Note: Descriptions are shown in the official language in which they were submitted.
CA 02528870 2005-12-09
-1-
DESCRIPTION
METHOD FOR PRODUCING TISSUE CELLS FROM
PLURIPOTENT STEM CELLS DERIVED FROM IRIS PIGMENTED
EPITHELIAL CELLS OF ANIMAL, AND TISSUE CELLS
OBTAINED BY METHOD
TECHNICAL FIELD
The present invention relates to a method for producing
tissue cells from pluripotent stem cells produced from iris
pigmented epithelial cells of an animal. The present invention
also relates to tissue cells obtained by the method.
BACKGROUND ART
Recently, attention has been paid to such regenerative
medical treatment that transplants cells built by using
pluripotency of brain- or spine-derived neural stem cells or that
of ES cells (embryonic stem cells).
Medical applications of the neural stem cells and the ES
cells raise many problems such as immunological rejection in
cell transplantation, ethical issues, and unbalance between
demand and supply of transplant cell sources.
Accordingly, when it becomes possible to use, as a
transplant source, cells derived from a transplant recipient per
se, autotransplantation becomes possible, thus solving the
foregoing problems.
An example of cells expected to serve as the transplant
sources is iris pigmented epithelial cells of an eyeball.
Iris pigmented epithelial cells are a component of an iris
serving as tissue for opening and narrowing a pupil in accordance
with an amount of light so as to adjust an amount of light which
CA 02528870 2005-12-09
-2-
reaches a retina.
The inventors of the present invention have reported in
Non-Patent Document 1 (Experimental Cell Res. (1998) 245,
245-251) that the inventors have successfully isolated and cultured
iris pigmented epithelial cells of a chick.
Furthermore, the inventors have made it possible to isolate
and culture mammalian iris cells (from mouse, rat, or human
embryo) by a method improved from the process of Non-Patent
Document 1 (see Non-Patent Document 2: Nature Neuroscience
(2001) 4 (12), 1163).
It is possible to collect part of iris pigmented epithelial cells
from a patient per se. Therefore, if it becomes possible to produce
tissue cells by using iris pigmented epithelial cells, regenerative
medical treatment using cells of the patient per se will be realized.
(To the best of the inventors' search, there is no document
concerning a method according to the present invention for
producing tissue cells from stem cells derived from iris pigmented
epithelial cells of an animal, and tissue cells obtained by the
method.)
However, no method for producing non-neural. cells from iris
pigmented epithelial cells of an animal has been established.
The present invention has been completed in consideration of
the foregoing problems and has an object to provide a method for
producing tissue cells derived from iris pigmented epithelial cells of
an animal, and tissue cells obtained by the method, the method
and the tissue cells making it possible to solve problems such as
immunological rejection in cell transplantation, ethical issues,
and unbalance between the demand and supply of transplant
cell sources.
DISCLOSURE OF INVENTION
As a result of diligently studying to attain the object, the
CA 02528870 2005-12-09
-3-
inventors have found that an aggregate is obtained by culturing
stem cells under specific culture conditions, the stem cells
being obtained by using a floated coagulated mass culturing
technique to selectively culture iris pigmented epithelial cells
isolated from an eyeball of an animal. Further, the inventors
have also found that the aggregate has an embryoid body
structure which contains various tissue cells such as muscle
cells, vascular endothelial cells, and other cells. Based on these
findings, the inventors have completed the present invention.
That is, the inventors have found that the stem cells are
pluripotent stem cells differentiable into various types of tissue,
the stem cells being obtained by selectively culturing the iris
pigmented epithelial cells by using the floated coagulated mass
culturing technique, the iris pigmented epithelial cells being
isolated from the eyeball of the animal.
In order to attain the foregoing object, a method of the
present invention for producing tissue cells includes the steps
of: obtaining pluripotent stem cells by selectively culturing iris
pigmented epithelial cells by a floated coagulated mass
culturing technique, the iris pigmented epithelial cells isolated
from an eyeball of an animal; and obtaining tissue cells from
the pluripotent stem cells by culturing the pluripotent stem
cells.
According to the foregoing arrangement, pluripotent stem
cells can be obtained by selectively culturing an iris pigmented
epithelium by using the floated coagulated mass culturing
technique, the iris pigmented epithelium being isolated from iris
tissue extirpated from an eyeball of an animal by using a
publicly known conventional method for isolating an iris
pigmented epithelium of an adult animal.
Moreover, by culturing the pluripotent stem cells, an
embryoid body structure can be formed.
CA 02528870 2005-12-09
-4-
The embryoid body is a structure which contains tissue
like an embryo, the tissue being made mainly from ES cells
subjected to differentiation induction. Since the embryoid body
contains tridermic cells, the iris-derived stem cells are believed
to have totipotency like ES cells. Accordingly, in the present
invention, the cells contained in the embryoid body structure
derived from iris epithelial cells are referred to as "tissue cells
derived from iris pigmented epithelial cells of an animal".
Pluripotent stem cells obtained during production of the
tissue cells according to the present invention have an
advantage of being collected and produced from autologous
tissue relatively easily and in a low invasive manner. That is,
unlike ES cells' conventionally used, the pluripotent stem cells
do not have problems such as immunological rejection and
ethical issues to which the use of ES cells face because ES cells
are derived from a fetal embryo. Further, unlike pluripotent
adult progenitor/ stem cells (MAPC), the pluripotent stem cells
do not require a highly invasive collection method such as bone
marrow puncture.
In the present invention, the animal is for example a
chicken, a mouse, a rat, or a human. Further, the animal may
be a fetal individual or a postnatal individual. What is meant by
the postnatal individual is an individual except for a prenatal
embryo. As the postnatal individual, a sexually matured adult,
a neonatal individual, and the like are exemplified. However, an
individual may be of any age.
Further, the pluripotent stem cells have at least either one
of the following characteristics (1) and (2).
(1) Oct-3/4 positive.
(2) tridermic differentiable.
The characteristics (1) and (2) will be described in Examples.
Further, the method of the present invention for
CA 02528870 2005-12-09
-5-
producing tissue cells is arranged so that, in the step of
obtaining the tissue cells from the pluripotent stem cells, the
pluripotent stem cells are differentiated into one or more types
of tissue cells by culturing the pluripotent stem cells under
differentiation inducing condition.
Here, what is meant by "culturing under the
differentiation inducing condition" is culturing under any
publicly known conventional condition designed to differentiate
cells. Specifically, it means a culturing method by which
culturing is conducted in a medium, to which serum and a
growth factor (e.g., FGF, EGF, CNTF, RA) is added, on a culture
dish coated with various extracellular substrate components.
Thus, the pluripotent stem cells are differentiable into one or
more types of tissue cells by being cultured under the
differentiation inducing condition.
It is preferable that the culturing under differentiation
inducing condition be conducted with use of a serum. The
serum is for example a fetal calf serum or an avian serum.
Further, in the culturing under the differentiation
inducing condition, a growth factor may be further used. As the
growth factor, for example, EGF (epidermal growth factor), FGF
(fibroblast growth factor), and the like can be used.
The tissue cells according to the present invention are
derived from iris pigmented epithelial cells part of which can be
collected from a patient per se. Therefore, according to the
present invention, the tissue cells which can be utilized as a
transplant source in regenerative medical treatment can be
produced from iris pigmented epithelial cells of an animal.
Further, the method of the present invention for
producing tissue cells is arranged so that the isolating of the
iris pigmented epithelial cells includes: an
iris-tissue-extirpating step of extirpating iris tissue from the
CA 02528870 2005-12-09
-6-
eyeball of the animal; and an
iris-pigmented-epithelial-cell-separating step of separating iris
pigmented epithelium from the iris tissue thus extirpated.
According to this arrangement, the tissue cells of the
present invention can be efficiently produced by efficiently
separating the iris pigmented epithelial cells of the animal.
Further, in the present invention, the
iris-tissue-extirpating step includes: an iris-tissue-excising
stage of excising only iris tissue from the eyeball of the animal;
an enzyme treatment stage of subjecting the excised iris tissue
to enzyme treatment; and an iris-tissue-restoring . stage of
restoring the enzyme-treated iris tissue.
According to this arrangement, the tissue cells of the
present invention can be efficiently produced by efficiently
extirpating only the iris tissue from the eyeball of the animal.
The tissue cells according to the present invention are
obtained by using a method of the present invention for
producing the tissue cells. As described above, the tissue cells
are derived from iris pigmented epithelial cells of an animal.
Therefore, the tissue cells of the present invention can be
provided as a transplant cell source which solves problems such
as immunological rejection caused by cell transplantation,
ethical issues, and unbalance between the demand and supply
of transplant cell sources.
Moreover, the tissue cells according to the present
invention are ectodermal cells or cells derived from ectoderm,
mesodermal cells or cells derived from mesoderm, or
endodermal cells or cells derived from endoderm. Furthermore,
the tissue cells of the present invention form tissue forming an
intravital organ. The "tissue forming an intravital organ"
specifically means nerve tissue, muscular tissue, heart tissue,
vascular tissue, and the like which form a neural organ, a
CA 02528870 2005-12-09
-7-
muscular organ, a heart, a blood vessel, and the like,
respectively.
For a fuller understanding of the nature and advantages
of the invention, reference should be made to the ensuing
detailed description taken in conjunction with the
accompanying drawings.
BRIEF DESCRIPTION OF DRAWINGS
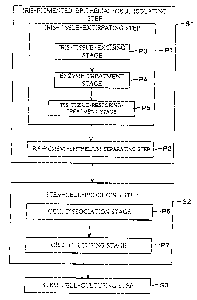
Fig. 1 is a schematic flow chart illustrating an example of
a method according to the present invention for producing
tissue cells.
Figs. 2 are diagrams illustrating states of stem cells
according to Example 1.
Fig. 3(a) and Fig. 3(b) are diagrams illustrating aggregates
labeled with desmin (muscle cell marker) antibody or DAPI
staining (nucleus) in Example 1.
Fig. 4 is a diagram illustrating emergence of myocardial
cells in each step of inducing each tissue cell from iris
pigmented epithelial cells in Example 2.
Fig. 5 is a diagram explaining an expression pattern of
Oct-3/4 in an initial developmental process of a mouse.
Fig. 6(a) is a diagram illustrating a result of labeling a
rat-obtained stem cell by Oct-3/4 antibody staining and DAPI
staining in Example 3.
Fig. 6(b) is a diagram illustrating a result of labeling a
rat-obtained stem cell by Oct-3/4 antibody staining and DAPI
staining in Example 3.
BEST MODE FOR CARRYING OUT THE INVENTION
One embodiment of the present invention will be
described below with reference to Fig. 1. The present invention
is not to be limited to the embodiment.
CA 02528870 2005-12-09
-8-
For the purpose of solving problems such as
immunological rejection in cell transplantation, ethical issues,
and unbalance between the demand and supply of transplant
cell sources, the inventors produced tissue cells derived from
iris pigmented epithelial cells of an animal.
A method according to the present embodiment for
producing tissue cells includes the steps of: obtaining
pluripotent stem cells by selectively culturing iris pigmented
epithelial cells by a floated coagulated mass culturing technique,
the iris pigmented epithelial cells being isolated from an eyeball
of an animal; and obtaining tissue cells from the pluripotent
stem cells by culturing the pluripotent stem cells.
That is, as shown in Fig. 1, the method of the present
embodiment for producing tissue cells at least includes:
iris-pigmented-epithelial-cell-isolating step (Step 1, hereinafter,
Step is abbreviated as S) of isolating iris pigmented epithelial
cells from an eyeball of an animal; the step of obtaining
pluripotent stem cells by using the floated coagulated mass
culturing technique to selectively culture the isolated iris
pigmented epithelial cells (hereinafter, this step is referred to as
stem-cell-producing step S2); and a step of obtaining tissue
cells from the pluripotent stem cells by culturing the
pluripotent stem cells with serum or the like (hereinafter, this
step is referred to as stem-cell-culturing step S3). The method
according to the present invention for producing a tissue cells is
not limited to the above arrangement and may include another
step. Further, the stem-cell-culturing step S3 can be referred to
also as tissue-cell-inducing step.
The animal may be a postnatal individual animal of any
age between a neonatal period and an adult period. That is, the
method according to the present invention for producing tissue
cells makes it possible to produce tissue cells derived from iris
CA 02528870 2005-12-09
-9-
pigmented epithelial cells of an adult animal as well as tissue
cells derived from iris pigmented epithelial cells of a neonatal
animal.
The iris-pigmented-epithelial-cell-isolating step S1 is not
particularly limited in terms of techniques and other features
concretely adopted therein, as long as the iris pigmented
epithelial cells can be obtained by the
iri s- pigmented- epithelial- cell-isolating step S1. Generally
speaking, a publicly known conventional technique may be
adopted so as to extirpate iris tissue from an eyeball of an
animal and isolate iris pigmented epithelial cells from the
extirpated iris tissue. It is preferable to use a method described
in Nature Neuroscience (2001) 4 (12), 1163 (Non-Patent
Document 2) so as to extirpate iris tissue from an eyeball of an
animal.
In the stem-cell-producing step S2, it is only necessary to
selectively culture only iris pigmented epithelial cells isolated
from an eyeball of an animal. A specific technique and the like
used in the step are not particularly limited. Generally speaking,
it is only necessary to use a publicly known conventional
technique so as to selectively culture only iris pigmented
epithelial cells isolated from an eyeball of an animal.
Here, the stem-cell-producing step S2 includes Process 6
and Process 7 (hereinafter, Process is abbreviated as P). P6 is a
cell dissociation stage at which iris pigmented epithelial cells,
isolated in the iris-pigmented-epithelial-cell-isolating step S1, is
dissociated from an aggregating state into an individual cell. P7
is a cell culturing stage at which only the isolated iris
pigmented epithelial cells are selectively cultured.
In the following, the stages P6 and P7 of the
stem-cell-producing step S2 will be described in detail. First, at
the cell dissociation stage P6, the isolated iris pigmented
CA 02528870 2005-12-09
-10-
epithelium cells arranged in a sheet-like form are dissociated
into individual cells.
For example, at the cell dissociation stage P6, a
commercially available trypsin solution is used to dissociate
into the individual cells the isolated iris pigmented epithelium
cells arranged in the sheet-like form. Further, for example, at
the cell dissociation stage P6, the isolated iris pigmented
epithelium cells arranged in the sheet-like form can be
dissociated into the individual cells also by pipetting operation
using a commercially available micropipette, without using the
trypsin solution.
The reagent and instrument used at the cell dissociation
stage P6 are not particularly limited, and it is possible to use a
publicly known conventional reagent and instrument which
make it possible to dissociate into individual cells the isolated
iris pigmented epithelial cells in the coagulated state.
At the cell culturing stage P7, the isolated iris pigmented
epithelial cells are cultured, in suspension, in a serum-free
medium to which FGF (fibroblast growth factor), LIF (leukemia
inhibitory factor), and SCF (human SCF (stem cell factor)) are
added either individually or in combination. This allows the iris
pigmented epithelial cells to grow without differentiation. This
growth of the iris pigmented epithelial cells without
differentiation leads to production of the tissue cells in higher
quantity. This stage is preferably arranged to employ the floated
coagulated mass culturing technique (neurosphere method),
described in Science 1992: 225; 1707-1710, so as to selectively
culture the iris pigmented epithelial cells isolated from the
eyeball of the animal.
For example, at the cell culturing stage P7, a mixture of a
commercially available serum-free medium and a commercially
available N2 supplement is used as a
CA 02528870 2005-12-09
- 11 -
floated-coagulated-mass-culturing culture medium. The iris
pigmented epithelial cells dissociated at the cell dissociation
stage P6 are cultured in the floated-coagulated-mass-culturing
culture medium with rotating by use of a commercially available
shaker. This makes it possible to selectively separate and collect
a cell population which contains a large number of pluripotent
stem cells.
The culture medium and reagent used at the cell culturing
stage P7 are not particularly limited, and it is possible to use a
publicly known conventional culture medium and reagent which
make it possible to obtain the stem cells.
Further, in the present embodiment, a culture period in
the cell culturing stage P7 may be set appropriately according to
need. However, if the culture period is too long, the resulting
aggregate may become overgrown thereby to be differentiated.
Accordingly, in the present embodiment, it is preferable to carry
out dissociation and passage of cells within three to four days.
Moreover, in the stem-cell-culturing step S3, the stem
cells obtained at the cell culturing stage P7 are cultured with
serum. The stem-cell-culturing step S3 is not particularly
limited in terms of techniques and other features concretely
adopted therein, as long as serum is used to culture the stem
cells. Generally speaking, a publicly known conventional
technique may be employed to culture the stem cells. Therefore,.
for example, the culturing of the stem cells may be carried out
by using a commercially available micropipette to transfer the
stem cells to a serum-containing medium.
As the serum, a fetal calf serum, an avian serum, and the
other serums can be exemplified. However, the serum is not to
be limited to these. Further, in the present embodiment, these
serums may be used solely or two or more of these serums may
be used according to need.
CA 02528870 2005-12-09
-12-
Further, a concentration of the serum is preferably 5 to
30% and more preferably 10 to 20%. When the culture is
conducted at such a serum concentration, a good result is
obtained. However, the present invention can be conducted at a
concentration lower or higher than that. Therefore, the serum
concentration is not limited as described above.
In the present embodiment, the stem cells may be
cultured with a growth factor in addition to the serum.
Specifically, as the growth factor, EGF (epidermal growth factor),
FGF (fibroblast growth factor), and the other growth factor can
be exemplified. In the present embodiment, these growth factors
may be used solely or two or more of these growth factors may
be used according to need.
Further, a concentration of the growth factor is not
particularly limited and may be set appropriately according to
need.
Further, in the present embodiment, in the
stem-cell-culturing step S3, a culture period to culture the stem
cells is preferably one to three months, albeit not particularly
limited. In the stem-cell-culturing step S3, when a culture
period is one month or shorter (particularly, two weeks or
shorter), differentiation induction efficiency undesirably
decreases. Conversely, when a culture period is three months or
longer, a cell survival rate within the aggregates may
undesirably decrease.
In the present embodiment, an embryoid body is obtained
by conducting the stem-cell-culturing step S3. The embryoid
body is derived from the iris pigmented epithelial cells of the
animal, and the iris pigmented epithelial cells are ectodermal
cells. Therefore, the embryoid body obtained in the present
embodiment encompasses not only neural stem cells but also
various tissue cells such as muscular cells and vascular
CA 02528870 2005-12-09
-13-
endothelial cells. Thus, according to the present embodiment,
the tissue cells derived from the iris pigmented epithelial cells of
an animal can be obtained by conducting the
stem-cell-culturing step S3.
The method according to the present embodiment for
producing tissue cells is arranged so that the isolating of the iris
pigmented epithelial cells includes: an iris-tissue-extirpating
step of extirpating iris tissue from the eyeball of the animal; and
an iris-pigmented-epithelial-cell-separating step of separating
an iris pigmented epithelium from the iris tissue thus extirpated.
Note that the method of the present embodiment for producing
tissue is not to be limited to this arrangement by may include
another step.
That is, as illustrated in Fig. 1, the method of the present
embodiment for producing tissue cells includes at least the
iris-pigmented-epithelial-cell-isolating step S1, the
stem-cell-producing step S2, and the stem-cell-culturing step S3.
Furthermore, the iris-pigmented-epithelial-cell-isolating step S1
includes an iris-tissue-extirpation step P1 and an
iris-pigmented-epithelial-cell-separating step P2. The method
according to the present embodiment for producing the tissue cells
is not limited to this arrangement and may include another step.
The iris-tissue-extirpating step P1 is not particularly limited
in terms of technique and the like concretely adopted, as long as
the iris tissue can be extirpated from the eyeball of the animal.
Generally speaking, a publicly known conventional technique may
be used so as to extirpate the iris tissue from the eyeball of the
animal. it is referable to use the method described in Nature
Neuroscience (2001) 4 (12), 1163 (Non-Patent Document 2), so as to
extirpate the iris tissue from the eyeball of the animal.
Here, as shown in Fig. 1, the iris-tissue-extirpation step P1
includes: an iris-tissue-excising stage P3 of excising only the iris
CA 02528870 2009-07-27
-14-
tissue from the eyeball of the animal; an enzyme treatment stage P4
of subjecting the extirpated iris tissue to an enzyme treatment; and
an iris-tissue-restoring treatment stage PS of restoring the
enzyme-treated iris tissue. The method according to the present
embodiment for producing the tissue cells is not limited to this
arrangement and may include another step.
In the following, each of the stages P3 to PS of the
iris-tissue-extirpating step P1 will be described in detail First, the
iris-tissue-excising stage P3 is not particularly limited in terms of
technique and the like concretely adopted, as long as the iris tissue
can be extirpated from the eyeball of the animal. Generally speaking.
a publicly known conventional technique may be used so as to
excise only the iris tissue ironer the eyeball of the animiL
For example, at the iris-tissue-exicising stage P3,
commercially available micro scissors are used to excise only iris
tissue from an eyeball of an animal.
The enzyme treatment stage P4 is desig< ed to subject the iris
tissue to the enzyme treatment in order to make it easy to separate
an iris pigmented epithelium from the iris tissue. The enzyme
treatment stage P4 is not particularly limited is terms of technique
and the like concretely adopted. Generally speaking, a publicly
known conventional technique may be used to subject the iris
tissue to the enzyme treatment in order to make it easy to separate
the his pigmented epithelium from the his tissue.
For example, in case of separating an iris pigmented
epithelium from an eyeball of a chicken, at the enzyme trestmggt
stage P4, iris tissue is allowed to react for 15 to 40 minuft* to a
disperse solution containing a commercially available e M
Thereafter, the his tissue is allowed to react for 20 to 30 minutes In
an EDTA solution containing a commercially available EDTA
tet}rylenediaminetetrancetic acid). The enzyme and reagent used at
the enzyme treatment stage P4 are not partici larly limited. It is
CA 02528870 2005-12-09
- 15-
possible to use a publicly known conventional enzyme and reagent
which make it possible to treat iris tissue in such a way as to make
it easy to separate the iris pigmented epithelium from the iris
tissue.
The iris-tissue-restoring-treatment stage P5 is designed to
restore the iris tissue weakened by enzyme treatment. The
iris-tissue-restoring-treatment stage P5 is not particularly limited in
terms of technique and the like concretely adopted. Generally
speaking, a publicly known conventional technique may be used so
as to restore the iris tissue weakened by enzyme treatment.
For example, at the iris-tissue-restoring stage P5, after the
reaction of the enzyme treatment stage P4, the iris tissue is. allowed
to react for 30 to 60 minutes in a culture medium containing a
commercially available fetal calf serum so as to restore the iris
tissue. The serum-containing culture medium and reagent used at
the iris-tissue-restoring-treatment stage P5 are not particularly
limited. It is possible to use a publicly known conventional culture
medium and reagent which make it possible to recover weakened
iris tissue.
Further, in the iris-tissue-extirpating step P1, the reaction
times at the enzyme treatment stage P4 and the reaction time at the
iris-tissue-restoring-treatment stage P5 are particularly important.
By adjusting the reaction time during which the iris tissue is
allowed to react in the dispase solution at the enzyme treatment
stage P4, the reaction time during which the iris tissue is allowed to
react in the EDTA solution at the enzyme treatment stage P4, and
the reaction time during which the iris tissue is allowed to react in
the fetal-calf-serum-containing culture medium at the
iris-tissue-restoring-treatment stage PS, it is possible to separate an
iris pigmented epithelium not only from the eyeball of the chicken
but also from an eyeball of an animal such as a mouse, a rat, or a
human being.
CA 02528870 2005-12-09
- 16-
In case of separating the iris pigmented epithelium from the
eyeball of the mouse, it is preferable that the iris tissue be allowed
to react in 1000 U/mL dispase solution at 25 to 37 C for 15 to 40
minutes, then in 0.05 to 0.1% EDTA solution at room temperature
for 16 to 40 minutes, and then in a culture medium with 8 to 10%
fetal calf serum content at room temperature for 30 to 120 minutes.
Further, in case of separating an iris pigmented epithelium
from an eyeball of a ten-day-old mouse, it is particularly preferable
that the iris tissue be allowed to react in 1000 U/mL dispase
solution at 37 C for 16 minutes, then in 0.05% EDTA solution at
room temperature for 20 minutes, and then in a culture medium
with 8 % fetal calf serum content at room temperature for 90
minutes.
Further, in case of separating, an iris pigmented epithelium
from an eyeball of a twelve-day-old mouse, it is particularly
preferable that the iris tissue be allowed to react in 1000 U/mL
dispase solution at 37 C for 20 minutes, then in 0.05% EDTA
solution at room temperature for 25 minutes, and then in a culture
medium with 8 % fetal calf serum content at room temperature for
60 minutes.
Further, in case of separating an iris pigmented epithelium
from an eyeball of a two-month-old mouse, it is particularly
preferable that the iris tissue be allowed to react in 1000 U/mL
dispase solution at 37 C for 30 minutes, then in 0.05% EDTA
solution at room temperature for 40 minutes, and then in a culture
medium with 8 % fetal calf serum content at room temperature for
30 minutes.
In case of separating an iris pigmented epithelium from an
eyeball of a rat, it is preferable that the iris tissue be allowed to
react in 1000 U/mL dispase solution at 37 C for 15 to 40 minutes,
then in 0.05 EDTA solution at room temperature for 15 to 60
minutes, and then in a culture medium with 8 to 10% fetal calf
CA 02528870 2005-12-09
- 17-
serum content at room temperature for 30 to 120 minutes.
In case of separating an iris pigmented epithelium from an
eyeball of a human embryo, it is preferable that the iris tissue be
allowed to react in 500 to 1000 U/mL dispase solution at 25 to
37 C for 15 to 30 minutes, then in 0.05 to 0.1% EDTA solution at
room temperature for 15 to 40 minutes, and then in a culture
medium with 8 to 10% fetal calf serum content at room
temperature for 10 to 60 minutes.
Further, in case of separating an iris pigmented epithelium
from an eyeball of a nineteen-week-old human after birth, it is
particularly preferable that the iris tissue be allowed to react in
1000 U/mL dispase solution at 37`C for 30 minutes, then in 0.05%
EDTA solution at room temperature for 30 minutes, and then in a
culture medium with 8% fetal calf serum content at room
temperature for 60 minutes.
As the culture medium, for example, a DMEM medium
(manufactured by Invitrogen Corporation) with a commercially
available fetal calf serum of an appropriate amount can be used.
The iris-pigmented-epithelium-separating step P2 is not
particularly limited in terms of technique an the like concretely
adopted, as long as the iris pigmented epithelium can be separated
from the iris tissue extirpated in the iris-tissue-extirpating step P1,
the iris tissue including iris stroma and the iris pigmented
epithelium. Generally speaking, a publicly known conventional
technique may be used so as to separate only the iris pigmented
epithelium from the iris tissue.
For example, the iris-pigmented-epithelium-separating step
P2 may be arranged so that the iris stroma and the iris pigmented
epithelium are separated by peeling and collecting the iris
pigmented epithelium from the restored iris tissue by using
commercially available micro forceps.
Thus, according to the present embodiment, the iris
CA 02528870 2005-12-09
- 18-
pigmented epithelial cells are isolated by the iris-tissue-extirpating
step PI and the iris-pigmented-epithelium-separating step P2. This
allows efficient separation of the iris pigmented epithelial cells,
thereby making it possible to efficiently produce the tissue cells.
As described above, the tissue cells according to the present
invention are derived from iris pigmented epithelial cells part of
which can be collected from a patient per se. Therefore, according
to the present embodiment, pluripotent stem cells differentiable into
various tissue cells can be produced from the iris pigmented
epithelial cells of the patient per se. Moreover, by culturing the
pluripotent stem cells under. various differentiation inducing
conditions, various tissue cells can be further produced which can
be utilized as transplant sources in regenerative medical treatment.
Further, use of the tissue cells of the present embodiment as a
transplant cell source can solve problems such as immunological
rejection in cell transplantation, ethical issue, and unbalance
between the demand and supply of transplant source cells.
[Examples]
In the following, the present invention will be described more
specifically with reference to examples and Figs. 2 to 6(b). The
examples are not to limit the present invention.
[Example 1]
(Isolation of Iris Pigmented Epithelial Cells)
Only iris tissue was excised from an eyeball of a chick by
using commercially available micro scissors. The iris tissue was
allowed to react in 1000 U/mL of a dispase solution ("dispase";
manufactured by Godo Seishu Co., Ltd.) for 15 to 40 minutes at
37 C. Thereafter, the iris tissue was allowed to react in 0.05% EDTA
(ethylenediaminetetraacetic acid) solution for 20 to 30 minutes at
room temperature.
After the reaction, the iris tissue was allowed to react for 30
to 60 minutes in a culture medium ("DMEM medium";
CA 02528870 2005-12-09
- 19-
manufactured by Invitrogen Corporation) with 8% fetal calf serum
content, thereby to restore the iris tissue. Thereafter, by peeling and
collecting only iris pigmented epithelium from the iris tissue by
using commercially available micro forceps, the iris pigmented
epithelium was separated from iris matrix.
(Floated Coagulated Mass Culturing Technique)
The separated iris pigmented epithelium was dissociated into
cells by using a commercially available trypsin solution. Thereafter,
the dissociated iris pigmented epithelial cells were selectively
cultured according to the floated coagulated mass culturing
technique (neurosphere method ) described in Science 1992: 225;
1707-1710.
Used as a floated-coagulated-mass-culturing medium was a
serum-free medium ("DMEM/F12 medium"; manufactured by
Invitrogen Corporation) either with the addition of a 1 / 100 volume
of an N2 supplement (manufactured by Invitrogen Corporation) and
20 ng/mL of an FGF2 (fibroblast growth factor-2; manufactured by
PeproTech Inc.), or with the addition of a I/ 100 volume of an N2
supplement (manufactured by Invitrogen Corporation), 1000 U/mL
of an LIF (leukemia inhibitory factor, ESGRO; manufactured by
CHEMICON International, Inc.), and 10 ng/mL of an SCF (human
SCF (stem cell factor); manufactured by DIACLONE).
With rotating by a commercially available shaker, the
trypsin-treated iris pigmented epithelial cells were cultured in the
floated-coagulated-mass-culturing medium in a CO2 incubator for
three to seven days. In this way, stem cells were obtained. The
obtained stem cells are illustrated in Fig. 2(a).
(Formation of an Aggregate by Stem Cell Culturing)
After the iris pigmented epithelial cells insolated from the
eyeball of the chick had been subjected to the floated coagulated
mass culturing, the stem cell culturing was conducted as follows.
The stem cells, derived from the iris pigmented epithelial cells
CA 02528870 2005-12-09
-20-
of the chick and obtained by the floated coagulated mass culturing
technique, were transferred to each of the following medium (b) and
(c), by using a commercially available micropipette.
(b) a DMEM medium (manufactured by Invitrogen Corporation)
containing fetal calf serum (8%) and avian serum (2%).
(c) a DMEM medium (manufactured by Invitrogen Corporation)
containing fetal calf serum (8%) and growth factors EGF and FGF2
(20 ng/mL each).
Moreover, the stem cells were cultured for 1 to 2 months by
respectively using the media (b) and (c). As a result, aggregates
illustrated Figs. 2( b) and 2( c ) were obtained.
The aggregates obtained by culturing the stem cells in the
medium (b) and (c) were labeled using desmin antibody (muscular
cell marker) and DAR staining (nucleus). The results are illustrated
in Figs. 3(a) and 3(b). Fig. 3(a) illustrates aggregates obtained by
culturing the stem cells in the medium of (b) in Fig. 2. Fig. 3(b)
illustrates aggregates obtained by culturing the stem cells in the
medium of (c) in Fig. 2. In Figs. 3(a) and 3(b), the white part
indicates an image of the aggregates labeled with desmin, and the
gray part indicates an image of the aggregates labeled by DAPI
staining (nucleus).
[Example 21
Iris pigmented epithelial cells were dissociated in the same
manner as in Example 1. The iris pigmented epithelial cells were
cultured for three days in a serum-free medium ("DMEM/F12
medium."; manufactured by Invitrogen Corporation), with a 1/100
volume of an N2 supplement (manufactured by Invitrogen
Corporation), and 20ng/mL of FGF2 (fibroblast growth factor-2p;
manufactured by PeproTech Inc.). Thereafter, the iris pigmented
epithelial cells were cultured for one to two months according to the
floated coagulated mass culturing technique in three types of
medium having the following compositions (1) to (3).
CA 02528870 2005-12-09
-21-
(1) Fetal calf serum (8%), EGF (20 ng/mL), and FGF2 (20 ng/mL).
(2) Fetal calf serum (8%) and avian serum (2%).
(3) Fetal calf serum (8%), avian serum (2%), EGF (20 ng/mL), and
FGF2 (20 ng/mL).
RNA was extracted from the obtained aggregates. RT-PCR
technique was used to examine absence and presence of gene
expression of the followings: fetoprotein a, which is an endodermal
marker; myosin and MEF2, which are mesodermal markers; and
pax 6 and tubulin J, which are ectodermal markers. As a result,
under any one of conditions (1) to (3), expression of the marker
genes was observed. This shows that the obtained aggregates
include cells differentiated into all the three types of tridermic
tissue.
The result showed that the aggregates have a similar
property to that of a cell structure called an embryoid body which
is formed mainly from ES cells by differentiation induction and
which contains various differentiated cells like an embryo does.
Although the iris pigmented epithelial cells are ectodermal cells, the
present example showed that it is possible to allow stem cells
derived from iris pigmented epithelial cells of an animal to be
differentiated into mesodermal cells and endodermal cells as well as
ectodermal cells. That is, the present embodiment showed that the
cells obtained by the stem-cell-producing step have tridermic
differentiation potency and can be differentiated into any one of a
mesoderm, an endoderm, and an ectoderm.
Further, Fig. 4 illustrates results of PT-PCR to confirm
expression induction of a gene specific for myocardial cells. RNA
were extracted from the cultured cells obtained at the stage of
culturing the cells on the serum-free media for three days (i.e., the
stem-cell-producing step S2) and from the cultured cells obtained
at the subsequent stage of culturing the cells for one to two months
(i.e., the stem-cell-culturing step S3).
CA 02528870 2005-12-09
-22-
Lane 1 illustrates the result obtained from a sample taken on
a second day of the culturing of the cell culturing stage S7 in the
stem-cell-producing step S2. Further, Lane 2 illustrates a result
obtained from a sample taken after two-month culturing under
condition (3) in the stem-cell-culturing step S3. From the results, it
was found that at the stem-cell-producing step (Lane 1) the
pluripotent stem cells were yet to be differentiated into tissue cells,
and in the stem-cell-culturing step (Lane 2), the pluripotent stem
cells were differentiated into the myocardial cells.
[Example 3]
Oct-3/4 is a molecule which is expressed specifically in
undifferentiated totipotent cells, and quite limited kinds of cells
show the presence of Oct-3/4 (see "system of expression of
Oct-3/4 in early development of mouse" shown in Fig. 5). It is
known that after birth Oct-3/4 is expressed only in
spermatogenous cells which are reproductive stem cells. It is
believed that Oct-3/4 is not expressed in another type of
somatic cell tissue after birth.
The present example examined expression of Oct-3/4 in iris
tissue of a postnatal mouse and a postnatal rat and in parts of stem
cells obtained from their iris tissue. The result is illustrated in Figs.
6(a) and 6(b). Fig. 6(a) illustrates stem cells obtained from an
eleven-day-old rat using the method according to the present
invention and labeled by Oct-3/4 antibody staining and DAPI
staining. The white color indicates the labeled portion. Fig. 6(b)
illustrates stem cells obtained from a three-week-old rat using
the method according to the present invention and labeled by
Oct-3/4 antibody staining and DAPI staining. The white color
indicates the labeled portion. From Figs. 6(a) and 6(b), it can be
understood that an Oct-3/4 gene and a gene product (Oct-3/4
protein) were expressed both in the iris tissue of the postnatal
mouse and the postnatal rat and in the parts of the stem cells
CA 02528870 2005-12-09
-23-
obtained from the iris tissue (i.e. they are all Oct-3/4 positive).
This result indicates that there is a high possibility that iris,
which is somatic cell tissue, also contain cells which still has
undifferentiated titopotency. If it becomes possible to purify and
culture the cells and to induce differentiation of the purified and
cultured cells under proper conditions, it will become possible to
produce various tissue cells therefrom. Research of regenerative
medical treatment involving application of ES cells is actively
carried out, but it presents huge ethical issues. In case of iris tissue,
it is possible to use cells of a patient per se. Therefore, it is expected
that use of iris tissue will lead to realization of regenerative medical
treatment using autotransplantation.
The invention being thus described, it will be obvious that
the same may be varied in many ways. Such variations are not
to be regarded as a departure from the spirit and scope of the
invention, and all such modifications as would be obvious to
one skilled in the art are intended to be included within the
scope of the following claims.
INDUSTRIAL APPLICABILITY
As described above, according to a method of the present
invention for producing tissue cells, pluripotent stem cells
differentiable into various tissue cells are produced from iris
pigmented epithelial cells of an animal, and various tissue cells
can be produced from the pluripotent stem cells. The tissue
cells can be used as a transplant source in regenerative medical
treatment.
Further, tissue cells obtained by the producing method of
the present invention can be provided as a transplant source,
which solves problems such as immunological rejection in cell
transplantation, ethical issues, and unbalance between the
demand and supply of transplant source cells.