Note: Descriptions are shown in the official language in which they were submitted.
CA 02529406 2005-12-14
WO 2004/112784 PCT/SE2004/000996
-1-
NEW USE I
RELATED APPLICATIONS
This application claims priority to Swedish application number 0301882-7,
filed on
June 25, 2003, the contents of which are incorporated herein by reference.
TECHNICAL FIELD
The present invention relates to the use of chemical compounds for wound
healing, said
compounds acting on the human 11-(3-hydroxysteroid dehydrogenase type 1 enzyme
(11 (3HSD1).
BACKGROUND ART
Cortisol performs a broad range of metabolic functions and other functions.
The
multitude of glucocorticoid action is exemplified in patients with prolonged
increase in
plasma glucocorticoids, so called "Cushing's syndrome". Patients with
Cushing's
syndrome have prolonged increase in plasma glucocorticoids and exhibit
impaired
glucose tolerance, type 2 diabetes, central obesity, and osteoporosis. These
patients also
is have impaired wound healing and brittle skin (1).
Glucocorticoids have been shown to increase risk of infection and delay
healing of open
wounds (2). Patients treated with glucocorticoids have 2-5-fold increased risk
of
complications when undergoing surgery (3).
The European patent application No. EP 0902288 discloses a method for
diagnosing the
status of wound healing in a patient, comprising detecting cortisol levels in
said wound.
The authors suggest that elevated levels of cortisol in wound fluid, relative
to normal
plasma levels in healthy individuals, correlates with large, non-healing
wounds (4).
In humans, the 11 (3-HSD catalyzes the conversion of cortisol to cortisone,
and vice
versa. The parallel function of 11(3-HSD in rodents is the interconversion of
CA 02529406 2005-12-14
WO 2004/112784 PCT/SE2004/000996
_2_
corticosterone and 11-dehydrocorticosterone (5). Two isoenzymes of 11 (3-HSD,
11 [3-
HSD 1 and 11 (3-HSD2, have been characterized, and differ from each other in
function
and tissue distribution (6). Like GR, 11 [3-HSD 1 is expressed in numerous
tissues like
liver, adipose tissue, adrenal cortex, gonads, lung, pituitary, brain, eye etc
(7-9). The
function of 11 (3-HSD 1 is to fine-tune local glucocorticoid action. 11 [3-HSD
activity has
been shown in the skin of humans and rodents, in human fibroblasts and in rat
skin
pouch tissue (10-13).
Wound healing consists of serial events including inflammation, fibroblast
proliferation,
secretion of ground substances, collagen production, angiogenesis, wound
contraction
and epithelialization. It can be divided in three phases; inflammatory,
proliferative and
remodeling phase (reviewed in (2)).
In surgical patients, treatment with glucocorticoids increases risk of wound
infection
is and delay healing of open wounds. It has been shown in animal models that
restraint
stress slows down cutaneous wound healing and increases susceptibility to
bacterial
infection during wound healing. These effects were reversed by treatment with
the
glucocorticoid receptor antagonist RU486 (14, 15). Glucocorticoids produce
these
effects by suppressing inflammation, decrease wound strength, inlubit wound
2o contracture and delay epithelialization (2). Glucocorticoids influence
wound healing by
interfering with production or action of cytokines and growth factors like
IGF, TGF-(3,
EGF, KGF and PDGF (16-19). It has also been shown that glucocorticoids
decrease
collagen synthesis in rat and mouse skin in vivo and in rat and human
fibroblasts (20).
2s WO 01/90090 discloses compounds of the formula (I) as defined hereinafter,
which
compounds inhibit the human 11(3-HSD1, and may be useful for treating
disorders such
as diabetes, obesity, glaucoma, osteoporosis, cognitive disorders and immune
disorders.
Other 11(3-HSD1 inhibitors axe disclosed in e.g. WO 01/90091; WO 01/90092; WO
01/90093; WO 01/90094; WO 03/044000; WO 03/044009; WO 03/043999; and
3o Swedish patent application No. SE 0301504-7, filed on May 21, 2003. WO
02/072084
relates to glycyrrhetinic acid derivatives, progesterone and progesterone
derivatives as
11 [3-HSD 1 inhibitors for wound healing. However, the use of the 11 [3-HSD 1
inhibitors
according to the present invention for wound healing has not previously been
disclosed.
CA 02529406 2005-12-14
WO 2004/112784 PCT/SE2004/000996
-3-
DISCLOSURE OF THE INVENTION
It has surprisingly been found that the present inhibitors of 11 (3-HSD 1 are
useful for the
promotion of wound healing. Consequently, in a first aspect this invention
provides a
method for promoting wound healing, said method comprising administering to a
mammal, including man, in need of wound healing an effective amount of an
inhibitor
of 11 (3-hydroxysteroid dehydrogenase type l, wherein the inhibitor of 11 (3-
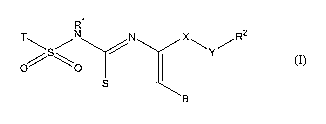
hydroxysteroid dehydrogenase type 1 is a compound of the formula (I):
to
R~
T~S/N /N X~Y/R2
O \O
S
(I)
wherein
is T is an aryl ring or heteroaryl ring or aryl-C2-alkenyl ring, optionally
independently
substituted by [R]n, wherein n is an integer 0-5, and R is hydrogen, aryl,
heteroaryl, a
heterocyclic ring, optionally halogenated C1_6-alkyl, optionally halogenated
Cl_6-alkoxy,
C1_6-alkylsulfonyl, carboxy, cyano, nitro, halogen, amine which is optionally
mono- or
di-substituted, amide which is optionally mono- or di-substituted, aryloxy,
arylsulfonyl,
2o arylamino, wherein aryl, heteroaryl and aryloxy residues and heterocyclic
rings can
further be optionally substituted in one or more positions independently of
each other by
C1_6-acyl, Cl_6-alkylthio, cyano, nitro, hydrogen, halogen, optionally
halogenated C1_6-
alkyl, optionally halogenated C1_6-alkoxy, amide which is optionally mono- or
di-
substituted, (benzoylamino)methyl, carboxy, 2-thienylmethylamino or ({[4-(2-
ethoxy-2-
2s oxoethyl)-1,3-thiazol-2-yl]amino]carbonyl);
Rl is hydrogen or C1_6-alkyl;
X is CHZ or CO;
CA 02529406 2005-12-14
WO 2004/112784 PCT/SE2004/000996
-4-
Y is CH2, CO or a single bond;
B is hydrogen or C1_6-alkyl;
Rz is selected from C1_6-alkyl, azido, arylthio, heteroarylthio, halogen,
hydroxymethyl,
2-hydroxyethylaxninomethyl, methylsulfonyloxymethyl, 3-oxo-4-
morpholinolinylmethylene, C1_6-alkoxycarbonyl, and 5-methyl-1,3,4-oxadiazol-2-
yl;
NR3R4, wherein R3 and R4 are each independently selected from hydrogen, C1_6-
alkyl,
optionally halogenated C1_6-alkylsulfonyl, C1_6-alkoxy, 2-methoxyethyl, 2-
hydroxyethyl,
l0 1-methylimidazolylsulfonyl, C1_6-acyl, cyclohexyhnethyl,
cyclopropanecarbonyl, aryl,
optionally halogenated arylsulfonyl, furylcarbonyl, tetrahydro-2-
furanylmethyl, N-
carbethoxypiperidyl and C1_6-alkyl substituted with one or more aryl or
heteroaryl, or
NR3R4 represent together heterocyclic systems which can be imidazole,
piperidine,
pyrrolidine, piperazine, morpholine, oxazepine, oxazole, thiomorpholine, 1,1-
Is dioxidothiomorpholine, 2-(3,4-dihydro-2(1H)isoquinolinyl), (1S,4S)-2-oxa-5-
azabicyclo[2.2.1]hept-5-yl, which heterocyclic systems can be optionally
substituted by
CI_6-alkyl, Cl_6-acyl, hydroxy, oxo, t-butoxycarbonyl;
OCONR3R4, wherein R3 and R4 are each independently selected from hydrogen,
C1_s-
alkyl or form together morpholinyl;
2o R50, wherein RS is hydrogen, optionally halogenated C1_6-alkyl, aryl,
heteroaryl, C1_6-
aryl, C1_6-alkylsulfonyl, arylcarbonyl, heteroarylcarbonyl, 2-
carbomethoxyphenyl; and
pharmaceutically acceptable salts, solvates, hydrates, geometrical isomers,
tautomers,
optical isomers, N-oxides and prodrug forms thereof.
2s It is preferred that:
T is selected from 5-chloro-1,3-dimethyl-1H-pyrazol-4-yl; 4-chloro-2,3,1-
benzoxadiazolyl; 5-(dimethylamino)-1-naphthyl; 1-methylimidazol-4-yl; 1-
naphthyl; 2-
naphthyl; (E)-2-phenylethenyl; 8-quinolinyl;
3o thienyl substituted with one or more of (benzoylamino)methyl, bromo,
chloro, 3-
isoxazolyl, 2-(methylsulfanyl)-4-pyrimidinyl, 1-methyl-5-
(trifluoromethyl)pyrazol-3-yl,
phenylsulfonyl, pyridyl;
CA 02529406 2005-12-14
WO 2004/112784 PCT/SE2004/000996
-5-
phenyl substituted with one or more of acetylamino, 3-acetylaminophenyl, 3-
acetylphenyl, benzeneamino, 1,3-benzodioxol-5-yl, 2-benzofuryl, benzylamino,
3,5-
bis(trifluoromethyl)phenyl, bromo, butoxy, carboxy, chloro, 4-carboxyphenyl, 3-
chloro-
2-cyanophenoxy, 4-chlorophenyl, 5-chloro-2-thienyl, cyano, 3,4-dichlorophenyl,
( f [4-
(2-ethoxy-2-oxoethyl)-1,3-thiazol-2-yl]amino}carbonyl), fluoro, 5-fluoro-2-
methoxyphenyl, 2-furyl, hydrogen, iodo, isopropyl, methanesulfonyl, methoxy,
methyl,
4-methyl-1-piperazinyl, 4-methyl-1-piperidinyl, 4-methylsulfanylphenyl, 5-
methyl-2-
thienyl, 4-morpholinyl, nitro, 3-nitrophenyl, phenoxy, phenyl, n-propyl, 4-
pyridyl, 3-
pyridylmethylamino, 1-pyrrolidinyl, 2-thienyl, 3-thienyl, 2-
thienylmethylamino,
io trifluoromethoxy, 4-trifluoromethoxyphenyl, and trifluoromethyl;
Rl is hydrogen or methyl;
X is CHZ or CO;
Y is CHZ, CO or a single bond;
B is hydrogen or methyl;
2o RZ is selected from
n-propyl, azido, bromo, chloro, 2-pyridinylsulfanyl, 3-oxo-4-
morpholinolinyhnethylene,
ethoxycarbonyl, 5-methyl-1,3,4-oxadiazol-2-yl;
NR3R4, wherein R3 and R4 are each independently selected from acetyl, 1,3-
benzodioxol-5-ylmethyl, benzyl, 3-chloro-2-methylphenylsulfonyl,
cyclohexylmethyl,
2s ethyl, 2-furylcarbonyl, 2-furylmethyl, hydrogen, 2-hydroxyethyl, 2-(1H-
indol-3-
yl)ethyl, isopropyl, methoxy, 2-methoxyethyl, methyl, 4-(1-
methylimidazolyl)sulfonyl,
methylsulfonyl, phenyl, propyl, trifluoromethylsulfonyl; or
NR3R4 represent together 4-acetylpiperazinyl, 4-t-butoxycarbonylpiperazinyl, 2-
(3,4-
dihydro-2(1H)isoquinolinyl), (2R,6S)-2,6-dimethylmorpholinyl, (2R)-2,4-
dimethyl-1-
so piperazinyl, 2-hydroxy-3-oxomorpholinyl, imidazolyl, 2-methyl-3-
oxomorpholinyl, 4-
methyl-2-oxopiperazinyl, 4-methylpiperazinyl, morpholinyl, (1S,4S)-2-oxa-5-aza-
bicyclo[2.2.1]hept-5-yl, 2-oxoimidazolinyl, 3-oxomorpholinyl, 3-oxo-1,4-
oxazepinyl, 2-
CA 02529406 2005-12-14
WO 2004/112784 PCT/SE2004/000996
-6-
oxooxazolinyl, piperazinyl; piperidinyl; pyrrolidinyl; pyrrolidonyl,
thiomorpholinyl;
1,1-dioxido-thiomorpholinyl;
OCONR3R4, wherein R3 and R4 are each independently selected from ethyl,
hydrogen
or form together morpholinyl;
R50, wherein RS is selected from acetyl, benzoyl, benzyl, ethyl, 2-
fluoroethyl, 2-
furylcarbonyl, hydrogen, isobutyryl, isopropyl, methyl, 2-carbomethoxyphenyl,
methylsulfonyl, phenyl, propionyl, 3-pyridinyl, and 2,2,2-trifluoroethyl.
The following compounds are especially preferred:
io
ethyl (2-{[(2,4-dichloro-5-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-
yl)acetate,
ethyl 2-(2-[[(4-chlorophenyl)sulfonyl]amino]-1,3-thiazole-4-yl)acetate,
ethyl 2-(2- { [ (4-chloro-2, 5-dimethylphenyl) sulfonyl] amino ~ -1,3-thiazol-
4-
yl)acetate,
~s ethyl2-(2-{[(2,4-difluorophenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
ethyl 2-(2-(((4-methylphenyl)sulfonyl)amino)-1,3-thiazol-4-yl)acetate,
ethyl 2-(2-{[(2,5-dichloro-3-thienyl)sulfonyl]amino-1,3-thiazol-4-yl)acetate,
ethyl (2-{[(2-chlorophenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
ethyl 2-(2- { [(3-chloro-2-methylphenyl)sulfonyl] amino }-1,3-thiazol-4-
yl)acetate,
2o ethyl 2-{2-[([ l,1'-biphenyl]-4-ylsulfonyl)amino]-1,3-thiazol-4-yl)
acetate,
ethyl 2-(2- { [ (3 -bromophenyl) sulfonyl] amino ) -1, 3-thiazol-4-yl)
acetate,
ethyl (2-{[(4-nitrophenyl)sulfonyl]amino)-1,3-thiazol-4-yl)acetate,
ethyl (2-{[(4-methoxyphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
ethyl (2-{[(3-nitrophenyl)sulfonyl]amino-1,3-thiazol-4-yl)acetate,
2s ethyl (2-{[(3-methylphenyl)sulfonyl]amino)-1,3-thiazol-4-yl)acetate,
ethyl (2-{[(3-chlorophenyl)sulfonyl]amino)-1,3-thiazol-4-yl)acetate,
ethyl (2-{[(4-fluorophenyl)sulfonyl]amino)-1,3-thia,zol-4-yl)acetate,
ethyl (2-{[(3-fluorophenyl)sulfonyl]amino)-1,3-thiazol-4-yl)acetate,
ethyl {2-[(phenylsulfonyl)amino]-1,3-thiazol-4-yl~ acetate,
3o ethyl (2-{[(4-isopropylphenyl)sulfonyl]amino)-1,3-thiazol-4-yl)acetate,
ethyl [2-({[3-({,[4-(2-ethoxy-2-oxoethyl)-1,3-thiazol-2-
yl] amino} carbonyl)phenyl] sulfonyl~ amino)-1,3-thiazol-4-yl] acetate,
CA 02529406 2005-12-14
WO 2004/112784 PCT/SE2004/000996
ethyl [2-( f [4-( { [4-(2-ethoxy-2-oxoethyl)-1,3-thiazol-2-
yl] amino} carbonyl)phenyl]sulfonyl} amino)-1,3-thiazol-4-yl] acetate,
ethyl (2- f [(2-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
ethyl [2-( f [2-(trifluoromethyl)phenyl]sulfonyl}amino)-1,3-thiazol-4-
yl]acetate,
s ethyl [2-(~[3-(trifluoromethyl)phenyl]sulfonyl}amino)-1,3-thiazol-4-
yl]acetate,
ethyl [2-( f [4-(trifluoromethyl)phenyl]sulfonyl}amino)-1,3-thiazol-4-
yl]acetate,
ethyl 2-(2- f [(4-bromophenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
ethyl (2- f [(2-nitrophenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
ethyl (2-~[(2,4-dichloro-6-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-
yl)acetate,
to ethyl (2- f [(2,4,6-trichlorophenyl)sulfonyl]amino}-1,3-thiazol-4-
yl)acetate,
ethyl (2- f [(2,4-dichlorophenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
ethyl (2-{[(5-fluoro-2-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
ethyl (2-~[(4-propylphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
ethyl (2-{[(2-methoxy-4-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
is ethyl (2- f [(3,5-dichlorophenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
ethyl [2-( f [4-(3-chloro-2-cyanophenoxy)phenyl]sulfonyl}amino)-1,3-thiazol-4-
yl]acetate, ,
ethyl (2-~[(3,4-dichlorophenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
ethyl (2-~[(4-butoxyphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
2o ethyl (2- f [(4-chloro-2-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-
yl)acetate,
ethyl [2-({[4-(acetylamino)phenyl]sulfonyl}amino)-1,3-thiazol-4-yl]acetate,
ethyl ~2-[(8-quinolinylsulfonyl)amino]-1,3-thiazol-4-yl}acetate,
ethyl (2- f [(3,4-dirnethoxyphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
ethyl (2-{[(4-iodophenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
2s ethyl (2-{[(3-chloro-4-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-
yl)acetate,
ethyl [2-( f [5-(dimethylamino)-1-naphthyl]sulfonyl}amino)-1,3-thiazol-4-
yl] acetate,
ethyl (2- f [(1-methyl-1H-imidazol-4-yl)sulfonyl]amino}-1,3-thiazol-4-
yl)acetate,
ethyl (2- f [(5-bromo-2-methoxyphenyl)sulfonyl]amino}-1,3-thiazol-4-
yl)acetate,
ethyl (2-~[(2,5-dimethoxyphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
ethyl ~2-[(2-naphthylsulfonyl)amino]-1,3-thiazol-4-yl}acetate,
ethyl f 2-[(mesitylsulfonyl)amino]-1,3-thiazol-4-yl}acetate,
ethyl (2-~[(3-bromo-5-chloro-2-thienyl)sulfonyl]amino}-1,3-thiazol-4-
yl)acetate,
CA 02529406 2005-12-14
WO 2004/112784 PCT/SE2004/000996
_g_
ethyl {2-[({5-[(benzoylamino)methyl]-2-thienyl}sulfonyl)amino]-1,3-thiazol-4-
yl } acetate,
ethyl {2-[({5-[1-methyl-5-(trifluoromethyl)-1H-pyrazol-3-yl]-2-
thienyl} sulfonyl)amino]-1,3-thiazol-4-yl} acetate,
s ethyl (2-{[(4-cyanophenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
ethyl {2-[({5-[2-(methylsulfanyl)-4-pyrimidinyl]-2-thienyl}sulfonyl)amino]-1,3-
thiazol-4-yl} acetate,
ethyl (2-{[(3-cyanophenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
ethyl (2-{[(2,4,5-trichlorophenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
to ethyl [2-({[(E)-2-phenylethenyl]sulfonyl}amino)-1,3-thiazol-4-yl]acetate,
ethyl (2-{[(2,3,4-trichlorophenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
ethyl (2-{[(4-bromo-2,5-difluorophenyl)sulfonyl]amino}-1,3-thiazol-4-
yl]acetate,
ethyl [2-({[4-(trifluoromethoxy)phenyl]sulfonyl}amino)-1,3-thiazol-4-
yl]acetate,
ethyl (2-{[(2,3-dichlorophenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
is ethyl (2-{[(2-bromophenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
ethyl (2-{[(4,5-dichloro-2-thienyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
ethyl [2-({[4-(phenylsulfonyl)-2-thienyl]sulfonyl}amino)-1,3-thiazol-4-
yl]acetate,
ethyl [2-({[5-(phenylsulfonyl)-2-thienyl]sulfonyl}amino)-1,3-thiazol-4-
yl]acetate,
ethyl (2-{[(2,6-dichlorophenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
2o ethyl (2-{[(2-cyanophenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
ethyl (2-({[4-(acetylamino)-3-chlorophenyl]sulfonyl}amino)-1,3-thiazol-4-
y1] acetate,
ethyl (2-{[(5-chloro-1,3-dimethyl-1H-pyrazol-4-yl)sulfonyl]amino}-1,3-thiazol-
4-
yl)acetate,
2s ethyl (2-{[(3-methoxyphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
ethyl (2-{[(4-bromo-5-chloro-2-thienyl)sulfonyl]amino}-1,3-thiazol-4-
yl)acetate,
ethyl 2-{2-[(1-naphthylsulfonyl)amino]-1,3-thiazol-4-yl} acetate,
ethyl (2-{[(2,5-dichlorophenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
ethyl (2-{[(4-methanesulfonylphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
so ethyl (2-{[(2-methanesulfonylphenyl)sulfonyl]amino}-1,3-thiazol-4-
yl)acetate,
ethyl (2-{[(4-bromo-2-fluorophenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
ethyl (2-{[(2-methyl-4-trifluoromethoxyphenyl)sulfonyl]amino}-1,3-thiazol-4
yl)acetate,
CA 02529406 2005-12-14
WO 2004/112784 PCT/SE2004/000996
-9-
ethyl (2-{[(2,3,4-trifluorophenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
ethyl (2- f [(4-chloro-2,3,1-benzoxadiazolyl)sulfonyl]amino}-1,3-thiazol-4
yl)acetate,
ethyl (2- f [(2,4,6-trifluorophenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
s ethyl (2-~[(S-carboxy-4-chloro-2-fluorophenyl)sulfonyl]amino}-1,3-thiazol-4-
yl)acetate,
ethyl (2- f [(2-(5-chlorothienyl))sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
ethyl (2-~[(2-chloro-4-fluorophenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
ethyl (2-{[(2-(5-(3-isoxazolo)thienyl))sulfonyl]amino}-1,3-thiazol-4-
yl)acetate,
to ethyl (2-~[(4-bromo-2-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-
yl)acetate,
ethyl (2-~[(4-phenoxyphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
ethyl (2- f [(4-chloro-2,6-dimethylphenyl)sulfonyl]amino}-1,3-thiazol-4-
yl)acetate,
ethyl (2-~[(2-methyl-4-trifluoromethoxyphenyl)sulfonyl]amino}-1,3-thiazol-4-
yl)acetate,
is ethyl (2-~[(2,4-bis(trifluoromethyl)phenyl)sulfonyl]amino}-1,3-thiazol-4-
yl)acetate, ~ . .
ethyl 2-{2-[[(3-chloro-2-methylphenyl)sulfonyl](methyl)amino]-1,3-thiazol-4-
yl} acetate,
ethyl oxo(2- f [(4-propylphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
2o ethyl (2-{[(3-chloro-2-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-
yl)(oxo)acetate,
ethyl oxo(2-{[(2,4,6-trichlorophenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
ethyl {2-[([1,1'-biphenyl]-4-ylsulfonyl)amino]-1,3-thiazol-4-yl}(oxo)acetate,
ethyl (2- f [(2,4-dichloro-6-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-
2s yl)(oxo)acetate,
2-(2- f [(4-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetic acid,
2-(2- f [(2,5-dichloro-3-thienyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetic acid,
(2-{[(2-chlorophenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetic acid,
2-(2-{[(3-chloro-2-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetic acid,
so isopropyl 2-(2- f [(3-chloro-2-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-
yl)acetate,
phenyl 2-(2- { [(3-chloro-2-methylphenyl)sulfonyl] amino } -1, 3-thiazol-4-yl)
acetate,
methyl (2- f [(3-chloro-2-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-
yl)acetate,
CA 02529406 2005-12-14
WO 2004/112784 PCT/SE2004/000996
-10-
methyl f 2-[([1,1'-biphenyl]-4-ylsulfonyl)amino]-5-methyl-1,3-thiazol-4-
yl ] acetate,
methyl (2-~[(4-chlorophenyl)sulfonyl]amino}-5-methyl-1,3-thiazol-4-yl)acetate,
methyl (2- f [(3-chloro-2-methylphenyl)sulfonyl]amino]-5-methyl-1,3-thiazol-4
s yl)acetate,
methyl [2-( f [4-(3-chloro-2-cyanophenoxy)phenyl]sulfonyl~amino)-5-methyl-1,3-
thiazol-4-yl]acetate,
methyl (5-methyl-2-{[(4-propylphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)acetate,
methyl (5-methyl-2- f [(2,4,6-trichlorophenyl)sulfonyl]amino}-1,3-thiazol-4-
1 o yl)acetate,
methyl (2- f [(2,4-dichloro-6-methylphenyl)sulfonyl]amino]-5-methyl-1,3-
thiazo1-
4-yl)acetate,
N-(2-methoxyethyl)-2-(2- f [(4-methylphenyl)sulfonyl]amino]-1,3-thiazol-4-
yl)acetamide,
Is 2-(2-~[(2,S-dichloro-3-thienyl)sulfonyl]amino)-1,3-thia,zol-4-yl)-N-
methylacetamide,
N-(1,3-benzodioxol-5-ylmethyl)-2-~2-[(1-naphthylsulfonyl)amino]-1,3-thiazol-4-
yl] acetamide,
N-(2-furylmethyl)-2- f2-[(1-naphthylsulfonyl)amino]-1,3-thiazol-4-
yl}acetamide,
20 2-(2- f [(2,4-difluorophenyl)sulfonyl]amino-1,3-thiazol-4-yl)-N-
ethylacetamide,
N-isopropyl-2- f 2-[(1-naphthylsulfonyl)amino]-1,3-thiazol-4-yl} acetamide,
N-[2-( 1 H-indo l-3-yl) ethyl]-2- ~2-[( 1-naphthylsulfonyl) amino]-1, 3-thi
azol-4-
yl] acetamide,
N-(cyclohexylmethyl)-2- f 2-[(phenylsulfonyl)amino]-1,3-thiazol-4-yl}
acetamide,
2s 2-(2- f [(3-chloro-2-methylphenyl)sulfonyl]amino]-1,3-thiazol-4-yl)-N-
methylacetamide,
2-(2- f [(3-chloro-2-methylphenyl)sulfonyl]amino]-1,3-thiazol-4-yl)-N-
ethylacetamide,
2-(2-{[(3-chloro-2-methylphenyl)sulfonyl]amino]-1,3-thiazol-4-yl)-N-
3o phenylacetamide,
2-(2-~[(4-chlorophenyl)sulfonyl]amino]-1,3-thiazol-4-yl)-N-(2-
furylmethyl)acetamide,
2-(2- f [(3-chloro-2-methylphenyl)sulfonyl]amino]-1,3-thiazol-4-yl)acetamide,
CA 02529406 2005-12-14
WO 2004/112784 PCT/SE2004/000996
-11-
2-(2-{[(3-chloro-2-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)-N,N-
diethylacetamide,
2- f 2-[([ l,1'-biphenyl]-4-ylsulfonyl)amino]-1,3-thiazol-4-yl}-N,N-
diethylacetamide,
s N,N-diethyl-2-(2-{[(4-propylphenyl)sulfonyl]amino}-1,3-thiazol-4-
yl)acetamide,
2-(2- ~ [(2,4-dichloro-6-methylphenyl)sulfonyl] amino } -1, 3-thiazol-4-yl)-
N,N-
diethylacetamide,
N,N-diethyl-2-(2-{[(2,4,6-trichlorophenyl)sulfonyl]amino}-1,3-thiazol-4-
yl)acetamide,
l0 2-f2-[([1,1'-biphenyl]-4-ylsulfonyl)amino]-1,3-thiazol-4-yl}-N,N-
diisopropylacetamide,
N,N-diisopropyl-2-(2- f [(4-propylphenyl)sulfonyl]amino}-1,3-thiazol-4-
yl)acetamide,
2-(2- ~ [(2,4-dichloro-6-methylphenyl) sulfonyl] amino } -1,3-thiazol-4-yl)-
N,N-
1 s diisopropylacetamide,
N,N-diisopropyl-2-(2- { [(2,4,6-trichlorophenyl)sulfonyl] amino}-1,3-thiazol-4-
yl)acetamide,
2-(2- ~ [(3-chloro-2-methylphenyl) sulfonyl] amino } -1, 3-thiazol-4-yl)-N,N-
diisopropylacetamide,
20 2-(2-{[(3-chloro-2-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)-N,N-
dipropylacetamide,
N-benzyl-2-(2-{[(3-chloro-2-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)-N-
ethylacetamide,
2-(2-~[(3-chloro-2-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)-N,N-
2s dimethylacetamide,
3-chloro-N- f 4-[2-(3,4-dihydro-2(1H)-isoquinolinyl)-2-oxoethyl]-1,3-thiazol-2-
yl}-2-methylbenzenesulfonamide,
2-(2- f [(3-chloro-2-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)-N-methyl-N-
phenylacetamide,
so 2-(2-{[(3-chloro-2-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)-N-
isopropyl-
N-methylacetamide,
2- f 2-[([l,1'-biphenyl]-4-ylsulfonyl)amino]-1,3-thiazol-4-yl}-N-isopropyl-N-
methylacetamide,
CA 02529406 2005-12-14
WO 2004/112784 PCT/SE2004/000996
-12-
N-ethyl-N-methyl-2-(2- f [(2,4,6-trichlorophenyl)sulfonyl]amino}-1,3-thiazol-4-
yl)acetamide,
2-(2- f [(2,4-dichloro-6-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)-N-
ethyl-
N-methylacetamide,
s N-ethyl-N-methyl-2-(2-~[(4-propylphenyl)sulfonyl]amino)-1,3-thiazol-4-
yl)acetamide,
2- f 2-[([ 1,1'-biphenyl]-4-ylsulfonyl)amino]-1,3-thiazol-4-yl)-N-ethyl-N-
methylacetamide,
2-(2- f [(3-chloro-2-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)-N-ethyl-N-
t o methylacetamide,
3-chloro-2-methyl-N- f 4-[2-oxo-2-(1-pyrrolidinyl)ethyl]-1,3-thiazol-2-
yl)benzenesulfonamide,
3-chloro-2-methyl-N- {4-[2-oxo-2-( 1-piperidinyl)ethyl]-1,3-thiazol-2-
yl)benzenesulfonamide,
is N-~4-[2-oxo-2-(1-piperidinyl)ethyl]-1,3-thiazol-2-yl)[l,l'-biphenyl]-4-
sulfonamide,
N-~4-[2-oxo-2-(1-piperidinyl)ethyl]-1,3-thiazol-2-yl}-4-
propylbenzenesulfonamide,
2,4-dichloro-6-methyl-N- f 4-[2-oxo-2-(1-piperidinyl)ethyl]-1,3-thiazol-2-
2o yl}benzenesulfonamide,
2,4,6-trichloro-N- f 4-[2-oxo-2-(1-piperidinyl)ethyl]-1,3-thiazol-2-
yl)benzenesulfonamide,
3-chloro-2-methyl-N-{4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-
yl)benzenesulfonamide,
2s 2,4,6-trichloro-N-~4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-
yl)benzenesulfonamide,
2,4-dichloro-6-methyl-N- {4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
N- f 4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-yl} [ 1,1'-biphenyl]-4-
3o sulfonamide,
N-~4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-yl)-4-
propylbenzenesulfonamide,
CA 02529406 2005-12-14
WO 2004/112784 PCT/SE2004/000996
-13-
2,4-dichloro-N- f 4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
4-chloro-2,6-dimethyl-N-~4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-
yl } b enzenesulfonamide,
s N-{4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-yl}-4-
phenoxybenzenesulfonamide,
2-methyl-N-~4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-yl}-4-
(trifluoromethoxy)benzenesulfonamide,
N- f 4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-yl}-2,4-
bis(trifluoromethyl)benzenesulfonamide,
4-bromo-2-methyl-N-{4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
4-(2-furyl)-N- f 4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-
yl} benzenesulfonamide,
is 4-(5-fluoro-2-methoxyphenyl)-N-{4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-
thiazol-
2-yl} benzenesulfonamide,
4-(5-methyl-2-thienyl)-N- f 4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
4-(3-acetylphenyl)-N-~4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-
2o yl}benzenesulfonamide,
4-(4-trifluoromethoxyphenyl)-N-~4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
4-(3,4-dichlorophenyl)-N- ~4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-
yl} benzenesulfonamide,
2s 4-(3,4-methylenedioxyphenyl)-N- f 4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-
thiazol-
2-yl}benzenesulfonamide,
4-(5-chloro-2-thienyl)-N- {4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-
yl } benzenesulfonamide,
4-(4-pyridyl)-N-{4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-
y1 } b enzenesulfonamide,
4-(3-acetylaminophenyl)-N- {4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
CA 02529406 2005-12-14
WO 2004/112784 PCT/SE2004/000996
- 14-
4-(3-thienyl)-N- {4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
4-(2-thienyl)-N- {4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
s 4-(4-carboxyphenyl)-N-{4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
4-(4-methylsulfanylphenyl)-N- {4-[2-(4-morpholinyl)-2-oxo ethyl]-1,3-thi azol-
2-
yl}benzenesulfonamide,
4-(3,5-bis(trifluoromethyl)phenyl)-N-{4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-
thiazol-2-yl}benzenesulfonamide,
4-(4-chlorophenyl)-N-{4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazo1-2-
y1 } b enzenesulfonamide,
4-(3-nitrophenyl)-N-{4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
is 4-(2-benzofuryl)-N-{4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
4-(1-pyrrolidino)-N-{4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
4-(4-methyl-1-piperidino)-N-{4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-
2o y1 } benzenesulfonasnide,
4-(benzeneamino)-N-{4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
4-(benzylamino)-N-{4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
2s 4-(2-thienylmethylamino)-N-{4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
4-(4-morpholinyl)-N- {4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
4-(4-methyl-1-piperazinyl)-N-{4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-
3o yl}benzenesulfonamide,
4-(3-pyridylmethylamino)-N-{4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
CA 02529406 2005-12-14
WO 2004/112784 PCT/SE2004/000996
-15-
2,4-dichloro-6-methyl-N- ~5-methyl-4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-
thiazol-2-yl}benzenesulfonamide,
N- ~5-methyl-4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-yl] [ 1,1'-
biphenyl]-
4-sulfonamide,
s 2,4,6-trichloro-N- f 5-methyl-4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
3-chloro-2-methyl-N-~5-methyl-4-[2-(4-morpholinyl)-2-oxoethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
3-chloro-N-(4- f 2-[(2R,6S)-2,6-dimethylmorpholinyl]-2-oxoethyl]-1,3-thiazol-2-
1 o yl)-2-methylbenzenesulfonamide,
3-chloro-2-methyl-N-(4- f2-[(1S,4S)-2-oxa-5-azabicyclo[2.2.1]kept-5-yl]-2-
oxoethyl}-1,3-thiazol-2-yl)benzenesulfonamide,
3-chloro-2-methyl-N- f 4-[2-oxo-2-(4-thiomorpholinyl)ethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
is N-f4-[2-oxo-2-(4-thiomorpholinyl)ethyl]-1,3-thiazol-2-yl}[1,1'-biphenyl]-4-
sulfonamide,
N-~4-[2-oxo-2-(4-thiomorpholinyl)ethyl]-1,3-thiazol-2-yl]-4-
propylbenzenesulfonamide,
2,4-dichloro-6-methyl-N- f 4-[2-oxo-2-(4-thiomorpholinyl)ethyl]-1,3-thiazol-2-
2o yl]benzenesulfonamide,
2,4,6-trichloro-N-~4-[2-oxo-2-(4-thiomorpholinyl)ethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
N- ~4-[2-( 1,1-dioxido-4-thiomorpholinyl)-2-oxoethyl]-1,3-thiazol-2-yl} -4-
propylbenzenesulfonamide,
2s tert-butyl 4-[(2- f [(3-chloro-2-methylphenyl)sulfonyl]amino]-1,3-thiazol-4-
yl)acetyl]-1-piperazinecarboxylate,
4-acetyl-(4-[(2- f [(3-chloro-2-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-
yl)acetyl])-1-piperazine,
4-[(2-~[(3-chloro-2-methylphenyl)sulfonyl]amino]-1,3-thiazol-4-yl)acetyl]-1-
3o piperazine trifluoroacetate,
3-chloro-2-methyl-N-{4-[2-(4-methyl-1-piperazinyl)-2-oxoethyl]-1,3-thiazol-2-
yl]benzenesulfonamide hydrochloride,
CA 02529406 2005-12-14
WO 2004/112784 PCT/SE2004/000996
-16-
2-methyl-N- f 4-[2-(4-methyl-1-piperazinyl)-2-oxoethyl]-1,3-thiazol-2-yl}-4-
(trifluoromethoxy)benzenesulfonamide,
2,4-dichloro-6-methyl-N- {4-[2-(4-methyl-1-piperazinyl)-2-oxoethyl]-1,3-
thiazol-
2-yl}benzenesulfonamide,
s 2,4-dichloro-N-~4-[2-(4-methyl-1-piperazinyl)-2-oxoethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
3-chloro-N-(4- f 2-[(2R)-2,4-dimethylpiperazinyl]-2-oxoethyl}-1,3-thiazol-2-
yl)-2-
methylbenzenesulfonamide,
2-(2- ~ [(3-chloro-2-methylphenyl)sulfonyl] amino}-1,3-thiazol-4-yl)-N-methoxy-
lo N-methylacetamide,
3-chloro-2-methyl-N-[4-(2-oxopentyl)-1,3-thiazol-2-yl]benzenesulfonamide,
4-chloro-N-[4-(2-hydroxyethyl)-1,3-thiazol-2-yl]benzenesulfonamide,
3-chloro-N-[4-(2-hydroxyethyl)-1,3-thiazol-2-yl]-2-methylbenzenesulfonamide,
3-chloro-N-[4-(2-ethoxyethyl)-1,3-thiazol-2-yl]-2-methylbenzenesulfonamide,
Is 3-chloro-N-[4-(2-isopropoxyethyl)-1,3-thiazol-2-yl]-2-
methylbenzenesulfonamide,
N- ~4-[2-(benzyloxy)ethyl]-1,3-thiazol-2-yl } -3-chloro-2-
methylbenzenesulfonamide,
3-chloro-N-[4-(2-methoxyethyl)-1,3-thiazol-2-yl]-2-riiethylbenzenesulfonamide,
ao 3-chloro-N-{4-[2-(2-fluoroethoxy)ethyl]-1,3-thiazol-2-yl}-2-
methylbenzenesulfonamide,
3-chloro-2-methyl-N- f 4-[2-(2,2,2-trifluoroethoxy)ethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
3-chloro-2-methyl-N- f 4-[2-(2-pyridinylsulfanyl)ethyl]-1,3-thiazol-2-
2s yl}benzenesulfonamide,
3-chloro-2-methyl-N- f 4-[2-(3-pyridinyloxy)ethyl]-1,3-thiazol-2-
yl } benzenesulfonamide,
3-chloro-2-methyl-N- f 4-[2-methylsalicylethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
2-(2- ~ [(3-chloro-2-methylphenyl)sulfonyl] amino } -1,3-thiazol-4-yl)ethyl
methanesulfonate,
2-(2- f [(3-chloro-2-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)ethyl
acetate,
CA 02529406 2005-12-14
WO 2004/112784 PCT/SE2004/000996
-17-
2-(2- f [(3-chloro-2-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)ethyl
propionate,
2-(2-~[(3-chloro-2-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)ethyl 2-
methylpropanoate,
s 2-(2-{[(3-chloro-2-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)ethyl2-
furoate,
2-(2- f [(3-chloro-2-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)ethyl
benzoate,
2-(2- f [(3-chloro-2-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)ethyl 4-
1 o morpholinecarboxylate,
2-(2-{[(3-chloro-2-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)ethyl
diethylcarbamate,
2-(2- f [(3-chloro-2-methylphenyl)sulfonyl]amino}-1,3-thiazol-4-yl)ethyl
ethylcarbamate,
is N-[4-(2-azidoethyl)-1,3-thiazol-2-yl]-3-chloro-2-methylbenzenesulfonamide,
N-[4-(2-aminoethyl)-1,3-thiazol-2-yl]-3-chloro-2-methylbenzenesulfonamide
hydrochloride,
4-chloro-N-~4-[2-(diethylamino)ethyl]-1,3-thiazol-2-yl}benzenesulfonamide
hydrochloride,
20 3-chloro-N-{4-[2-(diethylamino)ethyl]-1,3-thiazol-2-yl}-2-
methylbenzenesulfonamide hydrochloride,
3-chloro-N-{4-[2-(1-imidazolyl)ethyl]-1,3-thiazol-2-yl}-2-
methylbenzenesulfonamide hydrochloride,
3-chloro-2-methyl-N-~4-[2-(4-methyl-1-piperazinyl)ethyl]-1,3-thiazol-2-
2s yl}benzenesulfonamide dihydrochloride,
3-chloro-2-methyl-N-~4-[2-(4-morpholinyl)ethyl]-1,3-thiazol-2-
yl}benzenesulfonamide hydrochloride,
3-chloro-2-methyl-N-{4-[(4-morpholinyl)methyl]-1,3-thiazol-2-
yl}benzenesulfonamide hydrochloride,
30 2,4,6-trichloro-N-{4-[2-(4-morpholinyl)ethyl]-1,3-thiazol-2-
yl}benzenesulfonamide hydrochloride,
2,4-dichloro-N-~4-[2-(4-morpholinyl)ethyl]-1,3-thiazol-2-yl}benzenesulfonamide
hydrochloride,
CA 02529406 2005-12-14
WO 2004/112784 PCT/SE2004/000996
-18-
2,4-dichloro-6-methyl-N-{4-[2-(4-morpholinyl)ethyl]-1,3-thiazol-2
yl}benzenesulfonamide hydrochloride,
N- {4-[2-(4- morpholinyl)ethyl]-1,3-thiazol-2-yl~ -4-propylbenzenesulfonamide
hydrochloride,
s 3-chloro-N-{4-[2-(ethylamino)ethyl]-1,3-thiazol-2-yl}-2-
methylbenzenesulfonamide,
3-chloro-N-(4-{2-[(2-hydroxyethyl)amino]ethyl-1,3-thiazol-2-yl)-2-
methylbenzenesulfonamide,
N-[2-(2- { [(3-chloro-2-methylphenyl)sulfonyl] amino } -1,3-thiazo l-4-yl)
ethyl]-N-
i o ethylacetamide,
3-chloro-2-methyl-N-{4-[2-(3-oxo-4-morpholinyl)ethyl]-1,3-thiazol-2-
yl~benzenesulfonarnide,
3-chloro-N-{4-[2-(2-hydroxy-3-oxo-4-morpholinyl)ethyl]-1,3-thiazol-2-yl~-2-
methylbenzenesulfonamide,
2,4-dichloro-N- {4-[2-(3-oxo-4-morpholinyl)ethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
~,4-dichloro-N- {4-[2-(3-oxo-4-morpholinyl)ethyl]-1,3-thiazol-2-yl] -6-
methylbenzenesulfonamide,
N- {4-[2-(3-oxo-4-morpholinyl)ethyl]-1,3-thiazol-2-yl) -2,4~6-
2o trichlorobenzenesulfonamide,
4,5-dichloro-N-{4-[2-(3-oxo-4-morpholinyl)ethyl]-1,3-thiazol-2-yl]-2-
thienylsulfonamide,
N-{4-[2-(3-oxo-4-morpholinyl)ethyl]-1,3-thiazol-2-yl,~-4-
phenoxybenzenesulfonamide,
2s 3-fluoro-N-{4-[2-(3-oxo-4-morpholinyl)ethyl]-1,3-thiazol-2-
y1 } b enzenesulfonamide,
N-{4-[2-(3-oxo-4-morpholinyl)ethyl]-1,3-thiazol-2-yl~-5-(2-pyridyl)-2-
thienylsulfonamide,
4-acetylamino-3-chloro-N-{4-[2-(3-oxo-4-morpholinyl)ethyl]-1,3-thiazol-2-
s o y1 ~ benzenesulfonamide,
N-{4-[(3-oxo-4-morpholinyl)methyl]-1,3-thiazol-2-yl}-3-chloro-2-
methylbenzenesulfonamide,
CA 02529406 2005-12-14
WO 2004/112784 PCT/SE2004/000996
-19-
N-{4-[3-(3-oxo-4-morpholinyl)propyl]-1,3-thiazol-2-yl}-3-chloro-2-
methylbenzenesulfonamide,
N-{4-[2-(3-oxo-4-morpholinyl)ethyl]-1,3-thiazol-2-yl},N-methyl-3-chloro-2-
methylbenzenesulfonamide,
s 3-chloro-2-methyl-N-{4-[2-(2-methyl-3-oxo-4-morpholinyl)ethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
N-{4-[2-(acetylamino)ethyl]-1,3-thiazol-2-yl}-3-chloro-2-methyl-
benzenesulfonamide,
3-chloro-2-methyl-N- {4-[2-(4-(3-oxo-1,4-oxazepinyl))ethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
3-chloro-2-methyl-N-{4-[2-(1-pyrrolidonyl)ethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
3-chloro-2-methyl-N-{4-[2-(1-(2-oxoimidazolinyl))ethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
is 3-chloro-2-methyl-N-{4-[2-(1-(2-oxooxazolinyl)ethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
3-chloro-N-{4-[2-(N-(2-furylcarbonyl),N-2-hydroxyethylamino)ethyl]-1,3-
thiazol-2-yl}-2-methylbenzenesulfonamide, hydrochloride,
3-~chloro-2-methyl-N-{4-[2-(4-methyl-2-oxopiperazinyl)ethyl]-1,3-thiazol-2-
2o yl}benzenesulfonamide,
3-chloro-2-methyl-N-{4-[2-(methylsulfonylamino)ethyl]-1,3-thiazol-2
yl}benzenesulfonamide,
3-chloro-2-methyl-N-{4-[2-(N-methyl,N-methylsulfonamino)ethyl]-1,3-thiazol-2-
yl}benzenesulfonamide,
2s 3-chloro-2-methyl-N-{4-[2-(trifluoromethylsulfonylamino)ethyl]-1,3-thiazol-
2-
yl}benzenesulfonamide,
3-chloro-2-methyl-N-{4-[2-(N-methyl,N-trifluoromethylsulfonamino)ethyl]-1,3-
thiazol-2-yl} benzenesulfonamide,
3-chloro-2-methyl-N-{4-[2-(4-(1-methylimidazolyl)sulfonylamino)ethyl]-1,3-
3o thiazol-2-yl}benzenesulfonamide,
3-chloro-N- {4-[2-(N-(3-chloro-2-methylphenylsulfonyl),N-methylamino)ethyl]-
1,3-thiazol-2-yl}-2-methylbenzenesulfonamide,
N-[4-(2-bromoethyl)-1,3-thiazol-2-yl]-3-chloro-2-methylbenzenesulfonamide,
CA 02529406 2005-12-14
WO 2004/112784 PCT/SE2004/000996
-20-
3-chloro-N-[4-(2-chloroethyl)-1,3-thiazol-2-yl]-2-methylbenzenesulfonamide,
3-chloro-2-methyl-N-{4-[(5-methyl-1,3,4-oxadiazol-2-yl)methyl]-1,3-thiazol-2-
yl~benzenesulfonamide,
3-chloro-N-{4-[2-(ethoxycarbonyl)ethyl]-1,3-thiazol-2-yl}-2-
methylbenzenesulfonamide.
In one aspect of the invention, the said method is a method for the treatment
or
prophylaxis of a medical condition involving delayed or impaired wound
healing.
Examples of such medical conditions are diabetes, and conditions caused by
treatment
io with steroids, in particular glucocorticoids. The method according to the
invention is
also intended for the promotion of wound healing in chronic wounds, such as
diabetic
ulcers, venous ulcers or pressure ulcers.
The compounds referred to above may also be used in the manufacture of a
medicament
Is for promoting wound healing, e.g. for the treatment or prophylaxis of a
medical
condition involving delayed or impaired wound healing. Examples of such
medical
conditions are diabetes, and conditions caused by treatment with steroids, in
particular
glucocorticoids. The compounds referred to above may also be used for the
promotion
of wound healing in chronic wounds, such as diabetic ulcers, venous ulcers or
pressure
2o ulcers.
The various terms used, separately and in combinations, in the above
definition of the
compounds having the general formula (I) will be explained.
2s The term "aryl" in the present description is intended to include aromatic
rings
(monocyclic or bicyclic) having from 6 to 10 ring carbon atoms, such as phenyl
(Ph)
and naphthyl, which optionally may be substituted by C1_6-alkyl. Examples of
substituted aryl groups are benzyl and 2-methylphenyl.
3o The term "heteroaryl" means in the present description a monocyclic, bi- or
tricyclic
aromatic ring system (only one ring need to be aromatic) having from 5 to 14,
preferably 5 to 10 ring atoms such as 5, 6, 7, ~, 9 or 10 ring atoms (mono- or
bicyclic),
in which one or more of the ring atoms are other than carbon, such as
nitrogen, sulfur,
CA 02529406 2005-12-14
WO 2004/112784 PCT/SE2004/000996
-21 -
oxygen and selenium. Examples of such heteroaryl rings are pyrrole, imidazole,
thiophene, furan, thiazole, isothiazole, thiadiazole, oxazole, isoxazole,
oxadiazole,
pyridine, pyrazine, pyrimidine, pyridazine, pyrazole, triazole, tetrazole,
chroman,
isochroman, quinoline, quinoxaline, isoquinoline, phthalazine, cinnoline,
quinazoline,
s indole, isoindole, indoline, isoindoline, benzothiophene, benzofuran,
isobenzofuran,
benzoxazole, 2,1,3-benzoxadiazole, benzothiazole, 2,1,3-benzothiazole, 2,1,3-
benzoselenadiazole, benzimidazole, indazole, benzodioxane, indane, 1,2,3,4-
tetrahydroquinoline, 3,4-dihydro-2H 1,4-benzoxazine, 1,5-naphthyridine, 1,8-
naphthyridine, acridine, fenazine and xanthene. Examples of monocyclic
heteroaryl
1 o rings are pyrrole, imidazole, thiophene, furan, thiazole, isothiazole,
thiadiazole, oxazole,
isoxazole, oxadiazole, pyridine, pyrazine, pyrimidine, pyridazine, pyrazole,
triazole, and
tetrazole.
The term "heterocyclic" in the present description is intended to include
unsaturated as
1 s well as partially and fully saturated mono-, bi- and tricyclic rings
having from 4 to 14,
preferably 4 to 10 ring atoms, such as, for example, the heteroaryl groups
mentioned
above as well as the corresponding partially saturated or fully saturated
heterocyclic
rings. Exemplary saturated heterocyclic rings are azetidine, pyrrolidine~
piperidine,
piperazine, morpholine, thiomorpholine and 1,4-oxazepane.
C 1 _6-alkyl in the compound of formula (I) according to the present
application, which
may be straight or branched, is preferably C1_4-alkyl. Exemplary alkyl groups
include
methyl, ethyl, propyl, isopropyl, butyl, sec-butyl, tert-butyl, pentyl,
isopentyl, hexyl,
and isohexyl. For parts of the range "C1_6-alkyl" all subgroups thereof are
contemplated
2s such as C1_s-alkyl, C1_4-alkyl, C2_6-alkyl, CZ_s-alkyl, CZ_4-alkyl, Ca_3-
alkyl, Cs_s-alkyl, C4_
s-allcyl, etc.
C1-6-alkoxy, in the compound of formula (I) according to the present
application may
be straight or branched, is preferably C1_4-alkoxy. Exemplary alkoxy groups
include
3o methoxy, ethoxy, propoxy, isopropoxy, butoxy, sec-butoxy, tert-butoxy,
pentyloxy,
isopentyloxy, hexyloxy, and isohexyloxy. For parts of the range "C1_6-alkoxy"
all
subgroups thereof are contemplated such as C1_s-alkoxy, C1_4-alkoxy, CZ_6-
alkoxy, CZ_s-
alkoxy, C2_4-alkoxy, C2_3-alkoxy, C3_6-alkoxy, C4_s-alkoxy, etc.
CA 02529406 2005-12-14
WO 2004/112784 PCT/SE2004/000996
-22-
C 1 _6-acyl, in the compound of formula (I) according to the present
application may be
saturated or unsaturated and is preferably C1_q.-acyl. Exemplary acyl groups
include
formyl, acetyl, propionyl, butyryl, isobutyryl, valeryl, isovaleryl, butenoyl
(e.g. 3-
butenoyl), hexenoyl (e.g. 5-hexenoyl). For parts of the range "C1_6-acyl" all
subgroups
thereof are contemplated such as C1_5-acyl, C1_4-acyl, C2_~-acyl, C2_5-acyl,
C2_4-acyl, C2_
3-acyl, C3_~-acyl, C4_5-acyl, etc.
C2_~-alkenyl in the compound of formula (I) according to the present
application, which
Io ~ may be straight, branched or cyclic, is preferably C2_4-alkenyl.
Exemplary alkenyl
groups include vinyl, 1-propenyl, 2-propenyl, isopropenyl, 1-butenyl, 2-
butenyl, 1-
pentenyl, 2-pentenyl, 1-hexenyl, and 2-hexenyl. For parts of the range "CZ_6-
alkenyl" all
subgroups thereof are contemplated such as CZ_5-alkenyl, C2_4-alkenyl, C2_3-
alkenyl, C3_
6-alkenyl, C4_5-alkenyl, etc.
is
The term "halogen" in the present description is intended to include fluorine,
chlorine,
bromine and iodine.
The term "sulfanyl" in the present description means a thio group.
With the expression mono- or di-substituted is meant in the present
description that the
functionalities in question may be substituted with independently H, C1_6-
acyl, Cl_s-
alkenyl, C1_6-(cyclo)alkyl, aryl, pyridylmethyl, or heterocyclic rings e.g.
azetidine,
pyrrolidine, piperidine, piperazine, morpholine and thiomorpholine, which
heterocyclic
2s rings optionally may be substituted with C1_6-alkyl. With the expression
"optionally
mono- or disubstituted" is meant in the present description that the
functionalities in
question may also be substituted with independently hydrogen.
Combinations of substituents and variables envisioned by this invention are
only those
3o that result in the formation of stable compounds. The term "stable", as
used herein,
referes to compounds which possess stability sufficient to allow manufacture
and which
maintains the integrity of the compound for a sufficient period of time to be
useful for
CA 02529406 2005-12-14
WO 2004/112784 PCT/SE2004/000996
- 23 -
the purposes detailed herein (e.g., therapeutic administration to a subject
for the
treatment of disease, 11-(3-HSD1 inhibition, 11-(3-HSD1-mediated disease).
The term "prodrug forms" in the present description means a pharmacologically
acceptable derivative, such as an ester or an amide, which derivative is
biotransformed
in the body to form the active drug (see Goodman and Gilman's, The
Pharmacological
basis of Therapeutics, 8th ed., McGraw-Hill, Int. Ed. 1992, "Biotransformation
of Drugs,
p. 13-15).
to "Pharmaceutically acceptable" means in the present description being useful
in
preparing a pharmaceutical composition that is generally safe, non-toxic and
neither
biologically nor otherwise undesirable and includes being useful for
veterinary use as
well as human pharmaceutical use.
is "Pharmaceutically acceptable salts" mean in the present description salts
which are
pharmaceutically acceptable, as defined above, and which possess the desired
pharmacological activity. Such salts include acid addition salts formed with
organic and
inorganic acids, such as hydrogen chloride, hydrogen bromide, hydrogen iodide,
sulfuric acid, phosphoric acid, acetic acid, glycolic acid, malefic acid,
malonic acid,
20 oxalic acid, methanesulfonic acid, trifluoroacetic acid, fumaric acid,
succinic acid,
tartaric acid, citric acid, benzoic acid, ascorbic acid and the like. Base
addition salts may
be formed with organic and inorganic bases, such as sodium, ammonia,
potassium,
calcium, ethanolamine, diethanolamine, N-methylglucamine, choline and the
like.
2s The compounds of formula (I) can be prepared according to the methods
described in
WO 01/90090.
For use according to the invention, the compounds of formula (I) can
preferably be
topically administered. However, the compounds could also be administered by
other
3o routes, for instance orally, intraperitoneally, intraarticularly,
intracranially,
intradermally, intramuscularly, intraocularly, intrathecally, intravenously,
subcutaneously.
CA 02529406 2005-12-14
WO 2004/112784 PCT/SE2004/000996
-24-
EXAMPLES
EXAMPLE 1
Diabetic KKAy mice underwent surgery during anesthesia whereby a catheter was
inserted in the jugularis vein. Oral treatment twice daily (200 mg/kg/day)
with the 11 (3-
HSD1 inhibitor BVT.2733 (3-Chloro-2-methyl-N- f 4-[2-oxo-2-(1-
piperazinyl)ethyl]-
1,3-thiazol-2-yl}benzenesulfonamide; the trifluoroacetate of this compound is
disclosed
to as Example 172A in WO 01/90090), or vehicle started 4-6 days later and
continued for
3.5 days.
Advantageous effects on wound healing of the surgical wounds were observed
during
treatment. hi BVT.2733 treated mice, less complication were observed in and
around
is the wound area as compared to control mice. Examples of advantageous
effects were
less pus in the wound, as well as better wound strength. 5 ~ % of the vehicle
treated
animals showed complications during treatment period whereas complications
were
present in only 24 % of the BVT.2733 treated animals.
EXAMPLE 2
(a)
Advantageous effects of 11(3-HSD1 inhibitors (e.g. BVT.2733) on wound healing
are
2s confirmed in diabetic KKAY mice employing the excisional wound-healing
model. 1 cm
full-thickness wounds, including the panniculus carnosus muscle, are cut with
a scalpel
on the back of the mice. Mice are treated with BVT.2733 for 5 days. On day 2
and 9 of
treatment wounds are harvested, embedded and sectioned. Histological staining
of the
sections with hematoxylin/eosin are made to determine degree of re-
epithelialization
so and immunostaining against the von Willebrand factor to determine
revascularisation.
CA 02529406 2005-12-14
WO 2004/112784 PCT/SE2004/000996
-25-
(b)
Advantageous effects of 11 (3-HSD 1 inhibitors are confirmed in in vitro
studies.
Proliferation of human keratinocytes and fibroblasts, which are important cell
types in
the wound healing process, are studied after incubation with the 11 [3-HSD 1
inhibitor.
s
(c)
Effects on wound healing after treatment with 11 (3-HSD1 inhibitors are also
studied in
wounds on explants from human breast skin. The proliferative effect of the
substance
and the effect on re-epithelialization are determined.
to
REFERENCES
1. Ganong WF. Review of Medical Physiology. Eighteenth edition ed. Stamford,
Connecticut: Appleton & Lange; 1997.
2. Anstead GM. Steroids, retinoids, and wound healing. Adv Wound Care
1998;11 (6):277-85.
3. Diethelm AG. Surgical management of complications of steroid therapy. Ann
Surg 1977;185(3):251-63.
4. Hutchinson TC, Swaniker HP. Wound diagnosis by quantitating cortisol in
wound fluids. European patent application No. EP 0 902 288, published
17.03.1999.
5. Frey FJ, Escher G, Frey BM. Pharmacology of 11 beta-hydroxysteroid
dehydrogenase. Steroids 1994;59(2):74-9.
6. Albiston AL, Obeyesekere VR, Smith RE, Krozowski ZS. Cloning and tissue
distribution of the human 11 beta-hydroxysteroid dehydrogenase type 2 enzyme.
Mol
Cell Endocrinol 1994;105(2):R11-7.
7. Monder C, White PC. 11 beta-hydroxysteroid dehydrogenase. Vitam Horm .
1993;47:187-271.
8. Stewart PM, Krozowski ZS. 11 beta-Hydroxysteroid dehydrogenase. Vitam
Horm 1999;57:249-324.
9. Stokes J, Noble J, Brett L, Phillips C, Seckl JR, O'Brien C, et al.
Distribution of
glucocorticoid and mineralocorticoid receptors and llbeta-hydroxysteroid
CA 02529406 2005-12-14
WO 2004/112784 PCT/SE2004/000996
-26-
dehydrogenases in human and rat ocular tissues. Invest Ophthalmol Vis Sci
2000;41 (7):1629-38.
10. Hammami MM, Siiteri PK. Regulation of 11 beta-hydroxysteroid
dehydrogenase activity in human skin fibroblasts: enzymatic modulation of
glucocorticoid action. J Clin Endocrinol Metab 1991;73(2):326-34.
11. Cooper MS, Moore J, Filer A, Buckley CD, Hewison M, Stewart PM. 1 lbeta-
hydroxysteroid dehydrogenase in human fibroblasts: expression and regulation
depends
on tissue of origin. ENDO 2003 Abstracts 2003.
12. Teelucksingh S, Mackie AD, Burt D, Mclntyre MA, Brett L, Edwards CR.
Potentiation of hydrocortisone activity in skin by glycyrrhetinic acid. Lancet
1990;335(8697):1060-3.
13. Slight SH, Chilakamarri VK, Nasr S, Dhalla AK, Ramires FJ, Sun Y, et al.
Inhibition of tissue repair by spironolactone: role of mineralocorticoids in
fibrous tissue
formation. Mol Cell Biochem 1998;189(1-2):47-54.
14. Mercado AM, Quan N, Padgett DA, Sheridan JF, Marucha PT. Restraint stress
alters the expression of interleukin-1 and keratinocyte growth factor at the
wound site:
an in situ hybridization study. J Neuroimmunol 2002;129(1-2):74-83. .
15. Rojas IG, Padgett DA, Sheridan JF, Marucha PT. Stress-induced
susceptibility
to bacterial infection during cutaneous wound healing. Brain Behav Immun
2002;16(1):74-84.
16. Beer HD, Fassler R, Werner S. Glucocorticoid-regulated gene expression
during
cutaneous wound repair. Vitam Horm 2000;59:217-39.
17. Hamon GA, Hunt TK, Spencer EM. In vivo effects of systemic insulin-like
growth factor-I alone and complexed with insulin-like growth factor binding
protein-3
on corticosteroid suppressed wounds. Growth Regul 1993;3(1):53-6.
18. Laato M, Heino J, Kahari VM, Niinikoski J, Gerdin B. Epidermal growth
factor
(EGF) prevents methylprednisolone-induced inhibition of wound healing. J Surg
Res
1989;47(4):354-9.
19. Pierce GF, Mustoe TA, Lingelbach J, Masakowski VR, Gramates P, Deuel TF.
Transforming growth factor beta reverses the glucocorticoid-induced wound-
healing
deficit in rats: possible regulation in macrophages by platelet-derived growth
factor.
Proc Natl Acad Sci U S A 1989;86(7):2229-33.
CA 02529406 2005-12-14
WO 2004/112784 PCT/SE2004/000996
_27_
20. Oishi Y, Fu ZW, Ohnuki Y, Kato H, Noguchi T. Molecular basis of the
alteration in skin collagen metabolism in response to in vivo dexamethasone
treatment:
effects on the synthesis of collagen type I and III, collagenase, and tissue
inhibitors of
metalloproteinases. Br J Dermatol 2002;147(5):859-68.