Note: Descriptions are shown in the official language in which they were submitted.
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
1
Surface-modified zinc oxides
The invention relates to surface-modified zinc oxides, a
process for their preparation and their use.
One portion of the solar spectrum comprises wavelengths of
electromagnetic energy which range between about 290 and
3,000 nm. This range may be divided into different
regions, namely:
1. the ultraviolet region (290-400 nm)
2. the visible region (400-760 nm) and
3. the near-infrared region (> 760 nm).
The ultraviolet region has, moreover, been arbitrarily
divided into three bands, referred to as the UVA, UVB and
UVC bands.
The UVB band extends from 290 to 320 nm. It is the
principal cause of the sunburn reaction and it is also the
most effective in stimulating the tanning reaction in the
skin. UVC radiation (200-290 nm) from the sun does not
reach the surface of the earth, although one can encounter
radiation in this range from artificial sources such as
germicidal lamps and high and low pressure mercury arc
lamps. For purposes of the present invention however,
protection against UVC radiation is generally not a major
concern, i.e., in contrast to the dangers posed by UVA and
UVB radiation, The UVA band, which extends from 320-400
nm, can also cause the tanning reaction. UVA radiation can
also cause sunburns, but its capacity to do so is less
than that of UVB radiation.
The amount of OVA radiation exposure, however, is
increasing. This is due to the fact that most sunscreens
effectively block only UVB radiation. As stated above, UVB
radiation is more capable than UVA radiation of causing
the tanning and burning reactions. Therefore, if one is
using a sunscreen that blocks UVB radiation he/she will
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
2
tend to stay in the sun for an extended period of time
because the immediate effects of the sun tan/burn are not
evident. The problem is that UVA is still penetrating the
skin and although it is not causing any immediately
obvious effects, it is causing long term damage. In recent
years, it has been well documented that UVA radiation,
like UVB radiation, is harmful to the skin. In fact,
current data reveal that solar radiation containing these
wavelengths is the chief cause of skin cancer, which
presently accounts for 30-40 % of all new cancers each
year. In the United States alone, 500,000 new cases of
skin cancer will be reported this year and the number is
expected to keep rising in the future. UVA radiation has
been shown to promote skin cancer by inhibiting enzymes
that repair cells damaged by UVB radiation. UVA radiation
also penetrates more deeply into the skin than UVB
radiation and causes changes in blood vessels and
premature aging of the skin, thus adding to the damage
produced by UVB rays (see, e.g., Hurwitz, Sidney, The Sun
and Sunscreen Protection: Recommendations for Children"
Dermatol. Surg. Oncol; 14:6 (June 1988) P 657).
The goal of any sunscreen should thus be to protect the
user from both UVA and UVB radiation with a minimum of
side effects. This end has not been adequately achieved
with the use of presently available sunscreen products.
Sunscreen products can be grouped into two broad
categories, i.e.,
1. topical sunscreens and
2. oral sunscreens.
The present invention focuses upon the topical sunscreens,
which can be further differentiated into two
subcategories, namely
1. chemical sunscreens and
2. physical sunscreens.
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
3
Chemical sunscreens contain from about 3 to about 26 % of
one or more UV-absorbing chemicals. When applied to the
surface of the skin as a thin film, i.e., about 10-15 pm
in thickness, these chemicals act as a filler to diminish
the penetration of UV radiation to the cells of the
epidermis.
These sunscreens are typically applied in a cream, oil,
lotion, alcohol or gel vehicle and they are usually
colorless, because they do not contain any visibly light-
absorbing chemicals.
The most widely-used chemical sunscreens contain, for
example, para-aminobenzoic acid (PABA), PABA esters
(glyceryl PABA), amyldimethyl PABA and octyldimethyl
PABA), benzophenones (oxybenzone and sulisobenzone),
cinnamates (octylmethoxy cinnamate and cinoxate),
salicylates (homomethyl salicylate) and anthranilates.
To date, more than twenty-one such chemicals have been
approved by the United States Food and Drug Administration
as õsafe and effective" agents in protecting skin against
sunburn (see, e.g., Pathak, Madhu, õSunscreens: Topical
and Systemic Approaches for Protection of Human Skin
Against Harmful Effects of Solar Radiation", Continuing
Medical Education Series, J. Am. Acad. Dermat., 7:3
(September 1982) p. 285, 291).
Questions have recently been raised, however, by the
medical profession as to whether the chemical components
of these sunscreens are indeed inert and further, whether
repeated use of such sunscreens can result in significant
transdermal absorption of these chemicals. Because
chemical sunscreens are applied topically in relatively
high concentrations (i.e., up to 26 %), contact and
photocontact sensitization can occur, as well as
hypersensitivity, i.e., photoallergic reactions (see
Drumgoogle et al., ,Sunscreening Agent Intolerance:
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
4
Contact and Photocontact Sensitization and Contact
Urticania" J. Am. Acad. Dermatol., 1990:22, p. 1068).
Physical sunscreens, on the other hand, comprise particles
of a relatively physiologically inert sunblock,-i.e., UV-
absorbing, compound typically suspended in a cream or
lotion. Materials frequently utilized for this purpose
include kaolin, talc and two metal oxides, i.e., titanium
dioxide and zinc oxide. The latter two compounds are not
associated with the inflammatory reactions noted above.
The physical sunscreen products are, however, typically
messy and occlusive. Moreover, they additionally form a
visible, colored (e.g., white) layer on the surface of the
skin, which is cosmetically unacceptable to many that are
in need of sunscreen protection. This causes many such
individuals to forego the use of these products. The color
of these compositions is attributable to the optical
properties of the particles from which these materials are
formed. These properties are at least partially dependent
upon the size of these particles, which typically have a
fairly õstandard" range of diameters, measured in tenths
of a micron (i.e., about greater than about 0,7-0,7p).
In addition, presently available physical sunscreens are
not easily washed off of the user's body. Instead, they
typically melt off with the heat of the sun, thus
incidentally staining or otherwise discoloring the user's
clothing. Moreover, because they are applied as relatively
thick films (20-50 pm), use of these products may also
promote undesirable skin conditions, including miliaria, a
skin disease caused by an inflammation of the sweat
glands, and folliculitis, an inflammation of the hair
follicle, As such, these physical sunscreen products are
deemed cosmetically unacceptable by a large class of image
conscious persons, which primarily includes young people.
Unfortunately, this same group is the exact population
that needs solar protection the most.
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
It has stated that proper use of sunscreens prior to the
age of 18 would prevent 80 % of skin cancers (see e.g.,
Taylor et al., õPhotoaging/Photodamage and
Photoprotection" 22 J. Am. Acad. Dermatol., 9 (1990).
5 In one variant of the õtypical" prior art physical
sunblocks described above, certain commercial sunscreen
products containing titanium dioxide are made with what is
known as õmicronized" or õlarge surface area" particles of
the metal oxide.
It should be noted here that the term õmicronized" does
not denote a specific particle. size. Rather, the term is
only used to describe small particles having a large
surface area. The titanium dioxide particles utilized in
these sunblock products have a diameter an order of
magnitude smaller (i.e., measuring about 0,01 p) than the
õstandard" sized particles (measuring about greater than
about 0,7-0,9 p) described above.
One drawback to the use of this material, however, is that
titanium dioxide absorbs neither as much UV-radiation nor
transmits as much visible radiation as, for example, zinc
oxide, which is utilized by applicants in the present
invention (see, e.g., Brown, Harvey E., Zinc Oxide:
Properties and Applicants, pp. 11-12, FIG. 2-4 (1976)).
Thus, although the use of micronized titanium dioxide
particles does render the resultant product smoother and
less occlusive, it does not obviate the main drawback
faced with the use of this material, i.e., its
comparatively lower effectiveness (in contrast to ZnO) as
a sunblock agent.
Titanium dioxide-based products are also more opaque than
those formed with the zinc oxide of the present invention,
which is due to the fact that the crystalline structure of
the titanium dioxide material renders it only partially
transparent to visible wavelengths of light and thus not
generally as acceptable for cosmetic use.
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
6
Although it has been known to form micronized particles of
zinc oxide for very specialized uses in the rubber
industry, these particles contain substantial quantities
(i.e., greater than about 200 ppm) of trace metals such as
lead, mercury, arsenic and cadmium. The potential dangers
to human health caused by exposure to these materials is
well documented.
Thus, such zinc oxide particles containing these levels of
trace metals are not acceptable for topical application to
human skin.
Greater public awareness of the harmful effects of
exposure to excessive solar radiation has therefore
resulted in an increased use of sunscreen products by the
public, coupled with a call for improved sunscreen
materials free of the drawbacks described above by those
whose livelihood and/or leisure activities cause them to
be exposed to any substantial amounts of solar radiation.
To avoid these problems a topical formulation for
shielding skin from ultraviolet radiation is known which
comprises:
- a substantially colorless dermatologically acceptable
liquid carrier;
- micronized particles of zinc oxide, said particles
having an average particle diameter of less than about
0,2 microns and containg:
lead < 20 ppm;
arsenic < 3 ppm;
cadmium < 15 ppm; and
mercury < 1 ppm
said particles being substantially uniformly dispersed in
said substantially colorless dermatologically acceptable
liquid carrier to form a substantially visibly transparent
topical sunblock formulation, said particles being
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
7
dispersed in said carrier in an amount effective to shield
skin over which said substantially visibly transparent
topical sunblock formulation is applied from hazardous
effects of UVA and UVB radiation.
Zinc oxide is a reactive material which exhibits a wide
range of reactivity with alkaline as well as acidic
solutions, liquids and gases. In some applications the
reactive nature of the zinc oxide is desirable, for
example in paint applications, the reactivity of the
pigment results in adhesion into the polymer film. In many
applications, it is highly desirable to have zinc oxide in
a non-reactive form, that is to eliminate, or make
unavailable, the active sites present on the molecule.
Harvey Brown in his book Zinc Oxide Properties and
Applications (International Lead Zinc Research
Organization) states zinc oxide displays a high degree of
reactivity in water with a wide range of materials,
including acids, acid salts, and alkaline materials. Many
of the resulting compounds are complex structures because
of the variety of species furnished by zinc oxide in
aqueous solution. Brown goes on to state that zinc
oxychloride, zinc phosphates, zinc silicates, and a
variety of other materials can be formed in aqueous media.
One measure of the availability of reactive groups on the
zinc oxide is pH change associated with use of zinc oxide.
Zinc oxide containing reactive sites can increase the pH
of aqueous products. In some instances the increase can be
from an initial pH of 7 to a pH of 8,7. This increase is
not only a measure of the presence of reactive groups, but
is highly undesirable in the formulation.
It is therefore very desirable to produce a zinc oxide,
which has the pigment properties but lacks the reactivity
found in untreated zinc oxide.
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
8
One area in which zinc oxide has been used is in sunscreen
products. It protects the skin from sun.
The traditional materials used for protecting the skin
from the harmful effect of the sun are the organic
sunscreens. These include para amino benzoic acid and
other materials, which absorb ultra violet light.
Recently, studies have indicated that ultra violet light
is a major factor in the ageing of skin. This has resulted
in the incorporation of sunscreens in products, which are
not aimed specifically for use at the beach, like make up.
Additionally, there has been an increased interest in
providing higher levels of protection to the skin.
The called SPF system has been developed to evaluate
various materials for their effectiveness in protecting
the skin from the damaging affects of the sun. The quest
for higher and higher SPF values has resulted in the use
of greater levels of organic sunscreen. These materials
have a tendency to be irritating at high concentrations,
and have the affect of increasing the available organic
material for bacteria. This result in the need for more
preservative to protect the higher level of organic sun
screen agent from bacterial degradation. The higher levels
of preservative result in higher irritation levels, which
can be addressed by incorporation of irritation mitigates,
which themselves are degraded by bacteria.
The use of inorganic sunscreen agents like zinc oxide is a
good way around the use of organic sunscreens, since they
are not attacked by bacteria. However, their use does have
some other inherent problems. Specifically, these
materials are not easily formulated into stable products,
due to the reactivity issues raised above. Zinc oxide
tends to agglomerate in many finished formulations,
loosing it's effectiveness in the formulation and
resulting in unacceptable aesthetic results, most commonly
whitening and viscosity changes. Additionally, zinc oxide
tends to raise the pH of the formulation to about 8,5,
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
9
which is too high for many skin care formulations. These
formulations tend to be useful at a pH of 6-7. Zinc oxide
has limited usefulness as is due to these problems.
One approach has been to pre-disperse the zinc oxide in an
organic oil like Siltech's patented tri-
(octyldodecyl)citrate. While the dispersion is fairly
stable, the coating is not permanent since there is no
reaction between the oil and the zinc oxide. The oil also
disrupts the uniformity of the zinc oxide on the skin.
Traditionally, dispersing aids have been added to
formulations to minimize the disruptive effect upon the
film. These include phosphate esters, and lecithin. These
too suffer from the labile nature of the surface treatment
and dissociation between the particle and the oil. This is
especially evident when zinc oxide is exposed to extreme
mechanical or thermal stress as in the production of
plastics or stick cosmetics.
It is known to overcome the shortfalls of zinc oxide by
reacting a specific silicone compound under controlled
conditions to produce a stable, surface treated zinc oxide
which maintains it's state of dispersions and does not
contribute significantly to chemical instability in the
formulations.
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
According to US 5,486,631 it has been found that highly
effective system for hydrophobizing zinc oxide makes use
of a silicone compound conforming to the following
structure:
5 Me
R-Si-[-(-O-Si-)a-OR']3
Me
Me is methyl;
R is alkyl having one to ten carbon atoms;
10 R'is methyl or ethyl;
a is an integer ranging from 4 to 12.
The known process for hydrophobizing zinc oxide and the
resultant hydrophobic zinc oxide show the disadvantage
that the hydrophobazing agent produces a polymerized cover
on the surface of the zinc oxide.
It is one object of the invention to overcome the
disadvantage of the known hydrophobic zinc oxide.
The invention provides surface-modified zinc oxides, which
are characterized in that they have the following physico-
chemical characteristic data:
BET surface area: 18 5 m2/g
C content: 0.1 to 5.0 wt.%
The surface-modified zinc oxides according to the
invention can furthermore have a loss on drying of 0.1 to
0.2% and a loss on ignition of 0.8 to 1.4.
The surface-modified zinc oxide according to the invention
preferably has defined molecular groups on the surface.
CA 02534389 2011-02-10
10a
According to one aspect of the invention there is provided
a pyrogenically produced surface-modified zinc oxide powder
which is characterized by a BET surface area of 18 5 m2/g;
wherein the pyrogenically produced zinc oxide powder is
coated with an organosilane surface modifying agent which
provides a C content of 0.5 to 1.0 wt.% to the powder;
wherein the pyrogenically produced zinc oxide powder is a
nanoscale powder in the form of aggregates of anisotropic
primary particles, the aggregates having an average
diameter of 50 to 300 nm.
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
11
The invention also provides a process for the preparation
of the surface-modified zinc oxides according to the
invention, which is characterized in that the zinc oxides,
optionally after spraying with water, are sprayed with the
surface-modifying agent at room temperature and the
mixture is then heat-treated at a temperature of 50 to
4002C over a period of 1 to 6 h.
Alternatively, the surface-modified zinc oxides according
to the invention can be prepared by treating the zinc
oxides, optionally after spraying with water, with the
surface-modifying agent in vapour form and then heat-
treating the mixture at a temperature of 50 to 8002C over
a period of 0.5 to 6 h.
The heat treatment can be carried out under an inert gas,
such as, for example, nitrogen.
The surface modification can be carried out continuously
or batchwise in heatable mixers and dryers with spray
devices. Suitable devices can be, for example: plough
share mixers or plate, fluidized bed or flow-bed dryers.
Any desired zinc oxide can be employed as the hydrophilic
zinc oxide. For example, a zinc oxide which is known from
WO 92/13517 can be employed. A zinc oxide which is
described in the earlier Application according to DE 102
12 680 can preferably be employed.
This zinc oxide is a nanoscale, pyrogenically produced
zinc oxide powder having a BET surface area of 10 to
200 m2/g, characterised in that it is in the form of
aggregates of anisotropic primary particles and that the
aggregates display an average diameter of 50 to 300 nm.
The primary particles are understood to be the smallest
particles in high-resolution TEM images, which are
obviously unable to be broken down any further. Several
primary particles can congregate at their points of
contact to form aggregates. These aggregates are either
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
12
impossible or very difficult to break down again using
dispersing devices. Several aggregates can join together
loosely to form agglomerates, whereby this process can be
reversed again by suitable dispersion.
The term anisotropic means that the arrangement of atoms
differs along the three spatial axes. Anisotropic primary
particles include for example those that are acicular,
nodular or platelet-shaped. A cubic or spherical
arrangement, for example, would be isotropic.
Pyrogenic refers to the formation of oxides by flame
oxidation of metals or non-metals or compounds thereof in
the gas phase in a flame produced by reaction of a fuel
gas, preferably hydrogen, and oxygen. Highly disperse,
non-porous primary particles are initially formed which,
as the reaction continues, coalesce to form aggregates,
and these can congregate further to form agglomerates.
In a particular embodiment the aggregates can comprise a
mixture of nodular primary particles and acicular primary
particles, whereby the ratio of nodular to acicular
primary particles can be between 99:1 and 1:99.
The nodular primary particles preferably display an
average diameter of 10 to 50 nm and the acicular primary
particles preferably display a length of 100 nm to 2000 nm
and a width of 10 nm to 100 nm.
The aggregates in the powder can display a largely
anisotropic structure, defined by a shape factor F(circle)
of below 0.5. The variable F(circle) describes the
deviation of an aggregate from a perfect circular shape.
In a perfect circular object F(circle) equals 1. The lower
the value, the further removed the object structure from
the perfect circular shape. The parameter is defined
according to ASTM 3849-89.
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
13
The powder can display at its surface an oxygen
concentration as non-desorbable moisture in the form of
Zn-OH and/or Zn-OH2 units of at least 40%. It is determined
by XPS analysis (XPS = X-ray photoelectron spectroscopy)
of the oxygen signals at 532 to 533 eV and 534 to 535 eV.
The powder can preferably display a transmission of no
more than 60% at a wavelength of 310 nm and 360 nm.
In a particular embodiment the bulk density of the powder
is 40 to 120 g/l.
Te production of the powder is characterised in that zinc
powder is converted into zinc oxide powder in four
successive reaction zones, evaporation zone, nucleation
zone, oxidation zone and quench zone,
= whereby in the evaporation zone the zinc powder conveyed
there by an inert gas stream is evaporated in a flame of
air and/or oxygen and a fuel gas, preferably hydrogen,
under the proviso that the reaction parameters are
chosen such that oxidation of the zinc does not occur,
= and whereby in the nucleation zone, where the hot
reaction mixture, consisting of zinc vapour, water
vapour as a reaction product of the flame reaction and
optionally excess fuel gas, arrives from the evaporation
zone, it cools to temperatures below the boiling point
of zinc or is cooled by means of an inert gas,
= and whereby in the oxidation zone the mixture from the
nucleation zone is oxidised with air and/or oxygen,
= and whereby in the. quench zone the oxidation mixture is
cooled to temperatures of below 400 C by addition of
cooling gas (for example nitrogen, air, argon, carbon
dioxide).
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
14
The process can be performed in such a way that in the
evaporation zone an excess of fuel gas is used, expressed
in lambda values of 0.5 to 0.99, preferably 0.8 to 0.95.
In a particular embodiment the process can be performed in
such a way that the temperature in the evaporation zone is
preferably between 920 C and 2000 C. In the nucleation
zone the temperature can preferably be between 500 C and
900 C, particularly preferably between 700 C and 800 C.
Furthermore the cooling rate
= in the nucleation zone can preferably be between 100
Kelvin/seconds and 10000 Kelvin/seconds, particularly
preferably between 2000 Kelvin/seconds and 3000
Kelvin/seconds and
= in the quench zone the cooling rate can preferably be
between 1000 Kelvin/seconds and 50000 Kelvin/seconds,
particularly preferably between 5000 Kelvin/seconds and
15000 Kelvin/seconds.
The residence time of the reaction mixture in the
= evaporation zone can preferably be between 0.1 seconds
and 4 seconds, preferably between 0.5 seconds and 2
seconds,
= in the nucleation zone between 0.05 seconds and 1.00
seconds, preferably between 0.1 seconds and 0.2 seconds,
= in the oxidation zone between 5 milliseconds and 200
milliseconds, preferably between 10 milliseconds and 30
milliseconds,
= and in the quench zone between 0.05 seconds and 1.00
seconds, preferably between 0.1 seconds and 0.2 seconds.
The process can also be performed in such a way that air
and/or oxygen and the fuel gas can be supplied to one or
more points within the evaporation zone.
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
The zinc oxide powder can be separated from the gas stream
by means of a filter, cyclone, washer or other suitable
separators.
5 The following compounds can be employed as the surface-
modifying agent:
a) Organosilanes of the type (RO)3Si(CnH2n+1) and
(RO) 3Si (CnH2n-1)
R = alkyl, such as, for example, methyl-, ethyl-, n-
10 propyl-, i-propyl-, butyl-
n = 1 - 20
b) Organosilanes of the type R'X(RO)ySi(CnH2n+1) and
R'x(RO)ySi (CnH2n-1)
R = alkyl, such as, for example, methyl-, ethyl-,
15 n-propyl-, i-propyl-, butyl-
R' = alkyl, such as, for example, methyl-, ethyl-,
n-propyl-, i-propyl-, butyl-
R'=cycloalkyl
n = 1 - 20
x+y = 3
x = 1,2
y = 1,2
c) Halogeno-organosilanes of the type X3Si(CnH2n+1) and
X3Si (CnH2n-1)
X = Cl, Br
n = 1 - 20
d) Halogeno-organosilanes of the type X2 (R') Si (CnH2n+1) and
X2 (R' ) Si (CnH2n-1)
X = Cl, Br
R' = alkyl, such as, for example, methyl-, ethyl-,
n-propyl-, i-propyl-, butyl-
R'=cycloalkyl
n = 1 - 20
e) Halogeno-organosilanes of the type X(R')2Si(CnH2n+1) and
X ( R ' ) 2Si (CnH2n-1)
X = Cl, Br
R' = alkyl, such as, for example, methyl-, ethyl-, n-
propyl-, i-propyl-, butyl-
R'=cycloalkyl
n = 1 - 20
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
16
f) Organosilanes of the type (RO)3Si(CH2)m-R'
R = alkyl, such as methyl-, ethyl-, propyl-
m = 0,1 - 20
R' = methyl-, aryl (for example -C6H5, substituted
phenyl radicals)
-C4F9, OCF2-CHF-CF3, -C6F13, -O-CF2-CHF2
-NH2, -N3, -SCN, -CH=CH2, -NH-CH2-CH2-NH2,
-N- (CH2-CH2-NH2) 2
-OOC(CH3)C = CH2
-OCH2-CH(O)CH2
-NH-CO-N-CO-(CH2)5
-NH-COO-CH3, -NH-CO0-CH2-CH3, -NH-(CH2)3Si(OR)3
-Sx-(CH2)3Si(OR)3
-SH
-NR'R"R" ' (R' = alkyl, aryl; R" = H,
alkyl, aryl; R " = H, alkyl, aryl, benzyl,
C2H4NR " " R""' where R"'' = H, alkyl and
R" " ' = H, alkyl)
g) Organosilanes of the type (R")x(RO)ySi(CH2)m-R'
R" = alkyl x+y = 2
= cycloalkyl x = 1,2
y = 1,2
m = 0,1 to 20
R' = methyl-, aryl (for example -C6H5 , substituted
phenyl radicals)
-C4F9, -OCF2-CHF-CF3, -C6F13, -O-CF2-CHF2
-NH2 1 -N3, -SCN, -CH=CH2, -NH-CH2-CH2-NH2 ,
-N- (CH2-CH2-NH2) 2
-OOC (CH3) C = CH2
-OCH2-CH(O)CH2
-NH-CO-N-CO- (CH2) 5
-NH-COO-CH3, -NH-COO-CH2-CH3, -NH-(CH2)3Si(OR)3
-SX-(CH2)3Si(OR)3
-SH
- NR'R"R" ' (R' = alkyl, aryl; R" = H,
alkyl, aryl; R' ' = H, alkyl, aryl, benzyl,
C2H4NR" " Rilwhere R'''' = H, alkyl and
Rill'' = H, alkyl)
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
17
h) Halogeno-organosilanes of the type X3Si(CH2)m-R'
X = Cl, Br
m = 0,1 - 20
R' = methyl-, aryl (for example -C6H5, substituted
phenyl radicals)
-C4F9, -OCF2-CHF-CF3, -C6F13, -O-CF2-CHF2
-NH2, -N3, -SCN, -CH=CH2,
-NH-CH2-CH2-NH2
-N- (CH2-CH2-NH2) 2
-OOC (CH3) C = CH2
-OCH2-CH (0) CH2
-NH-CO-N-CO-(CH2)5
-NH-COO-CH3, -NH-COO-CH2-CH3, -NH-(CH2)3Si(OR)3
-SX- (CH2) 3Si (OR) 3
-SH
i) Halogeno-organosilanes of the type (R)X2Si(CH2)m-R'
X = Cl, Br
R = alkyl, such as methyl,- ethyl-, propyl-
m = 0,1 - 20
R' = methyl-, aryl (e.g. -C6H5, substituted
phenyl radicals)
-C4F9, -OCF2-CHF-CF3, -C6F13, -O-CF2-CHF2
-NH2, -N3, -SCN, -CH=CH2, -NH-CH2-CH2-NH2,
-N- (CH2-CH2-NH2) 2
-OOC (CH3) C = CH2
-OCH2 -CH (0) CH2
-NH-CO-N-CO-(CH2)5
-NH-COO-CH3, -NH-COO-CH2-CH3, -NH-(CH2)3Si(OR)3,
wherein R can be methyl-, ethyl-, propyl-,
butyl-
-SX-(CH2)3Si(OR)3, wherein R can be methyl-,
ethyl-, propyl-, butyl-
-SH
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
18
j) Halogeno-organosilanes of the type (R)2X Si(CH2)m-R'
X = Cl, Br
R = alkyl
m = 0,1 - 20
R' = methyl-, aryl (e.g. -C6H5, substituted
phenyl radicals)
-C4F9, -OCF2-CHF-CF3, -C6F13, -O-CF2-CHF2
-NH21 -N3, -SCN, -CH=CH2, -NH-CH2-CH2-NH2,
-N- (CH2-CH2-NH2) 2
-OOC(CH3)C = CH2
-OCH2-CH(O)CH2
-NH-CO-N-CO-(CH2)5
-NH-CO0-CH31 -NH-COO-CH2-CH3, -NH-(CH2)3Si(OR)3
-SX- (CH2) 3Si (OR) 3
-SH
k) Silazanes of the type R'R2Si-N-SiR2R'
H
R = alkyl, vinyl, aryl
R' = alkyl, vinyl, aryl
1) Cyclic polysiloxanes of the type D 3, D 4, D 5,
wherein D 3, D 4 and D 5 are understood as cyclic
polysiloxanes with 3, 4 or 5 units of the type -0-
Si(CH3)2-.E.g. octamethylcyclotetrasiloxane = D 4
CH3 CH3
~ Sim
H3\ /O~ 0\ $H3
Si
H3C N 0 0" Sk CH3
jS\
CH3 CH3
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
19
m) Polysiloxanes or silicone oils of the type
R R" m = 0,1,2,3,...00
I I n = 0,1,2,3.... oo
Y-O- Si-O - Si-O -Y u = 0,1,2,3,...oo
R' R'''
Y=CH3, H, CnH2n+1 n=1-20
1
m n u Y=Si(CH3)3, Si(CH3)2H
Si(CH3)20H, Si (CH3) 2 (OCH3) ,
Si(CH3)2(CnH2n+1) n=1-20
R = alkyl, such as CnH2n+1, wherein n = 1 to 20, aryl,
such as phenyl and substituted phenyl radicals,
(CH2)n-NH2, H
R' = alkyl, such as CnH2n+1, wherein n = 1 to 20, aryl,
such as phenyl- and substituted phenyl radicals,
(CH2)n-NH2, H
R '' = alkyl, such as CnH2n+1, wherein n = 1 to 20, aryl,
such as phenyl- and substituted phenyl radicals,
(CH2) n-NH2 , H
R... = alkyl, such as CnH2n+1, wherein n = 1 to 20, aryl,
such as phenyl and substituted phenyl radicals,
(CH2)n-NH2, H
The surface-modified zinc oxides according to the
invention can be used for the preparation of cosmetics, in
particular for the preparation of suncreen compositions.
The surface-modified zinc oxides according to the
invention have---the --f-ol--l-own- g--advan-tages :
They show as synergistic effect with cosmetic indredients
when used as sunprotecting agents.
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
Examples
Analytical methods
The BET surface area is determined according to DIN 66131.
The transmission electron micrographs were obtained with a
5 Hitachi transmission electron microscope, model H-75000-2.
Approximately 500 to 600 aggregates were analysed by means
of the CCD camera in the transmission electron microscope.
The variable F(shape) equals the quotient of the minimum
to the maximum aggregate diameter. The variable F(circle)
10 is calculated as F(circle) = 4n x average surface area)/2
(P), where P = circumference of the aggregates.
The variables F(shape) and F(circle) describe the
deviation of a particle from a perfect circular shape.
F(shape) and F(circle) are 1 for a perfect circular
15 object. The lower the value, the further removed the
object structure from the perfect circular shape.
The parameters are defined according to ASTM3849-89.
The surface properties are determined by large-area (1 cm2)
XPS analysis (XPS = X-ray photoelectron spectroscopy),
20 both in the original condition and after 30 minutes'
surface erosion by ionic bombardment (5 keV argon ions).
Fine structures of the oxygen signals are determined by
Gaussian/Lorentzian curve analyses for oxygen.
one-percent aqueous solutions are used for the
transmission measurements. Dispersion is performed by
means of an ultrasonic instrument from Bandelin
Elektronik. The sonication period is one minute. The
measurements are taken using a Perkin Elmer Lambda 2
UV/Vis Spectrometer.
The bulk density was determined in accordance with DIN-ISO
787/XI.
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
21
Examples
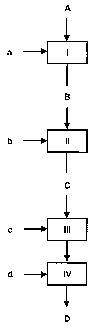
Figure 1 shows a flow diagram of the process according to
the invention with the process stages and the incoming and
outgoing mass flows.
There is: I = evaporation; II = nucleation; III =
oxidation; IV = quenching; A = zinc oxide powder + inert
gas; B = zinc vapour, water, (fuel gas); C = Zinc
particles, water, (inert gas, fuel gas); D = zinc oxide
particles, water, (inert gas); a = fuel gas, air/02; b =
cooling (inert gas); c = air/02; d = cooling gas.
Example 1:
Zinc powder (250 g/h, particle size =5 urn) is conveyed by
means of a nitrogen stream (1.5 m3/h) into an evaporation
zone, where a hydrogen/air flame (hydrogen: 4.25 m3/h, air:
8.40 m3/h, lambda = 0.82) is burning. The zinc powder is
evaporated here. The reaction mixture consisting of zinc
vapour, hydrogen, nitrogen and water is then cooled to a
temperature of 850 C by the addition of 1 m3/h nitrogen. 5
m3/h oxidation air and 34 m3/h quench air are then added,
whereby the reaction temperature falls to values below
400 C. The zinc oxide powder obtained is separated from
the gas stream by filtration.
Example 2
Same as Example 1, whereby the parameters are altered to
the values shown in Table 1.
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
22
Example 3 (comparative example)
Same as Example 1, except with an excess of air compared
to oxygen in the evaporation zone. The parameters are
altered to the values shown in Table 1.
Example 4 (comparative example)
Same as Example 1, except with no nucleation zone, the
temperature prior to oxidation does not fall below the
boiling point of zinc. The parameters are altered to the
values shown in Table 1.
The characterisation of the products obtained from these
examples is shown in Table 2.
Evaluation of the image analysis reveals the clearest
differences between the zinc oxide powders according to
the invention and the prior art for the average surface
area of the particles, the aggregate sizes and the shape
factor F(circle)
XPS analyses were performed of the zinc oxide powders
according to the invention from Examples 1 and 2. It was
found that the moisture content as non-desorbable oxygen
in the form of Zn-OH and Zn-OH2 units is 55.5 % (Example 1)
and 48.3 % (Example 2). The moisture is thus significantly
higher for example in the Nanotek Zinc Oxide product from
Nanophase Technologies.
Figure 2 shows a transmission electron micrograph of the
powder according to the invention. Aggregates of nodular
and acicular aggregates can clearly be seen.
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
sN
o Ln Ln rn Ln o '-o
In N l0 I I N
N d~ N r I O Ol Ln
M
Q) O 0
o
c
H O Ln N H N
N
ri O m k.0 O Ln O O 0 l>7
O 00 N Ol
M c-I d+ 01 O H 00 OM N
f1
ri O Ln M q N O O Ln
LIl 00 -1 Ln 4 co
N H d~ CO O CO LI) N
M
N 0) ri r r 0 E F. o
a
a.) ri
0) (d (1) U)
Ld b~ I~ CO 04
cad U) U) is cd -H ( ro
a o o ra -H Q~4 4J ) (0 O W )
0 4, rO i4 O U) U)
O U) x U) >
o N z x a U E+ 0 a H
a 0
-H 0 s~ b) (d
4) H 0 I~ Q
~=I (d 4) ,5u ?- " 0
0 raI b 9
1'W'I 4
> x
E W z 0 a
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
4J 0
rn ,~ N (d
N O r~ U)
N u-) ro b S-I
w N k.D %10 0 U) U) 0)
N r-I Ol - 0 lO ~-I U) 0)
r1 (n In N O O .-4 l0 pI rl (d
b
-a U)
M 4-)
(0 ro
U ~-1
ri
01 Q) rill
O (d r-I (0
Ln c) l0 In d * r l (D 4 )
= r1 CO m O 0 0 U) U)
N l0 r--I V1 O O Ol l0 rZ-~ ~,' 41
4--I
N 0
rc5
m U) bl >~
U) H cd
J--) -rl
4) U) U)
b) U) a) (1)
N U) -r-l r-l r-I
w H (N S-I U) r0 0
N M l0 M 0) 1-i U) r0
O In M N '.0 0) 0 U) 0
N H H N O O l0 Ln F:4 U 0
r--I
4-)
U)
41
r1 ~-I (d
x -I Q)
l0 M Lf) N m 0 0 ~I 0 0)
N m lf) N r-I 0 (D 00 L) W 0 cd
bl
i b) dA
a QI
w (0 X
ro a) a)
U N U) cd >, U
b rd (d b (1) = H 41
1U1 U) --I b) -H -rH 0 0 4) 0 (d En 4J a (0 U) C b, U U- MI -H
W b
4a S-I 0) (d (d a) 0 U) 0 Qa
N S-1 (1) U) (1) U) r-1 44 44 H 0) -r1 r-I E
3 0) b) 4-) b) O U ro Ei O O
41) U) rd (d a) (d =rI U) (1) 5-I U) '01 U
54 5-I si 5-I 40 04 Qa =rI .r4 I`'i P4
P U) U) (d U) ~ 4 (d (d U H (d 5-i ^
w > -r1 > (d 54 0 H
H f5 'x fgC b FC Qa v) v) W W E-
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
Surface modification
For the surface modification, the zinc oxides are
initially introduced into a mixer and, with intensive
mixing, optionally first sprayed with water and then
5 sprayed with the surface-modifying agent. When the
spraying has ended, after-mixing can be carried out for a
further 15 to 30 min, and then heat treatment for 1 to 4 h
at 50 to 4002C.
The water employed can be acidified with an acid, for
10 example hydrochloric acid, down to a pH of 7 to 1. The
silanizing agent employed can be dissolved in a solvent,
such as, for example, ethanol.
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
26
Table 1: Surface modification of the zinc oxide
preparation
Example 1 2 3
Oxide ZnO ZnO ZnO
Surface- octyl- octyl- polydi-
modifying trimethoxy- trimethoxy- methyl-
agent silane silane siloxane
Parts of
surface-
modifying agent 1.5 3 2
/ 100 parts of
oxide
Parts of H2O /
100 parts of 0 0.2 0
oxide
Temperature [ C] 120 120 350
Temperature time
2 2 2
[h]
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
27
Table 2: Physico-chemical data of the surface-modified
products from table 1
Example 1 2 3
BET surface 18 18 17
area [m2/g]
C content [%] 0.6 0.9 0.6
Loss on drying 0.1 0.1 0.2
[%]
Loss on 0.9 1.4 0.8
ignition [%]
pH 6.5 6.8 7.3
Use examples
The formulations according to the invention which, in the
combination of ZnO (w.c. = coating =
Trimethoxyoctylsilane), have shown a synergistic effect
with either OC = Octocrylene, OMC = Ethylhexyl
Methoxycinnamate, PISA = Phenylbenzimidazole Sulfonic Acid
or BEMT = Bis-Ethylhexyloxy Methoxyphenyl Triazine are
summarized in the following.
For statistical reasons it is assumed that the SPF should
be greater than or equal to two units higher than the
total SPF of the individual formulations if synergism is
to be referred to.
The SPF (sun protection factor) measurements are carried
out in vitro with an Optometrics SPF 290-S apparatus.
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
28
Examples 1-3
The standard recipe for W/O emulsions is used in these
examples. The nanoscale zinc oxide (with coating) is
introduced into the oily phase of the system. The
additional content of zinc oxide is subtracted from the
oily phase (Ethylhexyl Stearate and Mineral Oil).
1 Standard recipe W/O emulsion with ZnO (w.c.)
2 Standard recipe W/O emulsion with OC
3 Standard recipe W/O emulsion with ZnO (w.c.) and OC
Tab. 3: Build-up of the W/O recipes in examples 1-3 (data
in %)
Phase INCI 1 3 4
A Cetyl PEG/PPG -10/1 2.5 2.5 2.5
Dimethicone
Ethylhexyl Stearate 12.5 12.5 10.0
Mineral oil 12.5 12.5 10.0
Isostearic Acid 1.0 1.0 1.0
Hydrogenated Castor Oil 0.5 0.5 0.5
Microcrystalline Wax 1.0 1.0 1.0
Octocrylene 5.0 5.0
Zinc Oxide (w.c.) 5.0 5.0
Zinc Oxide (w/o.c.)
B Sodium Chloride 0.5 0.5 0.5
Aqua 64.45 64.45 64.45
2-Bromo-2-Nitropropane- 0.05 0.05 0.05
1,3-diol
SPF 2 3 6
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
29
Examples 4-7
The standard recipe for 0/W emulsions is used in these
examples. The nanoscale zinc oxide (with coating) is
introduced into the oily phase of the system. The
additional content of zinc oxide is subtracted from the
aqueous phase (Aqua). Isostearic acid is employed
experimentally as a surface modifier and pH stabilizer.
4 Standard recipe O/W emulsion with ZnO (w.c.)
5 Standard recipe 0/W emulsion with OC
6 Standard recipe O/W emulsion with Zn0 (w.c.) and OC
7 Standard recipe O/W emulsion with Zn0 (w.c.), OC and
isostearic acid
Tab. 4: Build-up of the 0/W recipes in examples 4-7 (data
in %)
Phase INCI 4 5 6 7
A Ceteareth-15, 2.5 2.5 2.5 2.5
Glyceryl Stearate
Glyceryl Stearate 1.0 1.0 1.0 1.0
Stearyl Alcohol 2.0 2.0 2.0 2.0
C12-15 Alkyl 14.5 9.5 9.5 8.5
Benzoate
Octocrylene 5.0 5.0 5.0
Zinc Oxide (w.c.) 10.0 10.0 10.0
Zinc Oxide (w/o.c.)
Isostearic Acid 1.0
B Glycerine 3.0 3.0 3.0 3.0
Aqua 66.5 76.5 66.5 66.5
Chloroacetamide 0.1 0.1 0.1 0.1
C Xanthan Gum 0.4 0.4 0.4 0.4
SPF 2 3 8 9
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
Examples 8-10
The standard recipe for W/O emulsions is used in these
examples. The nanoscale zinc oxide (with coating) is
introduced into the oily phase of the system. The
5 additional content of zinc oxide is subtracted from the
oily phase (Ethylhexyl Stearate and Mineral Oil).
8 Standard recipe W/O emulsion with ZnO (w.c.)
9 Standard recipe W/O emulsion with OMC
10 Standard recipe W/O emulsion with ZnO (w.c.) and OMC
10 Tab. 5: Build-up of the W/O recipes in examples 8-10 (data
in %)
Phase INCI 8 9 10
A Cetyl PEG/PPG -10/1 Dimethicone 2.5 2.5 2.5
Ethylhexyl Stearate 12.5 12.5 10.0
Mineral Oil 12.5 12.5 10.0
Isostearic Acid 1.0 1.0 1.0
Hydrogenated Castor Oil 0.5 0.5 0.5
Microcrystalline Wax 1.0 1.0 1.0
Ethylhexyl Methoxycinnamate 5.0 5.0
Zinc Oxide (w. c .) 5.0 5.0
Zinc Oxide (w/o.c.)
B Sodium Chloride 0.5 0.5 0.5
Aqua 64.45 64.45 64.45
2-Bromo-2-Nitropropane-1,3-diol 0.05 0.05 0.05
SPF 2 7 13
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
31
Examples 11-14
The standard recipe for ON emulsions is used in these
examples. The nanoscale zinc oxide (with coating) is
introduced into the oily phase of the system. The
additional content of zinc oxide is subtracted from the
aqueous phase (Aqua). Isostearic acid is employed
experimentally as a surface modifier and pH stabilizer.
11 Standard recipe 0/W emulsion with ZnO (w.c.)
12 Standard recipe 0/W emulsion with OMC
13 Standard recipe 0/W emulsion with ZnO (w.c.) and OMC
14 Standard recipe 0/W emulsion with ZnO (w.c.), OMC and
isostearic acid
Tab. 6: Build-up of the 01W recipes in examples 11-14
(data in %)
Phase INCI 11 12 13 14
A Ceteareth-15, Glyceryl 2.5 2.5 2.5 2.5
Stearate
Glyceryl Stearate 1.0 1.0 1.0 1.0
Stearyl Alcohol 2.0 2.0 2.0 2.0
C12-15 Alkyl Benzoate 14.5 9.5 9.5 8.5
Ethylhexyl Methoxycinnamate 5.0 5.0 5.0
Zinc oxide (w. c .) 10.0 10.0 10.0
Zinc oxide (w/o.c.)
Isostearic Acid 1.0
B Glycerine 3.0 3.0 3.0 3.0
Aqua 66.5 76.5 66.5 66.5
Chloroacetamide 0.1 0.1 0.1 0.1
C Xanthan Gum 0.4 0.4 0.4 0.4
SPF 2 6 11 16
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
32
Examples 15-17
The standard recipe for W/0 emulsions is used in these
examples. The nanoscale zinc oxide (with coating) is
introduced into the oily phase of the system. The
additional content of zinc oxide is subtracted from the
oily phase (Ethylhexyl Stearate and Mineral Oil).
Standard recipe W/O emulsion with ZnO (w.c.)
16 Standard recipe W/O emulsion with PISA
17 Standard recipe W/O emulsion with ZnO (w.c.) and PISA
10 Tab. 7: Build-up of the W/O recipes in examples 15-17
(data in %)
Phase INCI 15 16 17
A Cetyl PEG/PPG -10/1 Dimethicone 2.5 2.5 2.5
Ethylhexyl Stearate 12.5 15.0 12.5
Mineral Oil 12.5 15.0 12.5
Isostearic Acid 1.0 1.0 1.0
Hydrogenated Castor Oil 0.5 0.5 0.5
Microcrystalline Wax 1.0 1.0 1.0
Zinc Oxide (w. c .) 5.0 5.0
Zinc Oxide (w/o.c.)
B Sodium Chloride 0.5 0.5 0.5
Aqua 64.45 49.45 49.45
2-Bromo-2-Nitropropane-1,3-diol 0.05 0.05 0.05
Phenylbenzimidazole Sulfonic 15.0 15.0
Acid (20% Aqua)
SPF 2 5 9
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
33
Examples 18-21
The standard recipe for 0/W emulsions is used in these
examples. The nanoscale zinc oxide (with coating) is
introduced into the oily phase of the system. The
additional content of zinc oxide is subtracted from the
aqueous phase (Aqua). Isostearic acid is employed
experimentally as a surface modifier and pH stabilizer.
18 Standard recipe 0/W emulsion with ZnO (w.c.)
19 Standard recipe 0/W emulsion with PISA
20 Standard recipe O/W emulsion with Zn0 (w.c.) and PISA
21 Standard recipe 0/W emulsion with Zn0 (w.c.), PISA
and isostearic acid
Tab. 8: Build-up of the 0/W recipes in examples 18-21
(data in %)
Phase INCI 18 19 20 21
A Ceteareth-15, Glyceryl Stearate 2.5 2.5 2.5 2.5
Glyceryl Stearate 1.0 1.0 1.0 1.0
Stearyl Alcohol 2.0 2.0 2.0 2.0
C12-15 Alkyl Benzoate 14.5 14.5 14.5 13.5
Zinc Oxide (w. c .) 10.0 10.0 10.0
Zinc Oxide (w/o.c.)
Isostearic Acid 1.0
B Glycerine 3.0 3.0 3.0 3.0
Aqua 66.5 61.5 51.5 51.5
Chloroacetamide 0.1 0.1 0.1 0.1
Phenylbenzimidazole Sulfonic Acid 15.0 15.0 15.0
(20% Aqua)
C Xanthan Gum 0.4 0.4 0.4 0.4
SPF 2 5 11 15
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
34
Examples 22-24
The standard recipe for W/0 emulsions is used in these
examples. The nanoscale zinc oxide (with coating) is
incorporated into the oily phase of the system. The
additional content of zinc oxide is subtracted from the
oily phase (C12-15 Alkyl Benzoate).
22 Standard recipe W/O emulsion with ZnO (w.c.)
23 Standard recipe W/O emulsion with BEMT
24 Standard recipe W/O emulsion with ZnO (w.c.) and BEMT
Tab. 9: Build-up of the W/O recipes in examples 22-24
(data in %)
Phase INCI 22 23 24
A Cetyl PEG/PPG -10/1 Dimethicone 2.5 2.5 2.5
C12-15 Alkyl Benzoate 27.00 25.00 22.00
Isostearic Acid 1.0 1.0 1.0
Hydrogenated Castor Oil 0.5 0.5 0.5
Microcrystalline Wax 1.0 1.0 1.0
Bis-Ethylhexyloxyphenol 3.0 3.0
Methoxyphenyl Triazine
Zinc Oxide (w.c.) 5.0 5.0
B Sodium Chloride 0.5 0.5 0.5
Aqua 64.45 64.45 64.45
2-Bromo-2-Nitropropane-1,3-diol 0.05 0.05 0.05
SPF 2 8 13
CA 02534389 2006-01-30
WO 2005/019347 PCT/EP2004/009023
Examples 25 to 28
The standard recipe for 0/W emulsions is used in these
examples. The nanoscale zinc oxide (with coating) is
introduced into the oily phase of the system. The
5 additional content of zinc oxide is subtracted from the
aqueous phase (Aqua). Isostearic acid is employed
experimentally as a surface modifier and pH stabilizer.
25 Standard recipe 01W emulsion with ZnO (w.c.)
26 Standard recipe O/W emulsion with BEMT
10 27 Standard recipe O/W emulsion with ZnO (w.c.) and BEMT
28 Standard recipe 0/W emulsion with ZnO (w.c.), BEMT
and isostearic acid
Tab. 10: Build-up of the O/W recipes in examples 25-28
(data in %)
Phase INCI 25 26 27 28
A Ceteareth-15, Glyceryl Stearate 2.5 2.5 2.5 2.5
Glyceryl Stearate 1.0 1.0 1.0 1.0
Stearyl Alcohol 2.0 2.0 2.0 2.0
C12-15 Alkyl Benzoate 14.5 12.5 12.5 11.5
Bis-Ethylhexyloxyphenol 2.0 2.0 2.0
Methoxyphenyl Triazine
Zinc Oxide (w.c.) 10.0 10.0 10.0
Zinc Oxide (w/o.c.)
Isostearic Acid 1.0
B Glycerine 3.0 3.0 3.0 3.0
Aqua 66.5 76.5 66.5 66.5
Chloroacetamide 0.1 0.1 0.1 0.1
C Xanthan Gum 0.4 0.4 0.4 0.4
SPF 2 3 6 8