Note: Descriptions are shown in the official language in which they were submitted.
CA 02523486 2005-10-07
-1-
Title: JOINT-DIAGNOSTIC SPECTROSCOPIC AND BIOSENSOR METER
Field Of The Invention
[0001] The invention relates to meters, which measure fluid samples
contained in disposable microfluidic cartridges, using a combination of
spectroscopic and biosensor technology.
Background Of The Invention
[0002] There are many medical diagnostic tests that require a fluid, for
example without limitation, blood (sometimes referred to as whole blood, in
order to differentiate blood from serum and plasma), serum, plasma,
cerebrospinal fluid, synovial fluid, lymphatic fluid, calibration fluid, and
urine.
With respect to blood, a blood sample is typically withdrawn in either an
evacuated tube containing a rubber septum (a vacutainer), or a syringe, and
sent to a central laboratory for testing. The eventual transfer of blood from
the
collection site to the testing site results in inevitable delays. Moreover,
the red
blood cells are alive and continue to consume oxygen during any delay
period, which in turn changes chemical composition of the blood sample in
between the time the blood sample is obtained and the time the blood sample
is finally analyzed.
[0003] One example of a blood analysis technique that is affected by
the aforementioned sources of error is co-oximetry. Co-oximetry is a
spectroscopic technique that can be used to measure the different
Hemoglobin (Hb) species present in a blood sample. The results of co-
oximetry can be further evaluated to provide Hb Oxygen Saturation (Hb s02)
measurements. If the blood sample is exposed to air, the Hb s02
measurements are falsely elevated, as oxygen from the air is absorbed into
the blood sample. Co-oximetry also typically requires hemolyzing of the red
blood cells (hemolysis), using a sound generator, to make the blood sample
suitable for spectroscopic measurement. Hemolysis can also be
accomplished by chemical means. Parameters that can be measured in blood
by spectroscopic techniques (or spectrometry) are limited by the amount of
electromagnetic radiation (EMR) absorbed by the parameters measured. For
CA 02523486 2005-10-07
-2-
example, without limitation, hydrogen ions (which determine pH) and
electrolytes (sodium, potassium, chloride and bicabonate) do not absorb EMR
in the approximate wavelength range of about 300nm to 2500nm. Therefore,
if this wavelength range is used to conduct spectroscopic measurements of
Hb species for example, then these important parameters, i.e., hydrogen ions
and electrolytes, must be measured by other means.
[0004] Another example of a blood analysis technique that is affected
by the aforementioned sources of error is blood gases. Traditionally, blood
gas measurement includes the partial pressure of oxygen, the partial pressure
of carbon dioxide, and pH. From these measurements, other parameters can
be calculated, for example, Hb s02. Blood gas and electrolyte measurements
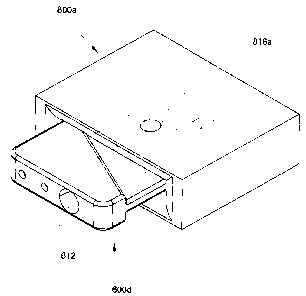
usually employ biosensors. Bench-top analyzers are available, which (1 )
measure blood gases, (2) perform co-oximetry, or (3) measure blood gases
and perform co-oximetry in combination. Some combinations of diagnostic
measurement instruments also include electrolytes, making such bench-top
analyzers even larger. Because these instruments are large and expensive,
they are usually located in central laboratories. Biosensor technology is also
limited by the blood parameters it can measure. To the inventor's knowledge,
biosensors are not currently available for measuring the Hb species measured
by co-oximeters.
[0005] Preferably, blood gases and co-oximetry are measured in
arterial blood collected in a syringe, since arterial blood provides an
indication
of how well venous blood is oxygenated in the lungs. There are many benefits
to providing these blood tests near or at the point of care of patients, but
these
are usually limited by the size and cost of the diagnostic measurement
instruments. Those skilled in the art will appreciate that, as a non-limiting
example, assessment of the acid-base status of a patient requires both the
measurement of hemoglobin (Hb) species in the blood and the blood pH.
[0006] Therefore, there is a need for small portable meters, which
combine spectroscopic technology with biosensor technology.
CA 02523486 2005-10-07
-3-
Summary Of The Invention
According to an aspect of an embodiment of the invention there is
provided a joint-diagnostic spectroscopic and biosensor meter for providing
results from analysis of a fluid sample or an extract from the fluid sample
within a disposable microfluidic cartridge. The meter comprises: (a) a
housing; (b) at least one source of electromagnetic radiation (EMR); (c) a
slot
in the housing of the meter for receiving the cartridge, the cartridge
comprising at least one optical chamber and at least one biosensor chamber,
wherein the at least biosensor chamber comprises at least one biosensor; (d)
at least one photodetector for measuring EMR transmitted through or
reflected from, the fluid sample or the extract; (e) a circuit board in
operative
association with the at least one biosensor and the at least one
photodetector;
and (f) a processor for preparing the results.
[0007] Other aspects and features of the present invention will become
apparent, to those ordinarily skilled in the art, upon review of the following
description of the specific embodiments of the invention.
Brief Description Of The Drawings
[0008] For a better understanding of the present invention, and to show
more clearly how it may be carried into effect, reference will now be made, by
way of example, to the accompanying drawings, which illustrate aspects of
embodiments of the present invention and in which:
[0009] Figures 1A is a schematic drawing showing details of a side
view of a spectroscopic and biosensor cartridge 600a that can be used with a
joint-diagnostic spectroscopic and biosensor meter according to an
embodiment of the invention;
[0010] Figure 1 B is a cross-sectional view through the spectroscopic
and biosensor cartridge 600a shown in Figure 1A along line B-B;
[0011] Figure 1 C is a perspective view of the spectroscopic and
biosensor cartridge 600a.
CA 02523486 2005-10-07
-4-
[0012] Figures 2A is a schematic drawing showing details of a top view
of a spectroscopic and biosensor whole blood and plasma cartridge 600b that
can be used with a joint-diagnostic spectroscopic and biosensor meter
according to an embodiment of the invention;
[0013] Figure 2B is a cross-sectional view through the spectroscopic
and biosensor whole blood and plasma cartridge 600b shown in Figure 2A
along line B-B;
[0014] Figure 2C is a cross-sectional view through the spectroscopic
and biosensor whole blood and plasma cartridge 600b shown in Figure 2A
along line C-C;
[0015] Figure 2D is a rear view of the spectroscopic and biosensor
whole blood and plasma cartridge 600b showning the electrical output
contacts from the biosensors;
[0016] Figure 2E is a cross-sectional view through the spectroscopic
and biosensor whole blood and plasma cartridge 600b shown in Figure 2D
along line E-E;
[0017] Figures 3A is schematic drawing showing details of the hollow
fiber bundle 660 (cartridge 600b shown collectively in Figures 2A-2E) shown
collectively in Figures 2A-2E;
[0018] Figure 3B is the left side-view of the hollow fiber bundle 660
shown in Figure 3A;
[0019] Figure 3C is the right side-view of the hollow fiber bundle 660
shown in Figure 3A;
[0020] Figure 3D is a cross-sectional view through the hollow fiber
bundle 660 shown in Figure 3A along line D-D;
[0021] Figure 3E is a perspective view of the hollow fiber bundle 660;
[0022] Figure 3F is a detailed view of the detail F shown in Figure 3D;
[0023] Figure 3G is an alternative perspective view of the hollow fiber
bundle 660;
CA 02523486 2005-10-07
-5-
[0024] Figure 4A is a schematic drawing showing details of a side-view
of an integrated needle and cartridge 600c that can be used with a joint-
diagnostic spectroscopic and biosensor meter according to an embodiment of
the invention (the needle and cartridge are shown collectively in Figures 5A-
5F and Figures 2A-2E respectively),
[0025] Figure 4B is a cross-sectional view through the integrated
needle and cartridge 600c shown in Figure 4A along line B-B;
[0026] Figure 4C is a perspective view of the integrated needle and
cartridge 600c;
[0027] Figure 5A is a schematic drawing showing details of a top view
of a needle 100 that can be used with the cartridge 600b shown collectively in
Figures 2A-2E, and the cartridge 600d shown collectively in Figures 9A-9C,
that can be used with a joint-diagnostic spectroscopic and biosensor meter
according to an embodiment of the invention;
[0028] Figure 5B is a left side-view of the needle 100 shown in Figure
5A;
[0029] Figure 5C is a right side-view of the needle 100 shown in Figure
5A;
[0030] Figure 5D is a cross-sectional view through the needle 100
shown in Figure 5A along line D-D;
[0031] Figure 5E is a perspective view of the needle 100;
[0032] Figure 5F is an alternative perspective view of the needle 100;
[0033] Figure 6A is a schematic drawing showing details of a top view
of a barrel 200 for a needle shown collectively in Figures 5A-5F;
[0034] Figure 6B is a left side-view of the barrel 200 shown in Figure
6A;
[0035] Figure 6C is a cross-sectional view through the barrel 200
shown in Figure 6A along line C-C;
CA 02523486 2005-10-07
-6-
[0036] Figure 6D is a right side-view of the barrel 200 shown in Figure
6A;
[0037] Figure 6E is an alternative cross-sectional view through the
barrel 200 shown in Figure 6A along line E-E;
[0038] Figure 6F is a perspective view of the barrel 200;
[0039] Figure 7A is a schematic drawing showing details of a top view
of an assembly 300 of the needle (shown collectively in Figures 5A-5F) and
the barrel (shown collectively in Figures 6A-6F), with the needle retracted
into
the barrel before and after use;
[0040] Figure 7B is a left side-view of the assembly 300 shown in
Figure 7A;
(0041] Figure 7C is a right side-view of the assembly 300 shown in
Figure 7A;
[0042] Figure 7D is a cross-sectional view through the assembly 300
shown in Figure 7A along line D-D;
[0043] Figure 7E is a perspective view of the assembly 300;
[0044] Figure 7F is an alternative perspective view of the assembly
300;
[0045] Figure 8A is a schematic drawing showing details of a front view
of a cartridge slot 800a, from a joint-diagnostic spectroscopic and biosensor
meter according to a first embodiment of the invention;
[0046] Figure 8B is a cross-sectional view through the cartridge slot
800a shown in Figure 8A along line B-B;
[0047] Figure 8C is a perspective view of the cartridge slot 800a;
[0048] Figure 9A is a schematic drawing showing details of a front view
of a cartridge 600d fully inserted in the cartridge slot 800a (shown
collectively
in Figures 8A-8C), from a joint-diagnostic spectroscopic and biosensor meter
according to the first embodiment of the invention;
CA 02523486 2005-10-07
_7_
[0049] Figure 9B is a cross-sectional view through the cartridge 600d
and the cartridge slot 800a shown in Figure 9A along line B-B;
[0050] Figure 9C is a perspective view of the cartridge 600d fully
inserted in the cartridge slot 800a;
[0051] Figure 10A is a schematic drawing showing details of a front
view of a cartridge slot 800b, from a joint-diagnostic spectroscopic and
biosensor meter according to a second embodiment of the invention;
[0052] Figure 10B is a cross-sectional view through the cartridge slot
800b shown in Figure 10A along line B-B;
[0053] Figure 10C is a perspective view of the cartridge slot 800b;
[0054] Figure 11A is a schematic drawing showing details of a front
view of a cartridge slot 800c, from a joint-diagnostic spectroscopic and
biosensor meter 900 (shown collectively in Figures 12A-12D) according to a
third embodiment of the invention;
[0055] Figure 11 B is a cross-sectional view through the cartridge slot
800c shown in Figure 11A along line B-B;
[0056] Figure 11 C is a perspective view of the cartridge slot 800c;
(0057] Figure 12A is a schematic drawing showing details of a front
view of a joint-diagnostic spectroscopic and biosensor meter 900 according to
the third embodiment of the invention;
[0058] Figure 12B is a cross-sectional view through the joint-diagnostic
spectroscopic and biosensor meter 900 shown in Figure 12A along line B-B;
[0059] Figure 12C is an alternative cross-sectional view through the
joint-diagnostic spectroscopic and biosensor meter 900 shown in Figure 12A
along line C-C; and
[0060] Figure 12D is a perspective view of the joint-diagnostic
spectroscopic and biosensor meter 900.
CA 02523486 2005-10-07
_$_
Detailed Description Of Preferred Aspects Of The Invention
[0061] Some embodiments of the invention provide a meter
(sometimes referred to as a reader) for a disposable microfluidic cartridge,
which is suitable for joint-diagnostic spectroscopic and biosensor
measurement of a fluid sample or an extract from the fluid sample. As an
example without limitation, whole blood is used as the sample, and plasma is
used as an example of an extract, wherein the plasma is extracted from the
blood sample within a particular embodiment of a disposable microfluidic
cartridge. Those skilled in the art will appreciate that although blood is
used
as an example of a fluid analyzed, measured or tested using the meter, other
fluids, for example without limitation, serum, plasma, cerebrospinal fluid,
synovial fluid, lymphatic fluid, calibration fluid, and urine, could also be
used
with the disposable microfluidic cartridges and the meter. Moreover, when a
fluid sample is mentioned, it should be understood that the sample could also
be an extract from the fluid.
[0062] Once the blood is transferred to the cartridge, the cartridge is
inserted into a cartridge slot (sometimes referred to as a slot) in a
diagnostic
measurement instrument, i.e., the meter or the reader, for rapid blood
analysis. Because the meter could be made as a small instrument, and no
pretreatment of the blood is necessary, the meter could be in the form of an
inexpensive hand-held instrument, for testing near the patients or at the
point
of patient care, commonly referred to as "point-of-care testing" or "near
patient testing".
[0063] In the specific embodiments of the invention, all the sample flow
paths are restricted within the microfluidic cartridges and therefore, there
is no
pump or fluid lines connecting the microfluidic cartridges to the meter, which
are usually seen in CO-oximeters and blood-gas instruments. Moreover,
there is no permanent installation of a cartridge in the meter, as is the case
with some CO-oximeters and blood-gas instruments.
[0064] When the cartridge is properly inserted in the slot of the meter,
the cartridge makes electrical connection with the electrical circuitry within
the
CA 02523486 2005-10-07
_g_
meter, and also the optical chamber of the cartridge becomes positioned in a
light path that is generated by a source of electromagnetic radiation (EMR)
within the meter. The EMR transmitted through the fluid sample in the
cartridge, or reflected from the fluid sample, impinges upon a photodetector
within the meter. Calibration algorithms for spectroscopic measurements and
biosensor measurements are installed within the processor of the meter, for
transforming the spectroscopic signals and the biosensor signals into analyte
measurements. The measurements are usually in concentration units, but
those skilled in the art will appreciate that other parameters can be
measured,
for example without limitations, the ratio of the concentrations of two
different
analytes.
[0065] Those skilled in the art will appreciate the various ways a
spectroscopic measurement instrument can be constructed, and various
elements that make up such instruments. Accordingly, for the sake of brevity,
description of basic spectroscopy and a list and function of the elements that
make up a spectroscopic apparatus will not be discussed here. However, it
should be noted that a joint-diagnostic spectroscopic and biosensor meter
according to the invention, requires at least one source of EMR, and the
preferred source of EMR is a tungsten lamp, but without limitation, the source
of EMR may be one or more than one Light Emitting Diode (LED), or one or
more than one laser. Also, with respect to the detection system, the preferred
detector is an array of photodiodes, but those skilled in the art will
appreciate
that a single photodiode or one or more than one charged coupled detector
(CCD) can be used.
[0066] With respect to spectroscopic measurements, the examples
shown describe an apparatus that operates in transmission mode. Those
skilled in the art will appreciate that the spectroscopic apparatus of a joint-
diagnostic spectroscopic and biosensor reader can also operate in reflectance
mode by placing a reflecting member in the cartridge slot, on one side of the
optical chamber 616 (Figure 1 B and Figure 9B), such that the EMR
transmitted through the sample would be reflected off the reflecting member,
CA 02523486 2005-10-07
-10-
and the reflected EMR would enter the sample for the second time.
Enamples of cartridge slots are shown schematically as 800a in Figure 8C,
800a in Figure 9C, 800b in Figure 10C and 800c in Figure 11 C and Figure
12B. In a diagnostic measurement instrument operating in the reflectance
mode, both the EMR source and the photodetector would be on the same
side of the optical chamber 616 (Figure 1 B and Figure 9B). Moreover, those
skilled in the art will also appreciate that instead of installing a
reflecting
member around the slot in the housing of the meter, one side of the wall
portions (624a or 624b, Figure 1A) of the optical chamber 616 (Figure 1 B and
Figure 9B) could be coated with a reflecting material.
[0067] In some very specific embodiments, the meter is provided with:
a housing (892);
at least one source of electromagnetic radiation (EMR);
a slot in the housing for receiving a disposable microfluidic cartridge;
at least one photodetector in the meter for measuring EMR transmitted
through or reflected from, the fluid sample or an extract from the fluid
sample
within the cartridge;
at least one electrical contact inside the slot, which mates with at least one
electrical contact in the microfluidic cartridge when the cartridge is
inserted
into the slot properly, the electrical contact in the cartridge being in
electrical
connectivity with a biosensor inside the cartridge;
a circuit board in operative association with at least one biosensor and at
least
one photodetector; and
a processor for preparing results from analysis of the fluid sample or an
extract of the fluid sample prepared within the microfluidic cartridge.
It will be appreciated by those skilled in the art that when the source of EMR
is
a single source, the single source could be split by a multi-channel optical
fiber for providing more than one light path.
CA 02523486 2005-10-07
-11-
[0068] In some embodiments, the joint-diagnostic spectroscopic and
biosensor meter further comprises a display screen for viewing the results and
aiding the operator in use of the meter, as well as buttons for manipulating
the
display function. Those skilled in the art will appreciate that the meter
could
be connected to a host computer. Therefore, some embodiments also
comprise at least one communication port for interfacing with other
instruments. Other non-limiting examples of other instruments are diagnostic
instruments like a pulse oximeter, or some other non-invasive testing
instrument. The optional communication port is also used to upgrade
information in the meter's processor, as well as to download information from
the meter's processor. Another optional port in the housing of some
embodiments of the joint-diagnostic spectroscopic and biosensor meter is
provided for charging the power supply within the meter.
[0069] Some embodiments of the joint-diagnostic spectroscopic and
biosensor meter comprise at least one photodetector (photodiode) assembled
as an array of detectors in a spectrometer, wherein the spectrometer
comprises a grating for dispersing EMR emerging from the fluid sample or the
extract, into component wavelengths. The meter optionally comprises a
focusing lens between the disposable cartridge and the spectrometer, show
as 870 in Figures 11a & 11c and also shown schematically in Figure 12A.
[0070] The disposable microfluidic cartridge comprises at least one
optical chamber and at least one biosensor chamber. Examples of cartridges
are illustrated collectively in Figures 1A-1C, 2A-2E and 9A-9C. The
disposable microfluidic cartridges comprise:
a housing (123);
an inlet within the housing for receiving the fluid sample;
at least one flow path within the housing, wherein the at least one flow path
is
fluidly connected to the inlet; and
CA 02523486 2005-10-07
-12-
at least one vent for facilitating airflow out of the at least one flow path.
Those skilled in the art will appreciate that a microfluidic cartridge can
comprise an optical chamber and a biosensor chamber in series along a
single flow path, with the biosensor chamber positioned either before or after
the optical chamber.
[0071] In some embodiments, the interior walls of the cartridges are
treated with a hydrophillic coating to promote even spreading of the blood
within the optical chamber, and to promote movement of blood along the flow
path.
[0072] The optical chamber is located along a flow path, and the optical
chamber has at least one optical window for spectroscopic analysis of the
fluid sample or the extract of the fluid sample, for example which should not
be considered limiting in any way, whole blood and plasma extracted from the
whole blood by a filtration system within a specific embodiment the disposable
microfluidic cartridge, illustrated collectively in Figures 2A-2E and Figures
3A-
3G. In this case, the fluid sample is whole blood and the extract from the
fluid
sample is plasma. Optionally, the disposable microfluidic cartridges contain
more than one flow path, and more than one optical chamber in one or more
than one flow path. The flow paths may also contain one or more reagents,
for example without limitation, an anticoagulant, or a reagent that reacts
with
an analyte to enhance the absorbance of EMR. The optical chamber is
specifically designed to reduce the average attenuation of EMR due to
scattering of EMR by the red blood cells in a blood sample, without having to
hemolyze the red blood cells using sound waves or hemolyzing chemicals.
Preferably the depth of the optical chamber, i.e., the internal distance
between
the optical windows, is about 0.1 mm, but those skilled in the art will
appreciate
that the depth of the optical chamber is preferably larger for plasma. An
average depth of an optical chamber is in an approximate range of about 0.02
mm to about 5 mm.
[0073] The biosensor chamber is located along a flow path, and the
biosensor chamber may have one or more than one biosensor for analyzing
CA 02523486 2005-10-07
-13-
the fluid or the extract. Optionally, the disposable cartridge contains more
than one biosensor chamber as illustrated collectively in Figures 2A-2E. A
flow path that includes a biosensor chamber is specifically designed with at
least one active surface of the biosensor exposed to the fluid sample. Those
skilled in the art will appreciate that biosensors may include various
transducer arrangements that convert at least one property of the fluid sample
into an electrical signal, wherein the transducer comprises at least one
active
surface for contacting the fluid sample. The at least one active surface is
one
of a chemical sensitive surface, or an ionic sensitive surface, and wherein
the
at least one biosensor comprises at least one of a transistor, an ion-
selective
membrane, a membrane-bound enzyme, a membrane-bound antigen, a
membrane-bound antibody, or a membrane-bound strand of nucleic acid.
The disposable cartridge also comprises at least one electrical output
contact,
and the cartridge slot of the meter also comprises at least one electrical
input
contact, wherein the electrical output contact mates with the electrical input
contact after the disposable cartridge is properly inserted into the slot, as
illustrated collectively in Figures 9A-9C. Although the example illustrated
collectively in Figures 9A-9C shows the cartridge electrical output contact in
a
male configuration, and also shows the slot electrical input contact in a
female
configuration, those skilled in the art will appreciate that the electrical
output
contacts can mate with the electrical input contacts in other ways.
[0074] In a specific embodiment of a disposable cartridge illustrated
collectively in Figures 2A-2E, the cartridge contains a filtration chamber,
which
comprises a hollow fiber bundle 660. More details on plasma extraction are
disclosed in Canadian Patent Application No. 2,507,323 (Samsoondar, the
entire contents of which are incorporated herein by reference). Details of the
hollow fiber bundle 660 are illustrated collectively in Figures 3A-3G.
Preferably the hollow fibers in the bundle 660 run approximately perpendicular
to the whole blood flow path.
[0075] The inlet 670 of the disposable cartridge, illustrated collectively
in Figures 2A-2E and Figures 9A-9C is dimensioned to encompass a male
CA 02523486 2005-10-07
-14-
end of a traditional syringe. The inlet of the cartridge is also dimensioned
to
resemble the end of a capillary tubing, illustrated collectively in Figures 1A-
1C
as 672, to receive the fluid sample from a pin prick drop of blood. As an
alternative, the inlet of the disposable cartridge is the sharp end 147 of a
needle, as illustrated collectively in Figures 4A-4C. The needle is allowed to
enter the lumen of a blood vessel for receiving the blood directly into the
disposable cartridge, eliminating the need of a syringe. The sharp end 147 of
the needle 100 is preferably encased in a moveable barrel 200, illustrated
collectively in Figures 6A-6F, for sheathing and unsheathing the sharp end, to
protect the user from accidental injury. An example of a needle, barrel, and
the assembly of the two, which should not be considered limiting in any way,
are illustrated collectively in Figures 5A-5F, Figures 6A-6F and Figures 7A-7F
respectively. Other embodiments of similar needles are disclosed in
Canadian Patent Application entitled, "Hollow Needle Assembly," filed August
26, 2005 (Samsoondar, the entire contents of which are incorporated herein
by reference). The outlet 171 of the needle assembly 300, illustrated
collectively in Figures 7A-7F, mates with the inlet 670 of the cartridges
illustrated collectively in Figures 2A-2E and Figures 9A-9C, eliminating the
need of a syringe. The cartridge could be inserted into the meter slot, with
the
needle still attached. More details on various other embodiments of
microfluidic cartridges are disclosed in US Patent Application Nos. 11/103,619
and 11/108,912 (Samsoondar, the entire contents of which are incorporated
herein by reference).
[0076] When an arterial blood sample is tested, the arterial blood is
usually collected in a traditional syringe. After the blood is collected, the
syringe needle must be removed and the end of the syringe must be capped
immediately to avoid atmospheric contamination. The arterial blood in the
syringe is usually transferred in ice to a central laboratory for testing.
Prior to
testing, the capped syringe must be uncapped before the sample is injected
or aspirated into the measurement instrument. The delays, potential for air
bubbles becoming trapped in the blood, the exposure of the healthcare
provider to blood, and the risk of infection through accidental needle stick,
are
CA 02523486 2005-10-07
-15-
all disadvantages in the current system. A further disadvantage is that when
the plunger of the syringe is pulled too forcefully, turbulence in the blood
flow
can cause air bubbles to develop in the blood. Moreover, the smaller the bore
or lumen of the needle, the greater the potential for hemolysis to occur. This
limits the minimum thickness of the needle; the smallest (thinnest) needle is
preferred to minimize pain experienced by the patient during an arterial
puncture. Also, because of the dead space inside a traditional syringe, a
minimum of about half to one milliliter of blood must be drawn, even though
the volume of blood required by the cartridge could be much less. The
aforementioned disadvantages are minimized with the use of the needle like
for example, the one collectively illustrated in Figures 7A-7F.
[0077] In some embodiment of a microfluidic cartridge, illustrated
collectively in Figures 4A-4C, there is provided an integrated cartridge and
needle 600c. The barrel 200 illustrated collectively in Figures 6A-6F is not
shown. The integrated cartridge and needle 600c is a safer and more
convenient alternative, to assembling a cartridge (shown collectively as
Figures 2A-2E) and a needle (shown collectively as Figures 7A-7F) before
use.
[0078] Some embodiment of a joint-diagnostic spectroscopic and
biosensor meter optionally comprises a barcode reader for reading the
barcode on the disposable cartridge (not shown), the barcode containing at
least information regarding calibration of a biosensor. The barcode also
optionally contains information about the joint-diagnostic spectroscopic and
biosensor meter. Alternatively, the disposable cartridge further comprises a
calibration pouch (not shown), containing a calibration fluid, that is
arranged in
fluid connection with the at least one biosensor chamber. More details on the
calibration pouch are disclosed in US Patent Application Nos. 11/103,619 and
11/108,912. For cartridges with calibration pouches, the joint-diagnostic
spectroscopic and biosensor meter further comprises a means for breaking
the calibration pouches, for example, which should not be considered limiting
in any way, a rotating cam, or a reciprocating plunger.
CA 02523486 2005-10-07
-16-
[0079] As mentioned previously, some embodiment of a disposable
cartridge for use with a joint-diagnostic spectroscopic and biosensor meter
comprises a flow-through filtration chamber for extracting plasma from whole
blood, and further comprises a first optical chamber along a whole blood flow
path, and a second optical chamber along the plasma flow path. In a similar
embodiment (not shown), the distance from the first optical chamber to its
adjacent edge of the disposable cartridge, is approximately equal to the
distance from the second optical chamber to its adjacent edge of the
disposable cartridge. An embodiment of a meter that operates with such a
cartridge is provided with at least one source of EMR and at least one light
path. For the embodiment of a meter that provides a single light path, the
single light path travels through the first optical chamber when the
disposable
cartridge is inserted properly in a first orientation. When the disposable
cartridge is inserted properly in a second orientation, the second orientation
being 180 degrees to the first orientation, the single light path is compelled
to
travel through the second optical chamber. Therefore, the plasma and the
whole blood are measured sequentially using the same light path. Because of
the absorbance signals for whole blood and plasma are so different, the
software in the meter is able to discriminate whole blood from plasma. Those
skilled in the art will appreciate that there are other methods of analyzing
the
plasma and whole blood using a single light path, for example, a prompt in the
display screen could provide appropriate instructions for cartridge insertion.
[0080] In embodiments of the cartridges shown as examples and in the
relevant incorporated references, the optical chamber is designed to spread
blood into a thin film, thereby reducing the incidences of trapped air bubbles
in
the blood sample in the optical chamber. Instead, air bubbles are pushed
through the optical chamber and guided out of the apparatus through a vent.
In the same embodiments, the second flow path includes at least one
biosensor. The optical chamber provides spectroscopic blood measurements
for determination of, for example without limitation, Hb species, and the
biosensor provides blood measurements for determination of, for example
without limitation, blood pH. The apparatus is particularly useful for, for
CA 02523486 2005-10-07
-17-
example without limitation, a combination of blood gas measurement and co-
oximetry.
[0081] Moreover, in these embodiments blood within the optical
chamber is further isolated from contamination by room air by providing an
inlet transition cavity and an overflow chamber at a respective entrance and
exit of the optical chamber. In use, blood in the inlet transition cavity and
the
overflow chamber serve as barriers between blood in the optical chamber and
room air, thereby isolating the blood in the optical chamber from oxygen
contamination. In the rare incident of a trapped air bubble, those skilled in
the
art will appreciate that various calibration algorithms for many specific
analytes measured in the blood sample can be developed that could
compensate for measurement inaccuracies caused by trapped air bubbles,
except for those analytes such as the partial pressure of oxygen and oxy-
hemoglobin, which become falsely elevated as a result of oxygen introduced
into the blood sample from the air bubble. Similarly in the same
embodiments, the biosensor chamber is also isolated from contamination by
room air by providing an inlet transition chamber and an overflow chamber at
a respective entrance and exit of the biosensor chamber. Those skilled in the
art will appreciate that a microfluidic cartridge could comprise an optical
chamber and a biosensor in series along a single flow path, with the
biosensor positioned either before or after the optical chamber. For a
cartridge with a single flow path, the overflow chamber of the biosensor
chamber could serve as the inlet transition chamber of the optical chamber,
and the overflow chamber of the optical chamber could serve as the inlet
transition chamber of the biosensor chamber.
[0082] Optionally the microfluidic cartridges also include at least one
visible fill line or indicator serving as a marker providing a user with a
visual
Boolean indicator relating to the sufficiency of the blood sample in the
optical
chamber and biosensor chamber. Briefly, in some embodiments, the visible fill
line is located in a position between the overflow chamber (618 Figure 1 B,
for
example) and the capillary break (622a Figure 1 B, for example), and is
CA 02523486 2005-10-07
-18-
indicative of whether or not a volume of blood drawn into the cartridge is
present in sufficient amount to: i) ensure that the blood in the optical
chamber
and biosensor chamber is substantially free from contaminants that may have
been introduced during the filling of the apparatus with blood; and/or, ii)
ensure that there is an effective amount of blood surrounding the optical
chamber and biosensor chamber to isolate the blood in the optical chamber
and biosensor chamber from room air.
[0083] Referring collectively to Figures 1A-1C, shown are schematic
drawings of a cartridge 600a suitable for attachment to a needle via the
internal threads in a female receptor of a needle (not shown), and the
matching threads in the inlet tubing 672 (Figure 1A and Figure 1 B). The
needle is similar to the needle assembly shown collectively in Figures 7A-7F
except that the needle outlet 137 contains internal threads that are
complimentary to the threads on the inlet tubing 672. Optionally, blood from a
pin prick can be drawn directly into the cartridge by inserting the opening
612
into the drop of blood. Referring to Figure 1A, shown is a side-view of the
cartridge 600a, with inlet 612, and an electrical output 654a from biosensor
652a (shown in Figure 1 B), and with optical wall portions 624a and 624b.
Referring to Figure 1C, shown is a perspective view of the cartridge 600a,
with the inlet 612 and the optical wall portion 624b.
[0084] Referring to Figure 1 B, shown is a cross-sectional view through
the cartridge 600a shown in Figure 1A along line B-B showing the sample
inlet 612 and the threaded inlet tubing 672. As already mentioned, capillary
blood obtained from a pinprick is allowed to flow into the cartridge 600a
through the inlet 612, arriving at first at the manifold 640; from the
manifold
640, the blood is distributed into the two main flow paths: the first flow
path
includes in series, the biosensor inlet transition chamber 642, the biosensor
chamber 674, the biosensor outflow chamber 620b, the biosensor capillary
break 622b, and terminating at the biosensor vent 137b; the second flow path
includes in series, the spectroscopic inlet transition chamber 614, the
optical
chamber 616, the spectroscopic overflow chamber 618, the spectroscopic
CA 02523486 2005-10-07
-19-
outflow chamber 620a, the spectroscopic capillary break 622a, and
terminating at the spectroscopic vent 137a. Two biosensors are shown as
652a and 652b, which are connected to their respective electrical output
contacts 654a and 654b, through respective electrical conductors 676a and
676b.
[0085] Referring collectively to Figures 2A-2E, shown are schematic
drawings illustrating details of the measurement cartridge 600b. The cartridge
600b is capable of extracting plasma from whole blood, and the measurement
technology includes spectroscopy with the optional use of one or more than
one reagent, and biosensor technology. Referring to Figure 2A is a top view
of the microfluidic cartridge 600b showing the sample inlet 612, the inlet
chamber 670, a whole blood optical chamber wall-portion 624a, a plasma
optical chamber wall-portion 626a, and three vents 137a, 137b, and 137c.
The cartridge 600b contain three flow paths illustrated in Figure 2E.
[0086] Referring to Figure 2E, shown is the sample inlet 612, the inlet
chamber 670. The cartridge can be filled with blood from a traditional
syringe,
after the male end of the syringe is inserted into the inlet chamber 670.
Alternatively, the male end 171 of the needle illustrated collectively in
Figures
5A-5F and Figures 7A-7F is first fitted into the cartridge inlet opening 670.
Then the sharp open end 147 of the needle is inserted into a blood vessel,
allowing the blood to flow into the cartridge 600b. Whether a traditional
syringe or the needle illustrated collectively in Figures 5A-5F and Figures 7A-
7F is used, the blood arrives at first at the manifold 640; from the manifold
640, the blood is distributed into the two main flow paths: the first flow
path
includes in series, the whole blood biosensor inlet transition chamber 642,
the
whole blood biosensor chamber 674, the whole blood biosensor outflow
chamber 620b, the whole blood biosensor capillary break 622b, and
terminating at the whole blood biosensor vent 137b; the second flow path
includes in series, the whole blood spectroscopic inlet transition chamber
614a, the whole blood optical chamber 616a, the filtration chamber 634 (for
extracting plasma from the whole blood using the hollow fiber bundle 660 with
CA 02523486 2005-10-07
-20-
closed flange 682 shown; details are shown collectively in Figures 3A-3G), the
filtration chamber outflow 620a, the filtration chamber capillary break 622a,
and terminating at the filtration chamber vent 137a. A third flow path is
defined as a plasma flow path, but is still in fluid connection with the
sample
inlet 612. The third flow path continues from the filtration chamber 634 at
the
plasma collection chamber 636, and includes in series the plasma biosensor
chamber 672, the plasma spectroscopic inlet transition chamber 614b, the
plasma optical chamber 616b, the plasma capillary break 622c, and
terminating at the plasma vent 137c. One plasma biosensor is shown as
652c, which is electrically connected through a medium 676c to the electrical
output contact 654c. Two whole blood biosensors are shown as 652a and
652b, which are connected to their respective electrical output contacts 654a
and 654b, through respective electrical conductors 676a and 676b.
[0087] Referring to Figure 2B, shown is a cross-sectional view through
apparatus 600b illustrated in Figure 2A along line B-B, showing parts already
identified for Figure 2E.
[0088] Referring to Figure 2C, shown is a cross-sectional view through
apparatus 600b illustrated in Figure 2A along line C-C, showing parts already
identified for Figure 2E.
[0089] Referring to Figure 2D, shown is a rear view of apparatus 600b
illustrated in Figure 2A, showing the three electrical contacts 654a, 654b,
and
654c for the three respective biosensors 652a, 652b, and 652c. For
convenience and as deemed appropriate, same reference numerals are used
as those used for the microfluidic cartridge illustrated collectively in
Figures
1A-1C
[0090] Referring collectively to Figures 3A-3G, shown are schematic
drawings illustrating details of the hollow fiber bundle 660 shown inside the
plasma extraction chamber 634 illustrated collectively in Figures 2A-2E. The
hollow fiber bundle 660 comprises several hollow fibers, held together by two
flanges 682 and 684. Referring to Figure 3A, shown is a top view of the
hollow fiber bundle 660, illustrating the closed flange 682, and the
perforated
CA 02523486 2005-10-07
-21 -
flanged 684, and identifying a single hollow fiber 696. Referring to Figure
3B,
shown is a left side-view of the hollow fiber bundle 660, illustrating the
closed
flange 682. Referring to Figure 3C, shown is a right side-view of the hollow
fiber bundle 660, illustrating the perforated flange 684, and identifying the
open end 690 of a single hollow fiber. Referring to Figure 3D, shown is a
cross-sectional view through the bundle 660 shown in Figure 3A along line D-
D. Referring to Figure 3F, shown is a detailed view of the cross-section of a
single hollow fiber, according to detail F identified in Figure 3D, showing
the
lumen 692 of the fiber, and the wall of the fiber 694. Referring to Figure 3E,
shown is a perspective view of the hollow fiber bundle 660, showing the
closed flange 682. Referring to Figure 3G, shown is an alternative
perspective view of the hollow fiber bundle 660, showing the perforated flange
684, and the open end 690 of a single hollow fiber. The hollow fibers are
inserted inside perforations in the flange 684 and sealed at the juncture of
the
hollow fiber and the flange. In some embodiments, the walls of the fiber
contain pores with an approximate distribution of diameters ranging from
about 0.1 micrometer to about 10 micrometers. In some embodiments, the
internal diameter of the hollow fibers (also referred to as hollow fiber
filter or
hollow fiber membrane) ranges approximately from about 0.1 mm to about 1
mm. Those skilled in the art will appreciate that blood flow decreases the
viscosity of the blood and therefore enhances separation (or filtration, or
extraction) of plasma from blood; extraction of plasma from blood also
increases by increasing the pore sizes of the membrane 694, by decreasing
thickness of the membrane 694, and increasing membrane surface area. The
surface area increases in proportion to the number of hollow fibers used.
[0091] Referring to Figure 4A, shown is a schematic drawing illustrating
a top view of an integrated needle and cartridge 600c, the hub of the needle
100 also comprising a cartridge 600b. Figure 4B illustrates a cross-sectional
view through the cartridge shown in Figure 4A along line B-B. Figure 4C is a
perspective view of the integrated needle and cartridge 600c shown in Figure
4A. Details of the cartridge 600b are already provided collectively with
reference to Figures 2A-E, and further details of the needle 100, showing the
CA 02523486 2005-10-07
-22-
sharp open end 147, will be provided collectively in Figures 5A-5F and
Figures 7A-7F. As already mentioned, the integrated needle and cartridge
eliminates the need of a traditional syringe.
[0092] Referring to Figure 5A, shown is a schematic drawing illustrating
a top view of a needle that can be used with the cartridge 600b illustrated
collectively in Figures 2A-2E and the cartridge 600d illustrated collectively
in
Figures 9A-9C. Figure 5B illustrates a left side-view of the needle 100 shown
in Figure 5A. Figure 5C illustrates a right side-view of the needle shown in
Figure 5A. Figure 5D illustrates a cross-sectional view through the needle
100 shown in Figure 5A along line D-D. Figure 5E illustrates a perspective
view of the needle 100, and Figure 5F illustrates an alternative perspective
view of the needle 100. Those skilled in the art will appreciate that other
suitable mating ends between needle and cartridge can be used, for example
without limitations, threads as illustrated collectively in Figures 1A-1C, and
Leuer lock mechanisms.
[0093] Still referring to Figure 5, the needle 100 comprises a shaft 143
and a hub with a front end 139 and a back end 123. It should be understood
that the front end refers to a general area of the hub, and does not
specifically
identify any point or local area. Similarly, it should be understood that the
back end refers to a general area of the hub, and does not specifically
identify
any point or local area. The shaft 143 has a sharp open end 147 and a
second end, which is mounted in the passage 145 of the hub. The sharp
open end 147 is usually the beveled end of the shaft, which is usually a
hollow
metal tube. The hollow portion is also referred to as the lumen (not shown).
The bevel provides a point for piercing the blood vessel. Also shown
collectively in Figure 5A and Figure 5F is the central axis 133a, which runs
through the center of the shaft 143, along its length. The section of the
shaft
143 mounted inside the hub is not shown. The passage 145 of the hub is
fluidly connected to the lumen of the shaft, and a flow path is defined by the
sharp open end 147, which leads into the lumen, which leads into the
passage 145 of the hub, and terminates at a blunt open end 137. The blunt
CA 02523486 2005-10-07
-23-
open end 137 is located at the back end 123 of the hub. The front end of the
hub 139 contains external threads 173 for mating with internal threads 175 in
a complementary barrel 200 illustrated collectively in Figures 6A-6F, and the
blunt open end 137 is housed in a tapered projection 171, wherein the
tapered projection resembles the male end of a syringe. In other
embodiments of a needle 100, the blunt open end 137 is threaded with
threads complementary to the threads in the inlet tubing 672 of a cartridge
collectively illustrated in Figures 1A-1C.
[0094] Referring to Figure 6A, shown is a schematic drawing illustrating
a top view of a barrel 200 for the needle illustrated collectively in Figures
5A-
F. Figure 6B illustrates a left side-view of the barrel 200 shown in Figure
6A.
Figure 6C illustrates a cross-sectional view through the barrel 200 shown in
Figure 6A along line C-C. Figure 6D illustrates a right side-view of the
barrel
200 shown in Figure 6A. Figure 6E illustrates an alternative cross-sectional
view through the barrel 200 shown in Figure 6A along line E-E. Figure 6F
illustrates a perspective view of the barrel 200. Also illustrated
collectively in
Figures 6A-6F is an opening 167 for the needle shaft 143 in the open anterior
end 159, an opening 165 (for the back end 123 of the hub) in the open
posterior end 161, and an axis 133b which runs through the center of the
barrel, along the length of the barrel. The barrel 200 comprises an internal
chamber 153 for housing the front end 139 of the hub. The central axis 133a
of the needle and axis 133b of the barrel are shown to be coaxial (illustrated
collectively in Figures 7A-7F), but the axes could also be parallel without
being coaxial for example, if the outer design of the barrel is not
cylindrical.
Also shown collectively in Figures 6A-6F are internal threads 175. In this
particular embodiment of the barrel 200, the threads 175 do not run
continuously throughout the length of the barrel, and prevents the front end
139 of the hub from moving beyond the threaded area in the barrel 200.
[0095] Referring to Figure 7A, shown is a schematic drawing illustrating
a top view of a needle and barrel assembly 300 with the needle shaft 143
retracted into the barrel 200. Figure 7B illustrates a left side-view of the
CA 02523486 2005-10-07
-24-
assembly 300 shown in Figure 7A. Figure 7C illustrates a right side-view of
the assembly 300 shown in Figure 7A. Figure 7D illustrates a cross-sectional
view through the assembly 300 shown in Figure 7A along line D-D. Figure7E
illustrates a perspective view of the assembly 300, and Figure 7F illustrates
an alternative perspective view of the assembly 300. The assembly 300
illustrated collectively in Figures 7A-7F is an assembly of the needle 100
illustrated collectively in Figures 5A-5F, and the barrel 200 illustrated
collectively in Figures 6A-6F, and accordingly, elements common to these
share common reference numerals.
[0096) Referring to Figure 8A, shown is a schematic drawing of a front
view of a cartridge slot 800a, for a joint-diagnostic spectroscopic and
biosensor meter according to a first embodiment of the invention. The
cartridge slot is shown schematically as a removable part of the joint-
diagnostic spectroscopic and biosensor meter, but those skilled in the art
will
appreciate that the slot could be an integral part of the meter, for example
as
illustrated collectively in Figures 12A-12D. Figure 8B is a cross-sectional
view
through the cartridge slot 800a shown in Figure 8A along line B-B, and Figure
8C is a perspective view of the cartridge slot 800a. Also shown is a top
aperture 816a and a bottom aperture 816b, wherein both apertures are
aligned with the optical chamber 616 as illustrated in Figure 9C, and wherein
EMR can either enter the sample through aperture 816a and exit through
aperture 816b, or enter the sample through aperture 816b and exit through
aperture 816a, depending on the location of the EMR source and the
photodetector. Those skilled in the art will appreciate that the apertures
816a
and 816b are not essential, as illustrated in the second and third
embodiments of the invention, shown collectively in Figures 10A-10C and
Figures 11A-11C respectively.
[0097) Referring to Figure 8A and Figure 8B, shown are points of
electrical contact 854a and 854b, between the respective biosensor electrical
output contacts 654a and 654b illustrated in Figure 9B, and the circuitboard.
Passages 876a and 876b are used to facilitate electrical connectivity between
CA 02523486 2005-10-07
-25-
respective points of electrical contact 854a and 854b and the circuitboard, by
means of an electrical conductor, which is well known to those skilled in the
art. Also shown are notches 812a and 812b, which are not essential, but can
ensure that cartridge 600d, illustrated in Figure 9C, is inserted in the
correct
orientation. Those skilled in the art will appreciate that there are other
means
for ensuring correct insertion of the cartridge 600d, and also that the
cartridge
slots must be designed to fit the various embodiments of microfluidic
cartridges. It should be noted that, as an example, the slot 800a was not
designed to accept the cartridge collectively illustrated in Figures 2A-2E.
[0098] Referring to Figure 8B and Figure 8C, the aperture 816a is
located in the wall 850a of the cartridge slot 800a, and aperture 816b is
located in the wall 850b of the cartridge slot 800a. Parts of the walls 850a
and 850b serve to hold the slot 800a together, and parts serve as sliding
tracks for insertion of the cartridge 600d shown in Figure 9C. Those skilled
in
the art will appreciate that the walls 850a and 850b are not essential, since
the slot 800a is a schematic representation of the slot, and in some
embodiments of the meter, the slot is an integral part of the housing 892 of
the meter 900, shown collectively in Figures 12A-12D.
[0099] Referring to Figure 9A, shown is a schematic drawing of a front
view of a cartridge 600d fully inserted inside the cartridge slot 800a shown
collectively in Figures 8A-8C, for a joint-diagnostic spectroscopic and
biosensor meter according to the first embodiment of the invention. The
cartridge 600d illustrated collectively in Figures 9A-9C is similar to the
cartridge 600a illustrated collectively in Figures 1A-1C, and accordingly,
elements common to them share common reference numerals. The primary
difference is that cartridge 600d does not have the inlet 612 in a piece
threaded capillary tubing 672. Instead, the inlet 612 shown collectively in
Figure 9B-9C is in an opening 670 that can accommodate the male end of a
traditional syringe, or the end 171 of the needle assembly 300 shown
collectively in Figures 7A-7F. Figure 9B is a cross-sectional view through the
cartridge 600d and the cartridge slot 800a shown in Figure 9A along line B-B,
CA 02523486 2005-10-07
-26-
and Figure 9C is a perspective view of the cartridge 600d fully inserted in
the
cartridge slot 800a.
[00100] Referring to Figure 10A, shown is a schematic drawing of a front
view of a cartridge slot 800b, for a joint-diagnostic spectroscopic and
biosensor meter according to a second embodiment of the invention. The
cartridge slot 800b illustrated collectively in Figures 10A-10C is similar to
the
cartridge slot 800a illustrated collectively in Figures 8A-8C, and
accordingly,
elements common to them share common reference numerals. The primary
difference is that aperture 816b in the wall 850b is replaced with a large
cutout
section shown as 860b in Figure 10B, and the surrounding section, identified
as 850c, still functions as an aid for insertion of the cartridge. Similarly,
the
aperture 816a in the wall 850a is replaced with a large cutout section shown
as 860a in Figure 10C. Therefore, there is no aperture to channel EMR from
the lamp (shown collectively in Figures 12A-12C as 880) to the cartridge, and
also there is no aperture to channel EMR emerging from the sample, to the
photodetector (shown in Figure 12A as 890). Figure 10B is a cross-sectional
view through the cartridge slot 800b shown in Figure 10A along line B-B, and
Figure 10C is a perspective view of the cartridge slot 800b.
[00101] Referring to Figure 11A, shown is a schematic drawing of a front
view of a cartridge slot 800c, for a joint-diagnostic spectroscopic and
biosensor meter according to a third embodiment of the invention. The
cartridge slot 800c illustrated collectively in Figures 11A-11C is similar to
the
cartridge slot 800a illustrated collectively in Figures 8A-8C, and
accordingly,
elements common to them share common reference numerals. The primary
difference is that aperture 816b in the wall 850b is replaced with a large
cutout
section shown as 860b in Figure 11 B, and the surrounding section, identified
as 850c, still functions as an aid for insertion of the cartridge. The second
difference is a focusing lens 870 located in the wall 850a of the cartridge
slot,
adjacent to the optical chamber of a properly inserted cartridge, for focusing
the EMR emerging from the sample, onto the detector 890 shown in Figure
12A and Figure 12C. Figure 11 B is a cross-sectional view through the
CA 02523486 2005-10-07
-27-
cartridge slot 800c shown in Figure 11A along line B-B, and Figure 11C is a
perspective view of the cartridge slot 800c.
[00102] Referring to Figure 12A, shown is a schematic drawing of a front
view of a joint-diagnostic spectroscopic and biosensor meter 900 according to
the third embodiment of the invention. By the third embodiment of the
invention, it is implied that there is no aperture for channeling the EMR from
the lamp 880 to the sample, and that there is a lens 870 for focusing EMR
emerging from the sample, unto the photodetector 890, as illustrated
collectively in Figures 11A-11 B. The cartridge slot 800c illustrated
collectively
in Figures 12A-12D is similar to the cartridge slot 800c illustrated
collectively
in Figures 11A-11C, and accordingly, elements common to them share
common reference numerals. Figure 12B is a cross-sectional view through
the joint-diagnostic spectroscopic and biosensor meter 900 shown in Figure
12A along line B-B, showing the slot 800c as an integral part of the body 892
of the meter 900. Figure 12C is an alternative cross-sectional view through
the joint-diagnostic spectroscopic and biosensor meter 900 shown in Figure
12A along line C-C. Figure 12D is a perspective view of the joint-diagnostic
spectroscopic and biosensor meter 900, showing the housing 892, a display
screen 892, three buttons 882a, 882b and 882c, for manipulating the display
functions.
[00103] While the above description provides example embodiments, it
will be appreciated that the present invention is susceptible to modification
and change without departing from the fair meaning and scope of the
accompanying claims. Accordingly, what has been described is merely
illustrative of the application of aspects of embodiments of the invention.
Numerous modifications and variations of the present invention are possible
in light of the above teachings. It is therefore to be understood that within
the
scope of the appended claims, the invention may be practiced otherwise than
as specifically described herein. Furthermore, the discussed combination of
features might not be absolutely necessary for the inventive solution.