Note: Descriptions are shown in the official language in which they were submitted.
CA 02533683 1999-11-30
-1-
MAGNETIC SEPARATION APPARATUS AND METHODS
Leon W. M. M. Terstappen
SUMMARY
According to one aspect of the present invention there is provided an
apparatus for
observing magnetically responsive microscopic entities suspended in a fluid
member. The
apparatus includes a vessel having a transparent wall and a chamber formed
therein for
containing the fluid medium; a ferromagnetic capture structure supported on
the interior
surface of the transparent wall; and magnetic means for inducing an internal
magnetic
gradient in the vicinity of the ferromagnetic capture structure, whereby the
magnetically
responsive entities are immobilized along the wall adjacent to the capture
structure;
wherein the ferromagnetic capture structure comprises a plurality of
ferromagnetic
members having a non-uniform spacing between adjacent members.
In particular, the invention related to methods for isolating, collecting,
immobilizing, and/or analyzing microscopic biological specimens or substances
which are
susceptible to immunospecific or non-specific binding with magnetic-responsive
particles
having a binding agent for producing magnetically-labeled species within a
fluid medium.
As used herein, terms such as "target entity" shall refer to such biological
specimens or
substances of investigational interest which are susceptible to such magnetic
labeling.
U.S. Patent No. 5,985,853 describes an apparatus and method wherein an
external
magnetic gradient is employed to attract magnetically labeled target entities
present in a
collection chamber to one of its surfaces, and where an internal magnetic
gradient is
employed to obtain precise alignment of those entities on that surface. The
movement of
magnetically labeled biological entities to the collection surface is obtained
by applying a
vertical magnetic gradient to move the magnetically labeled biological
entities to the
collection surface. The collection surface is provided with a ferromagnetic
collection
CA 02533683 2007-01-31
-2-
structure, such as plurality of ferromagnetic lines supported on an optically
transparent surface.
Once the magnetically labeled biological entities are pulled sufficiently
close to the surface by the externally applied gradient, they come under the
influence of an intense local gradient produced by the ferromagnetic
collection
structure and are immobilized at positions laterally adjacent thereto. The
local
gradient preferably exceeds adhesion forces which can hold the biological
entities
to the transparent surface after they collide with the surface. Alternatively,
the
adhesiveness of the surface must be sufficiently weak to allow the horizontal
magnetic force to move the magnetically labeled biological entities towards
the
ferromagnetic structures. The smoothness and the hydrophobic or hydrophilic
nature of the surface are factors that can influence the material chosen for
the
collection surface or the treatment of this surface to obtain a slippery
surface.
In accordance with the present invention, there are described further
alternative embodiments and improvements for the collection chamber, the
interior geometry of the collection chamber, and further useful techniques
that
may be accomplished by use of a vertical magnetic gradient separator
structure.
BRIEF DESCRIPTION OF THE FIGURES
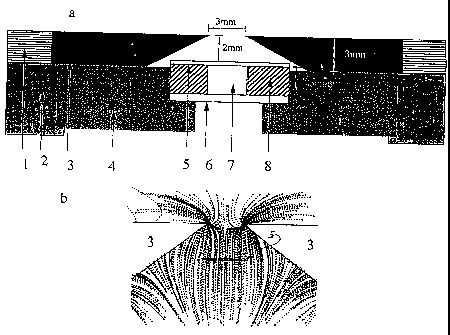
FIG. I A is a schematic diagram of a magnetic separator.
FIG. I B is a diagram showing the magnetic field provided in the magnetic
separator of FIG. 1 A
FIGS. 2A-C are microphotographs of specimens collected in a magnetic
separator.
FIGS. 3A-I are plan views of alternative ferromagnetic collection
structures for use in a magnetic separator.
FIG. 4 is a schematic diagram of an optical tracking and detection mechanism
for analyzing species collected in a magnetic separator.
FIGS. 5A-B are histograms of fluorescence signals obtained from a magnetic
separator (5A) and from a flow cytometer (5B) employed to quantify species in
identical fluid samples.
CA 02533683 2007-01-31
-3-
FIGS. 6A-6B are microphotographs of specimens collected in a magnetic
separator.
FIGS. 7A and 7B are successive schematic diagrams sowing a method of
charge-enhanced collection in a magnetic separator.
FIGS. 8A and 8B are respective cross-sectional and plan views of a
combined ferromagnetic and electrically conductive collection structure for a
magnetic separator.
FIGS. 9A-9C are successive schematic views showing a method of
particle separation in a magnetic separator.
FIGS. l0A and 10B are successive schematic views showing a method of
measuring particle density in a fluid having an unknown particle density.
FIG 10C is a histogram of cell density along a collection surface.
FIGS. 11A and 11B are sectional views of a separation vessel configured for
of multiple simultaneous analysis of fluids containing multiple target species
at
differing concentrations.
DETAILED DESCRIPTIONS
1. Vertical Gradient Collection and Observation of Target Entities
In a first embodiment of the invention, target entities such as cells are
collected against a collection surface of a vessel without subsequent
alignment
adjacent to a ferromagnetic collection structure. The collection surface is
oriented
perpendicular to a magnetic field gradient produced by external magnets. In
this
embodiment, magnetic nanoparticles and magnetically labeled biological
entities
are collected in a substantially homogeneous distribution on an optically
transparent surface while non-selected entities remain below in the fluid
medium.
This result can be accomplished by placing a chamber in a gap between two
magnets arranged as shown in FIG. lA, such that the chamber's transparent
collection surface is effectively perpendicular to a vertical field gradient
generated by external magnets 3. The magnets 3 have a thickness of 3 mm, and
are tapered toward a gap of 3 nun. The magnets 3 are held in a yoke 1, which
rests atop a housing 2. A vessel support 4 holds the vessel 6 in a region
between
CA 02533683 1999-11-30
-4-
the magnets where the lines of magnetic force are directed substantially
perpendicular to the collection surface 5 of the vessel 6. The collection
surface of
the vessel is preferably formed of a 0.1 mm thick polycarbonate member. The
collection surface is parallel to, and 2 mm below, the upper surface of the
external magnets 3. The space between the inner, top surface edges of the
magnets is 3 mm.
The taper angle of the magnets 3 and the width of the gap between the
two magnets determine the magnitude of the applied magnetic field gradient and
the preferable position of the collection surface of the vessel. The field
gradient
produced by the magnets can be characterized as having a substantially uniform
region, wherein the gradient field lines are substantially parallel, and
fringing
regions, wherein the gradient field lines diverge toward the magnets. FIG. 1B
shows mathematically approximated magnetic field gradient lines for such a
magnet arrangement. The magnetic field lines (not shown) are predominantly
parallel to the chamber surface while the gradient lines are predominantly
perpendicular to it. To collect a uniformly-distributed layer of the target
entities,
the vessel is positioned to place the chamber in the uniform region such that
there
are substantially no transverse magnetic gradient components which would cause
lateral transport of the magnetically labeled biological entities to the
collection
surface.
To illustrate the collection pattern of magnetic material on the collection
surface area, a chamber with inner dimensions of 2.5 mm height (z), 3 mm width
(x) and 30 nun length (y) was filled with 225 l of a solution containing 150
nm
diameter magnetic beads and placed in between the magnets as illustrated in
FIG.
lA. The magnetic beads moved to the collection surface and were distributed
evenly. When the vessel was elevated relative to the magnets, such that a
significant portion of the top of the vessel was positioned in a fringing
region,
significant quantities of the magnetic particles parallel toward and
accumulated at
respective lateral areas of the collection surface positioned nearest the
magnets.
In order to enhance uniformity of collection on the collection surface, the
surface material can be selected or otherwise treated to have an adhesive
CA 02533683 1999-11-30
-5-
attraction for the collected species. In such an adhesive arrangement,
horizontal
drifting of the collected species due to any deviations in positioning the
chamber
or deviations from the desired perpendicular magnetic gradients in the
"substantially uniform" region can be eliminated.
An example of the use of the present embodiment discussed device is a
blood cancer test. Tumor derived epithelial cells can be detected in the
peripheral
blood. Although present at low densities, 1- 1000 cells per 10 ml of blood,
the
cells can be retrieved and quantitatively analyzed from a sample of peripheral
blood using an anti-epithelial cell specific ferrofluid. FIG. 3 illustrates an
example of the use of the magnets and the chamber with no ferromagnetic
structure on the collection surface to localize, differentiate and enumerate
peripheral blood selected epithelial derived tumor cells. In this example, 5
ml of
blood was incubated with 35 pg of an epithelial cell specific ferrofluid
(EPCAM-FF, Immunicon Corp. Huntingdon Valley, PA) for 15 minutes. The
sample was placed in a quadrupole magnetic separator (QMS 17, Immunicon
Corp.) for 10 minutes and the blood was discarded. The vessel was taken out of
the separator and the collected cells present at the wall of the separation
vessel
were resuspended in 3 ml of a buffer containing a detergent to permeabilize
the
cells (Immunoperm, Immunicon Corp.) and placed back in the separator for 10
minutes. The buffer containing the detergent was discarded and the vessel was
taken out of the separator and the cells collected at the wall were
resuspended in
200 l of a buffer containing the UV excitable nucleic acid dye DAPI
(Molecular
Probes) and Cytokeratin monoclonal antibodies (identifying epithelial cells)
labeled with the fluorochrome Cy3. The cells were incubated for 15 minutes
after
which the vessel was placed in the separator. After 5 minutes the uncollected
fraction containing excess reagents was discarded, the vessel was taken out of
the
separator and the collected cells were resuspended in 200 l of an isotonic
buffer.
This solution was placed into a collection chamber and placed in the magnetic
separator shown in FIG. lA. The ferrofluid labeled cells and the free
ferrofluid
particles moved immediately to the collection surface and were evenly
distributed
along the surface as is shown in FIG. 2A. The figure shows a representative
area
CA 02533683 1999-11-30
-6-
on the collection surface using transmitted light and a 20X objective. In FIG.
2B
the same field is shown but now a filter cube is used for Cy3 excitation and
emission. Two objects can be identified and are indicated with I and 2. FIG.
2C
shows the same field but the filter cube is switched to one with an excitation
and
emission filter cube for DAPI. The objects at position 1 and 2 both stain with
DAPI as indicated at positions 3 and 5 confirm -their identity as epithelial
cells.
Additional non epithelial cells and other cell elements cells are identified
by the
DAPI stain; an example is indicated by the number 4.
II. Ferromagnetic collection structures producingcentral alignment of cells
To provide for spatially patterned collection of target entities, a
ferromagnetic collection structure can be provided on the collection surface
of the
vessel, in order to produce an intense local magnetic gradient.for
immobilizing
the target entities laterally adjacent to the structures. The various
ferromagnetic
structures described below have been made by standard lithographic techniques
using Nickel (Ni) or Penmalloy (Ni-Fe alloy). The thickness of the evaporated
metal layers was varied between 10 nm to 1700 nm. The 10 nm structures were
partially transparent. The immobilizing force of these thin structures was,
however, considerably less than those in the 200 - 700 nm thickness range.
Although immobilization and alignment of magnetically labeled biological
entities occurred sufficiently reliably, use of these moderately thicker
structures
was facilitated by a collection surface which had no or little adhesive force.
Collection structures thicknesses between 200 and 1700 nm were effective in
capturing the magnetically labeled biological entities and overcoming the
surface
adhesion.
FIGS. 3A through I show various magnets for ferromagnetic collection
structures.
In FIG 3B the ferromagnetic collection structure comprises Ni wires with
a spacing comparable to the cell diameter (nominally 10 microns). A decrease
in
the spacing between the wires shown in FIG. 3C, produces a much more uniform
CA 02533683 1999-11-30
-7-
cell position relative to the wire edge. Almost all cells appear to be
centrally
aligned. However, a portion of each cell overlaps, and is obscured by, the Ni
wire.
Cells collected along the ferromagnetic collection structures can be
detected by an automated optical tracking and detection system. The tracking
and
detection system, shown in FIG. 4, employs a computer controlled motorized
stage to move the magnets and chamber in the X and Y directions under a laser
beam having an elliptical 2-15, m spot. The maximum speed of the table is 2
cm/sec in the Y direction, and 1 mm/sec in the X direction. Two cylindrical
lenses 11 and 12 and a position adjustable objective 13 taken from a Sony
Compact Disc player were used to make a 2 x 15 m elliptical spot on the sample
with a 635 nm laser diode 14 as a light source (see inset 5). The light
reflected
from the sample was projected on a photomultiplier 16 through a dichroic
mirror
17 a spherical lens 18, a diaphragm 19 and band pass filters 110. Measurement
of
differences in the polarization direction of the light reflected from the
wires and
projected on a quadrant photodiode 111 through the mirror 112, the dichroic
mirror 17, a quarter-wavelength plate 113, a polarized beam splitter 114 a
cylindrical lens 115 and a spherical lens 116 were used to determine the
position
of the laser spot on the sample and to feed back a signal to the objective 13
to
correct its position for any deviations (see insert 17). A photodiode 118 was
positioned perpendicular to the sample and was used to measure light scattered
from the illuminated events. The feedback mechanism of the tracking system
were optimized such that the laser beam kept the same X and Z position with
respect to the lines while scanning in the Y direction with speeds up to 1
cm/sec.
At the end of the 2 cm long line the position of the objective was changed to
the
next line, this was repeated until all the lines of the chamber were scanned.
To evaluate the performance of the tracking and detection system and
compare it to that of a flow cytometer, 6 m polystyrene beads were prepared
which were conjugated to ferrofluid as well as to four different amounts of
the
fluorochrome Cy5. The beads were used at a concentration of l O5m1"i placed
into
a chamber with ferromagnetic collection structures of the type illustrated in
FIG
CA 02533683 1999-11-30
-8-
3C. The chamber was placed in the uniform gradient region between the two
magnets and all beads aligned between the lines. The tracking and detection
system was used to measure the fluorescence signals obtained while scanning
along the ferromagnetic wires. FIG. 5A shows a histogram of the fluorescence
signals of the bead mixture. Four clearly resolved peaks are discernible
representing the beads with no Cy5, dimly, intermediate and brightly labeled
with
Cy5. A mixture of the same beads was made and measured with a flow cytometer
also equipped with a 635 nm laser diode (FACScalibur, BDIS, San Jose CA).
The histogram of the fluorescence signals is shown in FIG. 5B and shows that
although four different populations were discemible, they are clearly less
resolved than in case samples were measured with the magnetic immobilization
cytometer of the present invention. These results demonstrate that the
alignment
of the beads obtained with the system described herein provides a sensitivity
and
accuracy of the measurement of fluorescent beads which is superior to that of
the
flow cytometer.
In applications where it is desired to simultaneously measure biological
entities with significant differences in size, the collection structure can be
configured to have a non-uniform geometry in order to centrally-align cells or
other species of differing sizes. An example of such a structure is shown in
FIG.
3D. A collection structure pattern was made with one area of the collection
surface having wires with a period of 10pm and a spacing of 7 m, and another
area having wires with a period of 25 m and a spacing of 7 m. This was used
to collect both the small platelets and the larger leukocytes from whole
blood.
Before collection, the blood was incubated with ferrofluids specific for
platelets
and leukocytes i.e. a ferrofluid labeled with the monoclonal CD41 and a
ferrofluid labeled with the monoclonal antibody CD45 respectively. The
leukocytes and platelets align along the wires in the respective areas of the
collection surface as is illustrated in FIG. 3D. The measurement of the
platelets
can be performed at the area with the small spaces between the wires and the
measurement of the leukocytes can be performed at the area with the larger
spaces between the wires. The variation of gap width along the length of the
CA 02533683 1999-11-30
-9-
ferromagnetic structure provides linear alignment of the collected cells of
different sizes along a common central axis.
Many more collection structure patterns are possible within the scope of
the invention for capturing and centrally aligning cells of varying sizes in a
single
sample. Four examples are illustrated in FIGS. 3E, 3F, 3G, 3H and 31. FIG. 3E
shows a similar wire spacing as shown in FIG -3C, but the wires have lateral
protrusions formed along the lengths thereof. For the geometry of FIG. 3E,
there
were two positions chosen by the cells - to the left or right of the
protrusions as
shown. Such a design induces a periodic positioning of the cells in both axes
of
the collection plane. Adding a asymmetric triangular "prong" edge shape
instead
of a "bar," as illustrated in FIG. 3F removes the slight (right-left) asymetry
observed in the FIG 3E. Adding a larger asymmetric triangular "prong" edge
shape as is illustrated in FIG. 3G is also effective for cells of varying
sizes. A
sharper triangular style is illustrated in FIGS. 3H. FIG. 31 shows an array of
isolated rectangles, with their spacing along one axis set to match the cell
size.
The spacing along the other axis exceeds the cell size, so that cells move
freely
toward the positions between more closely-spaced sides of the rectangles.
An example of the utilization of custom designed ferromagnetic structure
on the collection surface is a blood cancer test. Tumor derived epithelial
cells can
be detected in the peripheral blood and can be retrieved quantitatively from
peripheral blood using anti- epithelial cell specific ferrofluids. The
physical
appearance of the tumor derived epithelial cells is extremely heterogeneous
ranging from 2- 5 m size apoptotic cells to tumor cell clumps of 100 m size
or
more. To accommodate this large range of sizes, triangular shaped
ferromagnetic
structures as schematically illustrated in FIGS. 3G or 3H can be used. An
example of the positioning of peripheral blood derived cancer cells is
illustrated
in FIG. 6. In this example 5 ml of blood was incubated with epithelial cell
specific ferrofluid (EPCAM-FF, Immunicon Corp.) and processed using the same
method as described above. The final cell suspension was placed in the
magnetic
separator. The ferrofluid labeled cells and the free ferrofluid move
immediately to
the collection surface. FIG. 6A shows an area on the collection surface using
CA 02533683 1999-11-30
-10-
transmitted light and a 20X objective. The ferromagnetic collection structure
is
indicated with 1, the open wide collection space with 2, the narrow collection
space with 3 and a large object with 4. FIG. 6B shows the same area only now
UV excitation is used. The large object indeed is a large cell as confirmed by
the
staining with the nuclear dye indicator 5 and is nicely aligned. The tracking
system described in FIG. 4 was successfully used to scan along the
ferromagnetic
structures illustrated in FIGS. 3H and 6A.
III. Addressable ferromagnetic collection structures
In addition to using ferromagnetic structures to create high local magnetic
gradients, they also can serve as electronic conductors to apply local
electronic
fields charges. Furthermore, electronic conductors can be formed on the
collection surface to allow electronic manipulation of the collected target
entities.
The ability to first move biological entities to a specific location followed
by an
optical analysis is, schematically illustrated in FIG. 7A. Subsequent
application
of general or localized electronic charges, shown in FIG. 7B adds another
dimension to the utility of the described system. Useful applications of local
electronic charges for applications involving cells, RNA, protein and DNA are
known. A schematic drawing of one design of such a collection surface is
illustrated in FIG. 8. To optimize the control over the electronic charge one
can
first evaporate a specific pattern / layers of Aluminum 1 onto an optically
transparent substrate 4, which provides an electronic circuit to the
individual
ferromagnetic structures, 5 in FIG. 8B. The next layer of Ni or other
ferromagnetic material is evaporated onto the substrate, 2 in FIG. 8A, to
create
the individual ferromagnetic structures 5 in FIG. 8B. An insulating layer 3
can be
obtained by the evaporation of SiOZ or other insulating material. Magnetically
labeled biological entities 7 localize in between the ferromagnetic
structures.
Electronic charge can then be applied to improve the specificity of the
immunospecifc binding, change the orientation of the captured biological
entity
according to its electronic polarity, or to modify the entity properties (for
CA 02533683 1999-11-30
-11-
example, to"explode" it) by applying an electronic charge to the conductors.
The
biological entities can be studied before and/or after application of
electronic
charges.
IV. Porous Chamber Surfaces for Excess Particle Removal
When large initial volumes of fluid samples are processed and reduced to
smaller volumes by magnetic separation, the concentration of the nanometer
sized (<200 nm) magnetic labeling particles increases proportionally. The
collection surface in the chambers has a limited capacity for capturing
unbound
excess magnetic particles, and these particles may interfere with the
positioning
and observation of the magnetically labeled biological entities. An
arrangement
for separating unbound excess magnetic labeling particles from the magnetic
labeled biological entities is illustrated in FIG. 9. The collection chamber
comprises an outer compartment 1 and an inner compartment 2. The fluid sample
containing unbound magnetic particles 3 and magnetically labeled and non-
labeled biological entities 4 is placed in the inner compartment 2. At least
one
surface 5 of the inner chamber is porous, for example, a filter membrane
having a
pore size between 0.5 and 2 m. Magnetic nanoparticles can pass through the
pores, but the larger magnetically labeled cells cannot. The opposite surface
of
the inner chamber 6 consists of a transparent surface with or without
ferromagnetic collection structures as described above.
After the inner chamber is filled with the fluid sample, the outer chamber
is filled with a buffer. The vessel is then placed between the two magnets as
shown in FIG. 9B. The chamber is positioned so that respective lateral
portions
of the vessel extend into the fringing magnetic gradient region. The unbound
magnetic particles are transported by the magnetic gradient through the
membrane (5) and toward respective lateral regions 8 of the outer chamber (1).
This movement is consistent with the magnetic gradient field lines shown in
FIG.
I B. The lateral accumulation of the particles is effectively aided by the
CA 02533683 1999-11-30
-12-
horizontal movement of those nanoparticles which first hit the surface and
then
slide along the slippery surface (7').
Magnetically labeled biological entities such as cells also move according
to the gradient lines (9) until they reach the membrane, whereas non magnetic
biological entities settle to the bottom under the influence of gravity. After
the
separation of unbound particles is complete, the chamber is taken out of the
magnetic separator and inverted (10). The chamber is repositioned in the
uniform
gradient region to optimize the homogeneity of the distribution of the cells
at the
collection surface, FIG. 9C. The magnetically labeled cells move towards the
optically transparent surface (6) (indicated with 11 in FIG. 9B and 14 in FIG.
9C)
whereas the non magnetic biological entities settle to the membrane (5) under
the
influence of gravity. The free magnetic nanoparticles move vertically toward
the
surface 6. The free magnetic nanoparticles are no longer present in the
observation path and the magnetically labeled biological entities can be
examined. The system described above is especially suitable for applications
in
which the target cell number is low, in order to avoid clogging the membrane.
V. Long,itudinal Variation of chamber height
The height of the chamber in concert with the concentration of the target
entity determines the density of the distribution of target entities collected
at the
collection surface of a vessel such as described above. To increase the range
of
surface collection densities which are acceptable for accurate counting and
analysis, one can vary the height of the chamber to eliminate the need to
dilute or
concentrate the sample, for analysis of samples where the concentration may
vary
widely. In FIG. l OA, a cross section of a chamber is shown with a collection
surface 1, and six compartments having different heights. Target cells are
randomly positioned in the chamber. In FIG. 10B the same cross section is
shown
but now the cells have moved to the collection surface under the influence of
the
magnetic gradient. In the area of highest chamber depth, the density of the
cells is
to high to be accurately measured whereas in the area of the lowest chamber
= CA 02533683 2007-01-31
-13-
depth to few cells are present to provide an accurate cell count. To further
illustrate this principle, a histogram of the cell density along the
collection
surface is shown in FIG. l OC. Note that the number of cells in the area with
the
highest density is underestimated. The approach described here increases the
range of concentrations which can be accurately measured as compared to the
cell
number measurements traditionally used in hematology analyzers and flow
cytometers.
VI. Different compartments in the chamber
Different types of target entities present at different densities can be
present in the sample. To permit simultaneous multiple analyses, chambers can
be made with multiple compartments. An example of such a chamber is
illustrated in FIG. 11A. The collection surface I and two separate
compartments
2 and 3 in these chambers permit the usage of a different set of reagents. In
case
areas in the chamber are not separated by a wall as illustrated with 4 in FIG.
11B
in the reagents used will move all magnetically labeled cell types to the top.
An
example is for instance the simultaneous use of a leukocyte specific and a
platelet
specific ferrofluid. The density of the platelets is considerable larger than
that of
the leukocytes, measurement of the platelets would thus be done in the shallow
part of the chamber (which may have a relatively small line spacing on the
collection suiface) and measurement of the leukocytes would be performed in
the
deeper part of the chamber (which may have a relatively larger line spacing on
the collection surface; such as the arrangement shown in FIG. 3D.
The terms and expressions which have been employed are used as terms
of description and not of limitation. There is no intention in the use of such
terms
and expressions of excluding any equivalents of the features shown and
described
or any portions thereof. It is recognized, therefore, that various
modifications are
possible within the scope of the invention as claimed.