Note: Descriptions are shown in the official language in which they were submitted.
CA 02534080 2006-01-30
WO 2005/012456 PCT/US2004/024804
Background of the Invention
[0003] Drilling fluids used in the drilling of subterranean oil and gas wells
as well
as other drilling fluid applications and drilling procedures are known. In
rotary
drilling there are a variety of functions and characteristics that are
expected of
drilling fluids, also known as drilling muds, or simply "muds". The drilling
fluid is
expected to carry cuttings up from beneath the bit, transport them up the
annulus, and allow their separation at the surface while at the same time the
rotary bit is cooled and cleaned. A drilling mud is also intended to reduce
friction
between the drill string and the sides of the hole while maintaining the
stability of
uncased sections of the borehole. The drilling fluid is formulated to prevent
unwanted influxes of formation fluids from permeable rocks penetrated and also
often to form a thin, low permeability filter cake which temporarily seals
pores,
other openings and formations penetrated by the bit. The drilling fluid may
also
be used to collect and interpret information available from drill cuttings,
cores and
electrical logs. It will be appreciated that within the scope of the claimed
invention
herein, the term "drilling fluid" also encompasses "drill-in fluids".
[0004] Drilling fluids are typically classified according to their base
material. In
water-based muds, solid particles are suspended in water or brine. Oil can be
emulsified in the water or brine. Nonetheless, the water is the continuous
phase.
Oil-based muds are the opposite. Solid particles are suspended in oil and
water
or brine is emulsified in the oil and therefore the oil is the continuous
phase. Oil-
based muds that are water-in-oil emulsions are also called invert emulsions.
Brine-based drilling fluids, of course are a water-based mud in which the
aqueous component is brine.
[0005] Optimizing high performance water base mud design is commonly at the
forefront of many drilling fluid service and oil operating companies' needs
due to
the various limitations of invert emulsion fluids. Invert emulsion fluids
formulated
with traditional diesel, mineral or the newer synthetic oils are the highest
performing drilling fluids with regard to shale inhibition, borehole
stability, and
lubricity. Various limitations of these fluids, however, such as environmental
concerns, economics, lost circulation tendencies, kick detection, and geologic
evaluation concerns maintains a strong market for high performance water based
fluids. Increased environmental concerns and liabilities continue to create an
154-23110-CIP 1
CA 02534080 2008-10-23
WO 2005/012456 PCT/US2004/024804
industry need for water based drilling fluids to' supplement or replace the
performance leading invert emulsion mud performance.
[0006] A particular problem when drilling Into shale formations with water-
based
fluids is the pore pressure increase and swelling from penetration of the
shale by
the fluid. Shale stabilizers are typically added to the mud to inhibit these
phenomena and to stabilize the shale from being affected by the mud.
[0007] Reducing drilling fluid pressure invasion into the wall of a borehole
is one
of the most important factors in maintaining wellbore stability. It is
recognized that
sufficient borehole pressure will stabilize shales to maintain the integrity
of the
borehole. When mud or liquid invades the shale, the pressure In the pores
rises
and the pressure differential between the mud column and the shale falls. With
the drop in differential pressure, the shale is no longer supported and can
easily
break off and fall into the well bore. Likewise, the invasion of water into
the shale
matrix increases hydration or wetting of the partially dehydrated shale body
causing it to soften and to lose its structural strength. Chemical reactivity
can
also lead to instability. There is always a needfor a better composition and.
method to stabilize the shale formations.
[0008] In the drilling of depleted sands, there is also a need to prevent of
intrusion of drilling fluid through the borehole and into the formation.
Rather than
concern for formation stability, the loss of drilling fluid and resulting
higher
production costs are the more commonly the main concern. It would be
desirable to be able to reduce the loss of drilling fluid into depleted sands.
[0009] It is apparent to those selecting or using a drilling fluid for oil
and/or gas
exploration that an essential component of a selected fluid, is that it be
properly
balanced to achieve all of the necessary characteristics for the specific end
application. Because the drilling fluids are called upon to do a number of
tasks
simultaneously, this desirable balance is difficult to achieve.
[0010] It would be desirable if compositions and methods could be devised to
aid
and improve the ability of drilling fluids to simultaneously accomplish these
tasks.
Summary of the invention
[0011] Accordingly, it is anaspectof the present invention to provide methods
to
stabilize shale formations and avoid loss of fluids into depleted sands
formations
when drilling with water-based drilling fluids.
154-23110-CIP 2
CA 02534080 2010-02-26
[0012] It is another aspect of the present invention to provide water-based
drilling fluids
that reduce the rate of drilling fluid pressure invasion into the borehole
wall.
[0013] Still another aspect of the invention is to provide a composition and
method
that increase the pressure blockage, reliability, magnitude, and pore size
that can be
blocked with water-based fluids for stabilizing shale formations.
[0014] In carrying out these and other aspects of the invention, there is
provided, in one
form, a water-based drilling fluid including water and a polymer latex capable
of
providing a deformable latex film or seal on at least a portion of a
subterranean
formation.
[0014a] In accordance with a further aspect of the present invention there
provided
a method of inhibiting borehole wall invasion when drilling with a water-based
drilling fluid in a subterranean formation comprising depleted sands, the
method
comprising:
providing water-based drilling fluid comprising water and a polymer latex;
and
circulating the water-based drilling fluid comprising the sealing agent in
contact with a borehole wall comprising depleted sands, the polymer latex
producing a deformable latex seal on at least a portion of the subterranean
formation comprising depleted sands.
[0014b] In accordance with another aspect of the present invention, there is
provided a method of inhibiting borehole wall invasion when drilling with a
water-
based drilling fluid in a subterranean formation comprising depleted sands,
the
method comprising:
providing water-based drilling fluid comprising water and a polymer latex;
and
circulating the water-based drilling fluid in contact with a borehole wall
comprising depleted sands, the polymer latex producing a deformable latex seal
on at least a portion of the subterranean formation comprising depleted sands;
wherein the polymer latex is selected from the group consisting of
sulfonated styrene/butadiene copolymer, polyvinyl acetate/vinyl
chloride/ethylene
copolymer, polyvinyl acetate/ethylene copolymer, polydimethylsiloxane, and
mixtures thereof, where the polymer latex comprises particles that average
less
than 1 micron in size.
3
CA 02534080 2008-10-23
Brief Description of the Drawings
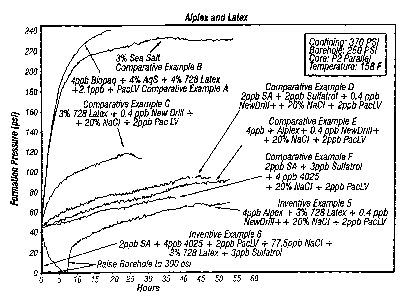
[0015] FIG. I shows a chart of the formation pressure as a function of time
for a
pressure invasion test using various Intermediate test formulations;
[0016] FIG. 2 is. a graph of the surfactant effect on GENCAL 7463 particle
size in
20% NaCI/1 Ib/bbl (2.86 g/I) NEWDRILL PLUS/1 lb/bbi (2.86 g/I) XAN-PLEX
D/0.5 Ib/bbI (1.43 g/1) sodium gluconate/3 Ib/bbl (8.58 g/l) NaAIO2/5% by
volume
GENCAL 7463;
[0017] FIG. 3 is a graph of the influence of polymer resins (3 lb/bbl, 8.58-
gA) on
GENCAL 7463 particle size distributions after 16 hours, 150 F (66 C) hot roll
in
20% NaCi/0.75 lb/bbl (2.15 g/l) XAN-PLEX D/0.5 Ib/bbI (1.43 gA) sodium D-
gluconate/0.4 Ib/bbl (1.14 g/1) NEW-DRILL PLUS/2 lb/bbl (5.72 gA) BIO-PAQ/3
lb/bbi (8.58 g/1) NaAIO2/3% GENCAL 7463/1 Ib/bbI (2.86 911) EXP-152;
[0018] FIG. 4 is a graphical comparison of the effects on mud properties for
EXP-
154 versus ALPLEX in 12 lb/gal (1.44 kg/1) mud; the base mud was 20%
Na000.5 lb/bbl (1.43 gA) XAN-PLEX D/2 Ib/bbl (5.72 gA) BIO-LOSE/1 lb/bb! (2.86
gll) NEW-DRILY PLUS/3% EXP-155/150 lb/bbl (429 g/l) MIL-BAR/27 Ib/bb1(77.2
g/I) Rev bust;
[0019] FIG..5 is a graph of PPT test results for ALPLEX, EXP-154/EXP-155, and
ISO-TEQ fluids;
[0020] FIG. 6 is a'graph showing the effect of circulation on EXP-154/EXP-155
mud performance;
[0021] FIG. 7 is a graph showing the effect of latex on mud properties in 9.6
lb/gal (1.15 kg/I) 20% NaCl fluid after 16 hours, 250 F (121 C) hot roll; the
base
fluid was 20% NaCI/1 lb/bbl (2.86 g/I) XAN-PLEX D/0.4 lb/bbl (1.14 gA) NEW-
3a
CA 02534080 2006-01-30
WO 2005/012456 PCT/US2004/024804
DRILL PLUS/2 Ib/bbl (5.72 g/I) BIO-PAQ/5 lb/bbl (14.3 g/l) EXP-154/10 lb/bbl
(28.6 g/I) MIL-CARB/27 lb/bbl (77.2 g/I) Rev Dust;
[0022] FIG. 8 is a graph showing the effect of latex on mud properties in 12
lb/gal
(1.44 kg/I) after hot rolling for 16 hours at 250 F (121 C); the base fluid
was 20%
NaCI/0.75 lb/bbl (2.15 g/I) XAN-PLEX D/0.4 Ib/bbl (1.14 g/l) NEW-DRILL PLUS/3
lb/bbl (8.58 g/l) BIO-PAQ/5 Ib/bbl (14.3 g/I) EXP-154/150 lb/bbl (429 g/I) MIL-
CARB/27 lb/bbl (77.2 g/I) Rev Dust;
[0023] FIG. 9 is a graph of 96 hour Mysidopsis Bahia range-finder results for
experimental products in 12 lb/gal (1.44 kg/I) fluids where the base fluid is
20%
NaCl/0.5 lb/bbl (1.43 g/I) XAN-PLEX D/0.4-1 Ib/bbl (1.14-2.86 g/I) NEW-DRILL
PLUS/2 lb/bbl (5.72 g/I) MIL-PAC LV (or BIO-PAQ)/150 Ib/bbl (429 g/I) MIL-BAR;
[0024] FIG. 10 is a graph of high temperature high pressure (HTHP) fluid loss
rate on 50 mD cement disk for the mud containing 3% latex polymer after being
hot rolled at 250 F for 16 hours; and
[0025] FIG. 11 is a photograph of an internal filter cake formed using the
method
of the present invention.
Detailed Description of the Invention
[0026] It has been discovered that a polymer latex added to a water-based
drilling fluid can reduce the rate the drilling fluid pressure invades the
borehole
wall of a subterranean formation during drilling. The polymer latex preferably
is
capable of providing a deformable latex film or seal on at least a portion of
a
subterranean formation. Within the context of this invention, the terms "film"
or
"seal" are not intended to mean a completely impermeable layer. The seal is
considered to be semi-permeable, but nevertheless at least partially blocking
of
fluid transmission sufficient to result in a great improvement in osmotic
efficiency.
In a specific, non-limiting embodiment, a submicron polymer latex added to a
high salt water-based mud containing an optional, but preferred
combining/precipitating agent, such as an aluminum complex will substantially
reduce the rate of mud pressure penetration into shale formations. The
pressure
blockage, reliability, magnitude and pore size that can be blocked are all
increased by the latex addition. Inhibiting drilling fluid pressure invasion
into the
wall of a borehole is one of the most important factors in maintaining
wellbore
stability.
154-23110-CIP 4
CA 02534080 2006-01-30
WO 2005/012456 PCT/US2004/024804
[0027] The essential components of the water-based drilling fluids of this
invention are the polymer latex and water, which makes up the bulk of the
fluid.
Of course, a number of other common drilling fluid additives may be employed
as
well to help balance the properties and tasks of the fluid.
[0028] The polymer latex is preferably, but not limited to a carboxylated
styrene/butadiene copolymer or a sulfonated styrene/butadiene copolymer. A
particular, non-limiting carboxylated styrene/butadiene copolymer is GENCAL
7463 available from Omnova Solution Inc. A particular, non-limiting sulfonated
styrene/butadiene copolymer is GENCEAL 8100 also available from Omnova
Solution Inc. Other suitable polymer latexes include, but are not limited to
polymethyl methacrylate, polyethylene, polyvinylacetate copolymer, polyvinyl
acetate/vinyl chloride/ethylene copolymer, polyvinyl acetate/ethylene
copolymer,
natural latex, polyisoprene, polydimethylsiloxane, and mixtures thereof. A
somewhat less preferred polymer latex is polyvinylacetate copolymer latex,
more
specifically, an ethylenevinyl chloride vinylacetate copolymer. While
polyvinylacetate copolymer latices will perform within the methods of this
invention, they generally do not perform as well as the carboxylated
styrene/butadiene copolymers. The average particle size of the polymer latex
is
preferably less than 1 micron or submicron and most preferably having a
diameter of about 0.2 microns or 0.2 microns or less. Other polymers in the
disperse phase may be found to work. It is anticipated that more than one type
of
polymer latex may be used simultaneously. The proportion of the polymer latex
in
the drilling mud, based on the total amount of the fluid may range from about
0.1
to about 10 vol.%, preferably from about 1 to about 8 vol.%, and most
preferably
from about 2 to about 5 vol.%.
[0029] The sulfonated latexes of the present invention have an added advantage
in that they can often be used in the absence of a surfactant. This can
simplify
the formulation and transportation of the drilling fluid additives to
production sites.
This can also reduce costs in some applications. In applications of drilling
in
depleted sands, there is often no need of a precipitating agent. In the
depleted
sands applications, there is also often no need of a surfactant for
carboxylated
styrene/butadiene copolymers for fresh water applications.
[0030] The optional salt may be any common salt used in brine-based drilling
fluids, including, but not necessarily limited to calcium chloride, sodium
chloride,
154-23110-CIP 5
CA 02534080 2006-01-30
WO 2005/012456 PCT/US2004/024804
potassium chloride, magnesium chloride, calcium bromide, sodium bromide,
potassium bromide, calcium nitrate, sodium formate, potassium formate, cesium
formate and mixtures thereof. By a "high salt content" is meant at least 20
weight
percent, and saturated brine solutions are preferred in one non-limiting
embodiment. It will appreciated that it is impossible to predict in advance
what
the salt content of a particular saturated brine solution will be since the
saturation
point depends on a number of factors including, but not limited to the kinds
and
proportions of the various components of the water-based fluid. The salt is
optional because the invention will perform without it, that is, using fresh
water.
[0031 ] Another optional component is precipitating agent. Suitable
precipitating
agents include, but are not limited to, silicates, aluminum complexes, and
mixtures thereof. Suitable aluminum complexes include, but are not limited to,
sodium aluminate, NaAI2O2, sometimes written as Na2OAl2 3, aluminum
hydroxide, aluminum sulfate, aluminum acetate, aluminum nitrate, potassium
aluminate, and the like, and mixtures thereof (especially at pH of >9 for
these
compounds to be soluble- in water). The proportion of the precipitating agent
in
the drilling mud, based on the total amount of the fluid may range from about
0.25 to about 20 lb/bbl (about 0.71 to about 57.2 g/I), preferably from about
1 to
about 10 lb/bbl (about 2.86 to about 28.6 g/I) and most preferably from about
2 to
about 7 Ib/bbl (about 5.72 to about 20 g/I). Without being limited to a
particular
theory, the precipitating agent is believed to chemically bound to the surface
of
the clay of the borehole and provide a highly active polar surface.
[0032] Another optional component of the composition of the invention is a
surfactant. I f the surfactant is present, the surfactant treated latex wets
the
surface strongly and accumulates to form a film or coating that seals
fractures
and defects in the shale. Suitable wetting surfactants include, but are not
limited
to, betaines, alkali metal alkylene acetates, sultaines, ether carboxylates,
and
mixtures thereof. It has been determined that surfactants are particularly
beneficial when salts are present in the drilling fluid, and are not as
preferred in
fresh water fluid systems.
[0033] The proportions of these components based on the total water-based
drilling fluid are from about 0.1 to 10 volume% of polymer latex, at least 1
wt % of
salt (if present), from about 0.25 to 20 lb/bbl (about 0.71 to about 57.2 g/I)
of
precipitating agent (if present), from about 0.005 to about 2 vol.% of
surfactant (if
154-23110-CIP 6
CA 02534080 2006-01-30
WO 2005/012456 PCT/US2004/024804
present), the balance being water. In a more preferred embodiment, the
proportions range from about 1 to 8 vol.% of polymer latex, at least 1 wt % of
salt
(if present), from about I to 10 lb/bbl (about 2.86 to about 28.6 g/I) of
precipitating agent (if present) from about 0.01 to about 1.75 vol. % of
wetting
surfactant (if present), the balance being water.
[0034] It is desired that the sodium aluminate or other precipitating agent be
in a
metastable form in the mud, which means that it is in suspension or solution,
but
precipitates out upon the borehole wall. Typically, aluminum compounds have
been added to the mud on site. If added to mud formulations earlier, they tend
to
be unstable and precipitate prematurely.
[0035] Since the development of pore pressure transmission (PPT) testing, the
effects of various chemical additives on pore pressure transmission rates have
been evaluated. Testing has focused primarily on the performance of salts,
glycols, and precipitating agents such as silicates and aluminum complexes.
Improvements in PPT test equipment and methods have accompanied the
general interest and search for increasing more efficient water-based mud
systems that approach the PPT test performance of invert emulsion fluids.
While
other investigators have found silicate fluids to be especially effective for
reduced
poor pressure transmission rates, silicate fluids have not been widely used
due to
limitations of these fluids. Although lower pore pressure transmission rates
have
been demonstrated for salts, glycols, and aluminum complexing agents, these
products still do not approach the performance of invert emulsion fluids.
[0036] A combination of a new formulation approach as well as modification to
the PPT test procedure was used to demonstrate the efficacy of an alternative
approach to enhance the performance of water-based mud systems. Water-
dispersible polymers were selected to provide sources of small, deformable
particles to provide a sealing and blocking effect on the shale. The first of
these
polymers was tested on the PPT test in a fluid with other products.
[0037] The invention will be further illustrated with respect to the following
examples, which are only meant to further illuminate the invention, and not
limit it in any way.
154-23110-CIP 7
CA 02534080 2006-01-30
WO 2005/012456 PCT/US2004/024804
EXAMPLE I
Fluid Intermediate Preparation
[0038] The following Example is the first preparation of the intermediate
compositions of this invention. Unless otherwise noted, the latex in the
Examples
is 728 Latex, a polyvinylacetate latex.
Component Grams per barrel Grams per 7 barrels
(per 159 I (per 1,113 I)
Tap water 310 2170
Sodium aluminate 2 14
LIGCO 2 14
AIRFLEX 728 10.5 73.5 (75 cc)
[0039] The mixture was hot rolled. After 6 days, the pH was 11.51. The
bottom of the jar was about 75% covered with 1/32 (0.79 mm) fines. The
following components were then added, again given in gram proportions for a
single barrel and 7 barrels, respectively:
154-23110-CIP 8
CA 02534080 2006-01-30
WO 2005/012456 PCT/US2004/024804
NEWDRILL PLUS 0.4 2.8
NaCI (20%) 77.5 540
MILPAC LV 2 14
[0040] The fluid with the latex and the NEWDRILL+ had a light brown color. LD8
was added to control foaming. The resulting mixture was hot rolled for four
hours
at 150 F (66 C). The final pH was 10.75.
EXAMPLE 2
Shale Pressure Penetration Determination
[0041] The pore pressure transmission (PPT) device is based on a 1500 psi
(10,300 kPa) Hassler cell designed for 2.5cm diameter core plugs from 2.5cm to
7.5cm in length. A Hassler cell is a cylinder with a piston inserted in each
end.
The core is held between the two pistons. A rubber sleeve is placed around the
core and the pistons to seal around the core and prevent flow around the core.
The outside of the sleeve is pressured to make a good seal. These tests use a
core 25 mm in diameter and 25mm long.
[0042] The low pressure side of the core (formation side) is fitted with a 1
liter,
2000 psi. (13,800 kPa), stainless steel accumulator to provide back pressure.
The high pressure side of the core is connected to two similar accumulators,
one
for pore fluid, and one for the test fluid. The pressure in each accumulator
is
controlled with a manual regulator fed by a 2200 psi (15,200 kPa) nitrogen
bottle.
[0043] All pressures are monitored with Heise transducers. The transducer
pressures are automatically computer logged at preset intervals.
[0044] The cell is enclosed in an insulated chamber and the temperature
maintained with a 200 watt heater. The heater is controlled with a Dwyer
temperature controller driving a Control Concepts phase angle SCR control
unit.
Temperature control is accurate to +/- 0.05 C.
[0045] A pressure is applied to one end of the core and the flow through the
core
is measured. The piston on the low pressure side is filled with liquid, and
blocked, so an increase in liquid pressure is measured rather than flow. A
very
small amount of liquid flow through the core will make a large rise in the
154-23110-CIP 9
CA 02534080 2006-01-30
WO 2005/012456 PCT/US2004/024804
pressure, making the cell sensitive enough to measure flow through shale.
Shale
has a very low permeability, so the flow of fluid through it is very small.
Pressure
is plotted versus time. Results are expressed as formation pressure (FP). If
the
FP increases over time, there is pressure penetration; if the formation
pressure
decreases over time there is not, and the latter is what is desired.
The fluid of Example 1 was used. Three 50% displacements of 50 cc each were
performed during and just after heating up of the test cell. One run was
started at
100% displacement and the temperature was difficult to control, so it was
decided starting at 50% was better.
Temperature = 155 F (68.3 C)
Borehole side pressure = 250 psi (1,720 kPa)
Confining pressure = 370 psi (2,550 kPa)
Formation Pressure
Time, hours:minutes Psi kPa
0 48.1 332
1:30 47.9 330
2:00 47.6 328
7:15 50.9 359
[0046] Eventually, 50 cc of fluid was displaced up to 50% within 2 F (1.1 C)
temperature variation. The pressure rose to 52.7 psi (363 kPa). Formation heat
was turned off, and the temperature was 147 F (64 C). Displacement pulled the
formation pressure down to 36 psi (248 kPa), then rose to 80.2 (553 kPa) over
the next two days. The initial formation pressure decrease demonstrated that
the
formulation of the invention inhibited pressure penetration.
154-23110-C I P 10
CA 02534080 2006-01-30
WO 2005/012456 PCT/US2004/024804
EXAMPLE 3
Fluid Intermediate Preparation - proportions in grams unless otherwise
noted
Component Per barrel (per 159j) Per 7 barrels (per 1,113 I)
Tap water 310 2170 cc
Sodium aluminate 2 14
LIGCO 2 14
AIRFLEX 728 Latex 10.5 75 cc
NEWDRILL PLUS 0.4 2.8
NaCl (20%) 77.5 540
MILPAC LV 2 14
[0047] The sodium aluminate and AIRFLEX 728 latex were mixed together and
allowed to stand over the weekend. The mixture was then hot rolled at 150 F
(66 C) for two hours. The salt and polymers were then added. The sequence of
addition to the sodium aluminate/latex mixture was: PHPA (partially hydrolyzed
polyacrylamide; NEWDRILL PLUS), followed by mixing; then half of the salt,
followed by MILPAC LV, followed by the other half of the salt. The mixture was
hot rolled overnight.
EXAMPLE 4
Shale Pressure Penetration Determination
Borehole side pressure = 250 psi (1,720 kPa)
Confining pressure = 370 psi (2,550 kPa)
Formation Pressure
Time, hours:minutes Psi kPa
0 46.3 319
5:49 2.3 16
7:36 0.6* 4.1
50:00 65.0 448
11
154-23110-CI P
CA 02534080 2006-01-30
WO 2005/012456 PCT/US2004/024804
* The confining pressure was raised to 410 psi (2,830 kPa) and the borehole
pressure was raised to 300 psi (2,070 kPa) at this point.
EXAMPLES 5 and 6, COMPARATIVE EXAMPLES A-F
[0048] Two other inventive formulations (Examples 5 and 6) and six comparative
Examples (A-F) were prepared and tested. The results are shown in FIG. 1. As
indicated the Inventive Examples 5 and 6 both gave the desired results of
decreasing formation pressure overtime. The comparative Examples undesirably
gave increasing formation pressures over time. The composition identities are
given on FIG. 1 itself. The designation "CORE: P2 PARALLEL" refers to the core
being Pierre Shale in parallel orientation.
[0049] These results verify the necessity of having all three components: the
salt, the latex, and the sodium aluminate (Examples 5 and 6). Use of the latex
alone (comparative Ex. A), use of salt only (comparative Ex. B), use of the
latex
together with salt only (comparative Example C), use of sodium aluminate and
the salt only (comparative Ex. D), use of the sodium aluminate- and salt only
(comparative Ex. E), and use of the sodium aluminate with salt only
(comparative
Ex. F) were all found to be ineffective, or at least certainly not as
effective as the
inventive composition.
[0050] Further experimental evidence indicates that some latex products
exhibit a
synergistic effect with aluminum complexes that results in improved pore
pressure transmission characteristics. Stable drilling fluid systems have been
formulated with latex that remain dispersed and flexible in highly saline
(high salt
content) fluids. Inventive drilling fluids provide pore pressure transmission
performance closer to oil-based fluids than what is exhibited by current
aluminum-based drilling fluids. Two features of this system are believed to be
the
main contributors to shale stabilization. First, the ultra-fine, deformable
latex
particles (having a preferable diameter of about 0.2 microns) mechanically
seal
shale micro-fractures and physically prevent further intrusion of drilling
fluids into
sensitive shale zones. Secondly, latex co-precipitation with precipitating
agents, if
present, such as aluminum complexes, produces a semi-permeable membrane
on shale surfaces that chemically improves the osmotic efficiency between the
fluid and the borehole.
154-23110-CIP 12
CA 02534080 2006-01-30
WO 2005/012456 PCT/US2004/024804
[0051] Three experimental additives were discovered for the inventive fluids:
EXP-1 53, EXP-1 54 and EXP-1 55. EXP-153 is a sulfonated polymer resin used to
control HTHP fluid loss in this system. EXP-154 is considered an alternative
to
aluminum complex product ALPLEX. Compared to ALPLEX, EXP-1 54 exhibits
much better compatibility with latex fluids. EXP-1 55 is a modified latex
product.
Compared to other commercially available latices EXP-155 displays less
sensitivity to electrolytes and does not flocculate in 20% sodium chloride
fluids at
temperatures up to 300 F (149 C). Furthermore, due to the wide temperature
range between its glass transition temperature (T9) and melting point (Tm),
the
particles of EXP-155 remain deformable and capable of plugging shale micro-
fractures at most application temperatures. The toxicities of all of these
products
meet the requirement for fluid disposal in the Gulf of Mexico.
Formulations and Fluid Properties
[0052] All fluids were mixed according to established Baker Hughes INTEQ
mixing procedures. The initial and final Bingham Plastic rheological
properties of
plastic viscosity, yield point, ten second gels, and ten minute gels were
measured
by Fann 35 viscometer at 120 F (49 C). The initial and final pH and API
filtrate
were recorded. HTHP fluid loss at 250 F (121 C) was measured after static and
dynamic aging for 16 hours at 250 F (121 C).
Latex Stability
[0053] The stability of the latex samples were first evaluated in 20% and 26%
NaCl solutions by the following procedure:
1. Add 332 ml 20% (or 26%) NaCl water solution into a mixer cup and start
mixing.
2. Slowly add 18 ml tested latex sample into the solution and adjust the
Prince Castle mixer to 4000 rpm with Variac and tachometer.
3. After stirring 5 minutes, slowly add 3 grams NaAIO2 into the above
solution and mix for a total of 20 minutes. During the mixing period it may
be necessary to add about 5 drops defoamer (LD-8) if foaming is
observed.
4. Put this fluid into a jar and statically age for 16 hours at 150 F (66 C).
154-23110-CIP 13
CA 02534080 2006-01-30
WO 2005/012456 PCT/US2004/024804
5. Remove the jar from the oven and cool to room temperature. Observe the
fluid for flocculation and separation.
6. If there is no separation or flocculation, sieve the fluid with a 100-mesh
(0.150 mm) screen. Observe sieve for amount of retained latex particles.
[0054] Additional evaluations were performed only for those samples having
passed the above screening test. A Malvern Mastersizer Particle Size Analyzer
was used to measure the particles size distributions of latex in formulated
fluids.
The small sample dispersion unit and the standard refractive index 50HD
(Particle R.I. = 1.5295, 0.1000 and Dispersant R.I. = 1.3300) were used in all
of
the particle size distribution tests. 20% NaCl water solution with pH adjusted
to
11.5.
Shale Inhibition Test
[0055] The shale inhibition characteristics were determined by shale
dispersion
tests that included static wafer test, and pore pressure (PPT) tests. In the
PPT
test, a preserved Pierre II shale core, 1 inch diameter by 0.9 inch long (2.54
cm x
2.29 cm long), is placed between two pistons, as described previously in
Example 2. The circumference of the shale and pistons are sealed with a rubber
sleeve. The plug i s oriented with the bedding planes i n the parallel o r
high
permeability direction. Drilling fluid at 300 psi (2,070 kPa) is displaced
through
the upstream piston (borehole side) and seawater at 50 psi (345 kPa) is
displaced through the downstream piston (formation side). The seawater in the
downstream piston is contained with a valve. As mud filtrate enters the
borehole
end of the plug, connate water in the shale is displaced into the formation
piston.
Latex Stability
[0056] As noted above, initial experiments indicated that some latex products
(emulsion polymers) produced synergistic effects with an aluminum complex,
resulting in improved pore pressure transmission characteristics of the
fluids.
This result revealed a new approach to the design of highly inhibitive, water-
based fluids. However, latex is generally considered to be a metastable
system.
The large surface of the particles is thermodynamically unstable and any
perturbation affecting the balancing forces stabilizing the polymer dispersion
154-23110-CIP 14
CA 02534080 2006-01-30
WO 2005/012456 PCT/US2004/024804
results in a change in the kinetics of particle agglomeration. Most commercial
lattices, which are designed for the production of synthetic rubber or the
application of painting/coating, are sensitive to increasing electrolytic
concentration and temperature.
[0057] As shown in Table I, among 16 latex samples tested in 26% and 20%
NaCl solutions, none of them is stable in 26% NaCl and only AIRFLEX 728 and
GENCAL 7463 are relatively stable in 20% NaCl. Clearly, for successful
applications of latex in drilling fluids, latex stability in high salt
environments and
at elevated temperatures must be improved. A common technique used to
increase latex stability in electrolyte solutions is the addition of some
surfactants.
FIG. 2 compares the effect of E XP-152 o n the particle size distributions of
AIRFLEX 728 with that of GENCAL 7463. These results indicate that a blend of
GENCAL 7463 and EXP-152 may be a stable product for drilling fluid
applications.
154-23110-CI P 15
CA 02534080 2006-01-30
WO 2005/012456 PCT/US2004/024804
TABLE I
Stability Test for Latex Products in NaCl Solution
Tg Stability After 16 Hours Static Aging
Ex. Latex Samples C 26% NaCI/3 lb/bbl 20% NaCI/3 lb/bbl
(8.58 q/I) NaAIO2 (8.58 q/I) NaAlO?
Vinyl Acetate/Ethylene Vinyl Chloride
7 AIRFLEX 728 0 Flocculation but pass 100 mesh Flocculation/Coagulation
Vinyl Acetate/Ethylene
8 AIRFLEX 426 0 J Flocculation/Coagulation Flocculation/Coagulation
9 AIRFLEX 7200 0 Flocculation/Coagulation Flocculation/Coagulation
VINAC XX-211 N/A Flocculation/Coagulation Flocculation/Coagulation
11 ELVACE 40722-00 N/A Flocculation/Coagulation Flocculation/Coagulation
Carboxvlated Styrene/Butadiene
12 GENCAL 7463 13 Flocculation but pass 100 mesh Floc. at 150 F (66 C) but
stable at 75 F (24 C)
13 GENCAL 7470 N/A Flocculation/Coagulation --
14 GENFLO 576 N/A Flocculation/Coagulation --
TYLAC 68219 N/A Flocculation but pass 100 mesh Flocculation but pass 100
mesh --
16 TYLAC CPS 812 N/A Flocculation/Coagulation --
17 TYCHEM 68710 N/A Flocculation/Coagulation --
18 ROVENE 9410 -56 Coagulation Coagulation
19 ROVENE 6140 -27 Coagulation Coagulation
Carboxylated Acrylic Copolymer
SYNTHEMUL CPS N/A Flocculation/Coagulation --
401
21 SYNTHEMUL N/A Flocculation/Coagulation --
97982
Styrene/Butadiene
22 ROVENE 4823L -51 Coagulation Coagulation
Aluminum Complex
5 [0058] Although a synergistic effect of ALPLEX with latex on stabilizing
shales
was confirmed by PPT test results, this system is fragile and very sensitive
to
increasing salt concentration and temperature. It was found that in 20% NaCI
solution, 3% AIRFLEX 728 or 3% G ENCAL 7463 were flocculated in a few
minutes by adding 4 Ib/bbl (11.4 g/I) ALPLEX. Prehydration of ALPLEX in fresh
10 water or addition of some surfactant (e.g. EXP-152) did improve the
stability of
this system at I ow temperatures, but the latex p article size was still
greatly
154-23110-CIP 16
CA 02534080 2006-01-30
WO 2005/012456 PCT/US2004/024804
influenced by ALPLEX. Those particles greater than 100 microns in the fluid
containing ALPLEX may have partially resulted from insoluble lignite (a
component of ALPLEX). A similar effect was also observed with GENCAL 7463.
Poor solubility and slow dissolution rate of the lignite in high salt
concentrations is
probably the main factor contributing to decreased latex stability.
[0059] In order to find a polymer resin that was compatible with a latex
system
additional tests were performed. FIG. 3 shows the effects of different polymer
resins on the particle size distributions of EXP-1 55. Among the tested
samples,
EXP-153 exhibited the best compatibility with this latex system.
[0060] A new aluminum complex product, EXP-154 (a blend of 45% NaAI 2,
45% EXP-1 53 and 10% sodium D-gluconate) was invented for the latex system.
FIG. 4 compares the effects on the mud properties for EXP-1 54 with ALPLEX in
12 lb/gal (1.44 kg/I) 20% NaCI/NEW-DRILL/EXP-1 55 fluids. The experimental
aluminum complex exhibits improved compatibility with latex and biopolymers.
Additionally, EXP-154 is found to control filtration, both API and HTHP,
better
than does ALPLEX.
Pore Pressure Transmission Testing
[0061] Borehole stability effects of the experimental latex system were
evaluated
with the pore pressure transmission (PPT) tester previously described. A
preserved Pierre 11 shale plug, 1 inch diameter by 0.9 inch long (2.54 cm x
2.29
cm long), is placed between two pistons, as described previously in Example 2.
The circumference of the shale and pistons sealed with a rubber sleeve. The
plug i s oriented with the bedding p lanes i n the parallel o r high
permeability
direction. Drilling fluid at 300 psi (2,070 kPa) is displaced through the
upstream
piston (borehole side) and seawater at 50 psi (345 kPa) is displaced through
the
downstream piston (formation side). The seawater in the downstream piston is
contained with a valve. As mud filtrate enters the borehole end of the plug,
connate water in the shale is displaced into the formation piston. This
additional
water compresses the water inside the piston causing the pressure to rise. The
pressure increase in the formation piston water is measured as formation
pressure (FP) rise.
[0062] The EXP-154/EXP-155 fluid produces the best PPT results to date as
shown in FIG. 5. The top curve is a standard salt/polymer. The next one down
is
154-23110-C I P 17
CA 02534080 2006-01-30
WO 2005/012456 PCT/US2004/024804
ALPLEX, the next curve is an EXP-1 54/AIRFLEX 728 formulation, below that is
the EXP-1 54/EXP-1 55 formulation, and finally at the bottom is a 80/20 ISOTEQ
fluid, 25% CaCl2, 6 ppb (17.2 g/I) CARBO-GEL, and 10 ppb (28.6 g/I) OMNI-
MUL. Without necessarily being limited to one explanation, the superior
performance of the EXP-154/EXP-155 fluid is believed to be due, at least in
part,
to its small particle size. As discussed previously, GENCAL 7463 was more
efficiently dispersed by the EXP-152 resulting in a much greater percentage of
particles smaller than one micron.
[0063] A synergistic effect between latex and aluminum complex has also been
observed in these tests. Such results may be related to the co-precipitation
behavior of EXP-155 and EXP-154. It was found that EXP-154 becomes
insoluble at pH <10. At this condition, EXP-155 alone does not precipitate.
However, when EXP-154 exists in this system, EXP-155 will be co-precipitated
with EXP-154. Because of their co-precipitation behavior, deposited particles
on
the shale surface are comprised of lipophilic and hydrophilic components. This
multiphase system is capable of creating a semi-permeable membrane, resulting
in a great improvement in osmotic efficiency. Another characteristic of EXP-
155
is that its ultra-fine particles are elastomer-like over a wide range of
temperatures. When subjected to differential hydraulic pressure, these ultra-
fine
particles do not shear or break, but deform and penetrate the hairline
fractures
and to form an impermeable seal. At the temperatures between Tg (glass
transition temperature) and Tm (melting point), most polymers will exhibit
rubber-
like elasticity. The glass transition temperature of EXP-1 55 is 52 F (11
C). From
the relationship between Tg and Tm plotted by Boyer, 1963, reproduced in
Billmeyer, Textbook of Polymer Science, Second Edition, Wiley- Interscience,
New York, 1971, p. 230, we can estimate that Tm of EXP-155 is about 300 F
(422 K). This temperature range covers most applications in drilling fluids.
[0064] Circulation of the fluid was found to be an important element of the
latex
plugging mechanism. This was explored in the tests with EXP-155. As the
formulation was only 1.5% latex particles by volume (EXP-1 55 is 50% active),
insufficient I atex was a vailable in the mud to produce plugging u nder
static
conditions. With circulation, however, the latex accumulated on the surface
and
formed a plugging film. Standard procedure is to circulate the mud about 7
hours
154-23110-CIP 18
CA 02534080 2006-01-30
WO 2005/012456 PCT/US2004/024804
followed by static exposure overnight. Four or five hours without circulation
elapses before the test is started in the morning. This static period
eliminates
pressure drift due to temperature effects by allowing temperature variation
from
circulation to equilibrium.
[0065] When the test started the formation pressure fell from 50 psi (345 kPa)
to
zero, increasing the differential pressure from 250 to 300 psi (1,720 to 2,070
kPa), as seen in FIG. 6. In about 30 hours, the plug began to leak and the
formation pressure rose. However, additional circulation sealed the leak in an
hour and the pressure again fell to zero. In previous tests the circulation
was
stopped after an hour, and the plug started leaking again after another 30
hours.
In this test, circulation was restarted after the pressure rose to 60 psi (414
kPa) in
70 hours (FIG. 6). However, circulation was maintained 5 hours instead of one
as
before. With a few hours of continued circulation after the greater pressure
differential was established, the seal was more stable. The pressure rose only
a
few psi in 45 hours.
[0066] Photomicrographs of the plug face showed latex accumulation along
microfractures in the shale. As the volume and velocity of filtration flow
into these
cracks is very small, filtration alone cannot account for the latex
accumulation at
the crack throat. Inside these cracks the clay surface area to filtrate volume
ratio
is very large resulting in heavy EXP-1 54 precipitation. The reason may relate
to
the co-precipitation behavior of EXP-1 54 and EXP-155 as discussed previous,
without being limited to any particular explanation. The precipitation of
aluminum
complex at pH <19 apparently enhances latex accumulation at the crack throat.
When sufficient latex is deposited to bridge the crack opening, the fracture
is
sealed and differential pressure is established across the latex. The
differential
pressure consolidates the latex deposit into a solid seal. Increasing the
differential pressure apparently causes this seal to deform over time (about
30
hours in the case of the FIG. 6 results) and/or grows additional cracks in the
shale and the shale begins to leak, although the inventors do not necessarily
want to be limited by this explanation. However, additional circulation
rapidly
sealed the leaks and reestablished the seal. Circulating after the full
differential
pressure was reached formed a stable seal with only a small pressure rise.
154-23110-CI P 19
CA 02534080 2006-01-30
WO 2005/012456 PCT/US2004/024804
Effect of Latex on Mud Properties
[0067] The previous results and discussions deal with latex stability in
drilling
fluids and its synergy with aluminum complex in improving mud inhabitability
to
shale formations. Besides, improved performance parameters achieved by the
latex products were also recognized. Two latex samples, Latex A (8:1 blended
AIRFLEX 728 and EXP-152) and EXP-155 (8:1 blended GENCAL 7463 and
EXP-152), were evaluated in 9.6 lb/gal (1.15 kg/I) 20% NaCl and 12 lb/gal
(1.44
kg/I) 20% NaCl fluids. The effects of adding 3% by volume of these latex
products are illustrated in FIGS. 7 and 8. Without obvious effect on the fluid
rheology, HTHP fluid loss at 250 F (121 C) decreased as much as 45% and 52%
in 9.6 lb/gal (1.15 kg/I) mud and 35% and 40% in 12 lb/gal (1.44 kg/I) mud by
Latex A and EXP-1 55, respectively. Again, EXP-1 55 presents better results
that
AIRFLEX 728. Additional tests with EXP-155 are listed in Table II.
154-23110-CIP 20
CA 02534080 2006-01-30
WO 2005/012456 PCT/US2004/024804
TABLE II
Typical Performance Parameters of 12 lb/gal 20% NaCI/EXP-155 Fluids
Formulation Example # 23 24
Water, bbl (I) 0.89 0.89 (141)
XAN-PLEX D, lb/bbl (/l) 0.5 (1.43 g/1) 0.5 (1.43 /I
BIO-PAQ, lb/bbl (g/l) 4(11.4) -
1310-LOSE, lb/bbl g/I - 4(11.4)
NEW DRILL PLUS, lb/bbl /I) 1 (2.86) 1 (2.86)
EXP-154, lb/bbl (g/I) 5 5 (14.3)
NaCl, lb/bbl (g/I 77.5 (222 77.5 222)
EXP-155, % by vol. 3 3
MIL-BAR, lb/unweighted bbl 150 (429) 150 (429)
(g/I)
Rev-Dust, lb/bbl (g/I) 27 (77.2) 27 (77.2)
Initial Properties
PV, cP 22 21
YP, lb/100ft26 (179) 20(138)
sec. gel, lb/100ft kPa 5(34) 4(28)
10 min. gel, Ib/100 kPa) 10 (69) 8 (56)
API, cm /30 min 2.5 1.4
pH 10.6 10.7
Density, lb/gal 12.2 12.2
after HR 16 hr @ 150 F 250 F -- 150 F 250 F --
66 C 121 C 66 C 121 C
after static aged 16 hr @ -- -- 300 F -- -- 300 F
149 C 149 C,
PV,cP 20 21 22 26 24 23
YP, lb/100ft (kPa) 24 29(200) 34(234) 17 21 (145) 22(152)
(165) (117)
5 (34) 5 (34)
10 sec. gel, Ib/100ft (kPa 6 (41) 7 (48) 10 (69) 4(28)
10 min. gel, lb/100ft kPa 9(62) 10(69) 13(90) 7(48) 7 48 7(48)
API, ml 2.8 3.7 2.8 2.2 2.6 1.8
pH 10.4 9.7 9.7 10.5 9.7 10.1
HTHP fluid loss, cm /30 min. 9.4 16.4 12 8.4 13 10.8
Toxicity Test
5 [0068] The 96 hour range-finder bioassay results of AIRFLEX 728, GENCAL
7463, EXP-1 52, EXP-1 54 and EXP-155 in 12 lb/gal (1.44 kg/I) 20% NaCI/NEW-
DRILL fluids are presented in FIG. 9. All products meet the requirement for
fluid
disposal in the Gulf of Mexico (30,000 ppm) and become less toxic after solids
contamination.
EXAMPLE 7
154-23110-CIP 21
CA 02534080 2006-01-30
WO 2005/012456 PCT/US2004/024804
[0069] Because latex polymers contain deformable colloidal particles, it can
provide an excellent bridging and sealing ability to reduce the permeability
of the
formation where the lost circulation of drilling fluids may encountered. Table
III
shows a typical formulation for testing the sealing ability of latex polymers
on
permeable formation. Without latex polymer, the fluid loss of this mud is out
control. However, an addition of 3% of a vinyl acetate/ethylene/vinyl chloride
latex polymer, available under the trade designation Airflex 728, into this
mud
results in the fluid loss decreasing sharply with time as shown in Figure 10.
Tables IV-VI display the data for Figure 10.
[0070] Figure 11 shows the section picture of a broken 50 milliDarcy (mD) disk
after testing for four hours at 300 F with the fluid containing 3% latex
polymer.
DFE-245 is an admixture of GenCal 7463 and Mirataine BET-030 at a volume
ratio of about 9:1. It can be clearly observed that the internal filter cake
was
formed inside of the 50 mD disk.
Table III. Mud Formulation for Testing Latex effect on High Pressure Fluid
Loss
Formulation # 1094-52-1
Water, bbl 0.89
NEW-DRILL PLUS, lb/bbl 0.4
MIL-PAC LV, lb/bbl 2
MAX-PLEX, lb/bbl 4
NaCl, lb/bbl 77.5
Airflex 728 (latex polymer), % by vol. 3
Maritaine BET-030, lb/bbl 1
Table IV. High Temperature High Pressure Fluid loss at 500 psi and 75 F on
50 mD disk for the mud containing 3% Airflex 728
Time interval, minutes HPHT FL, ml Average rate of HPHT FL,
m l/minutes
0-1 4.5 4.50
1-10 2 0.22
10-30 1.5 0.08
30-60 1.5 0.05
60-120 2.5 0.04
154-23110-CIP 22
CA 02534080 2006-01-30
WO 2005/012456 PCT/US2004/024804
Table V. High Temperature High Pressure Fluid loss at 500 psi and 250 F on
50 mD disk for the mud containing 3% Airflex 728
Time interval, minutes HPHT FL, ml Average rate of HPHT FL,
ml/minutes
0-1 6 6.00
1-10 4 0.44
10-30 6 0.30
30-60 4 0.13
60-120 4 0.07
Table VI. High Temperature High Pressure Fluid loss at 500 psi and 300 F
on 50 mD disk for the mud containing 3% Airflex 728
Time interval, minutes HPHT FL, ml Average rate of HPHT FL,
ml/minutes
0-1 10 10
1-10 13 1.44
10-30 8 0.4
30-60 6 0.20
60-120 10 0.17
120-180 5 0.08
-10 - [0071] In the foregoing specification, the- invention h as been
described with
reference to specific embodiments thereof, and has been demonstrated as
effective in providing a water-based drilling fluid that can effectively
reduce the
rate of drilling fluid pressure invasion of the borehole wall. However, it
will be
evident that various modifications and changes can be made thereto without
departing from the broader spirit or scope of the invention as set forth in
the
appended claims. Accordingly, the specification is to be regarded in an
illustrative
rather than a restrictive sense. For example, specific combinations of brines
and
latexes and with precipitating agents and/or wetting surfactants or salts
falling
within the claimed parameters, but not specifically identified or tried in a
particular
composition to reduce mud pressure penetration into shale, sand, and
otherformations, are anticipated to be within the scope of this invention.
GLOSSARY
4025-70 Low molecular weight amphoteric polymer sold by
Amoco, found to be ineffective (also abbreviated as
4025).
AIRFLEX 728 A polyvinylacetate latex (more specifically, an
ethylenevinyl chloride vinylacetate copolymer)
dispersion sold by Air Products.
154-23110-CI P 23
CA 02534080 2006-01-30
WO 2005/012456 PCT/US2004/024804
AIRFLEX 426 Vinyl acetate/ethylene copolymer available from
Air Products.
AIRFLEX 7200 Vinyl acetate/ethylene copolymer available from
Air Products.
ALPLEX Proprietary aluminum complex product available
from Baker Hughes INTEQ.
AqS Abbreviation for AQUACOL-S, a glycol available
from Baker Hughes INTEQ.
BIO-LOSE Derivatized starch available from Baker Hughes
INTEQ.
BIOPAQ Derivatized starch fluid loss additive available from
Baker Hughes INTEQ.
CARBO-GEL An amine-treated clay marketed by Baker Hughes
INTEQ.
CARBO-MUL Invert emulsion emulsifier marketed by Baker
Hughes INTEQ.
ELVACE 40722-00 Vinylacetate/ethylene copolymer latex available from
Reichhold.
EXP-152 Oleamidopropyl betaine surfactant.
EXP-153 Sulfonated polymer resin (or sulfonated humic acid
with resin) available from Baker Hughes INTEQ.
EXP-1 54 A mixture of 45% NaAIO2, 45% EXP-153 and 10%
sodium D-gluconate.
EXP-155 An 8:1 volume blend of GENCAL 7463 and EXP-
152.
FLOWZAN Biopolymer available from Drilling Specialties.
FT-1 A SULFATROL, 90% water-soluble sulfated asphalt
dispersion sold by Baker Hughes INTEQ.
GENCAL 7463 Carboxylated styrene/butadiene available from
Omnova Solution Inc.
GENCAL 7470 Carboxylated styrene/butadiene available from
Omnova Solution Inc.
GENFLO 576 Available from Omnova Solution Inc.
LD8 A commercial defoamer available from Baker
Hughes INTEQ.
LIGCO Lignite sold by Baker Hughes INTEQ.
MIL-BAR Barite weighting agent available from Baker
Hughes INTEQ.
MIL-CARB Calcium carbonate weighting agent available from
Baker Hughes INTEQ.
MILPAC LV Low viscosity polyanionic cellulose available from
Baker Hughes INTEQ (sometimes abbreviated as
PacLV).
MAX-PLEX An aluminum complex for shale stability available
from Baker Hughes INTEQ.
MIRATAINE BET-O-30 Betaine surfactant from Rhodia
NEWDRILL PLUS Partially hydrolyzed polyacrylamide available from
Baker Hughes INTEQ.
ROVENE 4823L Styrene/butadiene copolymer available from
Mallard Creek.
154-23110-CIP 24
CA 02534080 2006-01-30
WO 2005/012456 PCT/US2004/024804
ROVENE 6140 Carboxylated styrene/butadiene available from
Mallard Creek.
ROVENE 9410 Carboxylated styrene/butadiene available from
Mallard Creek.
SA Abbreviation for sodium aluminate.
SYNTHEMUL 97982 Carboxylated acrylic copolymer available from
Reichhold.
SYNTHEMUL CPS 401 Carboxylated acrylic copolymer available from
Reichhold.
TYCHEM 68710 Carboxylated styrene/butadiene copolymer
available from Reichhold.
TYLAC 68219 Carboxylated styrene/butadiene copolymer
available from Reichhold.
TYLAC CPS 812 Carboxylated styrene/butadiene copolymer
available from Reichhold.
VINAC XX-211 Vinyl acetate/ethylene copolymer available Air
Products.
XAN-PLEX D Biopolymer available from Baker Hughes INTEQ.
154-23110-CI P 25