Note: Descriptions are shown in the official language in which they were submitted.
CA 02525300 2005-11-09
WO 2004/104141 PCT/KR2004/001079
COMPOSITION FOR PREVENTING SCALING, EXCLUDING OF SOOT,
CLINKER AND SLUDGE, AND CONTROLLING FLAME IN COMBUSTION
APPARATUS
BACKGROUND OF THE INVENTION
(a) Field of the Invention
The present invention relates to a water-soluble fuel additive
composition comprising borax, sodium hydroxide, an amine-based stabilizer,
hydrogen peroxide, and water, which facilitates combustion, increases
thermal efficiency, reduces smoke generation, excludes soot and clinker
from a furnace, and controls flame, thereby improving the radiant heat
transfer system.
(b) Description of the Related Art
Conventionally, removal of soot and clinker in a furnace has been
performed physically, and air pollution control has been done by
post-treatment. Improvement of thermal radiation systems to enhance
thermal efficiency has focused on mechanical aspects. However, operation
status of furnaces and characteristics of fuels have caused problems.
To take a gas boiler as an example, hard solid materials such as
sludge, which are generated by whifiening and fihe like, may increase gas
consumption or cause explosions.
SUMMARY OF THE INVENTION
It is an object of the present invention to provide a fuel additive
CA 02525300 2005-11-09
WO 2004/104141 PCT/KR2004/001079
composition which is added to fuel, such as coal, oil, and gas, to facilitate
combustion, exclude impurities such as soot and clinker from a combustion
apparatus to facilitate heat transfer, and control flame size, thereby
improving the radiant heat transfer system.
BRIEF DESCRIPTION OF THE DRAWINGS
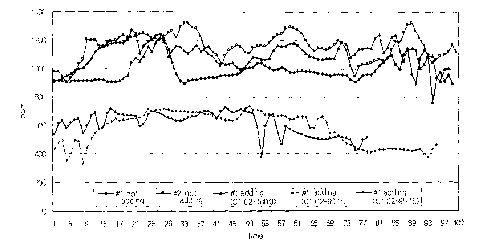
FIG. 1 is a graph showing the decrease of exhaust gas with time
when the fuel additive composition of the present invention has been added
to fuel.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The present invention provides a fuel additive composition
comprising 8 to 40 parts by weight of hydrogen peroxide, 8 to 40 parts by
weight of an amine-based stabilizer, 10 to 40 parts by weight of borax, 16 to
40 parts by weight of sodium hydroxide, and water.
The composition is prepared by dispersing it in water, so that the
content of the composition with respect to water ranges from 1:2 to 1:50 by
weight.
The fuel additive composition is added at 0.02 to 0.5 parts by weight
per 100 parts by weight of fuel.
The composition may further comprise methyl alcohol or a surfactant,
in which the content of the composition to them ranges from 1:1 to 1:3 by
weight.
The composition may further comprise one or more catalysts
selected from the group consisting of potassium carbonate, calcium
2
CA 02525300 2005-11-09
WO 2004/104141 PCT/KR2004/001079
carbonate, and sodium carbonate, in which the content of the composition to
a catalyst ranges from 1:0.03 to 1:0.3 by weight.
The present . invention also provides a method of preparing a fuel
additive composition, comprising the steps of:
mixing 16 to 40 parts by weight of sodium hydroxide in an aqueous
solution in which 10 to 40 parts by weight of borax has been dissolved;
adding 8 to 40 parts by weight of an amine-based stabilizer to the
resultant mixture; and
adding 8 to 40 parts by weight of hydrogen peroxide to the resultant
mixture.
The present invention further provides a use of the fuel additive
composition.
Hereinafter, the present invention is described in more detail.
The fuel additive composition of the present invention comprises
hydrogen peroxide, an amine-based stabilizer, borax; sodium hydroxide,
and water, and it facilitates combustion of fuel, thereby leading to complete
combustion.
Hydrogen peroxide generates oxygen radicals, and thus facilitates
combustion of fuel. Oxygen radicals are oxygen in an atomic state, and
they are very unstable. Thus, they exist for a very short time and are highly
reactive. In the composition of the present invention, hydrogen peroxide
generates oxygen radical, thereby facilitating combustion of fuel fed into the
furnace and the combustion tube. Therefore, the fuel may burn easily even
3
CA 02525300 2005-11-09
WO 2004/104141 PCT/KR2004/001079
with a small amount of oxygen. Also, the oxygen radicals reduce NOX
(thermal NO,~) and prevent generation of PM (parfiiculate matter) such as
SOx and CO in a combustion apparatus.
Because hydrogen peroxide produces oxygen radicals or oxygen
molecules even at room temperature, glycerin or an amine-based stabilizer
is used to inhibit it. As a result, radicals are generated in a large amount
at
about 400 C, which facilitates combustion of fuel. At about 800 C or
above, oxygen radicals from borax facilitate combustion.
The amine-based stabilizer is selected from the group consisting of
dimethanolamine, diethanolamine, trimethanolamine, and triethanolamine.
Using the amine-based stabilizer, decomposition is retarded even up
to about 180 C or higher. At about 180 C or higher, oxygen radicals are
generated in a large amount, so that combustion of the fuel is facilitated
even with a low oxygen content. The amine-based stabilizer also prevents
low-temperature corrosion and increases dispersibility in the aqueous
solution, thereby reducing differences of specific gravity of the matter.
Borax, or hydrated sodium borate (Na~B40~ ~ 10H20), excludes soot,
clinker, and sludge from the furnace of the combustion apparatus, thereby
increasing thermal conduction efficiency, and prevents corrosion of the
furnace, thereby extending the furnace life.
< 1 > Part of the borax mixed in the fuel is decomposed to generate oxygen
radicals, but intact borax is deposited on the furnace surface to form a film,
thereby preventing corrosion at elevated temperatures, reducing viscosity of
4
CA 02525300 2005-11-09
WO 2004/104141 PCT/KR2004/001079
ash, removing PM such as soot, clinker, and sludge, improving thermal
efiFiciency, and reducing air pollutants (dust, smoke, NOX, and SOX). If used
in a combustion apparatus, oxygen radicals generated from the fuel additive
composition of the present invention reduce generation of thermal NOX, and
sodium included in the mixture forms sodium sulfate, so that SOX exhausted
to the air is reduced.
Borax, which is powder, is added to and dissolved in water.
However, because deposition may occur over time, sodium hydroxide and
the amine-based stabilizer are added to increase solubility of the borax in
water and prevent the deposition.
The fuel additive composition is prepared by dispersing 8 to 40 parts
by weight of hydrogen peroxide, 8 to 40 parts by weight of borax, 10 to 40
parts by weight of the amine-based stabilizer, and 16 to 40 parts by weight of
sodium hydroxide, in water. The content of the composition to water
ranges from 1:2 to 1:50 by weight.
If the content of the components falls outside this range, combustion
may be retarded, which causes an increase of fuel use, and cleansing power
may be reduced or deposition may occur during dispersing.
The fuel additive composition may further comprise potassium
carbonate, calcium carbonate, or sodium carbonate to reduce smoke
generation during combustion. They induce low-temperature combustion
thereby reducing NOX generation, and control flame size during combustion
thereby improving the radiant heat transfer system and reducing fuel
5
CA 02525300 2005-11-09
WO 2004/104141 PCT/KR2004/001079
consumption. The content of the composition of the present invention to
the additive ranges from 1:0.03 to 1:0.3 by weight.
The content of the fuel additive composition of the present invention
may be controlled appropriately depending on the kind and quality of fuel,
operation status of the furnace, and degree of aging. Preferably, it is added
at 0.02 to 0.5 parts by weight per 100 parts by weight of fuel. The fuel
additive composition enhances cleansing power and prevents
low-temperature corrosion and high-temperature corrosion.
The fuel additive composition of the present invention is prepared by
the steps comprising:
mixing 10 to 40 parts by weight of borax in an aqueous solution in
which 16 to 40 parts by weight of sodium hydroxide have been dissolved;
adding 8 to 40 parts by weight of an amine-based stabilizer to the
resultant mixture; and
adding 8 to 40 parts by weight of hydrogen peroxide to the resultant
mixture.
Borax is added at 50 to 95 C to maximize its solubility, and
hydrogen peroxide is added at the last step to appropriately control the
oxygen radical content. If hydrogen peroxide is mixed along with borax in
the first step, oxygen radicals are generated excessively, so that foaming
occurs and oxygen radicals are lost. Also, the temperature of the resultant
fuel additive composition is elevated, which makes further processing
complicated and dangerous.
6
CA 02525300 2005-11-09
WO 2004/104141 PCT/KR2004/001079
Potassium carbonate, calcium carbonate, or sodium carbonate is
added after addition of hydrogen peroxide.
Any solid, liquid, or gas fuel may be used in the present invention.
For example, a solid fuel such as coal, coke, and charcoal, a liquid fuel such
as gasoline, kerosene, light oil, heavy oil, coal tar, oil sand, oil shale,
methanol, and ethanol, and a gaseous fuel such as natural gas, liquefied
petroleum gas, hydrogen, and acetylene may be used.
The fuel additive composition of the present invention burns carbon
particles (e.g. coal) before ashing, thereby preventing coagulation of carbon
particles with ashes and altering film formation of borax and the viscosity of
ashes, so that deposition of clinker, soot, and sludge in the furnace can be
prevented.
Particularly, under the reducing atmosphere in the furnace, the
ashing point is decreased. The fuel additive composition of the present
invention checks a decrease of the ashing point by oxygen radicals, thereby
preventing clinker generation. Also, borax, which penetrates into the pores
of coal, prevents coagulation of ashes by a glass bead reaction.
Undecomposed borax is deposited on the furnace surface to form a film,
thereby preventing high-temperature corrosion, interfering with clinker
deposition, and enhancing thermal efficiency.
In a preferred embodiment, if the fuel additive composition of the
present invention is employed in a gas boiler, it prevents generation of
sludge and reduces energy consumption.
7
CA 02525300 2005-11-09
WO 2004/104141 PCT/KR2004/001079
In a gas boiler, fine dust included in air which has been taken in for
combustion, may be hardened by whitening and the like to form solid
materials like sludge to a thickness of about 1 to 2 mm. When the fuel
additive composition of the present invention is used, it decreases the
melting point and flashing point, thereby removing the sludge or preventing
sludge generation. For example, when the fuel additive composition of the
present invention was dispersed in water in a proportion of 1:40 and
employed in a 20 ton/H gas/oil combined rotary boiler of H apartment, the
flame turned an orange color and elongated, and about 5% of energy
consumption was saved.
In another preferred embodiment, if the fuel additive composition of
the present invention is employed in a gas turbine, it removes dust attached
to the blade of the turbine. The dust induces vibration when the gas turbine
is operated at high speed. The fuel additive composition of the present
invention removes the dust and rapidly burns dust and soot, thereby
enabling effective high-speed operation and offering about 2% of thermal
efficiency improvement.
In another preferred embodiment, the fuel additive composition of
the present invention may be mixed with light oil for a diesel engine to
reduce energy consumption. To be specific, the fuel additive composition
is mixed with light oil for a diesel engine along with methyl alcohol or a
surfactant. As a result, dust generation during combustion can be reduced
and about 9% of energy consumption can be saved.
8
CA 02525300 2005-11-09
WO 2004/104141 PCT/KR2004/001079
In another preferred embodiment, the fuel additive composition of
the present invention is dispersed in water to increase the Hardgrove
grindability index (HGI) of fine coals by about 10%, reduce ash generation
by facilitating combustion, and enable recycling of coal ashes. Also, if the
composition is sprayed to or mixed with coal, briquettes, coke, or charcoal,
combustion is facilitated and smoke and noxious smells can be significantly
reduced. Especially, when potassium carbonate is mixed with the
composition, smoke generation is reduced, low-temperature combustion is
facilitated thereby decreasing discharge of such noxious exhaust gas as
NOX, and the radiant heat transfer system is improved during combustion of
gas fuel, thereby leading fo reduced fuel consumption.
In another preferred embodiment, the fuel additive composition of
the present invention may be a simple salt treated in a cement kiln to
improve combustion rate per unit area of a kiln and reduce clinker
productivity. In general, combustion rate is determined by flame length.
The fuel additive composition of the present invention induces complete
combustion and thus reduces flame length. And, in the case of porous
coal fuel, borax penetrates deep into the pores of the coal and generates
oxygen radicals at elevated temperatures. A melting point decrease and
porosity enhancement by calcium carbonate ~ or sodium carbonate
increases a contact area of oxygen thereby increasing the combustion rate,
reducing flashing temperature, and reducing smoke generation from a
Ringelmann turbidity of level 3 to level 1.
9
CA 02525300 2005-11-09
WO 2004/104141 PCT/KR2004/001079
In still another preferred embodiment, the fuel additive composition
of the present invention is employed in an oil boiler to induce complete
combustion of fuel, thereby reducing fly ash, improving dust collecting
efficiency, and extending catalyst life of a dust collector. To be specific,
when the composition was sprayed into the combustion chamber of an oil
boiler while being dispersed in water, about 3% of fuel consumption was
saved and sludge inside the boiler was removed. Also, generation of dust
and smoke was reduced and scale and sludge generated in the pre-heater
was removed.
Thus, the fuel additive composition of the present invention may be
used for a combustion apparatus to remove scale, to prevent corrosion, soot
generation, clinker generation, and sludge generation, and to control flames.
Hereinafter, the present invention is described in more detail through
examples. However, the following examples are only for the understanding
of the present invention and they do not limit the present invention.
EXAMPLES
Example 1
30 kg of borax and 20 kg of sodium hydroxide were dissolved in 50
kg of water at 70 °C . Then, 20 kg of triethylamine, 20 kg of hydrogen
peroxide, and 10 kg potassium carbonate were added to prepare a fuel
additive composition. The resultant fuel additive composition experienced
no precipitation or deposition of borax, and remained as a stable aqueous
solution.
CA 02525300 2005-11-09
WO 2004/104141 PCT/KR2004/001079
Comparative Example 1
A fuel additive composition was prepared as in Example 1, without
using sodium hydroxide. The resultant composition experienced
precipitation as time went by.
Comparative Example 2
A fuel additive composition was prepared as in Example 1, at a
temperature of 40 C . The resultant composition experienced precipitation
as time went by.
Comparative Example 3
A fuel additive composition was prepared as in Example 1, at a
temperature of 45 C. The resultant composition experienced precipitation
as time went by, as in Comparative Examples 1 and 2.
Comparative Example 4
30 kg of borax, 20 kg of sodium hydroxide, and 30 kg of hydrogen
peroxide were dissolved in 50 kg of water at 70 C . Then, 20 kg of
triethylamine, 20 kg of hydrogen peroxide, and 10 kg potassium carbonate
were added to prepare a fuel additive composition. The temperature of the
resultant fuel additive composition rapidly rose to 100 °C as time went
by.
This is because of excessive generation of oxygen radicals, which is caused
by addition of hydrogen peroxide.
Testing Example 1: Energy efficiency test
An energy efficiency test was performed for the composition of
Example 1.
CA 02525300 2005-11-09
WO 2004/104141 PCT/KR2004/001079
Coal having a moisture content of 1.73%, an ash content of 14.73%,
and a volatile content of 30.12% (calorific value = 6,977 kcal; ash fusion
temperature (FT) = 1,588 C), and a boiler having a steam production
capacity of 10 ton/h were used. The boiler was operated under a pressure
of 8 kPa with a load of 80%. As a result, 16.8% of coal consumption was
saved.
Testing Example 2: Pollution reduction test
Dust content, sulfur dioxide concentration, and Ringelmann turbidity
were measured for the composition. The results are shown in the following
Table 1.
Table 1
Test items Before After Measurement
measurementmeasurementresult
Average dust content Decreased by
1673 1082
8 3
(mg/Nm3) . . 35.3%
Average dust discharge Decreased by
42.5 26
9
amount (kg/h) . 36.7%
Average sulfur dioxide321 249 Decreased by
5 1
concentration (mg/ . . 22.5%
Nm3)
Average sulfur dioxide8,158 249.1 Decreased by
discharge amount (kg/h) 24.1
Ringelmann turbidity 1 1
(smoke
Same
concentration)
Testing Example 3: Fuel consumption saving test
The composition was employed in a combined heat and power plant
using bituminous coal. Fuel consumption saving effect due to reduction of
air pollutants and clinkers was determined.
12
CA 02525300 2005-11-09
WO 2004/104141 PCT/KR2004/001079
The composition was diluted to about 1,000:1, based on the coal
weight, in water, and sprayed on lump coal placed on a coal feeder. The
lump coal was crushed to 200 mesh or below and burnt with a burner.
A boiler having a steam production capacity of 120 tonlh and
Chinese Tatong bituminous coal (calorific value = 6,600 kcal/kg; ash fusion
temperature - 1,180 °C; sulfur content - 0.8%) were used. Fuel
consumption was 300 ton/h and temperature inside the furnace was 1,300 to
1,700 C. A horizontal firing type burner and a natural circulation type
boiler were used.
A. Air pollutants Generation reduction effect
Dust content, SOX concentration, NOX concentration, and CO
concentration were measured for 4 weeks.
1. Dust content: Measured with a cylindrical filter. Before addition
of the composition, average dust content was 19.4 mg/cm2. It decreased
by about 47.0%.
2. SOX concentration: Measured by precipitation titration method.
SOX concentration decreased by about 10.2%.
3. NOX concentration: Measured by zinc 1-naphthyldiamine method.
NOX concentration decreased by about 13.0%.
4. CO concentration: Measured by nondispersive infrared analysis
method. CO concentration decreased by about 27%.
B. Fuel efficiency improvement effect
Fly ash content decreased from 30.8% to 13.0%, which is about
13
CA 02525300 2005-11-09
WO 2004/104141 PCT/KR2004/001079
57.8%.
Additionally, the content of bottom ash, one of the clinkers,
decreased from 59.0% to 25.0%, which is about 57.6%.
C. Clinker removal effect
Although the coal used in the test had a lower ash fusion
temperature of 1,180 C than those commonly used (1,300 to 1,400 C), no
clinker was observed on the furnace wall or in the super heater. Also, there
was no fouling.
Testing Example 4: Fuel consumption saving test
The following test was performed to confirm clinker removal and
thermal efficiency improvement.
The composition diluted in water was sprayed on crushed coal.
The proportion of coal, water, and the composition was 1000:10:1. The
mixture was sprayed on lump coal on a coal feeder. The coal was crushed
to 200 mesh or below and burnt with a burner.
The coal had a moisture content of about 2.36%, an ash content of
about 27.89%, and a volatile content of about 17.97%. A horizontal firing
type boiler was used. Steam production capacity was 220 ton/hr, and
vapor pressure was 9.8 MPa. The temperature inside the furnace was
1,500 C to 1,700 C, the air ratio was about 4.8, the combustion
exhausting temperature was 120 °C, and the vapor temperature was about
540 °C .
14
CA 02525300 2005-11-09
WO 2004/104141 PCT/KR2004/001079
A. Fuel savings effect
Coal to which the fuel additive composition of the present invention
had been added was used for 14 days. 76,710 tons of vapor were
produced with 9,786.54 kg of coal. When only coal was used for 15 days,
68,462 tons of vapor were produced with 9,910.58 kg of coal. Therefore,
the fuel additive composition of the present invention offers better fuel
efficiency.
B. SOx reduction effect
Exhaust gas reduction efFect with time was evaluated for coal to
which the composition of Example 1 (C1) had been added, and the
composition (C2) which had been prepared by adding 10 wt% (10 kg) of
potassium carbonate and to which no additive had been added.
The test condition was as follows. The results are shown in FIG. 1.
Measuring device: Test 350M/XL (manufactured by TESTO)
Measuring method: Static potential chemical type
Measuring device type approval number: ASGAM-2001-6 (National
Institute of Environmental Research)
Measuring device performance test report: Prepared by Korea
Testing Laboratory
In FIG. 1, #1 and #2 (-o-, -O-) show SOx discharge when the
composition of Example 1 was not added. -~- is when C1 only was
added, -~- is when 10 wt% of C2 was added along with C1, and -~- is
when 15 wt% of C2 was added along with C1.
CA 02525300 2005-11-09
WO 2004/104141 PCT/KR2004/001079
Using the fuel additive composition of the present invention, fuel
exhaust gas discharge decreased from about 1,100 ppm to about 600
ppm on average. Therefore, SOX reduction effect was about 45%.
C: Fine particle removal in fly ash
Fine particle content was measured for 6 days for Boiler No. 1 to
which the composition of the present invention had been added, and Boiler
No. 2 to which the composition had not been added.
Fine particle content of Boiler No. 1 was 7.39% and that of Boiler No.
2 was 8.76%. Thus, fine particle content was decreased by about
15.64%.
As described above, the fuel additive composition of the present
invention reduces dust, SOX, and NOX generation and induces complete
combustion thereby reducing fuel consumption, and prevents soot, clinker,
sludge, and corrosion in a combustion apparatus, thereby enhancing heat
transfer efficiency and improving operation stability.
16