Note: Descriptions are shown in the official language in which they were submitted.
CA 02529516 2010-03-15
METHOD AND APPARATUS FOR SUPPLYING GAS TO AN AREA
FIELD OF THE INVENTION
The present invention relates generally to supplying a gas to an area.
BACKGROUND OF THE INVENTION
Throughout this application various publications are referenced -by arabic
numerals
within parentheses. Full citations for these publications may be found at the
end, of the
specification immediately preceding the claims. The disclosures of these
publications in their
entireties are hereby incorporated by reference into this application in order
to more fully
describe the state of the art to which this invention pertains.
The healing of wounds and the effect of oxygen tension has been intensively
studied
(1). Among the components important in the healing process are fibroblast
proliferation,
angiogenesis, collagen synthesis, and reepithelialization.
Soon after injury,=whether accidental or surgically induced, undifferentiated
mesenchymal cells transform to migratory fibroblasts, which migrate into and
across the
injured wound. It is known that fibroblasts are aerobic in nature. Fibroblasts
are stimulated
to produce collagen. While experiments from cultured fibroblasts suggest that
high lactate
and ascorbic acid concentration typical of hypoxic conditions may activate
some of the
fibroblast collagen-synthesizing enzymes, animal studies involving low,
normal, and high
oxygen tensions nevertheless demonstrate increased rates of collagen synthesis
under
hyperoxic rather than hypoxic conditions.
Angiogenesis, on the other hand, appears to be stimulated by a hypoxic tissue
gradient, with new capillaries extending in the direction of lower oxygen
concentration.
When a hypoxic gradient no longer exists, angiogenesis is minimized or static.
Epithelialization is also known to be related to oxygen tension, with higher
rates of epithelial
proliferation observed under hyperoxic as opposed to hypoxic conditions.
1
CA 02529516 2005-12-15
WO 2004/112649 PCT/US2004/019599
The supply of oxygen to healing wound tissue may be derived from three
sources:
oxygen chemically bound to hemoglobin in whole blood; oxygen dissolved in
plasma; and
oxygen which diffuses into plasma or tissue from the exterior. In deep wounds,
the latter is
of little importance. The studies of R. P. Gruber et al., for example,
indicate that oxygen
tension, measured polarographically, increases markedly at 3 bar of 100% 02 in
the
superficial dermis (0.30-0.34 mm), while the relative oxygen concentration of
the deep
dermis (1.8-2.2 mm) is unchanged under the same conditions (2).
In surface wounds, all sources of oxygen are important. In wounds of large
surface
area, however, for example ulcers, only the tissue at the edges of the ulcer
or at its base are
well supplied with blood, and the growing granulation tissue, in the absence
of oxygen
diffusing from the exterior, must be supplied by diffusion from blood vessels
and plasma, a
relatively inefficient process.
It is well established, also, that occlusive coverings that maintain a moist
environment
promote wound healing (3). Furthermore, it is well known that the changing of
wound
dressings may interfere with the healing process by disrupting the healing
tissue where
granulation and collagen synthesis has not imparted sufficient tensile
strength to avoid
rupture upon dressing removal. However, due to the inability of the blood and
plasma to
supply optimal oxygen concentration, and due to the further reduction in
oxygen from the
exterior brought about by the presence of the occluding dressing, a hypoxic
condition may
rapidly be reached. Although this condition may encourage angiogenesis, it
negatively
affects collagen synthesis and epithelialization. Moreover, various
clostridium species, e.g.,
C. perfringens and C. septicum, are induced to germinate under hypoxic
conditions, which
can also support other anaerobic flora (4). In addition to minimizing
anaerobic flora by
discouraging germination, hyperoxic conditions are known to reduce the
concentration of
other pathogens as well.
Past treatment of chronic ulcers and gangrenous tissue has, in many cases,
involved
extensive debridement in combination with antibiotics and systemic hyperbaric
oxygen.
Room size hyperbaric oxygen chambers or chambers sized for the individual
patient have
employed pure oxygen at pressures of 2 to 3 bar. Treatment time is limited, as
oxygen
toxicity and central nervous system (CNS) disorders may result from the
increased oxygen
content of the blood. Such treatments have met with a great deal of success,
but the success
may not be due to the increased systemic blood and plasma-derived oxygen
supply. The
blood and plasma already contain sufficient oxygen for the healing process.
Rather, it is the
diffusion-limited access of oxygen to the wound that limits the oxygen supply
required for
2
CA 02529516 2005-12-15
WO 2004/112649 PCT/US2004/019599
optimal healing and minimization of infection. The increased oxygen tension in
the wound
most likely results directly from increased diffusion into the wound surface
from the oxygen
in the chamber. Gruber, for example, indicates that rate of oxygen absorption
from the skin
is roughly proportional to oxygen concentration from nearly 0% to 30% (2).
Gruber further
indicates, however, that oxygen absorption tends to level off at higher oxygen
concentrations.
Due to the expense of large hyperbaric chambers and the systemic effects of
oxygen
toxicity that they may engender, topical hyperbaric chambers have been
proposed. Topical
chambers operating at "normal" hyperbaric pressures of 2-3 bar are difficult
to seal to the
body or extremity being treated, however, without interfering with blood
supply to the wound
locus. Thus, hyperbaric chambers operating at only modestly elevated pressure
have been
manufactured, such as a device operating at 22 mm Hg pure oxygen (1.03 bar)
(5). However,
such chambers are expensive and difficult to sterilize (6). Cross-infection is
stated to be
common.
Heng and others have proposed a simple hyperbaric oxygen treatment chamber
consisting of a polyethylene bag that may be secured to the body or extremity
with adhesive
tape (6), or a transparent nylon bag with straps and VELCRO closures (7).
Pressure is
maintained at between 20 mm Hg and 30 mm Hg. However, the leakage associated
with the
sealing of such bags requires a relatively high rate of oxygen flow. Thus,
this method is
useful only in facilities with sufficient oxygen supply, or in controlled home
environments
where a large oxygen tank is permissible. A disposable hyperbaric treatment
bag with
improved closure is disclosed in U.S. Pat. No. 5,029,579. Another disposable
hyperbaric
treatment bag,is disclosed in U.S. Pat. No. 5,478,3 10.
In U.S. Pat. No. 4,875,483, a combination layered dressing having an external
low
oxygen-permeability layer and an abutting internal oxygen permeable layer has
been
proposed. The relatively low permeability exterior layer is left attached for
3 to 72 hours
creating hypoxia, and hopefully stimulating angiogenesis, following which this
layer is
removed. However, although the remaining, and now exterior layer is oxygen
permeable, the
layer nevertheless decreases oxygen transport, and thus hyperbaric treatment,
by one of the
methods previously described, may be necessary to elevate oxygen levels
sufficiently to
provide optimal healing.
Ischemia compromises wound healing and wounds in aging populations are more
ischemic than those in younger populations (8). It has been demonstrated in
ischemic rabbit
ear models that topical or hyperbaric oxygen can convert a non-healing wound
into a healing
3
CA 02529516 2005-12-15
WO 2004/112649 PCT/US2004/019599
wound, and that growth factors (PDGF) provide a synergistic benefit when used
with oxygen
(9).
It is well known that the speed of epidermal migration on the normal wound is
critically dependent on the amount of oxygen available, and this is the rate-
limiting step. The
control of the local environment is dependent on the local blood supply and
the diffusion of
oxygen from the atmosphere. Any form of treatment that encourages an increase
in the
wound fluid and reduces the time during which the wound is non-perfused will
tend to
increase the rate of healing (10, 11).
It is generally agreed that the tissue surrounding a wound does not alone
supply
sufficient oxygen for wound repair, and that atmospheric oxygen is required
for the formation
of hydroxyproline, a key element in epidermal wound healing. It has been
demonstrated that
93% of the oxygen incorporated into the hydroxyl groups of newly synthesized
hydroxyproline is derived from the atmosphere (12).
It is further generally known that it is likely that oxygen reaches the
epidermal cells
directly by diffusion through the scab rather than via the vascular or tissue
supply. Prior
studies of wounds covered with plastic films found that the higher the oxygen
permeability of
the film, the greater the healing rate (13, 14). Furthermore, the films
prevented scab
formation, thereby altering the mode of epidermal regeneration. The use of
wound dressings
that prevent scab formation and have increased oxygen permeability are thought
to improve
wound healing. The increased presence of oxygen speeds the re-establishment of
epithelial
continuity. Direct access of pure oxygen to open wounds promotes epidermal
cell migration.
Kaufman et al. showed a continuum in wound healing improvement when changing
humidified oxygen levels from 21 to 60, and 80-96% on full thickness burns on
guinea pigs
(15). Niinikoski also suggested that collagen accumulation in the dead space
of animal
wounds increases with oxygen concentration of the environment, peaking at 70%
(16).
A review of topical oxygen and burn wound healing states that oxygen is
essential for
the contraction, the dominant healing process (17). Topical oxygen has also
been shown to
improve the healing rate of skin ulcers and wounds where an inadequate supply
of oxygen
results from peripheral vascular disease or local injury to the
microcirculation. Fischer
showed topical hyperbaric oxygen treatment improved epithelialization and
contraction of
decubitus ulcers (5).
Utkina demonstrated that moderate increases in oxygen levels at normal
atmospheric
pressure increases the closure rate of open wounds (18). He showed healing
rate improved
with continuous exposure to 45%.
4
CA 02529516 2005-12-15
WO 2004/112649 PCT/US2004/019599
A number of patents have been issued that disclose the use of local generation
of
oxygen at the wound site to treat wounds in bandage systems using chemical
reactions,
oxygen saturated solutions, or electrochemical generators (see U.S. Patent
Nos. 5,855,570,
5,578,022, 5,788,682, 5,792,090 and 6,000,403). These concepts have not been
commercialized. The present invention allows for gas to be contained simply
into the wound
dressing, which creates a wound environment with continuous exposure to preset
oxygen
levels, without need for a gas source such as a generator, saturated solution
or a chemical
reaction. Since the amount of oxygen consumed by metabolic processes in the
wound is
relatively small, the materials for the dressing and the volume of the oxygen
cavity in the
dressing can be selected to maintain the desired oxygen concentration for the
practical life of
the dressing
Prior to this invention, larger amounts of oxygen were believed to be required
to
benefit wound healing, which justified the need for an oxygen releasing source
However, the
actual amount of oxygen that the wound consumes in cell metabolism is quite
small, and
simply requires a design that assures a large diffusion gradient for oxygen
into the wound
during the healing period. Hyperbaric approaches that use elevated pressure to
further
enhance the oxygen diffusion gradients to transfer more oxygen into the tissue
are only used
briefly, and once the patient is withdrawn from the high-pressure environment,
the oxygen
levels in the wound drop down to pre-exposure limits quickly. The present
invention
operates as a hyperoxic environment without the need for using elevated
pressure to create
the oxygen diffusion gradient.
Supplying oxygen to a wound on a continuous and ambulatory basis is of benefit
to
speed healing and reduce infection. The oxygen dressing described below can be
complimentary to other therapies and can address a rate-limiting step for
various types of
wounds.
SUMMARY OF THE INVENTION
The present invention is an apparatus that is capable of providing one or more
gases
to a target area. One embodiment of the invention is a multi-layer wound
dressing comes
pre-filled with high levels of oxygen between the layers. The top layer is a
barrier film that
holds the oxygen over the wound, while the bottom layer is a high transfer
rate film, attached
over the wound. This self-contained dressing is applied to the wound like
conventional
wound dressings, and can be manufactured with a similar size, weight and feel
of
conventional dressings or transdermal patches. The dressing can also envelop
the target area,
5
CA 02529516 2005-12-15
WO 2004/112649 PCT/US2004/019599
such as an appendage, in a controlled environment. For example, the dressing
can be in the
form of a glove or mitten for hand treatments or wounds or in the form of a
sock for foot or
leg treatments or wounds.
The barrier layer holds the oxygen in the vicinity of the wound, while the
permeable
or porous layer allows the oxygen to diffuse into the wound fluid at a rate
proportional the
gradient, until the wound fluid is saturated. The dressing acts like an oxygen
reservoir, and
as oxygen is consumed by the wound, there is a local abundant supply to be
used as needed.
While oxygen is a rate-limiting component in the wound healing process, the
oxygen
transfer across intact skin is insignificant, and oxygen consumption by a
wound is a relatively
small number, estimated to be 10"4 cc/mL fluid-hr. Therefore the design of the
dressing is
influenced most significantly by the diffusion rates of the relevant gases
through the barrier
material, the target gas concentration range on the patient, the length of
time the dressing may
be worn, and the seal integrity of the dressing to itself and to the patient
The dressing would be removed by the user from a package that uses controlled
atmospheric packaging (CAP) to maintain the product integrity. CAP is
specifically a
package with high barrier properties that contains the desired ratio of gases
to preserve the
product. CAP is well known in the food industry and examples of the types of
CAP that may
be used are described in U.S. Patent No. 4,895,729 and in the published
literature (19, 20, 21,
22, 23).
The dressing will accelerate healing of acute and chronic wounds, as well as
provide
antibacterial and antifungal benefits.
BRIEF DESCRIPTION OF THE DRAWINGS
The accompanying drawings, which are incorporated in and constitute a part of
this
specification, illustrate one embodiment of the invention and, together with
the description,
explain one embodiment of the invention. In the drawings,
Figure 1 illustrates one embodiment of a dressing system.
Figure 2 illustrates one embodiment of a packaging system.
Figure 3 illustrates one embodiment of a gas emitting pouch system.
Figure 4 illustrates a flow diagram for utilizing a packaging system according
to one
embodiment of the invention.
Figure 5 illustrates a flow diagram for utilizing a dressing system according
to one
embodiment of the invention.
Figure 6 illustrates one embodiment of a pouch system.
6
CA 02529516 2005-12-15
WO 2004/112649 PCT/US2004/019599
Figure 7 illustrates the embodiment of a glove.
Figure 8 illustrates the embodiment of a sock.
DETAILED DESCRIPTION
The following detailed description of the invention refers to the accompanying
drawings. The detailed description merely provides exemplary embodiment of the
invention
and is not intended to limit the invention.
Figure 1 illustrates an apparatus for supplying one or more gases, also
referred to
herein as a dressing system 100. The dressing system 100 is shown as an
exemplary
perspective cut-away view to more clearly illustrate the invention. In one
embodiment, the
dressing system 100 is configured to contain a gas that is dispensed to a user
wearing the
dressing system 100. For example, the different gases contained within the
dressing system
100 may include but is not limited to oxygen, carbon dioxide, and/or nitrogen.
As used herein, the term "gas" includes any gas or volatile.
The dressing system 100 includes a seal 110, an external barrier (or top
layer) 120, a
reservoir 130, an absorbent ring 140, an adhesive backing 150, a permeable
film (or bottom
layer) 160, and a compliant porous insert 170.
The seal 110 is configured to bond the external barrier 120 and the permeable
film
160 together such that the reservoir 130 is formed.
The external barrier 120 is selected to be non-permeable to gases. For
example, the
external barrier 120 may be constructed of metallized polyester, ceramic
coated polyester,
polyvinylidene chloride laminates such as Saranex , EVOH laminates such as
Oxyshield ,
or polyamide laminates such as Capran . In one embodiment, the external
barrier 120 may
be configured to conduct heat or electrical stimulation from an external
source to the user.
For example, polyethylene or another infrared transmittable material may be
utilized as the
external barrier 120.
The permeable film 160 is configured to be permeable to gases. For example,
the
permeable film 160 may be constructed of polyurethane, silicone,
polyvinylchloride,
polyolefins, and the like, preferably ethylene vinyl alcohol (EVA) or
EVA/polyethylene.
The reservoir 130 is configured to store a gas while the dressing system 100
is worn
by a user. In one embodiment, the stored gas within the reservoir 130 is
controllably released
to the user through the permeable film 160.
7
CA 02529516 2005-12-15
WO 2004/112649 PCT/US2004/019599
The amount of gas released to the user while wearing the dressing system 100
may
vary according to the concentration of the gas contained within the reservoir
130 and the
material used as the permeable film 160. Other factors such as temperature and
atmospheric
pressure may also affect the amount of gas released to the user.
The absorbent ring 140 may be located adjacent to the permeable film 160 and
may
be configured to wick away moisture from the user.
The adhesive backing 150 is configured to adhere the dressing system 100 to
the user.
Further, the adhesive backing 150 may also be utilized to prevent the gas that
is delivered
through the permeable film 160 to the user from escaping. In one embodiment,
the adhesive
backing 150 may cover the perimeter of the dressing system 100. In another
embodiment,
the adhesive backing may cover the entire dressing system 100 and may be
integrated with
the permeable film 160.
Examples of the types of adhesive that may be used in the present invention
are
described in U.S. Patent Nos. 6,284,941 and 5,308,887. In one embodiment, the
adhesive
backing may be comprised of adhesive used in commercially available adhesive
bandages. In
another embodiment, the adhesive backing may be comprised of a gel adhesive.
The gel
adhesive may be comprised of a hydrogel. The gel adhesive may also be
reusable, such that
the dressing system could be removed from the user and replaced more than
once.
The compliant porous insert 170 is configured to prevent gas debt in areas
caused by
pressing the external barrier 120 directly on to the permeable film 160. In
one embodiment,
the compliant porous insert 170 placed within the reservoir 130 and between
the external
barrier 120 and the permeable film 160.
The elements comprising the dressing system 100 are shown for illustrative
purposes
only. Deletion or substitution of any shown elements does not depart from the
spirit and
scope of the invention. Similarly, the addition of new elements does not
depart from the
spirit and scope of the invention.
In one embodiment, the dressing system 100 is configured to be pre-filled with
high
levels of oxygen within the reservoir 130. In this embodiment, the dressing
system 100 is
configured to be placed over a wound of the user to help the wound heal. In
one
embodiment, the external barrier 120 is configured to hold the oxygen within
the dressing
system 100 and the permeable film 160 is a high transfer rate film and is
configured to
provide oxygen over the wound. In other words, the external barrier 120 holds
the oxygen in
the vicinity of the wound, while the permeable film 160 allows the oxygen to
diffuse into the
wound fluid at a rate proportional the gradient, until the wound fluid is
saturated.
8
CA 02529516 2005-12-15
WO 2004/112649 PCT/US2004/019599
Subsequent to the saturation, the dressing system 100 acts as an oxygen
reservoir; as
oxygen is consumed by the wound, there is a local abundant supply of oxygen to
be provided
to the wound as needed.
The proportions of the dressing system 100 may be influenced by the diffusion
rates
of the relevant gases through the permeable film 160, the target gas
concentration range on
the user, the length of time the dressing system 100 maybe worn, and the seal
integrity
between the dressing system 100 and the user. The dressing system 100 may
accelerate
healing acute and chronic wounds, as well as provide antibacterial and
antifungal benefits.
In another embodiment, in addition to providing gas to a user, the dressing
system 100
may be configured to deliver biologically beneficial agents such as drugs,
minerals, nutrition,
amino acids, pH modifiers, anti-microbials, growth factors, enzymes to the
user. In one
embodiment, integrating the delivery systems of the gas with the beneficial
agent additives
may lead to synergistic effects that are not achieved by just the gas or the
beneficial agent
additives alone. In one embodiment, these biologically beneficial agents may
be delivered as
microencapsulated agents incorporated in the adhesive backing 150. In another
embodiment,
the microencapsulated agents may be available in a gel matrix in the dressing
cavity 180,
accessible to the wound through pores or perforations, or using conventional
transdermal
technologies.
In an alternate embodiment, instead of filling the reservoir 130 with gas, a
substance
is included within the reservoir 130 to generate gas within the reservoir 130.
For example,
oxygen-releasing agents may be included within the reservoir 130. Oxygen
releasing agents
include oxygen releasing inorganic salts, hydrogen peroxide containing
formulations,
intercalated magnesium peroxide, sodium percarbonate, sodium carbonate and
hydrogen
peroxide, and the like.
In yet another embodiment, the permeable film 160 may be deleted and the
compliant
porous insert 170 may be utilized to hold a substance for generating a gas
within the dressing
system 100.
In yet another embodiment, the external barrier 120 is comprised of Saranex ,
the
permeable film 160 is a polyurethane high oxygen permeability film, these two
layers are
hermetically sealed around the perimeter, and the reservoir 130 contains 98%
oxygen.
One method of achieving the specified oxygen concentration in the reservoir
130 and to
create the controlled atmospheric packaging is to (1) assemble dressing,
sealing the reservoir
9
CA 02529516 2005-12-15
WO 2004/112649 PCT/US2004/019599
with normal atmospheric conditions (about 21% oxygen); (2) place the dressing
in the
metallized film package; (3) flush the package with 100% oxygen; and (4) seal
the package.
In storage, the gas in the reservoir 130 will come to equilibrium with the gas
in the package
via the permeable film 160. When the product is received by the customer and
opened, the
gas in the reservoir will achieve 98% oxygen. The materials and dimensions
used are
determined by taking into account these objectives.
In another embodiment, the dressing system as described herein may further
comprise
a septum, which is defined herein as a septum, a valve, a Luer-type fitting or
any resealable
opening through which one or more gases can be introduced into the dressing
system, then
resealed to prevent the one or more gases from escaping. The dressing system
of this
embodiment may be applied to the wound, and then the one or more gases in the
desired ratio
may be introduced into the dressing system, e.g., with a syringe. The septum
would also
allow for refilling of the dressing system, if desired.
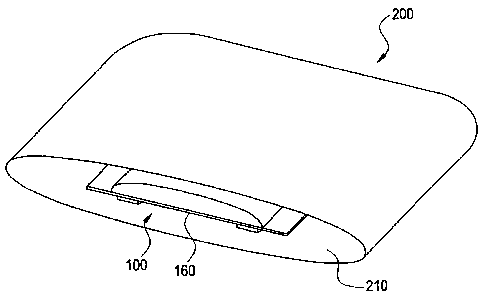
Figure 2 illustrates a packaging system 100. The packaging system 100 is shown
as
an exemplary perspective cut-away view to more clearly illustrate the
invention. In one
embodiment, the packaging system 200 is configured to contain a gas within an
enclosed
container 210, which is within the packaging system. For example, the
different gases
contained within the dressing system 100 may include but is not limited to
oxygen, carbon
dioxide, and/or nitrogen.
The enclosed container 210 is also configured to hold the dressing system 100
as
shown and described corresponding to Figure 1. Once the enclosed container 210
is sealed,
the enclosed container is substantially impermeable; the gas within the
enclosed container
210 substantially remains within the enclosed container 210. Further, the
enclosed container
210 utilizes controlled atmospheric packaging (CAP) to maintain the
environment within the
enclosed container 210. In one embodiment, CAP is a package with high barrier
properties
that contains the desired ratio of gases to preserve the internal environment.
The gas within the enclosed container 210 may penneate the dressing system 100
through the permeable film 160.
In one embodiment, the packaging system 200 may be utilized to store the
dressing
system 100 without degrading the gas stored within the reservoir 130 within
the dressing
system 100 when the gas within the reservoir 130 and the gas within the
enclosed container
210 are the same.
In another embodiment, the packaging system 200 may be utilized to change the
concentrations of gases in the dressing system 100. The gas constituents
stored within the
CA 02529516 2005-12-15
WO 2004/112649 PCT/US2004/019599
enclosed container 210, diffuse into the dressing system 100 when the
concentration of the
gas within the container 210 is higher in concentration compared to the gas
within the
dressing system 100. Similarly, the gas constituents stored within the
dressing system 100,
diffuse into the container 210 when the concentration of the gas within the
container 210 is
lower in concentration compared to the gas within the dressing system 100. The
gases may
diffuse through the permeable film 160 until the constituents reach
equilibrium, the same
concentrations on both sides of the permeable film.
Figure 3 illustrates a gas emitting pouch system 300. The gas emitting pouch
system
300 is shown as an exemplary perspective cut-away view to more clearly
illustrate the
invention. In one embodiment, the gas emitting pouch system 300 is configured
to contain a
gas that is dispensed to the local area surrounding the gas emitting pouch
system 300. For
example, the different gases contained within the gas emitting pouch system
300 may include
but is not limited to oxygen, carbon dioxide, and/or nitrogen.
The gas emitting pouch system 300 includes a first permeable film 310, a
second
permeable film 320, and a reservoir 330.
In one embodiment, the first permeable film 310 is coupled with the second
permeable film 320 and forms the reservoir 330 for storing gas within the gas
emitting pouch
system 300. For example, the first and second permeable films 310 and 330
maybe
constructed of polyurethane, polyethylene, silicone films, polyvinylchloride,
and the like.
The reservoir 330 is configured to store a gas while the gas emitting pouch
system
300 is being used. In one embodiment, the stored gas within the reservoir 330
is controllably
released to the area surrounding the gas emitting pouch system 300 through the
first and
second permeable films 310 and 320.
The amount and rate of gas released through the gas emitting pouch system 300
may
vary according to the concentration gradients of the gas across the permeable
films that
comprise the walls of reservoir 330 and the materials used as the first and
second permeable
films 310 and 320. 310 and 320 can be the same or different materials. The
amount and rate
of release of gas can be different on the opposite sides, this can occur when
310 and 320 have
different permeabilities. Other factors such as temperature, humidity, and
atmospheric
pressure may also affect the amount of gas released.
The elements comprising the gas emitting pouch system 300 are shown for
illustrative
purposes only. Deletion or substitution of any shown elements does not depart
from the spirit
and scope of the invention. Similarly, the addition of new elements does not
depart from the
spirit and scope of the invention.
11
CA 02529516 2005-12-15
WO 2004/112649 PCT/US2004/019599
In one embodiment, the gas emitting pouch system 300 is configured prefilled
with
the desired gas concentrations and is stored within the packaging system 200
(Figure 2) prior
to releasing gas into the surrounding environment, also prefilled with the
same gas
concentrations as in the gas emitting pouch, in order to maintain the levels
in the pouch. In
another embodiment, the gas within the reservoir 330 within the gas emitting
pouch system
300 comes to equilibrium within the packaging system 200 so that both the
pouch and the
package reach the target concentrations
In one embodiment, the gas emitting pouch system 300 is configured to be
placed in
an environment where the gas stored within the reservoir 330 is released
steadily into the
surrounding environment, as the gradient doesn't change appreciably. In
another
embodiment, the release rate of gas from the reservoir 330 into the
surrounding environment
slows as the surrounding environment becomes saturated with the gas.
Subsequent to the
saturation, the gas emitting pouch system 300 acts as a gas reservoir; as gas
is dissipated from
the surrounding environment, there is a local supply of gas within the
reservoir 330 to be
provided to the surrounding environment, governed by the transfer rate across
the permeable
film.
The gas emitting pouch 300 has many applications which may include non-
medical
applications such as applying the gas emitting pouch 300 to effect
environments in containers
for any purpose such as lab experiments, food preservation, to accelerate
degradation, to
prevent corrosion, and the like.
The flow diagrams as depicted in Figures 4, and 5 illustrate merely one
embodiment
of the invention. The flow diagrams in Figures 4 and 5 are one particular use
of the invention
based on a specific application. In other embodiments, the invention may be
utilized with
other applications. The blocks within the flow diagrams may be performed in a
different
sequence without departing from the spirit of the invention. Further, blocks
may be deleted,
added, or combined within each of the flow diagrams without departing from the
spirit of the
invention.
The flow diagram in Figure 4 illustrates an exemplary process of utilizing the
packaging system 200 according to one embodiment.
In Block 410, a gas-retaining object is placed within the packaging system
200. In
one embodiment, the gas-retaining object is the dressing system 100. In
another
embodiment, the gas-retaining object is gas emitting pouch system 300. In yet
another
embodiment, the gas-retaining object may be any item that is configured to
retain and
controllably release a gas from the object.
12
CA 02529516 2005-12-15
WO 2004/112649 PCT/US2004/019599
In Block 420, the packaging system 200 is flushed with a gas. In one
embodiment,
the packaging system 200 is flushed with the same gas contained with the gas-
retaining
object. For example, the dressing system 100 may be pre-filled with oxygen and
placed
within the packaging system. By flushing the packaging system 200 with oxygen,
the
packaging system 200 ensures that the dressing system 100 retains the pre-
filled oxygen
content.
In another embodiment, the packaging system 200 is flushed with a different
gas than
the gas contained with the gas-retaining object. For example, the dressing
system 100 may
contain air that contains other gases in addition to oxygen and may be placed
within the
packaging system 200. By flushing the packaging system 200 with pure oxygen,
the
packaging system 200 diffuses the dressing system 100 with additional oxygen
until the gas
within the packaging system 200 and the gas within the dressing system 100
have reached an
equilibrium.
In Block 430, the packaging system 200 is sealed after placing the gas-
retaining
object within the packaging system 200 and flushing the packaging system 200
with a gas.
In Block 440, if the gas within the gas retaining device and the gas within
the
packaging system 200 differ, then an exchange of gas occurs until an
equilibrium is achieved.
For example, by using the above example describing a dressing system 100 that
contains air
which is sealed within the packaging system 200 flushed with pure oxygen, the
oxygen
diffuses into within the dressing system 100, while nitrogen diffuses out of
the dressing
system 100 into the package 200 until an equilibrium is achieved between the
gas within the
dressing system 100 and the packaging system 200. In this embodiment, the gas
maybe
exchanged through the permeable film 160 (Figure 1).
In Block 550, the packaging system 200 may be opened to remove the gas-
retaining
object. The packaging system 200 may be utilized to store the gas-retaining
object without
degrading the gas within the gas-retaining object. In another embodiment, the
packaging
system 200 may be utilized to infuse the gas-retaining object with a gas.
The flow diagram in Figure 5 illustrates an exemplary process of utilizing the
dressing
system 100 according to one embodiment.
In Block 510, the dressing system 100 is removed from a packaging.
In Block 520, the dressing system 100 is adhered to a user. In one embodiment,
the
dressing system 100 may cover a wound or broken skin of the user. In one
embodiment, the
dressing system 100 utilizes the adhesive backing 150 to adhere the dressing
system 100 to
the user.
13
CA 02529516 2005-12-15
WO 2004/112649 PCT/US2004/019599
In Block 530, a seal is formed between the dressing system 100 and the user.
In one
embodiment, the adhesive backing 150 forms the seal between the dressing
system 100 and
the user.
In Block 540, gas is supplied from the dressing system 100 to the user. In one
embodiment, the penneable film 160 is positioned over the wound or broken skin
of the user
and allows the gas from the dressing system 100 to be supplied to wound of the
user.
In another embodiment, the permeable film 160 may be positioned over intact
skin of
the user and allows the gas from the dressing system 100 to be supplied to the
skin of the
user. There are numerous practical applications in supplying oxygen to intact
skin such as
treating sun or radiation damaged skin, exfoliated skin, dermabraded skin, or
providing
nourishment to aged skin. There may be a synergistic effect with topical
agents as well.
In Block 550, the gas within the reservoir 130 of the dressing system 100
maybe
stored until additional gas is supplied to the user through the permeable film
160.
Another embodiment of the packaging system comprises any of the packaging
systems described herein and further comprises a septum, which as defined
herein may be a
septum, a valve, Luer lock or any resealable opening, through which one or
more gases can
be introduced into the packaging system, then resealed to prevent gases from
escaping. The
packaging system may be charged with the one or more gases in the desired
ratio on site (e.g.,
hospital, doctor's office).
In another embodiment, the adhesive layer may comprise a gel. The gel may have
semi-adhesive properties, such that the same dressing system can be removed
and replaced
repeatedly. Examples of gels that may be used are described in U.S. Patent
Nos. 4,839,345,
5,354,790 and 5,583,114.
The foregoing descriptions of specific embodiments of the invention have been
presented for purposes of illustration and description
They are not intended to be exhaustive or to limit the invention to the
precise
embodiments disclosed, and naturally many modifications and variations are
possible in light
of the above teaching. The embodiments were chosen and described in order to
explain the
principles of the invention and its practical application, to thereby enable
others skilled in the
art to best utilize the invention and various embodiments with various
modifications as are
suited to the particular use contemplated.
Figure 6 illustrates a pouch system 600. The pouch system 600 is configured to
emit
gas into a local environment, similar to the gas emitting pouch system 300.
The pouch
system 600 includes a first layer 610 and a second layer 630. The first layer
610 and the
14
CA 02529516 2005-12-15
WO 2004/112649 PCT/US2004/019599
second layer 630 maybe permeable to gases. In one embodiment, the first layer
610 and the
second layer 630 are bonded through an intermediate layer 620. The
intermediate layer 620
provides the pouch system 600 a more resilient and durable seal between the
first layer 610
and the second layer 630 by diverting the load so that more robust shear force
is applied to a
higher bond strength seal rather than strictly a design that puts all the
internal pressure and
load on a peel strength surface. By adding the intermediate layer 620 with a
narrower
diameter than the first layer 610, the seal between the first layer 610 and
the second layer 630
is reinforced.
Another embodiment of the invention is an apparatus that envelops a target
area, such
as an appendage, in a controlled environment. Once the appendage requiring
treatment is
placed in the apparatus, the opening is secured around the appendage. For
example, the
apparatus can be in the form of a glove or mitten for hand treatments or
wounds or in the
form of a sock for foot or leg treatments or wounds. A glove or mitten would
be secured
around a patient's hand or arm and a sock would secured around a patient's
ankle or leg. The
apparatus can be prefilled in the reservoir, as described herein and packaged
in a CAP
environment. The apparatus can also be packaged in a CAP environment that
facilitates the
exchange of gases such that the reservoir achieves the target ratios of gases
passively through
diffusion in storage, as described herein. The apparatus can also be filled or
recharged
according to any of the methods described herein.
Figure 7 illustrates a glove for use in hand/arm treatments or wounds. A side
view
700A and a cross-section 700B of section A-A of the glove are shown. The inner
layer 710 is
a permeable film, as described herein, which is permeable to oxygen and/or
other gases. The
outer layer 720 is an external barrier, as described herein, selected to be
less permeable to
oxygen and/or other gases. The two layers form a reservoir 730 between them
that may
contain one or more gases, gel, fluid, cushioning material, resilient porous
material, or a
combination thereof, or as described herein.
Figure 8 illustrates a sock for use in foot/leg treatments or wounds. A top
view 800A,
a side view 800C and a front view 800D are shown. A cross-section 800B of
section A-A of
the sock is also shown. The inner layer 810 is a permeable film, as described
herein, which is
permeable to oxygen and/or other gases. The outer layer 820 is an external
barrier, as
described herein, selected to be less permeable to oxygen and/or other gases.
The two layers
form a reservoir 830 between them that may contain one or more gases, gel,
fluid, cushioning
material, resilient porous material, or a combination thereof or as described
herein.
CA 02529516 2005-12-15
WO 2004/112649 PCT/US2004/019599
A further embodiment of the invention is an apparatus wherein the inner and/or
outer
layers further comprise ribs. The ribs allow the one or more gases in the
reservoir to be
applied to the target area even when pressure is exerted on the apparatus from
an external
source.
Other embodiments of the invention can also be an apparatus in the form of a
blanket,
an oxygen mask, a wrap, or an eye patch. These other embodiments may be use to
supply
oxygen or other gases to target areas such as the face or eye by placing the
permeable layer
on the target area and securing the apparatus to a patient either externally
by mechanical
means, i.e., gravity, by wrapping a securing tape or material around it, or by
sealing the
apparatus directly to the target area with an adhesive seal around the
perimeter or other
surfaces.
Any of the embodiments of the invention described herein maybe used in
combination with existing technologies.
The present invention is useful for wound healing for human and animal
patients, for
use in laboratories, and anywhere a specific gas or combination of gases is
required to reach a
specific,'discrete site.
The foregoing descriptions of specific embodiments of the invention have been
presented for purposes of illustration and description. They are not intended
to be exhaustive
or to limit the invention to the precise embodiments disclosed, and naturally
many
modifications and variations are possible in light of the above teaching. The
embodiments
were chosen and described in order to explain the principles of the invention
and its practical
application, to thereby enable others skilled in the art to best utilize the
invention and various
embodiments with various modifications as are suited to the particular use
contemplated.
References:
1. Whitney JD, "Physiological Effects of Tissue Oxygenation on Wound", Heart &
Lung, Vol. 18., No. 5, pp. 466-474, Sep. 1989.
2. Gruber RP, et al., "Skin Permeability to Oxygen and Hyperbaric Oxygen",
ARCH.
SURG., Vol. 101, pp. 69-70, July 1970.
3. Eaglstein WH, "Experiences with Biosynthetic Dressings", J. AmM. Acad.
Dermatol., Vol. 12 (2 Pt 2), pp. 434-40, February 1985.
4. Niinikoski J, et al., "Combination of Hyperbaric Oxygen, Surgery, and
Antibiotics
in the Treatment of Clostridial Gas Gangrene", Infections in Surgery, pp. 23-
37, January
1983.
16
CA 02529516 2005-12-15
WO 2004/112649 PCT/US2004/019599
5. Fischer BH, "Treatment of Ulcers on the Legs with Hyperbaric Oxygen", J.
Derm.
Surg., Vol. 1, No. 3, pp. 55-58, Oct. 1975.
6. Heng M, et al., "A Simplified Hyperbaric Oxygen Technique for Leg Ulcers",
Arch Dermatol, Vol. 120, pp. 640-645, May 1984.
7. Olejniczak S, et al., "Topical Oxygen Promotes Healing of Leg Ulcers",
Medical
Times, Vol. 104, No. 12, pp. 114-121, Dec. 1976.
8. ' Transcript of United States Food & Drug Administration, Center for Drug
Evaluation & Research, Dermatologic and Ophthalmic Drugs Advisory Committee,
46th
Meeting, pp. 15-28, July 14, 1997
(http://www.fda.gov/ohnns/dockets/ac/97/transcpt/3308tl.pdf).
9. Zhao LL, Davidson JD, Wee SC, Roth S, Mustoe TA, "Effect of Hyperbaric
Oxygen and Growth Factors on Rabbit Ear Ischemic Ulcers," Arch Surg/Vol. 129,
Oct 1994.
10. Winter GD, Perins DJD, Proceedings of the 4th Intl Congress on Hyperbaric
Medicine, Igaku Shoin Ltd, p. 363, 1970.
11. Silver IA, in Wound Healing & Wound Infection, ed. Hunt TK, Appleton-
Century-Crofts, NY, p26, 1980.
12. "Prockop DJ, et al., "Oxygen-18 studies on the conversion of proline to
collagen
hydroxyproline", Arch Biochem BioPhys V101, p. 499, 1963.
13. Winter GD., Advances in Exp Med and Bio, V94, p. 673-8, July 4, 1977.
14. Silver IA, in Epidermal Wound Healing, ed. Maibach H & Rovee D, Year Book
Medical Publishers, Inc, Chicago, p. 291-305, 1972.
15. Kaufman T, et al., Surgical Forum V34, pp. 111-113,1983.
16. Niinikoski J, Clin Plast. Surg, V4, p. 361, 1977.
17. Kaufman T, et al., Burns, V9, pp.169-173,1983.
18. Utkina OT, Biol. Abstr., V45, 6289, 1964.
19. Brody AL, Food Technology, Vol. 55, No. 9, pp. 104-106, Sept. 2001.
20. Hoogenwerf SW, et al., Letters in Applied Microbiology, Vol. 35, Issue 5,
p. 419,
Nov. 2002.
21. Devlieghere F, et al., "Modified atmosphere packaging: state of the art",
http://www.ifis.co.uk/hottopics/MAParticle2.PDF, Sept. 2000.
22. Labell, "Controlled & Modified Atmosphere Packaging", Food Processing, p.
152, Jan. 1985.
23. "Biobased Packaging Materials for the Food Industry", ed. CJ Weber,
http://www.nf-2000.org//publications/f4046fin.pdf, Nov. 2000.
17