Note: Descriptions are shown in the official language in which they were submitted.
CA 02525672 2005-11-15
WO 03/097068 PCT/CA03/00738
1
TITLE OF THE INVENTION
Use of Peroxovanadium Compounds as Tumour Cell
Growth Suppressor
FIELD OF THE INVENTION
This invention relates to the use of low dosage levels of peroxovanadium
compounds, such as potassium bisperoxo(1,10-phenanthroline)oxovanadate
[bpV(phen)], potassium bisperoxo(pyridine-2-carboxylato)oxovanadate [bpV(pic)]
and potassium bisperoxo(2,2'-bipyridyl) oxovanadate [bpV(bipy)], for
preventing
and suppressing tumour growth in a mammal.
BACKGROUND OF THE INVENTION
Synthetic peroxovanadium (pV) compounds are structurally versatile molecules
which are potent inhibitors of phosphotyrosyl phosphatases (PTPs) (1 ), These
compounds contain one oxo ligand, one or two peroxo groups, one ancillary
ligand, all coordinated to vanadium. They are stable in aqueous solution at
'physiological pH when shielded from light. Their mode of action lies in the
modulation of the activity of cellular transduction pathways involved in the
progression of pathological conditions. Their effects are transitory and
disappear
within a few days after administration(2).
Phosphotyrosine phosphatases (PTPs) are enzymes which remove phosphates ,
from tyrosine residues of proteins. They are involved in several cell
functions
regulating proliferation, differentiation and metabolism. Their number is
estimated at about 100 in the human genome (3). These enzymes function by
engulfing in their catalytic site phosphates located on the tyrosine residues
of
target proteins. The mechanisms underlying the inhibition of PTPs and the
specificity of peroxo-anionic compounds towards inhibition of PTPs have been '
characterized. The inhibiting potential of PTPs by pVs is a 100 to a 1000
times
more powerful than that of oxovanadate (1 ). When compared to Known
CA 02525672 2005-11-15
WO 03/097068 PCT/CA03/00738
2
inhibitors of PTPs such as orthovanadate, molybdate, tungstate and zinc, the
increased inhibiting potential of pV can be explained by the presence of the
peroxide groups, which have the ability to irreversibly oxidize an essential
conserved cysteine residue located in the catalytic domain of practically all
PTPs
(4).
The possibility of manipulating the ancillary ligands of pVs is important in
regulating potency and specificity (1 ). The ancillary ligands are more or
less
hydrophilic or hydrophobic and provide the molecule with a specific mode of
action and distribution for the different PTPs. These ligands allow for the
specific
targeting of certain PTPs.
International Patent Publication WO 95/19177 teaches the use of vanadate
compounds for the treatment of proliferative disorders, metastasis and drug-
resistant tumours. This publication does not describe peroxovanadate
compounds. The publication further shows that an anti-tumour effect is
observed at dosages of vanadate higher than 5 mM. It is admitted that a
concentration of vanadate compound of 1.3 mM or lower has no apparent anti-
tumour effect.
Montesano et al. (5) teach, on the contrary, that vanadate compounds cause
endothelial cells to proliferateot anti-angiogenic.
US Patent 5,716,981 (Hunter et al.) mentions the use of vanadium compounds,
namely oxovanadate, orthovanadate and vanadyl compounds, in anti-angiogenic
applications.
International Patent Publication WO 01/12180 teaches the use of
peroxovanadium compounds for preventing angio-genesis, restenosis and the
production of endothelins in mammals. However, the publication teaches away ,
from the disclosure that peroxovanadium compounds are effective in preventing
or arresting the growth of tumour cells.
CA 02525672 2005-11-15
WO 03/097068 PCT/CA03/00738
3
International Patent Publication WO 00/57860 teaches the use of
peroxovanadium compounds as antineoplastic agents for the treatment of
cancer. However, this publication teaches that the peroxovanadium compounds
are cytotoxic to several human tumour types and cause cell death through
apoptosis. However, the main drawback of such use of peroxovanadium ,
compounds is that they must be administered in large quantities of 15-20 mg/kg
of body weight per day, or more. This is shown in Figure 8 of the publication.
In
such large quantities, the side-effect of the administration of the drugs are
deleterious. Indeed, transition metals such as vanadium are known to be toxic
(2).
In contrast, the use described in the present invention is aimed at obtaining
a
cytostatic effect meaning that tumour cells remain alive but stop dividing so
as to
prevent or arrest the growth of an invading tumour.
SUMMARY OF THE INVENTION
Surprisingly, it has been found that peroxovanadium compounds can obtain a
cytostatic effect at dosage levels much lower than those proposed in
International Patent Publication WO 00/57860 thereby minimising side-effecfis
of
the drug while improving the therapeutic outcomes of patients.
Thus, the present invention provides the use of peroxovanadium compounds, at
low dosages, as antitumorigenic agents, for example in the treatment of
cancer,
such as breast cancer and prostate cancer. It has been found that a low
dosages, these compounds can prevent or arrest further tumour growth.
Preferably the dosage levels will be 0.001 to less than 15 per mg of body
weight
per day and most preferably 1 to 10 mg/kg of body weight/day. In serum
concentration, the dosage levels are preferably between 1 and 50 pM.
Preferably, the molecules also contain an ancillary ligand, which includes any
molecule capable of binding the transition metal atom (usually, through bonds
CA 02525672 2005-11-15
WO 03/097068 PCT/CA03/00738
4
involving oxygen and nitrogen). Phenanthroline, picolinic acid, bipyridine,
oxalic
acid, 4,7-dimethyl-phenanthroline and peptides are examples of such ligands.
Peroxo vanadate complexes include complexes such as the following: '
methavanadate (V03 ), orthovanadate (VOq.3-), salts thereof, vanadyl
compounds (V02+) like vanadyl acetyl acetonate and vanadyl sulfate. Most
preferred peroxides comprise the following: t-butylhydroperoxide, benzoyl
peroxide, m-chloroperoxibenzoic acid, cumene hydroperoxide, peracetic acid,
hydroperoxiloneic acid, ethyl peroxide, pyridine peroxide and hydrogen
peroxide. '
The general structure of the compounds of the present invention is the
following:
Y
z V z~
L L'
wherein:
Y is oxygen or hydroxyl;
Z and Z' are independently selected from oxygen and peroxide and at least one
of them is peroxide; and
L and L' are any group which can donate an electron pair. ,
In a preferred embodiment, Y is oxygen, Z and Z' are peroxide and L and L' are
the nitrogen atoms of 1,10-phenanthroline.
In another preferred embodiment, Y is oxygen, Z and Z' are peroxide and L and
L' are nitrogen or oxygen atoms of picolinic acid.
CA 02525672 2005-11-15
WO 03/097068 PCT/CA03/00738
In yet another preferred embodiment, Y is oxygen, Z and Z' are peroxide and L
and L' are nitrogen atoms of 2,2'-bipyridine.
In one specific embodiment, the present invention relates to the inhibiting
action
5 on tumour growth of bpV(phen), demonstrating efficiency in vivo. In addition
it is
shown that bpV(phen) has also the capacity to inhibit the migration of tumour
cells in vitro.
Other objects, advantages and features of the present invention will become '
apparent upon reading the following non-restrictive description of preferred
embodiments thereof, with references to the accompanying drawings.
DESCRIPTION OF THE PREFERRED EMBODIMENTS OF THE PRESENT
INVENTION
This invention will now be described by referring to specific embodiments and
the appended Figures, which purpose is to illustrate the invention rather than
to
limit its scope.
BRIEF DESCRIPTION OF THE FIGURES
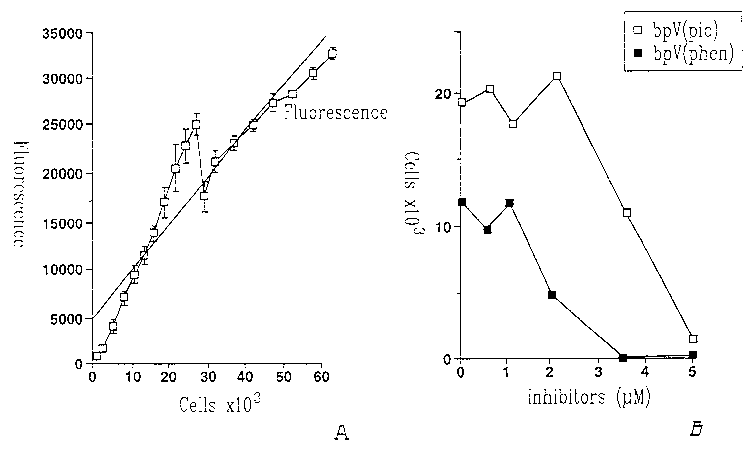
Figure 1: Peroxovanadium compounds inhibit the proliferation of endothelial
cells. Human umbilical vein endothelial cells (HUVECs) were extracted with
collagenase-controlled digestion. Pure HUVECs were used before the fourth
passage (trypsin-EDTA at each passage). The cells were analysed for their '
capacity to incorporate di-acetyl LDL and to be labelled with factor VIII-
related
antigen. Endothelial cells were plated at a density of 2500 cells/cm2 in a
sterile
plate coated with gelatin. Cells were cultured in complete medium (M199:
heparin (90mg/ml) or L-glutamine (2mM), bicarbonate, FBS (20%) and ECGS
(100mg/ml) for 24 hours to ensure cell adhesion. Then, cells were washed 3
times with PBS and culture medium was added according to experimental
conditions. The last PBS wash was considered as time t=0.. Cell proliferation
was evaluated with the amount of DNA present in the petri dishes. Each
CA 02525672 2005-11-15
WO 03/097068 PCT/CA03/00738
6
experiment was performed in triplicate. The culture medium was changed daily.
After 96 hours in culture, cells were lysed with Na-Citrate-SDS solution and '
incubated with Hoescht 33358. Samples were read at 365 nm with a
spectrofluorometer. The results show a dose-response inhibition of endothelial
cell proliferation with the pV compounds. The approximate ICSO is 2mM for
bpV(phen) and 3.5 mM for bpV(pic).
Figure 2: Illustration of the antitumour activity of bpV(phen) in vitro using
PC3
prostate cancer cells. Collagen gels containing the PC3 cells were prepared
according to the method of Esdale and Bard (1972) (reference 10). Briefly,
sfiock
collagen solution (3.5mg/ml in acetic acid 0.02N; Rat tail) was added to a
' mixture composed of culture medium (5x), fetal bovine serum (FBS),
bicarbonate (0.26M), and was neutralized with 0.1 N NaOH. A cell suspension
(1.42 x 106 cells/ml) was mixed into the collagen-medium mixture to obtain a
final concentration of 2.5x105 cells/ml.
A DMEM medium, having a normal concentration of glucose and without phenol
red, was used. After gelification (within 1 h) of the collagen mixture
containing the
cells, the gels were removed from their culture wells (mould) and interwoven
into
a receptor hole prepared in a fibrin gel, as previously described (6). The
fibrin gel
was made from a 0.3 % fibrinogen solution in Hank's balanced salt solution.
Fibrin was allowed to polymerize with thrombin (stock solution at 1.75mg/ml)
at a
ratio of 1:003 v/v fibrin to thrombin. The collagen-fibrin complexes were then
covered with serum-supplemented medium according to the cell types. An
inhibitor of plasminogen activator (Trasylol, Parke Davies) was added into the
medium at 10 pl/ml (100U/ml). Cell behavior was periodically monitored over 15
days of culture.
In this model, collagen is believed to mimic the tumour stroma, and fibrin is
well
recognized as the primary matrix for cancer cell expansion and migration (7).
bpV(phen) was used at 2, 5 and 10 p,M; the compound was renewed daily over a
2 week period. Phase contrast microscopic observations and micrographs were
taken after 15 days, and samples were prepared for histological sections.
CA 02525672 2005-11-15
WO 03/097068 PCT/CA03/00738
7
Histological sections were stained with periodic acid Shiff stain to enhance
the
matrix contrast.
Figure 3: Illustration of the antitumour activity of bpV(phen) in vitro using
ZR-75
breast cancer cells. Experiments were done as described for the figure 2
except
that DMEM medium having a high glucose concentration and without phenol red
was used. 10% FBS was used instead of 5% FBS. The media was
supplemented with 10-9 M estradiol (final concentration).
Figure 4; figures 4A and 4B, illustrate the antitumour activity of bpV(phen)
in
VIVO.
Figure 5: In vitro antitumour activity of lymphocytes pretreated with
bpV(phen).
PC-3 cancer cells embedded in a collagen gel, grew as a "primary tumour".
Some cells migrated from the primary tumour toward the fibrin gel, forming
front
edges that can be quantified. Small clumps of cells progressively appear in
the
fibrin gel and we assume that these cell extension are representative of the
invasive potential of the cancer cells. In the control panel (left), PC-3
cells
migrated slightly from the primary tumour and formed extensive secondary
tumours in the fibrin gel. In the presence of bpV(phen)-treated lymphocytes no
secondary tumour was observed and there was no migration front. In addition,
the primary tumours appeared less dense than in the control.
Definitions
In order to provide a clear and consistent understanding of terms used in the
present description, a number of definitions are provided hereinbelow.
Unless defined otherwise, the scientific and technological terms and
nomenclature used herein have the same meaning as commonly understood by
a person of ordinary skill to which this invention pertains.
CA 02525672 2005-11-15
WO 03/097068 PCT/CA03/00738
For the purposes of the present application, the term "animal" is meant to
signify
human beings, primates, domestic animals (such as horses, cows, pigs, goats,
sheep, cats, dogs, guinea pigs, mice, birds, fish etc.). ,
From the specification and appended claims, the term "therapeutic agent"
should
be taken in a broad sense so as to also include a combination of at least two
such therapeutic agents.
For administration to humans, the prescribing medical professional will
ultimately '
determine the appropriate form and dosage for a given patient, and this can be
expected to vary according to the chosen therapeutic regimen, the response and
condition of the patient as well as the severity of the disease.
Compositions within the scope of the present invention should contain the
active ,
agent (e.g. compound) in an amount effective to achieve the desired
therapeutic
effect while avoiding adverse side effects. Typically, the compounds of the
present invention can be administered to mammals (e.g. humans) in doses
ranging from 0.001 to less than 15 per kg of body weight per day of the mammal
which is treated and most preferably 1 to 10 mg/kg body weight/day.
Pharmaceutically acceptable preparations and salts of the active agent are
within the scope of the present invention and are well known in the art
(Remington's Pharmaceutical Science, 16th Ed., Mack Ed.). The dosage will be
adapted by the clinician in accordance with conventional factors such as the
extent of the disease and different parameters, from the patient. Typically,
0.001
to less than 15 mg/kg/day will be administered to the mammal.
A) PEROXOVANADIUM COMPOUNDS AND THE INHIBITION OF TUMOUR
GROWTH
Methods and cells
ZR-75: hormone-dependent cancer (ductal carcinoma) with oestrogen receptors
(ATCC, USA is depository)
CA 02525672 2005-11-15
WO 03/097068 PCT/CA03/00738
9
PC-3: adenocarcinoma (grade IV) with bone metastasis (ATCC, USA is
depository).
ZR-75-1 human breast cancer: ZR-75-1 human breast cancer cells obtained
from the American Tissue Culture Collection (Rockville, MD) were cultured in
phenol red-free RPMI 1640. The cells were supplemented with 2mM L-
glutamine, 1 mM Na-pyruvate, 100 IU penicillin/ml, 100p.g streptomycin/ml, and
10% (v/v) fetal bovine serum and incubated under a humidified atmosphere
comprised of 95% air and 5% C02 at 37°C.
r
Female homozygous HSD nu/nu athymic mice (50 days old) were obtained from
Harlan Sprague Dawley Inc. (Indianapolis, IN). Five mice were housed per vinyl
cage, which was equipped with air filter lids and kept in laminar air flow
hoods
under pathogen-limiting conditions. The photoperiod was composed of a period
of 14h of light and a period of 10h of darkness. Cages, bedding, and food
(Agway Pro-Lab R-M-H diet #4018) were autoclaved prior to use. Water was
acidified to pH of 2.8, autoclaved, and provided ad libitum. Bilateral
ovariectomy
(OVX) was performed on all animals one week prior to cell inoculation, under
2.5% isoflurane anesthesia mixed with oxygen. Simultaneously, an oestrogen
(E2) implant was inserted subcutaneously to stimulate initial tumour growth
and
appearance. E2 implants were prepared in 1-cm long silastic tubing (inside
diameter, 0.062 inch; outside diameter, 0.095 inch) containing 0.5 cm of
estradiol/cholesterol diluted at a ratio of 1:10 (w:w). One week after the
ovariectomy, 2.0 x 106 ZR-75-1 cells, in their logarithmic growth phase, were
harvested with 0.083% pancreatin/0.3 mM EDTA and inoculated s.c. in 0.1 ml of
RPMI 1640 culture medium containing 30% of Matrigel, from each flank of each
animal through a 2.5-cm-long 20-gauge needle. Four weeks after ZR-75-1 cell
inoculation, the E2 implants were replaced in all animals by estrone-
containing
implants (E~: cholesterol; 1:25 w:w) (7). Treatments consisting of increased
doses of bpV(phen) versus control were started 5 weeks after cell inoculation.
Mice bearing tumours of an average area of 15 mm2 were randomly assigned to
3 groups, each group containing more than 15 mice. OVX animals first received
CA 02525672 2005-11-15
WO 03/097068 PCT/CA03/00738
5
the most potent natural estrogen, to initiate cell proliferation and the
development of tumours. Thereafter, the E2 implants were replaced by E~
implants as a model for post-menopausal women in which . E~ is the main
circulating estrogen that is converted into E2 in peripheral tissues.
On day 0 of the experiment (5 weeks after inoculation), the E~-releasing
implants
were removed from the animals in Group 1 only. All mice received a daily
administration of bpV(phen) over 'a period of 42 days (i.p., 100p1, in a 2
blind
manner). Groups 1 and 2 received PBS, Group 3 received 2.5 mg/Kg
bpV(phen).
Human prostate adenocarcinoma (PC-3) in the athymic mice: Male Balb/c
nude (nu/nu) were purchased at 4-6 weeks of age from Charles Rivers Inc. Mice
were housed under pathogen free conditions and maintained on a 12-h lightl12-
h dark cycle with food and water supplied ad libitum. The hormono-independent
PC3 human tumour cells were from the American Tissue Cuture Collection.
Cells were grown in DMEM in the presence of 5 % foetal bovine serum. Cells
were collected at confluence, included in a matrix (1.0 X 106 cells /ml; 30
Matrigel). An equal volume of the tumour cell suspension was injected s.c. in
the
right flank of each mouse. After 5 days, a palpable tumour of approximately
5X5
mm was detected in the inoculated animals. Mice with palpable tumours were
divided into five groups (18 mice/group) for the treatment study. All mice in
each
treatment group had tumour of similar sire at the start of treatment. For
administration to mice, bpV(phen) was dissolved and diluted in phosphate
buffered saline (PBS) at pH 7.4. A 5mg/Kg dose of bpV(phen) was administed
daily by i.p. for 39 days. Taxol was used as a positive control, at the dose
of
20mg/Kg and injected i.p. at every three days. A control group of 10 animals
was
injected with PBS. The injection volume was kept constant at 100 pl/g body
weight. The mice were weighed three times during the experimental period to
assess toxicity of the treatment, and the tumours were measured twice weekly
using calipers. Tumour volume was calculated from the two-dimensional caliper
measurements using the following formula: tumour volume = length X (width)2 X
0.53.
CA 02525672 2005-11-15
WO 03/097068 PCT/CA03/00738
11
The treatment period was completed after 39 days, when the PBS treated group
of mice had large tumours, requiring that the animals be sacrificed according
to
the Animal Care Procedures. On the final day of the study, the mice were
sacrificed by carbon dioxyde inhalation. The s.c. tumours was removed and
weighed.
Statistical analysis: Tumour growth curves are presented in terms of treatment
group means and SEs. Statistical significance of treatment effect was assessed
by repeated measures ANOVA after applying a power transformation to equalize
residual variances and linearize the tumour growth curves.
Res a Its
1. Progression of tumour cells in vitro
The cells embedded in the collagen gel, grew as a "primary tumour". Some cells
migrated from the primary tumour towards the fibrin gel, forming front edges.
Small clumps of cells were observed in the fibrin gel; in this model they
represent "secondary tumours". Their extension and numbers are representative
of the invasive potential of the cancer cells.
PC-3: In control experiments, PC-3 cells migrated slightly from the primary
tumour and formed extensive secondary tumours in the fibrin gel. In the
presence of 2~,M bpV(phen), a decrease in the size of the secondary tumour
was observed and the migration front from the primary tumour was similar to
that
seen in the control gels. In the presence of 5 and 10 p,M bpV(phen), there was
no migration front and there were no secondary tumours in the fibrin gel. In
addition, the primary tumours appeared clearer than in the control (Figure 2).
ZR-75-1: In control experiments ZR-75-1 cells migrated into the fibrin as
small
spheroidal secondary tumours, with a limited and sparsely visible migration
front.
The presence of 2~;M bpV(phen) restricted the growth of the primary tumour. At
CA 02525672 2005-11-15
WO 03/097068 PCT/CA03/00738
12
and 10p.M bpV(phen) there were no secondary tumours and the primary
tumour had a lower cell density (Figure 3).
2. Inhibition of tumourproaression in vivo
5 ZR-75-1 human breast cancer: The tumour size in the control group, having
not
received E~ replacement therapy, did not increase. The tumour size in the
animals in the other control groups having received E~ replacement therapy was
found to have increased significantly (p<0.05) from 15 to 26 mm2 on day 42.
The
daily administration of bpV(phen) do not resulted in increase of tumour size
(p<0.05). The results show that bpV(phen) has the capacity to inhibit the
progression of tumours in vivo (Figure 4A).
Human prostate adenocarcinoma (PC-3) in the athymic mice: Daily
administration of bpV(phen) caused a significant (p<0.001) 59 % suppression of
the final tumour compared with PBS-treated control animals (Figure 4B).
No death were observed among the vehicle-treated controls or bpV(phen), and,
on average these mice gained 1.5 and 1.7 grams in body weight respectively,
relative to their weight at the initiation of the treatment.
B) THE USE OF INHIBITORS OF PROTEIN TYROSINE PHOSPHATASES
(PTP) FOR ANTI-TUMOUR IMMUNOTHERAPY
Lymphocytes with anti-tumour activity can be isolated from patients and grown
in
vitro for use in cell-tranfer therapies (5). The incubation of immune cells
with the
PTP inhibitor bpV(phen) augments their activation state (9). Therefore, the
re-administration of bpV(phen)-activated immune cells to cancer patients may
enhance the immune response towards tumour cells. The results described
below demonstrate the efficacy of a method in which a peroxometallic compound
(bpV(phen) is used ex vivo on autologous immune cells in order to stimulate
the
potency of these cells and once returned into blood circulation of cancer
patients
fight invasion malignant cells.
CA 02525672 2005-11-15
WO 03/097068 PCT/CA03/00738
13
Method
An in vitro cancer invasion system that has been previously designed was used.
Briefly, prostate cancer cells (PC-3; American Type Culture Collection,
Rockville
MD) were grown in DMEM medium with 5 °!° fetal bovine
serum, 2 mM
L-glutamine, and antibiotics. They were, incubated under a humidified
atmosphere of 95 % air/5% C02 at 37~C. Collagen gels containing the PC-3 cells
were prepared according the method of Esdale and Bard (10). The cell-
embedded collagen gels were laid down onto a layer of fibrin gel, and anchored
by a second layer of fibrin gel. The top of the collagen gel was not fully
covered
with fibrin gel in order to allow direct contacts between the cancer cells in
collagen and the splenocytes. The latter were directly seeded onto the top
lajrer
of the collagen and fibrin gel. Prior to the molding of the cancer invasion
system,
leucocytes were isolated from spleen of either healthy mice or mice bearing PC-
3 tumours. They were treated in vitro with bpV(phen) (25 NM) for 24 hr.
Thereafter, treated cells were washed, counted and seeded (106 ceNs per gel)
on
the cancer cells-embedded gels. Unfireated leukocytes seeded on the top of the
cancer invasion system (same concentration) were used as a control
experiment. Medium was renewed periodically. During the whole experiment,
most leucocytes remained on the top of the gels, and have a normal
morphology. Cell behavior was periodically observed for 7 days of culture,
then
recorded (by photography).
Results
Clumps of PC3 cells progressively appeared in the fibrin gel representing the
invasive potential of the cancer cells. In the control 3D culture system, PC-3
cells
migrated slightly from the primary site and formed extensive secondary
fiumours
in the fibrin gel as described in previous studies (11, 12). In contrast to
this, in
the presence of bpV(phen)-treated leucocytes, neither secondary tumours nor
migration front was observed. In addition, the primary tumours appeared less
dense than in the control, indicating a smaller number of growing cells
(Figure
5).
CA 02525672 2005-11-15
WO 03/097068 PCT/CA03/00738
14
It is to be understood that the use of bpV's as a therapeutic agent my be
alone
or in combination with other active ingredient such as interleukins or
chemokines.
It is also to be understood that the bpV dosage compositions of the present
invention may be used as vaccines for eliciting an immuno response from a
mammal.
Although the present invention has been described by way of preferred
embodiments thereof, these embodiments can be modified at will, within the
scope of the appended claims, without departing from the spirit and nature of
the
subject invention.
CA 02525672 2005-11-15
WO 03/097068 PCT/CA03/00738
LIST OF REFERENCES
1. Posner, B.I., Faure, R., Burgess, J.W., Bevan, A.P., Lachance, D., Zhan-
Sun, G., Fanfius, I.G., Ng, J.B., Hall, D.A., Lum, B.S. & Shaver, A. (1994).
5 Peroxovanadium compounds: a new class of potent phosphotyrosine-
phosphatase inhibitors which are insulin mimetics. J. Biol. Chem. 269:
4596-4604.
2. Faure, R., Vincent, M., Dufour, M., Shaver, A. & Posner, B. (1995). Arrest
at the G2lM transition of the cell-cycle by protein-tyrosine phosphatase
10 inhibition: Studies on a neuronal and a glial cell line. J. Cell. Biochem.
58:
389-401.
3. Tonks, N.K. and B.G. Heel Combinatorial control of the specificity of
. PTPs. Current opinion in cell Biology 2001 13(2): 182-195.
4. Huyer et al. (1997)
15 5. Montesano, R., Pepper, M.S., Belin, D., Vassalli, J.-D. & Orci, L.
(1988).
Induction of angiogenesis in vitro by vanadate, an inhibitor of
phosphotyrosine phosphatases. J. Cell. Physiol. 134: 460-466.
6. Janvier, R., Sourla, A., Koutsilieris, M. & Doillon, C. (1997). Stromal
Fibroblasts are required for PC-3 human prostate cancer cells to produce
capillary-like formation of endothelial cells in a three-dimensional co-
culture system. Anticancer res. 17: 1551-1558.
7. Couillard S, Gutman M, Labrie F, Candos B, Labrie C. (1999). Effect of
combined treatment with EM-800 and radiotherapy on the growth of
human 212-75-1 breast cancer xenografts in nude mice. Cancer Res.59:
4857-4863.
8. Rosenberg, S.A. Progress in human tumour immunology and
immunotherapy. Nature 411: 380-384. 2001.
CA 02525672 2005-11-15
WO 03/097068 PCT/CA03/00738
16
9. Olivier, M. Romero-Gallo B. J., St Laurent, R., Racette, N., Steven'son,
M., Jacobs, P., Posner, B., Tremblay, M., Faure, R. Modulation of INFg-
induced macrophage activation by phosphotyrosine phosphatase
inhibition. J. Biol. Chem. 273, 13944-13949 (1998).
10. Esdale, T., Bard, J. Collagen substrata for studies on cell behavior. J.
Cell. Biol. 54: 626-637 (1972).
11. Janvier, R., Sourla, A., Koutsilieris, M., Doillon, C. Stromal fibroblasts
are
required for PC-3 human prostate cancer cells to produce capillary -like
formation of endothelial cells in a three-dimensional co-culture system.
Anticancer Res. 17: 1551-1557 (1997).
12. Dvorak, H.F., Harvey, V.S., Estrella, P., Brown, L.F., Mc Donagh, J.,
Dvorak A.M. Fibrin containing gels induce angiogenesis. Implications for
stroma generartion and wound healing. Lab Invest. 57: 673-686 (1987).