Note: Descriptions are shown in the official language in which they were submitted.
CA 02534815 2006-02-03
WO 2005/015240 PCT/GB2004/003385
METHOD FOR THE DETECTION OF ABNORMALLY GLYCOSYLATED PROTEINS
'The present invention relates to a method of screening a sample for the
presence of
one or more abnormally glycosylated and/or expressed proteins. The present
invention is particularly, although not exclusively, concerned with screening
a
biological sample, for example a body fluid sample, for the presence of
abnormally O-
and/or N-glycosylated proteins, which may be indicative of disease or of
substance
abuse in an individual.
Most plasma membrane and secretory proteins are complex macromolecules
incorporating oligosacchaxides, which play an integral role in their
biological
function. Consequently, abnormal glycosylation or expression of proteins is
often
associated with disease. For example, defects in nine genes of the N-linked
glycosylation pathway are associated with serious medical conditions,
including
neurological dysfunction, collectively termed Congenital Disorders of
Glycosylation
(CDG, H. Freeze, Glycobiology 2001, 11, 1298-143R). Other conditions
associated
with abnormal glycosylation include Leroy disease, Wiskott-Aldrich syndrome
and
glycoproteinosis. Further, diseases resulting from exposure to some external
factor,
such as alcoholism, rheumatoid arthritis and cancer are also associated with
abnormal
glycosylation of proteins (see for example, G. Durand and N. Seta, Clin. Chem.
2000,
46, 795-805).
In addition, it is known (V. Skibeli et al., Blood 2001, 9~, 3626-3634) that
the
glycosylated form of recombinant erythropoietin (rEPO), which is used by
athletes to
boost performance, differs from the natural form in that it is deficient in
one type of
sialic acid linkage (NeuSAc(a2-6)Gal on N-glycans) and has supplementary poly-
N-
CA 02534815 2006-02-03
WO 2005/015240 PCT/GB2004/003385
2
acetyl lactosamine (Gal((31-4)GIcNAc(bl-3)) and glucosamine (GIcNAc((31-
4)Man((31-4)). Exogenous erythropoietin can therefore be distinguished from
the
endogenous protein.
Presently, methods for determining abnormal glycosylation rely on iso-electric
focusing (IEF) or Western blotting of blood extracts for different glycoforms
of
human serum transferin (H. Stibler et al., Acta. Paediatr. Scand. Suppl.,
1991, 375,
21-31; J. Jaeken et al., Clin, Chim. Acta, 1984, 144, 245 to 247; N. Seta et
al., Clin.
Chim. Acta 1996, 24, 131-140). Recently, however, abnormal glycosylation has
been
determined by lectin analysis of purified serum endocrine glycoproteins (M.
Ferrari et
al., Eur. J. Endocrinol., 2001, 144, 409-416).
The detection of illicit use of rEPO is currently based on IEF of urine
concentrates
followed by a double blotting procedure using antibodies (F. Lasne and J. de
Ceaurriz,
Nature 2000, 405, 635 and F. Lasne, J. T_m_m__unol. Methods, 2001, 253 (1-2),
125-131).
However, all of the above techniques require preliminary concentration,
purification
or isolation of a predetermined protein and can be time consuming and/or
expensive.
IEF involves a multitude of steps and can often take up to three days.
Further, it is not
specific in that it is based on net charge of the molecule so that different
defects,
leading to identical net charge can have substantially similar focusing
patterns.
International Patent Application WO 00/33076 discloses a method of diagnosis
of
human glycosylation disorders. A first, reagent, for example a lectin, binds
with an
oligosaccharide moiety present in a sample from an individual having a
glycosylation
CA 02534815 2006-02-03
WO 2005/015240 PCT/GB2004/003385
3
disorder and not present in a sample from a healthy individual. A second,
reagent
binds with an oligosaccharide present in a healthy individual but not present
in an
individual having a glycosylation disorder. A glycosylation disorder is
indicated
through binding of the first reagent and/or non-binding of the second reagent.
The method is, however, dependent on the presence or absence of a target
oligosaccharide moiety in the sample compared with a control sample. It is,
therefore,
unable to detect glycosylation disorders characterised by the relative extent
and/or
ratio of oligosaccharide moieties (or epitopes). There is consequently a need
for an
improved method for determining abnormal glycosylation or substance abuse in
individuals.
The present invention generally seeks to provide an improved method for
screening a
sample, in particular a body fluid sample, for one or more abnormally
glycosylated
proteins. The present invention also aims to improve diagnosis of disease
exhibiting
abnormal glycosylation and to improve testing for performance enhancing
protein
drugs in mammals.
The present invent'iori also seeks to provide an improved method for
determining the
presence of an abnormally expressed protein in a body fluid sample. Such a
method
may be of value to the diagnosis of disease, such as Alzeihemer's, BSE and the
like.
Accordingly, in a first aspect, the present invention provides a method of
screening a
sample for the presence of one or more abnormally glycosylated andlor
expressed
proteins comprising the steps of i) exposing said sample to two or more
different
CA 02534815 2006-02-03
WO 2005/015240 PCT/GB2004/003385
4
lectins and/or antibodies ii) determining the extent of binding of said sample
to at
least two of said lectins and/or antibodies and iii) comparing the determined
extent of
binding to said at least two lectins and/or antibodies with that of a control
sample.
The method is preferably used for screening biological samples, i.e. those
taken from
the human or animal body. However, it may also be used to screen other samples
such as those used during laboratory experimentation.
It will be understood that a particular sample may be exposed to the lectins
and/or
antibodies separately and sequentially. In a preferred embodiment, however,
the
sample is exposed to the said two or more lectins and/or antibodies
simultaneously.
In particular, the lectins and/or antibodies may be immobilised on a, solid
support
surface and arranged in an array and the sample sequentially and/or
simultaneously
exposed to them.
Although the determination of the extent of binding to a selection of
different lectins
and/or antibodies is of critical importance to the present invention, there is
no
requirement that more than two, or any one particular lectin or antibody is
used: All
that is required is that a pattern of the extent of binding is determined for
a particular
sample. Preferably, however, the extent of binding is determined for more than
two
lectins and/or antibodies.
Suitable lectins include those that are specific for sialic acid (NeuSAc),
galactose,
mannose, glucosamine and fucose containing oligosaccharides. Amongst these,
the
CA 02534815 2006-02-03
WO 2005/015240 PCT/GB2004/003385
commercially available lectins Sambucus nigra agglutinin (SNA), specific for a-
2, b-
linked NeuSAc and Maackia amure~csis agglutinin (MA.A), specific for a-2, 3-
linked
NeuSAc are preferred. Other suitable, lectins include TetYagoholobus
purpu~eaus
(TLP), Ahguila anguila (AA) and Ulex euYOpaeus I agglutinin (UEA) which are
5 specific for Fuc(al-3/4). Suitable antibodies may be raised by any technique
known
to the art.
As mentioned above, the extent of binding of a particular sample to a lectin
and/or
antibody array, for example, follows a unique pattern. The pattern, which can
be
thought of as a "fingerprint" or "signature" of the sample on the array,
characterises
the glycosylation environment of the sample, each lectin, for example, being
specific
to a group of related oligosaccharide epitopes.
'The fingerprint or signature of a particular sample may be expressed by one
or more
ratios of extent of binding to different lectins and/or antibodies. Thus, in a
preferred
embodiment the comparison with the control sample compares one or more ratios
(AB, A/C, B/C etc.) of extent of binding to different lectins and/or
antibodies (A, B,
C) for each sample.
It will be apparent, therefore, that the method is particularly suited to
screening
complex samples - which may be expected to exhibit binding to most or all of
the
lectins and/or antibodies.
The method can avoid the need for concentration, purification and isolation of
a
previously identified protein since, normally, it is the comparison of the
fingerprint or
CA 02534815 2006-02-03
WO 2005/015240 PCT/GB2004/003385
6
signature with that of a control sample that is indicative of the presence of
an
abnormally glycosylated or expressed protein. Thus, in one embodiment of the
present invention, a body fluid sample may be directly exposed to the array.
Of course, the selection of a particular body fluid for screening will be
based on the
amount of glycoprotein supposed or known to be directly available therein and
the
sensitivity of the method utilised in the determination step. Suitable body
fluids for
direct analysis include urine and blood serum.
It may, however, be desirable to concentrate or purify the sample. In another
embodiment, therefore, the method of the present invention comprises the
preliminary
step of isolating one or more proteins from the sample by capture with an
antibody or
antibodies. In this embodiment, the isolation of protein or proteins minimises
unwanted complications in the determination step - especially where the
concentration of the proteins approaches the limits of detection and
quantification.
The fingerprint or signature of a particular sample may be interrogated by any
suitable
means determining the extent of binding of the sample to said at least two
lectins
and/or antibodies. - Tn particular; the extent of binding of a particular
sample to a
particular lectin or antibody may be determined by colorimetric, fluorescence
or
optical techniques.
In a preferred embodiment, however, that the fingerprint or signature is
interrogated
by a technique allowing real-time monitoring of the binding of the sample to
the
array. Such techniques also allow kinetic characteristics such as binding or
CA 02534815 2006-02-03
WO 2005/015240 PCT/GB2004/003385
7
dissociation rates, to be determined providing further information as to the
presence of
abnormal glycosylated or expressed proteins.
In particular, the fingerprint or signature may be interrogated by an
evanescent optical
technique. Preferably, therefore, the solid support and array together
comprise an
optical biosensor. Suitable solid supports include surface plasmon resonance
(SPR)
chips and/or metal or dye clad leaky waveguide (MCLW/DCLW) chips, such as
those
described in our international applications WO 99144042 and PCT/GB02/045045.
Preferably, the optical interrogation utilises a flow technique. Suitable
apparatus,
therefore, include ordinary and optimised surface plasmon resonance apparatus
described in International Patent Application WO 01/42768.
The comparison of the interrogated fingerprint or signature with that of the
control
sample does not necessarily require that the investigator himself make an
exposure of
the control sample to an identical array.
In particular, the comparison may be made by reference to a library database
including fingerprint data from a number of control samples. Preferably the- --
comparison is computer aided. In this embodiment, the fingerprint data
deposited in
the database need only record salient features characterising health and/or
abnormal
glycosylation.
An exposure of the control sample may, however, be preferred in lectin-only
arrays
where the relatively weak lectin-glycoprotein interaction allows easy
regeneration.
CA 02534815 2006-02-03
WO 2005/015240 PCT/GB2004/003385
8
The control sample may be derived from a healthy individual or an individual
exhibiting abnormal glycosylation. In particular, the control sample may be
one or
more earlier samples taken from the same individual. Such a control sample is
especially useful for monitoring continuation of substance abuse or the
progress of
disease and/or treatment.
It will appreciated from the foregoing, that the present invention provides an
improved method of screening samples, in particular body fluid samples, in
that it
compares the ratio of binding to two or more lectins and/or antibodies. Thus
the
method is not dependent on the absence of certain epitopes in one or other of
the
samples and is less prone to false positives.
In addition, real-time monitoring based on optical techniques allows rapid
screening
of complex samples in a single exposure of the sample to a lectin and/or
antibody
array. Such techniques allow simultaneous determination of binding to each
lectin
and/or antibody in an array and may offer additional information which may
characterise the absence or presence of an abnormally glycosylated or
expressed
protein in a sample.
The method of the present invention may also be used to screen a body fluid
sample
for indication of use or abuse of glycoprotein drugs in humans or animals.
In a second aspect, therefore, the present invention provides a method for
determining
use of a glycoprotein drug in a human or animal comprising the steps of i)
taking a
body fluid sample ii) exposing said sample to two or more different lectins
and/or
CA 02534815 2006-02-03
WO 2005/015240 PCT/GB2004/003385
9
antibodies iii) determining the extent of binding of said sample to at least
two lectins
and/or antibodies and iii) comparing the determined extent of binding to said
at least
two lectins and/or antibodies with that of a control sample.
It will be understood that the glycoprotein drug is synthetic in origin and
differs from
the endogenous form in, for example, its glycosylation environment. The method
is
particularly suitable for determining abuse or illicit use of recombinant
performance
enhancing protein drugs, such as recombinant forms of erythropoietin,
chorionic
gonadotropin or human growth hormone.
The method provides for detection of exogenous protein through pattern
recognition
indicating the presence of abnormal glycoforms of the protein - even in the
presence
of endogenous protein.
Exposure of a urine sample to a lectin-only array, for example, and comparison
of the
interrogated fingerprint in relation and/or other indicia with that of a
control sample as
explained above can provide conclusive evidence of use. .
Embodiments of the second aspect of the present invention will be -apparent
from the
above and the claims appended hereto.
As mentioned above, the fingerprint or signature of a particular sample may be
informative as to a particular disease state in an individual when compared to
that of a
sample from a healthy individual.
CA 02534815 2006-02-03
WO 2005/015240 PCT/GB2004/003385
In a third aspect, therefore, the present invention provides a method for the
diagnosis
of acquired or inherited glycosylation disorders comprising the steps of i)
taking a
body fluid sample ii) exposing said sample to two or more different lectins
and/or
antibodies iii) determining the extent of binding of said sample to at least
two lectins
5 and/or antibodies and iii) comparing the determined extent of binding to
said at least
two lectins and/or antibodies with that of a control sample.
Embodiments of the third aspect of the present invention will also be apparent
from
the above and the claims appended hereto.
The method in this aspect of the present invention may, for example, be
particularly
suitable for diagnosis of disease in neonates, children or adults. The method
is not
necessarily limited to medical conditions which are attributable to abnormal
glyscosylation but may also be of value to conditions accompanied by abnormal
glycosylation.
In a further aspect, the present invention provides a kit of parts for use in
the
aforementioned methods comprising one or more lectins and/or antibodies and a
control sample and/or information relating to -nbrmal~ or expected-
glycosylation
binding patterns and/or characteristics for an individual type.
The lectins and/or antibodies may be supplied in vials or a suitable container
or
containers. Additionally, a solid support surface may be supplied for
attachment of a
selection of lectins and/or antibodies by any of the usual methods known to
the art.
Preferably, the solid support surface comprises an SPR, MCLW or DCLW chip.
CA 02534815 2006-02-03
WO 2005/015240 PCT/GB2004/003385
11
In a preferred embodiment, suitable for detecting the illicit use of a
performance
enhancing drug, such as erythropoietin, the kit may comprise only lectins.
The supplied information may also include information relating binding
patterns
and/or characteristics to candidate disease states. In particular, the
additional
information may categorise certain binding patterns and/or characteristics
according
to likely medical condition.
The present invention will now be described by reference to the following
examples
and drawings in which
Figure 1 is a putative bar graph illustrating the fingerprint or signature of
samples (a, b, c) on a four-fold lectin array;
Figures 2 a) and b) are SPR sensor-grams illustrating respectively the binding
of buffered rEPO and bovine fetuin solutions to a SNA/MAA array;
Figures 3 a), b) and c) are SPR sensor-grams illustrating respectively the
binding of a buffered solution of NESP (a rEPO), human EPO and human fetuin to
a
SNA/MAA array;
Figure 4 is a bar graph showing a fingerprint or signature for the samples of
Figures 3 a) to c);
Figures 5 a) to d) are SPR sensor-grams illustrating the binding of buffered
solutions of mixtures of human EPO/NESP and human EPO/human fetuin on a
SNA/MAA array;
Figure 6 is a graph showing the dissociation characteristics of human
EPO/NESP mixtures from MAA according to Figure 5 relative to the proportion of
EPO;
CA 02534815 2006-02-03
WO 2005/015240 PCT/GB2004/003385
12
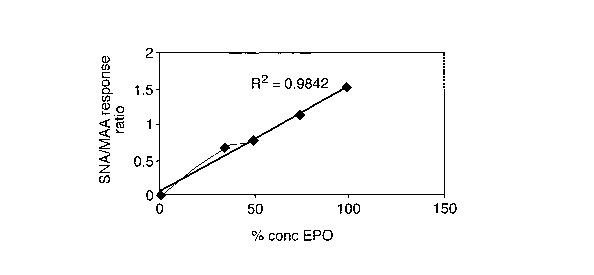
Figures 7 a) and b) are graphs showing the ratio of response to the SNA/MAA
array according to Figure 5 of human EPO/NESP mixtures and human EPO/human
fetuin mixtures relative to the proportion of EPO;
Figure 8 is a SPR sensor-gram illustrating the interaction of a urine sample
respectively with the SNAIMAA array; and
Figures 9 a) and b) are graphs highlighting the screening of human blood
serum samples for alcoholism or related diseases using a four-fold lectin
array.
Referring now to Figure 1, the putative response of four lectins (1 to 4) to a
control
sample a) and two samples b) and c) exhibiting an abnormal glycosylation
disorder
confer a fingerprint or signature to each sample (al, a2, a3, a4 etc). The
fingerprint or
signature of sample b), when compared to that of the control sample, indicates
the
disorder although it is not apparent from its interaction with lectin 2 alone.
Having regard to Figures 2 to 8, experiments were performed on a BIAcore~ 2000
four flow channel SPR instrument equipped with BIAevaluation software 3Ø
Lectins
Sambucus ~igra agglutinin and Maackia amurensis agglutinin (Sigma, Madrid)
were
each immobilised to part of an SPR chip (carboxymethylated dextran surface,
standard density CMS sensorchip; Pharmacia Biosensor AB, Uppsala~ Sweden) for
registration with each channel using known amine coupling techniques (S.R.
Haseley
et al., Anal. Biochem., 1999, 271, 203-210). Table 1 summarises the
immobilisation
parameters for the chip.
CA 02534815 2006-02-03
WO 2005/015240 PCT/GB2004/003385
13
Channel (Ch) Ch 1 Ch 2 Ch 3
Immobilisation None NaOAc, 10 mM, NaOAc, 10 mM,
Buffer (IB) pH 4.5 pH 4.5
Lectin SolutionBlank 4 ~,l of 5 mgml-'10 ~l of 2 mgml-1
(chemically MAA solution SNA solution
in in
activated for H20 diluted Ha0 diluted
lectin to 100 to 100
immobilisation)wIIB ~,IIB
Immobilisation 40 RU 9000 RU 14000RU
(Response Units
RU)
Table 1
Example 1
rEPO expressed in CHO cells (European Pharmacopeia, France) was made up to 5
~,gmf1 in distilled water. Bovine fetuin (Sigma, Madrid) was made up to l0~gm1-
1 in
distilled water. The interactions of 35 ~,l of each of the rEPO and bovine
fetuin
solution with the chip was studied in running buffer (pH 7.5; 100 mM HEPES,
150
mM NaCI, 2 mM CaCl2 and 2 mM MgCl2 in distilled water).
Referring now to Figure 2 a), the binding of rEPO to MAA is much greater than
to
SNA. rEPO, in, common with the natural form of EPO, has a significant number
of a-
2,3-NeuSAc linkages - specifically recognised by MAA but, in contrast to the
natural
form of EPO, only a single a-2,6-NeuSAc linkage - specifically recognised by
SNA.
The binding of rEPO with MAA shows a rapid uptake of the glycoprotein to the
lectin, which gradually levels off. A low level of disassociation of the
glycoprotein
follows - the residual level of binding remaining high.
CA 02534815 2006-02-03
WO 2005/015240 PCT/GB2004/003385
14
The binding of rEPO with SNA include an initial uptake of the glycoprotein,
which
almost immediately levels off. Residual binding of the glycoprotein tends to
zero
even though it contains one a-2,6-NeuSAc linkage.
Referring now to Figure 2 b), the binding of bovine fetuin, which is similar
to human
EPO in that it comprises significant numbers of both a-2,6-NeuSAc and a-2,3-
NeuSAc linkages, to MAA is gradual and low. By contrast, the binding to SNA is
rapid and sustained at high levels.
It will be appreciated that concentration effects and the presence of linkages
in
common do not permit one or other of these lectins alone to definitively
indicate
whether the sample contains exogenous protein in addition to the endogenous
protein.
However, these results suggest that the interaction of a sample with two or
more
lectins will, however, allow exogenous protein to be distinguished - even in
the
presence of the natural form.
Further, the presence of rEPO in a sample will be apparent not just through
analysis of
the ratio of MA.A/SNA binding compared with that from a healthy mammal but
also
through comparison of the analysis of uptake, equilibriurii and/or
dissociation
characteristics of each sample.
Example 2
Referring now to Figures 3 a) to c), the binding (after 7 min. of 5 ~,l miri 1
running
buffer TRIS, pH 7.2, 150 xnM NaCI, 2 mM CaCl2 and 2xnM MgCl2) of each of human
EPO (1 ~,1 from stock in 200 ~,1, b) and human fetuin (7.5 ~,gml'1 solution,
c), to a
CA 02534815 2006-02-03
WO 2005/015240 PCT/GB2004/003385
similar chip is greater to SNA than to MA.A. However, the binding of NESP (8
~l
from stock in 200 ~,1, a), a recombinant form of EPO, to SNA is almost
negligible
when compared to MAA.
5 Figure 4 shows the fingerprint or signature of each sample in relation to
both lectins.
As may be seen the ratio of binding having regard to both lectins
distinguishes the
samples from each other.
Refernng now to Figure 5, the interaction of each lectin with mixtures a) to
c) of
10 varying ratios of human EPO to NESP, are of interest not only in the ratio
of the level
of binding but also in the dissociation phase of each response. It will be
noted that the
fingerprints and the dissociation curves are different for each mixture as
well as for
the pure samples.
15 The linear relationship between the amount of EPO and kd for MAA and/or SNA
as
well as SNA/MAA binding ratio also suggests that the method may, in
combination
with other techniques, enable quantitative determination of rEPO in a sample.
Table 2 compares the ratio of response of the lectins and the rate of
dissociation lcd for
each sample. As is clear, especially in regard to Figures 6 and 7 a), the
ratio of
binding response SNA/MAA and the rate of dissociation from MAA varies linearly
with the amount of EPO in the mixture. These results suggest that NESP and,
indeed, human fetuin can be determined in the presence of human EPO.
CA 02534815 2006-02-03
WO 2005/015240 PCT/GB2004/003385
16
Sample Binding Ratio of ka/10
Response Residual 4
(10 s Binding
> Responses
dissociation
point)
SNA MAA SNA/MAA MAA/SNA SNA MAA
EPO (E) 234 160 1.463 0.684 3.30 3.09
NESP 0 157 0 - - -3.27
1:l E/N 215 282 0.762 1.312 ~ 2.16 0.05
.
3:1 E/N 401 361 1.111 0.900 2.81 2.08
1:2 E/N 200 305 0.665 1.525 1.34 0.29
Fetuin 245 18 13.611 0.073 0.70 -7.29
(F)
1:1 E/F 307 138 2.225 0.450 1.07 1.88
Table 2
Sensitivity experiments performed on the chip showed that the lower detection
limit
of rEPO by MAA was in the range of 5 ngml-1 (160 pmol.l'1) which is comparable
to
the reference solution used for IEF (380 pmol.l-1).
Referring now to Figure 8, the results of the interaction of a control urine
sample,
concentrated 20-fold, is shown. Here, the chip has in addition to a blank (-x-
line)
surface, has a surface in which the dextran surface is not activated for
lectin
immobilisation (-+-). As may be seen, glycoprotein interactions with MAA and
SNA
are present.
CA 02534815 2006-02-03
WO 2005/015240 PCT/GB2004/003385
17
Reference to values (0 to 5.3 pmol.l-I) published for EPO in urine suggest the
need for
a 200 fold increase in sensitivity. It is envisaged, however, that routine
optimisation
of the technique (by selection of lectins and running buffers) and/or
detection method
(for example, light or emitted from the array through fluorescent tagging)
will enable
urine samples to be directly screened without the need for a concentration or
isolation
step.
For the determination of abuse of rEPO large arrays including MAA, SNA and
lectins
specific for poly N-acetyl lactosamine repeat units (Gal((31-4)GIcNAc((31-3)
or lectins
specific for bisecting GIcNAc (GIcNAc((31-4)Man(~31-4) are expected to offer
significant improvements. Alternatively or additionally, arrays including
lectins or
antibodies specific for tetraantennary glycan moieties found mostly with the
recombinant form may also be of value.
Channel (Ch)Ch 1 Ch 2 Ch 3 Ch 4
Lectin BPA MAA RCAi2o SNA
Immobilisation9600 6500 1100 1100
(Response
Units RU)*
Table 3
Having regard now to Figures 9 a) and b), similar experiments were performed
on 24
human blood serum samples using an SPR chip including an array of four lectins
Bauhiuia pu~pu~eus agglutinin (BPA), Maackia amu~ensis agglutinin, Sambucus
CA 02534815 2006-02-03
WO 2005/015240 PCT/GB2004/003385
18
nigra agglutinin and Ricinus communis 120 agglutinin (RCAl2o). Table 3
summarises
the immobilisation responses of each lectin on the chip.
Example 3
Half of the serum samples were obtained from patients with alcoholism or
related
diseases, and so contained high (Hl-H12) levels of carbohydrate deficient
transferrin
(CDT) relative to the total content of transferrin. The other half of the
serum (control)
samples had normal (low, Ll to Ll2) levels of carbohydrate deficient
transferrin
(CDT) relative to the total content of transferrin.
The serum samples were simply diluted (1:100) in running buffer (10 mM
Tris.HCl,
pH 7.0; 150 mM NaCI; 2 mM CaCl2 and 2 mM MgCl2) and the interaction of each
diluted sample with the chip was studied at various time (t) intervals
At t = 150 s the high (between 3.5 to 9.2 % transferrin) and low (between 2.0
to 2.6
transferrin) CDT containing samples can be reliably distinguished by examining
one or more of the order of response in the lectin array as well as the
magnitude and
relative magnitude of these responses.
For example, high CDT samples were found to have, in general, an order of
response
Ch: 3, 4, 1, 2 or 3, 2, 4, 1 whereas the majority of low CDT samples gave an
order of
response 3, 4, 2, 1.
The difference (D, 4-2) in response of SNA and MAA to high CDT samples was
generally high or negative compared with low CDT samples. The ratio (R, 3/4)
in
CA 02534815 2006-02-03
WO 2005/015240 PCT/GB2004/003385
19
response of RCA120 to SNA to low CDT samples tended to be lower than high CDT
samples.
A plot of difference D, 4-2 against R, 3/4 (Figure 9 a) shows tight clustering
of the
majority of low CDT samples and two distinct clusters of high CDT samples. A
plot
of ratio R, 3/4 against ratio R, 4/2 (Figure 9 b) similarly reveals tight
clustering of low
CDT samples.
The present invention may be suitable for rapid recognition of a wide variety
of
inherited or congenital disorders associated with abnormal glycosylation.
Blood
serum or urine may be screened for other medical conditions, not mentioned
above.
In particular, it is envisaged that the method is suitable for prenatal
screening for
Down's syndrome in that concentrations of human chorionic gonadotropin (hCG)
or
sub-units thereof are high in the serum and urine of pregnant women.
It is also envisaged that a wide variety of other lectins may be used and that
the
interaction of a sample with large numbers of lectins will not be unduly
complicated -
especially where analysis is computer-aided.