Some of the information on this Web page has been provided by external sources. The Government of Canada is not responsible for the accuracy, reliability or currency of the information supplied by external sources. Users wishing to rely upon this information should consult directly with the source of the information. Content provided by external sources is not subject to official languages, privacy and accessibility requirements.
Any discrepancies in the text and image of the Claims and Abstract are due to differing posting times. Text of the Claims and Abstract are posted:
| (12) Patent Application: | (11) CA 2526151 |
|---|---|
| (54) English Title: | PROCESS AND EQUIPMENT TO PRODUCE PHENOL FROM BENZENE |
| (54) French Title: | PROCESSUS ET EQUIPEMENT POUR LA PRODUCTION DE PHENOL A PARTIR DE BENZENE |
| Status: | Deemed Abandoned and Beyond the Period of Reinstatement - Pending Response to Notice of Disregarded Communication |
| (51) International Patent Classification (IPC): |
|
|---|---|
| (72) Inventors : |
|
| (73) Owners : |
|
| (71) Applicants : |
|
| (74) Agent: | |
| (74) Associate agent: | |
| (45) Issued: | |
| (22) Filed Date: | 2005-10-24 |
| (41) Open to Public Inspection: | 2007-04-24 |
| Availability of licence: | N/A |
| Dedicated to the Public: | N/A |
| (25) Language of filing: | English |
| Patent Cooperation Treaty (PCT): | No |
|---|
| (30) Application Priority Data: | None |
|---|
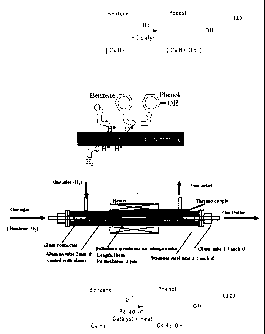
Existing phenol production via the cumene or other processes, are complicated,
multiple staged and
energy consuming, producing ketone by-products. The typical yield is about 5%
per pass by wt. A direct
oxidation process that would eliminate the need for any reagents such as H2,
gases, or Nitrous Oxide is
most sought whereby;
(see above formula)
A recent development for a single stage process uses a Palladium membrane as a
passive catalyst in a
reactor chamber, reporting up 16% yield per pass. However, this process also
generates hexane,
cyclohexane and toluene as by-products in reactor temperatures in the order of
250° C. This one step
process requires O2, & H2 gases and is very temperature sensitive. The volume
of gases introduced into
the reactor are also critical. In addition, the benzene must be delivered in
it's gaseous state. The
process demonstrates that the benzene ring is indeed reactive with an oxygen
ion, but not with the O2
gas molecule. This apparatus was developed by The National Institute of
Advanced Industrial Science and
Technology (AIST) Laboratory for Membrane Chemistry in Japan.
Note: Claims are shown in the official language in which they were submitted.
Note: Descriptions are shown in the official language in which they were submitted.
2024-08-01:As part of the Next Generation Patents (NGP) transition, the Canadian Patents Database (CPD) now contains a more detailed Event History, which replicates the Event Log of our new back-office solution.
Please note that "Inactive:" events refers to events no longer in use in our new back-office solution.
For a clearer understanding of the status of the application/patent presented on this page, the site Disclaimer , as well as the definitions for Patent , Event History , Maintenance Fee and Payment History should be consulted.
| Description | Date |
|---|---|
| Inactive: IPC from PCS | 2023-11-25 |
| Inactive: First IPC from PCS | 2023-11-25 |
| Inactive: IPC from PCS | 2023-11-25 |
| Inactive: Dead - Application incomplete | 2008-08-08 |
| Application Not Reinstated by Deadline | 2008-08-08 |
| Inactive: Adhoc Request Documented | 2008-07-30 |
| Deemed Abandoned - Failure to Respond to Maintenance Fee Notice | 2007-10-24 |
| Deemed Abandoned - Failure to Respond to Notice Requiring a Translation | 2007-08-08 |
| Inactive: Cover page published | 2007-05-16 |
| Inactive: Incomplete | 2007-05-08 |
| Application Published (Open to Public Inspection) | 2007-04-24 |
| Inactive: Cover page published | 2007-04-23 |
| Inactive: First IPC assigned | 2006-07-04 |
| Inactive: IPC assigned | 2006-07-04 |
| Inactive: IPC assigned | 2006-07-04 |
| Inactive: IPC assigned | 2006-07-04 |
| Inactive: Office letter | 2006-03-23 |
| Change of Address Requirements Determined Compliant | 2006-03-23 |
| Change of Address or Method of Correspondence Request Received | 2006-02-22 |
| Correct Applicant Requirements Determined Compliant | 2005-12-21 |
| Inactive: Inventor deleted | 2005-12-21 |
| Inactive: Applicant deleted | 2005-12-21 |
| Inactive: Filing certificate - No RFE (English) | 2005-12-19 |
| Filing Requirements Determined Compliant | 2005-12-19 |
| Application Received - Regular National | 2005-12-19 |
| Abandonment Date | Reason | Reinstatement Date |
|---|---|---|
| 2007-10-24 | ||
| 2007-08-08 |
| Fee Type | Anniversary Year | Due Date | Paid Date |
|---|---|---|---|
| Application fee - small | 2005-10-24 |
Note: Records showing the ownership history in alphabetical order.
| Current Owners on Record |
|---|
| HARRY LAUR |
| SAM TURTLE |
| ED MELCAREK |
| Past Owners on Record |
|---|
| None |