Note: Descriptions are shown in the official language in which they were submitted.
CA 02534957 2006-02-06
WO 2005/025556 PCT/US2004/025937
ANTIBIOTIC CYCLOALKYLTETRAHYDROQUINOLINE DERIVATIVES
RELATED APPLICATIONS
This application claims priority to and is a continuation of U.S. Application
No. 60/494,669, filed on August 13, 2003, the entire teachings of which are
incorporated herein by reference.
BACKGROUND OF THE INVENTION
In the last century, antibiotics were developed that led to significant
reductions
in mortality. Unfortunately, widespread use has led to the rise of antibiotic
resistant
bacteria, e.g., rnethicillin resistant Staphyloccocus au~eus (MRSA),
vancomycin
resistant er~te~~cocci (VRE), and penicillin-resistant Streptococcus
pneumoniae
(PRSP). Some bacteria are resistant to a range of antibiotics, e.g., strains
of
Mycobaete~°iunz tubef~culosis resist isoniazid, rifampin, ethambutol,
streptomycin,
ethionamide, kanamycin, and rifabutin. In addition to resistance, global
travel has
spread relatively unknown bacteria from isolated areas. to new populations.
Furthermore, there is the threat of bacteria as biological weapons. These
bacteria
may not be easily treated with existing antibiotics.
Infectious bacteria employ the peptidoglycan biosynthesis pathway, and in
particular, depend on MurA (phosphoefzolpyruvate:UDP-N acetyl-D-glucosarniue 1-
carboxyvinyltransferase, EC 2.1.5.7), to catalyze the transformation of
uridine
diphosphate-N acetyl-D-glucosamine and phosphoenolpyruvate into uridine
diphosphate-N acetyl-3-O-(1-carboxyvinyl)-D-glucosamine:
OH OH
o P;
Ho ° ~ -o~ ~o.~ - MurA Ho ' °
HO NH -°~P~~ ~ ~O ~ H~C~O NH
( Ac0-UDP O CHz CO - Ac0 UDP
2
UDP-N acetyl- UDP-N acet I-3-O- 1-carbox I
D-glucosamine phosphoenolpyruvate Y ~ Y~Y)
-D-glucosamine
CA 02534957 2006-02-06
WO 2005/025556 PCT/US2004/025937
_2_
MurA is conserved across both Gram positive and Crram negative bacteria, but
is not
present in mammalian systems, and is thus a desirable and selective target for
new
medications.
Therefore, there is a need fox new medications that target MurA, whereby
S infections from bacteria dependent on MurA can be treated.
SUMMARY OF THE 1NVENTTON
It has now been found that certain cycloalkyltetrahydroquinoline derivatives
strongly inhibit MurA, as shown in Example 3. A number of the disclosed
inhibitors
are found to have antibiotic activity against bacteria, including drug-
resistant
bacteria, as shown in Example 4. Furthermore, many of the disclosed MurA
inhibitors have low cytotoxicity, as shown in Example 5. Based on these
discoveries, compounds that are MurA inhibitors, methods of treatment with the
disclosed MurA inhibitors, and pharmaceutical compositions comprising the
disclosed MurA inhibitors, and methods for screening for MurA inhibitors are
provided herein.
A method of treating a subject for a bacterial infection includes
administering to
a subject in need of treatment for a bacterial infection an effective amount
of a
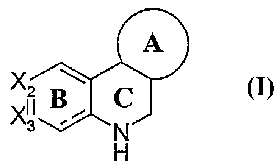
compound represented by structural formula I:
A
Xis B ~ C
N
or a pharmaceutically acceptable salt, solvate, or hydrate thereof.
Ring A is a 5 or 6 membered cycloalkyl or cycloalkenyl group, optionally
substituted with halogen or optionally halogenated C1-C3 alkyl or alkoxy.
2S ~i2 and ~3 are each carbon, or one is nitrogen and the othex is carbon.
Rings B and C are optionally and independently substituted at any
substitutable
ring carbon, pxovided that one ox two substitutable ring carbons in Rings B
and C
are substituted with an acidic group.
CA 02534957 2006-02-06
WO 2005/025556 PCT/US2004/025937
-3-
In another embodiment, the acidic group is selected from -(CO)OH, -(CS)OH,
-(SO)OH, -S03H, -OS03H, -P(ORa)(OH), -(PO)(ORa)(OH), -O(PO)(ORa)(OH), or
-B(ORa)(OH), wherein Ra is H or optionally substituted aryl, aralkyl,
hetexoaryl,
heteroaralkyl, or C1 to C4 alkyl. Typically, the acidic group is -(CO)OH, -
(CS)OH,
S -(SO)OH, -S03H, -OS03H, or preferably, -(CO)OH.
Another embodiment is a method of identifying a MurA inhibitor, including
contacting MurA with phosphoenolpyruvate and a test compound, under conditions
suitable for reaction between the MurA enzyme and the substrate
phosphoenolpyruvate, and determining a reaction rate between the
phosphoenolpyruvate and MurA. The test compound is identified as a MurA
inhibitor when the rate of reaction in the presence of the test compound is
less than a
reaction rate in the absence of the test compound. More preferably, the method
includes conducting the reaction in the presence of MurB and uridine S'-
diphospho-
N-acetylglucosamine. In a preferred embodiment, the method of identifying
compounds as MurA inhibitors is combined with one or more assays for
antibiotic
activity. Such assays are well known in the art, and can include, for example,
contacting bacteria of interest with a test compound under conditions
otherwise
suitable for bacterial growth, and determining if the test compound has
antibacterial
activity.
The invention is useful for treating (therapeutically or prophylactically)
bacterial
infections, particularly infections caused by bacteria that depend on the
peptidoglycan biosynthesis pathway, and more particularly, infections caused
by
bacteria that express the MurA enzyme. Furthermore, it can be useful against
bacteria that have developed antibiotic resistance, especially multiple drug
resistant
2S strains, because it is believed to act through a different mechanism than
existing,
widely used antibiotics.
DETAILED DESCRIPTION OF THE INVENTION
The invention is generally related to methods, compounds, and pharmaceutical
compositions for treating and preventing bacterial infections. In particular,
the
invention relates to substituted cycloalkyltetrahydroquinoline derivatives
that are
MurA inhibitors.
CA 02534957 2006-02-06
WO 2005/025556 PCT/US2004/025937
-4-
In preferred embodiments, the MurA inhibitor of the method is represented by
one of structural formulas I-a to I-c or I-a";
~B Ia ~ B I-b
N CO~H ~' N Y
H H02C H O
~ B ~ I-c I B I_a"
N CO H
HOZC H 2 z02C H CO H
S
In I-a", Z is -H or a C1 to C4 alkyl group.
In I-b, Y is optionally substituted C1 to C4 alkyl, C1 to C4 alkoxy, phenyl,
pyridyl, or NR~2, wherein each R~ is independently H, C 1 to C4 alkyl, aryl,
or
aralkyl, or NR~2 is a nonaromatic heterocycle.
In structural formulas I-a to I-c and I-a", Ring B is optionally substituted
at any
substitutable ring carbon.
In more preferred embodiments, the MurA inhibitor is represented by one of
structural formulas I-a' to I-c':
R1 R1 R1
R2 ~ R2 ~ R2
R3 I ~ N- 'C02H R3 I ~ N~Y HOzC I ~ N' _COaH
R4 H R4 H O R4 H
I-a' I-b' I-c'
The variables Rl, R2, R3 and R4 are independently -H, halogen, -NO2, -CN,
-(CO)Rb, -(C0)ORb, -(CO)0(CO)Rb, -(CS)ORb, -(CS)Rb, -(SO)ORb, -SO3Rb,
-OS03Rb, -P(ORb)Z, -(PO)(ORb)2, -0(PO)(ORb)2, -B(0Rb)2, -(CO)NR~2, -
NR°2,
-NRd(CO)Rb, -NRd(CO)ORb, -NRd(C0)NR°2, -SOZNR°Z, -NRdSO~Rb, or
an
optionally substituted aryl, aralkyl, heteroaryl, heteroaralkyl, C3 to C7
cycloalkyl,
nonaromatic heterocycle, C 1 to C4 alkyl, C 1 to C4 alkoxy, C 1 to C4 hydroxy
allcyl,
or C2 to C6 alkoxyalkyl; provided that, for I-b', at least one of Rl to R4 is
an acidic
CA 02534957 2006-02-06
WO 2005/025556 PCT/US2004/025937
-5-
group, e.g., -C02H. In a preferred embodiment of I-a', at least one of Rl to
R4 is -
COZH, and the remainder of Rl to R4 are as described above.
Each Rb and Rd is independently H or optionally substituted aryl, aralkyl,
heteroaxyl, heteroaralkyl, or Cl to C4 alkyl, and each R° is
independently H or
optionally substituted C1 to C4 alkyl, aryl, or axalkyl, or NR°z is an
optionally
substituted nonaromatic heterocycle. More typically, each Rb, R°, and
Rd is
independently -H, or optionally substituted C 1 to C4 alkyl or phenyl, ox each
NR°z
is an optionally substituted morpholinyl, piperidyl, or piperazyl. Preferably,
each Rb, ,
R° and Rd is independently H or Cl to C4 alkyl; or NR°z is
a nonaromatic
hetexocycle.
In preferred embodiments of I-a', I-b', and I-c', at least two of Rl to R4 are
H,
or more typically, at least two of Rl, R2, and R4 are H. More typically two,
and
preferably three of Rl to R4 are H, or two of Rl, R2, and R4 are -H.
More preferably for I-a', one or two of Rl to R4 are each independently
halogen
-(CO)Rb, -(CO)ORb, -(CO)NR°z, -NR°z, -NRd(c0)Rb, -NRd(CO)ORb,
-NRd(CO)NR°2, -NRd(CO)PhNRd(CO)Rb, or optionally substituted phenyl,
benzyl,
pyridyl, methylpyridyl, or optionally halogenated Cl to C4 alkyl or C1 to C4
alkoxy. In another preferable embodiment of I-a', Rl, R2, R3, and R4 are
independently -H, -(CO)Rb, -(CO)ORb, -(CO)O(CO)Rb, -(CS)ORb, -(CS)Rb,
-(SO)ORb, -S03Rb, -OSO~Rb, -P(ORb)z, -(PO)(ORb)z, -O(PO)(ORb)z, -B(oRb)2,
-NR°z, -NRd(CO)Rb, -NRd(CO)ORb, -NRd(CO)NR°z, -SO2NR°2, -
NRdSO2Rb, or an
optionally substituted aryl, aralkyl, heteroaryl, heteroaralkyl, C3 to C7
cycloalkyl, ox
nonaromatic heterocycle. More preferably, one or two of R1 to R4 are each
independently -(CO)Rb, -(CO)ORb, -(CO)NR°z, -NR°z, -NRd(CO)Rb, -
NRd(CO)ORb,
-NRd(CO)NR°z, -NR''(CO)PIzNRd(C~O)Rt', or optionally substituted
phenyl, benzyl,
pyridyl, or methylpyridyl;
More preferably, for I-b', Rl to R4 are as described in the preceding
paragraph,
provided that at least one of Rl to R4 is an acidic group, e.g., -COZH.
More preferably for I-c', Rl, R2, and R4 are independently -H, -F, -Cl, -Br,
-NOz, -CN, -(CO)Rb, -(CO)NR°2, -NR°z, -NRd(CO)Rb, -NRd(CO)ORb,
-NRd(CO)NR°z, -SOZNR°z, -NRdSO2Rb, or optionally halogenated C1
to C4 hydroxy
alkyl, G1 to C4 alkyl, or Cl to C4 allcoxy.
CA 02534957 2006-02-06
WO 2005/025556 PCT/US2004/025937
-6-
In other preferred embodiments of I-a' and I-b', at least one of R1 to R4 is
-(CO)ORb, e.g., -COZH or a C1-C4 carboxylic ester thereof. More typically, at
least
one of Rl to R4 is -COZH, or preferably, one of Rl to R3 is -C02H.
Specific examples of MurA inhibitors of the present invention are the
compounds in Table 1.
Also included in the present invention are pharmaceutical compositions
comprising the disclosed MurA inhibitors, (e.g., I-b, I-c, I-a' to I-c', and I-
a"). The
present invention also includes novel MurA inhibitors disclosed herein (e.g.,
I-b, I-
c, I-a' to I-c', and I-a"), or pharmaceutically acceptable, salts, solvates or
hydrates
thereof.
A "subject" includes mammals, e.g., humans, companion animals (e.g., dogs,
cats, birds, aquarium fish, reptiles, and the like), farm animals (e.g., cows,
sheep,
pigs, horses, fowl, farm-raised fish and the like) and laboratory animals
(e.g., rats,
mice, guinea pigs, birds, aquarium fish, reptiles, and the like).
Alternatively, the
subject is a warm-blooded animal. More preferably, the subject is a manunal.
Most
preferably, the subject is human.
A subject in need of treatment has a bacterial infection (or has been exposed
to
an infectious environment where bacteria are present, e.g., in a hospital) the
symptoms of which may be alleviated by administering an effective amount of
the
disclosed MurA inhibitors. For example, a subject in need of treatment can
have an
infection for which the disclosed MurA iWibitors can be administered as a
treatment. In another example, a subject in need of treatment can have an open
wound or burn injury, or can have a compromised immune system, for which the
disclosed MurA inhibitors can be administered as a prophylactic. Thus, a
subject can
be treated therapeutically or prophylactically. More preferably, a subject is
treated
therapeutically.
Typically, the subject is treated for a bacterial infection caused by a
bacteria of a
genus selected from Alloclzr-onzatium, Aeifzetobacter, Bacillus,
Campylobacter,
Clalamydia, Chlamydophila, Clostridium, CitrobacteY, Escherichia,
&ztef~obacter,
Erate~ococcus, Ft~ancisella, Haemophilus, Helicobacter, Klebsiella, Lister~ia,
Moraxella, Mycobacte~~iutn, Neisseria, Pnoteus, Pseudomohas, Salmonella,
Serratia,
CA 02534957 2006-02-06
WO 2005/025556 PCT/US2004/025937
- '7 _
Shigella, Stettotrophomonas, Staphyloccocus, Streptococcus, Synechococcus,
Tlibrio,
and Yersina.
More preferably, the subject is treated for a bacterial infection from
Allochromatiunt vinosum, Acinetobacter bautnartii, Bacillus anthracis,
Cantpylobacter jejuni, GlZlattaydia trachonZatis, Chlantydia pneumortiae,
Clostridium
spp., Citrobacter spp., Escherichia coli, Enterobacter spp., Enterococcus
faecalis.,
Enterococcus faecium, Francisella tularensis, Haetnophilus influenzae,
Helicobacter pylori, Klebsiella spp., Listeria ntonocytogenes, Moraxella
catarrhalis,
Mycobacterium tuberculosis, Neisseria meningitidis, Neisser is gonorrhoeae,
Proteus mirabilis, Proteus vulgaris, Pseudomonas aeruginosa, Salmonella spp.,
Serratia spp., Shigella spp., Stenotrophontonas ntaltophilia, Staphyloccocus
aureus,
Staphyloccocus epiderntidis, Streptococcus pneuntoniae, Streptococcus
pyogenes,
Streptococcus agalactiae, I'ersina pestis, and hetsina enterocolitica, and the
like.
Preferably, the subject is treated for a bacterial infection caused by a
bacterium
that expresses a peptidoglycan biosynthesis pathway, and in particular,
expresses the
enzyme encoded by the MurA/MurZ gene. Numerous studies have demonstrated
that the MurA gene and its paralog MurZ are conserved across a range of Gram
positive and Gram negative bacteria; see, for example, Schonbrunn E,
Eschenburg S,
Krekel F, Luger K, Amrhein N. (2000) Biochemistry. 2000 Mar 7;39(9):2164-73;
Baum EZ, Montenegro DA, Licata L, Turchi I, Webb GC, Foleno BD, Bush K.
(2001) Antimicrob Agents Chernother. 2001 Nov;45(11):3182-8; Kim DH, Lees
WJ, Kempsell KE, Lane WS, Duncan K, Walsh CT. (1996) Biochemistry. 1996 Apr
16;35(15):4923-8; and Skarzynski T, Mistry A, Wonacott A, Hutchinson SE, Kelly
VA, Duncan K. (1996) Structure. 1996 Dec 15;4(12):1465-74. The entire
teachings
of these documents are incorporated herein by reference.
As used herein, the term MurA, referring to the gene or the enzyme thereby
encoded, encompasses both MurA and its paralog MurZ. The enzymes are given
various names in the art, including, for example: MurA transferase; MurZ
transferase; UDP-N-acetylglucosamine 1-carboxyvinyl-transferase; UDP-N-
acetylglucosamine enoylpyruvyl transferase; UDP-N-acetyl glucosamine
enolpyruvyltransferase; enoylpyruvate transferase; phosphoenolpyruvate-UDP-
acetylglucosamine-3-enolpyruvyltransferase; phosphoenolpyruvate:UDP-2-
CA 02534957 2006-02-06
WO 2005/025556 PCT/US2004/025937
-g-
acetamido-2-deoxy-D-glucose 2-enoyl-1-carboxyethyltransferase;
phosphoenolpyruvate:uridine diphosphate N-acetyl glucosamine
enolpyruvyltransfexase; phosphoenolpyruvate:uridine-5'-diphospho-N-acetyl-2-
amino-2-deoxyglucose 3-enolpyruvyltransferase; phosphopyruvate-uridine
diphosphoacetylglucosamine pyruvatetransferase; pyruvate-UDP-acetyl
glucosamine transferase; pyruvate-uridine diphospho-N-acetyl glucosamine
transferase; pyruvate-uridine diphospho-N-acetyl-glucosamine transferase; or
pyruvic-uridine diphospho-N-acetylglucosaminyltransferase.
As used herein, the term MurB, referring to the gene or the enzyme thereby
encoded, is given various names in the art, including, fox example: UDP-N-
acetylinuramate dehydrogenase, MurB reductase; UDP-N-acetylenol pyruvoyl
glucosamine reductase; UDP-N-acetylglucosarnine-enoylpyruvate reductase; UDP-
GlcNAc-enoylpyruvate reductase; uridine diphosphoacetylpyruvoylglueosamine
reductase; uridine diphospho-N-acetylglucosamine-enolpyruvate xeductase;
uxidine-
5'-diphospho-N-acetyl-2-amino-2-deoxy-3-O-lactylglucose:NADP-oxidoreductase
The systematic name typically given fox MurAlMur2 is
phosphoenolpyruvate:UDP-N-acetyl-D-glucosamine 1-carboxyvinyltransferase, and
the ILTBMB systematic classification is EC 2.5.1.7. The systematic name
typically
given for MurB is UDP-N-acetylmuramate:NADP+ oxidoreductase, and the
ItJBMB systematic classification is EC 1.1.1.158. See International Union of
Biochemistry and Molecular Biology online at www.chem.qmuLac.uk/iubmb/.
In other embodiments, bacterial growth can be retarded, modulated, or
prevented
by employing an effective amount of the disclosed MurA inhibitors. Numerous
bacteria can express the MurA enzyme. Bacteria that express MurA can include,
for
example, actinobacteria, bacteroids, chlamydia, cyanobacteria; firmicutes,
e.g.,
bacillales, clostridia, and lactobacillales; fusobacteria; green sulfur
bacteria;
hyperthermophilic bacteria; proteobacteria, e.g., alpha, beta, delta, epsilon,
and
gamma; radioresistant bacteria; and spirochetes.
For example, actinobacteria can include, Bifidobacte~ium longurn,
CorynebacteYiurra e~cierts, Corynebaeteriuna glutamicurre,
Mycobacter°iurn bovis,
Mycobacteriurra lepr°ae, Mycobacterium tuberculosis (e.g., CDC1551
and H37Rv
CA 02534957 2006-02-06
WO 2005/025556 PCT/US2004/025937
_g_
(lab strain)), Streptotnyces coelicolor; Troplzeryma whipplei (e.g., Twist,
TW08/27);
and the like.
Examples of bacteroids include Bacteroides thetaiotaonzicr~ort. and the like.
Chlamydia can include, e.g., Chlarnydophila caviae, Chlanzydia >7iu>"idar~um,
Chlantydophila pneunzortiae (e.g., AR39, J138, CWL029, Chlarnydia
tt~aclaornatis,
and the like.
Examples of cyanobacteria can include Artabaena sp. PCC7120 (Nostoc sp.
PCC7120), Synechocystis sp. PCC6803, Ther~rnosynechococcus elongates, and the
like.
Firmicutes, e.g., bacillales can include Bacillus cereus, Bacillus halodurans,
Bacillus subtilis, Listeria irznocua, Listeria monocytogenes, Ocearzobaeillus
iheyerzsis, Staphylococcus aureus (e.g., MW2, N315, and Mu50), Staphylococcus
epiderntidis, and the like.
Firmicutes, e.g., clostridia, can include Clostridium acetobutylicurn,
Clostridium
perfr°irzgens, Clostridium tetani, Tlter~moartaer-obacter-
tehgcongensis, and the like.
Firmicutes, e.g., lactobacillales, can include Enterococcus faecalis,
Lactococcus
lactis, Lactobacillus plantar unt, Streptococcus agalactiae (e.g., 2603 and
NEM316),
Streptococcus mutarzs, Streptococcus pyogenes (e.g., MGAS315 (serotype M3),
SF370 (serotype Ml), SSI-1 (serotype M3), and MGAS8232 (serotype M18)),
Streptococcus pneuntorziae (e.g., TIGR4 and R6), and the like.
Fusobacteria can include Fusobacteriurrz nucleaturrz, and the like.
Green sulfur bacteria can include Clalorobiurn tepidurn, and the like
Iiyperthermophilic bacteria can include Aquzfex aeolieus, Tlzer~rzotoga
rrtaritinze,
and the like.
Examples of alpha proteobacteria can include Agr°obacteriurn
tumefaeiens C58
(Cereon), Bradyr°hizobium japorzicurn, Br~ccella rneliterzsis, Brueella
suis,
Caulobacter cr°escerztus, Mesorlaizobium loti, Riekettsia corzor~ii,
Rickettsia
prowazekii, Sinorhizobiurn meliloti, and the like.
Examples of beta proteobacteria can include Nitrosorrtonas europaea, Neisseria
rrteningitidis (e.g., Z2491 (serogroup A) and MC58 (serogroup B), Ralstaraia
solanacearurn, and the like.
CA 02534957 2006-02-06
WO 2005/025556 PCT/US2004/025937
-10-
Examples of delta/epsilon proteobacteria can include Campylobacter jejurzi,
Helicobacter pylori (e.g., J99 and 26695), and the like.
Examples of gamma proteobacteria can include Buchnera aplzidicola (e.g.,
Baizongia pistaciae), Buchatera aphidicola (e.g., Schizaphis
gr°antirturrt), Buchnera
sp. APS (e.g., Acyr-thosiphon pisum), Coxiella burnetii, Esclterichia coli
(e.g.,
CFT073, 0157 EDL933, K-12 W3110, K-12 MG1655, and 0157 Sakai),
Haentophilus irtfluenzae, Pseudornortas aer°uginosa, Pasteurella
multocida,
Pseudornortas putida, Pseudornonas syringae pv., Shigella flexneri 301
(serotype
2a), Shewanella oneidensis, Salmonella typhimuriunt, Salrnorzella typhi (e.g.,
Ty2,
GT18), Vibrio cholerae, Yibrio parahaemolyticus, T~ibrio vulnificus,
Wiggleswor°tlzia
br°evipalpis, Xarithontortas axortopodis, Xartthornonas campestris,
Xylella fastidiosa
(e.g., 9a5c and Temeculal), Yersinia pestis (e.g., C092 and KIM), and the
like.
Radioresistant bacteria can include Deinococcus r adiodurarts, and the like
Spirochetes can include Borrelia burgdorferi, Leptospira interrogarts,
Treponerrta pallidurn, and the like.
In one embodiment, a subject is also concurrently treated for a fungal
infection,
for example, a fungal infection caused by a pathogenic dermatophyte, e.g., a
species
of the genera Trichophytort, Tinea, Microsporum, Spider°mophyton and
the like; or a
pathogenic filamentous fungus, e.g., a species of genera such as Aspergillus,
Histoplasrna, Cryptococcus, Micr°osporurn, and the like; or a
pathogenic non-
filamentous fungus, e.g., a yeast, for example, a species of the genera
Candida,
Malassezia, Trichosporon, Rhodotor~ula, Tor~ulopsis, Blastorrtyces,
Paracoccidioides, Coccidioides, and the like. Preferably, the subject is
concurrently
treated for a fungal infection resulting from a species of the genera
Aspergillus or
Tr°iehophytort. Species of Tricltophytort include, for example, T.
rnentagrophytes, T.
r°ubr-um, T. schoenleinii, T. tonsur"arts, T. ver f°ucosum, and
T. violaceum. Species of
Aspergillus include, for example, A, funtigatus, A. flavus, A. niger, A.
arnstelodarrti,
A. cattdidus, A. car°rteus, A. nidulans, A oryzae, A. restrictus, A.
sydowi, A. terreus,
A. ustus, A. versicolor, A. caesiellus, A. clavatus, A. avenaceus, and A.
defl'eetus.
More preferably, the subject is concurrently treated therapeutically for a
fungal
infection caused by a species of the genus Asper gillus selected from A.
funtigatus, A.
flavus, A. niger, A. arrtstelodanti, A. cartdidus, A. carneus, A. nidularzs, A
oryzae, A.
CA 02534957 2006-02-06
WO 2005/025556 PCT/US2004/025937
-11-
z-estrictus, A. sydowi, A, terf~eus, A, ustus, A. versicoloz~, A. caesiellus,
A. clavatus, A.
avenaceus, and A. deflectus. Even more preferably the subject is concurrently
treated
therapeutically fox a fungal infection caused by Aspergillus fumigates or
Aspergillus
rziger, and most preferably, Aspet~gillus funzigatus.
An "effective amount" of a compound of the disclosed invention is the quantity
which, when administered to a subject in need of treatment, improves the
prognosis
of the subject, e.g., delays the onset of and/or reduces the severity of one
or more of
the subject's symptoms associated with a bacterial infection. The amount of
the
disclosed compound to be administered to a subject will depend on the
particular
disease, the mode of administration, co-administered compounds, if any, and
the
characteristics of the subject, such as general health, other diseases, age,
sex,
genotype, body weight and tolerance to drugs. The skilled artisan will be able
to
determine appropriate dosages depending on these and other factors. Effective
amounts of the disclosed compounds typically range between about 0.01 mg/kg
per
day and about 100 mg/kg per day, and preferably between 0.1 rng/kg per day and
about 10 mglkg/day. Techniques for administration of the disclosed compounds
of
the invention can be found in Refzziyzgtozz: the Scieizce azzd Practice of
Pha>"macy,
19th edition, Mack Publishing Co., Easton, PA (1995), the entire teachings of
which
are incorporated herein by reference.
A "pharmaceutically acceptable salt" of the disclosed compound is a product of
the disclosed compound that contains an ionic bond, and is typically produced
by
reacting the disclosed compound with either an acid or a base, suitable for
administering to a subject.
For example, an acid salt of a compound containing an amine or other basic
group can be obtained by reacting the compound with a suitable organic or
inorganic
acid, such as hydrogen chloride, hydrogen bromide, acetic acid, perchloric
acid and
the like. Compounds with a quaternary ammonium group also contain a
counteranion such as chloride, bromide, iodide, acetate, perchlorate and the
like.
Other examples of such salts include hydrochlorides, hydrobromides, sulfates,
methanesulfonates, nitrates, maleates, acetates, citrates, fumarates,
tartxates (e.g. (+)-
tartrates, (-)-tartrates or mixtures thereof including racemic mixtures),
succinates,
benzoates and salts with amino acids such as glutamic acid.
CA 02534957 2006-02-06
WO 2005/025556 PCT/US2004/025937
-12-
Salts of compounds containing a carboxylic acid or other acidic functional
group
can be prepared by reacting with a suitable base. Such a pharmaceutically
acceptable
salt may be made with a base which affords a pharmaceutically acceptable
cation,
which includes alkali metal salts (especially sodium and potassium), alkaline
earth
metal salts (especially calcium and magnesium), aluminum salts and ammonium
salts, as well as salts made from physiologically acceptable organic bases
such as
trimethylamine, triethylamine, morpholine, pyridine, piperidine, picoline,
dicyclohexylamine, N, N'-dibenzylethylenediamine, 2-hydroxyethylamine, bis-(2-
hydroxyethyl)amine, tri-(2-hydroxyethyl)amine, procaine, dibenzylpiperidine, N-
benzyl-~i-phenethylamine, dehydroabietylamine, N,N'-bisdehydroabietylamine,
glucamine, N-methylglucamine, collidine, quinine, quinoline, and basic amino
acid
such as lysine and arginine.
Certain compounds and their salts may also exist in the form of solvates, for
example hydrates, and the present invention includes each solvate arid
mixtures
thereof.
As used herein, a "pharmaceutical composition" is a formulation containing the
disclosed compounds in a form suitable for administration to a subject. The
pharmaceutical composition can be in bulk or in unit dosage form. The unit
dosage
form can be in any of a variety of forms, including, for example, a capsule,
an IV
bag, a tablet, a single pump on an aerosol inhaler, or a vial. The quantity of
active
ingredient (i.e., a formulation of the disclosed compound or salts thereof) in
a unit
dose of composition is an effective amount and may be varied according to the
particular treatment involved. It may be appreciated that it may be necessary
to
make routine variations to the dosage depending on the age and condition of
the
patient. The dosage will also depend on the route of administration. A variety
of
routes are contemplated, including topical, oral, pulmonary, rectal, vaginal,
parenternal, transdermal, subcutaneous, intravenous, intramuscular,
intraperitoneal
and intranasal.
The compounds described herein, and the pharmaceutically acceptable salts
thereof can be used in pharmaceutical preparations in combination with a
pharmaceutically acceptable Garner or diluent. Suitable pharmaceutically
acceptable
carriers include inert solid fillers or diluents and sterile aqueous or
organic solutions.
CA 02534957 2006-02-06
WO 2005/025556 PCT/US2004/025937
-13-
The compounds will be present in such pharmaceutical compositions in amounts
sufficient to provide the desired dosage amount in the range described herein.
Techniques for formulation and administration of the disclosed compounds of
the
invention can be found in Resrzihgtofa: the Science afad Practice of
Pham~aacy, above.
For oral administration, the disclosed compounds or salts thereof can be
combined with a suitable solid or liquid carrier or diluent to form capsules,
tablets,
pills, powders, syrups, solutions, suspensions and the like.
The tablets, pills, capsules, and the like contain from about 1 to about 99
weight
percent of the active ingredient and a binder such as gum tragacanth, acacias,
corn
starch or ,gelatin; excipients such as dicalcium phosphate; a disintegrating
agent such
as corn starch, potato starch or alginic acid; a lubricant such as magnesium
stearate;
and/or a sweetening agent such as sucrose, lactose or saccharin. When a dosage
unit
form is a capsule, it may contain, in addition to materials of the above type,
a liquid
carrier such as a fatty oil.
Various other materials may be present as coatings or to modify the physical
form of the dosage unit. For instance, tablets may be coated with shellac,
sugar or
both. A syrup or elixir may contain, in addition to the active ingredient,
sucrose as a
sweetening agent, methyl and propylparabens as preservatives, a dye and a
flavoring
such as cherry or orange flavor, and the like.
For parental administration of the disclosed compounds, or salts, solvates, or
hydrates thereof, can be combined with sterile aqueous or organic media to
form
injectable solutions or suspensions. For example, solutions in sesame or
peanut oil,
aqueous propylene glycol and the like can be used, as well as aqueous
solutions of
water-soluble pharmaceutically-acceptable salts of the compounds. Dispersions
can
also be prepared in glycerol, liquid polyethylene glycols and mixtures thereof
in oils.
Under ordinary conditions of storage and use, these preparations contain a
preservative to prevent the growth of microorganisms.
In addition to the formulations previously described, the compounds may also
be
formulated as a depot preparation. Suitable formulations of this type include
biocompatible and biodegradable polymeric hydrogel formulations using
crosslinked
or water insoluble polysaccharide formulations, polymerizable polyethylene
oxide
formulations, impregnated membranes, and the like. Such long acting
formulations
CA 02534957 2006-02-06
WO 2005/025556 PCT/US2004/025937
-14-
may be administered by implantation or transcutaneous delivery (for example
subcutaneously or intramuscularly), intramuscular injection or a transdermal
patch.
Preferably, they are implanted in, or applied to, the microenvirorunent of an
affected
organ or tissue, for example, a membrane impregnated with the disclosed
compound
can be applied to an open wound or burn injury. Thus, for example, the
compounds
may be formulated with suitable polymeric or hydrophobic materials, for
example,
as an emulsion in an acceptable oil, or ion exchange resins, or as sparingly
soluble
derivatives, for example, as a sparingly soluble salt.
For topical administration, suitable formulations may include biocompatible
oil,
wax, gel, powder, polymer, or other liquid or solid carriers. Such
formulations may
be administered by applying directly to affected tissues, for example, a
liquid
formulation to treat infection of conjunctival tissue can be administered
dropwise to
the subject's eye, a cream formulation can be administer to a wound site, or a
bandage may be;impregnated with a formulation, and the lilce.
For rectal administration, suitable pharmaceutical compositions are, for
example,
topical preparations, suppositories or enemas.
For vaginal administration, suitable pharmaceutical compositions are, for
example, topical preparations, pessaries, tampons, creams, gels, pastes, foams
or
sprays.
In addition, the compounds may also be formulated to deliver the active agent
by
pulmonary administration, e.g., administration of an aerosol formulation
containing
the active agent from, for example, a manual pump spray, nebulizer or
pressurized
metered-dose inhaler. Suitable formulations of this type can also include
other
agents, such as antistatic agents, to maintain the disclosed compounds as
effective
aerosols.
The term "pulmonary" as used herein refers to any part, tissue or organ whose
primary function is gas exchange with the external environment, i.e., Oz/C02
exchange, within a patient. "Pulinonary" typically refers to the tissues of
the
respiratory tract. Thus, the phrase "pulmonary administration" refers to
administering the formulations described herein to any part, tissue or organ
whose
primary function is gas exchange with the external environment (e.g., mouth,
nose,
pharynx, oropharynx, laryngopharynx, larynx, trachea, caring, bronchi,
bronchioles,
CA 02534957 2006-02-06
WO 2005/025556 PCT/US2004/025937
-15-
alveoli). For purposes of the present invention, "pulmonary" is also meant to
include
a tissue or cavity that is contingent to the respiratory tract, in particular,
the sinuses.
A drug delivery device for delivering aerosols comprises a suitable aerosol
canister with a metering valve containing a pharmaceutical aerosol formulation
as
described and an actuator lousing adapted to hold the canister and allow for
drug
delivery. The canister in the drug delivery device has a head space
representing
greater than about 15% of the total volume of the canister. Often, the polymer
intended for pulmonary administration is dissolved, suspended or emulsified in
a
mixture of a solvent, surfactant and propellant. The mixture is maintained
under
pressure in a canister that has been sealed~with a metering valve.
For nasal administration, either a solid or a liquid carrier can be used. The
solid
Garner includes a coarse powder having particle size in the range of, for
example,
from about 20 to about 500 microns and such formulation is administered by
rapid
inhalation through the nasal passages. Where the liquid Garner is used, the
formulation may be administered as a nasal spray or drops and may include oil
or
aqueous solutions of the active ingredients.
In addition to the formulations described above, a formulation can optionally
include, or be co-administered with one or more additional drugs, e.g., other
antibiotics, anti-inflammatories, antifungals, antivirals, immunomodulators,
antiprotozoals, steroids, decongestants, bronchodialators, and the like. For
example,
the disclosed compound can be co-administered with drugs such as such as
ibuprofen, prednisone (corticosteroid) pentoxifylline, Amphotericin B,
Fluconazole,
Ketoconazol, Itraconazol, penicillin, ampicillin, amoxicillin, and the like.
The
formulation may also contain preserving agents, solubilizing agents, chemical
buffers, surfactants, emulsifiers, colorants, odorants and sweeteners.
The term "aryl" group, (e.g., the aryl groups represented by Rl to R4) refers
to
carbocyclic aromatic groups such as phenyl, naphthyl, tetrahydronaphthyl,
anthracyl, and the like. The term. "heteroaryl" group (e.g., the
heteroaromatic groups
represented by Rl to R4) refers to heteroaromatic groups, for example
imidazolyl,
isoimidazolyl, thienyl, furanyl, pyridyl, pyrirnidyl, pyranyl, pyrazolyl,
pyrrolyl,
pyrazinyl, thiazolyl, isothiazolyl, oxazolyl, isooxazolyl, 1,2,3-triazolyl,
1,2,4-
triazolyl, tetrazolyl, benzo[1,3]dioxolyl, 2,3-dihydro-benzo[1,4]dioxine,
CA 02534957 2006-02-06
WO 2005/025556 PCT/US2004/025937
-16-
benzopyrimidyl, benzopyrazyl, benzofuranyl, indolyl, benzothienyl,
benzoxazolyl,
benzoisooxazolyl, benzothiazolyl, benzoisothiazolyl, quinolinyl,
isoquinolinyl,
benzimidazolyl, tetrahydroquinolinyl, and tetrahydroisoquinolinyl. Preferable
aryl
and heteroaryl groups include phenyl and pyridyl. The term "Ph" indicates a
phenyl
or a phenylene group, e.g., phenylene in -N:Rd(CO)PhNR'i(CO)R~', in Rl to R4.
The term "nonaromatic heterocycle" (e.g., the nonaromatic heterocyclic groups
represented by NR°Z or NR~2) refers to non-aromatic ring systems
typically having
three to eight members, preferably five to six, in which one or more ring
carbons,
preferably one to four, are each replaced by a heteroatom such as N, O, or S.
Examples of non-aromatic heterocyclic rings include 3-tetrahydrofuranyl, 2-
tetrahydropyranyl, 3-tetrahydropyranyl, 4-tetrahydropyranyl, [1,3]=dioxalanyl,
[1,3]-
dithiolanyl, [1,3]-dioxanyl, 2-tetrahydrothienyl, 3-tetrahydrothienyl, N-
morpholinyl,
2-morpholinyl, 3-morpholinyl, 4-morpholinyl, N thiomorpholinyl, 2-
thiomorpholinyl, 3-thiomorpholinyl, 4-thiomorpholinyl, 1-pyrrolidyl, 2-
pyrrolidyl,
3-pyrorolidyl, 1-piperazyl, 2-piperazyl, 1-piperidyl, 2-piperidyl, 3-
piperidyl, 4-
piperidyl, 4-thiazolidyl, diazolonyl, N-substituted diazolonyl, 1-pthalimidyl,
azetidyl, aziridyl, oxaziridyl, , oxazolidyl, isooxazolidyl, thiazolidyl,
isothiazolidyl,
oxazinanyl, thiazinanyl, azepanyl, oxazepanyl, and thiazepanyl. Typically, the
nonaromatic heterocycle groups represented by NR°z and NR~z are
selected from
optionally substituted pyrrolidyl, piperidyl, piperazyl, morpholinyl, and
thiomorpholinyl., or preferably, unsubstituted piperidyl or morpholinyl.
The disclosed compounds can contain one or more chiral centers. For example,
in
structural formula I, the carbons in common between Rings A and C, and the
carbon
in Ring C between the nitrogen and Ring A can each be a chiral center. The
presence
of chiral centers in a molecule gives rise to stereoisomers. For example, a
pair of
optical isomers, referred to as "enantiomers", exist for every chiral center
in a
molecule. A pair of diastereomers exist for every chiral center in a compound
having
two or more chiral centers. Where the structural formulas do not explicitly
depict the
stereochemistry of each chiral center, for example in structural formulas I-a
to I-c, I-
a' to I-c', I-a", I-m, and the compounds in Table l, it is to be understood
that these
formulas encompass enantiomers free from the corresponding optical isomer,
racemic
mixtures, mixtures enriched in one enantiomer relative to its corresponding
optical
CA 02534957 2006-02-06
WO 2005/025556 PCT/US2004/025937
-17-
isomer, a diastereomer free of other diastereomers, a pair of diastereomers
free from
other diasteromeric pairs, mixtures of diasteromers, mixtures of diasteromeric
pairs,
mixtures of diasteromers in which one diastereomer is enriched relative to the
other
diastereomer(s) and mixtures of diasteromeric paixs in which one
diastereomeric pair
is enriched relative to the other diastereorneric pair(s).
The term "alkyl" (e.g., the alkyl groups represented by Rl to R4, Ra to Rd,
and
R~), used alone or as part of a larger moiety (e.g., aralkyl, alkoxy,
alkylamino,
alkylaminocarbonyl, haloalkyl), is a straight or branched non-aromatic
hydrocarbon
which is completely saturated. Typically, a straight or branched allcyl group
has
from 1 to about 10 carbon atoms, preferably from 1 to about 5 if not otherwise
speciEed, Examples of suitable straight or branched alkyl group include
methyl,
ethyl, n-propyl, 2-propyl, r~-butyl, sec-butyl, tent-butyl, pentyl, hexyl,
heptyl or
octyl. A C1 to C10 straight or branched alkyl group or a C3 toC8 cyclic alkyl
group
can also be referred to as a "lower alkyl" group. An "allcoxy" group refers to
an alkyl
group that is connected through an intervening oxygen atom, e.g., methoxy,
ethoxy,
2-propyloxy, tef°t-butoxy, 2-butyloxy, 3-pentyloxy, and the like.
The terms "optionally halogenated alkyl", and "optionally halogenated alkoxy",
as used herein, includes the respective group substituted with one or more of -
F, -Cl,
-Br, or -I.
The terms "alkanoyl", "aroyl", and the like, as used herein, indicates the
respective group connected through an intervening carbonyl, for example,
-(CO)CHZCH3, benzoyl, and the like. 'The terms "alkanoyloxy", "aroyloxy", and
the
like, as used herein, indicates the respective group connected through an
intervening
carboxylate, for example, -O(CO)CHZGH3, -O(CO)C6Hs, and the like.
The term "cycloalkyl group" (e.g, the cycloalkyl groups represented by Ring A)
is a cyclic alkyl group having from 3 to about 10 carbon atoms, preferably
from 5 to
6. Examples of suitable cycloalkyl groups include cyclopropyl, cyclobutyl,
cyclopentyl, cyclohexyl, cycloheptyl and cyclooctyl. A "cycloalkoxy" group
refers
to a cycloalkyl group that is connected through an intervening oxygen atom,
e.g.,
cyclopentyloxy, cyclohexyloxy, and the like.
The term "cycloalkenyl" (e.g., the cycloalkyl groups represented by Ring A)
includes nonaromatic cycloalkyl groups that contain one or more units of
carbon-
CA 02534957 2006-02-06
WO 2005/025556 PCT/US2004/025937
-18-
carbon unsaturation, i.e., carbon-carbon double bonds. A cycloalkenyl group
includes, for example, cyclohexenyl or cyclopentenyl.
The terms "aralkyl", "heteroaralkyl", "cycloalkylalkyl", and "nonaromatic
heterocycloalltyl" refer to aryl, heteroaryl, cycloalkyl, and nonaromatic
heterocycle
groups, respectively, that are connected through an alkyl chain, e.g., benzyl,
-CHZHZ-pyridine, (3-cyclohexyl)propyl, and the like.
An "acyclic" group is a substituent that does not contain a ring. A
"monocyclic"
group contains only a single ring, 'for example, a phenyl ring that is not
fused to
another ring. A "polycyclic" group is a group that contains multiple fused
rings, for
example, naphthyl.
The term "derivative", e.g., in the term "cycloalkyltetrahydroquinoline
derivatives", refers to compounds that have a common core structure, and are
substituted with various groups as described herein. For example, all of the
compounds represented in Table 1 are cycloalkyltetrahydroquinoline
derivatives,
and have str~xctural formula I as a common core.
A line across a bond in a ring, for example, the line from H02C- in structural
formulas I-b and I-c, indicates that the represented bond can be connected to
any
substitutable atom in the ring.
A "substitutable atom" is any atom such as nitrogen or carbon that can be
substituted by replacing a hydrogen atom bound to the atom with a
substituent.A
"substitutable ring atom" in a ring, e.g., the substitutable ring carbons in
Rings A to
C, is any ring atom, e.g., a carbon or nitrogen, which can be substituted. For
example, when X2 is a carbon, it can be bound to -H or substituted, e.g., with
R2.
Suitable substituents are those that do not substantially interfere with the
pharmaceutical activity of the disclosed compound. A compound or group can
have
one or more substituents, which can be identical or different. Examples of
suitable
substituents for a substitutable carbon atom in an alkyl, cycloalkyl,
cycloalkenyl,
non-aromatic heterocycle, aryl, or heteroaryl group include -OH, halogen (-Br,
-Cl,
-I and -F), -R, -OR, -CHzR, - CHzCH2R, -OCH2R, -CH20R, -CH2CHZOR,
-CH20C(O)R, -O-COR, -COR, -SR, -SCHaR, - CHzSR, -SOR, -SOZR, -CN, -NOZ,
-COOH, -S03H, -NHS,, -NHR, -N(R)2, -COOR, -CHzCOOR, -CHZCHZCOOR,
-CHO, -CONH2, -CONHR, -CON(R)2, -NHCOR, -NRCOR, -NHCONHa,
CA 02534957 2006-02-06
WO 2005/025556 PCT/US2004/025937
-19-
-NHCONRH, -NHCON(R)2, -NRCONHa, -NRCONRH, -NRCON(R)z,
-C(=NH)-NH2, -C(=NH)-NHR, -C(--NH)-N(R)2, -C(--NR)-NHz, -C(=NR)-NHR,
-C(=NR)-N(R)2, -NH-C(=NH)-NH2, -NH-C(--NH) NIiR, -NH-C(=NH)-N(R)z,
-NH-C(=NR)-NH2, -NH-C(--NR)-NIiR, -NH-C(=NR)-N(R)z, -NRH-C(=NH)-NH2,
_NR_C(--NH)-NHR, _NR_C(=NI~_N(R)a, _NR_C(=NR)_NH2, _NR_C(--NR)-NHR,
-NR-C(--NR)-N(R)2, -SO~,NH2, -S02NHR, -S02NRa, -SH, -SOkR (k is 0, 1 or 2) and
-NH-C(=NH)-NH2. Each R is independently an alkyl, cycloalkyl, benzyl,
aromatic,
heteroaromatic, or phenylamine group that is optionally substituted.
Preferably, R is
unsubstituted. In addition, -N(R)2, taken together, can also form a
substituted or
unsubstituted heterocyclic group, (e.g., as for NR~2, and NR~2) such as
pyrrolidinyl,
piperidinyl, morpholinyl and thiomorpholinyl. Examples of substituents on
group
represented by R include amino, alkylamino, dialkylamino, aminocarbonyl,
halogen,
alkyl, alkylaminocarbonyl, dialkylaminocarbonyloxy, alkoxy, vitro, cyano,
carboxy,
alkoxycarbonyl, alleylcarbonyl, hydroxy, haloalkoxy, or haloalkyl.
Suitable substituents on the nitrogen of a heterocyclic group or
heteroaromatic
group include -R', -N(R')Z, -C(O)R', -C02 R~, -C(O)C(O)R', -C(O)CHZ C(O)R',
-SOZR', -SOZ N(R')2, -C(=S)N(R')2, -C(=NH)-N(R')Z, and -NR' S02R'. R' is
hydrogen, an alkyl, alkoxy, cycloalkyl, cycloalkoxy, phenyl, phenoxy, benzyl,
benzyloxy, heteroaromatic, or heterocyclic group that is optionally
substituted.
Examples of substituents on the groups represented by R' include amino,
alkylamino, dialkylamino, aminocarbonyl, halogen, alkyl, alkylaminocarbonyl,
dialkylaminocarbonyloxy, alkoxy, vitro, cyano, carboxy, alkoxycarbonyl,
alkylcarbonyl, hydroxy, haloalkoxy, or haloalkyl. Preferably, R' is
unsubstituted.
EXEMPLIFICATION
Example 1: Synthesis of MurA inhibitors of structural formula I-a
The disclosed compounds can be prepared by standard methods starting from
appropriate commercially available starting materials.
CA 02534957 2006-02-06
WO 2005/025556 PCT/US2004/025937
- 20 _
\ ~ \
+ OHCCOzMe +
NHz ,i N OMe
CO~H COZH O
Concentrated HCl (1.7 mL, 20 mmol) was added to a solution of 2-
aminobenzoic acid (2.74 g, 20 mmol) in 30 mL of methanol at 0°-
5° C. After
stirring for 15 min, glyoxylic acid methyl ester (2.8 M, 7.9 mL, 22 mmol) was.
The
mixture was stirred for 2 h at 0°-5° C, cyclopentadiene (1.6 mL,
20 mmol) was
added. After stirring an additional 2 h at 0°-5° C, the solid
product was collected by
filtration and purified by silica gel column chromatography ( petroleum ether-
ethyl
acetate, 2:1). The pure product was obtained as a white solid (2.6 g, 48%
yield).
See Ganem, B. 1989. Organizational Chemistry 2:127-128, the entire teachings
of
which are incorporated herein by reference.
Using the methods in the above example, compounds represented by structural
formula I, i.e., Compounds II to L~;XXIV and I-m (Table 1) were prepared by
starting from appropriate reagents. In Table 1, structures depicting unfilled
valences
on N or O, i.e., are understood to be bonded to -H.
Compounds that axe racemic, stereochemically enriched, or stereochemically
pure can be prepared by an appropriate combination of methods selected from
employing appropriate starting materials or reagents, crystallization, and
chromatographic purification. See, for example, Ahuja, S. "Chiral Separations
by
Chromatography", American Chemical Society, 2000; Ahuja, S. "Chiral
Separations: Applications and Technology", American Chemical Society, 1996,
and
references therein, the entire teachings of which are incorporated hexein by
reference.
Example 2: High Throughput Screen Identifies Likely lVIurA Inhibitors
A high throughput screen was employed on the compounds to identify the likely
MurA inhibitors depicted in Table 1 . The test conditions employed MurA and
MurB (UDP-N-acetylmuramate:NADP+ oxidoreductase, EC 1.1.1.158) coupled
enzymatic reactions carried in 96-well reaction plates.
CA 02534957 2006-02-06
WO 2005/025556 PCT/US2004/025937
-21 -
Using appropriate stock solutions, each well was prepared to contain a total
volume of about 100 ~,L, containing SOmM Tris-HCl
(Tris(hydroxymethyl)aminomethane-HC1, pH 8.0), 20mM KCI, 0.02% Brij~ 30
(Polyethylene glycol dodecyl ether), O.SmM DTT (dithiothreitol}, 0,1mM UDPAG
(Uridine S'-diphospho-N-acetylglucosamine), 0,1mM phosphoenolpyruvate {PEP),
O.lmM NADPH {nicotinamide adenine dinucleotide phosphate), I20ng MurA, and
40ng MurB. The preceding chemical reagents were obtained from Sigma, St. Louis
MO; the enzymes were produced in house.
The wells were prepared without substrate (PEP and UDPAG) incubated for a
half hour, combined with the substrate and each test compound, and the
evidence of
reaction was read after 1 hour of reaction time using a fluorescence
spectrometer at
355/460 nM for 0.1 second. Compounds that were associated with an increase in
fluorescence over control solutions were identified as likely MurA inhibitors.
CA 02534957 2006-02-06
WO 2005/025556 PCT/US2004/025937
-22-
Table 1: MurA Inhibitors of Structural Formula I
0
O ~ / N H ~ / ~ '0
I ~- "tJ N
O O
II V DC
/i
\ F O
\ \ \.
N ~ N 0 N
r
O ~0 O 0
III VI X
O O
F1
\o I \ \
N
N~O\H / N O \ r
I~I / /\ /O
~N
IV VII XI
F
O / N O \ ~ ~ ~ \ ~ N- o
w i w
N
O O
I-m VIII XII
CA 02534957 2006-02-06
WO 2005/025556 PCT/US2004/025937
- 23 -
Table 1(cont): MurA Inhibitors of Structural Formula I
~N \ \
I
N~OWH N \ 0 ( / N
O 0
0
F F p ~ \ ~N
I ~ °~ F~ / \ s ~"~
'N'
O
N
I\
I \/ ~ I ° ~ .\
- ~ N
N N
O' 'O
0 O
XV
O
I ~ \ I / N O ~H I \
N
0
O
CA 02534957 2006-02-06
WO 2005/025556 PCT/US2004/025937
-24-
Table 1(cont): MurA Inhibitors of Structural Formula I
~N \
\ I N~o o I / N o o I o ~N
O
/ / \
N:%~ / N~O\H N .
H
~O 0
O O
O O
XXVI XXXI XXXVI II
\ F \
0 / \ ~ ~ N O~H
,N, ~ o
XXVI I XXXV XLVI I
I
' \ \ N~ "~o \
N ~~ o / / W
~N' ~ H
XXVI I I XXXVI XLIX
CA 02534957 2006-02-06
WO 2005/025556 PCT/US2004/025937
-25-
Table 1 (coot): MurA Inhibitors of Structural Formula I
0
0 0
\ \
N / I / N ~ I ~ N- N
d
LII LX LXXVt
N~o ° I i
LVII LXI
0
0 0
w \
I
N
0
LVIII ' LXII
~N ° ~ ~ "~
'N' ~ ~ N
LIX LXXV
CA 02534957 2006-02-06
WO 2005/025556 PCT/US2004/025937
-26-
Example 3: Kinetic Assay of Disclosed Inhibitors Shows Potent MurA Inhibition
A series of IC50 Inhibition Concentration at 50 percent) assays were
performed in 96-well assay plates. About 60 ~.L of a buffer A1 was added into
each
well from column 1 to column 12. An additional 20 p,L of buffer A1 was added
into
column 12. Buffer A1 was prepared to contain 50 mM HEPES pH 7.5(4-(2-
hydroxyethyl)piperazine-1-ethanesulfonic acid), 20 mM KCl, 0.02% wt Brij 30,
0.001 mM UDPAG, 0.001 mM PEP, and 0.5 mM DTT.
Approximately 2 ~L of compound solution was transferred by serial dilution
from column 2 to column 1 l, resulting in a range of final compound
concentrations
from about 25 to about 0.049 ~g/mL.
Approximately 20 ~L of enzyme solution A2 was added into each well of
column 1 through 11. Buffer A2 was prepared to contain 50 mM HEPES pH 7.5, 20
mM KCI, 0.02% wt Brij 30, 0.001 mM UDPAG, 0.001 mM PEP, 0.5 mM DTT, and
6 ~,g/mL MurA.
The plated solutions were incubated for half hour, after which approximately
~.L of substrate solution B was added to each well, column 1 through 11, to
initiate the reaction. Buffer B is prepared as 2 mM UDPAG, 0.4 mM PEP, 50 mM
HEPES pH 7.5, 20 mM KCI, 0.02% wt Brij 30 and 0.5 mM DTT.
20 After reacting for 8 minutes, 150 ~,L of Malachite Green was added, the
resulting combination incubated for 15 minutes at ambient temperature, and the
reaction result was determined by measuring absorbance at 650 nrn with a
spectrometer.
The data were fit to a curve using ~lftt (ID Business Solutions, Cambridge,
MA)). The ICSO value was derived from the curve as the compound concentration
that gave 50% inhibition of the enzymatic reaction. The results are depicted
in Table
2.
CA 02534957 2006-02-06
WO 2005/025556 PCT/US2004/025937
Table 2: IC50 Inhibition Assay Reveals Potent MurA Inhibitors
# MURa IC50 # MIJRa IC50
II < 5 XXIV __>5, < 33
TII < 5 XXV >_5, < 33
IV < 5 XXVI >_5, < 33
I-m < 5 X~~VII >_S, < 33
V < 5 XXVIII >_5, < 33
VI < 5 XXX >_33
VII < 5 XXXI ~3
VIII < 5 ~;XXV >_33
IX < 5 XXXVI ?33
X < 5 ~XVII >33
XI < S XXXVIII ~3
XII < 5 XLVII >_33
XIII < 5 XLIX >_33
XIV < 5 LIT ?33
XV < 5 LVII ~3
< 5 LVIII ?33
XVII < 5 LIX ~3
XVIII < 5 LX ~ 3
XLX < 5 LXI ~3
< 5 LXII ?33
XXI < 5 LXXV >_33
XXII >_5, < 33 L~~XVI >33
XXIII >_5, < 33
CA 02534957 2006-02-06
WO 2005/025556 PCT/US2004/025937
-28-
While this invention has been particularly shown and described with references
to preferred embodiments thereof, it will be understood by those skilled in
the art
that various changes in form and details may be made therein without departing
from the scope of the invention encompassed by the appended claims.