Note: Descriptions are shown in the official language in which they were submitted.
CA 02530073 2005-12-20
WO 2005/002469 PCT/US2004/020219
ANNULOPLASTY CHAIN
BENEFIT CLAIMS TO PRIOR APPLICATIONS
This application claims the benefit of U.S. Provisional Application No.
601482,393, filed 25
June 2003.
GOVERMENT INTERESTS
This invention was made in part during work supported by the U.S. Government,
including
grants from the National Institutes of Health (NIH) E17-649 and HL52009. The
government may
have certain rights in the invention.
BACKGROUND OF THE INVENTION
1. Field of the Invention
This invention relates generally to the field of prostheses for cardiac valve
repair, and
specifically to an annuloplasty implant device incorporating a chain.
2. Description of Related Art
A frequently used method for eliminating some pathological alterations of the
mural and
tricuspid valves of the heart is that of reinstating the correct shape and
dimensions of the valve
annulus by means of surgical procedures known as annuloplasty. Annuloplasty
includes surgically
implanting a supporting prosthesis on the dilated or deformed annulus for the
purpose of reinstating
its dimensions andlor physiological shape in such a way as to allow the
cardiac valve to function
correctly.
Support prostheses utilized in valve repair operations are sometimes called
annuloplasty
rings. An annuloplasty ring can be implanted around the mural or tricuspid
heart valve for
reconstructive treatment of valvular insufficiency. Annular dilation or
degradation may influence
valve function causing cardiac insufficiency under specific pathologies.
Regeneration of the shape of the mitral annulus has been shown to be
beneficial in restoring
valve function. There are currently more than 25 different annuloplasty
devices on the market. The
main types of rings include rigid, flexible, "partial" flexible, and
adjustable.
Rigid type rings are widely employed with success, and reduce the dilatation
of the valve
annulus. Such rings generally include a metal core (for example, a titanium
alloy), an optional
sheath of cladding around the core, and an outer cladding of textile for
suturing. Rigid rings
generally do not allow the annulus of the valve to flex along the base of the
posterior cuspid in such
CA 02530073 2005-12-20
WO 2005/002469 PCT/US2004/020219
a way as to assist the cardiac muscle movements. Consequently, significant
stress is imposed on the
suture points subjected to torsion and traction, which prevents natural
behavior of the valve.
Unlike rigid rings, flexible rings follow the movements of the annulus during
the cardiac
cycle in a beneficial manner. Flexible rings interfere less with normal mural
valve motion, improve
peak velocity across the ring, and thus improve ventricular end-diastolic
diameter and volume.
However, they too have the disadvantage of not allowing the shape to be
reconstructed in an
optimal manner.
"Partial" flexible rings seek to unite the advantages of the rigid type with
those of the
completely flexible type while avoiding the disadvantages of each.
Theoretically, they are easier
and quicker to insert since no sutures have to be placed in the anterior
annulus.
Adjustable rings are designed to allow for adjustment of the annular length
during valve
testing:
Thus, while many conventional rings may restore annulus shape, annular
dynanucs are lost
when using rigid rings, and there remains controversy on the efficiency of
flexible rings in
preserving these dynamics.
Annular flexing and contraction is likely of importance in valve efficiency,
not only
mechanically, but functionally. Therefore, an annuloplasty device that
minimally interferes with
annular dynamics would be an improvement over current annuloplasty ring
technology. It can be
seen that there is a need in the art far an improved annuloplasty chain that
maximally preserves
annular dynamics in use.
Further, conventional rings are known to be hard to bend to distort their
shape so they can be
delivered. It would be beneficial to provide a device that is advantageous in
minimally invasive
procedures. Thus, it can also be seen that there is a need in the art for an
improved annuloplasty
chain that is easier to arrange than the conventional ring, so it fits in a
minimally invasive delivery
system.
Additionally, conventional rings are known to be difficult to use in beating
heart procedures.
It would be beneficial to provide a device that is advantageous in minimally
invasive procedures
that can thus be used in beating heart surgeries. It can therefore also be
seen that there is a need in
the art for an improved annuloplasty chain that can be used in a minimally
invasive delivery system
so that be implanted in procedures with' a beating heart. Beating heart
surgeries improve patient
survival and reduce surgical complications.
In essence, the present annuloplasty implant device opens new fields of
implants for
annuloplasty repair since conventional implants are rings.
CA 02530073 2005-12-20
WO 2005/002469 PCT/US2004/020219
BRIEF SUMMARY OF THE INVENTION
The present invention comprises an annuloplasty chain of metal, the chain
having a
surrounding shielding layer and a suturing layer. A re-sterilizable chain
holder can be used during
implant of the annuloplasty chain.
The present chain is a solution to the disadvantages inherent in conventional
rings. The
chain reconstructs the shape of the annulus, while maintaining the dynamics of
the valve through
appropriate flex and bend. The annuloplasty chain preserves a three-
dimensional perimeter,
enabling it to adjust the size of the annulus to a fixed quantity after
dilation or degradation.
The present chain can be implanted in minimally invasive procedures, and thus
in beating
heart procedures. The present chain can preferably achieve the complete saddle
shape of the
annulus with a 1/3 height-to-commissural diameter ratio, and has the ability
to maintain a normal
chc~rdal-force ~i~tr-Ibution.~s._i~~.bendi=ng is-dom~n-ated-by=its-mec-hanieal-
e~v3~owrnent.
In preferred embodiments, the present annuloplasty chain comprises a multilink
chain, a
solid link chain or a scaled chain. The chain is preferably fabricated from
metal having favorable
characteristics of wear under cyclic loading and friction, biocompatibility,
tensile strength, and MRI
safety. The links of the chain can include links of varying sizes and shapes
for improved function
with specific pathologies, or may include links of uniform sizes and shapes.
The chain is covered with a flexible, biocompatible polymer layer, which will
isolate blood
from the device. This shielding layer prevents blood damage, and therefore
thrombogenesis.
The shielding layer can be covered by a suturing layer of preferably cloth to
enable suturing
of the ring.
The chain holder dictates the. initial shape of the chain, and the size of the
implant. The
surgeon should be able to suture the chain completely around the valve before
retrieving the holder.
In vitro testing has been conducted to observe the mechanical and functional
implications of
a saddle-shaped annulus. Testing is also being conducted to elucidate the
importance of annular
dynamics on chordae tendinea mechanics. Initial results using embodiments of
the present
invention have shown that valve function is preserved for the range of annular
geometries generated
by the multilink chain saddle-shaped annulus. This implies that the extreme
geometries generated
by a multilink chain allow the valve to seal with no significant mural
regurgitation observed.
The annuloplasty implant of the present invention allows the surgeons to have
a truly
flexible instrument that will preserve the natural dynamic characteristics of
the mitral annulus, as
they have shown to be important in valve function.
CA 02530073 2005-12-20
WO 2005/002469 PCT/US2004/020219
Further, annuloplasty implant of the present invention can preferably achieve
the complete
saddle shape of the annulus with a 1/3 height-to-commissural diameter ratio.
The present annuloplasty chain can further be utilized as a delivery system.
The chain can
have a link, or a plurality of links, having an internal cavity or cavities,
or being formed of a
material with porosity or a material composition that will enable the links)
to store a
pharmaceutical agent or other substance necessary for patient treatment.
Substances can include
solids, liquids or gases that can be release from within the links) in a
controlled fashion after the
implantation of the device. The substance can include monitoring elements like
electronics to send
environmental characteristics about the chain or surrounding areas to a
doctor, and thus the
substance is not intended to exit the link, or a medicinal substance that is
designed to exit the
surface of a link, or from within a link. Alternatively, the substance can be
a refrigerant or the like
that simply keeps-.at-leapt ~orrions of the i-r-replant cool: Thin;-.one-
de~ivor--y ~ystem~~r-nbodim~rtt of
the annuloplasty chain can be a drug delivery system in addition to its normal
function as a cardiac
prosthesis. Other delivery systems can include the ability to provide
temperature control to
surrounding areas, or the chain can have monitoring means to deliver
monitoring characteristics to a
doctor.
These and other objects, features and advantages of the present invention will
become more
apparent upon reading the following specification in conjunction with the
accompanying drawing
figures.
BRIEF DESCRIPTION OF THE FIGURES
Fig. 1 illustrates the present annuloplasty implant device comprising a
multilink chain
according to a preferred embodiment of the present invention.
Fig. 2 illustrates the present annuloplasty implant device comprising a solid
link chain
according to a preferred embodiment of the present invention.
Fig. 3 illustrates the present annuloplasty implant device comprising a scaled
chain
according to a preferred embodiment of the present invention.
Fig. 4 illustrates the shielding layer and suturing layer of present
annuloplasty implant
device according to a preferred embodiment of the present invention.
Fig. 5 illustrates an attachment system with attachment devices of present
annuloplasty
implant device according to a preferred embodiment of the present invention.
Fig. 6(a) shows a mitral valve sutured on a flexible membrane.
Fig. 6(b) shows the saddle configuration is present when the basal chords are
extended as
observed by a tracing over the annulus.
CA 02530073 2005-12-20
WO 2005/002469 PCT/US2004/020219
Fig. 7 is a schematic of the Georgia Tech left heart simulator.
Fig. 8 is a schematic of the saddle shape configuration setup and local
orientation.
Fig. 9 is a diagram of an extended mitral valve identifying the chordae
tendineae selected
for tension measurements.
Fig. 10(a) is a diagram of the chordae tendineae insertion pattern.
Fig. 10(b) is a lateral diagram of the mitral valve with average chordal
lengths.
Fig. 11 are pressure and chordae tendineae tension curves for valve # 6 in
flat annulus
configuration.
Fig. 12 are pressure and chordae tendineae tension curves for valve # 6 in
saddled annulus
configuration.
Fig. 13 are pressure vectors acting on the mitral valve anterior leaflet in a
flat and saddled
cor~fi~uratior~~ Pressure v~ctflr-s ara redirected . to~vurds the sides of the
aalwe . in the s~dd~
configuration decreasing the resultant force in the direction of the anterior
strut.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
Referring now in detail to the drawing figures, wherein like reference
numerals represent
like parts throughout the several views, the present invention is a medical
device comprises an
annuloplasty chain 10 of metal, a shielding layer 60, a suturing layer 80, and
an attachment system
90 to facilitate attachment of the chain 10 to annulus tissue. The chain 10 is
capable of generating a
three-dimensional saddle shape while maintaining its perimeter relatively
constant. Thus, it
maintains annular dynamics while correcting annular size after valvular
dilatation.
The chain 10 maintains a relatively constant three-dimensional constant,
preferably
approximately 3% maximum deformation; thus, the present invention can correct
annular
degradation. The chain 10 is able to generate saddle-shaped annulus geometries
with a saddle
height to commissural ratio of up to approximately 25°Io.
The chain 10 is preferably fabricated from metal, but can be fabricated from
other materials,
or combinations of materials, that have favorable characteristics of wear
under cyclic loading and
friction, biocompatibility, tensile strength, and MRI safety.
In preferred embodiments, the present annuloplasty chain 10 comprises a
multilink chain 12,
a solid link chain 22 or a scaled chain 42. These specific designs preserve a
three- dimensional
perimeter.
Adjacent links of the chain can be movable relative to one another, have a
fixed orientation
to each other, or a single chain can have linlcs both movable and fixed. The
links can be fabricated
so movement between adjacent linlcs are controlled without additional means to
aid in such
CA 02530073 2005-12-20
WO 2005/002469 PCT/US2004/020219
6
movement, or the joints between links can incorporate additional means to
control such movement,
other than the contact points) between links. For example, the solid link
chain 22 embodiment can
utilize pins between adjacent links.
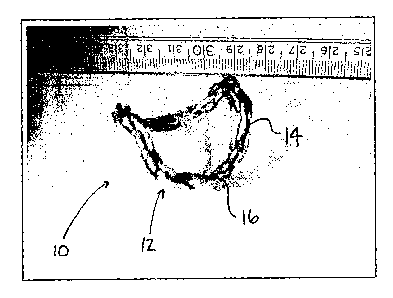
As shown in Fig. 1, a multilink chain 12 incorporates several links 14,
wherein the multilink
chain 12 is able to generate a saddle-shaped geometry while maintaining its
three-dimensional
perimeter significantly constant, wherein the perimeter variation is
approximately 3%. This chain
embodiment is of generally simple construction, but may use a large number of
joints 16, which can
be welded joints. Yet, welded joints can lead to a greater possibility of
failure if not welded
appropriately.
A solid link chain 22 is shown in Fig. 2. Chain 22 comprises solid links 24
joined at a pivot
26. The pivot 26 can incorporate cooperating members 28 from adjacent links 24
with a pin 32
« ».
r-otationall __~nnecti-n ::~o members .~ _~. _ : a . .
-y g 2 ~n another: The-term . olld=~li-nk -m-this embodiment
does not infer that the link 24 is solid throughout, but that it has a
distinguishing design from that of
an ordinary chain link 14 designed as a loop, as shown in Fig. 1. Solid link
24 may be solid
throughout its cross-section, although the links 24 may have cavities therein.
Such internal cavities
can be filled, partially or totally, with elastomeric material, in particular
silicone, polyurethane and
their mixtures.
The pivot direction can rotate from one linlc to another to allow three-
dimensional
deformations in order to produce the saddle configuration. This chain 22 has a
generally
approximately negligible variation in perimeter, which is defined by the fit
between the different
members 28. This design preferably has no welded joints, but the pins 32 used
with the members
28 are susceptible to wear because of the high frequency of the loading on the
chain 22.
In yet another embodiment, the present annuloplasty chain 10 comprises scaled
chain 42 as
shown in Fig. 3. This design resembles that used in a key chain, characterized
by a relatively
smooth surface 44 with the hinge points (not shown) within the surface 44.
Because this design has
a smooth surface 44, and its hinge points are not exposed, it causes less
blood damage due to
moving parts. The perimeter change for this design is on the order of
approximately 2%.
The chain 10 is preferably a self-lubricating metallic fabricated from
surgical steel or
titanium, having favorable characteristics of wear under cyclic loading and
friction,
biocompatibility, tensile strength, and MRI safety. The chain 10 can
alternatively be made from
materials such as Elgiloy (a cobalt-nickel alloy), titanium, or Nitinol (a
nickel-titanium alloy).
The present chain can further be utilized as a delivery system of monitoring
characteristics,
or drugs, or cooling, among other delivery embodiments. The chain can have a
link, or a plurality
CA 02530073 2005-12-20
WO 2005/002469 PCT/US2004/020219
of links, having a coating of a substance, or be at least partially filled
with a substance by
incorporating an internal cavity or cavities, or being formed of a material
with a substance in the
matrix, or formed of the material having porosity or a material composition
that will enable the
links) to store a pharmaceutical agent or other substance necessary for
patient treatment, and
allowing such substance to pass from within the link, to outside the link. The
substance thus can be
on the chain, or in the chain, or part of the material of the chain, for
delivery.
Substances can include solids, liquids or gases that can be released from the
outside surfaces
of the link(s), or from within the link(s), preferably in a controlled fashion
after the implantation of
the device. If the substance is monitoring equipment to provide one with
monitored characteristics
from within the body, such equipment can similarly be located within a link,
or on the surface of a
link, or make up a portion of the material of the link. For example, the
monitoring substance could
be--a-. f~=1m capable- of monitarir~g pre-selected- eh~r-actori~tic=s, -
including-far example temperat~ure~
stress, strain, and others.
The chain 10 preferably is at least partially covered with a shielding layer
60 as shown in
Fig. 4 being a flexible, biocompatible polymer layer. Biocompatible surfaces
lead to the success of
a continuously-increasing number of polymer applications in the biomedical
field. Surface
chemistry controls numerous chemical and physiological properties of a
polymer, including
thromboresistance, biostability, lubricity, permeability, and abrasion
resistance. Surface-modified
polymers need to be well characterized in order to correlate the surface
chemistry to the
biofunctionality of the application.
The shielding layer 60 can comprise various polymers that take into account
the design
characteristics previously mentioned, as well as crystallization and
calcification under cyclic
loading. The polymer should not fracture or increase porosity within the
mechanical environment
of the chain 10. Silicon based rubbers have been used in these types of
applications.
The surface of the chain 10 and/or of the shielding layer 60 can be clad
partially or totally
with a thin layer of hemocompatible carbon, for example turbostratic carbon.
This cladding
contributes to an improved hemocompatibility of the chain 10 and to a
controlled tissue growth of
the receiving organism.
The suturing layer 80 as shown in Fig. 4 provides a suitable material for
suturing or
otherwise attaching the chain 10 to the annulus tissue and promoting tissue
growth therein. The
suturing layer 80 can comprise a polyester knit or other fabric that is
appropriate for suturing. The
suturing layer 80 can comprise a biologically-compatible material such as,
without limitation,
Dacron (polyethylene terepthalate), polyester knit, PTFE knit, and ePTFE knit.
The knit is
CA 02530073 2005-12-20
WO 2005/002469 PCT/US2004/020219
beneficial because it provides a suitable surface gor suture penetration as
well as for tissue growth
after implantation, reducing the risk of dehiscence.
The suturing layer 80 can also be treated with a biologically-compatible
tissue growth factor
or other medicament to aid in treating the attachment area. The present
invention can reduce or
eliminate the occurrence of systolic anterior motion (SAM), wherein the
anterior leaflet of the
mitral valve bulges into the left ventricular outflow track (LVOT) thereby
obstructing blood flow
into the aorta. Suture pull-out testing must be within the ranges required by
international
biomedical standards for this type of implants.
Attachment system 90 as shown in Fig. 5 can facilitate attachment of the chain
10 to annulus
tissue. A number of attachment devices 92 can be positioned around the chain
10. The attachment
device 92 can comprise various tissue-engaging devices, including, for
example, needles, barbs, or
hooks. =A~~-acl~rent -d~vie-es-- 92 . preferabl--y -i~cor~or~te.
a.rbiolt~g~cally-co~npat~ble material such as ~ .
without limitation, stainless steel, titanium, or Nickel-Titanium alloy
(Nitinol).
A chain holder dictates the initial shape of the chain, and the size of the
implant. The
surgeon should be able to suture the chain completely around the valve before
retrieving the holder.
An embodiment of the present multilink chain 10 was tested in a physiological
left heart
simulator using human hearts. The results of the study showed that the ranges
of geometrical
variation used in this chain 10 induces geometries that do no present
significant mural
regurgitastion in normal valves under physiological conditions.
The multilink chain 10 tested was a constant annular three-dimensional
perimeter. To
maintain this perimeter, the annulus in the model was constructed with a
metallic multilink chain
that allowed for a maximum change in linear length of 3%. Measuring a segment
of the same
length as that used in the model, in a maximum contractile and then distended
state assessed this
percentage.
The chain was then joined at the ends to form a circle that in its flat state
had an approximate
area of 7 cm2. In the human heart, the annulus is not a perfect circle, and in
the mid anterior leaflet
area, the annulus tend to flatten out, generating a D-shaped configuration. To
simulate this
condition with the embedment of the present invention, a segment of the length
where the anterior
leaflet would be sutured was covered with resin to maintain a straight section
in the perimeter
(approximately l.7cm). This D-shaped chain was then sutured onto a flexible
elastic membrane.
The membrane stretched and adhered to the modified atrial model.
The modification to the model included the addition of two metal rods that
could be pushed
forward and fixed in position. The ends of the rods were joined to the
metallic annulus at the points
CA 02530073 2005-12-20
WO 2005/002469 PCT/US2004/020219
9
corresponding to the center of the commissural areas. Then by pushing the rods
forward, the
annulus would deform. The contact points of the rods and the annulus protruded
forward into the
ventricle generating a saddle. The nature of the metallic chain ensured a soft
curvature in the saddle
shape.
The test was to simulate just a change in shape without displacement of the
general
structure. Thus, considering the eccentrically deformation when generating a
saddle from a flat
fixed perimeter ring, the mid point of the anterior side was fixed rigidly to
the model, and the mid
point of the posterior annulus was restrained by using a sliding bar mechanism
that allowed for the
structure to deform radially, but with no movement in the atrium-ventricular
direction when force
was applied on the rods. The metallic chain was covered with Dacron to
facilitate the suturing of a
natural valve on to it.
The model was eapabie o~ simulating- a pear height ~f 1 em, fro rr-the lowest
p.o f. -the
saddle to the peaks of the commissural areas. This implied an approximate
height-to-diameter ratio
of 1/3, which is the approximate relation found in a healthy human heart.
Intermediate positions
with lower height-to-diameter ratios can also be obtained with the model to
simulate pathologic
conditions in the heart. As observed in Fig. 7, this design assured soft
curves in the three-
dimensional saddle. The constant perimeter also implied a change in the two-
dimensional projected
area. The change is approximately of 21% when the maximum saddle curvature is
applied. The
change in projected area occurred naturally with the distortion of the three-
dimensional shape.
Applicants reviewed the effects of a saddle-shaped annulus on nutral valve
function and
chordal force distribution in an in vitro study. Prior to Applicants' iyi
vitr~ study, studies had
concluded that the shape of the human mural valve annulus is a three-
dimensional saddle. The
objective of Applicants' study was to investigate the effects of a saddle-
shaped annulus on chordal
force distribution and mitral valve function.
In a synopsis of the study, eleven human mural valves were studied in a
physiological left
heart simulator with a variable shaped annulus (flat vs. saddle). Cardiac
output and transmitral
pressure were analyzed to determine mural regurgitation volume. In six
experiments, force
transducers were placed on six chordae tendineae to measure chordal force
distribution. Valves
were tested in normal and pathophysiologic papillary muscle positions.
When comparing the flat and saddle-shaped configurations, there was no
significant
difference in mitral regurgitation volume 11.2 ~ 24.7% (p=0.17). In the saddle-
shaped
configuration, the tension on the anterior strut chord was reduced 18.5 ~
16.1% (p<0.02), the
tension on the posterior intermediate chord increased 22.3 ~ 17.1% (p<0.03),
and the tension of the
CA 02530073 2005-12-20
WO 2005/002469 PCT/US2004/020219
commissural chord increased 59.0 ~ 32.2% (p<0.01). Annular shape also altered
the tensions on the
remaining chords.
The study shows that annular shape alone does not significantly affect mitral
regurgitation
caused by papillary muscle displacement. A saddle-shaped annulus redistributes
the forces on the
chords by altering coaptation geometry, leading to an optimally balanced
anatomic/physiologic
configuration.
The following detail of the Applicants' study uses the following Key Terms,
and
Abbreviations:
Ke,~ Abbreviation
Mitral Regurgitation MA - Mitral Annulus
Chordal Force PM - Papillary Muscles
Annulus Shape MV - Mitral Valve
~$____ ~n~titutio~rai-Review-Board-
FMR- Functional Mitral Regurgitation
STDEV- Standard Deviation
CTT- Chordae Tendineae Tension
VASAC- Variable Annular Shape
Atrial
Chamber
The mitral annulus is a dynamic component
of the mitral valve (MV) complex.
Although
the geometry and motion of the mitral annulus (MA) have been studied for
several decades, there is
still controversy over the exact geometry and dynamic characteristics of the
MA including the
origin of its shape. Sonomicrometry, magnetic resonance imaging, angiography,
and two and three-
dimensional echocardiographic techniques have been used to analyze the shape
and dynamics of the
MA in animal models and humans. Although there is still some disparity between
measurements,
current views tend to describe the annulus as a non-planar structure, which
varies geometrically
during the cardiac cycle.
The shape of the MA is described as a three-dimensional saddle because it
resembles a non-
planar, three-dimensional ellipse. In addition to its position, the area, the
eccentricity, and the non-
planarity or curvature of the MA vary during the cardiac cycle describing a
dynamic structure.
Mitral annular geometry and dynamics have been studied i.n vivo in animals and
humans,
both in normal and pathologic subjects. Mitral annular geometry is an
important factor in the
diagnosis of MV prolapse. Changes in annular geometry and dynamics (2D-area,
2D-perimeter,
saddle curvature, annular displacement, etc.) have been observed in patients
with functional mural
regurgitation, FMR, acute ischemic mitral regurgitation, and different types
of cardiomyopathies.
CA 02530073 2005-12-20
WO 2005/002469 PCT/US2004/020219
11
Although the exact origin and function of the shape of the MA is still
unknown, studies have
proposed that this shape may be important as part of the valve closure
mechanism and in the
distribution of stress on the mitral valve's anterior leaflet. The
understanding of these mechanic s
may prove vital in the design of new surgeries involving chordal cutting and
in the treatment of MV
related pathologies. Annular shape is also considered in the design of
different types of cardiac
implants such as annuloplasty rings.
An objective of the Applicants' ifs vitro study was to compare mitral
regurgitant volume and
chordal force distribution in human MVs under saddle and flat annulus
configurations in order to
elucidate the importance of the saddle shape in MV mechanics. The experimental
setup and
procedure were not designed to imitate the complete function of the heart, but
to isolate the effect of
annular shape, while controlling other variables such as PM position, trans-
mitral pressure, and flow
r--ate:
Materials and Method
Mitral Valves
Four fresh human MVs from Emory University in Atlanta, Georgia and seven MVs
from
frozen hearts provided by Corazon Technologies in California were used in this
study. The hearts
from Emory University were obtained from heart transplant recipients with IRB
approval following
the guidelines for the protection of study volunteers in research. Hearts
containing mural valve
pathology were excluded from the study. Valves with normal anatomical features
and similar
orifice areas (6.8 ~ 0.4cm2) were extracted.
The valves were extracted from the hearts preserving the complete mitral
apparatus. During
extraction, all chords that inserted into the leaflets or the. annulus from
the papillary muscles were
preserved. The PMs were then wrapped with Dacron cloth maintaining the chordae
distribution.
The Dacron cloth was then sutured onto holding disks designed to attach to the
Georgia Tech
(Atlanta, Georgia) left heart simulator.
Anatomical Measurements
After extraction, six valves were selected for anatomical measurements. Only
the last six
valves were measured since this section of the procedure was not included in
the initial protocol.
The selected valves were sutured to a flexible membrane held by a rigid
circular metallic ring. The
membrane was used to hold the valve, while enabling the annulus to deform
according to chordal
lengths. The papillary muscles were positioned so that there was no slack on
the chords inserting
near the annulus of the valve. The lengths of the individual basal chords were
measured from the
origin in each papillary muscle to their insertion. Only chords inserting into
the base of the leaflets
CA 02530073 2005-12-20
WO 2005/002469 PCT/US2004/020219
were measured in order to analyze the geometry generated on the annulus when
these chordae were
under tension. The length of the chords was recorded in an insertion map of
the valve. After these
initial measurements, the flexible membrane was moved away from the papillary
muscles to
observe the geometry generated on the annulus as illustrated in Fig. 6(a). The
membrane was then
removed from the valve before the ira vitro experiments. Fig. 6(b) shows the
saddle configuration is
present when the basal chords are extended as observed by the tracing 122 over
the annulus.
Ifz Vitro Flow Loop
The in vitro experiments were carried out in the modified Georgia Tech Left
Heart simulator
as shown in Fig. 7. This system is capable of physiologic and pathophysiologic
flow and pressure
waveforms. This simulator has been described in detail in previous studies.
Variable Shape Mitral Annulus Chamber (Flat - Saddle)
A -var-i-abie--annular shape- atr--i~l -c-h~~nber- (VA~AC) was=cons-t~=acted
t~ -obtain the di~f~r~~t
annular geometries during the ifa vitro experiments. This chamber was used
along with the Georgia
Tech Left Heart simulator. The atrial chamber was constructed of transparent
acrylic to enable
visualization and echocardiographic imaging of the valve through a frontal
window 5 cm away from
the annulus. The annulus chain was composed of a mufti-link chain which
deformed three-
dimensionally, but retained an approximately constant three-dimensional
perimeter (maximum
perimeter variation = 3%). A 2 cm section of chain links were welded together
to generate the D-
shaped geometry characteristic of the mitral annulus orifice. Two straight
control rods, connected
at one end to the center of the commissural sections of the annulus, were used
to modulate annular
shape. Moving the control rods in the forward direction pushed these sections
of the annulus
forward, transforming the initially flat chain into a geometry similar to that
of a saddle. The
annulus was held fixed at the middle of its anterior section and was connected
to a small metallic
piston at the midpoint of the posterior section. Because of this design, when
the rods were pushed
forward to generate the saddle, the commissural section protruded into the
ventricular cavity and the
anterior section of the annulus was fixed in place as shown in Fig. 8.
Since the perimeter was constant, the posterior section of the annulus moved
upward,
reducing the septal - lateral diameter of the valve. The piston was used so
that the posterior section
of the annulus did not move apically, only septal-laterally. This variation in
annular septal-lateral
diameter is observed in the native mitral valve when going from a semi-flat
structure in diastole to a
three-dimensional saddle in systole. The whole chain is wrapped in a Dacron
cloth allowing for
extra support and the suturing of the valve. Annular geometry varied from a
completely flat chain
with an approximate orifice area of 6.8 cm2 to a saddle-shaped geometry with
saddle height of 9
CA 02530073 2005-12-20
WO 2005/002469 PCT/US2004/020219
mm. This resulted in a reduction of septal lateral diameter of 3mm, and a
projected two-
dimensional orifice area of 5.4 cm2. A saddle height of 9mm was selected
because it represents an
intermediate point within a disparity of measurements recorded in previous
studies by other
researchers. The annular area and annular area variation were within ranges
observed clinically
during the cardiac cycle.
Strain Gauge Transducers & Force Rods
Miniature C-shaped force transducers were used to measure the tension on
individual
chordae tendinea during the dynamic testing of the valve. The sensitivity
(~0.5 NewtonslVolt) and
linearity of individual transducers was tested prior to and after each
experiment. The minimal
measurable difference in tension for these transducers was (0.5N1V*1.22mv =
6.1x10-4N) when
coupled to the DAQ 1200 PCMCIA data acquisition card (National Instruments,
TX, USA). The
vtalt-age-baseline--vas zer-oed Immedi-ately~bef~re :~~~g:
The modified Georgia Tech left heart simulator used force rods, which attached
to the
sutured PMs, enabling the system to measure the total force applied on each
PM. The rods were
used to define the normal PM position of the valve for both the saddle and
flat annulus
configurations. This system was used as a reference, ensuring a comparable
force on both PMs and
maintaining approximately the same force conditions when changing annular
shape. The
construction and function of these rods has been described in a previous
article.
From the initial eleven valves, six were instrumented with forces transducers
to measure
chordae tendineae force distribution. ~nly six valves were instrumented
because of C- ring
availability. Six C-rings were individually sutured onto the following chords:
anterior strut, anterior
marginal, posterior intermediate, posterior marginal, basal posterior, and
commissural (Fig. 9). It
was not possible to attach all C-rings onto chords extending from a single PM
because of spatial
constraints. Chords were selected according to thickness and implantation
feasibility. 5-0 sutures
(braided silk, Ethicon, NJ, USA) were used to fasten the C-rings to the chords
preventing the ring
from slippage or detachment.
Echocardiographic Imaging
A Diagnostic Ultrasound System SSA-270A with a 3.75MHz phased array transducer
(Toshiba Corporation, Japan) was used to evaluate valve performance. Color
Doppler velocity
mapping was used to monitor valve function and regurgitation. The imaging
depth of the
transducer was 5cm from the valve's annulus and reached an additional 6-8 cm
downstream of the
valve. Lateral views of the valve within the simulator were recorded in video.
The videos and echo
CA 02530073 2005-12-20
WO 2005/002469 PCT/US2004/020219
images of the valve can 14 be observed on our website:
http://www.bme. gatech.edu/,ar'ou~s/cfm~Jweb2/videos.html
Experimental Protocol
The atrial chamber containing the sutured MV was positioned in the left heart
simulator.
The PMs were attached to the force rods and the left heart simulator was then
filled with 0.9°70
saline solution. All transducers and c-rings were zeroed and connected to an
in-house interface box;
which was then connected to a laptop computer. An in-house data collection
program based on
LabVIEW 5.0 software was used to store the flow, pressure and chordal force
curves. This software
stored the curves representing ten cardiac cycles for each variable. These
were then averaged
offline.
After preparing the system, the valve was placed in the defined normal PM
position. The
normal~y o~i~~fln gas de-~-ine-day:
~ Lateral Locatiozz: The papillary muscles arranged parallel to each other and
directly
aligned with the valve's annulus on each commissure. The commissural chords
inserting in the
annulus were vertically perpendicular to the annular plane.
~ Septal-lateral location: The rods were moved septal-laterally until an even
extension
of the commissural chords inserting into the annulus was observed. Normally,
this point was a
couple of millimeters below the annular height midpoint.
~ Basal-Apical locatio~z: The PM rods were moved towards the annulus to a
point
where slack was observed in all the chordae tendineae. The papillary force
rods were zeroed at this
location. Each force rod was pulled apically until a change in voltage of 0.02
volts (0.092 Newtons)
was achieved for that particular rod. This was the minimal significant change
that may be observed
by the system. This defined a position with no slack or apparent tension on
the chordae tendineae.
Valve function at this location was confirmed under pulsatile flow by
observing appropriate
leaflet coaptation.
The simulator ran under physiologic conditions with the valve in the normal
position
(Cardiac output: 5 1/min, Peak trans-mitral pressure: 120mmHg, Cardiac rate:
70 BPM, Systolic
duration: approx. 300ms). Flow, tension, and pressure curves were saved for
offline processing.
After the initial set of recordings with the flat annulus, the shape of the
annulus was shifted
to the saddle-shaped configuration. The PMs were then displaced apically to
compensate for
movement of the commissural section of the annulus into the ventricle. The
force rods were used to
ensure that the same force was applied on the PMs in both the flat and saddle
configurations. All
the previously described data acquisition and Doppler recordings were
performed for this new
CA 02530073 2005-12-20
WO 2005/002469 PCT/US2004/020219
annulus configuration using the same physiolog c flow conditions. Both PMs
were then moved
5mm apically, 5 mm laterally, and 5 mm posteriorly from the normal position.
This constitutes the
symmetrically tethered PM position, which was used to induce mitral
regurgitation. All the
previously described data acquisition and Doppler recordings were performed at
this new PM
position using the same physiological flow conditions for both the flat and
saddle annulus
configurations.
Statistical Analysis
All data are reported as mean ~ 1 standard deviation (STDEV) unless otherwise
stated.
Chordae tendineae forces were normalized for statistical comparisons using the
flat annulus as
control. Means were compared using two-tailed t tests for paired comparisons.
Statistical analysis
was carried out using Minicab (version 13.32) software. A P-value < 0.05 was
considered
st~t~stie~lly si-grtifie-ant. - -
Results
Anatomical Observations
The anatomy of all valves showed dense chordae insertion in the commissural
sections near
the annulus when compared to the other areas of the MV. The midsections of the
base of the
anterior and posterior leaflets showed no direct insertions. The base of the
anterior leaflet presented
a larger area free from basal insertions when compared to the base of the
posterior leaflet, as shown
in Fig. 10(a) The chordae inserting into the central commissural areas
adjacent to the annulus were
significantly shorter than those inserting above and below this location
(35.8% Anterior PM, 44.7%
Posterior PM). These data are represented in Fig. 10(b). The MVs mounted onto
the flexible
membrane showed a saddle shape annular configuration when the PMs were moved
away from the
annulus (see Fig. 6(b)). The different lengths of the basal mural chords and
their insertion pattern
are responsible for this saddle curvature.
In Vitro Experiments
Hemodynamics
All eleven valves were tested at 120 ~ 2mmHg peak transmitral pressure and
average flow
rate of 5.03 ~ 0.1 1/min using the VASAC within the Georgia Tech left heart
simulator.
In the defined normal PM position for both the flat and saddle annular
configuration, the
valves coapted well showing no regurgitant orifices along the coaptation line
or leakage in the
Doppler images. Apical posterior lateral displacement of the PMs induced
tented leaflet
geometries, reproducing configurations observed clinically. Mural
regurgitation was calculated by
integrating the systolic negative volume in the flow curve, which included
both closing and leakage
CA 02530073 2005-12-20
WO 2005/002469 PCT/US2004/020219
volumes. Apical posterior lateral PM displacerln6ent was used to reproduce a
severe pathological
position. During apical posterior lateral PM displacement, the mean
regurgitant volume was 9.8 ~
3.84m1 for the saddle configuration and 10.9 ~ 3.52m1 for the flat
configuration. No significant
difference in mitral regurgitation between the saddle and flat annular
configurations was observed
(p=0.165, n=11).
Chordae Tendineae Tension
Chordal tension was compared using peak systolic values for individual chords.
Of the six
valves tested with c-rings, data from the posterior marginal chord from valve
1 was discarded
because of a strain gauge malfunction detected during the experiment and
confirmed after the
experiment with c-ring calibration. Peak systolic tension measurements under
O.O1N were
discarded, as they could not be distinguished from electrical crosstalk.
E.hordae=tenth=neae t~r~sion.~-~.TT).curves-were--plotted-against_tirr~e
d~ring.ane:car-disc cycle.
Diastolic tension was considered as baseline for the dynamic CTT curves. As
shown in Figs. 11
and 12, CTT curves paralleled the tracing of the transmitral pressure curve.
Fig. 11 are pressure
and chordae tendineae tension curves for valve # 6 in flat annulus
configuration. Fig. 12 are
pressure and chordae tendineae tension curves for valve # 6 in saddled annulus
configuration.
When comparing the systolic peak tensions on the different chords, the
secondary chords
(anterior strut and posterior intermediate chords) bore the larger loads on
each of their respective
leaflets when compared to the primary chords (anterior marginal and posterior
marginal chords).
The anterior strut chord had a tension 0.74 ~ 0.46 N higher than the anterior
marginal chord,
implying on average double the load observed on this marginal chord. The load
on the posterior
intermediate chord was 0.18 ~ 0.16N higher that the load on the posterior
marginal chord. The
commissural chord had a tension considerably smaller than that of the
secondary chords, but close
to that associated with the posterior basal chord.
Differences when comparing the peak systolic tensions in the two different
annular
configurations in the normal PM position, were measured as a percentage change
using the flat
annulus as a control. This eliminates to a certain extent the effects of the
natural variation between
valves. For all valves, the tension on the anterior strut chord was lower in
the saddle configuration
when compared to the flat configuration. The average difference of the force
on this chord was 18.5
~ 16.1 %, being statistically significant (p<0.02, n=6). The average
difference in the posterior
intermediate chord was 22.3 ~ 17.1%, with higher tensions being present in all
the valves for the
saddle configuration. This result was statistically significant, with all
valves showing the same
trend in force variation (p<0.03,n=5). Although all valves showed an increase
in tension for the
CA 02530073 2005-12-20
WO 2005/002469 PCT/US2004/020219
17
posterior marginal chord in the saddle configuration, this change was not
statistically significant
(p=0.12, n=4). Measurements on basal chords also showed an increase in tension
in the saddle
configuration for five valves. The average increase was 48.5 ~ 89.9%, although
not statistically
significant (p=0.12, n=6). In contrast, measurements on the commissural chord
showed a decrease
in tension in the saddle configuration for all valves. The average variation
in force for this chord
was 59.0 ~ 32.2% (p<0.01, n=5). For the anterior marginal chord, two valves
showed a decrease in
tension for the saddle configuration while four valves presented an increase
in tension for this same
configuration. The average increase in tension for the saddle configuration
was 58.5 ~ 111.4%.
However, because of the different tendencies in the results, this increase was
not statistically
significant (p=0.15, n=6).
When comparing the force distribution among the chordae tendineae, the flat
annulus
con=figur~tit~n -showed= a higher- -variak~llifiy- of -tensifln between the-
differ-ent chords -S~DEV=
0.47N, when compared to the saddle configuration STDEV= ~ 0.36N.
A summary of the peak systolic tensions on the chords and their variation from
one annular
configuration to the other are presented in Table 1.
CHORD NUMBER PEAK SYSTOLIC PEAK SYSTOLIC STATISTICAL
OF TENSION TENSION
DIFFERENCE
SPECIMENSFLAT ANNULUS SADDLED ANNULUS SIGNIFICANCE
Newtons (~/ )
( ) (Newtons) P-Value
Anterior Strut6 1.2210.52 0.950.35 18.516.1 0.018
Posterior Intermediate5 0.2510.14 0.3010.18 -22.317.10.022
Posterior Marginal4 0.030.04 0.060.05 -137.81188.60.12
basal posterior6 0.1910.10 0.3110.25 48.589.9 0.122
Anterior Marginal6 0.310.17 0.35f0.16 -58.51111.40.145
Commissural 5 0.1710.18 0.110.20 59.032.3 0.008
TABLE1
Discussion
Mitral Annulus Shape
The results describe increases in length of the basal chords from the
commissural to the
anterior and posterior segments of the annulus, which are larger than those
determined by
Pythagorean relations. When the chordae tendineae are extended and the annulus
is relatively free
to deform, the MA generates a saddle-shaped configuration. Chordae tendineae
lengths are
CA 02530073 2005-12-20
WO 2005/002469 PCT/US2004/020219
approximately constant during the cardiac cycle and there is a higher density
of basal chordae
inserting into the commissural section of the annulus. As a consequence, when
under systolic
pressure the mitral valve is pushed backwards. The free posterior and anterior
sections of the
annulus deflect into the atrium, while the commissural sections are relatively
held in place by the
PMs and corresponding chords. Annular flexinglbending and anatomical relations
may partially
explain the saddle shape of the annulus, but the shape of the mitral annulus
is not a simple
symmetric elliptical saddle, but a complex asymmetrical saddle structure.
Other phenomenon such
as myocardial contraction, aortic expansion, PM contraction and ventricular
motion may affect the
shape of the annulus. Therefore, the complex inter-relation between these
mechanisms warrants
further investigation.
Varve Function
-Gearnet~e~l-~ var-i-ations ~ -in mi-tra.l annular- shape- have- been obser-
ved in patient$ with-
pathologies such as functional mitral regurgitation, hypertrophic obstructive
and dilated
cardiomyopathy, and ischemic mitral regurgitation. Loss of saddle curvature
has been described as
a possible cause for mural regurgitation in animal and human studies. Patients
with FMR showed
loss of curvature in the saddled annulus, which subsequently may increase
annular area because of
reduced flexing. In-vitro studies have shown that only increases in projected
area over a factor of
1.75 will induce mitral regurgitation without PM displacement. Therefore, area
changes associated
with a loss of curvature are not sufficient to induce regurgitation. The loss
in curvature in FMR
patients may be related to changes in ventricular and PM dynamics since loss
of annular
displacement, curvature, and dynamical change have also been observed in
regurgitation associated
pathologies. Therefore, loss of annular curvature and regurgitation may not
hold a cause
consequence relationship, but both may have similar origins. This may explain
why variation of
annular shape alone (flat-saddle) did not induce mitral regurgitation as
represented by the results of
this study.
Chordae Tendineae Force Distribution
The results showed for both configurations, force distributions characterized
by the
secondary chords carrying most of the load on their respective leaflet. This
phenomenon has been
observed and analyzed by other researchers. The saddle configuration showed a
more evenly
distributed force as illustrated by the variance of the tensions on the
different chords. This
phenomenon may also be observed in Figs. 11 and 12 where the tension curves
for the different
chords are closer for the saddle configuration. Therefore, the saddle
configuration optimizes the
CA 02530073 2005-12-20
WO 2005/002469 PCT/US2004/020219
force distribution on the valve since a larger number of chords are extended
and the load is divided
more evenly among them.
The forces on the MV and its apparatus are determined by several factors:
valve geometry,
leaflet area, transmitral pressure, and contact forces along the coaptation
line. In parallel, leaflet
curvature has been shown to be important in valve mechanics, as billowing
(primary curvature) and
saddle curvature (secondary curvature) may reduce stress on leaflets. The
reduction in force for the
anterior strut chord may be explained by a redistribution of the force vectors
caused by pressure
because of the secondary curvature generated by the saddle (Fig. 13). Since
pressure acts
perpendicularly to the surface, due to the secondary curvature of the saddle,
more force vectors are
directed towards the commissural direction and less in the apical direction.
The anterior strut chord
is directed mostly in the apical direction implying that its tension will be
reduced if the apical force
component -gene-gated by pressure dec-re-asesChords ~ex~tent~rig -in ot-her-
d3reetions-- ~u°st then
balance the new redirected components of the force. These redirected vectors
may explain the
increase in force on the other chords.
The peak systolic tension of the anterior marginal chord did not vary
significantly, and this
variation followed different trends for different valves. The peak systolic
tension values for the
posterior marginal chord were small and near the limit of the c-ring crosstalk
range, all the values
above the crosstalk threshold followed the same trend of increased force. This
variability may be
explained by the fact that marginal chords insert on the outer edge of the
leaflet implying that
tension on this chord is predominantly determined by transmitral pressure,
contact forces, and
coaptation line geometry and location. Leaflet curvature may have a lesser
effect on marginal
chords than on secondary chords.
During coaptation, the posterior leaflet central scallop is extended for the
most part and it is
smaller than the anterior leaflet; therefore, the effects of~force
redistribution are probably less than
those seen on the anterior leaflet. Meanwhile, the relative distance from the
PMs to the anterior and
posterior segments of the annulus is increased by the saddle geometry. This
increase in length,
coupled with the decreased effect of the saddle curvature may explain the
increased tension on the
posterior intermediate chord. The basal posterior chord inserts directly above
the posterior section
of the annulus. The increased distance between the PMs and the annulus may
account for the
increased tension on this particular chord in the saddle configuration.
The clinical relevance of this study lies both in the cardiac implant field as
well as in the
surgical field. Considering the results, which clearly show that the shape of
the annulus alters the
force distribution among the chords, implants such as annuloplasty rings must
consider the effect of
CA 02530073 2005-12-20
WO 2005/002469 PCT/US2004/020219
their implantation on chordal force distribution Increased tension on the
chords under cyclic
loading implies under engineering standards a lower life expectancy for the
chords because of
possible tissue damage. On the other hand, reduction in force may increase the
life expectancy of
the chord, but if severe, this reduction may induce negative effects on valve
function. Several
authors have proposed chordal cutting as an alternative procedure for
pathologies such as ischemic
mitral regurgitation. For the most part surgeons have observed that cutting
the primary (marginal
chords) induces severe regurgitation, but that in some pathologies cutting the
secondary
(intermediate chords) may decrease leaflet tenting leading to better
coaptation and decreasing
regurgitation. Some surgeons are reluctant to use these procedures because
cutting the large
secondary chords may induce significantly higher loads on other chords that
may eventually fail due
to structural deterioration. As shown in our results, the secondary chords do
carry the highest loads
and- ~tllere-fore- .are-sir-u~t~ural~y .:r-elevent to rr~utral- vufv~--
function: 'Fher~fore, the.. mere-ased- -1«ad
generated by cutting these chords warrants further detailed/fundamental
studies, both itz vitro and in
vivo.
Limitations
There are several limitations associated with both the apparatus and the
procedure. An
initial limitation of this study was the limited population of human MVs.
Unfortunately, this is the
situation involving any study that utilizes human organs. Even though the
chordae were carefully
selected under a strict characterization protocol, their size and ramification
varied from valve to
valve. MV leaflet size and coaptation geometry also varied from valve to
valve. Coaptation line
location and geometry also varied from valve to valve although a standard
normal position was
used. Because of this natural variability and the reduced population, the
standard deviations for the
results were high.
The left ventricle heart simulator has several limitations, but it has been
used successfully in
several studies. Although the pressure and flow conditions generated in this
loop are physiological,
it does not reproduce phenomenon such as ventricular, atrial, or papillary
muscle contractions since
it is a rigid simulator. More important to this study, we used a static
annulus, which did not vary in
size or shape during the cardiac cycle. The VASAC was designed to mimic
geometrical conditions
found during peak systole when the saddles curvature is at its maximum.
Annular motion, which
has been to some extent related to mitral regurgitation was not modeled.
Measurement of tensions using the c-ring transducers had some technical
limitations. The
weight of these transducers even if minimal compared to other transducers may
affect readings of
absolute tension on the chords. However, for dynamic changes in tension, the
variation generated
CA 02530073 2005-12-20
WO 2005/002469 PCT/US2004/020219
21
by the weight of the transducers is not significant especially in chords with
high loads. The
level/noise/crosstalk range is high for these transducers when measuring the
forces on the minor
chords. Although the crosstalk margin persisted, acquiring data over ten
cardiac cycles and
averaging the readings over the cardiac cycle reduced level/noise error.
Future Work
Considering the limitations of the current VASAC, Applicants intend to
construct an
annulus model that will self modulate its annular shape during the cardiac
cycle. Data acquired
with the new model will be compared to present data to observe the difference
in force distribution
between a flexible annulus and a rigid annulus. This comparison could clarify
the difference in
force distribution generated on the mitral valve after the implant of a rigid
annuloplasty ring. The
protocol will be modified so that the chordae tendineae cross-sectional area
is available, this will
a~Io~-vs -to eaiculate-=the stress-~on -the differrent -chords -and -t-her-
efore -t~tain vulual~le -i3rforrnation
about chordae tendineae failure mechanics. Another important factor that
should be simulated in
future work is PM function including contractility. Therefore, a larger range
of physiological and
pathological conditions related to PM function could be studied. Finally,
after having a broad
understanding of normal MV mechanics, abnormal valves may be studied in order
to understand the
mechanics of their pathologies. Surgicalprocedures could be reproduced in
vitro using these
diseased valves, to observe the effectiveness of the proposed correction
methods.
Conclusions
Although not all conditions of mitral annular mechanics were replicated, this
study
simulated the effect of changing annular geometry from a flat ring to a three-
dimensional saddle on
chordae tendineae force distribution and mitral regurgitation due to PM
displacement. A saddle-
shaped geometry reduces mitral annulus orifice area by decreasing the septal-
lateral diameter of the
valve. However, annular shape alone does not significantly affect mitral
regurgitation due to
papillary muscle displacement because of the MVs redundant leaflet design.
Annular geometry directly affects tension on the basal chords by varying the
relative
distance from their insertion point to the PMs. The tension on the anterior
strut chord is
significantly reduced by a saddle-shaped annular geometry because the
secondary curvature of the
anterior leaflet causes redirection of the force vectors generated by
pressure. The natural
configuration of the MA is that of a three-dimensional saddle. In this
configuration more chords are
extended and a secondary curvature in the leaflets is induced. Therefore, the
saddle-shaped annulus
redistributes the forces on the chordae tendineae leading to a more even
distribution of tensions
among the chords.
CA 02530073 2005-12-20
WO 2005/002469 PCT/US2004/020219
22
While the invention has been disclosed in its preferred forms, it will be
apparent to those
skilled in the art that many modifications, additions, and deletions can be
made therein without
departing from the spirit and scope of the invention and its equivalents as
set forth in the following
claims.