agent, (e.g., a precursor, e.g., a larger iRNA agent which can be
processed into a sRNA
agent, or a DNA which encodes an iRNA agent, e.g., a double-stranded iRNA
agent, or sRNA
agent, or precursor thereof) to a subj ect, wherein the iRNA agent includes a
strand substantially
complementary to a target nucleic acid; evaluating an activity associated with
a protein encoded
by the target nucleic acid; wherein the evaluation is used to determine if a
second amount should
be administered. In a preferred embodiment the method includes administering a
second amount
of the composition, wherein the timing of administration or dosage of the
second amount is a
function of the evaluating. The method can include other features described
herein.
In another aspect, the invention features a method of administering a source
of a double-
stranded iRNA agent (ds iRNA agent) to a subject. The method includes
administering or
~5 implanting a source of a ds iRNA agent, e.g., a sRNA agent, that (a)
includes a double-stranded
region that is 19-25 nucleotides long, preferably 21-23 nucleotides, (b) is
complementary to a
target RNA (e.g., an endogenous RNA or a pathogen RNA), and, optionally, (c)
includes at least
one 3' overhang 1-5 nt long. In one embodiment, the source releases ds iRNA
agent over time,
e.g. the source is a controlled or a slow release source, e.g., a
microparticle that gradually
2o releases the ds iRNA agent. In another embodiment, the source is a pump,
e.g., a pump that
includes a sensor or a pump that can release one or more unit doses.
In one aspect, the invention features a pharmaceutical composition that
includes an iRNA
agent, e.g., a double-stranded iRNA agent, or sRNA agent, (e.g., a precursor,
e.g., a larger iRNA
agent which can be processed into a sRNA agent, or a DNA which encodes an iRNA
agent, e.g.,
25 a double-stranded iRNA agent, or sRNA agent, or precursor thereof)
including a nucleotide
sequence complementary to a target RNA, e.g., substantially and/or exactly
complementary. The
target RNA can be a transcript of an endogenous human gene. In one embodiment,
the iRNA
agent (a) is 19-25 nucleotides long, preferably 21-23 nucleotides, (b) is
complementary to an
endogenous target RNA, and, optionally, (c) includes at least one 3' overhang
1-5 nt long. In one
3o embodiment, the pharmaceutical composition can be an emulsion,
microemulsion, cream, jelly,
or liposome.
In one example the pharmaceutical composition includes an iRNA agent mixed
with a
topical delivery agent. The topical delivery agent can be a plurality of
microscopic vesicles. The
237
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
microscopic vesicles can be liposomes. In a preferred embodiment the liposomes
are cationic
liposomes.
In another aspect, the pharmaceutical composition includes an iRNA agent,
e.g., a
double-stranded iRNA agent, or sRNA agent (e.g., a precursor, e.g., a larger
iRNA agent which
can be processed into a sRNA agent, or a DNA which encodes an iRNA agent,
e.g., a double-
stranded iRNA agent, or sRNA agent, or precursor thereof) admixed with a
topical penetration
enhancer. In one embodiment, the topical penetration enhancer is a fatty acid.
The fatty acid can
be arachidonic acid, oleic acid, lauric acid, caprylic acid, capric acid,
myristic acid, palmitic acid,
stearic acid, linoleic acid, linolenic acid, dicaprate, tricaprate, monolein,
dilaurin, glyceryl 1-
monocaprate, 1-dodecylazacycloheptan-2-one, an acylcarnitine, an acylcholine,
or a Cl_io alkyl
ester, monoglyceride, diglyceride or pharmaceutically acceptable salt thereof.
In another embodiment, the topical penetration enhancer is a bile salt. The
bile salt can
be cholic acid, dehydrocholic acid, deoxycholic acid, glucholic acid,
glycholic acid,
glycodeoxycholic acid, taurocholic acid, taurodeoxycholic acid,
chenodeoxycholic acid,
ursodeoxycholic acid, sodium tauro-24,25-dihydro-fusidate, sodium
glycodihydrofusidate,
polyoxyethylene-9-lauryl ether or a pharmaceutically acceptable salt thereof.
In another embodiment, the penetration enhancer is a chelating agent. The
chelating
agent can be EDTA, citric acid, a salicyclate, a N-aryl derivative of
collagen, laureth-9, an N-
amino acyl derivative of a beta-diketone or a mixture thereof.
In another embodiment, the penetration enhancer is a surfactant, e.g., an
ionic or nonionic
surfactant. The surfactant can be sodium lauryl sulfate, polyoxyethylene-9-
lauryl ether,
polyoxyethylene-20-cetyl ether, a perfluorchemical emulsion or mixture
thereof.
In another embodiment, the penetration enhancer can be selected from a group
consisting
of msaturated cyclic areas, 1-alkyl-alkones, 1-alkenylazacyclo-alakanones,
steroidal anti-
inflammatory agents and mixtures thereof. In yet another embodiment the
penetration enhancer
can be a glycol, a pyrrol, an azone, or a terpenes.
In one aspect, the invention features a pharmaceutical composition including
an iRNA
agent, e.g., a double-stranded iRNA agent, or sRNA agent, (e.g., a precursor,
e.g., a larger iRNA
agent which can be processed into a sRNA agent, or a DNA which encodes an iRNA
agent, e.g.,
3o a double-stranded iRNA agent, or sRNA agent, or precursor thereof) in a
form suitable for oral
delivery. In one embodiment, oral delivery can be used to deliver an iRNA
agent composition to
a cell or a region of the gastro-intestinal tract, e.g., small intestine,
colon (e.g., to treat a colon
cancer), and so forth. The oral delivery form can be tablets, capsules or gel
capsules. In one
embodiment, the iRNA agent of the pharmaceutical composition modulates
expression of a
238
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
cellular adhesion protein, modulates a rate of cellular proliferation, or has
biological activity
against eukaryotic pathogens or retroviruses. In another embodiment, the
pharmaceutical
composition includes an enteric material that substantially prevents
dissolution of the tablets,
capsules or gel capsules in a mammalian stomach. In a preferred embodiment the
enteric
material is a coating. The coating can be acetate phthalate, propylene glycol,
sorbitan monoleate,
cellulose acetate trimellitate, hydroxy propyl methylcellulose phthalate or
cellulose acetate
phthalate.
In another embodiment, the oral dosage form of the pharmaceutical composition
includes
a penetration enhances. The penetration enhances can be a bile salt or a fatty
acid. The bile salt
1 o can be ursodeoxycholic acid, chenodeoxycholic acid, and salts thereof. The
fatty acid can be
capric acid, lauric acid, and salts thereof.
In another embodiment, the oral dosage form of the pharmaceutical composition
includes
an excipient. In one example the excipient is polyethyleneglycol. In another
example the
excipient is precirol.
In another embodiment, the oral dosage form of the pharmaceutical composition
includes
a plasticizes. The plasticizes can be diethyl phthalate, triacetin dibutyl
sebacate, dibutyl phthalate
or triethyl citrate.
In one aspect, the invention features a pharmaceutical composition including
an iRNA
agent and a delivery vehicle. In one embodiment, the iRNA agent is (a) is 19-
25 nucleotides
long, preferably 21-23 nucleotides, (b) is complementary to an endogenous
target RNA, and,
optionally, (c) includes at least one 3' overhang 1-5 nucleotides long.
In one embodiment, the delivery vehicle can deliver an iRNA agent, e.g., a
double-
stranded iRNA agent, or sRNA agent, (e.g., a precursor, e.g., a larger iRNA
agent which can be
processed into a sRNA agent, or a DNA which encodes an iRNA agent, e.g., a
double-stranded
iRNA agent, or sRNA agent, or precursor thereof) to a cell by a topical route
of administration.
The delivery vehicle can be microscopic vesicles. In one example the
microscopic vesicles are
liposomes. In a preferred embodiment the liposomes are cationic liposomes. In
another example
the microscopic vesicles are micelles.In one aspect, the invention features a
pharmaceutical
composition including an iRNA agent, e.g., a double-stranded iRNA agent, or
sRNA agent, (e.g.,
3o a precursor, e.g., a larger iRNA agent which can be processed into a sRNA
agent, or a DNA
which encodes an iRNA agent, e.g., a double-stranded iRNA agent, or sRNA
agent, or precursor
thereof) in an injectable dosage form. In one embodiment, the injectable
dosage form of the
pharmaceutical composition includes sterile aqueous solutions or dispersions
and sterile
239
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
powders. In a preferred embodiment the sterile solution can include a diluent
such as water;
saline solution; fixed oils, polyethylene glycols, glycerin, or propylene
glycol.
In one aspect, the invention features a pharmaceutical composition including
an iRNA
agent, e.g., a double-stranded iRNA agent, or sRNA agent, (e.g., a precursor,
e.g., a larger iRNA
agent which can be processed into a sRNA agent, or a DNA which encodes an iRNA
agent, e.g.,
a double-stranded iRNA agent, or sRNA agent, or precursor thereof) in oral
dosage form. In one
embodiment, the oral dosage form is selected from the group consisting of
tablets, capsules and
gel capsules. In another embodiment, the pharmaceutical composition includes
an enteric
material that substantially prevents dissolution of the tablets, capsules or
gel capsules in a
mammalian stomach. In a preferred embodiment the enteric material is a
coating. The coating
can be acetate phthalate, propylene glycol, sorbitan monoleate, cellulose
acetate trimellitate,
hydroxy propyl methyl cellulose phthalate or cellulose acetate phthalate. In
one embodiment,
the oral dosage form of the pharmaceutical composition includes a penetration
enhancer, e.g., a
penetration enhancer described herein.
~5 In one aspect, the invention features a pharmaceutical composition
including an iRNA
agent, e.g., a double-stranded iRNA agent, or sRNA agent, (e.g., a precursor,
e.g., a larger iRNA
agent which can be processed into a sRNA agent, or a DNA which encodes an iRNA
agent, e.g.,
a double-stranded iRNA agent, or sRNA agent, or precursor thereof) in a rectal
dosage form. In
one embodiment, the rectal dosage form is an enema. In another embodiment, the
rectal dosage
2o form is a suppository.
In one aspect, the invention features a pharmaceutical composition including
an iRNA
agent, e.g., a double-stranded iRNA agent, or sRNA agent, (e.g., a precursor,
e.g., a larger iRNA
agent which can be processed into a sRNA agent, or a DNA which encodes an iRNA
agents e.g.,
a double-stranded iRNA agent, or sRNA agent, or precursor thereof) in a
vaginal dosage form.
25 In one embodiment, the vaginal dosage form is a suppository. In another
embodiment, the
vaginal dosage form is a foam, cream, or gel.
In one aspect, the invention features a pharmaceutical composition including
an iRNA
agent, e.g., a double-stranded iRNA agent, or sRNA agent, (e.g., a precursor,
e.g., a larger iRNA
agent which can be processed into a sRNA agent, or a DNA which encodes an iRNA
agent, e.g.,
3o a double-stranded iRNA agent, or sRNA agent, or precursor thereof) in a
pulmonary or nasal
dosage form. In one embodiment, the iRNA agent is incorporated into a
particle, e.g., a
macroparticle, e.g., a microsphere. The particle can be produced by spray
drying, lyophilization,
evaporation, fluid bed drying, vacuum drying, or a combination thereof. The
microsphere can be
formulated as a suspension, a powder, or an implantable solid.
240
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
In one aspect, the invention features a spray-dried iRNA agent, e.g., a double-
stranded
iRNA agent, or sRNA agent, (e.g., a precursor, e.g., a larger iRNA agent which
can be processed
into a sRNA agent, or a DNA which encodes an iRNA agent, e.g., a double-
stranded iRNA
agent, or sRNA agent, or precursor thereof) composition suitable for
inhalation by a subject,
including: (a) a therapeutically effective amount of a iRNA agent suitable for
treating a condition
in the subject by inhalation; (b) a pharmaceutically acceptable excipient
selected from the group
consisting of carbohydrates and amino acids; and (c) optionally, a
dispersibility-enhancing
amount of a physiologically-acceptable, water-soluble polypeptide.
In one embodiment, the excipient is a carbohydrate. The carbohydrate can be
selected
from the group consisting of monosaccharides, disaccharides, trisaccharides,
and
polysaccharides. In a preferred embodiment the carbohydrate is a
monosaccharide selected from
the group consisting of dextrose, galactose, mannitol, D-mannose, sorbitol,
and sorbose. In
another preferred embodiment the carbohydrate is a disaccharide selected from
the group
consisting of lactose, maltose, sucrose, and trehalose.
~ 5 In another embodiment, the excipient is an amino acid. In one embodiment,
the amino
acid is a hydrophobic amino acid. In a preferred embodiment the hydrophobic
amino acid is
selected from the group consisting of alanine, isoleucine, leucine,
methionine, phenylalanine,
proline, tryptophan, and valine. In yet another embodiment the amino acid is a
polar amino acid.
In a preferred embodiment the amino acid is selected from the group consisting
of arginine,
2o histidine, lysine, cysteine, glycine, glutamine, serine, threonine,
tyrosine, aspartic acid and
glutamic acid.
In one embodiment, the dispersibility-enhancing polypeptide is selected from
the group
consisting of human serum albumin, a-lactalbumin, trypsinogen, and
polyalanine.
In one embodiment, the spray-dried iRNA agent composition includes particles
having a
25 mass median diameter (MlVm) of less than 10 microns. In another embodiment,
the spray-dried
iRNA agent composition includes particles having a mass median diameter of
less than 5
microns. In yet another embodiment the spray-dried iRNA agent composition
includes particles
having a mass median aerodynamic diameter (MMAD) of less than 5 microns.
In certain other aspects, the invention provides kits that include a suitable
container
3o containing a pharmaceutical formulation of an iRNA agent, e.g., a double-
stranded iRNA agent,
or sRNA agent, (e.g., a precursor, e.g., a larger iRNA agent which can be
processed into a sRNA
agent, or a DNA which encodes an iRNA agent, e.g., a double-stranded iRNA
agent, or sRNA
agent, or precursor thereof). In certain embodiments the individual components
of the
pharmaceutical formulation may be provided in one container. Alternatively, it
may be desirable
241
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
to provide the components of the pharmaceutical formulation separately in two
or more
containers, e.g., one container for an iRNA agent preparation, and at least
another for a carrier
compound. The kit may be packaged in a number of different configurations such
as one or
more containers in a single box. The different components can be combined,
e.g., according to
instructions provided with the kit. The components can be combined according
to a method
described herein, e.g., to prepare and administer a pharmaceutical
composition. The kit can also
include a delivery device.
In another aspect, the invention features a device, e.g., an implantable
device, wherein the
device can dispense or administer a composition that includes an iRNA agent,
e.g., a double-
stranded iRNA agent, or sRNA agent, (e.g., a precursor, e.g., a larger iRNA
agent which can be
processed into a sRNA agent, or a I~NA which encodes an iRNA agent, e.g., a
double-stranded
iRNA agent, or sRNA agent, or precursor thereof), e.g., a iRNA agent that
silences an
endogenous transcript. In one embodiment, the device is coated with the
composition. In
another embodiment the iRNA agent is disposed within the device. In another
embodiment, the
~ 5 device includes a mechanism to dispense a unit dose of the composition. In
other embodiments
the device releases the composition continuously, e.g., by diffusion.
Exemplary devices include
stems, catheters, pumps, artificial organs or organ components (e.g.,
artificial heart, a heart
valve, etc.), and sutures.
As used herein, the term "crystalline" describes a solid having the structure
or
2o characteristics of a crystal, i.e., particles of three-dimensional
structure in which the plane faces
intersect at definite angles and in which there is a regular internal
structure. The compositions of
the invention may have different crystalline forms. Crystalline forms can be
prepared by a
variety of methods, including, for example, spray drying.
The invention is further illustrated by the following examples, which should
not be
25 construed as further limiting.
242
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
EXAMPLES
Examples 1-442 represent typical syntheses of the compounds delineated in
Schemes 1-
17; 101-116; and 1001-1005.
Scheme la
HO ~Fmoc i SiiO N.Fmoc SiiO NH
~N --~ i ~/ - 1i-~ i ~/
HO~' ~ O~Si-O~ ~ O~Si-O.
1
HO-.,~, ,Fmoc ~i0 ''' N.Fmoc ~i0-''~ NH
N -~ S; I~ ii S~
O~Si-O ~ ~ O~Si-O
HO 4
a i O[('Pr)2SiC1j2, Imidazole, DMF; ii TEA/MeCN
15
243
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 2a
0II 0I'
~~O~NH i ~~O~N~O-R I' HO~N~O-R
O~Si-~~'' ~ ~ O~Si-~'
HO~
7a-I 8a-1
O OII
ii~ _Si-O-Si O~N~O-R iv _
O
'Siv H6~
9a-I
v'
Z = Me, Allyl or
p-cyanoethyl
O OI'
Si O Si O~N~O-R
O O
Sis O~N
11a-1 [O~ - ~H
o,
v
~H ~~., COOEt
H H .,aH
H
H _ _
a O : Fi fFi \ 11
H i
~~Of~ ~~.. COOEt
l ~n H ..nH v
b n=1-17
Ac0\ ~ H
Fi Fi
~O
In
c n = 1-17
O I W ~ H H
n ~ ~p
d n = 1-50 (mPEG) ~ - k
~O~O~ O O O I ~N
O ~ N NJ
HN
a n=1-50 IN ~
N
244
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
a (i) (1) 1,1'-Carbonyldiimidazole (CDR, DMAP / THF; (2) R-OH, (ii) TBAF /
THF; (iii)
Benzhydryloxybis(trimethylsilyloxy)silyl-Cl (BzHCI), diisopropylamine /
CHZC12; (iv) (a) Z =
Me: methyl tetraisopropyl phosphorodiamidite, 1 H tetrazole / CH2C12; (b) Z =
allyl:
diisopropylamie, (allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole /
CH3CN; (c) Z = ~-
cyanoethyl: 2-cyanoethyltetraisopropylphosphorodiamidite, N,N
diisopropylammonium
tetrazolide, CH3CN, rt; (v) (1) succinic anhydride, 1,2-dichloroethane, DMAP,
(CaHs)3N, rt; (2)
2-(lHbenzotriazole-1-yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBT~, 4-
methylmorpholine, DMF, aminoalkyl solid support, rt.
245
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 3a
0'I 0II
~/O °' NH ,O ''' N~O_R HO-~,,, N~O_R
i ~ i ii
>
O'Si_O O'Si-O HO
~ r ~ r 12a-1 13a-1
iii iv
-> >
.._. ~N~r'OZ
v vi ~~ 15a-1
Z = Me, Allyl or
p-cyanoethyl
O OI'
-Si-O-Si O-~,,
~N ~O-R
O - O
Siw O~N S
16a 1 IOI H
COOEt
W
H H .,vH
R- . - . . H
_ _
a O . F1 fFi \ hi
O Fi i
COOEt
~O
~~~n H ..,vH v
b n=1-17
Ac0\ ~ , H
f-1 hi
~O
'In
c n=1-17
H H
J' / O
d n =1-50 (mPEG)
h
~0~~ O O O ~ ~N
O O 4 I ~ N NJ
HN
a n=1-50 IN ~
N
246
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
a (i) (1) 1,1'-Carbonyldiimidazole (CDZ], DMAP / THF; (2) R-~H, (ii) TBAF /
THF; (iii)
Benzhydryloxybis(trimethylsilyloxy)silyl-Cl (BzHCl), diisopropylamine /
CH2Cla; (iv) (a) Z =
Me: methyl tetraisopropyl phosphorodiamidite, 1 H tetrazole / CHZCl2; (b) Z =
allyl:
diisopropylamie, (allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole /
CH3CN; (c) Z = (3-
cyanoethyl: 2-cyanoethyltetraisopropylphosphorodiamidite, N,N
diisopropylammonium
tetrazolide, CH3CN, rt; (v) (1) succinic anhydride, 1,2-dichloroethane, DMAP,
(C2H5)3N, rt; (2)
2-(1H benzotriazole-1-yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTT~,
4-
methylmorpholine, DMF, aminoallcyl solid support, rt.
247
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 4
i0-~~~ NH ~s0-~., ~NHFMoc ~~O-~~, JL(LNHZ
SI ~ t ~~N ~7P ii _~ I ' '~N ~7P
S "Si O 1~ (p= 1-15) ~Si-O
18
O H
HO e. N O. O
iii ~ .~ /N~P ~ R O
iv I I H
HO -~ 'Si-O-SI-O-....~N~N~O.R
19a-1
~ O
'Sip HO 20a-1
v
vi
-S NuO.R
a IIO
:, AIIyI or
[3-cyanoethyl
.,,. - _..
'.,vHv ~ '~,. COOEt
H H ."vH
Fi FiV . . H
R = \ a O _ H fH Ii Fi
Fi
COOEt
O H r' .vHv
n
b n = 1-1'T ~ H
Ac0 9 fi Fi
~O~ ' J
~ ~n
c n=1-17 I ~ H H
o,
n ~ k
d n = 1-50 (mPEG)
O ~ N
~0 0
n ~ HN
~O~nO~ I N
a n = 1-50
248
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
a (i) FmocGly, DCC, DMAP / DMF; (ii) Et3N /MeCN; (iii) a. (1) 1,1'-
Carbonyldiimidazole (CDI), DMAP / THF; (2) R-OH; b. TBAF / THF; (iv)
Benzhydryloxybis(trimethylsilyloxy)silyl-Cl (BzHCI), diisopropylamine /
CH2Cl2; (v) (a) Z =
Me: methyl tetraisopropyl phosphorodiamidite, 1 H tetrazole l CH2C12; (b) Z =
allyl:
diisopropylamie, (allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole /
CH3CN; (c) Z = [3-
cyanoethyl: 2-cyanoethyltetraisopropylphosphorodiamidite, N,N
diisopropylammonium
tetrazolide, CH3CN, rt; (vi) (1) succinic anhydride, 1,2-dichloroethane, DMAP,
(C2H5)3N, rt; (2)
2-(1H benzotriazole-1-yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTU),
4-
methylmorpholine, DMF, aminoalkyl solid support, rt.
249
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme Sa
~~O~NH eO~N~-NHFMoc ~~O~N~NHz
Si i S; P ii Si p
O~Si_0 ~ ~ ~ O~Si-~~ 23 (p =1-15) ~~ O~Si-O
~r ~r ~r 24
O H \ I \ I
HON~' N O.R O
ii "p ~ iv I I O H
HO ~ -Si-O-Si O~. N~N O.R
25a~1 I p V p
'Sip HO, 26a~1
v I vi
H
N~O.R
I'a
O N II O.R
O
~, Allyl or
p-cyanoethyl
28a~1
~~,.
~H ~~. COOEt
H H ."vH
H
R= \ H - -
Fi Fi
a O Fi f ~ Fi
O
n ~.., COOEt
H ..,vH
b n=1-17 ~ H
Ac0\ 9
Fi hi
~O j
1 ~n
c n = 1-17 I \ H H
\O~O~ . ~ O
- /n- /- ~ k
d n = 1-50 (mPEG)
-~/~ O \
~O~ ~O~ ~O I / N -
O
'~ ~\~-O ~ HN
\O /n ~ IN / ~ W /
a n = 1-50 N
250
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
a (i) FmocGly, DCC, DMAP / DMF; (ii) Et3N /MeCN; (iii) a. (1) 1,1'-
Carbonyldiimidazole (CDI), DMAP / THF; (2) R-OH; b. TBAF / THF; (iv)
Benzhydryloxybis(trimethylsilyloxy)silyl-Cl (BzHCI), diisopropylamine /
CH2C12; (v) (a) Z =
Me: methyl tetraisopropyl phosphorodiamidite, 1 H tetrazole / CHaCl2; (b) Z =
allyl:
diisopropylamie, (allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole /
CH3CN; (c) Z = (3-
cyanoethyl: 2-cyanoethyltetraisopropylphosphorodiamidite, N,1V
diisopropylammonium
tetrazolide, CH3CN, rt; (vi) (1) succinic anhydride, 1,2-dichloroethane, DMAP,
(C2H5)3N, rt; (2)
2-(1H benzotriazole-1-yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBT~, 4-
methylinorpholine, DMF, aminoalkyl solid support, rt.
251
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 6a
~iO~NH i ~iO~N~R " HON-R
~O~Si-o~ ~ ~O.Si-~' 1
29a-1 HO~ 30a-1
O
ii~ R iv = -Si-O-Si-O~N.R
O
'Siv O
,..,.-J / ~N~P~OZ
v
32a-1; Z = Me, Allyl or
O [3-cyanoethyl
-Si-O-Si-O~N-R
O OII
Siw O~N S
33a-1 IOI H
O O
..,vH I. .,vHv
H H / /
H O
f-i H ~ /
Ac0 a AcO
H g h
wO~O~CO X I O
n q
b n = 1-17; q = 0 - 4 FmocHN~CO
i X = Side chain of amino acid (Appropriately protected, Fmoc for
~O~-CO lys, orn, benzyl for asp, glu etc [base labile protecting groups])
n q
c n=1-17;q=0-4
FmocHN~CO
~CO j X = Side chain of amino acid (Appropriately protected, Fmoc for
d n = 0 -16 lys, orn, benzyl for asp, glu etc [base labile protecting groups])
O~~~ X H OfI '
FmocHN~CO FmocHN~N~N~
1 /n H nn
en=1-16
FmocHNUN k n = 0 - 20, X = Side chain of amino acid (Appropriately
I ~CO protected, Fmoc for lys, orn, benzyl for asp, glu etc [base labile
NH protecting groups])
fn=o-16
252
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
a (i) RCOOH, DCC, DMAP / DMF; (ii) TBAF / THF; (iii)
Benzhydryloxybis(trimethylsilyloxy)silyl-Cl (BzHCl), diisopropylamine /
CH2Cl2; (iv) (a) Z =
Me: methyl tetraisopropyl phosphorodiamidite, 1 FI tetrazole / CH2C12; (b) Z =
allyl:
diisopropylamie, (allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole l
CH3CN; (c) Z = (3-
cyanoethyl: 2-cyanoethyltetraisopropylphosphorodiamidite, N,N
diisopropylammonium
tetrazolide, CH3CN, rt; (v) (1) succinic anhydride, 1,2-dichloroethane, DMAP,
(CZHS)3N, rt; (2)
2-(1H benzotriazole-1-yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTI~,
4-
methylinorpholine, DMF, aminoalkyl solid support, rt.
253
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 7a
O-~.,. O-o, . R HO-.,,
~NH i i~ N ii ~N,R
~S~ ~ ~S~ ~ H j~'O
34a-1 35a-1
O
ii~ i~ -Si-O-Si-O-..,.~N.R
'rr,O
'Sis O
s i
.a-. ~N-P~OZ
v~
37a-1; Z = Me, Ailyl or
[3-cyanoethyl
O
-Si O-Si-O-e.,~N.R
\~'~O
S~~ O N S
38a-1 O H
O O
.,,,
..,,
."vH ~ .,~H
- H H i i
Fi f-1 ~ ~ O
H
Ac0 a Ac0
H g h
wO~O~CO X I O
n q
b n = 1-17; q = 0 - 4 FmocHN~CO
i X = Side chain of amino acid (Appropriately protected, Fmoc for
~O~-CO lys, orn, benzyl for asp, glu etc [base labile protecting groups])
n q
c n=1-17;q=0-4
FmocHN~CO
~CO j X = Side chain of amino acid (Appropriately protected, Fmoc for
d n = 0 -16 lys, orn, benzyl for asp, glu etc [base labile protecting groups])
O X H O
FmocHN~Cp FmocHN~N~N~
/n H nn
en=1-16
FmocHN~N ~ n = 0 - 20, X = Side chain of amino acid (Appropriately
I ~CO protected, Fmoc for lys, orn, benzyl for asp, glu etc [base labile
NH protecting groups])
fn=o-16
254
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
a (i) RCOOH, DCC, DMAP / DMF; (ii) TB.AF / THF; (iii)
Benzhydryloxybis(trimethylsilyloxy)silyl-Cl (BzHCl), diisopropylamine /
CH2C12; (iv) (a) Z =
Me: methyl tetraisopropyl phosphorodiamidite, 1 H tetrazole / CHaCl2; (b) Z =
allyl:
diisopropylamie, (allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole /
CH3CN; (c) Z = (3-
cyanoethyl: 2-cyanoethyltetraisopropylphosphorodiamidite, N,N
diisopropylammonium
tetrazolide, CH3CN, rt; (v) (1) succinic anhydride, 1,2-dichloroethane, DMAP,
(C2H5)3N, rt; (2)
2-(1H benzotriazole-1-yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTL~,
4-
methylinorpholine, DMF, aminoalkyl solid support, rt.
255
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 8a
0II 0
HO-~.,, ~ .R
O NH i i O N~H R ~ii ~ N H
O~Si- ~ ~ ~ O~Si- ~ ~ HO~
~ r ~ r 39a-g 40a~g
0 0I'
ii~ _Si-O-Si_O-,., N~N.R
O H
'Siv H
41 a-g
42a-g; Z = Me, Allyl or
p-cyanoethyl
R= AcO~ FmocHN~N~
an=0-16 dn=~~0 116 INI r~ ~~H
fn=0-16
FmocHN~ EtOOC,( y.[ H
YHN N
bn=0-16 en=0-16
F3COCHN~ 9 n = 0 -16; Y = H, Me, Et etc
l~ ~~n
cn=0-16
a (i) (1) 1,1'-Carbonyldiimidazole (CDl), DMAP / THF; (2) R-OH, (ii) TBAF /
THF; (iii)
Benzhydryloxybis(trimethylsilyloxy)silyl-Cl (BzHCI), diisopropylamine /
CHZC12; (iv) (a) Z =
Me: methyl tetraisopropyl phosphorodiamidite, 1 H tetrazole l CH2C12; (b) Z =
allyl:
diisopropylamie, (allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole /
CH3CN; (c) Z = (3-
cyanoethyl: 2-cyanoethyltetraisopropylphosphorodiamidite, N,N
diisopropylammonium
tetrazolide, CH3CN, rt; (v) (1) succinic anhydride, 1,2-dichloroethane, DMAP,
(CZHS)3N, rt; (2)
2-(1H benzotriazole-1-yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTl~,
4-
methylmorpholine, DMF, aminoalkyl solid support, rt.
256
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 9a
0II 0
NH i0 N~H-R I' HO~N~H.R
44a-g 45a-g
O
ii~ _Si_O_Si-O~N~N-R ~~ N-R
H
O H
'Si~ HO'
46a-g
i i
47a-g; Z = Me, Allyl or
(3-cyanoethyl
R
O a v
Sis O~N S
fl H
48a-g O
R = AcO~ FmocHN N
~~
an=0-16 0 16
dn=
fn=0-16
FmocHN~ EtOOC~ H
YHN N
=0
16
bn en=0-16
-
gn=0-16;Y=H,
Me, Etetc
F3COCHN~
C~ ~~
n
cn=0-16
a (i) (1) 1,1'-Carbonyldiimidazole (CDl), DMAP / THF; (2) R-OH, (ii) TBAF /
THF; (iii)
Benzhydryloxybis(trimethylsilyloxy)silyl-Cl (BzHCl), diisopropylamine /
CH2Cl2; (iv) (a) Z =
Me: methyl tetraisopropyl phosphorodiamidite, 1 H tetrazole / CHZC12; (b) Z =
allyl:
diisopropylamie, (allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole /
CH3CN; (c) Z = (3-
cyanoethyl: 2-cyanoethyltetraisopropylphosphorodiamidite, N,N
diisopropylammonium
tetrazolide, CH3CN, rt; (v) (1) succinic anhydride, 1,2-dichloroethane, DMAP,
(CZHS)3N, rt; (2)
2-(1H benzotriazole-1-yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTL~,
4-
methylmorpholine, DMF, aminoalkyl solid support, rt.
257
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 10a
0
O OII Et3Si O 'I
HO ~ Et3Si-O N~O-R ii ~N~O-R
~N O-R
O
HO~' H~ ~N~P~OZ
8a-I 49a-1
50a~I;Z = Me, Aliyl or
iii [3-cyanoethyl
OII
Et3Si-O~N~O-R
O
O~
~H~
51a~1 O
.,.,
,vH I a.,, COOEt
v
H ..,vH
R= . . H
- - H
H H
1-/ O O Fi f \ hi
i
0 ~. COOEt
... ,,vH
b n=1-17 H H
Ac0' ;
vJl~s~ g hi ti
~O
~ ~n
c n=1-17
.~ ~ H H
w0~0~
n n O_.
d n = 1-50 (mPEG) h k
N
O
0 0 0 I / N
'~ ~-O ~ HN
\O /n 1N / ~ v I /
a n = 1-50 N
a (i) Triethylsilyl chloride, imidazole / THF; (ii) (a) Z = Me: methyl
tetraisopropyl
phosphorodiamidite, 1 H tetrazole / CH2Cl2; (b) Z = allyl: diisopropylamie,
(allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole / CH3CN; (c) Z = (3-
cyanoethyl: 2-
cyanoethyltetraisopropylphosphorodiamidite, N,N diisopropylammonium
tetrazolide, CH3CN, rt;
(iii) (1) succinic anhydride, 1,2-dichloroethane, DMAP, (C2H5)3N, rt; (2) 2-
(1H benzotriazole-1-
yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTU), 4-methylmorpholine,
DMF,
aminoalkyl solid support, rt.
258
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme lla
0II
O ~ Et3Si-O-~,,~N~O-R
HO-.,,. N~O-R i Et3Si-O-.,,;~N O_R i ~~--~,i
O
HO HO rN'P~OZ
13a-1 52a-11
53a-1; Z = Me, Allyl or
iii p-cyanoethyl
OII
Et3Si-O ~,.
~N~O-R
~.J O
O II H
54a-1 O
COOEt
.vH I
H H .,~H
R= . . H
hi H H Fi
O O Fi f ~ i
COOEt
O~ o,..
n .,~H
b n=1-17 H H
Ac0\ ~ H hi
~O~ g j
~n
c n=1-17
H H
w0~0~ . ~ / O ._
n
d n =1-50 (mPEG) h k
~O I / I N HN ~N
~O~n I N / -~ N ~ d
a n=1-50
a (i) Triethylsilyl chloride, imidazole / THF; (ii) (a) Z = Me: methyl
tetraisopropyl
phosphorodiamidite, 1 H tetrazole / CH2Cl2; (b) Z = allyl: diisopropylamie,
(allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole / CH3CN; (c) Z = (3-
cyanoethyl: 2-
cyanoethyltetraisopropylphosphorodiamidite, N,N diisopropylammonium
tetrazolide, CH3CN, rt;
(iii) (1) succinic anhydride, 1,2-dichloroethane, DMAP, (C2H5)3N, rt; (2) 2-
(1H benzotriazole-1-
yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTL>], 4-methylmorpholine,
DMF,
aminoalkyl solid support, rt.
259
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 12a
O H O H
HO ., N~(~. N O.R Et3Si-O .. N N O.R
- ,.~ l7P O ~ - ,.~ ~ O
HO HO
19a-1 55a-1
ii ~ vi
O H
Et3Si-O .. l~~ ~~~/~.~~' N O.
- ./ N \/P O R OII H
O Et3Si-O-..,~N~N O.R
--~, OC7P O
N~P~OZ O
55a-1; Z = Me, Allyl or
p-cyanoethyl O
57a-1
.~~H ~, COOEt
H ..,~H
R= . . H
Ii Fi H
11 Fi =
a Fi hi
O H ~ i
COOEt
v
O
.,,H
b n=1-17 H H
Ac0' _ ;
Fi hi
~O )
'-In
c n=1-17
H H
~o-('~°~
n ~ O _=
d n = 1-50 (mPEG) b k
N
O
O O I / N
O ~ HN
N ~ ~ ~ ~
a n = 1-50 I N
a (i) Triethylsilyl chloride, imidazole / THF; (ii) (a) Z = Me: methyl
tetraisopropyl
phosphorodiamidite, 1 H tetrazole / CH2C12; (b) Z = allyl: diisopropylamie,
(allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole / CH3CN; (c) Z = (3-
cyanoethyl: 2-
cyanoethyltetraisopropylphosphorodiamidite, N,N diisopropylammonium
tetrazolide, CH3CN, rt;
(iii) (1) succinic anhydride, 1,2-dichloroethane, DMAP, (C2H5)3N, rt; (2) 2-
(1H benzotriazole-1-
yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTLI), 4-methylmorpholine,
DMF,
aminoalkyl solid support, rt.
260
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 13a
HO-~,,.. N O N O.R Et3Si-O-..,. N O N O~R
~~P O ~ ' ~~ O
HO HO
25a-1 58a-I
ii vi
O
Et3Si-O ~,, N O.
~N~P O~ R _ O H
O Et3Si-O ~, N~N~O,R
~N~P~OZ ,~~ O P IIO
O~H
59a~1; Z = Me, Allyl or
p-cyanoethyl O
60a-1
',, , .,vH ~~, COOEt
H ...~~H
H H
Fi Fi
Fi H =
a hi Fi
O H \ i
COOEt
O
b n=1-17 H H L.~~H
Ac0' ~ ;
hi Fi
~O
1-In
c n = 1-17
.~ ~ H H
w0~0~ ~ ~ ~ O
d n = 1-50 (mPEG) h k
_ N
O
O O O O O I / N
O ~ ~ HN
~n N ~
a n = 1-50 I N
~ (i) Triethylsilyl chloride, imidazole / THF; (ii) (a) Z = Me: methyl
tetraisopropyl
phosphorodiamidite, 1 H tetrazole / CH2C12; (b) Z = allyl: diisopropylamie,
(allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole / CH3CN; (c) Z = (3-
cyanoethyl: 2-
cyanoethyltetraisopropylphosphorodiamidite, N,N diisopropylammonium
tetrazolide, CH3CN, rt;
(iii) (1) succinic anhydride, 1,2-dichloroethane, DMAP, (C2H5)3N, rt; (2) 2-
(1H benzotriazole-1-
yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTL~, 4-methylmorpholine,
DMF,
aminoalkyl solid support, rt.
261
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 14a
Et3Si-O~N,R
HO~N.R i Et3Si-O~N.R ii _
-' - O.
HO~ HO'61 a~k ~N-P~OZ
30a-kk
iii 62a~k; Z = Me, Allyl or
p-cyanoethyl
Et3Si-O~N.R
O
O II H
O
63a-k
O O
e.,
a>,
a
..,~H ..,~H
R= H H
H Fi ~ ~ O
Fi Fi
Ac0 a Ac0
H g h
O CO
n q
b n =1-17; q = 0 - 4 FmocHN~CO
i X = Side chain of amino acid (Appropriately protected, Fmoc for
~O~-CO lys, om, benzyl for asp, glu etc [base labile protecting groups])
c n=1-17;q=0-4
FmocHN~CO
~CO j X = Side chain of amino acid (Appropriately protected, Fmoc for
d n = 0 - 16 lys, orn, benzyl for asp, glu etc [base labile protecting
groups])
O X H O
FmocHN~CO FmocHN~N~N~
en=1-1,6 TX 1H jO(~n T ~'X
FmocHN N k n = 0 - 20, X = Side chain of amino acid (Appropriately
~CO protected, Fmoc for lys, orn, benzyl for asp, glu etc [base labile
f n = off 16 Protecting groups])
a (i) Triethylsilyl chloride, imidazole / THF; (ii) (a) Z = Me: methyl
tetraisopropyl
phosphorodiamidite, 1 H tetrazole / CH2Cl2; (b) Z = allyl: diisopropylamie,
(allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole / CH3CN; (c) Z = (3-
cyanoethyl: 2-
cyanoethyltetraisopropylphosphorodiamidite, N,N diisopropylammonium
tetrazolide, CH3CN, rt;
(iii) (1) succinic anhydride, 1,2-dichloroethane, DMAP, (C2H5)3N, rt; (2) 2-
(1H benzotriazole-1-
yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTL~, 4-methylmorpholine,
DMF,
aminoalkyl solid support, rt.
262
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 15a
Et3Si-O-.,,~ .R
HO-°,, ~N,R i Et3Si-O °, ,R ii N
'~~J, - ~. N
HO
35a-k HO 64a~k ~N~P~OZ
65a-k; Z = Me, Allyl or
(3-cyanoethyl
Et3Si-O-.,,.~N, R
', O
O II H U
O
66a-k
O O
s,,
°o
U
.,vH ..,vH
R= . H . H
H Fi Ii ~ ~ O
Ac0 a Ac0 H g I i i
.~ ~- h
wO~O~CO X
n q
b n = 1-17; q = 0 - 4 FmocHN~CO
i X = Side chain of amino acid (Appropriately protected, Fmoc for
~O~CO lys, orn, benzyl for asp, glu etc [base labile protecting groups])
c n=1-17;q=0-4 X
FmocHN~CO
~CO j X = Side chain of amino acid (Appropriately protected, Fmoc for
d n = 0 -16 lys, om, benzyl for asp, glu etc [base labile protecting groups])
O X O
FmocHN~CO FmocHN~N~N~
en=1-1I6 TX 'H On TX ~'
FmocHN N k n = 0 - 20 X = Side chain of amino acid (Appropriately
~CO protected, Fmoc for lys, orn, benzyl for asp, glu etc [base labile
NH protecting groups])
fn=o-16
a (i) Triethylsilyl chloride, imidazole / THF; (ii) (a) Z = Me: methyl
tetraisopropyl
phosphorodiamidite, 1 H tetrazole / CHZCl2; (b) Z = allyl: diisopropylamie,
(allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole / CH3CN; (c) Z = (3-
cyanoethyl: 2-
cyanoethyltetraisopropylphosphorodiamidite, N,N diisopropylammonium
tetrazolide, CH3CN, rt;
(iii) (1) succinic anhydride, 1,2-dichloroethane, DMAP, (C2H5)3N, rt; (2) 2-
(1H benzotriazole-1-
yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTLJ), 4-methylmorpholine,
DMF,
aminoalkyl solid support, rt.
263
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 16a
0II
O O Et35i-O-~..~N~N.R
HO-.,,~N~N.R i Et3Si-O-.,,,~N~N,R ii H
H ~ ~(---~, H ~ O
HO 40a HO ~~ ~OZ
-g 64a-g
65a-g; Z = Me, Allyl or
iii (3-cyanoethyl
O R
Et3Si-O-~.,~N~-H~
\r, O
O II H
O 66a-g
R= AcO~ FmocHN~N~
n ~ ~~
an=0-16 dn=0-16 NH
fn=0-16
FmocHN~ EtOOC.( H
~ y.[ YHN N
~~ 1 ~
n n
bn=0-16
en=0-16
F3COCHN.( L.[ 9 n = 0 -16;
C~ ~~n Y = H, Me,
Et etc
cn=0-16
a (i) Triethylsilyl chloride, imidazole / THF; (ii) (a) Z = Me: methyl
tetraisopropyl
phosphorodiamidite, 1 H tetrazole / CH2C12; (b) Z = allyl: diisopropylamie,
(allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole / CH3CN; (c) Z = (3-
cyanoethyl: 2-
cyanoethyltetraisopropylphosphorodiamidite, N,1V diisopropylammonium
tetrazolide, CH3CN, rt;
(iii) (1) succinic anhydride, 1,2-dichloroethane, DMAP, (C2H5)3N, rt; (2) 2-
(1H benzotriazole-1-
1o yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTL>7, 4-
methylmorpholine, DMF,
aminoalkyl solid support, rt.
264
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 17a
0
O O Et3Si-O~N~N.R
HO~N~H.R i Et3Si-O~N~H.R ~ ~ H
O
HO 45a-g HO' ~~P~OZ
67a-g
68a-g; Z = Me, Allyl or
iii (i-cyanoethyl
OII R
Et3Si-O~N~H/
O
O II H
O 69a-g
R= ~ AcO~ FmocHN~N~
n ~~
an=0-16 dn=0-16 NH
fn=0-16
FmocHN~ EtOOC.(y[H
1 1 N
~ ~ YHN
~~ ~~
bn=0 ~
-
16
en=0 O
-
16
g n = 0 -
F3COOHN~ 16; Y = H,
~ ~~n Me, Et etc
cn=0-16
a (i) Triethylsilyl chloride, imidazole / THF; (ii) (a) Z = Me: methyl
tetraisopropyl
phosphorodiamidite, 1 H tetrazole / CHaCl2; (b) Z = allyl: diisopropylamie,
(allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole / CH3CN; (c) Z = (3-
cyanoethyl: 2-
cyanoethyltetraisopropylphosphorodiamidite, N,N diisopropylarnmonium
tetrazolide, CH3CN, rt;
(iii) (1) succinic anhydride, 1,2-dichloroethane, DMAP, (C2H5)3N, rt; (2) 2-
(1H benzotriazole-1-
1o yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBT~, 4-methylmorpholine,
DMF,
aminoalkyl solid support, rt.
265
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme lOla
0 0II
Bz0~ BzO~~O.R HO~~°.R
r--NH ii
Ac0", ~ Ac0"' ~~ HO"
101 102a-1 103a-1
R
ii~ i
° O~O.R
-Si-O-Si O '[N
O ~ O
Sis p
H
106a-1 O
i
105a-1; Z = Me, Allyl or
p-cyanoethyl
~ COOEt
."'H
~' 1
H H .,aH
H
R =_ ~ a O ; H fH \ Fi
H I ~~.. COOEt
~~.O
1-Jn , . 'vH
H H
b n = 1-17 ~ Ii Fi
Ac0'
~vJJI,,,,,,~~~~0
H H
c n=1-17
O_=
w0~0~ k
n
d n = 1-50 (mPEG)
N
~°~~ ~°~°~° ~ j N ~J
O O~ ~ HN
N
a n=1-50 I N
266
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
a (i) (1) 1,1'-Carbonyldiimidazole (CDI], DMAP / THF; (2) R-OH, (ii) LiOH /
THF-H20;
(iii) Benzhydryloxybis(trimethylsilyloxy)silyl-Cl (BzHCI), diisopropylamine /
CHaCl2; (iv) (a) Z
= Me: methyl tetraisopropyl phosphorodiamidite, 1 H tetrazole / CH2C12; (b) Z
= allyl:
diisopropylamie, (allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole /
CH3CN; (c) Z = (3-
cyanoethyl: 2-cyanoethyltetraisopropylphosphorodiamidite, N,N
diisopropylammonium
tetrazolide, CH3CN, rt; (v) (1) succinic anhydride, 1,2-dichloroethane, DMAP,
(C2H5)3N, rt; (2)
2-(1H benzotriazole-1-yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBT~, 4-
methylmorpholine, DMF, aminoalkyl solid support, rt.
267
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 102a
o _ o
Bz0-,, Bz0-.,, N~O.R HO °°~ N~O.R
~N~H i _ ~\ ~\
AcO~ ~ AcO~ ~-~ HO~
107 108a-I .__
O
O
ii~ -Si-O-Si-O- N~O.R
~Si ~ HO""~
110a-1
~ ~ v1
O O~O,R
-Si-O-Si-O-.. '(N
O .~ O
Sip p
H
112a-1 O
111 a-I; Z = Me, Allyl or
p-cyanoethyl
COOEt
~.."Hv 1
H H .'vH
H
Ii Ii
R = v a O . q ~ Fi
H i ~.~ COOEt
~O
1-/~n ~,,,~ I. ,'vH
~O~ H H
b n=1-17 H H
Ac0\
9 J
~O
1~ ~ H H
c n=1-17
O O O
k
d n = 1-50 (mPEG)
h
W ~N
O ~ ~ N 'N~
~O~ N ~ ~ HN 1 w
a n=1-50 I
N
268
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
a (i) (1) 1,1'-Carbonyldiimidazole (CDR, DMAP / THF; (2) R-OH, (ii) LiOH / THF-
H20;
(iii) Benzhydryloxybis(trimethylsilyloxy)silyl-Cl (BzHCI), diisopropylamine /
CHaCl2; (iv) (a) Z
= Me: methyl tetraisopropyl phosphorodiamidite, 1 H tetrazole / CHZCl2; (b) Z
= allyl:
diisopropylamie, (allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole /
CH3CN; (c) Z = (3-
cyanoethyl: 2-cyanoethyltetraisopropylphosphorodiamidite, N,N
diisopropylammonium
tetrazolide, CH3CN, rt; (v) (1) succinic anhydride, 1,2-dichloroethane, DMAP,
(C2H5)3N, rt; (2)
2-(1H benzotriazole-1-yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTL7],
4-
methylinorpholine, DMF, aminoalkyl solid support, rt.
269
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 103a
Bz0-. O~p NHFmoc O ~ ,R
NH Bz0-,, N ~p N O
~\ BzO-~,, N H
AcO
107 AcO
113a-1 (p = 1-15) Ac0 .~ ~e~_i
OII
O~N~O.R O
iii HO-°~~ N p N~O~R
H
HO 115ad
a-I
v ~ vi
0I'
~O.R ~ O '
O~ ~ .R
'~p H O
S
= Me, Allyl or O 118a-1
p-cyanoethyl
COOEt
~.."H v 1
H H ,,~H
H
hi hi
R = v a O . f R Fi
H i ~,.. COOEt
~O a
l~~n . .,~H
H H
b n = 1-17 11 Fi
Ac0\
_v l~~O
H H
c n =1-17 .
O_=
~O O k
n
d n = 1-50 (mPEG)
N
~°~,,,~ ~°~°~° ' % N ~J
O O~ ~ HN
N ~ ~ ~ ~ /
a n=1-50 I N
270
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
a (i) FmocGly, DCC, DMAP / DMF; (ii) Et3N /MeCN; (iii) a. (1) 1,1'-
Carbonyldiimidazole (CDn, DMAP / THF; (2) R-OH; b. LiOH / THF-H20; (iv)
Benzhydryloxybis(trimethylsilyloxy)silyl-Cl (BzHCl), diisopropylamine / CHZCh;
(v) (a) Z =
Me: methyl tetraisopropyl phosphorodiamidite, 1 H tetrazole l CHZCI~; (b) Z =
allyl:
diisopropylamie, (allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole /
CH3CN; (c) Z = (3-
cyanoethyl: 2-cyanoethyltetraisopropylphosphorodiamidite, N,N
diisopropylammonium
tetrazolide, CH3CN, rt; (vi) (1) succinic anhydride, 1,2-dichloroethane, DMAP,
(C2H5)3N, rt; (2)
2-(lHbenzotriazole-1-yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTL>7,
4-
1 o methylmorpholine, DMF, aminoalkyl solid support, rt.
271
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 104a
O~NHFmoc O ~ ,R
Bz0 NH Bz0 N ~p H O
BzO
AcO
101 Ac0
119a-1 (p= 1-15~ n,.ri' .__ .I
OII
O~N~O,R
'( ~~p H R
ii~ HO~ iv
Hd~121a-t
OII
N~O~ R
H
. = Me, Allyl or
p-cyanoethyl 124a-1
COOEt
r...aHv ~ '.vH
H H H
I-i Fi
a O . f \ Fi
H I ~~., COOEt
~ y0 v
l-In ..~~H
H H
b n=1-17
Ac0'
~vJl~ii~O
\ H H
c n=1-17 ~ ~
~ ~ O_=
~°'~\i0~./~ k
~n
d n = 1-50 (mPEG)
h
N
° ~°~°~° ~ , N ~J
O ° ~ HN
N ~ ~ ~ ~
a n=1-50 I N
272
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
a (i) FmocGly, DCC, DMAP / DMF; (ii) Et3N /MeCN; (iii) a. (1) 1,l'-
Carbonyldiimidazole (CDI), DMAP / THF; (2) R-OH; b. LiOH / THF-H20; (iv)
Benzhydryloxybis(trimethylsilyloxy)silyl-Cl (BzHCI), diisopropylamine /
CH~Cl2; (v) (a) Z =
Me: methyl tetraisopropyl phosphorodiamidite, 1 H tetrazole / CHZC12; (b) Z =
allyl:
diisopropylamie, (allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole /
CH3CN; (c) Z = (3-
cyanoethyl: 2-cyanoethyltetraisopropylphosphorodiamidite, N,N
diisopropylammonium
tetrazolide, CH3CN, rt; (vi) (1) succinic anhydride, 1,2-dichloroethane, DMAP,
(CZHS)3N, rt; (2)
2-(lHbenzotriazole-1-yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTL)],
4-
1o methylmorpholine, DMF, aminoalkyl solid support, rt.
273
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme lOSa
R R
Bz0 Bz0 N HO N
~N~H
AcO
101 Ac0 HO~
125a-1 126a-1
iii -Si-O-Si O N iv
I 1
128a-1; Z = Me, Allyl or
p-cyanoethyl
H
129a-1
O
... O
.,"H
H ..vH
H
H . . ~ ~ O
AcO a H H
w0 O CO Ac0 H g h
X I O
b n=1-17;q=0-4
FmocHN~CO
~O~CO i X = Side chain of amino acid (Appropriately protected, Fmoc for
n q lys, om, benzyl for asp, glu etc [base labile protecting groups])
c n=1-17;q=0-4 X
FmocHN~CO
~CO j X = Side chain of amino acid (Appropriately protected, Fmoc for
d n = 0 -16 lys, om, benzyl for asp, glu etc [base labile protecting groups])
O X H O
FmocHN~CO FmacHN~N~N~
en=1-16 H I ~~n
FmocHN N ~ n = 0 - 20, X = Side chain of amino acid (Appropriately
~CO protected, Fmoc for lys, orn, benzyl for asp, glu etc [base labile
NH protecting groups])
fn=o-16
274
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
a (i) RCOOH, DCC, DMAP / DMF; (ii) LiOH / THF-HaO; (iii)
Benzhydryloxybis(trimethylsilyloxy)silyl-Cl (BzHCl), diisopropylamine /
CHZCl2; (iv) (a) Z =
Me: methyl tetraisopropyl phosphorodiamidite, 1 H tetrazole / CH2C12; (b) Z =
allyl:
diisopropylamie, (allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole /
CH3CN; (c) Z = ~3-
cyanoethyl: 2-cyanoethyltetraisopropylphosphorodiamidite, N,N
diisopropylammonium
tetrazolide, CH3CN, rt; (v) (1) succinic anhydride, 1,2-dichloroethane, DMAP,
(C2H5)3N, rt; (2)
2-(1H benzotriazole-1-yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTL~,
4-
methylinorpholine, DMF, aminoalkyl solid support, rt.
275
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 106a
N R
Bz0-., NH Bz0-..,, HO--o,, N
~\ ii '
AoO
106 Ac0 HO
130a-1 130a-1
O R
iii _Si-O-Si-O-,,, N - iv
O ~
Siv HO qgla-I
v
O 132a-1; Z = Me, Allyl or
I R p-cyanoethyl
-Si-O-Si-O-~., N
.O ~ O
Sip O~
~H~
133a-1 O
O'
O
.,~H ~~,
v ~.
H ..,~H
_ / i v
R Ac0 ~ H = H = ~ w ~ O
a Fi Fi
O~CO Ac0 H g h
~n q X 1
b n=1-17;q=0-4 O
FmocHN~CO
~O~CO ; X = Side chain of amino acid (Appropriately protected, Fmoc for
c n = 1-17; q = 0 - 4 lys~ orn, bX zyl for asp, glu etc [base labile
protecting groups])
FmocHN~CO
~CO j X = Side chain of amino acid (Appropriately protected, Fmoc for
d n = 0 -16 lys, orn, benzyl for asp, glu etc [base labile protecting groups])
O X H O
FmocHN~CO FmocHN~N~N~
en=1-1/6 TX 'H On ~ ~'X
FmocHN N ~ n = 0 - 20, X = Side chain of amino acid (Appropriately
~CO protected, Fmoc for lys, orn, benzyl for asp, glu etc [base labile
f n = off 16 Protecting groups])
276
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
a (i) RCOOH, DCC, DMAP / DMF; (ii) LiOH / THF-H20; (iii)
Benzhydryloxybis(trimethylsilyloxy)silyl-Cl (BzHCl), diisopropylamine /
CHZCIa; (iv) (a) Z =
Me: methyl tetraisopropyl phosphorodiamidite, 1 H tetrazole / GHZC12; (b) Z =
allyl:
s diisopropylamie, (allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole /
CH3CN; (c) Z = ~3-
cyanoethyl: 2-cyanoethyltetraisopropylphosphorodiamidite, N,1V
diisopropylammonium
tetrazolide, CH3CN, rt; (v) (1) succinic anhydride, 1,2-dichloroethane, DMAP,
(C2H5)3N, rt; (2)
2-(1H benzotriazole-1-yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTL)),
4-
methylmorpholine, DMF, aminoallcyl solid support, rt.
277
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 107a
H H
O~N.R O~N.R
Bz0-.,
NH ~ Bz0-o,.~ i~ HO-,,,~
AcO ~~--~)~
106 Ac0 HO
134a-g 135a-g
t
~~R
R
iv
.a-g
w ~ w ~ /v
H 137a-g; Z = Me, Allyl or
O O~N.R
'[ p-cyanoethyl
-Si-O-Si-O-.., N
O ~ O
Sip p
H
O
138a-g
R= AcO~ FmocHN~N~
n ~/ ~~
an=0-16 dn=0-16 NH
fn=0-16
FmocHN~ EtOOC~
YHN N
bn=0 n
16
- en=0-16
O
F3COCHN.( y.[ g n = 0 - 16;
\~ ~~n Y = H, Me,
Et etc
cn=0-16
a (i) (1) l,l'-Carbonyldiimidazole (CDl), DMAP / THF; (2) R-OH, (ii) LiOH /
THF-
THF; (iii) Benzhydryloxybis(trimethylsilyloxy)silyl-Cl (BzHCI),
diisopropylamine / CH2Cl2; (iv)
(a) Z = Me: methyl tetraisopropyl phosphorodiamidite, 1 H tetrazole l CH2C12;
(b) Z = allyl:
diisopropylamie, (allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole l
CH3CN; (c) Z = (3-
cyanoethyl: 2-cyanoethyltetraisopropylphosphorodiamidite, N,N
diisopropylammonium
1o tetrazolide, CH3CN, rt; (v) (1) succinic anhydride, 1,2-dichloroethane,
DMAP, (C2H5)3N, rt; (2)
2-(lHbenzotriazole-1-yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTL~, 4-
methylmorpholine, DMF, aminoalkyl solid support, rt.
278
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 108a
H H
O~N.R O~N.R
Bz0 '( '(.
H I Bz0 N HO N
Ac0
101 AcO HO
139a~g ' -Oa-g
H
O O N.R
iii R
_S~-O-S~-O ~ i~
O
Siv H
141 a-a
142a-g; Z = Me, Allyl or
O~ N. p-cYanoethyl
'( R
-Si-O-Si-O N
p ~ O
Sip O
H
O
143a-g
R= AcO~ FmocHN~N~
~/ ~~n
an=0-16 dn=0-16 NH
fn=0-16
FmocHN~ EtOOC.( y.[
n YHN N
bn=0-16 en=0-16
O
F3COCHN.( y.[ 9 n = 0 -16; Y = H, Me, Et etc
l~ ~~n
cn=0-16
a (i) (1) l,l'-Carbonyldiimidazole (CDn, DMAP l THF; (2) R-OH, (ii) LiOH / THF-
THF; (iii) Benzhydryloxybis(trimethylsilyloxy)silyl-Cl (BzHCI),
diisopropylamine / CH2C12; (iv)
(a) Z = Me: methyl tetraisopropyl phosphorodiamidite, 1 H tetrazole / CHaCl2;
(b) Z = allyl:
diisopropylarnie, (allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole /
CH3CN; (c) Z = (3-
cyanoethyl: 2-cyanoethyltetraisopropylphosphorodiamidite, N,N
diisopropylammonium
1o tetrazolide, CH3CN, rt; (v) (1) succinic anhydride, 1,2-dichloroethane,
DMAP, (CZHS)3N, rt; (2)
2-(1H benzotriazole-1-yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTL)],
4-
methylinorpholine, DMF, aminoalkyl solid support, rt.
279
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 109a
O~O.R
O Et3Si-O
HO
~ R O
~-
-N
O'
Et
Si-O
~ .
i
3
(
)
HO" ---~ ~~O~R '
~
HO~'
103a-1 144a-1 OZ
O O, ~ 145a-1; Z =
R Me, Allyl
or
(3-cyanoethyl
Et3Si-O
'(
)
O
~
O
N S
O H
146ad
..,
COOEt
'' ."vH~
H H ,,vH
H
R - H hi H hi Fi
a O H f W
I %.. COOEt
~jn .. ,vHv
O
H H
b n = 1-17 H I-Iv
Ac0\
vJl~~~ A J
~O
~ ~n
c n=1-17 ~ / H H
w0~0~ . . k
d n = 1-50 (mPEG)
h
w
O l J4 '' I ~ N N
~O~ N ~ ~ HN ' w
a n = 1-50 I
N
a (i) Triethylsilyl chloride, imidazole / THF; (ii) (a) Z = Me: methyl
tetraisopropyl
phosphorbdiamidite, 1 H tetrazole / CH2C12; (b) Z = allyl: diisopropylamie,
(allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole / CH3CN; (c) Z = (3-
cyanoethyl: 2-
cyanoethyltetraisopropylphosphorodiamidite, N,N diisopropylammonium
tetrazolide, CH3CN, rt;
(iii) (1) succinic anhydride, 1,2-dichloroethane, DMAP, (C2H5)3N, rt; (2) 2-
(1H benzotriazole-1-
yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTiT), 4-methylmorpholine,
DMF,
aminoalkyl solid support, rt.
280
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 110a
O~O.R
O Et3Si-O-s.,~
HO-m. ~ ,R ~~--'JO
~N\ O i Et3Si-O- ~ ,R
HO~ ~ O -~ O
HO
107a-1 147a-1 ~ ~OZ
iii 148a-1; Z = Me, Allyl or
O~O.R p-cyanoethyl
Et3Si-O-o,~
O
O~
~H~
O 149a-1
COOEt
.vH ~ ~
H H .vH
H
R = ~ a O . H pH ~ f-i
H i ~~~. COOEt
.,,H v
H H
b n =1-17 Fi !1~
Ac0 g ~ j
~O~
H , H
c n = 1-17 ~ / .
O O O.
k
d n = 1-50 (mPEG)
h
O ~O O
a ~ ~ N N
~O~ N ~ ~ HN
a n = 1-50 1
a (i) Triethylsilyl chloride, imidazole / THF; (ii) (a) Z = Me: methyl
tetraisopropyl
phosphorodiamidite, 1 H tetrazole / CH2Cl2; (b) Z = allyl: diisopropylamie,
(allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole / CH3CN; (c) Z = (3-
cyanoethyl: 2-
cyanoethyltetraisopropylphosphorodiamidite, N,N diisopropylammonium
tetrazolide, CH3CN, rt;
(iii) (1) succinic anhydride, 1,2-dichloroethane, DMA.P, (CaHs)3N, rt; (2) 2-
(1H benzotriazole-1-
yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTT~, 4-methylmorpholine,
DMF,
aminoalkyl solid support, rt.
281
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme llla
OII p ~~NJ~O.R
O H~O-R O~N~O.R Et3Si-O-.,, IN ~~ H
HO-.,,. N Et3Si-O-o,.. 'N H
O
H0115a-I HO 150a-1 ~N~P~OZ
151a~1; Z = Me, Allyl or
iii p-cyanoethyl
OII
O~N~O,R
Et3Si-O-~,, IN ,' H
O
O II H
O
152a-1
COOEt
."vH v ~ .' .,,H
H H H
R = ~ a O _ H fH ~ Fi
H i ~,., COOEt
~-O
'/n ...vH
H H
b n = 1-17 ~ f1 Fi
Ac0\
~VJJI,,__ss~~~(O
H H
c n=1-17
O_=
O k
n
d n = 1-50 (mPEG)
N
~O~,,,~ ~O O O I ~ N
O O~ ~ HN
N / ~ v
a n = 1-50 I N
a (i) Triethylsilyl chloride, imidazole / THF; (ii) (a) Z = Me: methyl
tetraisopropyl
phosphorodiamidite, 1 H tetrazole / CHZC12; (b) Z = allyl: diisopropylamie,
(allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole / CH3CN; (c) Z = (3-
cyanoethyl: 2-
cyanoethyltetraisopropylphosphorodiamidite, N,N diisopropylammonium
tetrazolide, CH3CN, rt;
(iii) (1) succinic anhydride, 1,2-dichloroethane, DMAP, (C2H5)3N, rt; (2) 2-
(1H benzotriazole-1-
yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTL)], 4-methylinorpholine,
DMF,
aminoalkyl solid support, rt.
282
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 112a
O O v~Nl~O.R
O H~O.R O~N~O.R Et3Si-O~'( ~~ ~H
HO~ Et3Si-O~'( ~~ H ii _
O
HO~ Hd~ ~N~P~OZ
121a-1 153a-1 ~ 154a-1; Z = Me, Allyl or
iii p-cyanoethyl
O'I
O~N~O.R
Et3Si-O~l ,. H
O
O~H
O
155a-1
COOEt
' .~eH v I
H H .,vH
H
Fi Fi ' -
R =_ ~ a O _. f \ ti
H I <~.. COOEt
~O
/n ., ,vH
H H
b n = 1-17 Fi Fi
Ac0\
v~l ,~~0
H H
c n=1-17
O_=
w0 O k
n
d n = 1-50 (mPEG) h
N
O~,-~ ~O O O I ~ N
O O~ ~ HN
N
a n = 1-50 1 N
a (i) Triethylsilyl chloride, imidazole l THF; (ii) (a) Z = Me: methyl
tetraisopropyl
phosphorodiamidite, 1 H tetrazole / CH2C12; (b) Z = allyl: diisopropylamie,
(allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole / CH3CN; (c) Z = (3-
cyanoethyl: 2-
cyanoethyltetraisopropylphosphorodiamidite, N,N diisopropylammonium
tetrazolide, CH3CN, rt;
(iii) (1) succinic anhydride, 1,2-dichloroethane, DMAP, (CZHS)3N, rt; (2) 2-
(1H benzotriazole-1-
yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTI~, 4-methylmorpholine,
DMF,
aminoalkyl solid support, rt.
283
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 113a
R
Et3Si-O
HO N Et3Si O ~N
i ii
O
N~ P
H026a-k H0156a-k ~ OZ
157a-k; Z = Me, Allyl or
R ~~~ [3-cyanoethyl
Et3Si-O
O
O II H
O
158a-k
O
''' O
.,vH ~,,
H r. ,,vH , i
H
Ac0
a Fi Fiv
CO Ac0 H g h
~.n~C7q~ X i O
b n=1-17;q=0-4
FmocHN~CO
~O~-CO i X = Side chain of amino acid (Appropriately protected, Fmoc for
c n = 1-17; q = 0 - 4 IYs, om, bX zyl for asp, glu etc [base labile protecting
groups])
FmocHN~CO
~CO j X = Side chain of amino acid (Appropriately protected, Fmoc for
d n = 0 -16 lys, om, benzyl for asp, glu etc [base labile protecting groups])
O X H O
FmocHN~CO FmocHN~N~N~
1 ~n H nn
en=1-16
FmocHN N k n = 0 - 20, X = Side chain of amino acid (Appropriately
~CO protected, Fmoc for lys, om, benzyl for asp, glu etc [base labile
f n = off 16 Protecting groups])
a (i) Triethylsilyl chloride, imidazole / THF; (ii) (a) Z = Me: methyl
tetraisopropyl
phosphorodiamidite, 1 H tetrazole / CHZC12; (b) Z = allyl: diisopropylamie,
(allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole / CH3CN; (c) Z = (3-
cyanoethyl: 2-
cyanoethyltetraisopropylphosphorodiamidite, N,N diisopropylammonium
tetrazolide, CH3CN, rt;
(iii) (1) succinic anhydride, 1,2-dichloroethane, DMAP, (C2H5)3N, rt; (2) 2-
(1H benzotriazole-1-
1o yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTU7, 4-methylmorpholine,
DMF,
aminoalkyl solid support, rt.
284
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 114a
R
Et3Si-O-.,,,~
HO-,.,,, N Et3Si-O-..,,, N
O
N'P
H030a-k H0159a-k ~ OZ
160a~k; Z = Me, Allyl or
iii
R ~3-cyanoethyl
Et3Si-O-r.,~
O
O~
~H~
O
161 a-k
O
''' O
.,~~H ~,,
v ~.
H ~ .,vH
H
H . . W W O
Ac0 a Fi H
O~CO Ac0 H g b
/n q X I O
b n=1-17;q=0-4
FmocHN~CO
~O~-CO i X = Side chain of amino acid (Appropriately protected, Fmoc for
lys, orn, benzyl for asp, glu etc [base labile protecting groups])
c n=1-17;q=0-4 X
FmocHN~CO
~CO j X = Side chain of amino acid (Appropriately protected, Fmoc for
d n = 0 - 16 lys, orn, benzyl for asp, glu etc [base labile protecting
groups])
O X H O
FmocHN~CO FmocHN~N~N~
en=1-1/6 TX \H On T ~'X
FmocHN N k n = 0 - 20, X = Side chain of amino acid (Appropriately
~CO protected, Fmoc for lys, orn, benzyl for asp, glu etc [base labile
NH protecting groups])
fn=o-16
a (i) Triethylsilyl chloride, imidazole / THF; (ii) (a) Z = Me: methyl
tetraisopropyl
phosphorodiamidite, 1 H tetrazole / CHZCl2; (b) Z = allyl: diisopropylamie,
(allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole l CH3CN; (c) Z = (3-
cyanoethyl: 2-
cyanoethyltetraisopropylphosphorodiamidite,1V,N diisopropylammonium
tetrazolide, CH3CN, rt;
(iii) (1) succinic anhydride, 1,2-dichloroethane, DMAP, (CaHs)3N, rt; (2) 2-
(1H benzotriazole-1-
yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTLT), 4-methylmorpholine,
DMF,
aminoalkyl solid support, rt.
285
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 115a
O~N.R
H '(H
O~N.R N-R Et3Si-O-~,,~
HO-~,,.~ Et3Si-O-.,,.~
~~ --1! O
H035a-g HO 162a-g ~~P~OZ
iii 163a~g; Z = Me, Allyl or
H (3-cyanoethyl
O~N.R
Et3Si-O-~,,~
O
O II H
O
164a~g
R= ~ AcO~ FmocHN~N~
n I ~/ ~~
an=0-16 dn=0-16 NH
fn=0-16
FmocHN~ EtOOC
Can ~~ ~ YHN N
bn=0-16
en=0-16
F3COCHN.( y.[ 9 n = 0 -16;
~~ ~~n Y = H, Me,
Et etc
cn=0-16
a (i) Triethylsilyl chloride, imidazole / THF; (ii) (a) Z = Me: methyl
tetraisopropyl
phosphorodiamidite, 1 H tetrazole / CH2C12; (b) Z = allyl: diisopropylamie,
(allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole / CH3CN; (c) Z = (3-
cyanoethyl: 2-
cyanoethyltetraisopropylphosphorodiamidite, N,N diisopropylammonium
tetrazolide, CH3CN, rt;
(iii) (1) succinic anhydride, 1,2-dichloroethane, DMAP, (C2H5)3N, rt; (2) 2-
(1H benzotriazole-1-
1o yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTU), 4-methylmorpholine,
DMF,
aminoalkyl solid support, rt.
286
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 116a
H
H H O~N.R
O~N.R O~N.R Et3Si
HO 'N( Et3Si-O 'N ii
O
HO 140a-g HO ~~ ~OZ
165a-g
166a-g; Z = Me, Ailyl or
iii (3-cyanoethyi
H
O~N.R
Et3Si-O
O
O II H
O
167a-g
R= ~ AcO~ FmocHN~N~
I 1~ ~~n
an=0-16 dn=0-16 NH
fn=0-16
FmocHN~ EtOOC
~n ~~ ~ YHN N
bn=0-16 en=0-16
F3COCHN.( y.[ g n = 0 -16; Y = H, Me, Et etc
l~ ~~n
cn=0-16
a (i) Triethylsilyl chloride, imidazole / THF; (ii) (a) Z = Me: methyl
tetraisopropyl
phosphorodiamidite, 1 H tetrazole / CHZCl2; (b) Z = allyl: diisopropylamie,
(allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole / CH3CN; (c) Z = (3-
cyanoethyl: 2-
cyanoethyltetraisopropylphosphorodiamidite, N,1V diisopropylammonium
tetrazolide, CH3CN, rt;
(iii) (1) succinic anhydride, 1,2-dichloroethane, DMA.P, (CzHs)3N, rt; (2) 2-
(1H benzotriazole-1-
yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTU), 4-methylmorpholine,
DMF,
aminoalkyl solid support, rt.
287
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 1001a
R'
R"-Si-O
HO B R~~-Si-O B R.., O B
O ~ R... O
N. ,O DACE
HO OH HO DACE
1001a-s 1002a-s R', R = O-SiMe3; R"' = O-CH(C6H5)Z ~ O.X 1003a-s
X = allyl, methyl or
p-cyanoethyl
Et3Si- R"_Si-O
O B R... O B
~ O
HO DACE ~N~O OACE
1005a~s H - ~O 1004a-s
R', R", R"' = Ethyl
R', R" = O-SiMe3; R"' = O-CH(C6H5)2
Et3Si-O ACE = bis(2-acetoxyethoxy)methyl
B
~OACE
~ 1006a-s X= allyl, methyl or (3-cyanoethyl
~X
NH N(CH3)O N(CH3)2 N(CH3)Q N(CH3)2 NHO O
~N N ~ N ~ N i N ~ ~ N N
N> ~ N ~~N> ~ ~~ ~> L ~~~i N~ > ~ J
N b ( Nc ~ N d ~ N a ~ ~N f ~r Ng
NHCONHCH~COOQ"
OII HN O I ~N~> OI' HN O I ~N~> O HN O I ~> N ~ ~ J
O~N~ N O~N~ N ~ ~ N
H h ~ ( i ~ O N jN ~ k #
O CN O NHQ OI-IN NHO NHQ
I N ~ H~ ~ \ OII HN
O H N I ~ ~O H N m ~ ~p~N~N~~MeS N o
H
NHCOOCH3 Q'O
O H3COOC NHBz
/s N i QO
N \ N O \ \N I N \ I O NHO
~i
N N N~~ O
P
~O H N r
288
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
a(i) a. Tris(2-acetoxyethoxy)orthoformate, pyridniump-toluenesulfonate, 4-
(tert-
butyldimethylsilyloxy)-3-penten-2-one, dioxane, rt; b. TMEDA-HF, MeCN; c.
Benzhydryloxybis(trimethylsilyloxy)silyl-Cl (BzHCl), diisopropylamine /
CH2C12; (ii) (a) Z =
Me: methyl tetraisopropyl phosphorodiamidite, 1 H tetrazole / CH2C12; (b) Z =
allyl:
diisopropylamie, (allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole /
CH3CN; (c) Z = (3-
cyanoethyl: 2-cyanoethyltetraisopropylphosphorodiamidite, N,N
diisopropylammonium
tetrazolide, CH3CN, rt; (iii) (1) succinic anhydride, 1,2-dichloroethane,
DMAF, (C~HS)3N, rt; (2)
2-(1H benzotriazole-1-yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTL77,
4-
methylinorpholine, DMF, aminoalkyl solid support, rt. (iv) a. Tris(2-
acetoxyethoxy)orthoformate, pyridnium p-toluenesulfonate, 4-(tent-
butyldimethylsilyloxy)-3
penten-2-one, dioxane, rt; b. TMEDA-HF, MeCN; c.Triethylsilyl chloride,
imidazole / THF
289
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 1002a
R'
R~ R"-Si-O
HO R,~_Si-O R.., p B
O B R", p B ii
i ~
HO OH ~ ~.O DACE
HO OACE ~ 1009a-a
1007a~u 1008a-a R', R = O-SiMe3; R"' = O-CH(C6H5)z ~ p~X
iii X = allyi, methyl or
~iv
R~ (3-cyanoethyl
Et3Si-O R"-Si-O B
O B iii ~ R"' O
O
Hp OACE CpG N~O OACE
1011a-a H Ip 1010a-a
ii R', R", R"' = Ethyl
R', R" = O-SiMe3; R"' = O-CH(C6H5)z
Et3Si-O ACE = bis(2-acetoxyethoxy)methyl
O B
DACE
~ 1012a-a X = allyl, methyl or p-cyanoethyl
~X
B
NHBz NHBz OII O O O p'I
I I N F C~N ~~OAc S~N~pAc HN~N~R
N S S N SO N O
h ~ c ~ d ~ a f
I I ~~F ~I ~I
O HN \ HN ~ HN HN HN
HN~N~n NR'R" / I p I O / I p I S / I S
O
h S ~ i p $ j S ~ i~ p # I
NHBz N~ NHBz O NHBz NHBz NHBz
p~N I NJ ~ I IIN I N N ~ I ~ I eN ~ I NN
~m I O ~ n O N o ~ O N ~ N ~ N ~ N
NHBz NHBz p q r s
N/ I \ N/ I NJ
BzHN~N t ~ BzHN~N
a
290
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
a(i) a. O[('Pr)ZSiCI]2, imidazole, DMF; b. Tris(2-acetoxyethoxy)orthoformate,
pyridnium
p-toluenesulfonate, 4-(tent-butyldimethylsilyloxy)-3-penten-2-one, dioxane,
rt; c. TMEDA-HF,
MeCN; d. Benzhydryloxybis(trimethylsilyloxy)silyl-Cl (BzHCI), diisopropylamine
/ CH2Clz; (ii)
s (a) Z = Me: methyl tetraisopropyl phosphorodiamidite, 1 H tetrazole /
CH2Cl2; (b) Z = allyl:
diisopropylamie, (allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole /
CH3CN; (c) Z = (3-
cyanoethyl: 2-cyanoethyltetraisopropylphosphorodiamidite, N,N
diisopropylammonium
tetrazolide, CH3CN, rt; (iii) (1) succinic anhydride, 1,2-dichloroethane,
DMAP, (C2H5)3N, rt; (2)
2-(1H benzotriazole-1-yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBT~, 4-
methylinorpholine, DMF, aminoalkyl solid support, rt. (iv) a. O[('Pr)ZSiCI]2,
imidazole, DMF; b.
Tris(2-acetoxyethoxy)orthofonnate, pyridniump-toluenesulfonate, 4-(tert-
butyldimethylsilyloxy)-3-penten-2-one, dioxane, rt; c. TMEDA-HF, MeCN;
d.Triethylsilyl
chloride, imidazole / THF
291
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 1003a
R'
R, R~,_SI-O
HO B R"_S -O R," B
i R", B iii
N. ,O
HO Y HO Y ~ P 1015a~j
1013a~j Y = H or OH 1014a j R', R = O-SiMe3; R"' = O-CH(C6H5)Z, O'X
Y = H or DACE ~ iii X = allyl, methyl or
Iiv
R, p-cyanoethyl
Et3Si-O R"-S -O
B iii R," B
O
HO Y N O Y
1017a j H O 1016a j
ii R', R", R"' = Ethyl
R', R" = O-SiMe3; R"' = O-CH(C6H5)2
Et3Si- ACE = bis(2-acetoxyethoxy)methyl
~B
~O/~~Y/
~ 1018a j X = allyl, methyl or p-cyanoethyl
'X
B
NHBz NHO O O
NQQ" ~~ <N I N I
O N N N O N ~ O N N~NH
~ f O
O NHO NHO
~N I / NJ H ~~N~ ~ I J NIw I N,N
OHN N N O N N ~~NY
h ( H i
292
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
a(i) For Y = OH, a. Tris(2-acetoxyethoxy)orthoformate, pyridniump-
toluenesulfonate, 4-
(tef°t-butyldimethylsilyloxy)-3-penten-2-one, dioxane, rt; b. TMEDA-HF,
MeCN; c.
Benzhydryloxybis(trimethylsilyloxy)silyl-Cl (BzHCl), diisopropylamine /
CH2C12; For Y = H:
Benzhydryloxybis(trimethylsilyloxy)silyl-Cl (BzHCl), diisopropylamine /
CHaCl2; (ii) (a) Z =
Me: methyl tetraisopropyl phosphorodiarnidite, 1 H tetrazole / CH2C12; (b) Z =
allyl:
diisopropylamie, (allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole /
CH3CN; (c) Z = (3-
cyanoethyl: 2-cyanoethyltetraisopropylphosphorodiamidite, N,N
diisopropylammonium
tetrazolide, CH3CN, rt; (iii) (1) succinic anhydride, 1,2-dichloroethane,
DMAP, (C2H5)3N, rt; (2)
2-(1H benzotriazole-1-yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTIJ),
4
methylmorpholine, DMF, aminoalkyl solid support, rt. (iv) a. For Y = OH, a.
Tris(2
acetoxyethoxy)orthoformate, pyridnium p-toluenesulfonate, 4-(test-
butyldimethylsilyloxy)-3-
penten-2-one, dioxane, rt; b. TMEDA-HF, MeCN; c.
Benzhydryloxybis(trimethylsilyloxy)silyl-
Cl (BzHCl), diisopropylamine / CH2C12; For Y = H:
Benzhydryloxybis(trimethylsilyloxy)silyl-
Cl (BzHCI), diisopropylamine / CHZCl2;
293
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 1004a
F3COCHN~~~~
1019 1i
__ .., .. _ ......,.s, ., - x p-cYaiwemy
O-CH(C6H5)z
iii
a(i) For 8a: Triethylsilyl chloride, imidazole / THF; For 8b:
Benzhydryloxybis(trimethylsilyloxy)silyl-Cl (BzHCI), diisopropylamine /
CH2C12; (ii) (a) Z =
Me: methyl tetraisopropyl phosphorodiamidite, 1 H tetrazole / CH2C12; (b) Z =
allyl:
diisopropylamie, (allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole /
CH3CN; (c) Z = (3-
cyanoethyl: 2-cyanoethyltetraisopropylphosphorodiamidite, N,N
diisopropylammonium
tetrazolide, CH3CN, rt; (iii) (1) succinic anhydride, 1,2-dichloroethane,
DMAP, (C2H5)3N, rt; (2)
2-(1H benzotriazole-1-yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTI~,
4-
methylmorpholine, DMF, aminoalkyl solid support, rt.
294
R', R" = O-SiMe3; R"' = O-CH(C6H5)z
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Scheme 1005a
R'
R' R"_SI-O
HO O B R'~_S -O B R," O B
i R... p ii
Y
HO N.P~O
HO ~ 105a-z
1023a-z 1024a-z R', R = O-SiMe3; R"' = O-CH(C6H5)z ~ O~X 1025a1-c1
1023a1-c1 1024a1-c1 X = allyl, methyl or
iv iii p-cyanoethyl
R'
Et Si-O ~ R"-Si-O
3
B iii R", O B
O
H01026a-z ~N~01026a-z
1026a1-c1 H O 1026a1-c1
ii R', R", R"' = Ethyl
R', R" = O-SiMe3; R"' = O_CH(C6H5)z
Et3Si-O ACE = bis(2-acetoxyethoxy)methyl
B
~P~O 1027a-z X = allyl, methyl or p-cyanoethyl
O~X 1027a1-c1
B
O O O HzN H2N HzN HzN
HNI~ J ~ ~ , HN~ N HN \ N O O O~ O
'N N O N N N:~N> ~N~ ~~ / ~N / ~ N \1
a # 'z ~~~ .N
c i~ d ~ ~e ~p ~g ~h
NOz NOz NOz NOz NHQ O OzN
/N\ ~~ N N~ OzN ~ I N ~ I J ~ I N>H \ I N> y I NN
r~i ~j #k 1 f m # n # o ~ P
N CONHz N NOz
~N~CONH ~N~ / ~ I ~ \ I
( 9 z ~ r ~ S / N ~ i i
t ~ a v w
I~ F
N I I N N
I I I I ~ N I I Me I / I I ~ N ~ ~ ~ NHQ
O"N' zF NONO N
x y ~ # a1 # b1 ~ c1
295
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
a(i) Benzhydryloxybis(trimethylsilyloxy)silyl-Cl (BzHCI), diisopropylamine /
CHZCIz;
(ii) (a) Z = Me: methyl tetraisopropyl phosphorodiamidite, 1 H tetrazole /
CH2Cl2; (b) Z = allyl:
diisopropylamie, (allyloxy)bis(diisopropylamino)phosphine, 1 H tetrazole /
CH3CN; (c) Z = (3-
cyanoethyl: 2-cyanoethyltetraisopropylphosphorodiamidite, N,1V
diisopropylammonium
tetrazolide, CH3CN, rt; (iii) (1) succinic anhydride, 1,2-dichloroethane,
DMAP, (CZHS)3N, rt; (2)
2-(1H benzotriazole-1-yl)-1,1,3,3-tetramethyluroniumtetrafluoroborate (TBTU),
4-
methylinorpholine, DMF, aminoalkyl solid support, rt. (iv) Triethylsilyl
chloride, imidazole /
THF
Example 1
Compound 2 (Scheme 1): The diol 1 is prepared as reported in the literature
(Filichev
and Pedersen, Tetrahedron, 2001, 57, 9163-68). 1,3-Dichloro-1,1,3,3-
tetraisopropyldisiloxane
(0.9 mmol) is added into a solution of compound 1 (1 mmol) and imidazole (3
mmol) in DMF to
obtain compound 2 (Evans et al., Tetrahedron, 2000, 56, 3053-62).
Example 2
Compound 3 (Scheme 1): Compound 2 is stirred with triethylamine in
acetonitrile to
obtain compound 3 (Filichev and Pedersen, Tetrahedron, 2001, 57, 9163-68).
Example 3
2o Compound 6 (Scheme 1): The diol 4 is prepared according to the literature
reports by
Filichev and Pedersen (Tetrahedron, 2001, 57, 9163-68). Cyclic silylether is
prepared from
compound 4 according to literature procedure (Evans et al., Tetrahedron, 2000,
56, 3053-62).
Treatment of compound 5 with TEA in acetonitrile yields compound 6.
Example 4
Compound 7a (Scheme 2): Compound 3 (1 mmol) in anhydrous THF is stirred with
1,1'-carbonyldiimidazole (CDI, 1 mmol) in the presence of DMAP at ambient
temperature under
argon atmosphere for 2 h. Cholesterol (1 mmol) is added into the reaction
mixture after 2 h and
the stirring is continued for overnight to obtain compound 7a (Hernandez and
Hodges, J. Org.
Chent.,1997, 62, 3153-3157).
so Example 5
Compound 8a (Scheme 2): A solution of compound 7a in THF is treated with TBAF
for
1h at ambient temperature to yield compound 8a (Evans et al., Tetrahedron,
2000, 56, 3053-62).
Example 6
296
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Compound 9a (Scheme 2): The desired silyl ether 9a is obtained by the
treatment of
compound 8a with benzhydryloxybis(trimethylsilyloxy)silyl chloride (BzH-Cl) in
the presence
of diisopropylamine as reported by Chuff et al. (J. Org. Claem., 2002, 67,
8847-54).
Example 7
Compound 10a (Z = Me, Scheme 2): Treatment of compound 9a with methyl
tetraisopropyl phosphorodiamidite in the presence of 1 H tetrazole at ambient
temperature in
CHZC12 yields compound 10a (Chuff et al. J. O~g. Chem., 2002, 67, 8847-54).
Example 8
Compound 10a (Z = allyl, Scheme 2): To a solution of compound 9a (1 mmol) in
acetonitrile are added diisopropylamine (1.2 mmol),
(allyloxy)bis(diisopropylamino)phospine
(1.5 mmol) and 1 H tetrazole (1.2 mmol). The solution is stirred for 2 h at
ambient temperature
to obtain the desired phosphoramidite 10a (Hayakawa et al., .I. Am. Chem.
Soc.,1990,112,
1691-96).
Example 9
15 Compound 10a (Z = (3-cyanoethyl, Scheme 2): The desired phosphoramidite 10a
is
prepared from compound 9a and (3-cyanoethyl tetraisopropyl phosphorodiamidite
in the presence
of 1 H tetrazole diisopropylammonium salt in anhydrous acetonitrile as
reported in the literature
(Prakash et al., J. Org. Chem. 2002, 67, 357-369).
Example 10
2o Solid support 11a (Scheme 2}: Compound 9a (1 mmol) is mixed with succinic
anhydride (2 mmol) and dimethlyaminopyridine (1 mmol), and is dried over P205
in vacuo
overnight. Dichloromethne (0.9 mL) is added into the mixture, and the mixture
is stirred at
ambient temperature for 8 h. The reaction mixture is diluted with excess
dichloromethane and
the organic layer is subjected ice cold aqueous citric acid wash (10 %
solution) and brine. The
25 organic phase is dried over anhydrous NaZS04 and concentrated to dryness to
yield the
corresponding succinic acid derivative. The succinic acid derivative (1 mmol)
thus obtaned is
dried over P205 under vacuum overnight and suspended in anhydrous DMF, mixed
with 2-(1H
benzotriazole-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate (TBTU, 1
mmol) and 4-
methylmorpholine (2 mmol) with vortexing to give a clear solution. Calculated
amount of solid
3o support (118.9 ~,mol/g, particle size 80/120, mean pore diameter 569 A) is
added into the clear
solution, and the mixture is allowed to shake on a shaker at ambient
temperature for 18 h. An
aliquot of the support is withdrawn and washed with DMF, CH3CN and
diethylether, and dries in
vacuo. Loading capacity is determined by following standard procedure.
Functionalized solid
support is then washed with DMF, CH3CN, diethylether and dried in vacuo.
Unfunctionalized
297
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
sites on the SOLID SUPPORT are capped with acetic anhydride/collidine/N
methylimidazole in
THF (2 mL Cap A and 2 mL Cap B solutions from Perspective Biosystems Inc.) and
allows to
shake on a shaker for 2 h. The solid support is filtered, washed with CH3CN
followed by
diethlether, and dries in vacuo. The final loading capacity of 85a is
determined after capping
(Prakash et al., J. Org. Chern. 2002, 67, 357-369).
Example 11
Compound 10b (Z = Me, n =15, Scheme 2): The phosphoramidite lOb is prepared
from
compound 3 and 1,3-bis-O-(hexadecyl)glycerol according to the procedures
described in
Examples 4, 5, 6 and 7.
Example 12
Compound lOb (Z = allyl, n =15, Scheme 2): The phosphoramidite lOb is prepared
from compound 3 and 1,3-bis-O-(hexadecyl)glycerol according to the procedures
described in
Examples 4, S, 6 and 8.
Example 13
Compound lOb (Z = (3-cyanoethyl, n =15, Scheme 2): The phosphoramidite lOb is
prepared from compound 3 and 1,3-bis-O-(hexadecyl)glycerol according to the
procedures
described in Examples 4, 5, 6 and 9.
Example 14
2o Solid support 11b (n =15, Scheme 2): The solid support llb is prepared from
compound 9b as reported in Example 10. Compound 9b is obtained from compound 3
and 1,3-
bis-O-(hexadecyl)glycerol as reported in Examples 4, 5 and 6.
Example 15
Compound l Oc (Z = Me, n =15, Scheme 2): The phosphoramidite lOc is prepared
from
compound 3 and hexadecylglycerol according to the procedures described in
Examples 4, 5, 6
and 7.
Example 16
Compound lOc (Z = allyl, n =15, Scheme 2): The phosphoramidite lOc is prepared
from compound 3 and hexadecylglycerol according to the procedures described in
Examples 4,
5,6and8.
Example 17
Compound l Oc (Z = (3-cyanoethyl, n =15, Scheme 2): The phosphoramidite 10c is
prepared from compound 3 and hexadecylglycerol according to the procedures
described in
Examples 4, 5, 6 and 9.
298
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Example 18
Solid support llc (n =15, Scheme 2): The solid support llc is prepared from
compound 9c as reported in Example 10. Compound 9c is obtained from compound 3
and
hexadecylglycerol as reported in Examples 4, 5 and 6.
Example 19
Compound l Od (Z = Me, Scheme 2): The phosphoramidite 10d is prepared from
compound 3 and desired Me-O-PEG according to the procedures described in
Examples 4, 5, 6
and 7.
Example 20
Compound l Od (Z = allyl, Scheme 2): The phosphoramidite lOd is prepared from
compound 3 and desired Me-O-PEG according to the procedures described in
Examples 4, 5, 6
and ~.
299
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Example 21
Compound l Od (Z = ~i-cyanoethyl, Scheme 2): The phosphoramidite l Od is
prepared
from compound 3 and desired Me-O-PEG according to the procedures described in
Examples 4,
5, 6 and 9.
Example 22
Solid support lld (Scheme 2): The solid support 11d is prepared from compound
9d as
reported in Example 10. Compound 9d is obtained from compound 3 and desired Me-
O-PEG as
reported in Examples 4, 5 and 6.
Example 23
Compound 10e (Z = Me, Scheme 2): The phosphoramidite 10e is prepared from
compound 3 and desired branched Me-O-PEG according to the procedures described
in
Examples 4, 5, 6 and 7.
Example 23
~5 Compound 10e (Z = allyl, Scheme 2): The phosphoramidite 10e is prepared
from
compound 3 and desired branched Me-O-PEG according to the procedures described
in
Examples 4, 5, 6 and ~.
Example 24
Compound 10e (Z = (3-cyanoethyl, Scheme 2): The phosphoramidite 10e is
prepared
2o from compound 3 and desired branched Me-O-PEG according to the procedures
described in
Examples 4, 5, 6 and 9.
Example 25
Solid support lle (Scheme 2): The solid support lle is prepared from compound
9e as
reported in Example 10. Compound 9e is obtained from compound 3 and desired
branched Me-
25 O-PEG as reported in Examples 4, 5 and 6.
Example 26
Compound lOf (Z = Me, Scheme 2): The phosphoramidite l Of is prepared from
compound 3 and dihydrotestosteron according to the procedures described in
Examples 4, 5, 6
and 7.
300
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Example 27
Compound lOf (Z = allyl, Scheme 2): The phosphoramidite lOf is prepared from
compound 3 and dihydrotestosteron according to the procedures described in
Examples 4, 5, 6
and 8.
Example 28
Compound lOf (Z = ~3-cyanoethyl, Scheme 2): The phosphoramidite lOf is
prepared
from compound 3 and dihydrotestosteron according to the procedures described
in Examples 4,
5, 6 and 9.
1 o Example 29
Solid support llf (Scheme 2): The solid support 11f is prepared from compound
9f as
reported in Example 10. Compound 9f is obtained from compound 3 and
dihydrotestosteron as
reported in Examples 4, 5 and 6.
Example 30
~ 5 Compound lOg (Z = Me, Scheme 2): The phosphoramidite 10g is prepared from
compound 3 and borneol according to the procedures described in Examples 4, 5,
6 and 7.
Example 31
Compound lOg (Z = allyl, Scheme 2): The phosphoramidite lOg is prepared from
compound 3 and borneol according to the procedures described in Examples 4, 5,
6 and 8.
2o Example 32
Compound lOg (Z = (3-cyanoethyl, Scheme 2): The phosphoramidite l Og is
prepared
from compound 3 and borneol according to the procedures described in Examples
4, 5, 6 and 9.
Example 33
Solid support llg (Scheme 2): The solid support 11g is prepared from compound
9g as
25 reported in Example 10. Compound 9g is obtained from compound 3 and borneol
as reported in
Examples 4, 5 and 6.
301
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Example 34
Compound lOh (Z = Me, Scheme 2): The phosphoramidite lOh is prepared from
compound 3 and menthol according to the procedures described in Examples 4, 5,
6 and 7.
s Example 35
Compound lOh (Z = allyl, Scheme 2): The phosphoramidite l Oh is prepared from
compound 3 and menthol according to the procedures described in Examples 4, 5,
6 and 8
Example 36
Compound lOh (Z = (3-cyanoethyl, Scheme 2): The phosphoramidite lOh is
prepared
from compound 3 and menthol according to the procedures described in Examples
4, 5, 6 and 9.
Example 37
Solid support llh (Scheme 2): The solid support 11h is prepared from compound
9h as
reported in Example 10. Compound 9h is obtained from compound 3 and menthol as
reported in
Examples 4, 5 and 6.
~5 Example 38
Compound 10i (Z = Me, Scheme 2): The phosphoramidite 10i is prepared from
compound 3 and cholenic acid ethyl ester according to the procedures described
in Examples 4,
5, 6 and 7.
Example 39
2o Compound 10i (Z = allyl, Scheme 2): The phosphoramidite 10i is prepared
from
compound 3 and cholenic acid ethyl ester according to the procedures described
in Examples 4,
5,6and8.
Example 40
Compound 10i (Z = (3-cyanoethyl, Scheme 2): The phosphoramidite 10i is
prepared
25 from compound 3 and cholenic acid ethyl ester according to the procedures
described in
Examples 4, 5, 6 and 9.
302
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Example 41
Solid support lli (Scheme Z): The solid support lli is prepared from compound
9i as
reported in Example 10. Compound 9i is obtained from compound 3 and cholenic
acid as
s reported in Examples 4, 5 and 6.
Example 42
Compound l Oj (Z = Me, Scheme 2): The phosphoramidite lOj is prepared from
compound 3 and lithocholic acid ethyl ester according to the procedures
described in Examples
4, 5, 6 and 7.
Example 43
Compound lOj (Z = allyl, Scheme 2): The phosphoramidite l Oj is prepared from
compound 3 and lithocholic acid ethyl ester according to the procedures
described in Examples
4, 5, 6 and 8.
Example 44
15 Compound lOj (Z = (3-cyanoethyl, Scheme 2): The phosphoramidite lOj is
prepared
from compound 3 and lithocholic acid ethyl ester according to the procedures
described in
Examples 4, 5, 6 and 9.
Example 45
Solid support llj (Scheme 2): The solid support llj is prepared from compound
9j as
2o reported in Example 10. Compound 9j is obtained from compound 3 and
lithocholic acid ethyl
ester as reported in Examples 4, 5 and 6.
Example 46
Compound lOk (Z = Me, Scheme 2): The phosphoramidite 10k is prepared from
compound 3 and Vitamin E according to the procedures described in Examples 4,
S, 6 and 7.
2s Example 47
Compound lOk (Z = allyl, Scheme 2): The phosphoramidite lOk is prepared from
compound 3 and Vitamin E according to the procedures described in Examples 4,
5, 6 and 8.
303
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Example 48
Compound lOk (Z = [3-cyanoethyl, Scheme 2): The phosphoramidite l Ok is
prepared
from compound 3 and Vitamin E according to the procedures described in
Examples 4, 5, 6 and
9.
Example 49
Solid support llk (Scheme 2): The solid support llk is prepared from compound
9k as
reported in Example 10. Compound 9k is obtained from compound 3 and Vitamin E
as reported
in Examples 4, 5 and 6.
Example 50
Compound 101 (Z = Me, Scheme 2): The phosphoramidite 101 is prepared from
compound 3 and Hoechst 33258 - PEG conjugate according to the procedures
described in
Examples 4, 5, 6 and 7.
Example 51
Compound 101 (Z = allyl, Scheme 2): The phosphoramidite 101 is prepared from
compound 3 and Hoechst 33258 - PEG conjugate according to the procedures
described in
Examples 4, 5, 6 and 8.
Example 52
Compound 101 (Z = (3-cyanoethyl, Scheme 2): The phosphoramidite 101 is
prepared
2o from compound 3 and Hoechst 33258 -PEG conjugate according to the
procedures described in
Examples 4, 5, 6 and 9.
Example 53
Solid support 111 (Scheme 2): The solid support 111 is prepared from compound
91 as
reported in Example 10. Compound 91 is obtained from compound 3 and Hoechst
33258 - PEG
conjugate as reported in Examples 4, 5 and 6.
Example 54
Compound 15a (Z = Me, Scheme 3): The phosphoramidite 15a is prepared from
compound 6 and cholesterol according to the procedures described in Examples
4, 5, 6 and 7.
304
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Example 55
Compound 15a (Z = allyl, n =15, Scheme 3): The phosphoramidite 15a is prepared
from compound 6 and cholesterol according to the procedures described in
Examples 4, 5, 6 and
8.
Example 56
Compound 15a (Z = ~3-cyanoethyl, Scheme 3): The phosphoramidite 15a is
prepared
from compound 6 and cholesterol according to the procedures described in
Examples 4, 5, 6 and
9.
1 o Example 57
Solid support 16a (Scheme 3): The solid support 16a is prepared from compound
14a as
reported in Example 10. Compound 14a is obtained from compound 6 and 1,3-bis-O-
(hexadecyl)glycerol as reported in Examples 4, 5 and 6.
Example 58
~ 5 Compound 15b (Z = Me, n =15, Scheme 3): The phosphoramidite 15b is
prepared from
compound 6 and 1,3-bis-O-(hexadecyl)glycerol according to the procedures
described in
Examples 4, 5, 6 and 7.
Example 59
Compound 15b (Z = allyl, n =15, Scheme 3): The phosphoramidite 15b is prepared
2o from compound 6 and 1,3-bis-O-(hexadecyl)glycerol according to the
procedures described in
Examples 4, 5, 6 and 8.
Example 60
Compound 15b (Z = (3-cyanoethyl, n =15, Scheme 3): The phosphoramidite 15b is
prepared from compound 6 and 1,3-bis-O-(hexadecyl)glycerol according to the
procedures
25 described in Examples 4, 5, 6 and 9.
Example 61
Solid support 16b (n =15, Scheme 3): The solid support 16b is prepared from
compound 9b as reported in Example 10. Compound 14b is obtained from compound
6 and 1,3-
bis-O-(hexadecyl)glycerol as reported in Examples 4, S and 6.
305
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Example 62
Compound 15c (Z = Me, n =15, Scheme 3): The phosphoramidite 15c is prepared
from
compound 6 and hexadecylglycerol according to the procedures described in
Examples 4, 5, 6
and 7.
Example 63
Compound 15c (Z = allyl, n =15, Scheme 3): The phosphoramidite 15c is prepared
from compound 6 and hexadecylglycerol according to the procedures described in
Examples 4,
5,6and~.
Example 64
Compound 15c (Z = (3-cyanoethyl, n =15, Scheme 3): The phosphoramidite 15c is
prepared from compound 6 and hexadecylglycerol according to the procedures
described in
Examples 4, 5, 6 and 9.
Example 65
Solid support 16c (n =15, Scheme 3): The solid support 16c is prepared from
compound 14c as reported in Example 10. Compound 14c is obtained from compound
6 and
hexadecylglycerol as reported in Examples 4, S and 6.
Example 66
Compound 15d (Z = Me, Scheme 3): The phosphoramidite 15d is prepared from
2o compound 6 and desired Me-O-PEG according to the procedures described in
Examples 4, 5, 6
and 7.
Example 67
Compound 15d (Z = allyl, Scheme 3): The phosphoramidite 15d is prepared from
compound 6 and desired Me-O-PEG according to the procedures described in
Examples 4, 5, 6
and 8.
Example 68
Compound 15d (Z = (3-cyanoethyl, Scheme 3): The phosphoramidite 15d is
prepared
from compound 6 and desired Me-O-PEG according to the procedures described in
Examples 4,
5,6and9.
306
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Example 69
Solid support 16d (Scheme 3): The solid support 16d is prepared from compound
14d
as reported in Example 10. Compound 14d is obtained from compound 6 and
desired Me-O-PEG
as reported in Examples 4, 5 and 6.
Example 70
Compound 15e (Z = Me, Scheme 3): The phosphoramidite 15e is prepared from
compound 6 and desired branched Me-O-PEG according to the procedures described
in
Examples 4, 5, 6 and 7.
Example 71
Compound 15e (Z = allyl, Scheme 3): The phosphoramidite 15e is prepared from
compound 6 and desired branched Me-O-PEG according to the procedures described
in
Examples 4, 5, 6 and 8.
Example 72 -
Compound 15e (Z = (3-cyanoethyl, Scheme 3): The phosphoramidite 15e is
prepared
from compound 6 and desired branched Me-O-PEG according to the procedures
described in
Examples 4, 5, 6 and 9.
Example 73
Solid support 16e (Scheme 3): The solid support 16e is prepared from compound
14e as
2o reported in Example 10. Compound 14e is obtained from compound 6 and
desired branched Me-
O-PEG as reported in Examples 4, 5 and 6.
Example 74
Compound 15f (Z = Me, Scheme 3): The phosphoramidite 15f is prepared from
compound 6 and dihydrotestosteron according to the procedures described in
Examples 4, 5, 6
arid 7.
Example 75
Compound 15f (Z = allyl, Scheme 3): The phosphoramidite 15f is prepared from
compound 6 and dihydrotestosteron according to the procedures described in
Examples 4, 5, 6
and 8.
307
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Example 76
Compound 15f (Z = (3-cyanoethyl, Scheme 3): The phosphoramidite 15f is
prepared
from compound 6 and dihydrotestosteron according to the procedures described
in Examples 4,
5, 6 and 9.
Example 77
Solid support 16f (Scheme 3): The solid support 16f is prepared from compound
14f as
reported in Example 10. Compound 14f is obtained from compound 6 and
dihydrotestosteron as
reported in Examples 4, 5 and 6.
Example 78
Compound 15g (Z = Me, Scheme 3): The phosphoramidite 15g is prepared from
compound 6 and borneol according to the procedures described in Examples 4, 5,
6 and 7.
Example 79
Compound 15g (Z = allyl, Scheme 3): The phosphoramidite 15g is prepared from
~5 compound 6 and borneol according to, the procedures described in Examples
4, 5, 6 and 8.
Example 80
Compound 15g (Z = (3-cyanoethyl, Scheme 3): The phosphoramidite 15g is
prepared
from compound 6 and borneol according to the procedures described in Examples
4, 5, 6 and 9.
Example 81
2o Solid support 16g (Scheme 3): The solid support 16g is prepared from
compound 14g as
reported in Example 10. Compound 14g is obtained from compound 6 and borneol
as reported in
Examples 4, 5 and 6.
Example 82
Compound 15h (Z = Me, Scheme 3): The phosphoramidite 15h is prepared from
25 compound 6 and menthol according to the procedures described in Examples 4,
5, 6 and 7.
308
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Example 83
Compound 15h (Z = allyl, Scheme 3): The phosphoramidite 15h is prepared from
compound 6 and menthol according to the procedures described in Examples 4, 5,
6 and 8.
Example 84
Compound 15h (Z = (3-cyanoethyl, Scheme 3): The phosphoramidite 15h is
prepared
from compound 6 and menthol according to the procedures described in Examples
4, 5, 6 and 9.
Example 85
Solid support 16h (Scheme 3): The solid support 16h is prepared from compound
14h
as reported in Example 10. Compound 14h is obtained from compound 6 and
menthol as
reported in Examples 4, 5 and 6.
Example 86
Compound 16i (Z = Me, Scheme 3): The phosphoramidite 16i is prepared from
compound 6 and choleric acid ethyl ester according to the procedures described
in Examples 4,
15 5,6and7.
Example 87
Compound 15i (Z = allyl, Scheme 3): The phosphoramidite 15i is prepared from
compound 6 and choleric acid ethyl ester according to the procedures described
in Examples 4,
5, 6 and 8.
2o Example 88
Compound 15i (Z = (3-cyanoethyl, Scheme 3): The phosphoramidite 15i is
prepared
from compound 6 and choleric acid ethyl ester according to the procedures
described in
Examples 4, 5, 6 and 9.
Example 89
25 Solid support 16i (Scheme 3): The solid support 16i is prepared from
compound 14i as
reported in Example 10. Compound 14i is obtained from compound 6 and choleric
acid as
reported in Examples 4, 5 and 6.
309
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Example 90
Compound 15j (Z = Me, Scheme 3): The phosphoramidite 15j is prepared from
compound 6 and lithocholic acid ethyl ester according to the procedures
described in Examples
4,5,6and7.
Example 91
Compound 15j (Z = allyl, Scheme 3): The phosphoramidite 15j is prepared from
compound 6 and lithocholic acid ethyl ester according to the procedures
described in Examples
4,5,6and8.
Example 92
Compound 15j (Z = (3-cyanoethyl, Scheme 3): The phosphoramidite 15j is
prepared
from compound 6 and lithocholic acid ethyl ester according to the procedures
described in
Examples 4, 5, 6 and 9.
Example 93
~5 Solid support 16j (Scheme 3): The solid support 16j is prepared from
compound 14j as
reported in Example 10. Compound 14j is obtained from compound 6 and
lithocholic acid ethyl
ester as reported in Examples 4, 5 and 6.
Example 94
Compound 15k (Z = Me, Scheme 3): The phosphoramidite 15k is prepared from
2o compound 6 and Vitamin E according to the procedures described in Examples
4, 5, 6 and 7.
Example 95
Compound 15k (Z = allyl, Scheme 3): The phosphoramidite 15k is prepared from
compound 6 and Vitamin E according to the procedures described in Examples 4,
5, 6 and 8.
Example 96
25 Compound 15k (Z = (3-cyanoethyl, Scheme 3): The phosphoramidite 15k is
prepared
from compound 6 and Vitamin E according to the procedures described in
Examples 4, 5, 6 and
9.
310
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Example 97
Solid support 16k (Scheme 3): The solid support 16k is prepared from compound
14k
as reported in Example 10. Compound 14k is obtained from compound 6 and
Vitamin E as
reported in Examples 4, 5 and 6.
Example 98
Compound 151 (Z = Me, Scheme 3): The phosphoramidite 151 is prepared from
compound 6 and Hoechst 33258 - PEG conjugate according to the procedures
described in
Examples 4, 5, 6 and 7.
Example 99
Compound 151 (Z = allyl, Scheme 3): The phosphoramidite 151 is prepared from
compound 6 and Hoechst 33258 - PEG conjugate according to the procedures
described in
Examples 4, 5, 6 and 8.
Example 100
Compound 151 (Z = (3-cyanoethyl, Scheme 3): The phosphoramidite 151 is
prepared-
from compound 6 and Hoechst 33258 - PEG conjugate according to the procedures
described in
Examples 4, 5, 6 and 9.
Example 101
Solid support 111 (Scheme 3): The solid support 161 is prepared from compound
141 as
2o reported in Example 10. Compound 141 is obtained from compound 6 and
Hoechst 33258 -PEG
conjugate as reported in Examples 4, 5 and 6.
Example 102
Compound 17 (p =1, Scheme 4): Compound 6 (1 mmol, Scheme 1) is coupled to Fmoc-
Gly in the presence of DCC. A solution of Fmoc-Gly (1 mmol), DhbhOH (1.5 mmol)
and DCC
(1.1 mmol) in dry DMF (2 mL) is stirred at ambient temperature for 2 h after
which compound 6
(1.2 mmol) is added into the stirring reaction mixture. The reaction is
stirred for 8 h. DCU is
removed by filtration and DMF is removed under vacuum. The residue is
suspended in ethyl
acetate and is subjected bicarbonate wash to remove any unreacted acid
followed by water wash
and subsequently with KHS04 solution to remove excess amine. Standard workup
and
3o chromatographic purification is followed to obtain compound 17.
Example 103
Compound 18 (Scheme 4): Compound 17 is stirred in DMF-piperidine (9:1) for 30
min
to yield compound 18.
311
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Example 104
Compound 21a (Z = Me, Scheme 4): The phosphoramidite 21a is prepared from
compound 18 and cholesterol according to the procedures described in Examples
4, 5, 6 and 7.
Example 105
Compound 21a (Z = allyl, Scheme 4): The phosphoramidite 21a is prepared from
compound 18 and cholesterol according to the procedures described in Examples
4, 5, 6 and 8.
Example 106
Compound 21a (Z = (3-cyanoethyl, Scheme 4): The phosphoramidite 21a is
prepared
from compound 18 and cholesterol according to the procedures described in
Examples 4, 5, 6
1 o and 8.
Example 107
Solid support 22a (Scheme 4): The solid support 22a is prepared from compound
20a as
reported in Example 10. Compound 20a is obtained from compound 18 and 1,3-bis-
O-
(hexadecyl)glycerol as reported in Examples 4, 5 and 6.
15 Example 108
Compound 21b (Z = Me, n =15, Scheme 4): The phosphoramidite 21b is prepared
from
compound 18 and 1,3-bis-O-(hexadecyl)glycerol according to the procedures
described in
Examples 4, 5, 6 and 7.
Example 109
2o Compound 21b (Z = allyl, n =15, Scheme 4): The phosphoramidite 21b is
prepared
from compound 18 and 1,3-bis-O-(hexadecyl)glycerol according to the procedures
described in
Examples 4, 5, 6 and 8.
312
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Example 110
Compound 21b (Z = (3-cyanoethyl, n =15, Scheme 4): The phosphoramidite 21b is
prepared from compound 18 and 1,3-bis-O-(hexadecyl)glycerol according to the
procedures
s described in Examples 4, 5, 6 and 9.
Example 111
Solid support 22b (n =15, Scheme 4): The solid support 22b is prepared from
compound ZOb as reported in Example 10. Compound 20b is obtained from compound
18 and
1,3-bis-O-(hexadecyl)glycerol as reported in Examples 4, 5 and 6.
Example 112
Compound 21c (Z = Me, n =15, Scheme 4): The phosphoramidite 21c is prepared
from
compound 18 and hexadecylglycerol according to the procedures described in
Examples 4, 5, 6
and 7.
Example 113
15 Compound 21c (Z = allyl, n =15, Scheme 4): The phosphoramidite 21c is
prepared '
from compound 18 and hexadecylglycerol according to the procedures described
in Examples 4,
5,6and8.
Example 114
Compound 21c (Z = (3-cyanoethyl, n =15, Scheme 4): The phosphoramidite 21c is
2o prepared from compound 18 and hexadecylglycerol according to the procedures
described in
Examples 4, 5, 6 and 9.
Example 115
Solid support 22c (n =15, Scheme 4): The solid support 22c is prepared from
compound 20c as reported in Example 10. Compound 20c is obtained from compound
18 and
25 hexadecylglycerol as reported in Examples 4, 5 and 6.
Example 116
Compound 21d (Z = Me, Scheme 4): The phosphoramidite 21d is prepared from
compound 18 and desired Me-O-PEG according to the procedures described in
Examples 4, 5, 6
and 7.
313
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Example 117
Compound 21d (Z = allyl, Scheme 4): The phosphoramidite 21d is prepared from
compound 18 and desired Me-O-PEG according to the procedures described in
Examples 4, 5, 6
and 8.
Example 118
Compound 21d (Z = [3-cyanoethyl, Scheme 4): The phosphoramidite 21d is
prepared
from compound 18 and desired Me-O-PEG according to the procedures described in
Examples 4,
5, 6 and 9.
Example 119
Solid support 22d (Scheme 4): The solid support 22d is prepared from compound
20d
as reported in Example 10. Compound 20d is obtained from compound 18 and
desired Me-O-
PEG as reported in Examples 4, 5 and 6.
Example 120
y Compound 21e (Z = Me, Scheme 4): The phosphoramidite 21e is prepared from
compound 18 and desired branched Me-O-PEG according to the procedures
described in
Examples 4, 5, 6 and 7.
Example 121
Compound 21e (Z = allyl, Scheme 4): The phosphoramidite Zle is prepared from
2o compound 18 and desired branched Me-O-PEG according to the procedures
described in
Examples 4, 5, 6 and 8.
Example 122
Compound 21e (Z = (3-cyanoethyl, Scheme 4): The phosphoramidite 21e is
prepared
from compound 18 and desired branched Me-O-PEG according to the procedures
described in
Examples 4, 5, 6 and 9.
Example 123
Solid support 22e (Scheme 4): The solid support 22e is prepared from compound
20e as
reported in Example 10. Compound 20e is obtained from compound 18 and desired
branched
Me-O-PEG as reported in Examples 4, 5 and 6.
314
CA 02522349 2005-10-13
WO 2004/094345 PCT/US2004/011822
Example 124
Compound 21f (Z = Me, Scheme 4): The phosphoramidite 21f is prepared from
compound 18 and dihydrotestosteron according to the procedures described in
Examples 4, 5, 6
and 7.
Example 125
Compound 21f (Z = allyl, Scheme 4): The phosphoramidite 21f is prepared from
compound 18 and dihydrotestosteron according to the procedures described in
Examples 4, 5, 6
and 8.
Example 126
Compound 21f (Z = (3-cyanoethyl, Scheme 4): The phosphoramidite 21f is
prepared
from compound 18 and dihydrotestosteron according to the procedures described
in Examples 4,
5,6and9.
Example 127
Solid support 22f (Scheme 4): The solid support 22f is prepared from compound
20f as
reported in Example 10. Compound 20f is obtained from compound 18 and
dihydrotestosteron as
reported in Examples 4, 5 and 6.
Example 128
Compound 21g (Z = Me, Scheme 4): The phosphoramidite Zlg is prepared from
2o compound 18 and borneol according to the procedures described in Examples
4, 5, 6 and 7.
Example 129
Compound 21g (Z = allyl, Scheme 4): The phosphoramidite 21g is prepared from
compound 18 and borneol according to the procedures described in Examples 4,
5, 6 and 8.
Example 130
Compound 21g (Z = (3-cyanoethyl, Scheme 4): The phosphoramidite Zlg is
prepared
from compound 18 and borneol according to the procedures described in Examples
4, 5, 6 and 9.
315
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 315
NOTE : Pour les tomes additionels, veuillez contacter 1e Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 315
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME
NOTE POUR LE TOME / VOLUME NOTE: